Abstract
Background
Lipid‐lowering therapy is recommended for secondary prevention in people with coronary artery disease. It may also reduce cardiovascular events and/or local disease progression in people with lower limb peripheral arterial disease (PAD).
Objectives
To assess the effects of lipid‐lowering therapy on all‐cause mortality, cardiovascular events and local disease progression in patients with PAD of the lower limb.
Search methods
The authors searched The Cochrane Peripheral Vascular Diseases Group's Specialised Register (last searched February 2007) and the Cochrane Central Register of Controlled Trials (CENTRAL) (last searched Issue 2, 2007) for publications describing randomised controlled trials of lipid‐lowering therapy in peripheral arterial disease of the lower limb.
Selection criteria
Randomised controlled trials of lipid‐lowering therapy in patients with PAD of the lower limb.
Data collection and analysis
Three authors independently assessed trial quality and extracted data.
Main results
Eighteen trials were included, involving a total of 10,049 participants. Trials differed considerably in their inclusion criteria, outcomes measured, and type of lipid‐lowering therapy used. Only one trial (PQRST) reported a detrimental effect of active treatment on blood lipid/lipoprotein levels.
The pooled results from all eligible trials indicated that lipid‐lowering therapy had no statistically significant effect on overall mortality (Odds Ratio (OR) 0.86; 95% Confidence Interval (CI) 0.49 to 1.50) or on total cardiovascular events (OR 0.8; 95% CI 0.59 to 1.09). However, subgroup analysis which excluded PQRST showed that lipid‐lowering therapy significantly reduced the risk of total cardiovascular events (OR 0.74; CI 0.55 to 0.98). This was primarily due to a positive effect on total coronary events (OR 0.76; 95% CI 0.67 to 0.87). Greatest evidence of effectiveness came from the use of simvastatin in people with a blood cholesterol ≥ 3.5 mmol/litre (HPS).
Pooling of the results from several small trials on a range of different lipid‐lowering agents indicated an improvement in total walking distance (Mean Difference (MD) 152 m; 95% CI 32.11 to 271.88) and pain‐free walking distance (WMD 89.76 m; 95% CI 30.05 to 149.47) but no significant impact on ankle brachial index (WMD 0.04; 95% CI ‐0.01 to 0.09).
Authors' conclusions
Lipid‐lowering therapy is effective in reducing cardiovascular mortality and morbidity in people with PAD. It may also improve local symptoms. Until further evidence on the relative effectiveness of different lipid‐lowering agents is available, use of a statin in people with PAD and a blood cholesterol level ≥ 3.5 mmol/litre is most indicated.
Plain language summary
Lipid‐lowering for peripheral arterial disease of the lower limb
Atheroma (fatty deposits) in the walls of the arteries to the legs can lead to peripheral arterial disease with insufficient blood flow to the muscles and other tissues. People with peripheral arterial disease often do not have symptoms. The most common symptom is intermittent claudication, which is characterised by leg pain and weakness brought on by walking, with disappearance of the symptoms following a brief rest. Lipid‐lowering therapies may reduce cardiovascular events and worsening of local disease for people with lower limb peripheral arterial disease. They are recommended to people with coronary artery disease, for prevention of myocardial infarction and stroke.
Eighteen randomised controlled trials were included in the review, involving a total of 10,049 participants (78% were men) from seven different countries. The trials compared lipid‐lowering therapy with placebo or usual treatment for at least 90 days. They differed considerably in the inclusion criteria, outcomes measured, and type of lipid‐lowering therapy used. Lipid‐lowering therapies improved walking distance. The effect of lipid‐lowering therapy on death from any cause in people with peripheral artery disease was inconclusive. Using drugs to lower blood lipids had a beneficial effect on the incidence of total cardiovascular events, due primarily to an overall reduction in coronary events (OR 0.8; 95% Confidence Interval 0.7 to 0.9). The only type of drug for which consistent, clear evidence of a beneficial effect on total cardiovascular events, total coronary events and stroke was available, was the statins. The greatest evidence was with simvastatin in people with a blood cholesterol level of at least 3.5 mmol/litre. The evidence on side effects was inconclusive in these trials.
Background
Peripheral arterial disease (PAD) in the legs is caused by atheroma (fatty deposits) in the walls of the arteries leading to insufficient blood flow to the muscles and other tissues. Patients with PAD may have symptoms but are often asymptomatic. The commonest symptom, intermittent claudication, is characterised by leg pain and weakness brought on by walking, with disappearance of the symptoms following a brief rest. Approximately 1.5% of people over 50 years of age have a diagnosis of symptomatic peripheral arterial disease (predominantly intermittent claudication). A further 3% have undiagnosed intermittent claudication and even more (approximately 11%) have asymptomatic peripheral arterial disease (Fowkes 1991).
Patients diagnosed as having lower limb PAD, including those who are asymptomatic, have an increased risk of mortality, myocardial infarction and stroke (Dormandy 1999; Fowkes 1988; Heald 2006). Relative risks are two to three times that of age and sex matched groups without PAD. Thus PAD provides a potential opportunity for secondary prevention of cardiovascular events. Furthermore, patients with claudication can have a significantly reduced quality of life due to their restricted mobility (Dumville 2004). Careful consideration therefore needs to be given to evidence on drug and lifestyle management of claudication itself so that patients can achieve optimum mobility and quality of life within the limitations of their condition.
This review addresses the question of whether or not lipid‐lowering therapy, which has been shown to be of benefit for secondary prevention in coronary artery disease patients (SSS Study Group 1994; Sacks 1996; LIPID Study Group), is effective in reducing the incidence of subsequent cardiovascular events in people with PAD. The impact of lipid‐lowering therapy on local disease progression and symptoms of PAD (assessed by maximum and pain‐free walking distance) is also considered.
Objectives
To determine the effect of lipid‐lowering therapy on all‐cause mortality, cardiovascular events and local disease progression in patients with lower limb PAD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of lipid‐lowering therapy versus placebo, or other lipid‐lowering therapy or usual care.
Types of participants
Patients with lower limb PAD, including those with intermittent claudication (diagnosed either by questionnaire or clinically), critical limb ischaemia or asymptomatic disease (identified by validated techniques such as angiography or ankle brachial index). Trials in which all subjects were selected solely on the basis of previous peripheral vascular interventions were excluded.
Types of interventions
Any lipid‐lowering regime used with the aim of reducing blood lipids. The intervention could be dietary, a hypolipidaemic drug of the major classes currently listed in the British National Formulary (BNF) (fibrates, HMG‐CoA reductase inhibitors/statins, nicotinic acid group, anion exchange resins, Ezetimibe, omega‐3 fatty acid compounds) or any other agent specifically used to lower lipid levels.
Types of outcome measures
All‐cause mortality, fatal and non‐fatal cardiovascular events, revascularization (coronary and non coronary), and direct and indirect tests of local disease progression (maximum and pain‐free walking distance, ankle brachial index, angiographic measures) and side effects of treatment.
Search methods for identification of studies
Electronic searches
The Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched February 2007) and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (last searched 2007, Issue 2) for publications describing randomised controlled trials of lipid‐lowering therapy in peripheral arterial disease of the lower limb. For details of the search strategy used to search CENTRAL seeAppendix 1.
The PVD Group's Specialised Register contains citations of trials identified through back searching and continued prospective searching of MEDLINE (from 1960 to date), EMBASE (from 1980 to date), CINAHL (1982 to date) and from handsearching journals and conference proceedings.
The full list of journals that have been handsearched, as well as the search strategies used to search databases are described in the editorial information about the Cochrane PVD Group in The Cochrane Libraryhttp://www.mrw.interscience.wiley.com/cochrane/clabout/articles/PVD/frame.html.
Searching other resources
Additional trials were sought through cross‐referencing articles of lipid lowering, direct contact with principal investigators of trials in Europe, and (for the original review only) direct contact with pharmaceutical companies listed as manufacturing lipid‐lowering drugs in the British National Formulary. There were no restrictions for language of publication.
Data collection and analysis
Selection of trials
Three authors (PPA, HM and RJ) selected potential trials and assessed their eligibility for inclusion in the update of this review. Any disagreements were resolved by a fourth author (JP).
Assessment of trial quality
The quality of included trials was assessed for both allocation concealment and for internal and external validity using a standard scoring sheet developed by the PVD Group. For each included trial, information was collected on the method of randomisation, blinding and whether an intention‐to‐treat analysis had been done. For this update, three authors (PPA, HM and RJ) assessed trial quality and the results were cross‐checked by JP.
