Abstract
Cell migration is an essential process, both in unicellular organisms such as amoeba and as individual or collective motility in highly developed multicellular organisms like mammals. It is controlled by a variety of activities combining protrusive and contractile forces, normally generated by actin filaments. Here, we summarize actin filament assembly and turnover processes, and how respective biochemical activities translate into different protrusion types engaged in migration. These actin-based plasma membrane protrusions include actin-related protein 2/3 complex-dependent structures such as lamellipodia and membrane ruffles, filopodia as well as plasma membrane blebs. We also address observed antagonisms between these protrusion types, and propose a model – also inspired by previous literature – in which a complex balance between specific Rho GTPase signaling pathways dictates the protrusion mechanism employed by cells. Furthermore, we revisit published work regarding the fascinating antagonism between Rac and Rho GTPases, and how this intricate signaling network can define cell behavior and modes of migration. Finally, we discuss how the assembly of actin filament networks can feed back onto their regulators, as exemplified for the lamellipodial factor WAVE regulatory complex, tightly controlling accumulation of this complex at specific subcellular locations as well as its turnover.
Keywords: actin assembly, Arp2/3, contraction, formin, protrusion, WRC
Introduction
Cell migration is a key function of life. With a few exceptions, this process relies on the dynamic assembly and disassembly of actin filaments. These filaments organize into distinct subcellular domains, of which plasma membrane protrusions like lamellipodia, filopodia, and blebs are highly complementary to cell-substratum adhesion structures such as focal complexes or focal contacts, the latter of which serve as anchorage sites for yet another prominent actin structure relevant for migration, the so-called stress fiber. As possibly oversimplified, but still useful, general scheme, one can say that the protrusive structures best formed at the migrating front edge initiate the process of migration by exploring future space, while the contractile structures such as stress fibers (not always as prominently organized as distinct bundles as in migrating fibroblast or epithelial cells) deliver the forces onto cell-substratum adhesions to drag the rest of the cell body and cell rear forward. In this respect, efficient migration requires a well-balanced coordination of both protrusive and retractile activities, with overactivity of the latter frequently being counterproductive for efficient migration.
In this review, we first summarize actin filament assembly processes and the structures that they induce, as well as their turnover. We also aim for understanding how cells use the distinct molecular mechanisms known today to drive actin polymerization to form either one of the three major protrusion types. The discussion on similarities and differences between different protrusions are complemented by the ‘old’ question of how Rac and Rho GTPases control polarization and protrusion [1]. Finally, we ask ourselves how activities and turnover of the Rac effector WASP-family verprolin-homologous protein (WAVE) regulatory complex (WRC) might be tuned at lamellipodia. This is crucial for migration, last not least due to the essential functions found for WRC in lamellipodial actin polymerization.
The making and breaking of actin filaments
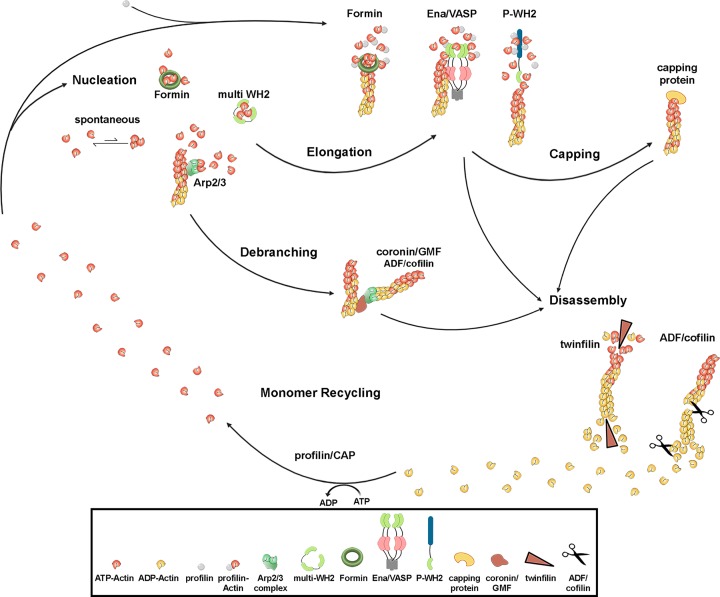
Polymerization of actin filaments at physiological concentrations is occurring rapidly in vitro. Thereby, filaments become polar, forming a fast polymerizing barbed (+) end and a slower polymerizing pointed (-) end. Cells, however, have been evolving various means to tightly control polymerization and depolymerization of actin filaments to their needs (Figure 1). It is commonly agreed that the vast majority of actin monomers is bound by monomer binding or sequestering factors such as members of the profilin family or thymosin β4, thereby strongly reducing the concentration of polymerization-compatible actin monomers in cells [2]. For nucleation of actin filaments, the most critical step is the formation of a trimer or tetramer. All actin nucleators have in common that they mimic this trimer/tetramer state. The most prominent factor to date nucleating actin filaments is the actin-related protein (Arp) 2/3 complex. This heteroheptameric protein complex comprises two actin related proteins, Arp2 and Arp3 that are highly similar to actin, as well as smaller subunits called ArpC1-ArpC5. Arp2 and Arp3 together with two actin monomers most efficiently form a nucleus for daughter filament generation, meaning that it must bind to a pre-existing filament, giving rise to large arrays of branched actin meshworks [3]. Arp2/3 complex is intrinsically inactive but can be activated by at least eight factors presently known in mammals, which frequently are regulated within protein complexes and are collectively called nucleation promoting factors (NPF) of class I. These have in common that they harbor a so-called WCA-domain. This module contains an actin monomer binding WH2- (or W-) region and Arp2/3 complex-binding connector (C) and acidic (A) domains [3,4]. Two NPF molecules most efficiently activate one Arp2/3 complex [5–7]. Other prominent examples for actin nucleators are proteins of the formin family. In contrast with Arp2/3 complex, they act by stabilizing an actin dimer using their conserved formin homology 2 (FH2) domain and subsequently recruit actin or profilin-actin complexes [8,9]. Yet another group of actin nucleators constitutes proteins with multiple WH2 domains or other actin-binding modules capable of bringing three or four actin monomers together, the close proximity of which can overcome the kinetically non-favorable nucleation [4].
Figure 1. Actin polymerization and turnover.
Diverse factors, such as Arp2/3 complex, various formins or proteins containing multiple actin monomer binding WH2 domains can catalyze nucleation of filaments from ATP-loaded actin monomers. Subsequently, elongation of nascent filaments is achieved by either formins, Ena/VASP proteins or proteins harboring a polyproline-WH2 (P-WH2) module. Elongation can be terminated by capping proteins, for instance heterodimeric capping protein. Filament branches, created by Arp2/3 complex, can be disconnected by proteins of the coronin/GMF as well as cofilin families, whereas twinfilin catalyzes the disassembly of filament ends. Moreover, ADF/cofilin family members are best known for promoting filament disassembly by severing and depolymerization. Recycling of ADP-actin to polymerization-competent ATP-actin is executed by profilin or cyclase-associated protein.
