Abstract
Background
Traction has been used to treat low‐back pain (LBP), often in combination with other treatments. We included both manual and machine‐delivered traction in this review. This is an update of a Cochrane review first published in 1995, and previously updated in 2006.
Objectives
To assess the effects of traction compared to placebo, sham traction, reference treatments and no treatment in people with LBP.
Search methods
We searched the Cochrane Back Review Group Specialized Register, the Cochrane Central Register of Controlled Trials (2012, Issue 8), MEDLINE (January 2006 to August 2012), EMBASE (January 2006 to August 2012), CINAHL (January 2006 to August 2012), and reference lists of articles and personal files. The review authors are not aware of any important new randomized controlled trial (RCTs) on this topic since the date of the last search.
Selection criteria
RCTs involving traction to treat acute (less than four weeks' duration), subacute (four to 12 weeks' duration) or chronic (more than 12 weeks' duration) non‐specific LBP with or without sciatica.
Data collection and analysis
Two review authors independently performed study selection, risk of bias assessment and data extraction. As there were insufficient data for statistical pooling, we performed a descriptive analysis. We did not find any case series that identified adverse effects, therefore we evaluated adverse effects that were reported in the included studies.
Main results
We included 32 RCTs involving 2762 participants in this review. We considered 16 trials, representing 57% of all participants, to have a low risk of bias based on the Cochrane Back Review Group's 'Risk of bias' tool.
For people with mixed symptom patterns (acute, subacute and chronic LBP with and without sciatica), there was low‐ to moderate‐quality evidence that traction may make little or no difference in pain intensity, functional status, global improvement or return to work when compared to placebo, sham traction or no treatment. Similarly, when comparing the combination of physiotherapy plus traction with physiotherapy alone or when comparing traction with other treatments, there was very‐low‐ to moderate‐quality evidence that traction may make little or no difference in pain intensity, functional status or global improvement.
For people with LBP with sciatica and acute, subacute or chronic pain, there was low‐ to moderate‐quality evidence that traction probably has no impact on pain intensity, functional status or global improvement. This was true when traction was compared with controls and other treatments, as well as when the combination of traction plus physiotherapy was compared with physiotherapy alone. No studies reported the effect of traction on return to work.
For chronic LBP without sciatica, there was moderate‐quality evidence that traction probably makes little or no difference in pain intensity when compared with sham treatment. No studies reported on the effect of traction on functional status, global improvement or return to work.
Adverse effects were reported in seven of the 32 studies. These included increased pain, aggravation of neurological signs and subsequent surgery. Four studies reported that there were no adverse effects. The remaining studies did not mention adverse effects.
Authors' conclusions
These findings indicate that traction, either alone or in combination with other treatments, has little or no impact on pain intensity, functional status, global improvement and return to work among people with LBP. There is only limited‐quality evidence from studies with small sample sizes and moderate to high risk of bias. The effects shown by these studies are small and are not clinically relevant.
Implications for practice To date, the use of traction as treatment for non‐specific LBP cannot be motivated by the best available evidence. These conclusions are applicable to both manual and mechanical traction.
Implications for research Only new, large, high‐quality studies may change the point estimate and its accuracy, but it should be noted that such change may not necessarily favour traction. Therefore, little priority should be given to new studies on the effect of traction treatment alone or as part of a package.
Keywords: Humans, Traction, Traction/adverse effects, Acute Pain, Acute Pain/therapy, Chronic Pain, Chronic Pain/therapy, Low Back Pain, Low Back Pain/complications, Low Back Pain/therapy, Pain Measurement, Randomized Controlled Trials as Topic, Sciatica, Sciatica/complications, Sciatica/therapy
Plain language summary
Traction for low‐back pain
We reviewed the evidence on the effect of traction on pain intensity, ability to perform normal daily activities, overall improvement and return to work among people with low back pain (LBP) in the acute (less than four weeks' duration), subacute (from four to 12 weeks' duration) or chronic (more than 12 weeks' duration) phase. Some patients also had sciatica. We examined the effects of traction immediately after the traction session, in the short‐term (up to three months after traction) and in the long‐term (around one year after traction).
LBP is a major health problem around the world and is a major cause of medical expenses, absenteeism and disability. One treatment option for LBP that has been used for thousands of years is traction, the application of a force that draws two adjacent bones apart from each other in order to increase their shared joint space. Various types of traction are used, often in combination with other treatments. The most commonly used traction techniques are mechanical or motorized traction (where the traction is exerted by a motorized pulley) and manual traction (in which the traction is exerted by the therapist, using his or her body weight to alter the force and direction of the pull).
The evidence is current to August 2012. The review included 32 studies and 2762 people with LBP. Most studies included a similar population of people with LBP with and without sciatica. The majority of studies included people with acute, subacute and chronic LBP. Most studies reported follow‐up of one to 16 weeks, and a limited number of studies reported long‐term follow‐up of six months to one year.
The included studies show that traction as a single treatment or in combination with physiotherapy is no more effective in treating LBP than sham (pretend) treatment, physiotherapy without traction or other treatment methods including exercise, laser, ultrasound and corsets. These conclusions are valid for people with and without sciatica. There was no difference regarding the type of traction (manual or mechanical).
Side effects were reported in seven of the 32 studies and included increased pain, aggravation of neurological signs and subsequent surgery. Four studies reported that there were no side effects. The remaining studies did not mention side effects.
The quality of the evidence ranged from very low to moderate. There was a scarcity of high‐quality studies, especially those that distinguished between people with different symptom patterns (with and without sciatica, with pain of different duration).
Summary of findings
Summary of findings for the main comparison. Traction compared with placebo, sham or no treatment for people with low‐back pain with and without sciatica.
| Traction compared with placebo, sham or no treatment for people with low‐back pain with and without sciatica | |||
|
Patient or population: people with low‐back pain with and without sciatica Settings: diverse Intervention: traction Comparison: placebo, sham or no treatment | |||
| Outcomes | Effects | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity VAS (0‐100 mm). Follow‐up 12‐16 weeks. |
1 trial showed that there was no difference in pain intensity between the 2 groups (MD ‐4, 95% CI ‐17.7 to 9.7). | 60 (1) |
⊕⊕⊕⊝
moderate Imprecision (< 400 participants) |
|
Functional status Oswestry Disability Index or Roland Morris Disability Questionnaire. Follow‐up 12‐16 weeks. |
Not measured. | ||
|
Global improvement Follow‐up 12‐16 weeks. |
1 trial showed that there was no difference in global improvement between the 2 groups (RD 0.06, 95% CI ‐0.16 to 0.28). | 81 (1) |
⊕⊕⊕⊝
moderate Imprecision (< 300 participants) |
|
Return to work Follow‐up 12‐16 weeks. |
Not measured. | ||
| Adverse effects | 1 trial reported aggravation of neurological signs in 28% of the traction group, 20% of the light traction group and 20% of the placebo group. | ||
| CI: confidence interval; MD: mean difference; RD: risk difference; VAS: visual analogue scale. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Note. Each 'Summary of findings' table presents evidence for a specific comparison and a set of prespecified outcomes. Therefore, the information presented in the tables is limited by the comparisons and outcomes reported in the included studies.
Summary of findings 2. Physiotherapy with traction compared with physiotherapy without traction for people with low‐back pain with and without sciatica.
| Physiotherapy with traction compared with physiotherapy without traction for people with low‐back pain with and without sciatica | |||
|
Patient or population: people with low‐back pain with and without sciatica Settings: physical medicine and rehabilitation outpatient clinic of a larger hospital Intervention: physiotherapy with traction Comparison: physiotherapy without traction | |||
| Outcomes | Effects | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity VAS (0‐100 mm). Follow‐up 12‐16 weeks. |
1 trial showed that there was no difference in pain intensity between the 2 groups (MD 5, 95% CI ‐5.7 to 15.7) in favour of the control group. | 39 (1) |
⊕⊕⊝⊝
low Study design (high risk of bias) Imprecision (< 400 participants) |
|
Functional status Oswestry Disability Index or Roland Morris Disability Questionnaire. Follow‐up 12‐16 weeks. |
2 trials showed that there was no difference in functional status between the 2 groups (SMD from 0.36 (95% CI ‐0.27 to 1.00) to 0.43 (95% CI ‐0.30 to 1.16)). | 69 (2) |
⊕⊕⊝⊝
low Study design (high risk of bias) Imprecision (< 400 participants) |
|
Global improvement Follow‐up 12‐16 weeks. |
1 trial showed no difference in global improvement, another trial did show a clinically significant difference in global improvement (RD 0.53, 95% CI 0.28 to 0.79). | 220 (2) |
⊕⊕⊝⊝
low Study design (high risk of bias) Imprecision (< 300 participants) |
|
Return to work Follow‐up 12‐16 weeks. |
Not measured. | ||
| Adverse effects | 1 study reported that 25% of the physiotherapy with traction group and 37% of the physiotherapy without traction group felt worse at 3 months' follow‐up. | ||
| CI: confidence interval; MD: mean difference; RD: risk difference. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Summary of findings 3. Traction compared with another type of traction for people with low‐back pain with and without sciatica.
| Traction compared with another type of traction for people with low‐back pain with and without sciatica | |||
|
Patient or population: people with low‐back pain with and without sciatica Settings: diverse Intervention: traction Comparison: another type of traction | |||
| Outcomes | Effects | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity VAS (0‐100 mm). Follow‐up 12‐16 weeks. |
Not measured. | ||
|
Functional status Oswestry Disability Index or Roland Morris Disability Questionnaire. Follow‐up 12‐16 weeks. |
Not measured. | ||
|
Global improvement Follow‐up 12‐16 weeks. |
Not measured. | ||
|
Return to work Follow‐up 12‐16 weeks. |
Not measured. | ||
| Adverse effects | 1 trial reported increased pain in 31% of the static traction group and 15% of the intermittent traction group. | ||
| VAS: visual analogue scale. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Summary of findings 4. Traction compared with any other treatment for people with low‐back pain with and without sciatica.
| Traction compared with any other treatment for people with low‐back pain with and without sciatica | |||
|
Patient or population: people with low‐back pain with and without sciatica Settings: diverse Intervention: traction Comparison: other treatment | |||
| Outcomes | Effects | No of participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity VAS (0‐100 mm). Follow‐up 12‐16 weeks. |
3 trials, of which 1 compared traction with 2 other types of treatment, showed no difference greater than 5 points on the VAS scale between the 2 groups (MD ‐2.90 (95% CI ‐8.53 to 2.93) to 4.50 (95% CI ‐0.45 to 9.45). | 304 (3) |
⊕⊕⊕⊝
moderate Imprecision (< 400 participants) |
|
Functional status Oswestry Disability Index or Roland Morris Disability Questionnaire. Follow‐up 12‐16 weeks. |
3 trials, of which 1 compared traction to 2 other types of treatment and used 2 types of questionnaires to assess functional status, showed no difference between the 2 groups (SMD ‐0.08 (95% CI ‐0.39 to 0.23) to 0.51 (95% CI ‐0.12 to 1.14)). | 350 (3) |
⊕⊕⊕⊝
moderate Imprecision (< 400 participants) |
|
Global improvement Follow‐up 12‐16 weeks. |
1 trial showed no difference in global improvement (RD 0.05, 95% CI ‐0.1 to 0.2). | 42 (1) |
⊕⊕⊝⊝
low Study design (high risk of bias) Imprecision (< 300 participants) |
|
Return to work Follow‐up 12‐16 weeks. |
Not measured. | ||
| Adverse effects | 1 trial reported temporary deterioration of low‐back pain in 17% of the traction group and 15% of the exercise group. | ||
| MD: mean difference; RD: risk difference; SMD: standardized mean difference. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Background
Description of the condition
Low‐back pain (LBP) is a major health problem around the world and a major cause of medical expenses, absenteeism and disability (Dagenais 2008; Lambeek 2011; Vos 2012). Although LBP is usually a self limiting and benign condition that tends to improve spontaneously over time, a large variety of therapeutic interventions is available for treatment (Chou 2007). Sciatica can result when the nerve roots in the lower spine are irritated or compressed. Most often, sciatica is caused when the L5 or S1 nerve root in the lower spine is irritated by a herniated disc. Degenerative disc disease may irritate the nerve root and cause sciatica, as can mechanical compression of the sciatic nerve, such as from spondylolisthesis, spinal stenosis or arthritis in the spine. For the purposes of this review, we define sciatica as pain radiating down the leg(s) along the distribution of the sciatic nerve (which is usually related to mechanical pressure, inflammation of lumbosacral nerve roots or both) (Bigos 1994).
Description of the intervention
One treatment for LBP and sciatica is traction, which has been used for thousands of years. It is used relatively frequently in North America (e.g. up to 30% of people with acute LBP and sciatica in Ontario, Canada) (Li 2001), and to a lesser extent in the UK, Ireland and the Netherlands (Harte 2005). Traction is often provided in combination with other treatment modalities (Harte 2005). The most commonly used traction techniques are mechanical or motorized traction (where the traction is exerted by a motorized pulley), manual traction (in which the traction is exerted by the therapist, using his or her body weight to alter the force and direction of the pull), and auto‐traction (where the person controls the traction forces by grasping and pulling bars at the head of the traction table). There are also less common forms, such as underwater (where the person is fixed perpendicularly in a deep pool, a bar is grasped under the arms and traction is applied), and gravitational traction (e.g. bed rest traction, in which the person is fixed to a tilted table or bed, and inverted traction, where the participant is held in an inverted position by the ankles and another part of the lower extremities and gravity provides the force).
Lumbar traction uses a harness (with Velcro strapping) that is fitted around the lower rib cage and around the iliac crest. Duration and level of force exerted through this harness can be varied in a continuous or intermittent mode. The force can be standardized only in motorized traction or in methods using computer technology. With other techniques, total body weight and the strength of the person or therapist determine the forces exerted. In the application of traction force, consideration must be given to counter forces such as lumbar muscle tension, lumbar skin stretch and abdominal pressure, which depend on the participant's physical constitution. If the person is lying on the traction table, the friction of the body on the table or bed provides the main counter force during traction.
How the intervention might work
The exact mechanism through which traction might be effective is unclear. It has been suggested that spinal elongation, by decreasing lordosis and increasing intervertebral space, inhibits nociceptive impulses, improves mobility, decreases mechanical stress, reduces muscle spasm or spinal nerve root compression (due to osteophytes), releases luxation of a disc or capsule from the zygo‐apophysial joint, and releases adhesions around the zygo‐apophysial joint and the annulus fibrosus.
A more recent rationale, adapted to available neurophysiological research, suggests that stimulation of proprioceptive receptors in the vertebral ligaments and in the mono segmental muscles may modify and halt what is being conceptualized as a 'dysfunction'. Dysfunction is a relatively generalized disturbance involving higher cerebral centres as well as peripheral structures for postural control. The dysfunction involves self maintaining pain‐provoking neuromuscular reflex patterns. In relation to benefits of traction, this rationale involves the 'shocking' of dysfunctional higher centres by means of relaying 'unphysiological' proprioceptive information centrally, and thus 'resetting' the dysfunction (Blomberg 2005). So far, none of the proposed mechanisms has been supported by sufficient empirical information.
Little is known about the adverse effects of traction. Only a few case reports are available, which suggest that there is some danger for nerve impingement in heavy traction (i.e. lumbar traction forces exceeding 50% of the total body weight). Other risks described for lumbar traction are respiratory constraints due to the traction harness or increased blood pressure during inverted positional traction. There is some debate about the effect of low traction forces. Beurskens 1997 says that a certain amount of force is required to achieve separation of the vertebra and widening of the intervertebral foramina. Forces below 20% of the participants' body weight do not achieve this goal and, therefore, can be considered to constitute a placebo or sham traction. Other reports say that these forces can still be expected to produce positive results, as even low traction forces can produce intervertebral separation due to flattening of lumbar lordosis, and relaxation of spinal muscles (Harte 2003; Krause 2000).
Why it is important to do this review
This systematic review updates our previous Cochrane review (Clarke 2006a). The 2006 review included 25 randomized controlled trials (RCTs) and was an update of a previous review of the effectiveness of traction for back and neck pain (Van der Heijden 1995). The previous review stated that traction was not likely to be effective for people with and without sciatica, due to inconsistent results and methodological problems in most studies. This update integrated new literature on the subject and was performed using the latest methods.
Objectives
The objective of this systematic review was to determine if traction was more effective than reference treatments, placebo, sham traction or no treatment for LBP with or without sciatica, with a focus on pain intensity, functional status, global improvement and return to work.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs.
Types of participants
We included RCTs involving the following types of participants: male or female; aged 18 years or older; treated for LBP; in the acute, subacute or chronic phases, with or without sciatica. We excluded studies involving people with LBP due to specific causes (e.g. tumour, metastasis, fracture, inflammation, osteoporosis, rheumatoid arthritis).
Types of interventions
We included RCTs using any type of traction, such as mechanical traction, manual traction (unspecific or segmental traction), computerized traction, auto‐traction, underwater traction, bed rest traction, inverted traction, continuous traction and intermittent traction. Additional treatment was allowed, provided that traction was the main contrast between the intervention and control groups. We included studies with any type of control group (i.e. those that used placebo, sham, no treatment or other treatments).
Types of outcome measures
The four primary outcome measures that we considered to be the most important were pain intensity (e.g. measured by a visual analogue scale (VAS) or a numerical rating scale (NRS)), back‐pain‐specific functional status (e.g. measured by the Roland Morris Disability Questionnaire or Oswestry Disability Index (ODI)), a global measure of improvement (e.g. overall improvement, proportion of participants recovered, subjective improvement of symptoms) and return to work (e.g. measured by return to work status or days off work). We also considered reported adverse effects.
These outcomes could be measured immediately after the end of one traction session, immediately after a course of traction sessions, in the short‐term after the end of the traction sessions (up to three months), or in the long‐term (around one year).
Search methods for identification of studies
Electronic searches
We used the results of the literature search listed in Appendix 1, updating the three previous versions of this review (Clarke 2006a; Clarke 2006b; Van der Heijden 1995a). This included a computer‐aided search the Cochrane Back Review Group Specialized Register (August 2012), the Cochrane Central Register of Controlled Trials (2012 Issue 8), MEDLINE (January 2006 to August 2012), EMBASE (January 2006 to August 2012) and CINAHL (January 2006 to August 2012).
Searching other resources
Furthermore, we screened reference lists of relevant reviews and identified RCTs, as well as references in personal files of the review authors.
Data collection and analysis
In this review, we followed the guidelines of the Cochrane Back Review Group (Furlan 2009), and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently selected the trials to be included in the systematic review using title, abstract and keywords. The same two review authors independently applied the selection criteria to the studies that were retrieved by our literature search. We used consensus to resolve disagreements concerning selection and inclusion of RCTs. There was the option to consult a third review author if disagreement had persisted, although this was not necessary. We only evaluated full papers and excluded papers written in languages other than English, Dutch, German, French and Swedish.
Data extraction and management
Two review authors (IW and ISW) independently extracted the data (using a standardized form) considering the study population (e.g. number of participants, age, gender, type and duration of back pain), the interventions (type, intensity, and frequency of index and reference interventions) and the primary outcomes (type and duration of follow‐up). We used consensus to resolve disagreements and we would have consulted a third review author (GH) if disagreement persisted, although this was not necessary. We summarized key findings in a narrative format. We did not blind data extraction.
