Abstract
Understanding co-activation patterns of the hypothalamic-pituitary-adrenal axis (HPA) and sympathetic adrenal medullary (SAM) during early adolescence may illuminate risk for development of internalizing and externalizing problems. The present study advances empirical work on the topic by examining SAM-HPA co-activation during both the reactivity and recovery phases of the stress response following acute stress exposure. Fourth and fifth grade boys and girls (N = 149) provided cortisol and alpha-amylase via saliva at seven times throughout a 95-minute assessment in which they were administered the modified Trier Social Stress Test. Parents reported on adolescents’ life stress, pubertal development, medication use, and externalizing problems. Adolescents reported their own internalizing symptoms. Multiple linear regressions tested both direct and interactive effects of SAM and HPA reactivity and recovery on internalizing and externalizing problems. Results from these analyses showed that whereas SAM and HPA reactivity interacted to predict internalizing symptoms, it was their interaction during the recovery phase that predicted externalizing. Concurrent high SAM and HPA reactivity scores predicted high levels of internalizing and concurrently low SAM and HPA recovery scores predicted high levels of externalizing. Implications of the findings for further study and clinical application are discussed.
Keywords: cortisol, stress reactivity, stress recovery, co-activation, internalizing, externalizing, alpha-amylase
Rates of depression, anxiety, substance use, and delinquency begin to rise in earnest during early adolescence and rates continue to climb throughout the second decade of life (e.g., Childs, Sullivan, & Gulledge, 2010; Salk, Petersen, Abramson, & Hyde, 2016). These increases co-occur with rising levels of stress across this developmental period, especially stressors arising from enhanced sensitivity to interpersonal relationships. Physiologic stress response systems such as the hypothalamic-pituitary-adrenal axis (HPA) and sympathetic adrenal medullary system (SAM) enter a phase of renewed plasticity and maturation during early adolescence as well; a process that both affects and is affected by burgeoning levels of stress (McEwen, 2007). Early adolescence clearly represents an important developmental period during which risk for stress-related problems intensifies and may constitute a critical time to intervene to prevent mental health problems. Knowledge of mechanisms of stress-related risk can facilitate development of appropriate interventions.
Atypical activation patterns (i.e., dysregulation) of the SAM system and HPA axis appear to be one such mechanism as they are associated with both stress exposure and emotional/behavioral problems (e.g., Rogosch, Dackis, & Cicchetti, 2011). As components of an inter-related stress response system, the SAM and HPA are hypothesized to work in coordination to facilitate adaptation to stress. Theoretical models of SAM-HPA co-activation have focused on the need to determine the extent to which these two systems optimally operate either in union or in tandem—in other words, when functioning “properly” are they active and online at the same time (symmetric activation) or are they sequentially active (asymmetric activation)? Extrapolating from this basic question, researchers have searched for evidence of dysfunction stemming from extreme symmetric or asymmetric co-activations—for example, that concurrent under-activation (i.e., symmetric) of both the SAM and HPA signals risk for externalizing problems (e.g., Bauer et al., 2002). A handful of studies have examined SAM-HPA co-activation (e.g., Gordis et al., 2006; Chen, Raine, & Granger, 2015), but there exists significant heterogeneity in the samples, methods, and findings of these investigations. Therefore, in this study we examine SAM-HPA co-activation effects on internalizing and externalizing problems during this critical developmental period. We also provide a review (see Table 1) of the existing studies examining the unique and combined contributions of SAM and HPA co-activation to symptoms of psychopathology in children and adolescents.
Table 1.
Characteristics and findings from existing empirical studies of SAM-HPA co-activation in children and adolescents.
| Study | Study Sample | Saliva Sampling | Biomarker Indices | Covariates | Findings: Associated with Psychopathology | |
|---|---|---|---|---|---|---|
| Symmetrical | Asymmetrical | |||||
| Allwood, Handwerger, Kivlighan, Granger, and Stroud (2011) | 56 children (ages 7-16) inclusion criteria: fluent in English exclusion criteria: use of oral contraceptives, thyroid medications, steroids, psychotropic medications, tobacco, drugs, and alcohol; history of psychological or behavioral problems; current physical illnesses. |
acute stress exposure: 7-9 saliva samples taken over baseline, stress, and recovery periods; stress tasks included 3 tasks, either TSST with mirror tracing or a peer rejection task; 6 time-matched samples were used to account for time-to-peak lag of cortisol and AA | use baseline measures and peak (maximum value of cortisol or AA post-stressor onset) | sex, age, and stressor type | Low AA reactivity in conjunction with high cortisol reactivity predicted greater parent reports of child total problems (attention problems and social problems) and greater internalizing problems. | |
| Bae, Stadelmann, Klein, Jaeger, Heimisch, Keiss et al., 2015 | 169 children (ages 8-14); selected on the basis of presence of internalizing (n=55) or externalizing (n=33) disorders; healthy control group (n=81) also included inclusion criteria: fluent in German; IQ > 80; no concurrent endocrine diseases or use of glucocorticoid medications |
circadian activity: saliva collected at wakening, 30 min after waking, and 30 min before bedtime over 3 days acute stress exposure: 8 saliva samples (3 pre-, 5 post) across TSST-C |
Calculated reactivity (difference between highest post-TSST-C and lowest pre-TSST-C) and recovery (difference between highest post- TSST-C and lowest post- TSST-C) | gender, age, height, BMI, Tanner pubertal stages | Low AA baseline and low cortisol reactivity was associated with more externalizing problems. | High AA recovery and low cortisol reactivity associated with more internalizing problems. |
| Chen, Raine, Rudo-Hutt, Glenn, Soyfer, & Granger, 2014 | 425 children (ages 11-12). Exclusion criteria: diagnoses of psychotic disorder, mental retardation, pervasive developmental disorder, current psychiatric treatment or treatment; current use of medication known to interfere with measurement of salivary analytes. |
circadian activity: 3 saliva samples collected across one morning in the laboratory, starting at approximately 9:19am (SD=20 minutes), then second 15 minutes later, and third 30 minutes later. | Levels of biomarkers modeled as latent variables with three corresponding sample analytes as indicators | pubertal stage, body mass index, household income, and ethnicity | For boys with high AA, as cortisol increased, the effect of harsh discipline on externalizing and internalizing problems decreased. No interaction for girls. | Among boys with low AA, as cortisol increased the effect of harsh discipline on externalizing and internalizing problems increased. No significant interaction for girls. |
| Chen, Raine, Soyfer, & Granger, 2015 | Same sample used in Chen et al, 2014. 429 children (ages 11-12). See Chen et al, 2014, for exclusion criteria. |
circadian activity: saliva collected four times across the day (3 in the morning at 15-minute intervals, and 4th sample in afternoon before series of lab tasks) | “Trait-like” indices of biomarkers modeled as latent variables with four corresponding sample analytes as indicators. | age, gender, ethnicity, saliva collected time | Children with low “trait-like” AA and cortisol together were more likely to have higher scores of externalizing problems. | Children with low sAA and high cortisol were significantly more likely to have lower levels of internalizing problems. |
| Chen, Raine, Glenn, & Granger, 2016 | Same sample used in Chen et al, 2014, with the addition of follow up assessments at 3-, 6-, and 12-month follow-up. 394 children (ages 11-12 at initial assessment). See Chen et al, 2014 for initial exclusion criteria. After initial assessment, children with elevated externalizing behavior were randomized into treatment or control group. Intervention administered between initial assessment and 3-month follow up. | circadian activity at initial assessment: see Chen et al 2014 for procedure | Levels of biomarkers modeled as latent variables with three corresponding sample analytes as indicators | age, sex, ethnicity, Tanner puberty stage, treatment assignment | Among children who had high latent cortisol at initial assessment, as AA decreased, the odds of having comorbid externalizing and internalizing problems across time points increased. For children with low latent cortisol, the probability of having stable comorbidity and high levels of symptoms was high regardless of latent AA level. |
|
| de Vries-Bouw, Jansen, Vermeiren, Doreleijers, van de Ven, & Popma, 2012 | 64 male adolescents (Mage = 18.4 years, SD=.9); recruited from delinquency diversion program (DP; n=48) or from community as matched controls (n=16). Of those boys in the delinquent group, 15 boys (DP+) had a DBD by self- or parent-report and 33 boys (DP−) did not. | acute stress response: 7 saliva samples (2 pre- and 4 post-stressor task, a public speaking task in front of a one-way screen with video recording) | basal: biomarker levels in saliva sample 2 (immediately before start of stress task) was used; reactivity: AUCi across samples 2-6 | nicotine use | Interaction between AA and cortisol not significantly associated with disruptive behavior. | |
