Abstract
Tumor necrosis factor (TNF) inhibitors are used for treatment of different autoimmune diseases. Interestingly they are also being noted to cause autoimmune side effects such as vasculitis, systemic lupus erythematosus, interstitial lung disease, etc. We describe one such case of granuloma annulare being treated with Adalimumab, who then developed pulmonary-renal syndrome form anti-neutrophilic cytoplasmic antibody (ANCA)-induced vasculitis. This was treated with steroids and immunosuppression, as well as discontinuation of the TNF inhibitor. However, he remains dependant on dialysis and immunosuppression. In this article, we review the existing literature on clinical presentation and course of TNF inhibitors-induced ANCA vasculitis. We also discuss the underlying mechanisms thought to be responsible for this.
Keywords: tnf inhibitor, anca vasculitis, adverse drug reactions
Introduction
Tumor necrosis factor (TNF) inhibitors are utilized for the treatment of a variety of autoimmune diseases, but have also been associated with the paradoxical emergence of autoimmune phenomena, including cutaneous vasculitis. There have also been several reported cases of systemic vasculitis following treatment with Adalimumab without mention of specific patient details or circumstances [1]. Anti-neutrophilic cytoplasmic antibody (ANCA) vasculitis has been rarely described.
Case presentation
We present the case of a 57-year-old male with past medical history significant for coronary artery disease, hypertension and granuloma annulare (GA) who was admitted with rapid decline in renal function and shortness of breath. GA was being treated with adalimumab for the last two years. Eight months prior to admission, he was noted to have an asymptomatic elevation in his blood urea nitrogen and creatinine (Table 1) which worsened five months later. Renal ultrasound was performed which showed bilateral echogenic kidneys. He was lost to follow up and represented to his primary care provider three months later with a one-week history of epistaxis, hemoptysis, anorexia and weight loss. He was asked to report to the emergency room.
