Abstract
Purpose
Targeted inhibitors and immunotherapy have entered the treatment landscape of metastatic prostate cancer. Genomic testing may uncover which patients benefit most from these therapies. We report the clinical utility and benefits of FoundationOne testing in men with advanced prostate cancer.
Patients and Methods
We retrospectively identified all men with prostate cancer who received tissue FoundationOne testing at our institution between January 2010 and April 2017. Genomic alterations, treatment selection based on FoundationOne results, and clinical outcomes including response and duration of therapy following matched targeted therapy were analyzed.
Results
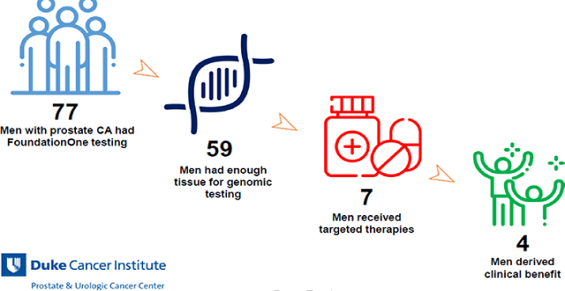
A total of 77 men with metastatic prostate cancer were referred for FoundationOne testing; 59 (77%) had sufficient tumor tissue for testing. Of these, 22% (17/77) of men had a targetable mutation and 9% (7/77) of men received matched off-label targeted therapy. Overall, 5% (4/77) of patients derived clinical benefit. One patient with a BRCA2 loss had a complete response on olaparib (>27 months) and three patients (ATM substitution, PALB2 frameshift, CDK12 frameshift) had stable disease with olaparib (10.3, 18.7, and 7.8 months respectively). 3 patients (BRCA2 frameshift, PDL1+PDL2 amplification, PMS2 missense) had progressive disease despite targeted therapy.
Conclusions
Tissue genomic testing can uncover patients who may benefit from targeted therapies such as PARP inhibitors or immunotherapy. In our limited single institution study, genomic testing led to clinical benefit in 5% of patients. Combined germline and circulating tumor DNA testing may be helpful to identify additional patients suitable for matched genomic therapies.
Keywords: Prostate Cancer, Tumor Genomic Profiling, Real World Outcomes, Precision Oncology
Graphical Abstract
1. Introduction
In the era of precision oncology, biomarker guided therapies have revolutionized treatment selection1–3. However, for men with metastatic castration resistant prostate cancer (mCRPC), the development of biomarker-driven therapies beyond targeting the androgen pathway remains challenging given limited actionable targets and limited access to metastatic tissue due to bone-predominant metastases. Current life-prolonging treatments have been developed in unselected patients, targeting the androgen receptor, microtubules, bone microenvironment, and the immune system4–9.
Two new classes of therapies are being evaluated in men with mCRPC which appear to have activity in molecularly defined subsets. First, poly(adenosine diphosphate[ADP]– ribose) polymerase (PARP) inhibitors such as olaparib have clear clinical activity in men with mCRPC and somatic or germline DNA homologous repair gene mutations10,11. Several studies are underway evaluating PARP inhibitors in preselected and unselected patients with mCRPC, alone and in combination with AR inhibitors and immunotherapies12–14.
A second class of therapy, the PD-1/PD-L1 immune checkpoint inhibitors, demonstrated a low response rate in unselected men with mCRPC (0%, 0/17) in early phase studies15. However, subsequent studies have reported dramatic responses to pembrolizumab, in particular for patients with high microsatellite instability (MSI)16,17. In addition, biallelic loss of the DNA repair enzyme CDK12 may also select for a subset of patients with tandem duplications and novel fusion neoantigens that may predict for response to PD-1 blockade18.
The above data demonstrate that the efficacy of single-agent PARP inhibitors and PD-1 checkpoint inhibitors for mCRPC patients rely on precise patient selection. The real-world benefits of next generation sequencing on unselected patients with mCRPC is unknown. This study describes the efficacy and clinical outcomes of a heavily pre-treated cohort of patients with mCRPC who are screened for biomarker directed therapies with the FoundationOne panel for somatic tumor tissue molecular profiling19. Our data supports the clinical utility of a precision medicine approach in an important but presently small subset of men with mCRPC.
2. Methods
We performed a single institution retrospective review of 77 men with metastatic prostate cancer treated at the Duke Cancer Center who received standard-of-care tumor tissue molecular profiling between January 2010 and April 2017. All patients were treated outside of a clinical trial as part of standard medical practice. We obtained IRB approval to review the charts of all patients in this series to abstract the clinical outcomes following FoundationOne testing.
