Abstract
Inflammation must be effective while limiting excessive tissue damage. To walk this line, immune functions are grossly compartmentalized by innate cells that act locally and adaptive cells that function systemically. But what about the myriad tissue-resident immune cells that are critical to this balancing act and lie on a spectrum of innate and adaptive immunity? We propose that mammalian perivascular adventitial ‘cuffs’ are conserved sites in multiple organs, enriched for these tissue-resident lymphocytes and dendritic cells, as well as lymphatics, nerves, and subsets of specialized stromal cells. Here, we argue that these boundary sites integrate diverse tissue signals to regulate the movement of immune cells and interstitial fluid, facilitate immune crosstalk, and ultimately act to coordinate regional tissue immunity.
Regional control of tissue immunity along the vascular tree
Immune responses extend well beyond simple host defense, including beneficial roles in organ development, tissue remodeling, damage response, and at epithelial barriers, maintaining host-commensal balance. But too much, or the wrong type, can promote pathology in a range of human maladies, including cancer, diabetes, allergy, autoimmunity, fibrosis, cardiovascular disease, and neurodegenerative disease. To accomplish this balancing act, immune cells are classically segregated into two major tiers: local and systemic. The most numerous effectors of local immunity are macrophages. Distributed throughout tissue parenchyma and present in all organs, they adapt to their local organ and microenvironmental milieu, constantly communicating with tissue stromal and parenchymal cells [1]. With tissue perturbation, rapidly recruited innate granulocytes (i.e. neutrophils, eosinophils) and monocyte-derived macrophages complement local macrophage functions. Concurrent with these local responses, interstitial fluid drains both antigen presenting cells (APCs) and unbound antigens from tissues to lymphatics, ultimately accumulating at secondary lymphoid organs (SLOs, lymph nodes/spleen) to engage B- and T cells -- the major effectors of systemic immunity.
However, tissue resident lymphocytes (TRLs, Box 1) are also present in resting tissues, expand after immune challenge, and are by definition, local, performing elements of both ‘innate’ and ‘adaptive’ immune function, while coordinating critical tissue remodeling events. TRLs are comprised of innate lymphoid cells (ILCs), innate-like T cells and B cells, and adaptive tissue-resident memory (TRM) T and B cells [2,3]. TRLs reinforce local macrophages while promoting effective adaptive immune responses when required, positioning them as critical regulators of tissue immunity. At well-studied barrier tissues (e.g. skin, gastrointestinal, upper respiratory, genitourinary tracts), epithelial- and subepithelial-associated TRLs cooperate with local macrophages and dendritic cells (DCs) to promote a balanced barrier response [4]. Epithelial barrier tissues are continuously challenged by microbes and are dysfunctional in diseases such as inflammatory bowel disease, atopic dermatitis, and psoriasis. However, many human maladies (e.g. metabolic, fibrotic, neuropsychiatric, and cardiovascular diseases) occur in organs that largely lack epithelial barriers to the outside microbial world, but still maintain TRLs. TRLs and other ‘bridging’ leukocytes such as DCs, preferentially reside at natural tissue boundaries. While this includes external epithelial borders, other internal, sterile boundaries have been less appreciated. The surface of organs is one such boundary site and includes thin mesothelial membranes at body cavities (e.g. heart, lung, peritoneum) as well as the capsules of many other organs (e.g. kidney, bladder, subcutaneous adipose tissue, portions of the colon and pancreas). However, we focus on the vascular tree, the most widely distributed tissue boundary site. We provide a novel perspective on how tissue microanatomic structure is an understudied variable that dictates local immune and stromal cell composition and function. We argue that the outermost adventitial layer of the vascular tree is a critical boundary site and regional immune hub (Figure 1). We discuss multiple immunologic aspects of the adventitia, including: (i) its unique cellular composition, (ii) the regulation of fluid and immune cell movement, (iii) the development of tertiary-lymphoid organs (TLOs), and (iv) alterations during immune responses and tissue pathology.
Box 1: Tissue-resident lymphocytes.
Tissue-resident lymphocytes (TRLs) include innate lymphoid cells (ILCs, NK cells), innate-like T cells (γδ T cells, MAIT cells, NKT cells) and tissue-resident memory T (TRM) cells that are developmentally deposited and contribute to local immune responses and tissue homeostasis [4]. TRM cells initially respond to their cognate antigen, but upon tissue residency and reactivation, acquire the ability to respond independently of antigen. TRLs are regulated by a broad range of tissue-derived signals, including locally produced cytokines, eicosanoids, hormones and neuropeptides, and produce signals that act on both hematopoietic and non-hematopoietic cells in their immediate environment. TRLs commonly reside in barrier tissues in proximity to the epithelial layer [2]. However, to what extent different populations of TRLs populate other regions remains unclear. In addition to promoting both beneficial and pathological immune responses, TRLs are an integral part of a range of physiological functions, including tissue development, metabolic function [31,79–81], remodeling [82,83] and damage repair [84].
Figure 1. Schematic representation of mammalian tissue interstitial fluid flow through three proposed immunological tiers.
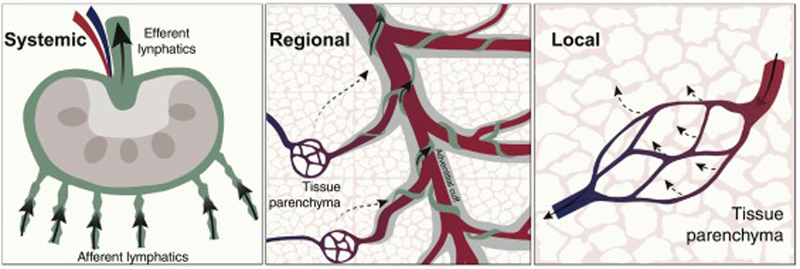
Arrows indicate interstitial fluid flow exiting the circulation in tissue parenchyma (Local; tier 3), flowing back via adventitial cuffs to enter lymphatic vessels (Regional; tier 2, adventitial spaces indicated in grey), and reaching lymph nodes through afferent lymphatics (Systemic; tier 1). The size of arrows indicates the volume of interstitial fluid flow.
