Abstract
Background:
Over 80% of cancer patients report taste changes. Despite the high prevalence of this symptom and its negative effects on health, few studies have assessed its association with other gastrointestinal (GI) symptoms.
Objectives:
Determine the occurrence, frequency, severity and distress of patient-reported “change in the way food tastes” (CFT) and identify phenotypic and GI symptoms characteristics associated with its occurrence.
Methods:
Patients receiving chemotherapy for breast, GI, gynecological, or lung cancer completed demographic and symptom questionnaires prior to their 2nd or 3rd cycle of chemotherapy. CFT was assessed using the Memorial Symptom Assessment Scale. Differences in demographic, clinical, and GI symptom characteristics were evaluated using parametric and nonparametric tests.
Results:
Of the 1,329 patients, 49.4% reported experiencing CFT in the week prior to their 2nd or 3rd cycle of chemotherapy. In the univariate analysis, patients who reported CFT had fewer years of education; were more likely to be Black or Hispanic, Mixed race, or other, and had a lower annual household income. A higher percentage of patients with CFT reported the occurrence of thirteen GI symptoms (e.g., constipation, diarrhea, abdominal cramps, feeling bloated). In a multivariable logistic regression analysis, Compared to patients with breast cancer, patients with lung (OR=0.55; p=0.004) had a decrease in the odds of being in the CFT group. Patients who received a neurokinin-1 receptor antagonist and two other antiemetics were at an increased odds of being in the CFT group (OR=2.51; p=0.001). Eight of the thirteen GI symptoms evaluated were associated with an increased odds of being in the CFT group.
Conclusions:
This study provides new evidence on the frequency, severity, and distress of CFT in oncology patients undergoing chemotherapy. These findings suggest that CFT is a important problem that warrants ongoing assessments and nutritional interventions.
Keywords: taste changes, chemotherapy, symptoms, nausea, constipation, diarrhea
INTRODUCTION
Taste changes occur in up to 84% of patients undergoing chemotherapy.1 The sense of taste is vital for nutritional intake and food choices.2 These changes begin within weeks of starting chemotherapy3 and recover approximately 8 weeks after the completion of therapy.4 However, changes can persist for years following treatment.5 Taste changes may involve a decrease in taste sensitivity (hypogeusia), an absence of taste sensation (ageusia), an alteration in normal taste (dysgeusia), or the occurrence of a taste perception without an external stimulus (phantogeusia).6 If the sense of taste and perception of food flavors are altered, patients may experience decrements in nutritional status and weight loss.7 These taste changes and nutritional deficits are associated with poorer responses to treatment as well as increases in adverse effects, morbidity, and mortality.8,9 Despite the clinical importance of taste changes, they are often overlooked by clinicians and studies of this symptom in patients receiving chemotherapy are limited.
An increased understanding of the phenotypic characteristics associated with changes in the way food tastes may identify modifiable risk factors. Findings from the general population suggest that age, sex, and race influence taste perceptions.10,11 For example, in two studies that used data from the U.S. National Health and Nutrition Examination Study (NHANES),12,13 in adults over 40 years of age, the prevalence of taste dysfunction decreased with age in women, but not in men. In addition, taste impairment was greater among non-Hispanic Black Americans compared to other ethnic groups.12 Other factors associated with self-reported taste alterations included: lower level of education, xerostomia, fair/poor health, and nose/facial injury.13
In terms of patients receiving chemotherapy, while several studies reported no relationships,14–20 in four studies,21–24 self-reported taste changes were more prevalent among women, younger patients, and those with higher levels of education. In contrast, patients who smoked or abused alcohol reported fewer taste changes.22
With regards to clinical characteristics, several studies reported no associations between taste changes and a variety of clinical characteristics (e.g., cancer diagnosis, time since diagnosis, chemotherapy regimen, prior cancer treatments).15–18,21,22 However, in one study of patients with lung, pancreatic, and colon cancer, compared to patients with the other two diagnoses, patients with colon cancer reported more severe taste alterations.22 In another study of patients with heterogeneous cancer diagnoses, prevalence rates for taste changes were highest among patients with colon cancer, followed by breast, lung, and lymphoma cancers.23 In terms of specific chemotherapy drugs, in one study,22 the highest rates of taste alterations were reported by patients who received irinotecan, followed by FOLFOX (i.e., 5-fluorouracil, leucovorin, and oxiplatin), and gemcitabine. In another study,21 the highest percentages of dysgeusia were associated with regimens that contained antimetabolites with platinum derivatives and antimetabolites with cytotoxic antibiotics. These inconsistent findings may be related to a number of factors including sample size, timing of the assessments, and methods used to evaluate taste changes (e.g., objective vs. subjective measures).
Information on the co-occurrence of other gastrointestinal (GI) symptoms may provide additional insights into the occurrence of taste changes in patients receiving chemotherapy. While the oral cavity gives rise to the perceptions of taste, as well as the flavors of foods and beverages, it is only the beginning of the digestive system that is impacted by chemotherapy. Only two studies were found that evaluated for associations between taste changes and four GI symptoms (i.e., nausea/vomiting, appetite loss, constipation, diarrhea) in patients receiving chemotherapy.23,25 Greater taste changes were associated with loss of appetite23,25 and increased severity of nausea and vomiting.23 A more in-depth analysis of the relationships between taste changes and other GI symptoms in patients receiving chemotherapy will provide increased insights into this clinically important problem. Given the paucity of research on this symptom, the purposes of the study, in a sample of oncology patients (n=1,329) receiving chemotherapy were to: determine the occurrence, frequency, severity, and distress associated with self-reported “change in the way food tastes (CFT) and to identify phenotypic and GI symptom characteristics associated with its occurrence.
