Abstract
Background
Chronic heart failure (HF) is a prevalent world‐wide. Angiotensin receptor blockers (ARBs) are widely prescribed for chronic HF although their role is controversial.
Objectives
To assess the benefit and harm of ARBs compared with ACE inhibitors (ACEIs) or placebo on mortality, morbidity and withdrawals due to adverse effects in patients with symptomatic HF and left ventricular systolic dysfunction or preserved systolic function.
Search methods
Clinical trials were identified by searching CENTRAL, HTA, and DARE, (The Cochrane Library 2010 Issue 3), as well as MEDLINE (2002 to July 2010), and EMBASE (2002 to July 2010). Reference lists of retrieved articles and systematic reviews were checked for additional studies not identified by the electronic searches.
Selection criteria
Double blind randomised controlled trials in men and women of all ages who have symptomatic (NYHA Class II to IV) HF and: 1) left ventricular systolic dysfunction, defined as left ventricular ejection fraction (LVEF) ≤40%; or 2) preserved ejection fraction, defined as LVEF >40%.
Data collection and analysis
Two authors independently assessed risk of bias and extracted data from included studies.
Main results
Twenty two studies evaluated the effects of ARBs in 17,900 patients with a LVEF ≤40% (mean 2.2 years). ARBs did not reduce total mortality (RR 0.87 [95% CI 0.76, 1.00]) or total morbidity as measured by total hospitalisations (RR 0.94 [95% CI 0.88, 1.01]) compared with placebo.
Total mortality (RR 1.05 [95% CI 0.91, 1.22]), total hospitalisations (RR 1.00 [95% CI 0.92, 1.08]), MI (RR 1.00 [95% CI 0.62, 1.63]), and stroke (RR 1.63 [0.77, 3.44]) did not differ between ARBs and ACEIs but withdrawals due to adverse effects were lower with ARBs (RR 0.63 [95% CI 0.52, 0.76]). Combinations of ARBs plus ACEIs increased the risk of withdrawals due to adverse effects (RR 1.34 [95% CI 1.19, 1.51]) but did not reduce total mortality or total hospital admissions versus ACEI alone.
Two placebo‐controlled studies evaluated ARBs in 7151 patients with a LVEF >40% (mean 3.7 years). ARBs did not reduce total mortality (RR 1.02 [95% CI 0.93, 1.12]) or total morbidity as measured by total hospitalisations (RR 1.00 [95% CI 0.97, 1.05]) compared with placebo. Withdrawals due to adverse effects were higher with ARBs versus placebo when all patients were pooled irrespective of LVEF (RR 1.06 [95% CI 1.01, 1.12]).
Authors' conclusions
In patients with symptomatic HF and systolic dysfunction or with preserved ejection fraction, ARBs compared to placebo or ACEIs do not reduce total mortality or morbidity. ARBs are better tolerated than ACEIs but do not appear to be as safe and well tolerated as placebo in terms of withdrawals due to adverse effects. Adding an ARB in combination with an ACEI does not reduce total mortality or total hospital admission but increases withdrawals due to adverse effects compared with ACEI alone.
Plain language summary
Are angiotensin receptor blockers (ARBs) an effective treatment for heart failure?
Drugs called angiotensin receptor blockers (ARBs), such as losartan (brand name: Cozaar), candesartan (Atacand), eprosartan (Teveten), irbesartan (Avapro), telmisartan (Micardis) and valsartan (Diovan) are commonly used to treat heart failure. We asked whether ARBs reduced death, or severe disability as assessed by hospital admission for any reason versus an inert substance (placebo) or another class of drugs called ACE inhibitors, such as ramipril (Altace), captopril (Capoten), enalapril (Vasotec), fosinopril (Monopril), lisinopril (Prinivil, Zestril), and quinapril (Accupril). We also asked whether combining an ARB with an ACE inhibitor is more effective than an ACE inhibitor alone in reducing death, disability, or hospital admission for any reason. The scientific literature was searched to find all trials that had assessed these questions.
We found 24 trials that randomly assigned participants to take either an ARB or control substance (placebo or ACEI). These trials evaluated ARBs in 25,051 patients with heart failure and followed them for 2 years. ARBs were no better than placebo or ACE inhibitors in reducing the risk of death, disability, or hospital admission for any reason. However, more patients stopped treatment early with ARBs than with placebo due to side effects. Adding an ARB to an ACEI also did not reduce the risk of death, disability, or hospital admission for any reason as compared to ACEI alone, although more patients taking the combination stopped early due to side effects.
Background
Chronic heart failure (HF) is a serious condition associated with high morbidity and mortality rates. Therefore, the goal of any pharmacological therapy is to lower the risk of adverse clinical outcomes associated with this chronic disease.
It is a well‐known fact that excess activation of the renin‐angiotensin system (RAS) contributes to the pathophysiology of heart failure. Activation of the RAS results in increased production of angiotensin I (AI), which is converted to angiotensin II (AII) by angiotensin‐converting enzyme (ACE). AII is a potent vasoconstrictor and also stimulates aldosterone secretion, which increases sodium and water retention (Erhardt 2005). AII and aldosterone are also implicated in other potentially deleterious effects on the cardiovascular system, including endothelial damage, sympathetic activation, collagen formation and decreased nitric oxide production (Erhardt 2005). Together, these effects put a strain on the heart, which can eventually lead to heart failure. With the understanding that the RAS plays such a vital role in the progression of heart failure, two drug classes, ACE inhibitors and angiotensin receptor blockers (ARBs), were developed to inhibit the RAS and thus provide a potentially beneficial therapeutic approach for the treatment of heart failure.
ACE inhibitors are indicated as first‐line treatment for HF (ACCF/AHA 2009; CCS 2006; ESC 2008) because they have been conclusively demonstrated in major clinical trials evaluating morbidity and mortality to reduce mortality as well as rates of reinfarction and hospitalizations for heart failure (Flather 2000). ACE inhibitors achieve their favourable effects by blocking the production of AII, thereby inhibiting its biological effects, such as enhanced vasoconstriction and excessive sodium and water retention. The favourable effect of ACE inhibitors may also be partly attributed to the fact that ACE is identical to another enzyme called kininase II that is responsible for kinin degradation. By retarding degradation of bradykinin, ACE inhibitors prolong its beneficial vasodilatory and antitrophic effects (Jong 2002). However, the accumulation of bradykinin in the lung probably causes the side effect of dry cough and possibly other side effects that force some patients to discontinue treatment with ACE inhibitors (McMurray 2005).
Initially, it was believed that ACE is the only enzyme that will catalyze the production of AII from AI. However, it is unclear how complete the blockade by ACE inhibitors is and if there is continuing angiotensin II formation during chronic treatment with ACE inhibitors (Wolny 1997). Numerous studies have now shown that some patients on long‐term treatment with ACE inhibitors eventually have AII levels return to pretreatment levels, demonstrating that the blockade of the RAS by the ACE inhibitor is incomplete. This phenomenon is referred to as “ACE escape” and it may be the result of AII formation through non‐ACE‐dependent pathways. For example, chymase, a serine protease found in the human heart and other tissues, is able to form AII from AI and is not blocked by ACE inhibitors (Wolny 1997). The physiological significance of these non‐ACE‐dependent pathways for AII formation is not known at the present time.
In contrast, RAS blockade with ARBs is achieved by inhibiting the binding of AII to the angiotensin II type 1 (AT1) receptor, which is believed to mediate the harmful cardiovascular effects of angiotensin II. ARBs are believed to provide a more effective means of blockade of the RAS than is possible with ACE inhibitors because this blockade at the receptor level is independent of the pathway for AII formation (Erhardt 2005). In addition, this drug class allows the displaced AII to continue to bind to the angiotensin II type II (AT2) receptors that are not blocked by ARBs. Since AT2 receptors are believed to mediate favourable vasodilatory and anti‐trophic effects, this unopposed stimulation of the AT2 receptors may confer a theoretical advantage with ARBs over ACE inhibitors [Azizi 2004]. Furthermore, ARBs may be better tolerated since they do not interfere with the degradation of bradykinin that is responsible for cough and possibly other side effects of ACE inhibitors.
Despite their theoretical benefits, several clinical practice guidelines recommend ARBs only in ACE‐intolerant patients because clinical trial evidence of their effectiveness in heart failure patients is less robust yet ARBs are still widely prescribed (ACCF/AHA 2009; CCS 2006; ESC 2008). A systematic review of existing trial data may therefore provide new insights on the use of this drug class in patients with HF.
Why it is important to do this review
In recent years, several meta‐analyses evaluating ARBs for HF have been published (Jong 2002; Lakhdar 2008; Lee 2004; Shah 2010; Sharma 2000; Shibata 2008). In fact, the authors who originally developed the protocol for this Cochrane review (Jong 2002b) decided to publish the results of their meta‐analysis elsewhere (Jong 2002). Subsequently a published meta‐analysis included all 17 studies from Jong 2002 as well as an additional five studies, totaling 22 studies evaluating ARBs in HF patients and LVEF ≤40% (Lee 2004). No new trials in patients with HF and LVEF ≤40% have been published since Lee 2004, which quantifies the effect of ARBs when compared with placebo (with and without background ACE inhibitors) and ACE inhibitors on all‐cause mortality and heart failure hospitalisations. This meta‐analysis concluded that there was a reduction in all‐cause mortality (RR 0.87 [95% CI 0.76 to 1.00]; ARR=7.1%, NNT=14, p=0.05) with ARBs and HF hospitalisations (RR 0.71 [95% CI 0.61 to 0.82]; ARR=7.9%, NNT=13, p<0.00001), as compared to placebo. For ARBs versus ACE inhibitors, all‐cause mortality (RR 1.05 [95% CI 0.91 to 1.22]) and HF hospitalisations (RR 0.96 [95% CI 0.83 to 1.11]) did not differ. For ARB plus ACE inhibitor combinations versus ACE inhibitors alone, all‐cause mortality was not reduced (RR 0.98 [95% CI 0.90 to 1.06]) but HF hospitalisations were reduced (RR 0.81 [95% CI 0.74 to 0.89]; ARR=4.4%; NNT=23; p<0.00001).
Of the 22 chronic HF trials included in the meta‐analysis, 17 were short‐term studies evaluating the effect of an ARB on cardiac haemodynamic and/or neurohormonal parameters. They were not designed to assess the long‐term impact of ARBs on mortality or morbidity and contribute very little (< 2% per trial) to the overall estimate of the effect of ARBs on all‐cause mortality and hospitalisations for HF.
Four large‐scale trials (CHARM‐Added 2003; CHARM‐Alternative 2003; ELITE II 2000; Val‐HeFT 2001) contribute nearly all of the data to the meta‐analysis. However, the Lee 2004 meta‐analysis is limited to only two outcomes (all‐cause mortality and HF hospitalisations), whereas each trial reports on other mortality and morbidity outcomes, as well as withdrawals due to adverse effects. Furthermore, a recent systematic review of two large‐scale studies evaluating ARBs in patients with HF and preserved systolic function (LVEF >40%) has also limited its meta‐analysis to all‐cause mortality and heart failure hospitalisations (Shah 2010).
Therefore, the purpose of this review is not only to provide an update on the available literature, but also to more completely evaluate the data from large‐scale clinical trials using a broader range of outcomes in order to get a more complete understanding of the benefit and harm of ARBs in the treatment of chronic heart failure.
Objectives
In patients with symptomatic HF (NYHA Class II to IV) and EF ≤40%, to assess the benefit and harm of:
ARBs versus placebo, in addition to standard therapy, with or without an ACE inhibitor.
ARBs versus ACEIs.
ARB plus ACEI combination therapy versus ACEI alone.
In patients with symptomatic HF (NYHA Class II to IV) and EF >40% (i.e. preserved EF), to assess the benefit and harm of:
ARBs versus placebo, in addition to standard therapy, with or without an ACE inhibitor.
ARBs versus ACEIs.
ARB plus ACEI combination therapy versus ACEI alone.
Methods
Criteria for considering studies for this review
Types of studies
Published double‐blind, randomised controlled trials enrolling patients with symptomatic HF with an ARB as the experimental intervention were considered.
Studies were included if:
Treatment assignments were randomised and administrated in parallel (i.e. no crossover). Studies with more than one ARB arm were permitted.
Mortality and/or morbidity rates were reported as either clinical or safety endpoints. Studies were counted even if no event of interest occurred during the study if this was explicitly reported. Endpoints were counted if they occurred outside of the period of randomised therapies. No maximum limit was imposed on the length of follow‐up.
Controlled interventions were either placebos or ACEIs. More than one control arm was allowed.
Duration of randomised therapy was at least four weeks (i.e. studies in which treatment consists of only a single one‐time dose of the ARB, such as in haemodynamic, pharmacodynamic dose‐response, or safety studies, were excluded).
Studies were excluded if:
Protocol included co‐administration of other non‐randomised investigational agents (e.g. angiotensin II, bradykinin).
Published only in abstract forms or non‐peer reviewed journals whereby no further or insufficient information could be procured from the authors.
Types of participants
For the purpose of this review, we relied on the investigators’ HF diagnosis. Men and women of all ages who had symptomatic HF with NYHA functional class II‐IV. Asymptomatic subjects with left ventricular dysfunction were excluded. Echocardiographic, radionuclide, or angiographic documentation of ventricular dysfunction was not required if the clinical diagnosis of HF has been established by the study investigators.
A LVEF cut‐off point of 40% was used to differentiate between HF patients with reduced EF from those with preserved EF.
Types of interventions
Experimental intervention with any ARB at any dose, including candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, valsartan, and other ARBs not currently marketed.
Studies with more than one control arm were allowed if at least one control intervention was either a placebo or an ACEI. If placebos were used as controls, both studies with and without background open‐label ACEI therapy were included.
The interventions could be administered as single agents, combination therapies (including ARB plus ACEI combo), and in fixed or stepped/titrated doses.
Types of outcome measures
All clinical events or other outcome measures reported post‐randomisation were included in this review. No maximum limit was imposed on the length of follow‐up.
