Abstract
Introduction:
Non-motor symptoms, including depression, anxiety, apathy and cognitive dysfunction, are common in Parkinson’s disease (PD). Although a link between mood symptoms and cognitive impairment in PD has been theorized vis-à-vis striatal dopamine depletion, studies have been inconsistent regarding the relationship between mood symptoms and cognitive function. Inconsistencies may reflect the cross-sectional nature of previous studies. The current study examined the bidirectional longitudinal relationship between mood and cognition.
Method:
Data were obtained from 310 individuals newly diagnosed with PD, who were followed up to four years (baseline, 1st, 2nd, 3rd and 4th annual follow-up). Apathy, anxiety, depressive symptoms, motor severity, and neurocognitive functioning were assessed at each annual assessment. The longitudinal relationship between apathy, anxiety, depressive symptoms, and cognition was analyzed with multi-level models.
Results:
Over the four-year period, more severe depressive symptoms were related to worse performance on tasks of processing speed, verbal learning and verbal delayed recall. Additionally, there was a significant depression X time interaction, suggesting that individuals with more severe depressive symptoms experience more rapid declines in global cognitive functioning and verbal learning. Apathy and anxiety were not significantly related to performance in any cognitive test. Lagged models revealed that changes in depression precede declines in working memory, verbal learning, delayed verbal recall and global cognition.
Conclusion:
Findings suggest depressive symptoms may be a harbinger for future cognitive decline among individuals with PD.
Keywords: Parkinson’s disease, depression, apathy, anxiety, cognition, cognitive impairment
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by motor dysfunction, as well as various neuropsychiatric symptoms (Alexander, DeLong & Strick, 1986; Kulisevsky et al., 2008). Anxiety, apathy and depression in PD may be partly attributable to disruption of dopaminergic neurons in the substantia nigra that leads to downstream disruption of frontal-striatal circuits important for various functions, such as emotional and cognitive functioning.
Traditionally, neurocognitive deficits in PD have been conceptualized as primarily representing a “frontal pattern” of impairment, and includes problems with working memory, executive function and slowed processing speed (Levin & Katzen, 1995; Zgaljardic et al., 1986). However, other studies have challenged this view and have emphasized the role of a “posterior pattern” of cognitive decline (e.g., impairments in construction or semantic fluency) in predicting future cognitive impairment and PD dementia (PDD; Williams-Gray et al., 2009). Regardless of the pattern, cognitive impairments in PD are common, as up to 80% of individuals will develop PDD within 15–20 years following initial symptom presentation (Hely et al., 2008).
Neuropsychiatric symptoms, such as anxiety, apathy and depression are also common in PD. These symptoms commonly overlap in their symptom presentation, but can be dissociable and may represent separate underlying mechanisms (Kirsch-Darrow et al., 2006; Kirsch-Darrow et al., 2011; Leentjens et al., 2011). Anxiety may be the most common mood symptom in PD. The prevalence rate of anxiety disorders and PD is between 20%–60% (Dissanayak et al., 2010; Pontone et al., 2009; Alzahrani & Venneri, 2015). Generalized anxiety disorder, panic disorder, and social anxiety are the most reported anxiety disorders (Dissanayak et al., 2010). Apathy is defined as impaired motivation and goal-oriented behavior and is more common among individuals with PD than in the general population (Starkstein et al., 1992). Depression is characterized by sad mood, loss of interest, difficulty concentrating, and alterations in sleep and appetite, and often overlaps with other symptoms of PD (American Psychiatric Association, 2013).
Regarding etiology, all symptoms have been linked to disruptions of circuits important for cognitive functioning. The relationship between anxiety and cognitive functioning is hypothesized to be mediated by: 1) dysregulation of frontal-striatal networks, and/or 2) disruption the hypothalamic-pituitary-adrenal (HPA) axis leading to elevated cortisol and hippocampal disruption (Pirogovsky et al., 2017). Apathy may be related to striatal dopamine depletion disrupting frontal-subcortical circuits, including prefrontal regions involved in motivation and initiation (Alexander, DeLong & Strick, 1986; Pagonabarraga et al., 2015; Starkstein et al., 1992). Neuroimaging studies have shown that depression is linked to changes within the limbic system, particularly regions important for memory and cognitive functioning such as the hippocampus and amygdala (Thobois et al., 2017). Additionally, depression in PD may be linked with disruptions of neurotransmitter systems, such as serotonin, norepinephrine, and acetylcholine (Maillet et al., 2016).
Two studies have investigated the relationship between anxiety, apathy, depression and cognitive functioning (Jones et al., 2016; Pirogovsky et al., 2017). Both studies provided preliminary evidence that anxiety and depression, but not apathy may be related to cognitive functioning. However, the findings in the literature are much more variable when studies only examine one or two of these constructs. For example, a number of cross sectional studies have examined the relationship between anxiety and cognitive functioning. Some studies exclusively measured anxiety, but not depression or apathy, and found that greater anxiety was associated with poorer set-shifting (Reynolds et al., 2017) or memory (Dissanayaka et al., 2017). Other cross-sectional studies have examined the independent effects of anxiety and apathy/depression and have found an independent relationship between anxiety and cognition (Ehgoetz Martens et al., 2016; Ehgoetz Martens et al., 2018; Ryder et al., 2002; Wan Mohamed, Din & Ibrahim, 2015). In general, these studies found that higher anxiety was related to worse performance across various cognitive domains, including general cognitive screeners and measures of attention/working memory, executive functioning, visuospatial abilities, learning and memory. There have been fewer longitudinal studies of anxiety and cognition, but findings have suggested baseline anxiety to be associated with worsening cognitive functions (Hu et al., 2014;) and others have found evidence for the inverse directionality-worse cognition at baseline predicts future anxiety (Petkus et al., 2019; Rutten et al., 2017).
Regarding apathy and depression, at least five past studies have examined the relationship/intersectionality between apathy, depression and cognition in PD (Butterfield et al., 2010; Jones et al., 2016; Pirogovsky et al., 2017; Szymkowicz et al., 2017; Varanese et al., 2011). However, findings have generally been inconsistent to whether cognition has a stronger relationship with apathy or depression. Two cross-sectional studies have found that apathy, but not depression, related to worse performance on tasks of immediate verbal memory recall and executive functioning (Butterfield et al., 2010; Varanese et al., 2011). Alternatively, three other studies have shown cognitive impairment to be related to depression, but not apathy (Jones et al., 2016; Pirogovsky et al., 2017; Szymkowicz et al., 2017).