METHODS
Patients and settings
This analysis is part of a larger study, funded by the National Cancer Institute, that evaluated the symptom experience of oncology outpatients receiving chemotherapy.26–28 Eligible patients were ≥18 years of age; had a diagnosis of breast, GI, gynecological, or lung cancer; had received chemotherapy within the preceding four weeks; were scheduled to receive at least two additional cycles of chemotherapy; were able to read, write, and understand English; and gave written informed consent. Patients were recruited from two Comprehensive Cancer Centers, one Veteran’s Affairs hospital, and four community-based oncology programs. Of the 2,234 patients approached, 1,343 consented to participate. For this analysis, 1,329 patients with data of CFT were included.
Instruments
Demographic questionnaire obtained information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. Patients completed the Karnofsky Performance Status (KPS) scale29 and the Self-Administered Comorbidity Questionnaire (SCQ)30 to evaluate functional status and comorbidity, respectively. Alcohol Use Disorders Identification Test (AUDIT) was used to assess alcohol consumption, alcohol dependence, and the consequences of alcohol abuse in the last 12 months.31,32
Memorial Symptom Assessment Scale (MSAS) was used to evaluate the occurrence, severity, frequency, and distress of 38 symptoms commonly associated with cancer and its treatment, including “change in the way food tastes” (CFT). For this analysis, the following GI symptoms were evaluated: CFT, constipation, diarrhea, feeling bloated, abdominal cramps, difficulty swallowing, weight gain, weight loss, increased appetite, lack of appetite, mouth sores, dry mouth, vomiting, and nausea.
Using the MSAS, patients were asked to indicate whether or not they had experienced each symptom in the past week (i.e., symptom occurrence). If they had experienced the symptom, they were asked to rate its frequency of occurrence, severity, and distress. The validity and reliability of the MSAS are well established.33
Study procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Board at each of the study sites. Eligible patients were approached by a research staff member in the infusion unit during their first or second cycle of chemotherapy,to discuss participation in the study. Written informed consent was obtained from all patients. Depending on the length of their chemotherapy cycles, patients completed questionnaires in their homes a total of six times over two cycles of chemotherapy. For this analysis, symptom occurrence data from the enrollment assessment that asked patients to report on their symptom experience for the week prior to the administration of their second or third cycle of chemotherapy were analyzed (i.e., recovery from previous chemotherapy cycle). Medical records were reviewed for disease and treatment information.
Data analysis
Data were analyzed using SPSS version 2334 and STATA version 14.35 Descriptive statistics and frequency distributions were calculated for demographic, clinical, and symptom characteristics. MAX 2 scores,36,37 emetogenicity of the chemotherapy regimen, and anti-emetic regimens were calculated as previously described.38 Differences in demographics, clinical, and symptom characteristics between patients who did and did not report CFT were evaluated using Independent sample t-tests, Chi Square analyses, and Mann Whitney U tests. In order to evaluate the association between select phenotypic and symptom characteristics and CFT group membership, a backwards, stepwise logistic regression analysis was done. The initial logistic regression model included eleven phenotypic characteristics (i.e., education, ethnicity, KPS score, number of prior cancer treatments, number of metastatic sites, exercise on a regular basis, cancer diagnosis, type of prior cancer treatments, MAX2 score, cycle length, emetogenicity of chemotherapy regimen, antiemetic regimen) that differed between the two groups (Table 1) and the occurrence of the 13 gastrointestinal symptoms on the MSAS. A p-value of <.05 was considered statistically significant.
Table 1.
Differences in Demographic and Clinical Characteristics Between Patients With and Without Self-Reported Change in the Way Food Tastes (n=1329)
| Characteristic | No Taste Changes (0) 50.6% (n = 673) | With Taste Changes (1) 49.4% (n = 656) | Statistics |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 57.50 (12.50) | 57.02 (12.15) | t = 0.72; p = 0.474 |
| Education (years) | 16.41 (3.09) | 15.97 (2.94) | t = 2.66; p = 0.008 |
| Body mass index (kg/m2) | 25.91 (5.31) | 26.52 (6.02) | t = −1.94; p = 0.052 |
| Karnofsky Performance Status score | 81.97 (12.20) | 78.10 (12.38) | t = 5.63; p < 0.001 |
| Number of comorbidities | 2.37 (1.41) | 2.46 (1.46) | t = −1.19; p = 0.236 |
