Abstract
Lung cancer is the leading cause of cancer-related mortality worldwide. Eighty-five percent of cases correspond to non-small cell lung cancer (NSCLC) and pivotal nonsurgical options for early-stage disease include percutaneous ablation and stereotactic body radiation therapy (SBRT). Microwave Ablation (MWA) is a locoregional treatment option that has many advantages over radiofrequency ablation and has been able to overcome the limitations of this technique in the treatment of early-stage NSCLC. In this review article, we highlight the current evidence supporting the use of MWA in patients with early-stage NSCLC and discuss the technical considerations of the procedure, including optimal patient selection and planning strategies, as well as the potential complications and reported outcomes. Finally, we mention future trends involving ablation in NSCLC, including its role in combination with SBRT in central tumors, management of post-SBRT local recurrence, and its potential as an adjuvant treatment option for patients with resistance to systemic therapy or in combination with checkpoint inhibitors.
Keywords: primary lung tumors, microwave ablation, NSCLC
Lung cancer is one of the leading causes of cancer-related morbidity in the United States with over 234,000 new cases diagnosed in 2018, surpassed only by breast cancer. It is estimated to be responsible for over 154,000 deaths in the United States in 2018, making it the most lethal cancer. 1 Globally, lung cancer was the leading cancer in incidence and mortality in 2018. 2
Lung cancer is divided into non-small cell lung cancer (NSCLC) (accounting for 85% of all cases) and small cell lung cancer. 3 4 NSCLC is pathologically divided into several subtypes, with adenocarcinoma, squamous cell carcinoma, and large cell carcinoma representing the vast majority of cases. 5
While NSCLC subtypes share several biological features, they are believed to develop through progressive pathologic changes from different cells of origin and exhibit different growth patterns, molecular pathways, and genetic aberrations. 5 6 The pathologic changes preceding squamous cell carcinoma of the lung beginning from hyperplasia, through squamous metaplasia, squamous dysplasia, and carcinoma in situ, are different from those preceding adenocarcinoma of the lung, for which atypical adenomatous hyperplasia is the only identified pathological precursor. 6 The progressive pathologic changes (multistep tumorigenesis) highlight the importance of early detection and treatment of NSCLCs and its impact in survival.
General Approach for Treatment of Early-Stage Disease
The cornerstone for the management of early-stage lung cancer has been surgical resection, specifically lobar resection. According to the National Comprehensive Cancer Network (NCCN) guidelines for 2018, surgery is the standard of care for patients with NSCLC up to stage IIb. 7 In more advanced stages, surgery is recommended in patients with no nodal involvement (N0) or involvement of ipsilateral peribronchial, hilar, and/or intrapulmonary lymph nodes (N1) even in patients with T3 or T4 disease. 7 The role of surgery in patients with stage IIIa NSCLC and above with involvement of ipsilateral mediastinal or subcarinal lymph nodes (N2) remains controversial. 7
The treatment options for stage Ia NSCLC have changed over the past few years and now include sublobar resection (wedge resection or segmentectomy), as well as stereotactic body radiation therapy (SBRT) and percutaneous ablative modalities. 7 Provided the patient is a surgical candidate with adequate cardiopulmonary reserve, surgical lobectomy remains the gold standard. A prospective randomized trial from 1995 comparing lobectomy with sublobar resection in patients with T1N0 (stage Ia) NSCLC concluded that sublobar resection showed a higher locoregional recurrence rate. 8 However, a multicenter randomized trial comparing sublobar resection to lobectomy in stage Ia NSCLC is currently ongoing, with expected primary completion date of 2021. This phase 3 trial (NCT00499330) will compare disease free survival, overall survival, rate of locoregional and systemic recurrence, and pulmonary function at 6 months postprocedure between the two treatment options ( Table 1 ). 9
Table 1. Reported long-term outcomes of different locoregional treatment options 12 27 44 49 .
