The use of viruses infecting bacteria (bacteriophages or phages) to treat bacterial infections has been ongoing clinically for approximately 100 years. Despite that long history, the growing international crisis of resistance to standard antibiotics, abundant anecdotal evidence of efficacy, and one successful modern clinical trial of efficacy, this phage therapy is not yet a mainstream approach in medicine.
KEYWORDS: bacteriophage therapy, phage clearance, phage movement, phage resistance, spectrum of activity, phage circulation
SUMMARY
The use of viruses infecting bacteria (bacteriophages or phages) to treat bacterial infections has been ongoing clinically for approximately 100 years. Despite that long history, the growing international crisis of resistance to standard antibiotics, abundant anecdotal evidence of efficacy, and one successful modern clinical trial of efficacy, this phage therapy is not yet a mainstream approach in medicine. One explanation for why phage therapy has not been subject to more widespread implementation is that phage therapy research, both preclinical and clinical, can be insufficiently pharmacologically aware. Consequently, here we consider the pharmacological obstacles to phage therapy effectiveness, with phages in phage therapy explicitly being considered to serve as drug equivalents. The study of pharmacology has traditionally been differentiated into pharmacokinetic and pharmacodynamic aspects. We therefore separately consider the difficulties that phages as virions can have in traveling through body compartments toward reaching their target bacteria (pharmacokinetics) and the difficulties that phages can have in exerting antibacterial activity once they have reached those bacteria (pharmacodynamics). The latter difficulties, at least in part, are functions of phage host range and bacterial resistance to phages. Given the apparently low toxicity of phages and the minimal side effects of phage therapy as practiced, phage therapy should be successful so long as phages can reach the targeted bacteria in sufficiently high numbers, adsorb, and then kill those bacteria. Greater awareness of what obstacles to this success generally or specifically can exist, as documented in this review, should aid in the further development of phage therapy toward wider use.
INTRODUCTION
Bacteriophage therapy (phage therapy) has remained for decades outside the scientific and clinical mainstream. Due to the rise in the frequency of multidrug-resistant bacterial infections (1, 2), often described as a “crisis” of antibiotic resistance (3, 4), phage therapy could potentially become more widespread as an antibacterial strategy (5). The therapeutic potential of phages has been highlighted in numerous recent reviews (2, 6–24), and the specific advantages of phage therapy over antibiotic therapy are abundant (25). They include (i) an ability of phages to increase in number in situ at the site of infection in the course of antibacterial treatment; (ii) the generally low toxicity of phages used for treatment; (iii) phage target specificity, which can limit the disruption of nontarget bacteria; (iv) the ability of phages to treat either antibiotic-resistant or antibiotic-tolerant infections; and (v) the typical ease of discovery of phages as agents with novel antibacterial activities.
It is not just antibiotic resistance that is a problem. In the past decade, we have observed a revolution in our understanding of the importance of the human microbiome in terms of its richness, variability, and key role in maintaining human health (26–31). The overuse of broad-spectrum antibiotics (32, 33), contributing to substantial changes in microbiome diversity (34–37), has reduced our ancestral microbial heritage, causing negative effects on human health. Industrialization and the Western lifestyle, which are widely associated with the overuse of antibiotics, correlate with increases in immune, metabolic, and cognitive diseases, including obesity, diabetes, asthma, allergies, inflammatory bowel disease, and autism (31, 37–39). Bacteriophages, in contrast to many antibiotics, are quite narrow in terms of their spectra of activity (40), and they thereby can be substantially more selective in terms of their impact on microbiomes. Consistently, high-throughput analyses of the microbiota in individuals treated with bacteriophages have revealed no substantial changes in the microbiome composition following treatment (41–45). This difference and others between bacteriophages and antibiotics make phages attractive as modern antibacterial therapeutics.
The phage features which can provide important advantages can also bring challenges and potential difficulties for the successful development of phage therapy. High specificity makes it difficult to find phages or even phage combinations that are able to treat wide ranges of bacterial pathogens. This makes empirical (presumptive) treatment using phages challenging. Phages are also large, replication-competent, nucleoprotein complexes. As a result, their pharmacokinetics in animals and humans are unlike those typically observed with small-molecule antibacterial drugs. Phages especially differ from classical antibacterials in terms of their ability to pass through various bodily barriers. Phages also differ in their potential to elicit immune responses, in contrast to small-molecule drugs, which immunologically may serve only as simple haptens (46–51).
Bacteriophages together represent the most diverse semiautonomous biological entities in the world (52–56). This makes it difficult to reach broad conclusions or identify general rules concerning phage-bacterium or phage-body interactions. As a result, there can exist numerous poorly appreciated obstacles to phage therapy success, obstacles which often can differ depending on the phage employed and the bacterium targeted and also in terms of the characteristics of the treated organism. At the same time, however, phages collectively represent an abundant source of naturally occurring antibacterial agents, possessing diverse mechanisms of antibacterial activity. Nevertheless, phage therapy efforts have resulted in microbiological as well as clinical successes, i.e., the elimination of targeted pathogens and/or substantial improvements in infection-associated signs and symptoms. This is evidenced by the successful phase I/II efficacy trial of Wright et al. (57), but also by the success of numerous clinical cases of phage treatment, both in recent times and over multiple decades in the past (6–12, 17, 18, 20, 22, 58–65).
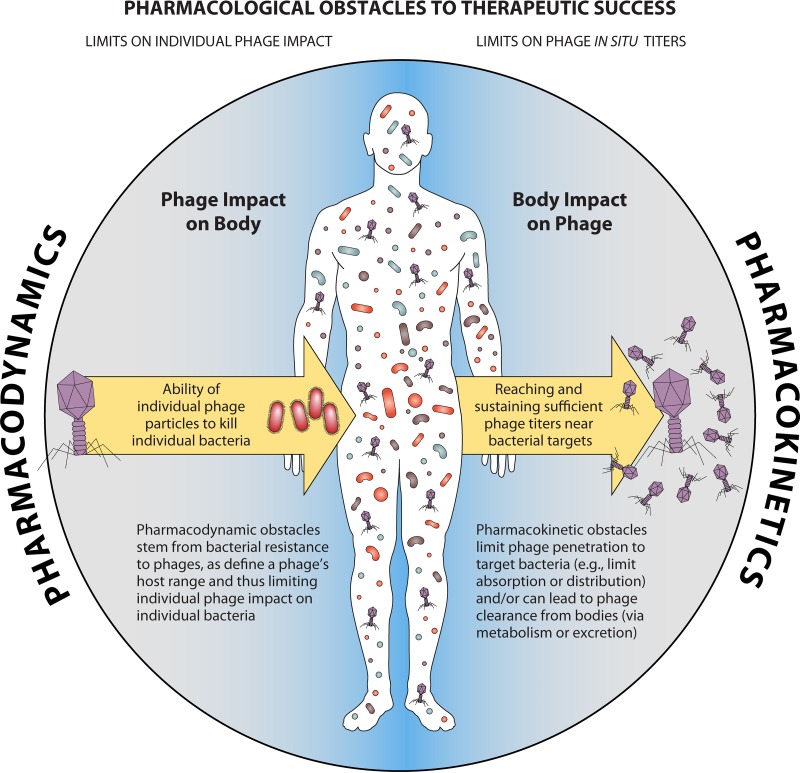
As with any endeavor, it can be useful to have a rational means of identifying and correcting deficiencies to, for example, improve phage treatments (20, 66, 67). Here, we provide a review of potential pharmacological obstacles to phage therapy success, in particular, to improve pharmacological awareness during experimentation with phage therapy (see the Appendix). This we do from both pharmacodynamic and pharmacokinetic perspectives. With pharmacodynamics, we consider the phage impact on body-associated bacteria, particularly in terms of the basic principles of the phage host range and the bacterial evolution of resistance to phages, as these factors can impact the antibacterial effectiveness of a given phage titer once that has been achieved in situ. With pharmacokinetics, we consider what is known about the potential for the body to impact the ability of phages to reach target bacteria at sufficient concentrations (titers). Emphasis there is on phage movement (pharmacokinetically known as absorption and distribution) as well as phage clearance (equivalent pharmacokinetically to metabolism and excretion). See Fig. 1 for a summary. We avoid assessment of the magnitude of the impacts of various factors, as these tend to vary from system to system, and such comparisons otherwise have not been well considered in the literature. The general goal is to help guide phage researchers and interested clinicians toward greater success with phage therapy.
FIG 1.
General principles of obstacles to phage therapy success. Pharmacokinetics refers to the impact of the body on drugs, while pharmacodynamics is described as the impact of the drug on bodies, with body in both cases including both body tissues and the associated microbiome. Pharmacokinetic obstacles are obstacles to drug movement, i.e., drug movement to the site of drug action, as well as problems with drug persistence within the body. Pharmacodynamic obstacles consist of bacterial mechanisms of resistance to phages, which can range from absolute (the infecting phage is inactivated, while the infected bacterium survives) to partial (neither the phage nor the bacterium survives) and which can consist of more subtle impacts of phage functionality (the bacterium does not survive but the phage infection vigor is compromised but not fully compromised, e.g., phage burst sizes are smaller or phages do not even adsorb to the phage-resistant bacterium). Not indicated in the figure are secondary pharmacodynamic issues, particularly, toxicities and side effects, which were not an emphasis of this review.
PHARMACODYNAMIC OBSTACLES
Pharmacodynamics is the study of the impact of drugs on the body, where “body” includes associated microorganisms (microbiota). These impacts can consist of efficacy effects as well as side effects. Phage-associated side effects may be reduced by ensuring a better purity of phage preparations, e.g., by removal of endotoxin (68–71), and by avoiding phages which encode bacterial virulence factors, e.g., by the use of bioinformatics (72–74). Bacterial virulence factor genes tend to not be associated with strictly or professionally lytic phages (75, 76). Efficacy effects are especially impacted by the phage host range, along with the bacterial evolution of phage resistance (Fig. 2), as addressed in this section. These are what bacteria are affected by phages (host range) and to what extent bacteria can change genetically in terms of their ability to be affected by phages (resistance). We consider these issues largely in terms of general principles. A more detailed look at phage host range is covered elsewhere (40, 77–79). Bacterial resistance mechanisms have also been recently reviewed (40, 80–83), as, too, has the bacterial evolution of phage resistance along with phage mechanistic and evolutionary responses (84–86).
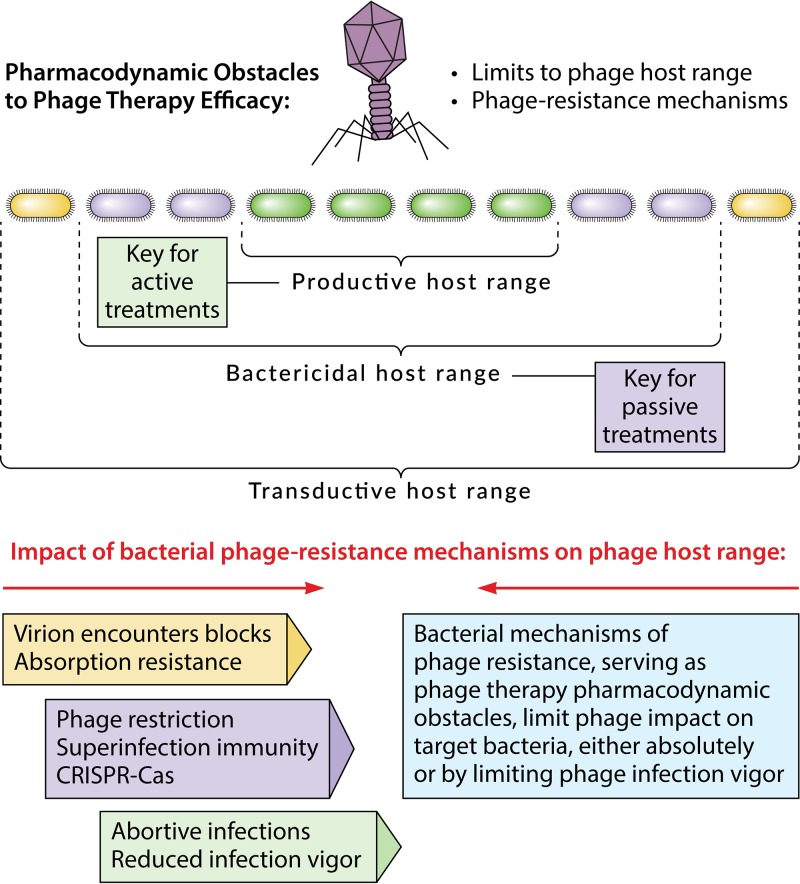
FIG 2.
Obstacles to phage action, given a phage-bacterium encounter. These pharmacodynamic obstacles collectively consist of bacterial mechanisms of resistance to phages. These in turn serve to define a given phage’s host range and, thereby, spectrum of activity. Three concepts of host range are presented, where phages can adsorb onto but may or may not kill or successfully infect the bacteria (but which may thereby still result in phage transduction of genetic material), where phages can kill bacteria but may or may not otherwise successfully infect the bacteria (referred to as a bactericidal host range; i.e., the phages do not necessarily produce new phage virions, but this bactericidal activity is still key for successful passive phage therapy), and where phages can kill the bacteria and, in the process, produce new virions (which is referred to as a productive host range and which is key for active phage therapy). Note that even if phages are allowed to produce virions, phage productivity while infecting a given bacterium, such as in terms of the phage burst size, may still be reduced to levels lower than expected or desired due to bacterial properties, i.e., productivity lower than may be desirable for phage therapy purposes. Shown to the lower left are various general mechanisms of bacterial resistance to phages, which are color coded to indicate what aspect of the host range they affect.
Phage Host Range
This section considers general principles of phage host range as they apply to phage treatments that either do or do not require phage population growth, in situ, to be successful. Theoretically, we can distinguish different aspects of phage host range in terms of phage functionality while infecting bacteria (40). For example, the range of bacteria that a phage is able to infect but not kill could be described as a transductive host range, i.e., those bacteria that a given phage is capable of transferring DNA to. For phage therapy, however, most relevant are a phage’s bactericidal host range and its productive host range. That is, at a minimum, phage therapy requires that phages kill bacteria. It can be helpful or even crucial, however, for phages to also be able to produce new phages in situ during treatments.
Payne and colleagues (46, 87) distinguished approaches to phage therapy into what can be described as “passive” versus “active” treatments. Passive treatments supply sufficient phage numbers to the targeted bacteria, i.e., inundative densities (49, 88–90), to kill a sufficient majority of these bacteria to control infection even absent phage production of new virions in situ. Active phage treatment, in contrast, explicitly involves the production of new phages during infection of the bacteria in situ. In terms of phage host range, passive treatments are dependent on phage bactericidal activity, while active treatments are also dependent on in situ phage virion production. For active treatments to be successful, sufficient numbers of bacteria individually supporting sufficiently large phage burst sizes to achieve inundative phage densities must also be present.
