Abstract
Introduction
Curcumin incorporation into polymeric fibers was tested for its antimicrobial properties and potential use in root canal disinfection.
Methods
Curcumin-modified fibers were processed via electrospinning and tested against a 7-day old established Actinomyces naeslundii (An) biofilm. The medicaments tested were as follows: curcumin-modified fibers at 2.5 and 5.0 mg/mL, curcumin-based irrigant at 2.5 and 5.0 mg/mL, saline solution (negative control), and the following positive controls: 2% chlorhexidine, 1% NaOCl, and triple antibiotic paste (TAP, 1 mg/mL). All medicaments, except for the positive controls, were allocated according to the light exposure protocol: photoactivation with an LED every 30 s for 4 min or without photoactivation. After treatment, the medicaments were removed and 1 mL of saline solution was added; the biofilm was scraped from the well and used to prepare a 1:2000 dilution. Spiral plating was done using anaerobic blood agar plates. After 24 h, colony-forming units (CFU/mL, n=11/group) were counted to determine the antimicrobial effects.
Results
Data exhibited significant antimicrobial effects on positive control groups, followed by the curcumin irrigants, and lastly, the photoactivated curcumin-modified fibers. There was a significant reduction of viable bacteria in curcumin-based irrigants, which was greater than the TAP-treated group. Curcumin-free fibers, saline, and the non-photoactivated curcumin-modified fibers did not display antimicrobial activity.
Conclusions
Curcumin seems to be a potential alternative to TAP when controlling infection, but it requires a minimal concentration (2.5 mg/mL) to be effective. Photoactivation of curcumin-based medicaments seems to be essential to obtain greater antibiofilm activity.
Keywords: electrospinning, curcumin, disinfection, endodontics, photodynamic therapy
Introduction
Complete root canal development of immature permanent teeth is dependent on the absence of infection within the root canal system, contributing to proper thickening of dentin walls and root apex closure (1). However, dental caries and/or trauma can lead to pulp necrosis, hampering tooth development. To solve this problem, clinical strategies have been advocated to continue root maturation, including conventional apexification and the recently introduced regenerative-based endodontic procedures. While the former induces the closure of open apices by formation of a hard tissue barrier (2), the latter allows for the replacement of damaged pulp by a non-infected living tissue, favoring physiological apex closure (3).
Regenerative endodontics combines the antimicrobial activity of a triple antibiotic paste (TAP) with the healing effects of an evoked bleeding (EB) procedure (4). Three antibiotics, namely, metronidazole (MET), ciprofloxacin (CIP), and minocycline (MINO) are mixed together to obtain TAP, which is placed inside the infected immature root canal for disinfection, followed by its removal and the EB procedure to induce the ingrowth of undifferentiated stem cells from the periodontal ligament and alveolar bone into the root canal. Although intracanal treatment with a high concentration of TAP may offer effective disinfection, it also poses disadvantages, such as stem cell toxicity (5) and significant tooth discoloration (6), probably due to its acidic nature (7). Alternatively, electrospun fibers containing lower amounts of antibiotics demonstrated pronounced disinfection ability and lower cytotoxicity than traditional TAP (7–9).
Electrospinning is a feasible process for obtaining nanofibers with a well-controlled drug release ability (1, 11). Antibiotics or any other therapeutic compound can be incorporated into polymer solutions, resulting in the fabrication of fibrous-based drug delivery systems (12–16). Recently, Bottino et al. (16) developed a patient-specific three-dimensional (3D) nanofibrous-based drug delivery construct that fits into the root canal of immature teeth; interestingly, this innovative disinfection strategy allowed for the formation of an appropriate environment that led to apex closure and the ingrowth of a thin layer of osteodentin-like tissue using a dog model.
