DEAR EDITOR,
A blind fish of Sinocyclocheilus (Cypriniformes: Cyprinidae) was caught in open water in the Three Gorges (Sanxia) reservoir, at a depth of 20 m in the mainstream of Yangtze River in Zigui County, Hubei Province, China. This fish can be easily distinguished from all other congeners by external morphological characteristics, and is estimated to have diverged from its sister group about 0.55 million years ago (Ma). The geologically well separated locality of this species has expanded the distribution of Sinocyclocheilus cavefish from around N25°(latitude) to above N30°. Herein, we describe this new species as Sinocyclocheilus sanxiaensis sp. nov., and discuss the possible reasons why the species appears, surprisingly, in the Three Gorges reservoir.
Cavefish, or hypogean fish, are a special group of aquatic organisms that spend much (or all) of their lives restricted to cave or subterranean river habitats (Ma et al., 2019). Many of these fishes possess distinct and highly specialized morphological troglomorphisms, such as rudimentary eyes and scales and loss of pigmentation (Yang et al., 2016). China has the highest number of cavefish in the world, with more than 150 species mainly distributing in the vast karst areas in southwestern China (Ma et al., 2019).
Cavefish belonging to Sinocyclocheilus are endemic, and also the most speciose cavefish group in China. Members of this genus are characterized by their compressed bodies, well-developed cephalic lateralis systems, two pairs of barbels, and especially, the extensively varied troglomorphisms. So far, all species of Sinocyclocheilus have been found only in Yunnan, Guizhou provinces, and Guangxi Zhuang Autonomous Region, within a very narrow distribution area (about 270 000 km2) at ~ N25° latitude (Zhao & Zhang, 2009).
Sinocyclocheilusspecies diversity was systematically reviewed by Zhao & Zhang (2009), who recognized a total of 49 valid species up to that date. However, the past ten years have witnessed many new species discoveries (Supplementary Figure S1), with most found in relatively inaccessible or formerly unexplored subterranean rivers. Alongside with the description of the new species, we briefly review the taxonomy and distribution of Sinocyclocheilus, which corroborates the high Sinocyclocheilus species diversity with an update of 75 valid species (Supplementary Table S1).
The type specimen of S. sanxiaensis was deposited in the Kunming Natural History Museum of Zoology, Chinese Academy of Sciences (KNHM). Measurements and counts were taken point-to-point with a digital caliper (0.1 mm precision) on the left side of the fish whenever possible. To facilitate comparisons to congeners, morphometric and meristic characters were selected following Zhao et al. (2006). Morphological data from congeners described before 2009 were taken from the review of Zhao & Zhang (2009), with checks of the original literature for any imprecise descriptions or discrepancies. For congeners described since 2009, we checked original descriptions for morphological comparisons. For morphologically similar species, we also examined specimens deposited in KNHM.
To clarify the phylogenetic position of the new species, and to estimate divergence time from its putative sister species, mitochondrial cytochrome b(cytb)was sequenced and analyzed. DNA was extracted from the pectoral fin on the right side of the fish. Primer pairs were selected according to Zhao et al. (2006). Thecyt b sequences of other species in Sinocyclocheilus were downloaded from GenBank, with one representative sequence selected for each species for the following phylogenetic analyses. Sequences were aligned using ClustalW in MEGA v7 (Kumar et al., 2016), and then inferred the phylogenetic relationship and estimated divergence time using BEAST v2.4.6 ( Bouckaert et al., 2014). As there are no suitable fossil records for Sinocyclocheilus, we estimated divergence time using a “standard clock” that roughly assumed an evolutionary rate of 2% per million years for mitochondrial DNA, which has been broadly applied in fish previously (Gharrett et al., 2001).
Holotype:KNHM 2019000001, 164.1 mm standard length (SL), 194.6 mm total length (TL); China: Hubei Province, Zigui County, Guojiaba Town; near the north bank of the Three Gorges (Sanxia) reservoir, mainstream of Yangtze River (N30°58′09.92″, E110°44′16.14″; about 144 m a.s.l.); caught on 28 February 2019 by Jie Li. Tissue of the pectoral fin (right side) was taken for DNA sequencing.