| Outcome | Radiofrequency ablation | Microwave ablation | Cryoablation | Stereotactic body radiation therapy |
|---|---|---|---|---|
| Overall survival rates | ||||
| 1 y | 85–89% | 79–100% | 88% | 84–87% |
| 3 y | 53–56% | 35–92% | 78% | 53–59% |
| 5 y | 32–41% | 16–50% | 67% | 36–45% |
| Local tumor control rates | ||||
| 1 y | 73–77% | 73–96% | 91% | 96–98% |
| 3 y | 55–62% | 65–80% | 87% | 86–90% |
| 5 y | 42–64% | 24–72% | 85% | 85–88% |
In patients who are medically inoperable or refuse surgery, the two main treatment options are SBRT and percutaneous ablation techniques, as they both have demonstrated safety and comparable survival in prospective trials. 10 11 Despite similar reported overall survival rates between ablation and SBRT, 12 13 it appears that local tumor control rates with SBRT are higher compared with ablation. 12 However, most literature includes radiofrequency ablation (RFA) data, as this was the most frequently utilized modality, without evaluating newer and possibly more effective techniques such as microwave ablation (MWA) and/or cryoablation. 12 In addition, the early RFA cases were performed by palpation in the operating room without image guidance, possibly impacting the outcomes of the therapy. The reported higher local recurrence rate after ablation compared with SBRT is countered by the fact that lung ablation can be repeated when local recurrence occurs. 14 This may also be confounded by the difficulty to accurately identify radiological evidence of local recurrence in the SBRT field compared with the ablation zone, which may account for underreporting or delayed reporting of local recurrence. 15 Recent evidence shows a higher pathological complete response rate reported for lung ablation (reported at 90.9%) 16 17 compared with SBRT (which was reported at 60%). 18 Taken together, the clinical significance of reported higher local recurrence rate in ablation remains unclear. Therefore, patients with high surgical risk but who are still operable (defined as patients who can tolerate sublobar resection, but not lobectomy) may undergo sublobar resection, SBRT, or ablation (recommendation category 2A). 7 19
Image-Guided Thermal Ablation for Primary Lung Malignancy
The first and most thoroughly studied ablation modality in the lung is RFA. Current ablation modalities frequently used include MWA, cryoablation, and irreversible electroporation (IRE). 20
RFA and MWA both achieve controlled heating at the tip of the applicator, which leads to tissue coagulation necrosis and tissue death. RFA achieves this through a rapid alternating electric current that is transmitted through the RFA applicator into the lesion and back to the RFA generator through grounding pads or a grounding electrode. This current causes agitation of ionic molecules in the tissue surrounding the applicator tip which generate heat. 21 MWA works in a similar way by applying an electromagnetic field that forces polar molecules to continuously realign with the oscillating field resulting in heat generation. 22
MWA has several advantages over RFA, the first of which is that it generates higher temperatures in a shorter period of time. 23 In addition, microwaves are capable of heating tissues with low electrical conductivity much better than RFA, which results in a larger ablation zone. 22 These advantages have led to the increased use of MWA over RFA in most centers, particularly with the increasing availability of long-term results for its use in lung ablation. 24
Indications
The current indications for image-guided ablation in NSCLC include the following: (1) initial treatment option in medically inoperable patients with stage Ia NSCLC, (2) patients with unresectable local recurrence, (3) patients with multiple primary lung cancers (based on biopsy-proven synchronous lesions or history of lung cancer) with solitary or multiple lesions suitable for definitive local therapy, 7 and (4) patients with stage Ia NSCLC with contraindications to surgery and SBRT. 25
Selection
Patient selection for MWA follows the same principles as RFA, with the exception that MWA is theoretically able to ablate larger tumors. MWA has the ability to use multiple applicators simultaneously and it also demonstrates a larger ablation zone per applicator. This allows more confidence in the use of MWA for tumors up to 3 cm in size (T1c) or extending greater than 3 cm in size in research settings. However, recent long-term follow-up data revealed that the probability of primary technical success is significantly lower in tumors greater than 3 cm in diameter, with a significantly higher incidence of complications and recurrences. 24
MWA is also less affected by “heat sink effect” in lesions close to large vessels compared with RFA. This is attributed to the ability to generate higher temperatures, which improves the possibility of obtaining complete ablations adjacent to such structures. 20 This comes with the caveat that MWA is theoretically more likely to injure surrounding vessels, though no significant differences in complications have been documented between the two heat-based ablation modalities. 26 27 Finally, MWA appears to be safer than RFA when used in patients with implantable cardiac devices. 28
Technique
Before the procedure, the trajectory and number of applicators to be used is planned based on the size and location of the lesion, as well as its proximity to pleural surfaces, fissures, and bronchovascular structures. The number of applicators used depends on the size of the lesion and the required ablation zone, with MWA providing more flexibility regarding ablation zone size owing to the ability to change ablation time and power settings. The trajectory is planned to traverse the least number of pleural surfaces possible, as this has been shown to decrease the rate of pneumothorax, 29 and to keep the pleural puncture site as far as possible from the ablation zone, as this has been shown to decrease the rate and severity of pneumothorax. 30 For lesions in the periphery of the lung ( Fig. 1 ), these considerations mean that it is often better to approach the lesion tangentially from a distant pleural puncture site rather than a perpendicular puncture directly through the pleura and into the nodule. This ensures that the probe is well anchored within the lesion and decreases the risk of it dislodging outside the lung during ablation. It also avoids involving the pleura in the ablation zone, which extends back along the MWA probe, known as “back burn,” which can lead to a bronchopleural fistula. 28 Lesions adjacent to bronchovascular structures ( Fig. 2 ) should be approached so that the ablation probe is parallel to the structure, rather than pointing toward it, avoiding injury to the structure, which may be caused by movement of the probe owing to changes in tissue volume induced by the ablation process. 28
Fig. 1.
