Surgical resection is regarded as the first-line curative treatment for early-stage hepatocellular carcinoma (HCC). However, the lack of sufficient future liver remnant (FLR) in many patients would preclude them from surgical candidacy. Yttrium-90 radiation lobectomy (RL) has been established over the past decade as an effective method to achieve tumor control as well as achieving FLR hypertrophy, while embedding a test-of-time. Most recently, Yttrium-90 radiation segmentectomy (RS) has been proven potentially curative in early-stage HCC. In this article, we highlight a modified radiation lobectomy (mRL) which is a combined approach of RL and RS that aims at concurrent achievement of complete tumor necrosis and FLR hypertrophy.
Radioembolization has historically been employed in the salvage setting for the treatment of unresectable hepatic malignancies. In this approach, lobar hepatic therapies have been found to offer palliative intent. 1 Observations of an atrophy–hypertrophy complex following this lobar therapy have led to a concept termed “radiation lobectomy”; the treated hepatic lobe will atrophy following radioembolization and the contralateral, untreated, hepatic lobe provides compensatory hypertrophy. This finding has allowed for the application of RL as an alternative to portal vein embolization (PVE), particularly in HCC, which can provide concurrent cancer therapy while promoting hypertrophy of the contralateral FLR for planned surgical resection in patients initially presenting with an FLR insufficient to sustain hepatic function postoperatively. 2 3 4
Described in 2010, RS 5 is a technique increasingly used to treat liver tumors in a segmental rather than lobar fashion. RS has demonstrated safety and efficacy, with good imaging response rates, long time to tumor progression posttreatment, and high rates of explant complete pathologic necrosis. 6 7 More recently, segmental radioembolization with glass microspheres has been described with curative intent in patients with unresectable HCC. 8
In this article, we highlight a modified approach to RL combining both lobar and segmental Y90 delivery to promote curative tumor therapy while inducing contralateral hypertrophy to facilitate surgical resection. These approaches have been most commonly performed and reported in the literature employing glass microsphere radioembolization (TheraSphere, Biocompatibles UK Ltd) for patients with unresectable HCC. 9
Modified Lobectomy Approaches
There are two mRL approaches toward achieving more complete tumor response in conjunction with contralateral lobar hypertrophy for future liver resection. The first approach (segmental + lobar approach) employs a segmental boost dose (>190 Gray [Gy]) delivered through segmental/subsegmental administration for tumor kill, followed by an ipsilateral lobar dose (80–120 Gy), for the promotion of FLR hypertrophy ( Fig. 1 ).
Fig. 1.
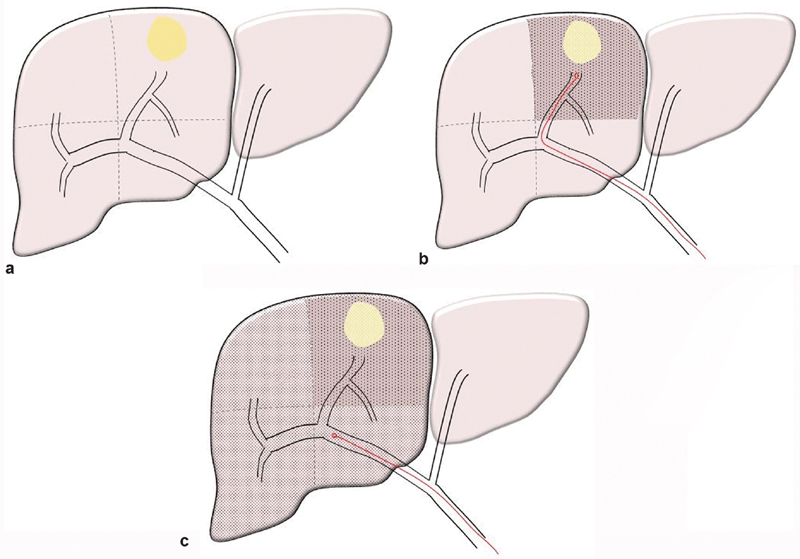
( a ) Diagram of solitary hepatoma located in segment 8. ( b ) Superselective administration of a segmental boost dose confined to the tumor bearing segment 8 to ensure maximal tumor necrosis. ( c ) Lobar administration of lobar dose to the right lobe to induce contralateral hypertrophy.