What are adventitial cuffs?
Blood vessels provide conduits for the trafficking of immune cells, delivery of oxygen and nutrients, and removal of tissue waste. Capillary endothelial cells are thin cells specialized for these critical exchange functions and are typically supported by a closely associated layer of stromal cells. In contrast, larger arteries and veins, as well as other tubular-shaped structures, contain three distinct layers: the intima -- the innermost layer comprised of endothelial cells (vessels) or epithelial cells (airways, ducts), the media -- comprised of smooth muscle cells that provides contractility, and the adventitia -- the external extravascular space, rich in connective tissue matrix, that provides structural support (Figure 2). Although adventitial regions are often ‘perivascular’, they are restricted to the extracellular matrix (ECM)-rich regions of intermediate-to-larger vessels. The term perivascular is also more broadly used to describe sites adjacent to capillary microvasculature -- a related but quite distinct tissue parenchymal site. Adventitial ‘cuffs’ (a term referencing the layer of material at the end of a shirt sleeve), were originally recognized in the lung over 30 years ago, described as expandable microanatomic spaces in models of pulmonary edema in dogs [5] and sheep [6]. Similar structures are present in multiple human tissues and described as dynamic, fluid-filled interstitial spaces, continuous with draining lymphatics, that are rich in unique collagens and fibroblast-like cells [7]. Approximately 15 years ago, immune aspects of lung bronchovascular cuffs were presciently discussed and which remain relevant today, including: (i) the spatial cuff-immune organization, (ii) fluid dynamics and cellular trafficking during active immune responses, and (iii) cuff remodeling during inflammation [8,9].
Figure 2. Schematic overview of the perivascular adventitial cuff region in cross-section.
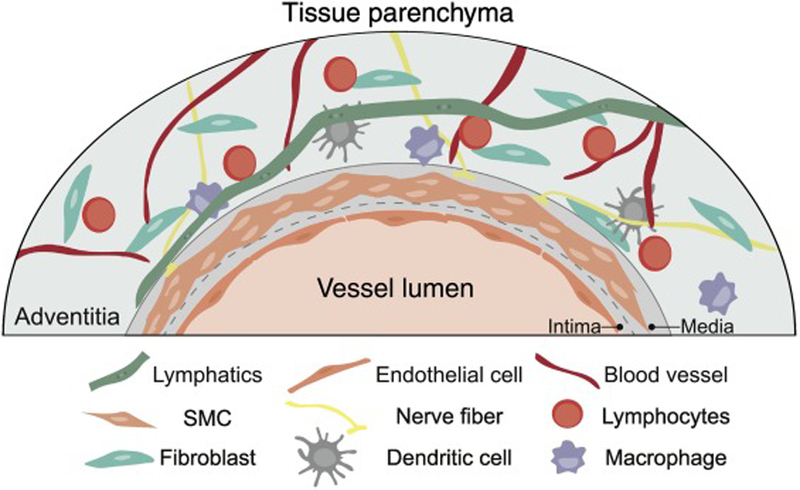
Adventitial cuffs are enriched for a variety of specialized hematopoietic and non-hematopoietic cells and structures, including lymphatics, smooth muscle cells (SMCs), fibroblasts, endothelial cells, peripheral nerves, lymphocytes, macrophages, and dendritic cells.
Adventitial cuffs host a variety of immune cells types, including macrophages and DC subset(s), and expand with immune cell infiltrates in the context of diverse challenges that include allergy, infection and organ transplantation [8,9]. Adventitial cuffs also maintain subsets of specialized stromal cells (Box 2) that produce and break down ECM, regulate vascular tissue remodeling [10], and include mesenchymal progenitor cells that can give rise to endothelial cells, smooth muscle cells, chondrocytes, adipocytes and osteocytes [11,12]. Adventitial cuffs contain their own distinct network of smaller blood vessels [13]. Lymphatics also travel in these regions [14] and peripheral nerves are enriched at these sites [10]. Adventitial cuffs have similarities to SLOs, including the presence of unique stroma and endothelial cells that regulate specialized leukocytes. For example, in mice, group 2 innate lymphoid cells (ILC2s) localize within adventitial cuffs of multiple tissues, together with DCs and T regulatory cells (Tregs) subset(s) [15], as well as a population of resident tissue macrophages during homeostasis [16,17]. Furthermore, T helper 2 resident memory (Th2 TRM) cells accumulate at these sites for many months following type 2 immune challenges, such as in helminthic ‘worm’ infections or allergic asthma models [15]. These findings suggest that adventitial cuffs function as dynamic hubs for regional tissue immunity in multiple organs. As most data supporting this hypothesis derives from mouse models, one area of future work will be to validate these concepts in humans.
Box 2: Stromal cells.
Multicellular organisms divide labor into macro-anatomic regions named ‘organs’, each of which has one or more ‘parenchymal’ tissue-specific cell types that perform the primary organ function(s). Adipose tissue has adipocytes, brain has neurons, and liver has hepatocytes. Within each organ there are a variety of supporting ‘stromal’ cells that maintain and regulate parenchymal cells. These supporting cells can have unique names in discrete organs (e.g. astrocytes in brain, hepatic stellate cells in liver, fibroblastic reticular cells in SLOs). Although the nomenclature is not entirely coherent, ‘stromal cells’ commonly refers to fibroblastic mesenchymal-derived cells that produce extracellular matrix components with varying degrees of multipotency.
The major stromal cell subsets in SLOs organize lymphocyte compartmentalization and facilitate adaptive responses by promoting interactions between DCs and antigen-specific T and B cells [85]. Recently, lymph node stromal cells were more thoroughly characterized by single-cell RNA sequencing, defining specialized subsets that form discrete functional niches [20]. In non-SLO tissues, stromal cells can be grouped into cells associated with small capillaries (pericytes), larger vessels (adventitial fibroblasts), and organ capsules or cavities (mesothelial cells, capsular fibroblastic cells).
