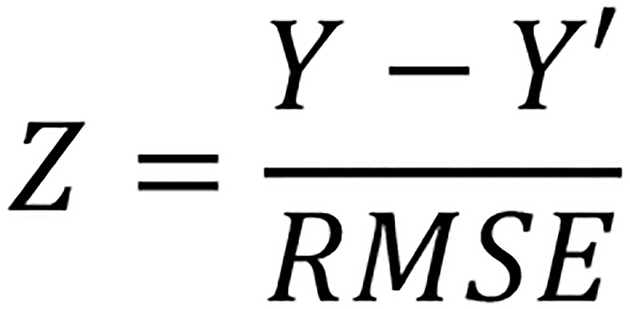Abstract
Objective:
Although researchers have documented the influence of cultural factors on neuropsychological test performance, few studies have examined the distribution of test scores among neurologically healthy older adults from different ethnic groups. The objective of this study was to determine if there are group differences in neuropsychological test score distributions with ethnicity specific norms for non-Hispanic White and Black / African American older adults.
Method:
Participants from the National Alzheimer’s Coordinating Center were selected if they were not diagnosed with dementia within 5 years (mean/SD: age = 75.26/6.98; education = 15.70/2.91). Groups were formed based on self-identified ethnicity of White (n= 5311) or Black/African American (n=1098). All participants completed neuropsychological testing including: Mini Mental State Exam, Logical Memory Immediate and Delayed, Digit Span Forward and Backward, Trail Making Test A & B, Animal Naming, Vegetable Naming, Digit Symbol, and Boston Naming Test.
Results:
Based on combined ethnicity norms, the scores of Black participants were overrepresented in the below average and low-average clinical ranges and the scores of White participants were overrepresented in the high-average and superior clinical ranges for all 11 neuropsychological measures. When group specific norms were used, the unbalanced pattern of score categorization was no longer present for any of the neuropsychological measures.
Conclusions:
These findings emphasize the importance of developing and using ethnically and culturally appropriate neuropsychological test norms, as well as the risk of interpreting some Black individual’s scores as below average when they likely are not.
Keywords: older adults, ethnicity, race, normal neuropsychological performance
Ethnic group differences in neuropsychological test performance have long been documented in research literature. For example, Campbell et al. (2002) researched the false positive rate among neurologically healthy African American adults who scored below normal performance cut offs according to published manuals. False positive rates were 21 to 46 percent higher among the African American participants than the normative population. Similarly, to evaluate the need for ethnicity corrections in their normative equation, two studies calculated false positive error rates for impairment on neuropsychological measures (Gladsjo et al., 1999; Norman et al., 2011). In both studies, false positive error rates were essentially reduced by half on all measures when applying ethnicity corrections. In a clinical group, White patients scored significantly higher than Black patients on many neuropsychological measures, even though the groups had similar diagnoses and age and education were controlled for (Boone, Victor, Wen, Razani, and Ponton, 2007). Differences in both clinical and healthy populations highlight the importance of cultural influences on neuropsychological test performance.
In a series of studies with older adults entitled Mayo’s Older African Americans Normative Studies (MOAANS), Lucas and colleagues (Lucas et al., 2005a, 2005b, 2005c, 2005; Pedraza et al., 2005; Rilling et al., 2005) developed neuropsychological norms for African Americans based on performance of about 300 cognitively normal older adults who lived near Jacksonville, Florida. While MOAANS is one of the largest normative sets for older African Americans, the sample was limited to adults who were primarily educated in southern segregated schools, which could greatly impact the generalization of their findings to African Americans outside that geographic region. One aim of MOAANS was to determine via factor structure if their assessment battery measured the same constructs in older African Americans and Whites (Pedraza et al., 2005). They found that a five-factor model including verbal comprehension, perceptual organization, attention/concentration, learning and retention was “nearly identical” to a model previously established with White participants. Two other groups similarly found that the factor structure for cognitive measures in African American older adults was similar to 5 factor theories that have been validated in studies using samples of predominately White participants (Whitfield, Allaire, Gamaldo, & Bichsel, 2010, Barnes et al. 2016).
Although neuropsychological tests measure the same cognitive constructs in different ethnic groups, ethnic based norms lead to more accurate clinical classification. African American participants in MOAANS scored two to four scale score points lower on the Mattis Dementia Rating Scale-2 when using standard norms compared to MOAANS (Rilling et al., 2005). When these lower scores were corrected with ethnicity adjusted norms, participants often crossed the threshold from an impaired classification to within normal limits (Rilling et al., 2005). Pedraza et al. (2009) found that White participants correctly named all but one item on the Boston Naming Test more frequently than Black participants. Pedraza et al. (2012) found that White cognitively normal research participants scored statistically significantly higher than Black participants on the MMSE despite adjusting for age and education level. However, when adjusting for age and quality of education as measured by Wide Range Achievement Test-3 (WRAT-3), the difference in scores was no longer statistically significant.
Many studies have replicated these results in other geographic regions of the United States and across additional cognitive domains (Manly et al., 1998; Manly et al., 2002; Mehta et al., 2004; Morgan, Marsiske, & Whitfield, 2008; Schwartz et al., 2004; Spering et al., 2012; Strickland, Longobardi, Alperson, & Andre, 2005). Each study found significantly lower scores among African American participants compared to White participants. Consistently, discrepancies persisted after controlling for socioeconomic variables such as education, occupation, and medical history. Accounting for quality of education, often measured with a standardized literacy measure such as WRAT-3, was best at attenuating discrepancies in neuropsychological performance across domains, but did not eliminate these differences.
Furthermore, theories of life course epidemiology have been suggested as explanations for ethnic differences in neuropsychological test performance. These theories explain that early life experiences, epigenetic influences such as childhood socioeconomic status, and social integration, are related to healthy cognitive aging and expression of neurodegenerative diseases (Glymour & Manly, 2008; Melrose et al., 2015). For example, hypertension is considered an expression of epigenetic influences that can impact cognition (Carvalho et al., 2014; Lezak et al., 2012; Mozaffarian et al., 2016; Schneider et al., 2015). There is an association between differential life experiences, such as socioeconomic status, and rates of hypertension beyond what is explained by genetic differences between ethnic groups (Manly & Echemendia, 2007). Rates of hypertension in African Americans are among the highest in the world, with a two to three fold higher risk for stroke compared to White Americans (Mozaffarian et al., 2016).
Manly et al. (1998) found that measures of acculturation were statistically related to lower scores on the Information subtest of Wechsler Adult Intelligence Scale-Revised (WAIS-R), Boston Naming Test, Trail Making Test B, and Story Memory Test. They concluded that lack of exposure to stimuli related to Information and Boston Naming Test likely explained lower performance among participants preferring African American culture because there were no differences in overall intellectual ability between those preferring African American culture more and those preferring it less. In another component of their study, Manly et al. (1998) matched Black and White participants on demographic variables and found that measures of acculturation accounted for ethnic group differences on Figure learning, WAIS-R Block Design, and Category Test, indicating that differences in performance on memory, construction and problem-solving tests are also culturally related.
