Abstract
Soy isoflavones are potentially beneficial phytoestrogens, but their tissue-selective effects in women are poorly understood. We tested the hypothesis that soy isoflavones affect bone mineral density (BMD), which may be influenced by individual differences in isoflavone metabolism and serum calcium levels. Ninety-nine healthy premenopausal women were randomized to isoflavones (136.6 mg aglycone equivalence) and 98 to placebo for 5 days per week for up to 2 years. BMD, serum calcium, and urinary excretion of daidzein and genistein were measured before and during treatment. In 129 adherent subjects, we found that isoflavone exposure, determined by urinary excretion levels, but not by dose assignment, interacted with serum calcium in affecting whole body BMD, but not hip and spine BMD. The regression coefficient was −0.042 for genistein excretion (GE) and 0.091 for the interaction between GE and serum calcium (all P<0.05). Daidzein excretion had similar but marginal effect. Genistein significantly decreased whole body BMD only at low normal serum calcium levels but increased whole body BMD at higher serum calcium levels. Comparing maximum to minimum GE, mean changes in whole body BMD were +0.033 and −0.113 g/cm2 at serum calcium levels of 10 and 8.15 mg/dL, respectively. These associations were not evident by intention-to-treat analysis, which could not model for inter-individual differences in isoflavone metabolism. In summary, soy isoflavones decrease whole body BMD only when serum calcium is low. Isoflavones are dietary substances that may influence calcium homeostasis by releasing calcium from bone while sparing the common fracture risk sites hip and spine.
Keywords: bone metabolism, isoflavones, daidzein, genistein, calcium homeostasis, hormone receptor modulators
1. Introduction
Estrogens play an important role not only in preserving bone health, but also in calcium homeostasis. Risk of osteoporosis increases after menopause as estrogen levels decline [1, 2]. Postmenopausal hormone replacement therapy (HRT) reduces this risk [3–6], but increases risk for hormone-sensitive cancers, coronary heart disease, stroke, and thromboembolic events [5, 7–9]. Phytoestrogens such as soy isoflavones are non-steroidal plant-derived compounds that have established estrogenicity in preclinical models, as evidenced by their causing infertility in sheep [10], and binding to estrogen receptors [11, 12]. Isoflavones have been investigated as alternatives to conventional HRT for preventing bone loss after menopause. Preclinical studies support such a role for soy isoflavones when administered with or without soy protein [13–20].
Daidzein and genistein are the major isoflavones in soy. They have similar as well as distinct molecular and cellular targets including endpoints for studying bone loss in preclinical models [14, 21–23]. Moreover, the dose response relationships of these two isoflavones are not always linear, but may be biphasic or plateau for many biological endpoints [13, 18, 21–23], and may be modified by endogenous estrogens [15, 17]. These factors may have contributed to inconsistent findings on effects of soy isoflavones on bone density in postmenopausal women in several randomized clinical trials (RCTs), even though some were adequately powered [20, 24–30].
The studies of the estrogenicity of soy isoflavones in premenopausal women are limited. It is not known whether isoflavones act as estrogens or anti-estrogens on bone in premenopausal women. This younger age group is of particular interest, because the timing of HRT initiation relative to onset of menopause appears to be critical for the complex pattern of its overall risks and benefits [5, 6, 31]. Some preclinical data also indicate that developmental stage at the time of exposure to soy may influence late-in-life bone mineral density [17]. Moreover, populations consuming soy and other legumes usually do so life-long and not just after menopause. We chose to investigate the effects of soy isoflavones on bone density in premenopausal women. Because of the well-known large inter-individual differences in soy isoflavone metabolism [32–34], we hypothesized that urinary excretion levels of isoflavones would be better than a categorical treatment assignment as exposure predictors of effects of soy isoflavones on BMD. Bone provides structural support for all organs, but is also the main endogenous calcium reservoir to maintain calcium homeostasis. Since we found that isoflavones increase serum calcium levels in this clinical trial samples [35], we also investigated whether effects of isoflavones on bone is modulated by levels of serum calcium. We tested the hypothesis that isoflavones affect bone mineral density and that the statistical significance of their treatment effects will depend on both individual differences in the metabolism of isoflavones (per urinary excretion measure) and on the levels of serum calcium.
2. Methods and materials
2.1. Study design
This was a single-site, parallel, two-arm, repeated measures, randomized, double blind, placebo-controlled study of premenopausal women to assess the risks and benefits of consuming soy isoflavones, as detailed previously [35, 36]. Breast density (the primary outcome) and bone density (a secondary outcome, reported here) were assessed at yearly intervals. Other variables such as blood chemistry and urinary excretion of isoflavones and riboflavin were measured at quarterly intervals [35, 36]. The study is registered at www.clinicaltrials.gov and the identifier is .
2.1.1. Ethical approval
The study protocol was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB), and written informed consent was obtained from all subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.1.2. Inclusion/exclusion criteria
Healthy women from the Houston-Galveston area within a range of 30–42 years of age and with regular monthly menstrual cycles were recruited. Women using hormonal contraceptive agents (oral, injection, or patch) or other exogenous hormones within the past 6 months were excluded, as were those who were peri- or post-menopausal, on medically prescribed diets, currently pregnant or lactating, or with a personal and family history of breast cancer.
2.1.3. Intervention agents
The isoflavone and placebo pills were identical in appearance [35, 36], and were designed and supplied at no charge by Dr. Brent Flickinger of Archer Daniel Midland Co. (Decatur, IL). Each isoflavone pill contained 246 mg of Nova soy from a single lot [providing 30 mg daidzein, 30 mg genistein, and 8.3 mg glycitein, totalling 68.3 mg as aglycone equivalence, of which 90 mol-% was as glycosides (daidzin, genistin, and glycitin) and 10 mol-% as aglycones]. Each placebo pill contained 246 mg of a carbohydrate filler. Both pills also contained 15 mg of riboflavin as a biomarker for adherence, 60 mg sorbitol, 3 mg magnesium stearate, and 676 mg dicalcium phosphate for a total of 1,000 mg per tablet.
2.2. Study procedures
2.2.1. Screening, enrollment and randomization
All study visits were scheduled only during the luteal phase of a menstrual cycle. There were four baseline visits, i.e. two paired visits during two separate luteal menstrual phases, not more than 6 months apart. Subjects who remained qualified after baseline evaluation, as determined by the investigative team, were randomly assigned at a 1:1 ratio in blocks of six to isoflavone or placebo groups, and then to one of three sub-groups for scheduling follow-up visits. The randomization list was generated in advance by the study statistician using the PLAN procedure in SAS©. Using this randomization list, the UTMB research pharmacy, which was blinded to all other aspects of the study, dispensed blinded pills in blister packs for distribution at each quarterly follow-up visit. A blister for each day provided two assigned study pills and one prenatal vitamin (Rugby Prenavite Prenatal Formula, Swanson Health Products, Duluth, GA) that met the required daily intakes of vitamins and minerals. Subjects were instructed to avoid any other vitamin supplements. They ingested these three pills daily for five days per week for up to 2 years. Subjects, research staff, and investigators were blinded to the treatment assignments.
