Abstract
Background
The creatine kinase system, the central regulatory system of cellular energy metabolism, provides ATP in situ at ATP‐ases involved in ion transport and muscle contraction. Furthermore, the enzyme system provides relative protection from tissue ischaemia and acidosis. The system could therefore be a target for pharmacologic intervention.
Objectives
To systematically evaluate evidence regarding the effectiveness of interventions directly targeting the creatine kinase system as compared to placebo control in adult patients with essential hypertension or cardiovascular disease.
Search methods
Electronic databases searched: Medline (1950 ‐ Feb 2011), Embase (up to Feb 2011), the Cochrane Controlled Trials Register (issue 3, Aug 2009), Latin‐American/Caribbean databank Lilacs; references from textbooks and reviews; contact with experts and pharmaceutical companies; and searching the Internet. There was no language restriction.
Selection criteria
Randomized controlled trials comparing creatine, creatine phosphate, or cyclocreatine (any route, dose or duration of treatment) with placebo; in adult patients with essential hypertension, heart failure, or myocardial infarction. We did not include papers on the short‐term use of creatine during cardiac surgery.
Data collection and analysis
The outcomes assessed were death, total myocardial infarction (fatal or non‐fatal), hospitalizations for congestive heart failure, change in ejection fraction, and changes in diastolic and systolic blood pressure in mm Hg or as percent change.
Main results
Full reports or abstracts from 1164 papers were reviewed, yielding 11 trials considering treatment with creatine or creatine analogues in 1474 patients with heart failure, ischemic heart disease or myocardial infarction. No trial in patients with hypertension was identified. Eleven trials (1474 patients, 35 years or older) comparing add‐on therapy of the creatine‐based drug on standard treatment to placebo control in patients with heart failure (6 trials in 1226 / 1474 patients ), or acute myocardial infarction (4 trials in 220 / 1474 patients) or 1 in ischemic heart disease (28 / 1474 patients) were identified. The drugs used were either creatine, creatine phosphate (orally, intravenously, or intramuscular) or phosphocreatinine. In the trials considering heart failure all three different compounds were studied; creatine orally (Gordon 1995, Kuethe 2006), creatine phosphate via intravenous infusion (Ferraro 1996, Grazioli 1992), and phosphocreatinine orally (Carmenini 1994, Maggi 1990). In contrast, the acute myocardial infarction trials studied intravenous creatine phosphate only. In the ischemic heart disease trial (Pedone 1984) creatine phosphate was given twice daily through an intramuscular injection to outpatients and through an intravenous infusion to inpatients. The duration of the study intervention was shorter for the acute patients, from a two hour intravenous infusion of creatine phosphate in acute myocardial infarction (Ruda 1988, Samarenko 1987), to six months in patients with heart failure on oral phosphocreatinine therapy (Carmenini 1994). In the acute myocardial infarction patients the follow‐up period varied from the acute treatment period (Ruda 1988) to 28 days after start of the symptoms (Samarenko 1987) or end of the hospitalization period (Zochowski 1994). In the other trials there was no follow‐up after discontinuation of treatment, except for Gordon 1995 which followed the patients until four days after stopping the intervention.
Only two out of four trials in patients with acute myocardial infarction reported mortality outcomes, with no significant effect of creatine or creatine analogues (RR 0.73, CI: 0.22 ‐ 2.45). In addition, there was no significance on the progression of myocardial infarction or improvement on ejection fraction. The main effect of the interventions seems to be on improvement of dysrhythmia.
Authors' conclusions
This review found inconclusive evidence to decide on the use of creatine analogues in clinical practice. In particular, it is not clear whether there is an effect on mortality, progression of myocardial infarction and ejection fraction, while there is some evidence that dysrhythmia and dyspnoea might improve. However, it is not clear which analogue, dose, route of administration, and duration of therapy is most effective. Moreover, given the small sample size of the discussed trials and the heterogeneity of the population included in these reports, larger clinical studies are needed to confirm these observations.
Keywords: Humans, Cardiovascular Diseases, Cardiovascular Diseases/drug therapy, Creatine, Creatine/analogs & derivatives, Creatine/therapeutic use, Creatine Kinase, Creatine Kinase/antagonists & inhibitors, Heart Failure, Heart Failure/drug therapy, Hypertension, Hypertension/drug therapy, Molecular Targeted Therapy, Molecular Targeted Therapy/methods, Myocardial Infarction, Myocardial Infarction/drug therapy, Myocardial Infarction/mortality, Myocardial Ischemia, Myocardial Ischemia/drug therapy, Phosphocreatine, Phosphocreatine/analogs & derivatives, Phosphocreatine/therapeutic use
Effectiveness of creatine analogues in cardiac disease
Creatine kinase is an enzyme that regenerates cellular ATP, which is used for energy demanding processes. This review evaluated existing therapeutic strategies that target the creatine kinase system in cardiovascular disease. The available trials indicate an improvement in abnormal heart rhythm and shortness of breath in heart disease. The effect on death and the size of myocardial infarction and the heart function were unclear. Given the small sample size of the discussed trials and the heterogeneity of the population included in these reports, larger clinical studies are needed to confirm these observations.
Summary of findings
Summary of findings for the main comparison.
Effects of creatine and creatine analogues on ejection fraction in patients with heart failure
| Patients or population: patients with heart failure Intervention: creatine phosphate or creatinine phosphate or creatine added to the conventional treatment Comparison: placebo Outcome: ejection fraction (p value as mentioned in article, we could not reproduce these findings) | |||||
| Intervention | Number of participants (studies) |
Illustrative comparative risk (per 1000 patients) |
Effect estimate | Quality of evidence (GRADE) | Comments |
| Creatine phosphate 1g/day | 13 (1) | Not applicable | 48 ± 12 → 53 ± 12 (p<0.05) Std. Mean Difference (IV, Random, 95% CI) 0.34 [‐0.44, 1.11] |
low | Ferraro 1996 |
| Creatine 20g/day for 6 weeks | 13 (1) | Not applicable | 28.8 ± 10.3% → 29.8 ± 8.6% (no significant difference) | low | Kuethe 2006 |
| Creatine 20g/day for 10 days | 17 (1) | Not applicable | 32 ± 4% → 32 ± 4.1%(no significant difference) | low | Gordon 1995 |
| Creatinine phosphate 1g/day for 6 months | 79 (1) | Not applicable | 35.9% (SD 8.87)→ 40.5% (SD 8.57) (P<0.001) | low | Carmenini 1994 |
| Creatinine phosphate 1g/day for 3 months | 88 (1) | Not applicable | 42.46% → 47.36% (P<0.01) | low | Maggi 1990 |
| CI: confidential interval, GRADE: GRADE Working Group grades of evidence. | |||||
Background
The creatine kinase system, the central regulatory system of cellular energy metabolism, is central to cardiovascular function (Brewster 2000). Creatine kinase regulates, buffers and transports, via creatine phosphate and creatine, ATP produced by glycolysis and oxidative phosphorylation, to sites of energy consumption such as myofibrils and membrane ion pumps. The enzyme substrate creatine is normally found in meat and fish, but it is also synthesized in the human body from dietary amino acids. Synthesis begins in the kidney with arginine and glycine forming guanidoacetic acid. This product is methylated in the liver, forming creatine (methylguanidine‐acetic acid). Normal creatine plasma levels are 40 ‐100 micromoles/liter (mcM/L); levels are about 25 mcM/L in vegetarians. The total adult body pool is approximately 120‐140 grams. About 95% of body stores are found in muscle; creatine is also found in the liver, kidney, sperm, brain, eyes and the nervous system (Wyss 2000). Myocytes use creatine (Cr) to make creatine phosphate (CrP) via creatine kinase. Creatine phosphate is used to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP). By using hydrogen ions to make ATP, creatine kinase also buffers intracellular hydrogen ions associated with lactate production and muscle fatigue during muscle contraction. There is evidence that the creatine kinase system protects the cardiovascular system from ischaemia and increases contractility. Cardiovascular implications are that high blood pressure is proposed to occur earlier and to be more severe with greater activities of this enzyme system (Brewster 2000; Brewster 2006). On the other hand, low CK activities are associated with heart failure (Ingwall 2004, Neubauer 2007). The system could therefore be a target for pharmacologic intervention in cardiovascular disease (Field 1996; Schaufelberger 1998).
Objectives
To systematically evaluate evidence regarding the effectiveness of interventions directly targeting the creatine kinase system as compared to placebo control in adult patients with essential hypertension or cardiovascular disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. There was no language restriction. Abstracts and reviews were excluded. Studies could have taken place in any care setting (in‐patient, outpatient, day‐care, or community).
Types of participants
Adults (over 18 years of age) with cardiovascular disease (essential hypertension, heart failure or myocardial infarction) were considered.
Types of interventions
Studies examining agents directly interfering with the creatine kinase energy system, such as creatine, creatine phosphate or other creatine analogues versus placebo were considered. The intervention could be addressed by any route, in any dose and for any duration. We did not include papers on the use of creatine during cardiac surgery.
Types of outcome measures
The outcomes assessed were death, total myocardial infarction (fatal or non‐fatal), hospitalization for congestive heart failure, change in ejection fraction, and changes in diastolic and systolic blood pressure in mm Hg or as percent change.
Search methods for identification of studies
The Database of Abstracts of Reviews of Effectiveness (DARE) was searched for related reviews.
The following electronic databases were searched for primary studies:
a) The Cochrane Central Register of Controlled Trials (CCTR) (2011, Issue 1)
b) Bibliographic databases, including MEDLINE (2005‐January 2011), EMBASE (2010‐January 2011), Latin American and Caribbean Health Sciences Literature (LILACS) (to August 2009), and the Cochrane Hypertension Group Specialised Register (all years). The Hypertension Group Specialised Register includes controlled trials from searches of AGRICOLA, Allied and Complementary Medicine (AMED), BIOSIS, CAB Abstracts, CINAHL, Cochrane Central Register of Controlled Trials, EMBASE, Food Science and Technology Abstracts (FSTA), Global Health, International Pharmaceutical Abstracts (IPA), LILACS, MEDLINE, ProQuest Dissertations & Theses, PsycINFO, SCIRUS, and Web of Science.
Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms relating to creatine and hypertension. No language restrictions were used. The MEDLINE search strategy (Appendix 1) was translated into EMBASE (Appendix 2), Cochrane CCTR (Appendix 3), and the Hypertension Group Specialised Register (Appendix 4) using the appropriate controlled vocabulary as applicable. Earlier searches designed by the authors were carried out in August 2009 (Appendix 5).
Other sources:
e) Handsearching of references from textbooks and reviews.
f) Reference lists of all papers and relevant reviews identified
g) Contact with experts and pharmaceutical companies
h) General Internet searches
Data collection and analysis
At least two reviewers independently assessed each eligible study unblinded. Risk of bias assessment was also performed independently by two reviewers. Disagreements were resolved through discussion. When there was no consensus between two reviewers, a third reviewer was asked for his or her opinion.
Judgement of validity We included only randomized controlled trials.
Data collection We developed a standard data abstract form for systematic collection of data on key trial characteristics, methodological quality, participants, comorbidity, intervention characteristics, drop outs, and outcomes. Two reviewers independently extracted data unblinded. Disagreements were resolved through discussion. We were unable to retrieve missing information. Analysis Statistical analysis was performed using Revman 5.1 software. Quantitative analysis of outcomes is based on intention to treat results (primary) and per protocol analysis (secondary). Our measure of effect for each study has been reported as relative risk for dichotomous data and weighted mean difference for continuous data.
Heterogeneity assessment Chi square tests for heterogeneity were used to assess outcome data for computability with the assumption of a uniform risk ratio (p > 0.05). If statistical heterogeneity was found across studies, the sources of the heterogeneity were to be explored and decision was made if studies should be aggregated. If so, the random effect model would then be used.
Sensitivity analysis If applicable, data was to be reanalyzed using both fixed and random effect models and using log odds ratio versus risk ratio.
Subgroup analysis Separate analysis of results were planned for patients with hypertension, myocardial infarction, and heart failure, and for gender and ethnicity if data was available.
Results
Description of studies
Trial retrieval
Full reports or abstracts from 1164 papers were reviewed, yielding 11 trials considering treatment with creatine or creatine analogues in 1474 patients with heart failure, ischemic heart disease or myocardial infarction. No trial in patients with hypertension was identified. Most trials were in English (n=6) and Italian (n=3). Other trials were in Polish (n=1) and Russian (n=1). A flow chart for trial retrieval and selection is provided in Figure 1.
Figure 1.
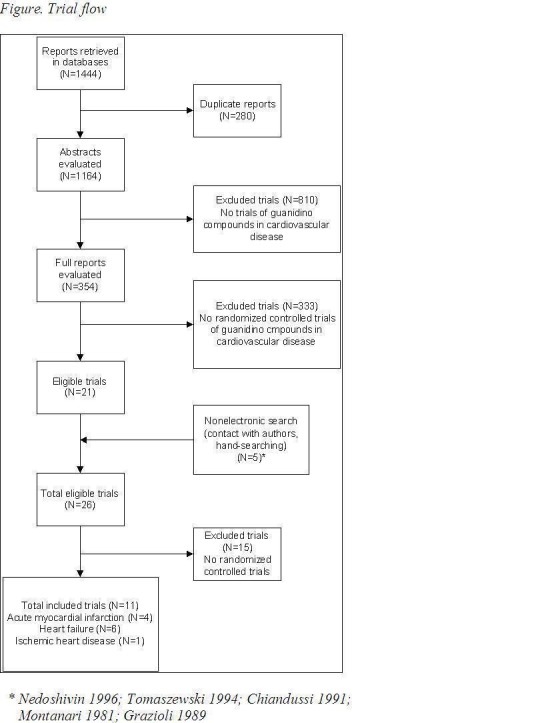
Flow chart trial retrieval
Description of included studies
All trials were randomized controlled trials of add‐on therapy of the creatine‐based drug to standard treatment, versus placebo. The methods, participants, interventions and outcomes of the included studies are listed in table 'Characteristics of included studies'. Eleven trials (1474 patients, 35 years or older) comparing add‐on therapy of the creatine‐based drug on standard treatment to placebo control. Four trials (220 / 1474 patients) considered patients in the acute stage of myocardial infarction (Samarenko 1987, Zochowski 1994, Ruda 1988, Pedone 1984‐2), six trials (1226 / 1474 patients) included patients with heart failure (Kuethe 2006, Ferraro 1996, Gordon 1995, Carmenini 1994, Grazioli 1992, Maggi 1990) and one (28 / 1474 patients) considered patients with ischemic heart disease (Pedone 1984). The drugs used were either creatine orally (Kuethe 2006, Gordon 1995); creatine phosphate (CrP) orally, intravenously, or intramuscular (Ferraro 1996, Zochowski 1994, Grazioli 1992, Ruda 1988, Samarenko 1987, Pedone 1984‐2), or phosphocreatinine orally (Carmenini 1994, Maggi 1990). In the heart failure trials all three different compounds were studied; creatine orally (Gordon 1995, Kuethe 2006), creatine phosphate via intravenous infusion (Ferraro 1996, Grazioli 1992), and phosphocreatinine orally (Carmenini 1994, Maggi 1990). The trials in patients with acute myocardial infarction only evaluated intravenous creatine phosphate. In the ischemic heart disease trial (Pedone 1984) creatine phosphate was given twice daily through an intramuscular injection to outpatients and through an intravenous infusion to inpatients. The duration of the study intervention was the shortest for the acute myocardial infarction patients, ranging from a two hour (Ruda 1988, Samarenko 1987) to a 24hr intravenous infusion of phosphocreatine (Zochowski 1994). In contrast, intervention periods in the heart failure trials ranged between ten days (Gordon 1995) and six months (Carmenini 1994). In the acute myocardial infarction patients the follow‐up period varied from the acute treatment period (Ruda 1988 24 hours, Pedone 1984 10 days) to 28 days after start of the symptoms (Samarenko 1987), between 24‐30 days after start of the symptoms (Pedone 1984‐2), or end of the hospitalization period (Zochowski 1994). In the heart failure trials there was no follow‐up after discontinuation of treatment, expect for Gordon 1995 which followed the patients until four days after stopping the intervention. Data in square brackets are standard deviations, unless otherwise specified.
Description of excluded studies
Sixteen studies were excluded because they were not randomized controlled trial. See table Characteristics of excluded studies.
Risk of bias in included studies
The results of the Risk of Bias assessment for each trial are outlined under Characteristics of included studies. Figure 2 and Figure 3 shows an overview of all trials. All trials were randomized controlled trials of add‐on therapy of the creatine‐based drug to standard treatment, versus placebo adding. Nine studies had a parallel design and two studies were crossover studies (Ferraro 1996; Pedone 1984). Three trials mentioned method of randomization (Carmenini 1994, Grazioli 1992, Ruda 1988). Allocation concealment was adequate in two trials (Grazioli 1992, Ruda 1988), and was unclear in nine trials. Blinding was adequate in six trials (Carmenini 1994, Ferraro 1996, Gordon 1995, Kuethe 2006, Maggi 1990, Pedone 1984) and was unclear in two trials (Pedone 1984‐2, Zochowski 1994). In the remaining three studies blinding was not adequate. Incomplete outcome data were found in six trials (Carmenini 1994, Kuethe 2006, Maggi 1990, Pedone 1984, Ruda 1988, Samarenko 1987). Nine trials were free of selective reporting, in two trials this was not clear (Grazioli 1992, Ruda 1988). None of the 11 trials were free of "other bias". Please see the Risk of bias in included studies table for further details.
Figure 2.
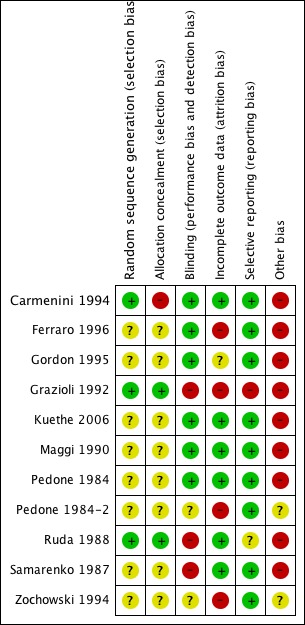
Risk of bias summary: review authors judgements about each risk of bias item for each included study
Figure 3.
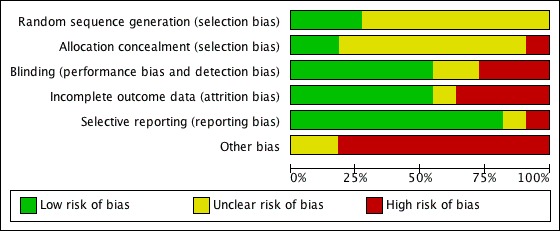
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Effects of interventions
See: Table 1
Trials in patients with hypertension
No randomized clinical trials were identified in patients with hypertension.
Trials in patients with acute myocardial infarction
Mortality Only two out of four trials (Samarenko 1987, Zochowski 1994) in patients (n=134) with acute myocardial infarction reported mortality outcomes during hospitalization, with no significant difference between creatine analogues and placebo. Samarenko 1987 reported a mortality rate of 10% of the patients in the CrP group (n=30) with 17% in the control group (n=30), that did not reach statistical significance with this small sample size (p > 0.05). Finally, Zochowski 1994 reported a 2.1% mortality rate in the intervention group (n=47), according to the authors, this was due to thrombolytic treatment complications; and no deaths in the control group (n=27). Pooling this data yields a point estimate for the relative risks of 0.73 (CI: 95% 0.22‐2.45), with this small samples size (Figure 4).