In sum, although factor analysis of neuropsychological tests indicates they measure similar constructs in different ethnic groups, for adults of all ages in the United States, research consistently shows Black participants score lower than White participants on neuropsychological measures across cognitive domains. Health status and environmental variables such as education, literacy, and financial status explain some of the differences in scores, but differences often persist even when controlling for these variables. This is of particular importance for older African-Americans for whom use of culturally inappropriate norms increases the risk of misdiagnoses of mild cognitive impairment or dementia. Few studies have examined differences in ethnic group performance utilizing a nationwide sample of neurologically healthy older adults. The objectives of this study are to: 1) determine if there are ethnic group differences in the distribution of neuropsychological test scores across five clinical ranges for a sample of older healthy older adults from 33 sites across the United States; 2) determine if ethnicity specific norms alter the distribution of scores across clinical ranges; and 3) identify the percentage of scores that change clinical range based on the norms applied.
Method
National Alzheimer’s Coordinating Center (NACC)
The National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) consists of longitudinal data collected from 33 past and present Alzheimer’s disease Centers (ADC). The aim is to develop a uniform database across ADCs in order to characterize mild Alzheimer’s disease and Mild Cognitive Impairment (MCI) based on common clinical observations such as neuropsychological assessment, neurological exam, and assessment of activities of daily living. (Morris et al., 2006). Data for this study were collected from September 2005 through May 2015.
Sample
Participants were recruited from each of the 33 past and present ADCs utilizing various recruitment strategies, depending on the ADC. Participants for this study were 6409 volunteers from ADCs (gender: 36% male; mean/SD: age = 75.26/6.98; education = 15.70/2.91) who were not diagnosed with dementia within five years and had no history of stroke, Parkinson’s Disease, imaging evidence of cerebrovascular disease, Huntington’s Disease, or traumatic brain injury with enduring cognitive deficits. Diagnoses of neurocognitive and mood status were based on trained clinician diagnosis or consensus of two or more clinicians. Clinicians were researchers and practitioners in psychiatry, psychology, or neurology. Participants whose primary language was not English were excluded. Written informed consents were obtained from participants at each ADC and approved by Institutional Review Board (IRB) of each ADC. Research using the NACC database was approved by the University of Washington IRB.
Descriptive statistics for the sample are presented in Table 1. Participants were divided into two groups of self-identified race or ethnicity, non-Hispanic White and non-Hispanic Black / African American, as defined by the NACC database. For brevity, the groups will be referred to as “White” for the non-Hispanic White group and “Black” for the non-Hispanic Black / African American group. There were 5311 White participants (gender: 39.3% male; mean/SD: age = 75.50/7.10; education = 16.00/2.74), 1098 Black participants (gender: 20.5% male; mean/SD: age = 74.09/6.26; education = 14.24/3.24). The groups’ age and education scores were compared using t tests. Chi-square was used to compare gender. The two racial groups were statistically significantly different on all demographic factors. However, examination of the data in Table 1 indicates that, while statistically significant, the only demographic differences that are clinically significant is the percentage of males and possibly level of education.
Table 1.
Summary of Descriptive Statistics by Racial/Ethnic Group
| Total Sample n=6409 | White n=5311 | Black n=1098 | Effect sizea | |
|---|---|---|---|---|
| Sex (%male) | 36.0 | 39.3 | 20.5 | .000 |
| Age (Mean/SD) | 75.26/6.98 | 75.50/7.10 | 74.09/6.26 | .168*** |
| Education (Mean/SD) | 15.70/2.91 | 16.00/2.74 | 14.24/3.24 | .621*** |
Hedges’ g for continuous variables, Cramer’s V for categorical variables
p < .05.
p < .01.
p < .001.
Assessment Procedures
Data for analyses were collected on the initial visit at an ADC. Follow up visits confirmed no change in neuropsychological status over 5 years. A complete description of neuropsychological measures was published by Weintraub et al. (2009). Neuropsychological Measures included Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975), Boston Naming Test (BNT, 30 odd-numbered items), Digit Symbol of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1987), Trail Making Test-A & B (Reitan & Wolfson, 1993), Digit Span Forward and Digit Span Backward of the WMS-R (Wechsler, 1987), Animal Naming, Vegetable Naming, Logical Memory immediate (LM I), and delayed (LM II) of the WMS-R (Wechsler, 1987).
Data Analysis
All neuropsychological test scores were coded as z-scores based on UDS normative data which was established using normal control participants and accounting for age, gender, and education (Weintraub et al., 2009). The mean score for each group was compared using independent samples t-tests, presented in Table 2.
Table 2.
Average z-score Performance on Measures by Race/Ethnic Group with UDS Norms
| Measure | White n=5311 Mean/SD | Black n=1098 Mean/SD | Effect size (g) |
|---|---|---|---|
| MMSE | 0.43/1.11 | −0.13/1.52 | .470*** |
| BNT | −0.17/.84 | −1.36/1.65 | 1.161*** |
| DS | 0.45/1.01 | −0.26/1.02 | .702*** |
| TMT-A | 0.08/.90 | −0.97/1.60 | .997*** |
| TMT-B | −0.00/.90 | −1.12/1.55 | 1.076*** |
| DSFT | −0.03/.98 | −0.34/.98 | .316*** |
| DSFL | −0.04/.97 | −0.30/.98 | .268*** |
| DSBT | 0.14/1.01 | −0.37/.96 | .509*** |
| DSBL | 0.12/1.00 | −0.38/.97 | .503*** |
| Animals | 0.03/.98 | −0.60/.88 | .654*** |
| Vegetables | 0.91/1.21 | 0.85/1.05 | .051 |
| LM I | 0.28/1.09 | −0.14/1.00 | .391*** |
| LM II | 0.30/1.10 | −0.12/1.00 | .388*** |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
p<.001.
The first aim was to determine if the groups’ scores were distributed proportionally across clinical performance ranges: below average, low-average, average, high-average, and superior. Chi-square analyses were used to determine if the proportion of scores falling in performance ranges was dependent on ethnicity using UDS norms. Clinical ranges were defined by the following percentile score parameters: 0–9 = below average; 10–24 = low-average; 25–74 = average; 75–90 = high-average; 91–100 = superior.