Among natural compounds with recognized antimicrobial and anti-inflammatory characteristics, curcumin is a plant-derived agent (turmeric root), which also exhibits antioxidant and anticancer effects, thus having significant clinical relevance related to the prevention and treatment of numerous illnesses (18). Curcumin has already been used in the fabrication of electrospun fibers for biomedical applications (e.g., skin tissue regeneration) (19–22), and more recently, it was employed as an intracanal irrigant during endodontic treatment, showing effective and promising disinfection results (23–24), probably explained by its permeabilization effects that cause damage of bacterial membranes (25). Moreover, curcumin is photosensitive (26) and, according to a study by da Frota et al. (27), infected root canals irrigated with curcumin, combined with photoactivation (5 min) using a light-emitting diode (LED) unit, effectively reduced contamination. However, increasing light exposure (10 min) did not result in any additional potential for disinfection, so that further studies investigating the effects of curcumin photoactivation used as irrigant or as bioactive-loaded fibers would contribute to a better understanding of the antimicrobial potential of this natural compound. To the best of our knowledge, electrospun curcumin-modified fibers have never been processed for endodontic purposes and, considering that bacterial elimination within the root canal system is paramount for successful pulp regeneration (28), we hypothesized that upon photoactivation, curcumin fibers would exhibit greater antibiofilm properties than TAP. Actinomyces naeslundii bacteria was used, since it is a prevalent bacterial specie found in traumatized immature teeth with necrotic pulps (29).
Materials and Methods
Synthesis of electrospun curcumin-modified polymer fibers
Polydioxanone polymer solutions (PDS II; Ethicon, Somerville, NJ, USA) were prepared by dissolving PDS in 1,1,1,3,3,3-hexafluoro-2-propanol at a concentration of 10 wt.% (8–10). Meanwhile, curcumin powder was dissolved in ethanol to obtain a stock solution (100 mg/mL), which was added into PDS solutions at different concentrations: 0 (curcumin-free), 2.5 (2.5 mg/mL), and 5.0 wt.%. (5.0 mg/mL); all solutions were stirred overnight. The polymer solutions were loaded into 5 mL plastic syringes (Becton, Dickson and Company; Franklin Lakes, NJ, USA) with 27G metallic blunt-tip needles (CML Supply, Lexington, KY, USA) and placed in a syringe pump (Legato 200; KD Scientific Apparatus, Holliston, MA, USA) with the following setting parameters flow rate of 1.5 or 2 mL/h for curcumin-modified and curcumin-free solutions, respectively; spinning distance of 18 cm; and distinct voltages (ES50P−-10W/DAM, Gamma High-Voltage Research, Inc., Ormond Beach, FL, USA) according to the solution (15 to 18 kV). The fiber mats were dried under vacuum for 48 h to ensure complete removal of any remaining solvent (8–10).
Characterization of the electrospun fibers
The electrospun fibers were observed under a field-emission scanning electron microscope (FE-SEM, Model JSM-6701F, JEOL, Tokyo, Japan) to evaluate the morphology and overall fiber architecture. Briefly, samples taken from the different electrospun mats were mounted on Al stubs and sputter-coated with Au-Pd prior to imaging. Image J software (National Institutes of Health Bethesda, MD, USA) was used to calculate fiber diameter (50 fibers/image and 3 images/group). Fourier-transform infrared spectroscopy (FTIR) was conducted using a Thermo Scientific™ Nicolet™ iS50 FTIR spectrometer (Nicolet Instrument Technologies, Inc., WI, USA) spanning the scanning range of 600–4000 cm−1 with a resolution of 2 cm−1.
Biofilm formation
Bacterial cultures were prepared by inoculating A. naeslundii (An, ATCC 43146) into 5 mL of brain heart infusion with yeast and 1% sucrose (BHI-YS) broth (Difco Laboratories Inc., Detroit, MI USA). The absorbance of the culture was monitored spectrophotometrically (SpectraMax® iD3 Molecular Devices, San Jose, CA, USA) using a wavelength of 450 nm, until an absorbance of 0.1 was reached (ca. 107/mL). After 48 h, 50 μL of the culture was inoculated into wells of 24-well plates containing 1.5 mL of BHI-YS. The plates were incubated in aerobic conditions (37°C and 5% CO2) for 7 days for biofilm formation. BHI-YS was replaced on day 3 to remove detached cells and ensure cell viability.
