STRUCTURED ABSTRACT
BACKGROUND:
Dermal regenerative matrices (DRMs) have been used for several decades in the treatment of acute and reconstructive burn injury. The objective of this study was to perform a systematic review of the literature to assess the clinical outcomes and safety profile of DRMs in full-thickness burn injury.
METHODS:
Comprehensive searches of MEDLINE, EMBASE, CINAHL, and Cochrane Library were performed from 1988–2017. Two independent reviewers completed preliminary and full-text screening of all articles. English-language articles reporting on DRM use in patients with full-thickness burn injury were included.
RESULTS:
Literature search generated 914 unique articles. Following screening, 203 articles were assessed for eligibility and 72 met inclusion criteria for analysis. DRM was applied to 1084 patients (74% acute burns, 26% burn reconstruction). Of the twelve studies that described changes in ROM, significant improvement was observed in 95% of reconstructive patients. The most frequently treated reconstructive sites were the neck, hand/wrist, lower extremity, and axilla. Vancouver scar scale was used in eight studies and indicated a significant improvement of the scar quality with DRM. The overall complication rate was 13%, most commonly infection, graft loss, hematoma formation, and contracture.
CONCLUSIONS:
While variability in functional and cosmetic outcomes was observed, DRM demonstrates improvements in ROM and scar appearance without objective regression. Essential demographic data was lacking in many studies, highlighting the need for future standardization of reporting outcomes in burns following application of dermal substitutes.
Keywords: Burn, burn injury, thermal injury, artificial skin, skin substitute
INTRODUCTION
Burn injuries represent one of the top causes of injury-related death, and its incidence varies worldwide.1 Significant advancements in burn care made over the past decades have led to improved patient survival and recovery, shifting the primary goal of management of severe burns from mere survival towards improving the “quality” of patient survival.2,3 Quality of life largely depends on how patients re-integrate into society, the scar quality and its appearance, and the perception of their own appearance. Autologous split thickness skin graft (STSG) is the current gold standard for the treatment of deep dermal and full thickness burn wounds2,4, however, there are numerous challenges associated with STSGs, including limited donor site availability, donor site morbidity, graft contracture, and an unpredictable or sometimes poor scarring process.5,6 In addition to human allograft, epidermal and/or dermal biologic, and synthetic skin substitutes have emerged in the last few decades.5 Dermal regenerative matrices (DRMs) are permanent skin substitutes used in the management of skin defect left after excision of burn wounds or release burn wound contractures.
Developed in 1981 by Burke et al.7, DRM (Integra, LifeSciences, Plainsboro, NJ, United States) is a bilayer dermal regeneration template composed of distinct dermal- and epidermal-like components. The dermal analog consists of cross-linked bovine collagen and shark chondroitin-6-sulphate, while the epidermal analog is composed of a thin silicone elastomer. After 2–3 weeks, a neodermis is formed, the temporary silicone layer is removed and replaced with a thin epidermal autograft.7,8 DRM is approved for use in acute burn surgery and burn reconstruction9 and has been shown to produce excellent functional and aesthetic results for both indications.6,10–14
In addition to its many benefits, DRM has also been cited to have several disadvantages, including the need for a two-stage procedure15–17, increased infection risk15,17, and high cost.14,17 Although the literature is abundant with studies related to DRM use, the majority lack power of the sample and are mainly case series. The aim of this study was to perform a systematic literature review of DRM use and its outcomes related to functional improvement and scar appearance, when used in acute burn surgery and burn reconstruction for patients with full-thickness burn injury. A review of the safety and efficacy will also be performed.
METHODS
Literature search and study selection
A systematic search of the literature was completed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with PROSPERO (CRD42018080191). Comprehensive searches of MEDLINE, EMBASE, CINAHL, and The Cochrane Library was performed for the period 1988–2017 inclusive. Medical Subject Headings (MeSH) were used where appropriate. The search strategies for all databases are included in Appendix 1. English-language, primary full-text articles describing human subjects with full-thickness burn injury treated with DRM were included for analysis. Following the literature search, duplicate citations were removed and preliminary screening (titles and abstracts) was performed to eliminate studies that did not meet the aforementioned eligibility criteria. Full-text articles were then obtained for all remaining studies, all of which were read in their entirety to identify relevant studies. Only articles that met all inclusion criteria were included for analysis. For the purpose of this systematic review, studies with insufficient information to extract data, review articles or animal studies, and for non-burn related indications were excluded. Two independent reviewers completed preliminary and full-text screening of all articles.
Data extraction and analysis
Extracted data included study characteristics (design, year of publication, authors affiliation), patient factors (demographics, extent of DRM reconstruction (%total body surface area (TBSA)), body regions affected, mechanism of burn injury), surgical factors (acute vs. reconstructive indications, application technique), and clinical outcomes (scar appearance and cosmesis, biomechanics, functional range of motion, complications, mortality). We defined the acute indications when DRM was used to resurface excised burn wounds during the initial admission after the thermal injury. Reconstructive burn surgery addresses functional and/or aesthetic problems (e.g. burn contractures, hypertrophic or keloid scars) that arise after all the burn wounds were treated and healed.8
RESULTS
Study selection and characteristics
The systematic literature search generated 2,205 articles, and an additional six were identified through article reference lists (Figure 1). After removal of duplicates, 914 unique articles remained for review. Following preliminary screening, 203 full-text articles were reviewed and 72 met inclusion criteria for data extraction and analysis.2–6,8,10–75 Included studies consisted of four randomized controlled trials (Level 1), four comparative studies (Level 2), five cohort studies (Level 3), two case-control studies (Level 3), 24 case series (Level 4), and 33 case reports (Level 5). Most of the studies were from Europe and North America (Table 1).
FIGURE 1.
Flow diagram depicting the screening of articles for inclusion for qualitative analysis, reported in accordance with the Preferred Reporting Systems for Systematic Reviews and Meta-Analysis (PRISMA) statement.
Table 1.
Authors’ affiliation
| Continent | No. of studies | No. of acute cases | No. of reconstructive cases |
|---|---|---|---|
| Europe | 37 | 107 | 216 |
| North America | 21 | 665 | 17 |
| Asia | 9 | 14 | 36 |
| Australia/Oceania | 4 | 7 | 8 |
| South America | 1 | 7 | 7 |
Patient demographics
DRM was applied to 1084 patients with full-thickness burn injuries, mainly for resurfacing after debridement of acute burns (800 patients, 74%) and 284 for burn reconstruction (26.%). From cumulative data, there was a wide age range (7 months to 93 years), with 333 adult patients (≥18 years) and 179 pediatric patients (<18 years). Age was unreported for the majority of patients (53%). Patient gender was available in only 56% of the cases (383 males and 221 females). The mean follow-up period for patients treated for acute burn injury and burn reconstruction was 21.8 and 21.1 months, respectively.
