Abstract
Background
Carpal tunnel syndrome is a common disorder for which several surgical treatment options are available.
Objectives
To compare the efficacy of the various surgical techniques in relieving symptoms and promoting return to work or activities of daily living and to compare the occurrence of side‐effects and complications in patients suffering from carpal tunnel syndrome.
Search methods
We updated the searches in 2006. We conducted computer‐aided searches of the Cochrane Neuromuscular Disease Group Trials Register (searched in June 2006), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 2), MEDLINE (January 1966 to June 2006), EMBASE (January 1980 to June 2006) and also tracked references in bibliographies.
Selection criteria
Randomised controlled trials comparing various surgical techniques for the treatment of carpal tunnel syndrome.
Data collection and analysis
Two review authors performed study selection, assessment of methodological quality and data extraction independently of each other.
Main results
Thirty‐three studies were included in the review of which 10 were newly identified in this update. The methodological quality of the trials ranged from fair to good; however, the use of allocation concealment was mentioned explicitly in only seven trials. Many studies failed to present the results in sufficient detail to enable statistical pooling. Pooling was also impeded by the vast variety of outcome measures that were applied in the various studies. None of the existing alternatives to standard open carpal tunnel release offered significantly better relief from symptoms in the short‐ or long‐term. In three studies with a total of 294 participants, endoscopic carpal tunnel release resulted in earlier return to work or activities of daily living than open carpal tunnel release, with a weighted mean difference of ‐6 days (95% CI ‐9 to ‐3 days).
Authors' conclusions
There is no strong evidence supporting the need for replacement of standard open carpal tunnel release by existing alternative surgical procedures for the treatment of carpal tunnel syndrome. The decision to apply endoscopic carpal tunnel release instead of open carpal tunnel release seems to be guided by the surgeon's and patient's preferences.
Keywords: Humans, Carpal Tunnel Syndrome, Carpal Tunnel Syndrome/surgery, Randomized Controlled Trials as Topic
Plain language summary
Surgical treatment options for carpal tunnel syndrome
There is no strong evidence for the replacement of standard open carpal tunnel release (OCTR) by alternative surgical procedures for the treatment of carpal tunnel syndrome. The decision to apply special, minimally invasive operations instead of standard OCTR seems to be guided by the surgeon's and patient's preferences.
Carpal tunnel syndrome is a common disorder causing pins and needles and pain in the hand due to compression of the median nerve in the carpal tunnel at the wrist. Its severity can range from mild to severe. Severe cases are generally treated surgically. This review aimed to compare different surgical options for the treatment of carpal tunnel syndrome. Current evidence from randomised controlled trials showed that none of the alternatives to standard open carpal tunnel release seem to offer better relief from symptoms in the short‐ or long‐term, although a special type of operation (endoscopic carpal tunnel release) seems to enable people to return to their work or daily activities sooner (on average approximately a week).
Background
Carpal tunnel syndrome (CTS) is a common compression neuropathy of the upper extremity. The annual incidence of CTS in primary care in the UK and the Netherlands is estimated at approximately 90 per 100,000 new presentations in men and 193 to 280 per 100,000 new presentations in women (Bongers 2007;Latinovic 2006). In an Italian population the prevalence of CTS was estimated at 1.9% (Salaffi 2005).
CTS is caused by compression of the median nerve in the carpal tunnel at the wrist and produces pain, paraesthesiae and hypoaesthesia in the hand. The severity of CTS ranges from mild to severe. Mild CTS presents as intermittent symptoms of paresthesiae and numbness, often at night. Severe CTS may cause permanent atrophy of the thenar muscles innervated by the median nerve and permanent loss of sensation in the median nerve distribution in the hand. CTS is a clinical diagnosis. Electrophysiological tests (nerve conduction studies) are often performed to support the clinical diagnosis.
For the treatment of CTS, both conservative and surgical options are available. Surgical treatment is generally preferred in severe cases of carpal tunnel syndrome whilst non‐surgical treatment is usually initiated for mild to moderate carpal tunnel syndrome (Duncan 1987). Surgical treatment is also indicated in cases where conservative management fails. Conservative treatment includes no intervention, wrist splints, corticosteroid injections and other non‐invasive options (Marshall 2002; O'Connor 2003). Surgery consists of dividing the transverse carpal ligament thereby increasing the canal volume (Ablove 1994; Richman 1989) and reducing the pressure on the nerve (Okutsu 1989). In 2000, operative treatment was undertaken in 31% of the new presentations of CTS in the UK (Latinovic 2006). In an earlier study in Denmark the estimated incidence of CTS operations was 0.61 per 1,000 per year (Ebskov 1997). In the United States approximately 40% of all CTS cases are treated surgically (Wilson 2003). Various surgical techniques are available and there has been considerable discussion about which method is the most effective.
Until recently, open carpal tunnel release (OCTR) with a long curvilinear palmar incision was the standard procedure (Taleisnik 1973). Later the incision was confined to the palm of the hand. A two to three cm long incision is now recommended for a standard OCTR without special designed instruments (MacKinnon 2005). In addition to cutting the transverse carpal ligament, the overlying structures from the skin to the median nerve are also divided. Sometimes an epineurotomy is performed because the nerve sheath (epineurium) is thickened (Fissette 1979). If there is scar tissue within the nerve an internal neurolysis may be performed to separate the involved fascicles from this fibrosis (Curtis 1973). Endoscopic carpal tunnel release (ECTR) is a relatively new procedure. Its proposed advantage is that by dividing the transverse carpal ligament from within the carpal tunnel the overlying structures are left intact. This might decrease post‐operative morbidity and hasten return to work. Two techniques are commonly used for ECTR: the single‐portal technique as described by Agee (Agee 1992; Agee 1994) and the two‐portal technique described by Chow (Chow 1989; Chow 1993). Since the introduction of ECTR various types of new incisions for OCTR have been introduced which further try to reduce surgical trauma and hence recovery time (Bromley 1994).
We previously published a systematic review comparing different operations for CTS (Gerritsen 2001) and have revised the review for publication in The Cochrane Library.
Objectives
We set out to systematically review the evidence from randomised controlled trials which compared the short‐term and long‐term efficacies of the various surgical treatments for relieving CTS symptoms and promoting return to work or resumption of activities of daily living (ADL). We also analysed the occurrence of side‐effects and complications.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials comparing different surgical techniques in patients with CTS and published as a full report. We did not apply a language restriction.
Types of participants
We included people with a clinical diagnosis of CTS with or without electrophysiological confirmation. We accepted the authors' definition of CTS and their views of what constituted electrophysiological confirmation.
Types of interventions
We included studies comparing any of the following interventions with each other:
standard open carpal tunnel release (OCTR);
endoscopic carpal tunnel release (ECTR);
open carpal tunnel release with additional procedures such as internal neurolysis, epineurotomy or tenosynovectomy; and
open carpal tunnel release using various incision techniques.
We did not include comparisons of surgical treatments with non‐surgical treatments or no treatment, which is the subject of another Cochrane review (Verdugo 2003).
Types of outcome measures
Primary outcomes
The primary outcome measure of interest was overall improvement (any measure in which patients indicated the intensity of their complaints compared to baseline).
Secondary outcomes
We also considered the following secondary outcome measures:
improvement of CTS symptoms (pain, paraesthesiae, nocturnal paraesthesiae);
time to return to work or to resume activities of daily living (ADL);
complications and side‐effects.
Overall improvement and improvement of CTS symptoms both in the short‐term (less than or equal to three months) and the long‐term (greater than three months) were taken into consideration.
Search methods for identification of studies
Electronic searches
The searches were updated in 2006. To identify publications we searched the Cochrane Neuromuscular Disease Group Trials Register (searched in June 2006), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 2), MEDLINE (January 1966 to June 2006), EMBASE (January 1980 to June 2006) and tracked references in bibliographies. We replaced the original generic Cochrane search for randomised controlled trials (RCTs) (Dickersin 1994) by a new, highly sensitive search (Robinson 2002). This search was combined with the following specific search for CTS: 'carpal tunnel syndrome [mesh]' OR 'carpal tunnel syndrome [tw]' OR 'carpal tunnel [tw]' OR 'carp* syndr* [tw]' OR 'carp* tunn* [tw]' OR 'tunn* syndr* [tw]' OR 'median nerve entrapment [mesh]' OR 'median nerve entrapment [tw]'. Finally, the resulting set was combined with the following specific search for surgical interventions: 'surgical [mesh]' OR 'surgical [tw]' OR 'surgery [mesh]' OR 'surgery [tw]' OR 'release [tw]' OR 'reconstruct* [tw]' OR 'epineurotomy [tw]'. To be included, a study had to meet the following criteria: (1) the study population consisted of patients with CTS; (2) different surgical techniques were compared; (3) the study was designed as an RCT; (4) the results were published as a full report. Two review authors independently selected studies and any disagreements were discussed to reach consensus.
For search strategies for MEDLINE and EMBASE see Appendix 1 and Appendix 2 respectively.
Data collection and analysis
Two review authors performed study selection, assessment of methodological quality and data abstraction independently of each other. Disagreements were resolved by discussion. We undertook assessment of methodological quality by following the list of internal validity criteria recommended for systematic reviews in the field of musculoskeletal disorders (Tulder 1997). The list was adapted for CTS with regard to prognostic indicators and outcome measures (Additional Table 1). In addition, because the intervention was surgery, the criteria 'care‐provider blinded' and 'compliance acceptable' were omitted from the original list. The criterion 'patient blinded' was only applied in studies comparing OCTR and OCTR with an additional procedure because with those techniques similar incisions were made. Criteria could be scored as positive ('yes'), negative ('no') or unclear ('don't know'). The criteria are shown in Additional Table 1. Studies with a negative score for 'adequate randomisation' were excluded from further analyses. Data from the articles were recorded on a standardised form. Information was collected on participants (age, gender, duration of symptoms, electrophysiological confirmation, number of patients treated bilaterally, number of patients or hands randomised), interventions (surgical technique), outcome measures, timing of the follow‐up measurements, and results (point estimates and measures of variability, number of patients or hands).
1. Criteria list for the assessment of methodological quality of included studies.
| Item ID | Description | Implementation |
| Patient selection | NOTE: All criteria were scored yes (+), no (‐) or don't know (?). | |
| b1 | Was an adequate method of randomisation applied? | A random (unpredictable) allocation sequence must have been applied. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation are not considered to be appropriate. |
| b2 | Was the treatment allocation concealed? | Allocation should have been performed by an independent person who is not responsible for determining eligibility for inclusion. This person has no information about the patients included in the trial and has no influence on the allocation sequence or the decision about eligibility for inclusion. |
| c | Were the groups similar at baseline with regard to the most important prognostic indicators? | Groups must be similar at baseline with regard to at least three of the four prognostic indicators of age, sex, duration of symptoms and value of main outcome measure(s). |
| Interventions | ||
| e | Were co‐interventions avoided or similar for all groups? | Co‐interventions should either have been avoided in the trial design or be similar in the groups. |
| f | Were the patients blinded for the intervention? | Adequate information about blinding must have been provided. |
| Outcome measurement | ||
| g | Was the outcome assessor blinded to the intervention? | Adequate information about blinding must have been provided. |
| j | Was the drop‐out/loss to follow‐up rate described and acceptable? | Included patients who did not complete the follow‐up period or were not included in the analysis, must have been described. If the percentage of drop‐outs and loss to follow‐up is < 20% for short‐term follow‐up and < 30% for long‐term follow‐up, and loss to follow‐up does not lead to substantial bias, a '+' is scored. (N.B. these percentages are arbitrary and not supported by empirical evidence). |
| l | Was the timing of the outcome assessment similar for all groups? | Timing of outcome assessment should have been started from the moment of treatment allocation and be identical for all intervention groups and for all important outcome assessments. |
| Statistics | ||
| n | Did the analysis include an intention‐to‐treat analysis? | For all randomised patients, the most important moments of effect measurement should have been reported/analysed (minus missing values), irrespective of non‐compliance and co‐interventions. |
Data synthesis
Results of studies that were both adequately randomised and clinically homogeneous (for which the participants, interventions, outcome measures and timing of the follow‐up measurements were considered to be sufficiently similar), were combined, initially using a fixed‐effect model. A random‐effects model was used if the studies were statistically heterogeneous. Studies that were clinically heterogeneous or did not present the data in sufficient detail to enable statistical pooling were summarised qualitatively. For dichotomous outcomes the relative risk was used as the measure of treatment effect and for continuous outcomes the mean difference was used. In all cases 95% confidence intervals were calculated. We attempted to perform all analyses according to the intention‐to‐treat principle.
Results
Description of studies
We updated the search in 2006 and, since the last update of 2003, we identified 13 new studies. A total number of 41 publications regarding various surgical treatment options for CTS met the inclusion criteria. Of these, 35 were found in MEDLINE, five publications were identified in EMBASE and one in CENTRAL. Two studies were published twice (in German and in English), so the results of both sets of papers were combined (Benedetti 1996 and Sennwald 1995; Brüser 1999 and Richter 1996). Similarly, data from two publications of the same trial, one reporting short‐term and the other long‐term data (Holmgren 1985 and Holmgren 1987), were combined for the purposes of this review. We excluded three studies because the participants were not adequately randomised (Borisch 2003; Foulkes 1994; Schäfer 1996), one cost‐analysis study (Leger 2000) and one study because it addressed the application of corticosteroid irrigation during surgery (Padua 2003). In the study by Agee and colleagues (Agee 1992), inadequate randomisation applied to the 25 patients with bilateral involvement, but not to the remaining 97 patients with unilateral involvement. Data regarding return to work were presented separately for those 97 patients and were included in our review. Thus, we included 33 studies in the review (among which 10 were new studies). Details of the participants, interventions and outcomes in these studies are presented in the Table 'Characteristics of included studies'.
