Advances in HIV-1 therapy have transformed the once fatal infection into a manageable, chronic condition, yet the search for a widely applicable approach to cure remains elusive. The ineffectiveness of antiretroviral therapy (ART) in reducing the size of the HIV-1 latent reservoir has prompted investigation into the mechanisms of HIV-1 latency and immune escape.
KEYWORDS: BCL-2 family, antiapoptosis, apoptosis, human immunodeficiency virus
SUMMARY
Advances in HIV-1 therapy have transformed the once fatal infection into a manageable, chronic condition, yet the search for a widely applicable approach to cure remains elusive. The ineffectiveness of antiretroviral therapy (ART) in reducing the size of the HIV-1 latent reservoir has prompted investigation into the mechanisms of HIV-1 latency and immune escape. One of the major regulators of apoptosis, the BCL-2 protein, alongside its homologous family members, is a major target of HIV-1-induced change. Recent studies have now demonstrated the association of this protein with cells that support proviral forms in the setting of latency and have helped identify BCL-2 as a novel and promising therapeutic target for HIV-1 therapy directed at possible cure. This review aims to systematically review the interactions of HIV-1 with BCL-2 and its homologs and to examine the possibility of using BCL-2 inhibitors in the study and elimination of the latent reservoir.
INTRODUCTION
First identified in 1983 (1), the human immunodeficiency virus (HIV) is responsible for the establishment of a chronic and incurable infection in humans. Following its clinical emergence in the latter half of the 20th century, HIV quickly achieved pandemic status and became one of the leading causes of morbidity and mortality worldwide.
Upon infection, HIV causes a progressive deterioration in the immune capabilities of the infected host, gradually resulting in a critically immune-deficient condition known as AIDS. HIV-1-associated CD4 T-cell depletion may occur as a direct result of the viral life cycle or as a result of the increased susceptibility of uninfected bystander cells to undergo cell death, initiated by soluble viral proteins or virally induced immune mediators, through mechanisms such as apoptosis, necroptosis, autophagy, or pyroptosis (2–5).
With the advent of HIV highly active antiretroviral therapy (HAART), it has been possible to reduce the once rapidly fatal illness to a chronic but manageable condition. The unfortunate caveat to viral suppression achievable with modern antiretroviral therapy (ART) is the rebound in plasma viremia observed with its interruption, facilitated by the survival of intact proviral forms in a pool of latently infected cells, referred to as the “viral reservoir” (6). The latent HIV reservoir refers to infected cells which harbor integrated replication-competent HIV-1 provirus but which do not actively produce the virus or viral proteins.
It is an apparent contradiction, then, that disease associated with HIV is characterized by increased cell death of CD4 T cells, and yet, the persistence of HIV in the setting of immune responses and antiretroviral therapy depends on the long-term survival of latently infected cells. Accurately identifying and targeting this latent viral reservoir is an important and ongoing goal for HIV research with the aim of developing a potential cure (7). Therefore, understanding how the latently infected cells survive in an otherwise proapoptotic milieu is critically important to develop successful viral eradication strategies.
Throughout the infective process, the HIV-1 virion is able to induce changes in the expression of BCL-2 and its homologs to induce both pro- and antiapoptotic states (2). The recent recognition that these proteins could serve as targets for therapy against the latent reservoir, thereby serving as a potential means of cure, necessitates an understanding of the interactions between virus and host and an examination of these virally induced modulations of BCL-2 expression and function.
THE BCL-2 FAMILY
Since it was first identified in 1984 (8), BCL-2 and its functions have been the subject of intense study. The functional quality of this protein to prolong cell survival (9) provided a new outlook to the understanding of cell proliferative disorders. The ascending burden of cellular overgrowth was recognized to be a result of not only uncontrolled proliferation but also the ability of the proliferating cells to evade programmed cell death events.
The discovery of the proapoptotic BCL-2 homologous protein BAX in 1993 (10) demonstrated that these proteins are members of a larger BCL-2 family of proteins that together are critical to defining the fate of individual cells and, thus, to the regulation of both cell survival and cell death. Subsequent studies have identified multiple such homologs with both pro- and antiapoptotic functions. Though the members of this family have been shown to be functionally involved in other cellular processes (reviewed in reference 11), this article focuses mainly on the members of the BCL-2 family in their role in apoptosis.
At homeostasis, an organism requires cellular machinery to manage physiological and pathological changes which may result in a phenotype unconducive to normal functioning. Cellular function may be lost or decreased due to a host of factors, such as aging, genetic defects, environmental insult, or infection. Cellular responses to these insults may result in cell death occurring through one of numerous mechanisms, such as autophagy, necroptosis, and apoptosis (reviewed in reference 12).
Apoptosis specifically refers to a form of programmed cell death (13) that serves to clear functionally impaired or infected cells and is essential to maintaining an appropriate cell number in an organism. Inadequate apoptosis has been identified as leading to cancer or autoimmunity, while abnormally excessive apoptosis has been observed under conditions such as immunodeficiency and infertility (14).
A variety of triggers may be responsible for the initiation of apoptosis, and these may be exogenous or endogenous to the cell. Depending on the point of initiation and the downstream substrates involved, the pathways that govern the apoptotic process have been classified into an extrinsic pathway, triggered by the binding of exogenous initiators, typically to their respective death receptors, and an intrinsic pathway, triggered by the release of mitochondrial proteins into the cytoplasm. A third pathway, termed the perforin-granzyme pathway, is utilized by effector T cells to induce apoptosis and may operate through a caspase-dependent or -independent mechanism (13).
The intermediaries for these pathways are a family of cysteine-dependent aspartic acid-specific proteases termed caspases (15). Caspase activation causes the cleavage of specific substrates, which may range from a few to a few hundred, depending on the caspase and the cell type (reviewed in reference 16), involved in structural integrity or nuclear functioning (14). Caspases generally cleave these target substrates at sites following aspartate but may also cause protein cleavage following glutamate or phosphoserine (16, 17). Though it is important to note that individual caspases have low redundancy with regard to their target substrates, the end proteolytic effect is generally similar across different cell types (16).
Though the intrinsic, extrinsic, and caspase-dependent granzyme B pathways differ in their initiation, they eventually converge, leading to the common final pathway and ultimately leading to cell death (15).
The BCL-2 family consists of a group of structurally homologous proteins that vary functionally to either potentiate or antagonize apoptosis. These proteins serve as important intermediaries in the intrinsic pathway of apoptosis. It is important to understand the properties and mechanistic activities of this family of proteins to better conceptualize the apoptotic process.
Classification
Members of the BCL-2 family may be classified based on their structure and function.
Structurally, BCL-2 contains four BCL-2 homology (BH) domains, referred to as BH1, BH2, BH3, and BH4. These domains are the defining feature of this family and are conserved across both pro- and antiapoptotic members (18). The multidomain members of this family exhibit a highly conserved structure, and, critically, all display a hydrophobic groove on their surface. This groove is essential for interactions between the different BCL-2 family members and is therefore key to determining the survival status of the cell (19).
Functionally, the multidomain homologous proteins BAX and BAK (and possibly BOK) serve to promote apoptosis, while the multidomain homologs, BCL-2, BCL-XL, BCL-W, MCL-1, Bfl1/A1, and BCL-B, protect against it (11).
The importance of the proapoptotic BCL-2 homologs to the intrinsic apoptotic process was evidenced with experiments which showed that cells that were doubly negative for both BAK and BAX were resistant to apoptosis induced by a variety of stimuli, such as growth factor deprivation; treatment with staurosporine, etoposide, or sodium butyrate; exposure to UV radiation; and endoplasmic reticulum stress (20, 21).
In addition to the multidomain proteins, a secondary group of related proteins consists of those with only the BH3 homology domain, but these proteins are capable of binding to and regulating the activity of the multidomain homologs (22) (Fig. 1).
FIG 1.
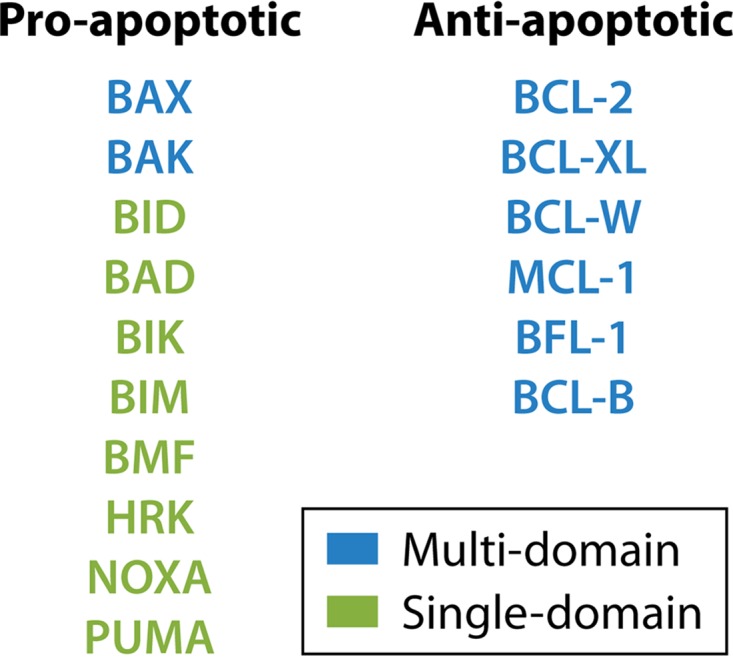
Classification of BCL-2 proteins. BCL-2 proteins may be classified based on structure and function. Structurally, they may be classified into multidomain proteins (consisting of four homology domains, BH1, BH2, BH3, and BH4) or single-domain (BH3 domain-only) proteins. Functionally, they may be grouped as having a proapoptotic or an antiapoptotic function.
The BH3 domain-only proteins, BID, BAD, BIK, BIM, BMF, HRK, NOXA, and PUMA, which are proapoptotic in nature, are responsible for adding a secondary layer of control for this system and act by promoting the proapoptotic effects of BAX and BAK.
The BH3 domain-only proteins exert their influence through two possible mechanisms: directly, by binding to and causing conformational changes in the proapoptotic homologs BAX and BAK, or indirectly, by inactivating the antiapoptotic (23) homologs BCL-2, BCL-XL, MCL-1, BCL-W, Bfl1/A1, and BCL-B. The BH3-only proteins tip the balance toward cell death when they reach concentrations sufficient to achieve both the inactivation of the antiapoptotic proteins and the activation of BAX and BAK (19).
Interestingly, the members of the BH3-only group express different affinities to their multidomain counterparts, depending on the sequences presented in the corresponding surface groove (19, 24).
The proteins BID, BIM, and PUMA were found to bind with a high affinity to all the antiapoptotic multidomain homologs, whereas BAD selectively bound BCL-2, BCL-XL, and BCL-W. NOXA was found to selectively bind MCL-1, and Bfl1/A1, BIK, and HRK were found to preferentially bind BCL-2, BCL-W, and Bfl1/A1 (24). Differential binding affinities have also been described among the multidomain homologs, with BAX binding to BCL-2, BCL-XL, MCL-1, or BCL-W and BAK preferentially binding to MCL-1 and BCL-XL (25, 26).
BCL-2 and BCL-XL have also been demonstrated to influence cell death through autophagy via the binding and inactivation of the BH3 domain-containing protein Beclin-1, a key molecule in the autophagic process (27–29).
Models of Interaction
The end result of proapoptotic BCL-2 family protein activation is mitochondrial outer membrane permeabilization (MOMP). The integrity of the mitochondrial outer membrane is essential for cell survival. Outer membrane depolarization results in the release of several proapoptotic proteins, such as cytochrome c, apoptotic protease-activating factor 1 (Apaf-1), apoptosis-inducing factor (AIF), and second mitochondrion-derived activator of caspases (SMAC)/DIABLO, which are usually stored between the inner and outer mitochondrial membranes. These proteins subsequently activate downstream caspases and result in cell death (30). This cell death pathway is referred to as the “intrinsic pathway” of cell death and is discussed in more detail below.
A growing understanding of the interactions between the pro- and antiapoptotic homologs of BCL-2 and how they influence the apoptotic process has led to the postulation of several models describing their mechanisms of activity. These models have been refined over time to include detail on new developments and have been reviewed extensively elsewhere (reviewed in reference 31). They are briefly summarized below.