| SCQ score | 5.33 (3.03) | 5.66 (3.36) | t = −1.85; p = 0.064 |
| AUDIT score | 3.12 (2.51) | 2.82 (2.43) | t = 1.73; p = 0.084 |
| Time since cancer diagnosis (years) | 2.26 (4.23) | 1.71 (3.50) | U; p = 0.037 |
| Time since cancer diagnosis (median, years) | 0.45 | 0.42 | |
| Number of prior cancer treatments | 1.70 (1.52) | 1.48 (1.48) | t = 2.66; p = 0.008 |
| Number of metastatic sites including lymph node involvement | 1.32 (1.22) | 1.16 (1.24) | t = 2.44; p = 0.015 |
| Number of metastatic sites excluding lymph node involvement | 0.86 (1.05) | 0.71 (1.04) | t = 2.56; p = 0.011 |
| MAX2 score | 0.16 (0.08) | 0.18 (0.08) | t = −4.01, p <.001 |
| % (n) | % (n) | ||
| Gender | χ2 = 4.20; p = 0.122 | ||
| Female | 75.8 (510) | 79.73 (523) | |
| Male | 24.2 (163) | 20.1 (132) | |
| Transgender | 0.0 (0) | 0.2 (1) | |
| Ethnicity | χ2 =19.14; p < 0.001 | ||
| White | 75.3 (499) | 64.4 (418) | 0 > 1 |
| Black | 5.6 (37) | 8.9 (58) | 0 < 1 |
| Asian or Pacific Islander | 10.7 (71) | 13.9 (90) | NS |
| Hispanic, Mixed, or Other | 8.4 (56) | 12.8 (83) | 0 < 1 |
| Married or partnered (% yes) | 66.2 (440) | 62.5 (403) | FE, p = 0.167 |
| Lives alone (% yes) | 20.0 (133) | 23.2 (150) | FE, p = 0.179 |
| Child care responsibilities (% yes) | 20.1 (133) | 23.9 (153) | FE, p = 0.108 |
| Care of adult responsibilities (% yes) | 6.7 (41) | 9.1 (54) | FE, p = 0.164 |
| Currently employed (% yes) | 35.7 (238) | 34.6 (224) | FE, p = 0.686 |
| Income | U, p = 0.020 | ||
| < $30,000 | 17.0 (102) | 19.9 (117) | |
| $30,000 to < $70,000 | 20.1 (121) | 22.2 (131) | |
| $70,000 to < $100,000 | 15.6 (94) | 17.8 (105) | |
| ≥ $100,000 | 47.3 (284) | 40.1 (236) | |
| Specific comorbidities (% yes) | |||
| Heart disease | 5.5 (37) | 6.1 (40) | FE, p = 0.725 |
| High blood pressure | 29.6 (199) | 31.4 (206) | FE, p = 0.475 |
| Lung disease | 12.8 (86) | 9.8 (64) | FE, p = 0.084 |
| Diabetes | 9.2 (62) | 9.0 (59) | FE, p = 0.924 |
| Ulcer or stomach disease | 4.0 (27) | 5.6 (37) | FE, p = 0.200 |
| Kidney disease | 1.5 (10) | 1.4 (9) | FE, p = 1.000 |
| Liver disease | 6.2 (42) | 6.7 (44) | FE, p = 0.739 |
| Anemia or blood disease | 11.1 (75) | 13.6 (89) | FE, p = 0.183 |
| Depression | 19.5 (131) | 18.9 (124) | FE, p = 0.835 |
| Osteoarthritis | 11.3 (76) | 13.3 (87) | FE, p = 0.278 |
| Back pain | 23.9 (161) | 27.7 (182) | FE, p = 0.117 |
| Rheumatoid arthritis | 2.8 (19) | 3.7 (24) | FE, p = 0.440 |
| Exercise on a regular basis (% yes) | 74.1 (492) | 67.6 (430) | FE, p = 0.010 |
| Smoking current or history of (% yes) | 36.8 (245) | 33.8 (217) | FE, p = 0.272 |
| Cancer diagnosis | χ2 = 16.57; p = 0.001 | ||
| Breast | 36.0 (242) | 44.5 (292) | 0 < 1 |
| Gastrointestinal | 30.2 (203) | 20.7 (204) | NS |
| Gynecological | 20.7 (139) | 14.3 (94) | 0 > 1 |
| Lung | 13.2 (89) | 10.1 (66) | NS |
| Type of prior cancer treatment | χ2 = 13.27; p = 0.004 | ||
| No prior treatment | 23.1 (151) | 26.9 (172) | NS |
| Only surgery, CTX, or RT | 39.8 (260) | 44.3 (283) | NS |
| Surgery & CTX, or Surgery & RT, or CTX & RT | 23.7 (155) | 16.0 (102) | 0 > 1 |
| Surgery & CTX & RT | 13.3 (87) | 12.8 (82) | NS |
| CTX cycle length | χ2 = 11.71; p = 0.003 | ||
| 14-day cycle | 38.2 (257) | 46.0 (301) | 0 < 1 |
| 21-day cycle | 52.7 (354) | 48.5 (317) | NS |
| 28-day cycle | 9.1 (61) | 5.5 (36) | 0 > 1 |
| Emetogenicity of CTX | χ2 = 16.41; p < 0.001 | ||
| Minimal/Low | 22.1 (149) | 16.8 (110) | 0 > 1 |
| Moderate | 62.4 (420) | 59.6 (390) | NS |
| High | 15.5 (104) | 23.5 (154) | 0 < 1 |
| Antiemetic regimens | χ2 = 37.27; p < 0.001 | ||
| None | 9.1 (60) | 5.0 (32) | 0 > 1 |
| Steroid alone or serotonin receptor antagonist alone | 22.2 (146) | 18.6 (119) | NS |
| Serotonin receptor antagonist and steroid | 50.7 (333) | 44.6 (285) | NS |
| NK-1 receptor antagonist and two other antiemetics | 18.0 (118) | 31.8 (203) | 0 < 1 |
Abbreviations: AUDIT = Alcohol Use Disorders Identification Test, CTX = chemotherapy, FE = Fisher’s Exact test, kg = kilograms, m2 = meter squared, NK-1 = Neurokinin-1, NS = not significant, RT = radiation therapy, SCQ = Self-administered Comorbidity Questionnaire, SD = standard deviation, X2 = Chi square test, U = Mann Whitney U test
Chi Square test done without the transgender participant
RESULTS
Sample characteristics
Of the 1,329 patients in this study, 49.4% reported experiencing CFT in the week prior to their second or third cycle of chemotherapy (Figure 1A). Of these 656 patients, 21.7% (n=141) rated the frequency of occurrence of CFT as “almost constantly” and 26.3% (n=171) rated it as “frequently” (Figure 1B). In terms of the severity of CFT, 45.4% (n=292) rated it as “moderate” and 20.4% (n=131) and 8.7% (n=56) rated it as “severe” or “very severe”, respectively (Figure 1C). Over 25% of the patients reported “quite a bit” (13.5%; n=86) or “very much” (12.9%; n=82) distress from CFT (Figure 1D).
