Abstract
Objectives:
To determine whether X-ray fused with MRI (XFM) is beneficial for select transcatheter congenital heart disease interventions.
Background:
Complex transcatheter interventions often require three-dimensional (3D) soft tissue imaging guidance. Fusion imaging with live X-ray fluoroscopy can potentially improve and simplify procedures.
Methods:
Patients referred for select congenital heart disease interventions were prospectively enrolled. Cardiac MRI data was overlaid on live fluoroscopy for procedural guidance. Likert scale operator assessments of value were recorded. Fluoroscopy time, radiation exposure, contrast dose, and procedure time were compared to matched cases from our institutional experience.
Results:
Forty-six patients were enrolled. Pre-catheterization, same day cardiac MRI findings indicated intervention should be deferred in nine patients. XFM-guided cardiac catheterization was performed in 37 (median age 8.7 years [0.5–63 years]; median weight 28 kg [5.6–110 kg]) with the following prespecified indications: pulmonary artery (PA) stenosis (n = 13), aortic coarctation (n = 12), conduit stenosis/insufficiency (n = 9), and ventricular septal defect (n = 3). Diagnostic catheterization showed intervention was not indicated in 12 additional cases. XFM-guided intervention was performed in the remaining 25. Fluoroscopy time was shorter for XFM-guided intervention cases compared to matched controls. There was no significant difference in radiation dose area product, contrast volume, or procedure time. Operator Likert scores indicated XFM provided useful soft tissue guidance in all cases and was never misleading.
Conclusions:
XFM provides operators with meaningful three-dimensional soft tissue data and reduces fluoroscopy time in select congenital heart disease interventions.
Keywords: device closure, fusion imaging, magnetic resonance imaging, transcatheter
1 |. INTRODUCTION
The complexity of transcatheter therapy for congenital heart disease has increased over recent years. To facilitate complex structural interventions, operators often perform pre-procedure three-dimensional (3D) imaging and use adjunctive procedural imaging such as transesophageal or intracardiac echocardiography. Fusion imaging, overlaying 3D images from cardiac MRI, CT, 3D rotational angiography (3DRA), or echocardiography on live fluoroscopy, may aid complex interventions.1 Patients with congenital heart disease have complex cardiac and post-surgical anatomy often resulting in relatively long procedure times requiring multiple contrast injections and adjunct 3D imaging to guide interventions. Many of these patients require multiple lifetime cardiac catheterizations and other examinations contributing to significant cumulative radiation exposure.2 Infants and children, who comprise a large proportion of congenital cardiac catheterization cases, are more susceptible to the damaging effects of ionizing radiation compared with adults and even low levels of radiation exposure are thought to contribute to long term risk of malignancy.3–5 Cardiac MRI (CMR) is used in routine clinical practice for pre-intervention assessment of congenital heart disease. CMR provides exquisite soft tissue data, additional hemodynamic information and is radiation sparing. CMR fusion with live X-ray fluoroscopy is promising for guidance of many congenital cardiac interventions, although there is limited published data confirming objective benefit compared to conventional X-ray guided procedures.6 We hypothesized that CMR fusion imaging, also known as X-ray fused with MRI (XFM), could simplify prespecified, select congenital cardiac interventions with associated reduction in fluoroscopy time, radiation exposure, and contrast dose.
2 |. METHODS
2.1 |. Subjects
Children and adults of any age with pulmonary artery stenosis, right ventricle to pulmonary artery (RV-PA) conduit dysfunction, coarctation of the aorta, or ventricular level intracardiac shunt referred to the Children’s National Medical Center Cardiac Catheterization Laboratory for elective interventions were prospectively enrolled in the study. Patients were excluded for estimated glomerular filtration rate of <30 mL/min/1.73 m2, pregnancy or nursing, or other ineligibility for CMR. The research protocol was approved by our institutional review board; and thus written informed consent, and assent where appropriate, was obtained in all cases.
2.2 |. X-ray fused with MRI
Pre-catheterization CMR was performed immediately before the elective cardiac catheterization case in our combined interventional CMR (iCMR) suite.7 Sedation level for CMR was assessed and instituted by anesthesiology as per clinical standard. Each patient underwent CMR imaging using a 1.5 T MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany), with sequence selection based on clinical indications. All patients had a contrast-enhanced magnetic resonance angiogram (MRA) performed with sequence parameters optimized by patient size (FOV 240–330 × 320–440 mm, matrix 211 × 230 – 352 × 384 pixels, PAT 2–3, res 0.9–1.1 mm isotropic, TE 1.07–1.16 ms, TR 3.12–3.29 ms, flip angle 25), a 3D steady-state free precession (3DSSFP) dataset that was either respiratory-navigated (FOV 210 × 320 mm, matrix 135 × 256 pixels, PAT 2, pixel 1.3 mm isotropic, TE 1.54 ms, TR 3.48 ms, flip angle 50) with a Cartesian acquisition or self-navigated (FOV 220 × 220 mm, matrix 192 pixels, PAT 1, res 1.1 mm isotropic, TE 1.53 ms, TR 3.05 ms, flip angle 50) with a radial acquisition and velocity-encoded MRI for measurement of blood flow. When appropriate, metal artifact-reducing imaging protocols were used.8 The MRA and 3DSSFP images were inspected for overall quality as well as quality of the region of interest, and the optimal 3D dataset was segmented and used to create the XFM overlay.