Host range-type obstacles to active treatment go beyond merely blocking phages from producing new virions. Specifically, the dynamics of phage population growth are such that a phage’s potential to reach bacterium-inundative densities, through in situ replication, is also dependent on the densities of those bacteria that are able to support phage population growth and is dependent as well on phage burst sizes; for detailed modeling of phage-bacterium dynamics, such as during phage therapy, see references 46, 47, 87, 91, and 92. For example, if achieving 108 phages/ml were required to inundate bacterial populations in a timely manner (49, 90), then this would be impossible given 105 bacteria/ml and a burst size of 100 or 106 bacteria/ml and a burst size of 10, each of which could generate peak phage titers of, at best, only about 107/ml. Thus, bacterial densities may or may not be sufficient to support phage population growth to inundative densities, even if the phages used can display, in situ, relatively large burst sizes.
Bacteria which are less physiologically active may not support phage burst sizes that are as large as those determined in the laboratory (93). Notably, changes in bacterial physiology or other changes in bacterial gene expression in response to specific in situ circumstances may affect the number of phages produced or reduce the number of receptor molecules required for phage adsorption that are found on bacterial surfaces (94). Furthermore, not all bacterial strains that may be treated using a given phage may be able to support relatively large burst sizes or rapid virion adsorption, even given optimal bacterial physiologies. Thus, even given sufficient densities of targeted bacteria and satisfactory phage growth characteristics, as determined in vitro, it may still be difficult to achieve adequate levels of phage population growth to result in successful active treatments in vivo.
Even with passive treatments, it can be helpful if phages are nonetheless able to produce new virions in the course of killing targeted bacteria, if only for the sake of being conservative in terms of maximizing phage numbers and, thereby, the impact of the phages on bacteria. That is, under most circumstances, for either passive or active treatments, it can be helpful to employ phages that have host ranges which include the targeted bacteria and that are productive under actual treatment conditions. To counter the issue of not all phages being able to adequately impact all targeted bacteria under all desired conditions, it is a common practice to gather up phages into multiphage-type cocktails. Such cocktails essentially are a form of phage-only combination therapy (10, 95, 96).
Bacterial Resistance to Phages
This section considers general principles of bacterial resistance evolution, especially as resistance can serve as an obstacle to the efficacy of phage therapy. Bacterial mechanisms of resistance to phages can be differentiated into those that can be described as innate (which consist of encounter blocks, adsorption resistance, penetration blocks, immunity to superinfection, abortive infection systems, restriction-modification systems, and phage growth limitation systems) and a minority that can be described as adaptive (i.e., CRISPR-Cas systems) (97). For phage therapy, there are two key issues with bacterial resistance and associated mechanisms (40, 80). The first issue is that bacterial resistance to phages limits the applicability of phages for presumptive use (as empirical treatments). This especially applies to monophage treatments but also can make it challenging to keep phage cocktails up to date. For organizational purposes, we refer to phage resistance that can dominate pathogen populations prior to phage treatment as “community resistance.” The second issue is bacterial resistance to phages, as this can evolve during the course of phage therapy (82, 83, 85, 98–100). We describe this as “treatment resistance.”
Community resistance.
Community resistance can be addressed in part via ongoing community monitoring, i.e., at the town, region, country level, etc., of locally circulating bacterial strains. This is a collective monitoring of patients in terms of the phage susceptibility characteristics of bacterial pathogens. In this way, phage resistance patterns may be recognized before large numbers of patients present with bacterial infections that are caused by newly community-prevalent strains. Thereby, standardized phage cocktails can be kept up to date, thus allowing for more effective presumptive treatment (10, 96). Though more complicated than targeting single bacterial species, it is also possible to keep phage cocktails targeting multiple possible pathogens current for presumptive use via community monitoring, as is the case with commercially available formulated phage products produced in the former Soviet Union (7, 8).
Bacterial testing for phage susceptibility—and, thereby, for phage resistance as well—can instead be undertaken immediately prior to the initiation of treatments, thus allowing for treatments that are not presumptive. The first approach is particularly applicable where delays in the start of treatments, such as on the order of days, would not be critical. This matter of timing is not necessarily absolute, however, as improvements in technology that deliver faster testing for bacterial susceptibility to treatment phages should eventually allow for the more rapid initiation of treatments, i.e., on the order of hours or even minutes rather than days. The testing of bacterial susceptibility to different phage types, in any case, can range from testing analogous to phage typing to determinations of plaque formation (101–103) and to other, more sophisticated phage-based technologies (104, 105).
Treatment resistance.
The underlying theory concerning treatment resistance is that a single round of phage therapy, whether it consists of a single or, instead, multiple phage dosings, may not succeed on its own in curing a bacterial infection, i.e., due to the evolution of phage-resistant bacterial strains (82). Though arguably logical, this concern that bacterial infections will tend to resist being cured due to phage resistance evolution is not always valid; i.e., even treatments with monophages can be clinically successful (58, 60, 62, 106) and are not necessarily associated with an increase in the numbers of phage-resistant bacteria, or this increase can be substantially delayed (107, 108). The evolution of phage resistance, however, is likely of greater concern when treating immunocompromised individuals (22, 109, 110). Importantly, it is crucial to keep in mind that there is a substantial difference between (i) phage-resistant bacteria arising during phage treatments, as they very often will (see next paragraph), and (ii) those phage-resistant bacteria flourishing following phage treatment, that is, to the point where these mutated bacteria contribute substantially to the persistence of the bacterial infection (79, 111, 112).
Based on mutation rates, it is nearly a certainty that most, though not all, bacterial populations making up bacterial infections contain small numbers of viable bacterial mutants which have acquired resistance to treatment phages even prior to phage application. In the absence of positive selection, however, these resistant populations will not tend to dominate bacterial populations and otherwise may not be problematic, should they display either reduced fitness or reduced virulence relative to phage-sensitive bacteria (79, 82, 85). Furthermore, the survival of such mutants can be of lower probability with treatment with phage cocktails than with treatment with monophages. That is, except in cases of cross-resistance, a bacterium would need to mutate twice or more to achieve resistance to the multiple phages making up a cocktail. Individual phage-resistant bacterial mutants are therefore likely to be infected and killed by a different phage making up the cocktail, despite having evolved resistance to one phage. This scenario represents the key underlying utility of combination therapies, like those routinely harnessed for treating cancers (99) or tuberculosis (100).
Optimizing for dealing with community versus treatment resistance.
We can consider, theoretically, the potential to generate cocktails which are effective for addressing community versus treatment resistance. Specifically, for a cocktail to be effective against treatment resistance, that cocktail must contain at least two phages whose host ranges relevantly include the targeted bacterium. This is because it is only with two active ingredients that the power of combination therapies against the evolution of resistance may be harnessed. In contrast, to be effective against community resistance, a cocktail needs to contain at least one phage that will kill a targeted bacterium.
It is certainly possible for cocktails to contain two phage types that are active against a given target bacterium. Nevertheless, a cocktail that has been optimized for a relatively broad spectrum of activity, e.g., by covering at least a single target species, and for a relative lack of excessive complexity (not too many constituent phages) will be difficult to optimize, especially if at least two phage types are required to target each possible bacterial target. In short, community resistance is logically more easily combatted than treatment resistance by employing preformulated phage cocktails. Regardless, successful treatment is dependent on the phages reaching the targeted bacteria in sufficient numbers, which is an issue of pharmacokinetics.
PHARMACOKINETIC OBSTACLES
Pharmacokinetics refers to the study of what happens to drugs, in terms of their movement and persistence, once they have entered a body. Pharmacokinetic obstacles therefore refer to those body aspects which limit the ability of a drug to build up to and then maintain effective concentrations over sufficient spans of time, ideally in association with targeted tissues. Traditionally, pharmacokinetics is differentiated into four categories. These are absorption, distribution, excretion, and metabolism. Absorption and distribution refer to movement within a body, with absorption describing movement into the blood and distribution referring to movement into other body tissues, particularly from out of the blood. Important as well is movement simply within body compartments, e.g., within the gastrointestinal tract, within the lungs, within bacterial biofilms, etc. Excretion also is movement but consists of movement out of the body, while metabolism refers to phage inactivation within the body. Collectively, we refer here to excretion and metabolism as “phage clearance,” which can also serve as a pharmacokinetic obstacle to phage therapy success. Failures in phage absorption or distribution or excessive phage excretion or inactivation represent obstacles to phage therapy success.
Accessibility of Bacteria to Phages within Bodies
Phages can be given orally, injected, or applied directly (locally) to selected organ or infection sites; e.g., they can be sprayed into the lungs or applied directly to an infected wound. To function as an antibacterial agent, the applied virions must then be able to physically reach the target bacteria in sufficient quantities. The pathway of such movement can be relatively simple, as is seen with some topical applications, or instead it can be somewhat complex, as is the case with systemic phage application targeting bacteria associated with nonblood tissues. In addition, with topical and oral administration, natural barriers decrease the amount of active phage entering the body. The study of such virion movement and its limitations, it should be noted, is what many consider to represent the essence of the study of the pharmacokinetics of phage therapy. Here, we discuss different routes of phage movement into and within the body, along with obstacles to that movement toward penetration to the target bacteria.
Absorption via injection.
Injections most easily overcome important body defensive barriers and have been demonstrated to be the most effective routes of systematic phage delivery. Injections in more modern approaches to phage therapy are commonly used, especially in preclinical animal models. These routes include the intraperitoneal (i.p.) (113–118), intramuscular (i.m.) (119–124), and subcutaneous (s.c.) (116, 125, 126) routes or direct injection into the blood intravenously (i.v.) (127–132). Administration by i.v. has also been applied to humans (133, 134) and has been reviewed by Speck and Smithyman (135).
Injections can be both efficient and very fast in delivering bacteriophages to the blood. Active phages are typically observed in blood circulation within the first hour and have even been observed in less than 5 min (136–142). Differences in the timing of phage access to the blood compartment can depend on the site of an injection; however, i.v. injections, of course, provide immediate delivery. Phage arrival in the blood was observed sooner after i.p. injection than after i.m. or s.c. injection. Injection by the i.p. route also resulted in higher phage titers than i.m. or s.c. injection, correlating with more effective protection of experimental animals from lethal septicemia (126). Together, these observations seem to imply, not surprisingly, that the fewest obstacles to phage absorption occur when phage is given by injection, though some injection routes (e.g., the i.v. route) present fewer obstacles than others. Alternatively, it is possible to inject phages directly into infected tissues (63). In that case, systemic circulation is bypassed and, thereby, distribution obstacles to phage movement are reduced, as are issues concerning phage clearance from the blood (see below).
Obstacles to per os (oral) delivery.
Among the various administration routes, oral delivery is usually the most convenient and most likely to be accepted by patients, as many drugs are administered orally. Oral administration of phages is not a consistently effective route of phage delivery to the systemic circulation, however, owing in part to unreliable phage absorption from the gastrointestinal tract. For example, Jun et al. (143) showed that i.p. injection allowed for the appearance of active phages in the blood 3 h sooner than oral application, with the maximum phage titer in the blood being reached as soon as 6 h prior to the peak being achieved by the orally administered phage (143). Analysis of the available experimental reports shows that only approximately 20% of oral applications were efficient in all investigated individuals, while 33% resulted in no penetration in any individuals of a studied group (42, 112, 113, 119, 123, 143–162). Consistently, no active bacteriophages were detected in blood in a human safety trial where T4 phages were given orally, despite the confirmed successful gut transit of the active phage (154), although phage presence in the blood was tested for only at the end of the trial.
Oral delivery is, of course, also fully applicable for targeting infections located in the gastrointestinal tract and can be considered an in situ delivery. Such delivery can still be inefficient, however, absent overcoming the stomach acid obstacle. Phages in particular are often sensitive to extreme pH values, such as those associated with stomach acidity, though they are also sensitive to the lower pH of the skin or vagina (163–166). Requirements for phage passage through gastric juices can thus serve as an obstacle to oral phage delivery, whether toward systemic delivery or toward in situ delivery. Consequently, acidity neutralizers are often applied or advised to improve phage transfer through the stomach (167).
Toward in situ oral phage treatment of cholera-infected patients, Monsur et al. (168) demonstrated that very high phage doses (1013 to 1014 PFU), together with phage propagation on infecting bacteria, resulted in phage concentrations in feces that were higher than 1011 PFU/g. The resulting phage absorption, however, was nevertheless still inefficient, reaching only approximately 102 PFU/ml of patient blood. These findings suggest that obstacles to phage absorption from the small intestine can be profound even when phage passage through the stomach is efficient. This problem of inefficient phage absorption following oral delivery can be counteracted to some extent by increasing the phage dose, since the efficacy of phage penetration from the gastrointestinal tract is positively correlated to the phage dose applied (158). In small-animal models, such as rodents and chickens, the minimum oral doses used for the systematic delivery of phages have been about 107 to 109 PFU per animal (108, 148, 158), but the concentrations achieved in blood were poor, and it is uncertain at what point increasing phage numbers might saturate absorption out of the gut.
In contrast, a high potential for transcytosis across the small intestine wall, thereby resulting in absorption, has been calculated from in vitro intestinal cancer cell cultures, with more than 3 × 1010 phages estimated to be absorbed every day (approximately 3.5 × 105 PFU per second) (169). The discrepancy between the seemingly easy translocation demonstrated in vitro and problems with achieving high phage titers in blood after oral administration suggests that in vivo obstacles to phage delivery from the gut to the blood are likely important determinants of this pharmacokinetic characteristic. The blocking role may specifically be played by the intestinal lymph node system, as postulated by Smith et al. (170). Lymph nodes are filters with a 70-kilodalton cutoff that prevent molecules from entering the parenchyma and high endothelial venules (171), whereas the sizes of most phages are measured in megadaltons (e.g., the T4 phage head is 194 MDa). Wolochow and colleagues (145, 148) demonstrated that in the gastrointestinal tract, phages are absorbed into the lymph nodes rather than into the blood. This pathway is consistent with the absorption pathways of large drug carriers (nanocapsules). These drug carriers, when absorbed from the gastrointestinal tract, also reach the blood and organs by first traveling through the lymphatic system (172–174). Phages may thus be predisposed to being filtered out by intestinal lymph nodes. Considering the results of phage oral delivery reported from in vivo models, however, we hypothesize that the filtering of phages by lymph nodes is not absolute. Thus, filtering could be inefficient enough to result in oral delivery exposing phages, despite this substantial obstacle to systemic delivery. Nevertheless, the possibility of the existence of other mechanisms of body resistance to phage absorption from the gastrointestinal tract cannot be excluded.
Topical delivery, absorption, and obstacles to phage distribution into biofilms.
Topical delivery is the application of phages to surfaces. Inhalation of phages, for example, can result in topical phage delivery (175), though it can also result in systemic delivery (i.e., absorption). Compared to i.p. and i.m. injections in animal models, however, inhalation has resulted in poor absorption efficacy (176, 177). Topical phage delivery to the lungs by inhalation, in contrast, has successfully been demonstrated many times to be capable of controlling respiratory tract infections (16, 178–185). Moreover, in mice with experimental pneumonia, the effectiveness of phage therapy was found to be dose dependent (186). Indeed, just as with direct phage application to a site of infection, we have an expectation that topical phage delivery, so long as phages are capable of reaching the targeted bacteria by such routes (as discussed especially in terms of biofilms below), should generally present fewer obstacles to phage penetration to bacteria than systemic delivery by any route.