Assessment of risk of bias in included studies
We used the Cochrane Back Review Group's 'Risk of bias' tool to assess the risk of bias of the included RCTs (Furlan 2009). The 12 criteria are listed in Appendix 2. Studies included in the previous version of the review had not been assessed using this tool. Therefore, we re‐assessed these studies according to the updated methods. We could not obtain two articles (Lind 1974; Reust 1988) and two articles were written in a language that the review authors did not master (Bihaug 1978; Walker 1982). We transformed the previous risk of bias assessments of these four trials to the new format without re‐assessing them. As a result, supporting statements for the risk of bias assessments are missing for these studies. Two review authors (IW and ISW) independently assessed the methodological quality. Review authors resolved their initial discrepancies during discussion; the presented results are based on their full consensus. We did not blind quality assessment with regard to the authors, institution and journal. We did not contact study authors for additional information, because half the trials were published in the late 1990s. If the article did not contain the required information for the scoring of a specific item, we scored the item as 'unclear'.
We scored the criteria as 'low risk', 'high risk' or 'unclear risk', and reported them in the 'Risk of bias' table. We defined a study with a low risk of bias as one fulfilling six or more of the criteria and having no fatal flaws. In the previous review, a sensitivity analysis was performed in which six was considered the cut‐off point for low risk of bias. A second sensitivity analysis was performed in which half of items that had been scored 'unclear' in each trial were included as 'positive'. The same cut‐off point of six for low risk of bias is supported by empirical evidence (Van Tulder 2009).
Blinding of participants and care providers to treatment allocation is nearly impossible in trials of traction therapy. Given that some of the primary outcomes assessed in this review are subjective measures (i.e. pain and functional status), any attempt to blind the outcome assessor regarding these outcomes can be considered irrelevant. However, most studies also assessed objective outcome measures. If the care provider assessing those outcomes was blinded, the item was scored as 'low risk'.
Measures of treatment effect
We analyzed dichotomous outcomes by calculating the risk difference. We analyzed continuous outcomes by calculating the mean difference (MD) when the same instrument was used to measure outcomes, or the standardized mean difference (SMD) when different instruments were used to measure the outcomes. We converted VAS or NRS scales to a 100‐point scale. We expressed uncertainty using with 95% confidence intervals (CI).
We grouped outcomes by timing when they were measured: immediately after, short term and long term.
Unit of analysis issues
In several studies, we compared more than two intervention groups. We included these studies by making pair‐wise comparisons between all possible pairs of intervention groups with traction being one of the intervention groups. The same group of participants was included more than once in these examples (e.g. underwater traction versus underwater massage and underwater traction versus balneotherapy in the study performed by Konrad 1992). These participants were not counted twice in the meta‐analysis.
Dealing with missing data
In cases where data were reported as a median with an interquartile range (IQR), we assumed that the median was equivalent to the mean and the width of the IQR equivalent to 1.35 times the standard deviation in accordance with Cochrane Handbook for Systematic Reviews of Interventions, section 7.7.3.5 (Higgins 2011). If standard deviations were not given, we calculated them from the 95% CIs, P values based on a two‐sided t‐test or standard errors. We did not include data reported in graphs in this review.
Assessment of heterogeneity
We tested heterogeneity using the Chi2 test and I2 statistic; however, the decision regarding heterogeneity was dependent upon the I2 statistic (Higgins 2011). We defined substantial heterogeneity as an I2 greater than 50%, and where necessary, the effect of the interventions were synthesised narratively when the I2 statistic was greater than 50%.
Assessment of reporting biases
We searched ClinicalTrials.org and ISRCTN.org for the protocols of included studies. When protocols were available, we checked studies for selective outcome reporting.
Data synthesis
A quantitative analysis had been planned, but most of the studies did not provide sufficient data to enable statistical pooling (e.g. some trials reported the mean score but not the standard deviation, other trials reported median and IQR; some trials reported only post‐intervention means and other trials reported mean change scores; some trials did not report any numerical data. Therefore, we used a descriptive analysis to summarize the data. In this analysis, we used a rating system of levels of evidence to summarize the results of the studies in terms of the strength of the scientific evidence. To accomplish this, we used the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted in the updated Cochrane Back Review Group method guidelines (Furlan 2009). The system consists of five levels of evidence, based on performance against five principal domains or factors:
high‐quality evidence ‐ consistent findings among at least 75% of RCTs with low risk of bias, consistent, direct and precise data and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence of the results;
moderate‐quality evidence ‐ one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low‐quality evidence ‐ two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very‐low‐quality evidence ‐ three of the domains are not met. We are very uncertain about the results;
no evidence ‐ no RCTs were identified that addressed this outcome.
Factors that may decrease the quality of the evidence are: study design and risk of bias (downgraded when > 25% of the participants were from studies with a high risk of bias), inconsistency of results, indirectness (downgraded when > 50% of the participants were outside the target group), imprecision (downgraded when the total number of participants was less than 400 for continuous outcomes and 300 for dichotomous outcomes) and other factors (e.g. reporting bias).
Because the majority of studies contained a mix of participants with acute, subacute and chronic LBP, we did not separate out these groups in our analyses, other than in several trials involving only people with chronic LBP. We categorized studies as including people 'with sciatica' if more than66% of the participants were described as having sciatica (this may or may not have included those with nerve root symptoms) or if there was a separate analysis of outcomes in those with sciatica.
Clinical relevance
Two review authors independently carried out an analysis of the clinical relevance of each study. Without using an arbitrary predefined threshold, studies were judged as to whether: participants were described in enough detail to allow practitioners to decide whether they were similar to those in their practices; interventions and treatment settings were described well enough to allow practitioners to provide the same treatment for their participants; clinically relevant outcomes were measured and reported; the size of the effect; and the treatment benefits were worth the potential harms (see Table 5).
1. Clinical relevance.
| Author | Participants | Interventions | Outcomes | Effect size | Benefits/harms |
| Beurskens 1997 | + | + | + | ‐ | ‐ |
| Bihaug 1978 | + | + | + | ‐ | ‐ |
| Borman 2003 | + | + | + | ‐ | ‐ |
| Coxhead 1981 | + | ‐ | + | ‐ | ‐ |
| Fritz 2007 | + | + | + | ‐ | ‐ |
| Gudavalli 2006 | + | + | + | ‐ | ‐ |
| Güvenol 2000 | + | + | + | ? | ‐ |
| Harte 2007 | + | + | + | ‐ | ‐ |
| Konrad 1992 | + | ? | + | ‐ | ‐ |
| Larsson 1980 | + | + | + | ‐ | ‐ |
| Letchuman 1993 | ‐ | + | + | ‐ | ‐ |
| Lidström 1970 | + | + | + | ? | ‐ |
| Lind 1974 | + | + | + | + | + |
| Ljunggren 1984 | + | + | + | ‐ | ‐ |
| Ljunggren 1992 | + | + | + | ‐ | ‐ |
| Mathews 1975 | + | + | + | ‐ | ‐ |
| Mathews 1988 | + | + | + | ‐ | ‐ |
| Ozturk 2006 | + | + | + | ‐ | ‐ |
| Pal 1986 | + | + | + | ‐ | ‐ |
| Reust 1988 | ‐ | + | + | ‐ | ‐ |
| Schimmel 2009 | + | + | + | ‐ | ‐ |
| Sherry 2001 | + | + | + | + | ? |
| Simmerman 2011 | + | + | + | ‐ | ‐ |
| Sweetman 1993 | + | + | + | ‐ | ‐ |
| Tesio 1993 | + | + | + | ? | ‐ |
| Unlu 2008 | + | + | + | ‐ | ‐ |
| Van der Heijden 1995 | + | + | + | ‐ | ‐ |
| Walker 1982 | + | + | + | ‐ | ‐ |
| Weber 1973 | ‐ | + | + | ‐ | ‐ |
| Weber 1984 (1) | ‐ | + | + | ‐ | ‐ |
| Weber 1984 (2) | ‐ | + | + | ‐ | ‐ |
| Werners 1999 | + | + | + | ‐ | ‐ |
+: yes; ‐: no; ?: unknown.
Subgroup analysis and investigation of heterogeneity
Predefined subgroup analyses included:
different types of comparison (traction versus placebo, sham or no treatment; physiotherapy with traction versus physiotherapy without traction; different types of traction and traction versus other treatments);
different symptom patterns in subjects (mixed population of people with LBP with and without sciatica; people with LBP with sciatica and people with LBP without sciatica).
However, we were not able to conduct these analyses, because of reasons stated above. Instead, the results were synthesized narratively. 'Summary of findings' tables were generated for all analyses of different types of comparison. Primary outcome measures at a follow‐up duration of 12 to 16 weeks were included in the 'Summary of findings' tables.
Sensitivity analysis
In the previous review, sensitivity analyses were carried out to determine the cut‐off for high‐quality studies. The cut‐off point was set at six criteria for risk of bias, which is supported by empirical evidence (Van Tulder 2009). We considered that studies that met six or more of the criteria for risk of bias carried low risk of bias, whereas studies that met fewer than six of the criteria carried high risk of bias. We did not plan or carry out any new sensitivity analyses.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
We identified 32 trials that fulfilled the inclusion criteria. Seven new trials were published since the publication of the previous review (Fritz 2007; Gudavalli 2006; Harte 2007; Ozturk 2006; Schimmel 2009; Simmerman 2011; Unlu 2008). We included all 25 trials discussed in the previous review in this review. The total number of studies retrieved by all search methods over time was not available. In this review, we included 32 studies, involving 2762 participants. Two of these studies were reported in one publication (Weber 1984); in four of the studies, there was more than one pertinent publication (Beurskens 1997; Gudavalli 2006; Mathews 1988; Van der Heijden 1995).
Presence of sciatica
Twenty‐three of the studies included a relatively homogeneous population of people with LBP and sciatica (Bihaug 1978; Coxhead 1981; Fritz 2007; Güvenol 2000; Harte 2007; Larsson 1980; Lidström 1970; Lind 1974; Ljunggren 1984; Ljunggren 1992; Mathews 1975; Mathews 1988; Ozturk 2006; Pal 1986; Reust 1988; Sherry 2001; Simmerman 2011; Sweetman 1993; Unlu 2008; Walker 1982; Weber 1973; two trials in Weber 1984). Eight studies included a greater mix of participants with and without sciatica (Beurskens 1997; Borman 2003; Gudavalli 2006; Konrad 1992; Letchuman 1993; Tesio 1993; Van der Heijden 1995; Werners 1999). There was only one study that exclusively involved people who did not have sciatica (Schimmel 2009).
Duration of low‐back pain
Ten studies included solely or primarily people with chronic LBP of more than 12 weeks (Borman 2003; Gudavalli 2006; Güvenol 2000; Ljunggren 1984; Schimmel 2009; Sherry 2001; Tesio 1993; Van der Heijden 1995; two in Weber 1984); in one study, participants were all in the subacute range (four to 12 weeks) (Konrad 1992); in 17 studies, the duration of LBP was a mixture of acute, subacute and chronic (Beurskens 1997; Bihaug 1978; Coxhead 1981; Fritz 2007; Harte 2007; Larsson 1980; Lidström 1970; Lind 1974; Ljunggren 1992; Mathews 1975; Mathews 1988; Ozturk 2006; Pal 1986; Simmerman 2011; Sweetman 1993; Unlu 2008; Walker 1982); in five studies duration was not specified (Letchuman 1993; Reust 1988; Weber 1973; and two in Weber 1984).
Comparisons
Thirteen studies compared traction with sham traction (Beurskens 1997; Letchuman 1993; Mathews 1975; Pal 1986; Reust 1988; Schimmel 2009; Van der Heijden 1995; Walker 1982; Weber 1973; and two in Weber 1984), with some kind of placebo (sham shortwave diathermy, Sweetman 1993; sham shortwave Lind 1974); or with no treatment (Konrad 1992). Fifteen studies compared traction with other treatments (Bihaug 1978; Coxhead 1981; Gudavalli 2006; Konrad 1992; Larsson 1980; Lidström 1970; Lind 1974; Ljunggren 1992; Mathews 1988; Sherry 2001; Simmerman 2011; Sweetman 1993; Unlu 2008; Werners 1999; Weber 1984). In one of these (Lind 1974), auto‐traction was compared with physiotherapy, in which Tru‐Trac traction was one of the range of treatments included. Five studies compared different types of traction (e.g. auto‐traction versus manual traction or passive traction, continuous versus intermittent traction, inversion traction versus conventional traction) (Güvenol 2000; Letchuman 1993; Ljunggren 1984; Reust 1988; Tesio 1993). Four studies compared a standard physiotherapy programme (not including traction) with the same treatment with traction (Borman 2003; Fritz 2007; Harte 2007; Ozturk 2006). One study compared different types of underwater therapy, underwater traction being one of them (Konrad 1992).
Length of follow‐up
Fourteen studies reported short‐term follow‐up (one week) (Fritz 2007; Gudavalli 2006; Harte 2007; Larsson 1980; Ljunggren 1984; Ljunggren 1992; Ozturk 2006; Pal 1986; Simmerman 2011; Sweetman 1993; Unlu 2008; Weber 1973; two in Weber 1984). Fifteen studies reported follow‐up at three to five weeks (Beurskens 1997; Bihaug 1978; Coxhead 1981; Fritz 2007; Konrad 1992; Lidström 1970; Lind 1974; Ljunggren 1984; Mathews 1975; Mathews 1988; Pal 1986, Reust 1988; Sherry 2001; Unlu 2008; Van der Heijden 1995). Fourteen studies reported follow‐up at nine to 16 weeks (Beurskens 1997; Bihaug 1978; Borman 2003; Coxhead 1981; Gudavalli 2006; Güvenol 2000; Harte 2007; Larsson 1980; Ljunggren 1984; Schimmel 2009; Tesio 1993; Unlu 2008; Van der Heijden 1995; Werners 1999). Five studies reported follow‐up at six months (Beurskens 1997; Gudavalli 2006; Harte 2007; Mathews 1988), or one year (Gudavalli 2006; Konrad 1992; Mathews 1988). One study did not report the timing at which the outcomes were measured (Walker 1982).
Risk of bias in included studies
See: Characteristics of included studies.
The results of the risk of bias analysis for the individual studies are summarized in Figure 1. Sixteen studies were considered to have a low risk of bias (Beurskens 1997; Fritz 2007; Gudavalli 2006; Larsson 1980; Letchuman 1993; Ljunggren 1984; Pal 1986; Schimmel 2009; Simmerman 2011; Sweetman 1993; Unlu 2008; Van der Heijden 1995; Weber 1973; both trials in Weber 1984; Werners 1999), representing 1568 (57%) participants. Overall, risk of bias scores ranged from two to 10 (maximum possible risk of bias score was 12). Some of the studies that were considered to have a low risk of bias based on the The Cochrane Collaboration's 'Risk of bias' tool were considered to have a high risk of bias in the previous review (Larsson 1980; Letchuman 1993; Ljunggren 1984; Pal 1986; Sweetman 1993; Weber 1973; Weber 1984). Overall completeness of data was assessed in this review, whereas previously, dropout during intervention and dropout during follow‐up were scored. Selective reporting and timing of outcome assessments were not assessed previously.
1.
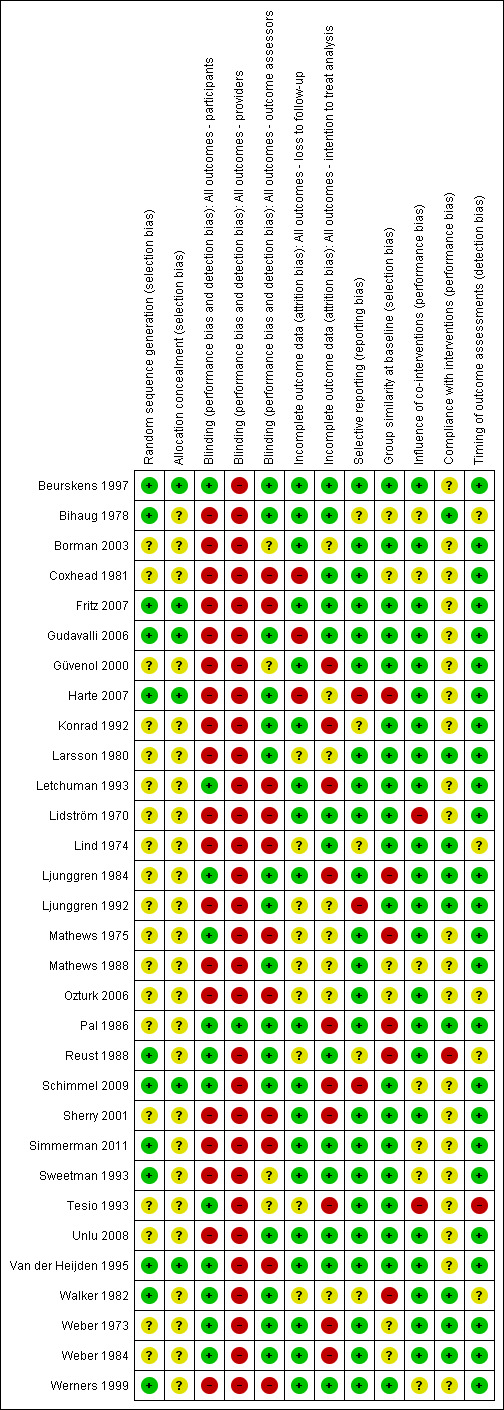
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The majority of the included studies did not properly report on their random and concealed allocation of treatment. In 20 of the included articles, there was no mention of the randomization procedure used and, in 26 of the included studies, it was unclear how concealment of treatment allocation was achieved. In six studies, both sequence generation and allocation procedure were conducted properly (Beurskens 1997; Fritz 2007; Gudavalli 2006; Harte 2007; Schimmel 2009; Van der Heijden 1991). In an additional six studies, the sequence generation was conducted properly, but the concealment of allocation was inadequately described (Bihaug 1978; Reust 1988; Simmerman 2011; Sweetman 1993; Walker 1982; Werners 1999). In the remaining studies, both randomization and allocation procedure were inadequately described or not mentioned at all. The authors claimed these studies were RCTs in the description of their methods and, therefore, these studies were included nevertheless.
Blinding
Blinding of outcomes was not achieved in the majority of the included studies. Blinding of the outcome assessor was achieved in 17 studies (Beurskens 1997; Bihaug 1978; Gudavalli 2006; Harte 2007; Konrad 1992; Larsson 1980; Ljunggren 1984; Ljunggren 1992; Mathews 1988; Pal 1986; Reust 1988; Schimmel 2009; Unlu 2008; Walker 1982; Weber 1973; both trials in Weber 1984), blinding of participants in 12 studies (Beurskens 1997; Letchuman 1993; Ljunggren 1984; Mathews 1975; Pal 1986; Reust 1988; Schimmel 2009; Tesio 1993; Van der Heijden 1995; Walker 1982; Weber 1973; Weber 1984), and blinding of care providers only in one study (Pal 1986). All of the studies that attempted to blind the participants to the assigned intervention did so by providing a sham treatment, with the exception of Tesio 1993. None of the studies evaluated the success of blinding post‐treatment. It should be noted that blinding of care providers of traction is impossible in most cases. It is disputable whether the outcome is likely to be influenced by a lack of blinding of care providers when it comes to assessing subjective measures such as pain intensity and functional status, as mentioned earlier. However, in the case of objective outcome measures, blinding is of importance.
Incomplete outcome data
In three studies, loss to follow‐up exceeded 20% of the study population (Coxhead 1981; Harte 2007), or significantly more subjects were lost to follow‐up in one treatment group compared the number of subjects that were lost to follow‐up in the other group (Gudavalli 2006). Loss to follow‐up never exceeded 23%. In nine of the included trials, it was not clear how many subjects were lost to follow‐up (Larsson 1980; Lind 1974; Ljunggren 1992; Mathews 1975; Mathews 1988; Ozturk 2006; Reust 1988; Tesio 1993; Walker 1982).