Sites of DRM Use
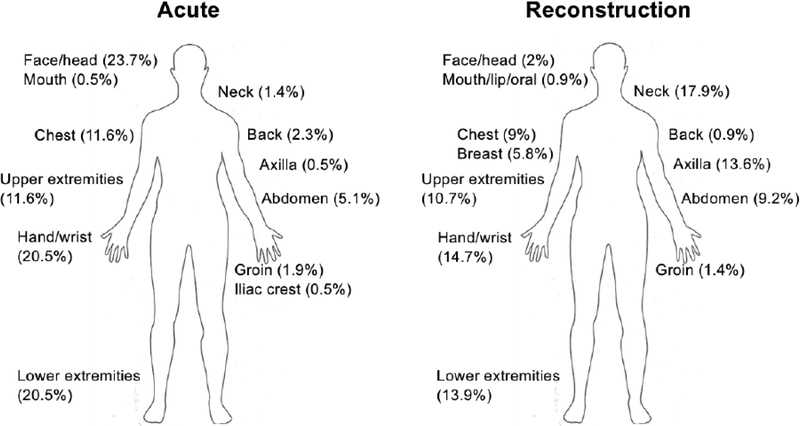
DRM was applied to a total of 1171 sites, covering a wide variety of body regions (Figure 2). The data lacks accuracy in reporting what body regions were resurfaced, with only 48% of sites specified (215 sites for acute, 346 for burn reconstruction). The most commonly treated body parts for acute burn injury were: face/head (24%), hand/wrist (21%), and lower extremities (21%). For burn reconstruction, the most commonly treated areas were: neck (18%), hand/wrist (15%), lower extremities (14%), and axilla (14%).
FIGURE 2.
Anatomic distribution of DRM application for both acute and reconstructive indications; reported for 561 treated sites (48% of all DRM-treated sites).
It is important to assess how much of the DRM reaches readiness for the skin grafting. The average DRM take for acute and reconstructive burn injuries was 86% (range 0–100%) and 95% (range 0–100%), respectively. STSG was applied over the DRM an average after 24.2 days (range 0–80 days) with an 90% take for acute burns, and 20.2 days (range 7–110 days) with 93% take for burn reconstruction. Cultured epidermal and keratinocyte autografts (CEA) were applied over DRM after 21 days, on average, (range 16–24 days). CEA was used alone for five patients31,68,71, and was combined with a STSG for one patient45.
Function and range of movement related outcomes
There was a wide range of reported outcomes related to function and range of motion (ROM). The improvements following DRM application were assessed with objective (four studies) or subjective methods (eight studies).
Objective outcomes related to function and ROM was reported in four studies for 42 patients, all of whom were treated with DRM for burn reconstruction.8,11,18,19 Post-operative improvements were seen in 40 of the 42 patients (95%) (Table 2).
Table 2.
Functional Outcome – Range of Motion (Objective) (n=4)
| Article | Body Site | Outcome |
|---|---|---|
| Chou et al., 2001 (n=13) | Elbow | +30°, n=6 |
| Axilla | +65°, n=2 | |
| Wrist | +31°, n=4 | |
| Upper arm | +45°, n=1 | |
| Figus et al., 2007 (n=1) | Axilla | 0°–30° → 0°–120° |
| Antecubital fossa | 120°–160° → 0°–160° | |
| Wrist | −160°– −140° → −45°– +45° | |
| Neck | 90° fixed flexion → 0°–90° | |
| Moieman et al., 2001 (n=20) | Axilla, neck, groin, thigh, knee, foot, chest, back, abdomen, ankle, arm | Patients reported a 72% increase in ROM |
| Young et al., 2004 (n=8) | Wrist | “Excellent” results (75–100% ROM); n=5 |
| Axilla | “Good” results (50–74% ROM); n=1 | |
| Elbow | “Poor” results (no change in ROM); n=2 | |
| Wrist: 2 sites FFD (+15°, +20°) | ||
| Elbow: 2 sites FFD (+55°, +90°) |
FFD=fixed flexion deformity
Subjective outcomes were reported in 111 patients from eight studies.4,20–26 Ten patients were treated for acute burn injury and 101 for burn reconstruction. No improvement or temporary improvement in ROM was reported in 16% of DRM-treated sites.23 Most patients had partial or significant improvements post-operatively, but minor limitations were still present in some (Table 3).
Table 3.