More familiarly in terms of topical delivery is delivery to the skin, mucous membranes, or, especially, wounds. Such delivery is usually not intended for phage absorption into the blood but also is distinct from the goal of phage distribution since the phages do not have to move to new locations in order to reach their target bacteria. On the other hand, in many cases with topical delivery, what these phages are being delivered to is biofilms, arguably requiring a distribution-like phage movement into those biofilms.
Obstacles to phage movement into biofilms are generally considered to consist of extracellular polymeric substances (EPS), which make up the biofilm matrix but which also, in the case of capsule material, can be associated with non-biofilm-associated bacteria as well. That EPS can serve as a bacterium-imposed barrier to phage adsorption, however, is not in fact certain. On the one hand, some phages exist which display EPS depolymerase enzymes in association with their virions (187), which would seem to be present to allow phages to reach bacterial surfaces that are otherwise unreachable (188). Alternatively, however, these enzymes may simply allow an acceleration of the adsorption process, and it has even been argued that they can allow EPS to serve as a primary receptor for phage interaction with bacteria (188, 189). On the other hand, biofilm clearance has been shown to be achieved without prior consideration of phage carriage of EPS depolymerase enzymes (190–195), or EPS depolymerases may not have much impact, if they are present (196). Whether or not biofilms can in fact consistently impose pharmacokinetic obstacles to phage access to bacterial surfaces therefore remains uncertain for a majority of treatment phages.
Tissue delivery (distribution).
Major organs that are commonly investigated for phage presence following their systemic delivery are the spleen and liver. Phages can enter these organs from the blood within minutes (197–199) and within 1 to 3 h can achieve high intraorgan titers that are often even higher than those in the blood (119, 124, 127, 129, 144). The spleen and liver are key elements of the mononuclear phagocyte system (MPS; which was previously also called the reticuloendothelial system [RES]) (119). Few obstacles to phage entrance into these organs from the blood thus exist, though it is nevertheless questionable whether phages truly remain available for adsorption onto extracellular bacteria after such sequestration. The acquisition of phages by these organs should thus generally be viewed more as a form of phage clearance from the blood rather than phage distribution to the spleen or liver.
Other organs can also be easily penetrated by phages, though they do not as readily accumulate phages. Plaque-forming units have been recovered from skeletal muscles, bone marrow, kidneys and bladder, heart, thymus, and the salivary glands and saliva following administration by various routes (50, 116, 119, 120, 144, 147, 155, 158, 161, 183, 197, 200–202). Thus, infections in these body sites can potentially be controlled by bacteriophages which have been delivered to systemic circulation, particularly to the extent that phages can leave the blood and move to sites of infection due to, for example, blood vessel damage. Phages can also penetrate from the blood to the gastrointestinal tract (119, 121, 144, 158, 197, 203), though this seems less practical, given the effectiveness of oral delivery for achieving considerable phage titers in the gastrointestinal tract. Thus, obstacles to phage distribution do not appear to be found in terms of phage movement though the blood vessels found in these organs. These obstacles, rather, block phage movement out of vessels, such as into interstitial fluid. Nevertheless, the efficiency of such movement and, in particular, the impact of bacterial infections breaching blood vessels sufficiently to allow phage movement locally out of the blood, thereby overcoming obstacles to phage distribution, are poorly understood.
Bacteriophages have been demonstrated in animal brains (114, 116, 120, 144, 183, 201, 204) and have also been used to successfully control intracerebral infections (204), in spite of a blood-brain barrier that is often challenging to drug distribution (205). Blood-to-tissue phage concentration ratios in rodents suggest that shortly after i.p. administration, i.e., after 2 and 6 h, most phages detected in brain tissue were probably still, in fact, in the blood, but at 24 h after administration, phages reportedly had effectively crossed the blood-brain barrier (116). The blood-brain barrier is thus an expected obstacle to phage distribution to the brain, not surprisingly, but a barrier which may also be breached, perhaps especially in the case of bacterial infection of the brain. The extent to which infections explicitly impact phage movement out of the blood and, indeed, the mechanism of such movement have nevertheless, to the best of our knowledge, not been well characterized.
Phages delivered systemically may penetrate into the lumen of the lungs (176, 201). The efficiency of this delivery, however, has been questioned by Takemura-Uchiyama et al. (118). They observed phages in bronchoalveolar lavage fluid (BALF) only in animals that had been challenged with bacteria and not in the unchallenged group, concluding that bacterial infection might cause micropores in tissues (including blood vessel endothelium), thereby facilitating phage diffusion from the bloodstream. Semler at al. (117) successfully controlled bacterial pulmonary infections by phage inhalation but not by i.p. administration. Thus, phage penetration from blood to the lumen of the respiratory tract is not necessarily efficient. As long as the infection does not involve the deeper tissue layers surrounding the lungs, inhalation seems to be the most efficient way of therapeutic phage delivery into the lumen of the lungs.
Intranasal administration to mice was efficient in the delivery of filamentous phages to the brain, though morphologically different forms of the same phages, which were described to be “spheroid” by the authors, were not able to penetrate in that way (206). This suggests that optimal delivery routes may be different for different phages as well as for different types of bacterial infections. Phage distribution as well as absorption is likely dependent at least on the general characteristics of the phage virion morphology but is potentially also dependent on other features not yet identified, such as the specific characteristics of the proteins making up the phage capsids. It is nevertheless of interest that the nasal delivery of certain phages may at least potentially present fewer obstacles to brain delivery than systemic delivery, possibly due to anatomical proximity. This superiority, as noted above, is likely limited in terms of what phages are applied and, indeed, might not be superior given the occurrence of brain bacterial infections.
Although phages seem to gain access to many tissues relatively easily, it is unknown if they are able to achieve inundative concentrations at sites of bacterial infection through movement (absorption and distribution) alone. Achieving inundative phage concentrations, in turn, is essential for achieving phage therapy success (87, 91). The potential for therapeutic phages to penetrate into specific organs or tissues can be increased by molecular engineering of phage virions, specifically by the display on phage surfaces of small peptides that promote phage accumulation in these tissues as so-called specific molecular addresses (207). This idea relates to the fundamental studies of Pasqualini and Ruoslahti (208) and Ruoslahti (209), who demonstrated the potential of homing peptides specific to selected organs. Notably, many peptides that can facilitate the delivery of nanoparticles to selected tissues were identified by the use of phage display libraries, i.e., pools of phages presenting short peptides on virions (153, 210–217). These observations directly indicate the applicability of phage display of short peptides for improving phage targeting inside the body, specifically for increasing phage delivery to the site of a localized infection, i.e., toward improved pharmacokinetics.
Clearance of Phages from Bodies
Metabolism and excretion are considered here primarily from the perspective that they serve as reducers of phage densities in situ and, thereby, as obstacles to the buildup and maintenance of phage densities. Collectively, we describe this as phage clearance from bodies. Metabolism as a phage means of inactivation involves actual virion breakdown or the binding of inactivating substances to virions. This generally occurs postabsorption or after local or in situ phage delivery. Excretion, in contrast, is drug movement out of the body in a drug-intact form.
In the gastrointestinal tract and on mucosal surfaces, phages can be affected by proteases. Experimental data available so far suggest, however, that bacteriophages demonstrate limited sensitivity to digestive enzymes (e.g., trypsin), but this differs between phage types. Notably, other proteases, such as proteinase K or papain, can easily inactivate phages resistant to trypsin (218–223). Unfortunately, so far there are no experimental reports available to assess the effects exerted on phages specifically by tissue proteases or those enzymes secreted onto the mucosal surfaces of the lungs. We have also discussed phage inactivation by gastric juices (see above), though this is more an inefficiency in phage delivery than inactivation postdistribution or postabsorption.
Here, we especially consider the impact of the vertebrate immune system on phage clearance, focusing on nonspecific versus antigen-specific mechanisms. These are phages as particles (nonspecific) and phages as protein complexes (specific), respectively, and also phage excretion (nonspecific) and phage metabolism (specific), respectively. Note that the potential for phage maintenance in distinct tissues is currently mostly unrecognized, except in the liver, spleen, and blood (119, 129). As a consequence, the impact of metabolism on phage clearance is generally considered from the perspective of as it occurs across whole bodies rather than as it occurs in specific locations, again, other than in association with the liver, spleen, and blood.
Nonspecific phage clearance via excretion.
Renal clearance is involved in the excretion of many drugs, with the result being removal of the drug into the urine. The molecular weight cutoff for glomerular filtration is thought to be 30 to 50 kDa, that is, for removal from the blood by kidney nephrons. The passage of larger objects has also been demonstrated, and with disease, size sieving by the kidneys can be substantially increased (224, 225). As evidenced by experimental reports, the nonspecific clearance of bacteriophages by excretion does not appear to be highly effective. Although phages have been detected in the kidneys and/or urine either in animals (116, 125, 137, 144, 150) or in humans (149, 159), in most cases phage excretion was not seen in all individuals. In addition, high levels of quantitative variability can be observed between individuals. In humans treated orally for various types of infections, phages were detected in the urine of 87.3% (n = 55) of treated children (159) and in that of 35% (n = 26) of treated adults (149). Urine titers, however, were a few orders of magnitude lower than blood titers in the same individuals (116, 125, 137, 144, 150). Schultz and Neva (226) proposed 109 PFU given by i.v. injection to be the minimum dose necessary to result in phage detection in the urine of mice.
Since bacteriophages can be stable in human and animal urine in vitro (201, 227, 228), low phage titers in urine simply suggest low rates of passage into the glomerular filtrate. From a practical perspective, in the case of phage therapy of bladder infections, phage administration directly into the urinary tract (229–231) will likely help to achieve higher therapeutic concentrations of therapeutic phages than systemic administration. The rates of renal excretion of phage virions nonetheless do not appear to result in substantial obstacles to maintaining phage densities elsewhere in the body.
Nonspecific phage clearance via phagocytosis (metabolism).
Poor phage removal via the renal route does not in itself result in the prolonged blood circulation of active phages. The phage half-life in the blood of mice, for example, has been estimated to be 2.2 to 4.5 h in different models (198, 199, 232). Reports of the half-lives from animal models of antibiotic pharmacokinetics cover a wide range, from approximately 0.5 h to more than 7 h (233–239), which seems comparable to the phage half-lives measured (198, 199, 232) (notably, half-lives in mice should translate to longer half-lives in humans, due to known differences between the two species). A simple explanation for why phages escape renal clearance can be derived from quantum dot studies that make use of particles of defined size. Large model particles (>8 nm) were not found in the bladder but instead were trapped by the mononuclear phagocyte system (MPS) (240). Thus, we can conjecture that the major feature directing phage particles to the MPS rather than renal filtration is the large size of phage particles, i.e., particles that are typically much larger than 8 nm. This affects the renal sieving cutoff that prevents the efficient excretion of phages, as well as the MPS capability to capture larger objects.
The potential correlation between phage size and its rate of clearance has been investigated only once, by Hajek (130). Phage T2 (Myoviridae, approximately 90 by 200 nm in size) was removed much faster than the phiX174 phage (Microviridae, approximately 30 nm in diameter) from newborn-pig circulation. This suggests that large phage virions could be easier to filter out in vivo, via the MPS, than smaller ones, and it further implies that small phages may be characterized by more favorable pharmacokinetics (i.e., they potentially can circulate and be bioavailable in the system for longer), while the large ones appear to be removed faster, at least from blood.
The liver and spleen are considered to be the main organs that immunologically filter out blood-circulating phages (119, 124, 127, 129, 144, 197–199). Since these organs are key elements of the MPS, as they are settled by numerous phagocytes, they are considered to be not only major phage traps but also organs which are actively engaged in phage neutralization. Only very scarce data allow for comparisons between the spleen and liver in terms of their effectiveness in phage neutralization, however. On the one hand, the spleen is the organ where active phages can be detected at the highest titers and for the longest periods, even for many days after administration (116, 119, 120, 137, 144, 198, 241, 242). Such high levels of measured trapping could be a result of the more effective filtration of phages, but they could also be due to less effective phage neutralization.
Inchley (129) investigated phage accumulation in tissues by the detection of radiation from 51Cr-labeled T4 phages rather than by the determination of the phage plaquing ability. He showed that 70 to 90% of the applied 51Cr was observed in the liver. Thus, even though both liver and spleen accumulate phage particles very effectively, it is probably the liver that most rapidly inactivates phages. Other parts of the MPS, such as the lymph nodes, are less often involved, but as suggested above, they are also capable of sequestering as well as neutralizing bacteriophages (50, 112). Phagocytosis can result not only in direct neutralization of a bacteriophage (50, 51, 119, 129, 243) but also, potentially, in antigen presentation by antigen-presenting cells, mainly dendritic cells and macrophages.
Nonspecific phage clearance via the complement system (metabolism).
A potentially underestimated element of innate immunity that contributes to phage neutralization in vivo is the serum complement system. This enzymatic cascade mediates the removal of invading microorganisms without specific recognition (244). Although phages are not pathogenic, bacterial viruses nevertheless are also susceptible to complement system activity (50, 136, 245). In addition to directly destroying viral particles, proteins of the complement cascade facilitate phagocytosis, thereby serving as opsonizing agents, which has the effect of enhancing phage sequestration as well as inactivation in vivo (130, 131, 246).
The degree of phage susceptibility to neutralization by the complement system affects the duration of active phage circulation in the animal or human body. Notably, long-circulating phages, which are mutants that remain actively circulating in the blood longer than the parental phages, have been shown to be more effective as therapeutic agents (115, 247). Typically, these phage variants are selected by repeated passage in living animals (by injection-recovery from blood), but Sokoloff et al. (248) linked phage susceptibility to neutralization in vivo to phage interaction with the serum complement system. They used phage display technology to identify amino acids that, when exposed on phage virions, caused a long-circulating phenotype in rats and then demonstrated that exposed peptides with a C-terminal arginine or lysine on phage virions resulted in decreased phage susceptibility to neutralization by serum complement. Thus, phages can be engineered to escape nonspecific neutralization, or if they are engineered without consideration of interaction with the complement system, they may become more sensitive to this form of neutralization (249).
Specifically, long-circulating phages selected by Vitiello et al. (250) had a mutation causing the substitution of glutamic acid for lysine in the phage major capsid protein. Display of this particular amino acid may decrease interactions of components of the complement system with the phage, equivalent to the findings for the modifications investigated by Sokoloff et al. (248). Thus, though the authors proposed lower phage susceptibility to the MPS (i.e., phagocytosis) as the causative mechanism of their longer circulation, a role for increased relative resistance of phages to neutralization by the complement system is possible as well.
Antigen-specific phage clearance.
Elements of the immune system can neutralize phage particles even when no specific response to bacteriophages has yet developed (see above), but the neutralization of phage virions is not limited to nonspecific responses. Indeed, the ability of phage virions to induce specific antibodies has been observed many times in animals (50, 108, 122, 178, 245, 251–255) and in humans (133, 256, 257). Antibodies are typically considered to potentially have a devastating effect on bacteriophages (50, 128, 133, 146, 178, 256, 258) and thereby potentially on phage therapy. As pointed out by Jerne (259) and Jerne and Avegno (260), however, only a fraction of phage virion-specific antibodies are expected to be virion neutralizing.