The primary objective of this analysis was to describe whether molecular testing led to a change in clinical management, and whether there were clinical responses in men with advanced mCRPC who received tumor molecular profiling with FoundationOne as described by PSA response and radiographic progression free survival (rPFS) as defined by RECIST 1.1 and PCWG2 criteria. Secondary objectives were to describe the specific genomic results of all patients and their clinical outcomes with matched targeted therapies. Clinical data (including pathologic and laboratory data) were recoded and secured in a password-protected, auditable, IRB-approved REDCap database.
For the patients who received molecularly matched targeted therapy, board-certified, fellowship-trained radiologists with expertise in abdominopelvic imaging and RECIST calculations (D.M and R.T.G) reviewed all imaging for response and radiographic progression free survival per RECIST 1.1 and PCWG2 criteria20,21.
All patients ≥18 years of age with histologically confirmed prostate adenocarcinoma who had tumor tissue (primary or metastatic) sent for FoundationOne testing within our timeframe were included in the analysis. Genomic alterations were classified for clinical utility based on the established OncoKB framework22 (Supplemental Table 1). AR amplification was considered non-informative as present inhibitors have activity independent of AR DNA genomic alterations. Microsatellite instability status (MSI) and tumor mutational burden (TMB) were not included on the FoundationOne panel at the time of our clinical testing – however, mismatch repair genes such as MLH1, MSH2, MSH6, PMS2, and EPCAM were included.
Duration of therapy was defined as the time of treatment initiation until discontinuation. No formal sample size calculation was necessary as this was a descriptive retrospective analysis.
3. Results
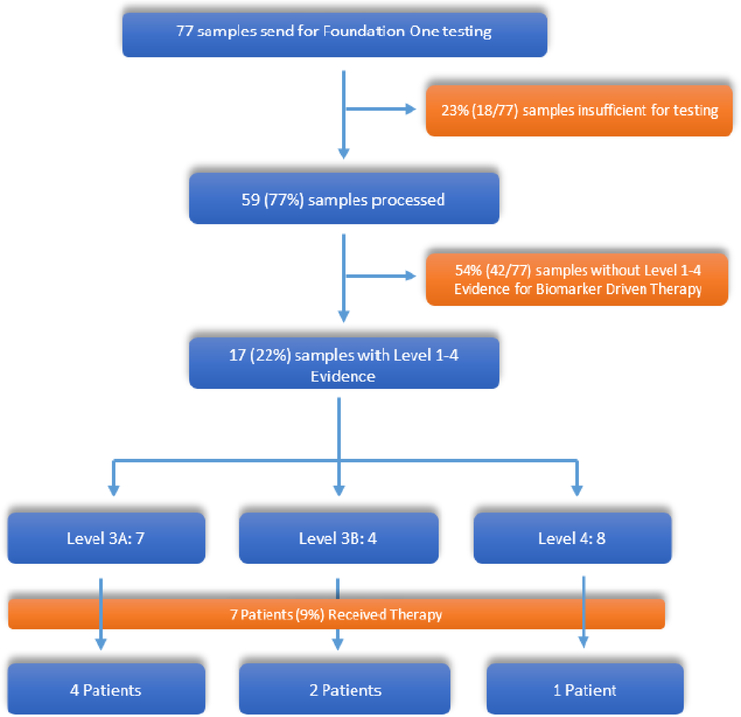
Between January 2010 and April 2017, a total of 77 individual patient tumor specimens were sent to FoundationOne for genomic profiling at Duke University. Of these 77 samples, 18 (23%) samples were deemed insufficient for processing due to low tumor content, and thus 59 (77%) samples were successfully processed and results were reviewed by the treating oncologist (Figure 1).
Figure 1:
Consort Diagram.
Levels of Evidence based on OncoKB precision Oncology knowledge Base There are currently no Level 1 or 2 precision oncology approaches for prostate cancer. Out of the 77 patients with Foundation One Testing, 7 patients(9%) received targeted therapy.
Baseline characteristics of our cohort at the time of genomic profiling are listed in Table 1. Median age was 69 years; 81% of patients were Caucasian and 14% were African American. Most patients had mCRPC (85%) while 15% had localized hormone sensitive prostate cancer (HSPC). Genomic testing using the FoundationOne platform occurred after a median of 3 prior lines of therapy (range 0–8). The most common therapies prior to genomic testing were enzalutamide (63%), docetaxel (58%), abiraterone (47%), and sipuleucel-T (47%).