CMR results were assessed, and XFM overlay was created only if the CMR findings confirmed that cardiac catheterization was indicated. Patients were transferred to the X-ray room, while a 3D map of the segmented cardiac MRI regions of interest was manually prepared to overlay onto biplane fluoroscopy using XFM as described.9,10 Live X-ray was fused with the 3D MRI roadmap (XFM) (MR fusion and overlay prototype software, Siemens, Forchheim, Germany) using automatic correction for gantry and table position. XFM was co-registered using internal angiographic markers, most frequently a biplane left innominate venogram (acquisition is institutional clinical standard).
2.3 |. Data analysis
Likert scale operator assessments of value were recorded following each case. XFM-guided intervention fluoroscopy time, radiation exposure by dose area product (DAP), contrast volume, procedure time, and sedation time were compared to age, weight, BSA, and intervention-matched controls identified from review of our 10-year institutional experience. Case complexity based on the subject’s cardiac or post-surgical anatomy, and exact location of the congenital lesion was matched to the extent possible. Weight and case complexity were the most heavily weighted factors in selecting appropriate controls. Preference was given to the most recently performed cases to limit the era effect of changes in radiation reduction practice. In rare instances, when more than one appropriate control was identified, the control with the lowest radiation DAP per weight was selected to reduce selection bias. XFM cases for which no appropriate control could be identified were excluded from statistical analysis. XFM data were compared to matched controls using two-tailed Wilcoxon Signed Rank test (SSPS v21). Results were considered significant if p < 0.05.
3 |. RESULTS
3.1 |. Study population
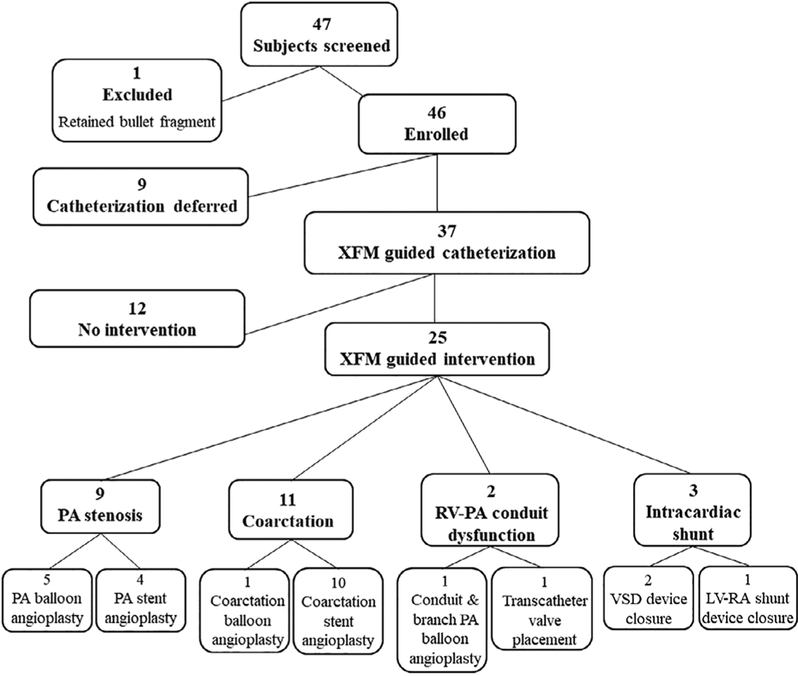
Referral screening identified 47 patients. One patient was excluded due to a retained bullet fragment which contraindicated CMR. Fortysix patients, aged 2 months to 63 years (median 8.1 years, IQR 3.5–16), were enrolled from November 2013 to November 2016 with diagnoses and treatment course as detailed in Figure 1.
FIGURE 1.
X-ray fused with MRI (XFM) study subject screening and treatment course. Abbreviations: XFM, x-ray fused with MRI; PA, pulmonary artery; RV-PA, right ventricle to pulmonary artery; VSD, ventricular septal defect; LV-RA, left ventricle to right atrium
CMR was clinically indicated in 35 patients (76%) (PA stenosis n = 18, conduit dysfunction n = 5, coarctation n = 9, intracardiac shunt n = 3) and performed solely for the research protocol in 11 patients (24%) (PA stenosis n = 2, conduit dysfunction n = 3, coarctation n = 6). Of those who had research only indications for CMR, nine patients had undergone a clinically indicated CMR in the preceding 12 months confirming need for cardiac catheterization, and two patients (2-year-old patient with PA stenosis and a 1-year-old patient with RV-PA conduit stenosis) had adequate echocardiographic data, as deemed by referring cardiologist, for cardiac catheterization. CMR was performed under sedation in 27 patients (59%), aged 2 months to 25 years (median 3.7 years, IQR 2.5–8), and non-sedated in 19 patients (41%), aged 7–63 years (median 17 years, IQR 9–23). Catheterization was deferred in nine patients (pulmonary artery stenosis = 5, coarctation of the aorta = 3, right ventricle to pulmonary artery conduit stenosis/insufficiency = 1) all of whom had clinical indications for the CMR which defined a lower degree of stenosis or insufficiency than had been estimated by echocardiography obviating the need for cardiac catheterization.