Among studies examining the relationship between mood symptoms and cognitive functioning, discrepant findings may be related to the fact that existing studies are primarily cross-sectional in nature and do not account for the high comorbidity among anxiety, depression and apathy. Therefore, it is impossible to ascertain the longterm bidirectional and independent effects of the mood symptoms on cognitive functioning in PD. The present study addressed this gap in the literature by examining the longitudinal relationships between anxiety, apathy, depression and cognition in a large sample of individuals newly diagnosed with PD over a four-year period. Additionally, we inspected the bidirectional cross-lagged relationship in order to determine if changes in apathy, anxiety and/or depressive symptoms precede changes in cognition, or vice-versa.
METHODS
Study Design
Data were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data).·The PPMI is a longitudinal multi-site study of newly diagnosed (two years or less), untreated PD patients. Further details of the study have been published (Marek et al., 2011). The study was approved by the institutional review board at each site and participants provided written informed consent.
The current sample included 310 individuals newly diagnosed with PD, who underwent cognitive testing and were followed for up to four years (baseline, 1st, 2nd, 3rd and 4th annual follow-up). We excluded participants <50 years of age (N=53) and non-PD controls. We excluded participants <50 years of age because the relationship between mood symptoms and cognitive functioning is hypothesized to be driven by similar mechanisms (i.e. disruption of similar neural circuits; Butterfield et al., 2010; Jones et al., 2016; Pirogovsky et al., 2017; Szymkowicz et al., 2017; Varanese et al., 2011); however there is evidence that young onset-PD patients (before the age of 50) experience greater psychosocial stressors (loss of employment, perceived stigma, family disruptions), which may contribute to depressive symptoms (Schrag et al., 2003). Therefore, we excluded participants <50 in order to remove the possible confound that depression is differentially driven by separate mechanisms (i.e. psychosocial contributors versus neurobiological contributors).
Neurocognitive Assessment
Participants completed neurocognitive tests at each annual assessment. The current study utilized tests of global cognitive functioning (Montreal Cognitive Assessment; MoCA; Nasreddine et al., 2005), working memory (Letter-Number Sequencing; LNS; Wechsler, 2008), processing speed (Symbol Digit Modalities Test; SDMT; Smith, 1982), visuospatial functioning (Judgment of Line Orientation test; JOLO; Benton et al., 1983), language/semantic fluency (Animal Fluency; Heaton et al., 2004), learning/immediate verbal memory (Hopkins Verbal Learning Test-Revised; HVLT-R trials 1–3; Brandt & Benedict, 2001), and delayed verbal recall (HVLT-R delayed free recall trial; Brandt & Benedict, 2001).
Depression, Anxiety, Apathy and Motor Severity
Depressive symptoms were assessed with the 15-item version of the Geriatric Depression Scale (GDS; Sheikh & Yesavage,1986). The GDS is a self-report questionnaire, whereby participants rate the presence or absence of depressive symptoms over the previous week. Total scores range from 0–15, with a score of ≥5 indicating clinically significant depression in PD (Weintraub et al., 2006).
Anxiety symptoms were assessed with the trait anxiety subscale of the State-Trait Anxiety Scale (STAI-T; Spielberger et al., 1983). The STAI-T consists of 20 self-report items assessing general/chronic symptoms of anxiety. A decision was made to only examine trait anxiety because the current paper focuses on relatively stable mood symptoms, as opposed to anxiety that is reactionary/transient. This approach is consistent with past studies of anxiety and clinical outcomes in PD (Jones et al., 2014a; Jones et al., 2014b; Jones et al., 2016).
Apathy was assessed with item four from the Unified Parkinson’s Disease Rating Scale (UPDRS). Item four assesses motivation/initiation on a five-point scale (0–4) with increasing scores indicating greater apathy. A review of apathy scales by the Movement Disorder Society classified UPDRS item four as a recommended measure of apathy, with a score of ≥2 indicating clinically significant apathy (Leentjens et al., 2008b; Starkstein & Merello, 2007). The UPDRS also contains single items assessing anxiety and depression. The depression and anxiety items were used in exploratory analyses.
Motor symptom severity was assessed with the Unified Parkinson’s Disease Rating Scale, part III (UPDRS-III), with higher scores indicating greater motor severity. Data about levodopa equivalent dose (LED) within the PPMI sample has previously been published (Eusebi et al., 2018).
Statistical Analyses
Multi-level models (MLM) were used to analyze the longitudinal relationship between apathy, anxiety, depression and cognitive functioning. Full-information, maximum-likelihood parameter estimation was used to account for missing data. Models were computed using IBM SPSS Statistics Version 24.
For Aim 1, MLM analyses were computed with the cognitive outcome (MoCA, LNS, SDMT, JOLO, animal fluency, HVLT-R learning and delayed recall) entered as the dependent variable, for a total of seven analyses. Predictors included: depressive symptoms, anxiety, apathy, time (baseline, 1st, 2nd, 3rd and 4th annual follow-up), UPDRS motor scores, age, education, and gender. In addition to the main effects of depressive symptoms, anxiety, apathy and time, we computed interaction terms: depression X time, anxiety X time, and apathy X time terms. These interaction terms were also included as predictors and examined whether participants with higher or lower depressive/anxiety/apathy symptoms had different trajectories of cognitive functioning over time (i.e., was the rate of cognitive decline faster or slower).
Aim 2 examined the cross-lagged relationship between depression, anxiety, apathy and cognitive functioning (i.e. do mood symptoms predict future changes in cognitive functioning, and/or does cognitive functioning predict future changes in mood symptoms). Similar MLM analyses were computed from Aim 1, except the depression, anxiety and apathy terms were replaced with a lagged depression, anxiety, and apathy term. The lagged variables were defined as depression/anxiety/apathy at the previous assessment (i.e. depression/anxiety/apathy at time 1 predicts cognition at time 2; depression/anxiety/apathy at time 2 predicts cognition at time 3, etc.). Age, education, gender, UPDRS motor scores, and time were also entered into the model. The lagged variables examined whether cognitive functioning was predicted by depressive symptoms, anxiety or apathy at the previous assessment (i.e., did changes in depressive symptoms/anxiety/apathy precede changes in cognitive functioning?). If a lagged mood variable (either depression, anxiety or apathy) significantly predicted cognitive functioning, then further MLMs were computed to examine the alternative directionality (i.e., did change in cognitive functioning predict future changes in depression/anxiety/apathy).