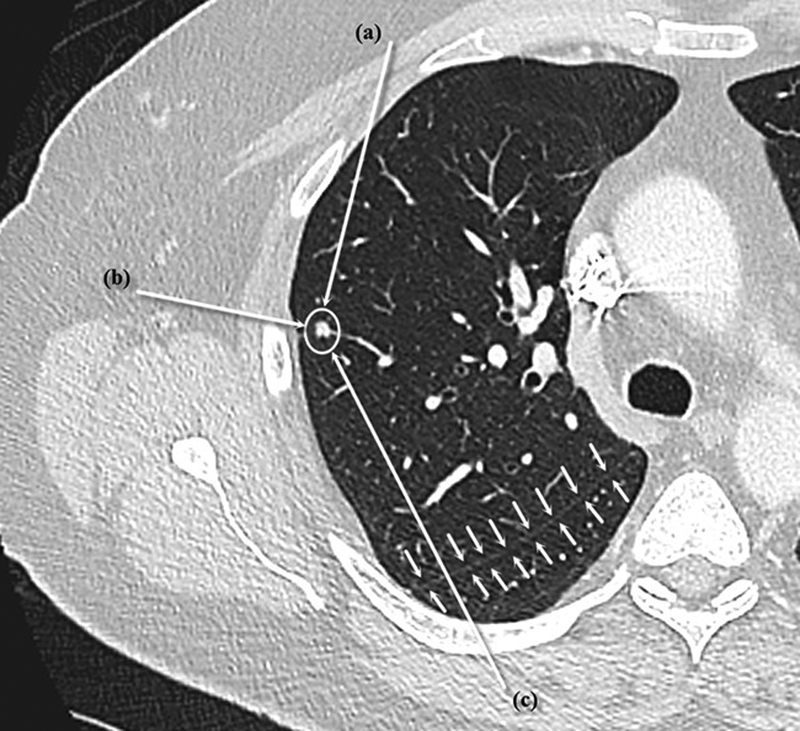
Possible approaches for ablation of a peripheral lesion (white circle). Approach (a) is preferred because it allows the ablation probe to be well seated within the lung and prevents “back burn” from damaging the pleura and possibly creating a bronchopleural fistula. Approach (b) is not advised because it risks the ablation probe becoming dislodged during ablation and may cause “back burn” into the pleura and adjacent chest wall. Approach (c) is not advised because it crosses a lung fissure (small arrows), which increases the risk of pneumothorax.
Fig. 2.
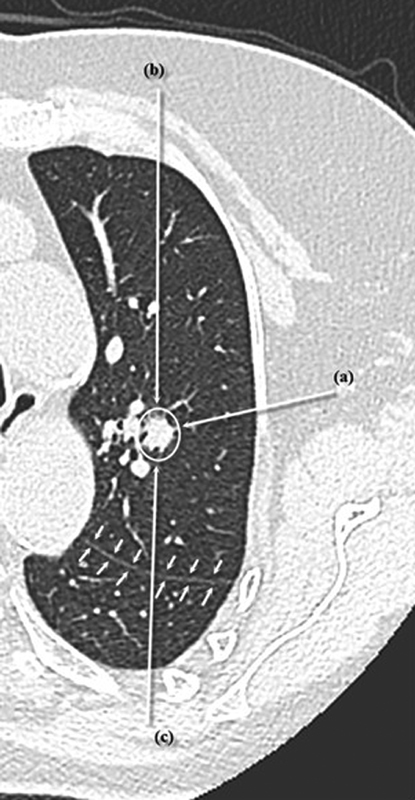
Possible approaches for ablation of a central lesion (white circle). Approach (a) is preferred because it is parallel to the vessels, which decreases the risk of bleeding during the procedure. Approach ( b ) is not advised because it is perpendicular to the vessels, which increases hemorrhage and “ghosting” of the lesion during the procedure. Approach ( c ) is not advised because it crosses a lung fissure (small arrows), which increases the risk of pneumothorax.
Lesions adjacent to the chest wall or the mediastinum carry a dual risk; if ablated completely, there is a risk of injury to the adjacent structure, and if ablated incompletely, there is a risk of residual tumor or recurrence. 31 One of the useful techniques to overcome both risks is instillation of artificial pneumothorax adjacent to the lesion, which moves it away from the adjacent structure and therefore decreases the risk of injury and allows complete ablation of the lesion. 31 This is done at our institution, when needed, under computed tomography (CT) guidance and using a 5-Fr centesis catheter needle connected to a three-way stopcock and a 60-mL syringe to allow one-way passage of air into the pleural space. Usually, 100 to 150 mL of air is needed to adequately separate the lung from the adjacent structure, but the amount varies according to the patient's size and the location of the lesion in relation to the patient's position. After completion of the ablation, the instilled air is removed using the same centesis catheter needle, which is kept in place during the procedure to quickly remove the air if needed. 32
The procedure can be done under sedation or general anesthesia, with preference for general anesthesia in some centers, as it is thought to improve the technique's feasibility, particularly in technically challenging cases where organ displacement or specific patient positioning is required. 20 Under CT guidance, real-time or stepwise according to the operator preference, the microwave applicator (or applicators) is advanced into the lesion. Once the desired position is reached, it is confirmed using CT with multiplanar reformats. The required ablation time and power are then set according to the required ablation zone as per the manufacturer's recommendations, and ablation is started and followed up with CT at intervals to ensure that the applicator does not move.