Diagnosis:This species can be distinguished from all other congeners by the following combination of characters: (1) albinotic body without pigmentation, (2) absence of normal eyes, (3) absence of dotted eyes, (4) absence of forehead horn, and (5) normal fusiform body with only slight uplift of back along and behind head, and two pairs of very short barbels (see more details in Table 1).
Table 1.
Meristics and morphometrics of S. sanxiaensisand its diagnosis relative to all other congeners
| Item | Value | Diagnosis |
|---|---|---|
| Dorsal fin | iii,8 | (1) Distinguished from most congeners (55) by presence of albinism (loss of pigmentation in the skin) vs. non-albinism, except for 19 species: S. altishoulderus,S. anatirostris, S. anophthalmus, S. anshuiensis,S. aquihornes, S. bicornutus, S. brevis, S. convexiforeheadus, S. flexuosdorsalis, S. furcodorsalis, S. hugeibarbus, S. hyalinus, S. jiuxuensis, S. lingyunensis, S. macrophthalmus, S. mashanensis, S. microphthalmus,S. tianlinensis, and S. xunlensis. |
| Anal fin | iii,5 | |
| Pectoral fin | I,14 | |
| Pelvic fin | I,7 | |
| Total vertebrae | 34 | |
| Predorsal vertebrae | 5 | |
| Caudal vertebrae | 18 | |
| Gill rakers | 7 | |
| Lateral line scales | 41 | |
| Scale row above lateral line | 16 | |
| Scale row below lateral line | 10 | |
| Circumpeduncular scale | 16 | |
| Standard length (mm) | 164.1 | |
| In % of standard length | (2) Distinguished from S. lingyunensis and S. macrophthalmus by absence of eyes vs. normal eyes. | |
| Body depth | 28.5 | |
| Predorsal length | 58.0 | |
| Dorsal fin base length | 15.7 | |
| Dorsal fin length | 21.6 | |
| Preanal length | 73.3 | (3) Distinguished from S. altishoulderus, S. bicornutus, S. brevis, S. hugeibarbus, S. jiuxuensis, S. mashanensis, andS. microphthalmus by absence of eyes vs. dotted eyes (reduced but visible). |
| Anal fin base length | 8.7 | |
| Anal fin length | 17.1 | |
| Prepectoral length | 34.2 | |
| Pectoral fin base length | 4.4 | |
| Pectoral fin length | 24.1 | |
| Prepelvic length | 54.8 | |
| Pelvic fin base length | 4.4 | (4) Distinguished from S. anatirostris, S. anshuiensis, S. aquihornes, S. convexiforeheadus, S. flexuosdorsalis, S. furcodorsalis, S. hyalinus, and S. tianlinensis by absence of forehead horn vs. forehead horn present (different types). |
| Pelvic fin length | 17.7 | |
| Caudal peduncle length | 18.6 | |
| Caudal peduncle depth | 11.6 | |
| Head length | 33.6 | |
| Head depth | 20.1 | |
| Head width | 17.4 | |
| Snout length | 16.4 | (5) Distinguished from S. anophthalmusand S. xunlensis by only slight uplift of back along and after head (vs. rapid) and very short barbels (vs. long). In addition, Sinocyclocheilus sanxiaensis can be further distinguished from S. anophthalmus by fewer vertebrae (34 vs. 38–39) and fewer lateral line scales (41 vs. 48–59); and from S. xunlensis by normal and subterminal mouth (vs. duckbilled mouth). |
| Eye diameter | 7.3 | |
| Interorbital width | 10.7 | |
| Prenostril length | 7.1 | |
| Width between posterior nostrils | 7.0 | |
| Upper jaw length | 8.9 | |
| Lower jaw length | 8.3 | |
| Mouth width | 9.7 | |
| Maxilla barbel length | 4.2 | |
| Rictal barbel length | 6.5 |
Description:Body compressed. Dorsal profile convex, ventral profile slightly concave, tapering gradually toward caudal fin. Greatest body depth slightly anterior to dorsal fin insertion.