Selective reporting
None of the included RCTs had a published protocol in any of the protocol databases that were searched. The study's prespecified (primary and secondary) outcomes as reported in the article itself were compared with the reported outcomes. One study indicated that VAS scores, overall improvement and improvement in the straight leg raising test had been recorded at three and six months but did not report this (Harte 2007), while in another study, improvement in mobility, activities of daily living and the straight leg raising test were measured but not reported (Ljunggren 1992), and similarly for all outcome assessments at two and six weeks in another study (Schimmel 2009).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Section (1) of the results describes those studies in which a mixed group of people with LBP is involved, i.e., some with and some without sciatica. In section (2), the participant populations include only people with LBP with sciatica. Section (3) describes the studies that included only people with LBP without sciatica. Studies that included more than 66% of participants with sciatica were categorized as studies that included people with sciatica.
(1) Traction for a mixed group of people with low‐back pain, some with and some without sciatica
(1a) Traction versus placebo, sham or no treatment
There was low‐quality evidence that decrease in pain intensity was greater in participants treated with traction at three to five weeks' follow‐up (MD 18.49 points on the VAS, 95% CI ‐24.12 to ‐12.87) (Beurskens 1997; Konrad 1992). However, the difference in pain intensity at one year' follow‐up had an MD of only 9 points on the VAS (95% CI ‐19.32 to 1.12), favouring traction (Konrad 1992). Moderate‐quality evidence indicated there was a small positive effect on functional status favouring the sham group at three to five weeks' follow‐up (1.3 points on the Roland Morris Disability Questionnaire (RMDQ), 95% CI ‐2.90 to 0.30) (Beurskens 1997). There was no difference in global improvement at three to five weeks (RD ‐0.03, 95% CI ‐0.17 to 0.12) (Beurskens 1997; Van der Heijden 1995), or at six to 12 weeks (RD 0.03, 95% CI ‐0.12 to 0.18) (Beurskens 1997; Van der Heijden 1995). Moderate‐quality evidence showed mean time to return to work in the traction group was two days earlier (Beurskens 1997).
(1b) Physiotherapy with traction versus physiotherapy without traction
There was low‐quality evidence that there was no difference in pain intensity at one to two weeks' follow‐up between the two groups (Borman 2003). There was a small mean difference of 5 points on the VAS (95% CI ‐5.67 to 15.67) in favour of physiotherapy at 12 to 16 weeks' follow‐up (Borman 2003). Short‐term and long‐term functional status as measured by the ODI was better in the traction group than the physiotherapy group (short term mean points: 4, 95% CI ‐1.91 to 9.71; long term: 95% CI ‐2.78 to 10.78) (Borman 2003). There was low‐quality evidence that global improvement at one to two weeks' follow‐up was the same for both groups, whereas at 12 to 16 weeks' follow‐up, global improvement was higher in the traction group (RD 0.53, 95% CI 0.28 to 0.79) (Borman 2003).
(1c) Different types of traction
One study with very‐low‐quality evidence showed that there was no difference in global improvement between participants undergoing static traction and participants undergoing intermittent traction (Letchuman 1993). Global improvement was higher in participants undergoing auto‐traction than in participants undergoing mechanical traction (RD 0.53, 95% CI 0.32 to 0.73) (Tesio 1993). Outcomes on pain intensity and functional status were reported only for those participants responding to treatment.
(1d) Traction versus other treatments
Six studies compared traction with another treatment (Bihaug 1978; Gudavalli 2006; Konrad 1992; Lind 1974; Sweetman 1993; Werners 1999). Traction was compared with varying other treatments: physiotherapy, exercise, short‐wave diathermy, interferential therapy, bed rest and analgesics.
There was low‐ to moderate‐quality evidence that pain intensity was slightly lower in participants treated with traction in the short‐term and the long‐term (Gudavalli 2006; Konrad 1992; Sweetman 1993; Werners 1999). MDs varied from 1 to 8 points on the VAS with a follow‐up duration varying from one week to one year. Moderate‐quality evidence showed that functional status as measured by the ODI or RMDQ was the same for both groups at one to two weeks, 12 to 16 weeks and one year' follow‐up (Gudavalli 2006; Werners 1999). There was a small difference in favour of the control group at three to five weeks (MD 0.2, 95% CI ‐0.05 to 0.46) and at six months (0.15 points, 95% CI ‐0.16 to 0.45) (Gudavalli 2006). There was a very small difference in global improvement favouring traction at 12 to 16 weeks (Bihaug 1978) (RD 0.05, 95% CI ‐0.11 to 0.20), for which there was high‐quality evidence. The difference in global improvement at three to five weeks was much higher with an RD of 0.14 (95% CI ‐0.08 to 0.36) (Bihaug 1978) and 0.87 (95% CI 0.67 to 1.07) favouring traction (Lind 1974). However, the quality of evidence supporting this difference was very low.
(2) Traction for people with low‐back pain and sciatica
(2a) Traction versus placebo, sham or no treatment for people with a mix of acute, subacute and chronic low back pain with sciatica
Low‐quality evidence suggested that there was a small effect on pain intensity in favour of the sham group (MD 2.93 points on the VAS scale, 95% CI ‐14.73 to 20.59) at one to two weeks' follow‐up (Pal 1986; Reust 1988), and at three to five weeks' follow‐up (Pal 1986). There was low‐ to moderate‐quality evidence that global improvement rates were higher in participants receiving traction at one to two weeks' follow‐up (RD 0.13, 95% CI 0.04 to 0.22) (Larsson 1980; Sweetman 1993; Weber 1973; Weber 1984), and three to five weeks' follow‐up (RD 0.27, 95% CI 0.12 to 0.43) (Larsson 1980; Lidström 1970). However, at 12 to 16 weeks' follow‐up, there was no significant difference in global improvement between the two groups (RD 0.06, 95% CI ‐0.16 to 0.28) (Larsson 1980). Moderate‐quality evidence suggested that more participants receiving traction returned to work compared with participants receiving sham treatment (RD 0.15, 95% CI ‐0.15 to 0.45) (Pal 1986).
(2b) Physiotherapy with traction versus physiotherapy without traction
Although moderate‐quality evidence showed a lower mean pain intensity in the traction group (a difference of 7.96 points on the VAS, 95% CI ‐16.53 to 0.61) at one to two weeks' follow‐up (Fritz 2007; Ozturk 2006), the difference in mean pain intensity between the two groups was 2.00 points (95% CI ‐10.02 to 14.02) in favour of the physiotherapy group at six weeks' follow‐up (Fritz 2007). Functional status was measured by both the ODI and the RMDI. There was low‐ to moderate‐quality evidence that there was no difference in functional outcome at one to two weeks', six to 12 weeks', 12 to 16 weeks and six months' follow‐up (Fritz 2007; Harte 2007). Low‐ to moderate‐quality evidence showed no difference in global improvement at one to two weeks' (Ozturk 2006), three to five weeks' (Coxhead 1981), six weeks' (Fritz 2007) and 12 to 16 weeks' (Coxhead 1981) follow‐up.
(2c) Different types of traction
We found three RCTs that compared two types of traction and reported on pain intensity (Ljunggren 1984; Reust 1988; Simmerman 2011). Reust 1988 compared auto‐traction with mechanical traction. There was a small effect in favour of auto‐traction (2.9 points on the VAS, 95% CI ‐14.73 to 20.59). Simmerman 2011 compared aquatic traction to a land‐based supine position at one to two weeks' follow‐up. There was a small effect in favour of auto‐traction at one to two weeks' follow‐up (8 points on the VAS, 95% CI ‐3.02 to 19.02). One RCT was identified that compared two types of traction, auto‐traction versus manual traction, and reported on global improvement (Ljunggren 1984). There was a small effect in favour of manual traction at one to two weeks' follow‐up (RD ‐0.16, 95% CI ‐0.40 to 0.09).
Although one more RCT compared two types of traction (Güvenol 2000), this study only reported P values.
(2d) Traction versus other treatments
Three RCTs compared traction with other treatments and reported varying outcome measures (Lidström 1970; Ljunggren 1992; Unlu 2008). Traction was compared with physiotherapy, exercise, laser, ultrasound, manipulation and corset treatment.
There was moderate‐quality evidence that mean pain intensity in the traction group was slightly lower at one to two weeks' follow‐up (Ljunggren 1992; Unlu 2008), and three to five weeks' follow‐up (Unlu 2008). The maximum MD in pain intensity was 4.9 points (95% CI ‐15.87 to 6.07) (Unlu 2008). However, at 12 to 16 weeks' follow‐up the mean pain intensity in the traction group was higher (maximum MD 4.4 points, 95% CI ‐5.40 to 14.20) (Unlu 2008). There was no difference in functional status measured by the ODI or RMDI between the two groups at one to two weeks', three to five weeks' and 12 to 16 weeks' follow‐up (Ljunggren 1992; Unlu 2008). There was low‐ to moderate‐quality evidence that there is only a very small difference in global improvement between the two groups at one to two weeks' follow‐up (RD 0.03, 95% CI ‐0.24 to 0.30) (Ljunggren 1992), and three to five weeks' follow‐up (RD 0.42, 95% CI 0.17 to 0.67) (Lidström 1970).
(3) Traction for people with low‐back pain and without sciatica
(3a) Traction versus sham treatment
There was moderate‐quality evidence that there is a very small difference in pain intensity between the two groups, favouring the traction group by 4 points on the VAS (95% CI ‐17.65 to 9.65) (Schimmel 2009).
Adverse effects
Of the 32 studies, four stated that there were no adverse effects (Gudavalli 2006; Konrad 1992; Schimmel 2009; Walker 1982); seven studies reported some adverse effects, for example, increased pain in 11 of 14 inversion traction participants versus 2 of 13 conventional traction participants, and anxiety during treatment with "almost all of the inversion traction patients" (Güvenol 2000); increased pain in 31% of static traction group and 15% of intermittent traction group (Letchuman 1993); temporary deterioration in 4 of 24 of traction and 4 of 26 of exercise group (Ljunggren 1992); subsequent surgery in 7 of 83 in lumbar traction group versus none in control group (Mathews 1988); aggravation of neurological signs in 5 of 18 of traction group, 4 of 20 of light traction group and 4 of 20 of placebo group (Reust 1988); aggravation of symptoms in 5 of 43 of traction and 1 of 43 of sham (Weber 1973). Borman 2003 reported that 25% of the group receiving traction as part of standard physiotherapy and 37% of the physiotherapy without traction group felt "probably or definitely worse" at three‐month' follow‐up. The remaining 21 studies did not report adverse effects.
Discussion
Summary of main results
Many studies were identified on the effect of traction on pain intensity, functional status, global improvement and return to work in people with LBP. However, most evidence was imprecise and inconsistent and numerous studies carried substantial risk of bias. Many of the studies seemed to have sample sizes that were too small to detect a clinically significant difference. Furthermore, the heterogeneity in comparisons, outcomes and follow‐up durations prohibited us, among other reasons, from pooling the data and, therefore, we used a descriptive analysis in this review. The sample sizes per comparison mostly did not reach the threshold of 400 for continuous outcomes and 300 for dichotomous outcomes (Furlan 2009; Higgins 2011). Therefore, we put little trust in positive effects that emerged.
The included studies largely differed in their population, outcome measures, and scales and duration of follow‐up. Some studies included hospitalized participants with demonstrated herniated discs, neurological findings and sciatica, while other studies included people recruited from primary care or workers recruited through internal company newspapers. Some studies used the ODI, while others used the RMDI. Some studies reported on all four primary outcomes (pain intensity, functional status, global improvement and return to work), whereas others only reported on one or two, which might suggest publication bias.
The studies showed small differences in effects between traction and other treatment options on pain intensity, functional status, global improvement and return to work at short term. The effect was even smaller at longer‐term follow‐up. Mostly the MD between the two groups favours the traction group, but not always. For most of the outcomes, no effects of traction were shown and when they were, the effects were too small to be clinically relevant. The minimum important difference (between groups) in changes (within groups) for pain intensity and functional status established by Ostelo 2008 were used to judge clinical relevancy. A clinically relevant effect was achieved in pain intensity at three to five weeks' follow‐up in people with and without sciatica undergoing traction when compared with sham treatment (Konrad 1992). A clinically relevant difference in changes in global improvement was seen in people with and without sciatica undergoing physiotherapy with traction at 12 to 16 weeks' follow‐up (RD 0.53) (Borman 2003), and in global improvement in people with and without sciatica undergoing traction when compared to other treatments at 12 to 16 weeks (RD 0.57) (Bihaug 1978; Lind 1974). However, in all of these cases, the effects did not reach statistical significance and they were based on low‐ to very‐low‐quality evidence, which means that we are very uncertain about the findings. Studies with a high risk of bias typically overestimate the effect compared to studies with a low risk of bias (Van Tulder 2009).
Two articles examined the level of physical force applied in the treatment and concluded that even a low level of force may be effective (Harte 2003; Krause 2000). Beurskens 1997 maintained that traction at levels below 25% of body weight and using a split table can be regarded as sham (or low‐dose) traction, and the sham traction group in their trial received treatment involving a force of 10% to 20% of the participant's body weight. In the other trials that classified their control groups as 'sham traction', the force applied varied (e.g. less than 25% of body weight in Van der Heijden 1995; 10 lb (4.5 kg) in Letchuman 1993; 1.8 kg in Pal 1986; 5 kg in Reust 1988; and a maximum of 20 lb (9 kg) in Mathews 1975). No differences between traction and sham traction were demonstrated in any of these trials.
Overall completeness and applicability of evidence
We minimized review bias by performing an extensive database search. Publication bias could be an issue. The many small RCTs are more likely to be published when positive. Authors possibly may refrain from publishing when results are negative. However, the review authors consider that it is unlikely that large trials on the subject were not published. Many of the published studies did not have a published protocol and, therefore, it is difficult to ascertain to what extent studies did not publish their findings because the results did not prove to be favourable.
Quality of the evidence
Sixteen of the 32 included studies demonstrated a low risk of bias. Items that were scored predominantly negatively or unclear were randomization, concealment and blinding. The majority of the included studies did not properly report on their random and concealed allocation of treatment. In 20 of the included articles, there was no mention of the randomization procedure used and, in 26 of the included studies, it was unclear how concealment of treatment allocation was achieved. Blinding of outcomes was not achieved in the majority of the included studies. Blinding of the outcome assessor was achieved in 17 studies and blinding of participants in 12 studies. The latter reflects the number of trials in which sham or simulated traction was used. Blinding of the care provider is virtually impossible given the nature of the intervention. As a result, only one study achieved blinding of the care provider.
Furthermore, relatively few participants were identified for any of the principal outcome measurements and, as a result, none of the findings should be considered robust.
Potential biases in the review process
Although content area experts may have inside knowledge, may be familiar with current interests in their field and may be aware of pressing questions in their field, they may also have personal prejudices and idiosyncrasies. Experts with strong opinions may make it difficult to prevent bias (Gotzsche 2012). To harness bias in this review, two non‐experts (IW and ISW) in this area, trained in reviewing literature, were involved in writing this review. Data from previous reviews were verified, checked and changed where necessary by these two review authors.
Agreements and disagreements with other studies or reviews
In general, the results and conclusions of this updated review are consistent with the previous version of the review, namely that traction is no better than standard interventions for (acute, subacute and chronic) LBP. In this review, we discussed one high‐quality study that included only people without sciatica that was not included in the previous review (Schimmel 2009). This study showed that traction in people without sciatica is no better than sham treatment. There was no significant difference between the traction and sham group in pain intensity or functional status.
Our findings were consistent with those reported in other systematic reviews on the subject (Chou 2007; Gay 2008). One review concluded there was insufficient data to draw firm conclusions on the clinical effect of traction (Van Middelkoop 2011). Only one RCT discussing the effect of traction was included in this review.
Authors' conclusions
Implications for practice.
Effects of traction alone or as part of a package for people with low‐back pain (LBP) with and without sciatica have not been shown. There are some randomized controlled trials (RCTs) showing benefit of traction, but the limited quality evidence from these small moderate to high risk of bias studies show very small effects that are not clinically relevant. In summary, to date the use of traction as treatment for non‐specific LBP is not supported by the best available evidence.
Implications for research.
New, large, high‐quality studies may change the point estimate and its accuracy, but it should be noted that such change may not necessarily favour traction. Therefore, little priority should be given to new studies on the effect of traction treatment alone or as part of a package.
Feedback
Personal experience with traction, 2 January 2010
Summary
Individual shared personal experience with traction as a positive alternative to surgery for his back pain.
Personal correspondence between Managing Editor and contributor. Not appropriate to include.
Reply
Contributor responded appreciatively to correspondence.
Contributors
Victoria Pennick, Managing Editor, Cochrane Back Review Group
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2013 | New citation required but conclusions have not changed | Conclusions not changed. |
| 13 May 2013 | New search has been performed | Review updated. Seven new RCTs were incorporated. The review was performed using the latest methods concerning risk of bias assessment and reporting as stated in the Handbook. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 14 January 2010 | Amended | Feedback added |
| 27 June 2008 | Amended | Converted to new review format. |
| 25 January 2007 | New citation required but conclusions have not changed | Conclusions were not changed by the addition of the newly identified trial. Author by‐line changed. |
| 31 October 2006 | New search has been performed | There was only one additional trial identified for this update. It did not change the conclusions. |
Acknowledgements
We acknowledge and thank Dr. J Clarke for her contribution to the previous review (Clarke 2006a), of which this is an update.
Appendices
Appendix 1. Search strategy
MEDLINE (Ovid) (1966 to August 2013)
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
exp Low Back Pain/
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
or/12‐22
exp Spine/
discitis.ti,ab.
exp Spinal Diseases/
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
spinal fusion.sh.
spinal neoplasms.sh.
(facet adj joints).ti,ab.
intervertebral disk.sh.
intervertebral disc.sh.
Intervertebral Disc Displacement.sh.
postlaminectomy.ti,ab.
arachnoiditis.ti,ab.
(failed adj back).ti,ab.
or/24‐38
23 or 39
11 and 40
exp Traction/
exp "Physical Therapy (Specialty)"/
42 or 43
exp Fractures, Bone/
44 not 45
11 and 41 and 46
EMBASE Ovid (1980 to August 2013)
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
human/
Nonhuman/
exp ANIMAL/
Animal Experiment/
33 or 34 or 35
32 not 36
31 not 36
37 and 38
38 or 39
dorsalgia.mp.
back pain.mp.
exp LOW BACK PAIN/
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
or/41‐50
exp SPINE/
discitis.mp.
exp Spine Disease/
(disc adj degeneration).mp.
(disc adj prolapse).mp.
(disc adj herniation).mp.
spinal fusion.mp.
spinal neoplasms.mp.
(facet adj joints).mp.
intervertebral disk.mp.
postlaminectomy.mp.
arachnoiditis.mp.