A case example is presented in Fig. 2 : A 73-year-old male with solitary 3.5 HCC located in hepatic segment 7. The patient's FLR at presentation was determined to be 29.5%. He underwent mRL using the segmental + lobar approach, where he received a radiation dose of 155 Gy to hepatic segment 7 followed by 90 Gy to the entire right hepatic lobe. This provided a tumor dose of 245 Gy. At 3-month follow-up imaging, the tumor showed complete response by Modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria to treatment and the FLR increased to 37%. The patient was offered surgical resection; however, he opted not to undergo surgical resection given the perceived morbidity and the complete tumor response to therapy. At 9-month follow-up, the patient maintained complete response status and FLR continued to increase in size (FLR = 51%).
Fig. 2.
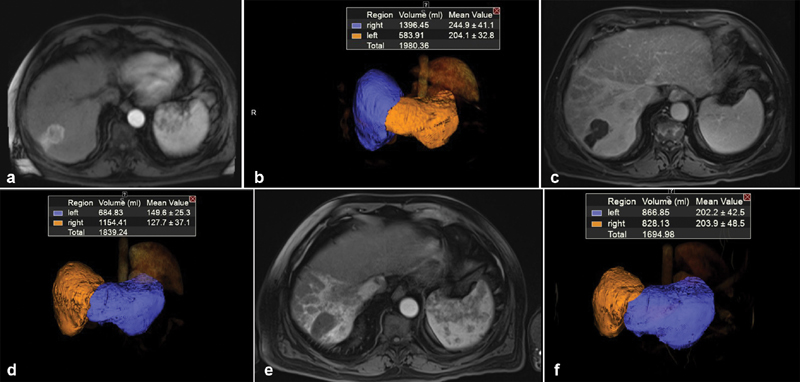
( a ) Baseline MRI of a 73-year-old male with solitary 3.5 HCC located in hepatic segment 7. ( b ) Volumetric assessment showing baseline FLR = 29.5%. ( c ) Three-month MRI follow-up post Y90 showing complete response of tumor by mRECIST criteria. ( d ) Volumetric assessment showing 3-month FLR = 37%. ( e ) Nine-month MRI follow-up post Y90 showing continuous complete response of tumor. ( f ) Volumetric assessment showing 9-month FLR = 51%.
Fig. 3 shows a 62-year-old male with a history of hepatitis C infection who presented with a 5-cm tumor in hepatic segment 7. He was not a surgical candidate given his FLR of 31.7%. The patient underwent radioembolization using the modified (segmental + lobar) approach that included segmental posterior right lobe treatment followed by right whole right lobe treatment. The posterior right lobe received a net radiation dose of 247 Gy, while the right lobe received a net radiation dose of 175 Gy. The patient achieved complete mRECIST response at 1-month follow-up; however, contralateral did not sufficiently hypertrophy at that time. At 6-month follow-up, the patient showed significant hypertrophy of contralateral lobe as well as atrophy of total right lobe volume yielding an FLR of 43%. The patient, subsequently, underwent surgical resection. Pathologic evaluation of liver explant revealed a completely necrotic tumor measuring 3.4 cm in greatest dimension.
Fig. 3.
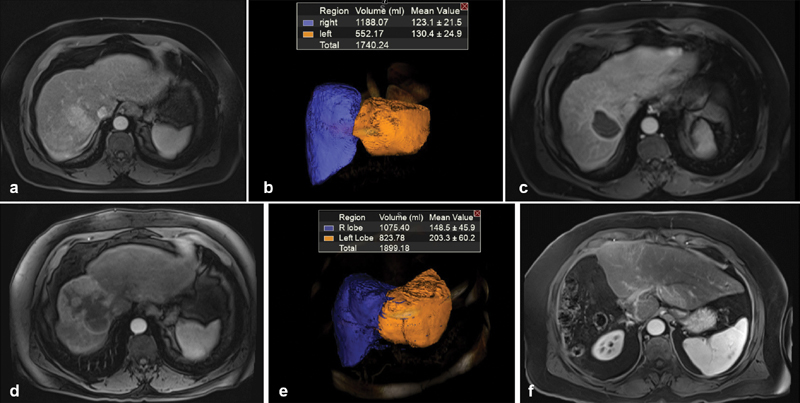
( a ) Baseline MRI of a 62-year-old male with a 5-cm tumor in hepatic segment 7. ( b ) Volumetric assessment showing baseline FLR = 31.7%. ( c ) One-month MRI follow-up post Y90 showing complete response of tumor by mRECIST criteria. ( d ) Six-month MRI follow-up showing continuous complete response of the treated tumor that is concurrent with right lobe atrophy and left lobe hypertrophy. ( e ) Volumetric assessment showing 6-month FLR = 43%. ( f ) One-month MRI follow-up scan postresection showing marked hypertrophy of the left liver lobe (liver remnant).