In humans, no phage-specific antibodies were detected in healthy volunteers treated with bacteriophages per os (154). Consistently, after therapeutic applications, antibody induction can be rather weak, thereby not affecting the phage ability to control bacterial infections (261). This corresponds to the fact that the efficient induction of specific antibodies requires sufficient time and antigen doses. Typically, a full response develops only weeks after phage exposure (108, 157, 262), and it can therefore be observed only many days after the time necessary to complete treatment (263). Consequently, antibodies seem to be a problem particularly envisioned for recurring phage applications, that is, when the same patient is treated repeatedly over long periods with the same phage (264). This perhaps is particularly a problem given systemic rather than topical exposure to phages for the treatment of systemic rather than gastrointestinal infections.
Phage cocktails are often recommended as a remedy to the problem of bacterial resistance to phages as well as to the challenge of developing ready-to-use phage preparations with a sufficiently broad spectrum of activity (see above). Mixed preparations of many phages, especially of somewhat unrelated phages, however, will obviously contain a wider collection of phage antigens than preparations of single phages. They therefore would be capable of inducing specific immune responses to more phages than monotherapy. Phage antigenicity should thus be considered when generating optimal phage cocktails. That is, the use of antigenically very similar phages, but phages that still have complementary host ranges, would be preferable for cocktail generation to the use of mixtures with antigenically distinct phages. Nevertheless, general knowledge of phage antigenicity is currently very limited (245) and does not allow even for estimation of whether cocktails containing antigenically similar natural phages, versus antigenically similar engineered phages, could be made available (265). With the development of therapeutic phage collections, identification of phage-specific epitopes could be an important part of phage characterization, adding to the well-established characterization of phage host range, and such characterization would also be able to screen for potentially undesirable genes in phage genomes.
Protection from clearance.
Phages seem to be able to counteract the neutralizing action of the immune response. That is, they show some immunity-silencing activity that could contribute to prolonged phage maintenance in the body, as can be concluded from ex vivo testing of immunological cells and mathematical modeling (68, 266). Phage pharmacokinetics can also be markedly changed by the encapsulation of bacteriophages. Most available reports documenting the use of encapsulated phages in vivo indicate the prolonged release of phages, which seems to be similar to constant release and different from the release achieved with bolus administration (166, 267, 268). Encapsulation not only delivers prolonged release but also protects bacteriophages from inactivation by possible chemical stress (e.g., gastric secretions) or by immunological factors. As has been demonstrated, encapsulated phages are able to remain active in a living system for longer (longer circulation), which can translate into antibacterial efficacy in vivo which is better than that of a relevant but nonencapsulated phage (166, 269).
Obstacles to successful phage therapy that may be related to high rates of phage neutralization by immune system factors can thus potentially be decreased by making phages less visible to the immune system, including by the use of various types of encapsulation. A prerequisite to this kind of solution is, of course, a lack of or a minimal impact of phage encapsulation on the ability of phages to infect bacteria, or at least an effect that is less than the beneficial effect of encapsulation on improving phage survival in vivo and the resulting better access of the phages to bacterial cells. Note that an earlier effort to protect virions from immune system effects involved particle PEGylation, i.e., conjugation to virions of monomethoxy-polyethylene glycol (132). One aspect common to all encapsulation technologies is that while they protect dosed phages, they have no impact on phage virions that are generated in situ, such as during the course of active treatments.
CONCLUSIONS
Obstacles to drug functioning can interfere with drug penetration into target tissues, drug retention in association with those tissues, and the impacts of the drug on those tissues once sufficient drug densities have been reached. Pharmacokinetic obstacles are those which interfere with drug density buildup and retention in the vicinity of target tissues, collectively, drug movement, while pharmacodynamic obstacles are ones that interfere with drug action once the concentration of the drug is built up and the drug is retained in association with target tissues. Approaching drug development in a pharmacologically aware manner thus requires appreciation of potential obstacles to pharmacokinetic as well as pharmacodynamic functioning. Here, we have catalogued such obstacles to the success of phage therapy that include, on the pharmacodynamic side, phage host range and bacterial resistance to phages and, on the pharmacokinetic side, the balance between phage movement and phage clearance from the body (Fig. 3). Both better awareness and better characterization of potential obstacles should result in more efficient development of phage therapy as a successful modern antibacterial strategy.
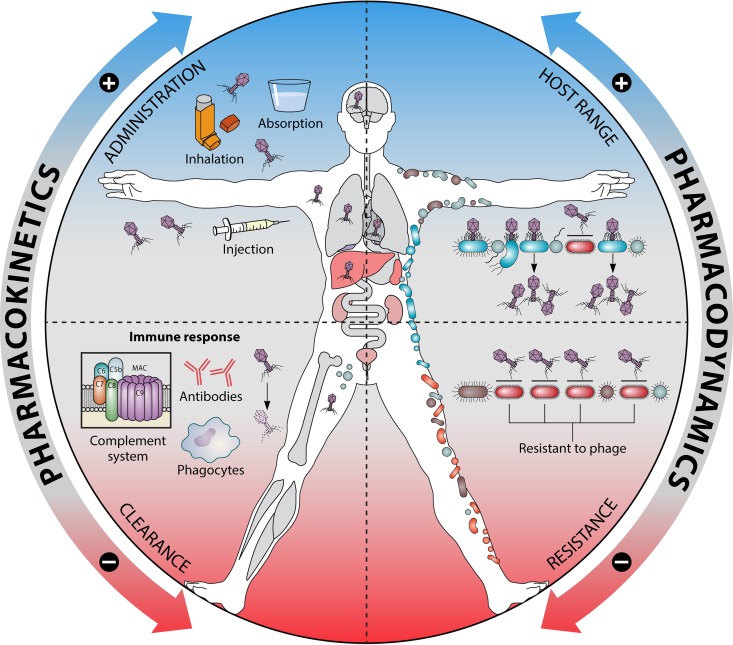
FIG 3.
Effects of pharmacokinetics and pharmacodynamics on bacteriophages in bodies. The ability of phages to penetrate into a bacterium-containing living system and to reach specific sites of bacterial infection is crucial for achieving an effective therapeutic concentration by the phage. The phage concentration decreases due to body responses, mainly due to the pressure of the immune system, with a prominent contribution of nonspecific mechanisms of inactivation. Phage host range defines the applicability of phage therapy at the practical level of the available phage strains. Bacterial resistance mechanisms limit a phage’s host range and related spectrum of activity as an antibacterial drug. MAC, membrane attack complex.
APPENDIX: PHAGE THERAPY PHARMACOLOGICAL AWARENESS
Selectively toxic antibacterial agents—agents toxic for the bacteria and not for the patient—are useful because of their potential to disrupt harmful bacteria without also harming bodies. Therefore, a major concern in antibiotic development, indeed, for drug development generally, is to identify agents which can be effective without at the same time excessively harming treated individuals (270). Broad therapeutic windows, i.e., the difference between effective doses and harmful doses, can, as a consequence, remove many of the constraints on pharmaceutical use and, thereby, on pharmaceutical development. Why try harder if patients, without such additional effort, are both cured and not significantly harmed? Thus, with phage therapy, which has generally been deemed nonharmful, there has been substantial technology development without a corresponding robust development of extensive pharmacological awareness. This is seen particularly in terms of pharmacokinetics, such as possible restrictions on phage movement to target bacteria. It is also relevant to phage therapy pharmacodynamics, especially considerations of how many phages must be delivered to the target bacteria to consistently achieve efficacy. Here, we consider the reasons that pharmacology has been a less prominent aspect of many phage therapy studies than it has been, e.g., for antibiotics.
One reason, as alluded to in the previous paragraph, is that during phage therapy phages have generally tended to be relatively safe, with few side effects (16, 42, 58, 271, 272), perhaps especially in modern, purified forms (61, 62, 64, 273, 274). This allows the exploration of phage therapy without excessive concerns over safety, thereby resulting in a strong emphasis on efficacy without much consideration of the subtleties of properly balancing antibacterial effectiveness against toxicity issues. Consequently, phage therapy development has focused almost entirely, first, on finding serviceable phages (77, 273, 274) and, second, on determining what delivery routes tend to result in the greatest effectiveness and/or convenience (11), and this is rather than focusing on reduced toxicity.
A second reason for the relative absence of pharmacological awareness in phage therapy development is that phages, unlike most drugs, are often able to increase their numbers in the course of effecting their bactericidal activity. This property has the consequence of further reducing the need to more formally explore phage therapy pharmacokinetics. That is, in many cases it seems that if only phages can reach the target bacteria, then phage therapy can be successful, even without necessarily reaching the bacterial targets at inherently inundative doses. Therefore, there is little need to worry about issues of phage delivery from a quantitative perspective; only a qualitative perspective is needed. Of course, phages still need to reach those bacteria at some level to be effective, so pharmacokinetics are not irrelevant. Furthermore, it is well understood that not all bacterial concentrations are sufficient to support adequate in situ phage population growth (41, 45–47, 91, 275).
A consequence of this second point—that phages in fact can replicate to higher densities while interacting with target bacteria in situ—is that with phage characterization for phage therapy, there is much less consideration of minimum inhibitory concentrations (MICs). Indeed, due to phage replication, it can be difficult even to conceptually define a phage MIC (89). Phages also display single-hit killing kinetics; that is, only a single phage is typically required to kill a single bacterium (92). This, too, makes the concept of MIC difficult to apply to phages. There also is little tradition in phage therapy of analyzing phage antibacterial performance beyond facile host range analyses (40), with a typical attitude being that if one phage isolate does not prove to be effective, then another phage may simply be substituted in its place (10, 96). Nonetheless, there is a growing movement to attempt to link phage in vitro properties with phage therapy success (181, 274, 276, 277), though clear limitations to this approach exist (278, 279).
Lastly, as an approximately 100-year-old technology, phage therapy was not developed using the standard, modern approach to drug development, that is, beginning with extensive preclinical testing, which is followed by multiple phases of clinical trials. For many decades, phage therapies instead were primarily developed in the course of clinical experimentation (7–9, 12, 18, 20). More recently, much of phage therapy clinical use has taken place under the guise of compassionate care, otherwise known as expanded access (58, 60–62, 280–282). Though animal models have certainly been relevant to phage therapy development in modern times (16, 20, 22), the focus of compassionate use understandably tends to be on curing patients, and this is rather than a focus on detailed microbiological or immunological analyses, iterative improvements to procedures, or detailed published documentation of individual phage treatments.
A combination of relatively low toxicity, an ability of phages to amplify in concentration (particularly in association with targeted tissues, in this case, in association with the targeted bacteria), difficulties in even defining standard measures of in vivo utility (such as MIC), and a tradition of testing in vivo in the clinic rather than in the laboratory has resulted in an often bypassing of many pharmacological norms in phage therapy development. Nevertheless, improvements in pharmacological awareness during phage therapy development should be useful, particularly toward obtaining a better understanding of the bases of treatment failures, especially in light of the need to fit phages into more standard routes toward regulatory approval (283, 284).
ACKNOWLEDGMENTS
K.D. is a recipient of grants from the National Science Centre in Poland (grants UMO-2012/05/E/NZ6/03314 and UMO-2018/29/B/NZ6/01659).
We are grateful to Anna Kukuła (Umi Studio Wrocław) for her valuable help with graphical work.
S.T.A. has consulted for and served on advisory boards for companies with phage therapy interests, holds an equity stake in a number of these companies, and maintains the websites phage.org and phage-therapy.org. The text presented, however, represents the perspectives of both authors alone, and no outside help was received in its writing.
Biographies

Krystyna Dąbrowska studies immune responses to phage and phage-derived proteins as they relate to therapeutic applications to animals and humans. She has been active in these studies for almost 20 years, with the title of her 2004 Ph.D. dissertation being “Studies of Phage-Mammalian Cell Interactions,” and her Ph.D. was awarded by the Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland. She has continued her research at the institute, with a major focus on molecular microbiology, immunology, new antibacterials derived from bacteriophages, and microbiomes. These serve as a basis for her studies of phage pharmacokinetics and bioavailability.

Stephen T. Abedon studies the ecology of bacteriophages, their evolutionary ecology, the history of their study, and their pharmacology, the last of which is in the context of phage use as antibacterial agents (phage therapy). He has been active in these studies since the late 1980s, with the title of his 1990 Ph.D. dissertation being “The Ecology of Bacteriophage T4.” Dr. Abedon has over 100 publications, mostly in these areas, including numerous edited volumes and monographs (as editor or coeditor) and one single-authored book. He was a founder of the currently topical study of phage-phage intercellular communication as well as the study of bacteriophage life history evolution. He also has been active in considering the ecology of phage interactions with spatially structured bacterial populations, such as those seen in the context of bacterial biofilms but also phage plaques.