(failed adj back).mp.
or/53‐65
52 or 66
40 and 67
exp traction therapy/
exp fracture/
69 not 70
68 and 71
CENTRAL (The Cochrane Library, 2012 Issue 8)
MeSH descriptor Back Pain explode all trees
dorsalgia
backache
MeSH descriptor Low Back Pain explode all trees
(lumbar next pain) or (coccyx) or (coccydynia) or (sciatica) or (spondylosis)
MeSH descriptor Sciatica explode all trees
MeSH descriptor Spine explode all trees
MeSH descriptor Spinal Diseases explode all trees
(lumbago) or (discitis) or (disc near degeneration) or (disc near prolapse) or (disc near herniation)
spinal fusion
facet near joints
MeSH descriptor Intervertebral Disk explode all trees
postlaminectomy
arachnoiditis
failed near back
MeSH descriptor Cauda Equina explode all trees
lumbar near vertebra*
spinal near stenosis
slipped near (disc* or disk*)
degenerat* near (disc* or disk*)
stenosis near (spine or root or spinal)
displace* near (disc* or disk*)
prolap* near (disc* or disk*)
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23)
MeSH descriptor Traction explode all trees
MeSH descriptor Physical Therapy (Specialty) explode all trees
(#25 OR #26)
MeSH descriptor Fractures, Bone explode all trees
(#27 AND NOT #28)
(#24 AND #29)
CINAHL (Ebsco) (January 2006 to August 2013)
S53 S49 and S52 S52 S50 NOT S51 S51 (MH "Fractures+") S50 (MH "Traction") OR "traction" S49 S47 or S48 S48 S35 or S43 or S47 S47 S44 or S45 or S46 S46 "lumbago" S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis") S44 (MH "Thoracic Vertebrae") S43 S36 or S37 or S38 or S39 or S40 or S41 or S42 S42 lumbar N2 vertebra S41 (MH "Lumbar Vertebrae") S40 "coccydynia" S39 "coccyx" S38 "sciatica" S37 (MH "Sciatica") S36 (MH "Coccyx") S35 S29 or S30 or S31 or S32 or S33 or S34 S34 lumbar N5 pain S33 lumbar W1 pain S32 "backache" S31 (MH "Low Back Pain") S30 (MH "Back Pain+") S29 "dorsalgia" S28 S26 NOT S27 S27 (MH "Animals") S26 S7 or S12 or S19 or S25 S25 S20 or S21 or S22 or S23 or S24 S24 volunteer* S23 prospectiv* S22 control* S21 followup stud* S20 follow‐up stud* S19 S13 or S14 or S15 or S16 or S17 or S18 S18 (MH "Prospective Studies+") S17 (MH "Evaluation Research+") S16 (MH "Comparative Studies") S15 latin square S14 (MH "Study Design+") S13 (MH "Random Sample") S12 S8 or S9 or S10 or S11 S11 random* S10 placebo* S9 (MH "Placebos") S8 (MH "Placebo Effect") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 triple‐blind S5 single‐blind S4 double‐blind S3 clinical W3 trial S2 "randomi?ed controlled trial*" S1 (MH "Clinical Trials+")
Appendix 2. Criteria for assessing risk of bias for internal validity
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for participant‐reported outcomes in which the participant was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and care providers (e.g. co‐interventions, length of hospitalization, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardized difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts are very large, imputation using even 'acceptable' methods may still suggest a high risk of bias) (Van Tulder 2003). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (Van Tulder 2003).
Selective Reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of participants with neurological symptoms) (Van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (Van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (Van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomized participants were reported/analysed in the group to which they were allocated by randomization.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (Van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Data and analyses
Comparison 1. Low‐back pain with/without radiation, traction versus placebo, sham or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 3‐5 weeks | 2 | 247 | Mean Difference (IV, Fixed, 95% CI) | ‐18.49 [‐24.12, ‐12.87] |
| 1.2 6‐12 weeks | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐9.91, 10.51] |
| 1.3 6 months | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐11.55, 10.55] |
| 1.4 1 year | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐9.10 [‐19.32, 1.12] |
| 2 Functional status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 3‐5 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 6‐12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global improvement | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 3‐5 weeks | 2 | 175 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 3.2 6‐12 weeks | 2 | 175 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.12, 0.18] |
| 3.3 6 months | 1 | 150 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.14, 0.18] |
| 4 Return to work (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 3‐5 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 6‐12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
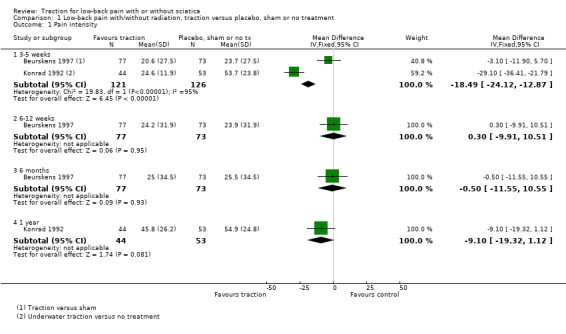
Comparison 1 Low‐back pain with/without radiation, traction versus placebo, sham or no treatment, Outcome 1 Pain intensity.
1.2. Analysis.
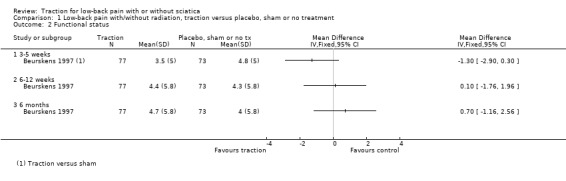
Comparison 1 Low‐back pain with/without radiation, traction versus placebo, sham or no treatment, Outcome 2 Functional status.
1.3. Analysis.
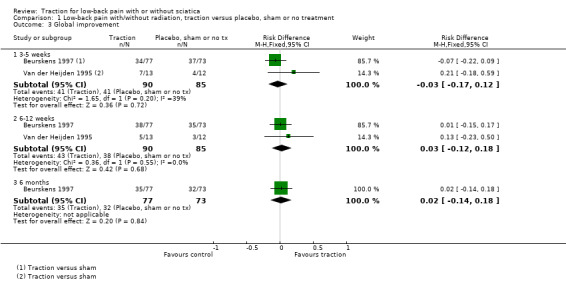
Comparison 1 Low‐back pain with/without radiation, traction versus placebo, sham or no treatment, Outcome 3 Global improvement.
1.4. Analysis.
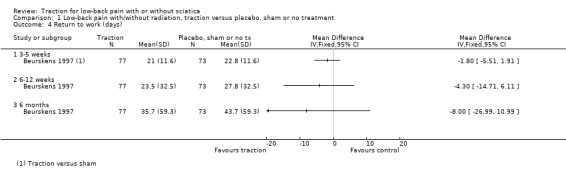
Comparison 1 Low‐back pain with/without radiation, traction versus placebo, sham or no treatment, Outcome 4 Return to work (days).
Comparison 2. Low‐back pain with/without radiation, physiotherapy with traction versus physiotherapy without traction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 1‐2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12‐16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 1‐2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 12‐16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global improvement | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 1‐2 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 12‐16 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
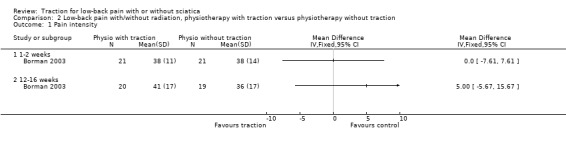
Comparison 2 Low‐back pain with/without radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 1 Pain intensity.
2.2. Analysis.
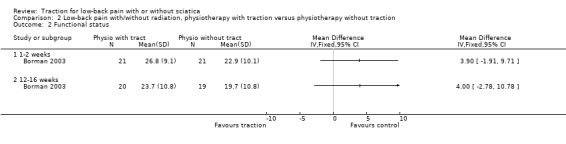
Comparison 2 Low‐back pain with/without radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 2 Functional status.
2.3. Analysis.
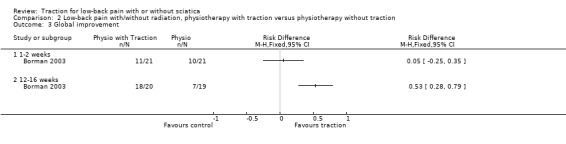
Comparison 2 Low‐back pain with/without radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 3 Global improvement.
Comparison 3. Low‐back pain with/without radiation, two types of traction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks | 2 | 93 | Risk Difference (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.54] |
3.1. Analysis.
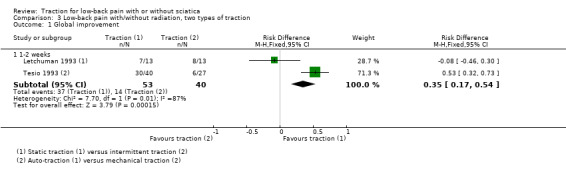
Comparison 3 Low‐back pain with/without radiation, two types of traction, Outcome 1 Global improvement.
Comparison 4. Low‐back pain with/without radiation, traction versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 1‐2 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3‐5 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 12‐16 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 1 year | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 1‐2 weeks | 1 | 138 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.40, 0.27] |
| 2.2 3‐5 weeks | 1 | 235 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.46] |
| 2.3 12‐16 weeks | 2 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.21] |
| 2.4 6 months | 1 | 168 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.16, 0.45] |
| 2.5 1 year | 1 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.25, 0.34] |
| 3 Global improvement | 3 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 1‐2 weeks | 2 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3‐5 weeks | 2 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 12‐16 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
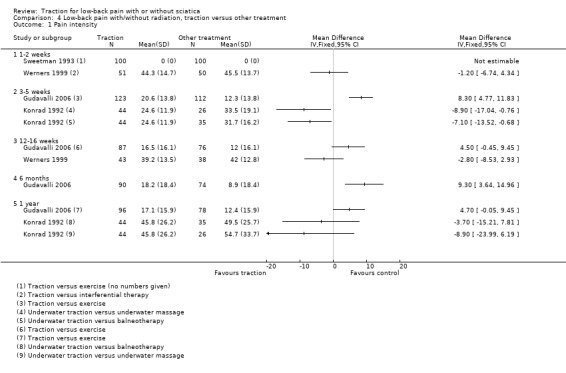
Comparison 4 Low‐back pain with/without radiation, traction versus other treatment, Outcome 1 Pain intensity.
4.2. Analysis.
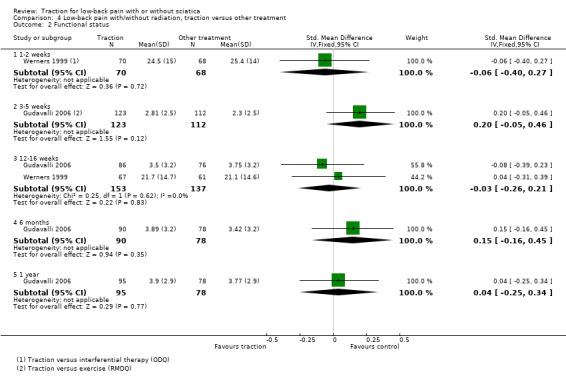
Comparison 4 Low‐back pain with/without radiation, traction versus other treatment, Outcome 2 Functional status.
4.3. Analysis.
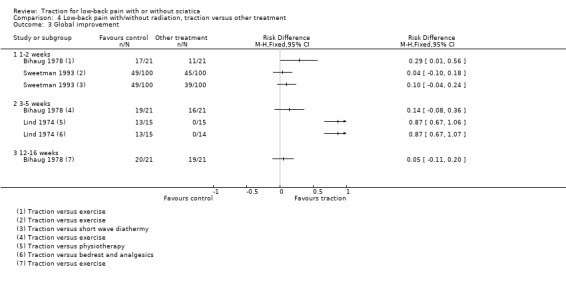
Comparison 4 Low‐back pain with/without radiation, traction versus other treatment, Outcome 3 Global improvement.
Comparison 5. Low‐back pain with radiation, traction versus placebo, sham or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [‐14.73, 20.59] |
| 1.2 3‐5 weeks | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Global improvement | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 1‐2 weeks | 4 | 398 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
| 2.2 3‐5 weeks | 2 | 123 | Risk Difference (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.43] |
| 2.3 12‐16 weeks | 1 | 81 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.16, 0.28] |
| 3 Return to work | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 years | 1 | 39 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [‐0.15, 0.45] |
5.1. Analysis.
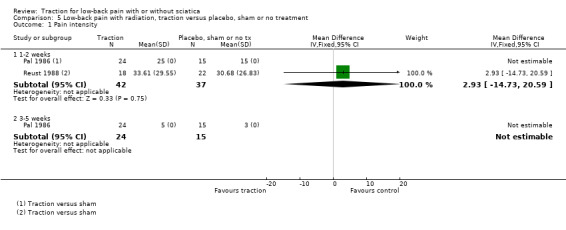
Comparison 5 Low‐back pain with radiation, traction versus placebo, sham or no treatment, Outcome 1 Pain intensity.
5.2. Analysis.
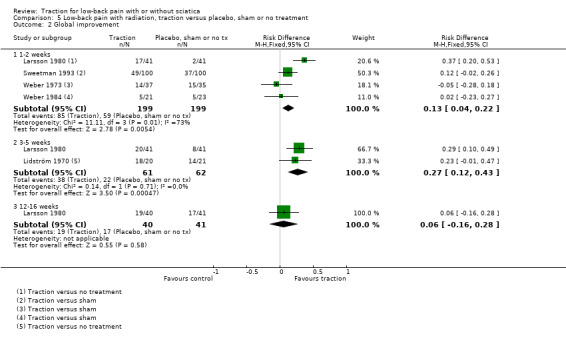
Comparison 5 Low‐back pain with radiation, traction versus placebo, sham or no treatment, Outcome 2 Global improvement.
5.3. Analysis.
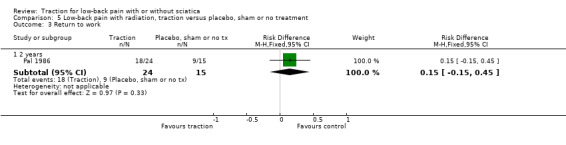
Comparison 5 Low‐back pain with radiation, traction versus placebo, sham or no treatment, Outcome 3 Return to work.
Comparison 6. Low‐back with radiation, physiotherapy with traction versus physiotherapy without traction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐7.96 [‐16.53, 0.61] |
| 1.2 6 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐10.02, 14.02] |
| 2 Functional status | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 1‐2 weeks | 2 | 94 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.49, 0.32] |
| 2.2 6‐12 weeks | 1 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.35, 0.63] |
| 2.3 12‐16 weeks | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.30, 1.16] |
| 2.4 6 months | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.54, 0.90] |
| 3 Global improvement | 3 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 1‐2 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3‐5 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 6 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 12‐16 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Return to work | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 3‐5 weeks | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
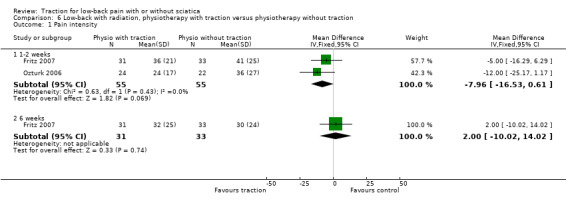
Comparison 6 Low‐back with radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 1 Pain intensity.
6.2. Analysis.
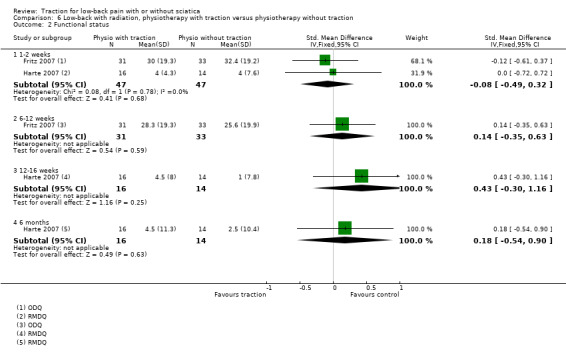
Comparison 6 Low‐back with radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 2 Functional status.
6.3. Analysis.
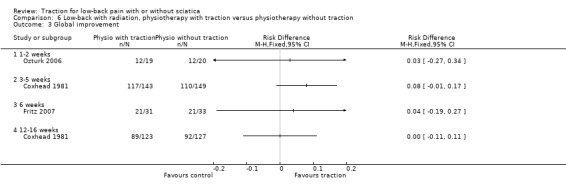
Comparison 6 Low‐back with radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 3 Global improvement.
6.4. Analysis.
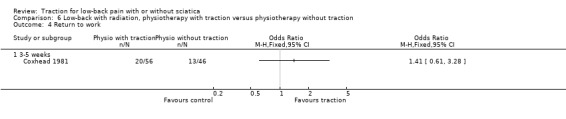
Comparison 6 Low‐back with radiation, physiotherapy with traction versus physiotherapy without traction, Outcome 4 Return to work.
Comparison 7. Low‐back pain with radiation, traction versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 1‐2 weeks | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3‐5 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 12‐16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 1‐2 weeks | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3‐5 weeks | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 12‐16 weeks | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global improvement | 2 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 1‐2 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3‐5 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
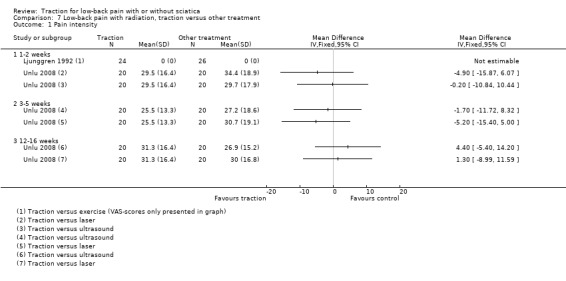
Comparison 7 Low‐back pain with radiation, traction versus other treatment, Outcome 1 Pain intensity.
7.2. Analysis.
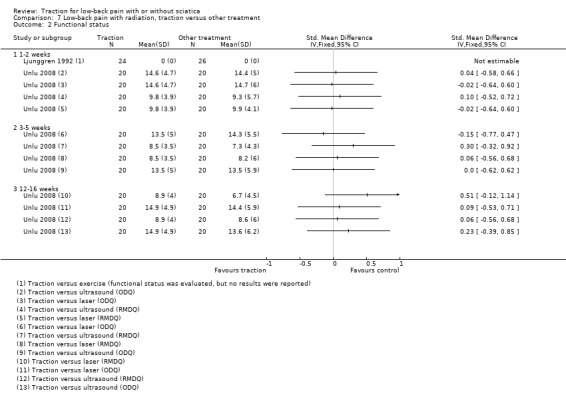
Comparison 7 Low‐back pain with radiation, traction versus other treatment, Outcome 2 Functional status.
7.3. Analysis.
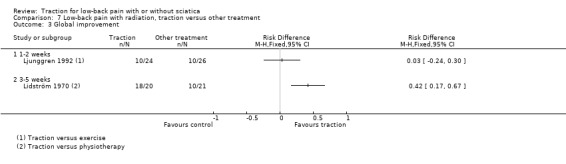
Comparison 7 Low‐back pain with radiation, traction versus other treatment, Outcome 3 Global improvement.
Comparison 8. Low‐back pain with radiation, two types of traction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks | 3 | 149 | Mean Difference (IV, Fixed, 95% CI) | 6.58 [‐2.77, 15.93] |
| 2 Global improvement | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 1‐2 weeks | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
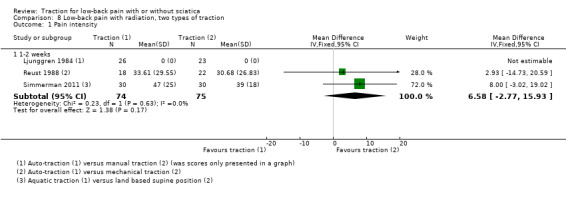
Comparison 8 Low‐back pain with radiation, two types of traction, Outcome 1 Pain intensity.
8.2. Analysis.
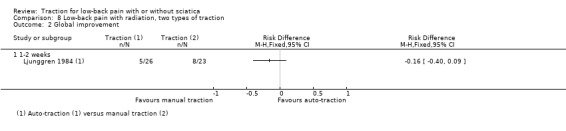
Comparison 8 Low‐back pain with radiation, two types of traction, Outcome 2 Global improvement.