Figure 1 –
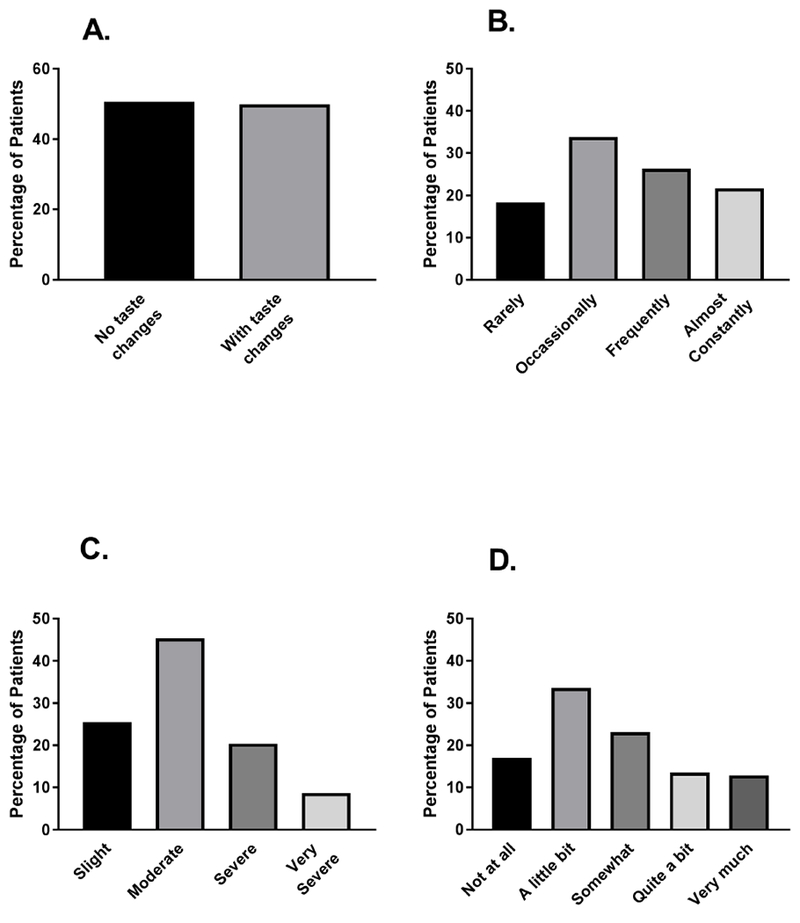
Percentages of patients with and without self-reported change in the way food tastes (CFT, A) and distribution of patients’ ratings of frequency (B), severity (C), and distress associated with CFT.
Differences in phenotypic and GI symptom characteristics
Patients who reported CFT had fewer years of education, were more likely to be Black or Hispanic, Mixed race, or other, and had a lower annual household income (Table 1). In addition, patients in the CFT group had lower KPS scores, were fewer years from their cancer diagnosis, had received fewer cancer treatments, and had fewer metastatic sites. Furthermore, a higher percentage of patients in the CFT group had breast cancer, had received chemotherapy on a 14-day cycle, had higher MAX2 scores, were receiving highly emetogenic chemotherapy, and were more likely to be receiving an antiemetic regimen that contained a neurokinin-1 (NK-1) receptor antagonist and two other antiemetics (e.g., serotonin (5HT3) receptor antagonist, steroid). Compared to the no CFT group, patients in the CFT group were less likely to exercise on a regular basis; less likely to have gynecological cancer; less likely to have received surgery and chemotherapy, or surgery and radiation therapy, or chemotherapy and radiation; less likely to have received chemotherapy on an 28-day cycle; less likely to have received minimal/low emetogenic chemotherapy, and more likely to have received an antiemetic.
With regard to the GI symptoms, a higher percentage of patients in the CFT group reported constipation, diarrhea, feeling bloated, abdominal cramps, difficulty swallowing, weight gain, weight loss, increased appetite, lack of appetite, mouth sores, dry mouth, vomiting, and nausea (Figure 2).
Figure 2 –
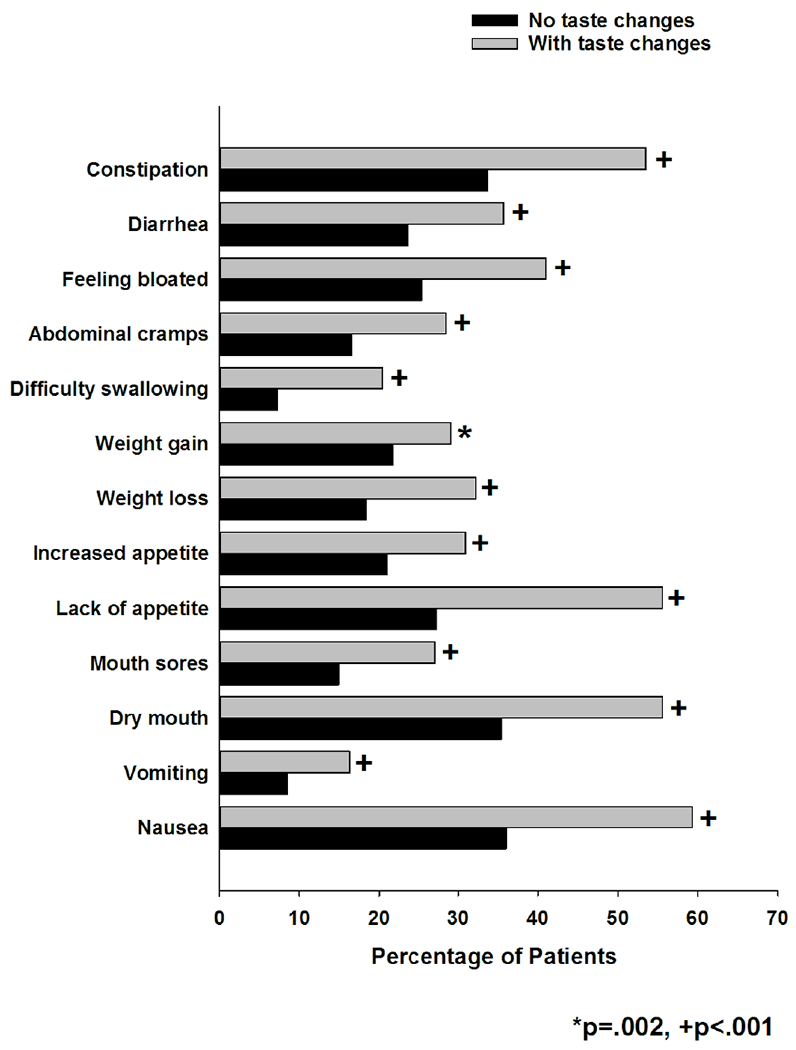
Differences in the occurrence rates for thirteen gastrointestinal symptoms between patients who did and did not report change in the way food tastes.