Table 1. Laboratory data on admission.
ANA: Anti-Nuclear Antibody; p-ANCA: perinuclear Anti-Neutrophilic Cytoplasmic Antibody; AI: Antibody Index; c-ANCA: cytoplasmic Anti-Neutrophilic Cytoplasmic Antibody.
| Laboratory Test | Results | ||
| During hospitalization | Three months prior | Eight months prior | |
| Blood urea nitrogen (7-18 mg/dl) | 136 | 51 | 32 |
| Creatinine (0.55-1.3 mg/dl) | 15.89 | 3.4 | 2.6 |
| Hemoglobin (13.5-18 gm/dl) | 6.1 | ||
| ANA Titre (Negative) | 1:80 (homogenous) | ||
| p-ANCA Titre (<1:20) | 1:40 | ||
| Proteinase 3 antibody (<1 AI) | <1 | ||
| Myeloperoxidase antibody (<1AI) | 5 | ||
| c-ANCA (Negative) | Negative | ||
| C3 complement (82-185 mg/dl) | 130 | ||
| C4 complement (15-35 mg/dl) | 34 | ||
| Hepatitis B surface antigen (Negative) | Negative | ||
| Hepatitis C virus antibody (Negative) | Negative |
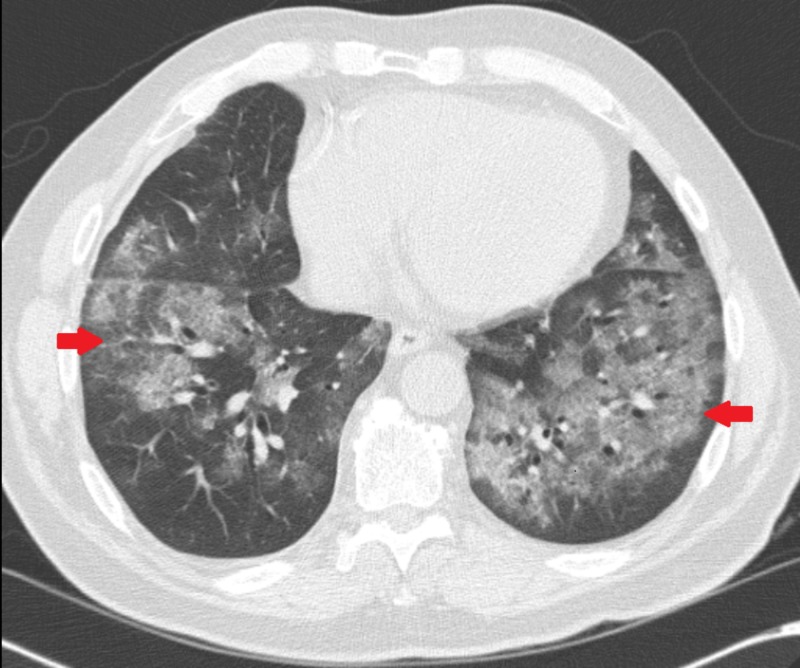
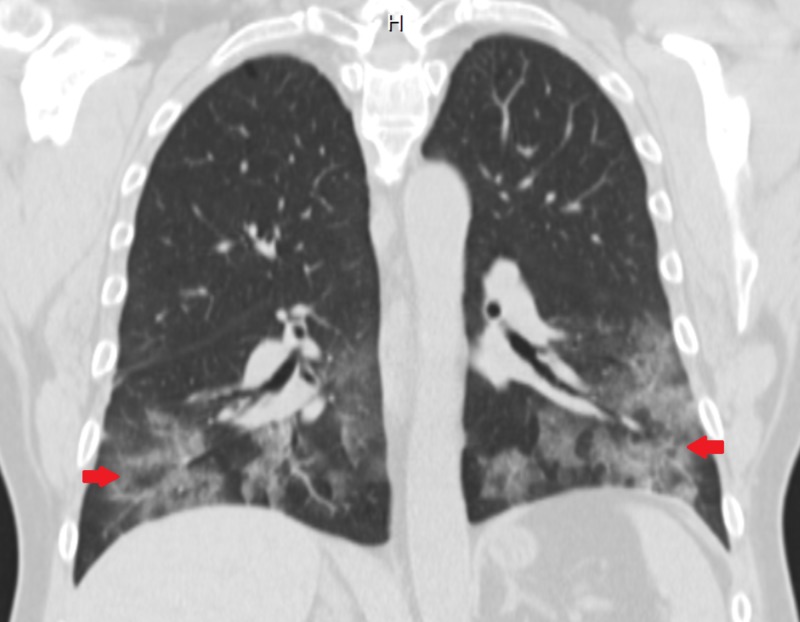
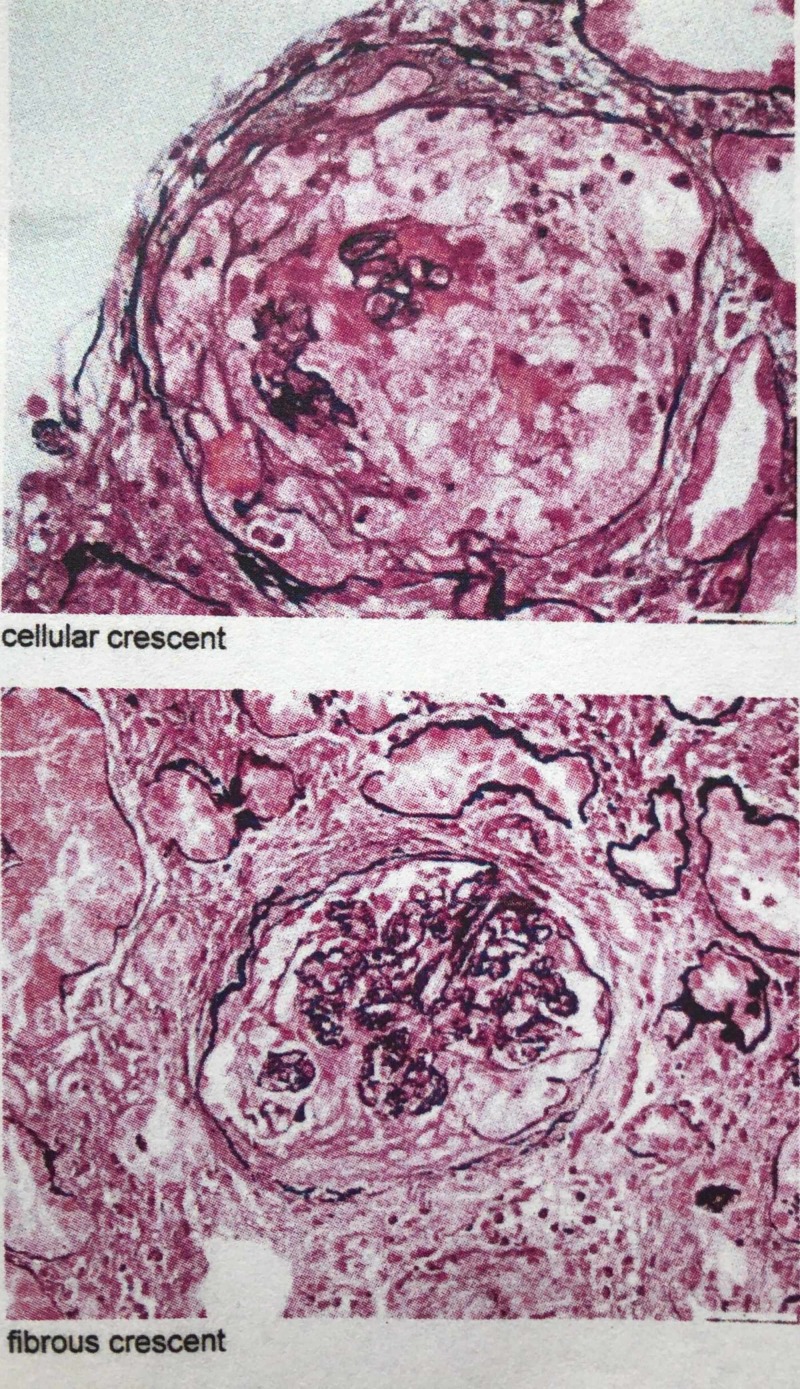
CT chest showed bilateral pulmonary consolidation and ground glass opacities (Figures 1, 2). Renal biopsy performed revealed pauci-immune, rapidly progressive glomerulonephritis with some fibrosis (Figure 3). ANCA with perinuclear staining and myeloperoxidase antibody were positive. He was started on hemodialysis immediately. He also received intravenous methylprednisolone 500 mg daily for three days followed by oral prednisone 60 mg daily, oral cyclophosphamide 125 mg daily (which was eventually transitioned to intravenous monthly pulses of cyclophosphamide) and trimethoprim-sulfamethoxazole for pneumocystis prophylaxis. Adalimumab was discontinued.
Figure 1. Axial CT chest image showing bilateral diffuse opacities (arrows).
Figure 2. Coronal CT chest image showing bilateral basilar opacities (arrows).
Figure 3. Renal biopsy images showing crescentic glomerulonephritis.
Two months after his hospitalization, pulmonary infiltrates have resolved, but there has been no recovery of renal function.
Discussion