Comparison 9. Low‐back pain without radiation, traction versus sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 12‐16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
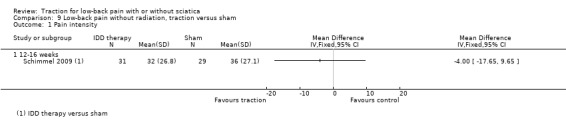
Comparison 9 Low‐back pain without radiation, traction versus sham, Outcome 1 Pain intensity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beurskens 1997.
| Methods | RCT; participants randomly allocated by computer, sealed envelopes prepared by independent person, containing treatment code. Stratified on duration of complaints (< 6 or > 6 months), and according to PT practices. | |
| Participants | 151 participants (85 male and 66 female, > 18 years old) recruited by physiotherapists and general practitioners in the Netherlands, with at least 6 wk of subacute and chronic non‐specific LBP, having never had any form of lumbar traction treatment. 150 completed 12‐wk follow‐up and 148 completed 6‐month follow‐up. | |
| Interventions | T) Traction: continuous mechanical traction with Eltrac, DIMEC Delft Instruments, the Netherlands. Traction force increased until participant indicated tolerance for pulling was reached, with minimum force of 35% and maximum of 50% of body weight. C) Comparison intervention: sham traction. Same as above except traction force was slowly increased until participant indicated feeling little pulling with maximum force of 20% body weight. Special brace worn around iliac crest, which became tighter in the back during treatment. Both groups treated 12 times in 5 wk for 20 min per session. | |
| Outcomes | At 5 wk: global perceived effect (number and %): T) 34 (44%), C) 37 (51%); first main complain (mean): T) 28.5, C) 28.4; second main complaint (mean): T) 27, C) 24.6; RMDQ (mean): T) 3.5, C) 4.8; pain at the moment (mean): T) 21.2, C) 22.5; pain last wk (mean): T) 20.6, C) 23.7; severity of LBP (mean): T) 1.6, C)1.8; ROM (mean): T) ‐2.1, C) 0.1; ADL disability (mean): T) 26.7, C) 33.8; work absence (days) (mean): T) 21, C) 22.8. No significant differences on any outcome measures. At 12 wk: global perceived effect‐recovery (number and %): T) 38 (50%), C) 35 (48%); first main complaint (mean): T) 33.7, C) 31.5; second main complaint (mean): T) 35.4, C) 30.7; RMDQ (mean): T) 4.4, C) 4.3; pain at the moment (mean): T) 28.5, C) 22.8; severity of LBP (mean): T) 2.3, C) 2.2; ROM: T) ‐1.1, C) 1.2; ADL disability (mean): T) 27.1, C) 29.4; work absence (days) (mean): T) 23.5, C) 27.8. At 6 months: global perceived effect (number and %): T) 35 (47%), C) 32 (44%); first main complain (mean): T) 36.7, C) 36.0; second main complaint (mean): T) 35.8, C) 32.8; RMDQ (mean): T) 4.7, C) 4.0; pain at the moment (mean): T) 23.8, C) 20.1; ADL disability (mean): T) 25.7, C) 25.8; work absence (days) (mean): T) 35.7, C) 43.7 No significant differences on any outcome measures. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation with the help of a random numbered list generated by computer. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by an independent person containing the treatment code. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Participants were blinded to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | After admission of a participant into the trial, the treating physiotherapist received a sealed envelope that contained the treatment code. The envelope was opened at the first treatment session and, therefore, the care provider was not blinded for the assigned treatment. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | Of the 151 participants, only 1 was lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | The 2 treatment groups had similar demographic and clinical baseline characteristics. |
| Influence of co‐interventions (performance bias) | Low risk | Co‐interventions, other than pain medication, were not allowed during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Bihaug 1978.
| Methods | RCT; method of randomization not described. | |
| Participants | 42 participants (23 male, 19 female, aged 19‐71 years (mean 44.1 years) referred from secondary care setting. All had radicular pain; in 32 radiating pain was below the knee. Pain duration was 3‐52 wk (mean 9.7 wk). 25 participants were on sick leave at baseline (1‐24 wk, mean 5.1 wk). 18 had severe pain, the remainder had moderate pain. 27 had neurological deficits (figures not given for the 2 different groups). | |
| Interventions | T) Traction: auto‐traction, using a combination of Lind's method and Myrin's method. Instead of pulling with the arms (as in Lind), participants pushed with 1 or both arms (according to Myrin/Spina‐Trac). 4‐12 sessions (mean 8.2), with interval of 3.1 days between sessions. (Force 70 kiloponds according to Lind.) All participants also received education in LBP/ biomechanics). C) Comparison intervention: exercise. Isometric exercises of the abdominal and pelvic floor muscles, to increase abdominal pressure (and, in turn, to increase intrinsic lumbar support) (Hume, Kendall and Jenkins; Fysioterapeuten number 3, Norway). 4‐12 sessions (mean 10.6) with interval of 4.1 days between sessions). | |
| Outcomes | Global improvement (symptom‐free; mild symptoms with ability to work; some or no improvement; deterioration) (n). At end of treatment series: T) 5, 12, 3, 1; C) 2, 9, 10, 0. At 1 month AT: T) 12, 7, 2, 0; C) 5, 11, 5, 0. At 3 months AT: T) 16, 4, 1, 0; C) 12, 7, 2, 0. | |
| Notes | Outcomes inappropriately dichotomized by authors, leading to P value < 0.05 at end of treatment series (ns at other follow‐up points). Without this dichotomization, group differences are not statistically significant at any follow‐up point. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Transformed from old format to new format. |
| Allocation concealment (selection bias) | Unclear risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Transformed from old format to new format. |
| Selective reporting (reporting bias) | Unclear risk | Transformed from old format to new format. |
| Group similarity at baseline (selection bias) | Unclear risk | Transformed from old format to new format. |
| Influence of co‐interventions (performance bias) | Unclear risk | Transformed from old format to new format. |
| Compliance with interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Timing of outcome assessments (detection bias) | Unclear risk | Transformed from old format to new format. |
Borman 2003.
| Methods | RCT; method of randomization not described. | |
| Participants | 42 participants (14 male, 28 female; age: T) 38.5 ± 8.4 years, C) 42.8 ± 10.5 years) with persistent (> 6 months) or recurring, non‐specific LBP, or both; outpatients in physical medicine and rehabilitation department of large hospital. Duration of pain (months): T) 27 ± 19.5, C) 34.09 ± 14.1. Ratio of participants with/without radiation: T) 14:7, C) 13:8. Excluded those with neurological deficits. | |
| Interventions | T) Traction and standard PT. Motorized traction (Eltrac 439, Enraf, the Netherlands), 10 x 20‐min sessions, participants lying on traction table in semi‐fowler position. Canvas braces attached around iliac crest and lower thoracic region, with force increased to maximum of 50% body weight. Traction applied between ultrasound therapy and exercise sessions in standard PT programme (as below). C) Comparison intervention: standard PT. Included hot packs (10 min), ultrasound (10 min), exercise (20 min). | |
| Outcomes | Pain (VAS) (mean, SD (range)): before: T) 5.7, 1.1 (3‐8); C) 5.6, 1.7 (2‐9); immediately after: T) 3.8, 1.1 (1‐6); C) 3.8, 1.4 (1‐7). Within‐group difference P value < 0.01; between‐group difference ns. 3 months. Follow‐up: T) 4.1, 1.7 (0‐7); C) 3.6, 1.7 (0‐6). ODI: (mean, SD (range)): before: T) 32.3, 9.6 (12‐44); C) 25.2, 10.4 (3‐41); immediately after: T) 26.8, 9.1 (4‐41); C) 22.9, 10.1 (3‐43). Within‐group differences P value < 0.01. 3 months. Follow‐up: T) 23.7, 10.8 (6‐38); C) 19.7, 10.8 (0‐32). Within‐group difference P value < 0.05; between‐group difference ns. Global improvement (complete/mild improvement, no change, no improvement and worse) (n): immediately after: T) 11, 6, 5; C) 10, 6, 5. 3 months follow‐up: T) 8, 7, 5; C) 7, 5, 7. Between‐group difference ns. Global satisfaction (n (%) of participants completely/somewhat satisfied; not satisfied): immediately after: T) 17 (80.9%), 4 (19%); C) 15 (71.4%), 6 (28.6%); 3 months' follow‐up: T) 12 (60%), 8 (40%); C) 11 (57.8%), 8 (42.1%). No differences were observed in outcomes for participants with and without radiation (P value > 0.05). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants were lost to follow‐up (9.5%): 2 in each group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is not clear whether an intention‐to‐treat analysis was used or not. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | There were no differences between groups in terms of age, sex, duration of pain, VAS and ODI scores at entry. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were allowed during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Coxhead 1981.
| Methods | RCT; randomly allocated treatment (method of randomization not described). The design was factorial ‐ there were 16 treatment groups, enabling a comparison of combinations of methods as well as of individual methods. | |
| Participants | 334 participants (185 men, 149 women, mean age 41.9 years) referred to the outpatient department with sciatic pain at least as far as the buttock crease, with/without back pain. Pain not due to malignant or infective disease, gynaecological disorders, sacroiliac disease, vertebral collapse or gross structural abnormality. Mean duration of symptoms 14.3 wk. | |
| Interventions | T) Traction: Tru‐Trac apparatus, giving intermittent traction at pre‐set forces and time intervals. Duration and intensity at the discretion of the physiotherapist. Comparison interventions: C1) Exercises based on a catalogue of exercises that brought in all ROM and muscle groups; C2) Manipulation by Maitland technique; C3) Corset ‐ a ready‐made fabric lumbar support available in 3 sizes. All participants received short‐wave diathermy and a standardized 30‐min "back school" lecture. For all interventions, participants treated daily for first wk, with decreasing frequency in the following 3 wk. | |
| Outcomes | Participant assessments at 4 wk, 16 wk (better): T) 82%, 72%; C1) 82%, 75%; C2) 80%, 69%; C3) 81%, 71%. Pain (‐100 to +100 VAS) at 4 wk: T) 50.1 (37.9); C1) 52.6 (36.9); C2) 49.0 (40.0); C3) 49.8 (37.9). Statistical significance in C1 only. ROW at 4 wk: T) 36%; C1) 36%; C2) 33%; C3) 33%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | High risk | At 4 months follow‐up only 78% of the included participants were assessed. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Unclear risk | No information provided on demographic characteristics at baseline. |
| Influence of co‐interventions (performance bias) | Unclear risk | Unclear whether co‐interventions were allowed during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Fritz 2007.
| Methods | RCT; computer‐generated random number lists and concealment of allocation by means of randomization envelopes. | |
| Participants | 64 participants (33 in the extension group, 31 in the traction plus extension group) with symptoms of pain or numbness (or both) extending distal to the buttocks and signs of nerve root compression in the past 24 hours. All had LBP, 76.5% sciatica. Exclusion criteria included non‐mechanical LBP and previous spinal fusion or spine surgery in the past 6 months. Mean age T) 41.7 years, C) 40.7 years. Duration of complaints: 47.5 days. | |
| Interventions | T) Traction: extension‐oriented treatment and mechanical traction using an adjustable table. Traction during first 2 wk of treatment, 4 sessions per wk, 12 min per session, with a traction force of 40‐60% of body weight. Extension‐oriented treatment included 9 sessions of exercise, mobilization and education during a 6‐wk treatment period. C) Comparison intervention: extension‐oriented treatment. |
|
| Outcomes | Assessment at 2 and 6 wk' post‐treatment. ODI (all measurements: MD): 2 wk 7.2 (95% CI 0.13 to 14.3), 6 wk 1.8 (95% CI ‐6.4 to 10.1). Pain rating: 2 wk 0.23 (95% CI ‐1.4 to 1.9), 6 wk ‐0.17 (95% CI ‐1.4 to 1.1). FABQ ‐ physical activity subscale: 2 wk 2.7 (95% CI 0.66 to 4.6), 6 wk 0.50 (95% CI ‐2.4 to 3.4). FABQ ‐ work subscale: 2 wk ‐1.1 (95% CI ‐4.2 to 1.9), 6 wk ‐3.1 (95% CI ‐6.5 to 0.36). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Randomization envelopes. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Outcome assessors did not participate in the subject's treatment and were blinded to the treatment allocation. However, blinding was lost for 15 subjects (20%). |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 8 participants were lost to follow‐up (12.5%). |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | There were no between‐group differences at baseline, other than a higher percentage of participants using prescription pain medication in the TRACT group. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions, other than analgesics, were allowed during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Gudavalli 2006.
| Methods | RCT; random number tables and concealment of allocation by means of randomization envelopes. | |
| Participants | 235 participants (123 in the flexion‐distraction group, 112 in the active trunk exercise programme) with LBP with a duration of at least 3 months. All had LBP, 22.8% sciatica. Mean age: T) 42.2 years, C) 40.9 years. | |
| Interventions | T) Traction: flexion‐distraction technique during 4 wk, 2‐4 sessions per wk, 9‐18 min of traction per session. C) Comparison intervention: active trunk exercise programme. Treatment duration of 4 wk, 2‐4 sessions per wk, 30‐45 min per session. |
|
| Outcomes | Assessment at 4 wk, 3 months and 12 months from baseline. VAS (mean change from baseline to time period indicated in MD (SE)): 4 wk: T) 20.57 (2.00), C) 12.34 (1.80); 3 months: T) 16.52 (2.95), C) 12.04 (2.53); 6 month: T) 18.26 (2.64), C) 8.92 (2.89); 12 months: T) 17.10 (2.55), C) 12.36 (2.43). RMDI: 4 wk: T) 2.81 (0.38), C) 2.30 (0.33); 3 months: T) 3.50 (0.50), C) 3.75 (0.51); 6 months: T) 3.89 (0.46), C) 3.42 (0.50); 12 months: T) 3.90 (0.53), C) 3.77 (0.44). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, manila envelopes. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded and all remained blinded for the entire study period. No incidents of unblinding were reported. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | High risk | Although total loss to follow‐up was only 16.6%, significantly more subjects in the active trunk exercise programme group dropped out of the study (T) 13, C) 25). |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were found at baseline. |
| Influence of co‐interventions (performance bias) | Low risk | Co‐interventions were not allowed during the treatment period. Analgesics were not allowed 24 hours prior to measurements. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Güvenol 2000.
| Methods | RCT; method of randomization not described. | |
| Participants | 29 participants (mean age: T1) 33.8 years, T2) 39.6 years) with LBP and lower extremity pain of not less than 1 month, and lumbar disc herniation diagnosed by CT. Mean duration of pain (months): T1) 28.5 ± 26.5 months, T2) 39.3 ± 39.2 months). None had history of spinal surgery. Pain not due to disease such as malignant, inflammatory, infectious, metabolic, congenital or developmental disorders. Disc pathology at 2 levels was present in 10 subjects, 5 from each treatment group. | |
| Interventions | Traction: T1) Inversion spinal traction. Traction used a modified tilt table (Sheffield 1996). With participant lying supine, ankle straps mounted to the foot of the table; lumbar strap allowed vertical slide only. Table rotated until participant was upside down (inverted). Inverted for 5 min on 1st day, 8 min on 2nd, 10 min on 3rd and onwards through 7 days (10 days total). T2) Conventional static traction. Initial force 30 kg, gradually increased up to 45 kg with 3‐kg increments daily, according to participant's tolerance. Both T1) and T2) also received 15 min of infrared radiation, with abdominal and gluteal isometric exercises. Participants were not allowed to take NSAIDS; bed rest was required of all participants. |
|
| Outcomes | Clinical parameters examined before, immediately after and 3 months after last treatment session. Pain cluster 1 ‐ combination of: morning pain; pain throughout the day; night pain; pain with Valsalva manoeuvre; radicular pain. Pain cluster 2 ‐ combination of: straight leg raising test pain onset; finger‐to‐floor distance; deep tendon reflex, sensory impairment, and motor strength; CT investigation. Results presented as P values only. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants were lost to follow‐up (14%): 2 from each group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | There was no significant difference between groups regarding any of the baseline characteristics. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions, other than analgesics, were allowed during the study period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Harte 2007.
| Methods | RCT; predetermined randomization table, concealment of allocation through sealed, opaque and sequentially numbered envelopes. | |
| Participants | 30 participants (16 in the traction group and 14 in the manual therapy group) with acute or subacute LBP accompanied with radiculopathy. Exclusion in case of previous spinal surgery, co‐existing conditions interventions within the last 3 months. Mean age T) 45.25 years, C) 42.79 years. Duration of complaints: T) 6.5 wk, C) 6 wk. | |
| Interventions | T) Traction: manual therapy (techniques described by Maitland or Cyriax), exercises, advice and motorized lumbar traction for 4‐6 wk, 2‐3 times per wk, 10‐20 min per session, traction force 5‐60 kg. C) Comparison intervention: manual therapy, exercises and advice. |
|
| Outcomes | Assessment at discharge, 3 months and 6 months post‐treatment (all measures median (IQR), T vs. C). RMDQ: at discharge: 4 (5.8) vs. 4 (10.3), 3 months: 4.5 (10.8) vs. 1 (10.5), 6 months: 4.5 (15.3) vs. 2.5 (14). MPQ‐PRI: at discharge: 4 (15.3) vs. 12 (16.5), 3 months: 6 (16.5) vs. 6 (21), 6 months: 10 (20.5) vs. 6.5 (21). SF36 PCS: at discharge: 38.5 (16.2) vs. 41.1 (21.1), 3 months: 41.6 (18.6) vs. 43.2 (24), 6 months: 40 (15) vs. 46 (22). SF36 MCS: at discharge: 52 (26.1) vs. 48.3 (25.6), 3 months: 49.5 (25.8) vs. 47.3 (21.3), 6 months: 51.8 (23) vs. 49.8 (19.8). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined randomization table. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque and sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded to treatment group allocation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | High risk | 7 participants were lost to follow‐up (23%). |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | High risk | Published results did not include all prespecified outcomes: VAS score, improvement and straight leg raising test. |
| Group similarity at baseline (selection bias) | High risk | Baseline characteristics varied between groups: off work due to LBP, history of episodes, participation in physical activity and presence of neurological signs. |
| Influence of co‐interventions (performance bias) | Low risk | Participants were not permitted to receive any other type of manual therapy or any additional interventions during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Konrad 1992.
| Methods | RCT; participants were randomly allocated to 1 of 4 groups in each factory. Method of randomization not described. | |
| Participants | 170 participants (95 female, 75 male, mean age of 41.5 years) from 3 factories in Budapest, with non‐specific back pain localized to the lumbosacral region, with or without radiation to the thigh. Duration of pain at least 1 month, but no longer than 3 months. A pain‐free year before onset of the current episode. Exclusion criteria: participants with pregnancy, back surgery, spondylolisthesis, infections, tumours, fractures, ankylosing spondylitis, osteoporosis and structural scoliosis. 12 participants dropped out (3 from the balneotherapy group and 9 from the underwater massage group) and were analyzed separately. | |
| Interventions | T) Traction: underwater traction. Participant fixed perpendicularly in special deep pool, bar grasped under the arms and traction applied. 1st treatment ‐ participant's own weight used. Then, in addition to traction due to gravity, traction belt applied to the pelvis with 3‐kg weight on both sides. Comparison interventions: C1) Balneotherapy. Participants immersed in thermal water with minerals. C2) Underwater massage. Same water, with massage and movement while a stream of hot water (37 °C, 1 atm, 10 cm) played on the affected part. C3) Control group (no treatment). All treatments done for 15 min, 3 times per wk, for 4 wk. All participants taught how to use their back correctly. Only NSAIDs were offered to participants in the control group. | |
| Outcomes | Number of analgesics taken on admission, at 4 wk, at 1 year: T) 5.1 (2.9), 2.2 (0.9), 2.1 (1.2); C1) 4.8 (3.2), 2.3 (1.3), 1.9 (1.8); C2) 4.9 (3.4), 1.8 (0.7), 2.3 (1.7); C3) 5.1 (2.8), 3.9 (2.7), 3.7 (1.9). At 1 month, statistically significant difference in all treatment groups compared to control (P value < 0.01). No significant difference in analgesic consumption between the treatment groups. Pain intensity (100 mm VAS) on admission, at 4 wk, at 1 year: T) 56.7 (28.2), 24.6 (11.9), 45.8 (26.2); C1) 63.4 (24.1), 31.7 (16.2), 49.5 (25.7); C2) 68.4 (31.8), 33.5 (19.1), 54.7 (33.7); C3) 61.5 (32.88), 53.7 (23.8), 54.9 (24.8). At 1 month, statistically significant pain reduction in all treatment groups (P value < 0.01). No significant difference in control group At 1 year, no difference between groups. Reduction in analgesic consumption well maintained in treatment groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | The investigator assessing the outcome was not aware of the treatment given. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 12 participants were lost to follow‐up (7%). |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | Published results did not include all prespecified outcomes: spinal ROM and straight leg raising. |
| Group similarity at baseline (selection bias) | Low risk | Groups were comparable at baseline regarding age, sex and medical history. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions, other than analgesics, were allowed during the study period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Larsson 1980.
