Abstract
Objectives:
While palliative care (PC) has been shown to improve symptoms and end-of-life (EOL) care for patients with cancer, data are lacking on the patterns of use and outcomes of PC consultations for hospitalized patients with liver disease. We sought to characterize the patterns of use and outcomes of PC consultations for hospitalized patients with liver disease compared to patients with cancer.
Methods:
We conducted an observational study using data from the Palliative Care Quality Network (PCQN). The PCQN contains prospectively collected data on 135,197 hospitalized patients receiving PC consultations at 88 PCQN sites between 1/2013–12/2017. The PCQN dataset includes patient demographics, processes of care, and patient-level clinical outcomes.
Results:
The cohort included 44,933 patients, of whom 4,402 (9.8%) had liver disease and 40,531 (90.2%) had cancer. Patients with liver disease were younger (58.9 years vs. 65.2 years, p<0.0001) and had higher in-hospital mortality (28% vs. 16.8%, p<0.0001). Patients with liver disease were more likely to receive PC consultations to address goals of care (81.7% vs. 67.9%, p<0.0001) as opposed to pain management (10.9% vs. 34.9%, p<0.0001). Both groups had similar rates of symptom improvement and change in resuscitation preferences after PC consultation.
Conclusion:
Hospitalized patients with liver disease were more likely to have a PC referral to address goals of care compared to those with cancer and were more likely to die in the hospital. Despite late PC consultations, patients with liver disease experienced improvement in symptoms and clarification of their goals of care, similar to those with cancer.
Keywords: Palliative care, cirrhosis, cancer, advance care planning, symptom management
INTRODUCTION
Liver disease is the twelfth-leading cause of mortality in the United States, resulting in almost 40,000 deaths in 2016.1,2 Due to symptoms such as fatigue, pruritus, muscle cramps, and pain, as well as complications such as ascites, hepatic encephalopathy, and variceal bleeding, patients with liver disease have a high symptom burden and limited life expectancy in the absence of liver transplantation.3–7 In addition, patients with advanced liver disease such as decompensated cirrhosis are frequently hospitalized during the last month of life and often receive intensive life-sustaining therapies such as mechanical ventilation and renal replacement therapy during their terminal hospitalizations.8–14 As such, patients with liver disease often experience poor quality of life and aggressive medical care at the end-of-life (EOL).
Given their significant morbidity and mortality, patients with liver disease have substantial palliative care (PC) needs, but they are rarely referred to specialty PC services.13–16 While strong evidence exists demonstrating that inpatient PC can improve symptom burden and quality of life for hospitalized patients with cancer, it remains unknown if similar improvements in symptom burden and quality of life occur for hospitalized patients with liver disease receiving PC consultations.17–21 Moreover, patterns of PC use for hospitalized patients with liver disease are currently unknown. While recent data from national observational studies have shown that PC consultations during the terminal hospitalizations of patients with liver disease are associated with lower healthcare utilization and costs, further work is needed to characterize the specific contribution of PC consultations to the improvement of other outcomes in this population.15,22
Using data from the Palliative Care Quality Network (PCQN), a national PC quality improvement collaborative, we sought to characterize current patterns of use and outcomes of PC consultations for hospitalized patients with liver disease compared to patients with cancer.
METHODS
The Palliative Care Quality Network:
The PCQN is a national collaborative of interdisciplinary PC teams in the United States collecting standardized data to benchmark processes of care and patient-level outcomes to identify best practices and promote quality improvement.23 As of December 2017, there were 88 teams in 17 states collecting and submitting data. PCQN member teams come from hospitals that vary in size (mean: 379 beds, range: 48–1126) and type (not-for-profit: 66%, teaching: 19%, public: 9%, for-profit: 1%, and other: 4%). For the purposes of the PCQN, a PC consultation is defined as the series of PC visits that occur for a single adult (age ≥ 18 years) patient during a single hospitalization.
Study Procedures:
The data for this observational study were extracted on March 6, 2018 and include 135,197 patient encounters conducted by 88 PCQN member teams between January 1, 2013 and December 31, 2017. The study was reviewed and approved by the University of California, San Francisco Institutional Review Board (#16–18596).