Thirty-seven patients had cardiac catheterization with XFM guidance, aged 6 months to 63 years (median 8.7 years, IQR 3.6–17); weight 5.6–110 kg (median 28 kg, IQR 15–57), with the following indications: pulmonary artery (PA) stenosis (n = 13), aortic coarctation (n = 12), conduit stenosis/insufficiency (n = 9), and intracardiac shunt (n = 3). XFM overlay creation was successful in all cases. Metallic artifact in cases (64%) with previously placed stents, coils, surgical clips, or sternal wires was minimal and did not preclude creation of XFM overlay.
Diagnostic cardiac catheterization data showed intervention was not indicated in 12 cases due to direct stenosis gradient measurements being lower than predicted (n = 7), concern for negative impact on surrounding structures, and/or newly identified indications for surgical repair (n = 5). Therefore, 25 patients aged 6 months to 63 years (median 9.5 years, IQR 3.8–17) with demographics detailed in Table 1, underwent the following XFM guided interventions: coarctation balloon angioplasty n = 1; coarctation stent angioplasty n = 10 (Supplemental Movie 1); PA balloon angioplasty n = 5; PA stent angioplasty n = 4 (Supplemental Movie 2); RV-PA conduit and bilateral PA angioplasty n = 1; RV-PA conduit stent angioplasty and transcatheter valve placement n = 1 (Supplemental Movie 3); VSD device closure n = 2; (Supplemental Movie 4) left ventricle to right atrium (LV-RA) shunt device closure n = 1. Examples of each case type XFM overlay are provided in Figures 2–6.
TABLE 1.
Wilcoxon-signed rank test (two-tailed) comparison of XFM-guided intervention group versus matched controls
| Parameter mean value (range) | XFM-guided intervention | Controls | p-value |
|---|---|---|---|
| Age (years) | 13 | 11 | 0.19 |
| (0.5–63) | (0.9–35) | ||
| Weight (kg) | 38 | 38 | 0.69 |
| (6–106) | (9–115) | ||
| BSA (m2) | 1.11 | 1.13 | 0.53 |
| (0.33–2.27) | (0.40–2.20) | ||
| Fluoroscopy time (min) | 18 | 25 | 0.04* |
| (6–44) | (9–81) | ||
| Radiation dose area product per kg (μGym2/kg) |
137 | 147 | 0.18 |
| (27–521) | (13–641) | ||
| Contrast volume per kg (mL/kg) |
4 | 4 | 0.07 |
| (1–7) | (1–9) | ||
| Catheterization procedure time (hr) |
2.0 | 2.1 | 0.67 |
| (0.9–3.4) | (0.9–3.3) |
Significance p < 0.05 considered significant.
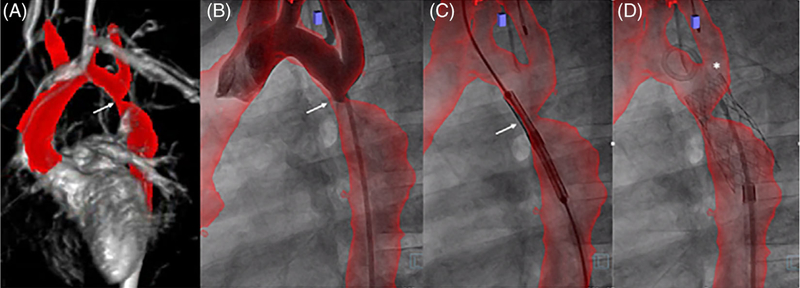
FIGURE 2.
XFM-guided coarctation stent angioplasty. XFM-guided coarctation stent angioplasty. (A) Cardiac MRI (CMR) delineating aorta, level of stenosis (arrows), and relationship to the head vessel origins segmented in red; (B) aortic angiogram in the straight lateral projection with XFM aortic overlay to confirm registration; (C) positioning of a stent in the region of coarctation of the aorta; (D) post intervention showing appropriately positioned stent at the previous site of coarctation and the relationship to the origin of the left subclavian artery (star). Case corresponds to Supplemental Movie 1
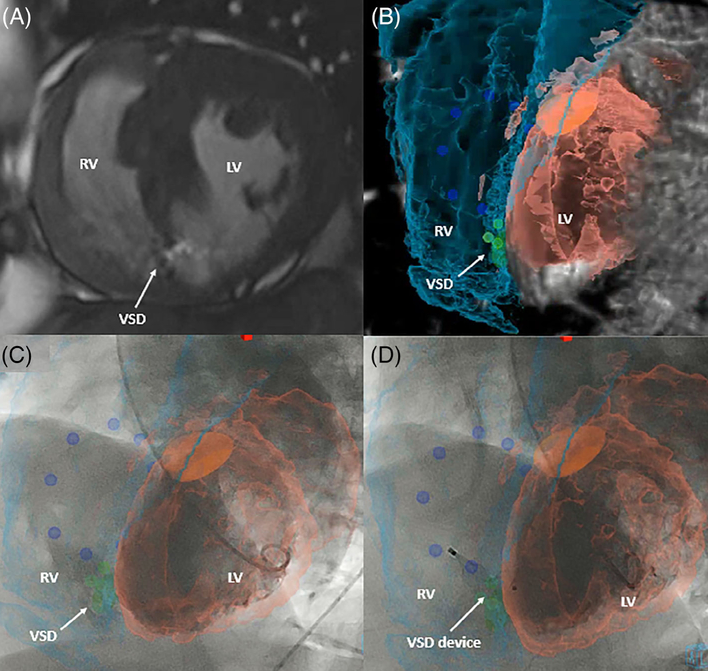
FIGURE 6.