The original rheostat model suggested that the ratio between pro- and antiapoptotic BCL-2 homologs determines the fate of the cell, with the overexpression of proapoptotic proteins driving the cell toward apoptosis. This model suggested that at homeostasis, the proapoptotic effects of BAX and BAK are directly inhibited by their sequestration by antiapoptotic homologs, such as BCL-2, preventing their interaction with the mitochondrial outer membrane and thereby preventing the resultant membrane depolarization and apoptosis (10). The direct model of activation incorporated the role of BH3-only proteins, classifying them into activators and sensitizers (22, 31). In this model, the BID-like proteins, which display binding affinity to both the pro- and antiapoptotic BCL-2 homologs, serve to directly activate BAK and BAX, while BAD-like proteins, which selectively bind only to the antiapoptotic homologs, serve to bind and inactivate these proteins, thereby allowing the BID-like proteins to be displaced and initiate apoptosis (31). The BID-like activator proteins are sequestered by the multidomain antiapoptotic proteins, like BCL-XL (32), and are limited in their activity until they are released upon the binding of sensitizer proteins. The direct model also proposed that the antiapoptotic function of multidomain homologs is accomplished by the sequestration of the BH3 activators and not BAX and BAK directly (32).
In contrast to the direct model, the displacement model of activation considers BAX and BAK to be constitutively active, with their proapoptotic activity being inhibited by sequestration with antiapoptotic homologs. The model suggests that upon the binding and inactivation of the multidomain proteins by BH3-only proteins, bound BAX and BAK are displaced, which initiates apoptosis (31).
The embedded-together model subsequently incorporated the involvement of the mitochondrial membrane in the conformational changes occurring in BAX and BAK. This model meshed the suggestions of the direct and displacement models by suggesting that while BAX and BAK can be activated by BH3-only activator proteins, they are also constitutively capable of initiating MOMP. It was also suggested that the interaction between the activator BH3 proteins and antiapoptotic proteins can result in either a proapoptotic or an antiapoptotic phenotype, with the survival of the cell being dictated by the relative cytoplasmic concentrations of each. An increase in activator concentration would allow for sequestration and inactivation of antiapoptotic proteins, thereby reducing their ability to bind BAX and BAK. Conversely, an increased relative concentration of antiapoptotic proteins would prevent BH3 activator effects on BAX and BAK activation (31, 32).
The subsequently proposed unified model classified antiapoptotic activity into two modes, with mode 1 being the sequestration of BH3-only proteins and mode 2 being the sequestration of BAX and BAK. The model states that mode 1 is a less effective inhibitor of MOMP, being easier to overcome than mode 2 (33).
The proapoptotic BCL-2 homologs may therefore act through a set of different mechanisms to achieve their end goal of MOMP. The fluidity of the interactions and their susceptibility to change based on relative cytoplasmic concentrations possess the potential not only to precipitate disease but also to serve as a target for therapeutic intervention.
PATHWAYS OF APOPTOSIS
The Extrinsic Pathway
There are three components required for the initiation of apoptosis through the extrinsic pathway: an exogenous ligand, a corresponding cell surface receptor for the ligand, and the respective cytoplasmic death domain associated with the receptor. The successful binding and association of all three components allow for the activation of an initiator caspase, such as caspase 8, which leads directly or indirectly to the activation of caspase 3 and the final common pathway, leading to apoptosis.
The ligand-receptor interactions that are most frequently involved are Fas ligand (FasL) binding to Fas receptor (FasR), tumor necrosis factor alpha (TNF-α) binding to tumor necrosis factor (TNF) receptor 1 (TNFR1), and TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) binding to either DR4 (TRAIL receptor 1) or DR5 (TRAIL receptor 2). The binding of these ligands to their cell surface receptors causes the respective intracellular adapter proteins to associate with the receptor Fas-associated death domain (FADD) to FasR and TNF-α-associated death domain (TRADD) to TNFR1 (13, 34). FADD also associates with TRAIL/DR4-DR5 binding (35). The death effector domain of FADD then associates with the zymogen form of caspase 8 and, possibly, caspase 10 (35, 36), leading to the formation of the death-inducing signaling complex (DISC) (13, 37). TRADD cannot directly induce DISC formation, relying on the recruitment of FADD to convey the signal downstream. Autoactivation of DISC-associated caspases, in turn, allows for caspase 3 activation and executioner pathway signaling, though this can be antagonized by the X-linked inhibitor of apoptosis protein (XIAP) (36).
The BCL-2 family interacts with the extrinsic pathway through caspase 8 activity. Caspase 8 possesses the ability to bind and activate the BH3-only protein BID. BID causes the activation of BAX and BAK and leads to mitochondrial permeabilization, as described above. One of the released proteins, second mitochondrion-derived activator of caspases (SMAC), can subvert the inhibitory activity of XIAP, potentiating apoptosis (36).
The Intrinsic Pathway
The intrinsic pathway functions through the BCL-2 homolog-mediated release of proapoptotic effectors into the cytoplasm. Initiation of the intrinsic pathway may be due to a variety of intra- or extracellular signals, such as DNA damage, withdrawal of growth factors, hypoxia, chemotherapy drugs, or a defective cell cycle (38, 39).
The BCL-2 homologous proteins BAX and BAK serve as a point of control for this system. Possessing an inherently proapoptotic role, these proteins possess the ability to effect changes in mitochondrial outer membrane permeability, allowing for the release of the apoptotic effectors.
BAX and BAK associate with a mitochondrial voltage-gated ion cannel (VDAC) and allow for the efflux of cytochrome c. This is antagonized under prosurvival conditions by antiapoptotic proteins, such as BCL-2 and BCL-XL (40). Under prosurvival conditions, the BCL-XL molecule binds to a 16-amino-acid sequence on the BH3 domain of BAK, rendering it inactive (41). Upon reception of proapoptotic signals, mediated by BH3-only proteins (42, 43), BAX undergoes a conformational change and inserts into the mitochondrial membrane as an active monomer. At this juncture, the binding of BAX by an antiapoptotic protein can still abort apoptosis (44). When BH3-only proteins reach the threshold level that allows for the binding and inactivation of all available antiapoptotic proteins, the BAX monomer goes unbound. This allows for the formation of a BAX homodimer and, subsequently, a homo-oligomer on the mitochondrial surface, with up to four BAX molecules associating with each other, leading to the formation of an apoptotic pore. The resultant alteration in mitochondrial membrane permeability allows for initiator protein efflux (19, 45).
This cascade results in the release of the mitochondrial proteins cytochrome c, Apaf-1, AIF, and SMAC/DIABLO into the cytoplasm (15, 46). Once in the cytoplasm, the cytochrome c interacts with the apoptotic protease-activating factor (Apaf-1), which activates the apoptosome, which in turn mediates the activation of procaspase 9 to caspase 9. Activated caspase 9 effects a sequential activation of the executioner pathway, ultimately leading to the death of the cell (47).
The Common Final Pathway
The executioner pathway is the final common denominator in the apoptotic cascade, with caspase 3 serving as the point of confluence for the intrinsic and extrinsic pathways. Activated caspase 3 activates CAD, an endonuclease, by cleaving its inhibitor, ICAD. This allows CAD to bind to and degrade chromosomal DNA. Caspase 3 also cleaves cytoskeletal proteins, such as actin, poly(ADP-ribose) polymerase 1 (PARP1), fodrin, laminin, and gelsolin, disrupting the cell structure and intracellular transport (13, 48). The end result of this process is cell shrinkage and DNA fragmentation, features that are described as the hallmarks of apoptotic cell death.
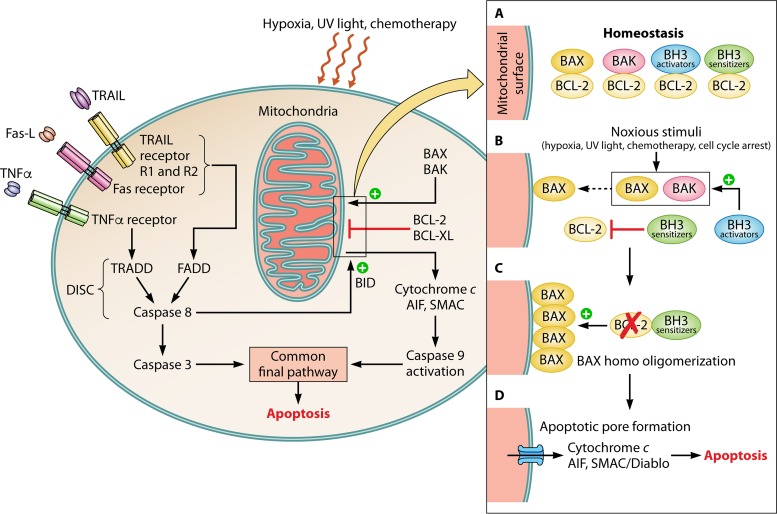
The pathways involved in the apoptotic process and the interactions of BCL-2 proteins involved are summarized in Fig. 2.
FIG 2.
Role of BCL-2 in the apoptotic process. (Left) Overview of the apoptotic pathways. The binding of an exogenous death-inducing ligand to its respective cell surface receptor leads to the formation of the death-inducing signaling complex (DISC), with caspase 8 activation leading either to BID cleavage, which acts upon BAX/BAK, or caspase 3 activation and apoptosis. Noxious external stimuli or an internal cellular dysfunction may lead to an imbalance between pro- and antiapoptotic members of the BCL-2 family. The resulting release of cytochrome c, SMAC/DIABLO, and AIF activates caspase 9, which activates caspase 3, leading to apoptosis. (Right) (A) At homeostasis, the proapoptotic proteins BAX and BAK and the activator BH3-only proteins are bound by the antiapoptotic proteins BCL-2 and BCL-XL. (B) Upon reception of noxious stimuli, BCL-2 dissociates from the bound proteins, allowing BAX and BAK to insert into the mitochondrial outer membrane. The BH3-only proteins may potentiate apoptosis either by directly activating the proapoptotic proteins or by inactivating antiapoptotic proteins. (C) BAX undergoes homo-oligomerization on the mitochondrial surface. (D) The resultant apoptotic pore allows for the release of cytochrome c, SMAC/DIABLO, and AIF, which activates caspase 9, which activates caspase 3, leading to apoptosis.
HIV-1 AND THE BCL-2 FAMILY
HIV-1 Infection and BCL-2 Modulation
At the cellular level, HIV-1 infection begins with the virus gaining cellular entry, integrating with the host genome, and utilizing the host machinery to translate viral proteins and to assemble viral progeny. The virus exhibits a predilection for the CD4+ T lymphocyte as the target cell of choice but is also capable of establishing infection in macrophages and monocytes, which also express the CD4 receptor. Though the nascent stages of infection see viral replication and dissemination occur in the peripheral circulation, the large majority of viral replication in early infection occurs in lymphoid follicles, with the gut lymph tissue serving as the major site (2).
The levels of BCL-2 and its homologs are modulated to different degrees during HIV infection, resulting in either a proapoptotic or an antiapoptotic phenotype, varying with the duration of infection and the type of infected cell (Table 1). Early infection is characterized by the widespread death of lymphocytes through a variety of viral and virally induced factors (reviewed in references 2 and 49), with the effects on BCL-2 expression playing a role. Viral replication affects BCL-2 homeostasis, favoring a proapoptotic profile. Peripheral blood lymphocytes from HIV-1-seropositive individuals show a correlation between the downmodulation of BCL-2 and active viral replication, with the rate of replication inversely correlating with the levels of BCL-2 (50). The following text attempts to summarize the modulations in the levels of BCL-2 and its homologous proteins throughout the infective process of HIV across the various immune cell types.
TABLE 1.