Data extraction
For this update PPA , HM and RJ independently extracted data and discrepancies were resolved in discussion with a fourth author (JP). We sought additional information to that appearing in published reports from the principal investigators when required.
We extracted data for the following outcomes and considered them for pooled analysis.
All‐cause mortality.
Cardiovascular events ‐ total, fatal and non‐fatal.
Coronary events ‐ total, fatal and non‐fatal.
Stroke ‐ total, fatal and non‐fatal.
Revascularizations (coronary and non‐coronary).
Walking distance ‐ pain free and total.
Ankle Brachial Index (ABI).
Disease progression assessed by angiogram.
Side effects.
Data analysis
Homogeneity between trial results was tested subjectively by clinical judgment of differences in patient populations, interventions and outcome assessments, and objectively using appropriate statistical tests. Where appropriate, trial results were pooled using relevant statistical techniques. We performed subgroup analyses for all trials excluding those reported as having a detrimental effect on lipid/lipoprotein parameters. We performed further subgroup analyses for trials with only the highest quality assessment and for trials confined to a single class of lipid‐lowering therapy. Although publication bias could not be excluded, there proved to be an insufficient number of trials for each outcome to assess this formally.
Results
Description of studies
In the original version of this review there were seven included studies and two excluded studies. We identified 62 new trials, of which 49 were excluded. In addition, we excluded two trials included in the original review (CLAS; Davis 1975). The former trial was excluded because participants included were not only those suffering from lower limb atherosclerosis but also unaffected subjects. The trial by Davis et al was excluded because only subjective symptom changes were reported and newer trials provide an increased quantity of evidence on objective measures. For additional information on excluded studies, seeCharacteristics of excluded studies table.
No studies were excluded from the review purely due to poor methods, but the results of methodological quality scoring were used to exclude trials from subgroups in the meta‐analysis. Four trials are awaiting further assessment (Borreani 1993; Degni 1973; Di Stefano 1984; Mayer 2001). Despite many attempts, two references have proved to be unobtainable (Degni 1973; Di Stefano 1984); the remaining two require detailed translation (Borreani 1993; Mayer 2001) (Characteristics of studies awaiting classification).
A total of 13 new studies involving a total of 10,049 subjects were included in this update, including one previously ongoing study (LEADER). The number of subjects participating in each study ranged from 19 to 6748.
The earliest study was published in 1973 (Nye 1973) and the most recent with relevant results in 2007 (HPS). Eight studies were from Italy, three from the UK, and three from Cuba. The remaining studies were from USA, The Netherlands, New Zealand and Sweden. Except for the LEADER trial, which was confined to men, there was no sex restriction. Men constituted 78% of the total number of study participants (n=7832). Duration of follow up ranged from 20 days to five years.
Trials differed in the criteria they used to define peripheral arterial disease of the lower limb (PAD) in trial participants. The majority used a combination of clinical symptoms (predominantly intermittent claudication) with or without confirmation using objective tests such as Doppler ultrasound, treadmill tests and/or angiography.
In the Nye study (Nye 1973) and HPS (HPS), patients who had undergone surgery for PAD were also included. HPS used a broad definition for PAD, including subjects with intermittent claudication, any peripheral revascularization procedure, or aortic aneurysm. Patients with diabetes and treated hypertension were also included in HPS but were excluded in some other studies. The LEADER trial used the Edinburgh Claudication questionnaire to identify PAD.
Five studies (HPS; LEADER; Mondillo 2003; PQRST; St Thomas' Trial) used minimum blood lipid and/or lipoprotein levels as inclusion criteria; one trial used a maximum LDL cholesterol level of ≤ 4.14 mmol/l (Mohler 2003).
All studies were on drug interventions except one study (Gans 1990) which used fish oil. The majority investigated a single drug against placebo and/or usual treatment. Three of these were on statins; two on simvastatin (HPS; Mondillo 2003), and one on atorvastatin (Mohler 2003); one on probucol, (probucol and cholestyramine versus cholestyramine alone) (PQRST); one on beta pyridil (Nye 1973); one on bezafibrate (LEADER); two on policosanol (fatty alcohol) (Castano 1999; Castano 2001); and seven on sulodexide (a glycosaminoglycan with lipid‐lowering properties) (Bonalumi 1986; Caramelli 1988; Coccheri 2002; Corsi 1985; Cospite 1985; Liguori 1993; Palmieri 1984). In addition, Castano 2003 compared policosanol with lovastatin; and in the St Thomas' Trial, participants in the active treatment group were allocated cholestyramine and/or nicotinic acid or clofibrate or nicotinic acid, depending on their type of hyperlipidaemia (versus usual care).
Three studies did not report on the effects of treatment on blood lipid profiles over the course of the trial (Caramelli 1988; Castano 1999; Coccheri 2002). The other studies (Bonalumi 1986; Castano 2001; Castano 2003; Corsi 1985; Cospite 1985; HPS; LEADER; Mohler 2003; Mondillo 2003; Nye 1973; St Thomas' Trial) reported a decrease in total and/or low density lipoprotein cholesterol levels and an increase in protective high density lipoprotein (HDL) cholesterol levels. The only exceptions were (i) Gans 1990 and Palmieri 1984 in which total and/or LDL cholesterol levels remained unchanged (in Gans 1990, HDL cholesterol increased, and in Palmieri 1984 triglyceride levels decreased) and (ii) PQRST, in which decreased total and LDL cholesterol levels were associated with a decrease also in HDL cholesterol levels.
For additional information on included studies, seeCharacteristics of included studies table.
Risk of bias in included studies
Methodological quality was assessed using the following criteria and in accordance with the guidelines provided by the Cochrane Peripheral Vascular Diseases Group based on the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook).
Method of allocation and concealment of allocation
Treatment and control group comparable at entry
Use of intention‐to‐treat analysis
Adequate blindness
For each criterion of internal validity, we graded studies as low risk of bias (A) if they fully met the criterion, moderate risk (B) if they partly met the criterion and high risk (C) if they failed to meet the criterion. Overall quality of each study was rated as A if the study scored A in all criteria, B if one or more of the criteria scored B and C if one or more of the criteria scored C. A detailed breakdown of the scoring is shown in the table of methodological quality assessment of included studies (Table 2).
1. Methodological quality assessment.
| Study ID | Allocation | Baseline comparable | Intention to treat | Blindness | Overall score | Loss to follow up | Inclusion criteria | Exclusion criteria | Outcome |
| Bonalumi 1986 | B | A | C | A (DB) | C | Not reported | B | C | A |
| Caramelli 1988 | B | A | B (not clear) | A (DB) | B | None | A | C | A |
| Castano 1999 | A | A | A | A (DB) | A | 16% | A | A | A |
| Castano 2001 | A | A | A | A (DB) | A | 28% | A | A | A |
| Castano 2003 | A | A | A | A (DB) | A | 3% | A | A | A |
| Cocheri 2002 | B | A | A | A (DB) | B | 18% | A | A | A |
| Corsi 1985 | A | A | B (not clear) | A (DB) | B | None | A | A | A |
| Cospite 1985 | A | A | B (not clear) | A (DB) | B | None | A | A | A |
| Gans 1990 | A | A | C | A (DB) | C | 14% | A | A | A |
| HPS | A | A | A | A (DB) | A | Compliance with statin use in treatment arm is 85%. % using statin in placebo arm is 17% | A | A | A |
| Leader trial | A | A | A | A (DB) | A | 45% | A | A | A |
| Liguori 1993 | B | A | B (not clear) | A (DB) | B | 9% | A | A | B |
| Mohler 2003 | B | A | C | A (DB) | C | 3% | A | A | A |
| Mondillo 2003 | B | A | A | A (DB) | B | None | A | A | A |
| Nye 1973 | B | A | B (not clear) | A (DB) | B | 32% | A | B | A |
| Palmieri 1984 | B | B | C | A (DB) | C | None | A | C | A |
| PQRST | B | A | B | A (DB) | B | 10% | A | A | A |
| St.Thomas | B | B | B | B (outcome assessor blinded) | B | 4% | A | A | A |
Information on provider blindness to the assignment status and observer variation in outcome measurements was not available for the included studies and therefore these quality criteria were not assessed in the review. Loss to follow up was also not used for quality assessment, as it was difficult to grade, but is described in the table of methodological quality assessment. External validity was also assessed by clear definition of inclusion and exclusion criteria as well as outcome reported but this was not used in the overall grading of studies.