Exploratory analyses were also conducted utilizing the UPDRS items assessing depression, anxiety and apathy. These analyses examined if differences in modality (i.e. a single rating item versus standardized questionnaire) explain why cognition may relate to some mood constructs but not others. MLM analyses were repeated similar to aim 1, but the UPDRS depression and anxiety items replaced the GDS and STAI-T variables.
For all analyses, all variables were transformed to a z-score metric, based on the sample’s mean and standard deviation. This approach preserves all within-person and between-person differences and produces coefficients that can be interpreted similar to traditional standardized regression coefficients (Bryk and Raudenbush, 1992).
RESULTS
Sample and Clinical Characteristics
Sample and clinical characteristics at baseline are displayed in Table 1 (see Jones et al., 2017 for description of attrition). Longitudinal clinical characteristics are displayed in Supplemental Table 1. In predictor-free models, the intraclass correlation coefficient [ICC; σ2 (between) / σ2 (between+within)] was computed to serve as an index of within-person and between-person variability to be explained in cognitive test (the ICC represents the between-person effect, and the inverse represent how much of the variance is a within-person effect). The ICC was 0.504 for the MoCA, 0.644 for working memory, 0.700 for processing speed, 0.552 for visuospatial functioning, 0.624 for semantic fluency, 0.600 for learning/immediate verbal memory, and 0.608 for delayed verbal recall; meaning that 49.6% (MoCA), 35.6% (working memory), 30.0% (processing speed), 44.8% (visuospatial functioning), 37.6% (semantic fluency), 40.0% (learning/immediate verbal memory), and 39.2% (delayed verbal recall) of the overall variance was a within-person (i.e., overtime) effect.
Table 1.
Mean Baseline Sample Characteristics
| N = 310 | ||
|---|---|---|
| Mean | Standard Deviation | |
| Age | 62.9 | 7.5 |
| Percent Male | 62.6 | --- |
| Education (years) | 15.6 | 3.0 |
| UPDRS-III | 20.1 | 9.0 |
| Montreal Cognitive Assessment | 27.3 | 1.8 |
| Letter Number Sequencing | 10.6 | 2.5 |
| Symbol Digit Modalities Test | 41.36 | 9.4 |
| Line Orientation | 13.0 | 1.9 |
| Animal Fluency | 21.2 | 5.6 |
| HVLT-R Learning | 24.8 | 4.7 |
| HVLT-R Delayed Recall | 8.5 | 2.4 |
| Geriatric Depression Scale | 2.4 | 2.7 |
| STAI-T | 33.0 | 11.1 |
| UPDRS- Apathy Item | 0.20 | 0.51 |
UPDRS-III = Unified Parkinson’s Disease Rating Scale motor score; HVLT-R = Hopkins Verbal Learning Test- Revised; STAI-T = State-Trait Anxiety Inventory- Trait subscale.
Aim 1: Relationship between Mood and Cognitive Functioning
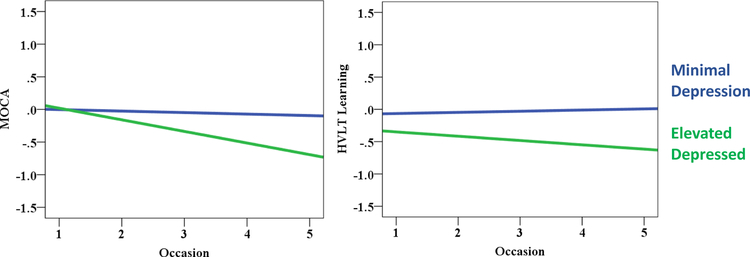
Table 2 displays the MLM results for depressive, anxiety and apathy symptoms predicting cognitive functioning. More severe depressive symptoms significantly predicted worse performance on tests of processing speed, verbal learning, and verbal delayed recall. Additionally, the depressive symptoms X time interaction term was a significant predictor of MoCA and verbal learning performance. Specifically, individuals with more severe depressive symptoms experienced a more rapid decline in MoCA and verbal learning scores (Figure 1).
Table 2.
Multilevel Models Predicting Cognition from Depression, Anxiety & Apathy
| Predictors | SDMT | HVLT Learning | HVLT Delay Recall | MoCA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | ||||||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| −0.073 | 0.026 | 0.007 | −0.078 | 0.031 | 0.015 | −0.091 | 0.035 | 0.011 | −0.012 | 0.038 | 0.746 | |
| Anxiety | −0.025 | 0.024 | 0.293 | 0.018 | 0.027 | 0.501 | −0.001 | 0.027 | 0.969 | −0.027 | 0.029 | 0.348 |
| Apathy | −0.034 | 0.021 | 0.112 | 0.005 | 0.024 | 0.846 | −0.030 | 0.025 | 0.230 | −0.045 | 0.027 | 0.102 |
| Depression X Occasion | −0.019 | 0.022 | 0.385 | −0.056 | 0.026 | 0.031 | 0.007 | 0.027 | 0.791 | −0.074 | 0.030 | 0.013 |
| Anxiety X Occasion | −0.024 | 0.019 | 0.203 | 0.031 | 0.022 | 0.159 | −0.010 | 0.023 | 0.669 | −0.021 | 0.025 | 0.400 |
| Apathy X Occasion | −0.025 | 0.022 | 0.262 | −0.002 | 0.023 | 0.918 | −0.043 | 0.023 | 0.061 | 0.016 | 0.027 | 0.563 |
| Time | −0.044 | 0.017 | 0.009 | −0.009 | 0.019 | 0.643 | −0.015 | 0.019 | 0.423 | −0.065 | 0.022 | 0.004 |
| Motor Severity | −0.118 | 0.023 | <0.001 | −0.023 | 0.027 | 0.385 | 0.015 | 0.027 | 0.587 | −0.056 | 0.029 | 0.056 |
| Age | −0.410 | 0.052 | <0.001 | −0.317 | 0.054 | <0.001 | −0.329 | 0.056 | <0.001 | −0.354 | 0.055 | <0.001 |
| Gender | 0.343 | 0.082 | <0.001 | 0.462 | 0.085 | <0.001 | 0.445 | 0.089 | <0.001 | 0.261 | 0.087 | 0.003 |
| Education | 0.208 | 0.039 | <0.001 | 0.193 | 0.040 | <0.001 | 0.196 | 0.042 | <0.001 | 0.104 | 0.041 | 0.012 |
| Between Pseudo R2 | 0.377 | Between Pseudo R2 | 0.354 | Between Pseudo R2 | 0.300 | Between Pseudo R2 | 0.213 | |||||
| Within Pseudo R2 | 0.212 | Within Pseudo R2 | 0.049 | Within Pseudo R2 | 0.098 | Within Pseudo R2 | 0.195 | |||||
Significant p values appear in bold; only models where depression, anxiety or apathy significantly predicted cognition are shown. SDMT = Symbol Digit Modality Test; HVLT = Hopkins Verbal Learning Test; MoCA = Montreal Cognitive Assessment.