XFM-guided ventricular septal defect (VSD) device closure. XFM- guided VSD closure in an adult patient. (A) VSD identification by CMR; (B) CMR segmentation of the right ventricle (turquoise), left ventricle (peach), aortic outflow (orange), tricuspid valve markers (dark blue), and VSD (green) demonstrating the ideal camera gantry angle for intervention; (C) left ventriculogram in the LAO 57/CRAN 15 projections with peak contrast opacification of VSD demonstrating difficulty in angiographic delineation of the shunt; and (D) post device closure of VSD with the device position corresponding to the XFM VSD marker. Case corresponds to Supplemental Movie 4
3.2 |. XFM-guided intervention versus matched controls
Comparison of procedural data between the XFM guided intervention group and controls is detailed in Table 1. A suitable control could not be identified for the previously reported XFM-guided LV-RA shunt device closure case and therefore was excluded from case–control analysis.11 Overall, there was no significant difference between control and XFM-guided intervention group age, weight, or BSA. Fluoroscopy time was significantly shorter in the XFM-guided intervention group compared to controls. There was no significant difference in the majority of other parameters including radiation DAP/kg, contrast volume/kg, or procedure time. Sedation time was significantly longer for the overall XFM-guided intervention group compared to controls but was not statistically different for the XFM sub-group (n = 10) who were only sedated for the catheterization portion of their case compared to matched controls.
Intervention based sub-group results are detailed in Supplemental Table 2. Statistical analysis was performed only for pulmonary artery and coarctation interventions due to insufficient sample size for intracardiac shunt and conduit intervention groups. There was a trend toward lower fluoroscopy times and contrast volumes but no statistically significant differences in fluoroscopy time, radiation DAP, contrast volume, and procedure time between XFM-guided PA subgroup or coarctation subgroup intervention cases and matched controls.
3.3 |. Operator assessment: XFM value
Likert operator scales for all XFM-guided catheterizations (diagnostic and interventional) revealed that XFM was never reported as misleading. Operators “strongly agreed” or “agreed” that XFM soft tissue data was additive in all cases. In addition, they felt XFM would have allowed omission of at least one traditional angiogram in 30% of cases. Operators felt that the intervention could have been performed appropriately without the additional information provided by XFM in most interventional cases (88%) and felt neutral about whether it could have been performed appropriately without XFM in three cases (all coarctation interventions). Cardiac and respiratory motion, for which XFM was not corrected in this series and caused overlay registration mismatch in Supplemental Movie 4, was reported to be irrelevant to the intervention in all cases.
Operators reported that XFM was most helpful in cases of intracardiac shunt with the XFM delineation of the shunt acting as a target for wire crossing to facilitate intervention. The XFM overlay was also useful in predicting camera gantry angles for angiography and interventions. Operators noted that stiff wires used for intervention distorted the vascular anatomy such that the XFM overlay did not register as accurately during the intervention compared to the diagnostic portion of cases as shown in Figures 2, 4, and 5 and Supplemental Movies.
FIGURE 4.
XFM-guided right pulmonary artery (RPA) balloon angioplasty. Right pulmonary artery (RPA) balloon angioplasty performed in a patient with D-transposition of the great arteries s/p repair with LeCompte maneuver such that both pulmonary arteries course anterior to the ascending aorta. CMR data showed RPA stenosis with 40% of blood flow to the right lung and 60% to the left lung. (A) CMR delineation of the right-sided cardiac structures; (B) CMR delineation of the pulmonary artery bifurcation showing RPA stenosis (arrows); (C) right ventriculogram in the straight lateral projection; and (D) pulmonary artery angiogram in the RAO 30/CRAN 30 projection with XFM overlay of the right ventricle and pulmonary arteries in blue and the aorta in red; (E) balloon positioning demonstrating wire deformation of the RPA; and (F) proximal RPA balloon angioplasty
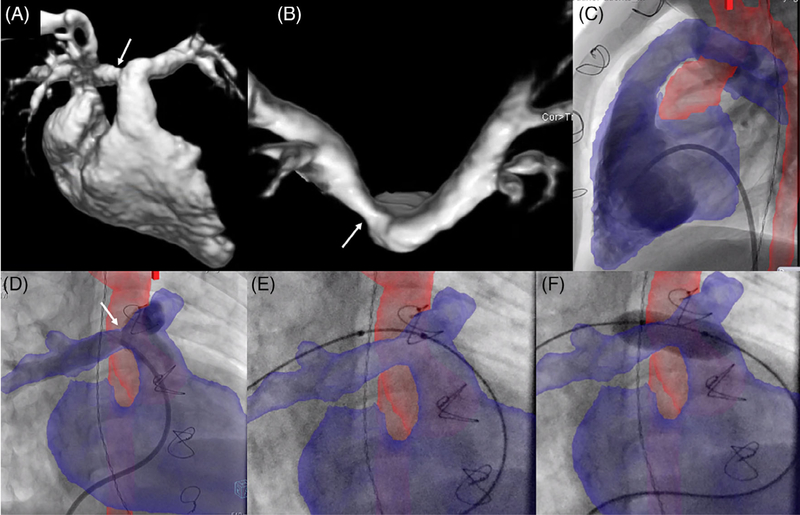
FIGURE 5.