In the setting of tissue fibrosis, activated ‘myofibroblasts’ (αSMA+) arise from pericytes and/or adventitial fibroblasts. While fibroblast-like stromal cells are well recognized for their contribution to tissue immunity, precisely where and how this occurs is not well described. Due to the complexity of stromal cell classification and nomenclature, we refer to these as ‘stromal cells’, broadly including mesenchymal-derived cells, while excluding epithelial, endothelial and tissue-specific parenchymal cells. Future studies will provide a better understanding of stromal cell localization, ontogeny, heterogeneity, and function.
Why organize regional immunity at adventitial cuffs?
Adventitial cuffs are continuously exposed to interstitial fluid, forming a collecting hub that drains parenchymal fluid into lymphatic vessels (Figure 1). Pre-lymphatics -- structures that channel interstitial fluid into lymphatic conduits [18]-- as well as the lymphatic conduits themselves, are enriched in the adventitia of the vascular structures and bronchial tree in the lung [14]; together, they create a focused site for interstitial fluid drainage and an initial meet-and-greet site for resident immune cells and tissue-derived signals (e.g. microbial products, cytokines, metabolites, hormones, neuropeptides, as well as changes in pH, and oxygen concentrations). As such, the adventitial cuff is well-suited for monitoring tissue homeostatic thresholds, integrating these signals to judge tissue health, coordinating appropriate regional immune responses and tissue remodeling, and recruiting blood-borne peripheral immune cells when thresholds are exceeded. There are several potential benefits to anatomically-restricting potent immune subset(s) from the tissue parenchyma while concentrating them in regional immune hubs, including: (i) increasing efficiency of immune monitoring (similar to SLOs), (ii) reducing excessive immune-mediated tissue destruction, (iii) conserving immune energy expenditure, and (iv) limiting inadvertent tissue autoimmune or autoinflammatory responses.
How do stromal cells crosstalk with local immune cells?
Subsets of stromal cells (Box 2) organize and communicate with immune cells in several tissues, both enhancing and restricting immunity depending on context [19]. Prior to focusing on adventitial stroma, we first review our current understanding of stromal-immune interactions. These interactions are best studied in SLOs, where diverse stromal cell subsets promote survival and positioning of immune cells to coordinate efficient adaptive immune responses [20]. Heterogenous stromal cells, often referred to as fibroblastic reticular cells (FRCs, PDGFRα+ gp38+ CCL19+), dynamically respond to inflammatory signals through numeric expansion and production of signals that can recruit and activate immune cells, as evidenced from mouse models of inflammation in the brain meninges [21], central nervous system-draining lymph nodes [22] and adipose tissue [23]. Conversely, mouse FRCs in intestinal Peyer’s patches can restrict group 1 ILC activity by controlling IL-15 concentrations, ultimately limiting excessive intestinal damage during enteric viral infection (e.g. mouse hepatitis virus (MHV)) [24]. In addition to regulating immune cell numbers and function, stromal cells may also be involved in shaping the tissue composition of TRLs during development. For example, a recent study identified a subset of mouse mesenteric adipose tissue stromal cells (PDGFRα+ gp38+) that supports the differentiation of ILC2s from embryonic ILC precursors [25] [15,26–30]. In adult mice, subset(s) of tissue stromal cells (PDGFRα+ gp38+ Sca1+) expressed interleukin (IL)-33 ( a central mediator of type 2 immunity and tissue repair), and supported lung ILC2s during allergic immune responses; they also promoted physiologic adipose tissue ILC2s and tissue-resident Treg numbers and functions, potentially conferring metabolic benefits in models of obesity-induced type 2 diabetes [15,26–30]. Similar stromal cells in the mouse pancreas support ILC2 residence and cytokine production, ultimately driving beneficial beta-cell insulin production in models of diabetes [31]. Together, these studies suggest that tissue stromal cell subset(s) may be critical regulators of ILC2s and other resident immune cells, acting during development, homeostasis, and pathologic perturbations. Although this cross-talk between stromal cells and immune cells appears to be an integral part of both SLO and tissue immunity, the precise stromal subsets and tissue locations that mediate these events are not well defined.
Are stromal cells in adventitial regions poised for immune crosstalk?
Adventitial stromal cells (ASCs), also referred to as adventitial or perivascular fibroblasts, are micro-anatomically defined stromal subset(s) that sense diverse local signals (e.g. hypoxia, mechanical stretch, cytokines, growth factors) and respond by producing cytokines, chemokines, growth factors, matrix components and metalloproteases to remodel and repair their environment [10]. However, a precise phenotypic and functional distinction between ASCs and other types of stromal cells (i.e. pericytes, mesenchymal stem/stromal cells, parenchymal fibroblasts, FRCs, see Box 2) is less clear, and thus, the nomenclature for similar populations is ambiguous. Aside from the expression of PDGFRα and gp38, the hedgehog family transcription factor Gli1 marks a subset of stromal cells that surround mouse lung bronchi and larger vessels in multiple organs [15,32,33]. Gli1+ stromal cells are transcriptionally and functionally distinct from parenchymal (alveolar-associated) stromal cells in the mouse lung [34]. Further, two subsets of lung stromal cells can be distinguished with a PDGFRαGFP reporter, with Sca1+ (Ly6A+) cells particularly enriched in the adventitial-associated fraction [15,35,36]. However, neither Gp38 nor Sca1 are specific markers for ASCs in all tissues. Transcriptionally similar subsets of PGDFRα+ stromal cells have been identified by single cell RNA sequencing of several other mouse tissues, suggesting that ASCs are a functionally conserved adventitial population across multiple organs [37,38], consistent with possible conserved immunologic functions that regulate regional immune responses in tissues. It is possible that stromal cells at other boundary sites (e.g. capsules, body cavities) are functionally similar to ASCs, and we suggest that ASCs are likely a stromal cell state that reflects adaptation to this unique boundary environment.