Sixteen studies compared ECTR with standard OCTR (Agee 1992; Atroshi 2006; Benedetti 1996 and Sennwald 1995; Brown 1993; Dumontier 1995; Eichhorn 2003; Erdmann 1994; Ferdinand 2002; Foucher 1993; Hoefnagels 1997; Jacobsen 1996; MacDermid 2003; Saw 2003; Stark 1996; Trumble 2002; Westphal 2000) and four studies compared ECTR with OCTR using a modified incision (Eichhorn 2003; Mackenzie 2000; Rab 2006; Wong 2003). Different types of ECTR were applied. All techniques were aimed at dividing the transverse carpal ligament from within the carpal tunnel but differed in the way in which this was achieved. Ten studies addressed Agee's one‐portal technique (Agee 1992; Benedetti 1996 and Sennwald 1995; Ferdinand 2002; Foucher 1993; Hoefnagels 1997; Mackenzie 2000; Saw 2003; Stark 1996; Trumble 2002; Westphal 2000) whereas in nine studies Chow's two‐portal technique was used (Atroshi 2006; Brown 1993; Dumontier 1995; Eichhorn 2003; Erdmann 1994; Jacobsen 1996; MacDermid 2003; Rab 2006; Wong 2003).
Fourteen studies solely addressed patients with electrophysiologically‐confirmed CTS (Agee 1992; Atroshi 2006; Benedetti 1996 and Sennwald 1995; Brown 1993; Eichhorn 2003; Erdmann 1994; Ferdinand 2002; Hoefnagels 1997; Jacobsen 1996; MacDermid 2003; Mackenzie 2000; Rab 2006; Trumble 2002; Wong 2003); one study addressed both patients with and without electrophysiologically‐confirmed CTS (Stark 1996); and two studies addressed patients with clinical CTS where electrophysiological confirmation was not required (Foucher 1993; Saw 2003). In two studies it was not clear how CTS was diagnosed (Dumontier 1995; Westphal 2000). One study also addressed patients with secondary CTS (Erdmann 1994). In four studies the type of CTS was not mentioned (Eichhorn 2003; Foucher 1993; Hoefnagels 1997; MacDermid 2003). In four studies only patients with bilateral CTS were included (Ferdinand 2002; Rab 2006; Stark 1996; Wong 2003). In two of those studies the first hand was randomised to either ECTR or OCTR and, after full recovery of the first hand (Stark 1996) or after at least six months (Rab 2006), the other hand was treated with the alternative treatment. In both studies the timing of the procedures was discarded and in one the analysis pertained to all hands, violating the assumption of independent observation (Stark 1996). In the other two studies ECTR was randomly allocated to one of both hands and the other hand was treated with the alternative procedure in the same session (Ferdinand 2002; Wong 2003). Three of the four studies with a matched design applied an appropriate statistical analysis (Ferdinand 2002; Rab 2006; Wong 2003).
Finally, in the study by Agee and colleagues (Agee 1992), randomisation of patients with bilateral CTS was discarded because patients who were randomised to ECTR refused to undergo OCTR as a second procedure. Therefore, the 25 patients with bilateral CTS have been omitted from further analysis.
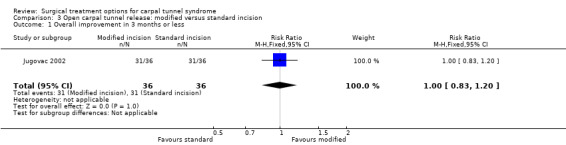
Six studies compared standard incision with a modified incision applied to OCTR (Brüser 1999 and Richter 1996; Citron 1997; Eichhorn 2003; Jugovac 2002; Nakamichi 1997; Nitzsche 1999). Although these studies used different types of modified incisions they were considered to be more or less similar because the purpose of all these new types of incisions was to provide an alternative to ECTR. These techniques aimed to reduce post‐operative morbidity (like in ECTR), but also allowed release of the transverse carpal ligament (partly) under direct vision (as in OCTR). One study addressed both patients with idiopathic and secondary CTS without requiring electrophysiological confirmation (Citron 1997) while three studies addressed only patients with electrophysiologically‐confirmed, idiopathic CTS (Brüser 1999 and Richter 1996; Eichhorn 2003; Nakamichi 1997). One study addressed patients with electrophysiologically‐confirmed CTS and excluded patients with traumatic CTS (Jugovac 2002) and one study did not mention the type of CTS patient (Nitzsche 1999). In this study additional epineurotomy was applied in both intervention groups.
One study compared standard OCTR with OCTR with lengthening of the flexor retinaculum (Dias 2004). This study involved 26 patients with either idiopathic or secondary, bilateral, electrophysiologically‐confirmed CTS. Lengthening was randomly allocated to one of both hands and the other hand was treated with standard OCTR in the same session. An appropriate matched statistical analysis was applied.
Three studies compared OCTR through a mini incision assisted by the Knifelight instrument with either standard OCTR (Bhattacharya 2004; Helm 2003) or OCTR with a limited incision (Cellocco 2005). One study included only electrophysiologically‐confirmed cases of CTS (Cellocco 2005) and in one study the type of CTS (idiopathic or secondary, clinical or electrophysiologically‐confirmed) was not mentioned (Helm 2003). The final study addressed 32 patients with bilateral, clinical CTS (Bhattacharya 2004). The hand with the most severe symptoms was randomly allocated to one of the procedures and the other hand was treated with the alternative procedure after six weeks. It was not clear whether an appropriate, matched analysis was performed in this study.
Three studies compared standard OCTR with additional internal neurolysis (Holmgren 1985 and Holmgren 1987; Lowry 1988; Mackinnon 1991). One study pertaining to idiopathic cases of CTS did not require electrophysiological confirmation of CTS for inclusion (Mackinnon 1991); one study addressed only severe cases of CTS (Lowry 1988); and one study did not mention the type of CTS (Holmgren 1985 and Holmgren 1987).
One study pertaining to patients with both idiopathic and secondary electrophysiologically‐confirmed CTS, compared standard OCTR with additional epineurotomy (Leinberry 1997).
The final study addressed patients with idiopathic, electrophysiologically‐confirmed CTS and compared standard OCTR with additional tenosynovectomy (Shum 2002).
Risk of bias in included studies
With the first release of this review, the two review authors initially agreed on 90% of the assessments of methodological quality. Disagreement was due to reading errors (65%) and interpretation differences (35%). With the 2003 update (seven additional studies), the two review authors initially agreed on 79 of 91 items (87%). With the 2006 update (10 additional studies), the two review authors initially agreed on 93 of 130 items (72 %). All disagreements were resolved by discussion. Additional Table 2 presents the scores on the internal validity items of the criteria list. The studies met between zero and all of the eight or nine internal validity criteria. Seventeen of the 33 studies fulfilled at least five of the validity criteria. Many studies reported on randomised treatment allocation but failed to describe either the exact procedure (b1) or whether concealed allocation had been performed (b2). The application of allocation concealment was mentioned explicitly in seven trials (Atroshi 2006; Benedetti 1996 and Sennwald 1995; Bhattacharya 2004; Dias 2004; Jugovac 2002; Mackinnon 1991; Saw 2003). Information on blinding of the outcome assessor (g) was often not provided.
2. Internal validity scores (b1, b2, c, e, f, g, j, l, n).
| Reference | b1 | b2 | c | e | f | g | j | l | n |
| Adequate randomisation | Adequate allocation concealment | Groups similar at baseline | Co‐interventions similar or avoided | Adequate patient blinding | Adequate assessor blinding | Dropout rate described and acceptable | Assessment timing similar for all groups | Intention to treat analysis included? | |
| ECTR versus OCTR | |||||||||
| Agee 1992 | ‐ | ? | ? | ? | n/a | ? | ? | + | ? |
| Atroshi 2006 | + | + | + | + | n/a | ‐ | + | + | + |
| Benedetti 1996 + Sennwald 1995 | + | + | + | + | n/a | ? | + | + | ‐ |
| Brown 1993 | + | ‐ | + | + | n/a | + | + | + | + |
| Dumontier 1995 | + | ‐ | ? | + | n/a | ? | ‐ | + | ‐ |
| Eichhorn 2003 | ? | ? | ? | ? | n/a | ? | ? | ? | ‐ |
| Erdmann 1994 | + | ? | + | ? | n/a | ? | ? | + | + |
| Ferdinand 2002 | + | ? | ? | + | n/a | + | + | + | ? |
| Foucher 1993 | ? | ? | ? | ? | n/a | ? | ? | + | ? |
| Hoefnagels 1997 | ? | ? | + | + | n/a | ? | + | + | ‐ |
| Jacobsen 1996 | ? | ? | + | + | n/a | ‐ | + | + | + |
| Macdermid 2003 | ? | ? | + | ? | n/a | + | ? | + | ? |
| Saw 2003 | + | + | + | + | n/a | + | + | + | + |
| Stark 1996 | ? | ? | ? | + | n/a | ? | + | + | + |
| Trumble 2002 | + | ? | + | + | n/a | + | + | + | ? |
| Westphal 2000 | ? | ? | + | ? | n/a | ? | ? | + | ? |
| ECTR versus OCTR with modified incision | |||||||||
| Eichhorn 2003 | ? | ? | ? | ? | n/a | ? | ? | ? | ‐ |
| Mackenzie 2000 | ? | ? | ? | + | n/a | ? | ? | + | ‐ |
| Rab 2006 | + | ? | + | + | n/a | ? | + | + | + |
| Wong 2003 | + | ? | + | + | n/a | ? | + | + | ? |
| OCTR: modified versus standard incision | |||||||||
| Brüser 1999 + Richter 1996 | + | ? | + | ‐ | n/a | ? | + | + | + |
| Citron 1997 | ? | ? | + | ? | n/a | ? | + | + | + |
| Eichhorn 2003 | ? | ? | ? | ? | n/a | ? | ? | ? | ‐ |
| Jugovac 2002 | + | + | ? | ? | n/a | + | + | + | + |
| Nakamichi 1997 | + | ? | ? | + | n/a | + | + | + | + |
| Nitzsche 1999 | ? | ? | ? | ? | n/a | ? | + | ? | ? |
| OCTR versus OCTR with lengthening of the flexor retinaculum | |||||||||
| Dias 2004 | + | + | + | + | + | + | + | + | + |
| OCTR with Knifelight versus OCTR alone | |||||||||
| Bhattacharya 2004 | + | + | + | + | n/a | ‐ | ? | + | ‐ |
| Helm 2003 | + | ? | + | ? | n/a | ‐ | + | + | ‐ |
| OCTR with Knifelight versus OCTR with limited incision | |||||||||
| Cellocco 2005 | ???? | ? | + | + | n/a | ? | ? | ‐ | ? |
| OCTR with internal neurolysis versus OCTR alone | |||||||||
| Holmgren 1985 + Holmgren 1987 | ? | ? | ? | + | ? | ? | + | + | + |
| Lowry 1988 | + | ? | ? | + | + | + | + | + | ‐ |
| Mackinnon 1991 | + | + | + | + | + | + | + | + | ? |
| OCTR with epineurotomy versus OCTR alone | |||||||||
| Leinberry 1997 | + | ? | + | + | ? | + | + | + | + |
| OCTR with tenosynovectomy versus OCTR alone | |||||||||
| Shum 2002 | + | ? | ? | ? | ? | ? | ? | ? | ? |
Effects of interventions
With a few exceptions, study results could not be combined because the studies were heterogeneous with regard to outcome measures (different types of symptoms assessed or different scales used; different definitions of time to return to work or activities of daily living (ADL); different complications; different timing of the follow‐up measurements). In addition, many studies suffered from poor data presentation or failed to present effect estimates with 95% confidence intervals (CI). We present quantitative results only when reported appropriately by the original authors.
Endoscopic versus open carpal tunnel release
Of the 16 studies, 11 presented the short‐term effects on various types of CTS symptoms expressed on different scales (Atroshi 2006; Brown 1993; Dumontier 1995; Erdmann 1994; Ferdinand 2002; Hoefnagels 1997; MacDermid 2003; Saw 2003; Stark 1996; Trumble 2002; Westphal 2000). In eight studies, no significant differences between the groups were found at three months or less (Brown 1993; Dumontier 1995; Erdmann 1994; Ferdinand 2002; Hoefnagels 1997; MacDermid 2003; Saw 2003; Westphal 2000) (Table 3). In two studies, a significantly better effect was noted in the ECTR group for at least one of the outcome measures (Atroshi 2006; Trumble 2002) (Table 3).