For further information on methodological quality assessment, see additional table (Table 2).
Effects of interventions
Trials comparing lipid‐lowering therapy with placebo and/or usual treatment for at least 90 days were included in meta‐analysis. There were insufficient trials comparing one lipid‐lowering therapy with another to enable pooling.
Many of the data on all‐cause mortality, fatal and non‐fatal cardiovascular events were obtained from information given on reasons for participant withdrawal from the trial, rather than forming a primary and prespecified outcome measure. In such cases, studies were generally under‐powered to investigate these outcomes. In many trials fatal and non‐fatal cardiovascular events were reported separately and it was not clear whether only the first event was recorded for a given individual. Therefore, where cardiovascular event rates were created by adding together fatal and non‐fatal cardiovascular events for the purposes of pooling results from different trials, it is possible that subjects with both a fatal and a non‐fatal event have been counted twice.
The included studies were not necessarily statistically diverse on tests of heterogeneity but were clinically varied. Therefore, we pooled results using a random‐effects model for meta‐analysis. We performed subgroup analyses excluding (i) those studies graded as having a high or moderate risk of bias and (ii) the PQRST trial, which had an adverse effect on lipoprotein profile (HDL cholesterol was reduced by 24% in those on probucol). We also performed subgroup analyses of studies using the same class of lipid‐lowering therapy for every outcome wherever possible. Only three classes of drugs ‐ statins, policosanol and sulodexide (the latter for walking distance only), were tested in more than one trial and were therefore subject to this sub‐group analysis
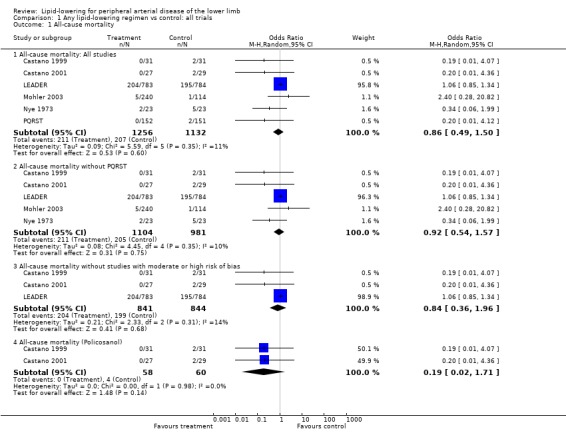
1. All‐cause mortality (Analysis 01/01)
Information on all‐cause mortality was reported in only six studies, of which three (Castano 1999; Castano 2001; Nye 1973) were not designed to look at this outcome.
The pooled result from all six studies indicated that there was no statistically significant effect of lipid‐lowering therapy on all‐cause mortality (odds ratio (OR) 0.86; 95% confidence interval (CI) 0.49 to 1.50, P = 0.60). Exclusion of PQRST from the analysis did not change this finding (OR 0.92; CI 0.54 to 1.57, P = 0.75). The result was similar when additional studies with high or moderate risk of bias were excluded (OR 0.84; CI 0.36 to 1.96, P = 0.68).
In a pooled subgroup analysis of the two small Castano studies of policosanol versus placebo a lower risk of all‐cause mortality among those treated with policosanol was not statistically significant (OR 0.19; 95% CI 0.02 to 1.71, P = 0.14).
2. Cardiovascular events
For further information on events contributing to vascular outcomes, see additional table (Table 3).
2. Events contributing to vascular outcomes.
| Study ID | Total cardiovascular | Non‐fatal cardiovasc | Fatal cardiovasc | Total coronary | Non‐fatal coronary | Total stroke | Source of data |
| Castano 1999 | Angina, TIA, non‐ fatal stroke; fatal stroke | Angina; TIA; non‐fatal stroke | Fatal stroke | Angina | Angina | Fatal and non‐fatal stroke | From adverse events |
| Castano 2001 | Fatal and non‐fatal cardiovascular | TIA; MI; unstable angina; non‐fatal stroke | Fatal stroke | MI; unstable angina | MI; unstable angina | Fatal and non‐fatal stroke | From withdrawals |
| Coccheri 2002 | Angina; acute leg ischaemia; non‐fatal MI; fatal stroke; fatal MI | Angina; acute leg ischaemia; non‐fatal MI | Fatal MI and stroke | Fatal and non‐fatal AMI; angina | Non‐fatal AMI; angina | Fatal and non‐fatal stroke | From serious adverse events |
| HPS | Non‐fatal MI or fatal CHD; strokes of any type and coronary or non‐coronary revascularisations | Predefined outcome | |||||
| Leader trial | Fatal and non‐fatal cardiovascular | Non‐fatal coronary heart disease; non‐fatal stroke | Fatal coronary heart disease and fatal stroke | Sudden coronary death; non‐fatal MI | Non‐fatal MI | Fatal and non‐fatal stroke | Predefined outcome |
| Mohler 2003 | Fatal and non‐fatal MI and stroke | MI; stroke | Fatal MI and stroke | Fatal MI and coronary heart disease; non‐fatal MI | Non fatal MI | Fatal and non‐fatal stroke | From discontinuation from studies |
| Nye | Severe coronary heart disease problem, | Severe coronary heart disease problem; carotid occlusion | Death from carotid occlusion, coronary thrombosis | From drop out information | |||
| PQRST | MI (acute, silent, possible silent); angina; arrhythmias; other heart disease, TIA; fatal MI | MI (acute, silent, possible silent); angina; arrhythmias; other heart disease; TIA | Fatal MI | Fatal and non‐fatal AMI including silent infarct; angina | Non fatal AMI; silent AMI; angina | From withdrawals |
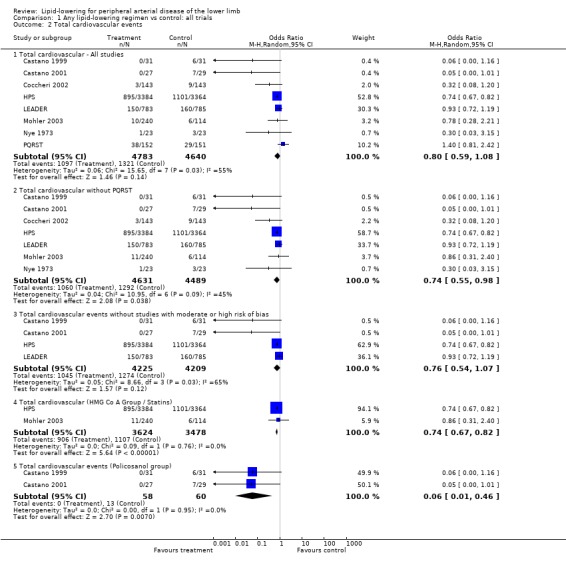
(a) Total cardiovascular events (fatal and non‐fatal) Analysis 01/02
A total of eight studies were included in this analysis, including the large PAD subgroup of the HPS study. On pooling the results of all studies, treatment was associated with a non‐significant reduction in the risk of total cardiovascular events (OR 0.80; 95% CI 0.59 to 1.08, P = 0.14). The HPS and LEADER trials contributed 53% and 30% of the weight to this result respectively. The pooled result was similar when all studies with moderate or high risk of bias were excluded (OR 0.76; 95% CI 0.54 to 1.07, P = 0.12). However, when the PQRST trial alone was excluded, the reduction in risk was statistically significant (OR 0.74; 95% CI 0.55 to 0.98, P = 0.04).
Two studies investigated the effects of statins on total cardiovascular events (simvastatin 40 mg daily in HPS and atorvastatin either 10 mg or 80 mg daily in Mohler 2003). The total number of participants was 7002 (95% in the HPS). A pooled subgroup analysis of these two studies demonstrated a statistically significant lower risk of a cardiovascular event among those taking HMG CO A lipid‐lowering therapy compared with placebo (OR 0.74; 95% CI 0.67 to 0.82, P < 0.00001). Since one third of the patients in HPS had undergone coronary or peripheral vascular surgery, it is possible that the effect of simvastatin on this group may differ from those without such co‐morbidity or advanced disease.
Pooled subgroup analysis of the two Castano studies (Castano 1999; Castano 2001) indicated a statistically significant lower risk of a cardiovascular event among those taking policosanol compared with placebo (OR 0.06; 95% CI 0.01 to 0.46, P = 0.007).