The second mRL (double segmental) approach is to apply different radioembolic doses to the anterior and posterior right hepatic lobes, with the dose to the tumor bearing segments intended to be ablative (>190), while the non–tumor-bearing segment(s) receive a nonablative dose (80–120 Gy) to ensure fibrosis induced contralateral hypertrophy ( Fig. 4 ).
Fig. 4.
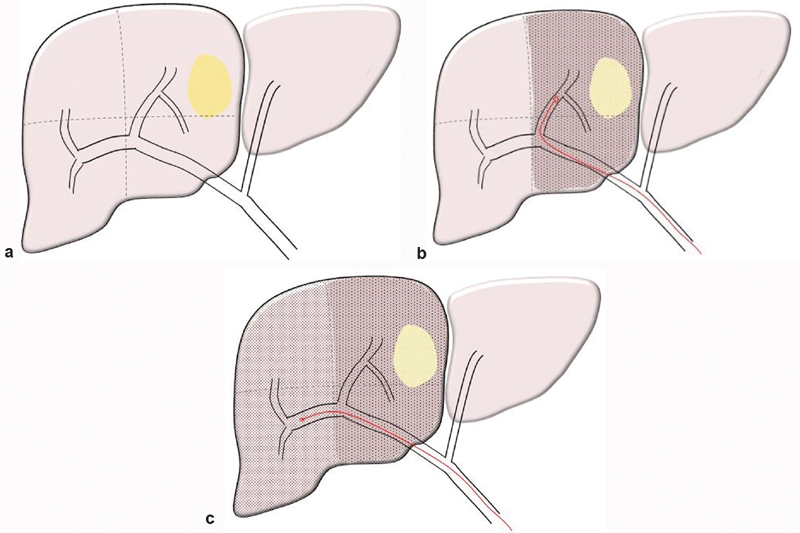
( a ) Diagram of solitary hepatoma located in segments 8 and 5. ( b ) Selective administration of a subsegmental boost dose (>190 Gy) confined to the tumor bearing segments 8 and 5 (anterior right lobe) to ensure maximal tumor necrosis. ( c ) Selective administration of lobar dose (80–120 Gy) to the non–tumor-bearing segments 6 and 7 (posterior right lobe) to induce contralateral hypertrophy.
A case example is presented in Fig. 5 : A 65-year-old male with a single 2.5-cm arterially enhancing mass in the anterior right lobe consistent with HCC. The tumor was unresectable due to the small size of the FLR; hence, it was planned to receive radioembolization to induce contralateral hypertrophy. During the treatment session, the patient received an ablative dose of 216 Gy to the right anterior segment bearing the tumor followed by radioembolization to the tumor-naive posterior segments 6/7 with a 120-Gy dose. The first postprocedural MRI exam at 30 days posttreatment demonstrated complete tumor response by mRECIST and an increase in left hepatic lobe volume from approximately 375 mL (30% FLR) to 484 mL (38.5% FLR). The patient underwent serial evaluation by MR imaging that showed sustained completed mRECIST tumor response and progressive left lobe hypertrophy. The patient elected not to undergo surgical resection once deemed a surgical candidate, given the sustained complete tumor response. The patient is now more than 4 years post-radioembolization; the treated tumor does not show viability and there is involution of the right hepatic lobe with compensatory hypertrophy of the left hepatic lobe ( Fig. 5d ).
Fig. 5.
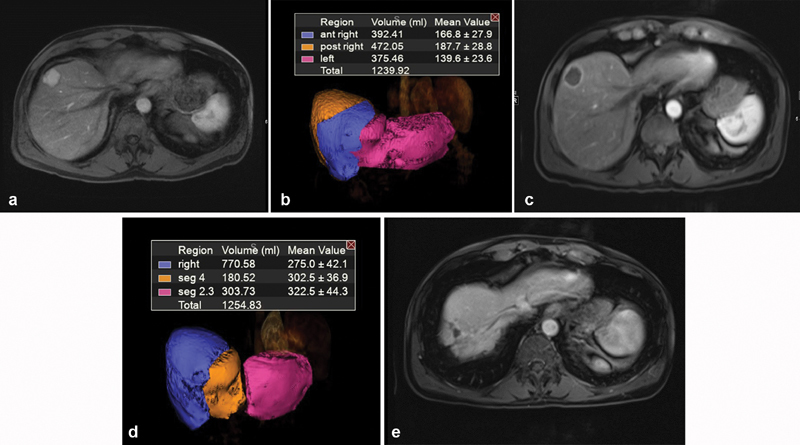
( a ) Baseline MRI of a 65-year-old male with a 2.5-cm tumor in the anterior right hepatic lobe. ( b ) Volumetric assessment showing baseline FLR = 30%. ( c ) One-month MRI follow-up post Y90 showing complete response of tumor by mRECIST criteria. ( d ) Volumetric assessment showing 1-month FLR = 38.5%. ( e ) MRI follow-up at 4 years post-Y90 showing sustained complete response.