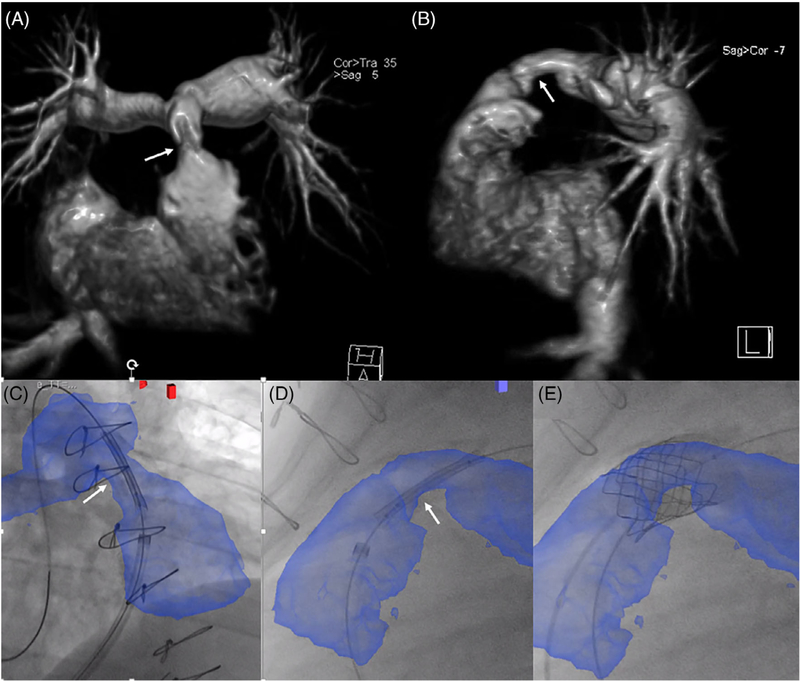
XFM-guided percutaneous pulmonary valve insertion. Right ventricle to pulmonary artery (RV-PA) conduit intervention in an adult patient with tetralogy of Fallot s/p repair. CMR confirmed RV-PA conduit stenosis (arrows) as shown in (A) coronal and (B) sagittal planes; XFM overlay of the RV-PA conduit and proximal branch pulmonary arteries in blue was used to guide initial stent positioning in (C) RAO 30/CRAN 20 and (D) straight lateral projections with subsequent transcatheter valve placement (E). Case corresponds to Supplemental Movie 3
4 |. DISCUSSION
This prospective study aims to determine whether XFM guidance is beneficial for prespecified select congenital heart disease interventions. Other groups have explored X-ray fused with CT, X-ray fused with 3DRA, and X-ray fused with ultrasound.6,12–15 Each has potential benefits and drawbacks. CT offers a 3D dataset with better spatial resolution than CMR but exposes the patient to additional radiation, additional contrast and can be difficult to acquire without repeat acquisitions particularly early in experience. Breath holds and pacing are required for 3DRA, and it may not be superior to conventional 2D contrast angiography roadmap procedural guidance. X-ray fused with ultrasound offers a real-time alternative but is best when planar and subject to variable acoustic windows dependent on patient and target anatomy such as the pulmonary arteries. We prefer XFM because CMR provides exquisite soft tissue data, additional hemodynamic information and is radiation sparing.
Based on our experience with XFM and catheter-based congenital heart disease intervention, we chose the following potential interventional case types a priori that we hypothesized there may be benefit: pulmonary artery stenosis, right ventricle to pulmonary artery (RV-PA) conduit dysfunction, coarctation of the aorta or ventricular level intracardiac shunt. Heterogeneity of congenital heart disease anatomy, patient age, and operator variability made fair assessment of benefit challenging; nevertheless, the study results indicate that XFM guidance is beneficial with reduction in fluoroscopy time compared to matched controls and favorable operator assessments.
4.1 |. Measured outcomes
The significant difference in fluoroscopy time between the XFM- guided intervention and control groups likely represents the utility of XFM as a roadmap during the diagnostic and interventional portions of the case. XFM provides a target for wire and catheter manipulation rather than “fishing” for target anatomy based on expected location and fluoroscopic landmarks or performing an angiogram to use as a comparative roadmap. XFM allows for selection of appropriately shaped catheters or creation of specific curves in wires and sheaths before or in lieu of angiography. The reduction in fluoroscopy time is a similar finding to previously published studies of XFM type guidance for cardiac catheterization which showed reduction in fluoroscopy time, radiation exposure, and contrast dose for congenital diagnostic cases with XFM guidance compared to matched controls16 and reduction in fluoroscopy time for coarctation intervention with 3D overlay guidance compared to matched controls.6
Contrast dose and radiation DAP are largely dependent on the number and characteristics of angiograms acquired during the case. Given that this was a pilot study of XFM for congenital interventions, the number of intervention related angiograms was not altered compared to clinical standard for conventional fluoroscopic guidance. XFM was used to provide guidance to accurately position stents and balloons in vessels before intervention but in most cases, given the pilot nature of this study, a hand injection of contrast via the sheath was also performed to confirm correct positioning before intervention. Our results showed a trend towards reduced contrast dose but this was not statistically significant. Presumably though, any potential mechanism of contrast reduction would be similar to fluoroscopy time reduction in that XFM provides a roadmap. Operators reported that the XFM overlay was never misleading and that at least one traditional angiogram could potentially be omitted in approximately one third of cases. Therefore, with increased operator XFM experience, it is conceivable that XFM would ultimately reduce contrast volume and radiation exposure in addition to fluoroscopy time. In the context of the high contribution of cardiac catheterization to the cumulative lifetime radiation dose of patients with congenital heart disease2 and in keeping with the ALARA principle, any potential measures to limit radiation exposure, even the modest benefit from decreased fluoroscopy time, may be prudent to pursue.