Functional Outcome – Range of Motion (Subjective) (n=8)
| Article | Acute / Reconstruction (n=number of patients) | Outcome |
|---|---|---|
| Cedidi et al., 2002 | Acute (n=1) | Functional ROM was complete and “excellent” |
| Verolino et al., 2008 | Acute (n=1) | Normal mobility with right hand, good thumb opposition |
| Active ROM was normal 2 years post-op | ||
| Active flexion of the IP joints was limited (5–15°) | ||
| Passive motion quasi normal in flexion and extension for all IP and MP joints | ||
| Pontini et al., 2015 | Acute (n=1) | Satisfactory oral ROM at 12 months |
| Frame et al., 2004 | Reconstruction (n=89) | Post-treatment ROM (rated by physicians): |
| Excellent (46%, 59 sites) | ||
| Good (29%, 37 sites) | ||
| Average (9%, 11 sites) | ||
| Below average (16%, 20 sites) | ||
| Post-treatment ROM (rated by patients): | ||
| 86% satisfied | ||
| Popescu et al., 2007 | Reconstruction (n=1) | Extension of neck was significantly improved |
| Mouth opening was almost normal | ||
| Lynch et al., 2008 | Reconstruction (n=1) | Contracture release improved the patient’s neck ROM |
| Park et al., 2009 | Reconstruction (n=3) | Significant improvement in shoulder abduction and extension |
| Improved ROM | ||
| Good ROM at ankle | ||
| Cuadra et al., 2012 | Acute (n=7), Reconstruction (n=7) |
Range of articular motion was complete in 15/17 cases (88%) |
| Moderate motility limitation in both active and passive movements of 5th digit; n=1 | ||
| Limited flexion of fingers; n=1 |
Scar related outcomes
Three studies investigated the reconstructed tissue quality.28,31,72 The average gross elasticity, elastic function, biological elasticity, and viscoelasticity among 21 DRM-treated sites were 0.40, 0.61, 0.82, and 0.69, respectively. No statistical differences in gross elasticity, elastic function, or biological elasticity was found between DRM-treated sites and healthy, unburned skin.28,72 One study reported reduced elastic stretch and greater viscous stretch with meshed autograft on DRM, with no difference in total stretch. These differences were not significant when cultured skin substitutes were applied over DRM.31
Vancouver scar scale (VSS) is a standardized instrument used to evaluate subjective parameters (burn scars) in an objective way, where a high score correlates with a worse scar (minimum score: 0 – maximum score: 13). The resultant scores are observer-dependent; thus, VSS is most valuable in identifying changes within an individual versus between individuals. Slight alterations in the scoring system exist; for example, additional options in the height and pigmentation categories, generating maximum possible scores of 14 and 15. These scar scales, among several other variations, are termed modified VSS.76 Nine studies used scar scales to evaluate scar appearance in a total of 125 patients who had DRM applied for acute (69 patients) or reconstructive indications (56 patients).2,6,8,10,18,27–30 The VSS was used in eight studies (original VSS in one, modified VSS in five, unknown for two), and the remaining study used the Hamilton-burn scar score. Post-operative improvements in VSS score was seen in all reconstructive patients,8,18,29,30 and ten acute patients (at 12 months vs. 3 months post-operatively).2 For the remaining acute burn injury patients, two studies provided only one mean post-operative VSS score (2.0828 and 3.1030) without earlier or later values for comparison. Only one study revealed a non-significant increase of the VSS score for the scars after DRM application, when compared with control sites (6 vs. 4).27 The mean post-operative VSS score was higher in the reconstruction versus acute burn injury group (4.7 vs. 2.3). The mean post-operative VSS score in control patients treated for acute burn injury was 2.9. No control subjects were available for comparison of postoperative VSS scores following burn reconstruction. (Table 4).
Table 4.
Scar Cosmesis (Scar Scales) (n=9)
| Article | Acute vs. Reconstruction | Outcome |
|---|---|---|
| (n=number of patients) | ||
| Anderson et al., 2011 | Acute (n=5 (DRM); n=8 (control)) | Median VSS DRM: 6 |
| Median VSS control (CEA +STSG): 4 | ||
| *Not a significant difference | ||
| Branski et al., 2007 | Acute (n= 10) | Mean Hamilton-burn scar score: |
| DRM: 4.2 | ||
| Autograft-allograft (control): 6.6 | ||
| Danin et al., 2012 | Acute (n=22) | Mean VSS: 2.08 |
| Lagus et al., 2013 | Acute (n= 10) | Mean ΔVSS: |
| DRM: 2.5 → 1.3 | ||
| STSG: 2.5 → 0.8 | ||
| Cellonex: 2.9 → 0.8 | ||
| Zajicek et al., 2017 | Acute (n=11) | Mean VSS score: |
| DRM: 1.4 | ||
| DE graft (control): 4 | ||
| Chou et al., 2001 | Reconstruction (n=13) | Mean ΔVSS: 8.7 → 2.5 |
| Moiemen et al., 2001 | Reconstruction (n=20) | Mean ΔVSS: 13.3 → 9 |
| Palao et al., 2003 | Reconstruction (n=12) | Mean ΔVSS: 8.1 → 2.5 |
| Dantzer et al., 2003 | Acute (n= 11), | Acute: Mean VSS of 3.1 |
| Reconstruction (n=11) | Reconstructive: Median ΔVSS from 10 → 2 |
Assessment of scar appearance and cosmesis was measured subjectively in thirty-six studies.2,4,8,10–13,15,20,21,23–25,29–51 All studies reported good or improved aesthetic outcomes in most patients. Suboptimal cosmetic outcomes were found for a subset of patients, including poor aesthetic result (n=4),10,15,37,40 pruritis, dryness, and hyper- or hypo-pigmentation (n=3).8,29,42 Less desirable cosmetic results were observed in 4.7% of patients (22 patients)2,43 when compared to controls, however these differences were not significant in ten patients. The incidence of “poor aesthetic outcome” was 2.2% (10 patients).10,15,37,40 Self-reported patient assessments were largely positive for both acute10,12,21,30,33 and reconstructive8,23–25,29,30,42,48 burn injuries. In one study of 106 acute burn surgery patients, 26% preferred the appearance of the DRM-treated sites, 64% found no difference, and only 10% preferred the control site (meshed autograft).12 A Visual Analogue Scale (VAS), where patients were asked to rate their overall satisfaction on a scale of 1–10, was used in two reconstructive studies8,29, with an average VAS score of 8 (range 6–9) reported in one study29. For the other study, the VAS revealed a 59% improvement in appearance compared to pre-operative states.8 The colour match of DRM-treated sites was found to be comparable to normal skin for acute burn injury2,28,38,43,50, except for one study which revealed pronounced hypopigmentation and small regions of hyperpigmentation in one patient31. For burn reconstruction, two patients developed a sustained colour mismatch.40,42 Palao et al.29 reported hyperpigmentation amongst all patients; however this improved in all patients at long-term follow-up (12–18 months). All other studies revealed comparable pigmentation between DRM-treated sites and healthy skin.8,24,40,42 Additionally, the majority of DRM-treated sites (>280) showed good or excellent texture match to autograft-treated sites, with the final skin being soft, supple, and pliable.4,8,11–13,20,23,24,29,31,33,39–42,46,50 A subset of 22 acute burn patients developed thicker skin on DRM-treated hands compared to healthy, non-burned hands (1.6 mm vs 1.18 mm), however this was not a statistically significant difference.28 Loss et al.45 reported subjectively thicker skin for one patient treated for acute burn injury (compared to non-burned skin). Two other studies reported inferior post-operative tissue quality at DRM- versus autograft-treated sites2,43, with one article reporting no statistical difference2.