The second aim of the study was to determine if ethnicity specific norms altered the distribution of scores across clinical ranges. Regression based normative equations were calculated for Black and White participants separately using age, gender, and education. All demographic variables were entered in one step for each group. Utilizing the same approach as Shirk et al. (2011) for the NACC UDS online normative calculator, z-score estimates were calculated by subtracting the predicted population mean (Y’) score from the individual raw score (Y) and dividing by the root square mean of the regression equation (RMSE) (Figure 1). Statistics for neuropsychological measures based on ethnicity specific norms are presented in Table 2. Finally, percentage of neuropsychological test scores that moved above or below the below average classification cutoff was calculated when using UDS combined ethnic norms compared to ethnic specific norms developed from participants in this study.
Figure 1.
Formula for z-score Estimates
Results
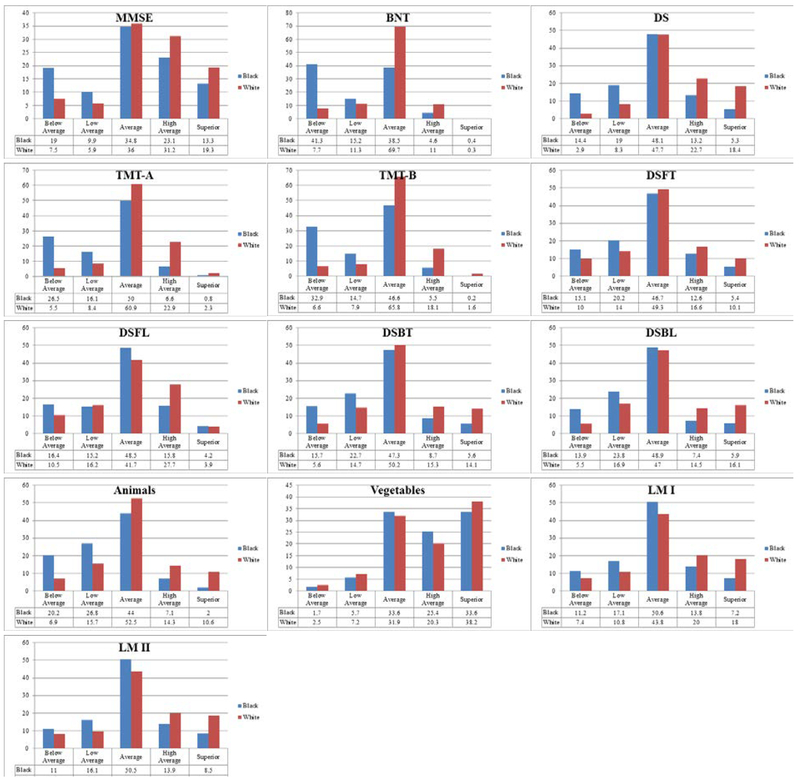
For the first aim, each analysis produced a significant χ2 value (χ2 (4) = 22.84–837.53, p<.001), indicating the proportion of scores across clinical performance ranges depended on ethnicity. Summary of Chi-square Statistics using UDS Norms are presented in Table 3 and histograms of proportion of scores across clinical performance ranges are presented in Figure 2. For all 11 neuropsychological measures, the Black participants’ scores were overrepresented in the below average and low-average categories and the White participants’ scores were overrepresented in the high-average and superior categories.
Table 3.
Summary of Performance Range Chi-Square Statistics Using UDS Norms
| Measure | X2 | Effect size (V) |
|---|---|---|
| MMSE | 181.97*** | .172 |
| BNT | 910.72*** | .387 |
| DS | 425.31*** | .278 |
| TMT-A | 620.37*** | .319 |
| TMT-B | 727.82*** | .346 |
| DSFT | 74 76*** | .111 |
| DSFL | 85 41*** | .118 |
| DSBT | 233.55*** | .196 |
| DSBL | 208.24*** | .185 |
| Animals | 325.32*** | .239 |
| Vegetables | 21 34*** | .059 |
| LM I | 135.08*** | .149 |
| LM II | 121.04*** | .141 |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
p < .05.
p < .01.
p < .001.
Figure 2.
Histograms of Performance Range Distributions for Each Ethnic Group Using UDS Norms
For the second aim of the study, a series of linear multiple regressions were conducted to establish regression based norms for each ethnic group separately and to determine the impact of age, gender, and education on neuropsychological measures in the two groups. Standardized regression coefficients are displayed in Table 4 and coefficients for regression based norms are presented in Tables 5 and 6. In both ethnic groups, age and education were statistically significant predictors on all measures; gender was a statistically significant predictor for Digit Symbol and measures of memory and language. Examination of regression equations reveals potential structural differences in the equations, indicating a difference in the relationship between the predictor variables and performance on neuropsychological measures in the two groups. For example, education was a stronger predictor and accounted for more variance on all measures among the Black participants compared to the White participants. Age was also a stronger predictor and accounted for more variance on measures of memory and language, with the exception of semantic fluency, for the Black participants. However, age was a stronger predictor and accounted for more variance on all attention and working memory measures except Digit Span Forward for the White participants. Among the White participants, age was a stronger predictor of performance on measures with a speeded component and education was a stronger predictor of performance on tasks without a speeded component. Education had the largest influence on performance across all measures for the Black participants, both with and without a speed component.
Table 4.
Summary of Regression Analyses
| Demographic Variable | |||||||
|---|---|---|---|---|---|---|---|
| Sex | Age | Education | |||||
| Measure | Group | β/p-value | % Variance | β/p-value | % Variance | β/p-value | % Variance |
| MMSE | Whites | .177/.000 | 2.3 | −.184/.000 | 4.6 | .212/.000 | 4.0 |
| Blacks | .143/.000 | 1.9 | −.207/.000 | 6.9 | .383/.000 | 17.0 | |
| BNT | Whites | −.071/.000 | 0.8 | −..259/.000 | 7.5 | .183/.000 | 4.9 |
| Blacks | −.066/.015 | 0.6 | −.257/.000 | 9.4 | .333/.000 | 13.8 | |
| DS | Whites | .138/.000 | 1.7 | −.373/.000 | 15.8 | .165/.000 | 3.3 |
| Blacks | .180/.000 | 2.7 | −.330/.000 | 14.5 | .380/.000 | 18.0 | |
| TMT-A | Whites | −.022/.100 | 0.1 | .348/.000 | 12.9 | −.098/.000 | 1.6 |
| Blacks | −.057/.043 | 0.3 | .258/.000 | 8.7 | −.250/.000 | 8.3 | |
| TMT-B | Whites | −.015/.258 | 0.0 | .347/.000 | 13.4 | −.195/.000 | 5.0 |
| Blacks | .002/.949 | 0.0 | .268/.000 | 9.8 | −.354/.000 | 15 | |
| DSFT | Whites | −.004/.751 | 0.0 | −.108/.000 | 1.5 | .134/. 000 | 2.1 |
| Blacks | −.001/.973 | 0.0 | −.149/.000 | 3.2 | .212/.000 | 5.5 | |
| DSFL | Whites | .002/. 890 | 0.0 | −.089/.000 | 1.0 | .122/.000 | 1.7 |
| Blacks | −.020/.497 | 0.1 | −.132/.000 | 2.6 | .206/.000 | 5.1 | |
| DSBT | Whites | .039/.006 | 0.0 | −.112/.000 | 1.8 | .188/.000 | 3.7 |
| Blacks | −.013/.659 | 0.0 | −.088/.003 | 1.6 | .258/.000 | 7.3 | |
| DSBL | Whites | .018/.199 | 0.0 | −.107/.000 | 1.6 | .178/. 000 | 3.5 |
| Blacks | −.033/.267 | 0.1 | −.074/.013 | 1.3 | .263/.000 | 7.5 | |
| Animals | Whites | .039/.004 | 0.0 | −.236/.000 | 6.8 | .226/.000 | 5.9 |
| Blacks | −.062/.026 | 0.5 | −.217/.000 | 6.9 | .308/.000 | 11.6 | |
| Vegetables | Whites | .365/.000 | 12.5 | −.224/.000 | 6.6 | .138/.000 | 1.0 |
| Blacks | .271/.000 | 7.0 | −.163/.000 | 3.7 | .233/.000 | 6.4 | |
| LM I | Whites | .197/.000 | 2.7 | −.087/.000 | 1.4 | .225/.000 | 4.0 |
| Blacks | .161/.000 | 2.4 | −.163/.000 | 3.9 | .251/.000 | 7.4 | |
| LM II | Whites | .197/.000 | 2.8 | −.108/.000 | 2.0 | .216/.000 | 3.7 |
| Blacks | .172/.000 | 2.8 | −.202/.000 | 5.6 | .255/.000 | 8.0 | |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
Table 5.
