Abstract
Background
Peripheral arterial disease affects five per cent of men and women by late middle age. Approximately 25% of those affected will develop critical limb ischaemia (rest pain, ulceration and gangrene) within five years. Naftidrofuryl is a vasoactive drug which may be beneficial in the treatment of critical limb ischaemia.
Objectives
To determine whether naftidrofuryl, when administered intravenously, is effective in alleviating symptoms and reducing progression of disease in patients with critical limb ischaemia.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched May 2012) and CENTRAL (2012, Issue 4). We searched the reference lists of articles. We also contacted pharmaceutical companies for any unpublished trials.
Selection criteria
All randomised controlled trials of critical limb ischaemia in which participants were randomly allocated to intravenous naftidrofuryl or control (either pharmacological, inert placebo or conservative therapy) were included. People with intermittent claudication were not included.
Data collection and analysis
Sixteen trials were identified, but eight were excluded because of poor methodology. The eight included trials involved a total of 269 participants from five different countries. The following outcomes were reported: pain reduction, rest pain/necrosis, progression of disease in terms of incidence of surgical reconstruction/amputation, mortality and side effects. On extraction of the data, odds ratios and mean differences were estimated where appropriate.
Main results
Treatment with naftidrofuryl tended to show reduction of pain evaluated by both analogue score and analgesic consumption, but the effect was statistically non‐significant (mean difference (MD): 0.42; 95% confidence interval (CI)1.19 to 0.35). Similarly, improvement in rest pain or skin necrosis occurred, but these effects were also non‐significant. The effect on mean ankle systolic pressure was inconclusive.
Authors' conclusions
Based on the results of these trials, it cannot be confirmed that intravenous naftidrofuryl is effective in the treatment of people with critical limb ischaemia. However, these results were based on trials of generally low methodological quality which had only a small number of participants, the duration of treatment was extremely short, and the methods varied between the trials. The wide range of endpoints effectively precluded any meaningful pooling of the results. Intravenous naftidrofuryl was withdrawn as a treatment for severe peripheral arterial disease in 1995 because of reported side effects.
Keywords: Female; Humans; Male; Amputation, Surgical; Amputation, Surgical/statistics & numerical data; Extremities; Extremities/blood supply; Infusions, Intravenous; Ischemia; Ischemia/drug therapy; Nafronyl; Nafronyl/administration & dosage; Nafronyl/therapeutic use; Pain; Pain/drug therapy; Pain Measurement; Pain Measurement/drug effects; Peripheral Vascular Diseases; Peripheral Vascular Diseases/drug therapy; Randomized Controlled Trials as Topic; Vasodilator Agents; Vasodilator Agents/administration & dosage; Vasodilator Agents/therapeutic use
Plain language summary
Intravenous naftidrofuryl for treating critical limb ischaemia
Peripheral arterial disease is relatively common, particularly in late middle age. Blockages in the leg arteries can reduce blood flow in the legs enough to cause cramping leg pain that limits walking (termed intermittent claudication). They can become severe and cause critical limb ischaemia, pain at rest, leg ulceration and gangrene that requires amputation. When a patient with critical limb ischaemia is being assessed for vascular surgery or if they are unsuitable or refuse surgery, they are treated conservatively as with bed rest. Drug therapy may be used to relieve symptoms and reduce progression of disease. Vasodilator drugs such as prostaglandins increase local blood flow to the leg but may not improve blockages (stenoses). Naftidrofuryl is also vasoactive and blocks serotonin. It has been used intravenously in severe critical limb ischaemia for rapid effect.
The review authors identified eight controlled trials that randomly allocated a total of 269 participants from five different countries to receive intravenous naftidrofuryl, other treatments, and placebo alone or with another treatment. There was no clear indication that short‐term intravenous naftidrofuryl significantly improves either symptoms of ischaemic rest pain or skin necrosis. Treatment with naftidrofuryl tended to reduce pain, measured using a scale or with analgesic consumption, and improve rest pain and skin necrosis but the effects were not clear (statistically significant). The trials were generally of low methodological quality, included small numbers of predominantly elderly participants with varying levels of severity of critical limb ischaemia and used different measures of effect. The duration of treatment was short, from three to 42 days, most often seven days. Other treatments were haemodilution, anti‐coagulant medication, prostaglandins, bed rest and reflex heating, and gingko biloba. Side effects included mild blood clotting (thrombophlebitis) at the injection site and in one trial two participants experienced renal insufficiency. Intravenous naftidrofuryl was withdrawn as a treatment for severe peripheral arterial disease in 1995 because of reported side effects.
Background
Description of the condition
Peripheral arterial disease is a relatively common condition and by late middle age about five per cent of men and women demonstrate symptoms of intermittent claudication. Approximately 25% of all claudicants referred to a peripheral vascular clinic progress to critical limb ischaemia (rest pain, ulceration and gangrene) within five years. Many of these people undergo major surgery and the amputation rate can be as high as five per cent (Leng 1993).
Description of the intervention
Conservative treatment, such as bed rest or exercise programmes, is initially used to treat the acute ischaemic symptoms in people who are either being assessed for revascularization, or are considered unsuitable for surgery. When standard therapy has been unsuccessful, or is not possible, drug therapy is an alternative option which may be considered. However, vasodilator drugs, such as prostanoids which increase local blood flow to the ischaemic limb, do not appear to improve haemodynamically significant stenoses.