Table 1:
Baseline characteristics of the 59 Patients with successful genomic testing
| Baseline Demographics | |
|---|---|
| Age (Years) | Median = 69, Range (46–82) |
| Race | White 81% (25/59), Black 14% (8/59), Unknown 5% (3/59) |
| Prior Prostatectomy | 42% (25/59) |
| Prior Prostate Radiation (Primary or Salvage) | 41% (24/59) |
| Histology | 98% Adenocarcinoma (58/59), 2% Small Cell (1/59) |
| Gleason (Radical Prostatectomy or Gleason) | Median = 8, Range (6–10) |
| PSA at Diagnosis (ng/mL) | Median = 15.9, Range (1.9 – 1232) |
| Disease state at the time Foundation One ordered | 85% (50/59) mCRPC, 15% (9/59) localized hormone sensitive prostate cancer |
| Lines of therapy before FoundationOne | Median = 3, Range (0–8) |
| Therapies Prior to FoundationOne | |
| Enzalutamide | 63% (37/59) |
| Docetaxel | 58% (34/59) |
| Abiraterone | 47% (28/59) |
| Sipuleucel-T | 47% (28/59) |
| Radium 223 | 47% (16/59) |
| Cabazitaxel | 29% (17/59) |
| Carboplatin | 14% (8/59) |
| Oxaliplatin | 3% (2/59) |
With regards to the tumor characteristics, 44% of samples were from the primary prostate and 54% of samples were from a metastatic site biopsy (Table 2). The most common metastatic sites analyzed were bone (14%), lymph nodes (14%), and liver (12%) (Table 2).
Table 2:
Sites of tissue used for genomic testing
| Tissue Location | Percent |
|---|---|
| Prostate | 44% (26/59) |
| Bone | 14% (8/59) |
| Liver | 12% (7/59) |
| Bladder | 8% (5/59) |
| Lymph Nodes | |
| Pelvic Lymph Node | 8% (5/59) |
| Axillary Lymph Node | 2% (1/59) |
| Supraclavicular Lymph Node | 2% (1/59) |
| Other | |
| Chest Wall | 2% (1/59) |
| Lung | 2% (1/59) |
| Peri-prostatic soft tissue | 2% (1/59) |
| Penis | 2% (1/59) |
| Rectum | 2% (1/59) |
| Unknown | 2% (1/59) |
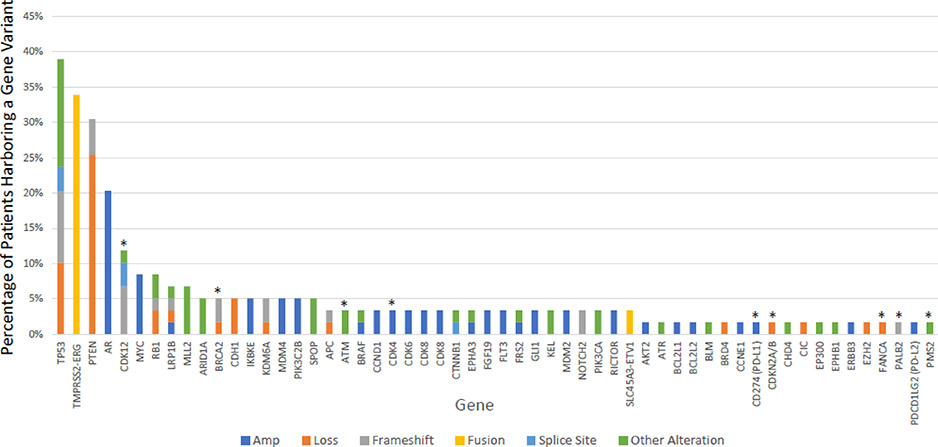
Of the 59 evaluable tissues with DNA sufficient for molecular profiling, a total of 209 genomic alterations were reported (Figure 2), with a median of 3 genomic alterations per patient (range 1–12). The most frequently reported alterations were TP53 mutation/loss (39%), TMPRSS2-ERG fusion (34%), PTEN loss (31%), and AR amplification (20%).
Figure2:
Genomic Landscape of 57 patients with mCRPC
The most common gene variants are included above. Those with an *above the bar denote genes with potentially actionable alterations.
Utilizing the OncoKB framework, somatic mutations were classified into four main levels of evidence of biomarker-guided therapy (Supplemental Table 1)22. A total of 17 of 59 (29%) patients had tumors with a total of 20 genetic alterations. Seven (12%) patients had tumors with a level 3A mutation (Table 3) – ATM (p.D2721N - missense, p.Q2414* - nonsense), BRCA2 (N986fs*2, K437fs*22, Loss), PALB2 (D616fs*12), and FANCA loss. 4 (7%) patients had a level 3B mutation – PD-L1 amplification (1), CDK4 amplification (2), and PMS2 mutation (p.V415M). 8 patients (14%) had Level 4 mutations: CDK12 loss (4 – frameshift, 2 – splice site, 1 - other) and CDKN2A/B loss (1). 19% (5/26) of samples processed from the prostate had a Level 1–4 mutation, compared with 36% (12/33) of samples processed from a metastatic site.