Regression Coefficients for White Participants and 95% Confidence Intervals
| Measure | Sex | Age | Education |
|---|---|---|---|
| MMSE | 0.49*** (0.42, 0.56) |
−0.04*** −0.04, −0.03) |
0.104** (0.09, 0.12) |
| BNT | −0.379*** (−0.52, −0.24) |
−0.10*** (−0.11, −0.08) |
0 17*** (0.15, 0.20) |
| DS | 3 17*** (2.58, 3.76) |
−0.58*** (−0.64 −0.56) |
0.67*** (0.57, 0.78) |
| TMT-A | −0.61 (−1.32, 0.12) |
0.67*** (0.62, 0.72) |
_0 477*** (−0.61, −0.35) |
| TMT-B | −1.32 (−3.60, 0.97) |
2.16*** (2.00, 2.32) |
−3 09*** (−3.49, −2.68) |
| DSFT | −0.02 (−0.13, 0.09) |
−0.03*** (−0.04, −0.02) |
0.10*** (0.08, 0.12) |
| DSFL | 0.00 (−0.06, 0.06) |
−0.01*** (−0.02, −0.01) |
0.05*** (0.04, 0.06) |
| DSBT | 0.17** (0.05, 0.29) |
−0.03*** (−0.04, −0.03) |
0 15*** (0.13, 0.17) |
| DSBL | 0.04 (−0.02, 0.11) |
−0.02*** (−0.02, −0.01) |
0.08*** (0.07. 0.09) |
| Animals | 0.43** (0.14, 0.73) |
−0.18*** (−0.20, −0.16) |
0 45*** (0.39, 0.50) |
| Vegetables | 3 17*** (2.95, 3.39) |
−0.13*** (−0.15, −0.12) |
0.21*** (0.17, 0.25) |
| LM I | 1.60*** (1.39, 1.82) |
−0.05*** (−0.06, −0.03) |
0.33*** 0.29, 0.37) |
| LM II | 1 76*** (1.52, 2.00) |
−0 07*** (−0.08, −0.05) |
0.34*** (0.30, 0.39) |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
p<.05.
p<.01.
p<.001.
Table 6.
Regression Coefficients for Black Participants
| Measure | Sex | Age | Education |
|---|---|---|---|
| MMSE | 0.71*** (0.45, 0.98) |
−0.07*** (−0.08, −0.05) |
0.24 (0.21, 0.27) |
| BNT | −0.86* (−1.58, −0.17) |
−0.22*** (−0.26, −0.17) |
0.55*** (0.46, 0.63) |
| DS | 5.47*** (3.95, 6.99) |
−0.64*** (−0.74, −0.54) |
1.43*** (1.24, 1.62) |
| TMT-A | −3.40* (−6.69, −0.10) |
0.97*** (0.76, 1.19) |
−1.84*** (−2.25, −1.42) |
| TMT-B | 0.33 (−9.93, 10.59) |
3.28*** (2.61, 3.94) |
−8.45*** (−9.75, −7.15) |
| DSFT | −0.01 (−0.30, 0.29) |
−0.05*** (−0.07, −0.03) |
0.13*** (0.10, 0.17) |
| DSFL | −0.06 (−0.21, 0.10) |
−0.02*** (−0.03, −0.01) |
0.07*** (0.05, 0.09) |
| DSBT | −0.07 (−0.37, .023) |
−0.03** (−0.05, −0.01) |
0.165*** (0.13, 0.20) |
| DSBL | −0.10 (−0.27, 0.07) |
−0.01* (−0.03, 0.00) |
0.10*** (0.08, 0.12) |
| Animals | −0.78* (−1.46, −0.10) |
−0.17*** (−0.22, −0.13) |
0.47*** (0.39, 0.56) |
| Vegetables | 2.61*** (2.08, 3.14) |
−0.10*** (−0.13, −0.07) |
0.28*** (0.21, 0.35) |
| LM I | 1 53*** (0.99, 2.06) |
−0.10*** (−0.13, −0.06) |
0.29*** (0.23, 0.36) |
| LM II | 1 77*** (1.20, 2.34) |
−0.132*** (−0.17, −0.10) |
0.33*** (0.254, 0.38) |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
p<.05.
p<.01.
p<.001.