Our group recently described crosstalk between stromal cells and ILC2s occurring within mouse adventitial cuffs [15]. In naïve mice, ASCs preferentially localize with ILC2s across multiple organs and confer superior in vitro survival to ILC2s and IL-5 producing Th2 TRM cells, in comparison with parenchymal-associated stromal cells. ASCs produce type 2 cytokines IL-33 and thymic stromal lymphopoietin (TSLP), and potentially other signal(s) that support ILC2s[15]. In turn, ILC2s can produce IL-13 that promotes stromal cell expression of IL-33 [15], CCL11 (Eotaxin1) [30] and possibly other cues to coordinate type 2 immunity. Because subsets of IL-33-responsive Tregs and DCs are also enriched at these sites, IL-33 appears to be at least one stroma-derived signal that coordinates immune cell composition of resting adventitial spaces[15]. These studies suggest that adventitial cuffs may be skewed towards mixed type 2 and regulatory immune responses in resting tissues -- a combination that is likely beneficial for promoting tissue homeostasis while restricting immune pathology. However, ASCs also express receptors for several cytokines (e.g. IL-1α/β, interferon (IFN)-γ, IL-17A, type I IFNs, IL-6, IL-22). Precisely how ASCs may respond to these distinct cytokine ‘flavors’ of immune responses is unclear, but suggests that ASCs are likely involved in coordinating other types of tissue immune responses. What other TRL subsets reside in the cuff during homeostatic or inflammatory states, remains to be clearly defined.
Are adventitial cuffs regional hubs that regulate tissue immunity?
Lymphatic vessels track in reverse along blood vessels in the adventitial space, draining tissue interstitial fluids, free antigens, DCs, and lymphocytes to lymph nodes [39]. DCs are tissue sentinel cells that both coordinate local immunity and help initiate adaptive immune responses by migrating to lymph nodes to prime naïve T cells. DCs are situated beneath epithelial layers in barrier tissues, but in non-barrier tissues, mouse DC subset(s) appear to be enriched in adventitial cuffs [15,40]. On the one hand, ILC2s localize to the same regions, and ILC2-DC crosstalk can promote DC migration to draining lymph nodes (dLNs), as well as DC expression of chemokines inducing the accumulation of Th2 TRM cells in mouse models of allergic asthma [41,42]. These results suggest that adventitial cuffs may help relay information to initiate and promote adaptive immune responses. On the other hand, Tregs act locally to limit excessive immune responses and are enriched in adventitial cuffs of resting mice [15]. IL-33 further drives tissue Treg accumulation [43–45] and ILC2s promote IL-33 mediated Treg expansion [28], suggesting that adventitial cuffs might also provide support for resident Tregs, and limit excessive tissue immunity. Another layer of control may be exerted via neurons, as ILC2-activity is both negatively and positively regulated by neuron-derived signals (e.g. VIP, NMU, CGRP, catecholamines) in mouse models of helminth-driven and allergic inflammation [43–45]. In summary, emerging evidence suggests that adventitial cuffs are sites that concentrate interstitial fluid drainage while maintaining unique subsets of immunoregulatory cells (ASCs, neurons) and immune cells (Tregs, DCs, ILC2s); this positions adventitial cuffs as strategic sites that integrate multiple signals of tissue status, and coordinate an appropriate immune response.
Are adventitial cuffs preferred sites for TLO formation?
In addition to its role in bridging tissue and lymph node immune responses, adventitial cuffs can also serve as a platform for further immune cell accumulation and organization. TLOs are heterogenous lymphoid aggregates in tissues, containing multiple immune, vascular, and stromal components that resemble SLOs. In contrast to SLO development, TLOs form in the absence of innate lymphoid tissue inducer (LTi) cells, and other TRLs enriched at adventitial cuffs may replace LTi function [46,47]. TLOs are not defined by the presence of any individual cell type, and likely represent a spectrum of lymphoid structures similar to cryptopatches and isolated lymphoid follicles in the intestine [48]; these can range from adventitial cuff TRLs present in loose clusters, to fully developed TLO structures with organized B cell follicles, follicular DC, and high endothelial venules. TLOs in the lung are also known as inducible bronchus-associated lymphoid tissue (iBALT) and primarily form in adventitial cuffs along the bronchovascular tree [49–53]. iBALTs are sufficient to mount protective antigen-specific memory T cells and humoral responses to influenza virus infection, independently of nodal and splenic based adaptive responses [54]; this is relevant, as it suggests that these regional immune hubs can compensate for a lack of systemic immunity.
TLOs also spontaneously form in visceral fat in peritoneal and pleural cavities, and are referred to as fat-associated lymphoid clusters (FALCs). Although FALCs are typically B cell-rich environments, spontaneous FALCs form in the absence of adaptive lymphocytes (Rag−/−) and are partially driven by the commensal flora via FRC-like stromal cells [23,46]. ILC2s are abundant in mesenteric adipose tissue [55] and make up the large majority (80%) of lymphocytes in mesenteric FALCs of Rag−/− mice [46]. After inflammation, mesenteric FALCs numerically increase and can be driven by invariant NKT cells and IL-4/IL-13 signaling [46,56]. While FALCs and other TLOs can be initiated independently of adaptive lymphocytes, expansion and maturation of TLOs are characterized by infiltration of B cells, not unlike peripheral lymph node development [57]. CXCL13 is a key chemokine that promotes accumulation of B cells and plays a role in both SLO and TLO development. CXCL13 can be induced in FRC-like stromal cells by a variety of different inflammatory cytokines, including type I IFN [58], IL-6 [59], IL-17 [52] and IL-22 [60]. Furthermore, IL-33 from stromal cells promotes B cell activation in pleural FALCs, possibly via ILC2-derived IL-5 [61]. Together, these studies suggest that diverse TRLs and stromal cells are enriched at adventitial cuffs and can cooperate to initiate and reinforce TLOs in both non-inflammatory and inflammatory conditions.
