Abstract
We recently developed a novel amine-reactive mass-defect-based chemical tag, dimethyl pyrimidinyl ornithine (DiPyrO), for quantitative proteomic analysis at the MS1 level. In this work, we further extend the application of the DiPyrO tag, which provides amine group reactivity, optical detection capability, and improved electrospray sensitivity, to quantify N-linked glycans enzymatically released from glycoproteins in the glycosylamine form. Duplex DiPyrO tags that differ in mass by 45.3 mDa were used to label the glycosylamine moieties of freshly released N-glycosylamines from glycoprotein standards and human serum proteins. We demonstrate that both MALDI-LTQ-Orbitrap and nano-HILIC LC/MS/MS Fusion Lumos Orbitrap platforms are capable of resolving the singly or multiply charged N-glycans labeled with mass-defect DiPyrO tags. Dynamic range of quantification, based on MS1 peak intensities, was evaluated across 2 orders of magnitude. With optimized N-glycan release conditions, glycosylamine labeling conditions, and MS acquisition parameters, the N-glycan profiles and abundances in human serum proteins of cancer patients before and after chemotherapy were compared. Moreover, this study also opens a door for using well-developed amine-reactive tags for relative quantification of glycans, which could be widely applied.
Graphical Abstract

Glycosylation is one of the most important post-translational modifications of proteins.1 Glycan moieties attached to glycoproteins play essential roles in many biological processes, such as cell–cell signaling and protein trafficking.1–4 Changes in glycan profiles are associated with a variety of diseases, including cancer, cardiovascular diseases, and neuro-degenerative diseases.5–9 However, high-throughput quantitative analysis of glycans remains challenging due to the high complexity of glycan structures, the lack of glycan standards, and the low ionization efficiency of native glycans by conventional mass spectrometry (MS) analysis. Therefore, it is urgent for researchers to develop innovative bioanalytical platforms for qualitative and quantitative glycomics analysis and to elucidate the roles of glycans in human diseases.
In order to improve the throughput and ionization efficiency for quantitative glycomics, several molecules have been used as chemical tags to label the reducing end of glycans using reductive amination. Some of the most commonly used tags include 2-aminobenzamide (2-AB), 2-aminobenzoic acid (2-AA), and 2-aminopyridine (PA).10 These tags are widely used in high-performance liquid chromatography (HPLC) with fluorescence detection or MS analysis for quantification, but they lack multiplexing capability for relative quantification. Several multiplexed isotopic (MS1-based)11–15 or isobaric (MS2-based)16–20 tags have been developed to improve throughput for quantification. However, multiplex isotopic tags increase spectral complexity, while isobaric tags can suffer from precursor isolation interference or sometimes poor fragmentation efficiency of reporter ions. Performing an additional MS3 scan could circumvent the deficiency in fragmenting reporter ions and improve quantitative performance of isobaric tags.16
Mass-defect-based quantification is a recently developed concept that allows multiplexed quantification at the MS1 level without the increase in spectral complexity that is inherent to mass-difference-based quantification and without the precursor isolation interference that is inherent to reporter-ion-based quantification at the MS2 level.21 This approach relies on isotopic mass defects, which refer to small mDa mass differences between elemental isotopes induced by differences in nuclear binding energy.21,22 Analytes are labeled with amino acids or chemical tags having the same nominal mass but differing isotopic compositions that lead to distinct masses differing by mere mDa. These small mass differences can be distinguished in MS1 scans at high resolution to permit relative quantification. Because all labeled ions are selected for fragmentation within the same MS2 isolation window, redundant sampling is avoided, and multiplexing is achieved without negatively impacting the duty cycle of the instrument. Moreover, since quantification is performed at the MS1 level, precursor coisolation, which affects the accuracy of reporterion-based quantification, is not a concern.23 This approach has been previously incorporated with permethylation using 13CH3I and 12CH2DI to quantify both N- and O-linked glycans in the Quantification by Isobaric Labeling (QUIBL) approach.24,25 However, the varying number of modification sites for different types of glycans leads to inconsistent mass shifts after derivatization and complicates data processing and data analysis. Moreover, the toxicity of the methyl iodide reagent used during derivatization necessitates extra attention during handling.