Variations in the levels of BCL-2 family members in cell subsets during HIV infectiona
| Cell subset | Level variation during: |
|||||
|---|---|---|---|---|---|---|
| Acute HIV infection |
Chronic HIV infection |
ART therapy |
||||
| BCL-2 | Other homologs | BCL-2 | Other homologs | BCL-2 | Other homologs | |
| CD4+ T cells | ||||||
| Proapoptotic | ↓BCL-2 | ↑BAX | ↓BCL-2 | |||
| Antiapoptotic | ↑BCL-2 | Levels restored to normal | ||||
| ↑BCL-2 in infected TFH cells | ↑BCL-2 in latently infected central memory CD4 cells | |||||
| ↑BCL-2 in infected TN, TReg, and PD-1low cells |
||||||
| CD8+ T cells | ||||||
| Proapoptotic | ↓BCL-2 | ↓BCL-2 | ↓BCL-XL | |||
| Antiapoptotic | ↑BCL-2 in elite controllers | ↑BCL-2 response to IL-7 | ||||
| Monocytes | ↓BCL-2 | ↑BCL-2, allowing for survival of infected cells | ||||
| Macrophages, antiapoptotic | ↑BCL-2 | ↑BCL-XL | ↑BIM (paradoxically increased survival) | |||
| ↓BAD and BAX | ||||||
| Dendritic cells | ||||||
| Myeloid | ↑BCL-2 in tissue | |||||
| ↓BCL-2 in circulation | ||||||
| Plasmacytoid | ↑BCL-2 in circulation | |||||
BCL-2 and its family members have demonstrated different susceptibilities to HIV-induced modulations in their levels across the different immune cell subsets at different stages of infection.TN, naive T cell; TReg, regulatory T cells; PD-1low, cells expressing low levels of the PD-1 ligand.
CD4+ T cells.
CD4+ T cells from untreated, infected patients have been observed to differentially express BCL-2 compared to CD4+ T cells from uninfected controls. These studies have consistently demonstrated that while the relative expression of BCL-2 in CD4 T cells may be up- or downregulated in comparison to its expression in the controls, infected cells are generally more susceptible to programed cell death events. It has been put forth that this finding may be explained by the concomitant upregulation of proapoptotic members of the BCL-2 family members in HIV-infected cells, altering the ratio between BCL-2 and its proapoptotic homologs, such as BAX. This finding was confirmed in in vitro infections of the CEM T-cell lymphoblastoid cell line. Additionally, it has been suggested that cells with low BCL-2 expression may experience rapid turnover and may therefore be detected at lower frequencies than cells with a relatively higher expression of BCL-2. Additionally, acute viral infection has been shown to demonstrate a decrease in BCL-2 in circulating CD4 T cells (51–53). BCL-2 levels have been shown to correlate inversely with the plasma viral load, with apoptotic HIV-1-infected CD4+ T cells consistently possessing decreased levels of BCL-2 (54). In infected individuals early in infection, Gag-specific CD4+ T cells exhibited decreased BCL-2 expression compared to cytomegalovirus (CMV)-specific CD4+ T cells from the same individuals (55). Similarly, the expression of BCL-2 in HIV-1-specific CD4+ T cells is decreased in chronic infection and is associated with increased rates of apoptosis (56). CD4+ T cells in the S phase of their life cycle demonstrated decreased levels of BCL-2 relative to other T cells in chronically infected patients and exhibited an increased susceptibility to apoptosis upon T-cell receptor (TCR) or interleukin-2 (IL-2) stimulation (57).
A recent study demonstrated that CD4 T cells isolated from patients on ART which express OX40 are preferentially infected by HIV in the in vitro setting (58). OX40 activity has clearly been demonstrated to upregulate BCL-2 and BCL-XL in CD4 T cells (59), and preferential infection of OX40hi cells may facilitate HIV persistence through BCL-2 overexpression.
Viral tropism is another factor that has been shown to impact BCL-2 levels. As mentioned earlier, during entry, the virus binds CD4 and one of two coexpressed receptors, CCR5 and CXCR4. Based on the preferential binding of the virus to either one or both of these receptors, the virus may be termed “CCR5 tropic,” “CXCR4 tropic,” or “dual tropic.” It is of interest to note that virally induced BCL-2 modulations may vary between CCR5- and CXCR4-tropic viruses. In vitro infections of follicular CD4+ T cells with both strains of virus demonstrated that the CCR5-producing follicular CD4+ T cells expressed larger amounts of BCL-2 than CXCR4-producing cells (60).
The decrease in the levels of BCL-2 was found to be reversible with the initiation of ART, with the levels returning to normal or even increasing in comparison to those in controls (54).
CD8+ T cells.
CD8+ cytotoxic T lymphocytes are responsible for the majority of antigen-specific immune effector functions. In untreated, HIV-1-infected individuals, CD8+ T cells displayed downmodulated BCL-2 expression profiles, which rendered them susceptible to apoptosis (51). The HIV-1-specific CD8+ T-cell subset demonstrated greatly reduced expression of BCL-2 and impaired induction of its homolog, BCL-XL, both of which resulted in increased rates of apoptosis (61). This population also exhibited reduced BCL-2 expression when activated (CD38+), with the levels of BCL-2 being lower than those in CMV-specific CD8+ T cells in both the activated and the inactivated states (62). This finding suggests that HIV-1-specific CD8+ T cells may inherently be at a survival disadvantage compared to CD8+ T cells specific to other antigens. Interestingly, acute HIV-1 infection was shown to cause an increase in the activation of CD8+ T cells targeted at other viruses, with Epstein-Barr virus-specific T cells exhibiting lower levels of BCL-2 than the controls (63). Similar to the findings in CD4+ T cells, the levels of BCL-2 in S-phase CD8+ T cells were also reduced; this reduction was found to be reversible upon TCR or IL-2 stimulation (57).
HIV-1-specific CD8+ T cells also exhibited lower levels of the IL-7 receptor (CD127) in acute infection, which was associated with lower levels of BCL-2. The early initiation of ART allowed for the establishment of a CD127-expressing memory phenotype pool which demonstrated an increased proliferative capacity, a finding that could be attributable to the IL-7-induced increase in BCL-2 expression in these cells (64). HAART was also shown to partially restore IL-7-induced BCL-2 expression in both CD4 and CD8 cells, though the response compared to that in healthy controls was still decreased (65).
The possible importance of BCL-2 to viral control is further illustrated by the finding that CD8+ T cells in elite controllers possessed higher levels of BCL-2 than CD8+ T cells in other chronically infected patient cohorts (66), providing an explanation for the superior immune surveillance and viral suppression observed in these patients.
Dendritic cells.
Though they serve as the major target, viral effects on BCL-2 and its homologs are not restricted solely to lymphocytes. Dendritic cells are immune effectors that participate in the innate immune response and may be characterized as plasmacytoid or myeloid.
Myeloid dendritic cells are antigen-presenting cells (APCs) that are responsible, in acute HIV-1 infection, for the transfer of the virus to the CD4+ lymphocyte (67). It has been observed that circulating myeloid dendritic cells in HIV-1-infected individuals show decreased levels of BCL-2, while, conversely, lymph node-derived myeloid dendritic cells show an increase in BCL-2 expression, creating a contrasting scenario of pro- and antiapoptotic profiles in different tissues (68).
Plasmacytoid dendritic cells, which are responsible for the release of TNF-α in response to stimulation, showed a decrease in BCL-2 levels in lymph tissue (68). The potential loss of survivability due to this decrease could possibly result in a blunted cytokine response. Considering the role of dendritic cells in the propagation of infection and considering that lymph tissue serves as the major site of replication (2), viral modulations to induce or repress the expression of BCL-2 would allow for sustained and effective viral dissemination.
Monocytes and macrophages.
Acute in vitro infection models utilizing monocytes showed a sharp decline in BCL-2 expression, followed by a rebound to basal levels. It was established that a decreased level of cellular BCL-2 is essential to HIV-1 replication, which was inhibited in cells with high BCL-2 expression. The acute decline was compensated for over time by increased, virally induced BCL-2 transcription, which allowed for the increased survival of infected cells (69).
HIV-1-infected macrophages were found to be resistant to apoptosis through an upregulation of BCL-2 and BCL-XL accompanied by a decrease in the proapoptotic BAD and BAX (70). In chronically infected patients, macrophages and microglia harboring latent virus were, paradoxically, found to exhibit increased survival, despite the paradoxical upregulation of the proapoptotic homolog BIM through an unknown mechanism (71).
Triggering receptor expressed on myeloid cells 1 (TREM-1) is a surface receptor expressed on macrophages which, when triggered, has been demonstrated to increase macrophage survival through an induction of BCL-2 (72). The HIV-1 proteins Tat and gp120 were found to upregulate the expression of the surface receptor TREM in macrophages and increase their survivability, allowing for viral persistence (73).
Myeloid precursor cells.
HIV-1 has been demonstrated to infect myeloid precursor (CD34+) cells both in vitro and in vivo (74). A positive correlation between CD34 positivity and the levels of BCL-2 has been observed in acute myeloid leukemia (75). Given that CD34+ cells were shown to express HIV-1 DNA in patients who were on ART (74), the upregulation of BCL-2 could provide a mechanism for the establishment of latency in this cell type.
Viral Modulators of BCL-2 Homologs
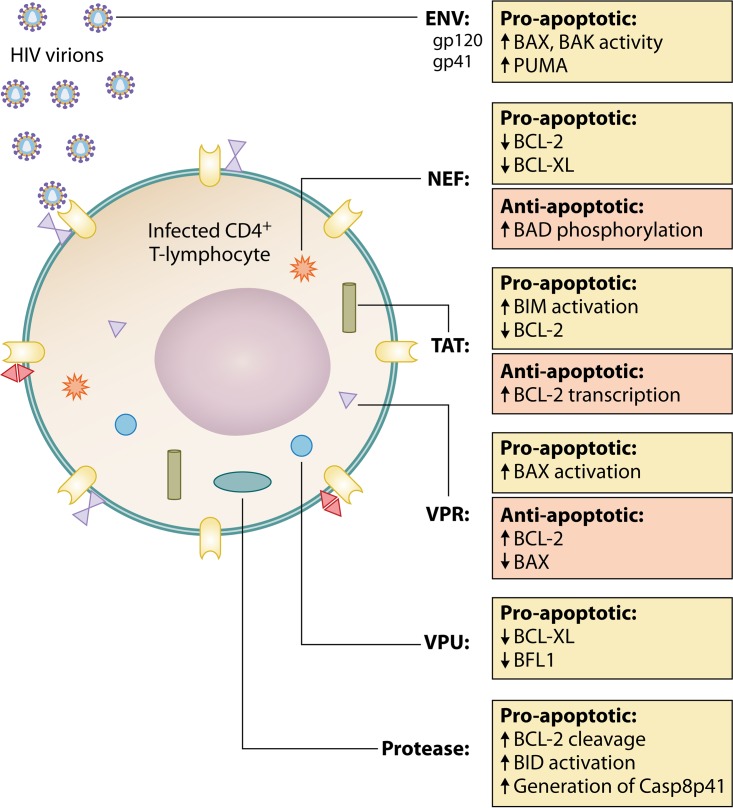
The various components of HIV-1 have been shown to exert independent, contrasting effects on apoptosis and BCL-2 expression both to favor and to oppose cell death (Fig. 3) (2). The effects of these proteins are summarized here.
FIG 3.
HIV protein effects on the BCL-2 family. HIV proteins may induce modulations in the levels of BCL-2 and its homologs through independent interactions.
Surface glycoproteins.
The viral Env protein has been implicated as a contributor to the widespread loss of cells during both acute and chronic infection (76). The Env protein, present on the viral surface, may also be expressed on the surface of the host cell following infection. Env-CD4 interactions may result in apoptosis through one of three mechanisms, each of which causes BCL-2 family modulation. (i) The first mechanism is direct interaction with the virus. gp120 cross-linking has been demonstrated to augment BAX and BAK activity by downmodulating the expression of BCL-2 in CD4+ T lymphocytes. Env may also increase the levels of BH3-only proteins, like PUMA, further potentiating BAX and BAK activity (77, 78). (ii) The second mechanism is cell surface expression of Env. Host cells expressing Env on their surface may cause the death of bystander cells in two ways: through an Env receptor interaction, where surface-expressed gp41 allows for a transfer of membrane lipids in a hemifusion event, resulting in caspase-independent cell death (79), or through the formation of a syncytium with bystander cells, leading to nuclear fusion and apoptosis (78). This syncytial pathway causes increased transcriptional activity of p53 and NF-κB, resulting in an increase in PUMA and BAX, leading to apoptosis (78, 80). (iii) The third mechanism is an interaction with Env proteins which may be present in a soluble form in plasma. Soluble Env protein gp120 has been observed to operate through a Fas/FasL- or BAX-dependent fashion (81) and has been documented as being responsible for cell death in a variety of cell types.
Viral proteins.
Upon gp120 anchoring of the virus to the cell surface, gp41 facilitates the transfer of viral proteins. These proteins contribute to the life cycle of HIV-1 and associated lymphocyte depletion, which may be accomplished through their interactions with BCL-2 homologs to modulate cell survival properties.