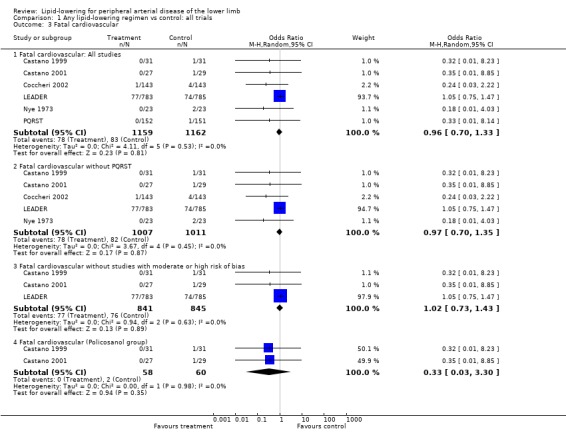
(b) Fatal cardiovascular events (Analysis 01/03)
Of the eight studies which reported on total cardiovascular events, one did not distinguish between fatal and non‐fatal events (HPS) and one other gave only non‐fatal cardiovascular events as a separate category (Mohler 2003). Therefore, only six studies were available for pooled analysis on this outcome, the result of which was heavily influenced by the LEADER trial (giving over 93% of the weight to the overall pooled result). There was no statistically significant difference in the risk of a fatal cardiovascular event between the treatment and placebo groups when all six studies were pooled (OR 0.96; 95% CI 0.70 to 1.33, P = 0.81), nor when PQRST was excluded (OR 0.97; 95% CI 0.70 to 1.35, P = 0.87), nor when additional studies with moderate or high risk of bias were excluded (OR 1.02; 95% CI 0.73 to 1.43, P = 0.89).
Pooled results from the two Castano studies showed no statistically significant difference in fatal cardiovascular events between policosanol and placebo (OR 0.33; 95% CI 0.03 to 23.30, P = 0.35).
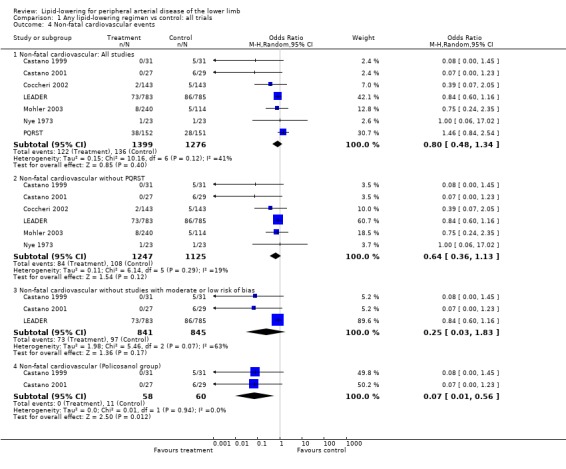
(c) Non‐fatal cardiovascular events (Analysis 01/04)
Of the seven studies which reported this outcome, the LEADER and PQRST trials contributed approximately 42% and 31% of weight to the overall pooled result respectively. There was no statistically significant difference in non‐fatal cardiovascular events between treatment and placebo groups when all seven studies were pooled (OR 0.80; CI 0.48 to 1.34, P = 0.40), nor when PQRST was excluded (OR 0.64; 95% CI 0.36 to 1.13, P = 0.12), nor when additional studies with moderate to high risk of bias were excluded (OR 0.25; 95% CI 0.03 to 0.56, P = 0.17).
In subgroup analysis of the two Castano trials, there was a statistically significant lower risk of a non‐fatal cardiovascular event among those receiving policosanol compared with placebo (OR 0.07; 95% CI 0.01 to 0.56, P=0.01).
3. Coronary events
(a)Total coronary events (Analysis 01/05)
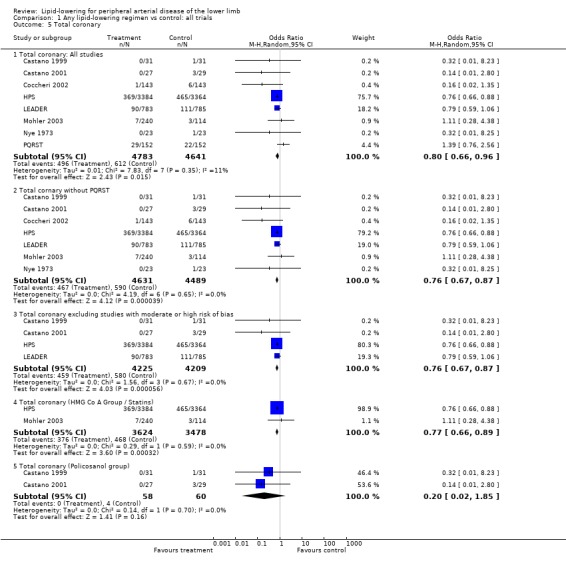
A total of eight studies were included in this analysis, including the large HPS and LEADER trials. On pooling the results of all studies, treatment was associated with a statistically significant reduction in the risk of total coronary events (OR 0.80; 95% CI 0.66 to 0.96, P = 0.01). The HPS and LEADER trials contributed 76% and 18% of the weight to this result respectively. The pooled result was similar when the PQRST trial alone was excluded (OR 0.76; 95% CI 0.67 to 0.87, P < 0.0001) and when additional studies with moderate or high risk of bias were excluded (OR 0.76; 95% CI 0.67 to 0.87, P < 0.0001).
A pooled subgroup analysis of the two studies investigating the effects of statins on total coronary events (HPS; Mohler 2003), demonstrated a statistically significant lower risk of a cardiovascular event among those taking HMG CO A lipid‐lowering therapy compared with placebo (OR 0.77; 95% CI 0.66 to 0.89, P = 0.0003).
Pooled subgroup analysis of the two Castano studies suggested a lower risk of a coronary event among those taking policosanol compared with placebo (OR 0.20; 95% CI 0.02 to 1.85, P = 0.16). However, the effect was not statistically significant.
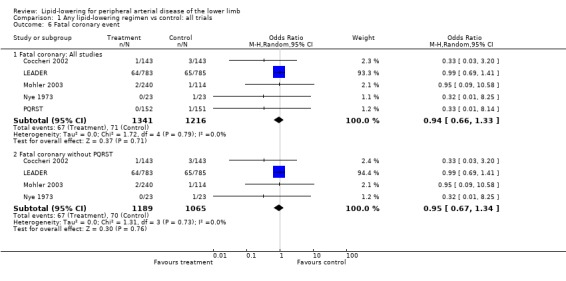
(b) Fatal coronary event (Analysis 01/06)
As with the total cardiovascular event outcome, the large HPS study did not differentiate between fatal and non‐fatal coronary events and was therefore not included in the analyses of coronary events by 'fatal' or 'non‐fatal'. Five studies did report on fatal coronary events separately, the pooled results for which were heavily influenced by the LEADER trial. There was no statistically significant difference in fatal coronary events between the treatment and placebo groups with all eligible trials included (OR 0.94; 95% CI 0.66 to 1.33, P = 0.71), nor when PQRST was excluded (OR 0.95; 95% CI 0.67 to 1.34, P = 0.76). Exclusion of studies with moderate to high risk of bias left only the LEADER trial.
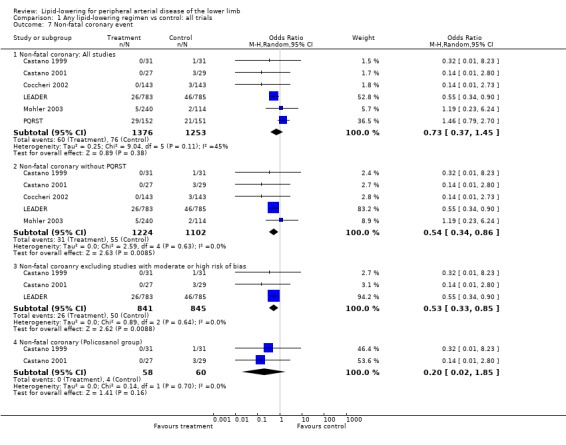
(c) Non‐fatal coronary events (Analysis 01/07)
Six studies reported this outcome separately. The pooled result showed no statistically significant difference in non‐fatal coronary events between the treatment and placebo groups (OR 0.73; 95% CI 0.37 to 1.45, P = 0.38). The LEADER trial contributed 53% of weight to this result. However, when PQRST was excluded, the pooled result showed a statistically significant lower risk of a non‐fatal coronary event among those treated with lipid‐lowering therapy compared with those taking placebo (OR 0. 54; 95% CI 0.34 to 0.86, P = 0.009). This result was maintained when additional studies with moderate to high risk of bias were excluded (OR 0.53; 95% CI 0.33 to 0.85, P = 0.009).