Dimethyl pyrimidinyl ornithine (DiPyrO) tags were conceived in our lab as a viable chemical tag for mass-defect-based multiplex quantitative analysis of amine-containing biomolecules.23 The DiPyrO labeling reagent consists of an amine-reactive triazine ester group and a dimethyl pyrimidinyl ornithine mass-defect tag that incorporates six stable isotopes in differing configurations to impart a 45.3 mDa mass difference between the light and heavy variants. We have shown previously that tryptic peptides labeled with duplex DiPyrO tags are resolvable and quantifiable at an Orbitrap resolving power of 120 K (at 400 m/z).23 While DiPyrO was first demonstrated for proteomics analysis, the triazine ester is also reactive toward glycosylamine moieties of N-glycans released from glycoproteins (Scheme 1), permitting the use of DiPyrO tag for relative quantification of glycans. N-glycans are released as glycosylamines in a slightly alkaline buffer, which allows amine-targeting reagents to be applied for glycan analysis, such as the RapiFluor labeling reagent.26 Due to differences in steric hindrance effects between N-glycans and peptides, labeling rates are likely to differ, requiring optimization of DiPyrO labeling conditions for glycosylamines. In contrast to the QUIBL approach, DiPyrO labeling derivatizes N-glycans with a single tag, resulting in a consistent nominal mass shift and a consistent mass difference between light- and heavy-labeled samples.
Scheme 1. DiPyrO Labeling of Glycosylaminea.
aRed dots represent heavy isotopic atoms (15N418O) in the light DiPyrO tag; blue dots represent heavy isotopic atoms (2H6) in the heavy DiPyrO tag.
Alteration of N-linked glycans, including increased branching, sialylation, and fucosylation, have been frequently reported during cancer cell invasion and metastasis.27,28 As a proof-of-principle study, N-glycan profiles and abundances of blood samples collected prior to and after chemotherapy from cancer patients diagnosed with acute lymphoblastic leukemia (ALL) were compared using this labeling approach. The relative abundances of most detected N-glycan species were found to be decreased one month after chemotherapy. We envision that this mass-defect-based labeling approach could be widely applied to facilitate glycan quantification in diverse biological and clinical specimens.
EXPERIMENTAL SECTION
Materials and Reagents.
Methanol (MeOH), acetonitrile (ACN), water, acetic acid (AA), and formic acid (FA) were purchased from Fisher Scientific (Pittsburgh, PA). Triethylammonium bicarbonate buffer (TEAB, 1.0 M), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Tris (2-carboxyethyl) phosphine hydrochloride (TCEP), dithiothreitol (DTT), iodoacetamide (IAA), N,N-dimethylformamide (DMF), 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate (DMTMM), and N-methylmorpholine (NMM) were purchased from Sigma-Aldrich (St. Louis, MO). PNGase F was purchased from Promega (Madison, WI). Bovine thyroglobulin (BTG), bovine lactoferrin (BLF), ovalbumin (OVA), RNaseB, and human serum protein mixture (HSP) were provided by Thermo Fisher Scientific (Rockford, IL). Oasis HLB 3 cm3 (60 mg) extraction cartridges were purchased from Waters Corporation (Milford, MA). Microcon-30 kDa centrifugal filters (30K MWCO) were purchased from Merck Millipore Ltd. (Darmstadt, Germany). PolyGLYCOPLEX A beads (3 μm) were purchased from PolyLC Inc. (Columbia, MD). Fused silica capillary tubing (inner diameter = 75 μm, outer diameter = 375 μm) was purchased from Polymicro Technologies (Phoenix, AZ). All reagents were used without additional purification.
Human Serum Protein Preparation.
Permission to perform this study was provided by the Health Sciences IRB of the University of Wisconsin–Madison (2015–0864). Serum samples from three pediatric patients diagnosed with B-cell acute lymphoblastic leukemia (B-cell ALL) were collected before induction chemotherapy and at 5 weeks, on the first day of consolidation chemotherapy. Treatment was conducted according to protocols AALL1131 or AALL0932. Serum samples were stored in −80 °C until analysis. Protein concentration of serum samples was measured by microBCA assay.
N-Glycan Release by Filter-Aided N-Glycan Separation (FANGS).