(i) Tat.
The Tat protein is involved in viral replication and was one of the first HIV-1-associated proteins that was shown to operate in a paracrine fashion, being expressed from infected cells and causing a myriad of effects, including the modulation of apoptosis regulators on both infected and bystander cells. The Tat protein has been demonstrated to both induce and repress the expression of BCL-2, resulting in both cell survival and death among different cell types.
In vitro, Tat effected an increase in BCL-2 promoter activity and mRNA expression both in transfected cell lines and in peripheral blood mononuclear cells treated with extracellular Tat, leading to the increased survivability in these cells (82). These effects were demonstrable with picomolar levels of Tat, which are similar to the levels observed in the plasma of infected individuals (83). Tat has also been shown to antagonize Fas-mediated apoptosis by upregulating BCL-2 and c-FLIP transcription (84). In monocytes, a dose-dependent increase in the levels of BCL-2 was observed and was shown to directly antagonize the effect of TRAIL and abrogate apoptosis (85).
Alternatively, Tat has been shown to cause mitochondrial membrane permeabilization and effect apoptosis facilitated by the proapoptotic homolog BIM in CD4 cells and has also been demonstrated to directly cause the upregulation of the BH3 domain-only, proapoptotic proteins BIM, PUMA, and NOXA through activation of the FOXO3a transcriptional activator (86, 87). Expression of the Tat protein in hematopoietic progenitors was found to correlate with the decreased transcription and translation of BCL-2, leading to apoptosis (88). In activated CD8+ T cells, exogenous Tat protein was shown to cause an increase in BCL-2 expression, accompanied by a downregulation of the IL-7 receptor (CD127) (89). Given the importance of IL-7 in BIM- and BCL-2-dependent lymphocyte homeostasis, the Tat protein may be able to effect increased rates of apoptosis in this cell type through this mechanism.
(ii) Nef.
The HIV-1 Nef protein has been shown to both induce and repress apoptosis. In vitro, Nef-transfected CEM lymphoblastoid T-cell lines expressed markedly reduced levels of the antiapoptotic proteins BCL-2 and BCL-XL, favoring a proapoptotic profile (90).
Conversely, Nef has also been shown to phosphorylate and inactivate the proapoptotic homolog BAD, preventing apoptosis (91). Simian immunodeficiency virus (SIV) Nef-expressing cells exhibited a significant increase in BCL-2 expression, accompanied by a decrease in susceptibility to Fas-mediated apoptosis (92). Nef has also been shown to downmodulate the expression of the tumor suppressor P53 and reduce the associated apoptosis (93). P53 is known to effect apoptosis through BCL-2-related mechanisms by both repressing BCL-2 transcription and promoting BAX and BH3-only protein transcription (94).
The HIV-1 Nef protein was also shown to antagonize the BCL-2-associated protein Beclin-1 and prevent cell death due to autophagy (95).
(iii) HIV-1 protease.
HIV-1 protease was shown to independently cause an increase in apoptosis in cells that were transfected with a plasmid coding for HIV-1 protease, and this was found to be associated with the cleavage of BCL-2 (96). BCL-2 was inversely associated with protease-mediated apoptosis, and overexpression was able to successfully inhibit activity (96). Protease was also shown to cleave procaspase 8 and activate the BCL-2 homolog BID, resulting in mitochondrial membrane depolarization (97). Protease-induced procaspase 8 cleavage may also generate the protein Casp8p41. Casp8p41 was shown to be highly specific to HIV-1-infected CD4 cells predominantly of the memory T cell subset and was shown to activate BAK and potentiate apoptosis (98, 99). The central memory subset of T cells also exhibited an increased BCL-2/procaspase 8 ratio, which allowed for cellular BCL-2 to bind and inactivate Casp8p41, allowing for the survival of the HIV-1-infected cell population (100).
(iv) Vpu.
Vpu is an HIV-1 viral protein involved in the budding and release of the mature virion from infected cells and in the degradation of CD4 (101, 102). Vpu has been demonstrated to possess apoptotic activity against CD4 lymphocytes, established through the inhibition of NF-κB-induced expression of the antiapoptotic BCL-2 homologs BCL-XL and Bfl1/A1 (103).
(v) Vpr.
The Vpr accessory protein, involved in HIV-1 replication, may exert pro- or antiapoptotic modulations. Vpr was shown to activate BAX and cause apoptosis, acting through an ATR-dependent mechanism (104). Vpr has also been shown to inhibit NF-κB activity in transfected cells, which, when considered in the context of the inherently proapoptotic property of Vpr and the effects of NF-κB inhibition, suggests a possible role for BCL-2 homolog downregulation (103, 105).
In contrast, in Jurkat cells transfected with Vpr, an increase in BCL-2 and a decrease in BAX accompanying decreased susceptibility to cytokine-induced apoptosis were observed (106).
Overall, the interactions of individual viral proteins and their effects on different cell types are largely variable and may not fully represent the effect of actual HIV infection on host cell expression of BCL-2 and its homologs. In the context of acute infection, the presence of HIV proteins in the circulation possesses the potential to differentially regulate BCL-2 and its homologs and to lead to bystander death or viral persistence.
In the era of modern ART therapy, it has been possible to largely limit the production of HIV virions and viral proteins; however, the importance of these effects on the BCL-2 family emerges with the concept of “leaky latency.” This term refers to the low-level expression of HIV RNA transcripts and viral proteins in patients with otherwise undetectable viremia. It has been demonstrated that in vitro infections of resting CD4 T cells resulted in the production of HIV Gag in the absence of activation or active viral production (107). It has also recently been demonstrated that a small proportion of cells from ART-treated HIV-infected individuals are translation competent and may produce HIV Gag in vivo. Some of these Gag-positive cells were also seen to be CD4−, which was suggested to be due to the expression of HIV Env or Nef, and it has been demonstrated that the low-level expression of Nef occurs in infections of resting CD4+ T cells in vitro (108). These findings suggest that low levels of translation of HIV protein may still be occurring in the absence of detectable viremia. Therefore, viral proteins may have important biological effects on BCL-2 expression even during ART-induced, clinical latency.
Nonviral Modulators of BCL-2 in HIV Infection
HIV infection induces an enormous number of changes to the levels of cytokines in the circulation. Modulations in BCL-2 members may also occur as a result of these virally induced differences in the expression of cytokines (reviewed in detail in reference 109). The important interactions of some of these cytokines with BCL-2 and its homologs are addressed here.
Interleukins are a set of secreted proteins that facilitate communication between immune effectors, regulating a myriad of effects, including cell proliferation, activation, and cycling (110). Some important BCL-2-centric interactions of interleukins in the setting of HIV infection are discussed below.
Acute HIV infection was found to be associated with increased levels of IL-4 and IL-10 and decreased levels of IL-2 in the circulation of infected patients. This profile was observed to be reversible with the initiation of HAART, with a steady increase in IL-2 and a gradual decrease in IL-4 and IL-10 being noted (111). Interestingly, IL-4 and IL-2 have been described to differentially regulate the expression of BCL-2 in a murine T-lymphocyte cell line, with IL-4 resulting in downregulation and IL-2 stimulating BCL-2 expression (112). IL-10 has been described to upregulate BCL-2 in T lymphocytes (113), though in the setting of HIV infection, it is seen to be directly associated with the viral load, suggesting that the upregulation of BCL-2 may be counteracted by concomitant downregulation by other factors (114).
Interleukin-6 (IL-6) is a cytokine associated with chronic inflammation which has been described as being upregulated in HIV infection even in the presence of suppressive ART. In patients with failure in immune reconstitution, increased IL-6 has been associated with low BCL-2 levels. This has been attributed to the IL-6-induced downregulation of CD127, which has been demonstrated to be essential for IL-7-induced BCL-2 expression in T lymphocytes (109, 115, 116). IL-1β is another cytokine upregulated in chronic infection which causes a similar downregulation of CD127 and the resultant IL-7 activity (116).
Lymphocyte homeostasis in normal settings is regulated by the ratio between BIM and BCL-2, with IL-7 stimulation resulting in the upregulation of BCL-2 expression (117, 118). IL-7 has been shown to facilitate viral reservoir survival and promote viral persistence in the presence of ART, suggesting a role for BCL-2 overexpression (119).
HIV-1 upregulates type I interferons (IFN-I), rendering the surrounding cells more susceptible to Fas-mediated apoptosis through the upregulation of both Fas production and the proapoptotic BCL-2 homolog BAK (120). It was observed that chronic exposure to type I interferons correlated with an increased viral load and progressive CD4+ T-cell depletion in SIV infection, suggesting that IFN-I-induced apoptosis may be a driving factor in the development of immunodeficiency in untreated individuals (109, 121). IFN-I levels were also seen to be elevated in the plasma of patients with chronic HIV infection but were seen to revert to undetectable levels in patients receiving ART (122).
BCL-2 IN HIV-1 LATENCY
Early viral infection is met with an attempted host immune response which is unable to achieve complete viral clearance. The drop in the levels of plasma viremia accompanies a switch in the immune system from active clearance to surveillance, allowing for the establishment of viral latency, with long-lived resting T cells serving as a reservoir (7, 123).
The role of BCL-2 in the establishment and maintenance of viral persistence has been well described in other chronic viral infective processes. B cells infected with Epstein-Barr virus have been demonstrated to have significantly improved survival and immune resistance resulting from viral protein-induced upregulation of BCL-2 (124). Similarly, CD34+ cells infected with latent cytomegalovirus (CMV) demonstrated increased levels of BCL-2 (125).
The major challenge facing HIV eradication efforts is the persistence of integrated, latent proviral forms despite HAART therapy. HIV preferentially establishes latency in CD4+ T cells (126). Multiple T-cell subsets have been described to possess significant quantities of integrated proviral HIV DNA, including central memory, naive, stem cell memory, transitional memory, effector memory, Th-1 polarized, and follicular helper CD4+ T cells (123, 127–133). However, the relative contribution of these T cell subsets to the overall reservoir of latently infected cells remains controversial. It has been demonstrated that CD4+ T cells from aviremic HIV-infected patients on HAART possess significantly higher levels of BCL-2 than CD4+ T cells from both viremic individuals and, interestingly, uninfected controls (134). It has also recently been demonstrated that BCL-2hi CD4+ T cells disproportionately harbor the HIV-inducible proviral reservoir and are resistant to immune clearance (135). Therefore, understanding the expression and activity of BCL-2 in these various T-cell subsets is important if eradication strategies based on BCL-2 inhibition are to be developed, and these are reviewed here.
Central memory T cells have been described in some studies to be the primary reservoir in latency and harbor significant quantities of HIV DNA (130). Central memory T cells have been shown to have higher levels of BCL-2 expression in conjunction with increased apoptosis resistance than effector memory T cells (100). Central memory cells from HIV-infected patients were also shown to demonstrate an altered BCL-2/procaspase 8 ratio, favoring an antiapoptotic profile (100).
Naive T cells are increasingly becoming recognized as an important reservoir in HIV latency. The percentage of naive T cells harboring HIV DNA remains constant even after ART initiation, in contrast to the steady decline observed in memory T cells, suggesting that these cells may be responsible for sustained viral persistence (129). Naive T cells from aviremic patients on HAART demonstrated an increased expression of BCL-2 compared to those from both viremic patients and uninfected controls. This was also seen to be accompanied by an increase in cell number (134). The recent demonstration that naive T cells also harbor amounts of latent, replication-competent HIV-1 comparable to those in memory T cells further illustrates the importance of this subset to latency (136). The increased BCL-2 levels seen in this subset may confer greater survivability in this subset and directly facilitate viral reservoir establishment and persistence.
Regulatory T cells (TReg) are another subset of CD4 T lymphocytes that have been identified to be an important reservoir (reviewed in reference 137). These cells are described as expressing high levels of CD25, CD62L, CTLA-4, CD103, and the glucocorticoid-induced TNF receptor-family related (GITR) gene, accompanied by the expression of FOXP3 gene, which has been described to control TReg function (138, 139).
T lymphocytes have been shown to be apoptosis resistant following CTLA-4 activation-induced BCL-2 upregulation (140), and CTLA-expressing PD-1− memory CD4 T cells isolated from lymph nodes were found to include a high frequency of TReg CD4 T lymphocytes and were shown to harbor a large and stable viral reservoir in ART-treated SIV-infected rhesus macaques. These cells were shown to express significantly higher levels of BCL-2 than other cell subsets. This subset was also shown to harbor large amounts of HIV DNA in ART-treated individuals (141).