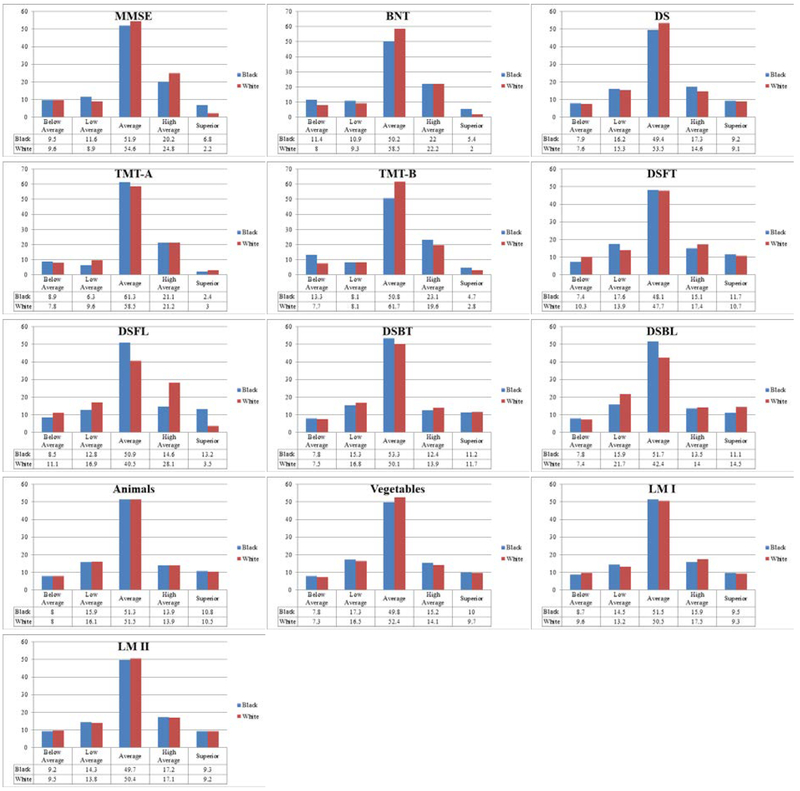
As expected, there were no group differences in average z-score using ethnicity specific norms. Summary of chi-square statistics using ethnicity specific norms are presented in Table 7 and histograms of proportion of scores across clinical performance ranges are presented in Figure 3. When considering the spread of performance in the two groups using ethnicity specific norms, the results from chi-square analyses were varied. Digit Symbol, Digit Span Backwards - total trials, Animal Fluency, Vegetable Fluency, Logical Memory-Immediate recall, and Logical Memory- Delayed recall no longer produced a significant χ2 value, indicating that the proportion of scores across performance ranges was not dependent on ethnicity. Chi-square analyses for MMSE, Boston Naming Test, Trails A, Trails B, Digit Span Forward - total trials and length of longest span, and Digit Span Backward - length of longest span produced a significant χ2 value (χ2(4) = 14.29–258.47, p<.001-.006), indicating the proportion of scores across clinical performance ranges continued to depend on ethnicity. Although a statistical difference is detected in the distribution of the scores across the 5 performance categories, the pattern of the Black participants’ scores being overrepresented in the below average and low-average categories and the White participants’ scores being overrepresented in the high-average and superior categories is no longer present with the use of ethnicity specific norms. Rather, there are small percentage differences in the spread of scores across clinical range classifications. For some neuropsychological tests, there is a higher percentage of the Black participants’ scores in above average clinical ranges. For other tests there is a higher percentage of the Black participants’ scores in below average clinical ranges. For yet other tests, while the total percentage of the Black and White participants’ scores was comparable for the two lowest clinical ranges combined (i.e. below average and low-average ranges combined), a significant Chi-square value resulted from the percentage of one group’s scores being greater in the low-average category and the percentage of the other group’s scores greater in the below average category.
Table 7.
Summary of Performance Range Chi-Square Statistics Using Ethnicity Specific Norms
| Measure | X2 | Effect size (V) |
|---|---|---|
| MMSE | 80.21*** | .114 |
| BNT | 64.05*** | .103 |
| DS | 7.50 | .036 |
| TMT-A | 14.29** | .048 |
| TMT-B | 63.68*** | .103 |
| DSFT | 19.15** | .056 |
| DSFL | 258.47*** | .206 |
| DSBT | 4.73 | .323 |
| DSBL | 39.32*** | .080 |
| Animals | 0.13 | .005 |
| Vegetables | 2.51 | .020 |
| LM I | 3.37 | .024 |
| LM II | 0.71 | .011 |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
p < .05.
p < .01.
p < .001.
Figure 3.
Histograms of Performance Range Distributions for Each Ethnic Group Using Ethnicity Specific Norms
From 28–74 percent of scores for the Black participants that originally fell in the below average range (less than 10th percentile) were no longer classified in the below average range when using ethnicity specific norms. From 0.76–7 percent of scores for the White participants were lowered from the average to below average performance category when using ethnicity specific norms. Percentage of scores that improved to above the 10th percentile for each measure among the Black participants and scores that declined to below the 10th percentile among the White participants are presented in Table 8. Conversely, there was also movement of the scores in the opposite direction of interest for this study, scores that declined to below the 10th percentile among the Black participants and scores that improved to above the 10th percentile among the White participants. From 0–8 percent of scores for the Black participants were downgraded from the average to below average performance category when using ethnicity specific norms. From 0–81 percent of scores for the White participants that originally fell in the below average range were no longer classified below average when using ethnicity specific norms.
Table 8.
Percentage of Scores that Fell Below or Above Impairment Classification with Ethnicity Combined Norms but not Ethnicity Specific Norms
| Black Participants’ Score Movement | White Participants’ Score Movement | |||
|---|---|---|---|---|
| Measure | % Combined below 10th percentile to Specific above 10th percentile- (n) | % Combined above 10th percentile to Specific below 10th percentile- (n) | % Combined below 10th percentile to Specific above 10th percentile- (n) | % Combined above 10th percentile to Specific below 10th percentile- (n) |
| MMSE | 50.00 (102) | 4.14 (36) | 8.38 (32) | 2.98 (140) |
| BNT | 74.13 (149) | 8.06 (70) | 81.40 (302) | 7.10 (330) |
| DS | 49.30 (74) | 0.67 (6) | 2.82 (4) | 4.86 (227) |
| TMT-A | 66.19 (186) | 0.00 (0) | 0.00 (0) | 2.39 (114) |
| TMT-B | 59.53 (203) | 0.00 (0) | 1.80 (6) | 1.26 (59) |
| DSFT | 50.90 (82) | 0.00 (0) | 4.94 (25) | 0.77 (35) |
| DSFL | 48.30 (84) | 0.00 (0) | 0.76 (4) | 0.76 (34) |
| DSBT | 50.30 (84) | 0.00 (0) | 20.59 (7) | 2.33 (98) |
| DSBL | 43.90 (65) | 0.00 (0) | 0.36 (1) | 1.98 (94) |
| Animals | 60.19 (130) | 0.00 (0) | 3.72 (13) | 1.52 (72) |
| Vegetables | 33.33 (6) | 6.78 (71) | 5.65 (7) | 5.00 (250) |
| LM I | 31.93 (38) | 1.27 (12) | 19.84 (73) | 4.03 (187) |
| LM II | 28.21 (33) | 1.48 (14) | 24.63 (101) | 3.71 (171) |
Note: MMSE = Mini Mental State Exam; BNT = Boston Naming Test; DS = Digit Symbol from WAIS-R; TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; DSFT = Digit Span Forward from WMS-R Total Trials; DSFL = Digit Span Forward from WMS-R Length of Longest Span; DSBT= Digit Span Backwards from WMS-R Total Trials; DSBL= Digit Span Backwards from WMS-R Length of Longest Span; Animals = Animal Naming; Vegetables = Vegetable Naming; LM I = Logical Memory-I- Immediate from WMS-R; LM II = Logical Memory-II-Delayed from WMS-R
Discussion
The Black participants scored significantly lower than the White participants on 10 of 11 neuropsychological tests. Chi-square analysis indicated these group differences were associated with different distributions of scores across clinical performance ranges that depended on ethnicity. For all 11 neuropsychological measures, the Black participants’ scores were overrepresented in the below average and low-average categories and the White participants’ scores were overrepresented in the high-average and superior categories. As expected, when using ethnicity specific regression based norms, there were no significant differences between the two groups average standardized test scores (i.e. z-scores). Chi-square analyses indicate that when these ethnicity specific norms were applied, the Black participants’ scores were no longer overrepresented in the below average and low-average ranges and the White participants’ scores were no longer overrepresented in the high-average and superior ranges. The distribution of scores across performance ranges was quite similar for the two groups. These results are consistent with previous research demonstrating Black participants score lower than White participants on neuropsychological tests (Campbell et al., 2002; Manly et al., 1998; Manly et al., 2002; Mehta et al., 2004; Morgan et al., 2008; Pedraza et al., 2009; Pedraza et al., 2012; Rilling et al., 2005; Schwartz et al., 2004; Spering et al., 2012; Strickland et al., 2005) and with previous research showing a substantial reduction in false positive rates among African American participants with ethnicity corrections (Gladsjo et al., 1999; Norman et al., 2011).