To our knowledge, there have only been nine previously reported cases of vasculitis and positive ANCA that were thought to be induced by TNF inhibitors [2-9]. See Table 2 for clinical presentation and treatment of each patient. One patient had atypical ANCA and lupus nephritis (Patient 8) and one patient had aortitis (Patient 9) which are not consistent with true ANCA-associated vasculitis. Of the remaining seven patients, four patients were females and six were being treated for rheumatoid arthritis. The mean age for patients was 51.4 years. Time of onset of symptoms after starting a TNF inhibitor varied from three months to four years. Four of these patients had positive c-ANCA, three had a positive p-ANCA. Six of seven patients had renal biopsies showing pauci-immune glomerulonephritis. Six patients were treated with intravenous methylprednisolone followed by oral prednisone. The TNF inhibitor was discontinued in all cases except patient 7. The most commonly used immunosuppressant was cyclophosphamide in six of seven patients. Four patients had persistent renal dysfunction and one patient died within nine months of presentation. Given the temporal sequence of events, a causal relationship might be present. One proposed mechanism is that anti-TNF drugs form immune complexes, activate complement and promote switching from a T-helper type 1 response (mediated by interleukin (IL)-1, TNF and interferon (IFN)-Y) to a T-helper type 2 response (IL-4, IL-5, IL-6, IL-10 and IL-13) leading to the production of autoantibodies [10].
Table 2. Vasculitis with positive ANCA induced by TNF- inhibitors.
ANCA: Anti-Neutrophilic Cytoplasmic Antibody; TNF-i: Tumor Necrosis Factor inhibitor; CD: Crohn’s Disease; GN: Glomerulonephritis; Hb: Hemoglobin; CRP: C-Reactive Protein; RBC: Red Blood Cell; PR-3: Proteinase-3; IV: Intravenous; MP: Methylprednisolone; RA: Rheumatoid Arthritis; UPC: Urine Protein Creatinine; ANA: Anti-Nuclear Antibody; dsDNA: double stranded Deoxyribonucleic Acid; anti-GBM: anti-Glomerular Basement Membrane; HCQ: Hydroxychloroquine; MTX: Methotrexate; HD: Hemodialysis; ESR: Erythrocyte Sedimentation Rate; RF: Rheumatoid factor; MPO: Myeloperoxidase; SS: Sjogren’s Syndrome; CrCl: Creatinine Clearance; PO: Per Oral; TMP: Trimethoprim; AZA: Azathioprine; RTX: Rituximab; Cr: Creatinine.
