Abstract
Goals:
We quantified cerebral blood flow response to a 500cc bolus of 0.9% normal saline (NS) within 96 hours of acute ischemic stroke (AIS) using diffuse correlation spectroscopy (DCS).
Materials and Methods:
Subjects with AIS in the anterior, middle, or posterior cerebral artery territory were enrolled within 96-hours of symptom onset. DCS measured relative cerebral blood flow (rCBF) in the bilateral frontal lobes for 15 minutes at rest (baseline), during a 30-minute infusion of 500cc NS (bolus), and for 15 minutes after completion (post-bolus). Mean rCBF for each time period was calculated for individual subjects and median rCBF for the population was compared between time periods. Linear regression was used to evaluate for associations between rCBF and clinical features.
Results:
Among 57 subjects, median rCBF (IQR) increased relative to baseline in the ipsilesional hemisphere by 17% (−2.0%, 43.1%), p<0.001, and in the contralesional hemisphere by 13.3% (−4.3%, 36.0%), p<0.004. No significant associations were found between ipsilesional changes in rCBF and age, race, infarct size, infarct location, presence of large vessel stenosis, NIH stroke scale, or symptom duration.
Conclusion:
A 500cc bolus of 0.9% normal saline produced a measurable increase in rCBF in both the affected and non-affected hemispheres. Clinical features did not predict rCBF response.
Keywords: cerebral blood flow, diffuse correlation spectroscopy, stroke, intravenous fluids, saline
Introduction
In the normal brain, cerebral autoregulation maintains cerebral blood flow (CBF) at a constant level despite variations in mean arterial pressure (MAP).(1) (2–4) In patients with acute ischemic stroke, autoregulation is thought to be impaired and CBF may vary with changes in MAP.(5–7) In acute stroke, clinical symptoms represent permanent damage to the infarct core, and reversible damage to surrounding tissue, the ischemic penumbra. Acute stroke therapies are intended to increase blood flow to the penumbra.
Simple approaches to increase CBF, such as lowering head of bed position and administration of intravenous 0.9% normal saline (NS) are used commonly in clinical practice, although their clinical utility is uncertain. Prior studies have shown that lowering head of bead has a small but measurable impact on CBF; although, a large cluster-randomized trial showed no difference in clinical outcomes between head of bed flat (0°) and elevated (≥30°).(8–11) Similarly, volume expansion and hemodilution have been shown to increase CBF, but not to improve clinical outcomes.(12) (13) Limited data suggest that bolus NS increases regional CBF in patients with subarachnoid hemorrhage.(14) Data on the CBF response to NS after ischemic stroke is limited.(15,16)
We aimed to quantify the CBF response to a 500cc bolus of NS administered within 96 hours of acute ischemic stroke. We employed diffuse correlation spectroscopy (DCS) to monitor changes in CBF. DCS is a transcranial noninvasive optical technique that uses near infrared light. DCS provides a real-time, continuous bedside measure of changes in CBF (i.e., relative CBF, rCBF). Unlike near-infrared spectroscopy (NIRS) or diffuse optical spectroscopy (DOS), which measures oxy- and deoxy-hemoglobin tissue concentration and uses blood volume as a surrogate for changes in blood flow, DCS, a newer technique, directly quantifies microvascular cerebral blood flow. Relative CBF measured with DCS has been validated against other blood flow imaging/monitoring technologies, and DCS has been used to measure changes in CBF in both healthy and stroke subjects.(8, 11, 17–25) The significance of this work is two-fold. First, by quantifying the rCBF response to bolus NS, this study provides information about the physiologic response to an intervention that is frequently used in clinical practice. Second, this study demonstrates the potential for DCS, a real-time continuous monitor of rCBF, to quantify individual response to an intervention over an extended period of time. With continued technical refinement, this technology holds the potential to enable clinicians to individualize care and optimize CBF.
Materials and Methods
Population
A single center, non-randomized, pilot, single arm time-series clinical trial was conducted at a single comprehensive stroke center and was approved by the University of Pennsylvania institutional review board. Inclusion criteria included: age ≥ 18 years, acute ischemic stroke in the territory of the anterior, middle, or posterior cerebral artery territory, and enrollment within 96 hours of symptom onset. Exclusion criteria included: infarct limited to the brainstem and/or cerebellum, bi-hemispheric infarcts or infarct in the contralesional hemisphere within the past 30 days, symptoms of active congestive heart failure (dyspnea, orthopnea, increased oxygen requirement), exacerbation of congestive heart failure requiring hospitalization within the past 30 days or severe systolic dysfunction with ejection fraction <20%, end-stage renal disease requiring hemodialysis or creatinine clearance <20 ml/min/1.7m2, hemicraniectomy or other skull defect, pregnant women, current enrollment in another clinical trial, and any other illness or condition that the enrolling investigator felt would pose a hazard to the subject from participating in the study.