| Methods | RCT; method of randomization not described. | |
| Participants | 82 participants (51 males and 31 female, age 20‐55 years) in 6 departments of orthopaedic surgery in Sweden, with lumbago‐sciatica with or without symptoms of neurological deficit. Duration of current episode at least 2 wk and not more than 3.5 months, positive straight leg raise test. | |
| Interventions | T) Traction: auto‐traction: up to 3 treatments within 1 wk as per Lind (1974). Pelvis fixed to the foot end of bench, participant grasps bars at end and performs traction himself by pulling his arms. Participant supplied with reinforced, high, fabric corset and special pillow. Sessions < 1 hour. Participants treated as outpatients were usually taken home by ambulance. Participants confined to bed for first few days, then mobilized gradually in corset. C) Comparison intervention: corset of same type as traction group and same instructions with respect to rest. Standard analgesics (paracetamol) prescribed when required for both groups. | |
| Outcomes | Complete recoveries 1 wk, 3 wk: T) 15%, 17% C) 0%, 7%. Partial recoveries 1 wk, 3 wk: T) 27%, 32% C) 4%, 12%. Statistically significant between group differences in participant's recovery at 1 wk. At 3 wk, ns for those "completely recovered" but significant for those "completely recovered or free from pain in the leg" and "completely recovered or free from pain in the leg or the back", with traction group having better results. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is unclear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is unclear whether intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Clinical characteristics were evenly distributed between the 2 groups at baseline. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions, other than analgesics, were allowed during the treatment period. |
| Compliance with interventions (performance bias) | Low risk | Participants were hospitalized, therefore, compliance with the given treatment was high. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Letchuman 1993.
| Methods | RCT, cross‐over. Subjects randomly assigned to 1 of the 2 experimental groups, with each subject serving as his/her own control in the control group (method of randomization not described). | |
| Participants | 26 subjects (16 male, 10 female, aged 26‐65 years) referred from physicians. Participants with LBP with/without lower extremity pain and neurological signs. Cough, sneeze or deep breaths did not cause severe pain, x‐rays, MRI or CT scan of lumbar spine taken within past 6 months | |
| Interventions | Traction: T1) Static (mechanical traction), continuous traction force (after sham treatment) for a 6‐min period at magnitude of 50% bodyweight. T2) Intermittent traction, for a 6‐min period (after sham treatment), with a 10‐sec hold period at a magnitude of 50% body weight, followed by a 10‐second rest period. C) Comparison intervention: sham treatment. 6 min of 'sham traction', using only 10 lb (4.5 kg) for a 10‐sec hold, and 0 lb for a 10‐sec rest. | |
| Outcomes | Pain intensity (0‐10 VAS). Decreased pain: T1) 53.9% (7 of 13 participants), T2) 61.5% (8 of 13 participants). Increased pain: T1) 30.8% (4 of 13 participants), T2) 15.4% (2 of 13 participants). | |
| Notes | Major thrust of study was to look at myoelectric activity for static or intermittent traction. Pain measures were recorded immediately after traction. Just 1 session of traction appears to have been given. Small sample size, frequency data only reported for pain measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | No mention of attempts to blind the participants. It is unlikely that the participants were aware of group assignment. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants were lost to follow‐up (13%): 2 in each group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Groups were similar at baseline with respect to age, sex and symptoms. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were used. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Lidström 1970.
| Methods | RCT, subjects were placed by a physiotherapist in 1 of 3 groups according to a randomization procedure decided before the experiment (method of randomization not described). | |
| Participants | 62 participants (29 male, 33 female, aged 21‐61 years) selected from an orthopaedic outpatient clinic. Participants had LBP and sciatic pain radiating down 1 leg for more than 1 month' duration. 32 participants had a history of pain > 1 year. Participants strongly suspicious of the presence of a disc prolapse were not accepted. | |
| Interventions | T) Traction: intermittent pelvic traction with a Tru‐Trac traction table for 20 min with 4‐sec hold intervals and a 2‐sec rest. Traction force was correlated to the participant's weight according to the given figures. Instruction on Fowler position, strengthening exercises, regimental dispositions, every day at home. Comparison interventions: C1) Conventional treatment, hot packs for 15 min, massage and mobilizing exercises. C2) Control, hot packs for a length of time corresponding with the mean for the other methods of treatment. | |
| Outcomes | Global measure ‐ participants opinion of noticeable improvement: T) 90% (18 of 20 participants), C1) 48% (10 of 21 participants), C2) 67% (14 of 21 participants). Need for analgesics before, after the treatments (of the 30 that were taking pills before the treatment): T) 9, 0; C1) 12, 7; C2) 9, 4. Traction appears to have reduced the subjective symptoms of the participants to a higher degree than the other methods. | |
| Notes | Authors stress the need for sufficient pull and duration of traction in order to influence the mechanical conditions of the spine effectively. No apparent follow‐up after the treatment had finished (i.e. other than post‐treatment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Both the care provider and a blinded outcome assessor took part in the assessment of the outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants (6.5%) did not complete follow‐up evaluation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | All 3 groups were similar at baseline. |
| Influence of co‐interventions (performance bias) | High risk | The traction group received isometrical training in conjunction with traction. The comparison group was not treated with isometrical training. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Lind 1974.
| Methods | RCT, method of randomization not reported. | |
| Participants | 45 participants (29 male, 16 female; aged 30‐50 years, mean 34.0 years) from waiting list of orthopaedic surgery department. All had several periods of attack, mean number 3.5. Participants with serious disorders (e.g. arteriosclerosis, hypertension) excluded. All had had some previous non‐surgical therapy. Included participants with or without neurological signs. | |
| Interventions | T) Traction: auto‐traction treatment followed initially by bed rest, correction of statico‐dynamic disorders and advice on spinal hygiene. No PT or medicine. 1 participant given cotton corset. Mean number of treatments, approximately 1 hour long, over 1‐3 wk: 3.7. Comparison interventions: C1) PT, with physiotherapist choosing individual treatment, including drugs. 12 of 15 participants received Tru‐Trac traction; other treatments included isometric muscle training (n = 14), ergonomic instruction (n = 11), shortwave therapy (n = 7), heat (n = 7), cycle machine (n = 10), bath (n = 4) and manipulation (n = 1). C2) Bed rest and analgesics (Paraflex comp, 3‐6 tablets/day), sham shortwave therapy. | |
| Outcomes | Disappearance of pain in lower back/legs without coughing/sneezing: T) 100%, C1) 53%, C2) 43%. Disappearance of pain in lower back/legs on coughing sneezing: T) 100%, C1) 50%, C2) 0%. Pain, mean distance radiated (initial radiation mean; at 3 wk; mean change score): T) 60 cm, 0 cm, 100%; C1) 66 cm; 23 cm, 65%; C2) 65 cm, 28 cm, 57%. Participant's own evaluation at 3 wk (1, 2, 3, 4, 0, ‐1 where 1 = highest improvement, 4 = unchanged, ‐1 = worse) T) 11, 2, 2, 0, 0, 0; C1) 0, 0, 6, 3, 5, 1; C2) 0, 2, 7, 3, 2, 0. (T vs. C1, P value < 0.000001; T vs. C2, P value < 0.0001) Recovery: T) 87%, C1) 0%, C2) 0%. P value < 0.00001 at 3 wk. Straight leg raising (% recovered) T) 100%, C1) 0%, C2) 0% (P value < 0.001). Regression of neurological deficits: auto‐traction more effective in effecting a regression of neurological deficits. | |
| Notes | Although no final conclusions were made by the authors, we can assume it had a positive conclusion considering the P values reported. This is an underpowered study that would need replication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Transformed from old format to new format. |
| Allocation concealment (selection bias) | Unclear risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Transformed from old format to new format. |
| Selective reporting (reporting bias) | Unclear risk | Transformed from old format to new format. |
| Group similarity at baseline (selection bias) | Low risk | Transformed from old format to new format. |
| Influence of co‐interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Compliance with interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Timing of outcome assessments (detection bias) | Unclear risk | Transformed from old format to new format. |
Ljunggren 1984.
| Methods | RCT (method of randomization not described) | |
| Participants | 52 hospitalized participants with lumbago‐sciatica and prolapsed lumbar intervertebral discs, admitted to neurological department, and considered for operation. Inclusion criteria: radicular signs L5 or S1 (or both) nerve root; symptoms aggravated or unchanged in last 2‐4 wk. | |
| Interventions | T1) Auto‐traction and modified Gertrud Lind: traction force between 33% and 100% of participant's body weight; each pull for some seconds and sometimes up to 2 min. Every treatment lasted about 1 hour. T2) Manual traction and modified manual therapy. Traction force scarcely reached 300 N. Static traction given twice, each pull lasting for 5 min. |
|
| Outcomes | Immediately AT: overall assessment: no effect (number) T1) 21, T2) 15. Moderate effect (number): T1) 2, T2) 4. Good effect (number) T1) 3, T2) 4. At 2 wk: overall assessment: no effect (number) T1) 21, T2) 16. Moderate effect (number): T1) 1, T2) 4. Good effect (number) T1) 4, T2) 3. At 3 months: identical to results at 2 wk. Pain intensity (VAS) median (SD): BT: T1) 1.3 (0.3‐3.5), T2) 3.5 (0.9‐6.0). AT: T1) 0.8 (0‐1.8), T2) 1.6 (0.2‐3.0). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Participants were not informed about their participation in a randomized investigation with 2 treatment modalities. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | There is no mention of blinding of the care providers, but it is unlikely that they were. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | The outcome assessor was blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 3 participants (5.8%) were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | High risk | Groups were not similar at baseline with regards to level of herniation, duration since first symptoms of sciatica and pain intensity in the lower back. |
| Influence of co‐interventions (performance bias) | Low risk | Participants were deprived of long‐term working analgesics later than hours prior to the traction session. |
| Compliance with interventions (performance bias) | Low risk | All participants were hospitalized, therefore, the compliance with the given treatment was high. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Ljunggren 1992.
| Methods | RCT (method of randomization not described) | |
| Participants | 50 participants (27 males, 23 females, aged 16‐62 years) admitted to the department of neurology were included. Inclusion criteria: radiating pain, neurological symptoms and signs confirmed by a myelogram. Participants with previous spinal surgery, spondylolisthesis and root entrapment were excluded. The males had a mean duration of symptoms for 4.8 months, and the females for 5.3 months. | |
| Interventions | T) Traction: continuous manual (static) traction. The therapist exerted traction by gently leaning backwards against a belt placed around the back or hips, and attached below the knees of the participant. The traction force reached approximately 300 N. Repeated relief of pain was guiding factor; once per day for 10 min (in a few cases twice per day for 5 min). C) Comparison intervention: isometric exercises for the abdominal, back, hip and thigh muscles. Education about importance of these muscles was given. Contractions 6‐8 sec, repeated 5‐10 times, daily session approximately 20 min. Following treatment, all participants were instructed to lie in the most comfortable positions for 2 hours. Treatment for all participants lasted 5‐7 days. | |
| Outcomes | Pain alleviation (1‐10 VAS): pain‐free or improved: T) 10 of 24 participants (41.6%), C) 10 of 26 participants (38.5%). Pain unchanged or worse: T) 14 of 24 participants (58.3%), C) 16 of 26 participants (61.5%). No significant difference between the 2 treatment groups found. 4 participants of each group deteriorated temporarily in connection with the treatment given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is not clear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is not clear whether an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | High risk | Published results did not include all prespecified outcomes: straight leg raising, mobility and ADL. |
| Group similarity at baseline (selection bias) | Low risk | Groups were similar at baseline with respect to age, sex, habits of physical therapy and symptoms. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were used, except for analgesics. |
| Compliance with interventions (performance bias) | Low risk | All participants were hospitalized. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Mathews 1975.
| Methods | RCT, participants were allocated at random to either control or treatment groups (method of randomization not described). | |
| Participants | 27 participants (9 female and 18 male, aged 20‐60 years). Participants had sciatica or cruralgia of at least 3 wk' duration with or without back pain. Back movement was required to be limited in at least 1 direction and either the sciatic or femoral nerve stretch test positive. All had root pain. Exclusion criteria: a recently acquired neurological deficit, psychological disturbance, were pregnant, a radiological evidence of sacro‐iliitis or osteoporosis, previous traction. | |
| Interventions | T) Traction: traction on a plain couch using a force of at least 36.3 kg applied through a pelvic harness, the trunk being restrained by a thoracic harness; 30 min per day, 5 days per wk, 3 wk. C) Comparison intervention: sham traction; same routine as above except the traction did not exceed 9.1 kg. | |
| Outcomes | Mean improvement in pain (VAS): T) 28.8%, C)18.9%. Not statistically significant. | |
| Notes | Control group was low force traction. Small sample. Authors cited an improvement but it was not statistically significant. Questioned whether larger trial would have shown significance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Participants were blinded. A sham condition was used. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | No mention of attempts to blind the outcome assessors. It is unlikely that the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is not clear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is not clear whether an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | High risk | Groups were not similar at baseline with regards to age and heavy work. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were used. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Mathews 1988.
| Methods | RCT, participants were allocated to treatment or control by the study methodologist, using a predetermined randomization system. | |
| Participants | 143 participants (63 females, 80 males, aged 20‐60 years), referred from a rheumatology clinic or general practitioner were included. Participants had low backache or pain, local tenderness, asymmetrical restriction of movement, limited straight leg raise and root pain with in the past 3 months. | |
| Interventions | T) Traction: continuous auto‐traction at level required to relieve pain (usually approximately 45 kg), for 30 min, 5 days per wk, until pain was relieved, but for a maximum of 3 wk. C) Comparison intervention: 3 times per wk infrared heat treatment to the low back area at 60 cm for 15 min. | |
| Outcomes | Participant's assessment of pain (6‐point scale). Number recovered (10‐18 days, 1 year): T) 40/77 (52%), 30/83 (36%); C) 27/54 (50%), 11/60 (18%). The 10‐18 day and 1 year outcomes are based on different numbers of participants in each group. On 8th day, more than twice the number treated people as controls were recovered (statistically significant) | |
| Notes | Data inconsistent between text and graph. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is not clear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is not clear whether an intention‐to‐treat analysis was used or not. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Unclear risk | No description of baseline characteristics given. No baseline table was added to the article. |
| Influence of co‐interventions (performance bias) | Unclear risk | It is not clear whether co‐interventions were part of treatment protocol or whether co‐interventions were allowed besides the treatment that was part of the protocol. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Ozturk 2006.
| Methods | RCT; method unknown. | |
| Participants | 46 participants (24 in the traction group, 22 in the control group) hospitalized with the diagnosis of lumbar disc herniation. Participants had LBP or sciatica, pain duration < 6 months and lumbar disc herniation verified by CT scan. People with LBP due to neoplastic, inflammatory, infectious or metabolic causes were excluded. Mean age: T) 40.2 years, C) 52.7 years. | |
| Interventions | T) Traction: physiotherapy programme, including hot pack, ultrasound and diadynamic current, and traction: continuous lumbar traction with Enraf Nonius Traction Eltrac 439. In total, 15 sessions, 5 sessions per wk, 15 min per session, traction force 255‐0% of body weight. C) Comparison intervention: physiotherapy programme without traction. |
|
| Outcomes | Assessment before and immediately AT. VAS for pain (mean (SD)) AT: T) 2.4 (1.7), C) 3.6 (2.7). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is not clear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | It is not clear whether an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Unclear risk | No mention of group characteristics at baseline. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were used during the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Unclear risk | Unclear at what time outcome assessments (for all intervention groups) were measured. |
Pal 1986.
| Methods | RCT, participants were randomly allocated to groups A and B (method of randomization not described). | |
| Participants | 39 participants (23 male (mean age 38 years) and 16 female (mean age 39 years) were admitted to hospital for back pain and sciatica. Mean duration of pain: T) 42 days, C) 56 days. Neurological deficits at baseline: T) 50% of participants, 73% of participants. | |
| Interventions | T) Traction: continuous mechanical traction of 5.5‐8.2 kg according to body weight, 2‐6 wk (n = 25). C) Comparison intervention: sham traction (continuous mechanical) of 1.4‐1.8 kg, 2‐6 wk (n = 14). Both methods were applied with the participant supine on a tilted bed by means of a pelvic harness pulled by metal weights over a pulley. | |
| Outcomes | Pain score (0‐100 VAS) baseline, 1 wk, 2 wk, 3 wk: T) 50, 25, 6, 5; C) 50, 15, 9, 3. No significant differences between groups. Number of participants returned to work, < 3 months, 3‐6 months, > 6 months: T) 7, 6, 5; C) 3, 4, 2. | |
| Notes | Used median scores. Timing or RTW measures not clear. Conclusion is that all recovered, may be due to enforces immobilization. Suggest that "minimal wt traction at home as compliment to complete bed rest may have important place". Data inconsistent between text and graph. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | The participants were not aware of the amount of traction and, therefore, were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | Low risk | The ward sister was responsible for allocation. All other care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | The outcome assessors were not aware of the amount of traction and, therefore, were blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 2 participants (4.9%) did not complete the trial: 1 participant in each group withdrew after a few days because of home circumstances. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | High risk | Groups were not similar at baseline. 24 participants were allocated to T and 15 participants were allocated to C. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were used. |
| Compliance with interventions (performance bias) | Low risk | Treatment was well tolerated by both groups. Participants were hospitalized. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Reust 1988.
| Methods | RCT, participants were randomized to 1 of 3 groups by a table of randomization. | |
| Participants | 60 participants (35 male, 25 female, mean age 50.8 years) hospitalized for back pain, with or without neurological deficits, were included. Exclusion criteria: previous traction, fast progressing neurological deficit, behavioural problems, or bone aliments that may have caused the back pain. Duration of back pain unknown. | |
| Interventions | Traction: T1) Continuous mechanical traction on an Eltrac 439. 5‐kg force on day 1, 10 kg on day 2, 15 kg on day 3, increasing 5 kg each day up to a maximum of 50 kg. 10 min per day, 12 sessions, 12 days. Participants also received medication, 20 min lumbar 'parafango' per day, 20 min massage per day and strict bed rest. T2) Same as above, except traction force of up to maximum of 15 kg. C) Comparison intervention: same as above, except traction force to maximum of 5 kg. | |
| Outcomes | Pain (100‐mm VAS): T1) 33.61 (29.55), T2) 30.68 (26.83), C) 30.25 (26.23). No significant difference between groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Transformed from old format to new format. |
| Allocation concealment (selection bias) | Unclear risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Transformed from old format to new format. |
| Selective reporting (reporting bias) | Unclear risk | Transformed from old format to new format. |
| Group similarity at baseline (selection bias) | High risk | Transformed from old format to new format. |
| Influence of co‐interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Compliance with interventions (performance bias) | High risk | Transformed from old format to new format. |
| Timing of outcome assessments (detection bias) | Unclear risk | Transformed from old format to new format. |
Schimmel 2009.