REFERENCES
- 1.Karam G, Chastre J, Wilcox MH, Vincent JL. 2016. Antibiotic strategies in the era of multidrug resistance. Crit Care 20:136. doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furfaro LL, Payne MS, Chang BJ. 2018. Bacteriophage therapy: clinical trials and regulatory hurdles. Front Cell Infect Microbiol 8:376. doi: 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neu HC. 1992. The crisis in antibiotic resistance. Science 257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 4.Martens E, Demain AL. 2017. The antibiotic resistance crisis, with a focus on the United States. J Antibiot (Tokyo) 70:520–526. doi: 10.1038/ja.2017.30. [DOI] [PubMed] [Google Scholar]
- 5.Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, Henderson IR, Hilpert K, Jones BV, Kadioglu A, Knowles D, Olafsdottir S, Payne D, Projan S, Shaunak S, Silverman J, Thomas CM, Trust TJ, Warn P, Rex JH. 2016. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis 16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 6.Housby JN, Mann NH. 2009. Phage therapy. Drug Discov Today 14:536–540. doi: 10.1016/j.drudis.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. 2010. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 8.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. 2011. Phage treatment of human infections. Bacteriophage 1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper DR, Anderson J, Enright MC. 2011. Phage therapy: delivering on the promise. Ther Deliv 2:935–947. doi: 10.4155/tde.11.64. [DOI] [PubMed] [Google Scholar]
- 10.Pirnay J-P, De Vos D, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M, Zizi M, Laire G, Lavigne R, Huys I, Van den Mooter G, Buckling A, Debarbieux L, Pouillot F, Azeredo J, Kutter E, Dublanchet A, Górski A, Adamia R. 2011. The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharm Res 28:934–937. doi: 10.1007/s11095-010-0313-5. [DOI] [PubMed] [Google Scholar]
- 11.Ryan EM, Gorman SP, Donnelly RF, Gilmore BF. 2011. Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J Pharm Pharmacol 63:1253–1264. doi: 10.1111/j.2042-7158.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 12.Chanishvili N. 2012. Phage therapy—history from Twort and d’Herelle through Soviet experience to current approaches. Adv Virus Res 83:3–40. doi: 10.1016/B978-0-12-394438-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 13.Krylov VN. 2014. Bacteriophages of Pseudomonas aeruginosa: long-term prospects for use in phage therapy. Adv Virus Res 88:227–278. doi: 10.1016/B978-0-12-800098-4.00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Verbeken G, Huys I, Pirnay JP, Jennes S, Chanishvili N, Scheres J, Gorski A, De Vos D, Ceulemans C. 2014. Taking bacteriophage therapy seriously: a moral argument. Biomed Res Int 2014:621316. doi: 10.1155/2014/621316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viertel TM, Ritter K, Horz HP. 2014. Viruses versus bacteria—novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 69:2326–2336. doi: 10.1093/jac/dku173. [DOI] [PubMed] [Google Scholar]
- 16.Abedon ST. 2015. Phage therapy of pulmonary infections. Bacteriophage 5:e1020260. doi: 10.1080/21597081.2015.1020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanishvili N. 2016. Bacteriophages as therapeutic and prophylactic means: summary of the Soviet and post Soviet experiences. Curr Drug Deliv 13:309–323. doi: 10.2174/156720181303160520193946. [DOI] [PubMed] [Google Scholar]
- 18.Górski A, Międzybrodzki R, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Rogóż P, Jończyk-Matysiak E, Dąbrowska K, Majewska J, Borysowski J. 2016. Phage therapy: combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front Microbiol 7:1515. doi: 10.3389/fmicb.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abedon ST. 2017. Bacteriophage clinical use as antibacterial “drugs”: utility and precedent. Microbiol Spectr 5:BAD-0003-2016. doi: 10.1128/microbiolspec.BAD-0003-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abedon ST. 2018. Phage therapy: various perspectives on how to improve the art, p 113–127. In López-Baena CMAF. (ed), Host-pathogen interactions. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 21.Sybesma W, Rohde C, Bardy P, Pirnay JP, Cooper I, Caplin J, Chanishvili N, Coffey A, De Vos D, Scholz AH, McCallin S, Puschner HM, Pantucek R, Aminov R, Doskar J, Kurtbke DI. 2018. Silk route to the acceptance and re-implementation of bacteriophage therapy—part II. Antibiotics (Basel) 7:E35. doi: 10.3390/antibiotics7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abedon ST. 3 July 2018. Use of phage therapy to treat long-standing, persistent, or chronic bacterial infections. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P, Singh HS, Shukla VK, Nath G, Bhartiya SK. 2019. Bacteriophage therapy of chronic nonhealing wound: clinical study. Int J Low Extrem Wounds 18:171–175. doi: 10.1177/1534734619835115. [DOI] [PubMed] [Google Scholar]
- 25.Loc-Carrillo C, Abedon ST. 2011. Pros and cons of phage therapy. Bacteriophage 1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. 2012. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol 10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Integrative HMP (iHMP) Research Network Cosnortium. 2014. The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 16:276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dąbrowska K, Witkiewicz W. 2016. Correlations of host genetics and gut microbiome composition. Front Microbiol 7:1357. doi: 10.3389/fmicb.2016.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Price J, Abu-Ali G, Huttenhower C. 2016. The healthy human microbiome. Genome Med 8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. 2019. Role of the microbiome in human development. Gut 68:1108–1114. doi: 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. 2018. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varughese CA, Vakil NH, Phillips KM. 2013. Antibiotic-associated diarrhea: a refresher on causes and possible prevention with probiotics—continuing education article. J Pharm Pract 26:476–482. doi: 10.1177/0897190013499523. [DOI] [PubMed] [Google Scholar]
- 35.Vangay P, Ward T, Gerber JS, Knights D. 2015. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17:553–564. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langdon A, Crook N, Dantas G. 2016. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med 8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaser MJ. 2018. Our missing microbes: short-term antibiotic courses have long-term consequences. Cleve Clin J Med 85:928–930. doi: 10.3949/ccjm.85gr.18005. [DOI] [PubMed] [Google Scholar]
- 38.Bello MGD, Knight R, Gilbert JA, Blaser MJ. 2018. Preserving microbial diversity. Science 362:33–34. doi: 10.1126/science.aau8816. [DOI] [PubMed] [Google Scholar]
- 39.Schulfer AF, Schluter J, Zhang Y, Brown Q, Pathmasiri W, McRitchie S, Sumner S, Li H, Xavier JB, Blaser MJ. 2019. The impact of early-life sub-therapeutic antibiotic treatment (STAT) on excessive weight is robust despite transfer of intestinal microbes. ISME J 13:1280–1292. doi: 10.1038/s41396-019-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyman P, Abedon ST. 2010. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol 70:217–248. doi: 10.1016/S0065-2164(10)70007-1. [DOI] [PubMed] [Google Scholar]
- 41.Sarker SA, McCallin S, Barretto C, Berger B, Pittet AC, Sultana S, Krause L, Huq S, Bibiloni R, Bruttin A, Reuteler G, Brussow H. 2012. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology 434:222–232. doi: 10.1016/j.virol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 42.McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, Krause L, Bibiloni R, Schmitt B, Reuteler G, Brüssow H. 2013. Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects. Virology 443:187–196. doi: 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Galtier M, De Sordi L, Maura D, Arachchi H, Volant S, Dillies MA, Debarbieux L. 2016. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ Microbiol 18:2237–2245. doi: 10.1111/1462-2920.13284. [DOI] [PubMed] [Google Scholar]
- 44.Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charton F, Bourdin G, McCallin S, Ngom-Bru C, Neville T, Akter M, Huq S, Qadri F, Talukdar K, Kassam M, Delley M, Loiseau C, Deng Y, El Aidy S, Berger B, Brüssow H. 2016. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarker SA, Berger B, Deng Y, Kieser S, Foata F, Moine D, Descombes P, Sultana S, Huq S, Bardhan PK, Vuillet V, Praplan F, Brussow H. 2017. Oral application of Escherichia coli bacteriophage: safety tests in healthy and diarrheal children from Bangladesh. Environ Microbiol 19:237–250. doi: 10.1111/1462-2920.13574. [DOI] [PubMed] [Google Scholar]
- 46.Payne RJ, Phil D, Jansen VA. 2000. Phage therapy: the peculiar kinetics of self-replicating pharmaceuticals. Clin Pharmacol Ther 68:225–230. doi: 10.1067/mcp.2000.109520. [DOI] [PubMed] [Google Scholar]
- 47.Levin BR, Bull JJ. 2004. Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2:166–173. doi: 10.1038/nrmicro822. [DOI] [PubMed] [Google Scholar]
- 48.Dabrowska K, Switała-Jeleń K, Opolski A, Górski A. 2006. Possible association between phages, Hoc protein, and the immune system. Arch Virol 151:209–215. doi: 10.1007/s00705-005-0641-7. [DOI] [PubMed] [Google Scholar]
- 49.Abedon ST. 2014. Phage therapy: eco-physiological pharmacology. Scientifica (Cairo) 2014:581639. doi: 10.1155/2014/581639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodyra-Stefaniak K, Miernikiewicz P, Drapała J, Drab M, Jończyk-Matysiak E, Lecion D, Kaźmierczak Z, Beta W, Majewska J, Harhala M, Bubak B, Kłopot A, Górski A, Dąbrowska K. 2015. Mammalian host-versus-phage immune response determines phage fate in vivo. Sci Rep 5:14802. doi: 10.1038/srep14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miernikiewicz P, Kłopot A, Soluch R, Szkuta P, Kęska W, Hodyra-Stefaniak K, Konopka A, Nowak M, Lecion D, Kaźmierczak Z, Majewska J, Harhala M, Górski A, Dąbrowska K. 2016. T4 phage tail adhesin Gp12 counteracts LPS-induced inflammation in vivo. Front Microbiol 7:1112. doi: 10.3389/fmicb.2016.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul JH, Sullivan MB, Segall AM, Rohwer F. 2002. Marine phage genomics. Comp Biochem Physiol B Biochem Mol Biol 133:463–476. doi: 10.1016/S1096-4959(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 53.Rohwer F. 2003. Global phage diversity. Cell 113:141. doi: 10.1016/s0092-8674(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 54.Paul JH, Sullivan MB. 2005. Marine phage genomics: what have we learned? Curr Opin Biotechnol 16:299–307. doi: 10.1016/j.copbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Hatfull GF. 2015. Dark matter of the biosphere: the amazing world of bacteriophage diversity. J Virol 89:8107–8110. doi: 10.1128/JVI.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adriaenssens EM, Wittmann J, Kuhn JH, Turner D, Sullivan MB, Dutilh BE, Jang HB, van Zyl LJ, Klumpp J, Lobocka M, Moreno Switt AI, Rumnieks J, Edwards RA, Uchiyama J, Alfenas-Zerbini P, Petty NK, Kropinski AM, Barylski J, Gillis A, Clokie MRC, Prangishvili D, Lavigne R, Aziz RK, Duffy S, Krupovic M, Poranen MM, Knezevic P, Enault F, Tong Y, Oksanen HM, Brister JR. 2018. Taxonomy of prokaryotic viruses: 2017 update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Arch Virol 163:1125–1129. doi: 10.1007/s00705-018-3723-z. [DOI] [PubMed] [Google Scholar]
- 57.Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 58.Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Szufnarowski K, Pawełczyk Z, Rogóż P, Kłak M, Wojtasik E, Górski A. 2012. Clinical aspects of phage therapy. Adv Virus Res 83:73–121. doi: 10.1016/B978-0-12-394438-2.00003-7. [DOI] [PubMed] [Google Scholar]
- 59.Abedon ST. 2018. Bacteriophage-mediated biocontrol of wound infections, and ecological exploitation of biofilms by phages In Shiffman M. (ed), Recent clinical techniques, results, and research in wounds. Springer, New York, NY. [Google Scholar]
- 60.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. 2016. Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J Wound Care 25:S27–S33. doi: 10.12968/jowc.2016.25.7.S27.26949862 [DOI] [Google Scholar]
- 61.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. 2018. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018:60–66. doi: 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferry T, Leboucher G, Fevre C, Herry Y, Conrad A, Josse J, Batailler C, Chidiac C, Medina M, Lustig S, Laurent F, Lyon BJI Study Group. 2018. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect Dis 5:ofy269. doi: 10.1093/ofid/ofy269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. 2018. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods Mol Biol 1693:159–170. doi: 10.1007/978-1-4939-7395-8_14. [DOI] [PubMed] [Google Scholar]
- 65.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, Gilmour KC, Soothill J, Jacobs-Sera D, Schooley RT, Hatfull GF, Spencer H. 2019. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 25:730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abedon ST. 2012. Phage therapy best practices, p 256–272. In Hyman P, Abedon ST (ed), Bacteriophages in health and disease. CABI Press, Wallingford, United Kingdom. [Google Scholar]
- 67.Abedon ST. 2017. Information phage therapy research should report. Pharmaceuticals (Basel) 10:E43. doi: 10.3390/ph10020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M. 2017. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep 7:8004. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boratyński J, Szermer-Olearnik B. 2017. Endotoxin removal from Escherichia coli bacterial lysate using a biphasic liquid system. Methods Mol Biol 1600:107–112. doi: 10.1007/978-1-4939-6958-6_10. [DOI] [PubMed] [Google Scholar]
- 70.Szermer-Olearnik B, Boratyński J. 2015. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One 10:e0122672. doi: 10.1371/journal.pone.0122672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Branston SD, Wright J, Keshavarz-Moore E. 2015. A non-chromatographic method for the removal of endotoxins from bacteriophages. Biotechnol Bioeng 112:1714–1719. doi: 10.1002/bit.25571. [DOI] [PubMed] [Google Scholar]
- 72.Casas V, Maloy S. 2011. Role of bacteriophage-encoded exotoxins in the evolution of bacterial pathogens. Future Microbiol 6:1461–1473. doi: 10.2217/fmb.11.124. [DOI] [PubMed] [Google Scholar]
- 73.Boyd EF. 2012. Bacteriophage-encoded bacterial virulence factors and phage-pathogenicity island interactions. Adv Virus Res 82:91–118. doi: 10.1016/B978-0-12-394621-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 74.Fortier LC, Sekulovic O. 2013. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 4:354–365. doi: 10.4161/viru.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hyman P, Abedon ST. 2008. Phage ecology of bacterial pathogenesis, p 353–385. In Abedon ST. (ed), Bacteriophage ecology. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 76.Hobbs Z, Abedon ST. 2016. Diversity of phage infection types and associated terminology: the problem with ‘lytic or lysogenic.’ FEMS Microbiol Lett 363:fnw047. doi: 10.1093/femsle/fnw047. [DOI] [PubMed] [Google Scholar]
- 77.Hyman P. 2019. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals (Basel) 12:35. doi: 10.3390/ph12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross A, Ward S, Hyman P. 2016. More is better: selecting for broad host range bacteriophages. Front Microbiol 7:1352. doi: 10.3389/fmicb.2016.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azam AH, Tanji Y. 2019. Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl Microbiol Biotechnol 103:2121–2131. doi: 10.1007/s00253-019-09629-x. [DOI] [PubMed] [Google Scholar]