Study Measurements and Intervention
After informed consent was obtained, demographic and clinical variables were recorded, including past medical history, infarct location, size, vascular imaging results, and the National Institute of Health stroke scale (NIHSS). The DCS probe was placed on the patient at the temporal margin of the forehead bilaterally (supplemental Figure 1).
The custom DCS instrument used in this study measures relative cortical cerebral blood flow using near infrared light. The details of this device have been described previously.(23) The instrument uses a laser (785nm, 80mW, CrystaLaser Inc., Reno NV) of constant intensity and custom fiber-optic detectors (Fiberoptic Systems, CA) integrated onto a 2cm x 5cm rubber probe. Light transmitted through tissue was detected at 1.0cm and 2.5cm source-detector separations. Unlike NIRS/DOS, which indirectly estimates changes in CBF based on measured changes in oxy and deoxy-hemoglobin, DCS provides a direct measure of microvascular CBF by quantifying temporal fluctuations of the intensity of light scattered by moving red blood cells. Temporal fluctuations in the detected light intensity are quantified using an intensity temporal autocorrelation function computed with custom software. An index of cerebral blood flow is derived by fitting the measured light intensity temporal autocorrelation functions to a solution of a two-layer light diffusion model through tissue.(26) (27)
After the DCS probes were placed and secured with a cloth headband, the patient was asked to remain in bed and to be still for the duration of the monitoring session. For the first fifteen minutes of the 60-minute monitoring session there was no normal saline infusion (baseline), then 500cc of 0.9% normal saline was infused over 30-minutes (bolus). Monitoring continued for 15-minutes after the completion of the bolus (post-bolus). During the monitoring session, heart rate and blood pressure were recorded every 15 minutes. The patient was assessed for adverse events during the monitoring session and 24 hours after the monitoring session.
Relative changes in cerebral blood flow were estimated for each subject. Details of this analysis methodology is presented in the online supplement. DCS intensity autocorrelation functions were pre-processed to remove and correct for motion artifacts. Intensity autocorrelation functions at 1.0 and 2.5 cm source detector separations were processed together to estimate changes in cerebral blood flow. We employed the modified Beer Lambert law for flow, adapted for a two-layer tissue photon diffusion model to account for the influence of blood flow changes in the scalp.(26, 27) As a final post processing step, rCBF measurements that were less than −100% or greater than 250% were considered non-physiologic, and were removed to reduce bias from measurement error. This correction affected only 1% of the measured CBF values.
Analysis
We hypothesized that bolus administration of NS would increase in CBF in the ipsilesional hemisphere, and that there would be no change in CBF in the contralesional hemisphere. Baseline demographics and clinical information were summarized using descriptive statistics. The NIHSS stroke scale was categorized as mild (0-4), moderate (5-9) and severe (10+). Infarct location was categorized as: anterior cerebral artery (ACA), posterior cerebral artery (PCA), anterior middle cerebral artery (MCA) with cortical involvement, posterior MCA with cortical involvement, and deep MCA territory without cortical involvement (subjects could be in multiple categories). Infarct volume was estimated using the ABC/2 method and categorized as <15cc, 15-30cc, and >30cc.(28, 29) Infarct etiology was classified as cardioembolism, large vessel atherosclerosis, small vessel, cryptogenic, and other/multiple. Ipsilesional large vessel stenosis was categorized as <50%, 50-95%, and near occlusion/occlusion. Baseline mean arterial pressure (MAP) was the mean of the MAP recorded at the start of monitoring (time 0 minutes) and immediately before initiation of NS bolus (time 15 minutes); post-bolus MAP was the mean of MAP recorded immediately after completion of the NS bolus (time 45 minutes) and at the end of monitoring (time 60 minutes).
The mean rCBF values in each time period (baseline, bolus, and post-bolus) were calculated for each subject. The median values for the entire population were summarized and signed rank test was used to compare rCBF at baseline and bolus as well as rCBF at baseline and post-bolus. Post-bolus rCBF was summarized across key clinical subgroups, including infarct size (<15cc and ≥15cc), ipsilesional large vessel stenosis (<50%, and 50-100%), infarct location (ACA and/or anterior MCA territory vs. all others), and NIHSS (0-4 vs 5+). Categories were dichotomized given the relatively small number of subjects. Finally, the change in mean rCBF from baseline to post-bolus was calculated for each subject. Univariate linear regression was used to quantify the relationship between clinical features and change in rCBF, with the plan to create a multivariable model including any variables associated at p<0.05 in the univariate analysis. We hypothesized that larger infarcts, ipsilesional large vessel stenosis, ACA and/or anterior MCA location, and higher NIHSS would be associated with a greater increase in rCBF. In an exploratory analysis we used linear regression to quantify the association between the change in rCBF and the change in MAP from baseline to post-bolus. Finally, mixed effects regression was used to estimate the relationship between rCBF and time as a linear relationship in the baseline and bolus time periods. This model used restricted maximum likelihood estimation, unstructured covariance, and included an interaction term to estimate the change in slope after the start of the NS bolus (time x NS bolus), which represents the relative increase in rCBF attributable to the saline infusion. All analyses were conducted separately in the ipsilesional and contralesional hemisphere using Stata version 15 (StataCorp LLC, College Station, TX). A priori power calculations, which assumed rCBF would be normally distributed, correlation between baseline and post-bolus rCBF of 0.6-0.8 and a standard deviation of 10-15%, suggested that 57 subjects would provide 80% power to detect a 5% increase in rCBF.