Table 3.
mCRPC patients with targetable genomic alterations and clinical responses to targeted therapies.
| ID | Mutation Status | Mutation | Prior Lines of Therapy | Targeted Therapy | Duration of Targeted Therapy (months) | rPFS (months) | Best Response by RECIST 1.1 | Best PSA Response |
|---|---|---|---|---|---|---|---|---|
| 1 | Level 3A | BRCA2 Loss | 1 | Olaparib | 27.7 | 27.7a | CR | −96%* |
| 2 | Level 3A | BRCA2 p.N986fs*2 | 6 | Olaparib | 2.1 | 2.6 | PD | +60% |
| 3 | Level 3A | ATM p.Q2414* (Nonsense) | 5 | Olaparib | 10.3 | 10.3b | Non-Cr/Non-PD | +75% |
| 4 | Level 3A | PALB2 D616fs*12 | 2 | Olaparib | 17.8 | 18.7c | Non-Cr/Non-PD | 0% |
| 5 | Level 3B | PDL2 Amplification | 2 | Pembrolizumab | 5.8 | 3.0 | PD | −11% |
| 6 | Level 3B | PMS2 p.V415M (Missense) | 0 | Pembrolizumab | 8 | 3.3 | PD | +83% |
| 7 | Level 4 | CDK12 Frameshift P955fs*18, Y246fs*2 | 6 | Olaparib | 7.8 | 7.8 | Non-Cr/Non-PD | +16% |
= confirmed PSA response
Patient currently on treatment break with no evidence of disease.
Patient switched therapy at 10.3 months due to clinical progression, without radiographic progression
Patient developed treatment related acute myeloid leukemia (AML)
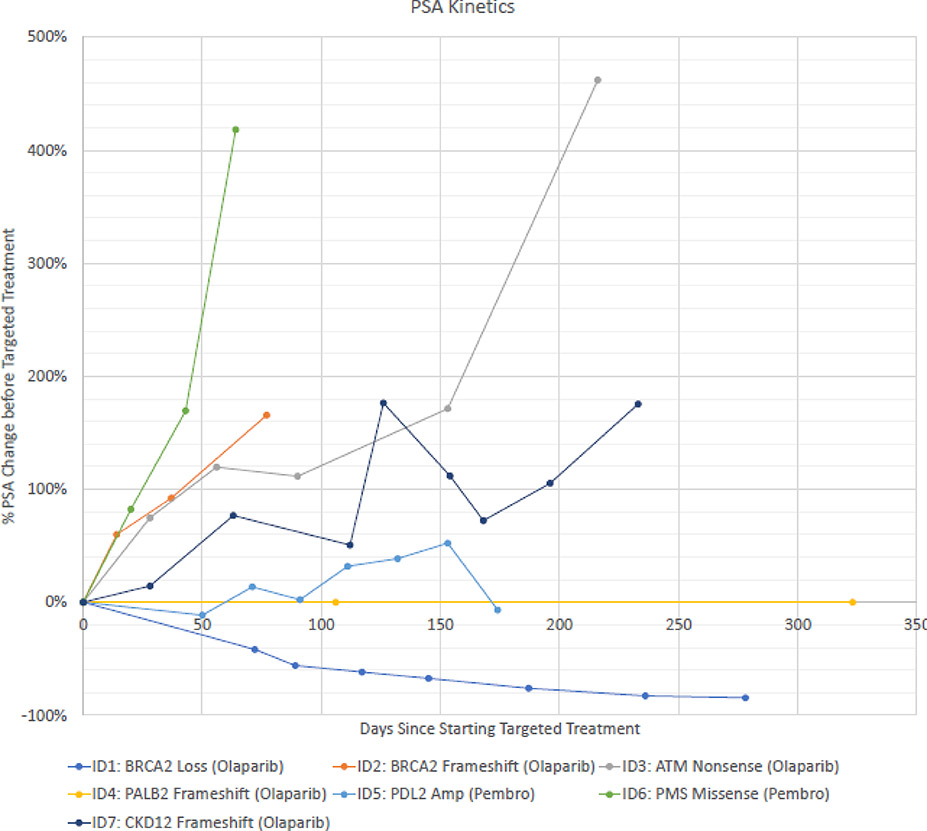
We next examined whether patients benefited from molecular profiling by examining the receipt and response to molecularly matched targeted therapies that these men would not have otherwise had access to as part of standard-of-care treatment. Treatment duration ranged from 2.1 – 27.7 months. Of the 7 patients with a Level 3A mutation, 4 patients received matched targeted therapy with olaparib for their DNA homologous repair defect mutation (Table 3) with one complete response (BRCA2 loss, duration >27 months), two with stable disease (10 and 19 months with ATM mutation and PALB2 frameshift mutation, respectively), and one patient with progressive disease (BRCA2 frameshift).