Phenotypic and GI symptom characteristics associated with CFT
The overall results of the logistic regression analysis were significant (X2=257.55, p<0.001, Table 2). None of the demographic characteristics were associated with CFT group membership. In terms of clinical characteristics, compared to patients with breast cancer, patients with lung cancer (OR=0.55; p=0.004) had a decrease in the odds of being in the CFT group. In terms of antiemetic regimen, compared to patients who did not receive any antiemetic, patients who received a NK-1 receptor antagonist and two other antiemetics were at an increased odds of being in the CFT group (OR=2.51; p=0.001). Eight of the 11 GI symptoms evaluated were significantly associated with an increased odds of being in the CFT group.
Table 2 –
Multiple Logistic Regression Analysis Predicting Change in the Way Food Tastes Group Membership (n = 1280)
| Predictor | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Cancer diagnosis | 0.019 | |
| Gastrointestinal vs. breast | 0.86 (0.63, 1.16) | 0.313 |
| Gynecological vs. breast | 0.70 (0.49, 0.99) | 0.046 |
| Lung vs. breast | 0.55 (0.36, 0.83) | 0.004 |
| Antiemetic regimen | 0.001 | |
| Steroid alone or serotonin receptor antagonist alone vs. none | 1.48 (0.86, 2.54) | 0.160 |
| Serotonin receptor antagonist and steroid vs. none | 1.45 (0.87, 2.41) | 0.149 |
| NK-1 receptor antagonist and two other antiemetics vs. none | 2.51 (1.47, 4.29) | 0.001 |
| Dry mouth | 1.44 (1.11, 1.86) | 0.006 |
| Nausea | 1.39 (1.07, 1.81) | 0.014 |
| Feeling bloated | 1.34 (1.02, 1.77) | 0.037 |
| Lack of appetite | 2.31 (1.76, 3.02) | <0.001 |
| Increased appetite | 1.56 (1.17, 2.08) | 0.003 |
| Difficult swallowing | 1.87 (1.26, 2.78) | 0.002 |
| Mouth sores | 1.57 (1.15, 2.16) | 0.005 |
| Constipation | 1.50 (1.16, 1.94) | 0.002 |
| Overall model fit: degrees of freedom = 17; X2 = 257.55, p < .001 | ||
Abbreviations: NK-1 = neurokinin 1, vs = versus
DISCUSSION
This study is the first and largest to describe phenotypic and GI symptom characteristics associated with CFT in cancer patients following at least one cycle of chemotherapy. Consistent with previously reported prevalence rates that ranged from 25%39 to 80%,40 almost 50% of our patients reported experiencing CFT in the week prior to their second or third cycle of chemotherapy. In prior reports that used the MSAS,41,42 the frequency of occurrence, as well as the severity, and distress related to CFT was highly variable. In terms of frequency, compared to the 21.7% of patients in our study who chose the rating “almost constantly”, in a previous study,42 only 11.5% of the patients used this rating. In terms of severity, compared to the 8.7% of our patients who rated CFT as severe or very severe, previous reports ranged from 31.9%41 to 36.8%.42 Finally, consistent with previous studies that found that between 18%41 and 35%43 of patients reported that CFT was “quite a bit or very much distressing”, 26.4% of our patients used these ratings. These wide ranges in occurrence, frequency, severity, and distress ratings may be related to differences in phenotypic characteristics of the samples, heterogeneity in the types of cancer treatments the patients received, and stage of the patients’ disease.
While in the univariate analysis, fewer years of education and ethnicity were associated with membership in the CFT group, none of the demographic characteristics remained significant in the multivariable model. While in a population-based study,12 a higher percentage of African Americans reported taste alterations, no studies were identified that found an association between ethnicity and CFT in oncology patients.
The only two clinical characteristics associated with CFT in the multivariable model were cancer diagnosis and antiemetic regimen. Compared to those with breast cancer, patients with lung cancer had a lower risk of being in the CFT group. While previous studies reported altered taste perceptions in patients with breast4,20,25,44, lung45,46, and gynecological25,47 cancers, no studies were identified that examined relative risk of CFT across cancer diagnoses.
As noted in the Introduction, two studies identified differences in the prevalence of taste changes among various chemotherapy regimens.21,22 Given the heterogeneity in cancer diagnoses and chemotherapy regimens in our study, we evaluated toxicity of the chemotherapy regimen (i.e., MAX2 score), the relative contribution of the emetogenicity of the chemotherapy regimen, cycle length, and the antiemetic regimen to membership in the CFT group. While all four characteristics were significant in the univariate analysis, only antiemetic regimen remained significant in the multivariable model.
In terms of the antiemetic regimen, being prescribed a NK-1 receptor antagonist with two other antiemetics (i.e., 5-HT-3 receptor antagonist being the most common additional antiemetic followed by a steroid) was associated with a 2.51-fold increased risk of being in the CFT group. Evidence exists to support a role for both of the agonists of the NK-1 (i.e, Substance P) and 5-HT3 (i.e., serotonin) receptors in taste perception48–53 and gut motility.54–56 In terms of taste, NK-1 receptors are present in the gustatory center and taste papillae at substance P-sensitive nerve fibers.57–59 The administration of an NK-1 receptor antagonist blocked the release of Substance P in a preclinical model.49 In addition, taste buds release 5-HT directly in response to sour stimuli and indirectly in response to bitter and sweet tasting stimuli. In a preclinical study,52 the administration of the 5-HT3 receptor antagonist, ondansetron, reduced taste nerve responses to acids and sucrose. Taken together, our findings are the first to suggest that multimodal anti-emetic regimens, which are the standard of care for the treatment of chemotherapy-induced nausea and vomiting,60,61 may contribute to taste changes in oncology patients undergoing chemotherapy.