Data Elements:
The 23-item core PCQN dataset includes patient demographics and clinical information, actions taken by the PC teams in the course of patient care, and patient-level clinical outcomes prospectively collected by PCQN members for every patient seen for an inpatient PC consultation.
Patient demographic and clinical information includes age, gender, primary diagnosis, location at the time of referral (e.g. medical/surgical unit, telemetry/stepdown unit, critical care unit, and others) and functional status as measured by the Palliative Performance Scale score (higher scores indicate greater physical ability).24 In the PCQN database, the PC clinician records the primary diagnosis prompting the referral. Data regarding patients with many different primary diagnoses are represented in the PCQN, including cancer, liver disease, heart disease, pulmonary disease, renal disease, neurological conditions, and others. In this study, we compared data for patients for whom liver disease was the primary condition prompting the PC consultation to those patients with cancer. In the PCQN dataset, liver disease is defined as the presence of end-stage liver disease, cirrhosis, acute liver failure, hepatitis B, hepatitis C, primary biliary cholangitis, and other primary hepatic conditions, while cancer is defined by the presence of a solid tumor in any organ regardless of the stage of disease.
In the PCQN database, palliative care process measures include date of the PC referral and reason(s) for the PC consultation categorized as discussing advance care planning and goals of care (ACP/GOC), symptom management, withdrawal of life-sustaining interventions, transition to comfort care, hospice referral, and providing patient/family with emotional and/or spiritual support. Multiple reasons for the PC consultation could be documented by the PC team. In addition, members of the PC team used screening questions directed at hospitalized patients and/or their families to identify PC needs at the time of consultation. PC needs were categorized as 1) symptom management; 2) psychosocial concerns; 3) spiritual concerns, and 4) ACP/GOC. Members of PCQN teams also documented when screening for PC needs was not possible due to patients’ inability to communicate, and/or their families declining or being unavailable for the discussion. Multiple PC needs could be screened by the PC team during the initial consultation.
PCQN members also collected data on resuscitation preferences during the hospitalization, as well as the completion of ACP documents such as advance directives (AD) and physician orders for life-sustaining treatment (POLST) forms. Data on services arranged at the time of hospital discharge, including referrals to hospice, home-based PC, outpatient PC, and home health care, were also collected.
Patient-level clinical outcomes data include the following: 1) patient-reported symptom severity scores for pain, anxiety, shortness of breath, and nausea assessed daily by the PC team during hospitalization on a 4–point scale ranging from none to severe; 2) resuscitation preferences at the time of PC consultation and at discharge; 3) survival to hospital discharge; and 4) disposition.
Because the PCQN is used primarily for quality improvement and data are collected by clinicians in the course of usual care, not all 23 data elements are entered for every patient seen. There is no effort to obtain missing data. A full list of the PCQN data elements is available at www.pcqn.org.
Statistical Analysis:
Descriptive statistics including frequencies, means, and standard deviations [SDs] were used to examine the distribution of measures. Chi-square (χ2) analysis was undertaken to examine bivariate associations between categorical variables and analysis of variance (ANOVA) was undertaken to examine associations between categorical and continuous variables. McNemar–Bowker test was conducted to examine change in resuscitation preferences, which were defined as full (patient preference was to receive all available resuscitative efforts including chest compressions, cardioversion, and intubation), do not resuscitate and do not intubate (DNR/DNI), and partial (any limitation in resuscitation efforts short of DNR/DNI status) from first consultation to discharge. We defined improvement in symptom severity as an improvement by at least one level of severity within 72 hours in patients who reported moderate or severe symptoms at the initial PC assessment.25,26 For all analyses, an alpha of < 0.05 was used to determine statistical significance. There was no adjustment or imputation for missing data; analyses were performed only for patients for whom data was available for each specific data element. The Statistical Package for the Social Sciences version 23 for Mac was used to conduct all analyses.
RESULTS
Patient Characteristics:
The cohort included 44,933 patients, of whom 40,531 (90.2%) had cancer and 4,402 (9.8%) had liver disease as the primary diagnosis leading to PC consultation. Patients with liver disease were younger (58.9 years vs. 65.2 years, p < 0.0001), had lower Palliative Performance Scale scores (34.6 vs. 42.3, p < 0.0001), and a higher percentage were men (59.6% vs. 49.1%, p < 0.0001) compared to patients with cancer (Table 1). Patients with liver disease were more likely to receive PC consultation in the critical care unit compared to those with cancer (27.9% vs. 12.5%, respectively). Patients with liver disease were significantly less likely to have an AD (14.7% vs. 21.2%, p < 0.0001) in the medical record at the time of the initial PC consultation.