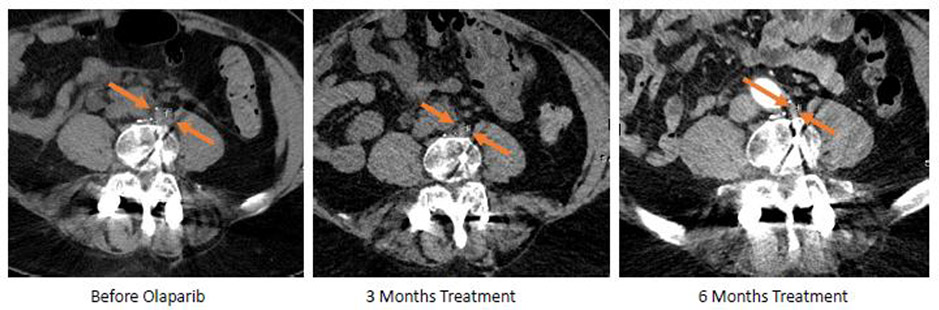
Patient 1 developed PSA recurrence after radiation for low risk PC and was treated with androgen deprivation therapy (ADT) for 4 years after which he developed mCRPC with bulky retroperitoneal and pelvic adenopathy and obstructive uropathy. He was treated with abiraterone and had a partial response but ultimately developed clinical/radiographic progression after 1 year. His genomic profiling revealed a somatic BRCA2 loss, RB1 loss, and TP53 loss; germline testing was negative. He was started on olaparib and reached a complete response by RECIST 1.1 with a corresponding 96% PSA decline from baseline (9.15 → 0.33) (Figure 3 and 4) which is ongoing after 27 months and is now disease free off therapy.
Figure 3:
PSA kinetivs
PSA change after initiation of targeted therapy shown above. See Table 3 for patient details ID1 was the only patint with a confirmed PSA response.
Figure 4:
patient ID1
Patient ID1 received olaparib for a pathogenic BRCA2 loss and above shoes the treatment response of one lymph node He achived a CR and a decrease in PSA by 96%.
Patient 2 presented with mHSPC, progressed to mCRPC after one year of ADT, and was subsequently treated with abiraterone, sipuleucel T, enzalutamide, radium-223, docetaxel, and cabazitaxel. His genomic testing revealed BRCA2 N986fs*2 mutation and was started on olaparib. Unfortunately, he did not respond and progressed clinically after 2 months of therapy.
Patient 3 had Gleason 9 mCRPC and had previously been treated with enzalutamide, sipuleucel-T, abiraterone, cabazitaxel, and two clinical trials before he was noted to have a somatic ATM p.Q2414* variant on FoundationOne testing. He was treated for 10.3 months with olaparib before switching therapy due to radiographic and PSA progression.
Patient 4 had a PALB2 frameshift alteration, detected in the setting of mCRPC and prior ADT, radiation, docetaxel, and carboplatin, with non-PSA producing disease. He was treated with olaparib with stable disease for 17.8 months. He subsequently developed fatal treatment related MDS-AML in follow-up, likely related to his prior therapy23.
Of the three patients with a Level 3A mutation who did not receive therapy, 2 (ATM mutation and BRCA2 deletion) were too ill for consideration of additional systemic therapy, and one patient (FANCA deletion) was enrolled into a clinical trial.
Both patients with a Level 3B mutation received pembrolizumab. Patient 5 had amplification of both PD-L1 and PD-L2 and Patient 6 had a missense mutation in PMS2, a mismatch repair gene. Both patients had progressive disease on pembrolizumab. Of note, patient 6 did not have subsequent MSI testing, and it is unknown at this time whether the alteration is pathogenic24. There were no confirmed PSA50 responses (Figure 3).
No patients with CDK12 deletions were treated with pembrolizumab, given that the data supporting CDK12 deletion with immune checkpoint response only emerged in 2018. Lastly, one patient with a Level 4 mutation (Patient 7) had a CDK12 frameshift (P955fs*18 and Y246fs*2) and received olaparib for 7.8 months with an overall response of progressive disease.
In summary, 12% (7/59) of evaluable patients received molecularly matched therapy and 5% (4/77) overall had clinical benefit from matched therapy for greater than 6 months. Of those receiving matched therapy, 57% (4/7) had complete imaging responses or stable disease for at least 6 months.
