Abstract
Background
Hemifacial spasm is characterised by unilateral involuntary contractions of muscles innervated by the facial nerve. The usual cause is a vessel touching the facial nerve near its origin from the brain stem. Although it is a benign condition it can cause significant cosmetic and functional disability. It is a chronic disease and spontaneous recovery is very rare. The two treatments routinely available are microvascular decompression and Botulinum Toxin type A (BtA) muscular injections.
Objectives
To determine whether botulinum toxin (BtA) is an effective and safe treatment for hemifacial spasm.
Search methods
We searched the Cochrane Movement Disorders Group trials register, the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2004), MEDLINE (1977 to December 2003), EMBASE (1977 to December 2003), and reference lists of articles. We also contacted drug manufacturers and researchers in the field.
Selection criteria
Randomised studies comparing BtA with placebo in people with hemifacial spasm.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data. Study authors were contacted for additional information. Adverse effects information was collected from the trials.
Main results
We found only one small randomised, placebo‐controlled trial involving 11 people. It was a crossover trial during which patients underwent four sets of injections, comparing placebo with three different doses of BtA ‐ formulation Botox(r) (low dose: one‐half of the intermediate dose; intermediate dose; and high dose: twice the intermediate dose), and one of placebo. In this trial BtA was superior to placebo.
Authors' conclusions
The findings of this single eligible trial support the results of large, open, case‐control studies showing a benefit rate between 76 and 100%. This effect size probably makes it very difficult to perform new large placebo controlled trials for hemifacial spasm. Despite the paucity of good quality controlled data, all the studies available suggest that BtA is effective and safe for treating hemifacial spasm. Future trials should explore technical factors such as the optimum treatment intervals, different injection techniques, doses, Bt types and formulations. Other issues include service delivery, quality of life, long‐term efficacy, safety, and immunogenicity. BtA should be compared with surgical microvascular decompression.
Keywords: Humans; Botulinum Toxins, Type A; Botulinum Toxins, Type A/therapeutic use; Hemifacial Spasm; Hemifacial Spasm/drug therapy; Neuromuscular Agents; Neuromuscular Agents/therapeutic use; Randomized Controlled Trials as Topic
Botulinum Toxin type A (BtA) muscular injections are beneficial in treating hemifacial spasm.
Hemifacial spasm is characterised by unilateral involuntary contractions of muscles innervated by the facial nerve. The usual cause is a vessel touching the facial nerve near its origin from the brain stem. Although it is a benign condition it can cause significant cosmetic and functional disability. It is a chronic disease and spontaneous recovery is very rare. The two treatments routinely available are microvascular decompression and Botulinum Toxin type A (BtA) muscular injections. This review demonstrated that BtA is effective and safe to treat hemifacial spasm.
Background
Hemifacial Spasm (HFS) is a condition characterised by involuntary paroxysmal contractions of muscles innervated by the facial nerve. Bilateral involvement (Jamjoon 1990) is rare. The involuntary contraction affects orbicularis oculi in the upper face, and orbicularis oris, platysma and other superficial muscles of the ipsilateral lower hemiface. Although HFS is not dangerous it usually causes significant cosmetic and functional disability. Its severity ranges from a slight unilateral blinking with no involvement of the lower hemiface, to intense spasm of the lower hemiface and neck with one eye closed (Cardoso 1995; Wang 1998), and a progressive facial weakness. HFS may interfere with the patient's professional and social life and have important health and economic implications (Serrano 1999). It is a chronic disease and recovery is rarely spontaneous.
HFS affects women more than men and it usually appears in the fourth to seventh decade of life. For the white population of the United States (Auger 1990) the average annual incidence is 0.78 per 100,000 population and the average prevalence 7.4 per 100,000 in men and 14.5 per 100,000 in women. Familial cases are rare (Carter 1990).