Primary:
-
Total mortality
Cardiovascular mortality
Non‐cardiovascular mortality
-
Cardiovascular morbidity
Myocardial infarction (MI)
Stroke
-
Total hospitalisations
Hospitalisations for HF (defined as a hospital admission for worsening signs or symptoms of HF, for complications relating to the treatment of HF, or for syncope or arrhythmias related to HF)
Other hospitalisations
Secondary:
Withdrawals due to adverse effects (WDAE)
Search methods for identification of studies
The protocol for this review was first published in Issue 2, 2001 of the Cochrane Database of Systematic Reviews (Jong 2002b). The authors published the results of their meta‐analysis a year later in another medical journal (Jong 2002). However, the full Cochrane review has never been published. In 2009, we took over the protocol in order to complete the systematic review according to the rigorous quality standards of The Cochrane Collaboration.
The strategies included in the published Cochrane protocol to search the bibliographic databases for potentially relevant studies (Jong 2002b) have been listed in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4). It is not possible to know if these exact search strategies have been used for the review since the published report did not include the search strategies (Jong 2002). Nevertheless, other systematic reviews have also been published in recent years (Lakhdar 2008; Lee 2004; Shah 2010; Shibata 2008), which have served to independently verify that Jong 2002 identified all relevant studies evaluating ARBs for HF up to May 2001. Therefore, we searched for additional reports of studies published from May 2001 onwards using updated electronic database search strategies (Appendix 5; Appendix 6; Appendix 7).
Electronic searches
Randomized controlled trials have been identified from previously published systematic reviews. This list of studies has been updated by searching the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2010 Issue 3, MEDLINE (May 2001 to July 2010), and EMBASE (May 2001 to July 2010). Health Technology Assessment (HTA) and Database of Abstracts of Reviews of Effects (DARE) databases have been searched via The Cochrane Library 2010 Issue 3.
Search strategies were designed with reference to those of the previous systematic review and in accordance with the Cochrane Heart Group methods and guidance. Electronic databases were searched using a strategy combining a modified form of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximizing version (Lefebvre 2011) with selected MeSH terms and free text terms relating to angiotensin receptor blockers and heart failure. The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary as applicable.
Searches have been limited to randomised controlled trials and a filter applied to limit by humans. No language restrictions have been applied. Consideration was given to variations in terms used and spellings of terms in different countries so that studies were not missed by the search strategy because of such variations.
See appendices for a list of the search strategies used for this review (Appendix 5; Appendix 6; Appendix 7).
Searching other resources
Reference lists of retrieved articles and systematic reviews and meta‐analyses were checked for any studies not identified by the electronic searches.
Data collection and analysis
Selection of studies
The titles and abstracts of citations identified by the electronic searches from May 2001 onwards were examined for possible inclusion by two reviewers (BSH & VM) working independently. Full publications of potentially relevant studies were retrieved (and translated into English where required) and two reviewers (BSH & VM) then independently determined study eligibility using a standardised inclusion form. Any disagreements about study eligibility were resolved by discussion and, if necessary, a third reviewer (KB) was asked to arbitrate.
Data extraction and management
Data from included studies were extracted by one reviewer (BSH or VM) using standardised data extraction forms and checked by a second reviewer (VM or BSH). If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were used because of possible measurement error when estimating from graphs. A second reviewer confirmed all numeric calculations and extractions from graphs or figures. Any discrepancies were resolved by consensus.
Data on patient characteristics (e.g. age, sex, race, NYHA class), details of the intervention (including drug name and dose), ACEI background therapy, and length of follow up were also extracted.
Assessment of risk of bias in included studies
Two reviewers (BSH, JMHC) independently assessed the risk of bias in included studies using the Cochrane Collaboration's recommended tool, which is a domain‐based critical evaluation of the following domains: sequence generation; allocation concealment; blinding of outcome assessment; incomplete outcome data; and selective outcome reporting (Higgins 2011). Assessments of risk of bias are provided in the risk of bias table for each study.
Measures of treatment effect
Dichotomous outcomes for each comparison have been expressed as relative risks with 95% confidence intervals (CI). If it was statistically significant then absolute risk difference, the associated number needed to treat/harm was calculated.
Three treatment comparisons have been made: 1) ARBs versus placebo, without background ACEI therapy; 2) ARBs versus ACEIs; and 3) ARB plus ACEI combination therapy versus ACEI alone. The latter comparison of combination therapy with ARBs and ACEIs against ACEIs alone is methodologically similar to a comparison of ARBs against placebos where background (open‐label) ACEI therapy is given. Trials with these latter two designs have been categorized as making the same type of treatment comparison.
Dealing with missing data
If there were multiple reports of the same study, the duplicate publications were scanned for additional data. Outcome results have been extracted at all follow up points post‐randomisation. Study authors were contacted where necessary to provide additional information.
Assessment of heterogeneity
In absence of substantial heterogeneity as judged by the I2 measure, a fixed effect model was used as the default model (Higgins 2011).
If there was substantial heterogeneity associated with an effect estimate, a random effects model was applied. This model provides a more conservative statistical comparison of the difference between intervention and control because a confidence interval around the effect estimate is wider than a CI around a fixed effect estimate. If a statistically significant difference was still present using the random effects model, the fixed effect pooled estimate and 95% CI have been reported because of the tendency of smaller trials, which are more susceptible to publication bias, to be over weighted with a random effects analysis (Heran 2008a; Heran 2008b).
Assessment of reporting biases
No language restrictions have been applied.
Data synthesis
Data have been processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data synthesis and analyses have been done using Review Manager 5.0 software.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis
Symptomatic HF patients have been divided into 2 subgroups according to baseline LVEF: 1) patients with LVEF ≤40%; and 2) patients with LVEF >40%.
Heterogeneity
Heterogeneity amongst included studies was explored qualitatively (by comparing the characteristics of included studies) and quantitatively (using the I2 statistic and chi‐squared test of heterogeneity). Where appropriate, data from each study have been pooled using a fixed effect model, except where substantial heterogeneity exists. The funnel plot and the Egger test were used to examine small study bias (Egger 1997).
Sensitivity analysis
Pooled effect estimates have been recalculated after exclusion of studies with a high risk of bias, i.e.: 1) unpublished studies; 2) studies in which participants with a previous intolerance of an ARB were excluded; or 3) studies with an ARB tolerability phase preceding randomisation. Each pooled treatment effect of ARBs will be considered qualitatively robust if the upper and lower confidence bounds for the pooled treatment effect remain unchanged in direction with respect to unity.
Results
Description of studies
Results of the search
Our search of the electronic databases yielded a total of 2622 records, and after de‐duplication 1911 records remained. After reviewing the titles and abstracts, we retrieved 55 full‐text articles for possible inclusion. A total of 21 studies (24 publications) were excluded: 10 reported no useful outcomes; five were not double‐blind; two were crossover studies; two studies included patients who were not all taking background ACE inhibitor therapy; one was not randomised; and one had an inappropriate control. Four non‐Cochrane systematic reviews were also excluded. Twenty four studies (27 publications) met the inclusion criteria and had extractable data to assess the effects of ARBs compared with ACE inhibitors or placebo on mortality and morbidity in patients with HF. The study selection process is summarized in the PRISMA flow diagram shown in Figure 1.
1.
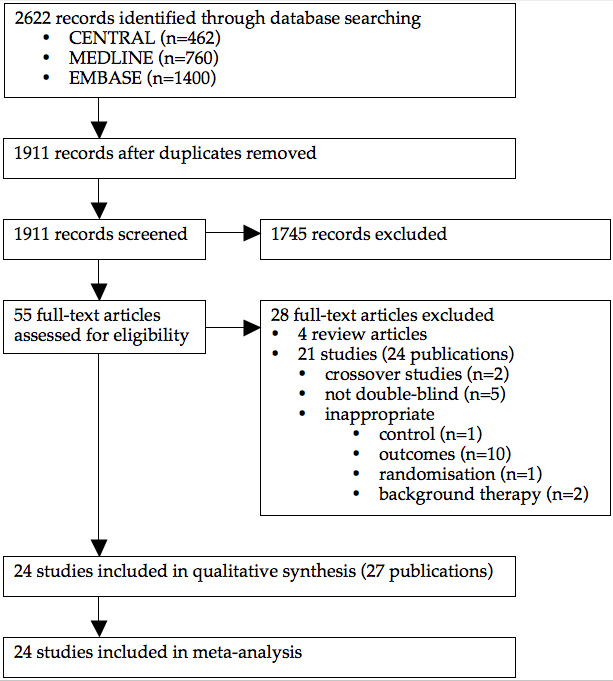
PRISMA flow diagram.
Included studies
The systematic review published in 2002 (Jong 2002) included a total of 17 studies, of which one was incorrectly judged to be a report of an independent study when, in fact, it was a review article (Weber 1997) that briefly summarized another included study (Crozier 1995). Thus, Weber 1997 has been listed as a duplicate publication of Crozier 1995 in this review.
In addition to the 16 studies (n=12,295) from Jong 2002 that have met the inclusion criteria of this review, eight studies (ARCH‐J 2003; CHARM‐Added 2003; CHARM‐Alternative 2003; CHARM‐Preserved 2003; HEAVEN 2002; I‐PRESERVE 2008; Mitrovic 2003; REPLACE 2001) have been identified by our search and have met the inclusion criteria. Thus, a total of 24 studies reporting data on a total of 25,051 patients with symptomatic HF have been included in this review. Twenty‐two studies randomised 17,900 patients with LVEF ≤40% and 2 studies randomised 7151 patients with LVEF >40%. Details of the studies included in the review are listed in the Characteristics of included studies table.
Eight duplicate publications of six included trials were identified. All 24 included studies were published in English, fifteen (63%) were multinational trials, and all but one (ADEPT 2001) were multicenter clinical trials. Twenty‐three (96%) of the included studies were industry‐sponsored while the remaining study did not report the source of funding.
Patients with LVEF >40%
In HF patients with preserved ejection fraction (EF) (i.e. >40%), only two placebo‐controlled studies have been published [CHARM‐Added 2003; I‐PRESERVE 2008). No studies comparing ARBs with ACE inhibitors or ARB plus ACE inhibitor combination with ACE inhibitor monotherapy have been identified. CHARM‐Added 2003 (candesartan) and I‐PRESERVE 2008 (irbesartan) randomised a total of 7151 patients (52% females) with mean age of 70 (range 67 to 72) years and LVEF 57% (range 54 to 60%) for a weighted mean duration of 176 (range 146 to 198) weeks.
Patients with LVEF ≤40%
In HF patients with LVEF ≤40%, eight were placebo‐controlled studies (ARCH‐J 2003; CHARM‐Alternative 2003; Crozier 1995; Mitrovic 2003; Sharma 2000, III‐Int'l; Sharma 2000, III‐US; SPICE 2000; STRETCH 1999), six studies compared ARBs with ACE inhibitors (Dickstein 1995; ELITE 1997; ELITE II 2000; HEAVEN 2002; Lang 1997; REPLACE 2001), and six studies compared ARB plus ACE inhibitor combination therapy with ACE inhibitor monotherapy (ADEPT 2001; CHARM‐Added 2003; Hamroff 1999; Tonkon 2000; V‐HeFT 1999; Val‐HeFT 2001). In addition, two were multi‐arm studies, of which one randomly assigned patients to an ARB, ACE inhibitor, and placebo (Mazayev 1998) and the other randomised patients to ARB plus ACE inhibitor combination therapy, ARB monotherapy, and ACE inhibitor monotherapy (RESOLVD 1999).
Of the 22 included trials, 18 were short‐term studies evaluating the effect of an ARB on cardiac haemodynamic and/or neurohormonal parameters. These studies were not designed to assess the long‐term impact of ARBs on mortality or morbidity and contribute very little (< 4% per trial) to the overall estimate of the effect of ARBs on total mortality and total hospitalisations. Four large‐scale trials (CHARM‐Added 2003; CHARM‐Alternative 2003; ELITE II 2000; Val‐HeFT 2001) contribute nearly all of the data to the meta‐analysis.
ARBs versus placebo
Nine studies with a weighted mean duration of 67 (range 4 to 135) weeks randomised a total of 4623 patients (29% females) with a mean age of 64 (range 53 to 65) years and LVEF 31% (range 23 to 35%). Trial sample sizes varied widely from 101 to 2028, with candesartan being the most studied ARB (5 studies; 3652 patients) followed by losartan (3 studies; 870 patients), and valsartan (1 study; 101 patients). The largest published trial was CHARM‐Alternative 2003 (n=2028), which contributes at least 90% of the data for this comparison of ARBs versus placebo.
ARBs versus ACEIs
Eight studies randomised 5201 patients (28% females) with a mean age of 70 (range 54 to 74) years and LVEF 30% (23 to 31%). The weighted mean duration of these studies was 56 (8 to 137) weeks. Sample sizes ranged from 90 to 3152, with losartan being the ARB most compared with an ACE inhibitor (4 studies; 4156 patients) followed by valsartan (2 studies; 231 patients), candesartan (1 study; 436 patients), and telmisartan (1 study; 378 patients). ELITE II 2000 (n=3152) contributes 80‐90% of the overall ARB versus ACE inhibitor data to the meta‐analysis.
ARB plus ACEI combination versus ACEI alone
Seven studies randomised a total of 8260 patients (20% females) with mean age of 63 (range 60 to 66) years and LVEF 27% (range 22 to 29%) for a weighted mean duration of 101 (range 4 to 144) weeks. Two large trials, CHARM‐Added 2003 (candesartan) and Val‐HeFT 2001 (valsartan), provide nearly all the data for this comparison.
Excluded studies
Twenty one studies (24 publications) were excluded for reasons listed in the Characteristics of excluded studies table, with the most common reason being a failure to report any of the pre‐specified outcomes of this review.
Risk of bias in included studies
Our judgements about each risk of bias item for each included study and about each risk of bias item presented as percentages across all included studies are summarized in Figure 2 and Figure 3, respectively.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Nearly all the trial publications simply reported that the trial was "randomised" but did not provide any details. Only 2/24 (8%) studies (I‐PRESERVE 2008; STRETCH 1999) reported details of appropriate sequence generation and 4/24 (17%) studies (CHARM‐Added 2003; CHARM‐Alternative 2003; CHARM‐Preserved 2003; I‐PRESERVE 2008) reported appropriate concealment of allocation.
Given the fact that many investigators use the term “randomised” when it is not justified, such vague reporting is insufficient to be confident that the allocation sequence was properly randomised and adequately concealed.
Blinding
Nine (38%) trials simply reported that the trial was “double‐blind” but did not provide any details about the blinding method. Thirteen (54%) trial publications described the blinding method as using “matching” or "matched" placebo. One trial described the method of blinding as "double dummy" (HEAVEN 2002). Due to the different dosage intervals of the drugs in Dickstein 1995, the authors stated that placebo tablets were provided in addition to secure blinding to treatment. The success of blinding in patients or investigators was not assessed in any of the included trials.