Complications
Complications associated with DRM were reported in 56% of studies (n=40) for 144 patients out of the total 1084 patients (13%). The most frequent complication was infection (n=27), reported in fifty patients (4.6%) – 32 for acute burn injury, 17 for burn reconstruction, one unknown. Five of the twenty-seven studies did not report an exact number of patients that developed an infection; however, infection rates were reported in three of these studies, at 10%,28 17%,52 and 20%23. The remaining two studies had either an unknown number,53 or a maximum of two patients develop infection54. On average, infection was reported to occur on post-burn day 22.4 (range 7–42). The organisms responsible were most commonly Staphylococcus aureus (including MRSA, ORSA) and Pseudomonas aeruginosa, and less frequently Staphylococcus epidermidis, Enterococcus, Aspergillus, Candida, Acinetobacter, Bacillus coliforms, Enterobacter, Klebsiella pneumoniae, and Serratia marcescens. Partial or total DRM, CEA, and STSG loss occurred in 22, one, and 11 cases, respectively. Other complications observed included: hematoma formation (n=11), contracture or re-contracture (n=10), seroma formation (n=5), and hypertrophic scar (n=5). Ninety-two patients developed a complication other than infection (8.5%), but the information was scarce and insufficient for reporting.
Ten studies reported mortality data,3,5,6,12,52,54–58 however only nine provided the specific number of deaths for DRM-treated patients. Death occurred for 89 patients treated for acute burn injury and one patient treated for burn reconstruction. The overall mean mortality rate seen amongst the nine studies was 30% (range 3.4%–100%). The cause of death was revealed for a subset of patients (15 patients) and included etiologies related to the severity of burn injury, such as multiresistant infection and sepsis5,6,56,58 (13 patients), inhalational injury5 (one patient), and multiple organ failure55 (one patient).Toxic shock syndrome was the cause of death for one patient.58
Safety of DRM Use
Forty-nine studies commented on safety of DRM. The majority of authors agreed that DRM is safe to use on full-thickness burn injuries where insufficient donor skin is available, both in the acute and reconstructive settings.4,6,8,10,12,14,15,17,18,21,24,29–32,34–38,42–44,47,48,51,52,59,60 Authors also frequently commented that in acute burn surgery, DRM with autograft is capable of achieving functionally and aesthetically similar results to that of autograft alone12,31 or unburned skin2,4. Three studies preferred DRM versus traditional options due to improved functional and cosmetic outcomes.10,13,14 These statements were applicable to a variety of body sites10,21–23,28–30,32,36,43,44,48,51,61 and age groups3,6,14,23,37,42,47,62.
For acute burn injury, two of the articles presented instances where application of DRM may be not be indicated, such as patients with multiple comorbidities where anesthesia places them at higher risk for a two-stage procedure,16 and eyelid burns43. When DRM is used for burn reconstruction, compliance with the treatment and adherence to frequent follow-ups is mandatory in order to achieve good results11,15. Additionally, several studies indicated that surgeon experience plays a role in the successful application of DRM.5,6,23,33
DRM Application – Tips & Tricks
Thirty-five percent of articles (n=25) made recommendations surrounding DRM application for improved effectiveness. There was variability in surgeon preference with respect to the use of meshed13,25,26,40,42,43 vs. un-meshed DRM37. Several studies reinforced the importance of adequate immobilization of DRM on the recipient wound bed to ensure successful take.15,30,35,40,47,51–53,62 Methods recommended include: tie-over dressings (especially when applied over joints)15, extensive hemostasis30, VAC dressings,25,26,35,47,52,53,62 an outer Biobrane dressing,51 and running sutures, single stiches, metal clips, K-wires, or conformed splints47. The importance of continuous wound care was also emphasized.8,24,26,42,43 With respect to infection control, some authors recommended antiseptic compresses56 and/or antimicrobial therapy55,56. Variability in the time to second stage procedure was observed. Two studies suggested a 2 week interval between DRM and epidermal grafting procedures, in order to reduce the risk of future hypertrophic scarring.30,40 In contrast, two other studies recommended delaying the second stage procedure until four weeks after initial DRM placement to ensure adequate vascularization.8,29 The thickness of the STSG varied between 0.05mm and 0.25mm, with a mean of 0.16mm.
DISCUSSION
DRM is one of several artificial skin substitutes available for wound coverage with indications in acute burn surgery and post-burn reconstruction. The body of literature currently available on DRM use in burn injury is variable with respect to study design, sample size, and reported outcomes. Age and sex was not reported for almost half of the total number of patients included for analysis. The outcomes across studies are not standardised, and subjective measurements are more frequently described than objective ones, making it challenging to quantify post-operative improvement.
Amongst the 72 studies included for analysis, DRM was applied successfully and safely to a variety of body sites in both acute and reconstructive settings. The literature review supports the clinical use of DRM as a valid alternative in the burn reconstruction patient population, both for its functional benefits, as well as its apparent reduction of recontracture rates compared to available alternatives.
Of the twelve studies that commented on post-operative changes in ROM, only a minority of patients (<16%) were found to have suboptimal functional outcomes and none experienced loss of function. An important consideration that may limit postoperative improvement in ROM is the severity of the initial injury. Given that DRM is recommended in instances where autograft is scarce, often with full-thickness burns covering >40% TBSA,6 the preoperative functional status of this patient population is likely to be quite limited. Thus, careful evaluation of a patient’s preoperative morbidity is paramount when drawing post-operative conclusions.
With successful application and take, DRM can produce excellent cosmetic results.12,21,30 The forty-five studies that commented on aesthetic outcomes indicate positive and encouraging results in acute and reconstruction burn patients. With respect to colour match, an important consideration is that the final result is more dependent on the epidermal skin graft than the DRM, therefore the area of the donor site was important. Other factors, such as time between first- and second-stage procedures may also play a role in affecting post-operative colouration. It has been proposed that shortening the time to autografting from 14 days to 7–10 days in regions of high vascularity (e.g. the face) may reduce complications of hyperpigmentation, due to faster neodermis formation.43
Invasive infection is the primary cause of death in the acute period following a burn injury, accounting for about 51% of mortality.77 It is unknown whether the risk of infection with DRM is related mainly to its intrinsic properties, or as a consequence of the patient population that it is applied to (severe, full-thickness burns). While both factors likely contribute, the severity of initial injury no doubt has an impact on a patient’s risk of developing an infection. In fact, one study included in our analysis was terminated early due to the high incidence of infection associated with DRM in patients with burns covering more than 45% TBSA.5 Based on these results, Pham et al.78 subsequently suggested that DRM may be better suited for patients with less extensive burn injuries. In contrast, numerous other studies included for analysis had amean %TBSA >45% without reporting infection as a complication. Thus, future work towards identifying specific patients at risk of developing infection with DRM is needed to help guide surgeon clinical decision making.