Processive filament elongation can also be achieved by many formin family members, which remain associated with and can thus ride on the growing, barbed ends of actin filaments. This activity appears to be dependent on the presence of profilin-actin complexes (profilin-actin) that are bound by the proline-rich FH1-domain of most formins [8]. However, proteins of the Ena/VASP family can processively elongate filaments as well, although as opposed to formins acting as dimers, Ena/VASP proteins operate in tetrameric complexes, thereby combining features of filament bundling and elongation, independently of profilin [10,11]. Aside from the role as Arp2/3 complex activators, NPFs have recently also been proposed to function in filament elongation, by accepting profilin-actin complexes and transferring actin monomers in a distributive and non-processive fashion onto barbed ends [12]. The activity counteracting filament elongation is most prominently represented by heterodimeric capping protein, capping filament barbed ends, and thus terminating their elongation [13].
Ageing of actin monomers incorporated into filaments, which is mediated by hydrolysis of bound ATP, favors the association with disassembly factors thereby driving disassembly toward filament pointed ends [14]. Disassembly has been described to occur through various means: Arp2/3 complex-containing networks can most prominently be debranched by proteins of the coronin and GMF families [15], but also by ADF/cofilin [16], although the latter protein family is best known for severing [17] or depolymerizing individual filaments [18]. Whereas ADF/cofilin is traditionally agreed to shift the balance toward disassembly at filament pointed ends [19], challenged though by a recent, divergent view [20], twinfilin family proteins are commonly thought to promote the disassembly at both filament ends [21,22]. Finally, ADP-bound actin monomers can be recharged by either profilin proteins [23–25] or by cyclase-associated protein (CAP) [26], thereby completing the cycle and providing fresh, polymerization-competent actin monomers (Figure 1).
How differential signaling and actin assembly factor activities translate into distinct protrusion types
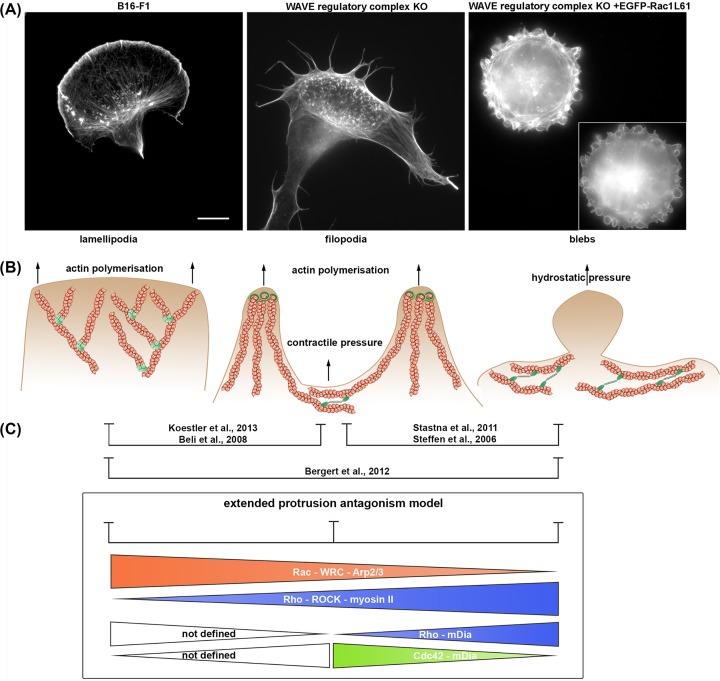
In principle, protrusion of the plasma membrane can be achieved by at least three different modes or mechanisms involving actin filaments (Figure 2). One mechanism is the generation of a branched Arp2/3 complex-containing network, such as the lamellipodium. Formation of this structure is frequently associated with mesenchymal migration. The lamellipodium constitutes a flat structure that can protrude on solid surfaces both in vitro and in vivo, but also lift up into the third dimension dependent on signaling conditions and adhesion capacity to the underlying substratum [14]. Such 3D lamellipodia are also termed ruffles, but are considered indistinguishable in mechanisms of actin turnover to lamellipodia protruding on flat surfaces. The lamellipodium is formed at the cell periphery by continuous branching of the Arp2/3 complex, which creates forces that overcome membrane tension and enable forward movement of the plasma membrane. In this structure, forces arising from actin polymerization driving protrusion are uncoupled from myosin II-based contractility [27]. Aside from ruffles at the cell periphery or those emerging as so-called circular ruffles at the dorsal cell surface, comparable structures involving Arp2/3 complex-dependent actin remodeling include the podosomes of hematopoietic cells and invadopodia of tumor cells [28,29]. In the latter two structures, however, which are occasionally grouped together as ‘invadosomes’, specific differences to lamellipodia and ruffles do exist. First, the molecular inventory of invadosomes is comparable, but not identical to lamellipodia [28,29], and second, the protrusive activity of invadosomes is tightly coupled not only to adhesion to the extracellular matrix, but also to degradation of the latter.
Figure 2. Types of actin-based protrusions.
(A) Representative examples of different types of plasma membrane protrusion, lamellipodia, filopodia, and blebs. Panels show B16-F1 cells wildtype (left) or upon specific experimental treatments (middle and right), as indicated on top of images, followed by fixation and staining for the actin cytoskeleton with phalloidin. Middle and right panels represent WAVE regulatory complex KO (Sra-1/PIR121 KO clone #3, as in [80]) and example of the same cell type transiently expressing EGFP-tagged, constitutively active Rac1, respectively (EGFP-Rac1L61; inset). Scale bar, 10 µm. (B) Mechanisms of formation of actin-dependent plasma membrane protrusions as shown in A. Protrusion of the plasma membrane is frequently achieved by forming an Arp2/3 complex-dependent lamellipodial actin network. Disruption of lamellipodia formation by for instance eliminating WRC expression (WRC KO) forces cells to migrate using a bunch of filopodial actin bundles, with the space between them being filled by contractile activity of myosin II (middle panel). Conversely, overexpression of active Rac in WRC KO cells interferes with filopodia formation and stimulates bleb formation (see also [80]). Plasma membrane blebbing relies on high myosin II activity in the contractile actin cortex creating intracellular, hydrostatic pressure. Local cortex – plasma membrane detachment or cortex rupture can lead to the protrusion of an actin-free plasma membrane bleb. (C) Models explaining antagonisms between protrusion types: we propose a model wherein Rac-WRC-Arp2/3 complex versus Rho-ROCK-myosin II signaling reciprocally control the formation of lamellipodia versus blebs, respectively [73]. On top of this, we suggest that spatial activation by either Rho or Cdc42 GTPases tunes output signals for mDia formins controlling filopodia or bleb formation at decreased Rac signaling conditions.
The second mechanism of plasma membrane protrusion involves the growth of small linear bundles of actin filaments, called filopodia, which also create forces toward the membrane through filament polymerization, but independent of Arp2/3 complex [30–33]. The space between individual filopodial bundles is commonly filled by the contractile activity mediated by myosin II [30]. As elaborated on in several, excellent reviews in more detail recently [34–36], this group of protrusions includes various types of bundled structures displaying significant differences in structural organization and dynamics, but ranging from sequentially protrusive and retractile or occasionally kinking filopodia to the much more ordered and apparently static microvilli of epithelia or the stereocilia in cochlear hair cells.