| El-Sheikh, Erath, Buckhalt, Granger, and Mize, (2008) | 64 children (Mage = 8.72, SD=0.47); exclusion criteria: parent report of child chronic/acute physical illness, clinically significant sleep disorders mental retardation, learning disabilities, ADHD | circadian activity: 2 saliva samples collected (1) 20 min after arrival, (2) 2 hours later, post-stressor (listen to interadult argument, complete star-tracing tasks; known to elicit SCL reactivity, but wouldn’t expect it to elicit cortisol); | samples were averaged to represent basal levels | gender, ethnicity, age, SES and time of saliva sampling | Concordantly high basal AA and cortisol predicted higher parent reports of child externalizing and internalizing problems. | High basal AA and low cortisol associated with lower levels of parent-reported externalizing and internalizing behaviors. |
| Gordis, Granger, Susman, & Trickett, 2006 | 67 youth (ages 10-14.5), with maltreated group (n=35) and non-maltreated (n=32). Exclusion criteria: use of synthetic corticosteroids or beta-adrenergic agonists or hormone contraceptives. | Acute stress response: 6 saliva samples (2 pre- and 4 post-TSST) | Acute response: Calculated AUC with respect to ground across 6 time points | maltreatment status, child sex, age, time of day | For children with low AA response, higher cortisol response predicted lower reports of child’s aggression. For children with high AA, there was no effect of cortisol on parents’ report of aggression. |
|
| Koss, George, Cummings, Davies, El-Sheikh, & Cicchetti 2014 | 176 families (mothers, fathers, and 2nd grade child; Mage = 7.99 years, SD=.53) participated in T1 (2nd grade) and T2 (early adolescence, mean grade=7th; Mage =12.55 years, SD=.56). Inclusion criteria: all 3 family members living together for a minimum of 3 years, families had a child in kindergarten at start of study, all family members proficient in English. | Acute Stress Response: 2 saliva samples collected pre- and 25-minutes post-stressor task, in which children viewed martial conflict vignettes. | Acute response: post-stressor biomarker levels (controlling for pre-stressor levels) | Assessment time, pre-task cortisol and AA levels, tape stimuli, child gender, child age, family income | Children with low AA-low cortisol in 2nd grade had more concomitant internalizing problems when in low conflict homes. This was not true for children in high conflict homes with the low AA-low cortisol pattern. 7th graders with high AA-high cortisol had more externalizing problems, but only in low conflict homes. |
Children from high conflict homes with high AA-low cortisol in 2nd grade had high levels of concurrent and prospective internalizing problems. Adolescents in 7th grade with high AA-low cortisol in 2nd grade had more externalizing problems when from high conflict homes. |
| Kreher, Powers, & Granger, 2012 | 109 female undergraduate students (Mage = 19). Inclusion criteria: native speaker of English, normal or corrected-to-normal vision. Participants received course extra credit for study participation. | Baseline: provided one late afternoon/early evening saliva sample upon arrival to the lab on three lab sessions. Appointments were scheduled to begin 8-10 hours after waking | Biomarker values were averaged across the three days. | medication and marijuana use | Among women with high AA, those with higher cortisol exhibited greater priming to negative words. | Among women with low AA, there was no significant relationship between cortisol and negative valence priming. |
| Platje, Jansen, Vermieren, Doreleijers, van Lier, Koot et al., 2017 | 197 adolescents (Mage = 17.31 years, SD=.44). Recruited for longitudinal Dutch population based cohort study, with oversampling of children with borderline clinical score on externalizing behaviors by teacher report at age 11. |
Cortisol awakening response: Collected 3 saliva samples, immediately after awakening, then 30 and 60 minutes later. Acute stress response: Provided 8 saliva samples, 2 pre-, 1 during, and 5 post-completion of the Leiden PST. |
CAR: Calculated AUC with respect to ground across the three morning samples. Acute response: Calculated AUC with respect to increase across time points 2-5 (just before start of task until 15 minutes after stressor). |
gender, nicotine use | The interaction approached significance (p=.076), but suggested that for youth with high AA, high cortisol reactivity was associated with higher externalizing behavior. | The interaction approached significance (p=.076), but suggested that for youth with low AA, high cortisol reactivity was associated with lower externalizing behavior. |
The Importance of the Preadolescent Developmental Period
The developing HPA (Romeo, 2010; Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2015) and hypothalamic-pituitary-gonadal systems work in tandem to bring about physical maturity (puberty) and at the same time lead to heightened attunement to social cues in the environment (Joos, Wodzinski, Wadsworth & Dorn, 2018). This increased awareness of social evaluation and the growing importance of interpersonal relationships contributes to increased perceived stress for the early adolescent. Coping with these normative stressful challenges “exercises” the stress response systems, contributing to their continued growth and development.
This confluence of developmental changes readies an individual for the increased demands of adulthood, and while normative, this process of stress adaptation goes more smoothly for some individuals than for others. Those with pre-existing biological, cognitive, or emotional vulnerabilities or those exposed to chronic stress and adverse life contexts can fall prey to negative psychological sequelae in the face of these normative challenges (Cicchetti & Rogosch, 2002). Mounting evidence suggests that independent and perhaps interdependent operation of the SAM and HPA systems constitutes a key proximal mechanism of risk (and resilience) for both internalizing and externalizing forms of psychopathology (e.g., Bae et al., 2015; Chen, Raine, Glenn, & Granger, 2016).
Physiologic Stress Reactivity and Recovery
At the outset of a stressful encounter, involuntary processes set in motion a cascade of activity that quickly mobilizes the SAM response, and activates the HPA axis eventually leading to the synthesis of cortisol. Associated with “fight/flight/freeze” response, activation of the SAM system in response to a stressor is a highly energy consumptive process (Hermans, Henckens, Joels, & Fernandez, 2014). Once a stressor is resolved, the cortisol produced by a well-functioning HPA axis will quickly help regulate the SAM and bring the organism back to baseline. Restoration of normal functioning in the SAM-activated cardiovascular, immunologic, metabolic, reproductive, and digestive systems will ensue. Alternatively, prolonged activation of the SAM stemming from protracted stress or a malfunctioning HPA taxes chronically engaged associated cardiovascular, metabolic, immunologic, and central nervous systems.
This finely coordinated system of triggers and feedback loops is well suited to periodic acute activations that resolve relatively quickly. The system is also highly adaptable and has been shown to calibrate its activity over time to match predominant environmental demands (Del Giudice, Ellis, & Shirtcliff, 2011). As such, the physiologic stress response systems of individuals living in the context of frequent, ongoing stressful events become calibrated to be optimally responsive in that context (Wadsworth, 2015). Hence, children growing up exposed to chronic, uncontrollable stressors such as poverty or maltreatment often develop SAM-HPA systems having lower activation thresholds and less responsive deactivation feedback loops, leading to chronic activation of the SAM-HPA systems. These re-calibrated stress responses are adaptive insofar as they help protect the organism by being vigilant to potential threat for example, and at the same time are maladaptive as they portend problems in other life areas such as physical and mental health (Del Giudice et al., 2011).
Multi-system Co-activation
To place our review of SAM-HPA co-activation in the context of the broader literature on stress response “dysregulation” effects, we first provide a brief overview of SAM and HPA main effects on symptoms of psychopathology in children and adolescents. In the studies reviewed here, HPA axis activity is indexed by the peripheral marker, salivary cortisol, and SAM activity is indexed by salivary alpha-amylase. Stress researchers generally refer to the initial activation of the acute stress response as “reactivity,” and quantify the size of the reactivity response as the change from baseline to peak levels of neuroendocrine hormones such as alpha-amylase (SAM) or cortisol (HPA). The post-stressor deactivation and restoration phase, conceptualized as the efficiency or rapidity with which an individual’s hormones return to baseline levels following the peak, is referred to as “recovery.” Distinguishing between reactivity and recovery is important because examination of the reactivity and recovery phases separately has proven helpful in specifying outcomes of different coping behaviors (e.g., Wadsworth et al., 2018; Zoccola & Dickerson, 2012), and in predicting psychopathology (Niermann et al., 2017). Hence, we include studies examining both reactivity and recovery phases of the stress response.