Stem cell memory T cells (TSCM) have recently been identified to be a significant reservoir of HIV proviral DNA (131, 132). This cell subset was identified within a group of CD45RA+, CCR7+, CD62L+, CD27+, CD28+, IL-7 receptor alpha-positive (IL-7Rα+) cells, which also expressed high levels of CD95, IL-7Rβ, CXCR3, and LFA-1 (142). The TSCM subset was characterized to overexpress BCL-2 in comparison to its expression in other T-cell subsets and demonstrated greatly enhanced survival and propagative potential (142, 143). These characteristics have brought members of this subset under increasing scrutiny as important players in the latency process (reviewed in reference 144). The contribution of the TSCM subset to the total latent reservoir was seen to gradually increase with long-term ART and, importantly, was recognized to be inversely associated with total HIV DNA in the CD4+ T-cell pool, indicating a stable and persistent reservoir. This cell type has also been demonstrated to be a reservoir for replication-competent HIV proviral forms in elite controllers (131, 145). The underlying mechanisms responsible for the persistence of latency in this subset warrant investigation, with the constitutively high BCL-2 expression possibly contributing toward the observed enhancement in cell survival.
Follicular T-helper cells are a subset of CD4+ T cells that have been demonstrated to serve as a reservoir for proviral DNA (133). Follicular T cells (CXCR5+) derived from tonsillar tissue were found to constitutively express higher levels of BCL-2 than extrafollicular cells (CXCR5−). Upon infection, it was demonstrated that CXCR5+ CD4 T cells expressed higher levels of BCL-2 than infected CXCR5− cells, with this finding being consistent regardless of viral tropism (60).
More recently, the role of PD-1-expressing follicular T-helper cells has come under increasing scrutiny as a result of the large and stable reservoir detected in this subset (146). It has been demonstrated that in chronic infection, PD-1 expression correlated inversely with the expression of BCL-2, with PD-1hi-expressing CD4 T cells from HIV-infected patients exhibiting decreased levels of BCL-2 compared to PD-1low-expressing and uninfected CD4 T cells. The initiation of ART was shown to revert PD-1 expression to levels comparable to those in uninfected controls (147). Though it is yet to be elucidated, it stands to reason that the phenotypic reconstitution observed in PD-1-expressing cells, in which the phenotype is similar to that in uninfected controls following the initiation of ART, would be accompanied by an increase in the levels of BCL-2 expression, possibly facilitating viral persistence.
It therefore stands to reason that ART may exist in an interesting duality, effecting viral clearance while also playing a critically important role in establishing the viral latent reservoir. The restoration of BCL-2 levels to physiological levels would allow for the survival of infected cells while simultaneously antagonizing viral release, thereby precipitating latency.
BCL-2 IN HIV LATENCY STUDIES
One of the challenges to studying HIV-1 latency is the difficulty in establishing an accurate and reproducible model of latency in vitro. This issue stems from the inadequacies of culture being able to simulate the conditions required for latency to occur. Latency models are largely grouped into pre- and postintegration models, depending on whether viral integration occurs, with the latter more closely mimicking in vivo latency. The different models of latency have been reviewed extensively elsewhere (reviewed in reference 148). The following sections attempt to examine the role of BCL-2 in the establishment of latency in the in vitro setting.
IL-7 Stimulation
The physiological change from the activated to the resting state occurs, in vivo, secondary to IL-7 stimulation (149). A model of latency was developed to more accurately mimic these changes and to include the effects of IL-7. Briefly, primary CD4 T cells were activated by exposure to monocyte-derived dendritic cells (MDDC) and subsequently infected with HIV. At day 24, the infected cells were sorted to exclude naive cells and subsequently cultured in low doses of IL-7 for up to 4 weeks. Phenotypic analysis of these cells revealed a largely central memory phenotype and demonstrated productive viral replication on restimulation (150). The role of IL-7 in the sustenance of resting CD4+ T cells has been attributed to the IL-7-induced upregulation of BCL-2 expression (118).
BCL-2 Transfection Model
BCL-2, which is a downstream protein to IL-7 signaling, has been successful in establishing a reproducible model of viral latency following transfection into primary CD4+ T cells in vitro (151, 152). The model was established based on the premise that the results of IL-7 signaling could be limited to purely the prosurvival effects by transfecting cells with BCL-2. This was shown to allow BCL-2-transfected cells to survive in the quiescent state necessary for latency establishment (152). Briefly, primary CD4 T cells were transduced with a lentivirus containing a BCL-2 overexpression construct. This BCL-2 overexpression allowed for the survival of subsequently HIV-infected cells for an extended period of time in the absence of cytokines, allowing for a spontaneous reversion to viral latency. The BCL-2-transfected model effectively replicates the cycle of CD4+ cell activation, infection, and a subsequent return to the resting state that is seen in in vivo HIV-1 infection (151). HIV-1 integration site characteristics in this model were also found to be similar to those observed in patient samples, though differences in gene ontology were observed between the integration sites seen in patient samples and those seen in the in vitro model (153).
Chemokine Stimulation Model
Chemokine stimulation of CD4 T cells prior to infection with the CCR7 agonists CCL19 and CCL21 has been recognized as another efficient and reproducible model of latency (154, 155). This model involves the incubation of isolated resting CD4 T cells with a CCR7 agonist—either CCL19 or CCL21—prior to infection with HIV-1. High levels of stable integration with low levels of reverse transcriptase activity were observed (154). It was also demonstrated that active viral production is inducible following mitogen-induced activation, similar to the findings for ex vivo samples from latently infected patients (155). CCR7 stimulation has canonically been described as affording cells an apoptosis-resistant phenotype across various cell types and, in CD4 T cells, a limitation of proliferation (156–159). The antiapoptotic effect of CCL21/CCR7 was demonstrated to be a direct result of BCL-2 overexpression and BAX downregulation in studies conducted in lung cancer cell lines (160). CCL21 presentation on breast cancer cell lines afforded interacting dendritic cells an antiapoptotic phenotype resulting from an overexpression of BCL-2 and a reduction in caspase 3 (158). CD8 T cells expressing CCR7 were seen to be more apoptosis resistant due to an overexpression of BCL-2 and decreased expression of the Fas ligand and BAX. CCL19 and CCL21 stimulation of these cells was seen to upregulate BCL-2 expression (161).
Though studies have yet to explicitly describe this correlation, the pretreatment of resting CD4 T cells with CCR7 agonists may be accompanied by an increase in the basal expression of BCL-2 or other antiapoptotic homologs and therefore facilitate cell survival in the setting of acute infection, allowing for the infected cells to transition to latency.
Ex Vivo Expansion
A more recent development in latency models involves the isolation and expansion of memory CD4 cells from infected patients on ART. Briefly, in this model, isolated memory (CD45RA−) CD4 T cells were cultured with phytohemagglutinin, IL-2, natural human IL-2/T-cell growth factor (TCGF), and irradiated feeder cells in the presence of antiretroviral therapy, allowing cellular expansion without new rounds of infection. The expanded cells were found to revert to a resting state in culture and were found to stably express proviral forms upon reactivation (162). As mentioned above, the HIV-infected CD4+ T cell reservoir expresses high levels of BCL-2 ex vivo and in vivo, which may help in the establishment and maintenance of the reservoir in this ex vivo expansion model.
THE THERAPEUTIC POTENTIAL OF BCL-2 INHIBITORS IN HIV-1 LATENCY REACTIVATION AND CURE
The major strategy under investigation for viral reservoir elimination is the shock-and-kill method, in which the latent virus is reactivated using a latency-reactivating agent (LRA), followed by viral elimination either through effective immune clearance or as a result of therapeutic agents (163). The major caveat to the shock-and-kill approach is the inadequate ability of current shock-and-kill therapy to achieve reactivation of all replication-competent proviral forms and achieve clearance (126, 164). It therefore becomes important to consider the administration of two or more concurrent therapies that would help clearance of virus from cells in which it is latent both through preferential apoptosis and through increased immune activity.
Given the dual role that BCL-2 homologs possess with regard to cell survival, increased cell apoptosis could be achieved either by a potentiation of the effects of proapoptotic molecules or by antagonism of antiapoptotic molecules. Both these effects may be achieved through chemical mimicry of BH3-domain proteins which possess the ability to achieve either outcome.
While the effects seen in preclinical studies are a significant proof of concept, the major disadvantage with BH3 mimetics which act on multiple BCL-2 homologs stems from the relative ubiquity of the BCL-2 family. This was illustrated during the development and testing of navitoclax (ABT263), which possesses similar activity against BCL-2 and BCL-XL but minimal activity against BCL-W (165), with the antagonism of BCL-XL being responsible for severe thrombocytopenia (166). This limiting side effect was overcome with the discovery of the selective BCL-2 inhibitor venetoclax (ABT199) (167).
Venetoclax has been shown to reduce the reservoir size and reduce the proviral load and quantitative viral outgrowth assay estimates of the viral reservoir size postreactivation in in vitro settings (168). Use of the combination of venetoclax with the LRA bryostatin against latently infected cells generated using the primary T-cell model was shown to greatly potentiate the clearance of replication-competent virus (169).
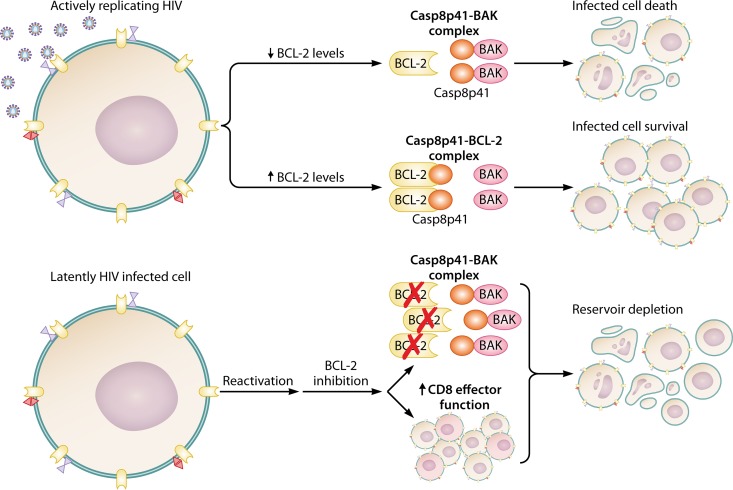
BCL-2 inhibition with venetoclax may also result in a preferential increase in apoptosis of infected cells through the potentiation of the proapoptotic, HIV-1-specific protein Casp8p41. Casp8p41 is preferentially bound and inactivated by BCL-2, and antagonism of BCL-2 resulted in Casp8p41 binding to and activation of BAK, resulting in apoptosis (99). Given the specificity of Casp8p41 for HIV-1-infected cells, BCL-2 inhibition was shown to achieve the targeted apoptosis of HIV-1-infected cells while resulting in minimal bystander cell death (168) (Fig. 4).
FIG 4.
BCL-2 inhibition assists viral clearance in HIV infection. HIV-infected central memory T cells express higher levels of BCL-2 and possess higher BCL-2/procaspase 8 ratios than uninfected cells, giving the infected cells a survival advantage over uninfected cells and allowing for the persistence of the viral reservoir. Increased levels of BCL-2 bind Casp8p41, facilitating cell survival, whereas decreased levels of BCL-2 allow for Casp8p41 to associate with BAK and induce cell death. Treatment with the BCL-2 antagonists have demonstrated the ability to (i) selectively induce apoptosis in infected cells through a Casp8p41-mediated mechanism, (ii) reduce proviral HIV DNA and viral outgrowth, suggesting a reduced viral reservoir size, and (iii) augment HIV-infected cell killing mediated by CD8+ T cells, causing reduced proviral HIV DNA and viral outgrowth.
This principle was tested in a recently conducted study which demonstrated that while the effects of venetoclax against viral latency generated by in vitro latency models were consistent and reproducible, similar effects were not seen against central memory T cells derived from patients on ART with venetoclax monotherapy. It was, however, observed in the same study that the addition of venetoclax greatly augmented HIV-1-specific CD8+ T-cell cytotoxic activity against reactivated virus from patient samples (135).