In terms of their effects on gut motility, Substance P plays a significant role in GI motility with resultant dysmotility, diarrhea, and edema.62,63 In terms of 5-HT3, information on its role in gut motility is evolving. While exogenous 5HT-3 may stimulate GI motility, the role of endogenous 5HT-3 in GI motility is under active investigation.55 However, the administration of NK-1 and 5-HT3 antagonists to patients receiving chemotherapy is associated with increased risk for constipation.64–66 Finally, both NK-1 and 5HT-3 receptor antagonists act on regions of the brain that are involved in nausea, vomiting, and eating behaviors that overlap with gustatory pathways.67,68 Therefore, it is possible that direct and indirect effects from both of these antiemetics contribute to CFT during the administration of chemotherapy.
Our study is the first to evaluate the associations among common GI symptoms to CFT in patients undergoing chemotherapy. Consistent with our findings regarding the effects of the antiemetic regimen on taste and GI motility, in a recent review of studies of patients receiving primary or adjuvant chemotherapy,69 a GI cluster was the most common symptom cluster identified. In studies that used the MSAS to evaluate for symptom clusters,70–72 the most common symptoms across the various GI clusters identified were: nausea, vomiting, lack of appetite, feeling bloated, and weight loss. In our study, CFT group membership was associated with dry mouth, nausea, feeling bloated, lack of appetite, increased appetite, difficulty swallowing, mouth sores, and constipation. The co-occurrence of these symptoms may be partially related to chemotherapy-induced alterations in the oral and intestinal mucosa.73,74 For example, chemotherapy-induced alterations in taste are mediated by the sonic hedgehog pathway75,76 which disrupts taste cell renewal in the oral cavity through the inhibition of progenitor cells.77,78 Undoubtedly, the mechanisms by which chemotherapy induces taste changes and co-occurring GI symptoms are extremely complex. In addition to the mechanisms cited above, activation of pro- and anti-inflammatory pathways79, as well as alterations in the gut microbiome80,81 could alter taste perceptions. Finally, taste alterations could result in dietary changes that contribute to additional GI symptoms. Longitudinal studies are needed to examine the associations between CFT and co-occurring GI symptoms during chemotherapy.
Some limitations warrant consideration. While the sample size was large, only a single item was used to evaluate “change in the way food tastes”. We acknowledge that “a change in the way food tastes”, goes beyond the traditional definition of taste (i.e., sweet, sour, bitter, salty, and umami) and likely incorporates changes in flavor perception.82,83 Therefore, studies that incorporate additional self-report measures, as well as quantitative psychophysical measures of taste are needed to identify which taste qualities are affected and to confirm our findings on the associations between CFT and GI symptoms and antiemetic regimens. The combination of both subjective and objective measures of taste and smell function would provide increased insights into patients’ experiences and draw connections with food behaviors and nutritional status. Moreover, the present study focuses on a single time point during chemotherapy treatment. A prospective longitudinal study would allow for the exploration of the effects that chemotherapy and antiemetic regimens have on CFT and GI symptoms throughout treatment and during recovery. In addition, studies that explore the molecular mechanisms that underlie CFT would provide information on inter-individual differences in this symptom in oncology patients. More information is needed to understand how CFT and GI symptoms influence changes in patients’ food behaviors, dietary intake, and nutritional status.
In summary, while CFT is a severe and distressing symptom, clinicians fail to assess it and its associated impact on patients’ nutritional status. Considering the high prevalence of CFT, ongoing assessment of this symptom is warranted. In addition, patients taking an NK-1 receptor antagonist as part of their antiemetic regimen may require additional symptom management interventions because their CFT and GI related symptoms may be more severe.
Acknowledgments
Disclosures: This study was supported by a grant from the National Cancer Institute (CA134900). Dr. Miaskowski is an American Cancer Society Clinical Research Professor and is funded by a K05 award from the National Cancer Institute (CA168960). Dr. Joseph is supported by the National Institute of Nursing Research (1ZIANR000035-01), the National Institutes of Health (NIH) Office of Workforce Diversity, the NIH Distinguished Scholars Award, and by the Rockefeller University Heilbrunn Nurse Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Gamper E-M, Zabernigg A, Wintner LM, et al. Coming to your senses: detecting taste and smell alterations in chemotherapy patients. A systematic review. J Pain Symptom Manage 2012;44:880–895. [DOI] [PubMed] [Google Scholar]
- 2.Kourouniotis S, Keast R, Riddell L, et al. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 2016;103:1–7. [DOI] [PubMed] [Google Scholar]
- 3.de Vries Y, Winkels R, van den Berg M, et al. Altered food preferences and chemosensory perception during chemotherapy in breast cancer patients: A longitudinal comparison with healthy controls. Food Qual Prefer 2018;63:135–143. [Google Scholar]
- 4.Boltong A, Aranda S, Keast R, et al. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PloS one 2014;9:e103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen SB, Mouridsen HT, Bergmann OJ, et al. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:217–226. [DOI] [PubMed] [Google Scholar]
- 6.Amezaga J, Alfaro B, Rios Y, et al. Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Support Care Cancer 2018;26:4077–4086. [DOI] [PubMed] [Google Scholar]
- 7.Kubrak C, Olson K, Jha N, et al. Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck 2010;32:290–300. [DOI] [PubMed] [Google Scholar]
- 8.Pressoir M, Desné S, Berchery D, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Brit J Cancer 2010;102:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrieta O, Ortega RMM, Villanueva-Rodriguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill CA, Beach M, Smith MC, Chen EYJJOH, Surgery N. Incidence of and factors associated with hypogeusia in healthy children. JAMA Otolaryngol Head Neck Surg 2016;142:229–233. [DOI] [PubMed] [Google Scholar]