How the intervention might work
The vasoactive drug, naftidrofuryl, can be administered either intravenously or orally. It is a serotonergic receptor antagonist which may be beneficial in the treatment of severe lower limb disease. Intravenous treatment has been preferred initially in severe critical limb ischaemia because of the more rapid conversion to active metabolites, and has been used at a higher dosage than the oral form. This drug increases oxidative metabolism and reduces lactic acidosis in ischaemic cells.
Why it is important to do this review
A meta‐analysis of clinical trials of the effect of oral naftidrofuryl on intermittent claudication found that walking distance was significantly improved in people treated with naftidrofuryl (Lehert 1990). Fewer trials have been conducted on more severe peripheral arterial disease, although naftidrofuryl has been widely prescribed in Europe for the treatment of critical limb ischaemia and was initially considered to have only minor side effects. This systematic review assessed the therapeutic effects of intravenous naftidrofuryl on critical limb ischaemia.
Objectives
To determine whether intravenous naftidrofuryl is effective in terms of alleviating symptoms and reducing progression of disease in people with critical limb ischaemia.
Methods
Criteria for considering studies for this review
Types of studies
The studies in this review include only those in which people with critical limb ischaemia (rest pain, ulceration or gangrene) were stated to be randomly allocated to either intravenous naftidrofuryl or control (either pharmacological, inert placebo or conservative therapy).
Types of participants
The participants were males and females of any age who had a diagnosis of critical limb ischaemia and who had been admitted to hospital for treatment. Participants were either unsuitable for reconstructive arterial surgery or were undergoing assessment for surgery or angioplasty. People with symptoms of intermittent claudication were not eligible for inclusion.
Types of interventions
Participants were randomly allocated to intravenous infusion of naftidrofuryl or control. Control interventions included conventional conservative therapy, e.g. bed rest, or other pharmacological therapy, or administration of an inert placebo such as isotonic saline.
Types of outcome measures
Primary outcomes
All cause mortality.
Reduction in pain assessed by analgesic requirements or pain analogue scales.
Progression of disease in terms of incidence of surgical reconstruction or amputation.
Secondary outcomes
Mean ankle systolic pressure.
Drug side effects.
Search methods for identification of studies
Electronic searches
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched May 2012) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 4, part of The Cochrane Library, www.thecochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
Additional articles were identified by reviewing the references of papers resulting from the initial search. We also contacted all the major pharmaceutical companies that manufacture naftidrofuryl for any unpublished trials.
Data collection and analysis
Selection of trials
Selection of trials was carried out by FBS and their eligibility for inclusion in the review was assessed independently by two of the review authors (FBS and FGRF). For studies in which eligibility was uncertain, a third review author (AWB) carried out a further independent assessment so that a consensus was reached. We obtained additional information, if required, from the principal investigators of all trials that appeared to meet the inclusion criteria.
Methodological quality
Information on the method of randomisation, blinding and whether an intention‐to‐treat analysis was performed, was extracted by FBS. Any doubts on the accuracy of information was discussed with a second author (FGRF). The quality of each trial was measured using the five point Jadad scale (Jadad 1996) to assess concealment of allocation, blinding and withdrawals.
Data extraction
Discrete and continuous data concerning outcome measures, extracted by FBS, were recorded on forms developed by the PVD Group.
Statistical analysis
Heterogeneity between trials was assessed subjectively by examining differences in patient populations, interventions and outcome assessments. The first draft of the review was produced by the first author (FBS) and detailed comments and amendments were made by AWB and FGRF.
Results
Description of studies
Sixteen trials examining the effect of intravenous naftidrofuryl on critical limb ischaemia were identified. Eight studies were not included in the review because they did not fulfil the inclusion criteria, or were of poor quality (seeCharacteristics of excluded studies). The eight included trials were relatively small and involved a total of 269 participants. Three trials were conducted in the UK, three in Germany and one each in France and Austria. There does not appear to be any other trials in progress.
The severity of critical limb ischaemia varied. Four of the trials used participants with rest pain and/or necrosis (Bohme 1994; Heiss 1985; Karnik 1986; Testart 1994), whereas the remainder used participants with rest pain only (Meehan 1982a; Negus 1987; Raftery 1982). In four trials, unsuitability for reconstructive surgery was used as an inclusion criterion. The majority of trials excluded people with other medical conditions. Most trials were conducted on predominantly elderly people of both sexes. In two of the trials, the sex of the people was not stated (Bohme 1994; Heiss 1985).
A combination of naftidrofuryl and other therapy, including haemodilution (Heiss 1985), bed rest and reflex heating (Meehan 1982a) and anti‐coagulant medication (Testart 1994), rather than treatment with naftidrofuryl alone was used in some of the trials. The dosage and duration of treatment also varied between the studies. The most commonly used dosage was 800 mg intravenous (i.v.) naftidrofuryl daily, but other trials used 600 mg (Karnik 1986) or 400 mg (Horsch 1998; Meehan 1982a; Raftery 1982). Duration of treatment varied between three and 42 days, but seven days was the most frequent length of treatment.