Hospitalization for congestive heart failure None of the acute myocardial infarction trials reported hospitalization for congestive heart failure.
Total myocardial infarction (fatal or non‐fatal) Data were inconclusive, in that Pedone 1984‐2 measured the progression of myocardial infarction scintigraphically in the intervention vs the placebo group between day 2‐6 vs day 24‐30 after the onset of symptoms. They found that the number of segments that were partially or totally revascularized between day 24‐30, were 15 / 40 (38%) with creatine phosphate (n=13) vs 5 / 46 (11%) with placebo (n=13). In addition they reported new abnormalities in 2 / 40 (5%) in the intervention vs 7 / 46 (15%) in the placebo group. However, Samarenko 1987 reported no significant differences in perfusion at day 27 or 28: the final size of the region of the myocardial perfusion defect was 20% (2) of the myocardium in the intervention group (n=30) vs 23% (2) in the control group (n=30) (p > 0.10).
Changes in diastolic and systolic blood pressure None of the acute myocardial infarction trials reported changes in blood pressure.
Changes in ejection fraction None of the acute myocardial infarction trials reported changes in ejection fraction.
Figure 4.
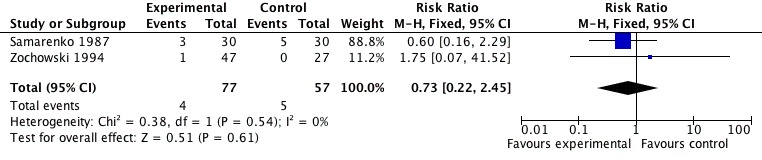
Phosphocreatine versus placebo. Outcome: mortality.
Trials in patients with heart failure
Mortality None of the heart failure trials reported mortality outcomes.
Hospitalization for congestive heart failure None of the heart failure trials reported hospitalization for congestive heart failure.
Total myocardial infarction (fatal or non‐fatal ) None of the heart failure trials reported total myocardial infarction.
Changes in diastolic and systolic blood pressure None of the heart failure trials reported changes in blood pressure.
Changes in ejection fraction Five trials reported ejection fraction as an outcome. Two out of five (Carmenini 1994, Ferraro 1996) reported a significant effect of intervention. Carmenini 1994 reported a significant improvement from 35.9% ejection fraction at baseline to 40.5% after six months in the intervention group (n=39) that were reported to be significantly different (p < 0.001) from the placebo group (n=40), although the data of the placebo group were not shown. Ferraro 1996 reported a significant improvement with creatine phosphate (n=13) vs placebo after acute and short term treatment (baseline: CrP 48% (SD 12) vs placebo 50% (12); acute: 53% (12) vs 48% (10); short term: 52% (11) vs 48% (12)). However, we could not reproduce the authors significant results. Out of the three remaining papers, two reported no significant effect of the intervention. Gordon 1995 after 10 days of treatment (no outcome data specified) and Kuethe 2006 after six weeks of treatment (Creatine 30% (9) vs placebo 29 ± 8%, n=13 in each group). Finally, Maggi 1990 did not report the difference between phosphocreatinine vs placebo. In conclusion, no study showed clear effect on ejection fraction.
Other outcomes as reported in publication of the included trials
The following outcomes were not prespecified in the review protocol, but were reported by authors.
Electrocardiographic findings
Reduction in dysrhythmia was found in the following trials. Three out of the four acute myocardial infarction (AMI) trials reported dysrhythmia as an outcome, with all three reporting a significant reduction in favour of the active intervention (Ruda 1988, Samarenko 1987, Zochowski 1994). Ruda 1988 reported a reduction in the total number of ventricular premature beats (VPBs) in the creatine phosphate group (n=30) vs placebo (n=30) with Holter monitoring (24hr ECG) during the 2hr treatment, CrP 690 (179) vs placebo 2468 (737), p < 0.02. Samarenko 1987 showed a decreased frequency of ventricular extrasystoles in the intervention group (n=30) vs the control group (n=30) during Holter monitoring simultaneously with treatment on the first day, CrP 690 (179) vs placebo 2468 (737), p < 0.02, without a significant decrease in supraventricular extrasystoles. Zochowski 1994 showed a significant difference in percentage reduction of dysrhythmia (not further specified) in CrP treated patients (n=47) vs controls (n=27) during Holter monitoring of the first 24hrs after the AMI (CrP ‐96,3% vs placebo ‐29,7%), but did not mention P‐value.
Furthermore, a reduction in dysrhythmias was found in the single trial including patients with ischaemic heart disease (Pedone 1984) and in two of the six heart failure trials (Grazioli 1992, Maggi 1990). Pedone 1984 reported a significant reduction in the number of VPBs in the CrP intramuscular group (n=10) and the CrP intravenously group (n=18) compared to placebo (resp. n=10 and n=18) with Holter monitoring on the 10th day (CrP i.m. ‐31,4% p <0.05, CrP i.v. ‐33,4% p < 0.001). Grazioli 1992 showed a significant difference in percentage reduction of VPBs in the CrP group (n=167) compared to placebo (n=150) after 15 days of treatment (CrP 68%, placebo 57%, p < 0.05). Maggi 1990 reported a reduction in the number dysrhythmias and atrioventricular blocks in the patients receiving creatine phosphate (n=30) compared to placebo (n=30) (CrP 50%, placebo 30%), but data on the exact time of measurement during the intervention period was not given.
Dyspnoea and orthopnoea
Four of the heart failure trials reported improvement in dyspnoea and orthopnoea (Carmenini 1994, Ferraro 1996, Grazioli 1992, Maggi 1990). Carmenini 1994 reported a reduction in dyspnoea (38.3%), orthopnoea (37.8%) and cough (32.8%) after six months in favour of the phosphocreatinine treatment (n=39), which all significantly differed from placebo (n=40) (p < 0.001). In addition, Ferraro 1996 reported a significant improvement of symptoms in the creatine phosphate group (n=13) compared to the controls (n=13) (p < 0.01), but did not further detail this data. Furthermore, Grazioli 1992 reported significant differences in dyspnoea after the 45 day treatment with creatine phosphate measured with a self‐monitoring four point scale: in the intervention group (n=508) 7% reported moderate to severe dyspnoea vs 23% in the control group (n=499) on the 45 day (p < 0.001). Finally, Maggi 1990 reported improvement on dyspnoea in favour of creatine phosphate (n=30) compared to controls (n=30), but not further detailed the data or mentioned the statistical significance. In contrast, Kuethe 2006 reported no significant differences on the Borg Scale of dyspnoea (measuring exhaustion on exercise) between creatine (n=13) and placebo (n=13), but did not provide further details.
All outcomes reported in the 11 included trials are summarized in Table 3.
Table 1.
Outcomes
| Study |
Cardiovascular disease |
Mortality |
Changes in blood pressure |
Changes in ejection fraction |
Total myocardial infarction |
Hospitalization for heart failure |
Other outcomes |
| Carmenini 1994 | Heart Failure | (‐) | (‐) | (+) 35.9% baseline to 40.5% 6 months. P<0.001 vs control reported, but data not shown by the authors | (‐) | (‐) | symptomatic parameters significantly decreased after six months (p < 0.001): dyspnoea ‐38.3%, orthopnoea ‐37%, asthenia ‐40.4% after six months, cough ‐32.8% after six months |
| Ferraro 1996 | Heart Failure | (‐) | (‐) | (+) baseline: CrP 48% (SD 12) vs placebo 50% (12); acute: 53% (12) vs 48% (10); short term: 52% (11) vs 48% (12). Significant difference reported by the authors, but not reproducible | (‐) | (‐) | significant difference comparing placebo and creatine phosphate treatment in end‐systolic volume (resp. 82 ± 23 vs 98 ± 48, p < 0.001) and end‐systolic diameter (resp. 4.2 ± 5 vs 4.5 ± 0.8, p < 0.001) after acute (15min) treatment, and in systemic vascular resistance (947.5±390.2 vs 975.4 ± 248.6, p<0.05) in the acute therapy and in the short‐term therapy (950.7 ± 394.3 vs 1121.7 ± 591.3, p<0.05) an increase in the acute phase in percent fractional shortening (baseline:25 ± 7, acute:28 ± 8%, p<0.05) significant improvement of symptoms (p<0.01), numbers not mentioned We could not reproduce the significance levels. |
| Gordon 1995 | Heart Failure | (‐) | (‐) | (+) no significant change, no outcome data specified | (‐) | (‐) | increase in total creatine compared with baseline (17 ± 4.0%, P<0.01) and compared to the placebo group (p<0.01), increase in creatine phosphate (12 ± 4.0%, p<0.01) compared to baseline and placebo (p<0.05) a significant increase in strength, measured through peak torque (+ 5 ± 1.5%) and one leg endurance tests (+ 21 ± 8.1%) which both significantly differed compared to baseline and placebo (p < 0.05). These improvements were linearly related to the increase in skeletal muscle creatine phosphate. |
| Grazioli 1992 | Heart Failure | (‐) | (‐) | (‐) | (‐) | (‐) | significant difference on dyspnoea after the 45 day treatment measured with a four point scale: in the intervention group 7% reported moderate to severe dyspnoea vs 23% in the control group (p < 0.001), pulmonary stasis (79 vs 84%, p<0.001) and peripheral edema (61 vs 64%, P<0.001) after 45 days of treatment, decrease in use of sublingual nitroglycerin after 15 days (from 19% to 2% in the intervention group), a decrease in ventricular premature beats in the intervention group of 68% vs. 57% in the control group after 15 days (p < 0.05) number of patients in NYHA classes III and IV decreased significantly (p < 0.01) more in the CrP group (9%) than in the control group (24%). |
| Kuethe 2006 | Heart Failure | (‐) | (‐) | (+) no significant change after 6 weeks of treatment (Creatine 30% (9) vs placebo 29 % (8) | (‐) | (‐) | a significant increase in body weight (85.9 ± 10.3 kg) compared to baseline (83.6 ± 10.6 kg) and placebo (84.3 ± 10.2 kg) and muscle strength (112,5 ± 14,8 mm Hg) compared to baseline (96.8 ± 26.5 mmHg) and placebo (88,3 ± 11,9 mmHg) after 6 weeks of oral supplementation of creatine (p < 0.05). no significant difference in left ventricular end diastolic diameter, no changes in the cardiopulmonary exercise tests no significant change in peak VO2, VO2AT, walking distance, Borg Scale levels of dyspnoea, and quality of life assessment |
| Maggi 1990 | Heart Failure | (‐) | (‐) | (+) ↑ 11.5% vs baseline, but no significant difference vs placebo | (‐) | (‐) | no differences in symptoms were reported except for the sense of dyspnoea in favour for phosphocreatinine as well as L‐carnitine; on the electrocardiogram the amount of first degree bundle branch blocks and arrhythmias was less in the phosphocreatinine (50%) and L‐carnitine (43%) group, but also in the control group (30%) The authors compared intervention to baseline and not placebo. |