N-glycans of glycoprotein standards and human serum protein mixtures were released with intact glycosylamine moieties using a simplified FANGS protocol12 with slight modifications. Briefly, glycoproteins were dissolved in 50 mM TEAB buffer to 2 μg/μL. Human serum protein samples were diluted with 50 mM TEAB buffer to 1 μg/μL, mixed with 4 μL of 0.5 M TCEP, and heat-denatured by alternating sample tubes between 100 °C and room temperature water baths for four cycles of 15 s each. The mixture was then added to a 30K MWCO filter and buffer exchanged with 200 μL of 0.5 M TEAB buffer for three cycles (centrifuged at 14 000g for 20 min).
The prepared protein samples were incubated with PNGase F (4 μL of PNGase F in 96 μL of 0.5 M TEAB per filter) and incubated at 37 °C overnight. The pH value of 7–8 was carefully controlled by using 0.5 M TEAB buffer during digestion in order to preserve the amino group for the subsequent labeling reaction. The released glycosylamines were separated from the deglycosylated protein by centrifuging at 14 000g for 20 min. The filter was washed with 100 μL of 0.5 M TEAB buffer. Both fractions were combined and dried in vacuo.
N-Glycan DiPyrO Labeling and Cleaning-Up.
DiPyrO tags were synthesized in-house, stored in the inactive carboxylic acid form, and activated to the amine-reactive triazine ester form immediately prior to labeling. Unless otherwise stated, activation was performed by combining DiPyrO in anhydrous DMF with DMTMM and NMM and vortexing at room temperature for 30 min to yield the active triazine ester. DiPyrO labeling reactions were optimized as specified in the Results and Discussion section. Labeling was performed by combining activated DiPyrO with released N-glycosylamine samples at a mass ratio of 25:1 (DiPyrO/glycoprotein) and vortexing at room temperature for 1 h. The labeling reaction was quenched by addition of hydroxylamine to a concentration of 0.25%.
An Oasis HLB 3 cm3 cartridge was used to remove excess labels and purify the labeled N-glycans. The cartridge was conditioned with 3 mL of 95% ACN, 1 mL of 50% ACN, and 3 mL of 95% ACN again. The crude reaction mixture was added to the conditioned cartridge, which was prefilled with 3 mL of 95% ACN for sample loading. The cartridge was then washed twice with 3 mL of 95% ACN, and the labeled N-glycans were eluted with 1 mL of 50% ACN. The eluting fraction was dried in vacuo, reconstituted in 30 μL of 80% ACN, and analyzed by LC/MS/MS immediately.
MALDI-MS Analysis for Labeling Efficiency Calculation.
Samples were prepared by premixing 1 μL of DiPyrO-labeled N-glycans with 1 μL of DHB matrix (2% N,N-dimethylaniline, 49% MeOH, and 49% water), and 1 μL of each matrix/sample mixture was spotted onto the MALDI target plate. A MALDI-LTQ-Orbitrap XL (Thermo Scientific, Bremen, Germany) was used for characterizing labeling efficiency to avoid ESI ionization bias. Ionization was performed using a laser energy of 20 μJ. Spectra were acquired in the Orbitrap mass analyzer within a mass range of m/z 900–4000 at a mass resolution of 30 K (at m/z 400).
LC/MS/MS Analysis.
A Dionex Ultimate 3000 nanoLC system was coupled to an Orbitrap Fusion Lumos Tribrid quadrupole–ion trap–Orbitrap mass spectrometer with a NanoSpray Flex ion source (Thermo Scientific, Bremen, Germany) for all LC/MS/MS analyses. A self-fabricated nano-HILIC column (20 cm, 75 μm i.d., 3 μm PolyGlycoPlex A HILIC beads) was used for glycan separation. Mobile phase A was water with 0.1% FA, and mobile phase B was ACN with 0.1% FA. The flow rate was set at 0.3 μL/min, and the injection volume was set to 3 μL. The following gradient was used (time, % mobile phase B) unless otherwise specified: (0 min, 80%), (20 min, 80%), (80 min, 20%), (80.1 min, 10%), (90 min, 10%), (90.1 min, 80%), (110 min, 80%).