Three trials compared naftidrofuryl with prostaglandins (Bohme 1994; Karnik 1986; Negus 1987), and four trials against placebo/conservative therapy. In the placebo‐controlled trials, three trials used placebo i.v. fluids, either alone (Raftery 1982), with haemodilution (Heiss 1985), or with anti‐coagulant medication (Testart 1994). In the Meehan trial, the control group received bed rest and reflex heating. The Horsch trial (Horsch 1998) compared naftidrofuryl with gingko biloba.
Evaluation of pain, either subjectively or objectively using either analogue scales or analgesic consumption was reported in all the trials, as was the reporting of side effects from naftidrofuryl. State/healing of skin necrosis was reported in two studies (Bohme 1994; Heiss 1985). Transcutaneous oxygen pressure was reported in two trials (Bohme 1994; Horsch 1998). There was one report of all‐cause mortality (Horsch 1998) and two reports of amputation as a non‐fatal outcome (Heiss 1985; Horsch 1998).
Risk of bias in included studies
Eight trials met our inclusion criteria. The methods used in these trials are described in detail in the 'Included Studies Table'. In general, the quality of the included trials was only just adequate. In five trials, the method of treatment allocation was described as 'random', but no further information was available (Heiss 1985; Horsch 1998; Karnik 1986; Meehan 1982a; Raftery 1982). Three trials were randomised by a randomisation list (Bohme 1994); by random numbers (Negus 1987); or by a random numbers table (Testart 1994). Double blinding occurred in four trials (Horsch 1998; Negus 1987; Raftery 1982; Testart 1994). The Meehan trial (Meehan 1982a) was blinded only in respect to outcome. It was unclear whether blinding was used in two trials (Bohme 1994; Karnik 1986). Losses to follow up occurred in most studies, particularly in the Heiss 1985 trial, in which 11 participants out of 40 were lost to follow up.
Effects of interventions
All cause mortality There was one report of all‐cause mortality (Horsch 1998). One patient in the naftidrofuryl group died from bronchopneumonia, which was unrelated to treatment.
Pain evaluation by analogue score Three trials which evaluated ischaemic pain by an analogue scale (Horsch 1998; Meehan 1982a; Testart 1994) found that pain decreased in those treated by naftidrofuryl compared to the control groups. Although the age of participants and the duration of the Meehan and Testart trials were similar, the dosage of intravenous naftidrofuryl used was twice as high in the Testart trial (800 mg) than in the Meehan trial (400 mg). In the Horsch trial, the dosage was 400 mg daily but duration was 21 days. These data were unable to be analysed in a meta‐analysis because no standard deviations were reported (Horsch 1998).
Pain evaluation by analgesic consumption Three trials evaluated pain using scores based on the amount and type of analgesia required to alleviate pain (Horsch 1998; Testart 1994; Raftery 1982). In two trials (Testart 1994; Raftery 1982), analgesic requirements were lower in the naftidrofuryl treated groups compared with the control groups after seven or eight days respectively, but the difference was statistically non‐significant (mean difference (MD) ‐0.42; (95% confidence interval (CI) ‐1.19 to 0.35). In the Horsch trial, the number of participants who reduced their analgesic intake was higher in the control group compared with the naftidrofuryl group. The Horsch data were unable to be included in the meta‐analysis since actual values were given rather than mean values and standard deviations.
Rest pain/skin necrosis Assessment of rest pain, and state of skin necrosis recorded photographically were reported in three trials (Bohme 1994; Heiss 1985; Horsch 1998). Rest pain was evaluated by five grades of severity according to Peitgen (Peitgen 1985) in the Heiss trial, and by changes in clinical symptoms in the Bohme trial. The trial conducted by Bohme used the prostaglandin, alprostadil, as control. In addition to naftidrofuryl or placebo, haemodilution (Rheomacrodex) was used as therapy in both groups in the Heiss trial.
In total, 83.3% of the treated subjects improved compared with 70.6% of the controls. However, improvement in symptoms using naftidrofuryl was not statistically significant in either trial.
In the Horsch trial, rest pain was measured using a visual analogue scale. There was no statistically significant difference in rest pain between the two groups. With regard to skin necrosis, there was a greater reduction in maximal length and width of necrosis in the naftidrofuryl group compared with the gingko biloba group (Horsch 1998).
Incident reconstructive surgery/amputation It was stated that in four of the trials (Bohme 1994; Heiss 1985; Negus 1987; Raftery 1982) participants were either unsuitable for reconstructive surgery, or had refused surgery. In the remainder, the incidence of reconstructive surgery was not reported. In one trial (Horsch 1998), one patient in the naftidrofuryl group dropped out following femoral amputation.
Only one study examined incidence of amputation after treatment with naftidrofuryl (Heiss 1985). This study found that the incidence of amputation within 45 days after hospitalisation was 7.5%, which increased to 21% after a follow‐up period of a maximum of two years. It was noted that only one of the nine participants who eventually underwent amputation had been treated by naftidrofuryl.
Mean ankle systolic pressure Mean ankle systolic pressures were measured before and after treatment in two trials. In the Bohme trial (Bohme 1994), ankle pressure in the naftidrofuryl group rose significantly to 40 mm Hg (standard deviation (SD) 20) during treatment from the initial level of 17 mm Hg (SD 19), P < 0.05, whereas the ankle systolic pressures of the group allocated to alprostadil dropped slightly from 43 mm Hg (SD 41) to 39 mm Hg (SD 41). There were no significant differences in ankle systolic pressure levels after treatment between the naftidrofuryl and control groups MD 1.00 (95% CI ‐30.2 to 32.2). However, the mean ankle pressure was much lower in the naftidrofuryl group than in the alprostadil group before treatment commenced. In the Karnik trial (Karnik 1986), ankle systolic pressures were slightly higher in the prostacyclin group compared to those treated by naftidrofuryl. After one week of treatment, there were no significant changes in either group.