Group allocation and antimicrobial evaluation
Biofilm cultures were categorized accordingly to 2 variable factors: “anti-biofilm treatment” and “light exposure”. Saline solution (negative control), specimens from the electrospun fibers (15 × 15 mm2), and curcumin irrigants were tested with and without photoactivation to investigate whether light exposure (photoactivation) would increase the antimicrobial effect. Positive controls were tested without photoactivation, since their components are not photosensitive. Photoactivation was performed using a light-emitting diode (LED) curing unit (Bluephase LED, Ivoclar-Vivadent, Schaan, Liechtenstein) with a wavelength range of 385–515 nm, which was kept vertical at a fixed distance from the well-plate; the irradiation intensity was 1200 mW/cm2. The wells were separated individually (using the wells on four corners of the 24-well plate) to prevent light scattering.
Curcumin-modified and curcumin-free fibers (15 × 15 mm2) attached to plastic inserts (CellCrown™, Scaffdex, Tampere, Finland) were soaked in 1.6 mL of 0.9% saline solution (12). For the photoactivated groups, light exposure was performed at 30 s intervals for 4 min (23). Care was taken to make sure the electrospun fibers were only wetted by the saline solution but not completely submerged. During the first 30 s, the irradiation was continuous without interruption, and for the next 30 s, the solution was stirred by moving the plate laterally. The wells were irradiated again for 30 s and this protocol was repeated for a total of 4 min. For the non-photoactivated group, electrospun specimens were immersed for 4 min in 0.9% saline. For the positive controls (2% chlorhexidine, 1% NaOCl, and TAP 1 mg/mL), each solution was prepared with the respective agent at the aforementioned concentrations.
After treatment, the respective solutions in each group were removed, 1 mL of sterile saline was pipetted into each well, and the biofilm scrapped. The dispersed biofilm cells were collected in microcentrifuge tubes (Fisherbrand, Fisher Scientific, Waltham, MA, USA) and 1:2000 dilutions were made. The dispersed bacteria were spiral plated using anaerobic blood agar plates. The colony forming units (CFU) was quantified after 24 h of incubation using an automated colony counter (Symbiosis Inc., Frederick, Maryland, USA).
Statistical analysis
One-way ANOVA, followed by pairwise comparisons using Fisher’s Protected Least Significant Differences, were used to compare among the groups for differences (CFU/mL). A 5% significance level was used for all tests. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Fiber morphology
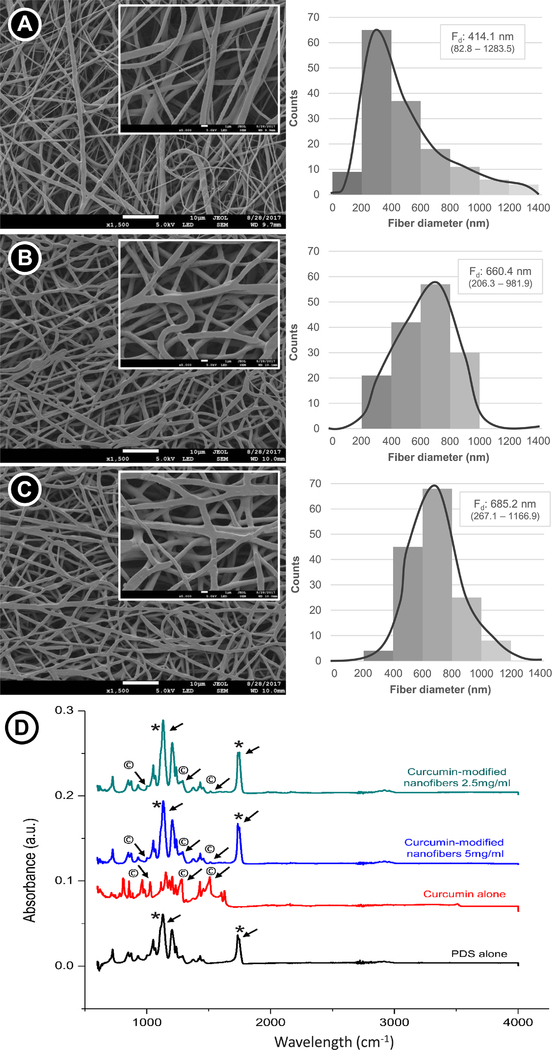
The fiber morphology and fiber diameter distribution are displayed in Figure 1 (A–C). A submicron median fiber diameter was observed for all groups, with the curcumin-modified fibers presenting slightly thicker fibers than the curcumin-free counterpart (p<0.001), but similar to each other. While the curcumin-free PDS fibers exhibited a wider distribution, the curcumin-modified fibers presented a narrower distribution (Gaussian distribution), with most of the fibers ranging from 400 to 800 nm.