How do immune cells traffic into adventitial cuffs?
Many immune cells accumulate in adventitial cuffs during inflammation [8], but it is not clear how they find their way to these sites. Although adventitial cuffs surround both arteries and veins, these larger vessels contain vascular walls composed of a network of endothelial cells, smooth muscle cells, and layers of elastic membranes. Thus, this is an unlikely route for direct immune cell trafficking. One potential path is via the vasa vasorum (VV, or ‘vessels of the vessels’) and VV-proximal venules. The VV is a separate network of small blood vessels present in larger arteries and veins. The VV provides the vascular wall with nutrients and oxygen where simple diffusion from the large vessel itself is not sufficient. In atherosclerosis and vascular disease, VV neovascularization protrudes into the media and intima and is associated with disease progression [62]. However, outgrowth of the VV network is also associated with inflammatory responses in the vessel wall, and an extended VV might be one of the mechanisms leading to increased accumulation of immune cells in the cuff during inflammation, as previously proposed [8]. Alternatively, or additionally, immune cells may enter tissues via venules in parenchymal regions and traffic towards adventitial cuffs via natural interstitial fluid flow or chemoattractant signals, as described below.
What signals attract immune cells to adventitial cuffs?
Chemokines guide migration and positioning of immune cells during development, homeostasis and inflammation. Adventitial stromal cells express a range of chemokines (e.g. Cxcl1, Cxcl12, Ccl2, Ccl7, Ccl8, Ccl11, Ccl19), and could influence trafficking and positioning of immune cells in adventitial cuffs. Several of these chemokines are known to recruit innate immune cells (neutrophils, monocytes, eosinophils) and control DC trafficking from tissues to lymph nodes [63]. CCL8 has been implicated in IL-5+ Th2 cell homing to mouse skin during allergic inflammation [64], as well as promoting IL-33-induced ILC2 motility in mouse lung adventitial cuffs [65]; tehse studies suggest that CCL8 can constitute one of the signals inducing ILC2s, Th2 TRM cells, and Treg subsets to preferentially localize to adventitial cuffs [15,66]. Further, mouse ASCs express both Cyp27a1 and Cyp7b1 -- enzymes necessary for processing cholesterol to generate oxysterol gradients, and known to guide DCs, B and T cells to their correct positions in lymph nodes to execute efficient adaptive immune responses [67–69]. As such, ASCs might also provide positional cues to resident lymphocytes and DCs expressing the oxysterol receptor EBI2, to coordinate cuff immune responses. If this applies, adventitial stromal cells might participate in the regulation of tissue immune cell recruitment and positioning, although further testing is warranted to assess this possibility.
How does the adventitial cuff change during inflammation?
The adventitial cuff is adaptable and responds rapidly to inflammatory signals, providing a transient niche for neutrophils, eosinophils, and monocytes [10]. Recent work also suggests that mouse ASCs expand and express elevated IL-33 concentrations for many months after helminthic ‘worm’ infection [15]. Similar to macrophages, innate lymphocytes, and other long-lived cells, stromal cells can also adapt to prior inflammatory insults, alter their functional characteristics, and display trained memory for subsequent insults, as recently discussed [70]. Lymphatics also expand following pulmonary infection -- primarily in adventitial cuffs -- and outgrown lymphatic networks persist long after the infection is cleared [14]. Lymphatic cell subset(s) express IL-7 in both humans and mice [15,51] and support the survival of lung Th2 TRM cells in response to allergic challenge [51].
CD4+ and CD8+ TRM cells are memory T cells with limited blood recirculation that mount local, protective immune responses upon re-challenge [2,71]. TRM cells are present in multiple tissues (e.g. lung, skin, gut, brain, female reproductive tract (FRT), kidney, liver) [72–77] and persist in tissues for extended periods post immune challenge [66,73,74,76]. Mouse and human TRM cells are best studied at epithelial barriers, where they commonly express CD69+ and CD103+ (an integrin associated with homing to epithelial sites). However, neither CD4+ nor CD8+ TRM cells universally express CD103 [2,71], implying that there are additional tissue-niches for TRM cell subset(s) that may include adventitial cuffs. Notably, IL-33 is one signal that promotes TRM accumulation in tissues [74], possibly via the proposed downregulation of S1PR1, in synergism with TGFβ and TNF signaling [78]. Similar to ILCs, TRM cells display phenotypic similarities that are tissue-specific, indicating that local niches likely influence the functional properties of these cells [72].
Expanded mouse ILC2s and Th2 TRM cells predominately localize within the cuff post-helminth infection, suggesting that ASCs may provide a niche for both of these long-lived cells. However, in helminth infection or allergic asthma models, these type 2 lymphocytes partially redistribute to tissue parenchyma, suggesting that novel, non-canonical stromal cell niches may also be formed [15,66]. What functional consequences TRL ‘niche re-localization’ may have remains to be explored, but could be significant in a variety of physiologic and disease states. Together, we suggest that adventitial cuffs remodel during prolonged periods after inflammatory encounters, and serve as a long-term expanded niche for ILC2s, Th2 TRMs, and possibly, other immune cell subsets (Key Figure, Figure 3).
Key Figure, Figure 3. Proposed adventitial cuff dynamics during development, homeostasis, inflammation, and resolution.