2.2.2. Follow-up visits
Treatment started on the second day of the menstrual period that immediately followed the fourth baseline visit. During treatment, study visits occurred approximately once every three menstrual cycles (i.e. roughly seasonally) except for the first treatment visit, which occurred after one, two, or three menstrual cycles of starting treatment, according to the pre-randomized three scheduling sub-groups, respectively, to accommodate the anticipated large number of study visits. The subjects reported to the study team by phone or email on every first day of menstruation, so a study visit could be scheduled to occur 20 to 24 days later during the luteal phase.
2.3. Variables: collections and analyses
At each study visit, subjects arrived after an overnight fast, and brought with them a 12-hr urine collection for measurement of riboflavin, daidzein, and genistein as reported previously [36], and provided fasting blood samples for analyses of blood chemistry by the certified UTMB hospital clinical laboratory using a VITROS® 5.1 FS analyzer (Ortho-Clinical Diagnostics, Rochester, NY). Demographic and reproductive information was obtained at the first visit and anthropometric values at each visit.
Total body mass, lean body mass, fat body mass, bone mineral content (BMC, g) and BMD (g/cm2) for whole body, left total hip, and lumbar spine (L1-L4) were measured using a dual energy x-ray absorptiometry (DXA) (Model Discovery A; Model QDR4500A, Hologic, Waltham, MA). DXA scans were obtained during scheduled visits by the same technician using the same densitometer once before and annually after starting the assigned supplementation. The densitometer was calibrated daily using a spine phantom and adjusted to within 1% of the reference value provided by the manufacturer. All DXA measurements were performed in duplicate (before and after repositioning) for reproducibility with subjects in the supine position, and duplicates with a CV <5% were averaged for statistical analyses. The radiation dose of one DXA scan is about 0.001 mSv equivalent to 3 hrs of natural background radiation exposure (www.radiology.info.org).
2.4. Statistical analyses
2.4.1. Sample size estimate
With an estimated attrition rate of 15%, 100 subjects per arm should provide 85 subjects who completed the study. We would have 80% power to detect 0.5 SD changes or greater with a 0.05 two-sided significance level using a two group t-test. The sample size estimate was performed for the primary outcome of breast density. For bone mineral density, based on the baseline characteristics of subjects (Table 1), we can detect 0.036 to 0.053 g/cm2 changes.
Table 1:
Baseline characteristics of all randomized subjects with dual x-ray absorptiometry (DXA) test
| Placebo | Isoflavone | |||
|---|---|---|---|---|
| Variables †, ‡ | n0 | Means ± SD; % | n1 | Means ± SD; % |
| Consent Age (years) | 96 | 37.35 ± 3.43 | 98 | 37.58 ± 2.96 |
| Weight (kg) | 96 | 75.06 ± 17.50 | 98 | 73.46 ± 16.70 |
| Race/ethnicity (%) | 96 | 49.48 | 96 | 50.52 |
| Hispanic | 39 | 44.04 | 50 | 44.96 |
| African American | 12 | 12.89 | 14 | 13.13 |
| White | 45 | 39.09 | 34 | 39.91 |
| Height (cm) | 96 | 160.10 ± 6.01 | 98 | 160.70 ± 7.67 |
| BMI (kg/m2) | 96 | 29.23 ± 6.38 | 98 | 28.45 ± 6.08 |
| Waist circumference (cm) | 95 | 90.84 ± 13.92 | 98 | 89.51 ± 13.18 |
| Hip circumference (cm) | 95 | 109.10 ± 13.07 | 98 | 108.10 ± 11.46 |
| Waist-to-hip | 95 | 0.83 ± 0.06 | 98 | 0.83 ± 0.06 |
| Riboflavin (μg/mL) | 91 | 10−3 ± 10−3 | 93 | 10−3 ± 10−3 |
| Daidzein (μg/mL) | 90 | 10−3 ± 4×10−4 | 89 | 10−3 ± 10−3 |
| Genistein (μg/mL) | 90 | 10−3 ± 10−3 | 89 | 10−3 ± 10−3 |
| Riboflavin (mg/h) | 91 | 0.05 ± 0.08 | 93 | 0.06 ± 0.12 |
| Daidzein (mg/h) | 90 | 0.06 ± 0.03 | 89 | 0.07 ± 0.04 |
| Genistein (mg/h) | 90 | 0.08 ± 0.05 | 89 | 0.08 ± 0.04 |
| Total protein (g/dL) | 96 | 7.53 ± 0.51 | 97 | 7.51 ± 0.45 |
| Albumin (g/dL) | 96 | 4.27 ± 0.34 | 98 | 4.28 ± 0.26 |
| Alkaline phosphatase (U/L) | 96 | 75.32 ± 18.33 | 98 | 72.07 ± 18.97 |
| Calcium (mg/dL) | 96 | 9.01 ± 0.35 | 98 | 9.05 ± 0.28 |
| Creatinine (mg/dL) | 96 | 0.75 ± 0.11 | 98 | 0.76 ± 0.12 |
| Potassium (mEq/L) | 96 | 4.04 ± 0.22 | 98 | 4.07 ± 0.24 |
| Sodium (mEq/L) | 96 | 140.50 ± 1.48 | 98 | 140.70 ± 1.50 |
| From DXA tests | ||||
| Fat (%) | 96 | 37.40 ± 6.92 | 98 | 36.31 ± 6.64 |
| Lean body mass (kg) | 96 | 44.12 ± 7.28 | 98 | 43.95 ± 7.08 |
| Fat body mass (kg) | 96 | 29.07 ± 11.40 | 98 | 27.57 ± 10.61 |
| Lean bone mineral content (kg) | 96 | 46.20 ± 7.47 | 98 | 46.05 ± 7.24 |
| Whole body bone mineral content (kg) | 96 | 2.08 ± 0.29 | 98 | 2.11 ± 0.28 |
| Hip bone mineral content (g) | 96 | 33.38 ± 6.17 | 98 | 33.82 ± 5.15 |
| Spine bone mineral content (g) | 96 | 60.32 ± 9.04 | 98 | 61.26 ± 9.87 |
| Hip bone mineral density (g/cm2) | 96 | 1.00 ± 0.13 | 98 | 1.01 ± 0.12 |
| Spine bone mineral density (g/cm2) | 96 | 1.06 ± 0.11 | 98 | 1.06 ± 0.11 |
| Whole body bone mineral density (g/cm2) | 96 | 1.07 ± 0.09 | 98 | 1.08 ± 0.09 |
| Study visit (number) | 96 | 8.73 ± 2.93 | 98 | 8.80 ± 3.08 |
A total of 197 subjects were randomized into placebo and isoflavone, respectively. Two placebo and one isoflavone subjects had breast images but no DXA tests, resulting 194 subjects in analysis.