We do not fully understand the pathophysiology of HFS. The motor nucleus of the facial nerve may be hyperexcitable in some patients (Cakmur 1999). Magnetic resonance imaging with special angiographic sequences shows that 65% (Bernardi 1993) to 100% (Hosoya 1995) of participants with HFS have a blood vessel touching the facial nerve at its root exit zone, the point at which it leaves the brainstem in the cerebellopontine angle. These vessels may cause focal demyelination with ephaptic transmission (current leakage and "cross‐talk") between axons (Viggo 1984a,b,c), and slow nerve conduction. Increasing nerve compression may be the cause of the progressive facial weakness.
The diagnosis is made by observation and clinical history. Radiological imaging is not important for the diagnosis but it may be worthwhile to exclude the rare cases associated with a tumour, aneurysm or arteriovenous malformation (Matsuura 1996; Nagata 1992; Sprik 1988; Wang 1998).
Although many surgical and pharmacological approaches to treatment have been reported, the two treatments routinely available are microvascular decompression of the facial nerve at the pons (Barker 1995), and intramuscular injections of Botulinum Toxin type A (BtA).
BtA causes a flaccid paralysis by blocking the release of acetylcholine at the neuromuscular junction. It is taken up by the nerve cells at the neuromuscular junction, and damages proteins within the nerve cell that are needed to fuse the synaptic vesicles containing acetylcholine with the cell membrane (Brin 2002).
Elston was the first to try BtA for HFS, in 1985 (Elston 1986). Subsequent large case series have consistently reported that BtA is clinically very effective in HFS. This systematic review covers the placebo‐controlled evidence for BtA in HFS, and aims to clarify how much benefit patients with hemifacial spasm derive from BtA, and its safety profile.
Objectives
To compare the clinical efficacy and safety of Botulinum Toxin Type A (BtA) versus placebo in the treatment of hemifacial spasm.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, controlled, double blind trials of BtA versus placebo. Trials in which allocation was not adequately concealed were excluded.
Types of participants
We sought trials with patients of any age with a clinical diagnosis of hemifacial spasm. We allowed previous therapy with BtA, whether patients were still responding to BtA (A‐responders) or not (A‐resistant), and we allowed concomitant medical therapies.
Types of interventions
Intramuscular injections of BtA versus placebo. We allowed all techniques (eg. EMG guided or not) and administration schedules of BtA.
Types of outcome measures
For each trial, we identified the number of patients originally randomised to each treatment group. For both groups, we sought outcome information for all the patients.
The primary outcomes were: Improvement in objective symptomatic rating scales (any).
Secondary Outcomes: (1) Changes in subjective evaluation of clinical status both by patients and clinicians, (2) Changes in quality of life assessments, (3) Adverse reactions (frequency and severity).
Search methods for identification of studies
We conducted searches from 1977, which was the first year that Bt was used therapeutically. We identified relevant trials from the following sources: (1) Cochrane Movement Disorders Group Specialised Register (December 2003); (2) Cochrane Controlled Trials Register (CENTRAL) (The Cochrane Library Issue 1, 2004); (3) MEDLINE (1977 to December 2003); (4) EMBASE (1977to December 2003).
We screened titles, keywords and abstracts of the citations downloaded from the electronic searches, and obtained full copies of reports of potentially suitable trials for further assessment. The search strategy also included: (5) Reference lists of located trials and BoNT review articles; (6) Handsearch of Movement Disorders Journal and international congress of movement disorders and botulinum toxins (1985 to June 2002); (7) Personal communication with other researchers in the field; (8) Contact with the drug manufacturer (Allergan and Ipsen). (9) If necessary, we contacted authors of published trials for further information and unpublished data.