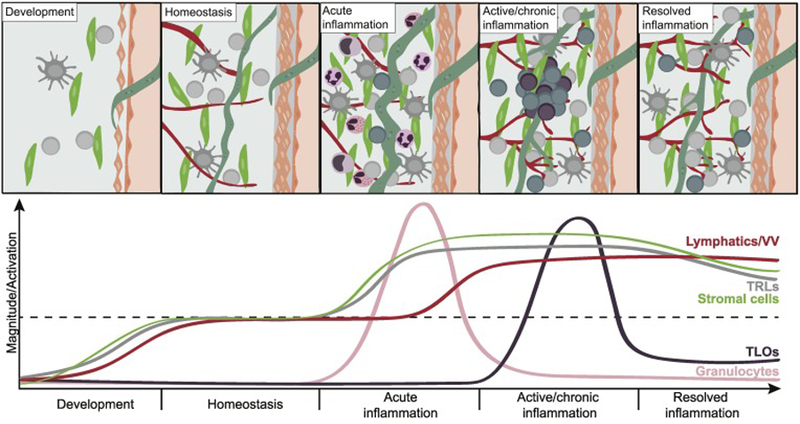
(A) During development: Subsets of tissue resident lymphocytes (TRLs) are deposited early and expand postnatally in parallel with local stromal cells. (B) During homeostasis: The cuff is occupied by a variety of hematopoietic cells, including TRLs, such as group 2 innate lymphoid cells (ILC2s) and regulatory T cells (Tregs), dendritic cells (DCs), and subsets of resident tissue macrophages. Stromal cells and lymphatics are sources of cytokines such as TSLP, IL-33 and IL-7, which provide support to resident immune cells, potentially skewing the milieu towards a resting type 2 immune tone. (C) During acute inflammation: Stromal cells produce chemokines that recruit innate immune cells such as neutrophils, monocytes and eosinophils. Cross-talk between TRLs and stromal cells have the ability to promote production of inflammatory cytokines by both subsets, DC activation and migration, and antigen drainage to lymph nodes. (D) During active/chronic inflammation: Adventitial cuffs serve as platforms for TRM accumulation and TLO formation, possibly because local stromal cells produce chemokines such as CXCL13 and provide support for infiltrating adaptive lymphocytes. The local microvasculature (VV) and lymphatics extend, facilitating both entry and exit of immune cells in and out of the cuff. (E) During resolution of inflammation: TLOs gradually reduce in number/size, but the extended lymphatic and vascular network persists, allowing increased trafficking and drainage. Innate TRLs are reinforced by the presence of TRMs, and trained stromal cells display an increased ability to respond to inflammatory signals, contributing to an elevated immunological tone in the adventitial cuff post-inflammation.
Concluding remarks
Here, we describe adventitial cuffs as regional outposts that integrate homeostatic and inflammatory signals, coordinating tissue immunity via crosstalk between stromal cells, nerves, TRLs, lymphatics, progenitor populations, macrophages and DCs. Adventitial cuffs are dynamic environments, separating vital organ function from potentially destructive immune responses, and relaying information to draining lymph nodes to coordinate systemic immunity. As hubs for regional immune responses, we propose that cuffs integrate diverse signals to regulate local immunity, shape systemic immunity, and communicate with tissue stromal and parenchymal cells to coordinate tissue growth and remodeling (see outstanding questions). Although described many years ago, we have only begun to dissect the cells and signals that define this complex site of regional immune organization. We anticipate that defining immune cell localization and stromal-immune crosstalk will lead to an improved understanding of normal tissue physiology and remodeling and help elucidate the immune mechanisms that impact conditions such as fibrosis, cardiovascular disease, metabolic disease, and allergic asthma.
Outstanding Questions Box:
How are adventitial immune responses regulated to protect the functionality of tissues?
Which signals activate adventitial stromal cells in adventitial cuffs? Physical? Microbial? Cytokines?
How are TRLs distributed to specific niches during development? How does this change following infection?
Are adventitial cuffs sites of immune cell trafficking at rest, or after inflammation?
Where do TRL subsets reside within non-barrier and barrier tissues at rest, and after inflammation, and how do resident stromal cells impact TRL distribution and function?
What are the functional consequences of TRL ‘niche redistribution’?
Highlights.
Perivascular, mammalian adventitial cuffs are enriched in specialized stromal cells and tissue resident immune cells, acting as outposts for regional tissue immunity.
Adventitial cuffs are dynamic environments that channel interstitial fluid from tissue parenchyma to lymphatics.
Stromal cells are heterogenous and shape discrete functional niches in tissues.
Stromal cell subset(s) can support the differentiation and maintenance of innate lymphocytes in peripheral tissues.
Acknowledgements
The authors wish to thank R.M. Locksley, A.V. Molofsky, and members of the A.B. Molofsky lab for their valuable input on the manuscript. This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, K08DK101604), the National Heart, Lung, and Blood Institute (NLHBI, R56HL142701, R01HL142701), the Larry L. Hillblom Foundation, the Sandler Asthma Basic Research Center, the Nina Ireland Program Foundation, the UCSF Department of Laboratory Medicine, the UCSF Program for Breakthrough Biomedical Research. M.W.D. is supported by the Swedish Society for Medical Research and the Swedish Research Council.