Side effects In the Bohme trial (Bohme 1994), one patient in each of the treatment groups suffered dizziness during infusion of the drugs.
Systemic side effects were reported in 35% of participants receiving naftidrofuryl/rheomacrodex therapy at a dosage of 800 mg daily, and in 32% of participants randomised to placebo/rheomacrodex in the Heiss study (Heiss 1985). These included renal insufficiency, nausea/vomiting, pruritus (itching), pulmonary congestion, somnolence (drowsiness), dyspnoea and depression. Seven participants in the naftidrofuryl group also developed thrombophlebitis at the injection site.
Minor side effects were recorded in three participants who received naftidrofuryl in the Karnik trial (Karnik 1986). These consisted of flushing (two participants) and pain in the extremities (one patient).
Nine cases of thrombophlebitis were noted out of a total of 323 infusions in the Meehan trial (Meehan 1982a).
A minor infection and a thrombosis occurred at the cannulation site of two participants (Negus 1987).
Side effects were not reported in the Raftery trial (Raftery 1982).
No specific side effects were mentioned in the Testart study (Testart 1994), but the participants were observed as showing 'good overall tolerance' of naftidrofuryl.
In the Horsch trial (Horsch 1998), one patient dropped out of the study because of a skin reaction to naftidrofuryl. Intravenous gingko biloba was well tolerated by all participants in that group.
Discussion
The trials included in this review involved a small number of participants and there was substantial variation in both treatment dosage of naftidrofuryl and in outcome measures. The duration of intravenous treatment was also very short. There was no clear indication that short‐term intravenous naftidrofuryl significantly improved either symptoms of ischaemic rest pain or skin necrosis. Although participants had reduced pain after treatment in comparison with controls, this was also statistically non‐significant.
Similarly, there was little clinical benefit from treatment by naftidrofuryl on ankle systolic pressures which if increased would indicate an improvement in circulation. This lack of effect may be due to the small numbers of participants, and the short duration of treatment.
Side effects were generally local and mild. However, in the trial conducted by Heiss (Heiss 1985), two participants were withdrawn from treatment using naftidrofuryl because of renal insufficiency.
Authors' conclusions
Implications for practice.
On the basis of these trials, there is no clear indication of clinical benefit from using intravenous naftidrofuryl in the treatment of critical limb ischaemia. Following a review by the UK and other European regulatory authorities, naftidrofuryl in the form of 200 mg/10 ml ampoules was withdrawn in 1995 as an intravenous treatment for severe peripheral arterial disease. This was because the risks of cardiac and neurological side effects were found to outweigh the possible benefit of intravenous naftidrofuryl in peripheral arterial disease.
Implications for research.
Although intravenous naftidrofuryl has been withdrawn as a treatment for severe peripheral arterial disease, oral treatment with this drug is permitted. In many of the trials included in this review assessing intravenous naftidrofuryl, oral naftidrofuryl was continued after the intravenous phase was completed. A systematic review of oral naftidrofuryl in the treatment of critical limb ischaemia is required.
What's new
| Date | Event | Description |
|---|---|---|
| 19 June 2012 | Review declared as stable | This Cochrane Review is no longer being updated because intravenous naftidrofuryl was withdrawn from the market as a treatment for severe peripheral arterial disease in 1995. Intravenous naftidrofuryl was withdrawn because of reported side effects. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 4 May 2012 | New citation required but conclusions have not changed | Searches re‐run, no new trials found. Minor copy edits made. The review was assessed as up to date. Conclusions not changed. |
| 4 May 2012 | New search has been performed | Searches re‐run, no new trials found. The review was assessed as up to date. |
| 3 September 2010 | New search has been performed | Searches re‐run. No new trials found. The review was assessed as up to date. |
| 30 July 2008 | New search has been performed | Searches re‐run. No new trials found. The review was assessed as up to date. |
| 30 May 2008 | Amended | Converted to new review format. |
| 5 January 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Consumer Network for providing the Plain Language Summary.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Arteriosclerosis, this term only | 893 |