Alpha amylase reactivity and recovery.
There is considerable inconsistency as to whether and how alpha-amylase reactivity and recovery are linked to clinically relevant outcomes in children or adolescents. On the one hand, heightened alpha-amylase reactivity has been associated with higher levels of anxiety in 7-16 year-old children (Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011), increased depression in early adolescents exposed to peer victimization (Rudolph, Troop-Gordon, & Granger, 2011), and more adolescent health and interpersonal problems (Afifi, Granger, Denes, Joseph, & Aldeis, 2011). On the other hand, lower alpha-amylase reactivity has been found to be related to problems such as rejection sensitivity (Chaudoir, Vergara-Lopez, & Stroud, 2017), post-traumatic stress disorder (Feldman, Vengrober, Eidelman-Rothman, & Zagoory-Sharon, 2013), and conduct problems (Susman et al., 2010). Few studies have looked specifically at alpha-amylase recovery following a stressor, though results seem to support that quicker recovery is beneficial (e.g., Chaudoir et al., 2017).
Cortisol reactivity and recovery.
There is substantially more research on associations between symptoms of psychopathology and cortisol reactivity and recovery. Cortisol reactivity to socio-evaluative stressors has been related to internalizing and externalizing symptoms during the preadolescent period in multiple samples. Most of these studies find an inverse relationship between HPA reactivity and externalizing problems (e.g., Hartman, Hermanns, de Jong, & Ormel, 2013; Northover, Thapar, Lamgley, Fairchild, & van Goozen, 2016), suggesting that an active HPA response to stress may protect against externalizing problems. In contrast, exaggerated cortisol reactivity is associated primarily with internalizing symptoms such as anxiety (Kryski, Smith, Sheikh, Singh, & Hayden, 2013) as well as broadband internalizing problems (Hartman et al., 2013; Hastings et al., 2011). Though the research on cortisol recovery is more limited, findings generally align with the theoretical premise that efficient return to baseline functioning (i.e., quick and complete) is optimal (Nederhof et al., 2015; Schoorl, van Rijn, de Wied, van Goozen, & Swaab, 2017).
Studies of Co-activation
Table 1 contains all of the published studies of SAM-HPA co-activation conducted with children or adolescents that were available at this writing. We here summarize the findings regarding interactions between alpha-amylase and cortisol in relation to internalizing and externalizing symptoms of psychopathology and consider potential sources of heterogeneity stemming from sample characteristics (sample age range, sampling population, sex) and measurement issues (reactivity vs. recovery vs. basal, calculation of activation).
Support for risk stemming from asymmetric SAM-HPA co-activation is limited, but three studies implicate risk for internalizing problems in particular. For the 8-14 year olds with clinical levels of internalizing symptoms in Bae and colleagues (2015) study, low basal alpha-amylase and high basal cortisol were inversely related (asymmetric co-activation). Similarly, low basal alpha-amylase and high basal cortisol were inversely related (asymmetric co-activation) to internalizing problems in the 11 and 12 year-olds in Chen and colleagues’ (2015) study. Finally, in response to a lab-based stress induction, low alpha-amylase reactivity and high cortisol reactivity (asymmetric co-activation) were linked to the highest internalizing problems in Allwood and colleagues’ (2011) community sample of 7-16 years olds. Hence, there is, albeit limited, converging evidence that concurrent low alpha-amylase and high cortisol activity portend risk for internalizing. This cross-study consistency is striking given the different ages, levels of clinical risk, and measurement strategies (basal versus reactivity to a stressor) used in the individual studies.
Support for symmetric low cortisol, low alpha-amylase co-activation as a risk for externalizing problems is relatively strong, although some studies also find risk stemming from symmetric high co-activation as well. Gordis and colleagues (2008) found an interaction between alpha-amylase and cortisol reactivity to the TSST in their sample of 10-14 year olds; at lower alpha-amylase reactivity, cortisol was inversely related to aggression (symmetric low activation), whereas at higher alpha-amylase reactivity, cortisol was not related to aggression. Chen and colleagues (2015) and Bae and colleagues (2015) both found that concurrent low levels of basal alpha-amylase and cortisol were associated with externalizing problems in their samples. Platje and colleagues’ (2017) found a trend consistent with risk stemming from concurrent low alpha-amylase and cortisol reactivity to the Leiden Public Speaking test in their older adolescent sample. Similarly, de Vries-Bouw et al. (2012) found that concurrent low levels of both alpha-amylase and cortisol predicted the highest levels of externalizing problems in their sample of older male adolescents.
There are a few findings that diverge from the above. For example, El-Sheikh and colleagues found risk for both internalizing and externalizing stemming from symmetrically high basal levels in their sample of third grade children (El-Sheikh et al., 2008)—findings which diverge from both patterns above. It is possible that this could represent a developmental phenomenon, as the children in this study were the youngest on average of any sample. However, a similar pattern emerged in the college students in Kreher and colleagues’ (2012) study where symmetric high basal alpha-amylase and cortisol were associated with increased priming of negative words, a putative risk factor for internalizing problems. Another anomaly was the asymmetric risk for parent-reported aggressive problems posed by high total alpha-amylase output and low total cortisol output (across both the reactivity and recovery phases of the stress response) in 10-14 year-olds in Gordis et al. (2006). The aggregation of alpha-amylase and cortisol across the full stress response (i.e., reactivity and recovery together) in the latter study makes it difficult to compare to other studies measuring either reactivity or basal levels. Finally, in two of the studies, co-activation effects were moderated by a third variable and as such only applied to boys receiving harsh discipline (Chen, Rudo-Hutt, Glenn, Soyfer, & Granger 2014) or youths exposed to high levels of marital conflict (Koss et al., 2014), for example. A third study (Chen et al., 2016) was specifically designed to detect co-morbidity in internalizing and externalizing problems.
1.2. The Current Study
The purpose of the study was to examine both singular and interactive contributions of SAM and HPA activation during both the reactivity and recovery phases of the acute stress response to levels of internalizing and externalizing problems in pre-adolescent boys and girls, accounting for known correlates of these physiologic systems (sex, pubertal status, medication use, time of day, and stress exposure). We reviewed the extant literature on SAM-HPA coactivation in children and adolescents in order to derive hypotheses regarding co-activation effects in our community sample of pre-adolescents who were oversampled for socioeconomic risk. We hypothesized that: (1) asymmetric reactivity co-activation would be associated with internalizing, specifically that a pattern of low alpha-amylase-high cortisol reactivity would be associated with internalizing symptoms; and (2) symmetric reactivity co-activation would be associated with externalizing symptoms, and that low alpha-amylase-low cortisol symmetric reactivity would represent risk for externalizing. The extent to which alpha-amylase and cortisol will interact in a meaningful way during the recovery phase is currently unknown and was also explored in this study.
2. Method
2.1. Participants
Participants were 149 fourth- and fifth-grade children (n = 76 males; Mage = 10.31, SD = 1.74) and one of their primary caregivers (85.2% mothers), who lived in either a large suburban area in the Western U.S. (n = 30) or a small suburban area in the Northeastern U.S. (n = 119). The majority of participants identified as White (94.6% of children and 93.2% of caregivers). Median household income for the sample was $70,500 USD.
2.2. Procedure
Participants were recruited through local elementary schools. Caregivers completed the online parent portion of the study in which they provided consent for their children to participate and completed online questionnaires. Preadolescents participated in 95-minute sessions that took place between the hours of 3:00 p.m. and 5:30 p.m. Upon arriving at the lab, participants provided written assent, rinsed their mouth out with a sip of bottled water, and provided the T1 saliva sample. Participants then completed questionnaires for 40 minutes, after which T2 saliva sample was taken. Next, participants were taken to a separate room and administered the modified Trier Social Stress Test (TSST-M; Yim, Quas, Cahill, & Hayakawa, 2010); T3 saliva sample was taken immediately after this task. Participants were then brought to a separate room for 10 minutes where they waited for their performance to be scored by the judges—half of the children waited in a “coping” room containing drawing materials and musical instruments and half waited in an empty room. After 10 minutes in the coping room T4 saliva sample was taken. T5, T6, and T7 saliva samples were taken at 10-minute intervals thereafter. All procedures were approved by the University of Denver and The Pennsylvania State University institutional review boards.