The third mechanism of plasma membrane protrusion is the one that does not require active actin polymerization [37,38]. The plasma membrane of eukaryotic cells is generally thought to be attached to a thin cortex of actin filaments. This cortex harbors contractile properties, based on myosin II activity, enabling cells at least within the animal kingdom to create hydrostatic pressure pushing against the plasma membrane [39]. A nucleus for a bleb can be formed, in principal, by two different ways. Either a local cortex-plasma membrane detachment or a rupture of the actin-cortex itself [37,38]. Anyhow, it appears that high myosin II activity is critical for the formation of blebs, indicating that intracellular, hydrostatic pressure is the driving force [37]. The type of migration mediated by these structures is called amoeboid motility.
Establishment of cell polarity by Rac and Rho GTPases
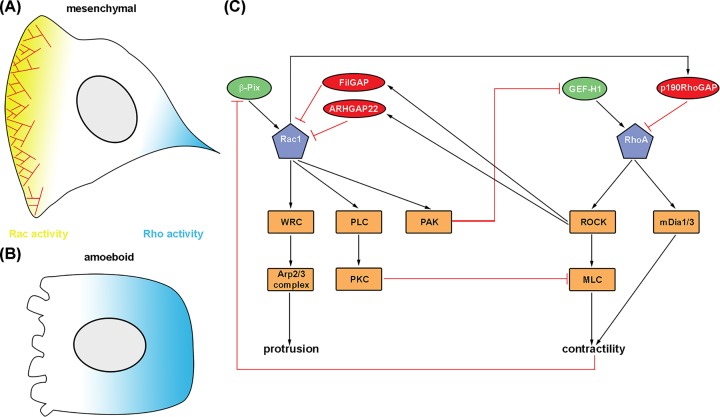
While exhibiting these three modes of protrusion, either exclusively or mixed at variable ratios, cells rely on their ability to polarize in order to allow the cell body to move forward. The small GTPases Rac1 and RhoA are crucial for initiating and maintaining cell polarity and directed movement, but appear to play opposing roles during these processes. Their spatiotemporal activities have been extensively studied using fluorescence resonance energy transfer microscopy, with both being active at leading edge and cell rear, in principle, although with differential patterns and magnitudes [40–42]. Although not absent in protrusion, which still constitutes a matter of vivid debate [42–45], it appears that RhoA signaling is at least enhanced at retraction sites [40,41]. Indeed, there is mutual inhibition at multiple levels between Rac1 and RhoA signaling, which can lead to a bistable system [46]. Figure 3 summarizes the antagonism between Rac1 and RhoA. At the leading edge, Rac likely dominates RhoA signaling and vice versa at the rear to achieve polarity and maintain directed movement. While Rac GTPases signal through WRC to achieve Arp2/3 complex-dependent lamellipodial protrusion, Rho signaling activates actomyosin-based contractility via ROCK and mDia formins at the cell rear [47–50]. However, there are multiple levels of cross-talk. For instance, polarization of myosin II activity to the rear appears to rely on WRC, as knockdown of the hematopoietic subunit Hem-1 results in activation of myosin II at the protruding front [51]. Likewise, acute inhibition of Arp2/3 complex causes rearrangement of lamellipodial actin filaments into antiparallel arrays sufficient to trigger myosin recruitment both in cells [52,53] and in vitro [54].
Figure 3. Establishment of polarity by Rac/Rho.
(A) The small GTPases Rac and Rho (or at least their best studied representatives in mammals, Rac1 and RhoA) ultimately control different migration modes, i.e. mesenchymal migration by Rac and amoeboid migration by Rho. During mesenchymal migration, Rac activity dominates at the leading edge (yellow), while Rho signaling is enhanced toward the cell rear (blue), leading to the formation of a lamellipodium at the leading edge. (B) Amoeboid movement is characterized by high levels of active Rho and decreased Rac signaling, causing elevated contractility giving rise to plasma membrane blebbing. (C) Rac and Rho signaling displays a mutual antagonism at multiple levels. For details see text.
On top of this, there is extensive negative cross-talk between Rac and Rho. This mutual antagonism is driven, at least in part, by activation or inactivation of GAPs and GEFs, respectively. For instance, Rac has been shown to directly activate p190RhoGAP [55] or to inhibit the RhoA-GEF GEF-H1 via its effector PAK [46,56]. On the contrary, the RhoA effector ROCK can activate the Rac-GAP FilGAP through phosphorylation [57] or indirectly activate the Rac-GAP ArhGAP22 [58]. Furthermore, the focal adhesion-localized Rac-GEF β-Pix is sensitive to tension and thereby negatively regulated by RhoA [59].
Despite the multitude of molecular mechanisms mediating antagonistic activities by directly impacting on Rho-GTPase regulation, phospholipase C gamma (PLC-γ) activity has also been shown to be essential for mesenchymal chemotaxis toward PDGF by selective inhibition of the respective downstream effector machinery of RhoA, more specifically myosin II at the leading edge [60]. Downstream of PLC-γ, this pathway involves PKCα activation through diacylglycerol production and subsequent inhibitory phosphorylation of myosin II. Although PLC isozymes are commonly activated downstream of G protein-coupled receptors, it is again Rac amongst the Rho-family GTPases that has also been shown to stimulate PLC-β and -γ activity in cells. Here, Rac operates by binding to the PH and split-PH domain of PLC-β2 and PLC-γ2 isozymes, respectively [61,62], while other Rho GTPases such as RhoA and Cdc42 fail to do so. The crystal structures of Rac GTPases in complex with PLC-β2 or PLC-γ2 revealed the structural basis for this selectivity [63,64]. In addition, the Rac-PLC-γ2 interaction has been shown to amplify B cell receptor-induced Ca2+ signaling by means of rescue in PLC-γ2-deficient cells with a Rac binding-deficient PLC-γ2 mutant [65]. Notwithstanding this, future studies are required to determine the precise role of Rac-PLC interactions for motile processes such as lamellipodia formation and chemotaxis.
In spite of the regulation of actin network polymerization and actomyosin contraction in a polar fashion, Rac and Rho also participate in the regulation of phosphoinositide asymmetry. There is a well-described feed forward loop between Rac and class I PI3K that favors the production of phosphatidylinositol-3,4,5-trisphosphate at the protruding cell front [66]. On the contrary, Rho signaling can activate the PI3K antagonist PTEN via ROCK [67,68].