Results
A total of 81 subjects were enrolled. A technical advancement in DCS fiber optic probe design which increased signal-to-noise ratio occurred after the first 8 subjects; these subjects were excluded because their data was not comparable to later subjects. There were 5 subjects who failed to complete the monitoring session and 11 subjects were excluded due to poor data quality (details in supplemental Table 1). The remaining 57 subjects were analyzed. Baseline characteristics of these subjects are summarized in Table 1.
Table 1.
Study Population
| Variable | N | |
|---|---|---|
| Age, median (IQR) | 57 | 61 (53-72) |
| Female | 26 | 46% |
| Race | ||
| White | 30 | 53% |
| Black | 26 | 46% |
| Other | 1 | 2% |
| Past Medical History | ||
| Hypertension | 41 | 72% |
| Diabetes | 10 | 18% |
| Hyperlipidemia | 28 | 49% |
| Atrial Fibrillation | 6 | 11% |
| Congestive Heart Failure | 2 | 4% |
| CAD/Prior MI | 8 | 14% |
| Chronic Kidney Disease | 2 | 4% |
| Peripheral Artery Disease | 4 | 7% |
| Prior stroke | 11 | 19% |
| Smoking Status | ||
| Current | 12 | 21% |
| Former | 20 | 35% |
| Time from onset, median (IQR) | 66(49-83) | |
| <48 hours | 14 | 25% |
| 48+ hours | 43 | 75% |
| NIHSS at monitoring, median (IQR) | 2 (1,7) | |
| NIHSS 0-4 | 39 | 68% |
| NIHSS 5-9 | 8 | 14% |
| NIHSS 10+ | 10 | 18% |
| Infarct Location | ||
| ACA | 2 | 4% |
| Anterior MCA, cortical involvement | 24 | 42% |
| Posterior MCA, cortical involvement | 14 | 25% |
| MCA, deep penetrator only | 20 | 35% |
| PCA | 4 | 7% |
| Infarct Volume, median (IQR) | 2 (1-9) | |
| <15cc | 47 | 84% |
| 15-30cc | 5 | 9% |
| >30cc | 4 | 7% |
| Infarct Etiology | ||
| Cardioembolism | 12 | 22% |
| Large vessel atherosclerosis | 10 | 18% |
| Small vessel lacune | 9 | 16% |
| cryptogenic | 18 | 32% |
| other/multiple | 8 | 14% |
| Ipsilesional large vessel stenosis | ||
| <50% | 43 | 75% |
| 50-95% | 5 | 9% |
| Near Occlusion/Occlusion | 9 | 16% |
MAP increased from median (IQR) of 100.1 mmHg (93.2-110.0 mmHg) at baseline to 104.8 (96.7-113.9) post-bolus period (p<0.001). Median rCBF (% change) across all subjects and its IQR is shown in Figure 1 for both the ipsilesional and contralesional hemispheres. All data points from all subjects are shown in supplemental Figure 2. Median rCBF by hemisphere and time period is shown in Table 2. The difference in rCBF between baseline and bolus, and between baseline and post-bolus was significant in both hemispheres (p<0.05 for all comparisons using Wilcoxon signed rank test). The median rCBF was numerically greater in the ipsilesional hemisphere, although there was not a statistically significant difference between the hemispheres in the bolus (rCBF ipsilesional = 13.8%, rCBF contralesional = 8.0%, p=0.39) or post-bolus time periods (rCBF ipsilesional = 17.0%, rCBF contralesional = 13.3%, p=0.64).