Incomplete outcome data
It is unlikely that attrition bias would have had an impact on the systematic review since most of the patients randomised in each trial completed the double blind treatment period. Those patients who were lost to follow up or dropped out prematurely were usually included in the clinical outcome or safety analysis of each trial.
Selective reporting
Eighteen (75%) of the included studies were not designed to assess treatment group differences in mortality and morbidity (as these were not the primary outcomes of these trials). Therefore, our assessment of selective reporting bias has been limited to the six long‐term health outcome trials (CHARM‐Added 2003; CHARM‐Alternative 2003; CHARM‐Preserved 2003; ELITE II 2000; I‐PRESERVE 2008; Val‐HeFT 2001). There is a potential for selective reporting bias in Val‐HeFT 2001 since this study only reported hospitalisations for HF but not total hospitalisations.
Other potential sources of bias
Selection bias
One of the exclusion criteria reported in one large‐scale trial was participants with a previous intolerance of an ARB (I‐PRESERVE 2008). This suggests that investigators have knowledge of each participant’s prior experience with this drug class and, thus, may select for patients who have responded favourably or have been found to tolerate treatment with ARBs. It was possible for us to prove selection bias in terms of WDAE (Analysis 1.9). Two included trials compared ARBs with placebo in patients with HF and a preserved EF (CHARM‐Preserved 2003; I‐PRESERVE 2008). In the CHARM‐Preserved 2003 trial, which did not exclude patients who demonstrated a previous ARB intolerance, there was a statistically significant increase in WDAE in patients treated with candesartan as compared to placebo (RR 1.32 [95% CI 1.12, 1.56]). In contrast, the increase in WDAE with irbesartan I‐PRESERVE 2008 trial did not reach statistical significance compared with placebo (RR 1.15 [95% CI 0.99, 1.33]).
1.9. Analysis.

Comparison 1: ARBs versus placebo, Outcome 9: WDAE
Two smaller studies (ARCH‐J 2003; Hamroff 1999) included an ARB tolerability phase and those patients with a confirmed intolerance of an ARB were excluded prior to randomisation. A sensitivity analysis was conducted by excluding these two studies and the results of the meta‐analysis were not affected.
Publication bias
Twenty three (96%) of the 24 included trials were sponsored by the manufacturer of the ARB being studied and one study did not report the source of funding. Given the fact that industry sponsored trials with positive results are selectively published, it is likely that this source of bias has had a significant impact on this review since it only included and appraised published trial evidence.
In order to test for the possibility of publication bias, a funnel plot was created for 9 of the placebo‐controlled studies (ARCH‐J 2003; CHARM‐Alternative 2003; Crozier 1995; Mazayev 1998; Mitrovic 2003; Sharma 2000, III‐Int'l; Sharma 2000, III‐US; SPICE 2000; STRETCH 1999) that reported total mortality in patients with EF ≤40%. CHARM‐Added 2003 and I‐PRESERVE 2008 looked at patients with preserved ejection (LVEF >40%) and were therefore excluded from the funnel plot analysis. There was no visual indication of funnel plot asymmetry and the Egger test was not statistically significant (Figure 4). However, due to the small number of studies the power was too low to reliably test for asymmetry.
4.
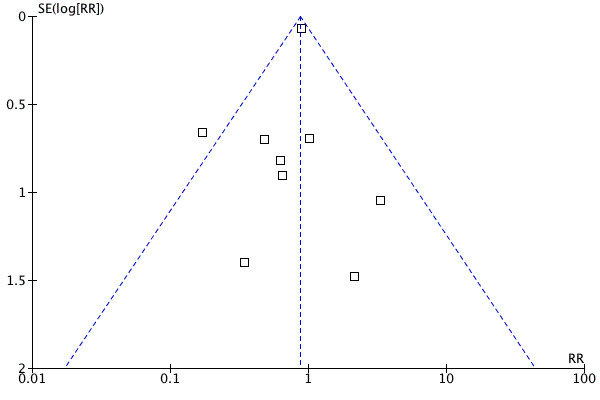
Funnel plot of ARBs versus placebo for total mortality in HF patients with EF ≤40%
Despite this limitation, there was still evidence of publication bias. One publication, a meta‐analysis of the effect of losartan on mortality in patients with HF, reported on deaths that occurred in two multicenter, placebo‐controlled studies (Sharma 2000, III‐Int'l; Sharma 2000, III‐US). Published reports of the complete data for these two multicenter studies were not identified by our comprehensive search.
Since the source of funding for these trials was almost always the manufacturer, the risk of bias is high. Therefore, we believe that this meta‐analysis may overestimate the benefits and underestimate the harms of ARB therapy. All studies, regardless of the findings, need to be published and accessible for secondary analysis in order to accurately assess the benefits and harms associated with this class of drugs.
Effects of interventions
ARBs versus placebo
Mortality
Patients with LVEF >40%
In 2 (n=7151) of the included studies in patients with preserved EF, ARBs did not reduce total mortality, cardiovascular mortality, or non‐cardiovascular mortality (Analysis 1.1; Analysis 1.2; Analysis 1.3).
1.1. Analysis.

Comparison 1: ARBs versus placebo, Outcome 1: Total mortality
1.2. Analysis.

Comparison 1: ARBs versus placebo, Outcome 2: Cardiovascular mortality
1.3. Analysis.

Comparison 1: ARBs versus placebo, Outcome 3: Non‐cardiovascular mortality
Patients with LVEF ≤40%
Nine (n=4643) of the included studies reported total mortality (Analysis 1.1). In these studies, the reduction in total mortality with ARB therapy was of borderline statistical significance at the 5% error level (RR 0.87 [95% CI 0.76, 1.00]). However, this estimate may be subject to bias in favour of ARBs as it includes two unpublished studies that have been summarized in a meta‐analysis sponsored by the manufacturer of losartan (Sharma 2000, III‐Int'l; Sharma 2000, III‐US). When the analysis is limited to the 7 trials with full reporting, the difference between ARBs and placebo is not statistically significant (RR 0.91 [95% CI 0.79 to 1.04]).
There were also no differences between ARBs and placebo for cardiovascular and non‐cardiovascular mortality (Analysis 1.2; Analysis 1.3).
Morbidity
Patients with LVEF >40%
None of the included studies reported stroke or MI.
Patients with LVEF ≤40%
Only 2 (n=2298) of the included trials studying candesartan reported total MI and stroke (Analysis 1.4; Analysis 1.5), of which CHARM‐Alternative 2003 contributes nearly all the data for these outcome measures. There was no statistically significant difference between candesartan and placebo for stroke, but candesartan increased the risk of MI (RR 1.44 [95% CI 1.03, 2.01]; ARI=1.9%; NNH=52) compared with placebo.
1.4. Analysis.

Comparison 1: ARBs versus placebo, Outcome 4: MI
1.5. Analysis.

Comparison 1: ARBs versus placebo, Outcome 5: Stroke
Hospitalisations
Patients with LVEF >40%
Two (n=7151) of the included studies reported total hospitalisations, hospitalisations for HF, and hospitalisations for other causes (Analysis 1.6; Analysis 1.7; Analysis 1.8). When this outcome was divided according to cause, the reduction in hospitalisations for heart failure with ARBs was of borderline statistical significance at the 5% error level (RR 0.90 [95% CI 0.81, 1.00]). This was offset by a non‐significant increase in other hospitalisations with ARB therapy (RR 1.05 [95% CI 0.99, 1.11]). Overall, total hospitalisations were not reduced with ARBs compared with placebo.
1.6. Analysis.

Comparison 1: ARBs versus placebo, Outcome 6: Total hospitalisations
1.7. Analysis.

Comparison 1: ARBs versus placebo, Outcome 7: Hospitalisations for heart failure
1.8. Analysis.

Comparison 1: ARBs versus placebo, Outcome 8: Other hospitalisations
Patients with LVEF ≤40%
Two (n=2298), 3 (n=2590), and 2 (n=2298) of the included studies reported total hospitalisations, hospitalisations for HF, and hospitalisations for other causes, respectively (Analysis 1.6; Analysis 1.7; Analysis 1.8). All data for these outcomes were from 3 candesartan studies, of which the long‐term CHARM‐Alternative 2003 trial contributed 90‐97% of the data. All‐cause hospitalisations were not reduced with candesartan compared with placebo. When this outcome was divided according to cause, candesartan reduced the risk of hospital admissions for HF (RR 0.71 [95% CI 0.61, 0.82]; ARR=8.0%; NNT=13) but increased the risk of hospital admissions for other causes (RR 1.12 [95% CI 1.00, 1.25]). CHARM‐Alternative 2003 contributed 98% of the data and demonstrated a statistically significant increase in other hospitalisations with candesartan (RR 1.13 [95% CI 1.01, 1.27]; ARI=4.6%; NNH=22).
Since an increase in hospitalisations for other causes can be attributed to a harmful effect of ARB therapy that is unrelated to the exacerbation of heart failure, we combined the data for all HF patients irrespective of LVEF (Analysis 1.8). There was a significantly increased risk of hospitalisations for other causes compared with placebo (RR 1.06 [95% CI 1.01, 1.12]; ARI=1.4%; NNH=72).
Withdrawals due to adverse effects
Patients with LVEF >40%
When the 2 large‐scale studies (n=7151) were pooled (Analysis 1.9), significantly more patients treated with ARBs withdrew due to adverse effects compared with placebo (RR 1.22 [95% CI 1.09, 1.36]; ARI=3%; NNH=33).
Patients with LVEF ≤40%
Six (n=3766) of the included studies reported WDAE (Analysis 1.9). When these trials were pooled, more patients in the ARB group discontinued therapy due to an adverse effect but the increase did not reach statistical significance (RR 1.14 [95% CI 0.97, 1.33]).
We pooled the data for all symptomatic HF patients irrespective of LVEF (Analysis 1.8) and WDAE were significantly higher in patients treated with ARBs (RR 1.19 [95% CI 1.09, 1.30]; ARI=1.4%; NNH=72).
ARBs versus ACE inhibitors
Mortality
Eight (n=5201), 4 (n=4131), and 4 (n=4131) of the included studies reported total mortality, cardiovascular mortality, and non‐cardiovascular mortality, respectively (Analysis 2.1; Analysis 2.2; Analysis 2.3). There was no difference between ARBs and ACEIs with regard to total mortality, cardiovascular mortality, or non‐cardiovascular mortality.
2.1. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 1: Total mortality
2.2. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 2: Cardiovascular mortality
2.3. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 3: Non‐cardiovascular mortality
Morbidity
MI and stroke were reported in two studies (n=3874) and 1 study (n=3152), respectively. There was no significant difference between treatment groups for these outcomes (Analysis 2.4; Analysis 2.5).
2.4. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 4: MI
2.5. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 5: Stroke
Hospitalisations
Four (n=4310) of the included studies reported total hospitalisations, hospitalisations for HF, and hospitalisations for other causes (Analysis 2.6; Analysis 2.7; Analysis 2.8). When these four studies were pooled, total hospitalisations, HF hospitalisations, and other hospitalisations did not differ between treatment groups.
2.6. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 6: Total hospitalisations
2.7. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 7: Hospitalisations for heart failure
2.8. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 8: Other hospitalisations
Withdrawals due to adverse effects
Six (n=3511) of the included studies reported WDAE (Analysis 2.9), which were significantly lower in patients treated with ARBs (RR 0.63 [95% CI 0.52, 0.76]; ARR=6.0%; NNT=17).
2.9. Analysis.

Comparison 2: ARBs versus ACE inhibitors, Outcome 9: WDAE
ARB plus ACEI versus ACEI alone
Mortality
Seven (n=8260), 2 (n=7558), and 2 (n=7558) of the included studies reported total mortality, cardiovascular mortality, and non‐cardiovascular mortality, respectively (Analysis 3.1; Analysis 3.2; Analysis 3.3). With respect to total mortality, cardiovascular mortality, or non‐cardiovascular mortality, there were no differences between combination therapy and ACEI monotherapy groups.
3.1. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 1: Total mortality
3.2. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 2: Cardiovascular mortality
3.3. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 3: Non‐cardiovascular mortality
Morbidity
MI and stroke were reported in only 1 large‐scale study, CHARM‐Added 2003 (n=2548). There was no significant difference between treatment groups for stroke (Analysis 3.5), but MI was reduced with combination therapy compared with ACEI alone (RR 0.64 [95% CI 0.44, 0.92]; ARR=2.0%; NNT=50) [Analysis 3.4].
3.5. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 5: Stroke
3.4. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 4: MI
Hospitalisations
Two (n=2989), 4 (n=8108), and 2 (n=2989) of the included studies reported total hospitalisations, hospitalisations for HF, and hospitalisations for other causes, respectively (Analysis 3.6; Analysis 3.7; Analysis 3.8). Total hospitalisations were not reduced with ARBs compared with placebo. When this outcome was divided according to cause, combination therapy reduced the risk of hospital admissions for HF (RR 0.81 [95% CI 0.74, 0.89]; ARR=4.4%; NNT=23) but this benefit was offset by a non‐significant increase in the risk of hospital admissions for other causes (RR 1.07 [95% CI 0.98, 1.18]).
3.6. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 6: Total hospitalisations
3.7. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 7: Hospitalisations for heart failure
3.8. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 8: Other hospitalisations
Withdrawals due to adverse effects
Four (n=7703) of the included studies reported WDAE (Analysis 3.9). When the data from these four trials were pooled, WDAE were significantly higher in patients receiving combination therapy (RR 1.34 [95% CI 1.19, 1.51]; ARI=3.7%; NNH=27).
3.9. Analysis.

Comparison 3: ARB plus ACEI versus ACEI alone, Outcome 9: WDAE
Discussion
Summary of main results
Jong 2002 included a total of 16 studies that randomly allocated 12,295 patients to either an ARB or control (placebo or ACEI). This review has allowed analysis of an increased number of patients from an additional eight studies published from 2001 to 2008, totaling 24 studies that enrolled 25,051 patients with symptomatic HF. A total of 17,900 patients with a LVEF ≤40% were studied in 22 studies (with a weighted mean follow‐up of 2.2 years in four large‐scale studies (CHARM‐Added 2003; CHARM‐Alternative 2003; ELITE II 2000; Val‐HeFT 2001) and 7151 patients with a LVEF >40% were studied in two large‐scale studies for a weighted mean duration of 3.7 years (CHARM‐Preserved 2003; I‐PRESERVE 2008).