Relation and interconversion between lamellipodia, filopodia, and bleb protrusions
There are several examples in the literature in which interference with lamellipodia formation promotes plasma membrane blebbing. Inhibition of lamellipodia through blocking WRC or Arp2/3 complex function promotes formation of plasma membrane blebs in various cell types in distinct organisms [69–74]. Interestingly, it was found that the induction of blebs upon knockdown of WRC depended on the formin mDia2 [74], which had previously been proposed to operate in cortical actin assembly [75]. On the contrary, inhibition of ROCK or myosin II that interferes with blebbing can enhance lamellipodia formation at least transiently [27,73,76]. However, there are also examples where disruption of Rac, WRC, or Arp2/3 complex, and hence removal of lamellipodia causes excessive filopodia formation instead of blebbing [30,77–79]. Interestingly, in the recent past, we have found means to switch the protrusive behavior of B16-F1 melanoma cells between all these three types of protrusion. While B16-F1 cells typically form lamellipodia when plated on laminin, knockout of Rac GTPases or WRC blocks lamellipodia and induces filopodia formation [77]. Intriguingly, however, when WRC-KO cells overexpress constitutively active Rac, the formation of filopodia appears to be impaired and cells start forming blebs ([80] and Figure 2A). A similar pattern has been found in spreading fibroblastoid cells, in which the Arp2/3 complex was disrupted [30]. Filopodia induced by this treatment appeared compromised upon additional inhibition with the antiformin compound SMIFH2, again leading to cell blebbing [30]. One explanation for this observation could be that Rac is interfering with filopodia/bundling formation. Indeed, microinjection of active Rac leads to immediate suppression of filopodia [78]. Interestingly, SMIFH2 treatments were also described to switch cell protrusion from lamellipodia to blebs [81]. Furthermore, specific isoforms of the formins mDia2 and FMNL1 are known to be capable of switching between filopodia and bleb induction [82,83]. In a ‘wildtype’ context, mDia2 is a well-established inducer of filopodia [84–86]. Whereas filopodia induction by WRC knockdown required mDia2, WAVE2 overexpression in these conditions promoted blebbing, again dependent on mDia2 [74]. This indicates that mDia2 activity might drive filopodia formation or membrane blebbing, dependent on context. Along these lines, the Rho GTPase Cdc42 was also observed to mediate a switch from bleb to filopodia formation, as its microinjection as active variant into blebbing, WRC-depleted cells triggered filopodia at the expense of blebbing ([31], and K.R., unpublished data). As already emphasized previously [87], mDia formins can be activated by both Cdc42 and RhoA, which have been linked to filopodia formation and actin cortex assembly, respectively. It is tempting to speculate that the spatial activation of respective mDia formins by either RhoA or Cdc42 and their subsequent engagement into locally established signaling complexes could determine whether they engage in filopodia formation or actin cortex assembly. Notably, as opposed to mDia2, active mDia1 accumulates at the rear and appears much less active than mDia2 concerning filopodia induction in otherwise comparable conditions [47,85,86]. Whether or not this can be solely explained by potential, differential regulation through RhoA versus Cdc42 signaling will have to be worked out in detailed, future studies. Irrespective of the outcome and inspired by previous models based on the apparent antagonism between Arp2/3-dependent, lamellipodia-based protrusion and contractility-dependent blebbing [73], we propose that specific cell context, meaning given protein repertoire and signaling status will define whether low Arp2/3 complex activity at the plasma membrane finally translates into blebbing or alternatively filopodia formation (Figure 2).
Regulation of WRC localization, activity, and turnover
Amongst the different mechanisms of protrusion at migrating cell fronts, the lamellipodium constitutes a structure particularly relevant for effective migration, at least in case of mesenchymal cells migrating on flat, adhesive structures, both in vitro and in vivo [28,88]. In recent years, we and others have thus systematically dissected the core actin assembly machinery in lamellipodia (i.e. Rac-WRC-Arp2/3 complex) because continuous protrusion of these structures and associated force development is driven by actin polymerization regulated by this machinery.
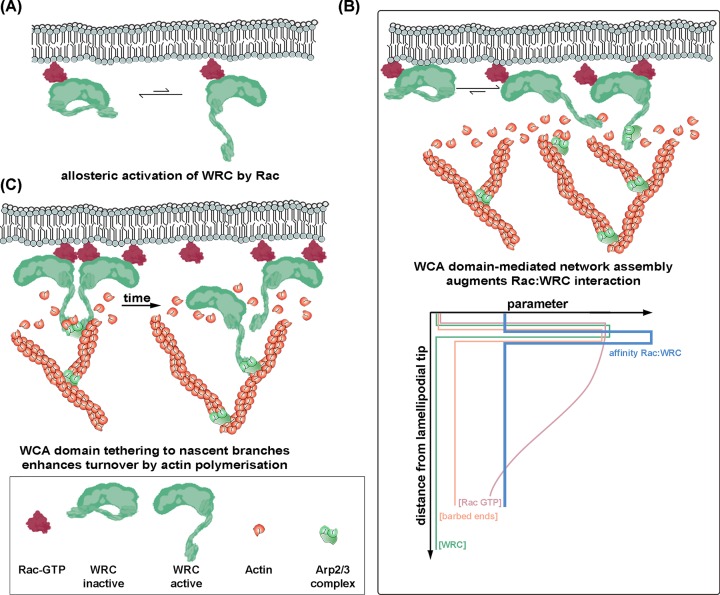
During initiation of lamellipodia protrusion, Rac function appears to be essential for activation of WRC. WRC possesses two binding sites for Rac [89,90] and although the so-called A site appears to be more crucial for allosteric activation in cells, both sites are required for optimal lamellipodial protrusion [77]. Furthermore, the Arp2/3 complex is thought to be most effectively activated by simultaneous engagement of two WCA domains [5–7], meaning four GTP-bound Rac molecules may be required for efficient activation of one Arp2/3 complex unit during the initiation phase of lamellipodia formation. Interestingly, Rac GTPase was recently shown to form nanoclusters in protruding regions of polarized cells [91,92]. It is tempting to speculate therefore that nanoclustering promotes protrusion by elevating local Rac concentrations above thresholds required for optimal Arp2/3 activation, while simultaneously enhancing the likelihood of spatially restricted signaling, ensuring establishment and maintenance of polarity.
Once an Arp2/3 complex-mediated lamellipodial actin network has formed, it still relies on continuous Rac/WRC signaling. While Rac appears active up to several micrometers from the edges of lamellipodia into more proximal parts of the plasma membrane [40,42], WRC shows specific accumulation at lamellipodia tips [70,77]. This suggests that active Rac alone is not sufficient for WRC localization at the lamellipodium tip. In fact, previously published work suggested interactions with both proteins and lipids to aid WRC accumulation at this site, e.g., with IRSp53, lamellipodin (Lpd) and the leading edge phospholipid phosphatidylinositol-3,4,5-trisphosphate [93–95]. Along these lines, recent single molecule tracking experiments of Rac at the lamellipodium tip confirmed surprisingly short residency times as compared to WRC, but they also revealed Rac immobilization to positively correlate with its activity state and effector binding, including WRC [96].
Although short-lived and local, continuous Rac interactions may thus still contribute to the maintenance of WRC mediating protrusion. Together, multivalency of WRC interactions, repeatedly either with Rac or additional WRC binding proteins and lipids appears crucial for its effective targeting and function as Arp2/3 complex activator in continuous lamellipodium protrusion.
In spite of aforementioned considerations, an alternative interpretation of existing data would be that Rac also contributes to localising WRC at the lamellipodium tip, last not least because constitutively active WRC lacking functional Rac binding sites shows strong impairment in lamellipodia induction and accumulation at their tips [77,80]. This conclusion is not necessarily incompatible with comparably transient residency times of Rac at lamellipodia tips [96]. Indeed, there is growing evidence that WRC accumulation and/or maintenance at protruding tips requires loose or transient interactions with lamellipodial building blocks, i.e. Arp2/3 complex and actin monomers that may positively feed back onto WRC-Rac interactions and can be considered crucial for the following reasons: I.) Actin monomers were described to accumulate at the tips of lamellipodia [97,98]. II.) Constitutively active WRC, i.e. WRC harboring a WCA domain released from transinhibition normally mediated by Sra-1/PIR121-binding shows strongly enhanced residency time at lamellipodial tips, which depended on both Rac binding sites [77]. III.) Constitutively active WRC has increased affinity for Rac [89,90]. IV. Immobilization of Rac at lamellipodial tips is mediated, at least in part, by WRC-binding [96]. Similar observations were previously made with the WAVE-related NPF N-WASP, reported to constitute a critical Arp2/3 complex activator in distinct subcellular structures. Its two actin binding WH2 domains had previously been implicated in filament barbed end capture [99], and WH2 domain-mutation decreased the dwell time of N-WASP in vaccinia virus-induced actin tails, which usurps this protein as essential Arp2/3 complex activator in viral actin tail formation functioning in viral spread at the plasma membrane [100–102]. Dwell time reduction of N-WASP close to the virus coincided with reduced tail formation frequency, indicating N-WASP stabilization to positively correlate at least with tail initiation [102].