The pooled result from the two Castano studies (Castano 1999; Castano 2001) suggested a reduction in non‐fatal coronary events in those taking policosanol (OR 0.20; 95% CI 0.02 to 1.85, P = 0.16) but the effect was not statistically significant.
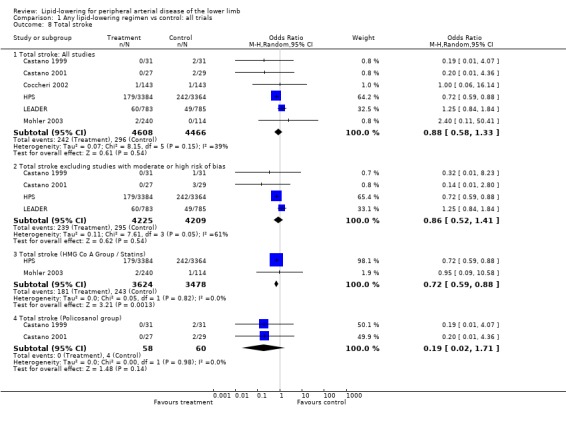
4. Total stroke (Analysis 01/08)
A total of six studies were included in this analysis, including the large HPS and LEADER trials. On pooling the results of all studies, there was no statistically significant effect of treatment on the risk of combined fatal and non‐fatal stroke (OR 0.88; 95% CI 0.58 to 1.33, P = 0.54). The HPS and LEADER trials contributed 64% and 33% of the weight to this result respectively. The pooled result was similar when all studies with moderate or high risk of bias were excluded (OR 0.86; 95% CI 0.52 to 1.41, P = 0.54).
A pooled subgroup analysis of the two studies that investigated the effects of statins on total stroke events (HPS; Mohler 2003), demonstrated a lower risk of statistically significant stroke among those taking lipid‐lowering therapy compared with placebo (OR 0.72; 95% CI 0.59, 0.88, P = 0.001).
Pooled subgroup analysis of the two Castano studies (Castano 1999; Castano 2001) indicated no statistically significant difference in risk of stroke between those taking policosanol and placebo (OR 0.19; 95% CI 0.02, 1.71, P = 0.14)
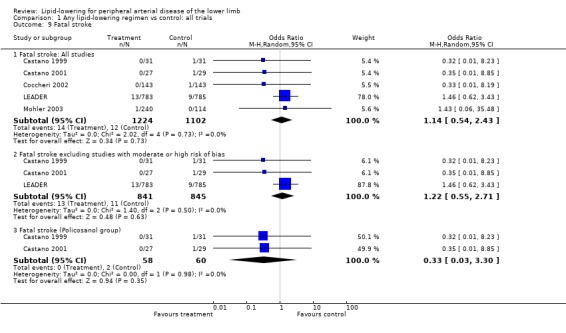
(a) Fatal stroke (Analysis 01/09)
The large HPS study did not differentiate between fatal and non‐fatal stroke and could not be included in the analyses of these outcomes separately. Five studies (but not the PQRST trial) did report on fatal stroke, the pooled results for which were once more heavily influenced by the LEADER trial. There was no statistically significant difference in risk of fatal strokes between the treatment and placebo groups when all trial were combined (OR 1.14; 95% CI 0.54 to 2.43, P = 0.73), nor when studies with moderate to high risk of bias were excluded (OR 1.22; 95% CI 0.55 to 2.71, P = 0.63).
Pooled result from the two Castano studies (Castano 1999; Castano 2001) showed no statistically significant difference in fatal stroke events between policosanol and placebo (OR 0.33; 95% CI 0.03 to 3.30, P = 0.35).
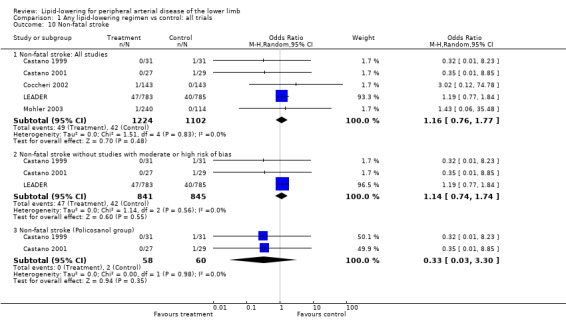
(b) Non‐fatal stroke (Analysis 01/10)
The same five studies reported this outcome, with the LEADER trial contributing over 90% of weight to the pooled result. The overall pooled result showed no statistically significant difference in risk of non‐fatal stroke between treatment and placebo (OR 1.16; 95% CI 0.76 to 1.77, P = 0.48). This remained the case when studies with moderate to high risk of bias were excluded from the analysis (OR 1.14; 95% CI 0.74 to 1.74, P = 0.55). In further subgroup analysis, the pooled result from the two Castano trials showed no statistically significant difference in risk of non‐fatal stroke events between policosanol and placebo (OR 0.33; 95% CI 0.03 to 3.30, P = 0.35).
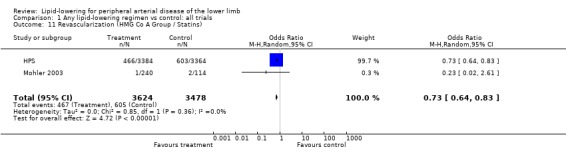
5. Revascularizations (Analysis 01/11)
Two studies (HPS; Mohler 2003) assessed the impact of lipid‐lowering therapy (HMG CO A group) on risk of coronary and non‐coronary procedures. The pooled result showed a statistically significant decrease in revascularization rate among those taking lipid‐lowering therapy compared with placebo (OR 0.73; 95% CI 0.64 to 0.83, P < 0.00001). The result was heavily influenced by HPS which contributed over 99% of weight to the result.
6. Walking distance
Eleven studies reported on walking distance (Bonalumi 1986; Caramelli 1988; Castano 1999; Castano 2001; Castano 2003; Corsi 1985; Cospite 1985; Gans 1990; Liguori 1993; Mondillo 2003; Palmieri 1984) of which only seven reported numerical data and were suitable for pooling by meta‐analysis. Of the remainder, Bonalumi et al (Bonalumi 1986) reported that treatment with sulodexide for 90 days doubled walking distance compared with no change in the placebo group but accurate numerical data could not be extracted from the graphs provided. Liguori 1993 reported that treatment with sulodexide for the same duration resulted in a statistically significant improvement over placebo (5.7 m in placebo group versus 28.8 m, 50 m and 38.9 m in sulodexide groups depending on dosage). Caramelli 1988 reported a statistically significant difference in pain‐free walking distance between the treatment group (mean 233.44 m, SD 115.02) and the placebo group (mean 156.60 m, SD 109.57, P < 0.05), but since the duration of treatment was limited to 20 days, this trial did not reach our criterion of a minimum of 90 days duration for inclusion in the meta‐analysis.
In the small (n = 28) trial by Castano (Castano 2003), both pain‐free and total walking distance increased more in the policosanol group (33.7% and 24.3% increases from baseline) compared with the lovastatin group (12.3% and 4.9% increases from baseline, P < 0.01 for comparison with policosanol group).
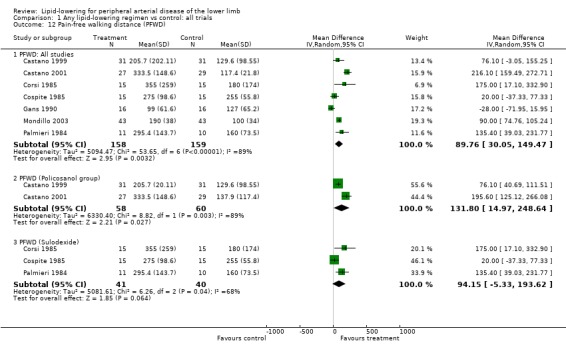
(a) Pain‐free walking distance (Analysis 01/12)
Seven studies were included in the analysis of this outcome (Castano 1999; Castano 2001; Corsi 1985; Cospite 1985; Gans 1990; Mondillo 2003; Palmieri 1984). The pooled result showed a statistically significant improvement in walking distance with lipid‐lowering therapy compared with placebo (mean difference (MD) 89.76 m; 95% CI 30.05 to 149.47, P = 0.003).