| #2 | MeSH descriptor Arteriolosclerosis, this term only | 0 |
| #3 | MeSH descriptor Arteriosclerosis Obliterans, this term only | 70 |
| #4 | MeSH descriptor Atherosclerosis, this term only | 370 |
| #5 | MeSH descriptor Arterial Occlusive Diseases, this term only | 750 |
| #6 | MeSH descriptor Intermittent Claudication, this term only | 700 |
| #7 | MeSH descriptor Ischemia, this term only | 740 |
| #8 | MeSH descriptor Peripheral Vascular Diseases explode all trees | 2125 |
| #9 | MeSH descriptor Vascular Diseases, this term only | 376 |
| #10 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 16465 |
| #11 | (arter*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 4829 |
| #12 | (vascular) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1326 |
| #13 | (vein*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 703 |
| #14 | (veno*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 972 |
| #15 | (peripher*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1340 |
| #16 | peripheral near3 dis* | 3124 |
| #17 | arteriopathic | 6 |
| #18 | (claudic* or hinken*) | 1416 |
| #19 | (isch* or CLI) | 16556 |
| #20 | dysvascular* | 15 |
| #21 | leg near4 (obstruct* or occlus* or steno* or block* or obliter*) | 180 |
| #22 | limb near4 (obstruct* or occlus* or steno* or block* or obliter*) | 229 |
| #23 | (lower near3 extrem*) near4 (obstruct* or occlus* or steno* or block* or obliter*) | 133 |
| #24 | (aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) near3 (obstruct* or occlus*) | 319 |
| #25 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) | 38109 |
| #26 | MeSH descriptor Nafronyl, this term only | 98 |
| #27 | MeSH descriptor Furans, this term only | 191 |
| #28 | (naftidrofur* or nafronyl* or naftifurin or naftirofuryl or naphthydrofur* or naphthohydrofur*) | 242 |
| #29 | (praxilene or dusodril or LS 121 or LS121 or artocoron or EU 1806 or EU1806 or gevatran or iridus or sodipryl or di‐actane or vascuprax) | 500 |
| #30 | artocoron or azunaftil or esedril or luctor or naftodril or naftiratiopharm or stimlor or vasolate | 3 |
| #31 | (#26 OR #27 OR #28 OR #29 OR #30) | 849 |
| #32 | (#25 AND #31) | 112 |
Data and analyses
Comparison 1. Naftidrofuryl versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain evaluation by analogue scale | 3 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Naftidrofuryl versus gingko | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Naftidrofuryl versus placebo | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Pain evaluation by analgesic consumption | 3 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.19, 0.35] |
| 2.1 Naftidrofuryl versus gingko | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Naftidrofuryl versus placebo | 2 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.19, 0.35] |
| 3 Mean ankle systolic pressure | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐30.20, 32.20] |
1.1. Analysis.
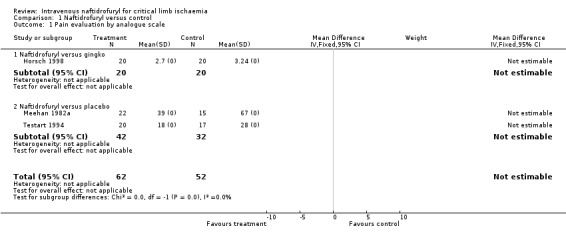
Comparison 1 Naftidrofuryl versus control, Outcome 1 Pain evaluation by analogue scale.
1.2. Analysis.
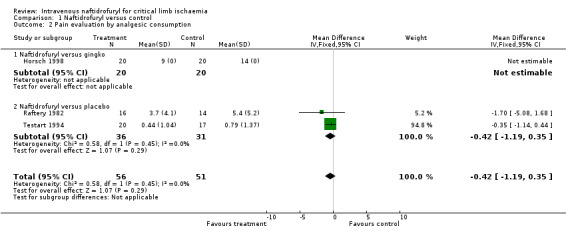
Comparison 1 Naftidrofuryl versus control, Outcome 2 Pain evaluation by analgesic consumption.
1.3. Analysis.
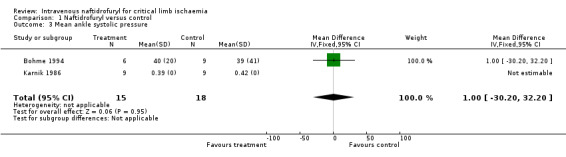
Comparison 1 Naftidrofuryl versus control, Outcome 3 Mean ankle systolic pressure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bohme 1994.
| Methods | Study design: Randomised controlled trial. Method of randomisation: Randomisation list, unclear if any blinding. Exclusions post‐randomisation: Not stated. Losses to follow up: 3 lost to assessment of rest pain follow up. |
|
| Participants | Country: Germany. Participants: 30 randomised. Age: median 77 years in naftidrofuryl group; 70 years in alprostadil group. Sex: Not stated. Inclusion criteria: Stage III and IV Fontaine PAD lasting more than 4 weeks. Stage III patients underwent neurological examination prior to randomisation, vascular surgery, catheter techniques, fibrinolysis not possible. Exclusion criteria: decompensated heart failure, poorly controlled hypertension (systolic more than 200 mmHg), unstable heart disease, renal failure (creatinine more than 21.2 mg/ 100 ml). |
|
| Interventions | Treatment: 400 mg intravenous naftidrofuryl twice daily. Control: 40 g intravenous alprostadil twice daily. Duration: 21 days. |
|
| Outcomes | State/healing of necrosis recorded by photograph at 7, 14 and 21 days, pain assessment, doppler pressures, transcutaneous oxygen pressures, side effects. | |
| Notes | Wash‐out phase of 2 to 4 days preceding treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Heiss 1985.