In the alternative view proposed here, transient interactions of WRC's WCA domain with filament barbed ends as well as Arp2/3 complex and actin monomers at the lamellipodium tip may thus delay or counteract its inactivation, thereby enhancing WRC affinity toward Rac (Figure 4B). Similar positive feedback mechanisms have been suggested for lamellipodin, VASP and FMNL2, all of which accumulate at lamellipodia tips and for which actin filament binding at least contributes to lamellipodial targeting ( [103,104], and Frieda Kage, personal communication).
Figure 4. WRC accumulation and turnover at lamellipodia.
(A) Allosteric activation of WRC requires active Rac GTPase prior to lamellipodia formation. (B) During lamellipodium protrusion, WRC’s WCA domain continuously binds actin and Arp2/3 complex monomers, likely inhibiting its inactivation, thereby in turn fostering binding to Rac, and enhancing its accumulation at the tips of protruding lamellipodia. The panel at the bottom displays gradients of Rac-GTP levels (rosy) versus concentrations of actin filament barbed ends (orange) or WRC (green) from distal to proximal lamellipodial regions, as indicated, proposed to cause an affinity increase between Rac and WRC at the lamellipodium tip (blue). Illustration inspired by models of actin or actin binding protein distributions at the leading edge published recently [12]. Note that sharp edges of parameter changes from distal to proximal lamellipodial regions are not intended to indicate true abrupt changes, but aid illustration purposes. (C) Replacement and thus turnover of WRCs upon successful branch formation may be achieved (or at least aided) by sustained binding of its WCA domain to nascent branches presumed to travel rearward during lamellipodium protrusion [108,109]. This may provide a mechanism for pulling WRC off the membrane and into the network, leading to its retrograde motion and inactivation (the latter not shown). Note that for the sake of simplicity, this model of WRC regulation by Rac, actin and Arp2/3 complex binding (for details see text) does not include WRC interactions with additional lamellipodial regulators, such as lamellipodin (Lpd, see [93]) or Ena/VASP family members, which were likewise reported to contribute to WRC activation and stabilization [110].
Finally, turnover of WRC at the lamellipodium, at least in part, may be regulated by a mechanism also involving its WCA domain. For daughter filament generation, NPF-bound Arp2/3 complex has to stably attach to a mother filament. Using elegant triple-colour TIRF assays, it was recently shown that N-WASP detachment from Arp2/3 complex at nascent branches takes several seconds in vitro [105]. Transferring this into a context of a growing network where the NPF stays attached to the protruding membrane such as in the lamellipodium would mean that WRC might be exposed to tension, created by the polymerising network, pulling it away from the lamellipodium tip. Consistent with this would be that a fraction of WRC was previously observed to undergo retrograde flow along with the lamellipodial network [106]. Furthermore, blocking actin polymerization was described to interfere with turnover of both WRC at the lamellipodium tip and N-WASP at Vaccinia virus surfaces, and deleting the Arp2/3 complex binding CA-region of N-WASP dramatically enhanced the residency time of the latter on the virus [102,107]. Together, all these data suggest that active, Arp2/3-dependent actin assembly may actually significantly contribute to NPF removal from sites of Arp2/3 complex activation, and thus directly contribute to NPF turnover at respective sites, but how this occurs precisely, remains to be established.
Summary
Actin filament assembly and disassembly is regulated by a variety of proteins in need of tight coordination.
Actin-dependent plasma membrane protrusions differentially involve polymerization of actin networks, bundles or contractile structures to exert forces onto and thus shape the plasma membrane.
Rac and Rho GTPases antagonize each other at multiple levels, and control cell polarization.
Accumulation and turnover of WAVE regulatory complex is controlled by complex positive and negative feedback loops, tuning the efficiency of lamellipodial actin network formation and turnover.
Acknowledgments
We would like to thank all members of our labs for continuous and fruitful discussions, and Frieda Kage for sharing unpublished data.
Abbreviations
- Arp
actin-related protein
- FRET
fluorescence resonance energy transfer
- NPF
nucleation promoting factor
- WASP
Wiskott–Aldrich syndrome protein
- WAVE
WASP-family verprolin-homologous protein
- WRC
WAVE regulatory complex
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported in part by the Deutsche Forschungsgemeinschaft [grant number GRK2223 to (K.R.)]. Research in the Giannone-lab is funded by the French Ministry of Research and CNRS, ANR grants Integractome and FastNano as well as the Fondation pour la Recherche Médicale.
References
- 1.Horwitz A.R. and Parsons J.T. (1999) Cell migration–movin’ on. Science 286, 1102–1103 10.1126/science.286.5442.1102 [DOI] [PubMed] [Google Scholar]
- 2.Skruber K., Read T.-A. and Vitriol E.A. (2018) Reconsidering an active role for G-actin in cytoskeletal regulation. J. Cell Sci. 131, jcs203760 10.1242/jcs.203760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotty J.D., Wu C. and Bear J.E. (2013) New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 14, 7–12 10.1038/nrm3492 [DOI] [PubMed] [Google Scholar]
- 4.dominguez R. (2016) The WH2 domain and actin nucleation: necessary but insufficient. Trends Biochem. Sci. 41, 478–490 10.1016/j.tibs.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boczkowska M., Rebowski G., Kast D.J. and Dominguez R. (2014) Structural analysis of the transitional state of Arp2/3 complex activation by two actin-bound WCAs. Nat. Commun. 5, 3308 10.1038/ncomms4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padrick S.B., Doolittle L.K., Brautigam C.A., King D.S. and Rosen M.K. (2011) Arp2/3 complex is bound and activated by two WASP proteins. Proc. Natl Acad. Sci. U.S.A. 108, E472–E479 10.1073/pnas.1100236108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padrick S.B., Cheng H.-C., Ismail A.M., Panchal S.C., Doolittle L.K., Kim S. et al. (2008) Hierarchical regulation of WASP/WAVE proteins. Mol. Cell 32, 426–438 10.1016/j.molcel.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitsprecher D. and Goode B.L. (2013) Formins at a glance. J. Cell Sci. 126, 1–7 10.1242/jcs.107250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kühn S. and Geyer M. (2014) Formins as effector proteins of Rho GTPases. Small GTPases 5, e983876 10.4161/sgtp.29513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bear J.E. and Gertler F.B. (2009) Ena/VASP: towards resolving a pointed controversy at the barbed end. J. Cell Sci. 122, 1947–1953 10.1242/jcs.038125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brühmann S., Ushakov D.S., Winterhoff M., Dickinson R.B., Curth U. and Faix J. (2017) Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays. Proc. Natl Acad. Sci. U.S.A. 114, E5815–E5824 10.1073/pnas.1703145114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bieling P., Hansen S.D., Akin O., Li T.-D., Hayden C.C., Fletcher D.A. et al. (2018) WH2 and proline‐rich domains of WASP‐family proteins collaborate to accelerate actin filament elongation. EMBO J. 37, 102–121 10.15252/embj.201797039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards M., Zwolak A., Schafer D.A., Sept D., Dominguez R. and Cooper J.A. (2014) Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 15, 677–689 10.1038/nrm3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small J.V., Stradal T., Vignal E. and Rottner K. (2002) The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120 10.1016/S0962-8924(01)02237-1 [DOI] [PubMed] [Google Scholar]