4. Discussion
Currently, there are six FDA approved therapies for mCRPC: docetaxel,25 cabazitaxel,7 abiraterone,26,27 enzalutamide,28,29 radium-223,8 and sipuleucel-T9. While these therapies are active in unselected patients, most men will progress within 1–2 years with treatment resistance. In a previously reported cohort of 150 patients, 20% of patients had a potentially clinically actionable mutation in the DNA repair pathway, suggesting a role for biomarker guided therapies in prostate cancer30. Here, we report our experience with the use of tissue molecular profiling in men with advanced prostate cancer and how genomic profiling impacted clinical care and eventual patient outcomes.
Our study demonstrates that genomic profiling utilizing a commercially available assay (FoundationOne) reveals that up to 22% (17/77) of patients may have a Level 1 to Level 4 actionable mutation, as defined by OncoKB. This is greater than the general estimates of benefit for genome-driven cancer therapy in the United States31, despite the fact that 23% of samples were insufficient for analysis. However, we found that in our mCRPC cohort, only 5% (4/77) of men had clear clinical benefit to a PARP inhibitor and no patients benefited from pembrolizumab based on duration of therapy. This represented 7% (4/59) of men with evaluable tissue, and is similar to another published report of precision oncology benefits in mCRPC patients32.
These data are important when counseling patients on the utility and outcomes of these tests, prior to genomic profiling. Based on this study, for the majority of patients for mCRPC, targeted gene panels will not change clinical decision making, as most tumors currently do not have any biomarker-driven therapeutic options. In the future, as emerging data supports the utility of genomic biomarkers to enrich for response to novel therapies, such as bi-allelic CDK12 deletion and MSI high mCRPC and response to PD-1 blockade, this proportion of men likely to benefit will increase18. In addition, as novel combination therapies are developed such as agents that target the PTEN/PI3K/Akt pathway, molecular alterations may become actionable and result in therapies that benefit patients33,34.
One strategy to improve the yield of genomic profiling may be earlier testing. First, earlier testing may enable additional lines of a targeted agent before a patient becomes too ill for therapy. One of the patients with a pathogenic BRCA2 mutation was unable to receive olaparib due to a declining functional status. Many patients at our institution who were not included in this chart review did not receive molecular testing at all, and thus did not have an opportunity for further matched treatment. A second reason for earlier testing is to allow for thoughtful sequencing of chemotherapy. Patient 4 died from treatment-related AML23. This patient had previously been treated with carboplatin, docetaxel, and radiation. Preliminary data in ovarian cancer suggests that patients exposed to earlier lines of platinum therapy have a higher incidence of developing treatment related AML to PARP inhibitors35.
Lastly, some patients with potentially actionable mutations did not receive matched therapy due to the inability to procure the treatment, either due to lack of prescription coverage or lack of expanded access use programs from the pharmaceutical company. However, most patients were granted therapy at no cost through the expanded access programs of AstraZeneca or Merck for olaparib and pembrolizumab, respectively. These programs that provide access to off-label therapies are crucial to our abilities to provide potentially efficacious treatment options for highly refractory patients who do not qualify for clinical trials.
There are several limitations to this study. First, tumor mutational burden (TMB) and microsatellite instability (MSI) status were not available at the time of testing, and knowledge of the association of MSI high disease or biallelic CDK12 loss with response to immune checkpoint blockade in mCRPC has only recently emerged. In our cohort, 7 (12%) patients had CDK12 loss but the FoundationOne assay does not currently report if these are biallelic18. These additional markers may increase the number of patients with actionable results, as both TMB and MSI status have been shown to be predictors of response to immunotherapy17,36,37. However, it is important to note that predictive biomarkers of immunotherapy remain imperfect for patients with prostate cancer. For MSI-high tumors (3% of all patients with prostate cancer), only about 50% of those patients will experience a PSA5038. Clinicians must exercise caution when interpreting biomarkers utilized outside of prostate cancer, such as PD-L1 amplification which is seen in less than 1% of prostate cancer39. In addition, careful analysis of each gene alteration is important as not all variants are pathogenic. In our study, patient 6 had a PMS2 p.V415M variant which has conflicting interpretations of pathogenicity, which may explain the lack of response to pembrolizumab.
A second limitation was that germline testing was not available for most of these men during the timeframe that this retrospective chart review was conducted. Our current practice, aligned with current NCCN guidelines, is to also include germline testing on all men with metastatic prostate cancer, given the prevalence of 12% or greater based on combined data from 7 different germline case series40. In the TOPARP trial, of the 7 patients who harbored BRCA2 loss, 3 had a previously unidentified pathologic germline mutation10. Preliminary data in prostate, ovarian and breast cancer suggest that patients with germline mutations in BRCA1 or BRCA2 benefit from PARP inhibitors, even in the absence of a somatic mutation41–43.