| Pedone 1984 | Ischemic heart disease | (‐) | (‐) | (‐) | (‐) | (‐) | reduction in premature ventricular beats (PVB) number was 31.4% for the group treated with 400 mg creatine phosphate daily (p < 0.05) and 33.4% for the group treated with 2000 mg daily (p < 0.01) compared to placebo simply improvement was seen in 8/10 (80 %) outpatients (p = 0.055) and 14/18 (p = 78 %) inpatients. PVB reductions of 50 % were seen in 5/10 (50 %) outpatients and 10/18 (55 %) inpatients.PVB reductions of 75% were seen in 2/10 (20 %) outpatients and 4/18 (22 %) inpatients. |
| Pedone 1984‐2 | Acute Myocardial Infarction (AMI) | (‐) | (‐) | (‐) | (+) | (‐) | the course of infarction size between the intervention and the placebo group imaged first between 2‐6 days after the onset of symptoms and secondly between day 24 and 30. The number of segments partially or totally revascularized after treatment with creatine phosphate 15 of the 40 segments, with placebo five of the 46 segments. New scintigraphy abnormalities in two segments in the intervention vs seven segments in the placebo group experienced symptoms: improvement in 6, worsening in 2 and unchanged in 5 patients in the intervention group; improvement in 3, worsening in 3 and 7 unchanged in the placebo group |
| Ruda 1988 | AMI | (‐) | (‐) | (‐) | (‐) | (‐) | the heart rate was unchanged the total number of ventricular premature beats (VPBs) during the Holter monitoring period (24 hours) was 690 ± 179 in the CrP group compared to 2468 ± 737 in the control group (p < 0.02); this was a result of a reduction in the number of complex forms of VPBs, the total number of single VPBs did not differ significantly between the two groups during the CrP infusion period the total number of VPBs (18 ± 6 intervention vs 111 ± 41 controls), single VPBs (15 ± 5 vs 85 ± 33) , and paired VPBs (1.0 ± 0.4 vs. 9 ± 3) were significantly reduced compared to control (p < 0.05) during the monitoring period a significant reduction in the number of ventricular tachycardia paroxysms (VT) was found in favour of the CrP group (6 ± 2 vs 97 ± 35, p < 0.01). During the infusion period the mean number of VTs in the CrP group was 0.3 ± 0.1 vs 2 ± 1 (p < 0.05) the incidence of supraventricular extrasystoles was 846 ± 315 in the CrP group and 1012 ± 711 in the control group (not significant, p > 0.05) |
| Samarenko 1987 | AMI | (+) 10% CrP vs 17% control (p>0.05) | (‐) | (‐) | (+) | (‐) | difference in CK activity (CrP 38 ± 4 Eq vs control 48 ± 4, 21% difference, p<0.01) no significant difference in myoglobin levels 35‐leads ECG showed lower STsegments on the first day of treatment with CrP compared to control, on the 5th day these differences were no longer significant CrP infusion in the early period of AMI resulted in the decreased frequency of ventricular extrasystoles in the intervention group (690 ± 179) vs the control group (2468 ± 737) (p < 0.02); no significant decrease in supraventricular extrasystoles treatment with CrP lowered likelihood of cardiac failure development no reliable influence of CrP on the central haemodynamics, heart rate and heart conduction total myocardial infarction was reported: scintigraphy did not show significant differences in the region of the myocardium showing perfusion defect at final examination at day 27‐28 (intervention group 20 ± 2 % of the myocardium vs control 23 ± 2%, p > 0.1) |
| Zochowski 1994 | AMI | (+) 2.1% CrP vs 0% control | (‐) | (‐) | (‐) | (‐) | Holter monitoring for 24 hours showed a significant reduction of dysrhythmia in CrP treated patients (‐96,3%) compared to controls (‐29,7%) compared to baseline (an average of 635,3 dysrhythmias) |
Discussion
High activity of the creatine kinase energy system has been associated with a greater risk to develop high blood pressure (Brewster 2000, Brewster 2006). On the other hand, too low activity might lead to heart failure and myocardial infarction (Ingwall 2004, Neubauer 2007) Although there is evidence that the creatine kinase system has a crucial role in the energy homeostasis, cardiovascular contractility, and ischaemic resistance (Brewster 2000, Brewster 2006, Ingwall 2004, Neubauer 2007), to our knowledge, the existing evidence on drugs directly targeting this system in humans has not been systematically reviewed previously. In this review, we found no trials of drugs targeting the CK system in patients with hypertension. The existing trials considered myocardial infarction and heart failure mainly. We found little evidence of a beneficial effect of creatine and creatine analogues in the prespecified outcomes, including ejection fraction and mortality. Pooling of the data resulted in a point estimate of 0.71 for mortality without evidence of heterogeneity, but this outcome did not reach statistical significance. With no significance on the progression of myocardial infarction or improvement on ejection fraction, the main effect seems to be on improvement of dysrhythmia. Ventricular premature beats, ventricular extrasystoles and atrioventricular blocks were reported to be significantly reduced with the use of creatine phosphate or phosphocreatinine.
Limitations of this review are the small sample size of the discussed trials and the clinical heterogeneity in patients, duration, mode of treatment (oral, intramuscular, or intravenous), and the drugs used (creatine, creatine phosphate, and phosphocreatinine). Therefore, larger clinical studies are needed to confirm these observations. In particular, the potential effect on mortality needs further study with a larger sample size. The importance of the creatine kinase system for cardiovascular functions renders the system an interesting therapeutic target for drug intervention in cardiovascular disease.
Authors' conclusions
There is inconclusive evidence to decide on the use of creatine analogues in clinical practice. In particular, it is not clear whether there is an effect on mortality, progression of myocardial infarction and ejection fraction. There is some evidence that dysrhythmia and dyspnoea might improve. However, it is not clear which analogue, dose, route of administration, and duration of therapy is most effective.
Larger clinical studies are needed to confirm the observations of beneficial effects of supporting the creatine kinase system with creatine analogues in heart disease. The importance of the creatine kinase system for cardiovascular performance under high energetic demands, renders the system an interesting target for therapeutic interventions in cardiovascular disease.
Acknowledgements
We would like to acknowledge the Cochrane Hypertension Group, in particular Dr. Vijaya Musini, for the advice and support provided.
The authors wish to thank Wiktor Majczak, Ph.D. for translating Zochowski 1994, Alexandra Bizzotto, MD for translating Maggi 1990, Olga A. Pougovkina, Ph.D. student for translating Samarenko 1987, Joanna Klopotowska for translating Tomaszewski 1994 and E. Pribyl for translating Weber 1995.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1948 to Present with Daily Update Search Date: 25 January 2011 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Creatine/ (12784) 2 exp Creatine Kinase/ (21355) 3 (creatine$ or creatinphosphate or neoton or phosphagen or phosphocreatine or cyclocreatine or guanidino or phosphorylcreatine).tw. (31509) 4 or/1‐3 (45658) 5 cardiovascular.mp. (268805) 6 exp heart failure/ (69282) 7 exp myocardial infarction/ (127268) 8 ((heart or cardiac or myocardial) adj2 (failure or infarc$ or attack$)).tw. (195498) 9 exp hypertension/ (183660) 10 hypertens$.tw. (253569) 11 exp blood pressure/ (220126) 12 (blood pressure or bloodpressure).tw. (176548) 13 or/5‐12 (885576) 14 randomized controlled trial.pt. (294538) 15 controlled clinical trial.pt. (80659) 16 randomized.tw. (217545) 17 placebo.tw. (123265) 18 drug therapy/ (28714) 19 randomly.tw. (148422) 20 trial.tw. (253090) 21 groups.ab. (989858) 22 or/14‐21 (1501255) 23 animals/ not (humans/ and animals/) (3399301) 24 22 not 23 (1222443) 25 4 and 13 and 24 (1904) 26 (2005$ or 2006$ or 2007$ or 2008$ or 2009$ or 2010$ or 2011$).ed. (4101559) 27 25 and 26 (476)
Appendix 2. EMBASE search strategy
Database: EMBASE <1980 to 2011 Week 03> Search Date: 25 January 2011 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 creatine/ (7977) 2 creatine kinase/ (25874) 3 (creatine$ or creatinphosphate or neoton or phosphagen or phosphocreatine or cyclocreatine or guanidino or phosphorylcreatine).tw. (34719) 4 or/1‐3 (50069) 5 cardiovascular.mp. (456627) 6 exp heart failure/ (199207) 7 exp heart infarction/ (197560) 8 ((heart or cardiac or myocardial) adj2 (failure or infarc$ or attack$)).tw. (242062) 9 exp hypertension/ (347389) 10 hypertens$.tw. (312985) 11 blood pressure.mp. (332721) 12 or/5‐11 (1234052) 13 randomized controlled trial/ (287964) 14 crossover procedure/ (29994) 15 double‐blind procedure/ (101029) 16 single‐blind procedure/ (13776) 17 random$.tw. (619966) 18 (crossover$ or cross‐over$).tw. (54707) 19 placebo$.tw. (154363) 20 (doubl$ adj blind$).tw. (115598) 21 allocat$.tw. (57846) 22 comparison.ti. (240099) 23 trial.ti. (106542) 24 or/13‐23 (1060546) 25 (animal$ not (human$ and animal$)).mp. (3246427) 26 24 not 25 (944315) 27 4 and 12 and 26 (1325) 28 (2010$ or 2011$).em. (1221440) 29 27 and 28 (137)
Appendix 3. Cochrane CCTR search strategy
Database: Wiley Cochrane Central Register of Controlled Trials (2011 Issue 1) Search Date: 25 January 2011 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MeSH descriptor Creatine explode all trees (560) #2 MeSH descriptor Creatinine, this term only (1074) #3 (creatine* or creatinphosphate or neoton or phosphagen or phosphocreatine or cyclocreatine or guanidino or phosphorylcreatine):ti,ab (2013) #4 #1 OR #2 OR #3 (2566) #5 cardiovascular:ti,ab,kw (7190) #6 MeSH descriptor Heart Failure explode all trees (4583) #7 MeSH descriptor Myocardial Infarction explode all trees (7538) #8 ((heart or cardiac or myocardial) near/2 (failure or infarc* or attack*)):ti,ab (5399) #9 MeSH descriptor Hypertension explode all trees (12544) #10 hypertens*:ti,ab in Clinical Trials (21602) #11 MeSH descriptor Blood Pressure explode all trees (20704) #12 (blood pressure or bloodpressure):ti,ab in Clinical Trials (32157) #13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (63540) #14 #4 AND #13 (716)
Appendix 4. Hypertension Group Specialised Register search strategy
Database: Cochrane Hypertension Group Specialized Register Search Date: 25 January 2011 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 All fields (creatine* or creatinphosphate or neoton or phosphagen or phosphocreatine or cyclocreatine or guanidino or phosphorylcreatine) (76)
Appendix 5. 2009 search strategies
Search strategies used by authors for key databases, with results (August 2009).