Except for race/ethnicity and study visits, all descriptive analyses on numerical variables were averages of 4 baseline visits. Study visit was average number of all baseline and follow-up visits. For numerical variables, the corresponding means and standard deviations (SDs) were stratified by groups. For categorical variable, percentages were presented.
All P > .05 for two group comparisons.
2.4.2. Baseline characteristics
Baseline characteristics of the subjects were summarized for each study group using means and standard deviations (SD) for continuous variables and then compared using independent t-tests, and frequencies and percentages for categorical variables and compared by chi-square tests.
2.4.3. Treatment effects
Outcomes were BMC and BMD for the hip, spine or whole body measured before and after 1 and 2 years of supplements. Predictors were treatment assignment (categorical data) or urinary excretion of soy isoflavones (continuous data, mg/h). Linear mixed effects (LME) regression models, accounting for inter-subject heterogeneity and intra-subject dependence on repeated measures, were applied.
Treatment effects were examined by two pre-specified conceptual approaches. The first was ‘intention-to-treat’ which assessed associations between treatment assignment (categorical) and measures in each of these outcomes during the treatment period by including interaction between type of treatment assignment and years of treatment as well as interaction between type of treatment assignment and serum calcium concentration in the models. The second strategy was a modified per protocol analysis that considered only the measured urinary excretion rates of daidzein and genistein ignoring treatment assignment as a main effect variable.
Urinary excretion of isoflavones and most variables (e.g., BMI, serum calcium) were measured four times during the baseline observation period and four times per year during treatment. DXA tests were done once at baseline and annually during treatment. Therefore, predictors were means of all four baseline measurements and means of the four quarterly treatment measurements between two DXA tests in statistical model analyses.
Interaction terms between soy isoflavones and calcium on bone outcomes, if significant, were investigated by the Johnson-Neyman procedure to fully explicate the nature of their conditional relationships [37]. To facilitate interaction-term result interpretation, data for hourly urinary excretion (mg/h) of isoflavones and calcium levels were mean-centered for statistical model analyses. Other covariates were not mean-centered.
All models were also adjusted for race and ethnicity, age at entry to the study, years on treatment, and BMI at each study visit. The corresponding assumption on LME models and the identification of potential outliers or influential points were also inspected through the residual analysis. The model fit of all LME models was assessed using the conditional Akaike information criterion (CAIC) [38, 39]. All tests of statistical significance were two-sided with a P < 0.05 indicating a significant difference. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Enrollment, treatment assignment and intervention
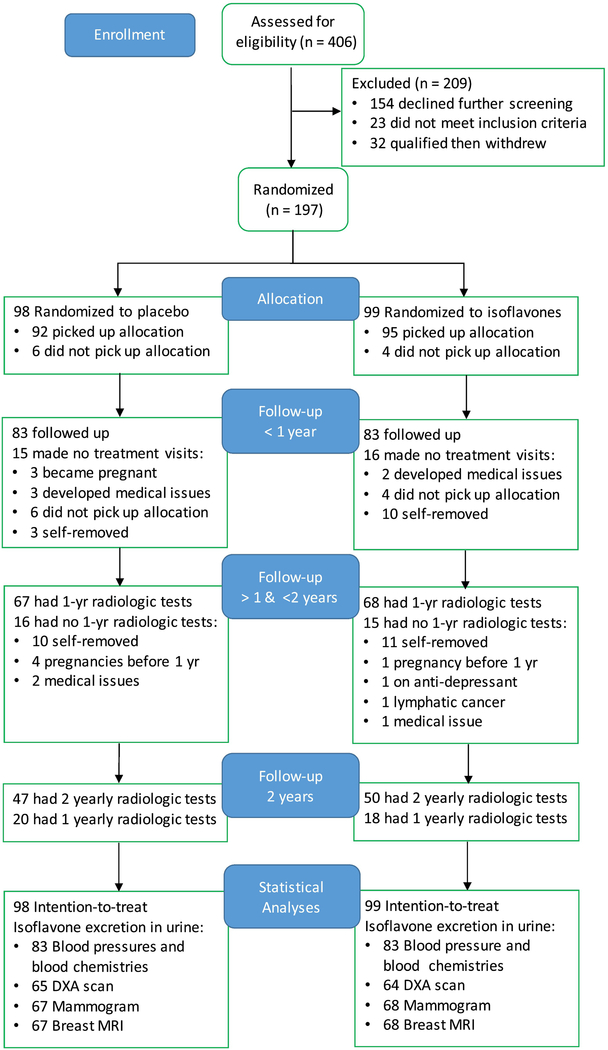
This single-site study was completed before August 2012 and was longer than expected, as described previously [35]. Figure 1 is a flow diagram of trial progress [40] and shows enrollment statistics for the main radiologic study outcomes. Subjects were randomized to isoflavones (N=99) or placebo (N=98) soon after completing at least one of the three planned radiologic tests (mammogram, breast magnetic resonance image, or DXA measurements). Three of the 197 randomized subjects completed baseline mammography and breast MRI, but did not have baseline DXA, leaving 194 subjects (96 placebo and 98 isoflavone subjects) for bone density analyses. The retention statistics (Figure 1), mean number of study visits (inclusive of all baseline and follow-up visits and a proxy for duration of participation in the study), DXA outcomes, and all other baseline characteristics (Table 1) were balanced between the two groups. This balance was not affected by drop-outs during the intervention phase (not shown). Four placebo and one isoflavone subjects became pregnant after their first DXA scans, and another placebo subject became pregnant after the second DXA scan and were removed from the study. Thirty-eight subjects removed themselves from the study after one year on supplement. Note that the retention statistics for outcomes measured at quarterly interval, e.g. blood chemistry, differ from those measured at yearly intervals, e.g. bone and breast density.
Figure 1:
Flow diagram of a blinded randomized trial comparing effects of soy isoflavones and placebo on bone density showing the enrollment of 30- to 42-year-old female subjects, allocation to treatment, follow-up for up to 2 years, and data analysis.