Figure 1.
Morphological SEM images illustrating the nanofiber architecture and submicron fiber diameter observation: (A) Curcumin-free PDS nanofiber. (B) PDS nanofibers containing curcumin at 2.5 mg/mL. (C) PDS nanofibers containing curcumin at 5.0 mg/mL. The median fiber diameter (Fd) and the minimum and maximum values for each group was expressed. The curcumin-modified nanofibers presented greater fiber diameter than the curcumin-free nanofibers (p<0.05; Kruskal-Wallis test – α=5%). (D) FTIR spectra confirming the incorporation of curcumin into the PDS fibers. Note the presence of characteristic peaks for curcumin (* and © denote PDS-, and curcumin-related peaks, respectively).
Fiber composition
Figure 1D shows the FTIR spectra for curcumin-free PDS fibers and for the PDS fibers containing curcumin at 2.5 and 5.0 mg/mL. The spectrum of curcumin-free fibers can be observed at bands of 1,732 cm−1 (ester carbonyl group), 1,125 cm−1 (C-O-C of ester with shoulder broadening at 1,100 cm−1 due to ether group), 1,066 cm−1 and 1,055 cm−1 (C-O bands of ester), and for aliphatic groups at 2,925 cm−1. For curcumin, the bands observed at 3085–3552 cm−1, 1588 cm−1, 1512 cm−1, 1265 cm−1, and 1143 cm−1 are respectively attributed to phenolic O-H stretching, stretching vibrations of the benzene ring, C-C vibrations, aromatic C-O stretching and C-O-C stretching modes. The spectra of the 2.5 and 5.0 mg/ml curcumin-containing fibers show the same features, comprising a composite of the PDS and curcumin spectra, and displaying all the characteristic bands of PDS and some peaks for curcumin. This indicates that few interactions occur between the two components.
Antimicrobial properties
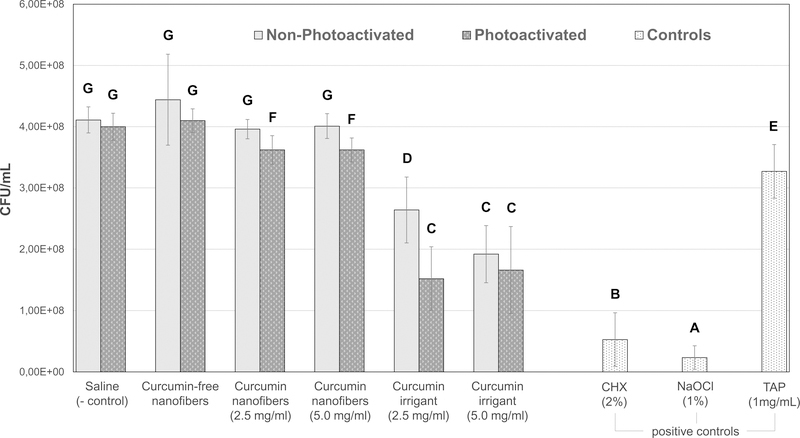
NaOCl and CHX were the most effective agents in reducing viable bacteria. TAP (1 mg/mL) was not as effective as the other positive controls. Concerning the curcumin-based strategies, the irrigants demonstrated greater antimicrobial activity than the nanofibers, which exhibited only a slight reduction in bacterial viability compared to the negative control. The antibiofilm activity of TAP was less effective than the curcumin irrigants, but more effective than the curcumin-modified fibers.