4.2 |. Subjective XFM benefit
Although the significant case–control benefits were limited to fluoroscopy time, operators conveyed subjective benefit in post-XFM procedure Likert score-based questionnaires. Five primary operators performed XFM cases with a wide operator experience range (<1 year to >20 years in practice post interventional fellowship training). We hypothesize that XFM might be most helpful to junior operators, although the cohort size precluded operator based subgroup analysis. XFM imaging shown in the Figures 2–6, and supplemental videos from this study illustrate XFM utility particularly for patients with complex congenital and post-surgical anatomy. Operator comments when reviewing the entire XFM experience were consistent revealing that XFM overlay was most useful for cases of intracardiac shunt. This was particularly evident in the LV-RA shunt case.11 Intracardiac echocardiography was used to help guide device placement as per clinical standard, and although, echocardiography-fluoroscopy fusion could have potentially provided a real-time overlay, XFM provided a simple soft tissue marker to use as a target for wire crossing without interfering with other fluoroscopic landmarks. As per other reports,16 XFM was found to be useful in predicting optimal camera gantry angles for angiography and interventions which is of particular importance in the congenital heart disease population who are unlikely to have anatomy that is accurately profiled by standard views. Although this study focused on prespecified, select congenital heart disease interventions, there are many other case types, including soft tissue interventions, where XFM may be invaluable for procedural guidance. Examples include targeted cardiac biopsy,17 recanalization of discontinuous vessels, transbaffle punctures for access to intracardiac structures in patients post Fontan or Mustard type surgical repairs, and transcatheter vessel anastomosis.18 These and other similar applications will likely be the target of future image fusion studies.
4.3 |. XFM limitations
As with any overlay technology using previously acquired 3D images, there are issues with imperfect registration. Dermal fiducial (external) markers can be used for patients undergoing CMR then left in place to aid overlay registration during same day interventions but these do not account for changes in patient position and interfere with the X-ray appearance therefore internal markers, registered to angiography or other structures, were used for registration in this study.9,19,20 Although baseline registration can be relatively straightforward to achieve, wire deformation of vessels required for interventions can result in noticeable discrepancies between live fluoroscopy and XFM registration. Operators in this study did not find this type of imperfect registration to be misleading likely due to knowledge of the predicted vessel deformation for the patient’s anatomy and intervention. Angiography may therefore still be necessary to confirm position of the target anatomy before intervention.
Another potential drawback of XFM is that it requires an additional imaging step which explains why there is no significant difference in overall procedure time despite a reduction in fluoroscopy time between XFM guided interventions and matched controls. For a subset of patients who are unable to comply with non-sedated CMR due to young age, there was significantly increased sedation time for XFM-guided intervention compared to matched controls. Given the high proportion of patients undergoing clinically indicated CMR (or same-sedation non-cardiac MRI for other indications) either before or on the day of catheterization the value of CMR structural and hemodynamic data to target catheterization procedures and guide therapy may justify this increased sedation time. For infants and children with complex congenital heart disease, every effort is taken to reduce the risks associated with anesthesia induction and therefore the strategy of combined CMR followed by cardiac catheterization under a single sedation procedure is appealing. For the purposes of this study, patients with previous CMR studies had a repeat CMR on the day of the procedure to create the XFM overlay but in many cases adequate XFM overlay can be created from clinically indicated CMR scans acquired before the day of the procedure.25
4.4 |. Study limitations
Changes in equipment and practice over the past decade in relation to radiation reduction, such as standard fluoroscopy frame rate decrease from 15 to 4 frames per second, precluded comparison of XFM guided cases to the complete historical institutional cohort. Case matching by selection from review of all contemporary interventional cases was therefore chosen to more accurately compare outcome measures. In comparison to the preclinical XFM-guided VSD device closure study which used the same animal model for both XFM and conventional catheterization to show that XFM reduces fluoroscopy time, radiation exposure, and contrast volume,9 the heterogeneous nature of congenital cardiac defects, interventions, and co-morbidities create challenges in comparing procedural data to historical controls even when matched to age, weight, and BSA. Some cases did not have an accurate intervention match such as the LV-RA shunt device closure for which the closest case identified was a complex VSD device closure in an adult patient of similar weight. The use of pilot overlay software in this study required maintenance of the clinical standard number of angiograms for the intervention being performed which include confirmatory angiography to determine stent positioning. This requirement significantly limited the ability to determine whether XFM could reduce the number of angiograms and therefore associated radiation exposure and contrast dose. It is conceivable that with XFM, some angiograms could have been replaced with stored fluoroscopy which contributes a much lower radiation dose or potentially omitted altogether. The number and different level of experience of XFM primary operators precludes analysis of whether there was an XFM learning curve but it is likely that as an operator’s experience with XFM increases there may be more marked improvements in the potential measures of benefit. As with almost all studies involving patients with congenital heart disease, our results and conclusions are limited by the relatively small and heterogeneous sample.