3. Endoscopic versus open carpal tunnel release.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work/ADL | Complications |
| Agee 1992 | Results of 97 adequately randomised patients with unilateral CTS not presented separately. | Results of 97 adequately randomised patients with unilateral CTS not presented separately. | Median 25 (ECTR) and 46.5 (OCTR) days (significant difference between the groups). | ECTR: re‐operation needed with OCTR in 2 of 82 patients; transient ulnar neurapraxia (2 patients). OCTR: injury to deep motor branch of ulnar nerve (1 patient); bowstringing of digital flexor tendons (1); wound dehiscence (2). |
| Atroshi 2006 | Mean symptom severity score (Levine) after 3 months: ECTR 1.5; OCTR 1.5. Mean functional status score (Levine) after 3 months: ECTR 1.3; OCTR 1.3. Difference in mean pain scores (0‐100) after 3 months ‐13.3 (95%‐CI ‐21.3 to ‐5.3) in favour of ECTR. | Mean symptom severity score (Levine) after 12 months: ECTR 1.4; OCTR 1.4 (NS). Mean functional status score (Levine) after 12 months: ECTR 1.3; OCTR 1.2 (NS). Difference in mean pain scores (0‐100) after 12 months ‐5.8 (95%‐CI ‐13.3 to ‐1.7) in favour of ECTR. | Not on sick leave before surgery: mean difference ‐5 days (95%CI ‐11.5 to 1.5) in favour of ECTR. On sick leave before surgery: mean difference 8 days (95%‐CI ‐62.5 to 78.5) in favour of OCTR. | Repeat surgery: ECTR 2/63 (3%); OCTR 1/65 (2%). No other complications. |
| Benedetti 1996 and Sennwald 1995 | Not assessed. | Not assessed. | Mean 24 (ECTR) and 42 (OCTR) days (significant difference between the groups). | 1 conversion to OCTR and 1 transient neurapraxia after ECTR. 1 painful hypertrophic scar and 1 reflex sympathetic dystrophy after OCTR. |
| Brown 1993 | Improvement in symptoms (paraesthesiae, numbness) in 99% (ECTR) and 98% (OCTR) after 12 weeks (difference 1% [95%CI ‐3 to 5%] ). | Not assessed. | Median 14 (ECTR) and 28 (OCTR) days (significant difference between the groups). | Significantly more scar tenderness after OCTR versus ECTR after 12 weeks (no significant differences after 3 and 6 weeks). No significant differences between the groups in tenderness of the thenar eminance at 3, 6 and 12 weeks. 1 partial transection of the superficial palmar arch, 1 digital‐nerve contusion, 1 ulnar‐nerve neurapraxia and 1 wound hematoma after ECTR. |
| Dumontier 1995 | Persisting paraesthesiae after 3 months: 7% (OCTR) versus 12% (ECTR). Persisting pain after 3 months: 43.3% (OCTR) versus 38.5% (ECTR). | Paraesthesiae completely disappeared in all patients after 6 months. Persisting pain after 6 months: 28% (OCTR) versus 25% (ECTR). | Percentage of patients returned to work (OCTR versus ECTR): 72% versus 45% after 1 month; 90% versus 72% after 3 months. | Transient reflex sympathetic dystrophy in 4 patients (2 in each group). |
| Eichhorn 2003 | Overall severity score (scale 1‐6) after > 1 year: OCTR 2.2; ECTR 2.1. | Two postoperative infections after OCTR; none in the ECTR group. Recurrences: ECTR 3/128 (2%), OCTR 4/60 (7%). Need for repeated surgery: ECTR 2/128 (2%); OCTR 3/60 (5%). | ||
| Erdmann 1994 | Significantly more improvement in carpal tunnel pain in favour of ECTR after 1, 2 and 4 weeks, but no significant difference between the groups after 3 months. | No significant difference in carpal tunnel pain between the groups after 6 and 12 months. | Mean 14 (ECTR) and 39 (OCTR) days (only patients not simultaneously operated on both hands) (significant difference between the groups). | 1 ulnar nerve paraesthesiae and 1 incomplete release after ECTR. 1 wound infection, 1 scar tethering and 5 scar hypertrophy after OCTR. |
| Ferdinand 2002 | After 12 weeks better endoscopic Jebson scores (75 vs 65). | After 12 months better endoscopic Jebson scores (59 vs 48). | Not applicable (all patients had bilateral CTS). | 3 conversions to OCTR after ECTR. 1 persisting wound pain in each group. 1 persisting symptoms and 1 superficial nerve injury after OCTR. |
| Foucher 1993 | No data presented. | No data presented. | No significant differences in time to return to work between the groups (all 17 days). | 1 algodystrophy and 2 conversions to OCTR after ECTR. |
| Hoefnagels 1997 | Mean symptom severity score after 3 months 1.6 ± 0.7 after ECTR; 1.5 ± 0.5 after OCTR (no significant difference). | Not assessed. | Longer than 4 weeks absence from work in 16% (ECTR); 13% (OCTR) (difference 3%, 95%CI ‐7 to 14). | Significantly less postoperative pain after ECTR versus OCTR after 1 week. 1 conversion to OCTR, 1 broken knife left in operation wound and 1 increased numbness in fingertips after ECTR. |
| Jacobsen 1996 | Not assessed. | Not assessed. | Mean 17 (ECTR) and 19 (OCTR) days (no significant difference between the groups). | 3 transient numbness on the radial side of the ring finger after ECTR. 1 prolonged wound secretion after OCTR. |
| Macdermid 2003 | After 12 weeks no significant differences in pain (McGill) (8 vs 12), symptom severity score (Levine) (1.8 vs 2.0) and functional status (SF‐36) (47 vs 42). | After a mean of 3.2 years lower satisfaction scores after ECTR (85% vs 93%). | No significant differences (no quantitative data presented). | No complications reported. Within 4 year in 5% of the ECTR cases re‐operation needed. |
| Saw 2003 | Area under the curve analysis of symptom severity score (Levine) after 3 months: ECTR 120 (IQR 21); OCTR 119 (IQR 19) (P = 0.70). Area under the curve analysis of functional status score (Levine) after 3 months: ECTR 109 (IQR 22); OCTR 108 (IQR 24) (P = 0.98). | Mean (SD) days off work ECTR 18 (11); OCTR 26 (14). Mean difference ‐8 (95% CI ‐13 to ‐2). | ECTR: 1 transient numbness index finger, 1 superficial wound infection, 1 repeat surgery. OCTR: 1 hyperaesthesia over scar area, 1 superficial wound infection, 1 superficial haematoma, 1 persistence of symptoms. | |
| Stark 1996 | (Matched pairs) Pain completely relieved in 20/20 (ECTR) vs. 15/20 hands (OCTR) after 3 months. Persisting paraesthesiae in 1/20 (ECTR) vs. 1/20 (OCTR) after 3 months. | (Matched pairs) Pain completely relieved in 20/20 (ECTR) vs 19/20 hands (OCTR) after 8 months. Persisting paraesthesiae in 1/20 (ECTR) vs 1/20 (OCTR) after 8 months. | Mean 20 (ECTR) versus 30 (OCTR) days (significant difference between the groups). | 1 subcutaneous hematoma and 1 loss of strength and mobility in the wrist after ECTR. 2 loss of strength and 1 swollen/stiff fingers after OCTR. |
| Trumble 2002 | After 12 weeks better scores for satisfaction (4.4 vs 4.0, non‐significant), symptom severity (Levine) (1.8 vs 2.5; significant) and functional status (Levine) (1.7 vs 2.4; significant.). | After 12 months no significant differences for satisfaction (4.6 vs 4.5), symptom severity score (Levine) (1.8 vs 1.8) and functional status score (Levine) (1.7 vs 1.7). | Median 18 (ECTR) and 38 (OCTR) days (significant difference between the groups). | After OCTR 2 reflex sympathetic dystrophy and 1 repeat procedure. |
| Westphal 2000 | Symptom severity score (variant of Levine) after 3 months: ECTR 11.0 (3.7); OCTR 10.6 (2.6). Mean functional status score (variant of Levine) after 3 months: ECTR 10.2 (4.5); OCTR 9.8 (4.4). | Mean 34.5 (ECTR) versus 36 (OCTR) days (no significant difference between the groups). |
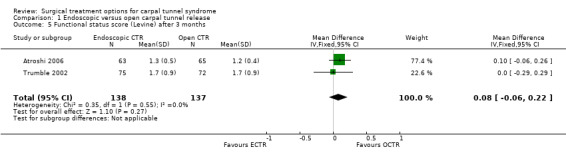
Eight studies addressed long‐term results on symptoms and no significant differences between the groups were found (Dumontier 1995; Eichhorn 2003; Erdmann 1994; Ferdinand 2002; MacDermid 2003; Stark 1996; Trumble 2002) except in one study (Atroshi 2006) in which there was a significant difference in mean pain scores (scale 0 to 100) after 12 months in favour of ECTR (mean difference (MD) ‐5.8, 95% CI ‐13.3 to ‐1.7).
Meta‐analysis was possible with three studies (Atroshi 2006; Hoefnagels 1997; Trumble 2002) that presented the symptom severity score and the functional status score of Levine (Levine 1993) after three months. ECTR resulted in a lower symptom severity score (MD ‐0.2; 95% CI ‐0.5 to 0.2) (see Comparison 01, Outcome 02) and a lower functional status score (MD ‐0.2; 95% CI ‐0.6 to 0.2) (see Comparison 01, Outcome 03). These differences were very small and in both meta‐analyses there was large heterogeneity with an I2 of approximately 90%, possibly due to the influence of one study (Trumble 2002). Two studies addressed the respective scores after one year (Atroshi 2006; Trumble 2002), resulting in MDs approaching zero (see Comparison 01, Outcome 04 and Outcome 05).
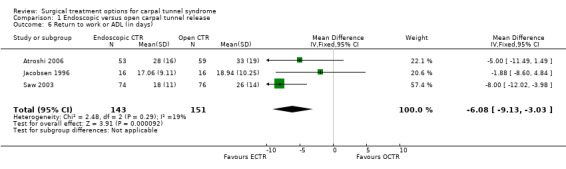
Fourteen studies reported results (expressed in many different formats) regarding return to work or ADL. Eight of those (Agee 1992; Atroshi 2006; Benedetti 1996 and Sennwald 1995; Brown 1993; Erdmann 1994; Saw 2003; Stark 1996; Trumble 2002) concluded that patients returned to work or ADL earlier after ECTR (Table 3), whereas one study found the opposite (Dumontier 1995). Five studies (Foucher 1993; Hoefnagels 1997; Jacobsen 1996; MacDermid 2003; Westphal 2000) found no significant difference. Overall, the mean difference in return to work ranged from zero to 25 days in favour of ECTR. Meta‐analysis was possible for three studies (Atroshi 2006; Jacobsen 1996; Saw 2003). The weighted mean difference in time to return to work was ‐6 days (95% CI ‐9 to ‐3) in favour of ECTR (see Comparison 01, Outcome 06).
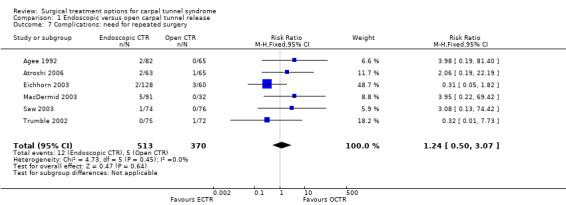
All studies comparing ECTR with OCTR reported complications in both groups, but no major complications resulting in permanent damage or major impairments were described (Table 3). It seems that ECTR gives more transient nerve problems (for example neurapraxia, numbness, paraesthesiae) and OCTR more wound problems (for example infection, hypertrophic scarring, scar tenderness). In a few cases, ECTR had to be abandoned and OCTR was performed instead. In one study (Trumble 2002) two cases of sympathetic reflex dystrophy were recorded out of 25 hands in the OCTR group. The need for repeated surgery was assessed in six studies (Agee 1992; Atroshi 2006; Eichhorn 2003; MacDermid 2003; Saw 2003; Trumble 2002); the relative risk was 1.2 (95% CI 0.5 to 3.1) in favour of OCTR. Repeated surgery was needed in 12 out of 513 ECTR procedures versus in 5 out of 370 OCTR procedures (see Comparison 01, Outcome 07).
Endoscopic carpal tunnel release versus open release with a modified incision
Four studies addressed this comparison (Eichhorn 2003; Mackenzie 2000; Rab 2006; Wong 2003). In one study a significant difference in pain score was found after two and four weeks but not after 8 and 16 weeks or 6 to12 months (Wong 2003). Another study involving 10 patients with bilateral CTS did not find a significant difference in pain score, the symptom severity score and the functional status score of Levine at 12 weeks and 12 months follow‐up (Rab 2006). No significant difference between the groups was observed after at least one year follow‐up in another study (Eichhorn 2003) (Table 4).
4. Endoscopic versus modified open carpal tunnel release.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Eichhorn 2003 | Mean overall severity score (scale 1‐6) after > 1 year: ECTR 2.1; mini incision 2.2. | Not assessed. | None. Recurrences: ECTR 2%, mini‐incision 14%. Need for repeated surgery ECTR 2%, mini‐incision 9%. | |
| Mackenzie 2000 | No quantitative data presented. | Not assessed. | Not assessed. | 1 pillar pain in each group. |
| Rab 2006 | At 12 weeks: mean symptom severity score (Levine) ECTR 14.7; modified OCTR 16.8 (P = 0.27). Mean functional status score (Levine) ECTR 10.3; modified OCTR 12.3 (P = 0.16). Pain (VAS) ECTR 0.3; modified OCTR 1.7 (P = 0.10). | At 12 months: mean symptom severity score (Levine) ECTR 14.0; modified OCTR 12.8 (P = 0.49). Mean functional status score (Levine) ECTR 11.1; modified OCTR 9.9 (P = 0.39). Pain (VAS) ECTR 0.6; modified OCTR 0.2 (P = 0.43). | Not assessed. | None. |
| Wong 2003 | Statistically significant difference in reduction of wound pain at 2 and 4 weeks in favour of ECTR, but not after 8 and 16 weeks. | At 12 months: complete relief or minimal symptoms ECTR 27/30 hands (90%); modified OCTR 27/29 hands (93%). Preference for ECTR 6, modified OCTR 13; no preference 10. | Not Assessed. | None. |
No study addressed return to work or ADL.
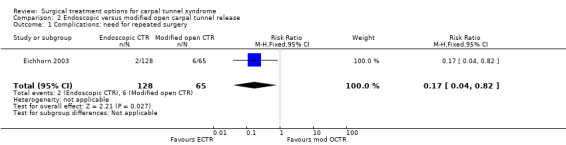
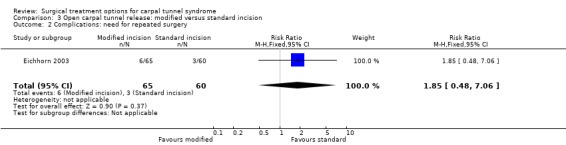
No complications were reported in two studies (Rab 2006; Wong 2003) and one study reported one instance of pillar pain in each group (Mackenzie 2000). Pillar pain is pain at the so‐called "pillars", the origins of the thenar and hypothenar muscle on either side of the surgical incision. In one study repeated surgery was necessary in two of 128 ECTR patients (1.5%) and in six of 65 patients treated with modified OCTR (9%; relative risk (RR) 0.2, 95% CI 0.04 to 0.8) (Eichhorn 2003).