Regarding the effect of light exposure on the antimicrobial properties of the strategies tested, the photoactivation of the curcumin-modified fibers and the irrigant containing 2.5 mg/mL of curcumin demonstrated greater bacterial viability reduction than their non-photoactivated counterparts. Light exposure did not significantly affect the antimicrobial potential of saline, curcumin-free fibers, and the irrigant containing 5.0 mg/mL of curcumin. Despite the slight decrease in bacterial viability obtained with the photoactivated curcumin-modified fibers, it was greater than that obtained from the saline and curcumin-free fiber groups.
Discussion
One of the most important stages of regenerative endodontics is proper disinfection of the root canal via intracanal application of medicaments (30). Prior to investigating the antimicrobial effects of the synthesized curcumin-modified fibers, they were characterized by means of morphology, fiber diameter distribution, and chemical analysis using FTIR. Curcumin incorporation led to the production of thicker fibers when compared to the curcumin-free fibers, probably due to the phenolic nature of curcumin (Figure 1D), which may have increased the viscosity of the polymer solution, favoring chain entanglement during electrospinning and, consequently, the generation of thicker fibers (31). Indeed, the hydroxyls found in curcumin may have allowed cross-linking with the polymer system via hydrogen bonding (32), producing fibers with increased diameters. This finding was not corroborated by previous studies (22, 33), which showed that greater curcumin content led to thinner fibers. One may suggest that the foregoing studies used poly(lactic acid) and poly(caprolactone) as polymer sources for fiber fabrication differently from our study that used PDS. Nevertheless, increased fiber diameters lead to longer drug release (e.g., from 72 h to 325 h) without an initial burst, since thick fibers make the diffusion passage more compact within the matrix (34). In turn, thicker fibers would contain a curcumin reservoir that would facilitate a much longer sustained release within the root canal system.
Concerning the morphology, curcumin-modified fibers seemed to present a flattened geometry, different from the round-shaped curcumin-free fibers. However, both fiber geometries may effectively contribute to proper drug release (22). Fiber diameter distribution was more homogeneous for the curcumin-modified fibers, indicating optimal solvent evaporation during electrospinning (35). Conversely, the less viscous solution of the curcumin-free polymer solution may have hampered solvent evaporation due to an imbalance in charge density, producing heterogeneous fibers with a broad diameter distribution.
This investigation aimed to determine the antimicrobial effects of curcumin-modified fibers against a 7-day old established A. naeslundii biofilm when compared to common antimicrobial agents used in endodontics. Unfortunately, the curcumin-modified fibers exhibited lower antimicrobial activity than the positive controls. Among the positive controls, NaOCl was the most effective agent, killing nearly all viable bacteria, followed by chlorhexidine. The use of NaOCl does not only disinfects the canal, it also provides dissolution of the necrotic pulp tissue. However, it is not biocompatible and can potentially reduce the survival rate of dental pulp stem cells, preventing cells from adhering to root canal surfaces. Hence, it has been advocated to thoroughly wash the canal using a saline solution to reduce the cytotoxic effects of NaOCl before application of intracanal medicaments, or use a low concentration of NaOCl (1.5%) to significantly increase the survival rate of stem cells when irrigating the canal for disinfection (36). Concerning TAP, it demonstrated limited antibiofilm activity, questioning the current AAE guidelines, which recommend the use of an antibiotic concentration in the range of 0.1 to 1 mg/mL. Interestingly, curcumin-based irrigants at both concentrations tested (2.5 and 5.0 mg/mL) provided greater antimicrobial effect than TAP (1 mg/mL), confirming curcumin’s antimicrobial potential.
It has been reported that curcumin has a broad-spectrum antimicrobial property, disrupting bacterial membranes by increasing bacterial cell wall permeability. Another study also concluded that photoactivated curcumin is as effective as TAP and was able to penetrate deeper into the dentinal tubules (24, 25). Curcumin has already been used in endodontics as an intracanal irrigant, demonstrating effective elimination of biofilm bacteria within the root canal system (23). Here it was hypothesized that curcumin incorporated into PDS electrospun fibers would also present a satisfactory antibiofilm activity; but considering the present findings, one can speculate that the antimicrobial protocol performed (i.e., soaking the fibers in saline for 4 min) was not sufficient to release the minimal inhibition concentration of curcumin into the solutions. Also, the release rate of curcumin-based polyethersulfone porous ultrafine fibers was dependent on the polymer structure of the fibers (37), with the greater the polymer’s hydrophobicity, the slower the curcumin release. PDS is a moderately hydrophobic polymer; as a result, the use of longer soaking times in our study would perhaps allow for proper curcumin release from the fibers, thus leading to improved antibiofilm activity.