| Patient No. | Age/Sex | TNF-i | Indication for TNF-i | Time of onset after starting TNF-i (months) | Clinical presentation | Labs | ANCA type | Other serologies | Pathology | Previous/Concomitant drugs | Treatment | Outcome |
| 54/M | Adalimumab | CD | 30 | Fever, asthenia, lower extremity edema, inflammatory arthritis, polyneuropathy and optic neuritis, anemia, GN | Hb: 9 gm/dl, CRP: 7.9 mg/dl, Urine studies: 1.2 gm protein/day >50 RBCs/hpf Granular casts | C-ANCA PR3 | - | Pauci-immune extracapillary GN (Kidney) | - | IV MP, IV CYC | Persistent renal dysfunction C-ANCA negative | |
| 62/F | Adalimumab | RA | 48 | Malaise, weight loss nasal stuffiness, visual blurring, rash, GN | Urine studies: 3+ blood 3+ protein UPC 5.9 g | C-ANCA PR3 | (+) ANA1:640 (-) dsDNA (-) anti-GBM (-) anti- Cardiolipin, Normal complements | Pauci-immune mild segmental sclerosis with no tubuloreticular lesions (Kidney) | HCQ, Sulfasalazine, MTX | IVMP, Plasma exchange, 1 HD PO prednisone, CYC | Improved UPC Persistent renal dysfunction Improved C-ANCA | |
| 67/F | Etanercept | RA | 3 | Painful, erythematous ulcerated nodules, nasal congestion, peripheral neuropathy, polyarthritis, scleritis, GN pulmonary parenchymal nodules, chronic sinusitis on CT | Hb 13 gm/dl, ESR 111 mm/hr, CRP 15.3 mg/dl, Urine Studies: Hematuria | C-ANCA | (+) RF (45 IU/ml) (+) ANA 1:320 homogenous | Leukocytoclastic (Skin) | MTX, Prednisolone | IVMP pulses, CYC 750/month Steroid taper | Good clinical response | |
| 33/F | Infliximab | RA | 16 | Synovitis anemia GN | Hb 8.8 gm/dl, Cr 0.6 mg/dl (CrCl 82.5 ml/min), ESR 56 mm/hr, CRP 2.5 mg/dl, Urine Studies: 3+ protein 3+ occult blood, 24 hr urine protein: 1.2 gm/day | MPO PR3 | (-) Anti-DNA (-) Anti-GBM Normal IgG, IgA, IgM Normal complement | IgM deposition (weak intensity) IgG, IgA, C3, C1q and kappa and Lambda chains (-)- Necrotizing GN (Kidney) | MTX, Sulfasalazine, Bucillamine, Cyclosporine | IVMP, PO prednisone | Good clinical response | |
| 31/M | Infliximab | RA | 8 | Synovitis rash GN | Cr 3.4 mg/dl (CrCl 54 ml/min), CRP 9.1 mg/dl, Urine Studies: 3+ blood 24 hr Urine protein 1.5 gm | C-ANCA PR3 | (+) ANA 1:320 (homogenous) (-) dsDNA (+) RF (-) HepB and C serology (-) Cryoglobulin Normal complement | Pauci-immune crescentic GN (Kidney), Non diagnostic (Skin) | MTX, Cyclosporine Sulfasalazine, HCQ leflunomide | TMP, 1 gm IVMP for 3 days, Oral CYC 2 mg/kg daily. AZA | Good clinical response Decreased PR3 | |
| 58/F | Adalimumab | RA | 48 | Asymptomatic rapidly progressive GN Alveolar hemorrhage with pulmonary biopsy showing pauci-immune vasculitis anemia | Hb 6.2 gm/dl, CRP < 10 mg/dl, Urine Studies: RBCs+, 2.47 gm spot urine protein | P-ANCA MPO | (-) GBM (-) dsDNA (+) RF (+) ANA 1:640 homogeneous (+) SS-A & SS-B | Pauci-immune necrotizing GN-extracapillary necrotizing GN (Kidney) | D-penicillamine, Gold, MTX, steroids | IVMP, PO prednisone, Plasma exchanges-7 over 2 weeks, IV CYC six courses HD, AZA | Persistent renal dysfunction | |
| 55/M | Etanercept | RA | 4 | Alopecia maculopapular rash lower extremity sensory neuropathy GN | Cr. 3 mg/dl, Urine Studies: 1+ protein >5 RBCs/hpf 5 WBCs/hpf no casts 24 hr urinary protein - 1 gm/day | P-ANCA | (+) ANA 1:320 (-) anti-dsDNA Normal C3 and C4 | Pauci-immune focal, segmental, necrotizing and crescentic GN (Kidney) | MTX | IV CYC | Died | |
| 52/F | Adalimumab | RA | 3 | Gross hematuria and acute renal failure, GN | Urine Studies: 3+ protein >20 RBC/hpf granular casts 3.8 gm proteinuria | Atypical ANCA | (+) RF (+) ANA 1:640 homogenous, (+) dsDNA 1:25 IgG (-) cryoglobulin Decreased C3 and C4 | Focal proliferative lupus nephritis (class 3) (Kidney) | Prednisone, MMF, Infliximab, MTX, HCQ Penicillamine, Gold | Pulse IVMP PO steroids for 1 month | Persistent renal dysfunction | |