The search strategy for MEDLINE and Central/CCTR is given below. The search strategy was modified for EMBASE. 1.Botulinum toxins/ 2.Botulinum Toxin Type A/ 3.botulin$ and tox$.tw 4.dyspor$ or oculinu$ or boto$.tw 5.or/1‐4 6.hemifacial spasm/ 7.hemif$ and spas$.tw 8.or/6‐7 9.5 and 8 10.limit 9 to human
Data collection and analysis
Three reviewers (Costa J, Borges A, Espírito‐Santo C) independently assessed the studies found by the search strategy, to identify potentially suitable trials for the review according to the criteria outlined above. We resolved disagreements about inclusions by discussion. We independently assessed the full papers for methodological quality by extracting details of randomisation methods, blinding of treatments and assessments, whether intention‐to‐treat analysis was possible from the published data, whether treatment groups were comparable with regard to demographics and clinical characteristics, the number of patients excluded or lost to follow‐up, definition of outcomes, and entry and exclusion criteria. They looked for sources of bias including: (1) selection bias, including randomisation and by chance differences in groups due to small sample sizes; (2) performance bias; (3) attrition bias; (4) detection bias; (5) selective reporting of results. The reviewers noted compliance, dropouts and other exclusions from analysis. They classified the analysis of trials as being on the basis of "intention‐to‐treat", or not.
Two reviewers (Costa J, Ferreira JJ) independently abstracted eligible data onto standardised forms, crosschecked for accuracy and amalgamated them. They resolved disagreements by discussion. All results were expressed as ordinal data. The various rating scales used were dichotomised using each author's own criteria for improvement or no improvement. If these criteria were not described, we defined 'improvement' as any beneficial change from baseline and 'no improvement' as no improvement from baseline which also included any deterioration from baseline.In order to dichotomise the results, we requested individual patient data if the results were presented as mean values for groups. We performed statistical analyses using the RevMan statistical software provided by the Cochrane Collaboration. We tested heterogeneity between trial results using a standard chi squared test. We reported the results as odds ratios (and 95% confidence intervals) for dichotomous outcomes, using the Peto fixed‐effect method. We calculated the significance of any differences between odds ratios using a standard method (Altman 1996). If we could not combine outcome data from different studies we gave a descriptive summary of the results.
Results
Description of studies
See also 'Characteristics of Included Studies'
We were able to include only one study (Yoshimura 1992). This was a randomised, double blind, study that enrolled only 11 patients with hemifacial spasm. It was a crossover trial during which patients underwent four sets of injections, comparing placebo with three different doses of BtA ‐ formulation Botox(r) (low dose: one‐half of the intermediate dose; intermediate dose; and high dose: twice the intermediate dose), and one of placebo. The randomisation method was not clear. Injections were given at least one month apart as clinically indicated. Selection of muscles to inject and of dose administered were based on clinical examination. For each participant, the site of injections was kept constant. Mean dose ranged from 2.5 to 10 BtA units per muscle. The total dose administered to participants at any one time varied between 5 and 90 BtA units. The study objectives were to estimate subjective and objective improvement, adverse events, and duration of improvement. All outcomes were assessed one month after injections. Subjective improvement was assessed with an analogue 10‐points scale where five was the preinjection baseline and values less than five represented improvement. Objective improvement was assessed with a categorical 10‐point scale to characterize the frequency of spasms (range, 0 to three), number of muscles involved (range, 0 to four), and severity of spasms (range, 0 to three). For both subjective and objective evaluations, a change of at least two points was considered substantial. Duration of benefit was defined as the time for the participant to return to subjective and objective baseline after each injection. One participant withdrew after two injections, because of concern about facial weakness caused by BtA. Two others withdrew for domestic reasons, after one and three injection sessions respectively. The data from these three participants were used in the analyses of treatment efficacy and adverse events.
After this blinded controlled trial, an additional 95 patients were enrolled in an open trial of BtA injections. We have not considered the results of this uncontrolled phase.