Glossary
- Adventitia
The outermost layer of connective tissue supporting larger vessels or other tubular structures
- Cryptopatches
Small lymphoid aggregates that develop postnatally in the small intestinal lamina propria
- Dendritic cells (DCs)
Professional antigen-presenting cells with the capacity to process and present antigen and prime naïve T cells. Subpopulations of DCs traffic from peripheral tissues to draining lymph nodes to initiate adaptive immune responses
- Fat-associated lymphoid clusters (FALCs)
non-classical lymphoid aggregates (TLOs) found in adipose tissues in both human and mouse
- Fibroblastic reticular cells (FRCs)
Heterogenous non-hematopoietic stromal cells residing in secondary lymphoid organs (SLOs), defined by the expression of PDGFRα and gp38 (podoplanin), and the absence of the endothelial marker CD31 (PECAM-1). Phenotypically and functionally similar populations of FRC-like stromal cells are recognized in diverse non-SLO tissues
- Inducible bronchus-associated lymphoid tissue (iBALT)
Ectopic lymphoid tissue (TLOs) induced by infection or inflammation in both human and mouse lungs
- Innate lymphoid cells (ILCs)
Lymphocytes that are predominantly tissue-resident, developmentally deployed, lack the expression of antigen specific receptors, and rapidly respond to tissue-derived signals. Divided into three major subgroups (ILC1–3) that functionally resembles the Th1, Th2 and Th17 subsets of corresponding CD4+ T helper cells
- Lymphatic vessels
Reabsorb excess interstitial fluid from peripheral tissue, transporting immune cells and antigen to draining lymph nodes; they can expand in response to inflammation. Lymphatic endothelial cells produce signals that can influence immune cell activation, survival, and trafficking
- Lymphoid tissue inducer (LTi) cells
a group of group 3 innate lymphoid cells that are essential for the formation of lymph nodes and intestinal Peyer’s patches via crosstalk with specialized stromal cells
- Mesenchymal stem/stromal cells (MSCs)
heterogenous adult, non-hematopoietic stromal cells with the potential to differentiate into all mesodermal lineages (bone, muscle, cartilage, fat) in vitro. Adult mesenchymal cells do not all have multipotency (MSC capability) and the in vivo multipotency of many tissue MSCs is not well established. MSCs are widely distributed in tissues and are studied for their immunomodulatory and reparative properties
- Pericytes
perivascular stromal cells that wrap around endothelial cells of small arterioles, venules and capillaries to maintain vascular integrity and to regulate blood flow
- Peyer’s patches
organized lymphoid structures found in the submucosal layer of the small intestine
- Secondary lymphoid organs (SLO)
Include spleen and lymph nodes that are embryonically formed and interconnected via the lymphatic system and blood circulation, allowing adaptive lymphocytes to recirculate systemically. SLOs are organized by specialized stromal cells to facilitate antigen encounter and coordinate efficient immune responses
- Stromal cells
Loosely defined group of non-hematopoietic cells often derived from the mesenchyme or mesodermal layer during development. Stromal cells lack the obvious cell polarity of an epithelial cell and produce extracellular fibers and ECM components. ‘Mesenchymal cells’ (see box 2) is a term often used interchangeably with ‘stromal cells’
- Tertiary lymphoid organs (TLOs)
Inflammation-induced lymphocyte aggregates that form postnatally in tissues, often in perivascular areas, and sometimes bear tissue-specific names (iBALT in lung, FALCs in fat, SALT in skin). They range in size from small (loose lymphoid aggregates), to fully organized structures (with B-cell follicles that resemble SLOs). Also referred to as tertiary lymphoid tissue (TLT)
- Tissue-resident memory cells (TRM cells)
Subpopulations of CD4+ and CD8+ effector memory T cells that remain in tissues following antigen clearance, and which provide increased protection against subsequent challenges. Commonly identified by CD69 expression, absence of CCR7 and variable expression of CD103. They adapt to their immediate environment and display striking similarities to tissue-resident innate lymphocytes
- Trained memory
enhanced responsiveness of previously activated cells that lacks antigen-specific receptors, including macrophages, innate lymphocytes, and stromal cells, upon re-challenge
- T regulatory (Tregs) cells
subset of CD4+ T cells that express FoxP3 and CD25 and suppress effector T cells, maintain tolerance to self-antigens, and prevent autoimmune responses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Okabe Y and Medzhitov R (2016) Tissue biology perspective on macrophages. Nat. Immunol 17, 9–17 [DOI] [PubMed] [Google Scholar]
- 2.Masopust D and Soerens AG (2019) Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol 37, 521–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allie SR et al. (2019) The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol 20, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan X and Rudensky AY (2016) Hallmarks of Tissue-Resident Lymphocytes. Cell 164, 1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel RP et al. (1986) Morphometry of the distribution of hydrostatic pulmonary oedema in dogs. Br J Exp Pathol 67, 865–877 [PMC free article] [PubMed] [Google Scholar]
- 6.Conhaim RL et al. (1989) Sequence of interstitial liquid accumulation in liquid-inflated sheep lung lobes. J. Appl. Physiol 66, 2659–2666 [DOI] [PubMed] [Google Scholar]
- 7.Benias PC et al. (2018) Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci Rep 8, 4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabst R and Tschernig T (2002) Perivascular capillaries in the lung: an important but neglected vascular bed in immune reactions? J. Allergy Clin. Immunol 110, 209–214 [DOI] [PubMed] [Google Scholar]
- 9.Pabst R (2004) The periarterial space in the lung: its important role in lung edema, transplantation, and microbial or allergic inflammation. Pathobiology 71, 287–294 [DOI] [PubMed] [Google Scholar]
- 10.Stenmark KR et al. (2013) The adventitia: essential regulator of vascular wall structure and function. Annu. Rev. Physiol 75, 23–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corselli M et al. (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21, 1299–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wörsdörfer P et al. (2017) The vascular adventitia: An endogenous, omnipresent source of stem cells in the body. Pharmacol. Ther 171, 13–29 [DOI] [PubMed] [Google Scholar]
- 13.Guntheroth WG et al. (1982) Pulmonary microcirculation: tubules rather than sheet and post. J Appl Physiol Respir Environ Exerc Physiol 53, 510–515 [DOI] [PubMed] [Google Scholar]
- 14.Baluk P et al. (2014) Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am. J. Pathol 184, 1577–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlgren MW et al. (2019) Adventitial Stromal Cells Define Group 2 Innate Lymphoid Cell Tissue Niches. Immunity DOI: 10.1016/j.immuni.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakarov S et al. (2019) Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 [DOI] [PubMed] [Google Scholar]
- 17.Lim HY et al. (2018) Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 49, 326–341.e7 [DOI] [PubMed] [Google Scholar]
- 18.Aharinejad S et al. (1995) Pulmonary lymphatics and their spatial relationship to venous sphincters. Anat. Rec 242, 531–544 [DOI] [PubMed] [Google Scholar]
- 19.Buechler MB and Turley SJ (2018) A short field guide to fibroblast function in immunity. Semin. Immunol 35, 48–58 [DOI] [PubMed] [Google Scholar]
- 20.Rodda LB et al. (2018) Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity 48, 1014–1028.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pikor NB et al. (2015) Integration of Th17- and Lymphotoxin-Derived Signals Initiates Meningeal-Resident Stromal Cell Remodelling to Propagate Neuroinflammation. Immunity 43, 1160–1173 [DOI] [PubMed] [Google Scholar]
- 22.Majumder S et al. (2019) IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat. Immunol 20, 534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Shibayama C et al. (2018) Fibroblastic reticular cells initiate immune responses in visceral adipose tissues and secure peritoneal immunity. Sci Immunol 3, eaar4539 [DOI] [PubMed] [Google Scholar]