The following MS parameters were used for all data acquisition unless otherwise noted. Samples were ionized in positive ion mode with a spray voltage of 3 kV, an S-lens RF level of 30, and a capillary temperature of 300 °C. Full MS scans were acquired at a resolving power of 500 K (at m/z 200) within a mass range of m/z 300–1500. Maximum injection time of 100 ms, automatic gain control (AGC) target value of 2e5, and one microscan were used for full MS scans. Data-dependent MS2 analysis was performed with rapid scan mode in the ion trap using a top 20 acquisition method with collision-induced dissociation (CID) operating with a normalized collision energy of 30. The first mass was fixed at m/z 110, and dynamic exclusion of acquired precursors was set to 15 s with a ±20 ppm tolerance.
N-Glycan Data Analysis.
DiPyrO-labeled N-glycans were manually identified by accurate mass matching. A peak list was exported based on an average spectrum and compared against an in-house database including most possible combinations of N-glycan units (Hexose (H), HexNAc (N), Fucose (F), and NeuAc (S)) with a mass tolerance of 10 ppm. Peak areas of extracted ion chromatograms were used for quantification. Microsoft Excel was used for calculations and statistical analyses.
RESULTS AND DISCUSSION
A quantitative glycomics strategy was developed in this study using multiplex mass-defect-based DiPyrO tags. The workflow of this study is illustrated in Figure 1, showing the microenvironment in Figure 1A and the stepwise experimental method in Figure 1B. The N-glycan release method, labeling conditions, and MS acquisition parameters were carefully optimized to achieve the best quantification performance. A proof-of-concept study was performed in a complex biological system by comparing the relative N-glycan abundance changes before and after chemotherapy in human serum proteins of cancer patients with ALL.
Figure 1.
Workflow for the relative quantification of DiPyrO-labeled N-glycans illustrating (A) the microenvironment and (B) the stepwise experimental method.
Labeling Chemistry Optimization.
Conventional approaches of releasing N-glycans involve a deamination step in acidic conditions after PNGase F digestion to produce N-glycans with oxidized reducing ends; however, by instead using slightly alkaline conditions, the transient glycosylamine moiety remains available for reaction with amine-reactive chemical tagging reagents designed for peptide labeling (Scheme 1). In this study, glycans were released from glycoproteins at pH 7–8 to produce N-glycans with free glycosylamines29 for labeling with DiPyrO tags and subsequent relative quantification by LC/MS/MS. Because the differing steric hindrance effects of glycosylamines compared to peptides could require unique labeling reaction conditions in order to achieve complete labeling, the PNGase F digestion protocol and DiPyrO labeling conditions and ratios were carefully optimized for N-glycosylamines. First, the release of N-glycans from glycoproteins by the traditional in-solution method or by FANGS, using either 10K or 30K MWCO filters, was tested using HEPES or TEAB buffer. The FANGS protocol yielded faster and more convenient N-glycan separation and enrichment compared to the in-solution approach. The 30K MWCO filter ensured that all N-glycans were collected, and TEAB buffer produced cleaner MS spectra compared to HEPES buffer.
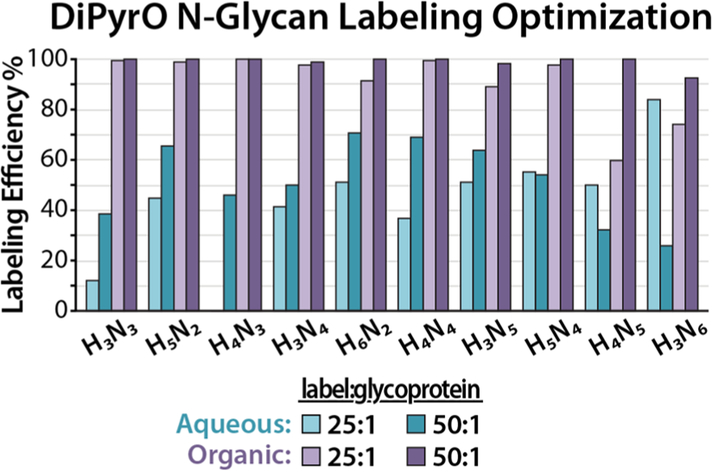
DiPyrO labeling conditions for N-glycan glycosylamines were carefully optimized using the N-glycans released from ovalbumin standard. N-glycans were labeled with or without aqueous buffer, either in 30:70 (v/v) 50 mM TEAB/DMF or anhydrous DMF only and analyzed by MALDI-MS. The label-to-glycoprotein mass ratios of 25:1 and 50:1 were compared, as lower mass ratios (5:1 and 10:1) resulted in incomplete labeling during preliminary tests. Labeling efficiencies were determined using the following equation: labeling efficiency = labeled peak intensity/(labeled peak intensity + unlabeled peak intensity) × 100%. The resulting labeling efficiencies of 4 different conditions for 10 N-glycans are shown in Figure 2. Although 30% TEAB buffer was used to dissolve peptides in proteomics analysis to achieve the optimum labeling efficiency, the existence of aqueous buffer in the N-glycan labeling