ARBs versus placebo
Treatment with ARBs in symptomatic HF patients with a LVEF ≤40% did not significantly reduce all‐cause mortality, stroke, or all‐cause hospital admission as compared to placebo. CHARM‐Alternative 2003, a large‐scale trial in which HF patients who had demonstrated intolerance to ACE inhibitors were treated with candesartan or placebo for a median duration of 33.7 months, contributed 90% or more of the data to the outcome of hospital admission. In this trial, candesartan reduced the risk of hospital admission for HF (RR 0.73 [95% CI 0.62, 0.85]; ARR=7.8%; NNT=13) but increased the risk of hospital admission for other causes (RR 1.13 [95% CI 1.01, 1.27]; ARI=4.6%; NNH=22). Candesartan also increased the risk of MI (RR 1.57 [95% CI 1.10, 2.23]; ARI=2.7%; NNT=37) compared with placebo, which may partly explain the significant increase in other hospitalisations observed in this trial. A recently published systematic review evaluating the effect of ARBs in all major trials reporting MI did not demonstrate an increased risk of MI with ARBs as compared to other drugs or placebo (Volpe 2009). However, we cannot be certain all unpublished trials are accounted for and data not reported in existing published trials are needed to confirm the findings of this meta‐analysis.
There is no available RCT evidence that demonstrates that any individual ARB is more or less effective than another in the treatment of HF. In fact, the data included in this review showed no reduction in total mortality or total hospitalizations when candesartan or valsartan studies were pooled. Elevated blood pressure is in most cases the predominant contributor to the development and progression of HF. ARBs or any other antihypertensive agents have not been shown to provide any benefit beyond BP lowering. The evidence (46 studies, n=13,451) from a Cochrane review of the BP lowering efficacy of ARBs for primary hypertension suggests that there are no clinically meaningful BP lowering differences between available ARBs (Heran 2008b).
In symptomatic HF patients with a LVEF >40%, ARB therapy did not significantly reduce all‐cause mortality or all‐cause hospital admission compared with placebo. A statistically marginal reduction in hospitalisations for HF with ARBs was counteracted by a non‐significant increase in other hospitalisations. Rates of stroke and MI were not reported.
We pooled the data for all HF patients, irrespective of LVEF, to fully examine the effect of ARBs on hospital admission for other causes and there was a significantly increased risk of hospitalisations for other causes compared with placebo (RR 1.06 [95% CI 1.01, 1.12]; ARI=1.4%; NNH=72). We also pooled WDAE data from all placebo‐controlled studies and significantly more patients in the ARB group stopped treatment early due to adverse effects (RR 1.19 [95% CI 1.09, 1.30]; ARI=1.4%; NNH=72). These findings are in contrast with the widely held belief in the literature that ARBs have a safety and tolerability profile similar to that of placebo (Mancia 2009; Smith 2008; White 2011).
ARBs versus ACEIs
We pooled trials that compared ARBs with ACE inhibitors in symptomatic HF patients with a LVEF ≤40% and there appears to be no difference between the two drug classes in total mortality, cardiovascular mortality, stroke, MI, total hospitalisations, or hospitalisations for HF. ARBs were found to be superior to ACE inhibitors in tolerability with a significantly lower rate of withdrawals due to adverse effects (RR 0.63 [95% CI 0.52, 0.76]; ARR=6.0%; NNT=17).
ARB plus ACEI versus ACEI alone
In symptomatic HF patients with a LVEF ≤40%, the addition of an ARB to an ACE inhibitor was not effective in reducing total mortality or cardiovascular mortality compared with ACE inhibitor therapy alone. Combination therapy reduced hospitalisations for HF (RR 0.81 [95% CI 0.74, 0.89]; ARR=4.4%; NNT=23) but a non‐significant increase in hospital admissions for other causes (RR 1.07 [95% CI 0.98, 1.18]) was also observed, resulting in no significant difference between combination and ACE inhibitor monotherapy in all‐cause hospital admissions. The risk of stroke was not reduced but the risk of MI (RR 0.64 [95% CI 0.44, 0.92]; ARR=2.0%; NNT=50) was reduced with combination therapy. More patients receiving ARB plus ACE inhibitor combination had to stop treatment prematurely because of adverse effects (RR 1.34 [95% CI 1.19, 1.51]; ARI=3.7%; NNH=27).
Our search did not identify any studies that compared ARB plus ACE inhibitor combination therapy with ACE inhibitor alone in HF patients with a LVEF >40%.
Overall completeness and applicability of evidence
There are important differences between our review and previously published meta‐analyses (Lee 2004; Shah 2010; Sharma 2000; Shibata 2008), including the non‐Cochrane meta‐analysis published by the authors who originally developed this protocol (Jong 2002). The major limitation with these other reviews is that they have limited their analyses to total mortality and hospital admission for worsening HF. Our review also analysed other clinical outcomes, such as cardiovascular mortality, total hospitalisations, hospitalisations for other causes, stroke, MI, and withdrawals due to adverse effects, in an attempt to fully elucidate the benefit and harm of ARB treatment in HF patients based on published data from large‐scale clinical trials. In particular, all‐cause hospital admission is a useful measure of total morbidity and was reported in three of four large‐scale studies. Other useful indexes of morbidity would be total length of hospital admission or days in ICU or on a ventilator; however, these outcomes were not reported in any of the studies.
By expanding our analysis, we have observed some effects of ARBs that have not been reported in other meta‐analyses. For instance, the reduction in hospitalisations for HF with ARBs compared with placebo has been reported previously. However, the increase in hospitalisations for other causes with ARBs observed in our review has not been reported before. Given that in these trials the majority of total hospital admissions is to due to reasons other than worsening symptoms of HF, and the fact that total hospitalisations for any cause are not reduced, this suggests that there is no net health benefit of ARBs. If this is true the use of ARBs in this setting is called into question.
It is commonly accepted that ACEI reduce mortality and morbidity and therefore have become standard therapy in HF patients (ACCF/AHA 2009; CCS 2006; ESC 2008). We therefore decided to review the findings of a meta‐analysis of data from individual patients with HF or left‐ventricular dysfunction that is frequently cited to support that claim (Flather 2000). The authors of this systematic overview collected individual patient data from three large‐scale studies (n=5966) that enrolled acute MI patients (AIRE 1993; SAVE 1992; TRACE 1995) and two studies (n=6797) that enrolled patients with chronic HF (SOLVD treatment 1991) or LV dysfunction (SOLVD prevention 1992) who were randomly assigned to an ACEI or placebo and continued treatment for at least a year.
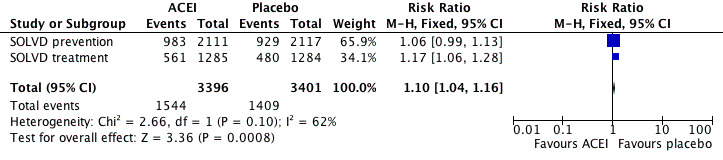
When the analysis was limited to the SOLVD treatment and prevention trials of enalapril in chronic HF, the ACEI group had lower rates of mortality (OR 0.87 [95% CI 0.78, 0.98]), reinfarction (OR 0.78 [95% CI 0.65, 0.92]) and readmission for HF (OR 0.63 [95% CI 0.56, 0.72]) than with placebo. The Flather 2000 meta‐analysis did not report total hospitalisations so we retrieved the published reports of SOLVD treatment 1991 and SOLVD prevention 1992 for these data. When these 2 studies were pooled, total hospitalisations (RR 0.96 [95% CI 0.92, 0.99]) and hospitalisations for HF (RR 0.69 [95% CI 0.63, 0.77]) were significantly reduced with enalapril (Figure 5; Figure 6). However, hospitalisations due to other causes were significantly increased (RR 1.10 [95% CI 1.04, 1.16]) with enalapril as compared to placebo (Figure 7), a finding consistent with what he have observed with ARBs.
5.
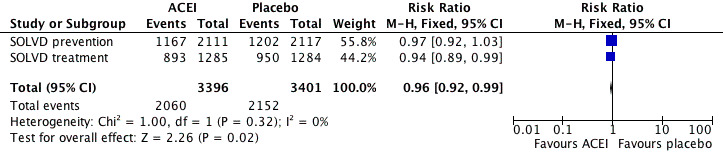
Forest plot of ACE inhibitor enalapril versus placebo for total hospitalisations.
6.
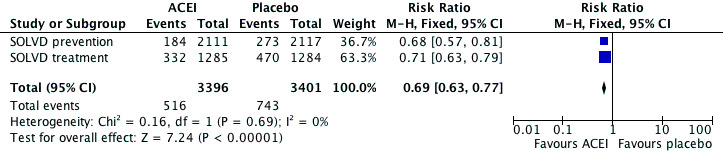
Forest plot of ACE inhibitor enalapril versus placebo for hospitalisations for heart failure.
7.
Forest plot of ACE inhibitor enalapril versus placebo for other hospitalisations.
Given this information, the finding in the present review that ARBs are not effective in terms or mortality and morbidity versus placebo is surprising and inconsistent with the finding that ACE inhibitors and ARBs are not different when compared directly. We cannot completely resolve this inconsistency; however, it is possible that we may have missed a modest benefit of ARBs compared to placebo in patients with HF and reduced ejection fraction. The mortality effect for ARBs (RR 0.87 [95% CI 0.76, 1.00] versus ACE inhibitors (OR 0.87 [95% CI 0.78, 0.98] and the total hospitalisations effect for ARBs (RR 0.94 [95% CI 0.88, 1.01] versus ACE inhibitors (RR 0.96 [0.92, 0.99] is not necessarily that different.
Where other meta‐analyses also failed to include WDAE as a safety outcome, we pooled all studies that reported this outcome and observed a higher rate in patients taking ARBs as compared to placebo. We also observed a higher rate of WDAE in patients taking ARB plus ACEI combination therapy versus an ACE inhibitor alone.
Overall, given the lack of proven benefit with ARBs in terms of total mortality and total hospitalisations as compared to placebo and a proven benefit of ACE inhibitors as compared to placebo, it is clear that ACE inhibitor should be the first choice class of drugs in HF patients.
Quality of the evidence
This review has revealed several significant limitations in the available RCT evidence, most notably the poor reporting of methodology in many trial publications. The method of randomisation, allocation concealment, and blinding was rarely described. Although the quality of reporting tended to be better in long‐term health outcome studies, details on the methodology was still incomplete.
All but one of the 24 included studies were sponsored by the manufacturer making a high risk of publication bias and other biases likely. It was not possible for us to determine the existence of publication bias by analysing funnel plots for asymmetry due to an insufficient number of included studies. However, we were able to find evidence of unpublished studies during our search for relevant studies. Sharma 2000 was an industry‐sponsored meta‐analysis of losartan for HF that included 2 unpublished placebo‐controlled, multicenter studies. The full reports of these 2 studies have not been published elsewhere. Therefore, it is possible that our inability to identify unpublished studies with negative results may have led to overestimation of treatment effects in our review (Egger 1997).
Furthermore, this meta‐analysis may under‐estimate the harms associated with ARB therapy as a result of patient selection bias. In one of two large‐scale trials in HF patients with preserved systolic function, participants with a previously documented intolerance of ARBs were excluded (I‐PRESERVE 2008). This source of bias clearly influenced the outcome of WDAE since in I‐PRESERVE 2008 trial there was no increase in WDAE with irbesartan compared with placebo, yet in the other trial (CHARM‐Preserved 2003) WDAE were significantly higher with candesartan, which did not select for patients who were tolerant of ARBs. Overall, significantly more patients treated with ARBs discontinued prematurely due to an adverse effect (RR 1.22 [95% CI 1.09, 1.36]; ARI=3%; NNH=33).
Authors' conclusions
Implications for practice.
ARBs versus placebo
ARBs do not reduce total mortality or all‐cause hospitalisations compared with placebo in the treatment of HF patients irrespective of LVEF. In HF patients with LVEF ≤40%, the benefit observed with ARBs in terms of a reduction in hospitalisations for HF was mitigated by an increase in hospitalisations for other causes. More patients treated with ARBs stopped treatment early due to adverse effects compared with placebo.
ARBs versus ACE inhibitors
ARBs do not reduce total mortality or all‐cause hospitalisations compared with ACE inhibitors in the treatment of symptomatic HF patients with LVEF ≤40%. ARBs reduced WDAE as compared to ACE inhibitors.
ARB plus ACE inhibitor combination versus ACE inhibitor alone
Adding an ARB to an ACE inhibitor does not reduce total mortality or all‐cause hospitalisations over an ACE inhibitor alone in the treatment of HF patients with LVEF ≤40%. Adding an ARB to an ACE inhibitor increases WDAE as compared to an ACE inhibitor alone in the treatment of HF patients with LVEF ≤40%.
Implications for research.
In placebo‐controlled trials, the higher rate of hospitalisations for other causes in patients treated with ARBs is a key observation. The majority of total hospital admissions in the included studies is to due to reasons other than worsening symptoms of HF. This potentially harmful effect of ARBs needs to be investigated further by exploring the causes of hospital admission using individual patient data.
RCTs are needed comparing ARBs and ACE inhibitors in HF patients with LVEF >40%.
What's new
| Date | Event | Description |
|---|---|---|
| 9 February 2021 | Review declared as stable | The one aspect of uncertainty within this topic is covered with a new review (CD012721). This review is therefore not planned to be updated. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 8 September 2008 | Amended | Converted to new review format. |
Notes
This review was supported by a National Institute for Health Research (NIHR) Cochrane Collaboration Programme Grant (CPGS10).
Acknowledgements
We would like to thank Phillip Jong, Catherine Demers, Robert S McKelvie, and Peter Liu for registering the title and developing the original protocol for this review.
We would also like to thank Doug Salzwedel for developing the search strategies and Gavin Wong for his translation services.
Appendices
Appendix 1. MEDLINE search strategy used by Jong 2002
exp Heart failure, congestive/
cardiomyopath:.tw.
(chf or hf ).tw.
(heart adj25 failure).tw.
(cardiac adj25 (failure or insufficiency)).tw.
or/1‐5
exp Receptors, angiotensin/
exp Losartan/
arb?.tw.
(angiotensin: adj25 receptor: adj25 (block: or antagon: or inhibit:)).tw
(candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan).tw.