Table 1.
Patient characteristics at time of referral to specialty palliative care for hospitalized patients with liver disease and patients with cancer
| Characteristic | Liver Disease | Cancer | P value |
|---|---|---|---|
| Age, mean (95% CI), y | 58.9 (58.5, 59.3) | 65.2 (65.0, 65.3) | <0.0001 |
| Mean Palliative Performance Scale score (95% CI) | 34.6 (34.0, 35.2) | 42.3 (42.2, 42.6) | <0.0001 |
| Other* | 381 (8.8) | 5,356(13.4) | |
| Support for patient/family | 985 (22.6) | 8,291 (20.9) | 0.01 |
| No. (%) | 624 (14.7) | 8,180(21.2) | <0.0001 |
| No. (%) | 356 (8.4) | 3,317(8.7) | 0.6 |
| Mean (95% CI), d | 6.8 (5.9, 7.7) | 3.4 (3.8, 4.0) | <0.0001 |
“Other” consists of emergency department, labor & delivery, skilled nursing facility, pediatrics, or acute rehabilitation. Abbreviations: AD, advance directive; LOS, length of stay; PC, palliative care; POLST, physician orders for life-sustaining treatment
Reasons for PC Consultation:
As compared to patients with cancer, patients with liver disease were significantly more likely to receive a PC consultation to address GOC/ACP (81.7% vs. 67.9%, p < 0.0001), withdrawal of life-sustaining interventions (3.5% vs. 1.5%, p < 0.0001) and transition to comfort care (7.6% vs. 5.6%, p < 0.0001). Patients with liver disease were significantly less likely to receive a PC consultation for pain management (10.9% vs. 34.9%, p < 0.0001) or other symptom management (10.2% vs. 21.7%, p < 0.0001).
Processes of Care:
As shown in Table 2, patients with liver disease were less likely to be screened for pain (80.5% vs. 88.4%, p < 0.0001) and non-pain symptoms such as anxiety, nausea, and dyspnea (80.2% vs. 85.6%, p < 0.0001), but were more likely to be screened for ACP/GOC needs (93.6% vs. 90.4%, p < 0.0001). There were no differences in the rates of screening for psychosocial and spiritual needs between the patient populations.
Table 2.
Patients’ symptoms and needs screened at the time of specialty palliative care referral
| Characteristic | Liver Disease | Cancer | P value |
|---|---|---|---|
| No. (%) | 2,473 (80.5) | 28,270 (88.4) | <0.0001 |
| No. (%) | 2,457 (80.2) | 26,695 (85.6) | <0.0001 |
| No. (%) | 2,723 (84.5) | 24,575 (83.3) | 0.08 |
| No. (%) | 2,025 (72.6) | 18,858 (72.4) | 0.8 |
| No. (%) | 3,472 (93.6) | 30,109(90.4) | <0.0001 |
Amongst those patients with liver disease who underwent symptom assessment screening during the initial PC consultation, 27.4%, 10.3%, 3.6%, and 5.8% reported moderate-to-severe pain, anxiety, nausea, and dyspnea symptoms, respectively. The proportion of patients with cancer who reported moderate-to-severe pain, anxiety, nausea, and dyspnea symptoms were 41.8%, 15.1%, 9.1%, and 10%, respectively.
Timing and Outcomes of PC Consultations:
Timing and outcomes of PC consultations are shown in Table 3. Patients with liver disease had a significantly longer average length of stay (LOS) in the hospital prior to the PC consult request compared to patients with cancer (6.8 days vs. 3.4 days, p < 0.0001) and they were less likely to receive “early” (within 24 hours of hospital admission) PC consultations than patients with cancer (34.1% vs. 49.0%, p < 0.0001). Patients with liver disease were also followed by the PC service for a longer period of time after initial consultation (6.0 days vs. 5.5 days, p = 0.02).
Table 3.