In subgroup analysis of the two Castano trials (Castano 1999; Castano 2001), there was a statistically significant improvement in pain‐free walking distance among patients treated with policosanol compared with those taking placebo (MD 131.80 m; 95% CI 14.97 to 248.64, P = 0.03). However, an improvement in pain‐free walking distance suggested by pooling the results of three trials using sulodexide was not statistically significant (MD 94.15 m; 95% CI ‐5.33 to 193.62, P = 0.06).
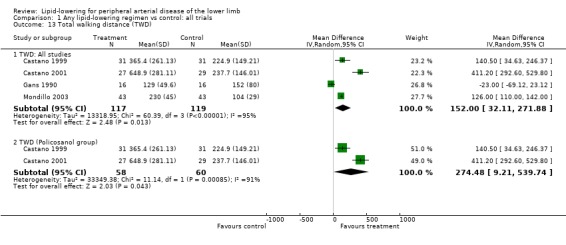
(b) Total walking distance (Analysis 01/13)
Four studies reported this outcome and were pooled (Castano 1999; Castano 2001; Gans 1990; Mondillo 2003). There was a statistically significant improvement in total walking distance with lipid‐lowering therapy compared with placebo (MD 152.00 m; 95% CI 32.11 to 271.88, P = 0.01).
Pooled subgroup analysis of the two Castano studies (Castano 1999; Castano 2001) also indicated a statistically significant improvement in total walking distance among those taking policosanol compared with placebo (MD 274.48 m; 95% CI 9.21 to 539.74, P = 0.04).
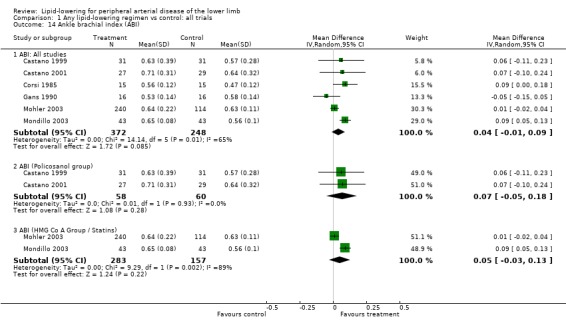
7. Ankle brachial index (ABI) (Analysis 01/14)
A total of six studies were included in this analysis and were pooled (Castano 1999; Castano 2001; Corsi 1985; Gans 1990; Mohler 2003; Mondillo 2003). There was no statistically significant difference in ABI following treatment between the intervention and placebo groups (MD 0.04; 95% CI ‐0.01 to 0.09, P = 0.09).
Pooled subgroup analyses indicated no significant effect on ABI of either policosanol (MD 0.07; 95% CI ‐0.05 to 0.18, P = 0.28) (Castano 1999; Castano 2001) or statin (MD 0.05; 95% CI ‐0.03 to 0.13, P = 0.22) (Mohler 2003; Mondillo 2003).
For the last three outcomes (PFWD, TWD and ABI), the two Castano studies were the only studies graded as A in quality assessment.
8. Disease progression assessed by angiogram
Two studies (PQRST; St Thomas' Trial) looked at this outcome. Meta‐analysis was not possible because of different summary measurements reported.
In the St Thomas' Trial, disease progression, assessed using femoral arteriography was reduced significantly in the treatment group (10/144 arterial segments) compared with the placebo group (27/156 arterial segments P < 0.01).
In the PQRST trial probucol did not show a favourable effect on femoral artery lumen volume (0.6% increase in volume from baseline, P = 0.61) compared with placebo (4.2% increase in volume from baseline, P < 0.001).
9. Side effects
No major side effects were detected in those taking sulodexide (Bonalumi 1986; Caramelli 1988; Corsi 1985; Liguori 1993), except for pain at the injection site and transient and sporadic epigastric heaviness and/or heartburn. No major side effects were experienced in subjects taking essential fatty acid supplements (Gans 1990). Betapyridyl carbinol produced gastrointestinal symptoms in seven out of the 23 patients at a dosage of 1800 mg daily (Nye 1973), but only three patients were unable to tolerate 1200 mg daily. In addition, two male patients developed liver toxicity in response to treatment. No major side effects were noted in patients receiving probucol therapy (PQRST). However, a reduction in the beneficial HDL cholesterol levels may be interpreted as an adverse reaction in those on probucol. Side effects were not given in the available reports from the St Thomas' Trial (Duffield 1982; Duffield 1983; Lewis 1985).
The Heart Protection Study reported that there was no increase in the incidence of cancer associated with simvastatin, except for a slight increase in the incidence of non‐melanoma skin cancer (HPS). The authors noted however that the incidence of this skin cancer did not increase with prolonged treatment, arguing against a causal relationship. The Leader trial reported no adverse effect on cancer incidence associated with bezafibrate, but a statistically significant higher number of patients on active treatment withdrew due to raised serum creatinine level compared with placebo (LEADER).
No information was provided on side effects for the trial using atorvastatin (Mohler 2003).
Policosanol was reported as well tolerated (Castano 1999; Castano 2001; Castano 2003). There were mild side effects (asthenia, muscular cramps/pain, and granuloma) reported for lovastatin 20 mg daily (Castano 2003).
Discussion
Thirteen additional studies were included in this first major update compared with the original version of the review . This was due to the results of studies still in progress at the time of the original review becoming available and an improved search facility which identified earlier studies (for example, Palmieri 1984) as well as the completion of new relevant trials.
Differences in included studies compared with original review
Compared with the original review, this update included two studies on statins: the Heart Protection Study which used simvastatin 40 mg daily (HPS) and a smaller study by Mohler (Mohler 2003) using atorvastatin 10 mg or 80 mg daily. Other groups of drugs which were added included sulodexide and policosanol, although these drugs are not currently listed as lipid‐lowering drugs in the British National Formulary (BNF).
Compared with studies included in the original review, the largest study added to this update used a wider definition of peripheral arterial disease (HPS). Of 6748 participants with a history of PAD, around one third had undergone previous peripheral arterial surgery, which included aortic revascularization procedures and aneurysm repair, angioplasty or a previous amputation (2%). We were unable to obtain the results of the HPS excluding those with aortic disease from the trial investigators. The Nye study (Nye 1973) , which was included in the original version of the review, also included some patients who had undergone peripheral arterial surgery in its study population. We had pre‐specified for this review that studies of lipid lowering solely in post‐procedural patients with PAD would be excluded, in part because these trials are concerned with re‐stenosis or procedure failure rates rather than long‐term cardiovascular morbidity and mortality. However, we made the decision to include studies with a mix of symptomatic and post‐procedural subjects due to the likely similar underlying pathology and consistency in outcomes measured with other included trials.
In terms of aortic disease, most of the included studies did not mention aortic disease or surgery in their inclusion or exclusion criteria. It was therefore decided to omit the clause 'aortic disease' from the exclusion criteria used in the original version of the review (which was originally intended to exclude entire studies on aortic disease).
In contrast with the original review, the CLAS study (CLAS) was excluded from this update because participants included not only those suffering from lower limb atherosclerosis but also unaffected subjects. Davis 1975 was also excluded because only subjective symptom changes were reported and newer trials provide an increased quantity of evidence on objective measures.
Studies which did not suggest a favourable effect on outcomes
Overall, the vast majority of individual trials suggested a favourable effect on outcomes although only some of these were statistically significant. Exceptions to this were the studies by Mohler (Mohler 2003), Gans (Gans 1990) and the LEADER and PQRST trials. It is worth noting that the study by Mohler (Mohler 2003) was not designed to detect all‐cause mortality or cardiovascular events and relatively few such events were recorded in either the treatment or placebo groups. Gans 1990 suggested a reduction in walking distance on active treatment (fish oil), but results were not statistically significant.
The PQRST trial was not designed to detect fatal and non‐fatal cardiovascular events but to assess disease progression measured by femoral angiography. Results showed that probucol (in addition to existing cholestyramine treatment) did not impact on femoral atherosclerosis. Probucol was found to have an adverse effect on the lipid profile in participants assigned active treatment. Thus, plasma high density lipoprotein (HDL) cholesterol was decreased by as much as 24% in the probucol group compared with those on placebo. The authors concluded that very low levels of HDL in probucol treated patients possibly counteracted the observed beneficial effect of treatment on plasma low density lipoprotein cholesterol level . Probucol is currently not listed as a lipid‐lowering drug in the British National Formulary (BNF).