Characteristics of excluded studies:
We found one other 'controlled' study of BtA versus placebo (Park 1993). In reality, this was a prospective case series of 101 participants with hemifacial spasm, which had first included in the protocol a randomized double‐blind phase. It is not clear whether any participants had previously received BtA treatments. Only eight patients with hemifacial spasm were enrolled in the controlled phase. There were no clear data for baseline characteristics, treatment program and results for BtA and placebo groups regarding these eight participants. We therefore excluded this study.
One randomised trial (Mezaki 1999) assessed the effects of different BtA doses. However it did not include a placebo arm. A randomised trial enrolling 42 participants with hemifacial spasm analysed the effectiveness and side effects of four different BtA treatment site applications (Price 1997). The authors found that the brow treatment was equally effective to standard treatment with fewer side effects. Another randomised controlled trial (Sampaio 1997) compared different BtA formulations (Botox(r) and Dysport(r) at a ratio of 1 to 4) in participants with blepharospasm and hemifacial spasm. No placebo group was included. Other prospective descriptive studies have looked at the long‐term results of BtA in the treatment of hemifacial spasm. One of these studies was an open non‐randomized trial that compared the effects of 2 different techniques of BtA injections (Jitpimolmard 1998). However, these comparisons are not within the scope of our review.
Risk of bias in included studies
The study included stated that it randomised participants to the different sets of injections. However, it did not give the precise method of randomisation and details of concealment of allocation, raising the possibility of detection bias since this was a cross‐over trial and we could not surely exclude that the assessment of the observer, whether the subject or the physician, were affected by their knowledge of what the injection consisted of. The trial was 'double blind' throughout, using an identical placebo. However, blinding may have been compromised as almost all participants had facial weakness after BtA. However, half the participants had some facial weakness before the study, and most were not aware of the facial weakness detected during the trial. These aspects make it less probable that there was important performance or attrition bias. There were three explicit withdrawals, and data from these patients were included in analysis, making attrition bias unlikely. The study clearly stated that its objective evaluation data were analysed blind, making detection bias unlikely.
Effects of interventions
Meta‐analysis was not required as there was only one eligible study. Because there were so few participants we present the results as absolute numbers and have not analysed them statistically. Note that in "any dose" the number given is not the number of patients (only 11 in total) but represent the total number of BtA injections. The total number of injections considered was 38 for subjective evaluation and 33 for objective evaluation (a smaller number because in five instances participants failed to attend evaluation after they had been injected). Subjective evaluation.
BtA Injection Session; Placebo Low dose, Intermediate dose, High dose, Any dose; Placebo Number of patients (%) with: Any improvement: 7 (70%), 7 (70%), 9 (100%), 23, (79%); 1 (11%) Substantial improvement: 6 (60%), 6 (60%), 9 (100%), 21 (72%); 1 (11%) No changes: 2 (20%), 3 (30%), 0, 5 (17%); 4 (44%) Worsening 1 (10%), 0, 0, 1 (3%); 4 (44%) Objective evaluation.
BtA Injection Session; Placebo Low dose, Intermediate dose, High dose, Any dose; Placebo Number of patients with: Any improvement: 7 (78%), 7 (88%), 7 (88%), 21 (84%); 3 (38%) Substantial improvement: 2 (22%), 4 (50%), 5 (63%), 11 (44%); 0 No changes: 1 (11%), 0, 1 (13%), 2 (8%); 2 (25%) Worsening: 1 (11%), 1 (13%), 0, 2 (8%); 3 (38%)
The mean duration of improvement considering any dose was 2.8 months (range, two weeks to six months). The authors reported adverse effects after BtA, affecting all 11 participants on a combined total of 30 occasions. These included facial weakness (29), bruising (6), diplopia (4), ptosis (2), headache (2), lagophthalmos, blurred vision, tearing, dysphagia, nausea, and flu‐like symptoms, all in one occasion. The distribution of adverse events between the various treatment groups and doses was not clear.