Open carpal tunnel release: modified versus standard incision
Six studies compared OCTR using a modified incision versus OCTR with a standard incision (Brüser 1999 and Richter 1996; Citron 1997; Eichhorn 2003; Jugovac 2002; Nakamichi 1997; Nitzsche 1999). Trivial to minor differences between the groups were found with regard to relief of symptoms (expressed in many different non‐combinable formats) in the 1.5 to 3 months after surgery (Brüser 1999 and Richter 1996; Jugovac 2002; Nakamichi 1997) and 0.5 to 2 years after surgery (Citron 1997; Eichhorn 2003; Nakamichi 1997) (Table 5).
5. Open carpal tunnel release: modified versus standard incision.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Brüser 1999 and Richter 1996 | No significant differences in night symptoms, tingling and numbness between the groups after 1, 2, 3, 6 weeks. | Not assessed. | Median (mean) 21 (17.6) days versus 18 (18.8) days (no significant difference between the groups). | No significant differences in scar tenderness and tenderness of the thenar and hypothenar eminances after 1, 2, 3, 6 weeks (differences ranging from 0.3 to 0.6 on 5 point scale). |
| Citron 1997 | No suitable data presented. | 96% (modified incision) and 89% (standard incision) completely free of symptoms after 12 months (difference 7%, 95%CI ‐9 to 22). | Not assessed. | No significant difference in pain of the thenar eminance between the groups. Significantly more recovery in scar sensitivity after modified incision compared with standard incision (differences ranging from 0.5 to 1.0 on a visual analogue scale). 1 delayed wound healing in both groups. |
| Eichhorn 2003 | Mean overall severity scores (scale 1‐6) after > 1 year: OCTR 2.2; mini incision 2.2 | Two postoperative infections after OCTR; none in the mini‐incision group. Recurrences: OCTR 7% and mini‐incision 14%. Need for repeated surgery: OCTR 5% and mini‐incision 9%. | ||
| Jugovac 2002 | No significant difference in relief (31/36 vs 31/36) between the groups after 3 months. | Not assessed. | Median 15 (5 to 45) days versus 30 (10 to 60) days (significant difference between the groups in favour of limited incision). | Scar tenderness: 3/36 (limited incision) versus 8/36 (standard incision). |
| Nakamichi 1997 | No significant differences in symptoms (numbness and paraesthesiae) between the groups after 3, 6, 13 weeks. | No significant differences in symptoms (numbness and paraesthesiae) between the groups after 6, 12, 24 months. 86% (modified incision) and 89% (standard incision) completely free of symptoms after 24 months (difference ‐3%, 95% CI ‐16 to 10). | Not assessed. | Standard incision significantly more scar pain and tenderness compared with modified incision after 3, 6, 13 weeks (differences ranging from 0.5 to 0.9 on 4 point scale). No other complications. |
| Nitzsche 1999 | No quantitative data reported. | No quantitative data reported. | No quantitative data reported. | No quantitative data reported. |
Two studies examined the time to return to work. One study found a significant earlier return to work in favour of the short incision group (Jugovac 2002) whereas the other study did not find a significant difference in effect between the two types of incisions (Brüser 1999 and Richter 1996).
No major complications (resulting in permanent damage or major impairments) were reported (Table 5). The standard incision resulted in slightly more scar pain or tenderness compared to the modified incision in three studies (Citron 1997; Jugovac 2002; Nakamichi 1997), but this was not confirmed in a fourth study (Brüser 1999 and Richter 1996). In one study two instances of wound infection were reported in the standard OCTR group; repeated surgery was necessary in six of 65 patients treated with modified OCTR (9%) and in three of 60 patients with standard OCTR (5%; RR 1.8, 95% CI 0.5 to 7.1) (Eichhorn 2003).
Open carpal tunnel release: with or without lengthening of the flexor retinaculum
One excellent study that fulfilled all methodological criteria was identified (Dias 2004). After 12 weeks and (on average) 26 weeks, no significant differences in symptom severity score and functional status score were found (Table 6). Return to work was not assessed because only patients with bilateral involvement were included. No complications were reported.
6. OCTR with versus without lengthening of the flexor retinaculum.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Dias 2004 | Mean symptom severity score (Levine) after 12 weeks: OCTR 1.2 (95% CI 1.1 ‐ 1.3); lengthening 1.2 (95% CI 1.1‐1.4). Mean functional status score (Levine) after 12 weeks: OCTR 1.2 (95% CI 1.1‐1.3); lengthening 1.2 (95% CI 1.0‐1.3). | Mean symptom severity score (Levine) after an average of 26 weeks: OCTR 1.3 (95% CI 1.2 ‐ 1.5); lengthening 1.3 (95% CI 1.1‐1.5). Mean functional status score (Levine) after an average of 26 weeks: OCTR 1.2 (95% CI 1.1‐1.3); lengthening 1.3 (95% CI 1.1‐1.5). | Not assessed. | None reported. |
Open carpal tunnel release: mini open technique assisted by the Knifelight instrument versus standard OCTR or OCTR with mini incision
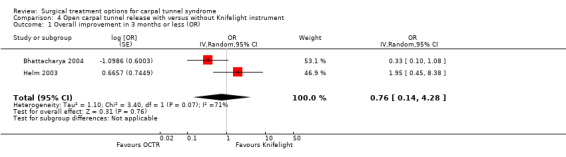
Three studies compared OCTR through a mini incision assisted by the Knifelight instrument with either standard OCTR (Bhattacharya 2004; Helm 2003) or with OCTR using limited open incision (Cellocco 2005). In two studies the follow‐up measurement after six weeks revealed no significant difference in the proportion of effectively treated patients between the groups (Bhattacharya 2004; Helm 2003) (see Comparison 04, Outcome 01). One study found a significant difference in the symptom severity score and the functional status score of Levine after a mean follow‐up period of 19 (range 12 to 28) months, in favour of the Knifelight instrument; but at a mean follow‐up of 30 (range 24 to 42) months no significant differences were observed (Cellocco 2005) (Table 7).
7. Mini open technique with Knifelight versus OCTR.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Knifelight versus OCTR | ||||
| Bhattacharya 2004 | Symptom relief after 6 weeks: Knifelight 17/26 (65%); OCTR 19/26 (73%). | Not assessed. | Median no. of weeks (range): Knifelight 2 (0‐6); OCTR 2 (0‐6) (P = 0.8). | Scar tenderness: Knifelight 8/26 (31%); OCTR 17/26 (65%). Postoperative numbness after Knifelight (1) |
| Helm 2003 | After 6 weeks: symptoms cured 36/39 (Knifelight) vs 37/43 (standard); none or mild scar tenderness 35/39 vs 21/43. | Not assessed. | Significant difference in time to return to work (20 vs 28 days). | 1 sympathetic reflex dystrophy, 2 conversions to OCTR (presumably analysed in the OCTR group), 1 superficial wound infection and 1 transient numbness of index finger after Knifelight. 1 partial wound dehiscence, 4 pillar pain, 1 unexplained thumb pain, 1 mild stiffness of fingers and 1 transient numbness of index finger after standard OCTR. |
| Knifelight versus OCTR (limited open) | ||||
| Cellocco 2005 | Not assessed. | Follow up 19 months (range 12 to 28 months): mean symptom severity score (Levine) 1.46 (Knifelight) versus 2.04 (OCTR limited open) (P < 0.001); mean functional status score (Levine) 2.02 (Knifelight) versus 2.53 (OCTR limited open) (P < 0.001). Follow up 30 months (range 24‐42 months): mean symptom severity score (Levine) 1.28 (Knifelight) versus 1.39 (OCTR limited open) (NS); mean functional status score (Levine) 1.87 (Knifelight) versus 1.73 (OCTR limited open) (NS). | Mean no. of days: Knifelight 16.6 (range 15 to 18); OCTR (limited open) 25.4 (range 23 to 29). | Recurrents disease Knifelight 5% vs OCTR (limited open) <1%. |
In two studies patients treated with the Knifelight instrument returned to work significantly earlier (eight to nine days) than the standard OCTR group (Cellocco 2005; Helm 2003), but in one study no difference was observed (Bhattacharya 2004) (Table 7).
In one study the following complications were reported: sympathetic reflex dystrophy (1), superficial wound infection (1), procedure replaced by standard OCTR (2) in the Knifelight group (39 patients); and pillar pain (4) and partial wound dehiscence (1) in the standard OCTR group (43 patients) (Helm 2003). We suspect that the two patients in whom the procedure could not be performed with the Knifelight instrument, were analysed in the standard OCTR group. Significantly, less scar tenderness was observed in the Knifelight group in one study (Bhattacharya 2004). In one study recurrent disease occurred more often in the Knifelight group (5%) compared to the limited open incision OCTR group (< 1%) (Cellocco 2005).
Open carpal tunnel release: with or without internal neurolysis
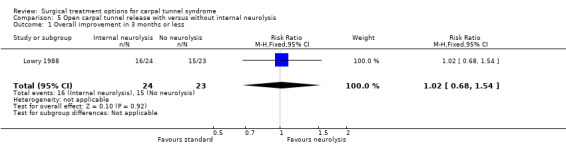
In the three studies that evaluated OCTR with or without internal neurolysis, no or trivial differences between the procedures were found with respect to improving CTS symptoms after three to four weeks (Holmgren 1985) and after three months (Lowry 1988) (Table 8). Similar long‐term results were found (Holmgren 1987; Mackinnon 1991). No study addressed return to work or ADL. One study reported a similar number of complications in both groups (Lowry 1988); one study merely stated that there were no complications ascribable to internal neurolysis (Holmgren 1985; Holmgren 1987) and the other study provided no information on complications (Mackinnon 1991). Pooling was not possible due to the use of different outcome measures or different scales.
8. OCTR with versus without internal neurolysis.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Holmgren 1985 and Holmgren 1987 | No significant differences in pain, paraesthesiae and hypoaesthesiae between the groups after 3‐4 weeks. | 89% totally free of symptoms in both groups after 6 months and 86% (with neurolysis) versus 75% (without neurolysis) after 3 to 4 years (difference 11%, 95% CI ‐15 to 36 ). | Not assessed. | No complications ascribable to internal neurolysis. |
| Lowry 1988 | Excellent or good clinical response (pain, sensory deficit, complications) according to neurologist in 67% (with neurolysis) and 65% (without neurolysis) after 3 months (difference 2%, 95% CI ‐26 to 29). | Not assessed. | Not assessed. | 4 persistent incisional pain, 1 hand swelling and 1 adhesive capsulitis in the group with internal neurolysis. 3 persistent incisional pain and 1 causalgia in the group without internal neurolysis. |
| Mackinnon 1991 | No data presented. | Improvement in symptoms in 81% (with neurolysis) and 88% (without neurolysis) after 12 months (difference ‐7%, 95% CI ‐25 to 11 ). | Not assessed. | No data presented. |
Open carpal tunnel release: with or without epineurotomy
Only one study was identified (Leinberry 1997). The follow‐up measurement after one year revealed no significant difference (56% versus 60%); Table 9) in the proportion of patients with complete relief of symptoms between the groups. No short‐term results or data regarding complications were presented and return to work was not assessed.
9. OCTR with versus without epineurotomy.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work /ADL | Complications |
| Leinberry 1997 | No data presented. | 56% (with epineurotomy) and 60% (without epineurotomy) totally free of symptoms (pain, altered sensibility, paraesthesias, loss of manual dexterity) after 1 year (difference ‐4% 95% CI ‐31% to 23%). | Not assessed. | No data presented. |
Open carpal tunnel release: with or without tenosynovectomy
One study was identified (Shum 2002). The follow‐up measurement after one year revealed no significant differences in symptom severity score and functional status score (Table 10). No short‐term results were presented and return to work was not assessed. No complications were observed.
10. OCTR with versus without tenosynovectomy.
| Reference | Symptoms <= 3 months | Symptoms > 3 months | Return to work / ADL | Complications |
| Shum 2002 | Not assessed. | After at least 12 months of follow‐up no significant differences between the groups with respect to symptom severity score (Levine: 1.6 (0.68) vs 1.6 (0.70)) and functional status score (Levine: 1.7 (0.71) vs 1.6 (0.62)). | Not assessed. | No wound infections. |
Discussion
As we revised this previously published systematic review (Gerritsen 2001) for publication in The Cochrane Library, not all the usual policies of the Cochrane Neuromuscular Disease Review Group were followed. We did not try to collect unpublished data or contact the original authors for information. For the updates, however, we did not apply a language restriction (unlike in the first version of our review).
The major limitation of this review was the limited availability of well‐presented, sufficiently homogeneous results. Not withstanding the fact that many patients were treated in an RCT that addressed the effect of some form of CTS treatment, formal meta‐analysis could only be performed for a few studies and outcomes. We cannot exclude that these meta‐analyses will be biased, due to possible selective presentation of the outcomes. This impeded the formulation of sound conclusions regarding the optimal treatment option for CTS. Therefore, we present the results of this systematic review in a predominantly qualitative way. For future research, we strongly agree with the recommendations made by others for developing consensus on a core set of outcomes to be addressed in RCTs regarding CTS (Gerritsen 2002; Jerosch‐Herold 2006).
This review included 33 randomised controlled trials comparing standard OCTR with various alternative surgical procedures for the treatment of CTS. Ten of the 33 trials included in this update were new trials.