In our study, 23% of patients were excluded due to insufficient tissue for genomic analysis. This is not uncommon for patients with mCRPC where the most common site of metastatic disease is bone, where it is technically challenging to biopsy and frequently yields inadequate tissue for genomic profiling44. In addition, many of our patients in the mCRPC setting only had tissue from their diagnostic biopsy or radical prostatectomy specimen, which was collected many years prior. Prostate cancer is known to evolve, with acquired homologous recombination, mismatch repair, and CDK12 mutations that may arise as treatment resistance mechanisms30,45. One solution to address inadequate metastatic tissue biopsies is the use of circulating tumor DNA (ctDNA) or circulating tumor cells (CTC)46,47. It is known that there may be intratumoral and intertumoral genomic heterogeneity in localized prostate cancer, and a sufficiently sensitive and contemporary “liquid biopsy” may help capture the true genomic diversity of a patient’s malignancy48. In addition to discovering actionable mutations, CTC assays may be able to help guide the sequencing of standard of care therapies. The first CTC assays have now been prospectively validated for patients with mCRPC in the PROPHECY study - these assays may help clinicians who are deciding between abiraterone/enzalutamide or chemotherapy based on the AR-V7 status of CTCs49.
Lastly, our study is limited by its size, retrospective nature, and patient population to make generalizations regarding the utility of genomic testing in all patients with mCRPC. Our study was completed at a major academic medical center, where many patients have been referred for a second opinion and have already completed several prior lines of therapy. It is unknown if the yield of genomic testing would be higher or more likely to change clinical outcomes if the testing had been done earlier in a patient’s treatment course. In the current era of precision oncology and basket trials, genomic profiling is important not only for primary treatment selection, but also enrollment in biomarker guided clinical trials. Several large prospective studies are underway such as the IRONMAN registry and the Metastatic Prostate Cancer Project, collecting data on genomics, treatments, and clinical outcomes50–52.
5. Conclusion
Tissue based genomic profiling utilizing the FoundationOne assay guided biomarker driven therapy in 9% (7/77) of men with advanced prostate cancer, with 5% (4/77) of men having prolonged and durable responses from this approach. These proportions can serve as benchmarks to judge the merits of future precision medicine-based approaches to treating men with mCRPC. Presently, all approved therapies (except pembrolizumab for MSI-high mCRPC) are available and approved for men irrespective of their molecular profile. To improve upon our current precision oncology efforts, earlier testing may capture a broader, more robust population of patients who may benefit from targeted therapy or enrollment into clinical trials.
Supplementary Material
Highlights.
We examined the clinical outcomes for patients with advanced prostate cancer who underwent tumor DNA testing with FoundationOne.
Analyzing tumor DNA gave additional information which changed therapy 9% (7/77) of the time
Of the patients who received targeted therapy, 57% (4/7) derived clinical benefit
Acknowledgements
This study is supported by the Duke Prostate and Urologic Cancer Center research team.
Research Support: Supported by the Duke Prostate and Urology Cancer Center, Duke Cancer Institute.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. [DOI] [PubMed] [Google Scholar]
- 2.Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. New England Journal of Medicine 2015;372:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman DM, Laetsch TW, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. American Society of Clinical Oncology; 2017. [Google Scholar]
- 4.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 2015;16:152–60. [DOI] [PubMed] [Google Scholar]
- 6.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. Journal of Clinical Oncology 2008;26:242–5. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–54. [DOI] [PubMed] [Google Scholar]
- 8.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- 9.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. [DOI] [PubMed] [Google Scholar]
- 10.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New England Journal of Medicine 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasso CS, Wu Y-M, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan CJ, Abida W, Bryce AH, et al. TRITON3: An international, randomized, open-label, phase III study of the PARP inhibitor rucaparib vs. physician’s choice of therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD). American Society of Clinical Oncology; 2018. [Google Scholar]
- 13.Karzai F, Madan RA, Owens H, et al. A phase 2 study of olaparib and durvalumab in metastatic castrate-resistant prostate cancer (mCRPC) in an unselected population. American Society of Clinical Oncology; 2018. [Google Scholar]
- 14.Gronberg H, Eklund M, Lindberg J, et al. ProBio II: An adaptive and randomized multi-arm biomarker driven phase 2 study in men with castrate resistant prostate cancer (CRPC). American Society of Clinical Oncology; 2018. [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamideresistant prostate cancer. Oncotarget 2016;7:52810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y-M, Cieślik M, Lonigro RJ, et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018;173:1770–82. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FoundationOne CDX. 2018. at https://www.foundationmedicine.com/genomictesting/foundation-one-cdx.)