3.2. Effects of soy isoflavones on bone by intention-to-treat analyses
A total of 194 randomized subjects with one baseline DXA scan were included in this analysis. Table 2 shows that assignment to the isoflavone group did not have statistically significant effects on any measures of bone content (whole body BMC, whole body BMD, hip BMD, or spine BMD) during 2 years of treatment. The interaction term between treatment assignment and years on treatment was insignificant in unadjusted Model 1 or in models adjusted for calcium, interaction between treatment assignment and calcium (Models 2–3) (P>0.05 for all models, Table 2), and age at study entry, race/ethnicity, or follow-up BMI (Model 3).
Table 2 –
Intervention effects of soy isoflavones on bone across 2 years of supplementation on all subjects as randomized
| Whole body bone mineral content, kg |
Whole body bone mineral density, g/cm2 |
Hip bone mineral density, g/cm2 |
Spine bone mineral density, g/cm2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Models * | Effect | β (95% CI) † | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Model 1 | Treatment × Year | −1.453 (−15.817, 12.910) | .843 | −0.001 (−0.008, 0.005) | .656 | −0.001 (−0.006, 0.005) | .827 | 0.003 (−0.002, 0.007) | .259 |
| Model 2 | Treatment × Year | 1.399 (−14.446, 17.245) | .863 | −0.001 (−0.007, 0.006) | .878 | 0.001 (−0.005, 0.007) | .789 | 0.003 (−0.002, 0.008) | .228 |
| Ca2+ (calcium) | 19.911 (−26.067, 65.889) | .397 | 0.006 (−0.012, 0.025) | .493 | −0.015 (−0.033, 0.003) | .106 | −0.002 (−0.016, 0.012) | .784 | |
| Treatment × Ca2+ | −32.160 (−102.566, 38.247) | .372 | −0.010 (−0.038, 0.018) | .492 | −0.011 (−0.039, 0.016) | .425 | −0.004 (−0.025, 0.018) | .724 | |
| Model 3 | Treatment × Year | −1.536 (−17.739, 14.667) | .853 | −0.001 (−0.008, 0.006) | .738 | −0.001 (−0.008, 0.005) | .655 | 0.002 (−0.002, 0.007) | .328 |
| Ca2+ | 10.649 (−35.524, 56.822) | .652 | 0.003 (−0.015, 0.021) | .759 | −0.014 (−0.032. 0.005) | .141 | −0.003 (−0.018, 0.011) | .641 | |
| Treatment × Ca2+ | −12.082 (−83.052, 58.889) | .739 | −0.005 (−0.033, 0.024) | .749 | 3 × 10−4 (−0.028, 0.029) | .982 | −3 × 10−4 (−0.022, 0.022) | .979 |
Intention to treat analysis was applied to 194 subjects (premenopausal women) using linear mixed effects regression analyses for measures repeated across 2 years of supplementation and accounting for inter-subject heterogeneity and intra-subject dependence. Exposure predictor was treatment assignment of isoflavone coded as ‘1’ and placebo coded as ‘0’.
Model 1 included treatment assignment (Treatment), years on the treatment (Year), and interaction term (Treatment × Year). Model 2 included all variables in Model 1 plus calcium (mean-centered) and interaction term (Treatment × Ca2+). Model 3 included all variables in Model 2 plus age at entry to the study, race/ethnicity, and BMI.
β, regression coefficient; 95% CI, 95% confidence interval and P value for effect in models indicated for various outcomes of bone.
3.2.1. Sensitivity analyses
Riboflavin was incorporated into both placebo and isoflavone tablets (15 mg/tablet, 30 mg/day for 2 tablets). As previously described [36], a urinary excretion of riboflavin ≥ or < 1.42 μg/ml (Youden index) was used to categorize subject adherence as ‘yes’ or ‘no’, respectively, to oral intake of study pills within 12 h of each scheduled study visit. Adherence rate was assessed from analyses of four urine samples from four scheduled study visits before each yearly DXA scan. Adjustment for adherence rate in the intention-to-treat analyses did not change the effects of isoflavone treatment on bone measures (results not shown).
As described previously [36], based on measurement of both isoflavones (treatment assignment markers) and riboflavin (an adherence marker) in up to 8 follow-up visit urine samples, an ‘as-observed’ treatment assignment in 143 subjects was confirmed to be consistent with their randomized assignment. These did not include twenty placebo subjects who were provided multiple erroneous batches of isoflavone-containing pills (i.e. urine positive for all three analytes), three subjects who were determined non-adherent (i.e. absence of all three analytes in all of their urine collections), and 31 subjects who provided no follow-up urine samples for analyses of the three analytes. However, they included four isoflavone subjects who each were provided one batch of placebo pills in error (i.e. high levels of urine riboflavin but negative for isoflavones), because ingestion of placebo pills by isoflavone subjects would have the same effect as not taking some assigned isoflavone pills. When the intention-to-treat models were restricted to this subset of 143 subjects, the interaction term between type of treatment assignment and years on treatment remained insignificant (results not shown).
3.3. Effects on bone of inter- and intra-individual variations in isoflavone excretion by a modified per protocol analysis
Ratios of urinary daidzein excretion (DE) to genistein excretion (GE) varied from 0.9 to 8.9 among adherent isoflavone subjects, even though the intake dose ratio of 1:1 (aglycone-equivalent weights) was the same for all subjects. The effects on bone content (as dependent variables) of these isoflavone excretion variations (as exposure predictors) were studied. This was a modified per protocol analysis because isoflavone excretion represented the combined influence of variations in adherence (traditional ‘per protocol analysis’) and isoflavone metabolism. All study visits were timed to occur during luteal phases of the menstrual cycle, and cycle lengths varied within and between subjects; the exact number of days (converted to years) on treatment was the time variable. Two placebo and four isoflavone subjects completed their first follow-up breast imaging visits but missed the required first follow-up DXA scans due to Hurricane Ike and other reasons. Therefore, 65 placebo and 64 isoflavone subjects (Figure 1) were included in the analysis of effects of DE and GE on bone mineral content. Twenty placebo and 18 isoflavone subjects withdrew themselves from further study after only one follow-up DXA test (Fig. 1).