After the ablation time is completed, CT is done to ensure that the ablation zone encompasses the lesion with a margin, evidenced by a halo of ground-glass opacity surrounding the lesion ( Fig. 3 ). This halo is better appreciated by waiting 5 to 10 minutes following the ablation. It has been shown that the ablation zone is a predictor for recurrence, with a threshold of 4.5 mm associated with a specificity for local recurrence of 100%. 33 That, along with evidence that tumors of the lung extend microscopically into adjacent lung parenchyma by 6 to 8 mm, indicates that a 10-mm margin of ground-glass opacity surrounding the lesion should be achieved. 34
Fig. 3.
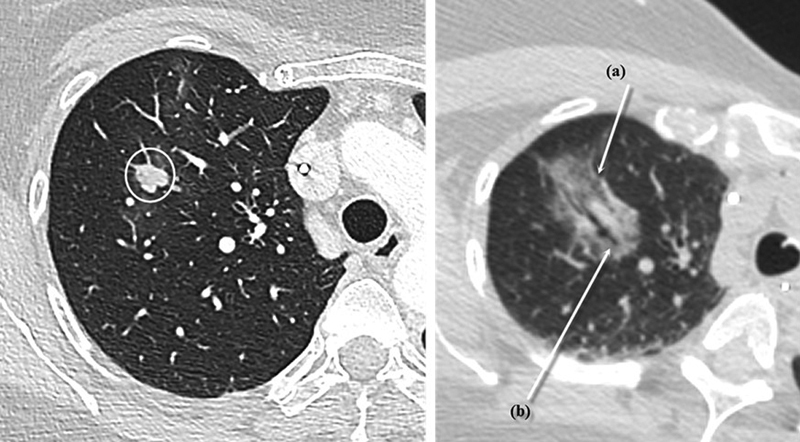
Conventional postablation appearance. Left: right upper lung lobe lesion prior to ablation. Right: the immediate postablation appearance of the same lesion, with (a) showing the expected ground-glass opacity encompassing the nodule with an hyperdense rim delimitating the ablation zone and (b) showing the ablation probe tract through the lesion. This typical appearance is easily noted in the 10-minute postablation scan.
Complications
Pneumothorax is the most common complication reported, seen in approximately 33.9% of patients. However, only approximately 11% of the patients will require intervention (chest tube placement). 27 Pleural effusion is reported in 9.6% of patients, with severe pleural effusion requiring intervention seen in only 0.3% of patients. 27 Life-threatening complications, such as severe hemoptysis or pseudoaneurysms, have been reported in 0.3 and 0.2% of patients, respectively, in a series of 1,000 ablations. 34 35 Other complications include pain (neuropathic pain), postablation syndrome, and infection, with the latter seen more commonly with MWA than with RFA due to higher ablation temperatures with increased incidence of cavitation ( Table 2 ). 24 36
Table 2. Most common complications of lung microwave ablation 24 42 54 .
| Complication | Incidence |
|---|---|
| Pain | 22% |
| Pneumothorax | |
| Not requiring intervention | 28–39% |
| Requiring intervention | 4–22% |
| Pleural effusion | |
| Not requiring intervention | 22% |
| Requiring intervention | 0–3% |
| Infection | |
| Pneumonia | 3% |
| Lung abscess | 0.5% |
Follow-up
Contrast-enhanced CT of the chest is preferred for follow-up over noncontrast CT, as it allows accurate detection of delayed complications (such as pseudoaneurysms), and can help detect residual or recurrent tumor in the ablation zone. 37 Follow-up is usually done by contrast-enhanced CT of the chest at 1, 3, 6, and 12 months following ablation with positron emission tomography (PET)/CT used as a problem-solving tool when CT findings are suspicious for recurrence. 38
The radiological appearance of the ablation zone transitions through three phases: (1) immediate and early phase (<24 hours to 1 week postablation), (2) intermediate phase (1 week to 2 months postablation), and (3) late phase (>2 months postablation). The immediate and early phase, which is usually done immediately postablation, is mostly used to confirm adequate coverage of the lesion by the ablation zone evidenced by the ground-glass opacity (as discussed previously) and to detect complications such as pneumothorax, pleural effusion, and hemothorax. 37 38
On imaging during the intermediate phase, which is typically done using a contrast-enhanced CT of the chest done at 1 month postablation, decrease in size of the ground-glass opacity surrounding the lesion compared with the immediate postprocedure CT is a reassuring finding, provided that it remains larger than the lesion prior to ablation. Cavitation of the ablation zone may also be seen, and has been considered a positive response to thermal ablation. 37 38 Mediastinal lymph node enlargement has also been reported, and can be a worrisome sign, but has not been associated with local recurrence. 36 Findings suspicious for local recurrence/residual disease include central or nodular enhancement exceeding 10 mm in diameter and/or 15 Hounsfield unit. Therein lies the importance of the use of contrast-enhanced CT of the chest in early follow-up, as stabilization and expansion of the ablation zone during the intermediate phase are not indicators for tumor recurrence or residual disease provided that an adequate ablation zone was achieved on the immediate postablation CT. 37
On late-phase imaging, the ablation zone should continue to decrease in size to be approximately equal to the size of the baseline tumor at 3 months ( Fig. 4 ) and should be smaller than the size of the baseline tumor at 6 months. An enlarging ablation zone at 3 months that continues to enlarge at 6 months warrants suspicion for recurrence, especially if a nodular growth pattern is seen ( Fig. 5 ). 38
Fig. 4.