Figure 1.
Relationship between cognitive functioning and depression status. Cognitive values are depicted in a z-score metric. Depression status was determined from the Geriatric Depression Scale (GDS) score (cut point = 5; Weintraub et al., 2006). Note: MLM analyses utilized GDS scores as a continuous variable. MOCA = Montreal Cognitive Assessment; HVLT = Hopkins Verbal Learning Test.
The main effect of apathy and anxiety, or the interaction terms (i.e. apathy X occasion or anxiety X occasion) did not significantly predict any cognitive domain (all p values > 0.05). Working memory, visuospatial and animal fluency performance were not significantly predicted by any mood construct (Supplementary Table 2).
Regarding additional predictors, the main effect of time predicted worsening processing speed, working memory, and total MoCA score (i.e., performance worsened over time); more severe motor symptoms predicted worse processing speed; male gender predicted worse performance on processing speed, verbal learning, verbal delayed recall, visuospatial functioning, and the MoCA; older age and fewer years of education predicted worse performance across all domains assessed.
Aim 2: Bidirectional Lagged Relationship between Depression, Anxiety and Apathy Symptoms and Cognitive Functioning
Results from the MLM analyses predicting cognitive functioning from lagged depression, anxiety and apathy terms are displayed in Table 3. The lagged depressive symptom term significantly predicted performance on working memory, verbal learning, delayed verbal recall and the MoCA. Specifically, more severe depressive symptom scores predicted declines in cognitive performance at the future/subsequent assessment. The lagged apathy and anxiety terms did not significantly predict performance in any cognitive domain (all p values > 0.05). Processing speed, visuospatial and animal fluency performance were not significantly predicted by any mood construct (Supplementary Table 3).
Table 3.
Lagged Model Predicting Cognition
| LNS | HVLT Learning | HVLT Delayed Recall | MoCA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | ||||||||||||
| Predictors | ||||||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| −0.089 | 0.030 | 0.003 | −0.096 | 0.032 | 0.003 | −0.078 | 0.033 | 0.017 | −0.126 | 0.034 | <0.001 | |
| Apathy Lagged | 0.002 | 0.021 | 0.930 | −0.003 | 0.024 | 0.893 | 0.001 | 0.022 | 0.950 | −0.014 | 0.028 | 0.621 |
| Anxiety Lagged | −0.001 | 0.019 | 0.955 | −0.005 | 0.022 | 0.808 | 0.015 | 0.021 | 0.486 | 0.018 | 0.022 | 0.429 |
| Occasion | −0.059 | 0.026 | 0.022 | 0.056 | 0.028 | 0.051 | −0.005 | 0.028 | 0.861 | 0.010 | 0.031 | 0.740 |
| Motor Severity | 0.006 | 0.029 | 0.829 | −0.038 | 0.031 | 0.231 | −0.003 | 0.032 | 0.926 | −0.047 | 0.033 | 0.162 |
| Age | −0.364 | 0.059 | <0.001 | −0.359 | 0.058 | <0.001 | −0.374 | 0.062 | <0.001 | −0.397 | 0.061 | <0.001 |
| Gender | 0.056 | 0.094 | 0.552 | 0.443 | 0.092 | <0.001 | 0.450 | 0.099 | <0.001 | 0.315 | 0.096 | 0.001 |
| Education | 0.111 | 0.044 | 0.013 | 0.182 | 0.043 | <0.001 | 0.185 | 0.046 | <0.001 | 0.090 | 0.045 | 0.049 |
| Between Pseudo R2 | 0.150 | Between Pseudo R2 | 0.228 | Between Pseudo R2 | 0.128 | Between Pseudo R2 | 0.033 | |||||
| Within Pseudo R2 | 0.160 | Within Pseudo R2 | 0.066 | Within Pseudo R2 | 0.115 | Within Pseudo R2 | 0.219 | |||||
Significant p values appear in bold; only models where depression, anxiety or apathy significantly predicted cognition are shown. LNS = Letter Number Sequencing; HVLT = Hopkins Verbal Learning Test; MOCA = Montreal Cognitive Assessment.
Additional MLMs examined the alternative directionality of the above analyses (i.e., did changes in cognitive performance precede changes in depressive symptoms). Results revealed that only lagged working memory performance was related to changes in depressive symptom scores (Table 4). Specifically, worse working memory performance predicted worsening of depressive symptoms at the subsequent assessment. No other lagged cognitive scores were found to be significant predictors of depressive symptom severity (verbal learning, delayed verbal recall or MoCA; all p values > 0.200).
Table 4.
Lagged Model Predicting Depression
| Predictors | Fixed Effects | ||
|---|---|---|---|
| B | SE | p | |
| −0.083 | 0.039 | 0.033 | |
| Occasion | −0.021 | 0.029 | 0.467 |
| Motor Severity | 0.105 | 0.033 | 0.001 |
| Age | −0.035 | 0.065 | 0.592 |
| Gender | −0.095 | 0.103 | 0.355 |
| Education | −0.119 | 0.049 | 0.015 |
Significant p values appear in bold; only models where depression, anxiety or apathy significantly predicted cognition are shown. LNS = Letter Number Sequencing
Exploratory Analyses: Relationship between Cognition and UPDRS Mood Items.