3.3.1. Comparison of effects of daidzein and genistein on bone as determined by excretion rates
As shown in Table 3, isoflavone excretion (mg/hr from a 12 hr urine collection) expressed as individual excretion rates (DE or GE), their sum (DE + GE), or their difference (DE – GE) was negatively associated with whole body BMD in all statistical models (n=129). Adjustment for serum calcium (Model 2) and the interaction term between isoflavone and calcium (Models 3–4) did not change β-estimates for any of these main effects (DE, GE, DE + GE and DE – GE) in all 129 adherent subjects (Table 3) or in 128 subjects after excluding one potentially influential data point. Effects of GE and DE + GE as predictors were significant (all P values <0.05), effects of DE marginal (P=0.08) and effects of DE – GE, insignificant (P=0.2). The β-estimates for the interaction terms between isoflavones and calcium were all positive (n=129 or 128) and significant for the interaction of GE and calcium on whole body BMD (P<0.05, n=129 and P=0.09, n=128). The CAIC criteria among models with the interaction terms show that GE or DE + GE were better predictors than DE or DE – GE.
Table 3 –
Excretion dependent effects of isoflavones (daidzein and genistein) and their interaction with serum calcium on whole body bone mineral density
| Model 1 †,‡ |
Model 2 †,‡ |
Model 3 †,‡ |
Model 4 †,‡ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Isoflavone | Effect * | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Daidzein (DE) | DE | −0.008 (−0.018, −0.002) | .077 | −0.008 (−0.017, 8 × 10−4) | .077 | −0.008 (−0.017, 5 × 10−4) | .067 | −0.008 (−0.017, 3 × 10−4) | .061 |
| Ca2+ | - ∥ | 0.001 (−0.013, 0.015) | .891 | 0.002 (−0.012, 0.016) | .748 | 0.003 (−0.011, 0.017) | .701 | ||
| DE × Ca2+ | - | - | 0.021 (−0.005, 0.048) | .118 | 0.023 (−0004, 0.050) | .095 | |||
| Genistein (GE) | GE | −0.041 (−0.070, −0.012) | .007 | −0.042 (−0.072, −0.012) | .007 | −0.042 (−0.072, −0.012) | .007 | −0.042 (−0.072, −0.012) | .007 |
| Ca2+ | - | 0.002 (−0.012, 0.016) | .776 | 0.004 (−0.010, 0.017) | .619 | 0.004 (−0.010, 0.018) | .582 | ||
| GE × Ca2+ | - | - | 0.090 (6 × 104, 0.180) | .050 | 0.095 (0.005, 0.185) | .040 | |||
| Sum (DE + GE) | DE + GE | −0.007 (−0.014, −2 × 10−4) | .044 | −0.007 (−0.014, −3 × 10−4) | .043 | −0.007 (−0.014, −5 × 10−4) | .038 | −0.007 (−0.014, −6 × 10−4) | .035 |
| Ca2+ | - | 0.001 (−0.013, 0.015) | .865 | 0.003 (−0.011, 0.017) | .712 | 0.003 (−0.011, 0.017) | .666 | ||
| (DE + GE) × Ca2+ | - | - | 0.018 (−0.003, 0.039) | .091 | 0.019 (−0.002, 0.040) | .073 | |||
| Difference (DE − GE) | DE − GE | −0.008 (−0.020, 0.004) | .196 | −0.008 (−0.020. 0.004) | .195 | −0.008 (−0.020, 0.004) | .172 | −0.009 (−0.021, 0.003) | .154 |
| Ca2+ | - | 0.001 (−0.014, 0.015) | .932 | 0.002 (−0.012, 0.016) | .814 | 0.002 (−0.012, 0.016) | .766 | ||
| (DE − GE) × Ca2+ | - | - | 0.024 (−0.012, 0.060) | .193 | 0.026 (−0.010, 0.063) | .158 |
A modified per protocol analysis was applied to 129 adherent premenopausal women using linear mixed effects regression analyses for measures repeated across 2 years of supplementation and accounting for inter-subject heterogeneity and intra-subject dependence. Exposure predictor was mean-centered urinary excretion rate (mg/h) of isoflavones and means for daidzein excretion (DE), 0.309; genistein excretion (GE), 0.146; DE + GE, 0.455; or DE − GE, 0.164.
Main effects included DE, GE, DE + GE, or DE − GE, Ca2+, and the interaction term between isoflavone and Ca2+.
All models included days on supplements and model 4 included additionally age at entry to the study, race/ethnicity, and BMI, but their β estimates are not shown.
β, regression coefficient; 95% CI, 95% confidence interval and P value for β estimate of effect indicated in models.
Effect (variable) not included in models.
Whole body BMD is derived from whole body bone mineral content (BMC) divided by the projected surface area (cm2) during DXA scanning. As expected, when whole body BMC was the dependent variable, hierarchical modeling patterned after the models in Table 3 showed that fitting patterns were very similar to those shown for whole body BMD in Table 3. For example, when modelled according to Model 4 in Table 3, the respective β-estimates for isoflavone (main effect, Pmain) and its interaction term with calcium (PCa-interac) are: (i) for DE, −18.73 (Pmain =0.10) and 73.58 (PCa-interac =0.03); (ii) for GE, −83.78 (Pmain =0.03) and 264.65 (PCa-interac =0.02); (iii) for DE + GE, −16.07 (Pmain =0.07) and 59.72 (PCa-interac =0.03); and (iv) for DE – GE, −20.57 (Pmain =0.18) and 90.86 (PCa-interac =0.05).
3.3.1.1. Explicating the nature (i.e. statistically significant region) of the interaction between isoflavones and calcium
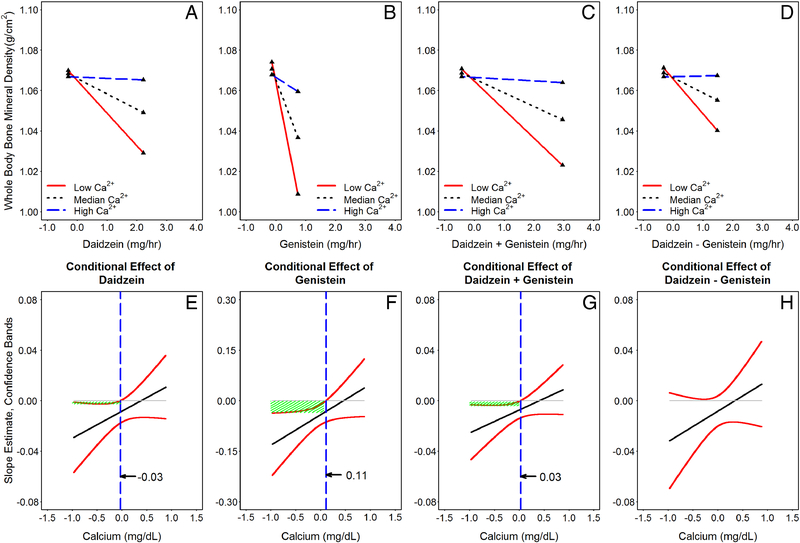
Simple slopes (Figures 2A–D) and their regions of significance (indicated by vertical dashed lines in Figures 2E–H) for hierarchical linear modeling 2-way interaction in all 129 adherent subjects (using Model 3 of Table 3 as examples) were studied by the Johnson-Neyman technique [37]. Note that scales for isoflavones (x-axis in Figures 2A–D) and serum calcium (x-axis in Figures 2E–H) were all confined to the actual values observed in our study samples. As shown, regardless whether the exposure predictor is DE (Figure 2A), GE (Figure 2B), DE + GE (Figure 2C) or DE – GE (Figure 2D), the slope estimates varied with serum calcium concentration as a moderator. When mean-centered serum calcium concentrations are ≤0.1104 mg/dL for GE (Figure 2F) and ≤0.0294 mg/dL for DE + GE (Figure 2H), the slope estimates were statistically significant, because the associated confidence bands (95% CI) all are below 0 (indicated by shaded areas). No statistically significant regions of calcium concentrations were found that interact with the effects of DE (Figure 2E) and DE – GE (Figure 2H) in their effects on whole body BMD. When serum calcium is higher than the median serum calcium for the group, isoflavones induced increases in whole body BMD, which however were not statistically significant within the calcium range of our study samples.