The methodological quality of the included studies was assessed by the use of a list of criteria consisting of nine items pertaining to internal validity. Included in the list were criteria for concealment of allocation and double blinding which are regarded as being most important in reducing bias (Schulz 1995). With few exceptions, the methodological quality of the RCTs was fair to good and 17 of the 33 studies fulfilled at least five of the criteria for internal validity. The application of allocation concealment, however, was mentioned explicitly in only seven trials.
When dealing with patients with bilateral involvement the statistical analysis is quite a challenge. Two problems may arise when considering hands instead of patients: violation of statistical assumptions and unit of analysis errors. Common statistical methods assume independency of observations. The observations in both hands of patients with bilateral CTS, however, are not independent. When the assumption of independency is violated the standard error of the effect estimate will be underestimated, resulting in a greater chance of a type I error and too narrow confidence intervals. Among the statistical methods that can address such dependent observations, are methods for repeated measurements (included matched analyses) and generalised estimating equations. These specialised statistical methods must be applied for outcomes where it makes sense for each hand to be assessed separately, such as for improvement of CTS‐specific symptoms, complications and side‐effects. Unit of analysis errors can be made when addressing outcomes such as overall improvement and time to return to work. For these outcomes it does not make sense to refer the result to one hand or the other. In that instance 'patients' should be the units of analysis. When 'hands' are chosen, as (wrong) units of analysis, the sample size will be inflated artificially which results in an erroneously small standard error of the effect estimate and thus too narrow a confidence interval. In our systematic review, hands were the units of analysis (instead of patients) in 13 studies (Agee 1992; Brown 1993; Cellocco 2005; Erdmann 1994; Foucher 1993; Jacobsen 1996; Leinberry 1997; Lowry 1988; Mackenzie 2000; Mackinnon 1991; Saw 2003; Shum 2002; Trumble 2002). In three of these studies the number of patients with bilateral CTS was small (Foucher 1993; Mackinnon 1991; Shum 2002) so no major effect was to be expected as a result of applying the wrong unit of analysis. In ten studies, however, the percentage of patients with bilateral CTS ranged from 10 to 48% (Agee 1992; Brown 1993; Cellocco 2005; Erdmann 1994; Jacobsen 1996; Leinberry 1997; Lowry 1988; Mackenzie 2000; Saw 2003; Trumble 2002), which may have led to the above‐mentioned problems with the analysis.
In six studies a matched design was applied among patients with bilateral CTS (Bhattacharya 2004; Dias 2004; Ferdinand 2002; Rab 2006; Stark 1996; Wong 2003). Four studies applied a statistical analysis that accounted for the dependency between both hands of the same patient (Bhattacharya 2004; Dias 2004; Rab 2006; Wong 2003) but in two studies the type of analysis was not clear (Ferdinand 2002; Stark 1996). In three of these studies (Dias 2004; Ferdinand 2002; Wong 2003) ECTR was randomly allocated to one of both hands and the other hand was treated with OCTR in the same session. In the other three studies a time lag was present between the two operations, ranging from six weeks to six months (Bhattacharya 2004; Rab 2006; Stark 1996), which seems to have been ignored in the analysis.
In the 16 studies with considerable bilateral involvement it was very difficult (if not impossible) to assess an unbiased effect regarding outcomes such as return to work. For example, in the study of Brown and colleagues (Brown 1993) patients returned to work earlier after ECTR. It was not clear how previous surgery in the contralateral hand affected the return to work when surgery was done in the remaining hand. An additional problem arose in three patients where both hands underwent simultaneous release of the carpal tunnel. It was not entirely clear from the report if these hands were included in the analysis (and how). A similar problem occurred in the study of Erdmann (Erdmann 1994). Information was presented on the days for return to work in a group of 46 patients. However, 55 hands were operated on in this group and it was not clear from the report if the days for return to work were given for patients or for hands. Agee and colleagues (Agee 1992) tackled the unit‐of‐analysis problem by presenting the results regarding return to work only for patients who had been treated unilaterally. The results of the studies could be re‐analysed by the use of statistical methods that address the dependency of the data. Because the required data for such analyses were generally not presented in the original publications, cooperation of the original authors in providing the necessary information would be needed.
Despite the identification of 10 new studies, none of the existing alternatives to standard OCTR (ECTR; OCTR with a modified type of incision; lengthening of the flexor retinaculum; additional internal neurolysis, epineurotomy or tenosynovectomy; or carpal tunnel release assisted by an operating device) seemed to offer better relief from CTS symptoms in either the short term or the long term. Possible advantages of ECTR would be earlier return to work or ADL and fewer wound problems, whereas possible disadvantages may be higher complication rates and costs. In this update a meta‐analysis of the average number of days to return to work was six days lower in favour of ECTR.
Until now, overall complication rates seemed to be small and similar for ECTR and OCTR (Boeckstyns 1999). This finding was confirmed in a recent review that found major complications (defined as structural damage to nerves, arteries or tendons) in only 42 of 22,327 (0.19%) endoscopic carpal tunnel releases and in 28 of 5,669 (0.49%) open releases (Benson 2006). In our review we found a (non‐significant) excess in the need for repeat surgery after ECTR. It should be noted that although the complication rates after ECTR may be similar or less, many problematic cases of CTS are generally excluded from ECTR, for example patients needing an adjuvant procedure (such as synovectomy in the case of inflammatory arthritis), and patients with space‐occupying lesions or with stiff (arthritic) wrists (Einhorn 1996; Erdmann 1994).
In 1999, a cost‐effectiveness study concluded that the two techniques had similar total costs but that ECTR was more costly if the difference between the techniques in mean time to return to work was less than 21 days (Vasen 1999). Among the studies comparing ECTR with other procedures two reported a difference of more than 21 days (Agee 1992; Erdmann 1994), whereas our meta‐analysis resulted in an average of six days.
In one of the studies included in this review ECTR turned out to be more cost‐effective in employed patients but not in the non‐employed (Saw 2003). Finally, in a randomised controlled trial of 194 CTS patients comparing minimally invasive carpal tunnel release with OCTR treatment costs were estimated from resource use and hospital financial data (Lorgelly 2005). Minimally invasive carpal tunnel decompression turned out to be more effective but more costly. The authors concluded that the additional expense for such a small improvement in function and no improvement in symptoms would not be regarded as value‐for‐money.
Authors' conclusions
Implications for practice.
There is no strong evidence supporting the need for replacement of standard OCTR by existing alternative surgical procedures for the treatment of CTS. The decision to apply ECTR instead of OCTR seems to be merely guided by the surgeon's and patient's preferences.
Implications for research.
With respect to ECTR compared to OCTR, the collection of missing data from the original authors should be pursued in order to enable meta‐analysis of results. If possible, an individual patient data meta‐analysis should be undertaken. There is no need for new RCTs comparing OCTR plus existing additional procedures or existing alternative incisions with OCTR alone for the treatment of CTS. Future research into interventions for CTS should use standardised outcome measures to enable results from various studies to be combined. In addition, a correct unit of analysis should be chosen (patients instead of hands) or adequate statistical methods should be applied which are able to deal with the dependency between the two hands of the same patient.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2014 | Amended | Revised Published notes |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 7 February 2012 | Review declared as stable | Information added to Published notes about the updating of this review. |
| 27 May 2008 | Amended | Converted to new review format. |
| 31 July 2007 | New citation required and conclusions have changed | This review was updated in February 2007. We redid the search completely in June 2006. Ten new trials were located. Compared to the previous version of this review, the conclusions have not altered substantially. |
Notes
This review will be updated through the publication of three new reviews and then withdrawn. The first of the new reviews, Endoscopic release for carpal tunnel syndrome, has been published (Vasiliadis 2014). Reviews of modified open carpal tunnel release, and open carpal tunnel release with adjuvant procedures will follow.
Acknowledgements
We thank the editor and four peer reviewers of the Cochrane Neuromuscular Disease Group for their constructive and most helpful comments regarding the first Cochrane version of this review. The co‐authorship by Annette Gerritsen and Dick van Geldere of earlier versions of this review is very much appreciated.
Appendices
Appendix 1. Ovid MEDLINE Search Strategy
| 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trials/ 4. random allocation/ 5. double‐blind method/ 6. single‐blind method/ 7. or/1‐6 8. animals/ not humans/ 9. 7 not 8 10. clinical trial.pt. 11. exp clinical trials/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab. 14. placebos/ 15. placebo$.ti,ab. 16. random$.ti,ab. 17. research design/ 18. or/10‐17 19. 18 not 8 20. 19 not 9 21. comparative study/ 22. exp evaluation studies/ 23. follow up studies/ 24. prospective studies/ 25. (control$ or prospectiv$ or volunteer$).ti,ab. 26. or/21‐25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. Carpal Tunnel Syndrome.mp. or Carpal Tunnel Syndrome/ 31. (carp$ tunn$ or tunn$ syndrom$ or carp$ syndrom$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 32. (nerve entrapment or nerve compression or entrapment neuropath$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 33. median nerve entrapment.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 34. nerve compression syndromes/ or nerve compression syndrom$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 35. or/30‐34 36. epineurotomy.mp. 37. reconstruct$.mp. 38. release.mp. 39. SURGERY/ or surgery.mp. 40. SURGICAL PROCEDURES, OPERATIVE/ or surgical.mp. 41. or/36‐40 42. 29 and 35 and 41 |
Appendix 2. Ovid EMBASE Search Strategy
| 1. Carpal Tunnel Syndrome.mp. or Carpal Tunnel Syndrome/ 2. (carp$ tunn$ or tunn$ syndrom$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. (nerve entrapment or nerve compression or entrapment neuropath$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 4. or/1‐3 5. epineurotomy.mp. or carpal tunnel release/ or epineurotomy/ 6. surgical approach/ or surgical technique/ 7. (surgery or surgical or operation or reconstruct$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 8. or/5‐7 9. Randomized Controlled Trial/ 10. Clinical Trial/ 11. Multicenter Study/ 12. Controlled Study/ 13. Crossover Procedure/ 14. Double Blind Procedure/ 15. Single Blind Procedure/ 16. exp RANDOMIZATION/ 17. Major Clinical Study/ 18. PLACEBO/ 19. Meta Analysis/ 20. phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 21. (clin$ adj25 trial$).tw. 22. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 23. placebo$.tw. 24. random$.tw. 25. control$.tw. 26. (meta?analys$ or systematic review$).tw. 27. (cross?over or factorial or sham? or dummy).tw. 28. ABAB design$.tw. 29. or/9‐28 30. human/ 31. nonhuman/ 32. 30 or 31 33. 29 not 32 34. 29 and 30 35. 33 or 34 36. 4 and 8 and 35 |
Data and analyses
Comparison 1. Endoscopic versus open carpal tunnel release.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement in 3 months or less | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.97, 1.05] |
| 2 Symptom severity score (Levine) in 3 months or less | 3 | 451 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.53, 0.20] |
| 3 Functional status score (Levine) in 3 months or less | 3 | 451 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.60, 0.16] |
| 4 Symptom severity score (Levine) after 3 months | 2 | 275 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.17, 0.17] |
| 5 Functional status score (Levine) after 3 months | 2 | 275 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.22] |
| 6 Return to work or ADL (in days) | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐6.08 [‐9.13, ‐3.03] |
| 7 Complications: need for repeated surgery | 6 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.07] |
1.1. Analysis.
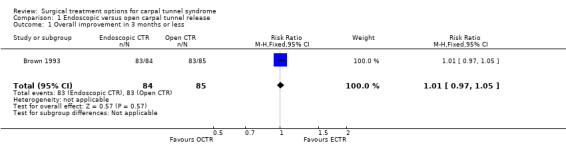
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 1 Overall improvement in 3 months or less.
1.2. Analysis.
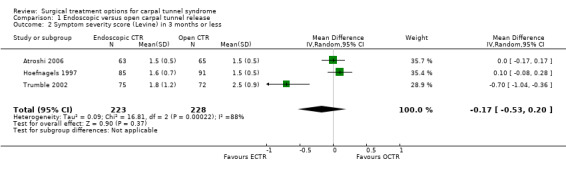
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 2 Symptom severity score (Levine) in 3 months or less.
1.3. Analysis.
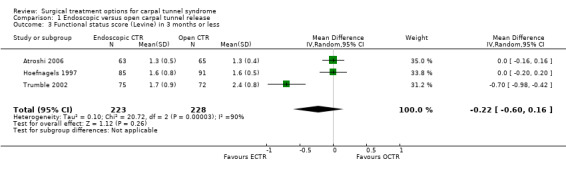
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 3 Functional status score (Levine) in 3 months or less.
1.4. Analysis.
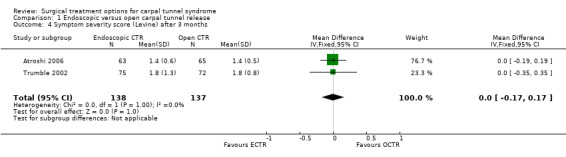
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 4 Symptom severity score (Levine) after 3 months.
1.5. Analysis.
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 5 Functional status score (Levine) after 3 months.
1.6. Analysis.
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 6 Return to work or ADL (in days).
1.7. Analysis.
Comparison 1 Endoscopic versus open carpal tunnel release, Outcome 7 Complications: need for repeated surgery.
Comparison 2. Endoscopic versus modified open carpal tunnel release.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications: need for repeated surgery | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.82] |
2.1. Analysis.
Comparison 2 Endoscopic versus modified open carpal tunnel release, Outcome 1 Complications: need for repeated surgery.
Comparison 3. Open carpal tunnel release: modified versus standard incision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement in 3 months or less | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.83, 1.20] |
| 2 Complications: need for repeated surgery | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.48, 7.06] |
3.1. Analysis.
Comparison 3 Open carpal tunnel release: modified versus standard incision, Outcome 1 Overall improvement in 3 months or less.