In aims 1 and 2, depression and anxiety were assessed by self-report questionnaires, but apathy was rated with a single item. Therefore, exploratory analyses were conducted to examine if differences in modalities (self-report questionnaire versus single rated item) confounded the relationship between apathy and cognition (Supplemental Table 4). Worse delayed verbal recall was significantly associated with worse depression (i.e. main effect of UPDRS-Depression item). Additionally, processing speed was significantly predicted by the UPDRS-Depression X Occasion interaction term; meaning that individuals with more severe depressive symptoms experienced a more rapid decline in processing speed.
The apathy and anxiety UPDRS items were not significantly related to any cognitive domain.
DISCUSSION
Past research investigating the relationship between mood symptoms and cognition in PD have primarily been cross-sectional in nature. Findings have generally been mixed with regards to whether anxiety, apathy or depressive symptoms more strongly predict cognitive functioning. Additionally, the majority of the past literature has focused on only one or two of these mood constructs, and therefore were unable to account for the high comorbidity of mood symptoms (Kirsch-Darrow et al., 2006; Kirsch-Darrow et al., 2011; Leentjens et al., 2011).
The current study expands upon the past literature by examining the bidirectional longitudinal relationship between apathy, anxiety, depressive symptoms, and cognitive functioning. In general, we found that cognitive functioning over a four-year period was related to depressive symptoms, but not to apathy or anxiety. Furthermore, findings generally suggest a uni-directional relationship, such that changes in depressive symptoms precede changes in cognitive functioning.
Multiple studies have found a link between cognitive functioning and depression in PD, with greater cognitive decline observed among depressed PD patients than those without depressive symptomatology (Hurtado-Gonzalez et al., 2018; Jones et al., 2016; Pirogovsky et al., 2017; Szymkowicz et al., 2017). Impairments are most commonly reported on tests of executive functioning, learning and memory. However, other studies have not found a significant relationship between cognitive functioning and depression (Ng et al., 2015). In the current study we found that depression (either the main effect or the interaction term) predicted performance on tests of speed, learning and delayed recall; furthermore, the lagged depression term also predicted performance in working memory, learning and delayed recall. On the other hand, depression was not predictive of performance on tasks of language and visuospatial functioning. These findings may highlight a contrast between a “frontal” versus a “posterior” pattern of cognitive impairment. A past study of newly diagnosed PD patients differentiated between a “posterior pattern” and “frontal pattern” of cognitive impairment (Williams-Gray et al., 2009). Specifically, tests of posterior functioning (semantic fluency and pentagon copying) were predictive of a genetic marker of tau pathology (MAPT H1/H1 genotype) and early dementia risk; whereas other tests (memory, speeded executive functioning and attention) were not predictive of these outcomes. The pattern of findings in the current study may suggest that depression is more predictive of a “frontal” (or frontal-temporal) profile as opposed to the “posterior” profile highlighted in the Williams-Gray et al., (2009) study.
Past neuroimaging studies also highlight the interplay between depression, cognitive functioning and fronto-temporal dysfunction. Longitudinal voxel-based morphometry studies revealed a significant association between depression and reduced amygdaloid, bilateral medial orbito-frontal cortex, and hippocampal volumes among individuals with PD (Goto et al., 2017). Similarly, some studies found a correlation between depression and reduced white and gray matter volume in orbitofrontal, temporal, and limbic regions (Feldmann et al., 2008; Wu et al., 2018). Functional imaging studies have also reported that depression was inversely correlated with connectivity in prefrontal-limbic circuits among individuals with PD (Cardoso et al., 2009; Wen et al., 2013).
This study shows evidence that changes in depression precede changes in certain cognitive domains (working memory, verbal learning and recall, and global cognitive functioning). These findings suggest that depression may be driving cognitive impairment and is consistent with some, but not all, studies of the healthy aging population. Specifically, four studies support the same directionality of findings in the current study but some mixed findings remain. At least four studies have found that depression predicts a decline in cognitive functioning in older adults (Chi & Chou, 2000; Gallagher et al., 2016; Pantzar et al., 2017; Bielack et al., 2007). One longitudinal study found that greater scores on the Center for Epidemiologic Studies Depression Scale (i.e. more symptoms of depression) at baseline predicted lower scores on a global measure of cognitive performance three years later (Chi & Chou, 2000). A second study found that higher more severe depressive symptoms at baseline significantly predicted declines in delayed recall and verbal fluency; the median duration of follow-up was 47 months later (Gallagher et al., 2016). Another longitudinal study found that processing speed, executive functioning, category fluency, and attention were predicted by depression, with those transitioning from a healthy to a depressed state showing the largest decline in cognitive functioning at three and six year follow-ups (Pantzar et al., 2017). A fourth study exclusively assessed processing speed with follow-up assessments at 2, 8, 11, and 15 years, and found that depressive symptoms predicted subsequent decline in perceptual speed (Bielack et al., 2007). Alternatively, one study found evidence of the reverse directionality. Specifically, poorer delayed recall at baseline, as well as steeper decline in processing speed, predicted worsening depression over a 13 year period (Brailean et al., 2017). Finally, one longitudinal study found that while cognitive functioning and depressive symptoms were related to each other, there was no evidence of directionality (i.e. depression did not predict future cognitive changes and vice-versa; Dzierzewski et al., 2015). Therefore, findings are quite mixed among studies examining the directional relationship between depression and cognition.
In PD, depression is commonly under-recognized and a large percentage of patients continue to report significant symptoms of depression despite receiving psychiatric treatment (Seppi et al., 2011). One clinical implication from the current study is that better recognition and treatment of depression may help reduce the risk for future cognitive decline. Improved control of depression and cognitive impairment may be particularly important to patients, as past studies have shown depression and cognitive functioning to be independently related to patients’ self-reported quality of life (Jones et al., 2014a; Jones et al., 2014b; Jones et al., 2015;).