4.5 |. Areas for further study
Given the significant training, personnel, and monetary costs associated with implementing new technology in the catheterization laboratory, further studies are indicated to better demonstrate the potential benefit of XFM and the exact type of applications for which the benefit would justify the investment. Multi-institutional studies, ideally representing all of the available vendor specific overlay software and incorporating use of pre-acquired clinically indicated MRI scans, would be particularly helpful in capturing enough patients for the assessment of congenital interventions.26 Registration issues may be partially ameliorated in the future by incorporation of cardiac and respiratory motion21,22 but XFM will always be limited by the fact that the over-lay is based on imaging that does not change in real-time with the patient’s condition and stage of intervention. Ultimately, if soft tissue visualization is necessary to guide interventions, rather than use a 3D image acquired before the intervention and then overlaid onto live fluoroscopy, it would be ideal to perform cases under real-time 3D soft tissue MRI-guidance. The clinical translation of real-time MRI procedures has until recently been limited by lack of available MRI- compatible and visible catheterization equipment for clinical use23; recent progress is promising.24
5 |. CONCLUSIONS
XFM provides adjunctive three-dimensional soft tissue data for procedural guidance in the familiar X-ray working environment. Fluoroscopy time is reduced for select congenital heart interventions, and XFM may also have the potential to reduce contrast volume and radiation exposure as operator experience increases. As the complexity of interventions increases from simple balloon angioplasty to transcatheter valve placement and beyond it is imperative that the imaging guidance for such procedures progresses in parallel. XFM is a step toward this goal. It has the potential to simplify complex interventions and could conceivably facilitate novel interventions in the future.
Supplementary Material
FIGURE 3.
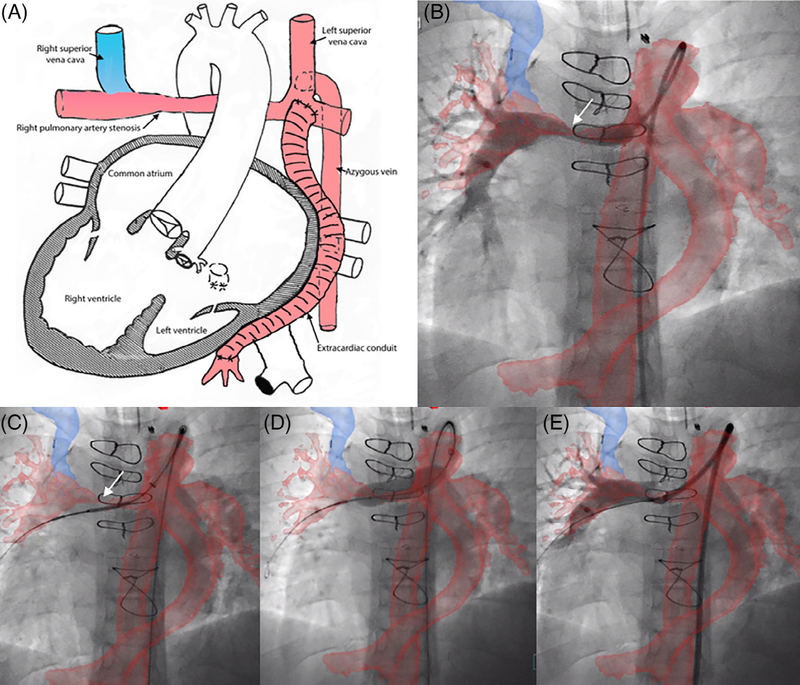
XFM-guided right pulmonary artery (RPA) stent angioplasty via Kawashima. Right pulmonary artery (RPA) stent angioplasty performed via the azygous vein continuation to left superior vena cava/Kawashima surgical repair of a patient with dextrocardia, complex congenital heart disease with interrupted inferior vena cava s/p single ventricle palliation bilateral superior cavopulmonary anastomosis and extracardiac conduit hepatic inclusion. (A) Diagrammatic representation of the cardiac anatomy with color coding corresponding to the XFM overlay. (B) RPA angiogram in the straight AP projection via a catheter advanced through from the femoral vein through the azygous vein and Kawashima confirming registration of the target anatomy (right pulmonary artery), although there is misalignment of the azygous vein and overlay; (C) positioning a stent across the stenotic RPA; (D) stent deployment; and (E) post RPA stent angioplasty angiogram confirming appropriate stent position. Case corresponds to Supplemental Movie 2
Funding information
National Heart Lung and Blood Institute, Grant/Award Numbers: Z01-HL006039, HHSN268201500001C; Siemens Medical, Grant/Award Number: CNMC-2013-AX-01–02-C213599
Footnotes
DISCLOSURES
Children’s National Medical Center and NHLBI have Master Research Agreements with Siemens Medical including X-ray fused with MRI.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Fagan TE, Truong UT, Jone PN, et al. Multimodality 3-dimensional image integration for congenital cardiac catheterization. Methodist Debakey Cardiovasc J. 2014;10:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JN, Hornik CP, Li JS, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;130:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(Suppl 2): 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health risks from exposure to low levels of ionizing radiation : Beir vii phase 2. Washington, D.C: National Academies Press; 2006. [PubMed] [Google Scholar]
- 5.Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–2708. [DOI] [PubMed] [Google Scholar]
- 6.Glockler M, Halbfabeta J, Koch A, Achenbach S, Dittrich S. Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease-a single-center, retrospective analysis of 78 cases. Catheter Cardiovasc Interv. 2013;82:436–442. [DOI] [PubMed] [Google Scholar]