Discussion
BtA received approval in US and Europe for hemifacial spasm treatment in the early 1990s. Although there are few high‐quality, placebo‐controlled trials, the data available were nonetheless considered sufficient to support the decision to consider it the treatment of choice for hemifacial spasm. A number of open case‐control studies have enrolled several thousand patients between them (Jost 2001). In all these studies BtA was considered highly effective, with a success rate of 76 to 100%. The mean duration of improvement ranged between 2.6 and four months. The most common adverse effects reported in these studies were: dry eye (7 to 18.1%), ptosis (2.8 to 23.3%), facial weakness (17.6 to 97%), tearing (5.5%) and diplopia (1 to 6%). All of these were transitory, and no systemic adverse effects were detected.
Bt therapy is probably the second most important discovery in movement disorders therapy after levodopa. Few drugs can match the obvious effect of Bt in some dystonias. The paucity of trials comparing BtA with placebo in hemifacial spasm is probably due to the very high success rate and degree of benefit reported in open studies. Although our review highlights this paucity of high‐quality placebo‐controlled data, we still believe that BtA is effective and safe in hemifacial spasm (Smith 2003).
For hemifacial spasm, the strength of the open‐label data is such that many researchers would find it ethically difficult to randomise patients to placebo or BtA in trials designed to examine simple efficacy. However, further controlled studies would be justifiable and valuable to compare different Bt types and formulations, techniques of injection, doses, long‐term efficacy and immunogenicity, and various models of service delivery.
In addition, the surgical option of posterior fossa microvascular decompression (MVD) is often successful and may be curative. To our knowledge, no randomized controlled study has compared MVD with BtA. Such a trial could be most helpful in determining the best long‐term management of HFS, especially in younger patients who otherwise face many years of BtA injections.
Authors' conclusions
All studies available strongly suggest that BtA is effective and safe for treating hemifacial spasm. Despite the absence of large RCTs, the efficacy of BtA for HFS is not in doubt.
Future controlled trials could usefully examine the effects of BtA in less common types of facial involuntary movements such as synkinesis, myokymia and continuous partial epilepsy unresponsive to anticonvulsants (Lozsadi 2004). BtA should be compared with surgery. Future trials should also explore technical factors such as the optimum treatment interval, injection technique, dose, Bt type and formulation. Other issues include service delivery, quality of life, long‐term efficacy, safety, and immunogenicity.
Data and analyses
Comparison 1.
Subjective
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 15.09 [6.42, 35.48] |
| 1.1 low | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.86 [1.67, 58.20] |
| 1.2 intermeidiate | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.86 [1.67, 58.20] |
| 1.3 high | 1 | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 29.96 [4.92, 182.55] |
| 1.4 any | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 17.35 [3.76, 80.13] |
| 2 Improvement substantial | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.69 [4.96, 27.56] |
| 2.1 low | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.32 [1.19, 45.04] |
| 2.2 intermeidiate | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.32 [1.19, 45.04] |
| 2.3 high | 1 | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 29.96 [4.92, 182.55] |
| 2.4 any | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.57 [2.60, 51.59] |
| 3 Unchanged or worse | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.07 [0.03, 0.16] |
| 3.1 low | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.10 [0.02, 0.60] |
| 3.2 intermeidiate | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.10 [0.02, 0.60] |
| 3.3 high | 1 | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.03 [0.01, 0.20] |
| 3.4 any | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.06 [0.01, 0.27] |
Analysis 1.1.
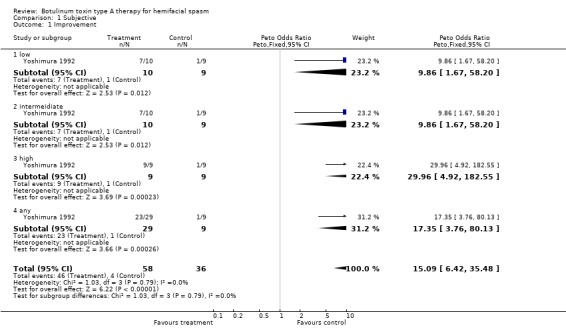
Comparison 1 Subjective, Outcome 1 Improvement.