- 11.Williams JA, Bartoshuk LM, Fillingim RB, Dotson CDJCs. Exploring ethnic differences in taste perception. Chem Senses 2016;41:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Zong G, Doty RL, Sun QJBO. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open 2016;6:e013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses 2016;41:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berteretche M, Dalix A, d’Ornano AC, et al. Decreased taste sensitivity in cancer patients under chemotherapy. Support Care Cancer 2004;12:571–576. [DOI] [PubMed] [Google Scholar]
- 15.Tantoy IY, Cooper BA, Dhruva A, et al. Changes in the occurrence, severity, and distress of symptoms in patients with gastrointestinal cancers receiving chemotherapy. J Pain Symptom Manage 2018;55:808–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV. Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 2011. ;41:673–683. [DOI] [PubMed] [Google Scholar]
- 17.Sözeri E, Kutluturkan S. Taste alteration in patients receiving chemotherapy. J Breast Health 2015;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spotten L, Corish CA, Lorton C, et al. Subjective and objective taste and smell changes in cancer. Ann Oncol 2017;28:969–984. [DOI] [PubMed] [Google Scholar]
- 19.IJpma I, Renken RJ, Gietema JA, et al. Changes in taste and smell function, dietary intake, food preference, and body composition in testicular cancer patients treated with cisplatin-based chemotherapy. Clin Nutr 2017;36:1642–1648. [DOI] [PubMed] [Google Scholar]
- 20.Steinbach S, Hummel T, Bohner C, et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J Clin Oncol 2009;27:1899–905. [DOI] [PubMed] [Google Scholar]
- 21.Bernhardson B-M, Tishelman C, Rutqvist LE. Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer 2008;16:275–283. [DOI] [PubMed] [Google Scholar]
- 22.Zabernigg A, Gamper E-M, Giesinger JM, et al. Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist 2010;15:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponticelli E, Clari M, Frigerio S, et al. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur J Cancer Care 2017;26(2). [DOI] [PubMed] [Google Scholar]
- 24.Imai H, Soeda H, Komine K, Otsuka K, Shibata H. Preliminary estimation of the prevalence of chemotherapy-induced dysgeusia in Japanese patients with cancer. BMC Palliat Care 2013;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamper EM, Giesinger JM, Oberguggenberger A, et al. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: prevalence, course of severity, and quality of life correlates. Acta Oncol 2012;51:490–496. [DOI] [PubMed] [Google Scholar]
- 26.Papachristou N, Puschmann D, Barnaghi P, et al. Learning from data to predict future symptoms of oncology patients. PloS one 2018;13:e0208808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kober KM, Cooper BA, Paul SM, et al. Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer 2016;24:1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright F, Melkus GDE, Hammer M, et al. Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Managet 2015;50:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnofsky D Performance scale, New York: Plenum Press, 1977. [Google Scholar]
- 30.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–163. [DOI] [PubMed] [Google Scholar]
- 31.Babor TF, Higgins-Biddle J, Saunders J, Monteiro M. The alcohol use disorders identification test (AUDIT): Guidelines for use in primary care. World Health Organization, Department of Mental Health and Substance Abuse 2001. [Google Scholar]
- 32.Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. In: Geneva, Switzerland: World Health Organization, 1992. [Google Scholar]
- 33.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30:1326–1336. [DOI] [PubMed] [Google Scholar]
- 34.IBM SPSS for Windows. Inc: 23 ed. Armonk, NY: SPSS, Inc, 2015. [Google Scholar]
- 35.StataCorp. Stat Statistical Software. In: College Station, TX: Stata Corperation, 2015. [Google Scholar]
- 36.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 37.Extermann M, Reich RR, Sehovic M. Chemotoxicity recurrence in older patients: Risk factors and effectiveness of preventive strategies-a prospective study. Cancer 2015;121:2984–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh KP, Kober KM, Dhruva AA, et al. Risk factors associated with chemotherapy-induced nausea in the week before the next cycle and impact of nausea on quality of life outcomes. J Pain Symptom Manage 2018;56:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam WWT, Law CC, Fu YT, et al. New insights in symptom assessment: the Chinese versions of the Memorial Symptom Assessment Scale Short Form (MSAS-SF) and the Condensed MSAS (CMSAS). J Pain Symptom Manage 2008;36:584–595. [DOI] [PubMed] [Google Scholar]
- 40.Pud D The psychometric properties of the Hebrew version of the Memorial Symptom Assessment Scale (MSAS-Heb) in patients with breast cancer. J Pain Symptom Manage 2015;49:790–795. [DOI] [PubMed] [Google Scholar]
- 41.Cheng KK, Wong EM, Ling W, Chan CW, Thompson DR. Measuring the symptom experience of Chinese cancer patients: a validation of the Chinese version of the memorial symptom assessment scale. J Pain Symptom Manage 2009;37:44–57. [DOI] [PubMed] [Google Scholar]
- 42.Yildirim Y, Tokem Y, Bozkurt N, et al. Reliability and validity of the Turkish version of the Memorial Symptom Assessment Scale in cancer patients. Asian Pac J Cancer Prev 2011;12:3389–3396. [PubMed] [Google Scholar]
- 43.Webber K, Davies AN. Validity of the memorial symptom assessment scale-short form psychological subscales in advanced cancer patients. J Pain Symptom Manage 2011;42:761–767. [DOI] [PubMed] [Google Scholar]
- 44.de Vries YC, Boesveldt S, Kelfkens CS, et al. Taste and smell perception and quality of life during and after systemic therapy for breast cancer. Breast Cancer Res Treat 2018;170:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belqaid K, Orrevall Y, McGreevy J, et al. Self-reported taste and smell alterations in patients under investigation for lung cancer. Acta Oncol 2014;53:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belqaid K, Tishelman C, Orrevall Y, Mansson-Brahme E, Bernhardson BM. Dealing with taste and smell alterations-A qualitative interview study of people treated for lung cancer. PLoS One 2018;13:e0191117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishijima S, Yanase T, Tsuneki I, Tamura M, Kurabayashi T. Examination of the taste disorder associated with gynecological cancer chemotherapy. Gynecol Oncol 2013;131:674–678. [DOI] [PubMed] [Google Scholar]