- 80.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 81.Dy RL, Richter C, Salmond GP, Fineran PC. 2014. Remarkable mechanisms in microbes to resist phage infections. Annu Rev Virol 1:307–331. doi: 10.1146/annurev-virology-031413-085500. [DOI] [PubMed] [Google Scholar]
- 82.Oechslin F. 2018. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 10:351. doi: 10.3390/v10070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rohde C, Resch G, Pirnay J-P, Blasdel B, Debarbieux L, Gelman D, Górski A, Hazan R, Huys I, Kakabadze E, Łobocka M, Maestri A, Almeida G, Makalatia K, Malik D, Mašlaňová I, Merabishvili M, Pantucek R, Rose T, Štveráková D, Van Raemdonck H, Verbeken G, Chanishvili N. 2018. Expert opinion on three phage therapy related topics: bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 10:E178. doi: 10.3390/v10040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samson JE, Magadan AH, Sabri M, Moineau S. 2013. Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11:675–687. doi: 10.1038/nrmicro3096. [DOI] [PubMed] [Google Scholar]
- 85.Scanlan PD, Buckling A, Hall AR. 2015. Experimental evolution and bacterial resistance: (co)evolutionary costs and trade-offs as opportunities in phage therapy research. Bacteriophage 5:e1050153. doi: 10.1080/21597081.2015.1050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pawluk A, Davidson AR, Maxwell KL. 2018. Anti-CRISPR: discovery, mechanism and function. Nat Rev Microbiol 16:12–17. doi: 10.1038/nrmicro.2017.120. [DOI] [PubMed] [Google Scholar]
- 87.Payne RJ, Jansen VA. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J Theor Biol 208:37–48. doi: 10.1006/jtbi.2000.2198. [DOI] [PubMed] [Google Scholar]
- 88.Abedon ST, Thomas-Abedon C. 2010. Phage therapy pharmacology. Curr Pharm Biotechnol 11:28–47. doi: 10.2174/138920110790725410. [DOI] [PubMed] [Google Scholar]
- 89.Abedon S. 2011. Phage therapy pharmacology: calculating phage dosing. Adv Appl Microbiol 77:1–40. doi: 10.1016/B978-0-12-387044-5.00001-7. [DOI] [PubMed] [Google Scholar]
- 90.Hagens S, Loessner MJ. 2010. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr Pharm Biotechnol 11:58–68. doi: 10.2174/138920110790725429. [DOI] [PubMed] [Google Scholar]
- 91.Cairns BJ, Timms AR, Jansen VA, Connerton IF, Payne RJ. 2009. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog 5:e1000253. doi: 10.1371/journal.ppat.1000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bull JJ, Regoes RR. 2006. Pharmacodynamics of non-replicating viruses, bacteriocins and lysins. Proc Biol Sci 273:2703–2712. doi: 10.1098/rspb.2006.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hadas H, Einav M, Fishov I, Zaritsky A. 1997. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology 143:179–185. doi: 10.1099/00221287-143-1-179. [DOI] [PubMed] [Google Scholar]
- 94.Hoyland-Kroghsbo NM, Maerkedahl RB, Svenningsen SL. 2013. A quorum-sensing-induced bacteriophage defense mechanism. mBio 4:e00362-12. doi: 10.1128/mBio.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan BK, Abedon ST. 2012. Phage therapy pharmacology phage cocktails. Adv Appl Microbiol 78:1–23. doi: 10.1016/B978-0-12-394805-2.00001-4. [DOI] [PubMed] [Google Scholar]
- 96.Chan BK, Abedon ST, Loc-Carrillo C. 2013. Phage cocktails and the future of phage therapy. Future Microbiol 8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 97.Abedon ST. 2012. Bacterial ‘immunity’ against bacteriophages. Bacteriophage 2:50–54. doi: 10.4161/bact.18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torres-Barcelo C. 2018. Phage therapy faces evolutionary challenges. Viruses 10:E323. doi: 10.3390/v10060323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. 2017. Combination therapy in combating cancer. Oncotarget 8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cottarel G, Wierzbowski J. 2007. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol 25:547–555. doi: 10.1016/j.tibtech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 101.Williams ML, LeJeune JT. 2012. Phages and bacterial epidemiology, p 76–85. In Hyman P, Abedon ST (ed), Bacteriophages in health and disease. CABI Press, Wallingford, United Kingdom. [Google Scholar]
- 102.Carlson K, Miller ES. 1994. Enumerating phage: the plaque assay, p 427–429. In Karam JD. (ed), Molecular biology of bacteriophage T4. ASM Press, Washington, DC. [Google Scholar]
- 103.Chanishvili NA. 2012. Literature review of the practical application of bacteriophage research. Nova Biomedical, New York, NY. [Google Scholar]
- 104.Cox CR. 2012. Bacteriophage-based methods of bacterial detection and identification, p 134–152. In Hyman P, Abedon ST (ed), Bacteriophages in health and disease. CABI Press, Wallingford, United Kingdom. [Google Scholar]
- 105.Schofield DA, Sharp NJ, Westwater C. 2012. Phage-based platforms for the clinical detection of human bacterial pathogens. Bacteriophage 2:105–283. doi: 10.4161/bact.19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fadlallah A, Chelala E, Legeais JM. 2015. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol J 9:167–168. doi: 10.2174/1874364101509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maura D, Morello E, Du Merle L, Bomme P, Le Bouguénec C, Debarbieux L. 2012. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ Microbiol 14:1844–1854. doi: 10.1111/j.1462-2920.2011.02644.x. [DOI] [PubMed] [Google Scholar]
- 108.Majewska J, Beta W, Lecion D, Hodyra-Stefaniak K, Kłopot A, Kaźmierczak Z, Miernikiewicz P, Piotrowicz A, Ciekot J, Owczarek B, Kopciuch A, Wojtyna K, Harhala M, Mąkosa M, Dąbrowska K. 2015. Oral application of T4 phage induces weak antibody production in the gut and in the blood. Viruses 7:4783–4799. doi: 10.3390/v7082845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roach DR, Leung CY, Henry M, Morello E, Singh D, Di Santo JP, Weitz JS, Debarbieux L. 2017. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22:38–47.e4. doi: 10.1016/j.chom.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 110.Leung CYJ, Weitz JS. 2017. Modeling the synergistic elimination of bacteria by phage and the innate immune system. J Theor Biol 429:241–252. doi: 10.1016/j.jtbi.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 111.Chan BK, Brown K, Kortright KE, Mao S, Turner PE. 2016. Extending the lifetime of antibiotics: how can phage therapy help? Future Microbiol 11:1105–1107. doi: 10.2217/fmb-2016-0133. [DOI] [PubMed] [Google Scholar]
- 112.Smith HW, Huggins MB. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol 129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 113.Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K. 1999. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Organ 37:33–41. doi: 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- 114.Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S. 2003. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J Infect Dis 187:613–624. doi: 10.1086/374001. [DOI] [PubMed] [Google Scholar]
- 115.Capparelli R, Ventimiglia I, Roperto S, Fenizia D, Iannelli D. 2006. Selection of an Escherichia coli O157:H7 bacteriophage for persistence in the circulatory system of mice infected experimentally. Clin Microbiol Infect 12:248–253. doi: 10.1111/j.1469-0691.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 116.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, Bingen E, Bonacorsi S. 2012. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother 56:3568–3575. doi: 10.1128/AAC.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Semler DD, Goudie AD, Finlay WH, Dennis JJ. 2014. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob Agents Chemother 58:4005–4013. doi: 10.1128/AAC.02388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takemura-Uchiyama I, Uchiyama J, Osanai M, Morimoto N, Asagiri T, Ujihara T, Daibata M, Sugiura T, Matsuzaki S. 2014. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes Infect 16:512–517. doi: 10.1016/j.micinf.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 119.Geier MR, Trigg ME, Merril CR. 1973. Fate of bacteriophage lambda in non-immune germ-free mice. Nature 246:221–223. doi: 10.1038/246221a0. [DOI] [PubMed] [Google Scholar]
- 120.Smith HW, Huggins MB. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol 128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 121.Barrow P, Lovell M, Berchieri A Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin Diagn Lab Immunol 5:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huff WE, Huff GR, Rath NC, Balog JM, Donoghue AM. 2003. Evaluation of aerosol spray and intramuscular injection of bacteriophage to treat an Escherichia coli respiratory infection. Poult Sci 82:1108–1112. doi: 10.1093/ps/82.7.1108. [DOI] [PubMed] [Google Scholar]
- 123.Prasad Y, Arpana, Kumar D, Sharma AK. 2011. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J Environ Biol 32:161–168. [PubMed] [Google Scholar]
- 124.Trigo G, Martins TG, Fraga AG, Longatto-Filho A, Castro AG, Azeredo J, Pedrosa J. 2013. Phage therapy is effective against infection by Mycobacterium ulcerans in a murine footpad model. PLoS Negl Trop Dis 7:e2183. doi: 10.1371/journal.pntd.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. 2007. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother 51:2765–2773. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McVay CS, Velasquez M, Fralick JA. 2007. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51:1934–1938. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uhr JW, Weissman G. 1965. Intracellular distribution and degradation of bacteriophage in mammalian tissues. J Immunol 94:544–550. [PubMed] [Google Scholar]
- 128.Nelstrop AE, Taylor G, Collard P. 1968. Studies on phagocytosis. I. Antigen clearance studies in rabbits. Immunology 14:325–337. [PMC free article] [PubMed] [Google Scholar]
- 129.Inchley CJ. 1969. The activity of mouse Kupffer cells following intravenous injection of T4 bacteriophage. Clin Exp Immunol 5:173–187. [PMC free article] [PubMed] [Google Scholar]
- 130.Hajek P. 1970. The elimination of bacteriophages ΦX 174 and T2 from the circulating blood of newborn precolostral pigs. Folia Microbiol (Praha) 15:125–128. doi: 10.1007/BF02880095. [DOI] [PubMed] [Google Scholar]
- 131.Taylor RP, Sutherland WM, Martin EN, Ferguson PJ, Reinagel ML, Gilbert E, Lopez K, Incardona NL, Ochs HD. 1997. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol 158:842–850. [PubMed] [Google Scholar]
- 132.Kim KP, Cha JD, Jang EH, Klumpp J, Hagens S, Hardt WD, Lee KY, Loessner MJ. 2008. PEGylation of bacteriophages increases blood circulation time and reduces T-helper type 1 immune response. Microb Biotechnol 1:247–257. doi: 10.1111/j.1751-7915.2008.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ochs HD, Davis SD, Wedgwood RJ. 1971. Immunologic responses to bacteriophage ϕX 174 in immunodeficiency diseases. J Clin Invest 50:2559–2568. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jennes S, Merabishvili M, Soentjens P, Pang KW, Rose T, Keersebilck E, Soete O, Francois PM, Teodorescu S, Verween G, Verbeken G, De Vos D, Pirnay JP. 2017. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—a case report. Crit Care 21:129. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Speck P, Smithyman A. 2016. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol Lett 363:fnv242. doi: 10.1093/femsle/fnv242. [DOI] [PubMed] [Google Scholar]
- 136.Sulkin SE, Finkelstein RA, Rosenblum ED. 1957. Effect of zymosan on bacteriophage clearance. Science 125:742–743. doi: 10.1126/science.125.3251.742. [DOI] [PubMed] [Google Scholar]
- 137.Keller R, Zatzman ML. 1959. Studies on the factors concerned in the disappearance of bacteriophage particles from the animal body. J Immunol 83:167–172. [Google Scholar]
- 138.Bartell PF, Thind IS, Orr T, Blakemore WS. 1963. The in vivo interaction between staphylococcus bacteriophage and Staphylococcus aureus. J Exp Med 118:13–26. doi: 10.1084/jem.118.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bartell PF, Geffen A, Orr T, Blakemore WS. 1965. Staphylococcus phage-bacterium in vivo interaction. Nature 205:474–475. doi: 10.1038/205474a0. [DOI] [PubMed] [Google Scholar]
- 140.Bogovazova GG, Voroshilova NN, Bondarenko VM. 1991. The efficacy of Klebsiella pneumoniae bacteriophage in the therapy of experimental Klebsiella infection. Zh Mikrobiol Epidemiol Immunobiol 1991:5–8. (In Russian.) [PubMed] [Google Scholar]
- 141.Bogovazova GG, Voroshilova NN, Bondarenko VM, Gorbatkova GA, Afanas’eva EV, Kazakova TB, Smirnov VD, Mamleeva AG, Glukharev IUA, Erastova EI. 1992. Immunobiological properties and therapeutic effectiveness of preparations from Klebsiella bacteriophages. Zh Mikrobiol Epidemiol Immunobiol 1992:30–33. (In Russian.) [PubMed] [Google Scholar]
- 142.Chhibber S, Kaur S, Kumari S. 2008. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 57:1508–1513. doi: 10.1099/jmm.0.2008/002873-0. [DOI] [PubMed] [Google Scholar]
- 143.Jun JW, Shin TH, Kim JH, Shin SP, Han JE, Heo GJ, De Zoysa M, Shin GW, Chai JY, Park SC. 2014. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3:K6 pandemic clinical strain. J Infect Dis 210:72–78. doi: 10.1093/infdis/jiu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keller R, Engley FB Jr. 1958. Fate of bacteriophage particles introduced into mice by various routes. Proc Soc Exp Biol Med 98:577–580. doi: 10.3181/00379727-98-24112. [DOI] [PubMed] [Google Scholar]
- 145.Hildebrand GJ, Wolochow H. 1962. Translocation of bacteriophage across the intestinal wall of the rat. Proc Soc Exp Biol Med 109:183–185. doi: 10.3181/00379727-109-27146. [DOI] [PubMed] [Google Scholar]
- 146.Bradley SG, Watson DW. 1963. Production of neutralizing antibody by mice injected with actinophage. J Immunol 90:782–787. [PubMed] [Google Scholar]
- 147.Hoffmann M. 1965. Animal experiments on the mucosal passage and absorption viremia of T3 phages after oral, tracheal and rectal administration. Zentralbl Bakteriol Orig 198:371–390. (In German.) [PubMed] [Google Scholar]
- 148.Wolochow H, Hildebrand GJ, Lamanna C. 1966. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis 116:523–528. doi: 10.1093/infdis/116.4.523. [DOI] [PubMed] [Google Scholar]
- 149.Weber-Dabrowska B, Dabrowski M, Slopek S. 1987. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch Immunol Ther Exp (Warsz) 35:563–568. [PubMed] [Google Scholar]
- 150.Reynaud A, Cloastre L, Bernard J, Laveran H, Ackermann HW, Licois D, Joly B. 1992. Characteristics and diffusion in the rabbit of a phage for Escherichia coli 0103. Attempts to use this phage for therapy. Vet Microbiol 30:203–212. doi: 10.1016/0378-1135(92)90114-9. [DOI] [PubMed] [Google Scholar]
- 151.Park SC, Shimamura I, Fukunaga M, Mori KI, Nakai T. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol 66:1416–1422. doi: 10.1128/aem.66.4.1416-1422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chibani-Chennoufi S, Sidoti J, Bruttin A, Kutter E, Sarker S, Brüssow H. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob Agents Chemother 48:2558–2569. doi: 10.1128/AAC.48.7.2558-2569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duerr DM, White SJ, Schluesener HJ. 2004. Identification of peptide sequences that induce the transport of phage across the gastrointestinal mucosal barrier. J Virol Methods 116:177–180. doi: 10.1016/j.jviromet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 154.Bruttin A, Brussow H. 2005. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Borie C, Albala I, Sanchez P, Sanchez ML, Ramirez S, Navarro C, Morales MA, Retamales AJ, Robeson J. 2008. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis 52:64–67. doi: 10.1637/8091-082007-Reg. [DOI] [PubMed] [Google Scholar]