Another goal of this study was to investigate the effects of light exposure on antibiofilm activity of the curcumin-modified fibers and curcumin irrigant. Considering that curcumin is photosensitive, it was anticipated that photoactivation would increase curcumin activity against A. naeslundii biofilm. Photoactivation of the curcumin-based strategies resulted in greater antimicrobial activity when compared to their non-photoactivated counterparts (Fig. 2), except for the irrigant containing 5.0 mg/mL of curcumin. It can be suggested that photoactivation acts at the molecular level of curcumin, increasing its antimicrobial activity at low concentrations. According to some studies, light exposure has been increasingly used as a photodynamic therapy (PDT) in dentistry. It is known that there is energy transfer from an activated photosensitizer to any available oxygen, resulting in the formation of toxic oxygen species, such as singlet oxygen and free radicals, which may cause rapid and selective destruction of microorganisms (38). Another advantage of curcumin as a photosensitizer is that it produces a lethal effect without attaching or being in close proximity to the bacteria and a recent report concluded this as a result of the formation of hydrogen peroxide as an intermediary compound upon photoactivation (24). However, it may be speculated that the positive role of photoactivation is concentration-dependent, since most concentrated curcumin irrigant (5.0 mg/mL) displayed a similar antibiofilm effect regardless of the light exposure condition performed. As a result, one should consider that the irradiation time used in our study was sub-optimal, i.e., 2 min in total (irradiation protocol comprised of cycles of 30 s cycles of photoactivation followed by 30 s of stirring without irradiation, for up to 4 min). However, da Frota et al. (27) demonstrated that increased times of photoactivation may not result in greater disinfection potential, and considering that the photoactivation protocol must not extensively delay endodontic treatment, a short irradiation time, comparable to that used here, could be considered as a more clinically-feasible procedure. Worth mentioning, the study by da Frota et al. (27) tested the effects of longer photoactivation times (e.g., 5 and 10 min) compared to our study, which makes us believe that our protocol would not pose a problem for its potential clinical translation.
Figure 2.
Antimicrobial effects of different treatments on Actinomyces naeslundii biofilm in CFU/mL (n=11). Similar uppercase letters next to the (±SD) indicate no significant differences at the p 0.05 level. NaOCl exhibited the strongest antimicrobial effect followed by CHX. There was a significant reduction of bacteria between curcumin-based irrigants when compared with TAP. No significant differences were observed between curcumin-free nanofibers, saline, and nonphotoactivated curcumin-modified fibers. There was a slight decrease of bacterial viability obtained with photoactivated curcumin-modified fibers when compared with the non-photoactivated curcumin-modified fiber and negative control groups.
In the present study, curcumin release from the processed fibers was intentionally provoked by soaking and stirring the fibers into saline. One may suggest that this protocol is not clinically possible; but when translating it to the clinics, the activation of the suspension with ultrasonic files or alternatives (e.g., GentleWave® System; Sonendo, Inc., Laguna Hills, CA, USA) could be feasible. Nevertheless, and according to a study by Neelakantan et al. (23), ultrasonic agitation of curcumin resulted in lower antibacterial efficacy than photoactivation of the curcumin-based solution, thus indicating that stirring is not the most important aspect under consideration. Despite this, the stirring protocol tested in our in vitro study must be further investigated in order to enhance curcumin release from the fibers, especially if an initial burst is not guaranteed due to the hydrophobic nature of the PDS fibers. Lastly, despite the more concentrated fibers (5.0 mg/mL) not showing improved disinfection upon photoactivation, the use of the less concentrated fibers (2.5 mg/mL), which were photo-sensitive due to the enhanced antibiofilm potential, could be beneficial in terms of stem cell survival (8, 13, 28), potentiating the biological effects of this alternative protocol.