Head compressed with no eyes. Eye orbit filled with fat tissue. Nares at 1/3 between snout tip and anterior margin of orbit. Anterior nares possessing anterior rim with posterior fleshy flap forming half-tube. Mouth subterminal, with slightly projecting upper jaw. Two pairs of very short barbels: maxillary barbel insertions in front of anterior nares, not extending to nares; rictal barbels slightly longer, but not reaching anterior margin of orbit. Gill opening large, opercular membranes not connected at isthmus. Joint of dentary-angular not close to each other at isthmus. Gill rakers developed, seven on first gill arch.
Pectoral fin long (24% SL), extending posterior to pelvic fin insertion and reaching to 1/3 of pelvic fin. Pelvic fin insertion slightly anterior to vertical through dorsal fin insertion, nearer to pectoral than to anal fin insertions; pelvic fin extending just beyond anus to anal fin insertion. Dorsal fin origin at 58% distance from snout tip to caudal-fin base and slightly posterior to vertical through pelvic fin insertion. Last unbranched spine of dorsal fin hard with serrations along posterior edge. Anal fin 17% SL, with insertion nearer to pelvic fin origin than to caudal fin base. Caudal fin bifurcate.
Lateral line complete and curved, descending greatly from posterior margin of operculum to point above pelvic fin insertion, descending slightly to point above anal fin insertion, then extending horizontally to end of caudal peduncle. Body covered by small soft scales, irregularly degenerated. Scales not easily visible to naked eye, partially embedded into epidermis. Lateral line scales similar in size to other scales. Lateral line scale: 41; scale row counts above lateral line, below lateral line, and circumpeduncular scales 16, 10, 16, respectively.
Coloration:In life, body generally white, with slightly flesh pink on posterior part (after dorsal fin); red block visible on lateral head, caused by red gills penetrating at posterior part of operculum. Holotype was fixed in 10% formalin and then preserved in 90% alcohol. In preservation, body generally light yellow; posterior part of operculum and all fins partially transparent.
Distribution and habitat:Known only the holotype. Although specimen was caught using a cage (without bait) in open water at a depth of 20 m, the birthplace (or real habitat) was still uncertain as it was a typical cavefish. The reservoir was gradually formed after the closure of the Three Gorges Dam in 1997. There are many karst landforms in the Three Gorges area. On 3–4 July 2019, we interviewed several local people, who described many caves and subterranean rivers near the type locality. One local had previously been told that blind fish existed in one cave. However, all caves mentioned were underwater according to our on-site survey.
Etymology:The name of the new species, sanxiaensis, is derivedfrom the Chinese name of the Three Gorges, 三峡(Sanxia).