Figure 2:
Probing the interactive effects of isoflavone(s) and calcium on whole body bone mineral density (using Model 3 of Table 3 as an example) by the Johnson-Neyman technique [37]. Panels A-D are plots of simple slopes for exposure predictor, isoflavones, on whole body bone mineral density as a function of serum calcium at 10th (Low Ca2+), 50th (Median Ca2+), and 90th (High Ca2+) percentiles of values found in our study samples. Panels E to H show the regions of significance and 95% confidence bands for the regression slope estimates for the conditional relation between bone and isoflavone concentrations as a function of serum calcium. The dashed vertical lines (----- in panels E-H) indicate calcium thresholds separating regions of statistical significances. Panels A & E for daidzein as predictor; B & F for genistein; C & G for sum excretion (daidzein and genistein); and D & H for difference excretion (daidzein minus genistein). Note that the lengths of all graph lines correspond to ranges of data found in our study samples.
3.3.2. Effects of isoflavones on whole body BMD as conditioned by the interaction of isoflavones and serum calcium
The effects of isoflavones as conditioned by calcium were estimated by comparing measurements obtained at two different levels of isoflavone excretion at specified serum calcium concentrations. Using Model 3 from Table 3 as an example, Table 4 shows that changes in whole body BMD are conditioned by both serum calcium concentration and the level of urinary genistein excretion. When serum calcium was at the 100th percentile for the group, the effects of GE at the 100th and 0th percentiles, respectively, on whole body BMD were 0.031 and −0.002 mg/dL, resulting in a net intervention effect of 0.033 mg/dL, an increase that was not statistically significant. In contrast, when the serum calcium concentration was at 0th percentile, the net intervention effect on whole body BMD when comparing maximum vs minimum excretion of genistein was −0.113 mg/dL, a decrease that was statistically significant. However, none of the isoflavones (DE, GE, DE + GE, or DE – GE) or their interaction terms with calcium predicted changes of BMC or BMD of the hip or spine (results not shown).
Table 4:
Examples of possible intervention effects on changes of whole body bone mineral density by soy isoflavones conditioning on serum calcium
| Calcium, mg/dL (percentile) |
|||||
|---|---|---|---|---|---|
| Genistein Excretion, mg/h (percentile) | 0.882 (100th) | 0.357 (90th) | 0.032 (50th) | −0.368 (10th) | −0.968 (0th) |
| 0.736 (100th) | 0.031* | −0.006 | −0.028 | −0.057 | −0.099 |
| 0.183 (90th) | 0.010 | −3×10−4 | −0.007 | −0.015 | −0.028 |
| −0.043 (50th) | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| −0.108 (10th) | −0.001 | 0.002 | 0.004 | 0.007 | 0.010 |
| −0.136 (0th) | −0.002 | 0.003 | 0.005 | 0.009 | 0.014 |
| Intervention Effect † | 0.033 | −0.008 | −0.034 | −0.065 | −0.113 |
Estimated changes centered on mean calcium (9.118 mg/dL) and mean GE (0.146 mg/h) using Model 3 of Table 3 where GE was the exposure predictor.
Difference between excreting maximum and minimum GE.
4. Discussion
We found that daidzein and genistein, the two main isoflavones in soy, affect whole body BMD and BMC, but not hip and spine bone density of the premenopausal women who took an isoflavone supplement for up to 2 years. Isoflavones interact with serum calcium in selectively altering whole body BMD and BMC, so that when serum calcium levels are at physiological low, isoflavones significantly decrease whole body BMD and BMC and when serum calcium levels are at physiologically high, isoflavones tend to increase whole body BMD and BMC, although the increase was not statistically significant. These effects were readily detected when urinary excretion of these two isoflavones rather than intake dose assignment was the exposure predictor and when serum calcium concentrations were considered in the statistical models. Therefore, we confirmed our hypotheses that soy isoflavone bioavailability, as determined by endogenous metabolism, affects BMD in premenopausal women, and that this effect is conditioned by the level of serum calcium.
We found that whole body BMD was significantly and inversely associated with measures of genistein exposure (GE and DE + GE, P<0.05, β-estimates negative in all models) and less significantly with daidzein exposure (DE, P=0.08; or DE – GE, P=0.2). These observations that isoflavones decrease whole body BMD when considered with our prior observations that isoflavones (DE, GE, DE + GE, and DE – GE) concurrently increased serum calcium levels support the suggestion that these micronutrients mobilize calcium from bone to plasma [35] and may contribute to maintain calcium homeostasis.
In addition to the main effect on bone discussed above, isoflavones and serum calcium also moderate the effects of each other on whole body BMD and BMC, with all β-estimates for their interaction terms being positive and statistically significant when GE is the exposure predictor. Johnson-Neyman analyses further showed that the effect of genistein to decrease whole body BMD is significant when absolute serum calcium concentrations are equal to or less than 9.23 mg/dL (0.11 mg/dL of mean-centered calcium + mean calcium of 9.12 mg/dL in 129 subjects). DE and DE + GE were not as good predictors as GE in decreasing bone density in these models. DE – GE did not interact with serum calcium to affect whole body BMD. We found that genistein is more effective than daidzein in mobilizing calcium from bone, and that daidzein may neutralize the BMD loss effect caused by genistein, as shown by the analyses of the effects of the sum and difference in their excretion. Therefore, daidzein and genistein appear to have somewhat different effects on whole body BMD.