An important finding of this study relies on the fact that TAP exhibited lower antibiofilm activity than the curcumin irrigants. Although it has been advocated that low TAP formulations should be used to maximize stem cell viability, the concentration of 1 mg/mL did not promote biofilm elimination. Meanwhile, the use of natural compounds, such as curcumin, could overcome the foregoing disadvantage. One could suggest the following protocol be translated to clinical use rather than employing TAP: first, disinfect the canal with NaOCl and allow the dissolution of necrotic pulp tissue, followed by further disinfection using a curcumin irrigant associated with light exposure; second, incorporate a long-lasting disinfection using the well-controlled curcumin-release approach described here with the less concentrated electrospun curcumin-modified fibers associated with photoactivation. Further studies are important to elucidate the minimal inhibitory concentration of curcumin when incorporated into polymer nanofibers, and the effect of the interaction between NaOCl and curcumin and other endodontic irrigants should be investigated. Lastly, the present study considered only one type of bacteria, so that other microorganisms need to be examined in order to elucidate whether the present disinfection protocols and curcumin concentrations would account for an effective intracanal antibacterial potential.
Highlights.
Medicaments of study showed antibiofilm activity against Actinomyces naeslundii
Curcumin-loaded fibers were prepared using electrospinning process
Curcumin-loaded fibers presented antibiofilm activity only upon photoactivation
Curcumin irrigants demonstrated greater antimicrobial properties than TAP
Chlorhexidine and NaOCl showed greater antibiofilm activity than TAP
Acknowledgements
The authors declare that they have no conflict of interest. We thank Dr. John A. Levon, Mr. George Eckert, and Dr. Grace F. Gomez for their significant support in the completion of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albuquerque MT, Valera MC, Nakashima M, et al. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 2014;93: 1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol 2012;28:33–41. [DOI] [PubMed] [Google Scholar]
- 3.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 2009;35:1343–49. [DOI] [PubMed] [Google Scholar]
- 4.Galler KM. Clinical procedures for revitalization: current knowledge and considerations. Int Endod J 2016;49:926–36. [DOI] [PubMed] [Google Scholar]
- 5.Ruparel NB, Teixeira FB, Ferraz CC, et al. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod 2012;38:1372–5. [DOI] [PubMed] [Google Scholar]
- 6.Porter ML, Munchow EA, Albuquerque MT, et al. Effects of novel 3-dimensional antibiotic-containing electrospun scaffolds on dentin discoloration. J Endod 2016;42:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria G, Rodrigues EM, Coaguila-Llerena H, et al. Influence of the vehicle and antibiotic formulation on cytotoxicity of triple antibiotic paste. J Endod 2018;44:1812–6. [DOI] [PubMed] [Google Scholar]
- 8.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res 2013;92:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palasuk J, Kamocki K, Hippenmeyer L, et al. Bimix antimicrobial scaffolds for regenerative endodontics. J Endod 2014;40:1879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pankajakshan D, Albuquerque MT, Evans JD, et al. Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endod 2016;42:1490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottino MC, Arthur RA, Waeiss RA, et al. Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig 2014;18:2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albuquerque MT, Valera MC, Moreira CS, et al. Effects of ciprofloxacin-containing scaffolds on Enterococcus faecalis biofilms. J Endod 2015;41:710–4. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque MT, Ryan SJ, Munchow EA, et al. Antimicrobial effects of novel triple antibiotic paste-mimic scaffolds on Actinomyces naeslundii biofilm. J Endod 2015;41:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamocki K, Nör JE, Bottino MC. Dental pulp stem cell responses to novel antibiotic-containing scaffolds for regenerative endodontics. Int Endod J 2015;48:1147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamocki K, Nör JE, Bottino MC. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-In vitro studies. Arch Oral Biol 2015;60:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waeiss RA, Negrini TC, Arthur RA, et al. Antimicrobial effects of drug-containing electrospun matrices on osteomyelitis-associated pathogens. J Oral Maxillofac Surg 2014;72:1310–9. [DOI] [PubMed] [Google Scholar]