The overall mortality observed amongst included studies was 8.3%, with a wide range of reported mortality rates amongst individual studies. Factors cited to have played a potential role in the death of patients were: higher (than usual) multiresistant infection rates6, relative surgeon inexperience in handling DRM5,6, burn immunosuppression5, and surgical site infections due to seeding from distant sites5. Although unlikely, there exists the potential for patients to develop toxic shock syndrome following DRM application – a complication that carries a high mortality risk amongst burn patients.58 Thus, burn care specialists must exercise caution when using DRMs, especially in non-life threatening situations. Strict adherence to infection control measures and prophylactic nasal Mupirocin or Chlorhexidine to reduce staphylococcal nasal carriage has been suggested to further reduce the likelihood of this outcome.58 In addition, increasing surgeon experience with DRM is paramount in improving take and reducing infection rates, ultimately leading to reduced mortality amongst burn patients. In a retrospective review of 1665 acute burn injury patients, Ryan et al.79 identified three risk factors associated with mortality: 1) age >60 years, 2) burn injury >40% TBSA, and 3) inhalational injury. In a separate study by Ryan et al.,57 equivalent mortality rates were noted amongst the control and DRM groups (30%), despite the DRM-treated patients having more baseline risk factors for death (significantly higher incidence of inhalational injury and %TBSA burned).
Despite some inconsistency in preferred application techniques, several common themes were identified. Authors unanimously preferred the use of thin autograft during the second stage procedure for improved cosmesis, and emphasized the importance of adequate immobilization of DRM to the wound bed to improve take. Various methods for immobilization were reported, with a heavy emphasis on the use of VAC techniques. Of course, surgeon experience with DRM has a significant impact on patient outcome. To facilitate this, Malic et al.9 have generated a nationwide protocol on wound healing and DRMs using expertise from several Canadian burn care specialists. Their expert panel has recommended certain minimum training requirements for surgeons without previous experience handling DRMs. A detailed protocol was also developed for reference, via nationwide specialist agreement. By applying the techniques recommended by those experienced with handling DRM in burn injury, one can expect substantial reductions in complication rates in the future.
Limitations and future directions
There are several limitations to this review. First, the quality of studies included for qualitative analysis was variable, with the majority being case series and case reports (79%). Consequently, this impacts the power of our conclusions. Second, there was inconsistency in reporting of demographic and outcome data amongst included studies, which complicated interpretation of the data and limited our ability to perform a meta-analysis. Amongst the four randomized controlled trials (RCTs), only two reported on objective measures of aesthetic outcome2,6 and only one commented subjectively12. None commented on functional outcome. Clearly, there is a need for future RCTs that document post-operative functional and cosmetic outcomes to better understand the role of DRM in full-thickness burn injuries. Third, the geographic distribution of articles included in this study was heavily skewed towards developed regions in North America and Europe (80.5%). This is likely attributable to its high cost and our analysis being restricted to include only English-language articles. Thus, this article is limited by pre-exclusion of data from other countries whose outcomes associated with DRM are reported in other languages. Lastly, none of the included articles conducted a cost-benefit analysis of DRM. This analysis is important to investigate moving forward, given that a major limitation of DRM use is its significant cost – both the material itself, and the lengthy hospital stay that is often required following application. Such analyses would provide burn specialists with the concrete data necessary to make more informed treatment decisions when allocating their healthcare system’s resources.
CONCLUSION
Since its introduction in 1981,7 DRM has been successfully used for a variety of applications, mainly burn injury. The objective of this work was to perform a systematic review of the literature to assess the clinical efficacy and safety of DRM use in patients with full-thickness burn injury. This review demonstrated improved functional and aesthetic outcomes amongst the majority of patients treated for acute burn injury as well as post-burn reconstruction. Both minor and major complications were observed across included studies with low incidence. Infection was the most commonly cited complication. We believe that the benefits of DRM from a survival, functional, and cosmetic point of view outweigh the risks of the aforementioned potential complications. In concordance with previous reports, DRM can be safely applied both in the acute and reconstructive phases of burn injury, at a variety of anatomic regions. A focus should be placed on improving certain, modifiable factors – namely surgical expertise in handling DRM, and post-operative patient compliance – in order to see further advancements in outcomes. Future, high level evidence studies with objective functional and cosmetic outcomes of DRM in burn injury are required, in order to improve our understanding of expected patient outcomes and provide patients with tangible, realistic goals for recovery.
APPENDICES
APPENDIX 1.
Systematic search strategies of databases
| MEDLINE | EMBASE | CINAHL | The Cochrane Library |
|---|---|---|---|
| 1. exp Burns/ | 1. burn/ | ((MH “Burns+”) OR | 1. burn.mp. |
| 2. (thermal adj3 injur*).tw. | 2. (thermal adj3 injur*).tw. | 2. integra.mp. | |
| (burn OR burns OR burned OR scald*)) | 3. artificial skin.mp. | ||
| 3. (burn or burns or burned or scald*).tw. | 3. (burn or burns or burned or scald*).tw. | AND ((MH “Skin, Artificial”) OR (integra) | 4. skin substitute.mp. |
| OR (skin substitute) OR (artificial skin)) | 5. 2 or 3 or 4 | ||
| 4. 1 or 2 or 3 | 4. thermal injury/ | 6. 1 and 5 | |
| 5. Skin, Artificial/ | 5. 1 or 2 or 3 or 4 | ||
| 6. (artificial* adj3 skin).tw. | 6. artificial skin/ | ||
| 7. (artificial* adj3 skin).tw. | |||
| (substitute adj3 skin).tw. | |||
| 8. (substitute adj3 skin).tw. | |||
| 8. integra.tw. | |||
| 9. 5 or 6 or 7 or 8 | 9. integra.tw. | ||
| 10. 4 and 9 | 10. 6 or 7 or 8 or 9 | ||
| 11. 5 and 10 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: An abstract of this work was accepted for poster presentation at the American Burn Association (ABA) Annual Meeting, April 2–5, 2019; Las Vegas, Nevada.