2.3. Measures
2.3.1. Salivary alpha-amylase and cortisol.
Physiologic functioning was assessed via repeated saliva samples. Participants were asked to refrain from brushing their teeth or consuming a large meal or dairy products within 60 minutes of their appointment and to refrain from sugary and acidic snacks within 20 minutes. Prior to the first saliva collection, participants rinsed their mouth with a small sip of water. Seven saliva samples were collected via passive drool through a straw directly into vials over the course of the 95-minute visit. Saliva samples were stored in a medical grade freezer and were transported on ice to the Behavioral Immunology and Endocrinology Lab at the University of Colorado.
Cortisol levels were determined using a commercial expanded-range high-sensitivity enzyme immunosorbent assay kit (No. 1-3002/1-3012; Salimetrics, LLC, State College, PA) that detects cortisol levels in the range of 0.003 to 3.0 μg/dL. All samples were run in duplicate. alpha-amylase levels were determined from the same saliva samples as were used to determine cortisol levels. Alpha-amylase levels were determined using a commercially available kinetic reaction assay kit (No. 1-1902; Salimetrics, LLC, State College, PA). The assay employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of alpha-amylase on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of alpha-amylase activity present in the sample is directly proportional to the increase (over a 2 minute period) in absorbance at 405 nm. Results are computed in U/mL of alpha-amylase using the formula: [Absorbance difference per minute × total assay volume (328 ml) × dilution factor (200)]/ [millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) × sample volume (.008 ml) × light path (.97)]. Samples were batched in the same order as random assignment.
2.3.2. Internalizing and externalizing symptoms.
The Behavior Assessment System for Children, Second Edition (BASC-2; Reynolds & Kamphaus, 2004), Parent Rating Scales (PRS) were used to assess children’s externalizing symptoms using the Externalizing broadband scale. Adolescents’ Self Report of Problems (SRP) assessed internalizing using the Internalizing broadband scale. Items were rated on a 4-point scale from 0 (Never) to 4 (Always). Cronbach’s α = .92 for both Internalizing and Externalizing. Analyses and plots of interactions use raw scores, but the mean t-scores are presented in Table 2 for ease of interpretation.
Table 2.
Correlations among covariates, alpha-amylase and cortisol reactivity and recovery, and internalizing and externalizing.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Life Stress | -- | |||||||||
| 2. Puberty | .03 | -- | ||||||||
| 3. Sex | .05 | .31** | -- | |||||||
| 4. Medication | −.05 | −.03 | .01 | -- | ||||||
| 5. Cortisol Reactivity | .17 | .11 | .08 | −.11 | -- | |||||
| 6. Alpha Reactivity | −.05 | .13 | .27** | −.02 | .38** | -- | ||||
| 7. Cortisol Recovery | .08 | .06 | .01 | −.14 | .73** | .15 | -- | |||
| 8. Alpha Recovery | −.07 | .05 | .07 | .02 | .01 | .70** | .03 | -- | ||
| 9. Internalizing | .23** | −.06 | −.01 | .01 | .11 | .16† | .17* | .15 | -- | |
| 10. Externalizing | .27** | −.07 | .03 | −.01 | .20* | .03 | −.03 | −.10 | .19* | -- |
| Mean | 4.89 | 1.66 | 0.32 | 0.11 | 67.88 | 0.07 | 49.31 | 45.56 | 51.78 | |
| SD | 3.28 | 0.54 | 1.17 | 0.16 | 74.44 | 0.07 | 59.08 | 7.14 | 9.60 | |
= p < .10,
= p < .05,
= p < .01
Note: Medication = Sum of cortisol affecting medications, Internalizing and externalizing mean and SD are t-scores.
2.4. Covariates
Child sex (0=boy, 1=girl), pubertal status, stressful life events, time of T1 saliva, peak cortisol timepoint, and cortisol-relevant medications were included as covariates. Perceived pubertal status was measured via the parent-report Physical Development Scale (PDS), which estimates adolescents’ Tanner staging (Petersen, Crockett, Richards, & Boxer, 1988). For females, parents indicated whether menstruation had begun and rated the extent of growth spurt, breast growth and body hair growth. For males, parents rated the extent of voice deepening, growth spurt, facial and body hair growth. All items were rated on either a 4- or 5-point Likert-type scale, aside from the menstruation item, which was scored dichotomously (no = 0; yes = 4). An average puberty score was computed from the four items for each child. Parents were asked to indicate all medications that the child takes regularly or frequently, their purpose, schedule, and dosing. All medications were classified according to guidelines established by Granger and colleagues as to whether they could affect cortisol levels (Granger, Hibel, Fortunato, & Kapelewski, 2009). A score was calculated for each participant which summed the number of cortisol-relevant medications that they take regularly. Stressful life events were measured using the parent report of a modified version of the Child and Adolescent Survey of Experiences: Parent Version (CASE-P; Allen & Rapee, 2009). The CASE-P is a 38-item checklist of life events that may have occurred over the last 12 months in the following domains: changes in household, illnesses, separation from family, moves, marital events, experiencing or witnessing traumatic events, family and peer interactions, and school achievement. Items assessing negative life events (n = 31) were selected.
2.5. Data Analytic Plan
2.5.1. Data reduction and preprocessing.
Participants missing a majority (e.g., > 3) of their alpha-amylase and cortisol data points (n = 3) were excluded from the present analyses. Extreme alpha-amylase and cortisol values (+/− 3 SD) were winsorized to 3 SD. The resulting raw alpha-amylase and cortisol values remained positively skewed and were corrected via a natural log (ln) transformation. MANOVA post-hoc tests of geographic location (western US, northeastern US) indicated locations differed only on covariate variables to be controlled for in all subsequent analyses. Coping room was examined as a possible covariate, but as it did not contribute to prediction in any analyses it was dropped from analyses to help preserve power. Due to cortisol’s customary time lagged appearance in saliva, SAM and HPA reactivity and recovery scores were calculated differently. SAM reactivity was calculated by subtracting T1 alpha-amylase level (ln) from T3 level (peak level for 61% of participants). SAM recovery was calculated by subtracting T7 from T3 alpha-amylase. Given typical heterogeneity in cortisol peak timing, we followed Miller and colleagues’ (2018) recommended approach, using each individual’s highest post-TSST-M cortisol level [T3 (10%), T4 (46%), T5 (25%)] to index peak. HPA reactivity scores were calculated by subtracting each individual’s lowest pre-TSST-M cortisol value from their peak value. Recovery scores were calculated by subtracting the lowest post-TSST-M value from the peak value.
2.5.2. Missing data.
The total percentage of missing values for key demographic and study variables was 3.6%. Little’s Missing Completely at Random (MCAR) test was non-significant (X2 (153) = 128.83, p = .92) indicating that the data could be MCAR.
2.5.3. Linear Multiple Regression Analyses.
Regression analyses were run using the Process macro in SPSS (Hayes, 2018). Regression models included sex, pubertal maturation level, use of potentially cortisol-affecting medications, time of day of T1 saliva sample, and life stress as covariates, as well as alpha-amylase reactivity and recovery scores, cortisol reactivity and recovery scores, and the interactions between reactivity alpha-amylase and cortisol and between recovery alpha-amylase and cortisol. Two initial models were run—one for internalizing and one for externalizing. In both models, the interaction of reactivity-phase alpha-amylase and cortisol tested reactivity-phase co-activation, while accounting for main effects of alpha-amylase and cortisol reactivity and recovery as well as recovery phase interaction of alpha-amylase and cortisol. Though exploratory, the interaction of recovery-phase alpha-amylase and cortisol tested recovery-phase activation accounting for the reactivity interaction, main effects, and covariates. Conditional effects of alpha-amylase (predictor) on internalizing and externalizing were plotted at the 16th, 50th, and 84th percentile values of cortisol (moderator), as recommended by Hayes (2018). Johnson-Neyman (J-N) plots were created to specify the region of significance for each interaction.