Figure 1:
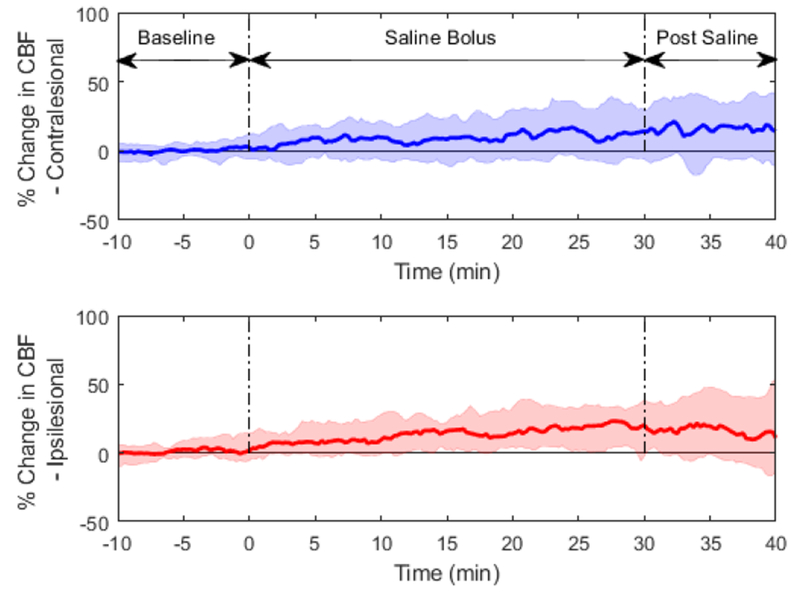
Time course of % change in CBF; solid lines are median responses averaged across all subjects, shaded regions are IQRs.
Table 2.
Percentage change in CBF, relative to baseline, by time period.
| Bolus | Post Bolus | |||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Ipsilesional Hemisphere | 13.8% | (5.3, 23.5) | 17.0% | (−2.0, 43.1) |
| Contralesional Hemisphere | 8.0% | (−2.1, 21.0) | 13.3% | (−4.3, 36.0) |
IQR=interquartile range
Post-bolus rCBF across clinical subgroups is summarized in Table 3. Univariate regression results, evaluating the association between clinical factors and the change in rCBF from baseline to post-bolus, is presented in Table 4. There were no statistically significant associations in the ipsilesional hemisphere. In the contralesional hemisphere, there was a smaller change in rCBF among subjects with an ACA and/or anterior MCA infarction compared to other locations. The data did not support our pre-existing hypotheses that larger infarcts, greater NIHSS, anterior MCA location, and ipsilesional large vessel stenosis would be associated with a greater increase in rCBF. Graphs of rCBF over time by clinical subgroup are presented in supplemental Figures 3–7. Multivariable regression was not performed due to lack of associations in the univariate analysis. We did not detect a significant association between the change in rCBF and the change in MAP from baseline to post-bolus (Figure 2A, ipsilesional hemisphere: β=0.1, 95% CI: −0.8-1.1, p=0.80; Figure 2B: contralesional hemisphere: β=−0.5, 95% CI: −1.5-0.5, p=0.35)
Table 3.
Percentage change in CBF, relative to baseline, in the post-bolus time period, by clinical subgroup
| Ipsilesional | Contralesional | ||||
|---|---|---|---|---|---|
| Subgroup | N | Median (IQR) | p-value | Median (IQR) | p-value |
| Infarct Size | |||||
| <15cc | 47 | 20.5% (−0.6, 43.6) | 0.06 | 13.3% (−4.3, 37.5) | 0.89 |
| 15cc+ | 9 | −2.0% (−15.1, 15.3) | 21.3% (5.9, 28.4) | ||
| Stenosis | |||||
| <50% | 43 | 13.5% (3.4, 23.3) | 0.99 | 9.7% (−6.0, 22.5) | 0.54 |
| 50-100% | 14 | 17.9% (10.0, 28.1) | 6.3% (1.3, 20.0) | ||
| Location | |||||
| Anterior Cortical MCA | 24 | 17.7% (−8.2, 36.1) | 0.69 | 3.0% (−22.4, 26.2) | 0.02 |
| All others | 33 | 17.0% (−0.6, 43.4) | 23.1% (5.9, 43.2) | ||
| NIHSS | |||||
| NIHSS 0-4 | 39 | 18.8% (−10.6, 43.4) | 0.93 | 21.3% (−4.3, 37.5) | 0.24 |
| NIHSS 5+ | 18 | 16.1% (1.1, 36.6) | 5.4% (−15.6, 32.6) | ||
| Time from onset | |||||
| <48 hours | 14 | 8.2% (−24.3, 29.1) | 0.19 | 3.0% (−15.58, 32.60) | 0.28 |
| 48+ hours | 43 | 18.8% (−0.6, 43.6) | 21.3% (1.77, 36.29) | ||
IQR=Interquartile Range
Table 4.