Patient-level outcomes after referral to specialty palliative care
| Outcomes | Liver Disease | Cancer | P value |
|---|---|---|---|
| No. (%) | 142 (3.5) | 1,375(3.6) | 0.7 |
| No. (%) | 526 (12.9) | 4,724 (12.4) | 0.4 |
| Dyspnea | 37 (62.7) | 935 (65.9) | 0.6 |
| No. (%) | 1,179(28.0) | 6,567(16.8) | <0.0001 |
| Other** | 449(15.2) | 3,159(9.9) | |
| No services | 3,878(19.8) | 368 (26.6) | <0.0001 |
Includes patients who reported moderate to severe symptoms at the initial PC assessment who reported symptom improvement by at least one level of severity at the second assessment occurring within 72 hours of the initial assessment.
“Other” consists of non-hospital inpatient setting, residential care, respite/shelter. Abbreviations: AD, advance directive; PC, palliative care; POLST, physician orders for life-sustaining treatment
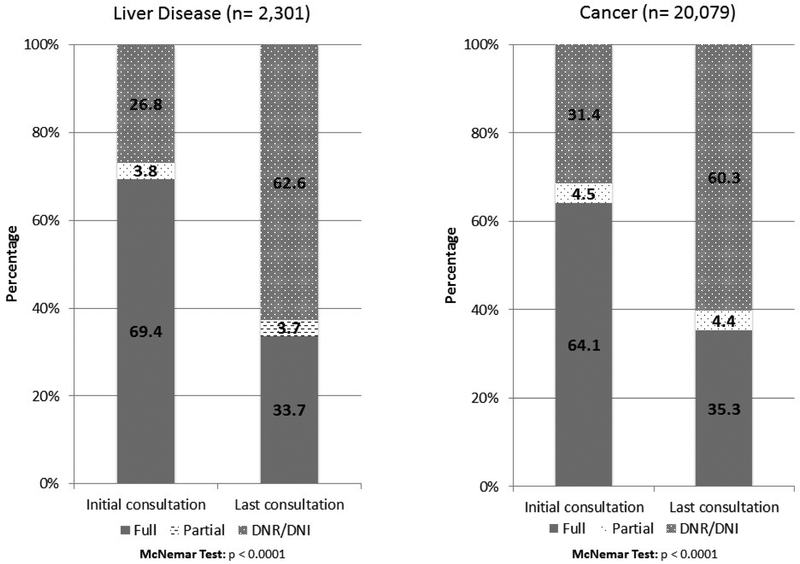
Changes in resuscitation preferences for patients with liver disease and cancer from the time of PC consultation to hospital discharge are shown in Figure 1. A large percentage of patients with liver disease and cancer had a significant change in their resuscitation preferences after PC consultation. Overall, 69.4% of patients with liver disease were full code at the time of PC consultation, and only 33.7% were full code at the time of discharge (p < 0.0001). Similar changes were seen for patients with cancer (from 64.1% to 32.5%, p < 0.0001). At the time of discharge, there were improved rates of ACP documentation in the form of POLST (12.9%) and AD (3.5%) for patients with liver disease, with similar rates of completion seen for patients with cancer.
Figure 1: Change in code status from time of palliative care consultation to hospital discharge for patients with liver disease versus patients with cancer.
DNR, do not resuscitate; DNI, do not intubate. Partial indicates any limitation in resuscitation efforts short of DNR/DNI status.
There were no differences in the proportion of patients experiencing improvement in pain, anxiety, nausea, or dyspnea symptoms after PC consultation between patients with liver disease and cancer (p ≥ 0.1).
A significantly higher proportion of patients with liver disease compared to patients with cancer who were seen by PC died during their hospitalization (28% vs. 16.8%, p < 0.0001). Patients with liver disease were also less likely to be discharged to home (48.6% vs. 64.7%) and more likely to be discharged to a long-term acute care hospital or extended care facility (20.7% vs. 15.2%). Amongst patients followed by the PC service who were discharged home, patients with liver disease were more likely to be referred to hospice (42.2% vs. 38.6%, p = 0.008) and less likely to be discharged with clinic-based (3% vs. 12.1%, p < 0.0001) or home-based (4.7% vs. 7.1%, p < 0.001) PC services.