- 24.Gil-Cruz C et al. (2016) Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs. Nat. Immunol 17, 1388–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga S et al. (2018) Peripheral PDGFRα+gp38+ mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med 215, 1609–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang SK et al. (2017) Stromal cell cadherin-11 regulates adipose tissue inflammation and diabetes. J. Clin. Invest 127, 3300–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahlakõiv T et al. (2019) Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol 4, eaax0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molofsky AB et al. (2015) Interleukin-33 and Interferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 43, 161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spallanzani RG et al. (2019) Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol 4, eaaw3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rana BMJ et al. (2019) A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J Exp Med 4, jem.20190689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalmas E et al. (2017) Interleukin-33-Activated Islet-Resident Innate Lymphoid Cells Promote Insulin Secretion through Myeloid Cell Retinoic Acid Production. Immunity 47, 928–942.e7 [DOI] [PubMed] [Google Scholar]
- 32.Peng T et al. (2015) Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526, 578–U282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramann R et al. (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C et al. (2018) Expansion of hedgehog disrupts mesenchymal identity and induces emphysema phenotype. J. Clin. Invest 128, 4343–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L et al. (2012) Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell Mol. Biol 47, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R et al. (2018) Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. Elife 7, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han X et al. (2018) Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 172, 1091–1107.e17 [DOI] [PubMed] [Google Scholar]
- 38.Schwalie PC et al. (2018) A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 [DOI] [PubMed] [Google Scholar]
- 39.Randolph GJ et al. (2017) The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol 35, 31–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabeza-Cabrerizo M et al. (2019) Tissue clonality of dendritic cell subsets and emergency DCpoiesis revealed by multicolor fate mapping of DC progenitors. Sci Immunol 4, eaaw1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halim TYF et al. (2014) Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halim TYF et al. (2016) Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat. Immunol 17, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardoso V et al. (2017) Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallrapp A et al. (2017) The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriyama S et al. (2018) β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 [DOI] [PubMed] [Google Scholar]
- 46.Bénézech C et al. (2015) Inflammation-induced formation of fat-associated lymphoid clusters. Nat. Immunol 16, 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang JY et al. (2016) Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol 7, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pabst O et al. (2005) Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol 35, 98–107 [DOI] [PubMed] [Google Scholar]
- 49.Woodland DL and Randall TD (2004) Anatomical features of anti-viral immunity in the respiratory tract. Semin. Immunol 16, 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eddens T et al. (2017) Pneumocystis-Driven Inducible Bronchus-Associated Lymphoid Tissue Formation Requires Th2 and Th17 Immunity. Cell Rep 18, 3078–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinoda K et al. (2016) Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc. Natl. Acad. Sci. U.S.A 113, E2842–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangel-Moreno J et al. (2011) The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol 12, 639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroda E et al. (2016) Inhaled Fine Particles Induce Alveolar Macrophage Death and Interleukin-1α Release to Promote Inducible Bronchus-Associated Lymphoid Tissue Formation. Immunity 45, 1299–1310 [DOI] [PubMed] [Google Scholar]
- 54.Moyron-Quiroz JE et al. (2006) Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25, 643–654 [DOI] [PubMed] [Google Scholar]
- 55.Moro K et al. (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 [DOI] [PubMed] [Google Scholar]
- 56.Nayar S et al. (2019) Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc. Natl. Acad. Sci. U.S.A 116, 13490–13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bovay E et al. (2018) Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med 215, 2760–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denton AE et al. (2019) Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med 48, jem.20181216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goya S et al. (2003) Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J. Pathol 200, 82–87 [DOI] [PubMed] [Google Scholar]
- 60.Barone F et al. (2015) IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc. Natl. Acad. Sci. U.S.A 112, 11024–11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson-Jones LH et al. (2016) Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun 7, 12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedding DG et al. (2018) Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front Immunol 9, 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokol CL and Luster AD (2015) The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 7, a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Islam SA et al. (2011) Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat. Immunol 12, 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puttur F et al. (2019) Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Sci Immunol 4, eaav7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner DL et al. (2018) Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J Immunol 200, 1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu E et al. (2017) Distinct oxysterol requirements for positioning naïve and activated dendritic cells in the spleen. Sci Immunol 2, eaal5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cyster JG et al. (2014) 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol 14, 731–743 [DOI] [PubMed] [Google Scholar]
- 69.Baptista AP et al. (2019) The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4+ T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity 50, 1188–1201.e6 [DOI] [PubMed] [Google Scholar]
- 70.Crowley T et al. (2018) Stroma: the forgotten cells of innate immune memory. Clin. Exp. Immunol 193, 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo PA et al. (2019) Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4, eaas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beura LK et al. (2019) CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med 216, 1214–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hondowicz BD et al. (2016) Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity 44, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slütter B et al. (2017) Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2, eaag2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinbach K et al. (2016) Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med 213, 1571–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinert EM et al. (2015) Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klicznik MM et al. (2019) Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci Immunol 4, eaav8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skon CN et al. (2013) Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol 14, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kohlgruber AC et al. (2018) γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol 19, 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brestoff JR et al. (2015) Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee M-W et al. (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bartemes KR et al. (2014) Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol 134, 671–678.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doisne J-M et al. (2015) Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol 195, 3937–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gadani SP et al. (2017) Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med 214, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller SN and Germain RN (2009) Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol 9, 618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]