Rates of scores that declined performance ranges for the Black participants and, for the most part, rates of scores that improved performance ranges for the White participants were low using ethnicity specific norms. The exceptions are around 20% of scores that improved above the 10th percentile for the White participants for three attention and memory tests, and 80% of scores that improved above the 10th percentile for the White participants for the Boston Naming test. Some participants in the present study were included in the Weintraub et al. (2009) normative data used for this study. It is not clear to what extent this overlap or methodological differences between our study and Weintraub et al’s (2009) may account for the higher than expected percent of scores that improved above the 10th percentile for the White participants on four measures. In any case, these somewhat anomalous findings for the White participants do not impact the main findings related to score improvement rates among the Black participants.
Age and education were statistically significant predictors on all neuropsychological measures in both racial groups. In both racial groups, gender was a statistically significant predictor only for measures of memory, language, and the processing speed measure Digit Symbol. Examination of regression equations reveals potential structural differences in the equations, indicating a difference in the relationship between the predictor variables and test performance in the two groups. For example, in general, education was a stronger predictor of test scores in Blacks and age was a stronger predictor in Whites. It is widely known that level of education can greatly impact performance on neuropsychological tests (Lezak, Howieson, Bigler, & Tranel, 2012), and research has shown that there is more to the story than years of education alone (Manly, Jacobs, Touradji, Small, & Stern, 2002; O’Bryant et al., 2007; O’Bryant, Schrimsher, & O’Jile, 2005). Multiple studies have found that discrepancies between reported years of education and measured reading level are larger among African American participants compared to White participants (Manly et al., 2002; O’Bryant, Schrimsher, & O’Jile, 2005; O’Bryant et al., 2007). These differences can most likely be attributed to differences in quality of educational experience as evidenced by disparities in average money spent on each student per year, teacher salary, number of books per student, and length of school day (Lucas et al., 2005). Manly (2005) concluded that disparities in educational experience likely lead to differences in knowledge, familiarity, and problem-solving strategies, which can impact performance on neuropsychological tests across cognitive domains and may account for potential structural differences in the equations for each group.
Strengths and Limitations of the Present Study.
A primary strength of this study is the utilization of a large national sample from many regions of the United States. To our knowledge, previous similar studies utilized geographically localized samples with the next most inclusive study utilizing six sites from Midwest and East Coast (Morgan et al., 2008). This study adds to the depth of the literature by replicating results in a sample from geographically diverse regions of the United States. However, because various recruitment strategies are utilized across the different NACC sites, participant equality cannot be assumed between the sites. Similarly, the normative equations utilized in the current study may not be appropriate for clinical use because of the various recruitment strategies utilized by different sites.
The current study also adds to the depth of the literature by including only participants with 5 years of follow up to confirm no diagnosis of dementia. To our knowledge, previous studies have not utilized a longitudinal approach to ensure that baseline assessments were not impacted by subthreshold cognitive decline. This helps to establish higher confidence that this sample was cognitively healthy. However, the participants are highly educated across both ethnic groups and may not be representative of the general population in this regard.
A primary benefit of studying ethnicity specific norms is better sensitivity and specificity for neuropsychological tests with the goal of improved diagnostic accuracy (Gasquoine, 2009; Manly, 2005; Manly & Echemendia, 2007). Ethnicity specific norms may lead to development of new measures that are more culturally valid for minority individuals. The percentage of minority individuals is predicted to continue to increase and without research to support clinical decisions, the field will be left without means for appropriate assessment.
It is important, however, to consider the limitations of ethnic classifications. Ethnic group categories are socially defined, amenable to change, and not rooted in science or genetics (Gasquoine, 2009; Manly, 2005). Furthermore, racial classifications imply an overarching assumption that phenotypic traits consistently correlate with genetic and cultural similarities (Gasquoine, 2009; Manly, 2005). In neuropsychological research and practice, however, race or ethnicity often serve as a proxy for variables that are known to impact cognitive functioning such as educational experiences and socioeconomic status (Manly & Echemendia, 2007). The current study adds to existing literature by demonstrating a change in performance range classification when education is weighted based for a single ethnic group.
A potential drawback to research examining ethnic differences in neuropsychological test performance is the possibility for harmful misinterpretation of results (Gasquoine, 2009; Manly, 2005). There are numerous examples throughout history of the utilization of cognitive tests to promote preferential treatment and views of inferiority for lower scoring groups or superiority for higher scoring groups. There is a significant social risk for over-attribution of discrepancies between races to biological or genetic causes and under-emphasizing cultural bias of tasks and questions. Misinterpretation could also lead to an increase in false negative errors, which could impede documentation of need for services or resources if an impairment is incorrectly considered intact (Gasquoine, 2009; Manly, 2005). Conversely, the current study only provides data for older adults and could be used to address the social risk of some Black older adults potentially losing autonomy because they are deemed as impaired or cognitively declining when they likely are not based on the results from this study and previous literature documenting lower baseline performance levels.