- 15.Sokolova O.S., Chemeris A., Guo S., Alioto S.L., Gandhi M., Padrick S. et al. (2017) Structural basis of arp2/3 complex inhibition by GMF, coronin, and arpin. J. Mol. Biol. 429, 237–248 10.1016/j.jmb.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan C., Beltzner C.C. and Pollard T.D. (2009) Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr. Biol. 19, 537–545 10.1016/j.cub.2009.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez C., Roland J., Boujemaa-Paterski R., Kang H., McCullough B.R., Reymann A.-C. et al. (2011) Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr. Biol. 21, 862–868 10.1016/j.cub.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni A.V. and Brieher W.M. (2014) Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends. Curr. Biol. 24, 2749–2757 10.1016/j.cub.2014.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanellos G. and Frame M.C. (2016) Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci. 129, 3211–3218 10.1242/jcs.187849 [DOI] [PubMed] [Google Scholar]
- 20.Wioland H., Guichard B., Senju Y., Myram S., Lappalainen P., Jégou A. et al. (2017) ADF/cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol. 27, 1956.e7–1967.e7 10.1016/j.cub.2017.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilton D.M., Aguilar R.M., Johnston A.B. and Goode B.L. (2018) Species-specific functions of twinfilin in actin filament depolymerization. J. Mol. Biol. 430, 3323–3336 10.1016/j.jmb.2018.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston A.B., Collins A. and Goode B.L. (2015) High-speed depolymerization at actin filament ends jointly catalysed by Twinfilin and Srv2/CAP. Nat. Cell Biol. 17, 1504–1511 10.1038/ncb3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldschmidt-Clermont P.J., Machesky L.M., Doberstein S.K. and Pollard T.D. (1991) Mechanism of the interaction of human platelet profilin with actin. J. Cell Biol. 113, 1081–1089 10.1083/jcb.113.5.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mockrin S.C. and Korn E.D. (1980) Acanthamoeba profilin interacts with G-actin to increase the rate of exchange of actin-bound adenosine 5’-triphosphate. Biochemistry 19, 5359–5362 10.1021/bi00564a033 [DOI] [PubMed] [Google Scholar]
- 25.Nishida E. (1985) Opposite effects of cofilin and profilin from porcine brain on rate of exchange of actin-bound adenosine 5’-triphosphate. Biochemistry 24, 1160–1164 10.1021/bi00326a015 [DOI] [PubMed] [Google Scholar]
- 26.Kotila T., Kogan K., Enkavi G., Guo S., Vattulainen I., Goode B.L. et al. (2018) Structural basis of actin monomer re-charging by cyclase-associated protein. Nat. Commun. 9, 1892 10.1038/s41467-018-04231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oakes P.W., Bidone T.C., Beckham Y., Skeeters A.V., Juan G.R.R.-S., Winter S.P. et al. (2018) Lamellipodium is a myosin-independent mechanosensor. Proc. Natl Acad. Sci. 115, 2646–2651 10.1073/pnas.1715869115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rottner K., Faix J., Bogdan S., Linder S. and Kerkhoff E. (2017) Actin assembly mechanisms at a glance. J. Cell Sci. 130, 3427–3435 10.1242/jcs.206433 [DOI] [PubMed] [Google Scholar]
- 29.Rottner K. and Schaks M. (2019) Assembling actin filaments for protrusion. Curr. Opin. Cell Biol. 56, 53–63 10.1016/j.ceb.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Suraneni P., Fogelson B., Rubinstein B., Noguera P., Volkmann N., Hanein D. et al. (2015) A mechanism of leading-edge protrusion in the absence of Arp2/3 complex. MBoC 26, 901–912 10.1091/mbc.E14-07-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffen A., Faix J., Resch G.P., Linkner J., Wehland J., Small J.V. et al. (2006) Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. MBoC 17, 2581–2591 10.1091/mbc.e05-11-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C., Asokan S.B., Berginski M.E., Haynes E.M., Sharpless N.E., Griffith J.D. et al. (2012) Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 148, 973–987 10.1016/j.cell.2011.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson‐Dykstra S.M. and Higgs H.N. (2008) Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes. Cell Motil. 65, 904–922 10.1002/cm.20312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquemet G., Hamidi H. and Ivaska J. (2015) Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 36, 23–31 10.1016/j.ceb.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Bornschlögl T. (2013) How filopodia pull: what we know about the mechanics and dynamics of filopodia. Cytoskeleton 70, 590–603 10.1002/cm.21130 [DOI] [PubMed] [Google Scholar]
- 36.Fischer R.S., Lam P.-Y., Huttenlocher A. and Waterman C.M. (2019) Filopodia and focal adhesions: an integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev. Biol. 451, 86–95 10.1016/j.ydbio.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paluch E.K. and Raz E. (2013) The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 25, 582–590 10.1016/j.ceb.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charras G. and Paluch E. (2008) Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730–736 10.1038/nrm2453 [DOI] [PubMed] [Google Scholar]
- 39.Charras G.T., Yarrow J.C., Horton M.A., Mahadevan L. and Mitchison T.J. (2005) Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365 10.1038/nature03550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin K., Reimann A., Fritz R.D., Ryu H., Jeon N.L. and Pertz O. (2016) Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics. Sci. Rep. 6, 21901 10.1038/srep21901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iseppon F., Napolitano L.M.R., Torre V. and Cojoc D. (2015) Cdc42 and RhoA reveal different spatio-temporal dynamics upon local stimulation with Semaphorin-3A. Front. Cell. Neurosci. [cited 2019 May 21];9. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2015.00333/full 10.3389/fncel.2015.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P. et al. (2009) Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103 10.1038/nature08242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isogai T. and Danuser G. (2018) Discovery of functional interactions among actin regulators by analysis of image fluctuations in an unperturbed motile cell system. Philos. Trans. Royal Soc. B 373, 20170110 10.1098/rstb.2017.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isogai T., van der Kammen R., Leyton-Puig D., Kedziora K.M., Jalink K. and Innocenti M. (2015) Initiation of lamellipodia and ruffles involves cooperation between mDia1 and the Arp2/3 complex. J. Cell Sci. 128, 3796–3810 10.1242/jcs.176768 [DOI] [PubMed] [Google Scholar]
- 45.Pertz O., Hodgson L., Klemke R.L. and Hahn K.M. (2006) Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069 10.1038/nature04665 [DOI] [PubMed] [Google Scholar]