Univariate Linear Regression
| Ipsilesional | Contralesional | |||||||
|---|---|---|---|---|---|---|---|---|
| β | Std Error | 95% CI | p-value | β | Std Error | 95% CI | p-value | |
| Age (per year) | 1.0 | 0.3 | (−0.7, 0.7) | 0.98 | −0.2 | 0.4 | (−0.9, 0.6) | 0.66 |
| Race | ||||||||
| White | Ref | Ref | ||||||
| Black/Other | 6.0 | 8.5 | (−11.1, 23.1) | 0.49 | 8.3 | 9.2 | (−10.2, 26.8) | 0.37 |
| Volume | ||||||||
| <15cc | Ref | Ref | ||||||
| 15cc+ | −19.6 | 11.5 | (−42.7, 3.5) | 0.10 | 0.1 | 12.4 | (−24.8, 24.9) | 1.00 |
| NIHSS | ||||||||
| 0-4 | Ref | Ref | ||||||
| 5+ | 4.3 | 9.2 | (−14.1, 22.6) | 0.65 | −11.7 | 9.8 | (−31.4, 8.0) | 0.24 |
| Location | ||||||||
| ACA or Anterior MCA | −5.9 | 8.6 | (−23.1, 11.4) | 0.50 | −19.7 | 9.0 | (−37.7, −1.7) | 0.03 |
| Other | Ref | Ref | ||||||
| Stenosis | ||||||||
| <50% | Ref | Ref | ||||||
| 50-100% | −1.1 | 9.9 | (−21.0, 18.8) | 0.91 | 13.1 | 10.6 | (−8.2, 34.3) | 0.22 |
| Time from onset | ||||||||
| <48 hours | Ref | Ref | ||||||
| 48+ hours | 17.8 | 8.6 | (−1.5, 37.1) | 0.07 | 11.2 | 10.7 | (−10.2,32.5) | 0.30 |
Figure 2:
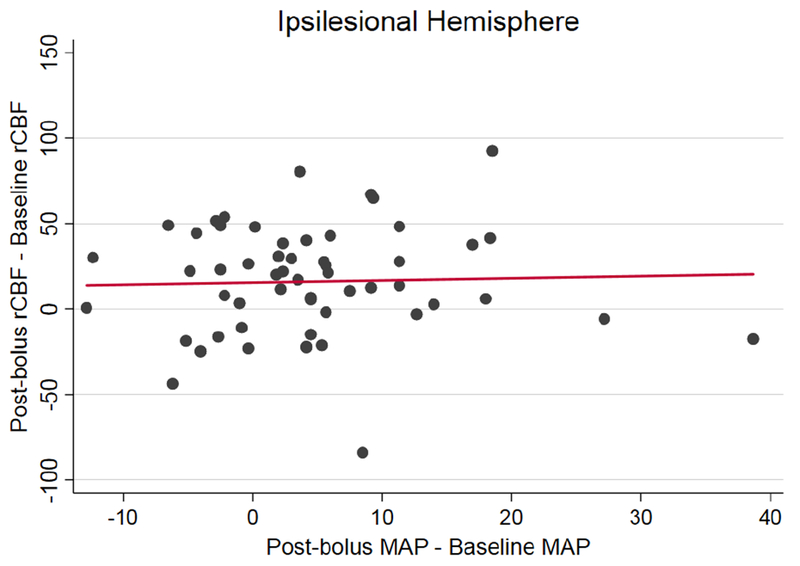

Change in rCBF (post-bolus mean rCBF – baseline mean rCBF) and change MAP (post-bolus MAP-baseline MAP) are graphed by subject. Linear regression showed no significant relationship in the ipsilesional hemisphere (figure 2A, β=0.1, 95% CI: −0.8-1.1, p=0.80) or the contralesional hemisphere (figure 2B, β=−0.5, 95% CI: −1.5-0.5, p=0.35).
In the mixed effects regression, the interaction term for time x NS bolus was significant in both the ipsilesional (β=0.05, 95% CI: 0.03-0.07, p<0.001) and contralesional hemispheres (β=0.08, 95% CI: 0.05-0.11, p<0.001), representing a significant change in slope after the start of the NS bolus. Over the 30-minute bolus time period this equates to a 6.0% increase in rCBF in the ipsilesional hemisphere (95% CI: 3.2-8.8) and a 9.4% increase in rCBF in the contralesional hemisphere (95% CI: 6.3-12.6). Estimates from the mixed effects regression differ from the primary analysis because the model attempts to account for changes in rCBF overtime in the baseline period.
The study intervention was well tolerated; no serious adverse events occurred. There were 3 adverse events which were possibly related to the study intervention: an IV infiltrated in 1 patient, monitoring stopped due to a systolic blood pressure >220 mm Hg in 1 patient, and 1 patient, who was in atrial fibrillation at the start of the monitoring session, had tachycardia during the monitoring session which was asymptomatic.