3.2. Analysis.
Comparison 3 Open carpal tunnel release: modified versus standard incision, Outcome 2 Complications: need for repeated surgery.
Comparison 4. Open carpal tunnel release with versus without Knifelight instrument.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement in 3 months or less (OR) | 2 | OR (Random, 95% CI) | 0.76 [0.14, 4.28] |
4.1. Analysis.
Comparison 4 Open carpal tunnel release with versus without Knifelight instrument, Outcome 1 Overall improvement in 3 months or less (OR).
Comparison 5. Open carpal tunnel release with versus without internal neurolysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement in 3 months or less | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.54] |
| 2 Overall improvement after 3 months | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.74, 1.14] |
5.1. Analysis.
Comparison 5 Open carpal tunnel release with versus without internal neurolysis, Outcome 1 Overall improvement in 3 months or less.
5.2. Analysis.
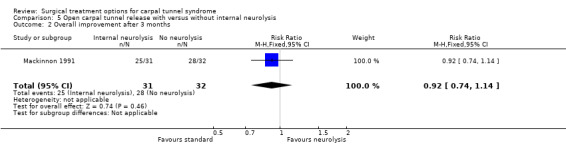
Comparison 5 Open carpal tunnel release with versus without internal neurolysis, Outcome 2 Overall improvement after 3 months.
Comparison 6. Open carpal tunnel release with versus without epineurotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall improvement after 3 months | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.50] |
6.1. Analysis.
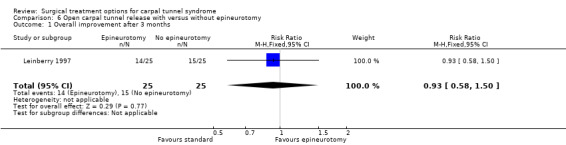
Comparison 6 Open carpal tunnel release with versus without epineurotomy, Outcome 1 Overall improvement after 3 months.
Comparison 7. Open carpal tunnel release with versus without tenosynovectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom severity score (Levine) after 3 months | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.29, 0.29] |
| 2 Functional status score (Levine) after 3 months | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
7.1. Analysis.
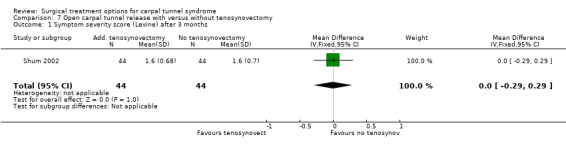
Comparison 7 Open carpal tunnel release with versus without tenosynovectomy, Outcome 1 Symptom severity score (Levine) after 3 months.
7.2. Analysis.
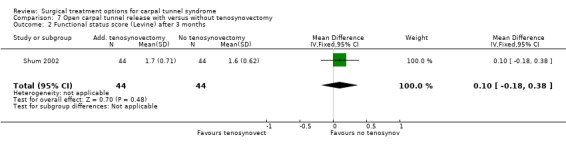
Comparison 7 Open carpal tunnel release with versus without tenosynovectomy, Outcome 2 Functional status score (Levine) after 3 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agee 1992.
| Methods | RCT | |
| Participants | Electrophysiologically confirmed CTS, idiopathic CTS, 25 of 122 patients treated bilaterally (147 procedures). | |
| Interventions | (1) ECTR one‐portal Agee technique (82 patients). (2) OCTR (65 patients). | |
| Outcomes | Symptoms (not specified). Return to work/ADL. Complications. | |
| Notes | After the start of the study, randomisation of patients with bilateral CTS was discarded because patients who were randomised to ECTR refused to undergo OCTR as a second procedure. These 25 patients have been omitted from further analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Atroshi 2006.
| Methods | RCT | |
| Participants | Employed participants only. Mean age 44 (25 to 59) years. 75% female. Mean duration of symptoms 36 (3 to 240) months. Electrophysiologically confirmed CTS, idiopathic CTS. Patients with CTS symptoms of the contralateral hand not relieved by a splint were excluded. | |
| Interventions | (1) ECTR: two‐portal extrabursal Chow technique (65 patients). (2) OCTR (63 patients). | |
| Outcomes | Severity of postoperative pain and limitations. Symptom severity score. Functional status score. Length of work absence. SF‐12 physical health score. Changes in hand sensation and strength. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Benedetti 1996.
| Methods | RCT | |
| Participants | Mean age 53 years. 79% female. Mean duration of symptoms 9 months. Electrophysiologically confirmed CTS, idiopathic CTS. | |
| Interventions | (1) ECTR: one‐portal Agee technique (23 patients). (2) OCTR (22 patients). | |
| Outcomes | Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bhattacharya 2004.
| Methods | RCT; matched design in patients with bilateral CTS. The hand with the most severe symptoms was randomly allocated to one of the procedures; after six weeks the other hand was treated with the other procedure. | |
| Participants | Mean age 48 years. 72% female. Mean duration of symptoms 24 months. Clinical CTS. All patients treated bilaterally. | |
| Interventions | (1) Mini open technique with Knifelight instrument (32 patients/hands). (2) OCTR (32 patients/hands). | |
| Outcomes | Symptom relief. Scar tenderness. Grip strength. Return to work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Brown 1993.
| Methods | RCT | |
| Participants | Mean age 56 years. 68% female. Mean duration of symptoms 24 months. Electrophysiologically confirmed CTS, idiopathic CTS. 24 of 145 patients treated bilaterally. | |
| Interventions | (1) ECTR: two‐portal extrabursal Chow technique (84 hands). (2) OCTR (85 hands). | |
| Outcomes | Improvement in symptoms (paraesthesiae, numbness). Return to work/ADL. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Brüser 1999.
| Methods | RCT | |
| Participants | Mean age 54 years. 78% female. Mean duration of symptoms 41 months. Electrophysiologically confirmed CTS, idiopathic CTS. | |
| Interventions | (1) OCTR with a short incision (2.5 cm) (38 patients). (2) OCTR with a long incision (4.5 cm, occasionally with epineurotomy) (42 patients). | |
| Outcomes | Nocturnal discomfort. Numbness. Paraesthesiae. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cellocco 2005.
| Methods | RCT | |
| Participants | Mean age 59 (29 to 85) and 60 (29 to 82) years. 73% female. Mean duration of symptoms 6.3 (2 to 15) and 7.7 (3 to 14) months. Electrophysiologically confirmed CTS. 20% treated bilaterally. | |
| Interventions | (1) Mini open technique with Knifelight instrument (82 patients, 99 hands). (2) OCTR with short incision (103 patients, 123 hands). | |
| Outcomes | Symptom severity score. Functional status score. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Citron 1997.
| Methods | RCT | |
| Participants | Mean age 52 years. 83% female, clinical CTS, both idiopathic and secondary CTS. | |
| Interventions | (1) OCTR with an ulnar L incision (23 patients). (2) OCTR (27 patients). | |
| Outcomes | Pain at rest. Numbness. Paraesthesiae (day and night). Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dias 2004.
| Methods | RCT; matched design in patients with bilateral CTS. Lengthening of the flexor retinaculum was randomly allocated to one hand; the other was treated with OCTR in the same session. | |
| Participants | Mean age 56 (23 to 84) years. 73% female. Electrophysiologically confirmed CTS, both idiopathic and secondary CTS. All patients treated bilaterally. | |
| Interventions | (1) OCTR with lengthening of the flexor retinaculum (26 hands). (2) OCTR (26 hands). | |
| Outcomes | Symptom severity score. Functional status score. Grip and pinch strength. Scar tenderness. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Dumontier 1995.
| Methods | RCT | |
| Participants | Mean age 52 years. 89% female, idiopathic CTS. | |
| Interventions | (1) ECTR: two‐portal extrabursal Chow technique (60 patients). (2) OCTR (43 patients). | |
| Outcomes | Pain. Paraesthesiae. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Eichhorn 2003.
| Methods | RCT with 3 treatment groups. Patients allocated to ECTR but undergoing OCTR were excluded from the study. | |
| Participants | Electrophysiologically confirmed CTS. 11% treated bilaterally. | |
| Interventions | (1) ECTR: two‐portal extrabursal Chow technique (110 patients, 125 hands). (2) OCTR (54 patients, 60 hands). (3) OCTR with modified incision (63 patients, 65 hands). | |
| Outcomes | Overall improvement. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Erdmann 1994.
| Methods | RCT | |
| Participants | Mean age 53 years. 73% female. Mean duration of symptoms 24 months. Electrophysiologically confirmed CTS, both idiopathic and secondary CTS. 34 out of 71 patients treated bilaterally (25 patients 1 hand ECTR, 1 hand OCTR simultaneously). | |
| Interventions | (1) ECTR: two‐portal extrabursal Chow technique (53 hands). (2) OCTR (52 hands). | |
| Outcomes | Pain. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ferdinand 2002.
| Methods | RCT; matched design in patients with bilateral CTS. ECTR was randomly allocated to one hand; the other hand was treated with OCTR in the same session. | |
| Participants | Mean age 55 (13) years. 80% female. Electrophysiologically confirmed CTS, idiopathic CTS. | |
| Interventions | (1) ECTR one‐portal Agee technique (25 hands). (2) OCTR (25 hands). | |
| Outcomes | Patient's satisfaction. Pain. Functional status (Jebson). Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Foucher 1993.
| Methods | RCT with 4 treatment groups | |
| Participants | Clinical CTS, 2 out of 249 patients treated bilaterally. | |
| Interventions | (1) ECTR: one‐portal Agee technique (54 hands). (2) OCTR (69 hands). (3) OCTR with anterior ligamentoplasty type I (59 hands). (4) OCTR with anterior ligamentoplasty type II (69 hands). | |
| Outcomes | Pain. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Helm 2003.
| Methods | RCT | |
| Participants | Mean age 54 (35 to 87) and 52 (29 to 77) years. 61% female. Mean duration of symptoms 31 (4 to 120) and 36 (10 to 240) months. | |
| Interventions | (1) Mini open technique with Knifelight instrument (39 patients). (2) OCTR (43 patients). | |
| Outcomes | CTS symptoms. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hoefnagels 1997.
| Methods | RCT | |
| Participants | Mean age 51 years. 74% female. Mean duration of symptoms 21 months. Electrophysiologically confirmed CTS. | |
| Interventions | (1) ECTR: one‐portal Agee technique (87 patients). (2) OCTR (91 patients). | |
| Outcomes | Symptom severity score. Functional status score. Satisfaction with result. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Holmgren 1985.
| Methods | RCT | |
| Participants | Mean age 50 years. 69% female. Electrophysiologically confirmed CTS. | |
| Interventions | (1) OCTR with internal neurolysis. (2) OCTR (the number of patients of each treatment group was not mentioned). | |
| Outcomes | Pain. Paraesthesiae. Hypoaesthesiae. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Holmgren 1987.
| Methods | See Holmgren 1985 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Long‐term follow‐up of previously published trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Jacobsen 1996.
| Methods | RCT | |
| Participants | Mean age 46 years. 72% female. Electrophysiologically confirmed CTS, idiopathic CTS. 3 out of 29 patients treated bilaterally. | |
| Interventions | (1) ECTR: two‐portal transbursal Chow technique (16 hands). (2) OCTR (16 hands). | |
| Outcomes | Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Jugovac 2002.
| Methods | RCT | |
| Participants | Mean age 59 (9) and 53 (10) years. 75% female. Electrophysiologically confirmed CTS, traumatic CTS excluded. No patients with bilateral CTS. | |
| Interventions | (1) OCTR with limited incision (36 patients). (2) OCTR (36 patients). | |
| Outcomes | Symptom relief. Return to work or ADL. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Leinberry 1997.
| Methods | RCT | |
| Participants | Mean age 65 years. 59% female, mean duration of symptoms 32 months. Electrophysiologically confirmed CTS, both idiopathic and secondary CTS. 6 out of 44 patients treated bilaterally. | |
| Interventions | (1) OCTR with epineurotomy (25 hands). (2) OCTR (25 hands). | |
| Outcomes | Symptoms (not specified). Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lowry 1988.
| Methods | RCT | |
| Participants | Electrophysiologically confirmed CTS, severe CTS. 9 out of 41 patients treated bilaterally. | |
| Interventions | (1) OCTR with internal neurolysis (25 hands). (2) OCTR (25 hands). | |
| Outcomes | Clinical response. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
MacDermid 2003.
| Methods | RCT | |
| Participants | Mean age 45 to 53 years. 68% female. Electrophysiologically confirmed CTS. | |
| Interventions | (1) ECTR: two‐portal transbursal Chow technique (91 patients). (2) OCTR (32 patients). | |
| Outcomes | Symptom severity scale. Pain. Functional status. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mackenzie 2000.
| Methods | RCT. Initial no. of patients not presented: only patients with complete follow‐up were included. | |
| Participants | 0% female. Electrophysiologically confirmed CTS, idiopathic CTS. 10 out of 26 patients treated bilaterally. | |
| Interventions | (1) ECTR one‐portal Agee technique (22 hands). (2) OCTR with short incision (14 hands). | |
| Outcomes | Severity of symptoms. Functional status. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mackinnon 1991.
| Methods | RCT | |
| Participants | Mean age 59 years. 81% female. Clinical CTS, idiopathic CTS. 4 out of 59 patients treated bilaterally. | |
| Interventions | (1) OCTR with internal neurolysis (29 patients/31 hands). (2) OCTR (30 patients/32 hands). | |
| Outcomes | Nocturnal discomfort. Response to treatment. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Nakamichi 1997.
| Methods | RCT | |
| Participants | Mean age 58 years. All female. Electrophysiologically confirmed CTS, idiopathic CTS. | |
| Interventions | (1) OCTR with short incision 1.0 to 1.5 cm with ultrasonographic assistance (50 patients). (2) OCTR (53 patients). | |
| Outcomes | Overall improvement. Numbness and paraesthesiae. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nitzsche 1999.