CONFLICT OF INTEREST STATEMENT
The authors have no personal, commercial, or financial conflicts of interest to disclose. None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. This study was supported by the National Institutes of Health R01-GM087285–01. CFI Leader’s Opportunity Fund: Project #25407 and Canadian Institutes of Health Research (CIHR) grant #123336. Government of Canada Summer Jobs Grant: ROMEO# 20180041. The aforementioned sponsors had no involvement in this study. Some of the Canadian guidelines for Integra usage arose from an Integra-Sponsored Symposium, the Canadian Burn Symposium (Winnipeg, September 2017). Integra Life Sciences had no influence on the outcome of the study.
Contributor Information
Marc Jeschke, Division of Plastic and Reconstructive Surgery, Department of Surgery, University of Toronto, Faculty of Medicine, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue., Room, D704; M4N3M5, Toronto, ON, Canada.
Claudia Malic, Division of Plastic and Reconstructive Surgery, Department of Surgery; University of Ottawa, Faculty of Medicine, Children’s Hospital of Eastern Ontario, 401 Smyth Road; K1H 8L1, Ottawa, ON, Canada.
REFERENCES
- 1.“Burns.” World Health Organization, World Health Organization, 6 March 2018, www.who.int/en/news-room/fact-sheets/detail/burns. [Google Scholar]
- 2.Lagus H, Sarlomo-Rikala M, Bohling T, Vuola J. Prospective study on burns treated with Integra®, a cellulose sponge and split thickness skin graft: comparative clinical and histological study – randomized controlled trial. Burns. 2013. December;39(8):1577–87. [DOI] [PubMed] [Google Scholar]
- 3.Tompkins RG, Hilton JF, Burke JF, et al. Increased survival after massive thermal injuries in adults: preliminary report using artificial skin. Crit Care Med. 1989. August;17(8):734–40. [DOI] [PubMed] [Google Scholar]
- 4.Cuadra A, Correa G, Roa R, et al. Functional results of burned hands treated with Integra®. J Plast Reconstr Aesthet Surg. 2012. February;65(2):228–34. [DOI] [PubMed] [Google Scholar]
- 5.Peck MD, Kessler M, Meyer AA, Bonham Morris PA. A Trial of the Effectiveness of Artificial Dermis in the Treatment of Patients with Burns Greater Than 45% Total Body Surface Area. J Trauma. 2002;52(5):971–8. [DOI] [PubMed] [Google Scholar]
- 6.Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Integra in primary burn management: A randomized pediatric clinical trial. Crit Care Med. 2007;35(11):2615–23. [DOI] [PubMed] [Google Scholar]
- 7.Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK. Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury. Ann Surg. 1981. October;194(4):413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moiemen NS, Staiano JJ, Ojeh NO, Thyway Y, Frame JD. Reconstructive surgery with a dermal regeneration template: clinical and histologic study. Plast Reconstr Surg. 2001. July;108(1):93–103. [DOI] [PubMed] [Google Scholar]
- 9.Malic C, Logsetty S, Papp A, et al. The development of a treatment pathway for dermal regenerative matrix (DRM). Burns. 2018. [DOI] [PubMed] [Google Scholar]
- 10.Zajicek R, Grossova I, Suca H, Kubok R, Pafcuga I. Experience with Integra® at the Prague Burns Centre 2002–2016. Acta Chir Plast. 2017. Summer;59(1):18–26. [PubMed] [Google Scholar]
- 11.Young RC, Burd A. Paediatric upper limb contracture release following burn injury. Burns. 2004. November;30(7):723–8. [DOI] [PubMed] [Google Scholar]
- 12.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi- center randomized clinical trial. Ann Surg. 1988. September;208(3):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshman S, King SR, Wallace RD. Case report: The use of dermal substitute in the reconstruction of full-thickness burns to the penis. Wounds. 2005. June;17(6):153–6. [Google Scholar]
- 14.Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008. April;121(4):1256–62. [DOI] [PubMed] [Google Scholar]
- 15.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg. 2001. December;54(8):659–64. [DOI] [PubMed] [Google Scholar]
- 16.Jeng JC, Fidler PE, Sokolich JC, et al. Seven years’ experience with Integra as a reconstructive tool. J Burn Care Res. 2007. Jan-Feb;28(1):120–6. [DOI] [PubMed] [Google Scholar]
- 17.Lohana P, Hassan S, Watson SB. Integra™ in burns reconstruction: our experience and report of an unusual immunological reaction. Ann Burns Fire Disasters. 2014. March 31;27(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Chou TD, Chen SL, Lee TW, et al. Reconstruction of burn scar of the upper extremities with artificial skin. Plast Reconstr Surg. 2001. August;108(2):378–84: discussion 385. [DOI] [PubMed] [Google Scholar]
- 19.Figus A, Leon-Villapalos J, Philip B, Dziewulski P. Severe multiple extensive postburn contractures: a simultaneous approach with total scar tissue excision and resurfacing with dermal regeneration template. J Burn Care Res. 2007. Nov-Dec;28(6):913–7. [DOI] [PubMed] [Google Scholar]
- 20.Cedidi C, Hierner R, Wilkens L, Berger A. The deeply burned upper extremity: functional graft distribution concept with selective use of a synthetic dermal substitute (Integra) and split-thickness skin grafts. Eur J Plast Surg. 2002. September;25(4):226–30. [Google Scholar]
- 21.Verolino P, Vasoli V, Masia D, Delia G, Isacu C, Castede JC. A skin substitute (Integra) in a successful delayed reconstruction of a severe injured hand. Burns. 2008. March;34(2):284–7. [DOI] [PubMed] [Google Scholar]
- 22.Pontini A, Reho F, Giatsidis G, Bacci C, Azzena B, Tiengo C. Multidisciplinary care in severe pediatric electric oral burn. Burns. 2015. May; 41(3):e41–6. [DOI] [PubMed] [Google Scholar]
- 23.Frame JD, Still J Lakhel-LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg. 2004. April 15;113(5):1330–8. [DOI] [PubMed] [Google Scholar]