Additionally, we found a bi-directional relationship between working memory and depression; suggesting a possible cyclical relationship where working memory impairment contributes to depression, which further contributes to worse working memory. A bidirectional relationship suggests that a combination of psychiatric and cognitive-enhancing therapies may lead to optimal control of each respective symptom. This idea is consistent with a past study of individuals with major depressive disorder, which showed that cognitive training combined with transcranial direct current stimulation lead to a better treatment response relative to either individual treatment modality (Segrave, et al., 2014).
In the current study, we did not find a significant relationship between anxiety, apathy and cognitive functioning. In regards to anxiety and cognitive functioning, past studies have linked anxiety symptoms (independent of depression and/or apathy) to worse cognitive functioning (Ehgoetz Martens et al., 2016; Ehgoetz Martens et al., 2018; Jones et al., 2016; Pirogovsky, et al., 2017; Ryder et al., 2002; Wan Mohamed, Din & Ibrahim, 2015). Specifically, Pirogovsky and others (2017) found that more severe anxiety symptoms at baseline were predictive of declines in visual learning (but not a 2-year period. In cross-sectional studies, anxiety is associated with a greater prevalence of mild cognitive impairment and worse performance on tests of working memory, executive functioning, language and memory (Ehgoetz Martens et al., 2016; Ehgoetz Martens et al., 2018; Jones et al., 2016; Pirogovsky, et al., 2017; Ryder et al., 2002; Wan Mohamed, Din & Ibrahim, 2015). Discrepancies between the current study and past studies may be due to differences in sample characteristics (the current sample consistent of newly diagnosed PD patients), study durations and/or study measures. Specifically, the current study was limited in measures of executive functioning. The current study was able to assess constructs (e.g., working memory, semantic fluency) that are sometimes subsumed under the executive functioning domain. However, anxiety is frequently associated with tests of set-shifting, behavioral inhibition and letter fluency, which were not available in this secondary data analysis (Ehgoetz Martens et al., 2016; Ehgoetz Martens et al., 2018; Reynolds, et al., 2017).
Regarding the possible relationship between apathy and cognition, previous authors have described apathy as an impairment of systems involved in planning, working memory, and set-shifting, which are required for self-initiation of goal-directed behaviors (Levy & Dubois, 2006). Similar to depression, findings regarding the relationship between apathy and cognition among PD patients are mixed (Butterfield et al., 2010; Jones et al., 2016; Szymkowicz et al., 2017; Varanese et al., 2011; Pirogovsky, et al., 2017). A recent meta-analysis found a moderate association between apathy and global cognition, delayed verbal memory, processing speed, attention, and executive functioning in patients with PD (D’lorio et al., 2018). It is worth noting that among studies examining the intersectionality of apathy, depression and cognitive functioning, the variability in findings may be accounted for by differences in assessment measures. Studies finding a significant association between apathy and cognitive functioning utilized the Apathy Evaluation Scale (AES; Butterfield et al., 2010; Varanese et al., 2011), whereas studies with a null relationship utilized the Apathy Scale (AS; Jones et al., 2016; Szymkowicz et al., 2017; Pirogovsky, et al., 2017). Although there is similarity between the AES and AS (i.e. both are self-report measures utilizing a four-point Likert scale), the AS was designed to reduce patient demands (Leentjens et al., 2008b), by containing fewer items (14 vs. 18) and having the questions read aloud to the participant. Additionally, a review by the Movement Disorders Society found the AS to have acceptable criterion validity in PD, while information on validity was not available for the AES (Leentjens et al., 2008a). Discrepancies between the current study and past studies of apathy and cognitive functioning may also be due to differences in cognitive measures (the current study was limited in measures of executive functioning, which may be particularly sensitive to apathy) or differences in patient population (the current study consisted exclusively of newly diagnosed PD patients).
Studies have generally hypothesized the relationship between apathy, anxiety and cognitive functioning is mediated by disruption of fronto-striatal circuits involving frontal cortical regions important for both cognitive functioning (such as the dorsolateral prefrontal cortex) and motivation/goal directed behavior (such as the anterior cingulate cortex; Kish et al., 1988; Owen, 2004). However, more recent studies have challenged the view that striatal-dopamine dysfunction is the primary driver of neuropsychiatrie symptoms in PD and have focused on the role of serotonergic systems in apathy (Maillet et al., 2016). Anxiety may additionally be related to dysregulation of the HPA axis, which subsequently leads to elevated cortisol and hippocampal dysregulation (Pirogovsky et al., 2017).
Exploratory analyses inspected if the lack of a significant relationship between apathy and cognitive functioning is partially related to differences in modalities (i.e. apathy was assessed with a single clinician rated item, while depression and anxiety were assessed with self-report questionnaires). Depression continued to be the only mood construct that predicted cognitive performance when the UPDRS mood items were utilized in the exploratory analyses. However, the depression item (either the main effect or the interaction term) was only predictive of 2/7 cognitive tests, as opposed to 4/7 when the full self-report questionnaire was used. This may suggest the null relationship between apathy and cognitive functioning may be partially, but unlikely fully, explained by utilization of a single rated item.
Limitations in this study include that apathy was assessed with a single-item, which may reduce variability and reduce our ability to parse out apathy symptoms from depressive symptoms. While the use of a single item may be a weakness, it is worth mentioning that a review of apathy measures by the Movement Disorder Society found that UPDRS item four had suitable psychometric properties (Leentjens et al., 2008b). Additionally, as is true with all PPMI studies, the sample consisted of participants newly diagnosed with PD. Findings may not generalize to the entire PD population. The current study was a secondary data-analysis limited to cognitive tasks administered as part of the PPMI. The current study was able to assess constructs (e.g., working memory, semantic fluency) that are sometimes subsumed under the executive functioning domain. However, future studies may benefit from utilizing additional tasks of executive functions (e.g., set-shifting, letter fluency, behavioral inhibition, novel-task problem solving) to fully parse apart the relationships between mood symptoms and this cognitive domain.
To the best of our knowledge, this is the largest longitudinal investigation of the independent effects of apathy, anxiety and depressive symptoms on cognitive functioning in PD. Findings suggest depression may be a harbinger for future cognitive decline among individuals with PD.