PubMed: [(creatine OR cyclocreatine OR phosphocreatin* OR guanidino OR Neoton)ti] AND (heart OR myocard* OR hypertensi* OR blood pressure OR cardiovascular); limit to "clinical trial" and "human"; 131 papers, 12 eligible trials; seven included in this review.
EMBASE: (creatine or cyclocreatine or phosphocreatin$ or guanidino or Neoton).m_titl. AND (heart or myocard$ or hypertensi$ or blood pressure or cardiovascular).mp. AND clinical trial; 104 papers, 10 new eligible trials not found in Pubmed systematic search; of which five are included in this review.
LILACS: (creatine OR cyclocreatine OR phosphocreatin$ OR guanidino OR Neoton)ti AND (heart OR myocard$ OR hypertensi$ OR blood pressure OR cardiovascular); 13 papers, no eligible trial that was not already found in Pubmed or Embase.
Cochrane Central Register of Controlled Trials (CCTR): [(creatine OR cyclocreatine OR phosphocreatine OR phosphocreatinine OR guanidino OR Neoton)ti] AND (heart OR myocard* OR hypertensi* OR blood pressure OR cardiovascular); 106 papers, no eligible trial that was not already found in PubMed or EMBASE.
Handsearching: six new eligible trials, not found in any database with systematic searching. This was either because the clinial trial was absent from the database (EMBASE and Cochrane), or the "clinical trial" identifier was missing (MEDLINE). However, none of these papers fulfilled the inclusion criteria for this review.
A total of 12 trials were included, please see Figure 1. The literature search was updated until January 2011 (Appendices 1‐4). No additional eligible trials were found.
Data and analyses
Comparison 1.
Phosphocreatine versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.22, 2.45] |
Analysis 1.1.
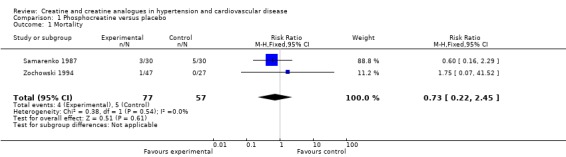
Comparison 1 Phosphocreatine versus placebo, Outcome 1 Mortality.
Differences between protocol and review
The title has been changed from 'Effects of creatine and analogues on blood pressure' to 'Creatine and creatine analogues in hypertension and cardiovascular disease'. The new title describes the inclusion criteria better.
Two new authors were added (D.L. Horjus and I. Oudman).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 79 subjects were randomized to receive phosphocreatinine or placebo for 6 months; check were performed on subjective and objective symptomatology and echocardiographic parameters were performed at three and at six months after the study started. There was no follow‐up period after withdrawal of treatment with phosphocreatinine. | |
| Participants | 79 patients affected by dilatative myocardiopathy, functional class II‐III NYHA | |
| Interventions | Six months treatment with oral phosphocreatinine 1 gram daily (n=39) vs placebo (n=40) added to standard therapy. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure and changes in blood pressure were not reported. Ejection fraction was significantly improved from 35.9% at baseline and 40.5% after six months in the intervention group. This improvement was significantly different from the compared to the placebo group (p < 0.001). The other outcomes reported were subjective and objective symptomatology and echocardiographic parameters of heart failure. Subjective and objective symptomatology and other echocardiographic parameters of heart failure showed a significant improvement after 3 and 6 months of treatment. |
|
| Notes | Cuore 1994. 11(2) (pp 187‐193). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "divisi at random in due gruppi." Another quote:"per quanto riguarda il tipo di trattamento effettuato per ciascun paziente sulla base della lista di randomizzazione (a randomization list was used)". Patients were randomly divided in groups with a randomization list. |
| Allocation concealment (selection bias) | High risk | They used a randomization list, so probably not done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "in doppio cieco (double‐blind)". Placebo: secondo modalita di trattamento analogo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out from creatine phosphate group: 5 cases, placebo group: 2 cases. Reasons are mentioned. Four cases due to personal reasons, 2 no show cases and 1 due to insufficient cardiac function. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | High risk | (Side) effect attribution difficult because simultaneous usage of standard therapy. |
| Methods | Compared the haemodynamic effects of creatine phosphate (CrP) and placebo in a double‐blind, crossover design study . All patients received conventional pharmacologic therapy for congestive heart failure (CHF); this was not changed during the study period. The study design consisted of two treatment periods (CrP or placebo and placebo or CrP, respectively) of 4 days each, separated by a 2‐day washout interval. Mono‐bidimensional echocardiographic examination (Hewlett Packard Sonos 1000, with a 2.5 MHz transducer) was performed at baseline, after acute infusion, and 12 h after the end of short‐term treatment. Data were analysed by ANOVA and Student's t‐test for paired data. There was no follow‐up period after withdrawal of treatment with CrP. | |
| Participants | 13 hospitalised patients (12 men, 1 woman, mean age 52 ± 8 years) with HF. NYHA class II‐III. | |
| Interventions | Treatment consisted of an intravenous infusion of 6 gram creatine phosphate or placebo, followed by a 4‐day treatment period during which the patients received 6 gram creatine phosphate or placebo daily in two intravenous administrations, diluted in 50ml NaCl 0.9%. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure and changes in blood pressure were not reported. Ejection fraction: a significant improvement (p < 0.01) compared to baseline after acute and short term treatment and compared to placebo. Baseline: 48 ± 12% ejection fraction, after acute infusion (53 ± 12%) and short‐term treatment of four days with creatine phosphate (52 ± 11%). Other outcomes: after treatment with CrP, a significant reduction of end‐systolic diameter (baseline: 4.5 ± 0.6; acute: 4.2 ± 0.5, (p < 0.001); short‐term 4.3 ± 0.6 cm, (p < 0.05)) and systemic vascular resistance (baseline: 1064.9 ± 483.7; acute: 947.5 ± 390.2 (p < 0.05); short‐term: 950.7 ± 394.3 dyne‐s‐cm‐5 (p < 0.05), and of percent fractional shortening [baseline: 25 ± 7; acute 28 ± 8 (p < 0.05); short‐term 28 ± 7% (p < 0.05)] was observed. After placebo treatment, no significant change was observed. |
|
| Notes | Clin Cardiol 1996 Sep;19(9):699‐703. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "it consisted of two randomized treatment periods". The method of randomization is not mentioned, so it is unclear if this was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", "the CrP or placebo was diluted in 50 ml of NaCl 0.9%". So CrP or placebo were indistinguishable. Not described who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not mentioned. |
| Selective reporting (reporting bias) | Low risk | All end points detailed. |
| Other bias | High risk | Cross‐over design, with a short washout period in between (two days). The discussion mentions: "the long‐lasting action of IV CrP" still observed after 12 hours. Placebo was mannitol, so a possible interference is not excluded. Small population with two types of pathophysiology for their heart failure 10 patients with ischemic and three with idiopathic dilated cardiomyopathy. (Side) effect attribution difficult because simultaneous usage of standard therapy. |
| Methods | A double‐blind, placebo‐controlled trial in which patients were treated with creatine vs placebo for 10 days. Before and on the last day of supplementation ejection fraction was determined by radionuclide angiography as was symptom‐limited 1‐legged knee extensor and 2‐legged exercise performance on the cycle ergometer. Muscle strength as unilateral concentric knee extensor performance (peak torque, Nm at 180degrees/s) was also evaluated twice with an interval of 14 days. Skeletal muscle biopsies were taken for the determination of energy‐rich phosphagens. Duration of follow‐up was not more than 14 days. | |
| Participants | 17 patients with heart failure (HF) aged 43‐70 years, ejection fraction (EF) < 40. | |
| Interventions | Patients were supplemented with creatine 20 g vs placebo per os daily during 10 days. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, or changes in blood pressure were not reported. Ejection fraction at rest (32 ± 4.1%) and during exercise (35 ± 5.7%) did not differ between groups and did not change after the ten day treatment in any of the two groups). Other outcomes: a significant increase in strength, measured through peak torque (+ 5 ± 1.5%) and one leg endurance tests (+ 21 ± 8.1%) which both significantly differed compared to baseline and placebo (p < 0.05). These improvements were linearly related to the increase in skeletal muscle creatine phosphate. |
|
| Notes | Cardiovascular Research. 30(3)(pp 413‐418), 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind randomization". Unclear because the method of randinomization is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind randomization", "Since glucose was added tot the creatine supplementation, there was no taste difference between the two substances." No further details on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Low risk | All end points detailed. |
| Other bias | High risk | Diverse pathophysiology of heart failure, and diverse standard therapy for heart failure, so possible baseline imbalance. (Side) effect attribution difficult because simultaneous usage of standard therapy. |
| Methods | A multicenter randomized controlled clinical trial in patients with heart failure to evaluate the effects of CrP in addition to conventional therapy. Patients were treated with CrP or placebo i.v. for 2 weeks, followed by CrP or placebo i.m. for 1 month. Clinical symptoms and NYHA classes, electrocardiographic signs of ischemia, and use of sublingual nitroglycerin were evaluated. There was no follow‐up after stopping treatment with CrP. | |
| Participants | 1007 patients hospitalised for heart failure; 508 patients were randomized to receive CrP and 499 patients were randomized to receive placebo. | |
| Interventions | One gram CrP i.v. daily added to conventional treatment or no added treatment for two weeks (acute treatment) followed by 500 mg CrP i.m. daily or no added treatment for 1 month. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, or changes in blood pressure were not reported. Ejection fraction at rest and at work did not change. The other outcomes reported were clinical symptoms of heart failure and angina pectoris with a 4‐point score, use of sublingual nitrogen, and rhythm, type and number of premature beats, ST segment, T wave, and atrioventricular conduction on the ECG. On dyspnoea a significant difference was reported after the 45‐day treatment measured with a four point scale: in the intervention group 7% reported moderate to severe dyspnoea vs 23% in the control group (p < 0.001) The number of patients in NYHA classes III and IV decreased significantly more in the CrP group (9%) than in the control group (24%). The clinical symptoms of heart failure, the main signs of ischaemia and a decrease in ventricular premature beats in the intervention group of 68% vs. 57% in the control group after 15 days (p < 0.05). |