- 24.Popescu S, Ghetu N, Grosu O, Nastasa M, Pieptu D. Integra – a therapeutic alternative in reconstructive surgery. Our first experience. Chirurgia (Bucur). 2007. Mar-Apr;102(2):197–204. [PubMed] [Google Scholar]
- 25.Lynch JB, Ismael TS, Kelly JL. Release of anterior neck burn contracture using artificial dermis and vacuum-assisted closure. Plast Reconstr Surg. 2008. January;121(1):352–3. [DOI] [PubMed] [Google Scholar]
- 26.Park CA, Defranzo AJ, Marks MW, Molnar JA. Outpatient reconstruction using integra* and subatmospheric pressure. Ann Plast Surg. 2009. February;62(2):164–9. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JR, Fear MW, Phillips JK, et al. A preliminary investigation of the reinnervation and return of sensory function in burn patients treated with INTEGRA®. Burns. 2011. November;37(7):1101–8. [DOI] [PubMed] [Google Scholar]
- 28.Danin A, Georgesco G, Touze AL, Penaud A, Quignon R, Zakine G. Assessment of burned hands reconstructed with Integra® by ultrasonography and elastometry. Burns. 2012. November;38(7):998–1004. [DOI] [PubMed] [Google Scholar]
- 29.Palao R, Gomez P, Huguet P. Burned breast reconstructive surgery with Integra dermal regeneration template. Br J Plast Surg. 2003. April;56(3):252–9. [DOI] [PubMed] [Google Scholar]
- 30.Dantzer E, Queruel P, Salinier L, Palmier B, Quinot JF. Dermal regeneration template for deep hand burns: clinical utility for both early grafting and reconstructive surgery. Br J Plast Surg. 2003. December;56(8):764–74. [DOI] [PubMed] [Google Scholar]
- 31.Boyce ST, Kagan RJ, Meyer NA, Yakuboff KP, Warden GD. The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J Burn Care Rehabil. 1999. Nov-Dec;20(6):453–61. [DOI] [PubMed] [Google Scholar]
- 32.Chou TD, Lee WT, Chen SL, Chen TM, Lee CH, Wang HJ. The management of complicated charcoal contact burns involving deep tissues. Burns. 2004. November;30(7):746–50. [DOI] [PubMed] [Google Scholar]
- 33.Fitton AR, Drew P, Dickson WA. The use of a bilaminate artificial skin substitute (Integra) in acute resurfacing of burns: an early experience. Br J Plast Surg. 2001. April;54(3):208–12. [DOI] [PubMed] [Google Scholar]
- 34.Foong DP, Evriviades D, Jeffery SL. Integra™ permits early durable coverage of improvised explosive device (IED) amputation stumps. J Plast Reconstr Aesthet Surg. 2013. December;66(12):1717–24. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez Alana I, Torrero Lopez JV, Martin Playa P, Gabilondo Zubizarreta FJ. Combined used of negative pressure wound therapy and Integra® to treat complex defects in lower extremities after burns. Ann Burns Fire Disasters. 2013. June 30;26(2):90–3. [PMC free article] [PubMed] [Google Scholar]
- 36.Gronovich Y, Maisel Lotan A, Retchkiman M. Post-burn breast reconstruction using an artificial dermis – a long-term follow-up. Burns Trauma. 2016. July 4;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groos N, Guillot M, Zilliox R, Braye FM. Use of an artificial dermis (Integra) for the reconstruction of extensive burn scars in children. About 22 grafts. Eur J Pediatr Surg. 2005. June;15(3):187–92. [DOI] [PubMed] [Google Scholar]
- 38.Hammer D, Rendon JL, Sabino J, Latham K, Fleming ME, Valerio IL. Restoring full-thickness defects with spray skin in conjunction with dermal regeneration template and split-thickness skin grafting: a pilot study. J Tissue Eng Regen Med. 2017. December;11(2):3523–9. [DOI] [PubMed] [Google Scholar]
- 39.Houle JM, Neumeister MW. A prefabricated, tissue-engineered Integra free flap. Plast Reconstr Surg. 2007. October;120(5):1322–5. [DOI] [PubMed] [Google Scholar]
- 40.Hunt JA, Moisidis E, Haertsch P. Initial experience of Integra in the treatment of post-burn anterior cervical neck contracture. Br J Plast Surg. 2000. December;53(8):652–8. [DOI] [PubMed] [Google Scholar]
- 41.Jaskille AD, Shupp JW, Jeng JC, Jordan MH. Use of Integra in the treatment of third degree burns to the penile shaft: a case series with 6-month follow-up. J Burn Care Res. 2009. May-Jun;30(3):524–8. [DOI] [PubMed] [Google Scholar]
- 42.Khan MR, El Faki HMA, Govila A, Rezq A, Aziz EA, Khadar R. Experience with integra in the management of post-burn hypertrophic scars and contractures. Eur J Plast Surg. 2007. November;30(3):101–106. [Google Scholar]
- 43.Klein MB, Engrav LH, Holmes JH, et al. Management of facial burns with a collagen/glycosaminoglycan skin substitute-prospective experience with 12 consecutive patients with large, deep facial burns. Burns. 2005. May;31(3):257–61. [DOI] [PubMed] [Google Scholar]
- 44.Kosutic D, Beasung E, Dempsey M, et al. Single-layer Integra for one-stage reconstruction of scalp defects with exposed bone following full-thickness burn injury: a novel technique. Burns. 2012. February;38(1):143–5. [DOI] [PubMed] [Google Scholar]
- 45.Loss M, Wedler V, Kunzi W, Meuli-Simmen C, Meyer VE. Artificial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns. 2000. November;26(7):644–52. [DOI] [PubMed] [Google Scholar]
- 46.Orgill DP, Straus FH 2nd, Lee RC. The use of collagen-GAG membranes in reconstructive surgery. Ann N Y Acad Sci. 1999. October 30;888:233–48. [DOI] [PubMed] [Google Scholar]
- 47.Stiefel D, Schiestl C, Meuli M. Integra Artificial Skin for burn scar revision in adolescents and children. Burns. 2010. February;36(1):114–20. [DOI] [PubMed] [Google Scholar]
- 48.Tsoutsos D, Stratigos A, Gravvanis A, Zapandioti P, Kakagia D. Burned breast reconstruction by expanded artificial dermal substitute. J Burn Care Res. 2007. May-Jun;28(3):530–2. [DOI] [PubMed] [Google Scholar]
- 49.Tymonova J, Adamkova M, Klosova H, Kadlcik M, Zamencikova I. Our first experience with Integra. Acta Chir Plast. 2005;47(1):5–9. [PubMed] [Google Scholar]
- 50.Wisser D, Steffes J. Skin replacement with a collagen based dermal substitute, autologous keratinocytes and fibroblasts in burn trauma. Burns. 2003. June;29(4);375–80. [DOI] [PubMed] [Google Scholar]