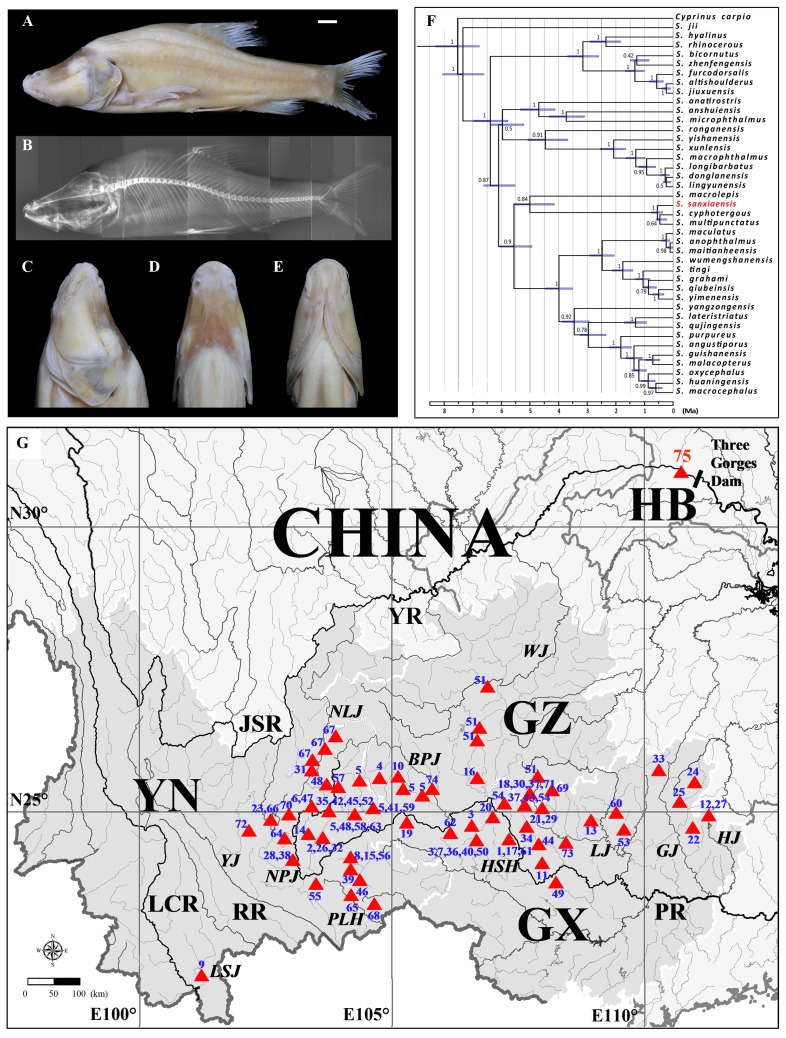
Phylogenetic position and divergence time:Phylogenetic reconstruction showed that S. sanxiaensis is the sister species of the subclade S. cyphotergous + S. multipunctatus, with strong support (1.0 Bayesian posterior probability, Figure 1F). The P-distances between S. sanxiaensis and S. cyphotergous, between S. sanxiaensis and S. multipunctatus were 1.5% and 1.3%, respectively (see more in Supplementary Table S2). These three species then grouped withS. macrolepis to form a major clade. Divergence time based on the constant evolutionary rate ofcyt b indicated that S. sanxiaensis separated from S. cyphotergous + S. multipunctatusat ~0.55 Ma (0.36–0.77, 95% highest posterior densities (HPDs)) and these three species separated from S. macrolepisat 4.86 Ma (4.15–5.57, 95% HPDs). Additionally, divergence time estimation showed the origin ofSinocyclocheilus to be 7.34 Ma (6.61–8.06, 95% HPDs).
Figure 1. Holotype, phylogenetic position and distribution of S. sanxiaensis .
Pictures show lateral view (A), X-ray lateral view (B), lateral head (C), dorsal head (D), and ventral head (E) of the holotype; Phylogenetic position of S. sanxiaensis (red) with the estimated divergence time in Sinocyclocheilus (F); Distributions of S. sanxiaensis (red, No. 75) and all other congeners (G). The abbreviations of rivers and numbers of other species (No. 1–74) are given according to Supplementary Table S1.
RemarksPhylogeny and origin of S. sanxiaensis
Although the Chinese cavefish in Sinocyclocheilus exhibit very high species diversity, our understanding of how these species evolved is far from resolved. This has been restricted due to a lack of well-resolved phylogenetic relationships. In combination of morphological characters and molecularcytb sequence, Zhao & Zhang (2009) proposed a four species group hypothesis, named by the representative species in each group: i.e., S. jii, S. angularis, S. tingi, and S. cyphotergous, and characterized by different morphological features and distribution in specific regions. Based on all available cytb sequences from GenBank, we reconstructed a phylogenetic tree from 40 species (Figure 1F). Although the phylogenetic relationship differed slightly from that of Zhao & Zhang (2009), Sinocyclocheilus sanxiaensis would be a member in the “ S. cyphotergous group” according to its phylogenetic position.