(139481‐59‐7 or 145040‐37‐5 or 133040‐01‐4 or 145216‐43‐9 or 138402‐11‐6 or 114798‐26‐4 or 124750‐99‐8 or 146613‐90‐3 or 146623‐69‐0 or 145733‐36‐4 or 144701‐48‐4 or 137862‐53‐4 or 145781‐32‐4).rn.
or/7‐12
6 and 13
Appendix 2. EMBASE search strategy used by Jong 2002
explode ’heart failure’ / all subheadings
explode ’congestive cardiomyopathy’ / all subheadings
cardiomyopath*
chf or hf
heart and failure
cardiac and (failure or insufficiency)
#1 or #2 or #3 or #4 or #5 or #6
explode ’angiotensin receptor antagonist’ / all subheadings
arb?
angiotensin* and receptor* and (block* or antagon* or inhibit*)
candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan
(139481‐59‐7 or 145040‐37‐5 or 133040‐01‐4 or 145216‐43‐9 or 138402‐11‐6 or 114798‐26‐4 or 124750‐99‐8 or 146613‐90‐3 or 146623‐69‐0 or 145733‐36‐4 or 144701‐48‐4 or 137862‐53‐4 or 145781‐32‐4) in rn
#8 or #9 or #10 or #11 or #12
#7 and #13
Appendix 3. CENTRAL search strategy used by Jong 2002
HEART‐FAILURE‐CONGESTIVE*:ME
CARDIOMYOPATH*
(CHF or HF)
(HEART and FAILURE)
(CARDIAC and FAILURE)
(CARDIAC and INSUFFICIENCY)
(((((#1 or #2) or #3) or #4) or #5) or #6)
RECEPTORS‐ANGIOTENSIN*:ME
LOSARTAN*:ME
(ARB or ARBS)
(ANGIOTENSIN* and (RECEPTOR* and ((BLOCK* or ANTAGON*) or INHIBIT*)))
(((((((((((CANDESARTAN or ELISARTAN) or EMBUSARTAN) or EPROSARTAN) or FORASARTAN) or IRBESARTAN) or LOSARTAN) or SAPRISARTAN) or TASOSARTAN) or TELMISARTAN) or VALSARTAN) or ZOLASARTAN)
((((#8 or #9) or #10) or #11) or #12)
(#7 and #13)
Appendix 4. Biological Abstracts & International Pharmaceutical Abstracts search strategy used by Jong 2002
clin* near trial*
(singl* or doubl* or trebl* or tripl*) near (blind* or mask*)
randomi*
#1 or #2 or #3
cardiomyopath*
chf or hf
heart near failure
cardiac near (failure or insufficiency)
#5 or #6 or #7 or #8
arb?
angiotensin* near receptor* near (block* or antagon* or inhibit*)
candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan
(139481‐59‐7 or 145040‐37‐5 or 133040‐01‐4 or 145216‐43‐9 or 138402‐11‐6 or 114798‐26‐4 or 124750‐99‐8 or 146613‐90‐3 or 146623‐69‐0 or 145733‐36‐4 or 144701‐48‐4 or 137862‐53‐4 or 145781‐32‐4) in rn
#10 or #11 or #12 or #13
#9 and #14
#4 and #15
Appendix 5. MEDLINE search strategy used for this review
exp heart failure/
heart failure.tw.
(chf or hf).tw.
((cardiac or mycardial) adj (failure or insufficiency)).tw.
cardiomyopath*.tw.
or/1‐5
exp receptors, angiotensin/
exp angiotensin II type 1 receptor blockers/
arb?.tw.
(angiotensin$ adj6 receptor$ adj6 (block$ or antagon$ or inhibit$)).tw.
azilsartan.mp.
candesartan.mp.
elisartan.mp.
embusartan.mp.
eprosartan.mp.
forasartan.mp.
irbesartan.mp.
losartan.mp.
olmesartan.mp.
saprisartan.mp.
tasosartan.mp.
telmisartan.mp.
valsartan.mp.
zolasartan.mp.
saralasin.mp.
cozaar.tw.
hyzaar.tw.
atacand.tw.
teveten.tw.
avapro.tw.
micardis.tw.
avalide.tw.
aprovel.tw.
amias.tw.
diovan.tw.
olmetec.tw.
or/7‐36
6 and 37
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.tw.
placebo.tw.
drug therapy/
randomly.tw.
trial.tw.
groups.tw.
or/39‐46
animals/ not (humans/ and animals/)
47 not 48
38 and 49
limit 50 to ed=20010501‐20100716
Appendix 6. EMBASE search strategy used for this review
exp heart failure/
heart failure.tw.
congestive cardiomyopathy/
cardiomyopath$.tw.
(chf or hf).tw.
((cardiac or myocardial) adj (failure or insufficiency)).tw.
or/1‐6
exp angiotensin receptor antagonist/
arb?.tw.
(angiotensin$ adj6 receptor$ adj6 (block$ or antagon$ or inhibit$)).tw.
azilsartan.mp.
candesartan.mp.
elisartan.mp.
embusartan.mp.
eprosartan.mp.
forasartan.mp.
irbesartan.mp.
losartan.mp.
olmesartan.mp.
saprisartan.mp.
tasosartan.mp.
telmisartan.mp.
valsartan.mp.
zolasartan.mp.
saralasin.mp.
cozaar.tw.
hyzaar.tw.
atacand.tw.
teveten.tw.
avapro.tw.
micardis.tw.
avalide.tw.
aprovel.tw.
amias.tw.
diovan.tw.
olmetec.tw.
or/8‐36
7 and 37
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross‐over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
crossover procedure/
double blind procedure/
randomized controlled trial/
single blind procedure/
39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53
(animal/ or nonhuman/) not human/
54 not 55
38 and 56
limit 57 to em=200105‐201008
limit 58 to embase
Appendix 7. CENTRAL search strategy used for this review
MeSH descriptor Heart Failure explode all trees
heart next failure in All Text
(chf in All Text or hf in All Text)
cardiomyopath* in All Text
(cardiac next failure in All Text or cardiac next insufficiency in All Text)
(myocardial next failure in All Text or myocardial next insufficiency in All Text)
(#1 or #2 or #3 or #4 or #5 or #6)
MeSH descriptor Angiotensin II Type 1 Receptor Blockers explode all trees
MeSH descriptor Receptors, Angiotensin explode all trees
arb* in All Text
(azilsartan in All Text or candesartan in All Text or elisartan in All Text or embusartan in All Text or eprosartan in All Text or forasartan in All Text or irbesartan in All Text or losartan in All Text or olmesartan in All Text or saprisartan in All Text or tasosartan in All Text or telmisartan in All Text or valsartan in All Text or zolasartan in All Text)
(saralasin in All Text or cozaar in All Text or hyzaar in All Text or atacand in All Text or teveten in All Text or avapro in All Text or micardis in All Text or diovan in All Text or avalide in All Text or amias in All Text)
(#8 or #9 or #10 or #11 or #12)
(#7 and #13)
Data and analyses
Comparison 1. ARBs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 1.1.2 Patients with LVEF ≤40% | 9 | 4643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 1.2 Cardiovascular mortality | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| 1.2.2 Patients with LVEF ≤40% | 4 | 3382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.03] |
| 1.3 Non‐cardiovascular mortality | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.25] |
| 1.3.2 Patients with LVEF ≤40% | 4 | 3382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.54] |
| 1.4 MI | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Patients with LVEF >40% | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.2 Patients with LVEF ≤40% | 2 | 2298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.03, 2.01] |
| 1.5 Stroke | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Patients with LVEF >40% | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.2 Patients with LVEF ≤40% | 2 | 2298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.27] |
| 1.6 Total hospitalisations | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.05] |
| 1.6.2 Patients with LVEF ≤40% | 2 | 2298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.01] |
| 1.7 Hospitalisations for heart failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.00] |
| 1.7.2 Patients with LVEF ≤40% | 3 | 2590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.82] |
| 1.8 Other hospitalisations | 4 | 9449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.01, 1.12] |
| 1.8.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.99, 1.11] |
| 1.8.2 Patients with LVEF ≤40% | 2 | 2298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.00, 1.25] |
| 1.9 WDAE | 8 | 10917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 1.9.1 Patients with LVEF >40% | 2 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |
| 1.9.2 Patients with LVEF ≤40% | 6 | 3766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
Comparison 2. ARBs versus ACE inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total mortality | 8 | 5201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.22] |
| 2.2 Cardiovascular mortality | 4 | 4131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 2.3 Non‐cardiovascular mortality | 4 | 4131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.34] |
| 2.4 MI | 2 | 3874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.62, 1.63] |
| 2.5 Stroke | 1 | 3152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.77, 3.44] |
| 2.6 Total hospitalisations | 3 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 2.7 Hospitalisations for heart failure | 3 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 2.8 Other hospitalisations | 3 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 2.9 WDAE | 6 | 3511 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.52, 0.76] |
Comparison 3. ARB plus ACEI versus ACEI alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total mortality | 7 | 8260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 3.2 Cardiovascular mortality | 2 | 7558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 3.3 Non‐cardiovascular mortality | 2 | 7558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.23] |
| 3.4 MI | 1 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 3.5 Stroke | 1 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.76, 1.72] |
| 3.6 Total hospitalisations | 2 | 2989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.05] |
| 3.7 Hospitalisations for heart failure | 4 | 8108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.89] |
| 3.8 Other hospitalisations | 2 | 2989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.18] |
| 3.9 WDAE | 4 | 7703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.19, 1.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ADEPT 2001.
| Study characteristics | ||
| Methods | Single‐center, prospective DBRCT (double blind randomised control trial) conducted in Glasgow, UK Follow‐up: 8 wks Background ACEI? Yes |
|
| Participants | N=36 patients ≥18 years of age with CHF, caused by IHD or nonischemic dilated cardiomyopathy, receiving ACEI therapy for ≥4 wks Mean age: 63.5 y Females: 19.4% NYHA Class: II–IV LVEF: ≤35% |
|
| Interventions | Eprosartan 200 mg BID to target 400 mg BID (n=18) Placebo (n=18) |
|
| Outcomes | Primary: LVEF Secondary: haemodynamics; neurohormones |
|
| Notes | Funding source: Smithkline Beecham Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal from study (6/36 patients) have been described. |
| Other bias | High risk | Funding source is manufacturer of eprosartan. |
ARCH‐J 2003.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in Japan Follow‐up: 6 mos Background ACEI? No |
|
| Participants | N=305 patients ≥20 years of age with CHF due to previous MI, hypertensive heart disease, dilated cardiomyopathy or valvular disease N=292 patients included in full analysis Mean age: 63.7 y Females: 22.5% NYHA Class: II–III LVEF: ≤45% |
|
| Interventions | Candesartan 8 mg OD (n=155; n=148 included in full analysis) Placebo (n=150; n=144 included in full analysis) |
|
| Outcomes | Primary: Composite outcome, 'confirmed progression of CHF', which included the following: 1) hospitalisation for management of CHF; or 2) addition of, or increase in, any medication(s) administered specifically for management of CHF in response to apparent aggravation of its manifestations Secondary: occurrence of CV event, including progression of CHF, cardiac death, life‐threatening arrhythmias, MI, coronary artery disease (defined as angina, coronary artery intervention or revascularization), stroke and transient Ischaemic attack |
|
| Notes | Funding source: Takeda Chemical Industries, Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data, i.e. total hospitalisations Patients excluded from full analysis [13/305 patients; candesartan (n=7), placebo (n=6)] are described in Table 1. |
| Other bias | High risk | Patient selection bias: Prior to randomisation, patients received 4‐mg test dose of candesartan to confirm tolerance of single dose of study drug. Eight patients were not randomised after test dose due to adverse event caused by test dose (n=7), such as marked hypotension, and one patient exited study immediately after having received test dose. Funding source is manufacturer of candesartan. |
CHARM‐Added 2003.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 3 y (mean); 41 mos (median) Background ACEI? Yes |
|
| Participants | N=2548 patients ≥18 years of age with symptomatic CHF, due to IHD (62.4%), idiopathic (26.2%), hypertensive cause (6.5%), or other cause (4.9%), and treated with ACEI at constant dose for ≥30 days Mean age: 64.1 y Females: 21.3% Race (white/black/other): 91%/5%/4% NYHA Class: II–IV (if class II, patients had to have admission to hospital for cardiac reason in previous 6 mos LVEF: ≤40% |
|
| Interventions | Candesartan, target dose 32 mg OD (n=1276) Placebo (n=1272) |
|
| Outcomes | Primary: CV death or unplanned admission to hospital for management of worsening CHF Secondary: CV death, hospital admission for CHF, or non‐fatal MI; CV death, hospital admission for CHF, non‐fatal MI, or non‐fatal stroke; CV death, hospital admission for CHF, non‐fatal MI, non‐fatal stroke, or coronary revascularization; all‐cause mortality or hospital admission for CHF; development of new diabetes |
|
| Notes | Funding source: AstraZeneca R&D | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomly assigned patients, in a double‐blind way, candesartan or matching placebo, which could be started at 4 or 8 mg once daily (figure 1), the assignment code being held at an independent centre and by the data safety monitoring board." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "We randomly assigned patients, in a double‐blind way, candesartan or matching placebo, which could be started at 4 or 8 mg once daily (figure 1), the assignment code being held at an independent centre and by the data safety monitoring board." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The analysis was done on an intention‐to‐treat basis and included all randomised patients." |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
CHARM‐Alternative 2003.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 33.7 mos (median) Background ACEI? No |
|
| Participants | N=2028 patients ≥18 years of age with symptomatic CHF, due to IHD (62.4%), idiopathic (26.2%), hypertensive cause (6.5%), or other cause (4.9%), and who had intolerance to ACEI. Mean age: 68.3 y Females: 31.9% Race (white/black/other): 88.6%/3.6%/7.8% NYHA Class: II–IV LVEF: ≤40% |
|
| Interventions | Candesartan, target dose 32 mg OD (n=1013) Placebo (n=1015) |
|
| Outcomes | Primary: CV death or unplanned admission to hospital for management of worsening CHF Secondary: CV death, hospital admission for CHF, or non‐fatal MI; CV death, hospital admission for CHF, non‐fatal MI, or non‐fatal stroke; CV death, hospital admission for CHF, non‐fatal MI, non‐fatal stroke, or coronary revascularization; all‐cause mortality or hospital admission for CHF; development of new diabetes |
|
| Notes | Funding source: AstraZeneca R&D | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomly assigned patients candesartan or matching placebo in a double‐blind way (figure 1), the assignment code being held by an independent centre and the data safety monitoring board." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "We randomly assigned patients candesartan or matching placebo in a double‐blind way (figure 1), the assignment code being held by an independent centre and the data safety monitoring board." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The analysis was based on intention to treat and included all randomised patients." |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