DISCUSSION
In this study, we examined current patterns of use and outcomes of PC consultations for hospitalized patients with liver disease compared to patients with cancer. Despite being younger on average than patients with cancer, patients with liver disease receiving inpatient PC consultations were sicker with poorer functional status, a higher likelihood of being in the critical care unit, and higher in-hospital mortality. These findings verify that patients with liver disease often receive PC referrals late in the course of illness to provide assistance when they are closer to death.16,27 Palliative care consultation for patients with liver disease were more likely to be requested to address EOL care needs such as discussing GOC/ACP, withdrawal of life-sustaining interventions, and referral to hospice, than pain and other symptom management as compared to patients with cancer. However, patients with liver disease who received a PC consultation did experience symptom improvement and change in resuscitation preferences which may suggest a potential supportive role for PC in this population.
Previous studies show and our study confirms that patients with liver disease suffer from substantial physical and psychological symptom distress and poor quality of life.5,28,29 Despite their high symptom burden and poor functional status, hospitalized patients with liver disease spent twice as long in the hospital (6.8 days vs 3.4 days) prior to receiving a PC consultation as patients with cancer. As compared to patients with cancer, patients with liver disease may be more likely to present in the setting of an acute deterioration from liver failure, variceal bleeding, sepsis, or hepatic encephalopathy, and thus the most immediate focus of their hospitalization would be on acute management of complications of liver disease, including expedited evaluation for liver transplantation. In turn, patients with liver disease might only be receiving PC consultations once efforts to treat their acute conditions have failed in order to support transitions to EOL care.14,16 Few data currently exist showing the benefit of PC involvement in addressing symptom burden in patients with liver disease. However, our study results demonstrate that patients with liver disease reported high rates of moderate-to-severe pain and anxiety symptoms and were equally likely as those with cancer to report symptom improvement, which suggests that reducing the time to PC consultation could improve their quality of care, regardless of their acuity of presentation or transplant eligibility. Given their significant unmet PC needs for symptom management, utilizing inpatient PC services for hospitalized patients with liver disease early and routinely in the illness trajectory, and not just for EOL care, may improve the quality of care delivered to this vulnerable patient population.
Our data demonstrate that despite being relatively young, patients with liver disease receiving inpatient PC consultations represent a very debilitated population receiving intense care with high rates of in-hospital death and substantial EOL care needs. Almost 30% of inpatients with liver disease receiving a PC consultation were seen in the intensive care unit, and they had a mean Palliative Performance Scale score of 34.6 at the time of consultation, corresponding to being completely bedbound and requiring total care.24 Despite their significant morbidity and higher in-hospital mortality, patients with liver disease had lower rates of ACP documentation at the time of PC referral compared to those with cancer. These findings may be attributed to several important factors. First, liver disease disproportionately affects adults under the age of 65, with recent striking increases in mortality seen in young adults aged 25–34 due to alcohol-related liver disease.31 Younger patients with incurable disease may find it difficult to accept the imminent loss of lifetime opportunities, which could delay ACP. Second, patients with liver disease often have an unpredictable clinical trajectory, in which progressive decline is frequently punctuated by acute and life-threatening decompensations due to complications of liver failure.28,32,33 Thus, the younger age and unpredictable illness trajectory in this population often makes prognostication difficult and may leave these patients and their families with little time to adequately prepare for EOL. Despite this, our findings demonstrate that inpatient PC consultations improved AD and POLST form completion underscoring the potential role of inpatient palliative care for helping patients with liver disease and their families clarify their preferences for EOL care.30 Futher randomized trials are needed to demonstrate the impact of PC referrals on ACP documentation in this patient population.