- 46.Byrne K.M., Monsefi N., Dawson J.C., Degasperi A., Bukowski-Wills J.-C., Volinsky N. et al. (2016) Bistability in the Rac1, PAK, and RhoA signaling network drives actin cytoskeleton dynamics and cell motility switches. Cell Systems 2, 38–48 10.1016/j.cels.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Litschko C., Brühmann S., Csiszár A., Stephan T., Dimchev V., Damiano-Guercio J. et al. (2019) Functional integrity of the contractile actin cortex is safeguarded by multiple Diaphanous-related formins. Proc. Natl Acad. Sci. U.S.A. 116, 3594–3603 10.1073/pnas.1821638116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramalingam N., Franke C., Jaschinski E., Winterhoff M., Lu Y., Brühmann S. et al. (2015) A resilient formin-derived cortical actin meshwork in the rear drives actomyosin-based motility in 2D confinement. Nat. Commun. 6, 8496 10.1038/ncomms9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe N., Kato T., Fujita A., Ishizaki T. and Narumiya S. (1999) Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136 10.1038/11056 [DOI] [PubMed] [Google Scholar]
- 50.Chrzanowska-Wodnicka M. and Burridge K. (1996) Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403–1415 10.1083/jcb.133.6.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner O.D., Rentel M.C., Ott A., Brown G.E., Jedrychowski M., Yaffe M.B. et al. (2006) Hem-1 complexes are essential for rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 4, e38 10.1371/journal.pbio.0040038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koestler S.A., Steffen A., Nemethova M., Winterhoff M., Luo N., Holleboom J.M. et al. (2013) Arp2/3 complex is essential for actin network treadmilling as well as for targeting of capping protein and cofilin. MBoC 24, 2861–2875 10.1091/mbc.e12-12-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q., Zhang X.-F., Pollard T.D. and Forscher P. (2012) Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J. Cell Biol. 197, 939–956 10.1083/jcb.201111052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reymann A.-C., Boujemaa-Paterski R., Martiel J.-L., Guérin C., Cao W., Chin H.F. et al. (2012) Actin network architecture can determine myosin motor activity. Science 336, 1310–1314 10.1126/science.1221708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bustos R.I., Forget M.-A., Settleman J.E. and Hansen S.H. (2008) Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 18, 1606–1611 10.1016/j.cub.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zenke F.T., Krendel M., DerMardirossian C., King C.C., Bohl B.P. and Bokoch G.M. (2004) p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized rho exchange factor. J. Biol. Chem. 279, 18392–18400 10.1074/jbc.M400084200 [DOI] [PubMed] [Google Scholar]
- 57.Saito K., Ozawa Y., Hibino K. and Ohta Y. (2012) FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. MBoC 23, 4739–4750 10.1091/mbc.e12-04-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S. et al. (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523 10.1016/j.cell.2008.09.043 [DOI] [PubMed] [Google Scholar]
- 59.Kuo J.-C., Han X., Hsiao C.-T., Yates Iii J.R. and Waterman C.M. (2011) Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asokan S.B., Johnson H.E., Rahman A., King S.J., Rotty J.D., Lebedeva I.P. et al. (2014) Mesenchymal chemotaxis requires selective inactivation of myosin II at the leading edge via a noncanonical PLCγ/PKCα pathway. Dev. Cell 31, 747–760 10.1016/j.devcel.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snyder J.T., Singer A.U., Wing M.R., Harden T.K. and Sondek J. (2003) The pleckstrin homology domain of phospholipase C-β2 as an effector site for rac. J. Biol. Chem. 278, 21099–21104 10.1074/jbc.M301418200 [DOI] [PubMed] [Google Scholar]
- 62.Piechulek T., Rehlen T., Walliser C., Vatter P., Moepps B. and Gierschik P. (2005) Isozyme-specific stimulation of phospholipase C-γ2 by Rac GTPases. J. Biol. Chem. 280, 38923–38931 10.1074/jbc.M509396200 [DOI] [PubMed] [Google Scholar]
- 63.Jezyk M.R., Snyder J.T., Gershberg S., Worthylake D.K., Harden T.K. and Sondek J. (2006) Crystal structure of Rac1 bound to its effector phospholipase C-β2. Nat. Struct. Mol. Biol. 13, 1135 10.1038/nsmb1175 [DOI] [PubMed] [Google Scholar]
- 64.Bunney T.D., Opaleye O., Roe S.M., Vatter P., Baxendale R.W., Walliser C. et al. (2009) Structural insights into formation of an active signaling complex between Rac and phospholipase C gamma 2. Mol. Cell 34, 223–233 10.1016/j.molcel.2009.02.023 [DOI] [PubMed] [Google Scholar]
- 65.Walliser C., Tron K., Clauß K., Gutman O., Kobitski A.Y., Retlich M. et al. (2015) Rac-mediated stimulation of phospholipase C-γ2 amplifies B cell receptor-induced calcium signaling. J. Biol. Chem. jbc.M115.645739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welch H.C.E., Coadwell W.J., Stephens L.R. and Hawkins P.T. (2003) Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546, 93–97 10.1016/S0014-5793(03)00454-X [DOI] [PubMed] [Google Scholar]
- 67.Vemula S., Shi J., Hanneman P., Wei L. and Kapur R. (2010) ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood 115, 1785–1796 10.1182/blood-2009-08-237222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z., Dong X., Wang Z., Liu W., Deng N., Ding Y. et al. (2005) Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7, 399 10.1038/ncb1236 [DOI] [PubMed] [Google Scholar]
- 69.Derivery E., Fink J., Martin D., Houdusse A., Piel M., Stradal T.E. et al. (2008) Free brick1 is a trimeric precursor in the assembly of a functional wave complex. PLoS ONE 3, e2462 10.1371/journal.pone.0002462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steffen A., Rottner K., Ehinger J., Innocenti M., Scita G., Wehland J. et al. (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23, 749–759 10.1038/sj.emboj.7600084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davidson A.J., Amato C., Thomason P.A. and Insall R.H. (2018) WASP family proteins and formins compete in pseudopod- and bleb-based migration. J. Cell Biol. 217, 701–714 10.1083/jcb.201705160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chikina A.S., Svitkina T.M. and Alexandrova A.Y. (2019) Time-resolved ultrastructure of the cortical actin cytoskeleton in dynamic membrane blebs. J. Cell Biol. 218, 445–454 10.1083/jcb.201806075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergert M., Chandradoss S.D., Desai R.A. and Paluch E. (2012) Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc. Natl Acad. Sci. U.S.A. 109, 14434–14439 10.1073/pnas.1207968109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beli P., Mascheroni D., Xu D. and Innocenti M. (2008) WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 10, 849–857 10.1038/ncb1745 [DOI] [PubMed] [Google Scholar]
- 75.Eisenmann K.M., Harris E.S., Kitchen S.M., Holman H.A., Higgs H.N. and Alberts A.S. (2007) Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr. Biol. 17, 579–591 10.1016/j.cub.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 76.Koestler S.A., Auinger S., Vinzenz M., Rottner K. and Small J.V. (2008) Differentially oriented populations of actin filaments generated in lamellipodia collaborate in pushing and pausing at the cell front. Nat. Cell Biol. 10, 306–313 10.1038/ncb1692 [DOI] [PubMed] [Google Scholar]