CHARM‐Preserved 2003.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 36.6 mos (median) Background ACEI? No |
|
| Participants | N=2548 patients ≥18 years of age with symptomatic CHF, due to IHD (56.5%), idiopathic (8.7%), hypertensive cause (22.7%), or other cause (12.1%), and had history of hospital admission for cardiac reason, and LVEF >40% Mean age: 67.2 y Females: 40.1% Race (white/black/other): 92%/4%/4% NYHA Class: II–IV LVEF: >40% |
|
| Interventions | Candesartan, target dose 32 mg OD (n=1514) Placebo (n=1509) |
|
| Outcomes | Primary: CV death or unplanned admission to hospital for management of worsening CHF Secondary: CV death, hospital admission for CHF, or non‐fatal MI; CV death, hospital admission for CHF, non‐fatal MI, or non‐fatal stroke; CV death, hospital admission for CHF, non‐fatal MI, non‐fatal stroke, or coronary revascularization; all‐cause mortality or hospital admission for CHF; development of new diabetes |
|
| Notes | Funding source: AstraZeneca R&D | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomly assigned patients, in a double‐blind way, candesartan or matching placebo (figure 1), which could be started at 4 or 8 mg once daily, the assignment code being held by an independent centre and the data safety monitoring board." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "We randomly assigned patients, in a double‐blind way, candesartan or matching placebo (figure 1), which could be started at 4 or 8 mg once daily, the assignment code being held by an independent centre and the data safety monitoring board." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The analysis was based on intention to treat and included all randomised patients. |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
Crozier 1995.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in US, Europe and New Zealand Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=134 patients with stable symptomatic HF (due to IHD in 64.3% of patients) Mean age: 60.9 y Females: 15.0% Race (white/hispanic/black/other): 83%/0%/7%/10% NYHA Class: II–IV LVEF: <40% |
|
| Interventions | Losartan 2.5 mg OD (n=28) Losartan 10 mg OD (n=29) Losartan 25 mg OD (n=29) Losartan 50 mg OD (n=22) Placebo (n=26) |
|
| Outcomes | Hemodynamics; neurohormones | |
| Notes | Funding source: Merck Research Study "HM" in Sharma 2000 meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of losartan. |
Dickstein 1995.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in US and Europe Follow‐up: 8 wks Background ACEI? No |
|
| Participants | N=166 patients with symptomatic HF due to IHD (69%), dilated cardiomyopathy (25%) or valvular disorder (4%) Mean age: 64.3 y Females: 22.3% Race (white/hispanic black/other): 99.3%/0%/0%/0.7% NYHA Class: III–IV LVEF: ≤35% |
|
| Interventions | Losartan 25 mg OD (n=52) Losartan 50 mg OD (n=56) Enalapril 20 mg OD (n=58) |
|
| Outcomes | Primary: Assessment of symptoms of heart failure, exercise capacity, and neurohormonal status | |
| Notes | Funding source: Merck, Sharpe and Dohme Research Laboratories Study "Phase II‐S" in Sharma 2000 meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Placebo tablets were provided in addition to secure blinding to treatment because of the different dosage intervals of the drugs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of losartan. |
ELITE 1997.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in US, Europe and South America Follow‐up: 48 wks Background ACEI? No |
|
| Participants | N=722 patients ≥65 years of age with symptomatic HF, due to IHD (68%) or non‐ischemic heart disease (32%), and no history of prior ACEI or ARB therapy Mean age: 73.5 y Females: 33.2% Race (white/black/other): 89.5%/4.7%/5.8% NYHA Class: II–IV LVEF: ≤40% |
|
| Interventions | Losartan, target dose 50 mg OD (n=352) Captopril, target dose 50 mg TID (n=370) |
|
| Outcomes | Primary: Safety measure of renal dysfunction, defined as persisting increase in serum creatinine of ≥26.5 μmol/L (≥0.3 mg/dL) on therapy Secondary: Composite of death and/or hospital admission for heart failure; total mortality; admission for heart failure; NYHA class; admission for MI or unstable angina |
|
| Notes | Funding source: Merck Research Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All deaths (including cause of death) and hospital admissions were adjudicated on by an independent Clinical Endpoint Adjudication Committee, blinded to study treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses of deaths and heart‐failure admissions (adjudicated endpoints) were based on an intention‐to‐treat population; all patients who discontinued prematurely were followed up to the specified 48 weeks." |
| Other bias | High risk | Funding source is manufacturer of losartan. Study "ELITE" in Sharma 2000 meta‐analysis |
ELITE II 2000.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in 46 countries Follow‐up: 1.5 y (median) Background ACEI? No |
|
| Participants | N=3152 patients ≥60 years of age with symptomatic HF, due to IHD (79%) or non‐ischemic heart disease (21%), and most patients were to be ACEI and ARB naive. Some patients were eligible if ACEI or ARB treatment had been recently started and exposure period was ≤7 days. Mean age: 71.5 y Females: 30.5% Race (white/black/asian/other): 82%/2%/5%/11% NYHA Class: II–IV LVEF: ≤40% |
|
| Interventions | Losartan, target dose 50 mg OD (n=1578) Captopril, target dose 50 mg TID (n=1574) |
|
| Outcomes | Primary: All‐cause mortality (classified as sudden cardiac death, progressive heart failure, fatal myocardial infarction, stroke, other cardiac causes, other vascular disease, non‐cardiovascular causes) Secondary: Composite of death and/or hospital admission for heart failure; total mortality; admission for heart failure; NYHA class; admission for MI or unstable angina |
|
| Notes | Funding source: Merck Research Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "(placebo) matched to losartan or captopril tablets"; "captopril‐matched placebo"; "losartan‐matched placebo" Judgment: Adequate double‐blinding method. Quote: "Results were reviewed and classified, according to prespecified criteria, by an independent clinical endpoint classification committee, unaware of treatment status and interim results." Judgment: Adequate blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Patients who discontinued treatment were followed up every 4 months by clinical assessment and mortality and morbidity data were collected until the end of the study." |
| Other bias | High risk | Patient selection bias: One of exclusion criteria was intolerance of ACEI or ARBs. Funding source is manufacturer of losartan. |
Hamroff 1999.
| Study characteristics | ||
| Methods | Prospective DBRCT conducted at 4 centers in US and France Follow‐up: 6 mos Background ACEI? Yes |
|
| Participants | N=33 patients with severe symptomatic CHF, due to IHD (30%) or non‐ischemic heart disease (70%), despite treatment with maximally recommended or tolerated doses of ACEI for ≥3 months Mean age: 60.8 y Females: 51.5% NYHA Class: III–IV |
|
| Interventions | Losartan 50 mg OD (n=16) Placebo (n=17) |
|
| Outcomes | Primary: Peak VO2; NYHA functional class Secondary: Laboratory safety parameters; doses of concomitant background medications |
|
| Notes | Funding source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Patient selection bias: All patients tolerated losartan 50 mg/day during single‐blind tolerability phase. |
HEAVEN 2002.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT in Sweden Follow‐up: 12 wks Background ACEI? Yes |
|
| Participants | N=141 patients ≥18 years of age with symptomatic CHF, due to IHD (61%) or non‐ischemic heart disease (39%), stabilized on an ACEI for ≥3 mos and able to perform 6‐minute walk test Mean age: 67.5 y Females: 25.5% NYHA Class: II–III LVEF: ≤45% |
|
| Interventions | Valsartan 160 mg OD (n=70) Enalapril 5 mg BID (n=71) |
|
| Outcomes | Primary: exercise capacity (6‐minute walk test) Secondary: QoL (Minnesota Living with Heart Failure Questionnaire); LVEF; dyspnea‐fatigue index score; left ventricular end diastolic diameter |
|
| Notes | Funding source: Novartis Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of valsartan. |
I‐PRESERVE 2008.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 49.5 mos Background ACEI? Yes, but only if considered essential for indication other than uncomplicated hypertension |
|
| Participants | N=4128 patients ≥60 years of age with stable symptomatic CHF, primarily due to IHD (25%) or hypertension (64%), with EF ≥45% Mean age: 72 y Females: 60% Race (white/black/asian/other): 93%/2%/1%/4% NYHA Class: II–IV LVEF: ≥45% |
|
| Interventions | Irbesartan, target dose 300 mg OD (n=2067) Placebo (n=2061) |
|
| Outcomes | Primary: Composite of total mortality or CV hospitalisation (worsening HF, MI, stroke, unstable angina, ventricular or atrial dysrhythmia, or MI or stroke that occurred during any hospitalisation) Secondary: Total mortality; CV hospitalisation; composite of death due to worsening HF or sudden death or hospitalisation due to worsening HF; QoL; composite of CV mortality, non‐fatal MI, or non‐fatal stroke; CV mortality |
|
| Notes | Funding source: Bristol‐Myers Squibb and Sanofi‐Aventis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was implemented with the use of an interactive voice‐response system. The randomisation block size was two and was stratified according to site." |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators and committee members who were involved in the conduct of the study (except for members of the data and safety monitoring board) were unaware of study‐group assignments." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...randomly assigned in a 1:1 ratio to receive irbesartan or matching placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data from all patients who underwent randomisation were analysed according to the intention‐to‐treat principle." "At the end of the study, vital‐status data were not available for 29 patients (1%) in the irbesartan group and 44 patients (2%) in the placebo group. If contact could not be made at end of study, data for these patients were censored from the analysis at the date they were last known to be alive." |
| Other bias | High risk | Patient selection bias: "Exclusion criteria included previous intolerance to an angiotensin‐receptor blocker;..." Funding source is manufacturer of irbesartan. |
Lang 1997.
| Study characteristics | ||
| Methods | Multicenter (15 centers from US and 1 from Canada), prospective DBRCT Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=116 patients with symptomatic CHF, primarily due to IHD (47%) or dilated cardiomyopathy (41%), previously treated with stable doses of ACEI and diuretic agents for a minimum of 6 weeks and 2 weeks, respectively, with or without concurrent digitalis and other vasodilators. Mean age: 57.8 y Females: 22% Race (white/black/hispanic/oriental): 71%/22%/5%/3% NYHA Class: II–IV LVEF: ≤45% |
|
| Interventions | Losartan 25 mg OD (n=38) Losartan 50 mg OD (n=40) Enalapril 10 mg BID (n=38) |
|
| Outcomes | Primary: Symptom‐limited treadmill exercise duration; 6‐minute walk test; dyspnea‐fatigue index; signs and symptoms of heart failure and functional class Secondary: Clinical and laboratory adverse events |
|
| Notes | Funding source: Merck Research Laboratories Study "Phase II‐US" in Sharma 2000 meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of losartan. |
Mazayev 1998.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT in Russia Follow‐up: 4 wks Background ACEI? No |
|
| Participants | N=116 patients aged 18‐80 years with stable CHF previously untreated with ACEI and who had pulmonary capillary wedge pressure ≥15 mm Hg Mean age: 56.0 y Females: 17.2% Race: All white NYHA Class: II–IV |
|
| Interventions | Valsartan 40 mg BID (n=24) Valsartan 80 mg BID (n=24) Valsartan 160 mg BID (n=27) Lisinopril 5 mg OD for 7 days followed by 10 mg OD for 3 wks (n=15) Placebo (n=26) |
|
| Outcomes | Primary: Change from baseline in mean pulmonary capillary wedge pressure Secondary: Changes from baseline in cardiac output and systemic vascular resistance |
|
| Notes | Funding source: Novartis Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients in the lisinopril group received active drug plus placebo, in order to have a twice daily regimen identical to that of valsartan. To maintain blindness, a matching technique was used so all three medications (valsartan, lisinopril, placebo) were identical." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 103/116 patients completed double‐blind treatment period; all randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of valsartan. |
Mitrovic 2003.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT in Europe and South Africa Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=218 patients aged 18‐75 years with symptomatic CHF (principal causes were coronary heart disease and/or cardiomyopathy) and pulmonary capillary wedge pressure ≥13 mm Hg at baseline Mean age: 54.0 y Females: 14.7% Race: All white NYHA Class: II–III LVEF: ≤40% |
|
| Interventions | Candesartan 2 mg OD (n=45) Candesartan 4 mg OD (n=46) Candesartan 8 mg OD (n=39) Candesartan 16 mg OD (n=44) Placebo (n=44) |
|
| Outcomes | Primary: pulmonary capillary wedge pressure; systemic vascular resistance; cardiac index Secondary: pulmonary arterial pressure; mean right arterial pressure; mean arterial blood pressure; heart rate; neurohormones; symptom scores for breathlessness, fatigue and ankle swelling; physicians' overall efficacy score; NYHA classification; QoL using validated measure of subjective health status SF‐36 questionnaire |
|
| Notes | Funding source: Takeda Europe R&D Centre Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 189/218 patients completed double‐blind treatment period; all randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
REPLACE 2001.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT in Europe Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=378 patients ≥21 years of age with stable symptomatic CHF, primarily due to IHD (78%), already taking diuretic plus ACEI (enalapril 10 mg BID), with or without digoxin Mean age: 64 y Females: 11% NYHA Class: II–III LVEF: ≤40% |
|
| Interventions | Telmisartan 10 mg OD (n=75) Telmisartan 20 mg OD (n=72) Telmisartan 40 mg OD (n=77) Telmisartan 80 mg OD (n=77) Enalapril 10 mg BID (n=77) |
|
| Outcomes | Primary: Change from baseline in bicycle exercise duration Secondary: LVEF; QoL; BP; neurohormonal changes; NYHA classification |
|
| Notes | Funding source: Boehringer‐Ingelheim Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of telmisartan. |