solution limited the labeling efficiency to approximately 50%. In contrast, the reaction in anhydrous DMF yielded approximately 100% labeling efficiency for 8 of the 10 N-glycans tested and was used for subsequent experiments. The reactivity of different types of glycans with DiPyrO is similar due to the commonly preserved chitobiose core structure of N-glycans. Satisfactory labeling efficiency was also observed for fucosylated and sialylated N-glycans. Both 25:1 and 50:1 labeling ratios provided approximately 100% labeling efficiency, with the exception of the larger N-glycans such as H4N5 and H3N6. Although the 50:1 labeling ratio was optimal, the 25:1 labeling ratio was chosen for subsequent experiments in order to achieve high labeling efficiency while conserving labeling reagent.
Figure 2.
Labeling efficiencies of 10 selected N-glycans released from OVA using 4 different labeling conditions: 30:70 50 mM TEAB/DMF solution with DiPyrO/glycoprotein mass ratio of 25:1 (light blue) or 50:1 (blue); or anhydrous DMF with mass ratio of 25:1 (light purple) or 50:1 (purple). On the x-axis: H = Hex, N = N-acetylhexosamine.
In order to maximize the separation of DiPyrO-labeled N-glycan mass-defect peak pairs, while minimizing ion coalescence, the mass resolution and automatic gain control (AGC) value were carefully optimized. Duplex DiPyrO-labeled N-glycan samples mixed at a 1:1 ratio between light and heavy were acquired on the Orbitrap Fusion Lumos instrument at resolving powers of 15K, 30K, 60K, 120K, 240K, and 500K (at m/z 200). At 15K, 30K, and 60K, peak pairs were unresolved, while at 120K, most peak pairs could be partially separated but not sufficiently resolved for quantification. At 240K, most peak pairs were sufficiently resolved at their full width at 10% maximum peak height, which is suitable for quantification. At 500K, peak pairs were completely resolved at baseline, so a resolution of 500K was used for subsequent experiments, while 240K could also be used if higher resolution instrument is not available. Peak pairs from DiPyrO-labeled N-glycans acquired at each resolving power are shown as examples in (Figure S1).
When excessively large ion populations accumulate in the Orbitrap mass analyzer, space-charge-induced ion coalescence occurs even when acquiring at high resolving powers.30 The AGC values were carefully optimized in order to minimize ion coalescence (Figure S2). AGC values of 1e5, 2e5, 5e5, and 1e6 were tested at a resolving power of 500K. No coalescence was observed at AGC values of 1e5 and 2e5, and the base peak was detected at similar intensity. At an AGC value of 5e5, peak coalescence started to occur, and at an AGC value of 1e6, the two peaks completely merged into one. An AGC value of 2e5 was used for subsequent experiments to ensure sufficient signal intensities and low frequency of ion coalescence.
Characterization of Quantification Performance.
The quantification accuracy and dynamic range of the duplex DiPyrO tags were characterized by labeling the N-glycans released from bovine lactoferrin glycoprotein standard and mixing at known light/heavy ratios. Released N-glycans with free glycosylamines were equally aliquoted into two portions, and each was labeled with either the light or heavy DiPyrO tag. The labeled N-glycan samples were then mixed at 10:1, 5:1, 1:1, 1:5, and 1:10 ratios. Three replicates were prepared for dynamic range characterization, and LC/MS/MS analysis was performed for each sample on the Orbitrap Fusion Lumos. The integrated area under the curve of the extracted ion chromatogram (XIC) for each ion was used to calculate the measured log2 ratio. In Figure 3, the resulting measured vs theoretical log2 ratio plot of selected N-glycans H9N2 (Figure 3A, m/z 1068.8461, z = 2+) and H5N4F3S (Figure 3B, m/z 1312.4421, z = 2+) is summarized. The dotted line is a linear equation of y = x, which represents the ideal situation where measured ratios match the theoretical ratios. Good matches between measured and theoretical ratios were observed in the range of 10:1 to 1:10, as all of the measured data points are located on the ideal projected dotted line with small standard deviation between replicates, demonstrating that this quantification approach can be used to accurately quantify glycans within a suitable dynamic range.