| Methods | Study design: Randomised, double blind controlled trial. Method of randomisation: Not stated. Exclusions post‐randomisation: Not stated. Losses to follow up: 4 lost in infusion phase, 7 in oral phase. |
|
| Participants | Country: Germany. Participants: 40 randomised. Age: mean 73.9 years in haemodilution/naftidrofuryl group; 70.3 years in haemodilution/placebo group. Sex: Not stated. Inclusion criteria: Stage IV Fontaine PAD, no indication for surgery or had refused surgery. Exclusion criteria: acute arterial occlusion, MI within previous 6 months, cardiac insufficiency at rest, renal insufficiency (creatinine more than 1.5 mg/ 100 ml), defibrinating, anti‐aggregatory or anti‐coagulant medication, non‐digitalis induced conduction disorder, vascular surgery or sympathectomy within previous 6 months. |
|
| Interventions | Treatment: 800 mg intravenous naftidrofuryl + hypervolaemic haemodilution (Rheomacrodex) therapy, then 600 mg naftidrofuryl orally. Control: intravenous placebo + Rheomacrodex. Duration: 42 days |
|
| Outcomes | State/healing of necrosis recorded by photograph at 7, 14, 21 days, analgesic consumption, pain evaluation, doppler ultrasound, oscillography, ECG, side effects. | |
| Notes | Wash‐out phase of 2 to 4 days preceding treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Horsch 1998.
| Methods | Study design: Stated as randomised, double blind. Method of randomisation: Not stated. Exclusions post randomisation: Not stated. Losses to follow up: 6. |
|
| Participants | Country: Germany. Participants: 40 randomised. Age: median 77.7 years in naftidrofuryl group; 70.0 years in gingko biloba group. Sex: male and female. Inclusion criteria: Stage III and IV Fontaine angiographically verified PAD. Exclusion criteria: ulcers exposing bones and sinew, indication for surgery, fibrinolysis or sympathectomy, myocardial infarction during previous 6 months, heart disease NYHA III or IV, severe high blood pressure (diastolic pressure more than 120 mmHg); severe kidney insufficiency (creatinine more than 2.0 mg/ 100 ml); severe liver function problems (transaminase increase more than 3 times normal), respiratory insufficiency, impairment through orthopaedic diseases (e.g.. arthritis); venous insufficiency at Grade II (Basler classification); poorly controlled diabetes mellitus; anaemia (haemoglobin less than 10 g/100 ml); expected poor compliance. Medications with similar indications to the test substances were also excluded. Only essential medication was allowed. Treatment with nitrates and calcium antagonists were permitted as long as they were taken right from the start of the study. |
|
| Interventions | Treatment: 400 mg intravenous naftidrofuryl. Control: 200 mg Ginko Special Extract EGb 761. Duration: 21 days. |
|
| Outcomes | Night pain assessment using analogue scores, transcutaneous oxygen pressures, amount and size of ulcers and necrosis, analgesic use, side effects, all cause mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Karnik 1986.
| Methods | Study design: Randomised cross‐over study. Method of randomisation: Not stated. Blinding unclear. Exclusions post randomisation: Not stated. Losses to follow up: one. |
|
| Participants | Country: Austria. Participants: 20 randomised. Age: Mean 74 years naftidrofuryl group, 67 years prostacyclin group. Sex: male and female. Inclusion criteria: Stage III and IV Fontaine PAD of at least 4 weeks duration. Exclusion criteria: stenoses/occlusion of iliac arteries. |
|
| Interventions | Treatment: 600 mg intravenous naftidrofuryl. Control: 5 ng/kg/min intravenous prostacyclin. Duration: 7 days naftidrofuryl, 5 days prostacyclin. |
|
| Outcomes | Pain + trophic evaluation, resting calf blood flow, venous plethysmography, reactive hyperaemia test, doppler systolic blood pressure, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Meehan 1982a.
| Methods | Study design: Stated as randomised. Method of randomisation: Not stated, single blind. Exclusions post randomisation: Not stated. Losses to follow up: 3. |
|
| Participants | Country: Scotland. Participants: 40 randomised. Age: mean 73 years naftidrofuryl group, 67 years conservative treatment group. Sex: male and female. Inclusion criteria: rest pain due to PAD and assessed for vascular reconstruction. Exclusion criteria: diabetes, previous reconstructive surgery, sympathectomy. |
|
| Interventions | Treatment: 400 mg intravenous naftidrofuryl daily + 600 mg oral naftidrofuryl daily + bed rest + reflex heating. Control: Bed rest + reflex heating. Duration: 7 days. |
|
| Outcomes | Changes in rest pain symptoms, mental state and well‐being assessment by analogue scales, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Negus 1987.
| Methods | Study design: Randomised controlled trial. Method of randomisation: random numbers, double blind. Exclusions post randomisation: Not stated. Losses to follow up: 3. |
|
| Participants | Country: England. Participants: 32 randomised. Age: mean 70 years naftidrofuryl group, 68 years prostacyclin group. Sex: male and female. Inclusion criteria: Ischaemic rest pain requiring analgesics, unsuitable for reconstructive surgery. Exclusion criteria: Not stated. |
|
| Interventions | Treatment: 0.02 mg/kg/min naftidrofuryl by arterial catheter. Control: 8 ng/kg/min prostacyclin by arterial catheter. Duration: 3 days. |
|
| Outcomes | Estimation of skin perfusion (histamine flare), relief of rest pain, analgesic consumption, amputation rate, ABPI, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Raftery 1982.