A limitation of the study design is inability to consider social interactions that impact cognitive test performance, considering ethnicity based norms cannot completely account for these variables. For instance, cognitive testing is conducted in a social situation involving many assumptions that are culturally dependent (Ardila, 2005; Sternberg, 2004). Examiners ask examinees to provide their “best performance” to obtain valid results. However, some research has shown that African Americans are more likely to believe that creative or expansive answers are rewarded more than obvious ones, and therefore, may select nonobvious answers in an effort to provide the best response. Also, it is assumed that the person who is being tested is culturally similar to the normative group that the test was originally validated on in several facets including the conceptualization of speed, the utilization of testing strategies, the impact of being in a one-to-one interaction, the impact of being in an isolated environment, and the assumed authority of the examiner administering tests.
Similarly, perceived discrimination by an examiner of a different race has been associated with poorer performance on memory tasks in a non-clinical sample of African Americans (Thames et al., 2013). African American participants exposed to stereotype threat had poorer neuropsychological performance across cognitive domains compared to individuals who were not exposed to stereotype threat (Thames et al., 2013). Functional imaging shows that when exposed to stereotype threat prior to the administration of a math working memory task, parts of the brain associated with social and emotional processing are more activated and areas of the brain more associated with math are less activated compared to individuals not exposed (Krendl, Richeson, Kelley, & Heatherton, 2008). Other studies have shown that test anxiety among African American individuals is more likely to be negatively correlated with performance on neuropsychological measures compared to European Americans (Thames et al., 2015). Concern about how poor performance would be perceived by others and self-image were the primary sources on anxiety for African Americans in the study, where European Americans in the study experienced anxiety related to not being well prepared for the test (Thames et al., 2015). Even outside of the testing situation, higher levels of perceived discrimination related chronic stress are associated with lower performance on tests of episodic memory and perceptual speed, even after controlling for vascular risk factors, age, sex, and education (Barnes et al., 2012).
Direction for Future Research.
Future studies could build on the current findings by comparing the long term, predictive power of ethnic combined versus ethnic specific norms: for example, predicting conversion from mild cognitive impairment to Alzheimer’s dementia. Additional analyses could be conducted to confirm demographic structural equation differences between the two groups as well. Follow up or replication studies could add to the power of these findings by adjusting the procedures to match groups on demographic variables, control for variance accounted for by site of participation, and control for health status differences, such as cerebrovascular disease load.
To our knowledge, few studies use the approach of creating two separate ethnic group normative equations rather than including ethnic group as a variable in a normative equation. Additional research is needed to validate the practice of using ethnic specific equations compared to including ethnicity as a variable a single equation. It would also be interesting to see if these results are replicated across education level or age cohorts. More importantly, it will likely be beneficial to deconstruct the concept of race and investigate variables that racial group classification serves as a proxy for in the practice of neuropsychology. In particular, studies could assess and describe the relationship between neuropsychological performance and quality of education, acculturation, socioeconomic status, health status, and examiner-examinee impact, such as perceived discrimination. Future studies should include additional ethnic groups as results from the two groups included in this study should not be generalized to other groups who may differ culturally.
Conclusions.
Neuropsychological tests and testing procedures are inherently culturally biased. The results of this study emphasize the importance of considering ethnicity and different life experiences related to ethnicity and culture when developing, norming, and interpreting neuropsychological tests in order to reduce the risk of interpreting some Black individual’s scores as impaired when they likely are not.
Public Significant Statement:
The current study utilized a sample of healthy older adults to demonstrate the potential for over-pathologizing Black patients’ neuropsychological performance compared to similar White older adults. The proportion of Black participant scores categorized as below average decreased when ethnicity specific norms were used. The results of this study emphasize the importance of considering ethnicity and culture when developing, norming, and interpreting neuropsychological tests in order to reduce the risk of interpreting some Black individual’s scores as impaired when they likely are not.
Acknowledgements:
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIAfunded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
References
- Ardila A (2005). Cultural values underlying psychometric cognitive testing. Neuropsychology Review, 15(4), 185–195. doi: 10.1007/s11065-005-9180-y [DOI] [PubMed] [Google Scholar]
- Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, & Wilson RS (2012). Perceived discrimination and cognition in older African Americans. Journal of the International Neuropsychological Society, 18(5), 856–865. doi: 10.1017/S1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Yumoto F, Capuano A, Wilson RS, Bennett DA, & Tractenberg RE (2016). Examination of the Factor Structure of a Global Cognitive Function Battery across Race and Time. Journal of the International Neuropsychological Society, 22(1), 66–75. doi: 10.1017/S1355617715001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone KB, Victor TL, Wen J, Razani J, & Ponton M (2007). The association between neuropsychological scores and ethnicity, language, and acculturation variables in a large patient population. Archives of Clinical Neuropsychology, 22, 355–365. doi: 10.1016/j.acn.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Campbell AL, Ocampo C, Rorie K, Lewis S, Combs S, Ford-Booker P, … Hastings A (2002). Caveats in the neuropsychological assessment of African Americans. Journal of the National Medical Association, 94, 591–601. [PMC free article] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, … Romero HR (2014). Deconstructing racial differences: The effects of quality of education and cerebrovascular risk factors. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(4), 545–556. doi: 10.1093/geronb/gbu086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gasquoine PG (2009). Race-norming of neuropsychological tests. Neuropsychological Review, 19, 250–262. doi: 10.1007/s11065-009-9090-5 [DOI] [PubMed] [Google Scholar]
- Gladsjo J, Schuman C, Evans J, Peavy G, Miller S, & Heaton R (1999). Norms for Letter and Category Fluency: Demographic corrections for age, education, and ethnicity. Assessment, 6(2), 147–178. [DOI] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). Boston Naming Test, Second Edition Philadelphia, Pennsylvania: PRO-ED Inc. [Google Scholar]