| Methods | RCT; computer‐generated random block lists and adequate allocation procedure. | |
| Participants | 60 participants randomly allocated to 2 treatment groups (31 to the traction group, 29 to the sham group). All participants had LBP for > 3 months. Exclusion criteria were previous surgical treatment and radicular leg pain. Mean age: T) 42 years, C) 46 years. | |
| Interventions | T) Traction: intervertebral differential dynamics therapy: 20 sessions during 6 wk, 25‐30 min per session, traction force 50% of body weight. After 2 wk a standard graded activity programme was added to the traction sessions, which consisted of 1‐hour training for 2 days per wk during a total of 12 wk. C) Comparison intervention: same as traction group, except for traction force of < 10% of body weight. |
|
| Outcomes | Assessment at 2, 6 and 14 wk. VAS LBP (mean change (SD)) at 14 wk: 32 (26.8) in the intervertebral differential dynamics group vs. 36 (27.1) in the sham group. Significant improvement during the treatment period in both intervertebral differential dynamics and sham group for the ODI, SF‐36 and VAS leg pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization through computer‐generated random block lists. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | The participant was not informed about the intervention received until after the 14 wk' follow‐up. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | The care provider was not blinded for the assigned treatment. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Follow‐up evaluation was carried out by an independent assessor, who was blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants were lost to follow‐up (7%): 1 from the T group, 3 from the C group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | High risk | Published results did not include all prespecified outcomes: outcome assessments at 2 and 6 wk were not included or could not be extracted from the graphs. |
| Group similarity at baseline (selection bias) | Low risk | No significant between‐group differences at baseline. |
| Influence of co‐interventions (performance bias) | Unclear risk | It is not clear whether co‐interventions were allowed during the treatment period or whether co‐interventions were part of treatment protocol. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Sherry 2001.
| Methods | RCT; participants randomized in sequential order and treatments determined by predefined central randomization list. | |
| Participants | 44 participants recruited through advertisements in local newspapers. Inclusion criteria: pain of > 3 months' duration, associated leg pain and confirmed disc protrusion or herniation on CT scan or MRI. (T) 11 male, 11 female; C) 12 male, 10 female; age (mean/range) T) 41/27‐57, C) 43/27‐55; chronicity (mean/range years) T) 8.4/0.25‐30, C) 6.2/0.5‐28. | |
| Interventions | T) Traction: VAX‐D: participant grasps handgrips with arms extended above head; pelvic harness connected to tensionometer, which provides feedback to programmed logic control and operating system; tension applied from baseline tension to therapeutic range of 50‐95 lbs, with sessions 30 min long, comprising 15 cycles of decompression and relaxation. 5 sessions/wk over 4 wk, then once/week for 4 wk. C) Comparison intervention: transcutaneous electrical nerve stimulation treatment 30 min per day for 20 days, then once per wk for 4 wk. | |
| Outcomes | Post‐treatment (8 wk): pain (10‐cm VAS: pre/post): T) 5.99/1.85, C) 5.44/5.97. Disability (4‐point self rating scale where 1 = cannot to, 4 = can do without limitation) (pre/post): T) 2.2/2.9, C) 2.2/2.2. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 2 participants (4.5%) did not complete the study: 1 participant from each group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Groups were similar at baseline. |
| Influence of co‐interventions (performance bias) | Low risk | Neither group received any physiotherapy modalities, epidural steroid injections or other treatments during the trial. Both groups were allowed to take non‐narcotic analgesics and anti‐inflammatory medication if necessary. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. |
Simmerman 2011.
| Methods | RCT; flip of a coin followed by an inadequate allocation procedure. | |
| Participants | 61 participants randomly allocated to 2 treatment groups (31 in the land‐based supine flexion first group, 30 to the aquatic vertical traction first group). All participants had LBP and sciatica. Participants with neurological disorders or vertebral fractures were excluded. Mean age: T) 59.9 years, C) 59.3 years. Mean duration of pain complaints: T) 1.7 years, C) 8.9 years. | |
| Interventions | T) Traction: 1 session of aquatic vertical traction for 15 min with the use of 2 x 2‐3 kg ankle weights, followed by 1 session of land‐based supine flexion. C) Comparison intervention: flexion group; 1 session of land‐based supine flexion, followed by 1 session of aquatic vertical traction. |
|
| Outcomes | Assessment at 2‐7 days following treatment. Decrease in pain (mean (SD)) on a numerical rating scale (0‐10 cm) after the first intervention: T) 2.7 (2.1), C) 1.7 (1.7). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Flip of a coin. |
| Allocation concealment (selection bias) | Unclear risk | Flip of a coin for the first subject, followed by assignment of all uneven‐numbered subjects to the land‐based supine flexion position as their first intervention and all even‐numbered subjects to the aquatic vertical traction position. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | No participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | There were no statistical differences between groups in terms of age, sex, body mass index, clinical signs and symptoms. |
| Influence of co‐interventions (performance bias) | Unclear risk | It is not clear whether co‐interventions were allowed during the treatment period or whether they were part of the treatment protocol. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Sweetman 1993.
| Methods | RCT, randomization was organized by placing the sequentially numbered treatment folders in a random order according to Documenta Geigy random number tables. | |
| Participants | 400 participants (200 males and 200 females, aged 14‐78 years) referred from general practice. Inclusion criteria: LBP of sufficient severity to warrant PT, pain for > 1 wk. Exclusion criteria: serious causes for back pain including fractures, infection and malignancy, pregnancy, inflammatory arthritis, bone diseases, where physician suspected that treatments may precipitate or exacerbate spinal cord or nerve root compromise, when other therapy was specifically indicated, recent steroid injections, intercurrent treatment other than routine oral medication. | |
| Interventions | T) Traction: continuous mechanical traction, constant pull (10 min), 1st wk 33% body weight, 2nd wk 50% body weight, 3 times per wk. Comparison interventions: C1) Shortwave diathermy: 20 min, 3 times per wk, 2 wk. C2) Sham shortwave diathermy: once participant felt heat, output was turned down to minimum, 20 min, 3 times per wk, 2 wk. C3) Extension exercises: hump and hollow, alternate leg raise, alternate arm raise, opposite leg and arm raise (prone kneeling). Bridging (crouch lying), alternate leg raise, clasp hands behind head and shoulder, and both leg raise, head and shoulder raise (prone lying), 3 times per wk, 2 wk. | |
| Outcomes | Participant opinion of overall effect (better) at 2 wk: T) 49, C1) 39, C2) 37, C3) 45. Not statistically significant. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 51 participants (12.8%) failed to attend for follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Groups were similar at baseline. |
| Influence of co‐interventions (performance bias) | Unclear risk | It is not clear whether co‐interventions were allowed during the treatment period or whether they were part of the treatment protocol. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Tesio 1993.
| Methods | RCT, participants allocated at random (method of randomization not described). | |
| Participants | 44 participants (25 males, 19 females, aged 23‐63 years), referred from an outpatient service of a rehabilitation unit in a large teaching hospital. Inclusion criteria: LBP with or without radiation, duration > 1 month, herniation or protrusion, failure of 1 or more conservative approaches. Exclusion criteria: neoplastic, inflammatory or metabolic causes of back pain, or indication for urgent surgery. | |
| Interventions | Traction: T1) Intermittent auto‐traction, participant provides traction force by pulling vigorously on the bar at the head of the table for a period of 3‐6 sec, 1 min rest, 30‐60 min session, every 2nd or 3rd day, total 3‐10 sessions. If the participant reported benefit, the treatment was continued for 3‐6 more sessions until no further improvement. T2) Passive traction. Traction force was adjusted approximately every 10 min, 35% of body weight, 45 min, daily bases for 5‐10 sessions. | |
| Outcomes | Immediate outcomes (improved): T1) 17 of 22 participants, T2) 4 of 22 participants (statistically significant). Cross‐over: non‐responders to either treatment were crossed over to the other modality after a delay of 4‐5 days. |
|
| Notes | Most results given for only auto‐traction responses (they openly favoured the treatment of the researchers). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | No mention of attempts to blind the participants. It is likely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Unclear risk | No mention of attempts to blind the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | It is not clear how many participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | No significant differences were found between groups with respect to sex, age, pain duration and score, presence of positive straight leg raise test or neural deficits, presence of more than 1 disc affected, presence of spinal stenosis, history of previous episodes and possible psychological bias. |
| Influence of co‐interventions (performance bias) | High risk | Co‐interventions were allowed. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | High risk | All important outcome assessments for all intervention groups were not measured at the same time. The auto‐traction group was evaluated after 3 sessions, whereas the passive traction group was assessed after 5 treatment sessions. |
Unlu 2008.
| Methods | RCT; method of randomization unclear. | |
| Participants | 60 participants (20 in the traction group, 20 in the ultrasound group and 20 in the low power laser group) with acute LBP and leg pain that was definitely being caused by lumbar disc herniation. All participants had complaints of sciatica. Mean age: T) 42.5 years, C1) 48.2 years, C2) 42.8 years. Symptom duration: T) 47.9 days, C1) 36.8 days, C2) 49 days. | |
| Interventions | T) Traction: standard motorized traction therapy system (Tru‐Trac 401) for 15 min per session, traction force 35‐50% of total body weight. Comparison interventions: C1) Ultrasound treatment, using 1 MHz at an intensity of 1.5 W/cm2, at the right and left sides of the lumbar region. The ultrasound head was moved using small, continuous, circular movements for 8 min. C2) Laser: a Gal‐Al‐As diode laser device (Endolaser 476) at power input of 50 mV and wavelength of 830 nm. Diameter of the laser beam was 1 mm. Stimulation time of 4 min at each point (both sides of the herniated disc). |
|
| Outcomes | Assessment BT, AT and at 1 and 3 months. VAS for LBP (mean (SD)): T) BT 58.2 (18.1), AT 29.5 (16.4), 1 month 25.5 (13.3), 3 months 31.3 (16.4); C1) BT 51.7 (18.7), AT 29.7 (17.9), 1 month 27.2 (18.6), 3 months 26.9 (15.2); C2) BT 54.0 (17.0), AT 34.4 (18.9), 1 month 30.7 (19.1), 3 months 30.0 (16.9). VAS for radicular pain (mean (SD)): T) BT 59.6 (15.4), AT 27.7 (15.4), 1 month 21.8 (15.4), 3 months 29.5 (16.7); C1) BT 56.0 (15.3), AT 29.1 (14.4), 1 month 26.8 (18.6), 3 months 25.2 (13.9); C2) BT 53.1 (25.9), AT 32.9 (23.6), 1 month 25.6 (21.1), 3 months 23.6 (17.7). RMDQ (mean (SD)): T) BT 14.2 (4.3), AT 9.8 (3.9), 1 month 8.5 (3.5), 3 month 8.9 (4.0); C1) BT 13.4 (4.5), AT 9.3 (5.7), 1 month 8.2 (6.0), 3 month 8.6 (6.0); C2) BT 12.5 (5.0), AT 9.9 (4.1), 1 month 7.3 (4.3), 3 months 6.7 (4.5). MODQ (mean (SD)): T) BT 19.3 (5.3), AT 14.6 (4.7), 1 month 13.5 (5.0), 3 months 14.9 (4.9); C1) BT 19.6 (6.4), AT 14.4 (5.0), 1 month 14.3 (5.5), 3 months 14.4 (5.9); C2) BT 18.4 (7.1), AT 14.7 (6.0), 1 months 13.5 (5.9), 3 months 13.6 (6.2). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | No mention of attempts to blind the participants. It is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessor was blinded to treatment allocation during the assessments. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | No loss to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | No statistically significant differences between groups. |
| Influence of co‐interventions (performance bias) | Low risk | Co‐interventions were not allowed during the treatment period. After the treatment period, participants were asked to restrict further treatment as much as possible. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Van der Heijden 1995.
| Methods | RCT (using sealed envelopes allocated from a list of random numbers) | |
| Participants | 25 participants (13 men, 12 women) recruited from hospital setting. Mean (SD) age: T) 46(8); C) 47(8). At baseline: mean duration: T) 18% < 6 months, 82% > 24 months; C) 17% < 6 months, 83% > 24 months. Severity: mean (SD) on pain VAS: T) 47 (27), C) 37 (23). Radiation: T) 73%, C) 58%. | |
| Interventions | T) Continuous traction: force slowly increased from 30% of body weight until participant indicated a distinct but tolerable pulling; maximum force 30‐50% of body weight. C) Comparison intervention: force slowly increased from zero until participant indicated a little pulling. Maximal force 25% of body weight. For both groups: 10‐12 sessions during 4 consecutive wk; also received leaflet about LBP and ADL. | |
| Outcomes | VAS at 5 wk (median improvement): T) 14, C) 16. Difference (95% CI): 2 (‐29 to 14). VAS at 9 wk (median improvement): T) 14, C) 4. Difference (95% CI): ‐10 (‐31 to 17). Global improvement/recovery at 5 wk (% recovered): T) 54, C) 34. Difference (95% CI): 20% (‐18% to 58%). Global improvement/recovery at 9 wk (% recovered): T) 38, C) 25. Difference (95% CI): 13% (‐25% to 51%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation with sealed envelopes with a code for either treatment group. Envelopes were prepared by an independent person. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Participants were blinded to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 4 participants (16%) were lost to follow‐up: 3 from the traction group and 1 from the comparison group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | Both groups were comparable with respect to age, sex and back pain history. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were allowed for the duration of the treatment period. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Walker 1982.
| Methods | RCT, methods of randomization judged adequate. | |
| Participants | 29 participants (18 male, 11 female, mean age: T) 37.8 years, C) 37.3) chosen by a specialist in neurology at the department of neurology in a hospital in Oslo, Norway. Non‐specific LBP and radiating pain, of mixed duration (18 subjects with pain > 12 wk; 11 with < 12 wk). | |
| Interventions | T) Traction: Spina‐Trac according to Myrin; 20 min daily with 2 hours rest afterwards, for 4‐8 days. 40‐70 kiloponds force. Other: "traditional regimen for sciatica: 1 wk of strict bed‐rest, back school, unspecified analgesics when needed (but never in morning BT sessions). C) Comparison intervention: sham traction. Same as (T) except that forces greater than 10 kiloponds not possible. | |
| Outcomes | Pain (number improved, unchanged or worse). T) 4, 13. C) 2, 10 (not statistically significant). Lasègue (number improved, unchanged or worse). T) 7, 10. C) 2, 10 (not statistically significant). Mobility (number improved, unchanged or worse). T) 4, 13. C) 2, 10 (not statistically significant). | |
| Notes | Underpowered study with invalid pain outcome measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Transformed from old format to new format. |
| Allocation concealment (selection bias) | Unclear risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Transformed from old format to new format. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Unclear risk | Transformed from old format to new format. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Unclear risk | Transformed from old format to new format. |
| Selective reporting (reporting bias) | Unclear risk | Transformed from old format to new format. |
| Group similarity at baseline (selection bias) | High risk | Transformed from old format to new format. |
| Influence of co‐interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Compliance with interventions (performance bias) | Low risk | Transformed from old format to new format. |
| Timing of outcome assessments (detection bias) | Unclear risk | Transformed from old format to new format. |
Weber 1973.
| Methods | No randomization methods mentioned. | |
| Participants | 72 participants (42 men, 32 women, 85% aged 30‐60 years), admitted to neurology department. All had radiating pains and neurological signs corresponding to a lesion in the L5 or S1 root (or both), positive radiculogram. Exclusion criteria: people with bladder paresis, strong persistent pains, acutely occurring pareses or considerable constraint of the spinal column (or both). Duration unknown. | |
| Interventions | T) Traction: intermittent mechanical traction, 33% of body weight, Tru‐Trac motor, 5‐sec pauses, 20 min once per day for 5‐7 days. C) Comparison intervention: sham traction with a force of up to 7 kg, 20 min once per day for 5‐7 days. | |
| Outcomes | Back pain (improved): T) 14 of 37 participants, C) 15 of 35 participants. Leg pain (improved): T) 19 of 37, C) 16 of 35. No difference between the groups. | |
| Notes | Did not test for statistical significance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Participants were not informed as to the amount of traction applied, therefore, they were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers. It is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | The investigator was not informed as to which participant belonged to which group, therefore, the outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 14 participants were lost to follow‐up: 6 in the traction group and 8 in the comparison group. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Unclear risk | No description of baseline characteristics, no baseline table included. |
| Influence of co‐interventions (performance bias) | Low risk | No co‐interventions were allowed/administered during the treatment period. |
| Compliance with interventions (performance bias) | Low risk | Participants were hospitalized during the course of treatment. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Weber 1984.
| Methods | RCT, allocation to the treatment groups was done by randomization (method of randomization not described). | |
| Participants | 94 participants (54 males, 40 females). All had sciatica, radiating pain, neurological symptoms and signs corresponding to a lesion of the L5 or S1 root and positive radiculogram. Exclusion criteria: spondylolisthesis or previous operations of the spine, root entrapment caused mainly by hypertrophic facet joints or a narrow bony canal in the last 3 studies. Duration unknown. | |
| Interventions | Traction:
T1) Spina‐Trac, intermittent manual traction, force 40‐70 Kp for 10‐12 sec followed by rest. 20 min once per day.
T2) Continuous manual traction, therapist exerted traction by gently leaning back against a belt placed below the knees of participant, force < 30 Kp.
Comparison intervention:
C1) Simulated traction (for comparison against Spina‐Trac).