- 156.Borie C, Sanchez ML, Navarro C, Ramirez S, Morales MA, Retamales J, Robeson J. 2009. Aerosol spray treatment with bacteriophages and competitive exclusion reduces Salmonella enteritidis infection in chickens. Avian Dis 53:250–254. doi: 10.1637/8406-071008-Reg.1. [DOI] [PubMed] [Google Scholar]
- 157.Denou E, Bruttin A, Barretto C, Ngom-Bru C, Brüssow H, Zuber S. 2009. T4 phages against Escherichia coli diarrhea: potential and problems. Virology 388:21–30. doi: 10.1016/j.virol.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 158.Oliveira A, Sereno R, Nicolau A, Azeredo J. 2009. The influence of the mode of administration in the dissemination of three coliphages in chickens. Poult Sci 88:728–733. doi: 10.3382/ps.2008-00378. [DOI] [PubMed] [Google Scholar]
- 159.Pagava KI, Gachechiladze KK, Korinteli IA, Dzuliashvili MG, Alavidze ZI, Hoyle N, Metskhvarishvili GD. 2011. What happens when the child gets bacteriophage per os? Georgian Med News 2011:101–105. (In Russian.) [PubMed] [Google Scholar]
- 160.Letarova M, Strelkova D, Nevolina S, Letarov A. 2012. A test for the “physiological phagemia” hypothesis—natural intestinal coliphages do not penetrate to the blood in horses. Folia Microbiol (Praha) 57:81–83. doi: 10.1007/s12223-011-0096-z. [DOI] [PubMed] [Google Scholar]
- 161.Christiansen RH, Dalsgaard I, Middelboe M, Lauritsen AH, Madsen L. 2014. Detection and quantification of Flavobacterium psychrophilum-specific bacteriophages in vivo in rainbow trout upon oral administration: implications for disease control in aquaculture. Appl Environ Microbiol 80:7683–7693. doi: 10.1128/AEM.02386-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jaiswal A, Koley H, Mitra S, Saha DR, Sarkar B. 2014. Comparative analysis of different oral approaches to treat Vibrio cholerae infection in adult mice. Int J Med Microbiol 304:422–430. doi: 10.1016/j.ijmm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 163.Georgakopoulos PA. 1968. Permeability of the female genital system to virus. Arch Gynakol 205:211–218. doi: 10.1007/bf00679856. (In German.) [DOI] [PubMed] [Google Scholar]
- 164.Jonczyk E, Klak M, Miedzybrodzki R, Gorski A. 2011. The influence of external factors on bacteriophages—review. Folia Microbiol (Praha) 56:191–200. doi: 10.1007/s12223-011-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Colom J, Cano-Sarabia M, Otero J, Aríñez-Soriano J, Cortés P, Maspoch D, Llagostera M. 2017. Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Sci Rep 7:41441. doi: 10.1038/srep41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malik DJ, Sokolov IJ, Vinner GK, Mancuso F, Cinquerrui S, Vladisavljevic GT, Clokie MRJ, Garton NJ, Stapley AGF, Kirpichnikova A. 2017. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv Colloid Interface Sci 249:100–133. doi: 10.1016/j.cis.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 167.Międzybrodzki R, Kłak M, Jończyk-Matysiak E, Bubak B, Wójcik A, Kaszowska M, Weber-Dąbrowska B, Łobocka M, Górski A. 2017. Means to facilitate the overcoming of gastric juice barrier by a therapeutic staphylococcal bacteriophage A5/80. Front Microbiol 8:467. doi: 10.3389/fmicb.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Monsur KA, Rahman MA, Huq F, Islam MN, Northrup RS, Hirschhorn N. 1970. Effect of massive doses of bacteriophage on excretion of vibrios, duration of diarrhoea and output of stools in acute cases of cholera. Bull World Health Organ 42:723–732. [PMC free article] [PubMed] [Google Scholar]
- 169.Nguyen S, Baker K, Padman BS, Patwa R, Dunstan RA, Weston TA, Schlosser K, Bailey B, Lithgow T, Lazarou M, Luque A, Rohwer F, Blumberg RS, Barr JJ. 2017. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 8:e01874-17. doi: 10.1128/mBio.01874-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith HW, Huggins MB, Shaw KM. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol 133:1111–1126. doi: 10.1099/00221287-133-5-1111. [DOI] [PubMed] [Google Scholar]
- 171.Hons M, Sixt M. 2015. The lymph node filter revealed. Nat Immunol 16:338–340. doi: 10.1038/ni.3126. [DOI] [PubMed] [Google Scholar]
- 172.Yu M, Yang Y, Zhu C, Guo S, Gan Y. 2016. Advances in the transepithelial transport of nanoparticles. Drug Discov Today 21:1155–1161. doi: 10.1016/j.drudis.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 173.Attili-Qadri S, Karra N, Nemirovski A, Schwob O, Talmon Y, Nassar T, Benita S. 2013. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci U S A 110:17498–17503. doi: 10.1073/pnas.1313839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dabrowska K. 2019. Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev 39:2000–2025. doi: 10.1002/med.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bodier-Montagutelli E, Morello E, L’Hostis G, Guillon A, Dalloneau E, Respaud R, Pallaoro N, Blois H, Vecellio L, Gabard J, Heuzé-Vourc’h N. 2017. Inhaled phage therapy: a promising and challenging approach to treat bacterial respiratory infections. Expert Opin Drug Deliv 14:959–972. doi: 10.1080/17425247.2017.1252329. [DOI] [PubMed] [Google Scholar]
- 176.Singla S, Harjai K, Katare OP, Chhibber S. 2015. Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae-induced lobar pneumonia. J Infect Dis 212:325–334. doi: 10.1093/infdis/jiv029. [DOI] [PubMed] [Google Scholar]
- 177.Carmody LA, Gill JJ, Summer EJ, Sajjan US, Gonzalez CF, Young RF, LiPuma JJ. 2010. Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J Infect Dis 201:264–271. doi: 10.1086/649227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huff WE, Huff GR, Rath NC, Balog JM, Donoghue AM. 2002. Prevention of Escherichia coli infection in broiler chickens with a bacteriophage aerosol spray. Poult Sci 81:1486–1491. doi: 10.1093/ps/81.10.1486. [DOI] [PubMed] [Google Scholar]
- 179.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. 2010. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 180.Cao Z, Zhang J, Niu YD, Cui N, Ma Y, Cao F, Jin L, Li Z, Xu Y. 2015. Isolation and characterization of a “phiKMV-like” bacteriophage and its therapeutic effect on mink hemorrhagic pneumonia. PLoS One 10:e0116571. doi: 10.1371/journal.pone.0116571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Henry M, Lavigne R, Debarbieux L. 2013. Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections. Antimicrob Agents Chemother 57:5961–5968. doi: 10.1128/AAC.01596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao F, Wang X, Wang L, Li Z, Che J, Wang L, Li X, Cao Z, Zhang J, Jin L, Xu Y. 2015. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed Res Int 2015:752930. doi: 10.1155/2015/752930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu KY, Yang WH, Dong XK, Cong LM, Li N, Li Y, Wen ZB, Yin Z, Lan ZJ, Li WP, Li JS. 2016. Inhalation study of mycobacteriophage D29 aerosol for mice by endotracheal route and nose-only exposure. J Aerosol Med Pulm Drug Deliv 29:393–405. doi: 10.1089/jamp.2015.1233. [DOI] [PubMed] [Google Scholar]
- 184.Gu J, Li X, Yang M, Du C, Cui Z, Gong P, Xia F, Song J, Zhang L, Li J, Yu C, Sun C, Feng X, Lei L, Han W. 2016. Therapeutic effect of Pseudomonas aeruginosa phage YH30 on mink hemorrhagic pneumonia. Vet Microbiol 190:5–11. doi: 10.1016/j.vetmic.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 185.Jeon J, Ryu CM, Lee JY, Park JH, Yong D, Lee K. 2016. In vivo application of bacteriophage as a potential therapeutic agent to control OXA-66-like carbapenemase-producing Acinetobacter baumannii strains belonging to sequence type 357. Appl Environ Microbiol 82:4200–4208. doi: 10.1128/AEM.00526-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Y, Mi Z, Niu W, An X, Yuan X, Liu H, Li P, Liu Y, Feng Y, Huang Y, Zhang X, Zhang Z, Fan H, Peng F, Tong Y, Bai C. 2016. Intranasal treatment with bacteriophage rescues mice from Acinetobacter baumannii-mediated pneumonia. Future Microbiol 11:631–641. doi: 10.2217/fmb.16.11. [DOI] [PubMed] [Google Scholar]
- 187.Pires DP, Cleto S, Sillankorva S, Azeredo J, Lu TK. 2016. Genetically engineered phages: a review of advances over the last decade. Microbiol Mol Biol Rev 80:523–543. doi: 10.1128/MMBR.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scholl D, Adhya S, Merril C. 2005. Escherichia coli K1’s capsule is a barrier to bacteriophage T7. Appl Environ Microbiol 71:4872–4874. doi: 10.1128/AEM.71.8.4872-4874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weinbauer MG. 2004. Ecology of prokaryotic viruses. FEMS Microbiol Rev 28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 190.Dickey J, Perrot V. 2019. Adjunct phage treatment enhances the effectiveness of low antibiotic concentration against Staphylococcus aureus biofilms in vitro. PLoS One 14:e0209390. doi: 10.1371/journal.pone.0209390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dvorackova M, Ruzicka F, Benesik M, Pantucek R, Dvorakova-Heroldova M. 2019. Antimicrobial effect of commercial phage preparation Stafal(R) on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol (Praha) 64:121–126. doi: 10.1007/s12223-018-0622-3. [DOI] [PubMed] [Google Scholar]
- 192.Gu Y, Xu Y, Xu J, Yu X, Huang X, Liu G, Liu X. 2019. Identification of novel bacteriophage vB_EcoP-EG1 with lytic activity against planktonic and biofilm forms of uropathogenic Escherichia coli. Appl Microbiol Biotechnol 103:315–326. doi: 10.1007/s00253-018-9471-x. [DOI] [PubMed] [Google Scholar]
- 193.Henriksen K, Rorbo N, Rybtke ML, Martinet MG, Tolker-Nielsen T, Hoiby N, Middelboe M, Ciofu O. 2019. P. aeruginosa flow-cell biofilms are enhanced by repeated phage treatments but can be eradicated by phage-ciprofloxacin combination. Pathog Dis 77:ftz011. doi: 10.1093/femspd/ftz011. [DOI] [PubMed] [Google Scholar]
- 194.Jamal M, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Ur Rahman S, Das CR. 2019. Isolation, characterization and efficacy of phage MJ2 against biofilm forming multi-drug resistant Enterobacter cloacae. Folia Microbiol (Praha) 64:101–111. doi: 10.1007/s12223-018-0636-x. [DOI] [PubMed] [Google Scholar]
- 195.Morris J, Kelly N, Elliott L, Grant A, Wilkinson M, Hazratwala K, McEwen P. 2019. Evaluation of bacteriophage anti-biofilm activity for potential control of orthopedic implant-related infections caused by Staphylococcus aureus. Surg Infect (Larchmt) 20:16–24. doi: 10.1089/sur.2018.135. [DOI] [PubMed] [Google Scholar]
- 196.Milho C, Andrade M, Vilas Boas D, Alves D, Sillankorva S. 2019. Antimicrobial assessment of phage therapy using a porcine model of biofilm infection. Int J Pharm 557:112–123. doi: 10.1016/j.ijpharm.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 197.Mukerjee S, Ghosh SN. 1962. Localization of cholera bacterio-phage after intravenous injection. Ann Biochem Exp Med 22:73–76. [PubMed] [Google Scholar]
- 198.Tiwari BR, Kim S, Rahman M, Kim J. 2011. Antibacterial efficacy of lytic Pseudomonas bacteriophage in normal and neutropenic mice models. J Microbiol 49:994–999. doi: 10.1007/s12275-011-1512-4. [DOI] [PubMed] [Google Scholar]
- 199.Cerveny KE, DePaola A, Duckworth DH, Gulig PA. 2002. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect Immun 70:6251–6262. doi: 10.1128/iai.70.11.6251-6262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dabrowska K, Zembala M, Boratynski J, Switala-Jelen K, Wietrzyk J, Opolski A, Szczaurska K, Kujawa M, Godlewska J, Gorski A. 2007. Hoc protein regulates the biological effects of T4 phage in mammals. Arch Microbiol 187:489–498. doi: 10.1007/s00203-007-0216-y. [DOI] [PubMed] [Google Scholar]
- 201.Nishikawa H, Yasuda M, Uchiyama J, Rashel M, Maeda Y, Takemura I, Sugihara S, Ujihara T, Shimizu Y, Shuin T, Matsuzaki S. 2008. T-even-related bacteriophages as candidates for treatment of Escherichia coli urinary tract infections. Arch Virol 153:507–515. doi: 10.1007/s00705-007-0031-4. [DOI] [PubMed] [Google Scholar]
- 202.Samoylova TI, Smith BF. 1999. Elucidation of muscle-binding peptides by phage display screening. Muscle Nerve 22:460–466. [DOI] [PubMed] [Google Scholar]
- 203.Capparelli R, Nocerino N, Iannaccone M, Ercolini D, Parlato M, Chiara M, Iannelli D. 2010. Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J Infect Dis 201:52–61. doi: 10.1086/648478. [DOI] [PubMed] [Google Scholar]
- 204.Dubos RJ, Straus JH, Pierce C. 1943. The multiplication of bacteriophage in vivo and its protective effect against an experimental infection with Shigella dysenteriae. J Exp Med 78:161–168. doi: 10.1084/jem.78.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Debinski W, Tatter SB. 2009. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother 9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Frenkel D, Solomon B. 2002. Filamentous phage as vector-mediated antibody delivery to the brain. Proc Natl Acad Sci U S A 99:5675–5679. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Górski A, Dąbrowska K, Hodyra-Stefaniak K, Borysowski J, Międzybrodzki R, Weber-Dąbrowska B. 2015. Phages targeting infected tissues: novel approach to phage therapy. Future Microbiol 10:199–204. doi: 10.2217/fmb.14.126. [DOI] [PubMed] [Google Scholar]
- 208.Pasqualini R, Ruoslahti E. 1996. Organ targeting in vivo using phage display peptide libraries. Nature 380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 209.Ruoslahti E. 2012. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater 24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E. 2002. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci U S A 99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. 2002. Steps toward mapping the human vasculature by phage display. Nat Med 8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 212.Kang SK, Woo JH, Kim MK, Woo SS, Choi JH, Lee HG, Lee NK, Choi YJ. 2008. Identification of a peptide sequence that improves transport of macromolecules across the intestinal mucosal barrier targeting goblet cells. J Biotechnol 135:210–216. doi: 10.1016/j.jbiotec.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 213.Giordano RJ, Edwards JK, Tuder RM, Arap W, Pasqualini R. 2009. Combinatorial ligand-directed lung targeting. Proc Am Thorac Soc 6:411–415. doi: 10.1513/pats.200903-014AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Budynek P, Dabrowska K, Skaradziński G, Górski A. 2010. Bacteriophages and cancer. Arch Microbiol 192:315–320. doi: 10.1007/s00203-010-0559-7. [DOI] [PubMed] [Google Scholar]
- 215.Li J, Feng L, Fan L, Zha Y, Guo L, Zhang Q, Chen J, Pang Z, Wang Y, Jiang X, Yang VC, Wen L. 2011. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 32:4943–4950. doi: 10.1016/j.biomaterials.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li J, Zhang Q, Pang Z, Wang Y, Liu Q, Guo L, Jiang X. 2012. Identification of peptide sequences that target to the brain using in vivo phage display. Amino Acids 42:2373–2381. doi: 10.1007/s00726-011-0979-y. [DOI] [PubMed] [Google Scholar]
- 217.Teesalu T, Sugahara KN, Ruoslahti E. 2013. Tumor-penetrating peptides. Front Oncol 3:216. doi: 10.3389/fonc.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kay D, Fildes P. 1960. The inactivation of a bacteriophage by a component of papain. Biochem J 75:139–145. doi: 10.1042/bj0750139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Northrop JH. 1964. The effect of proteolytic enzymes on E. coli phages and on native proteins. J Gen Physiol 48:73–78. doi: 10.1085/jgp.48.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chanda PK, Chatterjee SN. 1975. Properties of the cholera phage PL 163/10. Acta Virol 19:197–203. [PubMed] [Google Scholar]