3. Results
3.1. Descriptive Analyses
Means, standard deviations, and correlations among study variables are presented in Table 2. Alpha-amylase and cortisol reactivity scores are significantly correlated, as are alpha-amylase reactivity and recovery and cortisol reactivity and recovery. Recovery phase alpha-amylase and cortisol scores were not significantly correlated with each other. Parent-reported stress was significantly associated with both internalizing and externalizing problems. Sex and alpha-amylase reactivity were correlated, reflecting higher levels for girls. Finally, externalizing problems were positively correlated with cortisol reactivity scores. In terms of clinical risk, 38% of the sample had at-risk (T > 60) or clinically significant (T > 70) levels of BASC internalizing (parent or youth report) and/or externalizing problems (parent-report)—13% were at-risk or higher on both Internalizing and Externalizing.
3.2. Multiple Linear Regression Analyses
Due to the high correlations between alpha amylase reactivity and recovery scores and between cortisol reactivity and recovery scores, we examined variance inflation factors to detect possible problems with multicollinearity. Results indicated that removal of the non-significant alpha-amylase recovery score would reduce multicollinearity in the Internalizing model and that removal of the non-significant cortisol reactivity would reduce multicollinearity in the Externalizing model. Those scores were therefore removed from the model, as well as the respective non-significant interaction terms containing them. Reactivity and recovery alpha-amylase and cortisol scores were mean-centered prior to calculating interaction terms. Table 3 contains the results of the regression analyses for Internalizing and Externalizing. Table 4 contains the conditional effects of alpha-amylase reactivity on internalizing and alpha-amylase recovery on externalizing problems at low, medium, and high levels of cortisol reactivity and recovery respectively.
Table 3.
Multiple Linear Regressions Predicting Child-reported Internalizing and Parented-reported Externalizing Problems
| Internalizing Problems |
Externalizing Problems |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | se | t | p | Lower CI | Upper CI | B | Se | t | p | Lower CI | Upper CI | |
| Constant | 329.28 | 28.99 | 11.36 | .000 | 271.48 | 387.07 | 128.45 | 17.52 | 7.32 | .000 | 93.45 | 163.45 |
| Alpha Reactivity | 0.13 | 0.05 | 2.22 | .029 | 0.01 | 0.23 | 0.23 | 0.05 | 5.12 | .000 | 0.14 | 0.32 |
| Cortisol Reactivity | 189.71 | 62.91 | 3.02 | .003 | 64.29 | 315.11 | -- | -- | -- | -- | -- | -- |
| A X C Reactivity | −0.43 | 0.13 | −3.24 | .002 | −0.69 | −0.16 | -- | -- | -- | -- | -- | -- |
| Alpha Recovery | -- | -- | -- | -- | -- | -- | −0.12 | 0.05 | −2.46 | .016 | −0.22 | −0.02 |
| Cortisol Recovery | −230.18 | 87.57 | −2.63 | .010 | −404.76 | −55.61 | −128.29 | 37.99 | −3.37 | .001 | −204.18 | −52.42 |
| A X C Recovery | -- | -- | -- | -- | -- | -- | 2.28 | 0.74 | 3.09 | .003 | 0.81 | 3.76 |
| Puberty | −5.05 | 6.92 | −0.73 | .468 | −18.85 | 8.75 | −7.82 | 4.61 | −1.69 | .095 | −17.04 | 1.39 |
| Medications | 2.41 | 3.05 | 0.79 | .431 | −3.67 | 8.50 | 0.11 | 1.97 | 0.06 | .955 | −3.83 | 4.06 |
| Sex | 0.46 | 6.07 | 0.07 | .940 | −11.66 | 12.57 | −8.44 | 4.19 | −2.01 | .048 | −16.82 | −0.07 |
| Life Stress | 1.33 | 0.54 | 2.48 | .015 | 0.26 | 2.41 | 2.39 | 0.39 | 6.11 | .000 | 1.61 | 3.17 |
| Cortisol Peak | −9.24 | 5.51 | −1.68 | .090 | −20.23 | 1.74 | −0.40 | 3.47 | −0.12 | .908 | −7.33 | 6.52 |
| Time of Day | 0.27 | 3.37 | 0.08 | .935 | −6.44 | 6.98 | 4.11 | 2.26 | 1.82 | .073 | −0.39 | 8.63 |
Note. Alpha = Alpha-amylase. A X C = Alpha-amylase X Cortisol.
Table 4.
Conditional Effects of Alpha-amylase Reactivity on Internalizing and Alpha-amylase Recovery on Externalizing Problems at Low, Medium, and High Levels of Cortisol Reactivity and Recovery Respectively
| B | se | t | p | Lower CI | Upper CI | |
|---|---|---|---|---|---|---|
| Internalizing Problems | ||||||
| Low (−0.0902) | 0.161 | 0.059 | 2.71 | .008 | 0.043 | 0.279 |
| Medium (−.0456) | 0.142 | 0.057 | 2.49 | .015 | 0.028 | 0.256 |
| High (.0855) | 0.086 | 0.054 | 1.59 | .114 | −0.021 | 0.194 |
| Externalizing Problems | ||||||
| Low (−0.0517) | −0.237 | 0.061 | −3.86 | .000 | −0.360 | −0.115 |
| Medium (−.0238) | −0.174 | 0.051 | −3.38 | .001 | −0.277 | −0.071 |
| High (.0588) | 0.014 | 0.065 | 0.22 | .827 | −0.116 | 0.145 |
Note. Low = 16th percentile. Medium = 50th percentile. High = 84th percentile.
3.2.1. Internalizing problems.
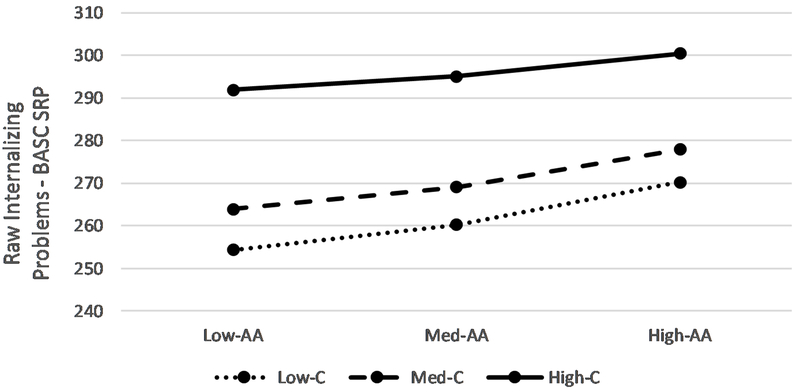
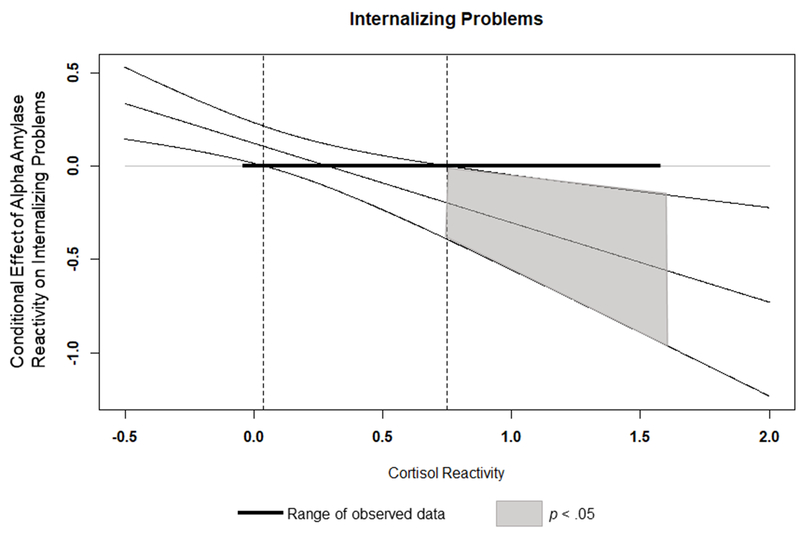
The overall model predicting internalizing problems was significant, F(10,72) = 2.13, p < .02; R2 = .228, MSE = 916.71. The test of the interaction between reactivity alpha-amylase and cortisol was also significant, F(1,72) = 10.48, p < .002; R2 change = .112. Parent-reported stressful events explained a significant portion of the variability in youth-reported internalizing. In addition, main effects of alpha-amylase and cortisol reactivity and cortisol recovery were found, such that more alpha-amylase and cortisol reactivity and less cortisol recovery were associated with higher levels of internalizing problems. These main effects were also qualified by the interaction between alpha-amylase and cortisol reactivity. As shown in Figure 1 and reported in Table 3, higher alpha-amylase reactivity is associated with more internalizing problems, and this effect is present primarily at higher levels of cortisol reactivity as indicated by the regions of significance shown in the J-N plot (≥ 0.75 standard deviations above the mean). Hence, we find support for risk for internalizing stemming from symmetric SAM-HPA reactivity co-activation.