|
| Notes | Current Therapeutic Research ‐ Clinical and Experimental. 52(2)(pp 271‐280), 1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to a randomization code". So sequence generation was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "According to a randomization code". |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reports on withdrawal, no missing data. |
| Selective reporting (reporting bias) | High risk | All outcomes mentioned in methods are reported. Nonsignificant outcomes only described as: "no statistically significant differences". No absolute numbers. |
| Other bias | High risk | (Side) effect attribution is difficult because simultaneous usage of standard therapy, e.g. "no side effects related to CrP treatment were reported". Possible differential diagnostic activity, because there was no blinding. |
| Methods | In a double‐blind, placebo‐controlled and crossover‐designed study, 20 patients with HF received creatine daily vs. placebo per os for 6 weeks and were crossed over for the following 6 weeks. Peak VO2, VO2 at the anaerobic threshold (VO2AT), EF, distance in 6‐minute‐walktest (6 min W), and muscle strength (Modified Sphygmomanometer (MS)) were determined at baseline, after 6, and after 12 weeks. Dyspnoea after 6‐minute‐walk‐test was measured using the Borg Scale. Quality of live was assessed with the Minnesota Living with Heart Failure Questionnaire (MLHFQ). There was no follow‐up period after withdrawal of creatine treatment. | |
| Participants | Twenty patients with a history of congestive heart failure of more than 6 months, NYHA II and III, and a peak oxygen uptake (peak VO2) of less than 20 ml/min/kg. | |
| Interventions | 4 x 5 g creatine daily vs. placebo per os for 6 weeks. For the following 6 weeks the patients were crossed over to receive creatine or placebo. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, or changes in blood pressure were not reported. Ejection fraction: no significant change in ejection fraction compared to baseline in the creatine (29.8 ± 8.6%) and the placebo group (28.7 ± 7.5%) after six weeks. Other outcomes: 13 of 20 patients finished the study. A significant increase in body weight (85.9 ± 10.3 kg) compared to baseline (83.6 ± 10.6 kg) and placebo (84.3 ± 10.2 kg) and muscle strength (112,5 ± 14,8 mm Hg) compared to baseline (96.8 ± 26.5 mmHg) and placebo (88,3 ± 11,9 mmHg) after 6 weeks of oral supplementation of creatine (p < 0.05). However, there was no significant change in peak VO2, VO2AT, ventricular end diastolic diameter, walking distance, Borg Scale levels of dyspnoea, and quality of life assessment. |
|
| Notes | Pharmazie. 61(3)(pp 218‐222), 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned "double‐blind placebo‐controlled and crossover designed study". So randomization method is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Only mentioned "double‐blind placebo‐controlled and crossover designed study". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", not further detailed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "13 of 20 patients finished the study." Reasons given. Not related to the treatment (evenly divided between the creatine and placebo group). Reasons were: 1 a cold, 1 a death, 1 newly diagnosed breast cancer, 1 acute decompensation, 3 stopped without giving reasons. |
| Selective reporting (reporting bias) | Low risk | All end points detailed. |
| Other bias | High risk | Cross‐over design: the measurements presented disregarding the sequence of creatine and placebo in the cross‐over regime. A washout period of four week was considered to "excluded an overlap of possible creatine effects". Small population (13). Relatively high amount of drop‐outs (7/20). Possible baseline imbalance due to different co‐medication at baseline. |
| Methods | A randomized trial with 90 subjects with heart failure, NYHA II en III, comparing L‐carnitine (30 patients), phosphocreatinine (30 patients) and placebo (30 patients) addition to the conventional treatment for 3 months. At baseline and after 3 months clinical parameters of heart failure, laboratory parameters, ECG parameters and echocardiographic parameters were evaluated. There was no follow‐up period after withdrawal of treatment with phosphocreatinine. | |
| Participants | 90 subjects, aged 61,9 ± 5 years, 57 men and 33 women, with established heart failure (class II and III NYHA scale) on medical treatment in form of cardiokinetic (digitalis) and/or diuretic drugs. | |
| Interventions | Thirty subjects were given 500 mg phosphocreatinine twice a day per os, a second group of thirty subjects was given 1 gram L‐carnitine per os twice a day, and the control group was given a placebo twice a day per os during 3 months. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, or changes in blood pressure were not reported. In the phosphocreatinine group there was a significant improvement of ejection fraction in the intervention group; increasing from 42.46% at baseline to 47.36% after three months (p < 0.01). Other outcome measures: reported symptoms, blood parameters, ECG measurements and echocardiography. With all three treatment, significant control and reduction of all cardiopathology symptoms was reached (p < 0.01). No differences in symptoms between treatments were reported except for the sense of dyspnoea in favour for phosphocreatinine compared to the L‐carnitine and placebo group. On the electrocardiogram (no time of measurement mentioned) the amount of first degree bundle branch blocks and arrhythmias was less in the phosphocreatinine (50%) and L‐carnitine (43%) group, but also in the control group (30%). |
|
| Notes | Maggi et al., Acta toxicologica et therapeutica, 1990, 11 (pp173‐184). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Lo studio policentrico e stato condotto in doppio cieco randomizzato (this is a double‐blind randomized multicenter tial)". Further methods of randomization are not mentioned. So it's unclear if this was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", "the boxes containing the three products in use were identical and indistinguishable from each other". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two drop‐outs mentioned, reason: lack of compliance. |
| Selective reporting (reporting bias) | Low risk | All end points detailed. |
| Other bias | High risk | Continuous scale used for overall opinion of participant and doctor: very subjective outcome measurement. (Side) effect attribution difficult because simultaneous usage of standard therapy. Possible baseline imbalance because of different baseline medication. Three treatment groups. |
| Methods | The effectiveness of CrP (Neoton) was investigated in a double blind placebo‐controlled cross‐over trial with 28 patients with coronary heart disease and frequent premature ventricular beats (PVBs). Ten of the patients were outpatients and received 200 mg CrP intramuscular twice daily for 10 days. The other 18 patients were inpatients and received 1000 mg CrP by i.v. infusion. The 10th day of the study all patients were subjected to continuous ECG monitoring for 24 hours. After the first ten days, all 28 patients were crossed‐over. A washout period between the two treatment periods is not mentioned. There was no follow‐up period after withdrawal of CrP treatment. | |
| Participants | 28 patients with ischemic heart disease and frequent ventricular extrasystoles. | |
| Interventions | Ten outpatients received intramuscular CrP 200 mg twice daily whilst 18 inpatients received CrP by intravenous infusion 1000 mg twice daily. All patients received both CrP and placebo for 10 day periods in randomized order. | |
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, ejection fraction, or changes in blood pressure were not reported. The main outcomes of the study were 1) the total number of PVBs in the treated group compared to the placebo group, and 2) the percentage of patients with a significant reduction in PVBs. 1) The overall reduction in PVB number was 31.4% for the group treated with 400 mg daily (p < 0.05) and 33.4% for the group treated with 2000 mg daily (p < 0.01) compared to placebo. 2) Simply improvement was seen in 8/10 (80 %) outpatients (p = 0.055) and 14/18 (p = 78 %) inpatients. PVB reductions of 50 % were seen in 5/10 (50 %) outpatients and 10/18 (55 %) inpatients. PVB reductions of 75% were seen in 2/10 (20 %) outpatients and 4/18 (22 %) inpatients. No other effects nor adverse effects of treatment were noted. |
|
| Notes | Clinical Trials Journal. 21(2)(pp 91‐99), 1984. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the double‐blind trial was carried out in all the patients with placebo". So blinding was probably adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals. |
| Selective reporting (reporting bias) | Low risk | All end points detailed. |
| Other bias | High risk | Cross‐over design, no washout period between the two treatment phases. Possible baseline imbalance because of use of different anti‐arrhytmic drugs and different frequency of premature beats. Baseline measurements of PVBs not done. (Side) effect attribution difficult because simultaneous usage of standard therapy. |
| Methods | A randomized controlled trial in which the effectiveness of CrP (Neoton) is investigated in 26 patients recovering from acute myocardial infarction, i.e.. 2‐6 days after the start of the symptoms. Thirteen patients received 1000 mg CrP intravenously twice daily during 4 weeks. The other 13 patients received placebo. At baseline and 24‐30 days after start of the symptoms evolution of the myocardial necrotic area was measured with scintigraphy. There was no follow‐up thereafter. | |
| Participants | In total 26 patients with a AMI. Thirteen patients received 1000 mg CrP intravenously twice daily during 4 weeks. The other 13 patients received placebo. | |
| Interventions | CrP 1000 mg i.v. twice daily vs placebo during 4 weeks besides the usual therapy. | |
| Outcomes | Outcomes on death, hospitalization for heart failure, ejection fraction, or changes in blood pressure were not reported. The main outcome was the scintigraphic evolution of the AMI during the first 4 weeks after the onset of symptoms when treated with CrP vs placebo. Total myocardial infarction: described the course of infarction size between the intervention and the placebo group imaged first between 2‐6 days after the onset of symptoms and secondly between day 24 and 30. The number of segments partially or totally revascularized after treatment with creatine phosphate 15 of the 40 segments, with placebo five of the 46 segments. New scintigraphy abnormalities in two segments in the intervention vs seven segments in the placebo group. |