| Methods | RCT | |
| Participants | Details not reported. | |
| Interventions | (1) OCTR with 2 short incisions and epineurotomy (50 patients). (2) OCTR with epineurotomy (50 patients). | |
| Outcomes | Details not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rab 2006.
| Methods | RCT; matched design in patients with bilateral CTS. The first hand was randomly allocated to one procedure; after at least 6 months, the other hand underwent the alternative procedure. | |
| Participants | Mean age 56 (8) years. 40% female. Electrophysiologically confirmed CTS, idiopathic CTS. All patients treated bilaterally. | |
| Interventions | (1) ECTR: two‐portal Chow technique (10 hands). (2) OCTR with 2 mini incisions (10 hands). | |
| Outcomes | Pain (VAS). Symptom severity score. Functional status score. Grip, pinch and key grip strength. Two‐point discrimination. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Richter 1996.
| Methods | See Brüser 1999 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Double publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Saw 2003.
| Methods | RCT; patients with bilateral CTS (n = 27; 22%) had their releases sequentially with the more affected hand first. Once these patients felt that they could use the operated hand normally they underwent the second procedure (shortest interval between the two procedures 7 months). | |
| Participants | Mean age 54 (15) and 50 (15) years. 73% female. Clinical CTS, idiopathic CTS. | |
| Interventions | (1) ECTR: one‐portal Agee technique (74 hands). (2) OCTR (76 hands). | |
| Outcomes | Symptom severity score. Functional status score. Grip strength. Sick leave. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sennwald 1995.
| Methods | See Benedetti 1996 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Double publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shum 2002.
| Methods | RCT | |
| Participants | Mean age 58 (16 to 97) years. 83% female. Electrophysiologically confirmed CTS, idiopathic CTS. 23% bilateral involvement. | |
| Interventions | (1) OCTR with tenosynovectomy. (2) OCTR (the number of patients of each treatment group was not mentioned). | |
| Outcomes | Symptom severity score. Functional status score. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Stark 1996.
| Methods | RCT; matched design in patients with bilateral CTS. The first hand of 20 patients with bilateral CTS was randomised to one of the treatments; after recovery the other hand was treated with the alternative procedure. | |
| Participants | Mean age 53 years. 55% female. Mean duration of symptoms 29 months, 15 patients with electrophysiologically confirmed CTS, 5 patients clinical CTS, idiopathic CTS, all 20 patients treated bilaterally. | |
| Interventions | (1) ECTR: one‐portal Agee technique (20 hands). (2) OCTR (20 hands). | |
| Outcomes | Pain. Paraesthesiae. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Trumble 2002.
| Methods | RCT | |
| Participants | Mean age 56 (24 to 74) years. 65% female. Mean duration of symptoms 32 (4 to 132) months. Electrophysiologically confirmed CTS, idiopathic CTS, 45 of 147 patients treated bilaterally. | |
| Interventions | (1) ECTR: one‐portal Agee technique (75 patients, 97 hands). (2) OCTR (72 patients, 95 hands). | |
| Outcomes | Patient's satisfaction score. Symptom severity score. Functional status score. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Westphal 2000.
| Methods | RCT | |
| Participants | Idiopathic CTS. | |
| Interventions | (1) ECTR: one‐portal technique (45 patients). (2) OCTR (35 patients). | |
| Outcomes | CTS‐complaints. Functional status. Return to work. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Wong 2003.
| Methods | RCT; matched design in patients with bilateral CTS. The dominant hand was randomly allocated to one of the procedures; the other was treated with the alternative procedure in the same session. | |
| Participants | Mean age 47 (35 to 73) years. 93% female. Mean duration of symptoms 50 (42) and 60 (43) months. Electrophysiologically confirmed CTS, idiopathic CTS. All patients treated bilaterally. | |
| Interventions | (1) ECTR: two‐portal Chow technique (30 patients/hands). (2) OCTR with limited incision (30 patients/hands). | |
| Outcomes | Overall measure of improvement. (Pillar) pain (VAS). Presence of hypoesthesias or paresthesias. Weakness of m. abductor pollicis brevis. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borisch 2003 | No adequate randomisation. Patients were allocated according to odd or even year of birth. |
| Foulkes 1994 | No adequate randomisation. Patients were allocated according to odd or even birth month. |
| Leger 2000 | Cost analysis only. No clinical results. |
| Padua 2003 | RCT addressing the application of corticosteroid irrigation during OCTR versus OCTR alone. |
| Schäfer 1996 | No adequate randomisation. Patients were allocated according to odd or even day of the week. |
Contributions of authors
A contribution to all aspects of the review was made by Annette Gerritsen (initial review), Riekie de Vet and Rob Scholten (both updates). Aebele Mink van der Molen and Bernard Uitdehaag performed assessment of methodological quality and data extraction. Dick van Geldere performed data abstraction (initial review). All authors contributed to the content and commented on earlier and final drafts of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Health Care Insurance Council, Netherlands.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Agee 1992 {published data only}
- Agee JM, McCarroll HR Jr, Tortosa RD, Berry DA, Szabo RM, Peimer CA. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. The Journal of Hand Surgery. American volume. 1992;17(6):987‐95. [DOI] [PubMed] [Google Scholar]
Atroshi 2006 {published data only}
- Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ 2006;332(7556):1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedetti 1996 {published data only}
- Benedetti RB, Sennwald G. Agee endoscopic decompression of the median nerve: a prospective study in comparison with the open decompression [Endoskopische Dekompression des N. medianus nach Agee: Prospektive Studie mit Vergleich zur offenen Dekompression]. Handchirurgie, Mikrochirurgie, Plastiche Chirurgie 1996;28(3):151‐5. [PubMed] [Google Scholar]
Bhattacharya 2004 {published data only}
- Bhattacharya R, Birdsall PD, Finn P, Stothard J. A randomized controlled trial of Knifelight and open carpal tunnel release. Journal of Hand Surgery. British volume. 2004;29(2):113‐5. [DOI] [PubMed] [Google Scholar]
Brown 1993 {published data only}
- Brown RA, Gelberman RH, Seiler JG 3rd, Abrahamsson SO, Weiland AJ, Urbaniak JR, et al. Carpal tunnel release. A prospective randomized assessment of open and endoscopic methods. The Journal of Bone and Joint Surgery. American volume. 1993;75(9):1265‐75. [DOI] [PubMed] [Google Scholar]
Brüser 1999 {published data only}
- Brüser P, Richter M, Larkin G, Lefering R. The operative treatment of carpal tunnel syndrome and its relevance to endoscopic release. European Journal of Plastic Surgery 1999;22(2‐3):80‐4. [Google Scholar]
Cellocco 2005 {published data only}
- Cellocco P, Rossi C, Bizzarri F, Patrizio L, Costanzo G. Mini‐open blind procedure versus limited open technique for carpal tunnel release: a 30‐month follow‐up study. Journal of Hand Surgery. American volume 2005;30(3):493‐9. [DOI] [PubMed] [Google Scholar]
Citron 1997 {published data only}
- Citron ND, Bendall SP. Local symptoms after carpal tunnel release. A randomized prospective trial of two incisions. Journal of Hand Surgery. British volume. 1997;22(3):317‐21. [DOI] [PubMed] [Google Scholar]
Dias 2004 {published data only}
- Dias JJ, Bhowal B, Wildin CJ, Thompson JR. Carpal tunnel decompression. Is lengthening of the flexor retinaculum better than simple division?. Journal of Hand Surgery. British volume. 2004;29(3):271‐6. [DOI] [PubMed] [Google Scholar]
Dumontier 1995 {published data only}
- Dumontier C, Sokolow C, Leclercq C, Chauvin P. Early results of conventional versus two‐portal endoscopic carpal tunnel release. A prospective study. Journal of Hand Surgery. British volume. 1995;20(5):658‐62. [DOI] [PubMed] [Google Scholar]
Eichhorn 2003 {published data only}
- Eichhorn J, Dieterich K. Open versus endoscopic carpal tunnel release. Results of a prospective study. Chirurgische Praxis 2003;61(2):279‐83. [Google Scholar]
Erdmann 1994 {published data only}
- Erdmann MWH. Endoscopic carpal tunnel decompression. Journal of Hand Surgery. British volume. 1994;19(1):5‐13. [DOI] [PubMed] [Google Scholar]
Ferdinand 2002 {published data only}
- Ferdinand RD, MacLean JGB. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. The Journal of Bone and Joint Surgery. British volume. 2002;84(3):375‐9. [DOI] [PubMed] [Google Scholar]
Foucher 1993 {published data only}
- Foucher G, Buch N, Overstraeten L, Gautherie M, Jesel M. Carpal tunnel syndrome. Can it still be a controversial topic? [Le canal carpien. Peut‐il être encore sujet de controverse?]. Chirurgie 1993‐1994;119(1‐2):80‐4. [PubMed] [Google Scholar]
Helm 2003 {published data only}
- Helm RH, Vaziri S. Evaluation of carpal tunnel release using the Knifelight(R) instrument. Journal of Hand Surgery. British volume. 2003;28(3):251‐4. [DOI] [PubMed] [Google Scholar]
Hoefnagels 1997 {published data only}
- Hoefnagels WAJ, Kleef JGF, Mastenbroek GGA, Blok JA, Breukelman AJ, Krom MCTFM. Surgical treatment of carpal tunnel syndrome: endoscopic or classical (open) surgery? A prospective randomized study [Operatieve behandeling wegens carpaletunnelsyndroom: endoscopisch of klassiek (open)? Een prospectief gerandomiseerd onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1997;141(18):878‐82. [PubMed] [Google Scholar]
Holmgren 1985 {published data only}
- Holmgren‐Larsson H, Leszniewski W, Linden U, Rabow L, Thorling J. Internal neurolysis or ligament division only in carpal tunnel syndrome‐results of a randomized study. Acta Neurochirurgia 1985;74(3‐4):118‐21. [DOI] [PubMed] [Google Scholar]
Holmgren 1987 {published data only}
- Holmgren H, Rabow L. Internal neurolysis or ligament division only in carpal tunnel syndrome II. A 3 year follow‐up with an evaluation of various neurophysiological parameters for diagnosis. Acta Neurochirurgia 1987;87(1‐2):44‐7. [DOI] [PubMed] [Google Scholar]
Jacobsen 1996 {published data only}
- Jacobsen MB, Rahme H. A prospective, randomized study with an independent observer comparing open carpal tunnel release with endoscopic carpal tunnel release. Journal of Hand Surgery. British volume. 1996;21(2):202‐24. [DOI] [PubMed] [Google Scholar]
Jugovac 2002 {published data only}
- Jugovac I, Burgic N, Micovic V, Radolovic‐Prenc L, Uravic M, Golubovic V, et al. Carpal tunnel release by limited palmar incision vs traditional open technique: randomized controlled trial. Croatian Medical Journal 2002;43(1):33‐6. [PubMed] [Google Scholar]
Leinberry 1997 {published data only}
- Leinberry CF, Hammond NL 3rd, Siegfried JW. The role of epineurotomy in the operative treatment of carpal tunnel syndrome. The Journal of Bone and Joint Surgery. American volume. 1997;79(4):555‐7. [DOI] [PubMed] [Google Scholar]
Lowry 1988 {published data only}
- Lowry WE Jr, Follender AB. Interfascicular neurolysis in the severe carpal tunnel syndrome. A prospective, randomized, double‐blind, controlled study. Clinical Orthopaedics and Related Research 1988;227:251‐4. [PubMed] [Google Scholar]
MacDermid 2003 {published data only}
- MacDermid JC, Richards RS, Roth JH, Ross DC, King GJW. Endoscopic versus open carpal tunnel release: A randomized trial. The Journal of Hand Surgery. American volume. 2003;28(3):475‐80. [DOI] [PubMed] [Google Scholar]
Mackenzie 2000 {published data only}
- Mackenzie DJ, Hainer R, Wheatley MJ. Early recovery after endoscopic vs. short‐incision open carpal tunnel release. Annals of Plastic Surgery 2000;44(6):601‐4. [DOI] [PubMed] [Google Scholar]
Mackinnon 1991 {published data only}
- Mackinnon SE, McCabe S, Murray JF, Szalai JP, Kelly L, Novak C, et al. Internal neurolysis fails to improve the results of primary carpal tunnel decompression. The Journal of Hand Surgery. American volume. 1991;16(2):211‐8. [DOI] [PubMed] [Google Scholar]
Nakamichi 1997 {published data only}
- Nakamichi K, Tachibana S. Ultrasonographically assisted carpal tunnel release. The Journal of Hand Surgery. American volume. 1997;22(5):853‐62. [DOI] [PubMed] [Google Scholar]
Nitzsche 1999 {published data only}
- Nitzsche T, Steen M. Open and modified open surgery technique to split the retinaculum flexorum in treatment of carpal tunnel syndrome. Deutsche Gesellschaft Fur Chirurgie 1999;Suppl Kongressband II:999‐1001. [Google Scholar]
Rab 2006 {published data only}
- Rab M, Grunbeck M, Beck H, et al. Intra‐individual comparison between open and 2‐portal endoscopic release in clinically matched bilateral carpal syndrome. Journal of Plastic, Reconstructive and Aesthetic Surgery 2006;59(7):730‐6. [DOI] [PubMed] [Google Scholar]
Richter 1996 {published data only}