Figure 1.
Interaction between Alpha Amylase Reactivity and Cortisol Reactivity on Internalizing Problems. Dashed vertical lines indicate bounds of significance. Outer diagnonal lines represent 95% confidence intervals. AA=Alpha-amylase. C = cortisol.
3.2.2. Externalizing problems.
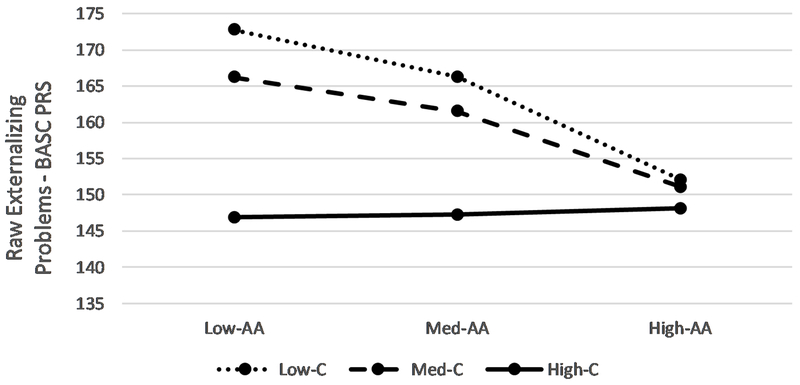
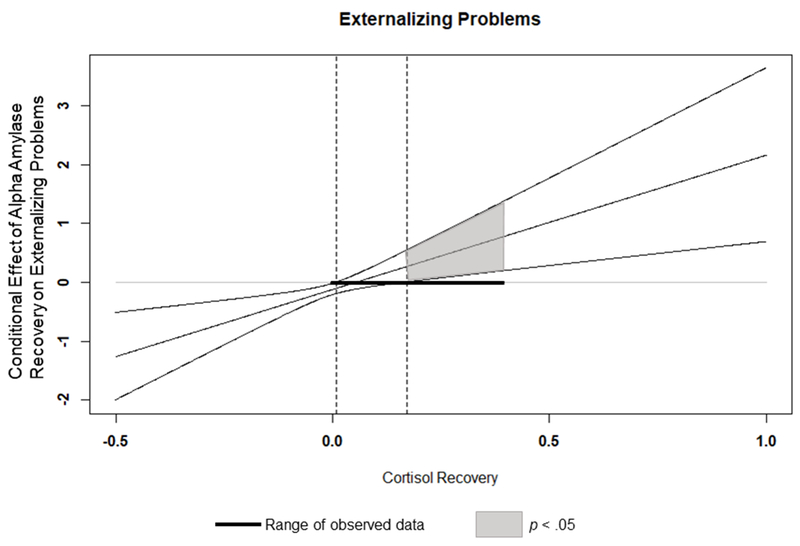
The overall model predicting externalizing problems was significant, F(10,65) = 6.49, p < .000; R2 = .496, MSE = 385.78. The test of the interaction between recovery alpha-amylase and cortisol was also significant, F(1,65) = 9.56, p < .003; R2 change = .074. High levels of life stress predicted more externalizing, as did being a boy. Main effects were evident for alpha-amylase reactivity and both alpha amylase and cortisol recovery, such that larger alpha-amylase reactivity and smaller cortisol and alpha amylase recovery scores were associated with higher levels of symptoms. These main effects were, however, qualified by a significant interaction between recovery alpha-amylase and cortisol. As shown in Figure 2 and reported in Table 3, the symmetric combination of low cortisol and alpha-amylase recovery was associated with increased externalizing problems, though as shown in the J-N plot, this effect is restricted to low-to-moderate levels of cortisol recovery (0.17 – 0.5 SD above the mean). This shows the risk associated with symmetric combination of low-moderate alpha-amylase and low-moderate cortisol for externalizing problems.
Figure 2.
Interaction between Alpha Amylase Recovery and Cortisol Reactivity on Externalizing Problems. Dashed vertical lines indicate bounds of significance. Outer diagnonal lines represent 95% confidence intervals. AA=Alpha-amylase. C = cortisol.
4. Discussion
Considering early adolescence as a period in which rates of emotional and behavioral problems begin to rise, this study examined how simultaneous modeling of changes in the SAM and HPA biomarkers alpha-amylase and cortisol in response to acute stress could help distinguish between risk for internalizing and externalizing problems. Partial support for the reactivity phase hypotheses was found and results of recovery phase analyses generally align with theoretical propositions that efficient recovery is beneficial. Higher levels of reactivity alpha-amylase and lower levels of recovery cortisol were associated with both internalizing and externalizing problems. In addition, interactions between alpha-amylase and cortisol pinpointed risk specific to stress response phase and type of emotional-behavioral problem. In particular, the combination of high levels of both alpha-amylase and cortisol during the reactivity phase was associated with higher internalizing and the combination of low levels of both alpha-amylase and cortisol during recovery was associated with more externalizing problems.
4.1. Internalizing
The effect of high cortisol reactivity on internalizing symptoms was clear and large—across all levels of alpha amylase, high cortisol was associated with the highest levels of symptoms. Our findings also suggest that reactivity alpha-amylase adds valuable predictive information on internalizing problems, especially at lower levels of cortisol, which is consistent with studies finding higher alpha-amylase reactivity is linked to anxiety and depression (Allwood et al., 2011; Afifi et al., 2011). Additionally, there was an inverse association between recovery cortisol and internalizing problems, similar to a pattern previously observed in adolescents (Nederhof et al., 2015). As for our hypothesis that asymmetric reactivity would characterize children with higher internalizing problems, our results did not support this proposition. Rather, high alpha-amylase reactivity and high cortisol reactivity both predicted higher levels of internalizing problems. We based our hypothesis on three existing studies that have shown evidence of asymmetric SAM-HPA co-activation predicting internalizing problems. Two studies have shown that low basal alpha-amylase and high basal cortisol characterize children with more internalizing problems (Bae et al., 2015, Chen et al., 2015), while a third found that low reactivity alpha-amylase and high reactivity cortisol together were linked to more internalizing problems (Allwood et al., 2011). Our findings are most consistent with those of El-Sheikh and colleagues (2008), who found that high basal alpha-amylase enhances the risk provided by high basal cortisol, despite our different measures of activation (basal vs reactivity).
It is not entirely clear why our findings align with the latter study, though both of these samples had much smaller age ranges than either the Allwood or Bae study. This area of inquiry is presently too sparse to examine developmental patterns in risk from symmetry of co-activation but given the powerful influence of the pubertal transition on stress physiology and behavior, there may be substantial differences between 7-year-olds and 16-year-olds. Additionally, our sample and that of El-Sheikh were among the youngest of those found in Table 1, and both may therefore capture peri-pubertal co-activation risk that is inherently different from post-pubertal risk. Alternatively, in one of the few studies to measure both basal and reactivity SAM-HPA co-activation, Reeves, Fisher, Newman, and Granger (2016) found that baseline asymmetry and post-stressor (reactivity) symmetry characterized the adults with Generalized Anxiety Disorder in their sample. Hence, our symmetric reactivity-phase co-activation risk for internalizing aligns with Reeves and colleagues’ anxiety-related reactivity pattern.
It is interesting that internalizing problems were linked to symmetric SAM-HPA co-activation in the reactivity phase only, begging the question of why the reactivity but not recovery phase mattered for co-activation effects on internalizing problems? It is possible that this reflects anticipatory emotional reactivity in the face of uncertainty, a risk process associated specifically with the development of anxiety disorders (Grupe & Nitschke, 2013). This finding highlights the need for revised theoretical work on SAM-HPA co-activation that integrates empirical findings and provides testable hypotheses about the role of reactivity and recovery phases, particularly with respect to different forms of developmental psychopathology. Additionally, while significant reactivity co-activation was evident in our sample, cortisol during the recovery period also explained variability in children’s internalizing problems, which aligns with the limited research to date on recovery cortisol (Nederhof et al., 2015; Schoorl et al., 2017).