The LEADER trial was designed to detect the combined incidence of coronary heart disease and stroke (fatal and non‐fatal) as its primary outcome and separate fatal and non‐fatal coronary and stroke events as secondary outcomes. Bezafibrate did not reduce the incidence of fatal events but did reduce the incidence of non‐fatal coronary events. In post‐hoc subanalysis, bezafibrate also decreased total coronary events (RR 0.13; 95% CI 0.03 to 0.56) among those aged under 65 years. Several factors need to be taken into account in interpreting the result of the LEADER trial, which heavily influenced the pooled results on fatal and non‐fatal cardiovascular events, and all‐cause mortality in this review. Firstly, there was an increased use of statins in the placebo group; more subjects from the placebo group (109 out of 403 subjects) compared with those on active treatment (42 out of 369) withdrew because they were prescribed lipid‐lowering therapy (predominantly a statin). Secondly, the primary outcome measure of combined fatal and non‐fatal cardiovascular events did not reach the level required by the author's sample size calculation; it was estimated that 33% of the placebo group would develop a primary end‐point during four years follow up whereas the actual observed event rate was 20.4%. Although bezafibrate improved the blood lipid profile of participants (reducing plasma total cholesterol, low density lipoprotein cholesterol and triglycerides and increasing high density lipoprotein cholesterol), it was also reported that bezafibrate increased plasma homocysteine concentrations, which may be a risk factor for vascular diseases in high concentrations.
Authors' conclusions
Implications for practice.
The effect of lipid‐lowering therapy on all‐cause mortality in people with PAD was inconclusive, probably due to lack of large scale and long duration trials measuring this outcome. However, lipid‐lowering therapy had a beneficial effect on the incidence of total cardiovascular events due primarily to a reduction in coronary events. Lipid‐lowering therapy also improved walking distance. There was insufficient evidence to compare the effectiveness of different lipid‐lowering regimens. However, the only class of drug for which consistent and statistically significant evidence of a beneficial effect on total cardiovascular events, total coronary events and stroke was available, was the statins. This evidence came from the HPS, in which simvastatin was assigned to subjects with PAD and a random blood total cholesterol level of at least 3.5 mmol/l. As such, lipid‐lowering therapy with a statin is recommended for patients with peripheral arterial disease and total cholesterol levels > 3.5 mmol/l.
Implications for research.
Further research is indicated to compare the relative effectiveness of different lipid‐lowering therapies in people with PAD. However, because of increasing use of statins in clinical practice, the opportunities to undertake such trials may be limited. Because of the relatively large number of people with asymptomatic PAD (detected for instance using measures of ankle brachial index), research into the effectiveness of lipid‐lowering therapy in such subjects should be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 24 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 3, 1996
| Date | Event | Description |
|---|---|---|
| 12 July 2007 | New citation required and conclusions have changed | Review substantively updated. 1) The title of this review has been amended from 'Lipid‐lowering for lower limb atherosclerosis' to reflect more accurately the content of the review. 2) Thirteen new included studies and 51 new excluded studies have been added. 3) There is a new contact author and an additional co‐author. |
Acknowledgements
We would like to thank the following trialists who provided further information on included and excluded studies: Dr Rosa Mas (Castano trials) Dr Jane Armitage (Heart Protection Study). We would also like to thank Dr Janet Wale (Consumer Network) for providing the Plain Language Summary.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Arterial Occlusive Diseases, this term only #2 arter* near occlus* #3 periph* near arter* #4 obstruct* near arter* #5 PAOD #6 PAD #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Intermittent Claudication, this term only #9 intermitt* near claud* #10 claud* #11 (#8 OR #9 OR #10) #12 limb ischem* #13 critical near limb #14 (#12 OR #13) #15 (#11 OR #14) #16 lipid near lower* #17 statin* or simvastatin* or atorvastatin* or fibrate* or HMG‐COA #18 hydroxymethylglutaryl‐coa‐reduct* or probucol* or niacin* or nicotin* near acid* #19 MeSH descriptor Hydroxymethylglutaryl CoA Reductases explode all trees #20 MeSH descriptor Probucol, this term only #21 MeSH descriptor Niacin, this term only #22 (#16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#15 AND #22)
Data and analyses
Comparison 1. Any lipid‐lowering regimen vs control: all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All‐cause mortality: All studies | 6 | 2388 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.50] |
| 1.2 All‐cause mortality without PQRST | 5 | 2085 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.57] |
| 1.3 All‐cause mortality without studies with moderate or high risk of bias | 3 | 1685 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.36, 1.96] |
| 1.4 All‐cause mortality (Policosanol) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.71] |
| 2 Total cardiovascular events | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Total cardiovascular ‐ All studies | 8 | 9423 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.59, 1.08] |
| 2.2 Total cardiovascular without PQRST | 7 | 9120 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.98] |
| 2.3 Total cardiovascular events without studies with moderate or high risk of bias | 4 | 8434 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.4 Total cardiovascular (HMG Co A Group / Statins) | 2 | 7102 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.67, 0.82] |
| 2.5 Total cardiovascular events (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.46] |
| 3 Fatal cardiovascular | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fatal cardiovascular: All studies | 6 | 2321 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.33] |
| 3.2 Fatal cardiovascular without PQRST | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.35] |
| 3.3 Fatal cardiovascular without studies with moderate or high risk of bias | 3 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.73, 1.43] |
| 3.4 Fatal cardiovascular (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.30] |
| 4 Non‐fatal cardiovascular events | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Non‐fatal cardiovascular: All studies | 7 | 2675 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.34] |
| 4.2 Non‐fatal cardiovascular without PQRST | 6 | 2372 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.13] |
| 4.3 Non‐fatal cardiovascular without studies with moderate or low risk of bias | 3 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 1.83] |
| 4.4 Non‐fatal cardiovascular (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.56] |
| 5 Total coronary | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Total coronary: All studies | 8 | 9424 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.96] |
| 5.2 Total cornary without PQRST | 7 | 9120 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 5.3 Total coronary excluding studies with moderate or high risk of bias | 4 | 8434 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.67, 0.87] |
| 5.4 Total coronary (HMG Co A Group / Statins) | 2 | 7102 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.89] |
| 5.5 Total coronary (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.85] |
| 6 Fatal coronary event | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Fatal coronary: All studies | 5 | 2557 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.66, 1.33] |
| 6.2 Fatal coronary without PQRST | 4 | 2254 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 7 Non‐fatal coronary event | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Non‐fatal coronary: All studies | 6 | 2629 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.37, 1.45] |
| 7.2 Non‐fatal coronary without PQRST | 5 | 2326 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 7.3 Non‐fatal coroanry excluding studies with moderate or high risk of bias | 3 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 7.4 Non‐fatal coronary (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.85] |
| 8 Total stroke | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Total stroke: All studies | 6 | 9074 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.33] |
| 8.2 Total stroke excluding studies with moderate or high risk of bias | 4 | 8434 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.52, 1.41] |
| 8.3 Total stroke (HMG Co A Group / Statins) | 2 | 7102 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.88] |
| 8.4 Total stroke (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.71] |
| 9 Fatal stroke | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Fatal stroke: All studies | 5 | 2326 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.43] |
| 9.2 Fatal stroke excluding studies with moderate or high risk of bias | 3 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.55, 2.71] |
| 9.3 Fatal stroke (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.30] |
| 10 Non‐fatal stroke | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Non‐fatal stroke: All studies | 5 | 2326 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.76, 1.77] |
| 10.2 Non‐fatal stroke without studies with moderate or high risk of bias | 3 | 1686 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.74] |
| 10.3 Non‐fatal stroke (Policosanol group) | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.30] |
| 11 Revascularization (HMG Co A Group / Statins) | 2 | 7102 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.64, 0.83] |
| 12 Pain‐free walking distance (PFWD) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 PFWD: All studies | 7 | 317 | Mean Difference (IV, Random, 95% CI) | 89.76 [30.05, 149.47] |
| 12.2 PFWD (Policosanol group) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 131.80 [14.97, 248.64] |
| 12.3 PFWD (Sulodexide) | 3 | 81 | Mean Difference (IV, Random, 95% CI) | 94.15 [‐5.33, 193.62] |
| 13 Total walking distance (TWD) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 TWD: All studies | 4 | 236 | Mean Difference (IV, Random, 95% CI) | 152.00 [32.11, 271.88] |
| 13.2 TWD (Policosanol group) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 274.48 [9.21, 539.74] |
| 14 Ankle brachial index (ABI) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 ABI: All studies | 6 | 620 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 14.2 ABI (Policosanol group) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.18] |
| 14.3 ABI (HMG Co A Group / Statins) | 2 | 440 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.03, 0.13] |
1.1. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 1 All‐cause mortality.