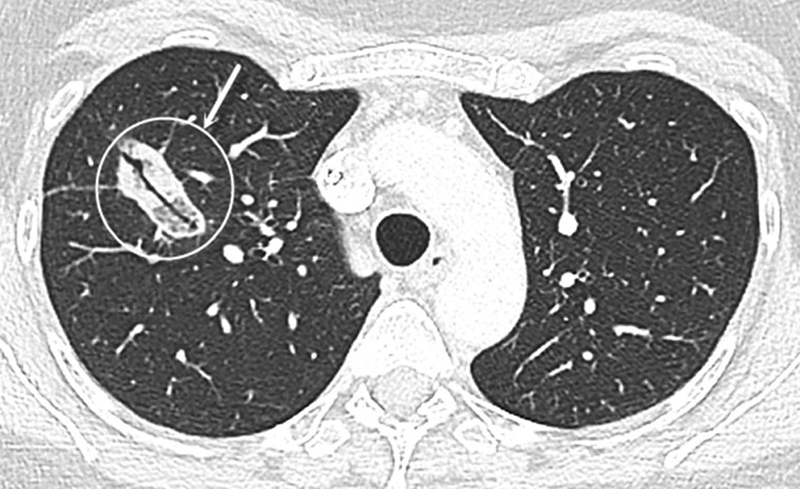
Expected 1 month CT appearance. The ablated right upper lung lobe lesion at 1-month follow-up shows involution and increased density of the ablation zone, with no signs suspicious of local recurrence and persistent nonnodular margin.
Fig. 5.
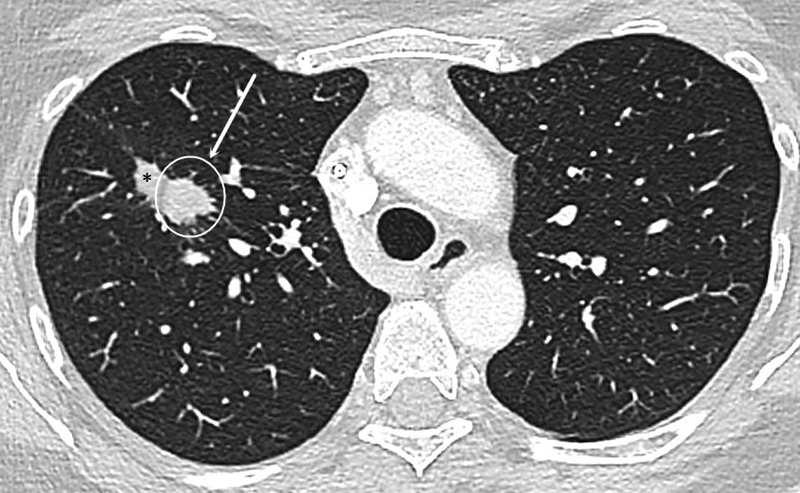
Example of recurrence. CT shows the ablated right upper lung lobe lesion at 6-month follow-up with a nodular contour of the anterior-medial aspect of the ablation zone (white circle). Note the involution of the adjacent ablation zone (*).