|
| Notes | Clin. Ter. 111:531‐538, 1984. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "uno schema randomizzato", but the method of randomization is not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The placebo is described as, quote: "una sostanza di riferimento (placebo)". No futher description on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No report of incomplete data or withdrawals. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Possible information bias due to unclear blinding policies. (Side) effect attribution of intervention difficult to estimate, because simultaneous usage of standard therapy. |
| Methods | In this randomized controlled trial 60 patients diagnosed with acute myocardial infarction at the time of admission (clinical signs in combination with ECG changes typical for myocardial infarction) received CrP (Neoton) or placebo as a 2 gram bolus injection, followed by a 2‐hour infusion at the rate of 4 gram / hour. AMI diagnosis was subsequently verified by ECG, total CK, and scintigraphic scans. 24‐hour Holter monitoring was begun immediately after patients were enrolled in the study and modified ECG leads V1 and V5 were monitored. There was no follow‐up thereafter. |
|
| Participants | 60 patients, mean age 56 year with AMI admitted within 6 hours of the onset of chest pain. | |
| Interventions | CrP 2 g bolus injection followed by 2‐hour infusion at the rate of 4g/h, a total of 10 gram vs isotonic sodium chloride infusion. Patients also received morphine for pain relief in both groups. |
|
| Outcomes | Outcomes on death, total myocardial infarction, hospitalization for heart failure, changes in blood pressure or ejection fraction were not reported. Main outcome: ventricular arrhythmia measured simultaneously with treatment with creatine phosphate or placebo. The heart rate was unchanged. The total number of ventricular premature beats (VPBs) during the Holter monitoring period (24 hours) was 690 ± 179 in the CrP group compared to 2468 ± 737 in the control group (p < 0.02); this was a result of a reduction in the number of complex forms of VPBs, the total number of single VPBs did not differ significantly between the two groups. During the CrP infusion period the total number of VPBs (18 ± 6 intervention vs 111 ± 41 controls), single VPBs (15 ± 5 vs 85 ± 33) , and paired VPBs (1.0 ± 0.4 vs. 9 ± 3) were significantly reduced compared to control (p < 0.05). During the monitoring period a significant reduction in the number of ventricular tachycardia paroxysms (VT) was found in favour of the CrP group (6 ± 2 vs 97 ± 35, p < 0.01). During the infusion period the mean number of VTs in the CrP group was 0.3 ± 0.1 vs 2 ± 1 (p < 0.05). The incidence of supraventricular extrasystoles was 846 ± 315 in the CrP group and 1012 ± 711 in the control group (not significant, p > 0.05). |
|
| Notes | Am Heart J. ;116(2 Pt 1):393‐7, Aug 1988 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomized by means of sealed envelops". Method not further described but probably adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons of exclusion after randomization mentioned. Reason for inaccessible data mentioned. Quote: "Six tapes could not be analyzed because of technical problems; in two patients in the control group infusion of lidocaine was begun after ventricullar fibrillation, and the results were excluded from the analysis." |
| Selective reporting (reporting bias) | Unclear risk | The aim of the study was to assess the ability of CrP to reduce the incidence of arrythmias. Further specification is not mentioned in methods. |
| Other bias | High risk | (Side) effect attribution difficult because simultaneous usage of standard therapy. |
| Methods | In this randomized study in 60 patients with AMI, 30 patients received CrP intravenously during 5 days, the other 30 patients received placebo (isotonic NaCl solution). Arrhytmia was monitored by Holter registration. To evaluate the size of infarction 35‐leads ECG, determination of CK, CKMB and myoglobins, and perfusion scintigraphy (day 12‐14 and day 27‐28) were performed. There was no follow‐up after the last perfusion scintigraphy at day 27‐28. | |
| Participants | 60 patients with acute myocardial infarction (AMI) admitted to hospital in the first 6 hours of the disease. 30 patients received CrP and 30 patients received placebo. | |
| Interventions | 30 patients received a bolus of 2 gram CrP intravenously, followed by a 2‐hour continuous infusion of CrP 4 grams / hour. During the next 5 days they received CrP 2 grams twice daily. The control group of 30 patients received similar infusion with isotonic NaCl solution. | |
| Outcomes | Outcomes hospitalization for heart failure, changes in blood pressure or ejection fraction were not reported. Death during hospitalization was reported: 10% in the CrP group and 17% in the control group (p > 0.05). Total myocardial infarction was reported. Scintigraphy did not show significant differences in the region of the myocardium showing perfusion defect at final examination at day 27‐28 (intervention group 20 ± 2 % of the myocardium vs control 23 ± 2%, p > 0.1). There a was difference in CK activity (CrP 38 ± 4 Eq vs control 48 ± 4, 21% difference, p<0.01). There was no significant difference in myoglobin levels. 35‐leads ECG showed lower STsegments on the first day of treatment with CrP compared to control. At the fifth day these differences were no longer significant. Ventricular arrhythmia was another outcome of the study. CrP infusion in the early period of AMI resulted in the decreased frequency of ventricular extrasystoles in the intervention group (690 ± 179) vs the control group (2468 ± 737) (p < 0.02) during the 24 hour Holter monitoring; no significant decrease in supraventricular extrasystoles. Treatment with CrP lowered likelihood of cardiac failure development. No reliable influence of CrP on the central haemodynamics, heart rate and heart conduction was reported. |
|
| Notes | Biull Vsesoiuznogo Kardiol Nauchn Tsentra AMN SSSR. 1987. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided in two groups". Method not further mentioned, so unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method not further mentioned, so unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Although the control group recieved the same infusion with isotonic NaCl, they do not mention blinding. So treatment was probably not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients excluded after randomization. |
| Selective reporting (reporting bias) | Low risk | All endpoints detailed. |
| Other bias | High risk | Differences in medical history, differences in other medication not further mentioned so possible selection bias. |
| Methods | Randomized controlled trial in 27 patients with acute myocardial infarction (based on ECG and laboratory parameters, hospitalized < 6 hours since the first pain) comparing CrP with placebo infusion. The number and kind of arrhythmias, cardiac conduction defects, laboratory parameters and echographic parameters were evaluated. Follow‐up was till the end of the hospitalization period. | |
| Participants | In total 74 patients with AMI. 47 patients (33 men, 14 women), age 25‐74 y, received CrP. 27 patients (20 men, 7 women), age 34‐65 y, received placebo. |
|
| Interventions | Actively treated patients received an intravenous bolus injection of 2 gram CrP not later than 6 hours after the onset of symptoms, followed by an infusion at the rate of 4 g/hr for 2 hours. In the following 5 days, they received 2 grams intravenously twice a day. Other interventions included 1500000 U streptokinase i.v. for all patients, and heparin, lidocaine nitroglycerin diuretics and analgesics when needed. | |
| Outcomes | Outcomes total myocardial infarction, hospitalization for heart failure, changes in blood pressure or ejection fraction were not reported. Mortality: one death in the intervention group during hospitalization (n=47) due to thrombolytic treatment complications and no deaths in the control group (n=27). Ventricular arrhythmia, cardiac conduction defects on ECG, and dimensions of heart cavities, contractility and the number of akinetic segments with ultrasound. Holter monitoring for 24 hours showed a significant reduction of dysrhythmia in CrP treated patients (‐96,3%) compared to controls (‐29,7%) compared to baseline (an average of 635,3 dysrhythmias). |
|
| Notes | Pol Tyg Lek; 20‐27;49(25‐26):576‐8, Jun 1994. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled patients were randomly divided into 2 groups." Used randomization method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method not further mentioned, so this is unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding method not detailed described, except that "the control group received the same quantity of isotonic NaCl solution". The standard therapy was given equally to both groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No withdrawal mentioned. No participants baseline data given. |
| Selective reporting (reporting bias) | Low risk | All endpoints described in methods reported as a figure next to description in the result secton. |
| Other bias | Unclear risk | (Side) effect attribution difficult because simultaneous usage of standard therapy. |
RCT=randomized controlled trial
h=hour; w=week; m=months; y=year
AMI= acute myocardial infarction
HF= heart failure
Cr= creatine
CrP= creatine phosphate
VPB: ventricular premature beats
VT= ventricular tachycardia
ECG= electrocardiogram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andreev 1992 | Not a randomized controlled trial |
| Cafiero 1994 | Not a randomized controlled trial |
| Chiandussi 1991 | Not a randomized controlled trial |
| Divitiis 1986 | Not a randomized controlled trial |
| Golikov 1993 | Not a randomized controlled trial |
| Grazioli 1989 | Not a randomized controlled trial |
| Montanari 1981 | Not a randomized controlled trial |
| Nedoshivin 1996 | Not a randomized controlled trial |
| Perepech 1993 | Not a randomized controlled trial |
| Perepech 1997 | Not a randomized controlled trial |
| Perepech 2001 | Not a randomized controlled trial |
| Reimers 1994 | Not a randomized controlled trial |
| Stejfa 1993 | Not a randomized controlled trial |
| Tomaszewski 1994 | Not a randomized controlled trial |
| Weber 1995 | Not a randomized controlled trial |
| Weinbergova 1996 | Not a randomized controlled trial |
Contributions of authors
L.M. Brewster: wrote the protocol and the search strategy, extracted the data, and entered the data into RevMan.