The experimental model compared the activity of in vitro-generated HIV-1-specific CD8+ T cells against central memory T cells from patients virally suppressed with ART with and without therapy with venetoclax. The study observed a significant reduction in HIV-1 DNA across both conditions but did not see a difference in the size of the inducible reservoir in the untreated group, consistent with previously published findings (169). The addition of venetoclax, however, significantly augmented CD8+ T-cell clearance of the viral reservoir, illustrating that the addition of venetoclax may result not only in the preferential apoptosis of HIV-1-infected cells but also in increased immune efficacy against the viral reservoir (135, 169).
Potential resistance to BH3 mimetics, however, is a factor to be considered in their application in the setting of HIV-1. Lymphoma cell lines that were initially responsive to treatment were seen to develop resistance following chronic treatment with the BH3 mimetic ABT737. This was attributed to a stable overexpression of Bfl1/A1 and MCL-1, accompanied by a dynamic increase in both proteins following reexposure (170). Furthermore, consideration of the suggestion that resistance to BCL-2 inhibitors may be conferred by upregulation of any of the antiapoptotic homologs, including those that serve as specific targets of the BH3 mimetic (47), presents an important caveat to the usage of these agents in the treatment of HIV-1, given the ability of the virus to upregulate not only BCL-2 but also a plethora of homologs.
To overcome similar challenges in oncology, combination therapies were considered and were shown to be effective (171). Combination therapy with navitoclax and histone deacetylase (HDAC) inhibitors like vorinostat was shown to overcome BCL-2 homolog-mediated antagonism in cancer cells (172). In the context of HIV-1, the latency reversal properties of HDAC inhibitors have seen them become exciting prospects for clinical use in viral reactivation (reviewed in reference 173). Given that vorinostat has been demonstrated to effectively reverse HIV-1 latency but minimally impact the total latent reservoir (174), combination therapy with BH3 mimetics might serve to accomplish viral reactivation and latency clearance while also avoiding homolog-mediated treatment resistance. Panobinostat is another HDAC inhibitor which was similarly shown to achieve viral reactivation while having a minimal impact on the total number of latently infected cells (175). Panobinostat has been demonstrated to have a profile similar to that of vorinostat in the context of BCL-2 homolog antagonistic activity and has been shown to potentiate venetoclax activity in acute myeloid leukemia (176). Given the independent activity of these agents in HIV-1 infection, combination therapy might effectively accomplish both aspects of the shock-and-kill adage.
Proteasome inhibitors have been described to independently induce latency reactivation and reduce viral titers (177, 178). Interestingly, in melanoma, synergistic effects were seen with the proteasome inhibitor bortezomib and ABT737 through antagonism of MCL-1 via upregulation of NOXA (179). The proteasome inhibitor ixazomib has also been shown to effect a similar upregulation of NOXA (180). Both ixazomib and bortezomib were observed to prevent Casp8p41 degradation and selectively induce cell death in HIV-1-infected cells (178). Given that a similar potentiation of Casp8p41-induced apoptosis was observed with the BCL-2 inhibitor venetoclax, combination therapy may achieve synergism and greater viral reservoir clearance, while it may also provide protection against MCL-1-mediated venetoclax resistance.
Another avenue for combination therapy is through the use of selective MCL-1 inhibitors (171). Numerous compounds currently under study are able to specifically target MCL-1 (reviewed in reference 181). Combination therapy with navitoclax and MCL-1 inhibitors has been demonstrated to have increased efficacy in cancer and may provide another alternative avenue for HIV-1 therapy (182).
CONCLUDING REMARKS
As the battle against HIV continues and conventional ART fails to target latently infected cells, the exploration of alternative therapies is necessary to achieve a final cure. BCL-2 and its homologs undergo numerous modulations over the course of the HIV infective process. Studies are required, however, to clearly define the differences in BCL-2 expression between T-cell subsets in lymphoid tissues and the efficacy of anti-BCL-2 therapy in targeting these cells. With the advent of highly specific and tolerable BCL-2 inhibitors, the clinical applicability of these agents increases in relevance. These compounds have shown specific activity against HIV-infected cells in vitro both in the context of acute infection and in the shock-and-kill approach in the setting of latency. A challenge facing the clinical application of these compounds, however, is the relatively low tissue penetrability that these agents currently possess.
As studies progress in an attempt to answer these issues, the application of BCL-2 inhibition in the setting of HIV infection and latency might represent a novel strategy to bring HIV cure closer to reality.
ACKNOWLEDGMENTS
We thank the innumerable persons living with HIV who have participated in research studies, without which many of the described observations would not have been possible.
Portions of this work were funded through grants (grants AI110173 and AI120698) from the National Institute of Allergy and Infectious Diseases of the NIH and the Mayo Clinic Foundation.
The opinions expressed are solely of the authors and do not necessarily represent the opinions of the funding organization(s).
Biographies

Aswath P. Chandrasekar, M.B.B.S., is a researcher in the HIV/Immunology laboratory under the Department of Infectious Diseases at the Mayo Clinic in Rochester, MN. Dr. Chandrasekar received his M.B.B.S., with First Class, from the K. S. Hegde Medical Academy, Mangalore, India, and aspires to pursue a career in clinical infectious disease and immunology while maintaining a strong interest in basic science research. His current ongoing projects explore the influence of antiapoptotic proteins in HIV persistence and their effect on cytotoxic activity mediated by immune effectors.

Nathan W. Cummins, M.D., is a Consultant in Infectious Diseases, Chair of Infectious Diseases Research, and Assistant Professor of Medicine at the Mayo Clinic in Rochester, MN. He completed medical training at the University of Kentucky, internal medicine and infectious diseases training at the University of Cincinnati, and transplant infectious diseases training at the Mayo Clinic. He has been an HIV clinician and researcher for over 10 years. He has authored or coauthored >40 peer-reviewed publications and has been awarded Fellowship in the Infectious Diseases Society of America (FIDSA).

Andrew D. Badley, M.D., is the H. H. Sheikh Khalifa Bin Zayed Al-Nahyan Professor of Infectious Diseases, honoring Dr. W. Wilson, and a Professor of Molecular Medicine at the Mayo Clinic in Rochester, MN. He received his M.D. degree from Dalhousie University in Halifax, Nova Scotia, Canada, in 1990 and completed his internal medicine residency and clinician investigator training in infectious diseases at Mayo Clinic, Rochester, MN. Dr. Badley’s research focus investigates the regulation of cell death and cell survival during infectious diseases, notably, HIV infection, and how understanding these processes can lead to novel therapeutic strategies to reduce morbidity and mortality and contribute to a cure of HIV infection. Dr. Badley is a past Associate Dean of Research and currently serves as the Founder and Director of the Office of Translation to Practice and Chair of Research in the Department of Medicine at Mayo Clinic.
REFERENCES
- 1.Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Cummins NW, Badley AD. 2010. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis 1:e99. doi: 10.1038/cddis.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinkins C, Arko-Mensah J, Deretic V. 2010. Autophagy and HIV. Semin Cell Dev Biol 21:712–718. doi: 10.1016/j.semcdb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan T, Wu S, He X, Luo H, Zhang Y, Fan M, Geng G, Ruiz VC, Zhang J, Mills L, Bai C, Zhang H. 2014. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS One 9:e93944. doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey RT Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siliciano RF, Greene WC. 2011. HIV latency. Cold Spring Harb Perspect Med 1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. 1984. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 9.Vaux DL, Cory S, Adams JM. 1988. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 10.Oltvai ZN, Milliman CL, Korsmeyer SJ. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick JM, Soane L. 2013. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 5:a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK-M, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin K-M, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. 2014. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Hengartner MO. 2000. The biochemistry of apoptosis. Nature 407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 16.Julien O, Wells JA. 2017. Caspases and their substrates. Cell Death Differ 24:1380–1389. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 18.Cory S, Adams JM. 2002. The BclII family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Adams JM, Cory S. 2018. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ 25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cost GJ, Freyvert Y, Vafiadis A, Santiago Y, Miller JC, Rebar E, Collingwood TN, Snowden A, Gregory PD. 2010. BAK and BAX deletion using zinc-finger nucleases yields apoptosis-resistant CHO cells. Biotechnol Bioeng 105:330–340. doi: 10.1002/bit.22541. [DOI] [PubMed] [Google Scholar]
- 21.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183–192. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 23.Bouillet P, Strasser A. 2002. BH3-only proteins—evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci 115(Pt 8):1567–1574. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 27.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. 1998. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 72:8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. 2007. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. 2005. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Chipuk JE, Bouchier-Hayes L, Green DR. 2006. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ 13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 31.Shamas-Din A, Kale J, Leber B, Andrews DW. 2013. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol 5:a008714. doi: 10.1101/cshperspect.a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leber B, Lin J, Andrews DW. 2007. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. 2011. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu H, Xiong J, Goeddel DV. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone RW, Frew AJ, Smyth MJ. 2008. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 36.Tummers B, Green DR. 2017. Caspase-8: regulating life and death. Immunol Rev 277:76–89. doi: 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan KH, Blanco-Codesido M, Molife LR. 2014. Cancer therapeutics: targeting the apoptotic pathway. Crit Rev Oncol Hematol 90:200–219. doi: 10.1016/j.critrevonc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Wu CC, Bratton SB. 2013. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal 19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu S, Narita M, Tsujimoto Y. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 41.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. 1997. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 42.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. 2010. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell 40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson CJ, Davids MS. 2015. BCL-2 antagonism to target the intrinsic mitochondrial pathway of apoptosis. Clin Cancer Res 21:5021–5029. doi: 10.1158/1078-0432.CCR-15-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. 2013. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X, Wang X. 2004. Cytochrome c-mediated apoptosis. Annu Rev Biochem 73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 47.Schenk RL, Strasser A, Dewson G. 2017. BCL-2: long and winding path from discovery to therapeutic target. Biochem Biophys Res Commun 482:459–469. doi: 10.1016/j.bbrc.2016.10.100. [DOI] [PubMed] [Google Scholar]
- 48.Sakahira H, Enari M, Nagata S. 1998. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 49.Cummins NW, Badley AD. 2014. Making sense of how HIV kills infected CD4 T cells: implications for HIV cure. Mol Cell Ther 2:20. doi: 10.1186/2052-8426-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Re M, Gibellini D, Aschbacher R, Vignoli M, Furlini G, Ramazzotti E, Bertolaso L, La Placa M. 1998. High levels of HIV-1 replication show a clear correlation with downmodulation of Bcl-2 protein in peripheral blood lymphocytes of HIV-1-seropositive subjects. J Med Virol 56:66–73. doi:. [DOI] [PubMed] [Google Scholar]
- 51.Boudet F, Lecoeur H, Gougeon ML. 1996. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol 156:2282–2293. [PubMed] [Google Scholar]
- 52.Dobmeyer TS, Klein SA, Dobmeyer JM, Raffel B, Findhammer S, Hoelzer D, Helm EB, Rossol R, Kabelitz D. 1998. Differential expression of bcl-2 and susceptibility to programmed cell death in lymphocytes of HIV-1-infected individuals. Clin Immunol Immunopathol 87:230–239. doi: 10.1006/clin.1997.4465. [DOI] [PubMed] [Google Scholar]