Supplementary Material
Public Significance Statement:
Non-motor symptoms such as depression, anxiety, apathy and cognitive impairment are common in Parkinson’s disease. The current study demonstrated that more severe symptoms of depression are related to worse cognitive functioning over the four-year study period. Findings suggest that depression may be a harbinger for future cognitive decline among individuals with Parkinson’s disease.
Acknowledgements/ Study Funding
Jacob Jones, Ph.D. was supported by NIH T32MH019535.
Taylor Kuhn, Ph.D. was supported by NIH 1U01AG052564–01.
Joseph Bunch was supported by NIH T34GM083883.
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen Idec, BioLegend, Bristol-Meyers Squibb, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer Inc., Piramal Imaging, Roche group, Sanofi-Genzyme, Servier, Takeda, TEVA, and UCB.
We would like to thank the California State University San Bernardino Institute for Child Development and Family Relations, and the Faculty Center for Excellence for supporting the publication of this paper with funded writing time.
References
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience, 9(1), 357–381. [DOI] [PubMed] [Google Scholar]
- Alzahrani H, & Venneri A (2015). Cognitive and neuroanatomical correlates of neuropsychiatrie symptoms in Parkinson’s disease: a systematic review. Journal of the neurological sciences, 356(1–2), 32–44. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric Pub. [Google Scholar]
- Benton AL, Hamsher KD, Varney NR, & Spreen O (1983). Judgment of line orientation. New York: Oxford University Press. [Google Scholar]
- Brandt J, & Benedict RHB (2001). Hopkins Verbal Learning Test - Revised, Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Bryk AS, & Raudenbush SW (1992). Hierarchical linear models for social and behavioral research: Applications and data analysis methods.
- Butterfield LC, Cimino CR, Oelke LE, Hauser RA, & Sanchez-Ramos J (2010). The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology, 24(6), 721. [DOI] [PubMed] [Google Scholar]
- Cardoso EF, Maia FM, Fregni F, Myczkowski ML, Melo LM, Sato JR,… & Amaro E Jr (2009). Depression in Parkinson’s disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage, 47(2), 467–472. [DOI] [PubMed] [Google Scholar]
- D’lorio A, Maggi G, Vitale C, Trojano L, & Santangelo G (2018). “Pure apathy” and cognitive dysfunctions in Parkinson’s disease: A meta-analytic study. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- Dissanayaka NN, Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK,… & ICICLE-PD study group. (2017). Anxiety is associated with cognitive impairment in newly-diagnosed Parkinson’s disease. Parkinsonism & related disorders, 36, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka NN, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, … & Mellick GD (2010). Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Movement Disorders, 25(7), 838–845. [DOI] [PubMed] [Google Scholar]
- Ehgoetz Martens KA, Silveira CR, Intzandt BN, & Almeida QJ (2018). State anxiety predicts cognitive performance in patients with Parkinson’s disease. Neuropsychology, 32(8), 950. [DOI] [PubMed] [Google Scholar]
- Ehgoetz Martens KA, Szeto JYY, Muller AJ, Hall JM, Gilat M, Walton CC, & Lewis SJG (2016). Cognitive function in Parkinson’s disease patients with and without anxiety. Neurology research international, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi P, Romoli M, Paoletti FP, Tambasco N, Calabresi P, & Parnetti L (2018). Risk factors of levodopa-induced dyskinesia in Parkinson’s disease: results from the PPMI cohort, npj Parkinson’s Disease, 4(1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann A, Illes Z, Kosztolanyi P, Illes E, Mike A, Kover F,… & Nagy F (2008). Morphometrie changes of gray matter in Parkinson’s disease with depression: a voxel-based morphometry study. Movement Disorders, 23(1), 42–46. [DOI] [PubMed] [Google Scholar]
- Goto M, Kamagata K, Hatano T, Hattori N, Abe O, Aoki S, … & Gomi T (2018). Depressive symptoms in Parkinson’s disease are related to decreased left hippocampal volume: correlation with the 15-item shortened version of the Geriatric Depression Scale. Acta Radiologica, 59(3), 341–345. [DOI] [PubMed] [Google Scholar]
- Hu MT, Szewczyk-Królikowski K, Tomlinson P, Nithi K, Rolinski M, Murray C, … & Ben-Shlomo Y (2014). Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Movement Disorders, 29(3), 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM „ & Morris JG (2008). The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disorders, 23(6), 837–844. [DOI] [PubMed] [Google Scholar]
- Hurtado-Gonzalez CA, Trivino O, Rinco A, Seminec D, & Olayo BRI (2018). Influence of depression on the executive functioning of patients with advanced idiopathic Parkinson’s disease without dementia. Biomedical Research, 29(7). [Google Scholar]
- Jones JD, Butterfield LC, Song W, Lafo J, Mangal P, Okun MS, & Bowers D (2014a). Anxiety and depression are better correlates of Parkinson’s disease quality of life than apathy. The Journal of Neuropsychiatry and Clinical Neurosciences, 27(3), 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Hass C, Mangal P, Lafo J, Okun MS, & Bowers D (2014b). The Cognition and Emotional Well-being indices of the Parkinson’s disease questionnaire-39: What do they really measure?. Parkinsonism & Related Disorders, 20(11), 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Mangal P, Lafo J, Okun MS, & Bowers D (2016). Mood differences among Parkinson’s disease patients with mild cognitive impairment. The Journal of Neuropsychiatry and Clinical Neurosciences,28(3), 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Marsiske M, Okun MS, & Bowers D (2015). Latent growth-curve analysis reveals that worsening Parkinson’s disease quality of life is driven by depression. Neuropsychology, 29(4), 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Fernandez HF, Marsiske M, Okun MS, & Bowers D (2006). Dissociating apathy and depression in Parkinson disease. Neurology, 67(1), 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Marsiske M, Okun MS, Bauer R, & Bowers D (2011). Apathy and depression: separate factors in Parkinson’s disease. Journal of the International Neuropsychological Society, 17(6), 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, & Hornykiewicz O (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. New England Journal of Medicine, 318(14), 876–880. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, Garcia-Sánchez C, Gironell A, & Trapecio Group Study. (2008). Prevalence and correlates of neuropsychiatrie symptoms in Parkinson’s disease without dementia. Movement Disorders, 23(13), 1889–1896. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, … & Stebbins GT (2008a). Anxiety rating scales in Parkinson’s disease: critique and recommendations. Movement disorders: official journal of the Movement Disorder Society, 23(14), 2015–2025. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, … & Stebbins GT (2008b). Apathy and anhedonia rating scales in Parkinson’s disease: critique and recommendations. Movement Disorders, 23(14), 2004–2014. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Richard IH, Starkstein SE, & Martinez-Martin P (2011). Anxiety rating scales in Parkinson’s disease: a validation study of the Hamilton anxiety rating scale, the Beck anxiety inventory, and the hospital anxiety and depression scale. Movement Disorders, 26(3), 407–415. [DOI] [PubMed] [Google Scholar]