4.2. Externalizing
As with internalizing, recovery cortisol was inversely related to externalizing problems (Nederhof et al., 2015; Schoorl et al., 2017), in further support of the utility of cortisol activity during the recovery period. Partially consistent with hypothesis 2, low symmetric SAM-HPA co-activation was linked to greater externalizing problems, however, it was co-activation during the recovery phase rather than the predicted reactivity phase. These findings complement most existing studies involving children and adolescents, which show that under-activation in either the SAM or HPA is associated with conduct problems. Our findings go further to pinpoint that concurrent low activations in both SAM and HPA during recovery indexes particularly high risk for externalizing problems. These findings confirm and extend current theorizing regarding externalizing behavior problems, that an inability to mount an appropriate SNS response in the face of stress may lead to sensation seeking and insensitivity to environmental cues (Hawes, Brennan, & Dadds, 2009).
High levels of recovery activation in either the SAM or HPA appears to provide robust protection against externalizing problems, emphasizing the importance of the ability to self-regulate following stress, especially for externalizing. In contrast to internalizing symptoms which can constitute an immediate response to stress, externalizing problems generally occur in a time-delayed space following reactivity. Poor recovery from stress may leave a youth agitated and more prone to act out on the environment in negative ways.
4.3. Summary
These findings support Bauer and colleagues’ additive model (2002), which posits that optimal functioning of the SAM and HPA systems is achieved by complementary co-activation, and that risk for emotional-behavioral problems comes from symmetrically high (internalizing) or low (externalizing) co-activation. This study extends our understanding of such risks by separately examining the reactivity and recovery phases of the stress response. Both the main and interaction effects are informative here. It is interesting that co-activation interactions showed differential phase specificity to the two types of emotion-behavioral problems studied.
In fact, across the board, and likely reflecting the different functions of the reactivity and recovery phases, hormone levels had opposite effects in the reactivity versus recovery phases. Thus, analytic approaches that average across the phases could cancel out phase-specific hormone effects entirely and likely mask potentially important information. In addition, a single measurement of alpha-amylase or cortisol, especially at a random timepoint is not likely to unearth such nuanced effects. At the very least, resting levels of hormones should not be labelled as reactivity, as it is clear that resting and post-stress (peak reactivity) levels are not the same (e.g., Reeves et al., 2016).
Reactivity reflects the readiness to act and respond in the face of a potentially life-threatening challenge. Therefore, activation of the stress response is necessary and appropriate during this phase, but excessive activation characterized by very high levels of both SAM and HPA hormones appears to reflect an imbalanced response. Recovery reflects the ability to return to healthy homeostasis once it is safe to do so—timely down-regulation is therefore essential and very low levels of recovery hormones also appear to index an imbalanced response. Both scenarios may leave the individual vulnerable to experiencing negative sequelae.
Both the main and interaction effects support the importance of cortisol activation in particular during recovery. As noted by Villada, Hidalgo, Almela and Salvador (2016), in the context of recovering from acute stress, cortisol readies the individual to cope with stress, and is thereby beneficial. This recovery period is a time when coping can be enacted, for example and therefore HPA activation should be beneficial. The explicit separation of the phases to precisely capture changes over time enhanced our ability to detect these differences. It seems therefore critical that research in this area of inquiry calculate hormone changes separately for the two phases of acute stress response. This may help advance understanding of adaptive and maladaptive recovery profiles that can protect against or accentuate damage done by stress during childhood and should help dispel common misconceptions about cortisol as a harmful hormone. Why the reactivity phase is particularly relevant for internalizing and the recovery phase for externalizing remains an open question at this juncture.
4.4. Limitations and Future Directions
In addition to several conceptual and methodological advances, the present study has some limitations that point to areas for future research. First, the question of how high (or low) is too high (or low) remains largely unanswered. However, the non-significant conditional effects at the 84th percentile of cortisol in both models show that the low and medium cortisol levels are essentially indistinguishable from each other and that both are distinguished from the highest levels of cortisol in terms of conferring risk or protection for internalizing and externalizing in their respective phases. Importantly, alpha-amylase activations exacerbate extreme cortisol activations. Still, the absence of a clear threshold limits clinical applicability of findings such as these currently. The young adolescents in this study had higher than average levels of internalizing and externalizing symptoms, with close to 40% scoring as at-risk or clinically significant on internalizing, externalizing or both. This sample thereby had sufficient variability in symptoms to detect meaningful patterns. However, replicating these findings in a sample containing adolescents with clinical diagnoses would be needed to move forward with identifying thresholds, which would be of great clinical utility.
Second, sample size limitations precluded examination of patterns by important study covariates such as sex and pubertal status. Given the sex differences that emerge in rates of psychopathology (Hankin, Mermelstein, & Roesch, 2007) and in HPA functioning (Gunnar, Talge, & Herrera, 2009) during adolescence for example, it will be important to determine how patterns of adaptation to stress may converge or differ across sex (Susman et al., 2010). It is also a limitation that the data analyzed here were cross-sectional, though findings from Koss and colleagues (2014), for example show substantial temporal consistency across a 5-year timespan. Finally, we followed the recommended practice of using parent report of externalizing problems and youth report of internalizing problems (Aebi et al., 2017). While this practice ensures that the best estimate of a youth’s problem behaviors is captured, it does preclude inclusion of additional behaviors that may be observed by different reporters in different contexts.
In sum, this study adds to and expands upon a small group of studies of SAM-HPA co-activation in childhood. Our findings converge with the vast majority of studies of externalizing problems and find that concurrent low activation of SAM and HPA indexes risk for externalizing problems. We extend the extant literature and specify that this effect seems located primarily during the recovery phase of the stress response. Our findings regarding internalizing align primarily with a study by El-Sheikh and colleagues and await replication in additional research measuring separate reactivity and recovery effects. A major strength of this study is that recovery phase effects were examined while accounting for reactivity phase effects and vice versa, and hence these findings do suggest strongly that the reactivity phase is especially critical for understanding internalizing and that the recovery phase is critical for understanding externalizing. The field will benefit from further studies examining SAM-HPA co-activation, separating out and examining both phases of the stress response, and especially studies which (1) use best practices for measuring acute alpha-amylase and cortisol activations, (2) use either small sample age ranges to zero in on a particular developmental period or include sufficiently large sample sizes in order to examine different developmental periods within a sample, and (3) include critical covariates known to affect cortisol levels, including, for example, pubertal maturity, stress, and cortisol-relevant medication use. Better understanding of SAM-HPA mechanisms underlying risk for internalizing and externalizing problems may provide critical information regarding targets for biologically potent preventive and treatment interventions during this stress sensitive developmental period.