- Krendl AC, Richeson JA, Kelley WM, & Heatherton TF (2008). The negative consequences of threat: A functional magnetic resonance imaging investigation of the neural mechanisms underlying women’s underperformance in math. Psychological Science, 19, 168–175. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological assessment (5th ed.). New York, NY: Oxford University Press. [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, & Graff-Radford NR (2005a). A brief report on WAIS-R normative data collection in Mayo’s Older African Americans Normative Studies. The Clinical Neuropsychologist, 19(2), 184–188. doi: 10.1080/13854040590945283 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, & Graff-Radford NR (2005b). Mayo’s Older African Americans Normative Studies: Norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, Wrat-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. The Clinical Neuropsychologist, 19(2), 243–269. doi: 10.1080/13854040590945337 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, & Graff-Radford NR (2005c). Mayo’s Older African Americans Normative Studies: WMS-R Norms for African American elders. The Clinical Neuropsychologist, 19(2), 189–213. doi: 10.1080/13854040590945292 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Willis FB, Ferman TJ, Smith GE, Parfitt FC, … Graff-Radford NR (2005). Mayo’s Older African Americans Normative Studies: Normative Data for Commonly Used Clinical Neuropsychological Measures. The Clinical Neuropsychologist, 19(2), 162–183. doi: 10.1080/13854040590945265 [DOI] [PubMed] [Google Scholar]
- Manly JJ (2005). Advantages and disadvantages of separate norms for African Americans. The Clinical Neuropsychologist, 19(2), 270–275. doi: 10.1080/13854040590945346 [DOI] [PubMed] [Google Scholar]
- Manly JJ & Echemendia RJ (2007). Race-specific norms: Using the model of hypertension to understand issues of race, culture, and education in neuropsychology. Archives of Clinical Neuropsychology, 22, 319–325. doi: 10.1016/j.acn.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Sano M, Bell K, Mechant CA, Small SA, & Stern Y (1998). Cognitive test performance among nondemented elderly African Americans and Whites. Neurology, 50, 1238–1245. doi: 10.1212/WNL.50.5.1238 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, & Stern Y (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society, 8(3), 341–348. doi: 10.1017/S1355617702813157 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Miller SW, Heaton RK, Byrd D, Reilly J, Velasquez RJ, … Grant I (1998). The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. Journal of the International Neuropsychological Society, 4, 291–302. [PubMed] [Google Scholar]
- Mehta KM, Simonsick ED, Rooks R, Newman AB, Pope SK, Rubin SM, & Yaffe K (2004). Black and White differences in cognitive function test scores: what explains the difference?. Journal Of The American Geriatrics Society, 52(12), 2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Brewster P Marquine MJ, MacKay-Brandt A, Reed B, Farias ST, & Mungas D (2015). Early life development in a multiethnic sample and the relation to late life cognition. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(4), 519–531. doi: 10.1093/geronb/gbt126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AA, Marsiske M, & Whitfield KE (2008). Characterizing and explaining cognitive test performance between African American and European American older adults. Experimental Aging Research, 34(1), 80–100. doi: 10.1080/03610730701776427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, … Kukull WA (2006). The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Disease and Associated Disorders, 20(4), 210–216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Turner MB (2016). Heart disease and stroke statistics- 2016 update: A report from the American Heart Association. Circulation, 133, 38–360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, … Heaton RK (2011). Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test–Revised, Brief Visuospatial Memory Test–Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Yest 64-card version. Journal of Clinical and Experimental Neuropsychology, 33(7), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Lucas JA, Willis FB, Smith GE, Graff-Radford NR, & Ivnik RJ (2007). Discrepancies between self-reported years of education and estimated reading level among elderly community-dwelling African Americans: Analysis of the MOAANS data. Archives of Clinical Neuropsychology, 22, 327–332. doi: 10.1016/j.acn.2007.01.007. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Schrimsher GW, & O’Jile JR, (2005). Discrepancies between self-reported years of education and estimated reading level: Potential implications for neuropsychologists. Applied Neuropsychology, 12(1), 5–11. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Clark JH, O’Bryant SE, Smith GE, Ivnik RJ, Graff-Radford NR, … Lucas JA (2012). Diagnostic validity of age and education corrections for the Mini-Mental State Examination in older African Americans. Journal of the American Geriatrics Society, 60(2), 328–331. doi: 10.1111/j.1532-5415.2011.03766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O, Graff-Radford NR, Smith GE, Ivnik RJ, Willis FB, Petersen RC, & Lucas JA (2009). Differential item functioning of the Boston Naming Test in cognitively normal African American and Caucasian older adults. Journal of the International Neuropsychological Society, 15(5), 758–768. doi: 10.1017/S1355617709990361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O, Lucas JA, Smith GE, Willis FB, Graff-Radford NR, Ferman TJ, … Ivnik RJ (2005). Mayo’s older African American Normative Studies: Confirmatory factor analysis of a core battery. Journal of the International Neuropsychological Society, 11(2), 184–191. doi: 10.1017/S1355617705050204 [DOI] [PubMed] [Google Scholar]
- Rilling LM, Lucas JA, Ivnik RJ, Smith GE, Willis FB, Ferman TJ, … Graff-Radford NR (2005). Mayo’s Older African American Normative Studies: Norms for the Mattis Dementia Rating Scale. The Clinical Neuropsychologist, 19(2), 229–242. doi: 10.1080/13854040590945328 [DOI] [PubMed] [Google Scholar]
- Reitan R, & Wolfson D (1993). The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. (2nd ed.). Tucson, AZ: Neuropsychology Press. [Google Scholar]
- Schneider BC, Gross AL, Bangen KJ, Skinner JC, Benitez A, Glymour MM, … & Luchsinger JA (2015) Association of vascular risk factors with cognition in a multiethnic sample. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70(4), 532–544. doi: 10.1093/geronb/gbu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, … Bandeen-Roche K (2004). Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives, 112(3), 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, & Atri A (2011). A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s Research & Therapy, 3(6), 32 10.1186/alzrt94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering CC; Hobson V, Lucas JA, Menon CV, Hall JR, & O’Bryant SE (2012). Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer’s disease in ethnically diverse highly educated individuals: an analysis of the NACC database. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 67(8), 890–896. doi: 10.1111/jgs.13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg RJ (2004). Culture and intelligence. American Psychologist, 59(5), 325–338. doi: 10.1037/0003-066X.59.5.325 [DOI] [PubMed] [Google Scholar]
- Strickland TL, Longobardi PG, Alperson BL, & Andre K (2005). Mini-Mental State and Cognistat performance in an older African American sample. Clinical Neuropsychologist, 19(1), 87–98. doi: 10.1080/13854040490887243 [DOI] [PubMed] [Google Scholar]
- Thames AD, Hinkin CH, Byrd DA, Bilder RM, Duff KJ, Mindt MR, … Streiff V (2013). Effects of stereotype threat, perceived discrimination, and examiner race on neuropsychological performance: simple as Black and White?. Journal of the International Neuropsychological Society, 19(5), 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Panos SE, Arentoft A, Byrd DA, Hinkin CH, & Arbid N (2015). Mild Test Anxiety Influences Neurocognitive Performance Among African Americans and European Americans: Identifying Interfering and Facilitating Sources. Cultural Diversity & Ethnic Minority Psychology, 21(1), 105–113. doi: 10.1037/a0037530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler A (1987). Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale-Revised Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford N, Chui H, … Morris JC (2009). The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Disease and Associated Disorders, 23(2), 91–101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield K, Allaire J, Gamaldo A, & Bichsel J (2010). Factor Structure of Cognitive Ability Measures in Older African Americans. Journal Of Cross-Cultural Gerontology, 25(3), 271–284. doi: 10.1007/s10823-010-9120-z [DOI] [PubMed] [Google Scholar]
- Whitfield KE, FIllenbaum GG, Pieper C, Albert MS, Berkman LF, Blazer DG, Rowe JW, & Seeman T (2000). The effect of race and health-related factors on naming and memory. Journal of Aging and Health, 12, 69–89. doi: 10.1177/089826430001200104 [DOI] [PubMed] [Google Scholar]