Our study has limitations. For one, we are unable to ascertain the timing of the PC referral relative to the timing of patients’ primary diagnosis – patterns of PC utilization may differ between patients presenting with acute vs. chronic disease. Furthermore, because data collection is performed by the PC teams, there may be opportunity for bias in reporting outcomes of PC consultations. Notably, the PCQN database only contains information on patients who received PC consultations during their hospital admissions. As such, information on the PC needs and outcomes of hospitalized patients with liver disease and cancer who were not followed by the PC team service are not known. Additionally, the PCQN inpatient dataset does not include information about symptoms such as fatigue, muscle cramps, insomnia, depression, and others that are associated with poor health-related quality of life in patients with liver disease, thereby underestimating the disease burden for these patients.14,34–36 The PCQN dataset is purposely focused on the most prevalent symptoms to reduce the burden of data collection and is not meant to limit the number of symptoms that PC teams assess. Because symptom management is a core tenet of PC practice, it is likely that PC teams evaluate and treat more symptoms than are included in the dataset, further supporting the potential benefits of PC consultation for this patient population. Importantly, the database does not include data on patients’ transplant candidacy, medical comorbidities, disease complications, social backgrounds, psychosocial support, stage of illness, or Model for End-Stage Liver Disease scores, and additionally it is unknown whether patients with hepatocellular carcinoma were classified as having liver disease versus cancer. The impact of transplant candidacy in particular on patterns of PC use for hospitalized patients with liver disease is an important area meriting further study. These data would help us to understand better the needs of specific populations of patients with liver disease and target PC services accordingly. Lastly, we did not assess whether patterns of PC utilization differed based on clinical setting, such as academic or community hospitals, an important area that will require future study.
In conclusion, our study demonstrates that hospitalized patients with liver disease receiving a PC consultation were younger on average than patients with cancer and suffered from poorer functional status and higher in-hospital mortality. When compared to patients with cancer, patients with liver disease were more likely to receive a referral to PC later in their hospital course, and consults were requested in order to address goals of care rather than symptom management. Despite this difference, patients with liver disease referred for PC consultations had similar improvements in their symptoms and documentation of their goals of care as patients with cancer.
These data suggest that routine screening of hospitalized patients with liver disease for PC needs and referring those with significant needs to PC teams early in their hospital course may improve symptoms, clarify preferences, and improve the quality of care for this very sick population of patients.
Acknowledgment:
We would like to thank PCQN advisory board members and all members of the PCQN, who inspire us with their dedication to working together to improve care for seriously ill patients and their families.
Financial Support: We would like to acknowledge the foundations that have generously supported the PCQN including the Archstone Foundation, the California HealthCare Foundation, the Gordon and Betty Moore Foundation, the James Irvine Foundation, the Kettering Family Foundation, the Stupski Foundation, and the UniHealth Foundation. This work was also supported by the National Institutes of Health (K24DK078772 to RTC, T32DK007191 to NNU, and K23DK114526 to NDE).
Abbreviations:
- PC
palliative care
- EOL
end-of-life
- PCQN
Palliative Care Quality Network
- ACP
advance care planning
- GOC
goals of care
- AD
advance directive
- POLST
physician orders for life-sustaining treatment
- DNR
do not resuscitate
- DNI
do not intubate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Competing Interests: The authors have no financial disclosures or conflicts of interest to declare related to the topic of this manuscript.
REFERENCES
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep.2016;65(4):1–122. [PubMed] [Google Scholar]
- 2.Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49(8):690–696. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Gralnek IM, Hays RD, et al. Health-Related Quality of Life Predicts Mortality in Patients With Advanced Chronic Liver Disease. Clinical Gastroenterology and Hepatology. 2009;7(7):793–799. [DOI] [PubMed] [Google Scholar]
- 4.Dan AA, Kallman JB, Wheeler A, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26(6):815–820. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–178. [DOI] [PubMed] [Google Scholar]
- 6.Orr JG, Homer T, Ternent L, et al. Health related quality of life in people with advanced chronic liver disease. Journal of Hepatology. 2014;61(5):1158–1165. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. [DOI] [PubMed] [Google Scholar]