Acknowledgments
This study was funded by grant 1R21 HD078753 from the National Institute of Child Health and Human Development awarded to the first author.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Aebi M, Kuhn C, Banaschewski T, Grimmer Y, Poustka L, Steinhausen HC, & Goodman R (2017). The contribution of parent and youth information to identify mental health disorders or problems in adolescents. Child and adolescent psychiatry and mental health, 11, 23. doi: 10.1186/s13034-017-0160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi TD, Granger DA, Denes A, Joseph A, & Aldeis D (2011). Parents’ communication skills and adolescents’ salivary α-amylase and cortisol response patterns. Communication Monographs, 78(3), 273–295. [Google Scholar]
- Allen JL, & Rapee RM (2009). Are reported differences in life events for anxious children and controls due to comorbid disorders? Journal of Anxiety Disorders, 23(4), 511–518. [DOI] [PubMed] [Google Scholar]
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, & Stroud LR (2011). Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology, 88(1), 57–64. 10.1016/j.biopsycho.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YJ, Stadelmann S, Klein AM, Jaeger S, Hiemisch A, Kiess W, … Döhnert M (2015). The hyporeactivity of salivary cortisol at stress test (TSST-C) in children with internalizing or externalizing disorders is contrastively associated with α-amylase. Journal of Psychiatric Research, 71,78–88. 10.1016/j.jpsychires.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, & Boyce WT (2002). Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics, 23(2), 102–113. 10.1097/00004703-200204000-00007 [DOI] [PubMed] [Google Scholar]
- Chaudoir SR, Vergara-Lopez C, & Stroud LR (2017). Links between rejection sensitivity and biobehavioral response to laboratory stress in youth. Personality and Individual Differences, 114, 86–91. 10.1016/j.paid.2017.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FR, Raine A, Glenn AL, & Granger DA (2016). Hypothalamic pituitary adrenal activity and autonomic nervous system arousal predict developmental trajectories of children’s comorbid behavior problems. Developmental Psychobiology, 58(3), 393–405. 10.1002/dev.21379 [DOI] [PubMed] [Google Scholar]
- Chen FR, Raine A, & Granger DA (2015). Tactics for modeling multiple salivary analyte data in relation to behavior problems: Additive, ratio, and interaction effects. Psychoneuroendocrinology, 51, 188–200. 10.1016/j.psyneuen.2014.09.027 [DOI] [PubMed] [Google Scholar]
- Chen FR, Raine A, Rudo-Hutt A, Glenn AL, Soyfer L, & Granger DA (2015). Harsh discipline and behavior problems: The moderating effects of cortisol and alpha-amylase. Biological Psychology, 104, 19–27. 10.1016/j.biopsycho.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Childs KK, Sullivan CJ, & Gulledge LM (2010). Delinquent behavior across adolescence: Investigating the shifting salience of key criminological predictors. Deviant Behavior, 32(1), 64–100. [Google Scholar]
- Cicchetti D, & Rogosch FA (2002). A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology, 70(1), 6–20. 10.1037//0022-006X.70.1.6 [DOI] [PubMed] [Google Scholar]
- de Vries-Bouw M, Jansen L, Vermeiren R, Doreleijers T, van de Ven P, & Popma A (2012). Concurrent attenuated reactivity of alpha-amylase and cortisol is related to disruptive behavior in male adolescents. Hormones and Behavior, 62(1), 77–85. 10.1016/j.yhbeh.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, & Mize J (2008). Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology, 36(4), 601–611. 10.1007/s10802-007-9204-6 [DOI] [PubMed] [Google Scholar]
- Feldman R, Vengrober A, Eidelman-Rothman M, & Zagoory-Sharon O (2013). Stress reactivity in war-exposed young children with and without posttraumatic stress disorder: Relations to maternal stress hormones, parenting, and child emotionality and regulation. Development and Psychopathology, 25(4), 943–955. 10.1017/S0954579413000291 [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, & Trickett PK (2006). Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31(8), 976–987. 10.1016/j.psyneuen.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, & Trickett PK (2008). Salivary alpha amylase–cortisol asymmetry in maltreated youth. Hormones and Behavior, 53(1), 96–103. 10.1016/j.yhbeh.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953–967. 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, & Roesch L (2007). Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Development, 78(1), 279–295. 10.1111/j.1467-8624.2007.00997.x [DOI] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, & Ormel J (2013). Self- or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: A systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biological Psychology, 94(1), 175–184. 10.1016/j.biopsycho.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, … Zahn-Waxler C (2011). Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology, 23(4), 1149–1165. 10.1017/S0954579411000538 [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, & Dadds MR (2009). Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Current Opinion in Psychiatry, 22(4), 357–362. 10.1097/YCO.0b013e32832bfa6d [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to Mediation, Moderation, and Conditional Process Analysis Second Edition: A Regression Approach. New York: Guilford Press. [Google Scholar]
- Hermans EJ, Henckens MJAG, Joëls M, & Fernández G (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304–314. 10.1016/j.tins.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Joos CM, Wodzinski AM, Wadsworth ME, & Dorn LD (2018). Neither antecedent nor consequence: Developmental integration of chronic stress, pubertal timing, and conditionally adapted stress response. Developmental Review, 48, 1–23. 10.1016/j.dr.2018.05.001 [DOI] [Google Scholar]
- Koss KJ, George MRW, Cummings EM, Davies PT, El-Sheikh M, & Cicchetti D (2014). Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology, 56(4), 836–849. 10.1002/dev.21156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher DA, Powers SI, & Granger DA (2012). The relationship between cortisol, salivary alpha-amylase, and cognitive bias in young women. Behavioral Neuroscience, 126(1), 157–166. 10.1037/a0026654 [DOI] [PubMed] [Google Scholar]
- Kryski KR, Smith HJ, Sheikh HI, Singh SM, & Hayden EP (2013). HPA axis reactivity in early childhood: Associations with symptoms and moderation by sex. Psychoneuroendocrinology, 38(10), 2327–2336. doi: 10.1016/j.psyneuen.2013.05.002 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews, 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- Miller R, Wojtyniak J, Weckesser LJ, Alexander NC, Engert V, & Lehr T (2018). How to disentangle psychobiological stress reactivity and recovery: A comparison of model-based and non-compartmental analyses of cortisol concentrations. Psychoneuroendocrinology, 90, 194–210. doi: 10.1016/j.psyneuen.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Nederhof E, van Oort FVA Bouma EMC, Laceulle OM, Oldehinkel AJ, & Ormel J (2015). Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychological Medicine, 45(11), 2403–2412. 10.1017/S0033291715000392 [DOI] [PubMed] [Google Scholar]
- Niermann HCM, Figner B, Tyborowska A, van Peer JM, Cillessen AHN, & Roelofs K (2017). Defensive freezing links hypothalamic-pituitary-adrenal-axis activity and internalizing symptoms in humans. Psychoneuroendocrinology, 82, 83–90. 10.1016/j.psyneuen.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Northover C, Thapar A, Langley K, Fairchild G, & van Goozen Stephanie H. M. (2016). Cortisol levels at baseline and under stress in adolescent males with attention-deficit hyperactivity disorder, with or without comorbid conduct disorder. Psychiatry Research, 242, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Platje E, Jansen LMC, Vermeiren RRJM, Doreleijers TAH, van Lier Pol A. C., Koot HM, … Popma A (2017). Adolescent antisocial behavior explained by combining stress-related parameters. Journal of Psychophysiology, 31(3), 107–115. 10.1027/0269-8803/a000173 [DOI] [Google Scholar]
- Reeves JW, Fisher AJ, Newman MG, & Granger DA (2016). Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology, 53(6), 951–957. 10.1111/psyp.12634 [DOI] [PubMed] [Google Scholar]
- Reynolds C, & Kamphaus R (2004). BASC-2: Behavior assessment system for children. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Dackis MN, & Cicchetti D (2011). Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology, 23(4), 1107–1124. 10.1017/S0954579411000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD (2010). Adolescence: A central event in shaping stress reactivity. Developmental Psychobiology, 52(3), 244–253. https://search.proquest.com/docview/622188805?accountid=13158 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, & Granger DA (2011). Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology, 214(1), 209–219. 10.1007/s00213-010-1879-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, & Essex MJ (2015). Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology, 57(6), 688–704. 10.1002/dev.21138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk RH, Petersen JL, Abramson LY, & Hyde JS (2016). The contemporary face of gender differences and similarities in depression throughout adolescence: Development and chronicity. Journal of affective disorders, 205, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoorl J, van Rijn S de Wied M van Goozen SHM, & Swaab H (2017). Neurobiological stress responses predict aggression in boys with oppositional defiant disorder/conduct disorder: a 1-year follow-up intervention study. European Child & Adolescent Psychiatry, 26(7), 805–813. 10.1007/s00787-017-0950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, & Dorn LD (2010). Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology, 35(4), 557–569. 10.1016/j.psyneuen.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villada C, Hidalgo V, Almela M, & Salvador A (2016). Individual differences in the psychobiological response to psychosocial stress (trier social stress test): The relevance of trait anxiety and coping styles. Stress and Health: Journal of the International Society for the Investigation of Stress, 32(2), 90–99. 10.1002/smi.2582 [DOI] [PubMed] [Google Scholar]
- Wadsworth ME (2015). Development of maladaptive coping: A functional adaptation to chronic, uncontrollable stress. Child Development Perspectives, 9(2), 96–100. 10.1111/cdep.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, Bendezú JJ, Loughlin-Presnal J, Ahlkvist JA, Tilghman-Osborne E, Bianco H, … Hurwich-Reiss E (2018). Unlocking the black box: A multilevel analysis of preadolescent children’s coping. Journal of Clinical Child and Adolescent Psychology, 47(4), 527–541. 10.1080/15374416.2016.1141356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, & Hayakawa CM (2010). Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology, 35(2), 241–248. 10.1016/j.psyneuen.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Zoccola PM, & Dickerson SS (2012). Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research, 73(1), 1–9. 10.1016/j.jpsychores.2012.03.007 [DOI] [PubMed] [Google Scholar]