Discussion
The role of RL is evolving in the bridge to resection setting in patients with unresectable liver cancer, especially those with HCC. PVE has been considered the gold standard for non-HCC patients with potentially resectable liver cancer but insufficient FLR. PVE can promote significant hypertrophy of the contralateral lobe prior to surgical resection, 10 but some studies suggest that PVE may cause tumor progression due to production of angiogenic factors and altered vascular flow. 11 12 Furthermore, should an HCC patient receive PVE, surgical resection is important as some patients will develop progressive cirrhosis and portal hypertension, making thrombosis of a lobar portal vein suboptimal for future treatment considerations.
RL has gained interest as an alternative to PVE as a means to control tumor while promoting FLR hypertrophy, especially in patients with HCC. While the rate of FLR hypertrophy is likely more rapid with PVE, there are several proposed advantages of RL over PVE: (1) Tumor control is secured during time interval of contralateral hypertrophy. (2) Tumor shift from major vessels can be achieved, thus optimizing R0 resection. (3) Neoadjuvant radioembolization followed by surveillance for contralateral hypertrophy embeds a biologic test of time aiming to identify patients with good tumor biology and therefore potentially decreasing the chances of tumor recurrence rates after resection. (4) Achieving tumor necrosis might be of oncologic value in mitigating tumor spread during mobilization/manipulation of the liver intraoperatively. (5) Induction of liver hypertrophy with concurrent tumor necrosis prior to resection might limit risk of postoperative tumor progression induced by rapid postoperative regenerative activity.
Modified approaches to RL allow for individualized and aggressive treatment of unresectable liver cancer. In the scenario of mRL, both described approaches allow for curative intent delivery of ablative radiation dosing to the tumor while also promoting the atrophy–hypertrophy complex to facilitate potential surgical resection. The advancement in the mRL approach is this capacity to deliver curative intent therapy to the tumor. If patients undergo resection, there is theoretically less risk of tumor spread during liver mobilization and manipulation. Furthermore, if patients are ultimately deemed not surgical candidates or choose not to undergo surgery, the portal vein remains patent and the tumor well controlled.
In conclusion, modified lobar radioembolization approaches facilitate a more individualized approach to liver-directed therapy, combining historical lobar principles with evolving segmental curative use of this therapy. These should be considered when using RL to convert patients to liver resection candidacy.
Footnotes
Conflict of Interest None declared.
References
- 1.Salem R, Lewandowski R J, Mulcahy M F et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(01):52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Gaba R C, Lewandowski R J, Kulik L M et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16(06):1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski R J, Donahue L, Chokechanachaisakul A et al. (90) Y radiation lobectomy: outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol. 2016;114(01):99–105. doi: 10.1002/jso.24269. [DOI] [PubMed] [Google Scholar]
- 4.Vouche M, Lewandowski R J, Atassi R et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59(05):1029–1036. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riaz A, Gates V L, Atassi B et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79(01):163–171. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 6.Salem R, Thurston K G. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17(08):1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 7.Padia S A, Kwan S W, Roudsari B, Monsky W L, Coveler A, Harris W P. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. J Vasc Interv Radiol. 2014;25(07):1067–1073. doi: 10.1016/j.jvir.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski R J, Gabr A, Abouchaleh N et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(03):1050–1058. doi: 10.1148/radiol.2018171768. [DOI] [PubMed] [Google Scholar]
- 9. TheraSphere ® Yttrium-90 Glass Microspheres [package insert]. Farnham, Surrey, UK: Biocompatibles UK Ltd., Rev 14. Available at: https://www.btg-im.com/BTG/media/TheraSphere-Documents/PDF/TheraSphere-Package-Insert_USA_Rev-14.pdf. Accessed September 5, 2019
- 10.Farges O, Belghiti J, Kianmanesh R et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(02):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graaf W, van den Esschert J W, van Lienden K P, van Gulik T M. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16(02):423–430. doi: 10.1245/s10434-008-0222-6. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Baba Y, Ueno K et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48(07):721–727. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]