Discussion
In patients with acute ischemic stroke, 500cc of 0.9% normal saline administered over 30 minutes was associated with a measurable increase in rCBF in both the ipsilesional and contralesional hemispheres. There was not a statistically significant difference between hemispheres.
Fluid resuscitation is a common treatment strategy in acute ischemic stroke. American Fleart Association guidelines recommend correction of hypovolemia, but note “there are no data to guide volume and duration of parenteral fluid delivery.(30)’(31) Our study provides evidence that a 500cc bolus of NS results in increased CBF. This builds on prior reports showing increased cerebral perfusion measured using MRI during induced hypertension from isotonic fluids and confirms a physiologic rationale for the use of bolus isotonic fluids in the early management of ischemic stroke and/or in patients with fluctuating symptoms.(32–34)
Our results raise several important questions. First, it is uncertain whether the measured changes in rCBF could have a meaningful clinical impact. Prior studies of hemodilution and/or volume expansion have failed to demonstrate an improvement in clinical outcomes, and overly aggressive fluid resuscitation has the potential to be harmful.(13, 35) Second, there was wide variability in rCBF response to bolus NS across subjects, as illustrated by the wide IQR for rCBF. This may be due in part to the heterogenous patient population. Also, studies have questioned the extent of autoregulation impairment after stroke, suggested that the range of autoregulation is narrower than classical models, and suggested that autoregulation curves may vary across subjects; all of which may also be contributing to the observed variability. (36)’(2, 37–42) Finally, although we expected the contralesional hemisphere to serve as a control, with no change in CBF during saline infusion, we observed increased CBF bilaterally. This change in CBF was was not associated with changes in MAP. There is limited evidence from studies in healthy subjects that changes in cardiac output can lead to changes in cerebral blood flow that is independent of changes in MAP.(43, 44) Unfortunately, we did not directly measure cardiac output or cerebral autoregulation in this study. Further study is necessary to address all of these important questions in order to better understand the CBF response to normal saline infusion, and to determine if there is any potential therapeutic benefit.
Still, our study highlights the potential utility of a real-time continuous CBF monitor, such as DCS, to inform and individualize stroke care. If individual CBF response to interventions cannot be inferred by clinical factors or measurement of MAP, then direct measurement of CBF is required to maximize therapeutic benefit. This logic applies to a range of possible therapeutic approaches beyond NS, including head of bed position, volume expansion, cerebral vasodilators, or induced hypertension.
This study has limitations. Although the measured changes in rCBF were modest overall, some individual measurements were larger than would be expected given the intervention. DCS is a direct measure of microvascular CBF, which is more sensitive to arterial than venous contributions.(45, 46) DCS CBF measurements have been validated against MRI techniques, Xenon CT, TCD, and fluorescent microspheres in both healthy controls and neurologically injured populations.(17–19, 21) Still, in the setting of extended monitoring across a wide range of subjects, measurement error could conceivably be biasing the results. DCS is an evolving technology and advancements in the field of optical imaging should help to improve its accuracy and utility in future studies. Key recent advancements include the ability to use a real-time software correlator to improve the temporal resolution of DCS measurements and the use of a pressure modulation algorithm to more accurately separate cerebral from extracerebral signals.(25) Additionally, with the use of indocyanine green as a dynamic contrast agent, DCS can be calibrated to generate absolute, rather than relative, estimates of CBF.(47, 48) The current study predates some of these advances. Additionally, the DCS probes used in this study measure rCBF in the frontal poles, and may not be sampling penumbra in many cases. Ongoing work is aimed at monitoring other cortical territories (i.e. through hair).
The monitoring session was not sufficiently long to know the duration of rCBF elevation after NS bolus. It is also possible that response to bolus NS depends on intravascular volume status, which was not objectively measured. Because most patients were enrolled >24 hours after admission, it is likely that they had received some fluid resuscitation prior to enrollment. Given our relatively small sample size and the heterogenous study population, there was limited power to detect significant differences across clinical subgroups. In particular, we had very few subjects with large infarcts and/or an ipsilesional large vessel occlusion. Our comparison of change in rCBF to change in MAP is limited by the fact that blood pressure was measured only twice in the baseline and post-bolus time periods. Continuous blood pressure monitoring would allow for a more rigorous analysis of the relationship between rCBF and MAP. Despite these limitations, it is notable that we were able to measure a significant change in rCBF in a population that was predominately comprised of minor strokes. These results highlight the power of DCS to detect small changes in CBF.
Conclusions
A 500cc bolus of 0.9% normal saline, administered over 30 minutes, resulted in a measurable increase in rCBF in subjects with acute ischemic stroke. There was significant inter-individual variability in rCBF response, which was not associated with measured clinical factors or with change in MAP. This study highlights a potential physiologic rationale for the use of isotonic fluid resuscitation in acute ischemic stroke, although more study is needed to better understand the physiologic response to saline infusion and to determine whether observed changes in rCBF are clinically meaningful.