| Methods | Study design: Stated as randomised. Method of randomisation: Not stated, double blind. Exclusions post randomisation: Not stated. Losses to follow up: None. |
|
| Participants | Country: England. Participants: 30 randomised. Age: mean 66 years naftidrofuryl group, 64 years placebo group; sex: male and female. Inclusion criteria: rest pain associated with PAD, patients unsuitable for reconstructive surgery. Exclusion criteria: diabetes mellitus, congestive cardiac failure, collagen disease, previous medication with naftidrofuryl, gangrene more than 1 digit, ischaemic ulceration. |
|
| Interventions | Treatment: 400 mg intravenous naftidrofuryl daily + 100 mg naftidrofuryl orally daily. Patients who showed improvement after 7 days were given 200 mg orally for maximum 3 months. Control: identical placebo. Duration: 7 days. |
|
| Outcomes | Analgesic consumption, improvement after 7 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Testart 1994.
| Methods | Study design: Randomised controlled trial. Method of randomisation: Four‐factor random number table, double blind. Exclusions post randomisation: Not stated. Losses to follow up: 3. |
|
| Participants | Country: France. Participants: 37 randomised. Age: mean 69.8 years. Sex: male and female. Inclusion criteria: permanent ischaemia associated with PAD with or without trophic disorders. Exclusion criteria: Not stated. |
|
| Interventions | Treatment: 800 mg intravenous naftidrofuryl daily + anticoagulant therapy (heparin or vitamin K antagonists). Control: identical intravenous placebo + anticoagulant therapy. Duration: 8 days. |
|
| Outcomes | Assessment of pain using analogue scales, consumption of analgesics, clinical evaluation, side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECG: electromyocardiograph MI: myocardial infarction NYHA: New York heart association PAD: peripheral arterial disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bianchi 1974 | This trial was not restricted to patients with critical limb ischaemia and included some patients with intermittent claudication It was also not stated if the study was randomised. |
| Fellmann 1989 | This study was probably not a randomised trial. In addition, the group of 30 patients treated with naftidrofuryl included 11 patients with intermittent claudication. There was no separate analysis on patients with symptoms of critical limb ischaemia. |
| Greenhalgh 1981 | This study of 21 patients was not a randomised controlled trial. The placebo was given first and then naftidrofuryl was subsequently administered over an eight day period. |
| Horsch 1988 | This trial simply compares two dose regimes of naftidrofuryl with no control group. |
| Meehan 1982b | Although this appeared to be an adequately designed randomised controlled trial of naftidrofuryl versus conservative therapy, measurement of tissue pH was the only outcome reported. This was considered to have little relevance in the assessment of the clinical efficacy of naftidrofuryl in the treatment of critical limb ischaemia. This study was not therefore included in the review. |
| Mustapha 1984 | This study was conducted on 20 patients with ischaemia of the lower limb and a group of healthy volunteers. There was, however, no randomisation of treatment in patients with PAD and for this reason the study was considered unsuitable for inclusion into the review. |
| Taggart 1989 | Twenty patients were randomised to either naftidrofuryl or saline. Each group, however, was not confined to those with rest pain but included seven patients with intermittent claudication, which made the trial unsuitable for inclusion in the review. |
| Wong 1980 | This was not a randomised controlled trial. The study consisted of treatment with naftidrofuryl, analgesia and bed rest. There was no control group and all patients received the same treatment. |
PAD: peripheral arterial disease
Contributions of authors
Felicity Smith: Identified potentially relevant trials, assessed their eligibility for inclusion in the review, assessed quality of trials, extracted data, and wrote the text of the review.
Gerry Fowkes: Assessed trial eligibility for inclusion in the review and addressed questions concerning accuracy of information available in trial papers.
Andrew Bradbury: Read and provided comments on the review and acted as arbiter in cases where study eligibility was uncertain.
The Peripheral Vascular Diseases Review Group assisted with developing the search strategy and running the searches for trials for this review.
Sources of support
Internal sources
University of Edinburgh, UK.
External sources
-
Chief Scientist Office, Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
-
Merck (Lipha Pharmaceuticals), Middlesex, UK.
Supplementary financial support was provided by Merck (Lipha Pharmaceuticals) for the original review. The review was conducted independently without input from Merck other than the provision of references and protocols. Subsequent updates were completed without financial support.
Declarations of interest
Felicity Smith: Supplementary financial support was provided by Merck (Lipha Pharmaceuticals) for the original review. The review was conducted independently without input from Merck other than the provision of references and protocols. Subsequent updates were completed without financial support. Gerry Fowkes: None known Andrew Bradbury: None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bohme 1994 {published data only}
- Bohme H, Hartel U, Walter H. Naftidrofuryl versus alprostadil in stage III and IV of peripheral arterial occlusive disease. Medizinische Welt 1994;45(5):209‐13. [Google Scholar]
Heiss 1985 {published data only}
- Heiss HW, Peitgen A, Hasenfuss G, Just H. Treatment of arterial occlusive disease Stage IV with naftidrofuryl [Behandlung der arteriellen Verschlusskrankheit im Stadium IV mit Naftidrofuryl]. Berichtsband der 5. Jahrestagung der angiologischen Gesellschaften der BRD, Östereichs und der Schweiz (Report of the Berlin 1985 5th Joint Annual Meeting of the Angiological Associations of West Germany, Austria and Switzerland). Demeter, Gräfelfing, 1985:S335.