Outcomes
The reported primary technical success rate for MWA ranges from 80 to 100%. 24 39 The lower margin of this technical success rate is compensated by the fact that the ablation can be repeated multiple times until adequate margins are achieved. The reported median time to recurrence was as high as 39.7 months in one study, with strong association with original tumor diameter. Patients with original maximal tumor diameter exceeding 3 cm showed a median time to recurrence of 17.3 months, while those with original maximal tumor diameter below 3 cm showed a median time to recurrence of 62.1 months. 24
One factor that may affect local recurrence after ablation, and should be considered, is the histologic subtype of the tumor. A study on 53 patients who underwent thermal ablation for lung adenocarcinoma found that lesions with micropapillary and/or solid histological components were associated with a shorter time to local recurrence. 40 This is similar to the data from the surgical literature, which showed that solid predominant histologic subtype was one of the factors associated with poorer recurrence-free survival. 41 The implications of this finding regarding subtypes of other NSCLCs is unknown, but reinforces the importance of histopathologic evidence prior to treatment as opposed to relying on imaging diagnosis of NSCLC. 42
Regarding overall survival, a meta-analysis of 53 studies of RFA and MWA in primary and secondary lung tumors showed a median survival time of 24.4 months in a subgroup analysis focusing mainly on MWA in primary lung tumors. This result should be handled with caution as long-term follow-up data are scarce, and this meta-analysis included studies that enrolled patients with NSCLC beyond stage I disease. 27 One study that was not included in this meta-analysis showed that median time until death could not be reached for MWA in patients with NSCLC, with a first quartile value of 40.5 months and a range reaching up to 102.1 months. 24
Other Ablation Methods
Other ablation methods that have been studied in NSCLC include cryoablation and IRE, with varying results. Cryoablation relies on the Joule–Thompson effect to rapidly decrease the temperature of the cryoprobe and the surrounding tissues, leading to cellular damage initially through formation of ice crystals in the extracellular space, cellular dehydration and later through formation of ice crystals in the intracellular space, and immediate cell death. 43 The results of cryoablation in NSCLC have been promising. A study retrospectively evaluating the long-term survival of medically inoperable patients with early-stage NSCLC who underwent cryoablation showed a 5-year overall survival rate of 67.8% and a 5-year progression-free survival rate of 87.9%. 44 These results are comparable to RFA in early-stage NSCLC, and a study comparing sublobar resection, cryoablation, and RFA in 64 patients with stage I NSCLC found no statistically significant difference in overall survival, cancer-free survival, or cancer-specific survival at 3 years between the three groups. 45
IRE is a nonthermal ablation tool that utilizes short, intense, pulsing electric fields created across a well-defined area to destroy cells by causing permanent damage to the lipid bilayer of the cell membrane. In animal studies, it has demonstrated the ability to ablate sharply demarcated areas with sparing of surrounding major blood vessels and preserved connective tissue architecture. 46 The results of IRE in lung ablation, on the other hand, have not been as promising. A prospective, single-arm, phase II trial assessing the safety and efficacy of IRE in lung malignancies was stopped prematurely after interim analysis showed that the technique did not show adequate efficacy, with a local control rate of only 39%. The authors hypothesized that the reduced efficacy is related to increased impedance of current flow caused by the air within the lungs. 47 Therefore, IRE is not a recommended ablative technique in the lung.
Future Trends
While ablation and SBRT have proven comparable efficacy and safety to sublobar resection in the management of NSCLC in patients who are medically inoperable or have high surgical risk, studies have started comparing them to lobar resection. 48 A retrospective study comparing MWA and lobectomy for stage I NSCLC showed similar overall survival and disease-free survival, with a significantly lower complication rate in the MWA group, which may pave the way for prospective studies comparing the two treatment strategies. 49
Another area of future investigation is the use of combination therapy (percutaneous ablation and SBRT) for the management of central primary lung tumors, which are not adequately managed by either modality. A prospective phase 2 study including 16 patients with central tumors who received lower dose SBRT followed by RFA or MWA showed local control rates at 1 and 2 years of 93 and 81%, respectively, and concluded that percutaneous ablation can be a reasonable supplement to SBRT in central tumors. 50
Studies have highlighted the usefulness of percutaneous ablation in addressing local recurrence of NSCLC within a radiation field, 51 with a newer study showing that percutaneous ablation is useful in addressing local recurrence following SBRT. 52 In this retrospective study, the authors concluded that patients who received salvage treatment for local recurrence (which included percutaneous ablation) showed similar life expectancy to patients who did not show local recurrence, which again questions the significance of local recurrence if the treatment can be repeated. 52
Percutaneous ablation has also been recently proven to be useful in patients with epidermal growth factor receptor mutant NSCLC who experience oligoprogression while on tyrosine kinase inhibitors (TKIs). 53 These patients typically progress through the development of acquired resistance in one or few distant sites from the original tumor, and a study on 71 of these patients showed that percutaneous ablation provided an additional 10 months of disease control when combined with continuous TKI treatment. 53
Immuno-oncology will also impact the practice of local ablative techniques. Currently, the addition of cryoablation to immune checkpoint inhibitors for the treatment of advanced lung cancer is currently under clinical investigation (NCT03290677).
Conclusion
In conclusion, MWA is a proven safe and effective tool in the management of medically inoperable patients with early-stage NSCLC. Prospective trials comparing it to anatomical resection and SBRT with long-term follow-up are needed to accurately assess its location in the treatment algorithm provided to medically operable patients with early-stage NSCLC. It is also expected to play an expanding role in NSCLC patients following, or in conjunction with, SBRT as well as in those patients who develop oligoprogression while on optimal systemic therapy. In addition, the potential synergy with immunotherapy is yet to be studied.
Footnotes
Conflict of Interest None declared.