- 48.Dill MJ, Shaw J, Cramer J, Sindelar DK. 5-HT1A receptor antagonists reduce food intake and body weight by reducing total meals with no conditioned taste aversion. Pharmacol Biochem Behav 2013;112:1–8. [DOI] [PubMed] [Google Scholar]
- 49.Grant J Tachykinins stimulate a subset of mouse taste cells. PLoS One 2012;7:e31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang AY, Wu SY. Substance P as a putative efferent transmitter mediates GABAergic inhibition in mouse taste buds. Br J Pharmacol 2018;175:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaber L, Zhao FL, Kolli T, Herness S. A physiologic role for serotonergic transmission in adult rat taste buds. PLoS One 2014;9:e112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larson ED, Vandenbeuch A, Voigt A, et al. The role of 5-HT3 receptors in signaling from taste buds to nerves. J Neurosci 2015;35:15984–15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onaga T Tachykinin: recent developments and novel roles in health and disease. Biomol Concepts 2014;5:225–243. [DOI] [PubMed] [Google Scholar]
- 54.Ge X, Pan J, Liu Y, et al. Intestinal crosstalk between microbiota and serotonin and its impact on gut motility. Curr Pharm Biotechnol 2018;19:190–195. [DOI] [PubMed] [Google Scholar]
- 55.Keating DJ, Spencer NJ. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol Res 2019;140:50–55. [DOI] [PubMed] [Google Scholar]
- 56.Neuhuber W, Worl J. Monoamines in the enteric nervous system. Histochem Cell Biol 2018;150:703–709. [DOI] [PubMed] [Google Scholar]
- 57.Kinnman E, Aldskogius H. The role of substance P and calcitonin gene-related peptide containing nerve fibers in maintaining fungiform taste buds in the rat after a chronic chorda tympani nerve injury. Exper Neurol 1991;113:85–91. [DOI] [PubMed] [Google Scholar]
- 58.Nagy J, Goedert M, Hunt S, Bond A. The nature of the substance P-containing nerve fibres in taste papillae of the rat tongue. Neuroscience 1982;7:3137–3151. [DOI] [PubMed] [Google Scholar]
- 59.Chang GQ, Vigna SR, Simon SA. Localization of substance P NK-1 receptors in rat tongue. Regul Pept 1996;63:85–89. [DOI] [PubMed] [Google Scholar]
- 60.Molassiotis A, Aapro M, Herrstedt J, Gralla R, Roila F. MASCC/ESMO Antiemetic Guidelines: Introduction to the 2016 guideline update. Support Care Cancer 2017;25:267–269. [DOI] [PubMed] [Google Scholar]
- 61.Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–v133. [DOI] [PubMed] [Google Scholar]
- 62.Holzer P, Holzer-Petsche U. Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol 2001;1:583–590. [DOI] [PubMed] [Google Scholar]
- 63.Lecci A, Capriati A, Altamura M, Maggi CA. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Auton Neurosci 2006;126-127:232–249. [DOI] [PubMed] [Google Scholar]
- 64.Li Q, Wang W, Chen G, et al. Evaluation of a neurokinin-1 antagonist in preventing multiple-day cisplatin-induced nausea and vomiting. Open Med 2018;13:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Wu Y, Wang W, et al. Effectiveness and safety of combined neurokinin-1 antagonist aprepitant treatment for multiple-day anthracycline-induced nausea and vomiting. Curr Probl Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 66.Yuan DM, Li Q, Zhang Q, et al. Efficacy and safety of neurokinin-1 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting: Systematic review and meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev 2016;17:1661–1675. [DOI] [PubMed] [Google Scholar]
- 67.Harrison TA, Hoover DB, King MS. Distinct regional distributions of NK1 and NK3 neurokinin receptor immunoreactivity in rat brainstem gustatory centers. Brain Res Bull 2004;63:7–17. [DOI] [PubMed] [Google Scholar]
- 68.Tuerke KJ, Limebeer CL, Fletcher PJ, Parker LA. Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT(3) receptor in the posterior and anterior insular cortex. J Neurosci 2012;32:13709–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward Sullivan C, Leutwyler H, Dunn LB, Miaskowski C. A review of the literature on symptom clusters in studies that included oncology patients receiving primary or adjuvant chemotherapy. J Clin Nurs 2018;27:516–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molassiotis A, Wengstrom Y, Kearney N. Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manage 2010;39:847–58. [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Gu L, Zhang L, et al. Symptom Clusters in Ovarian Cancer Patients With Chemotherapy After Surgery: A Longitudinal Survey. Cancer Nurs 2016;39:106–116. [DOI] [PubMed] [Google Scholar]
- 72.Suwisith N, Hanucharururnkul S, Dodd M, et al. Symptom clusters and functional status of women with breast cancer. Thai J Nurs Res 2008;12:153–165. [Google Scholar]
- 73.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995–2025. [DOI] [PubMed] [Google Scholar]
- 74.Stringer AM, Gibson RJ, Bowen JM, Keefe DM. Chemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of change. Curr Drug Metab 2009;10:79–83. [DOI] [PubMed] [Google Scholar]
- 75.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci 2009;30:303–312. [DOI] [PubMed] [Google Scholar]
- 76.Kumari A, Ermilov AN, Allen BL, et al. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophys 2015;113:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwatsuki K, Liu H-X, Grónder A, et al. Wnt signaling interacts with Shh to regulate taste papilla development. PNAS 2007;104:2253–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miura H, Barlow LA. Taste bud regeneration and the search for taste progenitor cells. Arch Ital Biol 2010;148:107. [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci 2009;1170:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner A, Veysey M, Keely S, et al. Interactions between bitter taste, diet and dysbiosis: Consequences for appetite and obesity. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyte M, Fodor AA, Chapman CD, et al. Gut microbiota and a selectively bred taste phenotype: A novel model of microbiome-behavior relationships. Psychosom Med 2016;78:610–619. [DOI] [PubMed] [Google Scholar]
- 82.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exper Brain Res 2005;166:345–357. [DOI] [PubMed] [Google Scholar]
- 83.Boltong A, Keast R. The influence of chemotherapy on taste perception and food hedonics: A systematic review. Can Treat Rev 2012;38:152–163. [DOI] [PubMed] [Google Scholar]