- Richter M, Brüser P. Surgical treatment of carpal tunnel syndrome: a comparison between long and short incision and endoscopic release [Die operative Behandlung des Karpaltunnelsyndroms: ein Vergleich zwischen langer und kurzer Schnittfürung sowie endoskopischer Spaltung]. Handchirurgie, Mikrochirurgie, Plastiche Chirurgie 1996;28(3):160‐6. [PubMed] [Google Scholar]
Saw 2003 {published data only}
- Saw NL, Jones S, Shepstone L, Meyer M, Chapman PG, Logan AM. Early outcome and cost‐effectiveness of endoscopic versus open carpal tunnel release: a randomized prospective trial. Journal of Hand Surgery. British volume. 2003;28(5):444‐9. [DOI] [PubMed] [Google Scholar]
Sennwald 1995 {published data only}
- Sennwald GR, Benedetti R. The value of one‐portal endoscopic carpal tunnel release: a prospective randomized study. Knee Surgery, Sports Traumatology, Arthroscopy 1995;3(2):113‐6. [DOI] [PubMed] [Google Scholar]
Shum 2002 {published data only}
- Shum C, Parisien M, Strauch RJ, Rosenwasser MP. The role of flexor tenosynovectomy in the operative treatment of carpal tunnel syndrome. The Journal of Bone and Joint Surgery. American volume. 2002;84(2):221‐5. [DOI] [PubMed] [Google Scholar]
Stark 1996 {published data only}
- Stark B, Engkvist‐Löfmark C. Endoscopic operation or conventional open surgical technique in carpal tunnel syndrome: a prospective comparative study [Endoskopische Operation oder konventionelle offene Operationstechnik bei Karpaltunnelsyndrom: eine prospektive, vergleichende Studie]. Handchirurgie, Mikrochirurgie, Plastiche Chirurgie 1996;28(3):128‐32. [PubMed] [Google Scholar]
Trumble 2002 {published data only}
- Trumble TE, Diao E, Abrams RA, Gilbert‐Anderson MM. Single‐portal endoscopic carpal tunnel release compared with open release :‐ a prospective, randomized trial. The Journal of Bone and Joint Surgery. American volume. 2002;84(7):1107‐15. [DOI] [PubMed] [Google Scholar]
Westphal 2000 {published data only}
- Westphal KP, Bayat M, Wustner‐Hofmann M, Hofmann A. Course of clinical symptoms before and after surgical decompression in carpal tunnel surgery. Lymphologie in Forschung Und Praxis 2000;4(2):69‐73. [Google Scholar]
Wong 2003 {published data only}
- Wong KC, Hung LK, Ho PC, Wong JM. Carpal tunnel release. A prospective, randomised study of endoscopic versus limited‐open methods. The Journal of Bone and Joint Surgery. British volume. 2003;85‐B(6):863‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Borisch 2003 {published data only}
- Borisch N, Haussmann P. Neurophysiological recovery after open carpal tunnel decompression: comparison of simple decompression and decompression with epineurotomy. Journal of Hand Surgery. British volume. 2003;28(5):450‐4. [DOI] [PubMed] [Google Scholar]
Foulkes 1994 {published data only}
- Foulkes GD, Atkinson RE, Beuchel C, Doyle JR, Singer DI. Outcome following epineurotomy in carpal tunnel syndrome: a prospective, randomized clinical trial. The Journal of Hand Surgery. American volume. 1994;19(4):539‐47. [DOI] [PubMed] [Google Scholar]
Leger 2000 {published data only}
- Leger O, Bustamente K. Influence of operative technique on the surgical treatment in carpal tunnel syndrome. Chirurgie De La Main 2000;19(2):94‐9. [DOI] [PubMed] [Google Scholar]
Padua 2003 {published data only}
- Padua R, Padua L, Bondi R, Campi A, Ceccarelli E, Padua S. Intrasurgical use of steroids on carpal tunnel syndrome: A randomized, prospective, double‐blind controlled study. Journal of Orthopaedics & Traumatology 2003;4(2):76‐80. [Google Scholar]
Schäfer 1996 {published data only}
- Schäfer W, Sander KE, Walter A, Weitbrecht WU. Agee endoscopic operation of carpal tunnel syndrome in comparison with open surgical technique [Endoskopische Operation des Karpaltunnelsyndroms nach Agee im Vergleich mit der offenen Operationstechnik]. Handchirurgie, Mikrochirurgie, Plastiche Chirurgie 1996;28(3):143‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Azzara 1995 {published data only}
- Azzara A, Altissimi M, Mancini GB. Carpal tunnel release: transverse, carpal ligament reconstruction versus simple section [abstract]. The Journal of Bone and Joint Surgery. British volume. 1995;77‐B(Suppl II):148. [Google Scholar]
Azzara 1997 {published data only}
- Azzara A, Altissimi M, Mancini GB, Romeo F. Carpal tunnel release: transverse carpal ligament reconstruction vs simple section [abstract]. Journal of Hand Surgery. British volume. 1997;22(Suppl):162. [Google Scholar]
DeJong 1997 {published data only}
- DeJong ES, Bagg MR, Milnor WH, Cancio L. Carpal tunnel release with versus without release of the antibrachial fascia: a prospective, randomized study [abstract]. Orthopaedic Transactions 1997;21(4):1364‐65. [Google Scholar]
Giele 2000 {published data only}
- Giele H. Bilateral simultaneous carpal tunnel release to prospectively compare endoscopic and open techniques [abstract]. Journal of Hand Surgery. British volume. 2000;25(Suppl 1):128‐9. [Google Scholar]
Hartley 1999a {published data only}
- Hartley R, Chamberlain ST, Koukou C, Bradley M, Burke FD. The results of a prospective randomised study comparing open carpal tunnel decompression with decompression via a limited open incision using a subcutaneous tome. The Journal of Bone and Joint Surgery. British volume 1999;81(Suppl 3):284. [Google Scholar]
Hartley 1999b {published data only}
- Hartley R, Chamberlain ST, Koukou C, Bradley M, Burke FD. Results of a prospective randomized trial comparing open carpal tunnel decompression with decompression using a subcutaneous tome [abstract]. Journal of Hand Surgery. British volume. 1999;24(Suppl 1):4‐5. [Google Scholar]
Koskella 1996 {published data only}
- Koskella KR, Alexander C. A comparison between open and endoscopic carpal tunnel release [abstract]. Orthopaedic Transactions 1996;20(4):1102. [Google Scholar]
Sorensen 1997 {published data only}
- Sorensen AI, Boeckstyns M, Nielsen NS, Haugegard M. Carpal tunnel release, a comparison of 3 methods ‐ a preliminary report [abstract]. Acta Orthopaedica Scandinavica 1997;68(Suppl 277):24‐5. [Google Scholar]
Tetik 2002 {published data only}
- Tetik C, Erol B. Comparison of the alternative methods used in the surgical treatment of carpal tunnel syndrome. Artroplasti Artroskopik Cerrahi 2002;13(1):5‐9. [Google Scholar]
Werber 1996 {published data only}
- Werber KD, Braver RB, Richtarsky I. Endoscopic carpal tunnel release versus open procedure: a prospective randomized study [abstract]. Journal of Hand Surgery. British volume. 1996; Vol. 21‐B, issue Suppl 1:11‐2.
Additional references
Ablove 1994
- Ablove RH, Peimer CA, Diao E, Oliverio R, Kuhn JP. Morphologic changes following endoscopic and two‐portal subcutaneous carpal tunnel release. The Journal of Hand Surgery. American volume. 1994;19(5):821‐6. [DOI] [PubMed] [Google Scholar]
Agee 1994
- Agee JM, McCarroll HR, North ER. Endoscopic carpal tunnel release using the single proximal incision technique. Hand Clinics 1994;10(4):647‐59. [PubMed] [Google Scholar]
Benson 2006
- Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy 2006;22(9):919‐24. [DOI] [PubMed] [Google Scholar]
Boeckstyns 1999
- Boeckstyns MEH, Sørensen AI. Does endoscopic carpal tunnel release have a higher rate of complications than open carpal tunnel release? An analysis of published series. Journal of Hand Surgery. British volume. 1999;24(1):9‐15. [DOI] [PubMed] [Google Scholar]
Bongers 2007
- Bongers FJ, Schellevis FG, Bosch WJ, Zee J. Carpal tunnel syndrome in general practice (1987 and 2001): incidence and the role of occupational and non‐occupational factors. British Journal of General Practice 2007;57(534):36‐9. [PMC free article] [PubMed] [Google Scholar]
Bromley 1994
- Bromley GS. Minimal‐incision open carpal tunnel decompression. The Journal of Hand Surgery. American volume. 1994;19(1):119‐20. [DOI] [PubMed] [Google Scholar]
Chow 1989
- Chow JCY. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy 1989;5(1):19‐24. [DOI] [PubMed] [Google Scholar]
Chow 1993
- Chow JCY. The Chow technique of endoscopic release of the carpal ligament for carpal tunnel syndrome: four years of clinical results. Arthroscopy 1993;9(3):301‐14. [DOI] [PubMed] [Google Scholar]
Curtis 1973
- Curtis RM, Eversmann WW Jr. Internal neurolysis as an adjunct to the treatment of the carpal‐tunnel syndrome. The Journal of Bone and Joint Surgery. American volume. 1973;55(4):733‐40. [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 1987
- Duncan KH, Lewis RC, Foreman KA, Nordyke MD. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: results of a questionnaire. The Journal of Hand Surgery. American volume. 1987;12(3):384‐91. [DOI] [PubMed] [Google Scholar]
Ebskov 1997
- Ebskov LB, Boeckstyns MEH, Sørensen AI. Operative treatment of carpal tunnel syndrome in Denmark. Results of a questionnaire. Journal of Hand Surgery. British volume 1997;22(6):761‐3. [DOI] [PubMed] [Google Scholar]
Einhorn 1996
- Einhorn N, Leddy JP. Pitfalls of endoscopic carpal tunnel release. Orthopaedic Clinics of North America 1996;27(2):373‐80. [PubMed] [Google Scholar]
Fissette 1979
- Fissette J, Onkelinx A. Treatment of carpal tunnel syndrome. Comparative study with and without epineurolysis. Hand 1979;11(2):206‐10. [DOI] [PubMed] [Google Scholar]
Gerritsen 2002
- Gerritsen AA, Vet HC, Scholten RJ, Tulder MW, Bouter LM. Enabling meta‐analysis in systematic reviews on carpal tunnel syndrome. The Journal of Hand Surgery. American volume. 2002;27(5):828‐32. [DOI] [PubMed] [Google Scholar]
Jerosch‐Herold 2006
- Jerosch‐Herold C, Leite JC, Song F. A systematic review of outcomes assessed in randomized controlled trials of surgical interventions for carpal tunnel syndrome using the International Classification of Functioning, Disability and Health (ICF) as a reference tool. BMC Musculoskeletal Disorders 2006;7(Dec 5):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latinovic 2006
- Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. Journal of Neurology, Neurosurgery, and Psychiatry 2006;77(2):263‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self‐administrered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. The Journal of Bone and Joint Surgery. American volume. 1993;75(11):1585‐92. [DOI] [PubMed] [Google Scholar]
Lorgelly 2005
- Lorgelly PK, Dias JJ, Bradley MJ, Burke FD. Carpal tunnel syndrome, the search for a cost‐effective surgical intervention: a randomised controlled trial. Annals of the Royal College of Surgeons of England 2005;87(1):36‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKinnon 2005
- MacKinnon SE, Novak CB. Compressive Neuropathies. In: Green DP, Pederson WC, Hotchkiss RN, Wolfe SW editor(s). In: Green's Operative Hand Surgery. 5th Edition. Vol. One, Pennsylvania: Churchill‐Livingstone, 2005:1016‐7. [Google Scholar]
Marshall 2002
- Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI] [PubMed] [Google Scholar]
O'Connor 2003
- O'Connor D, Marshall S, Massy‐Westropp N. Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okutsu 1989
- Okutsu I, Ninomiya S, Hamanaka I, Kuroshima N, Inanami H. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. The Journal of Bone and Joint Surgery. American volume. 1989;71(5):679‐83. [PubMed] [Google Scholar]
Richman 1989
- Richman JA, Gelberman RH, Rydevik BL, Hajek PC, Braun RM, Gylys‐Morin VM, et al. Carpal tunnel syndrome: morphologic changes after release of the transverse carpal ligament. The Journal of Hand Surgery. American volume. 1989;14(5):852‐7. [DOI] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3. [DOI] [PubMed] [Google Scholar]
Salaffi 2005
- Salaffi F, Angelis R, Grassi W. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community‐based study. I. The MAPPING study. Clinical and Experimental Rheumatology 2005;23(6):819‐28. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Taleisnik 1973
- Taleisnik J. The palmar cutaneous branch of the median nerve and the approach to the carpal tunnel. An anatomical study. The Journal of Bone and Joint Surgery. American volume. 1973;55(6):1212‐7. [PubMed] [Google Scholar]
Tulder 1997
- Tulder MW van, Assendelft WJJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine 1997;22(20):2323‐30. [DOI] [PubMed] [Google Scholar]
Vasen 1999
- Vasen AP, Kuntz KM, Simmons BP, Katz JN. Open versus endoscopic carpal tunnel release: a decision analysis. The Journal of Hand Surgery. American volume. 1999;24(5):1109‐17. [DOI] [PubMed] [Google Scholar]
Vasiliadis 2014
- Vasiliadis HS, Georgoulas P, Shrier I, Salanti G, Scholten RJPM. Endoscopic release for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008265.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verdugo 2003
- Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non‐surgical treatment for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI] [PubMed] [Google Scholar]
Wilson 2003
- Wilson JK, Sevier TL. A review of treatment for carpal tunnel syndrome. Disability and Rehabilitation 2003;25(3):133‐19. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gerritsen 2001
- Gerritsen AAM, Uitdehaag BMJ, Geldere D van, Scholten RJPM, Vet HCW de, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. British Journal of Surgery 2001;88(10):1285‐95. [DOI] [PubMed] [Google Scholar]