- 8.Altaii H, Al-Kindi SG, Yaqoob Z, Al-Khazaraji A, Romero-Marrero C. Place of Death and Hospice Utilization Among Patients Who Die From Cirrhosis in the United States. Clin Gastroenterol Hepatol. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Brown CL, Hammill BG, Qualls LG, Curtis LH, Muir AJ. Significant Morbidity and Mortality Among Hospitalized End-Stage Liver Disease Patients in Medicare. Journal of Pain and Symptom Management. 2016;52(3):412–419.e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology.2011;54(5):1864–1872. [DOI] [PubMed] [Google Scholar]
- 11.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. [DOI] [PubMed] [Google Scholar]
- 13.Patel AA, Walling AM, May FP, Saab S, Wenger N. Palliative Care and Healthcare Utilization for Patients with End-Stage Liver Disease at the End of Life. Clin Gastroenterol Hepatol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients With Cirrhosis and Denied Liver Transplants Rarely Receive Adequate Palliative Care or Appropriate Management. Clinical Gastroenterology and Hepatology. 2014;12(4):692–698. [DOI] [PubMed] [Google Scholar]
- 15.Rush B, Walley KR, Celi LA, Rajoriya N, Brahmania M. Palliative Care Access for Hospitalized Patients with End Stage Liver Disease Across the United States. Hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 16.Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World Journal of Transplantation. 2016;6(3):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Majzoub I, Qdaisat A, Chaftari PS, et al. Association of emergency department admission and early inpatient palliative care consultation with hospital mortality in a comprehensive cancer center. Support Care Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter JG, McDarby M, Smith D, Johnson M, Thorpe J, Ersek M. Associations between Timing of Palliative Care Consults and Family Evaluation of Care for Veterans Who Die in a Hospice/Palliative Care Unit. J Palliat Med. 2017;20(7):745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grudzen CR, Richardson LD, Johnson PN, et al. Emergency Department-Initiated Palliative Care in Advanced Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Jawahri A, Traeger L, Greer JA, et al. Effect of Inpatient Palliative Care During Hematopoietic Stem-Cell Transplant on Psychological Distress 6 Months After Transplant: Results of a Randomized Clinical Trial. J Clin Oncol. 2017;35(32):3714–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. 2016;316(20):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel AA, Walling AM, Ricks-Oddie J, May FP, Saab S, Wenger N. Palliative Care and Health Care Utilization for Patients With End-Stage Liver Disease at the End of Life. Clin Gastroenterol Hepatol. 2017;15(10):1612–1619.e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantilat SZ, Marks AK, Bischoff KE, Bragg AR, O’Riordan DL. The Palliative Care Quality Network: Improving the Quality of Caring. J Palliat Med. 2017;20(8):862–868. [DOI] [PubMed] [Google Scholar]
- 24.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5–11. [PubMed] [Google Scholar]
- 25.Bischoff KE, O’Riordan DL, Fazzalaro K, Kinderman A, Pantilat SZ. Identifying Opportunities to Improve Pain Among Patients With Serious Illness. J Pain Symptom Manage. 2018;55(3):881–889. [DOI] [PubMed] [Google Scholar]
- 26.Grubbs V, O’Riordan D, Pantilat S. Characteristics and Outcomes of In-Hospital Palliative Care Consultation among Patients with Renal Disease Versus Other Serious Illnesses. Clin J Am Soc Nephrol. 2017;12(7):1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ufere NN, Donlan J, Waldman L, et al. Barriers to Use of Palliative Care and Advance Care Planning Discussions for Patients with End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langberg KM, Kapo JM, Taddei TH. Palliative Care in Decompensated Cirrhosis: A Review. Liver Int. 2017. [DOI] [PubMed] [Google Scholar]
- 29.Tillmann HL, Wiese M, Braun Y, et al. Quality of life in patients with various liver diseases. Journal of Viral Hepatitis. 2011;18(4):252–261. [DOI] [PubMed] [Google Scholar]
- 30.Dying in America: improving quality and honoring individual preferences near the end of life. Mil Med. 2015;180(4):365–367. [DOI] [PubMed] [Google Scholar]
- 31.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimbell B, Boyd K, Kendall M, Iredale J, Murray SA. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open. 2015;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd K, Kimbell B, Murray S, Iredale J. Living and dying well with end-stage liver disease: Time for palliative care? Hepatology. 2012;55(6):1650–1651. [DOI] [PubMed] [Google Scholar]
- 34.Chatrath H, Liangpunsakul S, Ghabril M, Otte J, Chalasani N, Vuppalanchi R. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125(10):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimbell B, Murray SA. What is the patient experience in advanced liver disease? A scoping review of the literature. BMJ Supportive & Palliative Care. 2013:bmjspcare-2012–000435. [DOI] [PubMed] [Google Scholar]
- 36.Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Alimentary Pharmacology & Therapeutics. 2011;34(9):1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]