Calcium is essential for life, because it is either a first [41] or second messenger that initiates numerous cellular and physiological processes [42]. Its plasma level is tightly controlled and maintained within a very narrow range by multiple mechanisms involving, most notably calcitonin, vitamin D, parathyroid hormone (PTH), and estrogens. Results from this clinical trial suggest that micronutrients such as soy isoflavones are a new class of compounds that can participate in calcium homeostasis. This is evident in this study since there is a statistically significant interaction between isoflavones and serum calcium on changes of whole body BMD. A significant interaction implies that isoflavones will have a completely opposite effect on whole BMD depending on serum calcium levels. Analysis by Johnson-Neyman technique [37] showed that a statistically significant decrease in whole body BMD occurred only when serum calcium level is physiologically low. In contrast, when serum calcium level is physiologically high, whole BMD increases, although this change was not statistically significant. Bone serves as a major calcium reservoir, and the decrease in whole body BMD in this study is consistent with our prior observation in the same subjects of an increase in serum calcium after isoflavone exposure [35]. Taken together, we suggest that these isoflavones participate in calcium homeostasis by mobilizing calcium from bone to the circulation, and in doing so may contribute to maintaining serum calcium levels and calcium-dependent physiological processes. Further studies are needed to determine whether this effect of soy isoflavones on BMD is adverse or not, since clinically important fracture sites hip and spine were not affected. Among hormones [43–45] involved in calcium homeostasis, PTH mobilizes calcium from bone and also increases renal reabsorption of calcium when serum calcium is low. Vitamin D and 17β-estradiol increase intestinal absorption of calcium. Whether isoflavones affect intestinal absorption and renal reabsorption of calcium warrants investigation.
The absence of effects of soy isoflavones on hip and spine BMD in premenopausal women while consistent with prior studies of postmenopausal women the underlying reasons for null effects in pre- and post-menopausal women may differ. There are a number of challenges in detecting treatment effects of a mixture of soy isoflavones by intention-to-treat analyses. Firstly, an up to 9-fold variation in urinary excretion ratios of these two isoflavones [32–34, 46] suggest that considering these differences may provide a more sensitive exposure predictor of response than the categorical treatment assignment in the intention to treat analyses. Secondly, our study of premenopausal women shows that genistein and daidzein do not have identical effects in mobilizing calcium from bone, and their effects may to some degree neutralize each other, which is consistent with their varying spectra of biological effects observed in preclinical models [20, 47, 48]. Intention to treat analysis cannot consider such differences in effects and interactions between different components of a multicomponent supplement. Lastly, prior clinical trials of isoflavone supplementation have not considered in statistical models the moderation of isoflavone effects by calcium, which we have demonstrated in this study.
Strengths of this study include a sensitive statistical analysis strategy that considered individual differences in isoflavone excretion, which represents differences in both isoflavone metabolism and adherence, and effect modification by calcium levels. Additional strengths included stringent inclusion criteria, quality control of randomization, frequent measurements of predictors, timing of sample collections to the luteal phase of the menstrual cycle, and inclusion of riboflavin as a marker for ingestion adherence. We considered it important to study soy effects in premenopausal women because dietary exposure to soy is usually life-long.
Weaknesses of the study include providing a mixture of isoflavones (genistein, daidzein, and glycitein in their native forms as found in soybeans) instead of individual isoflavone to different groups, which would be a more costly study. We did not analyze the contributions of glycitein, which is a minor isoflavone component in soy, or equol, a metabolite of daidzein whose effects would be anticipated to show high collinearity with daidzein. A narrow age range in our study limits inferences to other age groups including postmenopausal women. Dropout rates were high but were balanced between the two comparison groups. We also found errors in pills provided to some subjects. Because this was the only variable not balanced by randomization, such errors more likely occurred during pill manufacturing rather in dispensing. The impact of this error was minimized by the pre-planned strategy to use urinary excretion data as exposure predictors.
In summary, we have shown that soy isoflavones decrease whole body BMD in premenopausal women and spare the most common fracture sites of hip and spine. These effects differ for genistein and daidzein. Importantly, these effects are dependent on serum calcium concentrations and are significant only when serum calcium falls to low normal levels. When serum calcium is high normal, isoflavones tend to preserve whole body BMD. Taken together with our prior observation of isoflavone-induced increases of serum calcium levels [35], we suggest that dietary isoflavones can contribute to mobilizing calcium from bone to the circulation when there is a physiological need. These novel findings suggest that micronutrients such as isoflavones should be recognized as a new class of compounds that participate in calcium homeostasis in premenopausal women. Whether isoflavones play a similar role in estrogen deficient postmenopausal women needs future investigation.
Highlights.
Phytoestrogens may be considered as natural selective estrogen receptor modulators and alternatives for estrogen replacement.
Phytoestrogens daidzein and genistein differentially mobilize calcium from bone when physiologically needed.
They do so by mobilizing calcium from sites other than hip and spine, and preserving the bone mineral density of hip and spine, perhaps, analogous to that occurring during lactation.
Effects of isoflavones on bone may depend on serum calcium levels and deserves further studies.
Because isoflavones appear to participate in calcium homeostasis in response to physiological needs so that when serum calcium is high, isoflavones increase bone mineral density but when serum calcium is low, isoflavones decrease whole body bone mineral density perhaps to maintain many calcium-dependent physiological reactions.
Acknowledgment
The authors wish to acknowledge the nursing staff of the Institute for Translational Sciences-Clinical Research Center (ITS-CRC). We are also very grateful for the women who volunteered as subjects in this study for up to 2 years. This research was supported by the National Institute of Health [grant numbers R01 CA095545 and CA065628]; and National Center for Advancing Translational Sciences [grant number UL1TR000071)]; the National Institute of Environmental Health Science [grant number, 2 P30 ES06676]; and by the Institute for Translational Sciences at UTMB. All authors declare that they have no conflict of interest.