Analysis 1.2.

Comparison 1 Subjective, Outcome 2 Improvement substantial.
Analysis 1.3.

Comparison 1 Subjective, Outcome 3 Unchanged or worse.
Comparison 2.
Objective
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.16 [2.79, 18.37] |
| 1.1 low | 1 | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.78 [0.73, 31.26] |
| 1.2 intermeidiate | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [1.04, 52.46] |
| 1.3 high | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [1.04, 52.46] |
| 1.4 any | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.71 [1.67, 56.47] |
| 2 Improvement substantial | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.67 [3.43, 27.28] |
| 2.1 low | 1 | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.50 [0.43, 131.96] |
| 2.2 intermeidiate | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.18 [1.36, 109.00] |
| 2.3 high | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 15.29 [1.97, 118.44] |
| 2.4 any | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.82 [1.29, 35.98] |
| 3 Unchanged or worse | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.05, 0.36] |
| 3.1 low | 1 | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.03, 1.37] |
| 3.2 intermeidiate | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.02, 0.96] |
| 3.3 high | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.02, 0.96] |
| 3.4 any | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.10 [0.02, 0.60] |
Analysis 2.1.

Comparison 2 Objective, Outcome 1 Improvement.
Analysis 2.2.

Comparison 2 Objective, Outcome 2 Improvement substantial.
Analysis 2.3.
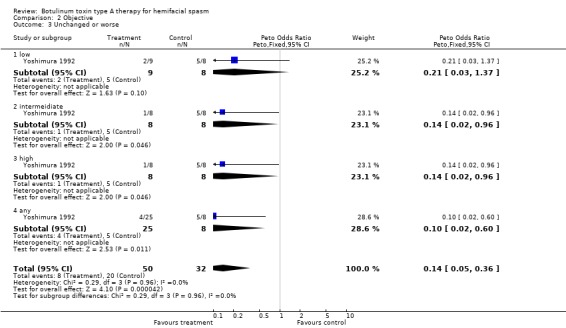
Comparison 2 Objective, Outcome 3 Unchanged or worse.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 25 October 2004 | New citation required and conclusions have changed | Substantive amendment |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, cross‐over study.Method of randomisation: not mentioned.Data analysed on an intention to treat.Location: one centre in US.Duration: cross‐over at 1 month (minimum). | |
| Participants | Eleven participants were enrolled. 3 participants were male and 8 participants were female. Mean age was 50 years (37‐68). Mean duration of symptoms was 5.2 years (1.5‐12). Six participants had facial weakness ipsilateral to the spasm. None of the participants had received previous treatment with Botulinum toxin type A (BtA). All had been treated with medications without success. One participant had undergone surgical microvascular decompressive procedures without lasting benefit.Inclusion criteria: not mentioned.Exclusion criteria: not mentioned | |
| Interventions | The participants were randomly assigned to 4 sets of injections. Three of these injections were of BtA (formulation Botox) using 3 different doses (low, intermediate, and high dose), and one injection was of placebo (saline). Muscles were selected for injection based on clinical involvement. For each participant, the site of injections was kept constant. BtA dose was determined for each participant on the basis of the number of muscles involved, frequency and severity of the spasms. The average dose ranged from 2.5 to 10 units per muscle. Doses of one‐half (low dose) and twice this dose (high dose) were administered on different occasions. The total dose administered to participants at any one time varied between 5 and 90 units. BtA was diluted to a concentration of 2.5 to 5 units per 0.1ml. Participants were not reinjected until any response to the previous injection (as determined by both the patients and physicians) had been lost. | |