- 7.Ratnayaka K, Kanter JP, Faranesh AZ, et al. Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Reson. 2017;19:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivieri LJ, Cross RR, O’Brien KE, Ratnayaka K, Hansen MS. Optimized protocols for cardiac magnetic resonance imaging in patients with thoracic metallic implants. Pediatr Radiol. 2015;45: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratnayaka K, Raman VK, Faranesh AZ, et al. Antegrade percutaneous closure of membranous ventricular septal defect using X-ray fused with magnetic resonance imaging. JACC Cardiovasc Interv. 2009;2: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva R, Gutierrez LF, Raval AN, McVeigh ER, Ozturk C, Lederman RJ. X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction. Circulation. 2006;114:2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant EK, Faranesh AZ, Cross RR, et al. Image fusion guided device closure of left ventricle to right atrium shunt. Circulation. 2015;132: 1366–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliger C, Jelnin V, Sharma S, et al. CT angiography-fluoroscopy fusion imaging for percutaneous transapical access. J Am Coll Cardiol Img. 2014;7:169–177. [DOI] [PubMed] [Google Scholar]
- 13.Berman DP, Khan DM, Gutierrez Y, Zahn EM. The use of three- dimensional rotational angiography to assess the pulmonary circulation following cavo-pulmonary connection in patients with single ventricle. Catheter Cardiovasc Interv. 2012;80:922–930. [DOI] [PubMed] [Google Scholar]
- 14.Jone PN, Ross MM, Bracken JA, Mulvahill MJ, Di Maria MV, Fagan TE. Feasibility and safety of using a fused echocardiography/- fluoroscopy imaging system in patients with congenital heart disease. J Am Soc Echocardiogr. 2016;29:513–521. [DOI] [PubMed] [Google Scholar]
- 15.Hadeed K, Hascoet S, Karsenty C, et al. Usefulness of echocardiographic-fluoroscopic fusion imaging in children with congenital heart disease. Arch Cardiovasc Dis. 2018;111:399–410. [DOI] [PubMed] [Google Scholar]
- 16.Abu Hazeem AA, Dori Y, Whitehead KK, et al. X-ray magnetic resonance fusion modality may reduce radiation exposure and contrast dose in diagnostic cardiac catheterization of congenital heart disease. Catheter Cardiovasc Interv. 2014;84:795–800. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez LF, Silva R, Ozturk C, et al. Technology preview: X-ray fused with magnetic resonance during invasive cardiovascular procedures. Catheter Cardiovasc Interv. 2007;70:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratnayaka K, Moore JW, Rios R, Lederman RJ, Hegde SR, El-Said HG. First-in-human closed-chest transcatheter superior cavopulmonary anastomosis. J Am Coll Cardiol. 2017;70:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuirt D, Mazal J, Rogers T, et al. X-ray fused with magnetic resonance imaging to guide endomyocardial biopsy of a right ventricular mass. Radiol Technol. 2016;87:622–626. [PMC free article] [PubMed] [Google Scholar]
- 20.Dori Y, Sarmiento M, Glatz AC, et al. X-ray magnetic resonance fusion to internal markers and utility in congenital heart disease catheterization. Circ Cardiovasc Imaging. 2011;4:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faranesh AZ, Kellman P, Ratnayaka K, Lederman RJ. Integration of cardiac and respiratory motion into MRI roadmaps fused with x-ray. Med Phys. 2013;40:032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer P, Faranesh A, Pohl T, et al. An MR-based model for cardiorespiratory motion compensation of overlays in X-ray fluoroscopy. IEEE Trans Med Imaging. 2018;37:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Washburn AE, Tavallaei MA, Pop M, et al. Real-time MRI guidance of cardiac interventions. J Magn Reson Imaging. 2017;46: 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell-Washburn AE, Rogers T, Stine AM, et al. Right heart catheterization using metallic guidewires and low SAR cardiovascular magnetic resonance fluoroscopy at 1.5 tesla: first in human experience. J Cardiovasc Magn Reson. 2018;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goreczny S, Dryzek P, Morgan GJ, Lukaszewski M, Moll JA, Moszura T. Novel three-dimensional image fusion software to facilitate guidance of complex cardiac catheterization: 3D image fusion for interventions in CHD. Pediatr Cardiol. 2017;38:1133–1142. [DOI] [PubMed] [Google Scholar]
- 26.Goreczny S, Dryzek P, Kim S, Sandoval JP, Morgan GJ, von der Wettern JM, Moszura T Three-Dimensional Image Fusion to Facilitate Guidance of Complex Cardiac Catheterizations in Congenital Heart Defects - Results from an International Multi-center Registry 52nd Annual Meeting of the Assoication for European Paediatric and Congenital Cardiology. Athens, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.