Figure 3.
Dynamic range test of duplex DiPyrO-labeled N-glycans released from BTG. DiPyrO labeling ratios (light/heavy) of 10:1, 5:1, 1:1, 1:5, and 1:10 were tested with three replicates. The results of (A) H9N2 (m/z 1068.8461, z: 2+) and (B) H5N4F3S (m/z 1312.4421, z: 2+) are plotted as measured ratios vs theoretical ratios at the log2 scale. Error bars represent the standard deviation of three replicates. The dotted line represents a linear equation of y = x, the ideal situation where measured ratios match the theoretical ratios.
Representative XIC and MS spectra are shown in Figure 4 for a duplex DiPyrO-labeled neutral complex N-glycan H3N4F at a 1:1 mixing ratio. An MS1 spectrum along with associated XIC is shown in Figure 4A. The light and heavy peaks were baseline resolved with intensities in agreement with the 1:1 mixing ratio. No significant retention time shift was observed between the light- and heavy-labeled species. An HCD MS2 spectrum of the DiPyrO-H3N4F is shown in Figure 4B. Abundant fragment ions from HCD cleavage of glycosidic bonds permitted confident identification of the labeled glycan. Figure S3 (Supporting Information) shows two other examples of duplex DiPyrO-labeled N-glycans: high-mannose N-glycan H5N2 (Figure S3A) and acidic complex N-glycan H5N4FS (Figure S3B) mixed at 1:2 ratios. The light and heavy DiPyrO-labeled N-glycans eluted at the same time with no significant retention time shift. The light and heavy peaks were baseline resolved with intensities in agreement with the 1:2 mixing ratio.
Figure 4.
Evaluation of quantification performance of duplex DiPyrO tags for glycan analysis. (A) An MS1 spectrum of duplex DiPyrO-labeled glycan H3N4F at 1:1 mixing ratio acquired at a resolving power of 240 K (at m/z 200). (B) An HCD MS2 spectrum of DiPyrO-H3N4F. Fragment ion peaks are annotated with putative glycan fragment structures.
In-source fragmentation of the glycosidic bond is commonly observed for protonated native and sometimes even reducing-end-labeled glycans during positive-mode ESI, which often causes decreased sensitivity for quantification. For the protonated DiPyrO-labeled glycans generated from ESI, protons can be localized to the two basic amine groups (one tertiary amine and one secondary amine) of the DiPyrO tag, making the glycosidic bond less labile when the ions travel through the atmospheric region. By careful optimization of the ionization source parameters, especially the S-lens RF level, we noticed that in-source fragmentation was insignificant for the DiPyrO-labeled glycans. In LC/MS analysis of DiPyrO-labeled glycan released from human serum protein, ions resulting from in-source fragmentation were detected at less than 2.5%, which has minimal impact on accurate quantitative glycomics (Figure S4, Supporting Information).
Relative Quantification of N-Glycan Profiles Affected by Chemotherapy.
The ultimate goal of this study was to apply the mass-defect-based quantification method to a complex biological system and to accurately and precisely quantify the N-glycan profiles at different biological states. With an established collaboration between our lab and the Departments of Neurology and Pediatrics, we compared the N-glycan changes in human serum protein of three B-cell ALL pediatric patients before induction and on the first day of consolidation chemotherapy as a proof-of-principle study. Duplex DiPyrO tags were used to label N-glycans released from equal amounts of human serum protein samples before (light) and after (heavy) induction chemotherapy using the same workflow shown in Figure 1 with FANGS, labeling, and LC/MS/MS analysis.