Abbreviations used
- HRT
hormone replacement therapy
- DE
daidzein excretion
- GE
genistein excretion
- CAIC
conditional Akaike information criterion
- LME
linear mixed effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. Journal of Clinical Endocrinology & Metabolism. 1998;83(7):2239–43. [DOI] [PubMed] [Google Scholar]
- [2].Cauley JA. Estrogen and bone health in men and women. Steroids. 2015. July;99(Pt A):11–5. [DOI] [PubMed] [Google Scholar]
- [3].Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Annals of Internal Medicine. 1995;122(1):9–16. [DOI] [PubMed] [Google Scholar]
- [4].Anonymous. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI [see comments]. JAMA. 1996;276(17):1389–96. [PubMed] [Google Scholar]
- [5].Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002. July;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- [6].Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013. January;121(1):172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998. August;280(7):605–13. [DOI] [PubMed] [Google Scholar]
- [8].Beral V, Collaborators MWS. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003. August;362(9382):419–27. [DOI] [PubMed] [Google Scholar]
- [9].Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013. October;310(13):1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J. 1946;22:2–12. [DOI] [PubMed] [Google Scholar]
- [11].Miksicek RJ. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994. June;49(2–3):153–60. [DOI] [PubMed] [Google Scholar]
- [12].Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. [DOI] [PubMed] [Google Scholar]
- [13].Anderson JJB, Ambrose WW, Garner SC. Biphasic effects of genistein on bone tissue in the ovariectomized, lactating rat model. Proceedings of the Society for Experimental Biology & Medicine. 1998. March;217(3):345–50. [DOI] [PubMed] [Google Scholar]
- [14].Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P, et al. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. Journal of Nutrition. 2000;130(7):1675–81. [DOI] [PubMed] [Google Scholar]
- [15].Ishimi Y, Arai N, Wang X, Wu J, Umegaki K, Miyaura C, et al. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem Biophys Res Commun. 2000. August;274(3):697–701. [DOI] [PubMed] [Google Scholar]
- [16].Arjmandi BH, Smith BJ. Soy isoflavones’ osteoprotective role in postmenopausal women: mechanism of action. J Nutr Biochem. 2002. March;13(3):130–7. [DOI] [PubMed] [Google Scholar]
- [17].Kaludjerovic J, Ward WE. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J Nutr. 2010. April;140(4):766–72. [DOI] [PubMed] [Google Scholar]
- [18].Bitto A, Polito F, Squadrito F, Marini H, D’Anna R, Irrera N, et al. Genistein aglycone: a dual mode of action anti-osteoporotic soy isoflavone rebalancing bone turnover towards bone formation. Curr Med Chem. Netherlands 2010:3007–18. [DOI] [PubMed] [Google Scholar]
- [19].Yamaguchi M Nutritional factors and bone homeostasis: synergistic effect with zinc and genistein in osteogenesis. Mol Cell Biochem. 2012. July;366(1–2):201–21. [DOI] [PubMed] [Google Scholar]
- [20].Zheng X, Lee SK, Chun OK. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J Med Food. 2016. January;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Migliaccio S, Anderson JJ. Isoflavones and skeletal health: are these molecules ready for clinical application? Osteoporos Int. 2003. June;14(5):361–8. [DOI] [PubMed] [Google Scholar]
- [22].Reinwald S, Weaver CM. Soy isoflavones and bone health: a double-edged sword? J Nat Prod. 2006. March;69(3):450–9. [DOI] [PubMed] [Google Scholar]
- [23].Dang ZC, Lowik C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol Metab. 2005. July;16(5):207–13. [DOI] [PubMed] [Google Scholar]
- [24].Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial. Menopause. 2004. 2004 May-Jun;11(3):246–54. [DOI] [PubMed] [Google Scholar]
- [25].Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004. July;292(1):65–74. [DOI] [PubMed] [Google Scholar]
- [26].Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. United States 2007:839–47. [DOI] [PubMed] [Google Scholar]
- [27].Wong WW, Lewis RD, Steinberg FM, Murray MJ, Cramer MA, Amato P, et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr. 2009. November;90(5):1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alekel DL, Van Loan MD, Koehler KJ, Hanson LN, Stewart JW, Hanson KB, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010. January;91(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011. August;171(15):1363–9. [DOI] [PubMed] [Google Scholar]
- [30].Tai TY, Tsai KS, Tu ST, Wu JS, Chang CI, Chen CL, et al. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: a 2-year randomized double-blind placebo-controlled study. Osteoporos Int. 2012. May;23(5):1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choi SD, Steinberg EM, Lee HH, Naftolin F. The Timing Hypothesis remains a valid explanation of differential cardioprotective effects of menopausal hormone treatment. Menopause. 2011. February;18(2):230–6. [PubMed] [Google Scholar]
- [32].Lu LJ, Lin SN, Grady JJ, Nagamani M, Anderson KE. Altered kinetics and extent of urinary daidzein and genistein excretion in women during chronic soya exposure. Nutr Cancer. 1996;26(3):289–302. [DOI] [PubMed] [Google Scholar]
- [33].Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. Journal of Nutrition. 1994;124(6):825–32. [DOI] [PubMed] [Google Scholar]
- [34].van der Velpen V, Hollman PC, van Nielen M, Schouten EG, Mensink M, van’t Veer P, et al. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur J Clin Nutr. 2014. 10//print;68(10):1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu L-JW, Chen N-W, Nayeem F, Ramanujam VMS, Kuo Y-F, Brunder DG, et al. Novel effects of phytoestrogenic soy isoflavones on serum calcium and chloride in premenopausal women: A 2-year double-blind, randomized, placebo-controlled study. Clinical Nutrition. 2018 2018/December/01/;37(6, Part A):1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramanujam VS, Nayeem F, Anderson KE, Kuo YF, Chen NW, Ju H, et al. Riboflavin as an independent and accurate biomarker for adherence in a randomized double-blind and placebo-controlled clinical trial. Biomarkers. 2017. September;22(6):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bauer DJ, Curran PJ. Probing Interactions in Fixed and Multilevel Regression: Inferential and Graphical Techniques. Multivariate Behav Res. 2005;40(3):373–400. [DOI] [PubMed] [Google Scholar]
- [38].Vaida F, Blanchard S. Conditional Akaike information for mixed-effects models. Biometrika. 2005;92(2):351–70. [Google Scholar]
- [39].Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology. 2011 2011/01/January;65(1):13–21. [Google Scholar]
- [40].Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery. 2012. //;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- [41].Spitzer NC. Calcium: first messenger. Nat Neurosci. 2008. March;11(3):243–4. [DOI] [PubMed] [Google Scholar]
- [42].Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000. October;1(1):11–21. [DOI] [PubMed] [Google Scholar]
- [43].Goltzman D, Mannstadt M, Marcocci C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front Horm Res. 2018;50:1–13. [DOI] [PubMed] [Google Scholar]
- [44].Christakos S, Prince R. Estrogen, vitamin D, and calcium transport. J Bone Miner Res. 2003. October;18(10):1737–9. [DOI] [PubMed] [Google Scholar]
- [45].Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011. December;347(1–2):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). Journal of Nutrition. 1998;128(10):1710–5. [DOI] [PubMed] [Google Scholar]
- [47].Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol. 2009. May;304(1–2):30–42. [DOI] [PubMed] [Google Scholar]
- [48].Hall WL, Rimbach G, Williams CM. Isoflavones and endothelial function. Nutr Res Rev. 2005. June;18(1):130–44. [DOI] [PubMed] [Google Scholar]