Supplementary Material
Acknowledgements
We acknowledge Malavika Chandra, Julien Menko and Ken Abramson from the Laboratory for Research on the Structure of Matter at the University of Pennsylvania for technical assistance and Melissa Yates and Nichole Gallatti for assistance with clinical research coordination, data collection, and data management.
Funding:
This work was funded by NINDS R01 NS060653 “Diffuse Optics for Acute Stroke Management,” NIH P41-EB015893 “A Resource for Magnetic Resonance and Optical Imaging (AY, JD, SK, MM, AP, WB) and NIH R25 NS065745 (JD, CF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration: clinicaltrials.gov identifier
Disclosures:
Arjun Yodh and John Detre are inventors on patents submitted on behalf of the Trustees of the University of Pennsylvania that have at least some overlap with this research: US10,064,554, US8,082,015, and provisional patent 2016036101717 (AY and JD) and US6,076,010 (AY).
References
- 1.Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J Neurosurg. 1984;61(2):241–53. [DOI] [PubMed] [Google Scholar]
- 2.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183–238. [DOI] [PubMed] [Google Scholar]
- 3.Strandgaard S Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976;53(4):720–7. [DOI] [PubMed] [Google Scholar]
- 4.Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14(3):332–41. [DOI] [PubMed] [Google Scholar]
- 6.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16(1):69–75. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz S Effects of Induced Hypertension on Intracranial Pressure and Flow Velocities of the Middle Cerebral Arteries in Patients With Large Hemispheric Stroke. Stroke. 2002;33(4):998–1004. [DOI] [PubMed] [Google Scholar]
- 8.Durduran T, Zhou C, Edlow BL, Yu G, Choe R, Kim MN, et al. Transcranial optical monitoring of cerebrovascular hemodynamics in acute stroke patients. Opt Express. 2009;17(5):3884–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64(8):1354–7. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AJ, Snodgrass SJ, Quain D, Parsons MW, Levi CR. HOBOE (Head-of-Bed Optimization of Elevation) Study: association of higher angle with reduced cerebral blood flow velocity in acute ischemic stroke. Phys Ther. 2011;91(10):1503–12. [DOI] [PubMed] [Google Scholar]
- 11.Favilla CG, Mesquita RC, Mullen M, Durduran T, Lu X, Kim MN, et al. Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation. Stroke. 2014;45(5):1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarria VV, et al. Cluster-Randomized, Crossover Trial of Head Positioning in Acute Stroke. N Engl J Med. 2017;376(25):2437–47. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12(11):1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jost SC, Diringer MN, Zazulia AR, Videen TO, Aiyagari V, Grubb RL, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J Neurosurg. 2005;103(1):25–30. [DOI] [PubMed] [Google Scholar]
- 15.Jauch EC, Saver JL, Adams HP Jr., Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 16.Bonoczk P, Panczel G, Nagy Z. Vinpocetine increases cerebral blood flow and oxygenation in stroke patients: a near infrared spectroscopy and transcranial Doppler study. Eur J Ultrasound. 2002;15(1–2):85–91. [DOI] [PubMed] [Google Scholar]
- 17.Buckley EM, Hance D, Pawlowski T, Lynch J, Wilson FB, Mesquita RC, et al. Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging. J Biomed Opt. 2012;17(3):037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MN, Durduran T, Frangos S, Edlow BL, Buckley EM, Moss HE, et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit Care. 2010;12(2):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Floyd TF, Durduran T, Zhou C, Wang J, Detre JA, et al. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt Express. 2007;15(3):1064–75. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Eucker SA, Durduran T, Yu G, Ralston J, Friess SH, et al. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J Biomed Opt. 2009;14(3):034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zirak P, Delgado-Mederos R, Marti-Fabregas J, Durduran T. Effects of acetazolamide on the micro- and macro-vascular cerebral hemodynamics: a diffuse optical and transcranial doppler ultrasound study. Biomed Opt Express. 2010;1(5):1443–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado-Mederos R, Gregori-Pla C, Zirak P, Blanco I, Dinia L, Marin R, et al. Transcranial diffuse optical assessment of the microvascular reperfusion after thrombolysis for acute ischemic stroke. Biomed Opt Express. 2018;9(3):1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesquita RC, Durduran T, Yu G, Buckley EM, Kim MN, Zhou C, et al. Direct measurement of tissue blood flow and metabolism with diffuse optics. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parthasarathy AB, Gannon KP, Baker WB, Favilla CG, Balu R, Kasner SE, et al. Dynamic autoregulation of cerebral blood flow measured non-invasively with fast diffuse correlation spectroscopy. J Cereb Blood Flow Metab. 2018;38(2):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Parthasarathy AB, Baker WB, Gannon K, Kavuri V, Ko T, et al. Fast blood flow monitoring in deep tissues with real-time software correlators. Biomed Opt Express. 2016;7(3):776–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker WB, Parthasarathy AB, Busch DR, Mesquita RC, Greenberg JH, Yodh AG. Modified Beer-Lambert law for blood flow. Biomed Opt Express. 2014;5(11):4053–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker WB, Parthasarathy AB, Ko TS, Busch DR, Abramson K, Tzeng SY, et al. Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts. Neurophotonics. 2015;2(3):035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luby M, Hong J, Merino JG, Lynch JK, Hsia AW, Magadan A, et al. Stroke mismatch volume with the use of ABC/2 is equivalent to planimetric stroke mismatch volume. AJNR Am J Neuroradiol. 2013;34(10):1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 31.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. [DOI] [PubMed] [Google Scholar]
- 32.Lee AW, Davis C, Puttgen HA, Hillis AE. Urgent intervention to reduce functional deficits after postoperative stroke. Nat Clin Pract Neurol. 2007;3(3):173–7. [DOI] [PubMed] [Google Scholar]
- 33.Hillis AE, Wityk RJ, Beauchamp NJ, Ulatowski JA, Jacobs MA, Barker PB. Perfusion-weighted MRI as a marker of response to treatment in acute and subacute stroke. Neuroradiology. 2004;46(1):31–9. [DOI] [PubMed] [Google Scholar]
- 34.Hillis A Reperfusion of Specific Brain Regions by Raising Blood Pressure Restores Selective Language Functions in Subacute Stroke. Brain and Language. 2001;79(3):495–510. [DOI] [PubMed] [Google Scholar]
- 35.Aichner FT, Fazekas F, Brainin M, Polz W, Mamoli B, Zeiler K. Hypervolemic hemodilution in acute ischemic stroke: the Multicenter Austrian Hemodilution Stroke Trial (MAHST). Stroke. 1998;29(4):743–9. [DOI] [PubMed] [Google Scholar]
- 36.Jordan JD, Powers WJ. Cerebral autoregulation and acute ischemic stroke. Am J Hypertens. 2012;25(9):946–50. [DOI] [PubMed] [Google Scholar]
- 37.Tan CO, Taylor JA. Integrative physiological and computational approaches to understand autonomic control of cerebral autoregulation. Exp Physiol. 2014;99(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones SC, Radinsky CR, Furlan AJ, Chyatte D, Qu Y, Easley KA, et al. Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected]. Anesthesiology. 2002;97(2):488–96. [DOI] [PubMed] [Google Scholar]
- 40.Kharlamov A, Brown BR, Easley KA, Jones SC. Heterogeneous response of cerebral blood flow to hypotension demonstrated by laser speckle imaging flowmetry in rats. Neurosci Lett. 2004;368(2):151–6. [DOI] [PubMed] [Google Scholar]
- 41.Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of Cerebral Autoregulation Patterns with Near-infrared Spectroscopy during Pharmacological-induced Pressure Changes. Anesthesiology. 2015;123(2):327–35. [DOI] [PubMed] [Google Scholar]
- 42.Sanders RD, Degos V, Young WL. Cerebral perfusion under pressure: is the autoregulatory ‘plateau’ a level playing field for all? Anaesthesia. 2011;66(11):968–72. [DOI] [PubMed] [Google Scholar]
- 43.Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015;123(5):1198–208. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa Y, Iwasaki K, Aoki K, Shibata S, Kato J, Ogawa S. Central hypervolemia with hemodilution impairs dynamic cerebral autoregulation. Anesth Analg. 2007;105(5):1389–96, table of contents. [DOI] [PubMed] [Google Scholar]
- 45.Boas DA, Sakadzic S, Selb J, Farzam P, Franceschini MA, Carp SA. Establishing the diffuse correlation spectroscopy signal relationship with blood flow. Neurophotonics. 2016;3(3):031412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker WB, Parthasarathy AB, Gannon KP, Kavuri VC, Busch DR, Abramson K, et al. Noninvasive optical monitoring of critical closing pressure and arteriole compliance in human subjects. J Cereb Blood Flow Metab. 2017;37(8):2691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott JT, Diop M, Morrison LB, d’Esterre CD, Lee TY, St Lawrence K. Quantifying cerebral blood flow in an adult pig ischemia model by a depth-resolved dynamic contrast-enhanced optical method. Neuroimage. 2014;94:303–11. [DOI] [PubMed] [Google Scholar]
- 48.Diop M, Verdecchia K, Lee TY, St Lawrence K. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed Opt Express. 2011;2(7):2068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