- 221.Granboulan P. 1983. The tail fibre of bacteriophage T4 is sensitive to proteases at elevated temperatures. J Gen Microbiol 129:2217–2228. doi: 10.1099/00221287-129-7-2217. [DOI] [PubMed] [Google Scholar]
- 222.Lu Z, Breidt F, Plengvidhya V, Fleming HP. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Appl Environ Microbiol 69:3192–3202. doi: 10.1128/aem.69.6.3192-3202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Verthe K, Possemiers S, Boon N, Vaneechoutte M, Verstraete W. 2004. Stability and activity of an Enterobacter aerogenes-specific bacteriophage under simulated gastro-intestinal conditions. Appl Microbiol Biotechnol 65:465–472. doi: 10.1007/s00253-004-1585-7. [DOI] [PubMed] [Google Scholar]
- 224.Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, Manova-Todorova K, Deen WM, Scheinberg DA, McDevitt MR. 2010. Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci U S A 107:12369–12374. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, Wilder J, Li F, Miner JH, Berg UB, Smithies O. 2017. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A 114:2958–2963. doi: 10.1073/pnas.1616457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schultz I, Neva FA. 1965. Relationship between blood clearance and viruria after intravenous injection of mice and rats with bacteriophage and polioviruses. J Immunol 94:833–841. [PubMed] [Google Scholar]
- 227.Schultz I, Frohlich E. 1965. Viruria and viraliquoria in the dog after intravenous injection of T5 bacteriophage. Proc Soc Exp Biol Med 118:136–138. doi: 10.3181/00379727-118-29778. [DOI] [PubMed] [Google Scholar]
- 228.Pereira S, Pereira C, Santos L, Klumpp J, Almeida A. 2016. Potential of phage cocktails in the inactivation of Enterobacter cloacae—an in vitro study in a buffer solution and in urine samples. Virus Res 211:199–208. doi: 10.1016/j.virusres.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 229.Perepanova TS, Darbeeva OS, Kotliarova GA, Kondrat’eva EM, Maiskaia LM, Malysheva VF, Baiguzina FA, Grishkova NV. 1995. The efficacy of bacteriophage preparations in treating inflammatory urologic diseases. Urol Nefrol (Mosk) 1995:14–17. (In Russian.) [PubMed] [Google Scholar]
- 230.Khawaldeh A, Morales S, Dillon B, Alavidze Z, Ginn AN, Thomas L, Chapman SJ, Dublanchet A, Smithyman A, Iredell JR. 2011. Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J Med Microbiol 60:1697–1700. doi: 10.1099/jmm.0.029744-0. [DOI] [PubMed] [Google Scholar]
- 231.Ujmajuridze A, Chanishvili N, Goderdzishvili M, Leitner L, Mehnert U, Chkhotua A, Kessler TM, Sybesma W. 2018. Adapted bacteriophages for treating urinary tract infections. Front Microbiol 9:1832. doi: 10.3389/fmicb.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Molenaar TJ, Michon I, de Haas SA, van Berkel TJ, Kuiper J, Biessen EA. 2002. Uptake and processing of modified bacteriophage M13 in mice: implications for phage display. Virology 293:182–191. doi: 10.1006/viro.2001.1254. [DOI] [PubMed] [Google Scholar]
- 233.Griffith DC, Harford L, Williams R, Lee VJ, Dudley MN. 2003. In vivo antibacterial activity of RWJ-54428, a new cephalosporin with activity against gram-positive bacteria. Antimicrob Agents Chemother 47:43–47. doi: 10.1128/aac.47.1.43-47.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Oshida T, Onta T, Nakanishi N, Matsushita T, Yamaguchi T. 1990. Activity of sub-minimal inhibitory concentrations of aspoxicillin in prolonging the postantibiotic effect against Staphylococcus aureus. J Antimicrob Chemother 26:29–38. doi: 10.1093/jac/26.1.29. [DOI] [PubMed] [Google Scholar]
- 235.Miyazaki S, Fujikawa T, Matsumoto T, Tateda K, Yamaguchi K. 2001. Efficacy of azithromycin, clarithromycin and beta-lactam agents against experimentally induced bronchopneumonia caused by Haemophilus influenzae in mice. J Antimicrob Chemother 48:425–430. doi: 10.1093/jac/48.3.425. [DOI] [PubMed] [Google Scholar]
- 236.Girard AE, Girard D, Gootz TD, Faiella JA, Cimochowski CR. 1995. In vivo efficacy of trovafloxacin (CP-99,219), a new quinolone with extended activities against gram-positive pathogens, Streptococcus pneumoniae, and Bacteroides fragilis. Antimicrob Agents Chemother 39:2210–2216. doi: 10.1128/aac.39.10.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Petersen EA, Grayson JB, Hersh EM, Dorr RT, Chiang SM, Oka M, Proffitt RT. 1996. Liposomal amikacin: improved treatment of Mycobacterium avium complex infection in the beige mouse model. J Antimicrob Chemother 38:819–828. doi: 10.1093/jac/38.5.819. [DOI] [PubMed] [Google Scholar]
- 239.Chang HR, Comte R, Piguet PF, Pechere JC. 1991. Activity of minocycline against Toxoplasma gondii infection in mice. J Antimicrob Chemother 27:639–645. doi: 10.1093/jac/27.5.639. [DOI] [PubMed] [Google Scholar]
- 240.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. 2007. Renal clearance of quantum dots. Nat Biotechnol 25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Muir WR, Blakemore WS. 1960. Staphylococcal bacteriophage in experimental infection in mice. Surg Forum 10:339–342. [PubMed] [Google Scholar]
- 242.Guang-Han O, Leang-Chung C, Vellasamy KM, Mariappan V, Li-Yen C, Vadivelu J. 2016. Experimental phage therapy for Burkholderia pseudomallei infection. PLoS One 11:e0158213. doi: 10.1371/journal.pone.0158213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kantoch M. 1961. The role of phagocytes in virus infections. Arch Immunol Ther Exp (Warsz) 9:261–340. [PubMed] [Google Scholar]
- 244.Rus H, Cudrici C, Niculescu F. 2005. The role of the complement system in innate immunity. Immunol Res 33:103–112. doi: 10.1385/IR:33:2:103. [DOI] [PubMed] [Google Scholar]
- 245.Dąbrowska K, Miernikiewicz P, Piotrowicz A, Hodyra K, Owczarek B, Lecion D, Kaźmierczak Z, Letarov A, Górski A. 2014. Immunogenicity studies of proteins forming the T4 phage head surface. J Virol 88:12551–12557. doi: 10.1128/JVI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hajek P, Mandel L. 1966. Antibody response of young animals to bacteriophages of different immunological behaviour: Φ X 174 and T2. Folia Microbiol (Praha) 11:282–289. doi: 10.1007/bf02878898. [DOI] [PubMed] [Google Scholar]
- 247.Merril CR, Biswas B, Carlton R, Jensen NC, Creed GJ, Zullo S, Adhya S. 1996. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci U S A 93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sokoloff AV, Bock I, Zhang G, Sebestyen MG, Wolff JA. 2000. The interactions of peptides with the innate immune system studied with use of T7 phage peptide display. Mol Ther 2:131–139. doi: 10.1006/mthe.2000.0110. [DOI] [PubMed] [Google Scholar]
- 249.Hodyra-Stefaniak K, Lahutta K, Majewska J, Kazmierczak Z, Lecion D, Harhala M, Keska W, Owczarek B, Jonczyk-Matysiak E, Klopot A, Miernikiewicz P, Kula D, Gorski A, Dabrowska K. 2019. Bacteriophages engineered to display foreign peptides may become short-circulating phages. Microb Biotechnol 12:730–741. doi: 10.1111/1751-7915.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vitiello CL, Merril CR, Adhya S. 2005. An amino acid substitution in a capsid protein enhances phage survival in mouse circulatory system more than a 1000-fold. Virus Res 114:101–103. doi: 10.1016/j.virusres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 251.Uhr JW, Finkelstein MS, Baumann JB. 1962. Antibody formation. III. The primary and secondary antibody response to bacteriophage ϕX 174 in guinea pigs. J Exp Med 115:655–670. doi: 10.1084/jem.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Uhr JW, Finkelstein S, Franklin EC. 1962. Antibody response to bacteriophage ΦX 174 in non-mammalian vertebrates. Proc Soc Exp Biol Med 111:13–15. doi: 10.3181/00379727-111-27691. [DOI] [PubMed] [Google Scholar]
- 253.Chang CC, Winter AJ, Norcross NL. 1981. Immune response in the bovine mammary gland after intestinal, local, and systemic immunization. Infect Immun 31:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Smith HW, Huggins MB, Shaw KM. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol 133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 255.Huff WE, Huff GR, Rath NC, Donoghue AM. 2010. Immune interference of bacteriophage efficacy when treating colibacillosis in poultry. Poult Sci 89:895–900. doi: 10.3382/ps.2009-00528. [DOI] [PubMed] [Google Scholar]
- 256.Kucharewicz-Krukowska A, Slopek S. 1987. Immunogenic effect of bacteriophage in patients subjected to phage therapy. Arch Immunol Ther Exp (Warsz) 35:553–561. [PubMed] [Google Scholar]
- 257.Fogelman I, Davey V, Ochs HD, Elashoff M, Feinberg MB, Mican J, Siegel JP, Sneller M, Lane HC. 2000. Evaluation of CD4+ T cell function in vivo in HIV-infected patients as measured by bacteriophage phiX174 immunization. J Infect Dis 182:435–441. doi: 10.1086/315739. [DOI] [PubMed] [Google Scholar]
- 258.Huff WE, Huff GR, Rath NC, Balog JM, Donoghue AM. 2003. Bacteriophage treatment of a severe Escherichia coli respiratory infection in broiler chickens. Avian Dis 47:1399–1405. doi: 10.1637/7041. [DOI] [PubMed] [Google Scholar]
- 259.Jerne NK. 1956. The presence in normal serum of specific antibody against bacteriophage T4 and its increase during the earliest stages of immunization. J Immunol 76:209–216. [PubMed] [Google Scholar]
- 260.Jerne NK, Avegno P. 1956. The development of the phage-inactivating properties of serum during the course of specific immunization of an animal: reversible and irreversible inactivation. J Immunol 76:200–208. [PubMed] [Google Scholar]
- 261.Łusiak-Szelachowska M, Żaczek M, Weber-Dąbrowska B, Międzybrodzki R, Letkiewicz S, Fortuna W, Rogóż P, Szufnarowski K, Jończyk-Matysiak E, Olchawa E, Walaszek KM, Górski A. 2017. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol 12:109–117. doi: 10.2217/fmb-2016-0156. [DOI] [PubMed] [Google Scholar]
- 262.Clark L, Greenbaum C, Jiang J, Lernmark A, Ochs H. 2002. The antibody response to bacteriophage is linked to the lymphopenia gene in congenic BioBreeding rats. FEMS Immunol Med Microbiol 32:205–209. doi: 10.1111/j.1574-695X.2002.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 263.Wang J, Hu B, Xu M, Yan Q, Liu S, Zhu X, Sun Z, Reed E, Ding L, Gong J, Li QQ, Hu J. 2006. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J Mol Med 17:309–317. [PubMed] [Google Scholar]
- 264.Bochkareva SS, Aleshkin AV, Ershova ON, Novikova LI, Karaulov AV, Kiseleva IA, Zul’karneev ER, Rubal’skiy EO, Zeigarnik MV. 2017. Anti-phage аntibody response in phage therapy against healthcare-associated infections (HAIs). Infekc Bolezni 15:35–40. doi: 10.20953/1729-9225-2017-1-35-40. [DOI] [Google Scholar]
- 265.Goodridge LD. 2010. Designing phage therapeutics. Curr Pharm Biotechnol 11:15–27. doi: 10.2174/138920110790725348. [DOI] [PubMed] [Google Scholar]
- 266.Van Belleghem J, Dąbrowska K, Vaneechoutte M, Barr J, Bollyky P. 2018. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 11:E10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Colom J, Cano-Sarabia M, Otero J, Cortes P, Maspoch D, Llagostera M. 2015. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl Environ Microbiol 81:4841–4849. doi: 10.1128/AEM.00812-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stanford K, McAllister TA, Niu YD, Stephens TP, Mazzocco A, Waddell TE, Johnson RP. 2010. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J Food Prot 73:1304–1312. doi: 10.4315/0362-028X-73.7.1304. [DOI] [PubMed] [Google Scholar]
- 269.Singla S, Harjai K, Katare OP, Chhibber S. 2016. Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLoS One 11:e0153777. doi: 10.1371/journal.pone.0153777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pankey GA, Sabath LD. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis 38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 271.Olszowska-Zaremba N, Borysowski J, Dabrowska K, Górski A. 2012. Phage translocation, safety, and immunomodulation, p 168–184. In Hyman P, Abedon ST (ed), Bacteriophages in health and disease. CABI Press, Wallingford, United Kingdom. [Google Scholar]
- 272.Pirnay JP, Blasdel BG, Bretaudeau L, Buckling A, Chanishvili N, Clark JR, Corte-Real S, Debarbieux L, Dublanchet A, De Vos D, Gabard J, Garcia M, Goderdzishvili M, Gorski A, Hardcastle J, Huys I, Kutter E, Lavigne R, Merabishvili M, Olchawa E, Parikka KJ, Patey O, Pouilot F, Resch G, Rohde C, Scheres J, Skurnik M, Vaneechoutte M, Van Parys L, Verbeken G, Zizi M, Van den Eede G. 2015. Quality and safety requirements for sustainable phage therapy products. Pharm Res 32:2173–2179. doi: 10.1007/s11095-014-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gill JJ, Hyman P. 2010. Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol 11:2–14. doi: 10.2174/138920110790725311. [DOI] [PubMed] [Google Scholar]
- 274.Lobocka M, Hejnowicz MS, Gagala U, Weber-Dabrowska B, Wegrzyn G, Dadlez M. 2014. The first step to bacteriophage therapy: how to choose the correct phage In Borysowski JRM, Górski A (ed), Phage therapy: current research and applications. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 275.Weiss M, Denou E, Bruttin A, Serra-Moreno R, Dillmann M-L, Brüssow H. 2009. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology 393:16–23. doi: 10.1016/j.virol.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 276.Bull JJ, Gill JJ. 2014. The habits of highly effective phages: population dynamics as a framework for identifying therapeutic phages. Front Microbiol 5:618. doi: 10.3389/fmicb.2014.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lindberg HM, McKean KA, Wang IN. 2014. Phage fitness may help predict phage therapy efficacy. Bacteriophage 4:e964081. doi: 10.4161/21597073.2014.964081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tsonos J, Oosterik LH, Tuntufye HN, Klumpp J, Butaye P, De Greve H, Hernalsteens JP, Lavigne R, Goddeeris BM. 2014. A cocktail of in vitro efficient phages is not a guarantee for in vivo therapeutic results against avian colibacillosis. Vet Microbiol 171:470–479. doi: 10.1016/j.vetmic.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 279.Atterbury RJ, Van Bergen MA, Ortiz F, Lovell MA, Harris JA, De Boer A, Wagenaar JA, Allen VM, Barrow PA. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl Environ Microbiol 73:4543–4549. doi: 10.1128/AEM.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kutter EM, Kuhl SJ, Abedon ST. 2015. Re-establishing a place for phage therapy in Western medicine. Future Microbiol 10:685–688. doi: 10.2217/fmb.15.28. [DOI] [PubMed] [Google Scholar]
- 281.Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A. 2018. Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11:E18. doi: 10.3390/v11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McCallin S, Sacher JC, Zheng J, Chan BK. 2019. Current state of compassionate phage therapy. Viruses 11:E343. doi: 10.3390/v11040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pirnay JP, Merabishvili M, Van Raemdonck H, De Vos D, Verbeken G. 2018. Bacteriophage production in compliance with regulatory requirements. Methods Mol Biol 1693:233–252. doi: 10.1007/978-1-4939-7395-8_18. [DOI] [PubMed] [Google Scholar]
- 284.Aziz RK, Ackermann HW, Petty NK, Kropinski AM. 2018. Essential steps in characterizing bacteriophages: biology, taxonomy, and genome analysis. Methods Mol Biol 1681:197–215. doi: 10.1007/978-1-4939-7343-9_15. [DOI] [PubMed] [Google Scholar]