- Levin BE, & Katzen HL (1995). Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. Advances in Neurology, 65, 85–95. [PubMed] [Google Scholar]
- Levy R, & Dubois B (2005). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex, 16(7), 916–928. [DOI] [PubMed] [Google Scholar]
- Maillet A, Krack P, Lhommée E, Metereau E, Klinger H, Favre E, … & Fraix V (2016). The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain, 139(9), 2486–2502. [DOI] [PubMed] [Google Scholar]
- Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, … & Poewe W (2011). The parkinson progression marker initiative (PPMI). Progress in Neurobiology, 95(4), 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221 [DOI] [PubMed] [Google Scholar]
- Ng A, Chander RJ, Tan LC, & Kandiah N (2015). Influence of depression in mild Parkinson’s disease on longitudinal motor and cognitive function. Parkinsonism & related disorders, 21(9), 1056–1060. [DOI] [PubMed] [Google Scholar]
- Owen AM (2004). Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. The Neuroscientist, 10(6), 525–537. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J, Strafella AP, & Krack P (2015). Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. The Lancet Neurology, 14(5), 518–531. [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Filoteo JV, Schiehser DM, Gomez ME, & Petzinger G (2019). Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: A bidirectional analysis. Neuropsychology, 33(1), 35. [DOI] [PubMed] [Google Scholar]
- Pirogovsky-Turk E, Moore RC, Filoteo JV, Litvan I, Song DD, Lessig SL, & Schiehser DM (2017). Neuropsychiatrie predictors of cognitive decline in parkinson disease: a longitudinal study. The American Journal of Geriatric Psychiatry, 25(3), 279–289. [DOI] [PubMed] [Google Scholar]
- Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SA, Grill S,… & Rabins PV (2009). Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Movement disorders, 24(9), 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GO, Hanna KK, Neargarder S, & Cronin-Golomb A (2017). The relation of anxiety and cognition in Parkinson’s disease. Neuropsychology, 31(6), 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten S, van der Ven PM, Weintraub D, Pontone GM, Leentjens AF, Berendse HW,… & van den Heuvel OA (2017). Predictors of anxiety in early-stage Parkinson’s disease-Results from the first two years of a prospective cohort study. Parkinsonism & related disorders, 43, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder KA, Gontkovsky ST, McSwan KL, Scott JG, Bharucha KJ, & Beatty WW (2002). Cognitive function in Parkinson’s disease: Association with anxiety but not depression. Aging, Neuropsychology, and Cognition, 9(2), 77–84. [Google Scholar]
- Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S,… & Stebbins GT (2007). Depression rating scales in Parkinson’s disease: critique and recommendations. Movement Disorders, 22(8), 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Hovris A, Morley D, Quinn N, & Jahanshahi M (2003). Young-versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Movement disorders: official journal of the Movement Disorder Society, 18(11), 1250–1256. [DOI] [PubMed] [Google Scholar]
- Segrave RA, Arnold S, Hoy K, & Fitzgerald PB (2014). Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain stimulation, 7(2), 325–331. [DOI] [PubMed] [Google Scholar]
- Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R,… & Sampaio C (2011). The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Movement Disorders, 26(S3), S42–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, & Yesavage JA (1986). Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. [Google Scholar]
- Smith A (1982). Symbol Digit Modalities Test (SDMT) Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory, STAI (Form Y): Self-evaluation questionnaire. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi T, Andrezejewski P, Leiguarda R, & Robinson RG (1992). Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci,4(2), 134–139. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, & Merello M (2007). The Unified Parkinson’s Disease Rating Scale: validation study of the mentation, behavior, and mood section. Movement disorders, 22(15), 2156–2161. [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, Dotson VM, Jones JD, Okun MS, & Bowers D (2018). Symptom Dimensions of Depression and Apathy and Their Relationship With Cognition in Parkinson’s Disease. Journal of the International Neuropsychological Society, 24(3), 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobois S, Prange S, Sgambato-Faure V, Tremblay L, & Broussolle E (2017). Imaging the etiology of apathy, anxiety, and depression in Parkinson’s disease: implication for treatment. Current Neurology and Neuroscience Reports, 77(10), 76. [DOI] [PubMed] [Google Scholar]
- Varanese S, Perfetti B, Ghilardi MF, & Di Rocco A (2011). Apathy, but not depression, reflects inefficient cognitive strategies in Parkinson’s disease. PLoS One, 6(3), e 17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Mohamed W, Din NC, & Ibrahim N (2015). Cognitive Profiles in Parkinson’s Disease and their Correlation with Dementia, Anxiety and Depression: A Preliminary Study. The Malaysian journal of medical sciences: MJMS, 22(Spec Issue), 29. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weintraub D, Oehlberg KA, Katz IR, & Stern MB (2006). Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. The American Journal of Geriatric Psychiatry, 14(2), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Wu X, Liu J, Li K, & Yao L (2013). Abnormal baseline brain activity in nondepressed Parkinson’s disease and depressed Parkinson’s disease: a resting-state functional magnetic resonance imaging study. PloS one, 8(5), e63691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW,…& Barker RA (2009). The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain, 132(11), 2958–2969. [DOI] [PubMed] [Google Scholar]
- Wu JY, Zhang Y, Wu WB, Hu G, & Xu Y (2018). Impaired long contact white matter fibers integrity is related to depression in Parkinson’s disease. CNS neuroscience & therapeutics, 24(2), 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, & Mattis P (2003). A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology, 16(4),193–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.