- 53.Scheuring UJ, Sabzevari H, Corbeil J, Theofilopoulos AN. 1999. Differential expression profiles of apoptosis-affecting genes in HIV-infected cell lines and patient T cells. AIDS 13:167–175. doi: 10.1097/00002030-199902040-00004. [DOI] [PubMed] [Google Scholar]
- 54.Regamey N, Harr T, Battegay M, Erb P. 1999. Downregulation of Bcl-2, but not of Bax or Bcl-x, is associated with T lymphocyte apoptosis in HIV infection and restored by antiretroviral therapy or by interleukin 2. AIDS Res Hum Retroviruses 15:803–810. doi: 10.1089/088922299310700. [DOI] [PubMed] [Google Scholar]
- 55.Zaunders JJ, Munier ML, Kaufmann DE, Ip S, Grey P, Smith D, Ramacciotti T, Quan D, Finlayson R, Kaldor J, Rosenberg ES, Walker BD, Cooper DA, Kelleher AD. 2005. Early proliferation of CCR5(+) CD38(+++) antigen-specific CD4(+) Th1 effector cells during primary HIV-1 infection. Blood 106:1660–1667. doi: 10.1182/blood-2005-01-0206. [DOI] [PubMed] [Google Scholar]
- 56.Yue FY, Kovacs CM, Dimayuga RC, Gu XX, Parks P, Kaul R, Ostrowski MA. 2005. Preferential apoptosis of HIV-1-specific CD4+ T cells. J Immunol 174:2196–2204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 57.Sieg SF, Bazdar DA, Lederman MM. 2008. S-phase entry leads to cell death in circulating T cells from HIV-infected persons. J Leukoc Biol 83:1382–1387. doi: 10.1189/jlb.0907643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer CS, Duette GA, Wagner MCE, Henstridge DC, Saleh S, Pereira C, Zhou J, Simar D, Lewin SR, Ostrowski M, McCune JM, Crowe SM. 2017. Metabolically active CD4+ T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett 591:3319–3332. doi: 10.1002/1873-3468.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. 2001. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 60.Haas MK, Levy DN, Folkvord JM, Connick E. 2015. Distinct patterns of Bcl-2 expression occur in R5- and X4-tropic HIV-1-producing lymphoid tissue cells infected ex vivo. AIDS Res Hum Retroviruses 31:298–304. doi: 10.1089/aid.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrovas C, Mueller YM, Dimitriou ID, Bojczuk PM, Mounzer KC, Witek J, Altman JD, Katsikis PD. 2004. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J Immunol 172:4444–4453. doi: 10.4049/jimmunol.172.7.4444. [DOI] [PubMed] [Google Scholar]
- 62.Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar P, Mounzer KC, Altman JD, Katsikis PD. 2007. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8(+) T cells. Blood 109:2505–2513. doi: 10.1182/blood-2006-05-021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, Venet A. 2004. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol 173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 64.Lecuroux C, Girault I, Boutboul F, Urrutia A, Goujard C, Meyer L, Lambotte O, Chaix ML, Martinez V, Autran B, Sinet M, Venet A. 2009. Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV-specific CD8+ T cells. AIDS 23:1649–1658. doi: 10.1097/QAD.0b013e32832e6634. [DOI] [PubMed] [Google Scholar]
- 65.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Jacod S, Delfraissy JF, Theze J. 2006. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. J Acquir Immune Defic Syndr 42:277–285. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 66.Yan J, Sabbaj S, Bansal A, Amatya N, Shacka JJ, Goepfert PA, Heath SL. 2013. HIV-specific CD8+ T cells from elite controllers are primed for survival. J Virol 87:5170–5181. doi: 10.1128/JVI.02379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derby N, Martinelli E, Robbiani M. 2011. Myeloid dendritic cells in HIV-1 infection. Curr Opin HIV AIDS 6:379–384. doi: 10.1097/COH.0b013e3283499d63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dillon SM, Friedlander LJ, Rogers LM, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. 2011. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol 85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aillet F, Masutani H, Elbim C, Raoul H, Chêne L, Nugeyre MT, Paya C, Barré-Sinoussi F, Gougerot-Pocidalo MA, Israël N. 1998. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines. J Virol 72:9698–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guillemard E, Jacquemot C, Aillet F, Schmitt N, Barré-Sinoussi F, Israël N. 2004. Human immunodeficiency virus 1 favors the persistence of infection by activating macrophages through TNF. Virology 329:371–380. doi: 10.1016/j.virol.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 71.Castellano P, Prevedel L, Eugenin EA. 2017. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci Rep 7:12866. doi: 10.1038/s41598-017-12758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan Z, Syed MA, Panchal D, Joo M, Colonna M, Brantly M, Sadikot RT. 2014. Triggering receptor expressed on myeloid cells 1 (TREM-1)-mediated Bcl-2 induction prolongs macrophage survival. J Biol Chem 289:15118–15129. doi: 10.1074/jbc.M113.536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan Z, Fan X, Staitieh B, Bedi C, Spearman P, Guidot DM, Sadikot RT. 2017. HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1. Sci Rep 7:42028. doi: 10.1038/srep42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, Bixby D, Savona MR, Collins KL. 2010. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med 16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lauria F, Raspadori D, Rondelli D, Ventura MA, Fiacchini M, Visani G, Forconi F, Tura S. 1997. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia 11:2075–2078. doi: 10.1038/sj.leu.2400854. [DOI] [PubMed] [Google Scholar]
- 76.Garg H, Mohl J, Joshi A. 2012. HIV-1 induced bystander apoptosis. Viruses 4:3020–3043. doi: 10.3390/v4113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto F, Oyaizu N, Kalyanaraman VS, Pahwa S. 1997. Modulation of Bcl-2 protein by CD4 cross-linking: a possible mechanism for lymphocyte apoptosis in human immunodeficiency virus infection and for rescue of apoptosis by interleukin-2. Blood 90:745–753. [PubMed] [Google Scholar]
- 78.Perfettini JL, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, Lazar V, Ciccosanti F, Nardacci R, Penninger J, Piacentini M, Kroemer G. 2004. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med 199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanco J, Barretina J, Ferri KF, Jacotot E, Gutiérrez A, Armand-Ugón M, Cabrera C, Kroemer G, Clotet B, Esté JA. 2003. Cell-surface-expressed HIV-1 envelope induces the death of CD4 T cells during GP41-mediated hemifusion-like events. Virology 305:318–329. doi: 10.1006/viro.2002.1764. [DOI] [PubMed] [Google Scholar]
- 80.Castedo M, Ferri KF, Blanco J, Roumier T, Larochette N, Barretina J, Amendola A, Nardacci R, Metivier D, Este JA, Piacentini M, Kroemer G. 2001. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med 194:1097–1110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, Kroemer G. 2005. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ 12(Suppl 1):916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 82.Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M, Capitani S. 1995. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 86:3823–3834. [PubMed] [Google Scholar]
- 83.Zauli G, Gibellini D. 1996. The human immunodeficiency virus type-1 (HIV-1) Tat protein and Bcl-2 gene expression. Leuk Lymphoma 23:551–560. doi: 10.3109/10428199609054864. [DOI] [PubMed] [Google Scholar]
- 84.López-Huertas MR, Mateos E, Sánchez Del Cojo M, Gómez-Esquer F, Díaz-Gil G, Rodríguez-Mora S, López JA, Calvo E, López-Campos G, Alcamí J, Coiras M. 2013. The presence of HIV-1 Tat protein second exon delays Fas protein-mediated apoptosis in CD4+ T lymphocytes: a potential mechanism for persistent viral production. J Biol Chem 288:7626–7644. doi: 10.1074/jbc.M112.408294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng L, Yang Y, Guocai L, Pauza CD, Salvato MS. 2007. HIV Tat protein increases Bcl-2 expression in monocytes which inhibits monocyte apoptosis induced by tumor necrosis factor-alpha-related apoptosis-induced ligand. Intervirology 50:224–228. doi: 10.1159/000100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen D, Wang M, Zhou S, Zhou Q. 2002. HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J 21:6801–6810. doi: 10.1093/emboj/cdf683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dabrowska A, Kim N, Aldovini A. 2008. Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes. J Immunol 181:8460–8477. doi: 10.4049/jimmunol.181.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sastry KJ, Marin MC, Nehete PN, McConnell K, el-Naggar AK, McDonnell TJ. 1996. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene 13:487–493. [PubMed] [Google Scholar]
- 89.Sforza F, Nicoli F, Gallerani E, Finessi V, Reali E, Cafaro A, Caputo A, Ensoli B, Gavioli R. 2014. HIV-1 Tat affects the programming and functionality of human CD8(+) T cells by modulating the expression of T-box transcription factors. AIDS 28:1729–1738. doi: 10.1097/QAD.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 90.Rasola A, Gramaglia D, Boccaccio C, Comoglio PM. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J Immunol 166:81–88. doi: 10.4049/jimmunol.166.1.81. [DOI] [PubMed] [Google Scholar]
- 91.Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d'Aloja P, Schürmann A, Baur AS. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med 7:1217–1224. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- 92.Ndolo T, Dhillon NK, Nguyen H, Guadalupe M, Mudryj M, Dandekar S. 2002. Simian immunodeficiency virus Nef protein delays the progression of CD4+ T cells through G1/S phase of the cell cycle. J Virol 76:3587–3595. doi: 10.1128/jvi.76.8.3587-3595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greenway AL, McPhee DA, Allen K, Johnstone R, Holloway G, Mills J, Azad A, Sankovich S, Lambert P. 2002. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J Virol 76:2692–2702. doi: 10.1128/jvi.76.6.2692-2702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hemann MT, Lowe SW. 2006. The p53-Bcl-2 connection. Cell Death Differ 13:1256–1259. doi: 10.1038/sj.cdd.4401962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V. 2009. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186:255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strack PR, Frey MW, Rizzo CJ, Cordova B, George HJ, Meade R, Ho SP, Corman J, Tritch R, Korant BD. 1996. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci U S A 93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nie Z, Phenix BN, Lum JJ, Alam A, Lynch DH, Beckett B, Krammer PH, Sekaly RP, Badley AD. 2002. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ 9:1172–1184. doi: 10.1038/sj.cdd.4401094. [DOI] [PubMed] [Google Scholar]
- 98.Nie Z, Bren GD, Vlahakis SR, Schimnich AA, Brenchley JM, Trushin SA, Warren S, Schnepple DJ, Kovacs CM, Loutfy MR, Douek DC, Badley AD. 2007. Human immunodeficiency virus type 1 protease cleaves procaspase 8 in vivo. J Virol 81:6947–6956. doi: 10.1128/JVI.02798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sainski AM, Dai H, Natesampillai S, Pang YP, Bren GD, Cummins NW, Correia C, Meng XW, Tarara JE, Ramirez-Alvarado M, Katzmann DJ, Ochsenbauer C, Kappes JC, Kaufmann SH, Badley AD. 2014. Casp8p41 generated by HIV protease kills CD4 T cells through direct Bak activation. J Cell Biol 206:867–876. doi: 10.1083/jcb.201405051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cummins NW, Sainski AM, Dai H, Natesampillai S, Pang Y-P, Bren GD, de Araujo Correia MCM, Sampath R, Rizza SA, O'Brien D, Yao JD, Kaufmann SH, Badley AD. 2016. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol 90:4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montal M. 2003. Structure-function correlates of Vpu, a membrane protein of HIV-1. FEBS Lett 552:47–53. doi: 10.1016/s0014-5793(03)00849-4. [DOI] [PubMed] [Google Scholar]
- 102.Raja NU, Jabbar MA. 1996. The human immunodeficiency virus type 1 Vpu protein tethered to the CD4 extracellular domain is localized to the plasma membrane and is biologically active in the secretory pathway of mammalian cells: implications for the mechanisms of Vpu function. Virology 220:141–151. doi: 10.1006/viro.1996.0294. [DOI] [PubMed] [Google Scholar]
- 103.Akari H, Bour S, Kao S, Adachi A, Strebel K. 2001. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors. J Exp Med 194:1299–1311. doi: 10.1084/jem.194.9.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersen JL, DeHart JL, Zimmerman ES, Ardon O, Kim B, Jacquot G, Benichou S, Planelles V. 2006. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog 2:e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med 3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 106.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. 1998. The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J Exp Med 187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pace MJ, Graf EH, Agosto LM, Mexas AM, Male F, Brady T, Bushman FD, O'Doherty U. 2012. Directly infected resting CD4+ T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog 8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeMaster LK, Liu X, VanBelzen DJ, Trinité B, Zheng L, Agosto LM, Migueles SA, Connors M, Sambucetti L, Levy DN, Pasternak AO, O'Doherty U. 2016. A subset of CD4/CD8 double-negative T cells expresses HIV proteins in patients on antiretroviral therapy. J Virol 90:2165–2179. doi: 10.1128/JVI.01913-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freeman ML, Shive CL, Nguyen TP, Younes S-A, Panigrahi S, Lederman MM. 2016. Cytokines and T-cell homeostasis in HIV infection. J Infect Dis 214(Suppl 2):S51–S57. doi: 10.1093/infdis/jiw287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, Frei R, Garbani M, Globinska A, Hess L, Huitema C, Kubo T, Komlosi Z, Konieczna P, Kovacs N, Kucuksezer UC, Meyer N, Morita H, Olzhausen J, O'Mahony L, Pezer M, Prati M, Rebane A, Rhyner C, Rinaldi A, Sokolowska M, Stanic B, Sugita K, Treis A, van de Veen W, Wanke K, Wawrzyniak M, Wawrzyniak P, Wirz OF, Zakzuk JS, Akdis CA. 2016. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol 138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 111.Imami N, Antonopoulos C, Hardy GA, Gazzard B, Gotch FM. 1999. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: impact of highly active antiretroviral therapy. AIDS Res Hum Retroviruses 15:1499–1508. doi: 10.1089/088922299309784. [DOI] [PubMed] [Google Scholar]