References
- 1.Siegel R L, Miller K D, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(01):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel R L, Torre L A, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(06):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Molina J R, Yang P, Cassivi S D, Schild S E, Adjei A A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(05):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?-a review. Transl Lung Cancer Res. 2016;5(01):26–38. doi: 10.3978/j.issn.2218-6751.2016.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pikor L A, Ramnarine V R, Lam S, Lam W L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82(02):179–189. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Kadara H, Scheet P, Wistuba I I, Spira A E. Early events in the molecular pathogenesis of lung cancer. Cancer Prev Res (Phila) 2016;9(07):518–527. doi: 10.1158/1940-6207.CAPR-15-0400. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Guidelines.Non-Small Cell Lung CancerVersion 3; February 21, 2018. Available at:https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed October 4, 2019
- 8.Ginsberg R J, Rubinstein L V; Lung Cancer Study Group.Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer Ann Thorac Surg 19956003615–622., discussion 622–623 [DOI] [PubMed] [Google Scholar]
- 9.Al Torki N.Comparison of Different Types of Surgery in Treating Patients with Stage IA Non-Small Cell Lung CancerAvailable at:https://clinicaltrials.gov/ct2/show/NCT00499330. Accessed October 4, 2019
- 10.Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuy D E, Fernando H C, Hillman S et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491–3498. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi N, Shedden K, Zheng X, Kong F S. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable Stage I non-small cell lung cancer: a systemic review and pooled analysis. Int J Radiat Oncol Biol Phys. 2016;95(05):1378–1390. doi: 10.1016/j.ijrobp.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlig J, Ludwig J M, Goldberg S B, Chiang A, Blasberg J D, Kim H S. Survival rates after thermal ablation versus stereotactic radiation therapy for Stage 1 non-small cell lung cancer: a national cancer database study. Radiology. 2018;289(03):862–870. doi: 10.1148/radiol.2018180979. [DOI] [PubMed] [Google Scholar]
- 14.Pereira P L, Masala S; Cardiovascular and Interventional Radiological Society of Europe (CIRSE).Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors Cardiovasc Intervent Radiol 20123502247–254. [DOI] [PubMed] [Google Scholar]
- 15.Frakulli R, Salvi F, Balestrini D et al. Radiological differential diagnosis between fibrosis and recurrence after stereotactic body radiation therapy (SBRT) in early stage non-small cell lung cancer (NSCLC) Transl Lung Cancer Res. 2017;6 01:S1–S7. doi: 10.21037/tlcr.2017.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clasen S, Krober S M, Kosan B et al. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer. 2008;113(11):3121–3129. doi: 10.1002/cncr.23882. [DOI] [PubMed] [Google Scholar]
- 17.Yasui K, Kanazawa S, Sano Y et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231(03):850–857. doi: 10.1148/radiol.2313030347. [DOI] [PubMed] [Google Scholar]
- 18.Palma D A, Nguyen T K, Louie A V et al. Measuring the integration of stereotactic ablative radiotherapy plus surgery for early-stage non-small cell lung cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(05):681–688. doi: 10.1001/jamaoncol.2018.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree T, Puri V, Timmerman R et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033) J Thorac Cardiovasc Surg. 2013;145(03):692–699. doi: 10.1016/j.jtcvs.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 20.de Baere T, Tselikas L, Catena V, Buy X, Deschamps F, Palussière J. Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging. 2016;97(10):1019–1024. doi: 10.1016/j.diii.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Rose S C, Thistlethwaite P A, Sewell P E, Vance R B.Lung cancer and radiofrequency ablation J Vasc Interv Radiol 20061706927–951., quiz 951 [DOI] [PubMed] [Google Scholar]
- 22.Lubner M G, Brace C L, Hinshaw J L, Lee F T., JrMicrowave tumor ablation: mechanism of action, clinical results, and devices J Vasc Interv Radiol 201021(8, Suppl):S192–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brace C L, Hinshaw J L, Laeseke P F, Sampson L A, Lee F T., Jr Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(03):705–711. doi: 10.1148/radiol.2513081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healey T T, March B T, Baird G, Dupuy D E. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28(02):206–211. doi: 10.1016/j.jvir.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Postmus P E, Kerr K M, Oudkerk M et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 04:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 26.Shi F, Li G, Zhou Z et al. Microwave ablation versus radiofrequency ablation for the treatment of pulmonary tumors . Oncotarget. 2017;8(65):109791–109798. doi: 10.18632/oncotarget.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Z, Wang Y, Zhang J, Zheng J, Li W. A Meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol. 2019;16(03):302–314. doi: 10.1016/j.jacr.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Abtin F, De Baere T, Dupuy D E et al. Updates on current role and practice of lung ablation. J Thorac Imaging. 2019;34(04):266–277. doi: 10.1097/RTI.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 29.Moreland A, Novogrodsky E, Brody L et al. Pneumothorax with prolonged chest tube requirement after CT-guided percutaneous lung biopsy: incidence and risk factors. Eur Radiol. 