The sister relationship of S. sanxiaensis and (S. cyphotergous + S. multipunctatus) is understandable as S. multipunctatusis the only species that has been recorded as far north as the Yangtze River (specimens have been reported in Wujiang, a tributary of the Yangtze River in Zunyi County of Guizhou Province). The divergence time between S. sanxiaensisand its sister group was estimated to be very young (0.55 Ma) relative to the origin time of Sinocyclocheilus (7.35 Ma, Figure 1F). The complete channelization of the Yangtze River is generally thought to have formed by the lower reach capturing the middle and then the upper reaches, which was associated with the stepwise growth of the Qinghai-Tibetan Plateau (Clark et al., 2004; Zhang et al., 2018; Zheng et al., 2013). However, the start of the Yangtze throughflow and the key point of the incision of the Three Gorges have variously been argued to postdate 0.75 Ma at one extreme and to be as early as 40–45 Ma at the other (Zheng et al., 2013). If the speciation of S. sanxiaensis (~0.55 Ma) was the direct product of the formation of the Three Gorges, the time of the incision of the Three Gorges would be very late, as proposed recently (0.3–0.12 Ma, Zhang et al., 2018). However, we cannot exclude the greater possibility of an occasionally regional capture scenario later than the incision of the Three Gorges: some northward tributary of the Wujiang River captured a previously southward tributary of the Pearl River, which could have possibly transported the ancestor of S. sanxiaensis from the Pearl River into the Wujiang and also into the Yangtze River basin. This could have happened anytime because of the well-developed subterranean rivers in the board areas between Guizhou and Guangxi, which would withdraw any direct links to the time of incision of the Three Gorges.
Possible reason for occurrence of S. sanxiaensis
Our discovery does raise the question as to how the new species arrived in the Three Gorges reservoir of the mainstream of the Yangtze River. Given the massive karst landforms and abundant underground water systems found within the Three Gorges area (Xia et al., 2010), we advocate that the new species likely came from a subterranean river draining into the Yangtze River. The rise of water after the impoundment of the Three Gorges Dam may have facilitated a more direct link between some subterranean rivers and the Yangtze River. Not only did the dam increase water height and coverage area, it also slowed the velocity of water flow. Interestingly, there were many deep channels found in the Yangtze River before the construction of Three Gorges Dam, especially in the Xiling Gorge (Jing & Liu, 1985), which includes the river reach where we collected this species. Our on-site habitat survey further confirmed underground rivers with several karst caves near the type locality. Of note, one local resident claimed that blind fish are known to exist in a nearby cave (about 4 km from type locality). Although these underground rivers and caves have been submerged with the impoundment of the Three Gorges Dam, it is reasonable to infer that this species could come from an exit of these underground river systems. However, whether the species exited actively or passively remains unknown. The date when we collected the specimen (28 February 2019) was during the spawning season of most Sinocyclocheilus species; therefore, it is possible that the fish actively exited in an attempt to explore a new environment (cave-like deep water) to meet breeding requirements. However, turbulence due to geological activities within original underground rivers could also result in possible passive spread.
In addition, we still know almost nothing about the life history of this species. However, the discovery of this single specimen should encourage further efforts to survey, study, and protect this rare species. The existence of this fish also indicates that junction areas within the Chongqing, Hubei, Hunan, and Guizhou provinces likely harbor other undiscovered Sinocyclocheilus cave species (Figure 1G); thus far, only three cavefish in the genus Triplophysa have been reported (Huang et al., 2019).
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5–8.6 of the Code). This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/.
Publication LSID:
urn:lsid:zoobank.org:pub: AC302B8C-E445-48A0-9086-C90C40279690.