- 112.Gómez J, Martínez AC, González A, García A, Rebollo A. 1998. The Bcl-2 gene is differentially regulated by IL-2 and IL-4: role of the transcription factor NF-AT. Oncogene 17:1235–1243. doi: 10.1038/sj.onc.1202049. [DOI] [PubMed] [Google Scholar]
- 113.Cohen SB, Crawley JB, Kahan MC, Feldmann M, Foxwell BM. 1997. Interleukin-10 rescues T cells from apoptotic cell death: association with an upregulation of Bcl-2. Immunology 92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, Mangioni D, Gori A, Jacobson JM, Brooks AD, Hardacre J, Ammori J, Estes JD, Schacker TW, Rodriguez B, Lederman MM. 2014. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis 210:619–629. doi: 10.1093/infdis/jiu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shive CL, Clagett B, McCausland MR, Mudd JC, Funderburg NT, Freeman ML, Younes S-A, Ferrari BM, Rodriguez B, McComsey GA, Calabrese LH, Sieg SF, Lederman MM. 2016. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr 71:483–492. doi: 10.1097/QAI.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. 2007. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med 204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bradley LM, Haynes L, Swain SL. 2005. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol 26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 119.Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy JP, Sekaly RP, Chomont N. 2013. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, Hope JL, Kathuria N, McGettigan SE, Lewis MG, Giavedoni LD, Jacobson JM, Katsikis PD. 2013. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog 9:e1003658. doi: 10.1371/journal.ppat.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, Debernardo R, Rabin RL, Lederman MM, Harding CV. 2013. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One 8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. 1991. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 125.Poole E, McGregor Dallas SR, Colston J, Joseph RS, Sinclair J. 2011. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34(+) progenitors. J Gen Virol 92:1539–1549. doi: 10.1099/vir.0.031377-0. [DOI] [PubMed] [Google Scholar]
- 126.Kim Y, Anderson JL, Lewin SR. 2018. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe 23:14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee GQ, Orlova-Fink N, Einkauf K, Chowdhury FZ, Sun X, Harrington S, Kuo H-H, Hua S, Chen H-R, Ouyang Z, Reddy K, Dong K, Ndung'u T, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2017. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J Clin Invest 127:2689–2696. doi: 10.1172/JCI93289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hiener B, Horsburgh BA, Eden JS, Barton K, Schlub TE, Lee E, von Stockenstrom S, Odevall L, Milush JM, Liegler T, Sinclair E, Hoh R, Boritz EA, Douek D, Fromentin R, Chomont N, Deeks SG, Hecht FM, Palmer S. 2017. Identification of genetically intact HIV-1 proviruses in specific CD4(+) T cells from effectively treated participants. Cell Rep 21:813–822. doi: 10.1016/j.celrep.2017.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, Ho YS, Saksena NK, Hoy J, Crowe SM, Cameron PU, Lewin SR. 2010. Both CD31+ and CD31− naive CD4+ T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis 202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 130.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sékaly R-P. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2014. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jaafoura S, de Goer de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, Delfraissy JF, Goujard C, Taoufik Y. 2014. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T cells. Nat Commun 5:5407. doi: 10.1038/ncomms6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Airo P, Torti C, Uccelli MC, Malacarne F, Palvarini L, Carosi G, Castelli F. 2000. Naive CD4+ T lymphocytes express high levels of Bcl-2 after highly active antiretroviral therapy for HIV infection. AIDS Res Hum Retroviruses 16:1805–1807. doi: 10.1089/08892220050195766. [DOI] [PubMed] [Google Scholar]
- 135.Ren YH, Patel S, Magat D, Macedo AB, Durga R, Zale E, Mota T, Truong R, Rohwetter T, McCann CD, Kovacs CC, Benko E, Wimpelberg A, Cannon C, Hardy WD, Bosque A, Bollard CM, Jones RB. 9 May 2019. BCL-2 antagonism sensitizes CTL-resistant HIV reservoirs to elimination ex vivo. SSRN Electronic J doi: 10.2139/ssrn.3385137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW, Sluis-Cremer N. 7 February 2019. Naive CD4+ T cells harbor a large inducible reservoir of latent, replication-competent HIV-1. Clin Infect Dis doi: 10.1093/cid/ciz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rocco J, Mellors JW, Macatangay BJ. 2018. Regulatory T cells: the ultimate HIV reservoir? J Virus Erad 4:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fehervari Z, Sakaguchi S. 2004. CD4+ Tregs and immune control. J Clin Invest 114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 140.Pandiyan P, Gartner D, Soezeri O, Radbruch A, Schulze-Osthoff K, Brunner-Weinzierl MC. 2004. CD152 (CTLA-4) determines the unequal resistance of Th1 and Th2 cells against activation-induced cell death by a mechanism requiring PI3 kinase function. J Exp Med 199:831–842. doi: 10.1084/jem.20031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L, Benne C, Ryan ES, Balderas R, Jean S, Easley K, Marconi V, Silvestri G, Estes JD, Sekaly RP, Paiardini M. 2017. CTLA-4(+)PD-1(−) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 47:776–788.e5. doi: 10.1016/j.immuni.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat Med 17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. 2005. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 144.Chahroudi A, Silvestri G, Lichterfeld M. 2015. T memory stem cells and HIV: a long-term relationship. Curr HIV/AIDS Rep 12:33–40. doi: 10.1007/s11904-014-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Walker BD, Yu XG. 2013. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 13:487–498. doi: 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- 146.Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux J-M, de Leval L, Pantaleo G, Perreau M. 2016. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- 147.Breton G, Chomont N, Takata H, Fromentin R, Ahlers J, Filali-Mouhim A, Riou C, Boulassel M-R, Routy J-P, Yassine-Diab B, Sékaly R-P. 2013. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol 191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hakre S, Chavez L, Shirakawa K, Verdin E. 2012. HIV latency: experimental systems and molecular models. FEMS Microbiol Rev 36:706–716. doi: 10.1111/j.1574-6976.2012.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med 198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marini A, Harper JM, Romerio F. 2008. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol 181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 151.Kim M, Hosmane NN, Bullen CK, Capoferri A, Yang HC, Siliciano JD, Siliciano RF. 2014. A primary CD4(+) T cell model of HIV-1 latency established after activation through the T cell receptor and subsequent return to quiescence. Nat Protoc 9:2755–2770. doi: 10.1038/nprot.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang H-C, Xing S, Shan L, O'Connell K, Dinoso J, Shen A, Zhou Y, Shrum CK, Han Y, Liu JO, Zhang H, Margolick JB, Siliciano RF. 2009. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest 119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sunshine S, Kirchner R, Amr SS, Mansur L, Shakhbatyan R, Kim M, Bosque A, Siliciano RF, Planelles V, Hofmann O, Ho Sui S, Li JZ. 2016. HIV integration site analysis of cellular models of HIV latency with a probe-enriched next-generation sequencing assay. J Virol 90:4511–4519. doi: 10.1128/JVI.01617-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. 2007. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 155.Saleh S, Wightman F, Ramanayake S, Alexander M, Kumar N, Khoury G, Pereira C, Purcell D, Cameron PU, Lewin SR. 2011. Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells. Retrovirology 8:80. doi: 10.1186/1742-4690-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ziegler E, Oberbarnscheidt M, Bulfone-Paus S, Forster R, Kunzendorf U, Krautwald S. 2007. CCR7 signaling inhibits T cell proliferation. J Immunol 179:6485–6493. doi: 10.4049/jimmunol.179.10.6485. [DOI] [PubMed] [Google Scholar]
- 157.Kochetkova M, Kumar S, McColl SR. 2009. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ 16:664–673. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 158.Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei P, Li W, Wang M, Zhu H, Huang B, Huang L, Shen G. 2008. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology 213:417–426. doi: 10.1016/j.imbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 159.Banas B, Wornle M, Berger T, Nelson PJ, Cohen CD, Kretzler M, Pfirstinger J, Mack M, Lipp M, Grone HJ, Schlondorff D. 2002. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol 168:4301–4307. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
- 160.Xu Y, Liu L, Qiu X, Liu Z, Li H, Li Z, Luo W, Wang E. 2012. CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS One 7:e33262. doi: 10.1371/journal.pone.0033262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim JW, Ferris RL, Whiteside TL. 2005. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res 11:7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 162.Takata H, Kessing C, Sy A, Lima N, Sciumbata J, Mori L, Jones RB, Chomont N, Michael NL, Valente S, Trautmann L. 2019. Modeling HIV-1 latency using primary CD4+ T cells from virally suppressed HIV-1-infected individuals on antiretroviral therapy. J Virol 93:e02248-18. doi: 10.1128/JVI.02248-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deeks SG. 2012. HIV: shock and kill. Nature 487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 164.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. 2008. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 166.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. 2017. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16:273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 167.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. 2013. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 168.Cummins NW, Sainski-Nguyen AM, Natesampillai S, Aboulnasr F, Kaufmann S, Badley AD. 2017. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J Virol 91:e00012-17. doi: 10.1128/JVI.00012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang SH, Ren Y, Thomas AS, Chan D, Mueller S, Ward AR, Patel S, Bollard CM, Cruz CR, Karandish S, Truong R, Macedo AB, Bosque A, Kovacs C, Benko E, Piechocka-Trocha A, Wong H, Jeng E, Nixon DF, Ho YC, Siliciano RF, Walker BD, Jones RB. 2018. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 128:876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yecies D, Carlson NE, Deng J, Letai A. 2010. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Opydo-Chanek M, Gonzalo O, Marzo I. 2017. Multifaceted anticancer activity of BH3 mimetics: current evidence and future prospects. Biochem Pharmacol 136:12–23. doi: 10.1016/j.bcp.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 172.Nakajima W, Sharma K, Hicks MA, Le N, Brown R, Krystal GW, Harada H. 2016. Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer. Cancer Biol Ther 17:27–35. doi: 10.1080/15384047.2015.1108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bashiri K, Rezaei N, Nasi M, Cossarizza A. 2018. The role of latency reversal agents in the cure of HIV: a review of current data. Immunol Lett 196:135–139. doi: 10.1016/j.imlet.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 174.Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM. 2017. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 127:3126–3135. doi: 10.1172/JCI92684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L, Sogaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 176.Schwartz J, Niu X, Walton E, Hurley L, Lin H, Edwards H, Taub JW, Wang Z, Ge Y. 2016. Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo. Am J Transl Res 8:3893–3902. [PMC free article] [PubMed] [Google Scholar]
- 177.Miller LK, Kobayashi Y, Chen CC, Russnak TA, Ron Y, Dougherty JP. 2013. Proteasome inhibitors act as bifunctional antagonists of human immunodeficiency virus type 1 latency and replication. Retrovirology 10:120. doi: 10.1186/1742-4690-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Natesampillai S, Cummins NW, Nie Z, Sampath R, Baker JV, Henry K, Pinzone M, O'Doherty U, Polley EC, Bren GD, Katzmann DJ, Badley AD. 2018. HIV protease-generated Casp8p41, when bound and inactivated by BclII, is degraded by the proteasome. J Virol 92:e00037-18. doi: 10.1128/JVI.00037-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reuland SN, Goldstein NB, Partyka KA, Smith S, Luo Y, Fujita M, Gonzalez R, Lewis K, Norris DA, Shellman YG. 2012. ABT-737 synergizes with bortezomib to kill melanoma cells. Biol Open 1:92–100. doi: 10.1242/bio.2011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chauhan D, Tian Z, Zhou B, Kuhn D, Orlowski R, Raje N, Richardson P, Anderson KC. 2011. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res 17:5311–5321. doi: 10.1158/1078-0432.CCR-11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wan Y, Dai N, Tang Z, Fang H. 2018. Small-molecule Mcl-1 inhibitors: emerging anti-tumor agents. Eur J Med Chem 146:471–482. doi: 10.1016/j.ejmech.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 182.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJ, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. 2015. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]