- 77.Schaks M., Singh S.P., Kage F., Thomason P., Klünemann T., Steffen A. et al. (2018) Distinct interaction sites of Rac GTPase with WAVE regulatory complex have non-redundant functions in vivo. Curr. Biol. 28, 3674.e6–3684.e6 10.1016/j.cub.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steffen A., Ladwein M., Dimchev G.A., Hein A., Schwenkmezger L., Arens S. et al. (2013) Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J. Cell Sci. 126, 4572–4588 10.1242/jcs.118232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suraneni P., Rubinstein B., Unruh J.R., Durnin M., Hanein D. and Li R. (2012) The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J. Cell Biol. 197, 239–251 10.1083/jcb.201112113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaks M., Döring H., Kage F., Steffen A., Klünemann T., Blankenfeldt W. et al. (2019) RhoG and Cdc42 can contribute to Rac-dependent lamellipodia formation through WAVE regulatory complex-binding. Small GTPases 0, 1–11 10.1080/21541248.2019.1657755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizvi S.A., Neidt E.M., Cui J., Feiger Z., Skau C.T., Gardel M.L. et al. (2009) Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem. Biol. 16, 1158–1168 10.1016/j.chembiol.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stastna J., Pan X., Wang H., Kollmannsperger A., Kutscheidt S., Lohmann V. et al. (2012) Differing and isoform-specific roles for the formin DIAPH3 in plasma membrane blebbing and filopodia formation. Cell Res. 22, 728–745 10.1038/cr.2011.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han Y., Eppinger E., Schuster I.G., Weigand L.U., Liang X., Kremmer E. et al. (2009) Formin-like 1 (FMNL1) is regulated by n-terminal myristoylation and induces polarized membrane blebbing. J. Biol. Chem 284, 33409–33417 10.1074/jbc.M109.060699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pellegrin S. and Mellor H. (2005) The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 15, 129–133 10.1016/j.cub.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 85.Block J., Stradal T.E.B., Hänisch J., Geffers R., Köstler S.A., Urban E. et al. (2008) Filopodia formation induced by active mDia2/Drf3. J. Microsc. 231, 506–517 10.1111/j.1365-2818.2008.02063.x [DOI] [PubMed] [Google Scholar]
- 86.Yang C., Czech L., Gerboth S., Kojima S., Scita G. and Svitkina T. (2007) Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 5, e317 10.1371/journal.pbio.0050317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mellor H. (2010) The role of formins in filopodia formation. Biochim. Biophys. Acta 1803, 191–200 10.1016/j.bbamcr.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 88.Krause M. and Gautreau A. (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 10.1038/nrm3861 [DOI] [PubMed] [Google Scholar]
- 89.Chen Z., Borek D., Padrick S.B., Gomez T.S., Metlagel Z., Ismail A.M. et al. (2010) Structure and control of the actin regulatory WAVE complex. Nature 468, 533–538 10.1038/nature09623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen B., Chou H.-T., Brautigam C.A., Xing W., Yang S., Henry L. et al. (2017) Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. Elife 6, 10.7554/eLife.29795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maxwell K.N., Zhou Y. and Hancock J.F. (2018) Rac1 nanoscale organization on the plasma membrane is driven by lipid binding specificity encoded in the membrane anchor. Mol. Cell Biol. 10.1128/MCB.00186-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Remorino A., De Beco S., Cayrac F., Di Federico F., Cornilleau G., Gautreau A. et al. (2017) Gradients of Rac1 nanoclusters support spatial patterns of Rac1 signaling. Cell Rep. 21, 1922–1935 10.1016/j.celrep.2017.10.069 [DOI] [PubMed] [Google Scholar]
- 93.Law A.-L., Vehlow A., Kotini M., Dodgson L., Soong D., Theveneau E. et al. (2013) Lamellipodin and the Scar/WAVE complex cooperate to promote cell migration in vivo. J. Cell Biol. 203, 673–689 10.1083/jcb.201304051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oikawa T., Yamaguchi H., Itoh T., Kato M., Ijuin T., Yamazaki D. et al. (2004) PtdIns(3,4,5)P 3 binding is necessary for WAVE2-induced formation of lamellipodia. Nat. Cell Biol. 6, 420 10.1038/ncb1125 [DOI] [PubMed] [Google Scholar]
- 95.Suetsugu S., Kurisu S., Oikawa T., Yamazaki D., Oda A. and Takenawa T. (2006) Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP3, and Rac. J. Cell Biol. 173, 571–585 10.1083/jcb.200509067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehidi A., Rossier O., Schaks M., Chazeau A., Binamé F., Remorino A. et al. (2019) Transient activations of Rac1 at the lamellipodium tip trigger membrane protrusion. Curr. Biol. 10.1016/j.cub.2019.07.035 [DOI] [PubMed] [Google Scholar]
- 97.Lee C.W., Vitriol E.A., Shim S., Wise A.L., Velayutham R.P. and Zheng J.Q. (2013) Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr. Biol. 23, 1046–1056 10.1016/j.cub.2013.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vitriol E.A., McMillen L.M., Kapustina M., Gomez S.M., Vavylonis D. and Zheng J.Q. (2015) Two functionally distinct sources of actin monomers supply the leading edge of lamellipodia. Cell Rep. 11, 433–445 10.1016/j.celrep.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Co C., Wong D.T., Gierke S., Chang V. and Taunton J. (2007) Mechanism of actin network attachment to moving membranes: barbed end capture by N-WASP WH2 domains. Cell 128, 901–913 10.1016/j.cell.2006.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snapper S.B., Takeshima F., Antón I., Liu C.-H., Thomas S.M., Nguyen D. et al. (2001) N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. 3, 897 10.1038/ncb1001-897 [DOI] [PubMed] [Google Scholar]
- 101.Leite F. and Way M. (2015) The role of signaling and the cytoskeleton during Vaccinia Virus egress. Virus Res. 209, 87–99 10.1016/j.virusres.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 102.Weisswange I., Newsome T.P., Schleich S. and Way M. (2009) The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature 458, 87–91 10.1038/nature07773 [DOI] [PubMed] [Google Scholar]
- 103.Hansen S.D. and Mullins R.D. (2015) Lamellipodin promotes actin assembly by clustering Ena/VASP proteins and tethering them to actin filaments. Lappalainen P, editor. eLife 4, e06585 10.7554/eLife.06585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loureiro J.J., Rubinson D.A., Bear J.E., Baltus G.A., Kwiatkowski A.V. and Gertler F.B. (2002) Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. MBoC 13, 2533–2546 10.1091/mbc.e01-10-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith B.A., Padrick S.B., Doolittle L.K., Daugherty-Clarke K., Corrêa I.R. Jr, Xu M.-Q. et al. (2013) Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. Sundquist W, editor. eLife 2, e01008 10.7554/eLife.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Millius A., Watanabe N. and Weiner O.D. (2012) Diffusion, capture and recycling of SCAR/WAVE and Arp2/3 complexes observed in cells by single-molecule imaging. J. Cell Sci. 125, 1165–1176 10.1242/jcs.091157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weiner O.D., Marganski W.A., Wu L.F., Altschuler S.J. and Kirschner M.W. (2007) An actin-based wave generator organizes cell motility. PLoS Biol. 5, e221 10.1371/journal.pbio.0050221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwasa J.H. and Mullins R.D. (2007) Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr. Biol. 17, 395–406 10.1016/j.cub.2007.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai F.P., Szczodrak M., Block J., Faix J., Breitsprecher D., Mannherz H.G. et al. (2008) Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 27, 982–992 10.1038/emboj.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X.J., Squarr A.J., Stephan R., Chen B., Higgins T.E., Barry D.J. et al. (2014) Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev. Cell 30, 569–584 10.1016/j.devcel.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]