Selected N-glycan fold changes are summarized in Figure 5 for high-mannose N-glycan (H5N2, H6N2), complex/neutral N-glycans (H4N4F, H4N5F), and acidic N-glycans with one or two sialic acids. Most detected N-glycans revealed a trend of consistently decreasing after induction of chemotherapy (Figure 5A). Representative MS spectra of duplex DiPyrO-labeled N-glycan peak pairs are shown in Figure 5B–E, and a full list of N-glycans and their fold changes are reported in Table S1 (Supporting Information). A total of 36 N-glycans were quantified by this method, all of which showed decreased abundance in serum after induction chemotherapy. Of these, 19 N-glycans were detected in all 3 patient samples. The high labeling efficiency, broad linear range, and accurate quantification results make DiPyrO labeling a reliable and accurate method for glycomic analysis in complex samples. Also, DiPyrO tags offers an additional advantage of increased multiplexing for population-based and clinical specimen analysis research areas.23
Figure 5.
(A) Selected N-glycan relative quantification of equal amounts of human serum protein from ALL patients before (light) and after (heavy) induction chemotherapy. Ratios represent areas under the curve of light/heavy-labeled N-glycan XICs. Error bars represent the standard deviation of three biological replicates. Representative MS spectra of duplex DiPyrO-labeled N-glycan peak pairs are shown: (B) H5N2 (z: 2+), (C) H6N4FS (z: 2+), (D) H4N4F (z: 2+), (E) H5N4S2 (z: 2+).
While it might be premature to conclude that chemotherapy reduces the relative N-glycan amount in human serum protein based on one proof-of-principle study, the observed results in these patients could be explained by the profound reduction or elimination of blasts after induction chemotherapy. Cancer cell invasion and metastasis can alter N-glycans by increasing branching, sialylation, and fucosylation,27,28 and reverting such processes could lead to the decrease in abundance of such N-glycans. In fact, the N-glycans with fucosylation or sialylation tend to decrease significantly in these patients’ serum proteins after chemotherapy, including H4N4F (4.5-fold decrease), H6N3S (4.8-fold decrease), and H5N4S2 (3.6-fold decrease).
CONCLUSIONS
In this study, an innovative strategy was developed for quantitative glycomics using multiplexed mass-defect-based DiPyrO tags. The N-glycan release method, labeling conditions, and MS acquisition parameters were carefully optimized to achieve the best quantification performance. This strategy was also applied to a complex biological system to compare the N-glycan profile of B-cell ALL patients prior to and after induction chemotherapy. It was found that most N-glycan abundances had been relatively decreased after chemotherapy, which could be associated with cancer cell reduction. While more in-depth studies are currently ongoing to elucidate the association between N-glycan profile and chemotherapy, this novel quantification technique is suitable for implementation in many biological and clinical applications. This study also unlocks the potential of using various well-developed proteomics tags for quantifying both N- and O-linked glycans. In conclusion, we envision that this new quantitative glycomics method can be widely applied by other research endeavors in a variety of biological and clinical specimens.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by the National Institutes of Health grants R21AG055377, R01AG052324, R01DK071801, the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427, and a Robert Draper Technology Innovation Fund grant with funding provided by the Wisconsin Alumni Research Foundation (WARF). The Orbitrap instruments were purchased through the support of an NIH shared instrument grant (NIH-NCRR S10RR029531) and Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin–Madison. A.R.B. acknowledges an NIH General Medical Science NRSA Fellowship (1F31GM119365) for funding support. L.L. acknowledges a Vilas Distinguished Achievement Professorship and Janis Apinis Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin–Madison School of Pharmacy.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.anal-chem.8b00548.