2016;26(10):3483–3491. doi: 10.1007/s00330-015-4200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K S, Takaki H, Yarmohammadi H et al. Pleural puncture that excludes the ablation zone decreases the risk of pneumothorax after percutaneous microwave ablation in porcine lung. J Vasc Interv Radiol. 2015;26(07):1052–1058. doi: 10.1016/j.jvir.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraki T, Gobara H, Fujiwara H et al. Lung cancer ablation: complications. Semin Intervent Radiol. 2013;30(02):169–175. doi: 10.1055/s-0033-1342958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia H, Tian J, Liu B, Meng H, Pan F, Li C. Efficacy and safety of artificial pneumothorax with position adjustment for CT-guided percutaneous transthoracic microwave ablation of small subpleural lung tumors. Thorac Cancer. 2019;10(08):1710–1716. doi: 10.1111/1759-7714.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Baere T, Farouil G, Deschamps F. Lung cancer ablation: what is the evidence? Semin Intervent Radiol. 2013;30(02):151–156. doi: 10.1055/s-0033-1342956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith S L, Jennings P E.Lung radiofrequency and microwave ablation: a review of indications, techniques and post-procedural imaging appearances Br J Radiol 201588(1046):2.0140598E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashima M, Yamakado K, Takaki H et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol. 2011;197(04):W576–W580. doi: 10.2214/AJR.11.6408. [DOI] [PubMed] [Google Scholar]
- 36.Wolf F J, Grand D J, Machan J T, Dipetrillo T A, Mayo-Smith W W, Dupuy D E. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(03):871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 37.Chheang S, Abtin F, Guteirrez A, Genshaft S, Suh R. Imaging features following thermal ablation of lung malignancies. Semin Intervent Radiol. 2013;30(02):157–168. doi: 10.1055/s-0033-1342957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abtin F G, Eradat J, Gutierrez A J, Lee C, Fishbein M C, Suh R D. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics. 2012;32(04):947–969. doi: 10.1148/rg.324105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng A, Ye X, Yang X, Huang G, Gai Y. Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol. 2016;27(12):1806–1814. doi: 10.1016/j.jvir.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Gao S, Stein S, Petre E N et al. Micropapillary and/or solid histologic subtype based on pre-treatment biopsy predicts local recurrence after thermal ablation of lung adenocarcinoma. Cardiovasc Intervent Radiol. 2018;41(02):253–259. doi: 10.1007/s00270-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J, Wang R, Han B et al. Solid predominant histologic subtype and early recurrence predict poor postrecurrence survival in patients with stage I lung adenocarcinoma. Oncotarget. 2017;8(04):7050–7058. doi: 10.18632/oncotarget.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsakok M T, Little M W, Hynes G et al. Local control, safety, and survival following image-guided percutaneous microwave thermal ablation in primary lung malignancy. Clin Radiol. 2019;74(01):8.0E20–8.0E27. doi: 10.1016/j.crad.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Erinjeri J P, Clark T WI.Cryoablation: mechanism of action and devices J Vasc Interv Radiol 201021(8, Suppl):S187–S191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore W, Talati R, Bhattacharji P, Bilfinger T. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26(03):312–319. doi: 10.1016/j.jvir.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Zemlyak A, Moore W H, Bilfinger T V. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211(01):68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Lee E W, Chen C, Prieto V E, Dry S M, Loh C T, Kee S T. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(02):426–433. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 47.Ricke J, Jürgens J HW, Deschamps F et al. Irreversible electroporation (IRE) fails to demonstrate efficacy in a prospective multicenter phase II trial on lung malignancies: the ALICE trial. Cardiovasc Intervent Radiol. 2015;38(02):401–408. doi: 10.1007/s00270-014-1049-0. [DOI] [PubMed] [Google Scholar]
- 48.Tian S, Higgins K A, Curran W J, Cassidy R J. Stereotactic body radiation therapy vs. surgery in early-stage non-small cell lung cancer: lessons learned, current recommendations, future directions . J Thorac Dis. 2018;10(03):1201–1204. doi: 10.21037/jtd.2018.01.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao W, Lu M, Fan W et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia. 2018;34(08):1329–1336. doi: 10.1080/02656736.2018.1434901. [DOI] [PubMed] [Google Scholar]
- 50.Sandler K A, Abtin F, Suh R et al. A prospective phase 2 study evaluating safety and efficacy of combining stereotactic body radiation therapy with heat-based ablation for centrally located lung tumors. Int J Radiat Oncol Biol Phys. 2018;101(03):564–573. doi: 10.1016/j.ijrobp.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Leung V A, DiPetrillo T A, Dupuy D E. Image-guided tumor ablation for the treatment of recurrent non-small cell lung cancer within the radiation field. Eur J Radiol. 2011;80(03):e491–e499. doi: 10.1016/j.ejrad.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 52.Brooks E D, Sun B, Feng L et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early-stage lung cancer. JAMA Netw Open. 2018;1(04):e181390. doi: 10.1001/jamanetworkopen.2018.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ni Y, Liu B, Ye X et al. Local thermal ablation with continuous EGFR tyrosine kinase inhibitors for EGFR-mutant non-small cell lung cancers that developed extra-central nervous system (CNS) oligoprogressive disease. Cardiovasc Intervent Radiol. 2019;42(05):693–699. doi: 10.1007/s00270-018-02153-x. [DOI] [PubMed] [Google Scholar]
- 54.Zheng A, Wang X, Yang X et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98(01):243–248. doi: 10.1016/j.athoracsur.2014.03.008. [DOI] [PubMed] [Google Scholar]