D.L. Horjus: contributed to the protocol, data extraction, and entering data into RevMan.
I. Oudman: contributed to the search strategy, extracting the data and entering data into RevMan.
G. van Montfrans: wrote the protocol and contributed to the data extraction.
All authors contributed to writing the final report.
Sources of support
Internal sources
Academic Medical Centre, University of Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Deborah L Horjus, Inge Oudman and Lizzy M Brewster: none known. Gert A van Montfrans has received payments from Merck, Novartis and Daichii Sankyo for lectures and from Daichii Sankyo for development of a course on difficult to treat hypertension. He has also received royalties from Stafleu, Bonn for authoring a book chapter on hypertension and employment income from an outpatient hypertension clinic. He declares that none of these financial activities are related to the subject of this review.
New
References
References to studies included in this review
- Carmenini G. Controlled multicentric clinical study with placebo in patients with dilatative myocardiopathy functional class II‐III N.Y.H.A. treated with oral phosphocreatinine. CUORE RIVISTA DI CARDIOCHIRURGIA E CARDIOLOGIA 1994;11(2):187‐193. [Google Scholar]
- Ferraro S, Codella C, Palumbo F, Desiderio A, Trimigliozzi P, Maddalena G, Chiariello M. Hemodynamic effects of creatine phosphate in patients with congestive heart failure: a double‐blind comparison trial versus placebo. Clin Cardiol 1996 Sep;19(9):699‐703. [DOI] [PubMed] [Google Scholar]
- Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf CJ, Nyquist O, Sylven C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res 1995 Sep;30(3):413‐8. [PubMed] [Google Scholar]
- Grazioli I, Melzi G, Strumia E. Multicenter controlled study of creatine phosphate in the treatment of heart failure. Current Therapeutic Research 1992;52:271‐80. [Google Scholar]
- Kuethe F. Creatine supplementation improves muscle strength in patients with congestive heart failure. Die Pharmazie 2006;61(3):218‐22. [PubMed] [Google Scholar]
- Maggi GC, Lomanto B, Mazzola C. Double‐blind multicenter clinical trial on the efficacy of phosphocreatinine vs L‐carnitine and placebo in a group of patients affected by heart disease [Studio clinico in doppio cieco multicentrico sull'efficacia di fosfocreatinina vs L‐carnitina e placebo in un gruppo di cardiopatici]. Acta Toxicol Ther 1990;11(2):173‐184. [EMBASE: 1990393758] [Google Scholar]
- Pedone V. An assessment of the activity of creatine phosphate (Neoton) on premature ventricular beats by continuous ECG monitoring in patients with coronary cardiac disease. Clinical trials journal 1984;21(2):91‐99. [Google Scholar]
- Pedone V, Corbelli C, Frondini C, Franchi R, Luppi M, Lonardo E, Balboni A, Monetti N, Biase G. Myocardial T1‐201 scintigraphy in the study of the development of acute myocardial infarction. Evaluation of the effects of a drug with metabolic action: creatine phosphate [la scintigrafia miocardica con T1‐201 nello studio dell'evolutione dell'infarto miocardico acuto (AMI): Valutazione degli effetti di un farmaco ad azione metabolica: la creatina fosfato]. La Clinica Terapeutica 1984;111(6):531‐8. [PubMed] [Google Scholar]
- Ruda MYa, Samarenko MB, Afonskaya NI, Saks VA. Reduction of ventricular arrhythmias by phosphocreatine (Neoton) in patients with acute myocardial infarction. Am Heart J 1988 Aug;116(2 Pt 1):393‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Samarenko, M B. [Initial experience with using phosphocreatine in patients in the early (up to 6 hours) period of myocardial infarction]. Biull Vsesoiuznogo Kardiol Nauchn Tsentra AMN SSSR 1987;10(2):75‐81. [PubMed] [Google Scholar]
- Zochowski RJ, Sobieszczanska‐Malek M, Komuda K. Use of phosphocreatinine in treatment of acute heart failure [Zastosowanie fosfokreatyny w leczeniu ostrego zawalu serca]. Pol Tyg Lek 1994 Jun 20‐27;49(25‐26):576‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
- Andreev N. Effect of phosphocreatine in congestive heart failure. Current Therapeutic Research 1992;51(5):649‐60. [Google Scholar]
- Cafiero M, Strumia E, Pirone S, Pacileo S, Santoro R. [The efficacy of creatine phosphate in the treatment of patients with heart failure. Its echographic evaluation after acute and protracted treatment]. Clin Ter 1994 Apr;144(4):321‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Chiandussi. Multicenter clinical study of phosphocreatinine effects and tolerability. Riforma medica 1991;106(1):17‐21. [Google Scholar]
- de Divitiis, O. [Evaluation of the effects on the peripheral blood circulation of i.v. administration of high doses of phosphocreatinine in patients with arterial diseases]. Minerva cardioangiologica 0026‐4725 1986;34(10):671‐80. [PubMed] [Google Scholar]
- Golikov, A P. [Neoton in the treatment of myocardial infarct and unstable stenocardia]. Kardiologiia 1993;33(7):15‐17. [PubMed] [Google Scholar]
- Grazioli I, Ettore S. Treatment with creatine phosphate in patients with heart failure: a multicenter study. [Terapia con creatina fosfato nel paziente con insufficienza cardiaca in fase di scompenso: studio policentrico.]. Giornale Italiano Di Ricerche Cliniche E Terapeutiche 1989;10:39‐45. [Google Scholar]
- Montanari. Cardiodynamic alterations induced by treatment with phosphocreatinine. Gazzetta medica italiana 1981;140(6):285‐291. [Google Scholar]
- Nedoshivin A. [Neoton (exogenic phosphocreatinine) in combined therapy of chronic cardiac failure]. Klin Med 1996;74(9):45‐8. [PubMed] [Google Scholar]
- Perepech, N B. [Exogenous phosphocreatine in the prevention and treatment of cardiac insufficiency in patients with myocardial infarction]. Klin Med 1993;71(1):19‐22. [PubMed] [Google Scholar]
- Perepech NB. Prophylactic use of Neoton in cardiac failure in patients with myocardial infarction]. Klin Med (Mosk) 1997;75(10):52‐4. [PubMed] [Google Scholar]
- Perepech NB, Nedoshivin AO, Nesterova IV. [Neoton and thrombolytic therapy of myocardial infarction] [Neoton i tromboliticheskaia terapiia pri infarkte miokarda]. Ter Arkh 2001;73(9):50‐5. [PubMed] [Google Scholar]
- Reimers B, Maddalena F, Cacciavillani L, resta M, marzari A, Dalla Volta S. Phosphocreatine in acute myocardial infarction: multicenter randomized study [La fosfocreatina nell'infarto miocardico acuto: studio randomizzato multicentrico]. Il Cuore 1994;11(4):345‐254. [Google Scholar]
- Stejfa, M. [The effect of creatine phosphate (Neoton) in acute myocardial infarct (a prospective multicenter pilot study]. Vnitr Lek 1993;39(2):136‐142. [PubMed] [Google Scholar]
- Tomaszewski A, Markiewicz M, Drabowski P. Assessment of cardiac arrythmias in patients with newly diagnosed myocardial infarction treated with Neoton (phosphocreatine). Medical Journal 1994;47:15‐16. [Google Scholar]
- Weber, P. Use of creatine phosphate in treatment of cardiocerebral syndrome associated with acute myocardial infarct in the aged. Cas Lek Cesk 1995;134(2):53‐6. [PubMed] [Google Scholar]
- Weinbergová, O. Dynamics of some biochemical indices in myocardial infarction treated with thrombolysis and creatine phosphate.. Acta Universitatis Palackianae Olomucensis Facultatis Medicae 1996;140:91‐4. [PubMed] [Google Scholar]
Additional references
- Brewster LM, Clark JF, Montfrans GA. Is greater tissue activity of creatine kinase the genetic factor increasing hypertension risk in black people of sub‐Saharan African descent?. J Hypertens 2000 Nov;18(11):1537‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brewster LM, Mairuhu G, Bindraban NR, Koopmans RP, Clark JF, Montfrans GA. Creatine kinase activity is associated with blood pressure. Circulation 2006 Nov 7;114(19):2034‐9. [DOI] [PubMed] [Google Scholar]
- Field ML. Creatine supplementation in congestive heart failure. Cardiovasc Res 1996 Jan;31(1):174‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 2004;95(2):135‐45. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart‐‐an engine out of fuel. N Engl J Med 2007;356(11):1140‐51. [DOI] [PubMed] [Google Scholar]
- Schaufelberger M, Swedberg K. Is creatine supplementation helpful for patients with chronic heart failure?. Eur Heart J 1998 Apr;19(4):533‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wyss M, Kaddurah‐Daouk. Creatine and creatinine metabolism. Physiol Rev 2000;80(3):1107‐213. [DOI] [PubMed] [Google Scholar]