Figures S1–S4 and Table S1 PDF
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Varki A; Lowe JB Biological Roles of Glycans In Essentials of Glycobiology, 2nd ed.; Varki A, Cummings RD, Esko JD, et al. , Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, U.S.A., 2009. [PubMed] [Google Scholar]
- (2).Moremen KW; Tiemeyer M; Nairn AV Nat. Rev. Mol. Cell Biol 2012, 13, 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dwek RA Chem. Rev 1996, 96, 683–720. [DOI] [PubMed] [Google Scholar]
- (4).Defaus S; Gupta P; Andreu D; Gutierrez-Gallego R Analyst 2014, 139, 2944–2967. [DOI] [PubMed] [Google Scholar]
- (5).Alley WR; Mann BF; Novotny MV Chem. Rev 2013, 113, 2668–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).An HJ; Kronewitter SR; de Leoz MLA; Lebrilla CB Curr. Opin. Chem. Biol 2009, 13, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Arnold JN; Saldova R; Hamid UMA; Rudd PM Proteomics 2008, 8, 3284–3293. [DOI] [PubMed] [Google Scholar]
- (8).Jankovic MJ Med. Biochem 2011, 30, 213–223. [Google Scholar]
- (9).Taniguchi N Mol. Cell Proteomics 2008, 7, 626–627. [PubMed] [Google Scholar]
- (10).Ruhaak LR; Zauner G; Huhn C; Bruggink C; Deelder AM; Wuhrer M Anal. Bioanal. Chem 2010, 397, 3457–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chen Z; Zhong X; Tie C; Chen B; Zhang X; Li L Anal. Bioanal. Chem 2017, 409, 4437–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hecht ES; McCord JP; Muddiman DC Anal. Chem 2015, 87, 7305–7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tie C; Zhang X-X Anal. Methods 2012, 4, 357–359. [Google Scholar]
- (14).Walker SH; Budhathoki-Uprety J; Novak BM; Muddiman DC Anal. Chem 2011, 83, 6738–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Walker SH; Taylor AD; Muddiman DC J. Am. Soc. Mass Spectrom 2013, 24, 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen B; Zhong X; Feng Y; Snovida S; Xu M; Rogers J; Li L Anal. Chem 2018, 90, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hahne H; Neubert P; Kuhn K; Etienne C; Bomgarden R; Rogers JC; Kuster B Anal. Chem 2012, 84, 3716–3724. [DOI] [PubMed] [Google Scholar]
- (18).Yang S; Wang M; Chen L; Yin B; Song G; Turko IV; Phinney KW; Betenbaugh MJ; Zhang H; Li S Sci. Rep 2015, 5, 17585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yang S; Yuan W; Yang WM; Zhou JY; Harlan R; Edwards J; Li SW; Zhang H Anal. Chem 2013, 85, 8188–8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhong X; Chen Z; Snovida S; Liu Y; Rogers JC; Li L Anal. Chem 2015, 87, 6527–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hebert AS; Merrill AE; Bailey DJ; Still AJ; Westphall MS; Strieter ER; Pagliarini DJ; Coon JJ Nat. Methods 2013, 10, 332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Merrill AE; Hebert AS; MacGilvray ME; Rose CM; Bailey DJ; Bradley JC; Wood WW; El Masri M; Westphall MS; Gasch AP; Coon JJ Mol. Cell. Proteomics 2014, 13, 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Frost DC; Buchberger AR; Li L Anal. Chem 2017, 89, 10798–10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Atwood JA; Cheng L; Alvarez-Manilla G; Warren NL; York WS; Orlando RJ Proteome Res 2008, 7, 367–374. [DOI] [PubMed] [Google Scholar]
- (25).Botelho JC; Atwood JA; Cheng L; Alvarez-Manilla G; York WS; Orlando R Int. J. Mass Spectrom 2008, 278, 137–142. [Google Scholar]
- (26).Lauber MA; Yu YQ; Brousmiche DW; Hua ZM; Koza SM; Magnelli P; Guthrie E; Taron CH; Fountain KJ Anal. Chem 2015, 87, 5401–5409. [DOI] [PubMed] [Google Scholar]
- (27).Norton PA; Comunale MA; Krakover J; Rodemich L; Pirog N; D’Amelio A; Philip R; Mehta AS; Block TM J. Cell. Biochem 2008, 104, 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhao J; Qiu WL; Simeone DM; Lubman DM J. Proteome Res 2007, 6, 1126–1138. [DOI] [PubMed] [Google Scholar]
- (29).Oyama T; Yodohsi M; Yamane A; Kakehi K; Hayakawa T; Suzuki SJJ Chromatogr. B: Anal. Technol. Biomed. Life Sci 2011, 879, 2928–2934. [DOI] [PubMed] [Google Scholar]
- (30).Gorshkov MV; Fornelli L; Tsybin YO Rapid Commun. Mass Spectrom 2012, 26, 1711–1717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.