- 51.Yeong EK, Huang HF, Chen YB, Chen MT. The use of artificial dermis for reconstruction of full thickness scalp burn involving the calvaria. Burns. 2006. May;32(3):375–9. [DOI] [PubMed] [Google Scholar]
- 52.Greenhalgh DG, Hinchcliff K, Sen S, Palmieri TL. A ten-year experience with pediatric face grafts. J Burn Care Res. 2013. Sep-Oct;34(5):576–84. [DOI] [PubMed] [Google Scholar]
- 53.Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil. 1990. Jan-Feb;11(1):7–13. [DOI] [PubMed] [Google Scholar]
- 54.Machens HG, Berger AC, Mailaender P. Bioartificial skin. Cells Tissues Organs. 2000;167(2–3):88–94. [DOI] [PubMed] [Google Scholar]
- 55.Muangman P, Deubner H, Honari S, et al. Correlation of clinical outcome of Integra application with microbiologic and pathological biopsies. J Trauma. 2006. November;61(5):1212–7. [DOI] [PubMed] [Google Scholar]
- 56.Papp A, Harma M. A collagen based dermal substitute and the modified Meek technique in extensive burns. Report of three cases. Burns. 2003. March;29(2):167–71. [DOI] [PubMed] [Google Scholar]
- 57.Ryan CM, Schoenfeld DA, Malloy M, Schulz JT 3rd, Sheridan RL, Tompkins RG. Use of Integra artificial skin is associated with decreased length of stay for severely injured adult burn survivors. J Burn Care Rehabil. 2002. Sep-Oct;23(5):311–7. [DOI] [PubMed] [Google Scholar]
- 58.Shirley R, Teare L, Dziewulski P, Frame J, Navsaria H, Myers S. A fatal case of toxic shock syndrome associated with skin substitute. Burns. 2010. September;36(6):e96–8. [DOI] [PubMed] [Google Scholar]
- 59.Yeong EK, Chen SH, Tang YB. The treatment of bone exposure in burns by using artificial dermis. Ann Plast Surg. 2012. December;69(6):607–10. [DOI] [PubMed] [Google Scholar]
- 60.Clayman MA, Clayman SM, Mozingo DW. The use of collagen-glycosaminoglycan copolymer (Integra) for the repair of hypertrophic scars and keloids. J Burn Care Res. 2006. May-Jun;27(3):404–9. [DOI] [PubMed] [Google Scholar]
- 61.Kakagia D, Kyriopoulos E, Zapandioti P, Tsoutsos D. Natural expansion of artificial dermal template by successful full-term pregnancy. J Burn Care Res. 2012. May-Jun;33(3):e166–8. [DOI] [PubMed] [Google Scholar]
- 62.McEwan W, Brown TL, Mills SM, Muller MJ. Suction dressings to secure a dermal substitute. Burns. 2004. May;30(3):259–61. [DOI] [PubMed] [Google Scholar]
- 63.King P Artificial skin reduces nutritional requirements in a severely burned child. Burns. 2000. August;26(5):501–3. [DOI] [PubMed] [Google Scholar]
- 64.Leffler M, Horch RE, Dragu A, Bach AD. The use of the artificial dermis (Integra) in combination with vacuum assisted closure for reconstruction of an extensive burn scar – a case report. J Plast Reconstr Aesthet Surg. 2010. January;63(1):e32–5. [DOI] [PubMed] [Google Scholar]
- 65.Michaeli D, McPherson M. Immunologic study of artificial skin used in the treatment of thermal injuries. J Burn Care Rehabil. 1990. Jan-Feb;11(1):21–6. [DOI] [PubMed] [Google Scholar]
- 66.Moiemen NS, Yarrow J, Kamel D, Kearns D, Mendonca D. Topical negative pressure therapy: does it accelerate neovascularization within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns. 2010. September;36(6):764–8. [DOI] [PubMed] [Google Scholar]
- 67.Moyer HR, Yezhelyev M, Ingram WL. Bilayer dermal matrix for the treatment of painful burn scars. Wounds. 2011. August;23(8):236–42. [PubMed] [Google Scholar]
- 68.Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD. Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue- engineered dermal template (Integra). Plast Reconstr Surg. 2004. March;113(3):978–81. [DOI] [PubMed] [Google Scholar]
- 69.Lorenz C, Petracic A, Hohl HP, Wessel L, Waag KL. Early wound closure and early reconstruction. Experience with a dermal substitute in a child with 60 per cent surface area burn. Burns. 1997. September;23(6):505–8. [DOI] [PubMed] [Google Scholar]
- 70.Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S. Exposed tibial bone after burns: Flap reconstruction versus dermal substitute. Burns. 2016. March;42(2):e31–7. [DOI] [PubMed] [Google Scholar]
- 71.Matsumura H, Gondo M, Imai R, Shibata D, Waanabe K. Chronological histological findings of cultured epidermal autograft over bilayer artificial dermis. Burns. 2013. June;39(4):705–13. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen DQ, Potokar TS, Price P. An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns. 2010. February;36(1):23–8. [DOI] [PubMed] [Google Scholar]
- 73.O’Neill TB, Rawlins J, Rea S, Wood F. Complex chemical burns following a mass casualty chemical plant incident: how optimal planning and organisation can make a difference. Burns. 2012. August;38(5):713–8. [DOI] [PubMed] [Google Scholar]
- 74.Chan ES, Lam PK, Liew CT, Yen RS, Lau JW. The use of composite biodegradable skin graft and artificial skin for burn reconstruction. Plast Reconstr Surg. 2000. February;105(2):807–8. [DOI] [PubMed] [Google Scholar]
- 75.Sasidaran R, Dorai AA, Sulaiman WA, Halim AS. Use of dermal regeneration template (INTEGRA) in reconstructive burn surgery. Med J Malaysia. 2008. July;63 Suppl A:29. [PubMed] [Google Scholar]
- 76.Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010. June 21;10:e43. [PMC free article] [PubMed] [Google Scholar]
- 77.Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in Burns. Surg Infect (Larchmt). 2016. April 1;17(2):250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pham C, Greenwood G, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007. December;33(8):946–57. [DOI] [PubMed] [Google Scholar]
- 79.Ryan CM, Schoenfeld DA, Thorpe WP, Sheridan RL, Cassem EH, Tompkins RG. Objective Estimates of the Probability of Death from Burn Injuries. N Engl J Med. 1998. February 5;338(6):362–6. [DOI] [PubMed] [Google Scholar]