| Outcomes | The primary efficacy outcome was patient self assessment of global improvement on a 10‐point scale. Secondary efficacy outcomes included the physician assessment of improvement (using a 10‐point scale to characterize the frequency of spasms, number of muscles involved, and severity of spasms), and adverse events. All outcomes data were collected at week 4 after injections. Author's also looked at duration of improvement (defined as the time for the patient to return to subjective and objective baseline after each injection). | |
| Notes | There was 1 withdrawal after 2 injections due to concerns about facial weakness caused by BtA. Two other withdrawals occurred because of domestic reasons after 3 and 1 injection sessions, respectively.After this blinded controlled trial, an additional 95 participants were enrolled in an open trial of BtA injections. The results of this uncontrolled phase are not considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Contributions of authors
Protocol ‐ Costa J, Ferreira JJ, Miguel C, Sampaio C Literature search ‐ Costa J, Borges A, Espírito‐Santo C Literature selection ‐ Costa J, Borges A, Espírito‐Santo C Papers quality assessment ‐ Costa J, Ferreira JJ Data collection from papers ‐ Costa J, Ferreira JJ Interpretation of data ‐ Costa J, Ferreira JJ, Moore P, Sampaio C Review writing ‐ Costa J, Ferreira JJ, Moore, Sampaio C
Declarations of interest
Costa J, Ferreira JJ, Sampaio C, and Miguel C had been investigators in clinical trials sponsored by Elan, Allergan, and Ipsen. Ferreira JJ and Sampaio C were speakers in symposia promoted by Elan, Allergan, and Ipsen. Moore P has received fees from various companies marketing botulinum toxin for speaking at meetings and for advice. His unit has received funds for research.
Edited (no change to conclusions)
References
References to studies included in this review
- Yoshimura DM, Aminoff MJ, Tami TA. Botulinum toxin therapy for Hemifacial Spasm. Neurology 1990;40 Suppl 1:381(974S). [Google Scholar]; Yoshimura DM, Aminoff MJ, Tami TA, Scott AB. Treatment of Hemifacial Spasm with botulinum toxin. Muscle Nerve 1992;15:1045‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Jitpimolmard S, Tiamkao S, Laopaiboon. Long term results of botulinum toxin type A (Dysport) in the treatment of hemifacial spasm: report of 175 cases. Journal of Neurology, Neurosurgery and Psychiatry 1998;64(6):751‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezaki T, Kaji R, Kimura J, Ogawa N. Treatment of Hemifacial Spasm with Type A Botulinum Toxin (AGN 191622): A Dose Finding Study and the Evaluation of Clinical Effect with Electromyography. No To Shinkei 1999;51:427‐32. [PubMed] [Google Scholar]
- Park YC, Lim JK, Lee DK, Doe Yi S. Botulinum A toxin treatment of hemifacial spasm and blepharospasm. Journal of Korean Medical Science 1993;8(5):334‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Farish S, Taylor H, O'Day J. Blepharospasm and Hemifacial Spasm. Randomized trial to determine the most appropriate location for botulinum toxin injections. Ophthalmology 1997;104(5):865‐8. [DOI] [PubMed] [Google Scholar]
- Sampaio C, Ferreira JJ, Simoes F, Rosas MJ, Magalhaes M, Correia AP, et al. DYSBOT: a single‐blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of botulinum toxin type A‐‐Dysport and Botox‐‐assuming a ratio of 4:1. Movement Disorders 1997;12(6):1013‐8. [DOI] [PubMed] [Google Scholar]; Sampaio C, Ferreira JJ, Simões F, Rosas MJ, Magalhães M, Martins R, Bastos‐Lima A, Castro‐Caldas A. Dysbot: a single blind, randomized clinical trial to compare two different formulations of botulinum toxin type A. Movement Disorders 1995;10(3):387. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman DG, Matthews JN. Statistics notes. Interaction 1: Heterogeneity of effects. BMJ 1996;313(7055):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Archives of Neurology 1990;47(11):1233‐4. [DOI] [PubMed] [Google Scholar]
- Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. Journal of Neurosurgery 1995;82:201‐10. [DOI] [PubMed] [Google Scholar]
- Bernardi B, Zimmerman RA, Savino PJ, Adler C. Magnetic resonance tomographic angiography in the investigation of hemifacial spasm. Neuroradiology 1993;35(8):606‐11. [DOI] [PubMed] [Google Scholar]
- Brin. Scientific and Therapeutic Aspects of Botulinum Toxin. Philadelphia: Lippincott Williams & Wilkins, 2002. [Google Scholar]
- Cakmur R, Tataroglu C, Idiman F. Electrophysiologic observations on pathogenic mechanisms of hemifacial spasm: F‐waves of the facial muscles and silent period evoked by transcranial magnetic stimulation. European Journal of Neurology 1999;6 Suppl:136. [Google Scholar]
- Cardoso F, Jankovic J. Blepharospasm. Handbook of Dystonia. Tsui J, Calne D. New York: Marcel Dekker, 1995: 129‐42. [Google Scholar]
- Carter JB, Patrinely JR, Jankovic J, et al. Familial hemifacial spasm. Archives of Opthalmology 1990;108:249‐50. [DOI] [PubMed] [Google Scholar]
- Elston JS. Botulinum toxin treatment of hemifacial spasm. Journal of Neurology, Neurosurgery and Psychiatry 1986;49:827‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T, Watanabe N, Yamaguchi K, Saito S, Nakai O. Three‐dimensional‐MRI of neurovascular compression in patients with hemifacial spasm. Neuroradiology 1995;37(5):350‐2. [DOI] [PubMed] [Google Scholar]
- Jamjoon AB, Anderson RL, Jordan DR, Patrinely JR. Bilateral hemifacial spasm. Journal of Clinical Neuro‐ophthalmology 1990;10:153‐154. [PubMed] [Google Scholar]
- Jost WH, Kohl A. Botulinum toxin: evidence‐based medicine criteria in blepharospasm and hemifacial spasm. Journal of Neurology 2001;248 Suppl 1:I/21‐I/24. [DOI] [PubMed] [Google Scholar]
- Lozsadi DA, Hart IK, Moore AP. Botulinum toxin A improves involuntary limb movements in Rasmussen syndrome. Neurology 2004;62(7):1233‐4. [DOI] [PubMed] [Google Scholar]
- Matsuura N, Kondo A. Trigeminal neuralgia and hemifacial spasm as false localizing signs in patients with a contralateral mass of the posterior cranial fossa. Journal of Neurosurgery 1996;84:1067‐71. [DOI] [PubMed] [Google Scholar]
- Nagata S, Matsushima T, Fujii K, Masashi F, Kuromatsu C. Hemifacial spasm due to tumor, aneurysm, or arteriovenous malformation. Surgical Neurology 1992;38:204‐9. [DOI] [PubMed] [Google Scholar]
- Serrano‐Duenas M. Hemifacial spasm, quality of life and depression. Revista de Neurologia 1999;29(12):1108‐11. [PubMed] [Google Scholar]
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ 2003;327(7429):1459‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprik C, Wirtschafter JD. Hemifacial spasm due to intracranial tumor. An international survey of botulinum toxin investigators. Ophthalmology 1988;95:1042‐45. [DOI] [PubMed] [Google Scholar]
- Viggo Kamp N. Pathophisiology of hemifacial spasm: I. Ephatic transmission and ectopic excitation. Neurology 1984;34:418‐26. [DOI] [PubMed] [Google Scholar]
- Viggo Kamp N. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology 1984;34:427‐31. [DOI] [PubMed] [Google Scholar]
- Viggo Kamp N, Jannetta PJ. Pathophysiology of hemifacial spasm: III. Effects of facial nerve decompression. Neurology 1984;34:891‐97. [DOI] [PubMed] [Google Scholar]
- Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve 1998;21:1740‐47. [DOI] [PubMed] [Google Scholar]