| 65/F | Etanercept | AS | 36 | Worsening cervical pain Severe and extensive aortitis on CTA chest and abdomen | Hb 9 mg/dl, ESR > 100 mm/hr, CRP 23.9 mg/dl | C-ANCA MPO | Borderline ANA (-) RF Normal complements | IVMP 1 g for 3 days, RTX, Prednisone | Good clinical response |
Conclusions
TNF-induced ANCA vasculitis is exceedingly rare. These cases in conjunction with ours suggest a possible relationship between anti-TNF use and induction of ANCA vasculitis. To the best of our knowledge, our case is the 4th to describe TNF-induced pulmonary renal syndrome as a manifestation of ANCA-vasculitis. It is difficult to conclusively prove that it is not just a spontaneous emergence of ANCA in a patient predisposed to autoimmunity. However, the biological plausibility of shifting towards a T-helper 2 type response in a susceptible individual leading to the emergence of these antibodies among others, remains. Additionally, this subset of patients appears to have a predilection for rapidly progressive kidney injury with long-term impairment despite discontinuation of the anti-TNF agent. This highlights the need for further studies looking into recognizing risk factors for the development of this rare but significant complication.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Ramos-Casals M, Brito-Zeron P, Soto MJ, Cuadrado MJ, Khamashta MA. Medicine. 2007;86:242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 2.Antineutrophil cytoplasmic antibody associated vasculitis in a patient treated with adalimumab for a rheumatoid arthritis. Fournier A, Nony A, Rifard K. Nephrol Ther. 2009;5:652–657. doi: 10.1016/j.nephro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Antineutrophil cytoplasmic antibodies associated vasculitis in patient with Crohn's disease treated with adalimumab. Martin Varas C, Heras M, Saiz A, et al. Nefrologia. 2017;37:560–561. doi: 10.1016/j.nefro.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 4.ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Simms R, Kipgen D, Dahill S, Marshall D, Rodger RS. Am J Kidney Dis. 2008;51:11–14. doi: 10.1053/j.ajkd.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Stokes MB, Foster K, Markowitz GS, et al. Nephrol Dial Transplant. 2005;20:1400–1406. doi: 10.1093/ndt/gfh832. [DOI] [PubMed] [Google Scholar]
- 6.Development of myeloperoxidase-antineutrophil cytoplasmic antibody-associated renal vasculitis in a patient receiving treatment with anti-tumor necrosis factor-alpha. Hirohama D, Hoshino J, Hasegawa E, et al. Mod Rheumatol. 2010;20:602–605. doi: 10.1007/s10165-010-0339-x. [DOI] [PubMed] [Google Scholar]
- 7.Developing of granulomatosis with polyangiitis during etanercept therapy. Ortiz-Sierra MC, Echeverri AF, Tobon GJ, Canas CA. Case Rep Rheumatol. 2014;2014:3. doi: 10.1155/2014/210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.C-ANCA positive systemic vasculitis in a patient with rheumatoid arthritis treated with infliximab. Ashok D, Dubey S, Tomlinson I. Clin Rheumatol. 2008;27:261–264. doi: 10.1007/s10067-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 9.Etanercept treatment-related c-ANCA-associated large vessel vasculitis. Ginsberg S, Rosner I, Slobodin G, et al. Clin Rheumatol. 2016;35:271–273. doi: 10.1007/s10067-015-3134-4. [DOI] [PubMed] [Google Scholar]
- 10.Tumor necrosis factor-alpha blockade and the risk of vasculitis. Guillevin L, Mouthon L. https://www.ncbi.nlm.nih.gov/pubmed/15468348. J Rheumatol. 2004;31:1885–1887. [PubMed] [Google Scholar]