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 21.Sonpavde G, Pond GR, Armstrong AJ, et al. Radiographic progression by Prostate Cancer Working Group (PCWG)‐2 criteria as an intermediate endpoint for drug development in metastatic castration‐resistant prostate cancer. BJU international 2014;114:E25–E31. [DOI] [PubMed] [Google Scholar]
- 22.Chakravarty D, Gao J, Phillips S, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precision Oncology 2017:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Tucker M, Wang E, et al. Acute Myeloid Leukemia After Olaparib Treatment in Metastatic Castration-Resistant Prostate Cancer. Clinical genitourinary cancer 2017;15:e1137–e41. [DOI] [PubMed] [Google Scholar]
- 24.NM_000535.6(PMS2):c.1243G>A (p.Val415Met). (Accessed 05/25/19, at https://www.ncbi.nlm.nih.gov/clinvar/variation/142561/.)
- 25.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. The New England journal of medicine 2004;351:1502–12. [DOI] [PubMed] [Google Scholar]
- 26.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. The New England journal of medicine 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- 30.Robinson D, Van Allen EM, Wu Y-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquart J, Chen EY, Prasad V. Estimation of The Percentage of US Patients With Cancer Who Benefit From Genome-Driven Oncology. JAMA oncology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltran H, Eng K, Mosquera JM, et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA oncology 2015;1:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bono JS, De Giorgi U, Nava Rodrigues D, et al. Randomized Phase II Study of Akt Blockade With or Without Ipatasertib in Abiraterone-Treated Patients With Metastatic Prostate Cancer With and Without PTEN Loss. Clinical Cancer Research 2018. [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, George DJ, Armstrong AJ. Precision medicine approaches when prostate cancer Akts up. Clinical Cancer Research 2018. [DOI] [PubMed] [Google Scholar]
- 35.Korach J, Turner S, Milenkova T, et al. Incidence of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients (pts) with a germline (g) BRCA mutation (m) and platinum-sensitive relapsed ovarian cancer (PSR OC) receiving maintenance olaparib in SOLO2: Impact of prior lines of platinum therapy. American Society of Clinical Oncology; 2018. [Google Scholar]
- 36.Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Molecular Cancer Therapeutics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subudhi SK, Aparicio A, Troncoso P, et al. Linking tumor mutational load to clinical responses to ipilimumab (IPI) in men with advanced prostate cancer (PCa). American Society of Clinical Oncology; 2017. [Google Scholar]
- 38.Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA oncology 2019;5:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman AM, Piccioni D, Kato S, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA oncology 2018;4:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. New England Journal of Medicine 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennington KP, Walsh T, Harrell MI, et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clinical Cancer Research 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA½ Mutation. Journal of Clinical Oncology 2015;33:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meisner E, Rollins R, Ensor J, et al. Efficacy of olaparib monotherapy in patients (pts) with HER2-negative metastatic breast cancer (MBC) with germline BRCA mutation (g BRCAm) or lesional BRCA mutation (l BRCAm). American Society of Clinical Oncology; 2018. [Google Scholar]
- 44.Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology 2013;269:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S, Li J, Kemeny G, et al. Whole Genomic Copy Number Alterations in Circulating Tumor Cells from Men with Abiraterone or Enzalutamide-Resistant Metastatic Castration-Resistant Prostate Cancer. Clinical Cancer Research 2017;23:1346–57. [DOI] [PubMed] [Google Scholar]
- 47.Belic J, Graf R, Bauernhofer T, et al. Genomic alterations in plasma DNA from patients with metastasized prostate cancer receiving abiraterone or enzalutamide. International journal of cancer 2018;143:1236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei L, Wang J, Lampert E, et al. Intratumoral and intertumoral genomic heterogeneity of multifocal localized prostate cancer impacts molecular classifications and genomic prognosticators. European urology 2017;71:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong AJ, Halabi S, Luo J, et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. Journal of Clinical Oncology 2019:JCO. 18.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W, Kwiatkowski K, Brown J, et al. An observational study profiling biospecimens from 10,000 metastatic prostate cancer (mPCa) patients to screen for molecular alterations. American Society of Clinical Oncology; 2018. [Google Scholar]
- 51.Metastatic Prostate Cancer Project. at https://mpcproject.org/about-us.)
- 52.Prostate Cancer Outcomes: An International Registry to Improve Outcomes in Men With Advanced Prostate Cancer (IRONMAN) (IRONMAN). at https://clinicaltrials.gov/ct2/show/NCT03151629.)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.