RESOLVD 1999.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 43 wks Background ACEI? No |
|
| Participants | N=768 patients ≥21 years of age with symptomatic CHF, due to IHD (72%), idiopathic dilated cardiomyopathy (17%), or other cause (11%), with a 6‐minute walk distance <500 m Mean age: 63.1 y Females: 16.4% NYHA Class: II–IV LVEF: <40% |
|
| Interventions | Candesartan 4, 8 or 16 mg OD (n=327) Candesartan 4 or 8 mg OD plus enalapril 10 mg BID (n=332) Enalapril 10 mg BID (n=109) |
|
| Outcomes | Primary: Change in 6‐minute walk distance Secondary: Neurohormone levels; ventricular function; QoL; NYHA functional classification |
|
| Notes | Funding source: Astra Hassle, AB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
Sharma 2000, III‐Int'l.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=385 patients with symptomatic CHF who had never received ACEI or who had discounted ACEI for ≥12 wks Mean age: 60.3 y Females: 30% Race (white/hispanic/black/other): 59%/31%/5%/5% NYHA Class: II–IV LVEF: ≤40% |
|
| Interventions | Losartan, target dose 50 mg (n=254) Placebo (n=131) |
|
| Outcomes | Primary: Exercise capacity (maximal treadmill exercise time) | |
| Notes | Funding source: Merck Research Laboratories Phase IIII‐Int'l study information and results reported in retrospective meta‐analysis conducted and published by manufacturer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...patients were randomised to losartan or matching placebo in a 2:1 ratio." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of losartan. |
Sharma 2000, III‐US.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT in US Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=351 patients with symptomatic CHF who had never received ACEI or who had discounted ACEI for ≥6 wks Mean age: 65.0 y Females: 33% Race (white/hispanic/black/other): 83%/1%/15%/1% NYHA Class: II–IV LVEF: ≤40% |
|
| Interventions | Losartan, target dose 50 mg (n=237) Placebo (n=114) |
|
| Outcomes | Primary: Exercise capacity (maximal treadmill exercise time) | |
| Notes | Funding source: Merck Research Laboratories Study information and results reported in retrospective meta‐analysis conducted and published by manufacturer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...patients were randomised to losartan or matching placebo in a 2:1 ratio." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of losartan. |
SPICE 2000.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=768 patients with symptomatic CHF, due to IHD (71.5%), idiopathic dilated cardiomyopathy (15.9%), or other cause (12.6%), and history of discontinuing ACEI because of intolerance Mean age: 65.7 y Females: 31.1% NYHA Class: II–IV LVEF: <35% |
|
| Interventions | Candesartan, target dose 16 mg OD (n=179) Placebo (n=91) |
|
| Outcomes | Primary: Tolerability, defined as % of randomised population completing 12‐wk DB treatment period with candesartan 4, 8 or 16 mg Secondary: NYHA functional class; 6‐minute walk test; QoL, laboratory tests |
|
| Notes | Funding source: Astra Hassle, AB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...candesartan or matching placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Major cardiovascular clinical events that occurred during the study period were ascertained for all patients." |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
STRETCH 1999.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 12 wks Background ACEI? No |
|
| Participants | N=844 patients aged 21‐80 years with symptomatic CHF, primarily due to coronary heart disease (70.9%) Mean age: 61.9 y Females: 31.6% Race: All white except for 2 Oriental patients NYHA Class: II–III LVEF: 30‐45% |
|
| Interventions | Candesartan 4 mg OD (n=208) Candesartan 8 mg OD (n=212) Candesartan 16 mg OD (n=213) Placebo (n=211) |
|
| Outcomes | Primary: Total exercise time at study end point determined by bicycle ergometry Secondary: Signs and symptoms of CHF, NYHA functional class, cardiothoracic ratio, and neuroendocrine parameters |
|
| Notes | Funding source: Takeda Europe R&D Centre Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients entered a double‐blind treatment phase and were randomised to candesartan cilexetil 4, 8, or 16 mg or matching placebo for 12 weeks through a computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Eligible patients entered a double‐blind treatment phase and were randomised to candesartan cilexetil 4, 8, or 16 mg or matching placebo for 12 weeks through a computer‐generated randomisation list. All medication was identical in appearance to maintain blinding." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of candesartan. |
Tonkon 2000.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in US Follow‐up: 12 wks Background ACEI? Yes |
|
| Participants | N=109 patients ≥18 years of age with stable symptomatic CHF, due to IHD (53%), idiopathic (40%), or other cause (7%), who were already receiving stable doses of ACEI (≥6 wks) and diuretics (≥2 wks) before and throughout study Mean age: 63.9 y Females: 23.9% Race (white/black/other): 82%/12%/6% NYHA Class: II–III LVEF: ≤40% |
|
| Interventions | Irbesartan, target dose 150 mg OD (n=57) Placebo (n=52) |
|
| Outcomes | Primary: Symptom‐limited exercise tolerance time Secondary: NYHA functional class; LVEF |
|
| Notes | Funding source: Bristol‐Myers Squibb Pharmaceutical Research Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind therapy was administered as capsules of irbesartan or matching placebo." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data, i.e. total hospitalisations Quote: "Data from all randomised patients were included in the safety analysis." |
| Other bias | High risk | Funding source is manufacturer of irbesartan. |
Val‐HeFT 2001.
| Study characteristics | ||
| Methods | Multinational, multicenter, prospective DBRCT Follow‐up: 23 mos Background ACEI? Yes |
|
| Participants | N=5010 patients ≥18 years of age with stable symptomatic HF, primarily due to coronary heart disease (57%), idiopathic (31%), hypertension (7%), or other cause (5%), and had to have been receiving for ≥2 wks a fixed‐dose regimen that could include ACEI, diuretics, digoxin, and beta‐blockers Mean age: 62.7 y Females: 20.1% Race (white/black/other): 90%/7%/3% NYHA Class: II–IV LVEF: <40% |
|
| Interventions | Valsartan 160 mg BID (n=2511) Placebo (n=2499) |
|
| Outcomes | Primary: Mortality; combined end point of mortality and morbidity, which was defined as cardiac arrest with resuscitation, hospitalisation for heart failure, or administration of intravenous inotropic or vasodilator drugs for 4 hours or more without hospitalisation Secondary: Change from baseline in LVEF; NYHA functional classification; QoL; signs and symptoms of heart failure |
|
| Notes | Funding source: Novartis Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Eligible patients...were randomly assigned to receive oral valsartan or matching placebo." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data, i.e. total hospitalisations All randomised patients who discontinued prematurely included in analysis. |
| Other bias | High risk | Funding source is manufacturer of valsartan. |
V‐HeFT 1999.
| Study characteristics | ||
| Methods | Multicenter, prospective DBRCT conducted in US Follow‐up: 4 wks Background ACEI? Yes |
|
| Participants | N=83 patients with stable symptomatic HF, primarily due to IHD (55%), and PCWP ≥15 mm Hg already receiving ACEI therapy Mean age: 64.1 y All males Race (white/black/other): 65%/27%/8% NYHA Class: II–IV |
|
| Interventions | Valsartan 80 mg BID (n=28) Valsartan 160 mg BID (n=27) Placebo (n=28) |
|
| Outcomes | Primary: Change from baseline in PCWP Secondary: Neurohormones; haemodynamics |
|
| Notes | Funding source: Novartis Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in safety analysis. |
| Other bias | High risk | Funding source is manufacturer of valsartan. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belousov 2005 | No useful outcomes reported. |
| Berezin 2001 | No useful outcomes reported. |
| Blanchet 2005 | No useful outcomes reported. |
| De Tommasi 2003 | No useful outcomes reported. |
| Ellis 2002 | Not double‐blind. |
| Guazzi 1999 | Crossover study. |
| HEAAL 2009 | No parallel control (placebo or ACEI) arm. |
| Hikosaka 2002 | Not double‐blind. No useful outcomes reported. |
| Houghton 2000 | No useful outcomes reported. |
| Kasama 2003 | Not double‐blind. No useful outcomes reported. |
| Kasama 2005 | No useful outcomes reported. |
| Ki 2008 | No useful outcomes reported. |
| Nakamura 2002 | All patients were not receiving background ACEI therapy. No useful outcomes reported. |
| Parthasarathy 2009 | All patients were not receiving background ACEI therapy. |
| SADKO‐CHF 2005 | No useful outcomes reported. |
| Tang 2003 | No useful outcomes reported. |
| Tsutamoto 2000 | Not double‐blind. No useful outcomes reported. |
| Vaile 2001 | Crossover study. |
| Vizir 2000 | Not double‐blind. |
| Vizir 2002 | Not randomised; control arm comprised patients who refused therapy with ARB instead of being randomised to appropriate control. |
| Ye 2005 | No useful outcomes reported. Non‐equivalent doses studied; benazepril 20 mg vs. benazepril 10 mg plus valsartan 80 mg combination vs. valsartan 160 mg. |
Contributions of authors
All authors were involved in the conception and design of the review, as well as the analysis and interpretation of the data. BSH and VM performed study selection, data extraction and risk of bias assessment. BSH wrote the first draft of the review, and all authors revised it critically for important intellectual content. All authors have given final approval of the published version of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR, UK Cochrane Collaboration Programme Grant, UK
Declarations of interest
None.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
ADEPT 2001 {published data only}
- Murdoch DR, McDonagh TA, Farmer R, Morton JJ, McMurray JJV, Dargie HJ. ADEPT: Addition of the AT1 receptor antagonist eprosartan to ACEI inhibitor therapy in chronic heart failure trial: Hemodynamic and neurohormonal effects. American Heart Journal 2001;141(5):800-7. [DOI] [PubMed] [Google Scholar]
ARCH‐J 2003 {published data only}
- Matsumori A. Efficacy and safety of oral candesartan cilexitil in patients with congestive heart failure. European Journal of Heart Failure 2003;5:669-77. [DOI] [PubMed] [Google Scholar]
CHARM‐Added 2003 {published data only}
- McMurray JJV, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:762-71. [DOI] [PubMed] [Google Scholar]
- Weir RAP, McMurray JJV, Puu M, Solomon SD, Olofsson B, Granger CB, Yusuf S, Michelson EL, Swedberg K, Pfeffer MA. Efficacy and tolerability of adding an angiotensin receptor blocker in patients with heart failure already receiving an angiotensin-converting inhibitor plus aldosterone antagonist, with or without a beta blocker. Findings from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-Added trial. European Journal of Heart Failure 2008;10:157-63. [DOI] [PubMed] [Google Scholar]
CHARM‐Alternative 2003 {published data only}
- Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772-6. [DOI] [PubMed] [Google Scholar]
CHARM‐Preserved 2003 {published data only}
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Östergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-81. [DOI] [PubMed] [Google Scholar]
Crozier 1995 {published data only}
- Crozier I, Ikram H, Awan N, Cleland J, Stephen N, Dickstein K, Frey M, Young J, Klinger G, Makris L, Rucinska E. Losartan in heart failure. Hemodynamic effects and tolerability. Circulation 1995;91(3):691-7. [DOI] [PubMed] [Google Scholar]
- Klinge R, Polis A, Dickstein K, Hall C. Effects of angiotensin II receptor blockade on N-terminal proatrial natriuretic factor plasma levels in chronic heart failure. Journal of Cardiac Failure 1997;3(2):75-81. [DOI] [PubMed] [Google Scholar]
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
- Weber M. Clinical safety and tolerability of losartan. Clinical Therapeutics 1997;19(4):604-16. [DOI] [PubMed] [Google Scholar]
Dickstein 1995 {published data only}
- Dickstein K, Chang P, Willenheimer R, Haunso S, Remes J, Hall C, Kjekshus J. Comparison of the effects of losartan and enalapril on clinical status and exercise performance in patients with moderate or severe chronic heart failure. Journal of the American College of Cardiology 1995;26(2):438-45. [DOI] [PubMed] [Google Scholar]
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
ELITE 1997 {published data only}
- Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan In the Elderly Study, ELITE). Lancet 1997;349:747-52. [DOI] [PubMed] [Google Scholar]
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
ELITE II 2000 {published data only}
- Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial - the Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-7. [DOI] [PubMed] [Google Scholar]
Hamroff 1999 {published data only}
- Hamroff G, Katz SD, Mancini D, Blaufarb I, Bijou R, Patel R, Jondeau G, Olivari MT, Thomas S, Le Jemtel TH. Addition of angiotensin II receptor blockade to maximal angiotensin-converting enzyme inhibition improves exercise capacity in patients with severe congestive heart failure. Circulation 1999;99:990-2. [DOI] [PubMed] [Google Scholar]
HEAVEN 2002 {published data only}
- Willenheimer R, Helmers C, Pantev E, Rydberg E, Lofdahl P, Gordon A. Safety and efficacy of valsartan versus enalapril in heart failure patients. International Journal of Cardiology 2002;85:261-70. [DOI] [PubMed] [Google Scholar]
I‐PRESERVE 2008 {published data only}
- Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. New England Journal of Medicine 2008;359(23):2456-67. [DOI] [PubMed] [Google Scholar]
- Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation 2010;121:1393-405. [DOI] [PubMed] [Google Scholar]
Lang 1997 {published data only}
- Lang RM, Elkayam U, Yellen LG, Krauss D, McKelvie RS, Vaughan DE, Ney DE, Makris L, Chang PI. Comparative effects of losartan and enalapril on exercise capacity and clinical status in patients with heart failure. Journal of the American College of Cardiology 1997;30(4):983-91. [DOI] [PubMed] [Google Scholar]
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
Mazayev 1998 {published data only}
- Mazayev VP, Fomina IG, Kazakov EN, Sulimov VA, Zvereva TV, Lyusov VA, Orlov VA, Olbinskaya LI, Bolshakova TD, Sullivan J, Spormann DO. Valsartan in heart failure patients previously untreated with an ACE inhibitor. International Journal of Cardiology 1998;65:239-46. [DOI] [PubMed] [Google Scholar]
Mitrovic 2003 {published data only}
- Mitrovic V, Willenbrock R, Miric M, Seferovic P, Spinar J, Dabrowski M, Kiowski W, Marks DS, Alegria E, Dukat A, Lenz K, Arens HA. Acute and 3-month treatment effects of candesartan cilexitil on hemodynamics, neurohormones, and clinical symptoms in patients with congestive heart failure. American Heart Journal 2003;145(3):D1-D9. [DOI] [PubMed] [Google Scholar]