Horsch 1998 {published data only}
- Horsch S, Holscher U. Infusion therapy with ginkgo biloba special extract EGb 761 (TM) in patients with PAOD fontaine stages III and IV ‐ A reference‐controlled double‐blind clinical trial [Infusionstherapie mit Ginkgo‐biloba‐Spezialextrakt EGb 761 (TM) bei pAVK ‐ Eine referenzkontrollierte Doppelblindstudie an Patienten im Fontaine‐Stadium III und IV]. Munchener Medizinische Wochenschrift 1998;140(16):232‐6. [Google Scholar]
Karnik 1986 {published data only}
- Karnik R, Valentin A, Slany J. Prostacyclin versus naftidrofuryl: A randomised study in patients with peripheral arterial occlusive disease (PAOD) Stage III and IV [Prostacyclin versus Naftidrofuryl: Eine randomisierte Studie an Patienten mit peripherer arterieller Verschlußkrankheit (PAVK) Stadium III und IV]. Herz Kreislauf 1986;18(12):644‐7. [Google Scholar]
Meehan 1982a {published data only}
- Meehan SE, Preece PE, Walker WF. The usefulness of naftidrofuryl in severe peripheral ischaemia ‐ a symptomatic assessment using linear analogue scales. Angiology 1982;33(10):625‐34. [DOI] [PubMed] [Google Scholar]
Negus 1987 {published data only}
- Negus D, Irving JD, Friedgood A. Intra‐arterial prostacyclin compared to praxilene in the management of severe lower limb ischaemia: a double blind trial. Journal of Cardiovascular Surgery 1987;28(2):196‐9. [PubMed] [Google Scholar]
Raftery 1982 {published data only}
- Raftery AT. A controlled trial of naftidrofuryl (Praxilene) in ischaemic rest pain. British Journal of Intravenous Therapy 1982;July:7‐10. [Google Scholar]
Testart 1994 {published data only}
- Testart J, Guidicelli H, Glanddier G, Mosnier M. Evaluation of the analgesic effect of naftidrofuryl in permanent ischaemia in the patient with peripheral arterial disease: placebo‐controlled double‐blind study [Evaluation de l'effet antalgique du naftidrofuryl dans l'ischemie permanente chez l'arteriopathe: Etude double‐aveugle contre placebo]. Annales de Cardiologie et d' Angeiologie 1994;43(9):542‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Bianchi 1974 {published data only}
- Bianchi G. Double blind clinical experiences with L.S. 121 (praxilene) in patients with chronic ischemic peripheral syndromes [Esperienze cliniche in doppio cieco con L.S. 121 (praxilene) in pazienti portatori di sindromi ischemiche croniche periferiche]. Minerva Chirurgica 1974;29(15‐16):885‐90. [PubMed] [Google Scholar]
Fellmann 1989 {published data only}
- Fellman N, Fabry R, Coudert J. Calf sweat lactate in peripheral arterial occlusive disease. American Journal of Physiology 1989;257(2 Pt 2):H395‐8. [DOI] [PubMed] [Google Scholar]
Greenhalgh 1981 {published data only}
- Greenhalgh RM. Naftidrofuryl for ischaemic rest pain: a controlled trial. British Journal of Surgery 1981;68(4):265‐6. [DOI] [PubMed] [Google Scholar]
Horsch 1988 {published data only}
- Horsch S. Naftidrofuryl in Stages III/IV of peripheral arterial occlusive disease [Über den Einsatz von Naftidrofuryl im Stadium III/IV einer peripher arteriellen Verschlußkrankheit]. Vasa ‐ Supplementum 1988;24:38‐43. [PubMed] [Google Scholar]
Meehan 1982b {published data only}
- Meehan SE, Walker WF. Naftidrofuryl for severe ischaemia: assessment using skin pH micro‐electrode measurements. Current Medical Research & Opinion 1982;7(10):690‐9. [Google Scholar]
Mustapha 1984 {published data only}
- Mustapha NM, Jain SK, Dudley P, Redhead RG. The effect of oxygen inhalation and intravenous naftidrofuryl on the transcutaneous partial oxygen pressure in ischaemic lower limbs. Prosthetics & Orthotics International 1984;8(3):135‐8. [DOI] [PubMed] [Google Scholar]
Taggart 1989 {published data only}
- Taggart I, Wishart GC, Cuschieri RJ, MacBain GC. Effect of intravenous naftidrofuryl on transcutaneous oxygen pressure in severe peripheral vascular disease. Angiology 1989;40(10):895‐8. [DOI] [PubMed] [Google Scholar]
Wong 1980 {published data only}
- Wong AL, McBain GC. The importance of personality measurement in the assessment of response to treatment for ischaemic rest pain. British Journal of Surgery 1980;67(7):509‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Lehert 1990
- Lehert P, Riphagen FE, Gamand S. The effect of naftidrofuryl on intermittent claudication: a meta‐analysis. Journal of Cardiovascular Pharmacology 1990;16(Suppl 3):S81‐6. [PubMed] [Google Scholar]
Leng 1993
- Leng GC, Fowkes FGR. The epidemiology of peripheral arterial disease. Vascular Medicine Review 1993;4(1):5‐18. [Google Scholar]
Peitgen 1985
- Peitgen A. Therapy for arterial closure disease stage IV with haemodilution and naftidrofuryl [Therapie der arteriellen verschlusskrankheit im stadium IV mit hamodilution und naftidrofuryl]. Doctoral Thesis, Freiburg 1985.
References to other published versions of this review
Smith 2000
- Smith FB, Bradbury A, Fowkes G. Intravenous naftidrofuryl for critical limb ischaemia. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002070] [DOI] [PubMed] [Google Scholar]