C2) Isometric exercises (for comparison against continuous manual traction). Duration of treatment unknown. |
|
| Outcomes | Improved (overall assessment): T1) 5 of 21 participants, T2) 10 of 24 participants, C1) 5 of 23 participants, C2) 10 of 26 participants. No significant difference between T1 and C1. No significant difference between T2 and C2. Temporary, immediate relief of pain obtained in the manual traction group, but not in the exercise group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | Low risk | Study 1: participants were blinded for treatment allocation. Study 2: no mention of attempts to blind the participants, but it is unlikely that the participants were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | No mention of attempts to blind the care providers, but it is unlikely that the care providers were blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | Low risk | Study 1: without knowledge of the method used, a neurologist recorded the results. Study 2: without knowledge of the method used, a physiotherapist recorded the results. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | Study 1: 4 participants (9.1%) were lost to follow‐up: 6 from the treatment group and 8 from the control group. Study 2: 1 participant (2%) was lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | High risk | No intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Unclear risk | No description of baseline characteristics, no baseline table provided. |
| Influence of co‐interventions (performance bias) | Low risk | Except for analgesics, no co‐interventions were allowed during the treatment period. |
| Compliance with interventions (performance bias) | Low risk | Participants were hospitalized. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
Werners 1999.
| Methods | RCT, randomization was done by the orthopaedic practitioner using a minimization computer program. | |
| Participants | 147 participants (79 males, 68 females, mean age 38.75 years). Entry criterion was LBP severe enough to warrant seeking the help of an orthopaedic general practitioner. Participants with sciatica not excluded. No participant had objective neurology Exclusion: age < 20, > 60 years, previous surgery, significant medical condition and spinal disorder demonstrable on plain x‐ray. | |
| Interventions | T) Traction: motorized, intermittent lumbar traction, with simultaneous massage applied by 2 motorized, mechanical wheels moving up and down the spine while the participant is lying on their back, 10‐20 kg, 6 sessions, 2‐3 wk. C) Comparison intervention: interferential therapy, standard Galva electrotherapy system, 6 sessions, 2‐3 wk. | |
| Outcomes | ODI 1st, 2nd, 3rd visit: T) 29.5 (14.8), 24.5 (15.0), 21.7 (14.7); C) 29.7 (15.1), 25.4 (14.0), 21.1 (14.6). Pain (VAS 1‐100) 1st, 2nd, 3rd visit: T) 50.6 (15.1), 44.3 (14.7), 39.2 (13.5); C) 49.7 (13.3), 45.5 (13.7), 42.0 (12.8). No differences between groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization computer program with stratification. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes ‐ participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ providers | High risk | Care providers were not blinded. |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ loss to follow‐up | Low risk | 24 participants (16%) were lost to follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes ‐ intention to treat analysis | Low risk | Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | Published results included all prespecified outcomes. |
| Group similarity at baseline (selection bias) | Low risk | The demographics of the participants entering were similar for both groups with respect to age, sex, type of work, sick leave, weight, height and previous treatment for back pain. |
| Influence of co‐interventions (performance bias) | Unclear risk | It is not clear whether co‐interventions were allowed during the treatment period or whether they were part of the treatment protocol. |
| Compliance with interventions (performance bias) | Unclear risk | Not mentioned. |
| Timing of outcome assessments (detection bias) | Low risk | All important outcome assessments for all intervention groups were measured at the same time. |
ADL: activities of daily living; AT: after treatment; BT: before treatment: C: comparison; CI: confidence interval; CT: computed tomography; FABQ: Fear‐Avoidance Beliefs Questionnaire; IQR: interquartile range; LBP: low‐back pain; MD: mean difference; in: minute; MODQ: Modified Oswestry Disability Questionnaire; MPQ‐PRI: McGill Pain Questionnaire ‐ Pain Rating Index; MRI: magnetic resonance imaging; ns: not significant; NSAID: non‐steroidal anti‐inflammatory drug; ODI: Oswestry Disability Index; PT: physiotherapy; RCT: randomized controlled trial; RMDQ: Roland Morris Disability Questionnaire; ROM: range of motion; RTW: return to work; SD: standard deviation; SE: standard error; sec: second; SF36 MCS: Short Form‐36 Mental Component Summary; SF36 PCS: Short Form‐36 Physical Component Summary; T: traction; VAS: visual analogue scale; wk: week.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cevik 2007 | Study was not randomized. |
| Gose 1998 | Study was not an RCT. |
| Hansen 1993 | Used low‐force traction as a sham treatment and included regular traction as 1 component of a physiotherapy programme. |
| Moret 1998 | Article described a feasibility study, not a full trial. |
| Olah 2008 | Study was not randomized. |
| Ramos 1994 | Study is not an RCT; outcome is intradiscal pressure. |
| Van der Heijden 1991 | Pilot study only, in preparation for Van der Heijden 1995. |
RCT: randomized controlled trial.
Contributions of authors
All authors were involved in writing the protocol and the final manuscript. I Wegner, IS Widyahening and GJMG van der Heijden were involved in the quality assessment, data extraction processes and the data analysis.
Sources of support
Internal sources
Institute for Work & Health, Canada.
EMGO+ Institute for Health and Care Research, VU University Medical Centre, Netherlands.
Department of Public Health and Caring Sciences, Family Medicine, Uppsala Science Park, Sweden.
Northwestern Health Sciences University, Wolff‐Harris Center for Clinical Studies, USA.
Julius Centre for Health Sciences and Primary Care, University Medical Center Utrecht, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Two review authors (GJMG van der Heijden, HCW de Vet) were also authors of two included studies. They did not participate in the quality assessment or data extraction processes in these studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beurskens 1997 {published data only}
- Beurskens AJ, Heijden GJ, Vet HCW, Koke AJ, Lindeman E, Regtop W, et al. The efficacy of traction for lumbar back pain: design of a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 1995;18:141‐7. [PubMed] [Google Scholar]
- Beurskens AJ, Vet HC, Köke AJ, Lindeman E, Regtop W, Heijden GJ, et al. Efficacy of traction for non‐specific low back pain: a randomised clinical trial. Lancet 1995;346:1596‐600. [DOI] [PubMed] [Google Scholar]
- Beurskens AJ, Vet HC, Köke PT, Regtop W, Heijden GJ, Lindeman E, et al. Efficacy of traction for nonspecific low back pain: 12‐week and 6‐month results of a randomized clinical trial. Spine 1997;22(23):2756‐62. [DOI] [PubMed] [Google Scholar]
Bihaug 1978 {published data only}
- Bihaug O. [Autotraksjon for ischialgpasienter: en kontollert sammenlikning mellom effekten av Auto‐traksjon‐B og isometriske ovelser ad modum Hume endall og enkins]. Fysioterapeuten 1978;45:377‐9. [Google Scholar]
Borman 2003 {published data only}
- Borman P, Keskin D, Bodur H. The efficacy of lumbar traction in the management of patients with low back pain. Rheumatology International 2003;23:82‐6. [DOI] [PubMed] [Google Scholar]
Coxhead 1981 {published data only}
- Coxhead CE, Inskip H, Meade TW, North WR, Troup JD. Multicentre trial of physiotherapy in the management of sciatic symptoms. Lancet 1981;1(8229):1065‐8. [DOI] [PubMed] [Google Scholar]
Fritz 2007 {published data only}
- Fritz JM, Lindsay W, Matheson JW, Brennan GP, Hunter SJ, Moffit SD, et al. Is there a subgroup of patients with low back pain likely to benefit from mechanical traction? Results of a randomized clinical trial and subgrouping analysis. Spine 2007;32(26):E793‐800. [DOI] [PubMed] [Google Scholar]
Gudavalli 2006 {published data only}
- Cambron JA, Gudavalli MR, McGregor M, Jedlicka J, Keenum M, Ghanayem AJ, et al. Amount of health care and self‐care following a randomized clinical trial comparing flexion‐distraction with exercise program for chronic low back pain. Chiropractic & Osteopathy 2006;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudavalli MR, Cambron JA, McGregor M, Jedlicka J, Keenum M, Ghanayem AJ, et al. A randomized clinical trial and subgroup analysis to compare flexion‐distraction with active exercise for chronic low back pain. European Spine Journal 2006;15:1070‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Güvenol 2000 {published data only}
- Güvenol K, Tüzün Ç, Peker O, Goktay Y. A comparison of inverted spinal traction and conventional traction in the treatment of lumbar disc herniations. Physiotherapy Theory and Practice 2000;16:151‐160. [Google Scholar]
Harte 2007 {published data only}
- Harte AA, Baxter GD, Gracey JH. The effectiveness of motorised lumbar traction in the management of LBP with lumbo sacral nerve root involvement: a feasibility study. BMC Musculoskeletal Disorders 2007;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konrad 1992 {published data only}
- Konrad K, Tatrai T, Hunka A, Vereckei E, Korondi I. Controlled trial of balneotherapy in treatment of low back pain. Annals of the Rheumatic Diseases 1992;51:820‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 1980 {published data only}
- Larsson U, Choler U, Lidström A, Lind G, Nachemson A, Nilsson B, et al. Auto‐traction for treatment of lumbago‐sciatica: a multicentre controlled investigation. Acta Orthopaedica Scandinavica 1980;51:791‐8. [DOI] [PubMed] [Google Scholar]
Letchuman 1993 {published data only}
- Letchuman R, Deusinger RH. Comparison of sacrospinalis myoelectric activity and pain levels in patients undergoing static and intermittent lumbar traction. Spine 1993;18(10):1361‐5. [DOI] [PubMed] [Google Scholar]
Lidström 1970 {published data only}
- Lidström A, Zachrisson M. Physical therapy on low back pain and sciatica. Scandinavian Journal of Rehabilitation Medicine 1970;2:37‐42. [PubMed] [Google Scholar]
Lind 1974 {published and unpublished data}
- Lind GAM. Auto‐traction, Treatment of Low Back Pain and Sciatica. An Electromyographic, Radiographic and Clinical Study [thesis]. Linköping: University of Linköping, 1974. [Google Scholar]
Ljunggren 1984 {published data only}
- Ljunggren AE, Weber H, Larsen S. Autotraction versus manual traction in patients with prolapsed lumber intervertebral discs. Scandinavian Journal of Rehabilitation Medicine 1984;16:117‐24. [PubMed] [Google Scholar]
Ljunggren 1992 {published data only}
- Ljunggren AE, Walker L, Weber H, Amundsen T. Manual traction versus isometric exercises in patients with herniated intervertebral lumbar discs. Physiotherapy Theory and Practice 1992;8:207‐13. [Google Scholar]
Mathews 1975 {published data only}
- Mathews JA, Hickling J. Lumbar traction: a double‐blind controlled study for sciatica. Rheumatology and Rehabilitation 1975;14(4):222‐5. [DOI] [PubMed] [Google Scholar]
Mathews 1988 {published data only}
- Mathews JA, Mills SB, Jenkins VM, Grimes SM, Morkel MJ, Mathews W, et al. Back pain and sciatica: controlled trials of manipulation, traction, sclerosant and epidural injections. British Journal of Rheumatology 1987;26(6):416‐23. [DOI] [PubMed] [Google Scholar]
- Mathews W, Morkel M, Mathews J. Manipulation and traction for lumbago and sciatica: physiotherapeutic techniques used in two controlled trials. Physiotherapy Practice 1988;4:201‐6. [Google Scholar]
Ozturk 2006 {published data only}
- Ozturk B, Gunduz OH, Ozoran K, Bostanoglu S. Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. Rheumatology International 2006;26:622‐6. [DOI] [PubMed] [Google Scholar]
Pal 1986 {published data only}
- Pal B, Mangion P, Hossain MA, Diffey BL. A controlled trial of continuous lumbar traction in the treatment of back pain and sciatica. British Journal of Rheumatology 1986;25(2):181‐3. [DOI] [PubMed] [Google Scholar]
Reust 1988 {published data only}
- Reust P, Chantraine A, Vischer TL. Treatment of lumbar sciatica with or without neurological deficit using mechanical traction. A double‐blind study. [Traitment par tractions mécaniques des lombosciatalgies avec ou sans déficit neurologique]. Schweizerische Medizinische Wochenschrift 1988;118(8):271‐4. [PubMed] [Google Scholar]
Schimmel 2009 {published data only}
- Schimmel JJP, Kleuver M, Horsting PP, Spruit M, Jacobs WCH, Limbeek J. No effect of traction in patients with low back pain: a single centre, single blind, randomized controlled trial of Intervertebral Differential Dynamics Therapy. European Spine Journal 2009;18:1843‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherry 2001 {published data only}
- Sherry E, Kitchener P, Smart R. A prospective randomized controlled study of VAX=D and TENS for the treatment of chronic low back pain. Neurological Research 2001;23:780‐4. [DOI] [PubMed] [Google Scholar]
Simmerman 2011 {published data only}
- Simmerman SM, Sizer PS, Dedrick GS, Apte GG, Brismée JM. Immediate changes in spinal height and pain after aquatic vertical traction in patients with persistent low back symptoms: a crossover clinical trial. PM & R: The Journal of Injury, Function, and Rehabilitation 2011;3(5):447‐57. [DOI] [PubMed] [Google Scholar]
Sweetman 1993 {published data only}
- Sweetman BJ, Heinrich I, Anderson JAD. A randomized controlled trial of exercises, short wave diathermy, and traction for low back pain, with evidence of diagnosis‐related response to treatment. Journal of Orthopaedic Rheumatology 1993;6:159‐66. [Google Scholar]
Tesio 1993 {published data only}
- Tesio L, Merlo A. Autotraction versus passive traction: an open controlled study in lumbar disc herniation. Archives of Physical Medicine and Rehabilitation 1993;74:871‐6. [DOI] [PubMed] [Google Scholar]
Unlu 2008 {published data only}
- Unlu Z, Tasci S, Tarhan S, Pabuscu Y, Islak S. Comparison of 3 physical therapy modalities for acute pain in lumbar disc herniation measured by clinical evaluation and magnetic resonance imaging. Journal of Manipulative and Physiological Therapeutics 2008;31:191‐8. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1995 {published data only}
- Heijden GJMG, Beurskens AJHM, Dirx MJM, Bouter LM, Lindeman E. Efficacy of lumbar traction: a randomised clinical trial. Physiotherapy 1995;81(1):29‐35. [Google Scholar]
Walker 1982 {published data only}
- Walker L, Svenkerud T, Weber H. [Traksjonbehandling ved lumbago‐ischias: en kontrollert undersolske med Spina‐trac]. Fysioterapeuten 1982;49:161‐3, 177. [Google Scholar]
Weber 1973 {published data only}
- Weber H. Traction therapy in sciatica due to disc prolapse. Journal of the Oslo City Hospitals 1973;23:167‐76. [PubMed] [Google Scholar]
Weber 1984 {published data only}
- Weber H, Ljunggren AE, Walker L. Traction therapy in patients with herniated lumbar intervertebral discs. Journal of the Oslo City Hospitals 1984;34:61‐70. [PubMed] [Google Scholar]
Werners 1999 {published data only}
- Werners R, Pynsent PB, Bulstrode CJK. Randomized trial comparing interferential therapy with motorized lumbar traction and massage in the management of low back pain in a primary care setting. Spine 1999;24(15):1579‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cevik 2007 {published data only}
- Cevik R, Aslan B, Gur A, Sarac AJ, Yildiz H, Nas K, et al. Effect of new traction technique of prone position on distraction of lumbar vertebrae and its relation with different application of heating therapy in low back pain. Journal of Back and Musculoskeletal Rehabilitation 2007;20:71‐7. [Google Scholar]
Gose 1998 {published data only}
- Gose E, Naguszewski K, Naguszewski K. Vertebral axial decompression therapy for pain associated with herniated or degenerated discs or facet syndrome: an outcome study. Neurological Research 1998;20:186‐90. [DOI] [PubMed] [Google Scholar]
Hansen 1993 {published data only}
- Hansen FR, Bendix T, Skov P, Jensen CV, Kristensen JH, Krohn L, et al. Intensive, dynamic back‐muscle exercises, conventional physiotherapy, or placebo‐control treatment of low‐back pain: a randomized, observer‐blind trial. Spine 1993;18(1):98‐107. [DOI] [PubMed] [Google Scholar]
Moret 1998 {published data only}
- Moret NC, Stap M, Hagmeijer R, Molenaar A, Loes BW. Design and feasibility of a randomized clinical trial to evaluate the effect of vertical traction in patients with a lumber radicular syndrome. Manual Therapy 1998;3:203‐11. [Google Scholar]
Olah 2008 {published data only}
- Olah M, Molnar L, Dobai J, Olah C, Feher J, Bender T. The effects of weightbath traction hydrotherapy as a component of complex physical therapy in disorders of the cervical and lumbar spine: a controlled pilot study with follow‐up. Rheumatology International 2008;28:749‐56. [DOI] [PubMed] [Google Scholar]
Ramos 1994 {published data only}
- Ramos G, Martin W. Effects of vertebral axial decompression on intradiscal pressure. Journal of Neurosurgery 1994;81:350‐3. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1991 {published data only}
- Heijden GJMG, Bouter LM, Terpstra‐Lindeman E, Essers AHM, Waltjè EMH, Köke AJA, et al. [De effectiviteit van tractie bij lage rugklachten: de resultaten van een garandomisserde en geblindeerde pilotstudy]. Nederlands Tijdschrift voor Fysiotherapie 1991;101(2):37‐41. [Google Scholar]
Additional references
Bigos 1994
- Bigos SJ, Bowyer OR, Braen GR, Brown K, Deyo R, Haldeman S, et al. Acute Low Back Problems in Adults. Rockville, Maryland: U.S. Department of Health and Human Services, 1994. [AHCPR Publication No. 95‐0642] [Google Scholar]
Blomberg 2005
- Blomberg S. A new pragmatic management strategy for low‐back pain ‐ an integrated multimodal programme. In: Hutson M, Ellis R editor(s). Textbook of Musculoskeletal Medicine. Corby, UK: Oxford University Press, 2005:1‐20. [ISBN 0‐19‐263050‐4] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58:1233‐40. [DOI] [PubMed] [Google Scholar]
Chou 2007
- Chou R, Huffman LH, American Pain Society, American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Annals of Internal Medicine 2007;147(7):492‐504. [DOI] [PubMed] [Google Scholar]
Dagenais 2008
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal 2008;8(1):8‐20. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board CBRG. 2009 Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Gay 2008
- Gay RE, Brault JS. Evidence‐informed management of chronic low back pain with traction therapy. Spine 2008;8(1):234‐42. [DOI] [PubMed] [Google Scholar]
Gotzsche 2012
- Gotzsche P, Ioannidis J. Content area experts as authors: helpful or harmful for systematic reviews and meta‐analyses?. BMJ 2012;345:e7031. [DOI] [PubMed] [Google Scholar]
Harte 2003
- Harte AA, Baxter GD, Gracey JH. The efficacy of traction for back pain: a systematic review of randomized controlled trials. Archives of Physical Medicine and Rehabilitation 2003;84:1542‐53. [DOI] [PubMed] [Google Scholar]
Harte 2005
- Harte AA, Gracey JH, Baxter GD. Current use of lumbar traction in the management of low back pain: results of a survey of physiotherapists in the United Kingdom. Archives of Physical Medicine and Rehabilitation 2005;86:1164‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Krause 2000
- Krause M, Refshauge KM, Dessen M, Boland R. Lumbar spine traction: evaluation of effects and recommended application for treatment. Manual Therapy 2000;5(2):72‐81. [DOI] [PubMed] [Google Scholar]
Lambeek 2011
- Lambeek LC, Tulder MW, Swinkels IC, Koppes LL, Anema JR, Mechelen W. The trend in total cost of back pain in the Netherlands in the period 2002 to 2007. Spine 2011;36(13):1050‐8. [DOI] [PubMed] [Google Scholar]
Li 2001
- Li LC, Bombardier C. Physical therapy management of low back pain: an exploratory survey of therapist approaches. Physical Therapy 2001;81(4):1018‐28. [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Devo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33(1):90‐4. [DOI] [PubMed] [Google Scholar]
Sheffield 1996
- Sheffield FJ. Adaptation of tilt table for lumbar traction. Arch Phys Med Rehabil 1964;45:469‐72. [PubMed] [Google Scholar]
Van Middelkoop 2011
- Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, et al. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non‐specific low back pain. European Spine Journal 2011;20(1):19‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in The Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Van Tulder 2009
- Tulder MW, Suttorp M, Morton S, Bouter LM, Shekelle P. Empirical evidence of an association between internal validity and effect size in randomized controlled trials of low‐back pain. Spine 2009;34(16):111‐6. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clarke 2006a
- Clarke JA, Tulder MW, Blomberg SEI, Vet HCW, Heijden GJMG, Bronfort G. Traction for low‐back pain with or without sciatica. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003010.pub4] [DOI] [PubMed] [Google Scholar]
Clarke 2006b
- Clarke J, Tulder M, Blomberg S, Vet H, Heijden G, Bronfort G. Traction for low back pain with or without sciatica: an updated systematic review within the framework of The Cochrane Collaboration. Spine 2006;31(14):1591‐9. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1995a
- Heijden GJMG, Beurskens AJHM, Koes BW, Assendelft WJJ, Vet HCW, et al. The efficacy of traction for back and neck pain: a systematic, blinded review of randomized clinical trials methods. Physical Therapy 1995;75(2):18‐29. [DOI] [PubMed] [Google Scholar]