- 17.Bottino MC, Albuquerque MTP, Azabi A, et al. A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J Biomed Mater Res B Appl Biomater 2019;107:1576–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 2017;57:2889–95. [DOI] [PubMed] [Google Scholar]
- 19.Sun XZ, Williams GR, Hou XX, et al. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr Polym 2013;94:147–53. [DOI] [PubMed] [Google Scholar]
- 20.Gandhimathi C, Venugopal JR, Bhaarathy V, et al. Biocomposite nanofibrous strategies for the controlled release of biomolecules for skin tissue regeneration. Int J Nanomedicine 2014;9:4709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Ma C, Wu Z, et al. Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber: In vitro and in vivo study. Nanoscale Res Lett 2015;10:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouthuy PA, Somogyi Skoc M, Cipak Gasparovic A, et al. Investigating the use of curcumin-loaded electrospun filaments for soft tissue repair applications. Int J Nanomedicine 2017;12:3977–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelakantan P, Cheng CQ, Ravichandran V, et al. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal dentin. Photodiagnosis Photodyn Ther 2015;12:108–14. [DOI] [PubMed] [Google Scholar]
- 24.Devaraj S, Jagannathan N, Neelakantan P. Antibiofilm efficacy of photoactivated curcumin, triple and double antibiotic paste, 2% chlorhexidine and calcium hydroxide against Enterococcus faecalis in vitro. Sci Rep 2016;6:24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyagi P, Singh M, Kumari H, et al. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PlosOne 2015;10:e0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahzad M, Sherry L, Rajendran R, et al. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int J Antimicrob Agents 2014;44:269–73. [DOI] [PubMed] [Google Scholar]
- 27.da Frota MF, Guerreiro-Tanomaru JM, Tanomaru-Filho M, et al. Photodynamic therapy in root canals contaminated with Enterococcus faecalis using curcumin as photosensitizer. Lasers Med Sci;30:1867–72. [DOI] [PubMed] [Google Scholar]
- 28.Albuquerque MTP, Nagata JY, Diogenes AR, et al. Clinical perspective of electrospun nanofibers as a drug delivery strategy for regenerative endodontics. Curr Oral Health Rep 2016;3:209–20. [Google Scholar]
- 29.Nagata JY, Soares AJ, Souza-Filho FJ, et al. Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod 2014;40:778–83. [DOI] [PubMed] [Google Scholar]
- 30.Bottino MC, Pankajakshan D, Nor JE. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent Clin North Am 2017;61:689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dargaville BL, Vaquette C, Rasoul F, et al. Electrospinning and crosslinking of low-molecular-weight poly(trimethylene carbonate-co-(L)-lactide) as an elastomeric scaffold for vascular engineering. Acta Biomater 2013;9:6885–97. [DOI] [PubMed] [Google Scholar]
- 32.Manolova Y, Deneva V, Antonov L, et al. The effect of the water on the curcumin tautomerism: a quantitative approach. Spectrochim Acta A Mol Biomol Spectrosc 2014;132:815–20. [DOI] [PubMed] [Google Scholar]
- 33.Guo G, Fu S, Zhou L, et al. Preparation of curcumin loaded poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cells. Nanoscale 2011;3:3825–32. [DOI] [PubMed] [Google Scholar]
- 34.Jannesari M, Varshosaz J, Morshed M, et al. Composite poly(vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomedicine 2011;6:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahma A, Munir MM, Khairurrijal, et al. Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl(pyrrolidone) fiber. Biol Pharm Bull 2016;39:163–73. [DOI] [PubMed] [Google Scholar]
- 36.Martin DE, de Almeida JFA, Henry MA, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod 2014;40:51–5. [DOI] [PubMed] [Google Scholar]
- 37.Wei Z, Xiong C, Liu Z, et al. Release characteristics and processing-structure-performance relationship of electro-spinning curcumin-loaded polyethersulfone based porous ultrafine fibers. J Biomater Sci Polym Ed 2018;29:1825–38. [DOI] [PubMed] [Google Scholar]
- 38.Tennert C, Feldmann K, Haamann E, et al. Effect of photodynamic therapy (PDT) on Enterococcus faecalis biofilm in experimental primary and secondary endodontic infections. BMC Oral Health 2014;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]