Nomenclatural act LSID:
urn:lsid:zoobank.org:act: 7844B164-1AE4-4617-BC23-49745BE21ED4.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys in Hubei Province was granted by the Ministry of Agriculture and Rural Affairs of China (MOA). Project approval (No. 171821301354052150) was issued by the Bureau of Fisheries of MOA.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
J.L. collected the fish. J.X.Y. and J.B.C. designed the study. J.L., W.S.J., and Y.Z.H. carried out a later on-site habitat survey. W.S.J. examined the fish and performed morphological comparisons and molecular analysis. W.S.J. and J.L. wrote the draft. X.Z.L., Z.R.W., J.X.Y., and J.B.C. revised the manuscript. All authors read and approved the final manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31672282; U1702233), Species Resources Protection Project (Fishery, 171821301354052150), Agricultural Basic Long-term Observation and Monitoring Project (ZX08S182057), and Youth Innovation Promotion Association of the Chinese Academy of Sciences to W.S.J.
REFERENCES
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10( 4): e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MK, Schoenbohm LM, Royden LH, Whipple KX, Burchfiel BC, Zhang X, Tang W, Wang E, Chen L. 2004. Surface uplift, tectonics, and erosion of eastern Tibet from large‐scale drainage patterns. Tectonics, 23(1): 1006–1029. [Google Scholar]
- Gharrett AJ, Gray AK, Brykov V. 2001. Phylogeographic analysis of mitochondrial DNA variation in Alaskan coho salmon, Oncorhynchus kisutch . Fishery Bulletin, 99(4): 528–545. [Google Scholar]
- Huang TF, Zhang PL, Huang XL, Wu T, Gong XY, Zhang YX, Peng QZ, Liu ZX. 2019. A new cave-dwelling blind loach, Triplophysa erythraea sp . nov. (Cypriniformes: Nemacheilidae), from Hunan Province, China. Zoological Research, 40(4): 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing C, Liu C. 1985. The features of the karst landforms in the area of the Three Canyons of the Changjiang river. Journal of Central China Normal University, 3: 85–92. (in Chinese) [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7): 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhao Y, Yang J. 2019. Cavefish of China. In: White WB, Culver DC, Pipan T. Encyclopedia of Caves (3rd edn). London: Academic Press, 1225. [Google Scholar]
- Xia KS, Yuan DX, Xie SY, Chu YC. 2010. Preliminary study on morphology features of karst in the lower reaches of Wujiang River—a case in Wulong, Chongqing and the surrounding area. Carsologica Sinica, 29: 196–204. (in Chinese) [Google Scholar]
- Yang JX, Chen XL, Bai J, Fang DM, Qiu Y, Jiang WS, Yuan H, Bian C, Lu J, He SY, Pan XF, Zhang YL, Wang XA, You XX, Wang YS, Sun Y, Mao DQ, Liu Y, Fan GY, Zhang H, Chen XY, Zhang XH, Zheng LP, Wang JS, Cheng L, Chen JM, Ruan ZQ, Li J, Yu H, Peng C, Ma XY, Xu JM, He Y, Xu ZF, Xu P, Wang J, Yang HM, Wang J, Whitten T, Xu X, Shi Q. 2016. The Sinocyclocheilus cavefish genome provides insights into cave adaptation . BMC Biology, 14(1): 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XB, Liu Y, Wang SJ, Liu WM, Xue WX. 2018. On the chronology of the Yellow Rivers and the Yangtze Rivers. Mountain Research, 36( 5): 661–668. (in Chinese) [Google Scholar]
- Zhao YH, Watanabe K, Zhang CG. 2006. Sinocyclocheilus donglanensis, a new cavefish (Teleostei: Cypriniformes) from Guangxi, China . Ichthyological Research, 53(2): 121–128. [Google Scholar]
- Zhao YH, Zhang CG. 2009. Endemic Fishes of Sinocyclocheilus (Cypriniformes: Cyprinidae) in China—Species Diversity, Cave Adaptation, Systematics and Zoogeography . Beijing: Science Press; (in Chinese) [Google Scholar]
- Zheng HB, Clift PD, Wang P, Tada R, Jia J, He MY, Jourdan F. 2013. Pre-Miocene birth of the Yangtze River. Proceedings of the National Academy of Sciences of the United States of America, 110(19): 7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.