REPLACE 2001 {published data only}
- Dunselman PHJM. Effects of the replacement of the angiotensin converting enzyme inhibitor enalapril by the angiotensin II receptor blocker telmisartan in patients with congestive heart failure. The replacement of angiotensin converting enzyme inhibition (REPLACE) investigators. International Journal of Cardiology 2001;77:131-8. [DOI] [PubMed] [Google Scholar]
RESOLVD 1999 {published data only}
- McKelvie RS, Yusuf S, Pericak D, Avezum A, Burns RJ, Probstfield J, Tsuyuki RT, White M, Rouleau J, Latini R, Maggioni A, Young J, Pogue J. Comparison of candesartan, enalapril, and their combination in congestive heart failure. Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. Circulation 1999;100:1056-64. [DOI] [PubMed] [Google Scholar]
Sharma 2000, III‐Int'l {published data only}
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
Sharma 2000, III‐US {published data only}
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. American Journal of Cardiology 2000;85:187-92. [DOI] [PubMed] [Google Scholar]
SPICE 2000 {published data only}
- Granger CB, Ertl G, Kuch J, Maggioni AP, McMurray J, Rouleau JL, Stevenson LW, Swedberg K, Young J, Yusuf S, Califf RM, Bart BA, Held P, Michelson EL, Sellers MA, Ohlin G, Sparapani R, Pfeffer MA. Randomized trial of candesartan cilexitil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. American Heart Journal 2000;139:609-17. [DOI] [PubMed] [Google Scholar]
STRETCH 1999 {published data only}
- Riegger GAJ, Bouzo H, Petr P, Munz J, Spacek R, Pethig H, Behren V, George M, Arens HJ. Improvement in exercise tolerance and symptoms of congestive heart failure during treatment with candesartan cilexitil. Circulation 1999;100:2224-30. [DOI] [PubMed] [Google Scholar]
Tonkon 2000 {published data only}
- Tonkon M, Awan N, Niazi I, Hanley P, Baruch L, Wolf RA, Block AJ. A study of the efficacy and safety of irbesartan in combination with conventional therapy, including ACE inhibitors, in heart failure. International Journal of Clinical Practice 2000;54:11-8. [PubMed] [Google Scholar]
Val‐HeFT 2001 {published data only}
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. New England Journal of Medicine 2001;345:1667-75. [DOI] [PubMed] [Google Scholar]
V‐HeFT 1999 {published data only}
- Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Circulation 1999;99:2658-64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Belousov 2005 {published data only}
- Belousov IB, Upnitskii AA, Khanina NI. [Effect of long-term therapy with contemporary drugs on diastolic cardiac function in patients with chronic heart failure]. Kardiologiia 2005;45:26-32. [PubMed] [Google Scholar]
Berezin 2001 {published data only}
- Berezin AE. Angiotensin-II receptor antagonist losartan dose-dependently improves the left ventricular remodelling in patients with congestive heart failure. Journal of Clinical & Basic Cardiology 2002;5(Issue 1):83-6. [Google Scholar]
- Berezin AE. Losartan in the therapy of heart failure patients. Asian Cardiovascular & Thoracic Annals 2001;9:302-7. [Google Scholar]
Blanchet 2005 {published data only}
- Blanchet M, Sheppard R, Racine N, Ducharme A, Curnier D, Tardif JC, Sirois P, Lamoureux MC, De Champlain J, White M. Effects of angiotensin-converting enzyme inhibitor plus irbesartan on maximal and submaximal exercise capacity and neurohumoral activation in patients with congestive heart failure. American Heart Journal 2005;149:938.e1-7. [DOI] [PubMed] [Google Scholar]
De Tommasi 2003 {published data only}
- De Tommasi E, Iacoviello M, Romito R, Ceconi C, Guida P, Massari F, Francolini G, Bertocchi F, Ferrari R, Rizzon P, Pitzalis MV. Comparison of the effect of valsartan and lisinopril on autonomic nervous system activity in chronic heart failure. American Heart Journal 2003;146:E17. [DOI] [PubMed] [Google Scholar]
Ellis 2002 {published data only}
- Ellis GR, Nightingale AK, Blackman DJ, Anderson RA, Mumford C, Timmins G, Lange D, Jackson SK, Penney MD, Lewis MJ, Frenneaux MP, Morris-Thurgood J. Addition of candesartan to angiotensin converting enzyme inhibitor therapy in patients with chronic heart failure does not reduce levels of oxidative stress. European Journal of Heart Failure 2002;4:193-9. [DOI] [PubMed] [Google Scholar]
Guazzi 1999 {published data only}
- Guazzi M, Palermo P, Pontone G, Susini F, Agostoni P. Synergistic efficacy of enalapril and losartan on exercise performance and oxygen consumption at peak exercise in congestive heart failure. American Journal of Cardiology 1999;84:1038-43. [DOI] [PubMed] [Google Scholar]
HEAAL 2009 {published data only}
- Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GAJ, Malbecq W, Smith RD, Guptha S, Poole-Wilson PA. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet 2009;374(9704):1840-8. [DOI] [PubMed] [Google Scholar]
- Konstam MA, Poole-Wilson PA, Dickstein K, Drexler H, Justice SJ, Komajda M, Malbecq W, Martinez FA, Neaton JD, Riegger GAJ, Guptha S. Design of the heart failure endpoint evaluation of AII-antagonist losartan (HEAAL) study in patients intolerant to ACE-inhibitor. European Journal of Heart Failure 2008;10:899-906. [DOI] [PubMed] [Google Scholar]
Hikosaka 2002 {published data only}
- Hikosaka M, Yuasa F, Yuyama R, Mimura J, Kawamura A, Motohiro M, Iwasaki M, Sugiura T, Iwasaka T. Candesartan and arterial baroreflex sensitivity and sympathetic nerve activity in patients with mild heart failure. Journal of Cardiovascular Pharmacology 2002;40:875-80. [DOI] [PubMed] [Google Scholar]
Houghton 2000 {published data only}
- Houghton AR, Harrison M, Cowley AJ, Hampton JR. Combined treatment with losartan and an ACE inhibitor in mild to moderate heart failure: results of a double-blind, randomized, placebo-controlled trial. American Heart Journal 2000;140:e25. [DOI] [PubMed] [Google Scholar]
Kasama 2003 {published data only}
- Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Addition of valsartan to an angiotensin-converting enzyme inhibitor improves cardiac sympathetic nerve activity and left ventricular function in patients with congestive heart failure. Journal of Nuclear Medicine 2003;44:884-90. [PubMed] [Google Scholar]
Kasama 2005 {published data only}
- Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. Journal of the American College of Cardiology 2005;45(5):661-7. [DOI] [PubMed] [Google Scholar]
Ki 2008 {published data only}
- Ki HL, Myung HJ, Young KA, Woo SL, Dae HJ, Jeong GC, Jong CP, Jung CK, Seok KO, Nam HK, Kyung HY, Nam JY, Moon Y, Jay YR, Ji HL, Seong HJ, Ok YP, Seung UL, Dong GK. [The comparative clinical effects of valsartan and ramipril in patients with heart failure]. Korean Circulation Journal 2008;38:101-9. [Google Scholar]
Nakamura 2002 {published data only}
- Nakamura M, Saito S, Yoshida H, Arakawa N, Sugawara S, Hiramori K. Effects of chronic subdepressor dose of angiotensin II type 1 receptor antagonist on endothelium-dependent vasodilation in patients with congestive heart failure. Journal of Cardiovascular Pharmacology 2002;40(3):411-9. [DOI] [PubMed] [Google Scholar]
Parthasarathy 2009 {published data only}
- Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. European Journal of Heart Failure 2009;11:980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
SADKO‐CHF 2005 {published data only}
- Skvortsov AA, Nasonova SN, Sychev AV, Arbolishvili GN, Baklanova NA, Mareev V, Belenkov N. [Combined therapy with quinapril, an ACE inhibitor, and valsartan, a type 1 angiotensin II receptors blocker, for moderate chronic cardiac failure may raise the degree of neurohormonal block and improve 24-h heart rate variability compared to the effect of monotherapy (data from the trial SADKO-CHF)]. Terapevticheski Arkhiv 2005;77(8):34-43. [PubMed] [Google Scholar]
- Skvortsov AA, Nasonova SN, Sychev AV, Orlova A, Baklanova NA, Masenko VP, Mareev V, Belenkov N. [Effects of long term therapy with angiotensin converting enzyme inhibitor quinapril, antagonist of receptors to angiotensin II valsartan, and combination of quinapril and valsartan in patients with moderate chronic heart failure. Main results of the SADKO-CHF study]. Kardiologiia 2006;46:33-51. [PubMed] [Google Scholar]
Tang 2003 {published data only}
- Tang B, Sun H, Ma Y. Effect of losartan and captopril on congestive heart failure. Journal of Xingjiang Medical University 2003;26(5):424-6. [Google Scholar]
Tsutamoto 2000 {published data only}
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. Journal of the American College of Cardiology 2000;35(3):714-21. [DOI] [PubMed] [Google Scholar]
Vaile 2001 {published data only}
- Vaile JC, Chowdhary S, Osman F, Ross HF, Fletcher J, Littler WA, Coote JA, Townend JN. Effects of angiotensin II (AT1) receptor blockade on cardiac vagal control in heart failure. Clinical Science 2001;101:559-66. [PubMed] [Google Scholar]
Vizir 2000 {published data only}
- Vizir VA, Berezin AE. [Losartan in therapy of chronic heart failure]. Klinicheskaia Meditsina 2000;78(2):36-9. [PubMed] [Google Scholar]
Vizir 2002 {published data only}
- Vizir VA, Berezin AE. [Effect of long-term treatment with enalapril, losartan and their combination on the quality of life of patients with congestive heart failure]. Terapevticheski Arkhiv 2002;74(1):52-5. [PubMed] [Google Scholar]
Ye 2005 {published data only}
- Ye JF, Liu DS. Effects of benazepril combined with valsartan on congestive heart failure. Journal of First Military Medical University 2005;25(11):1441-2, 1447. [PubMed] [Google Scholar]
Additional references
ACCF/AHA 2009
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. Focused Update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977-2016. [DOI] [PubMed] [Google Scholar]
AIRE 1993
- Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993;342:821-8. [PubMed] [Google Scholar]
Azizi 2004
- Azizi M, Menard J. Combined blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 antagonists. Circulation 2004;109:2492-9. [DOI] [PubMed] [Google Scholar]
CCS 2006
- Arnold JM, Liu P, Demers C, Dorian P, Giannetti N, Haddad H, Heckman GA, Howlett JG, Ignaszewski A, Johnstone DE, Jong P, McKelvie RS, Moe GW, Parker JD, Rao V, Ross HJ, Sequeira EJ, Svendsen AM, Teo K, Tsuyuki RT, White M, Canadian Cardiovascular Society. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: Diagnosis and management. Canadian Journal of Cardiology 2006;22(1):23-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 9-13-1997;315(7109):629-634. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erhardt 2005
- Erhardt LR. A review of the current evidence for the use of angiotensin receptor blockers in chronic heart failure. International Journal of Clinical Practice 2005;59(5):571-8. [DOI] [PubMed] [Google Scholar]
ESC 2008
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Stromberg A, Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. European Heart Journal 2008;29:2388-2442. [DOI] [PubMed] [Google Scholar]
Flather 2000
- Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, Torp-Pedersen C, Ball S, Pogue J, Moye L, Braunwald E. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000;355(9215):1575-81. [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003823. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003822. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.. In: The Cochrane Collaboration. Available from www.cochrane-handbook.org: Wiley-Blackwell, 2011. [Google Scholar]
Jong 2002b
- Jong P, Demers C, McKelvie RS, Liu P. Angiotensin receptor blockers for heart failure. Cochrane Database of Systematic Reviews 2001, Issue 2. Art. No: CD003040. [DOI: 10.1002/14651858.CD003040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakhdar 2008
- Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. Journal of Cardiac Failure 2008;14(3):181-8. [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Annals of Internal Medicine 2004;141:693-704. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Galnville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s) Cochrane Handbook for Systematic Reviews of Interventions Vetsion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.
Mancia 2009
- Mancia G, Corrao G. Targeting blood pressure in the management of total cardiovascular risk. European Heart Journal Supplements 2009;11(Supplement F):F27-32. [Google Scholar]
McMurray 2005
- McMurray JJV, Pfeffer MA. Heart failure. Lancet 2005;365:1877-89. [DOI] [PubMed] [Google Scholar]
SAVE 1992
- Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown Jr EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. New England Journal of Medicine 1992;327(10):669-77. [DOI] [PubMed] [Google Scholar]
Shah 2010
- Shah RV, Desai AS, Givertz MM. The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. Journal of Cardiac Failure 2010;16(3):260-7. [DOI] [PubMed] [Google Scholar]
Sharma 2000
- Sharma D, Buyse M, Pitt B, Rucinska EJ. Meta-analysis of observed mortality data from all-controlled, double-blind, multiple-dose studies of losartan in heart failure. Am J Cardiol 2000;85(2):187-192. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shibata 2008
- Shibata M C, Tsuyuki R T, Wiebe N. The effects of angiotensin-receptor blockers on mortality and morbidity in heart failure: a systematic review. International Journal of Clinical Practice 2008;62(9):1397-402. [DOI] [PubMed] [Google Scholar]
Smith 2008
- Smith DHG. Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs 2008;68(9):1207-25. [DOI] [PubMed] [Google Scholar]
SOLVD prevention 1992
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. New England Journal of Medicine 1992;327:685-91. [DOI] [PubMed] [Google Scholar]
SOLVD treatment 1991
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine 1991;325:293-302. [DOI] [PubMed] [Google Scholar]
TRACE 1995
- Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auglert L, Pauly NC, Aliot E, Persson S, Camm AJ. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. New England Journal of Medicine 1995;333(25):1670-6. [DOI] [PubMed] [Google Scholar]
Volpe 2009
- Volpe M, Toccia G, Sciarretta S, Verdecchia P, Trimarco B, Mancia G. Angiotensin II receptor blockers and myocardial infarction: an updated analysis of randomized clinical trials. Journal of Hypertension 2009;27(5):941-6. [DOI] [PubMed] [Google Scholar]
Weber 1997
- Weber M. Clinical safety and tolerability of losartan. Clinical Therapeutics 1997;19(4):604-16. [DOI] [PubMed] [Google Scholar]
White 2011
- White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension 2011;57:413-20. [DOI] [PubMed] [Google Scholar]
Wolny 1997
- Wolny A, Clozel J, Rein J, Mory P, Vogt P, Turino M, et al. Functional and biochemical analysis of angiotensin II forming pathways in the human heart. Circulation Research 1997;80:219-27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jong 2002
- Jong P, Demers C, McKelvie RS, Liu PP. Angiotensin receptor blockers in heart failure: Meta-analysis of randomized controlled trials. Journal of the American College of Cardiology 2002;39(3):463-70. [DOI] [PubMed] [Google Scholar]