1.2. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 2 Total cardiovascular events.
1.3. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 3 Fatal cardiovascular.
1.4. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 4 Non‐fatal cardiovascular events.
1.5. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 5 Total coronary.
1.6. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 6 Fatal coronary event.
1.7. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 7 Non‐fatal coronary event.
1.8. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 8 Total stroke.
1.9. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 9 Fatal stroke.
1.10. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 10 Non‐fatal stroke.
1.11. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 11 Revascularization (HMG Co A Group / Statins).
1.12. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 12 Pain‐free walking distance (PFWD).
1.13. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 13 Total walking distance (TWD).
1.14. Analysis.
Comparison 1 Any lipid‐lowering regimen vs control: all trials, Outcome 14 Ankle brachial index (ABI).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonalumi 1986.
| Methods | Study design: double blind, randomised controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: not stated. Losses to follow up: not stated. Intention to treat: no. | |
| Participants | Country: Italy. Setting: hospital. No: 30 (15 per group). Age: (mean) sulodexide 60.1 ± 8.6; placebo 60.4 ± 9.6. Sex: sulodexide, males 14, females 1; placebo, males 11, females 4. Inclusion Criteria: "patients with PVD". Exclusion criteria: not stated. | |
| Interventions | Treatment: sulodexide 2 vials i.m. per day for 20 days followed by 4 capsules daily for 70 days. Control: placebo i.m. twice daily followed by 4 placebo capsules daily for 70 days. Duration: 90 days | |
| Outcomes | Walking distance, symptom scores (pain, cramps, tingling, dulled sensations) biochemical and hamatological parameters. | |
| Notes | Biochemical and haematological parameters measured pre, during and at end of study. Active treatment associated with reduced tChol and VLDL and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Caramelli 1988.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: randomisation list. Concealment of allocation: not stated. Exclusions post randomisation: none. Losses to follow up: none. Intention to treat: not stated in the article but all patients accounted for in analysis | |
| Participants | Country: Italy. Setting: hospital. No: 60. Age: (mean) 60.01 ± 8.27. Sex: males 52; females 8. Inclusion criteria: PAD assessed by Doppler, plethysmography. Exclusion criteria: none stated. | |
| Interventions | Treatment: sulodexide one 300 LRU vial of i.m. twice daily. Control: placebo i.m. vials twice daily. Duration: 20 days. | |
| Outcomes | Pain‐free and total walking distance; subjective parameters (night pain intensity, sense of coldness in lower limbs) confirmed using Doppler. | |
| Notes | Biochemical outcomes measured pre and post study but not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Castano 1999.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: computer generated using balanced blocks without stratification (randomisation ratio 1:1). Concealment of allocation: details of medication sealed in sequentially numbered identical containers according to progressive inclusion order. Exclusions post randomisation: none. Losses to follow up: policosanol 3; placebo 7 Intention to treat: yes. | |
| Participants | Country: Cuba. Setting: hospital outpatients. No: 62. Age: (mean) policosanol 59 ± 11; placebo 60 ± 11. Sex: policosanol, males 27, females 4; placebo, males 23 females 8. Inclusion criteria: ICD 50 to 300 metres; absolute claudication distance < 500 metres. Exclusion criteria: > 80 years; MI; vascular surgery; unstable angina or stroke 3 months before the study; severe hypertension; occlusive thromboangiitis; congenital or acquired haemorrhagic diseases; pregnant or nursing women. | |
| Interventions | Treatment: policosanol 10 mg tablets twice daily. Control: placebo tablets twice daily. Duration: 6 months. | |
| Outcomes | Primary: initial claudication distance; absolute claudication distance; ABPI. Secondary: pain; parethesia and coldness in lower limbs. Side effects: adverse effects and tolerability. | |
| Notes | Some patients had hypercholesterolemia. Biochemical outcomes not measured. ABPI recorded as ankle/arm pressure ratio of the worst lower limb. Authors used terms initial (ICD) and absolute claudication distance (ACD). For comparison we used pain‐free and total walking distance. Authors contacted for additional information on trial. Response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Castano 2001.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: computer generated using balanced blocks without stratification (randomisation ratio 1:1). Concealment of allocation: details of medication sealed in sequentially numbered identical containers according to progressive inclusion order. Exclusions post randomisation: none. Losses to follow up: policosanol 4; placebo 12. Intention to treat: yes. | |
| Participants | Country: Cuba. Setting: hospital outpatients. No: 56. Age: (mean) policosanol 59 ± 11; placebo 59 ± 11. Sex: policosanol, males 24, females 3; placebo, males 22, females 7. Inclusion criteria: ICD 50 to 300 metres. Exclusion criteria: > 80 years; MI; vascular surgery unstable angina or stroke 3 months before the study; severe hypertension; occlusive thromboangiitis; congenital or acquired haemorrhagic diseases; pregnant or nursing women. | |
| Interventions | Treatment: policosanol 10 mg tablets twice daily. Control: placebo tablets twice daily. Duration: 24 months. | |
| Outcomes | Primary: initial claudication distance; absolute claudication distance; ABPI. Secondary: pain; parethesia and coldness in lower limbs. Side effects: adverse effects and tolerability. | |
| Notes | Some patients had hypercholesterolemia. Biochemical outcomes measured and recorded pre, during, and post study. ABPI recorded as ankle/arm pressure ratio of the worst lower limb. Authors used terms initial (ICD) and absolute claudication distance (ACD). For comparison we used pain‐free and total walking distance. Authors contacted for additional information on trial. Response received. Active treatment associated with reduced tChol and LDL and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Castano 2003.
| Methods | Study design: double blind, randomised, controlled, pilot trial. Method of randomisation: computer generated using balanced blocks without stratification (randomisation ratio 1:1). Concealment of allocation: details of medication sealed in sequentially numbered identical containers according to progressive inclusion order. Exclusions post randomisation: none. Losses to follow up: policosanol 0; lovostatin 1. Intention to treat: yes. | |
| Participants | Country: Cuba. Setting: hospital outpatients. No: 28. Age:(mean) policosanol 60 ± 9; lovostatin 65 ± 9. Sex: policosanol, males 10, females 4; lovastatin, males 11, females 3. Inclusion criteria: ICD 50 to 300 metres; ACD < 500 metres. Exclusion criteria: > 80 years MI; vascular surgery unstable angina or stroke 3 months before the study; severe hypertension; occlusive thromboangiitis; congenital or acquired haemorrhagic diseases; pregnant or nursing women. | |
| Interventions | Treatment: policosanol 10 mg tablets once daily. Control: lovostatin 20 mg tablets once daily. Duration: 20 weeks. | |
| Outcomes | Primary: initial claudication distance; absolute claudication distance; ABPI. Secondary: lipid profile and fibrinogen levels. Side effects: tolerability. | |
| Notes | Some patients had hypercholesterolemia. Biochemical and haematological outcomes measured and recorded pre, during, and post study. ABPI recorded as ankle/arm pressure ratio of the worst lower limb. Authors used terms initial (ICD) and absolute claudication distance (ACD). For comparison we used pain‐free and total walking distance. Authors contacted for additional information on trial. Response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Coccheri 2002.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: none. Losses to follow up: sulodexide 17; placebo 34. Intention to treat: yes. | |
| Participants | Country: Italy. Setting: hospital outpatients. No: 286 sulodexide 143; placebo 143. Age: sulodexide 64.7 ± 7.6; placebo 66.2 ± 7.5. Sex: males, 230 sulodexide 120, placebo 110; females 56, sulodexide 23, placebo 33. Inclusion criteria: age 45 to 75 years; presence of chronic PAD diagnosed by echo‐colour Doppler ultrasound; history of intermittent claudication for at least 6 months (no acute deterioration in previous 3 months); maximum walking distance 100 to 300 m, measured by standard treadmill test; repeated treadmill test after 2 week wash our and run‐in period to check stability of claudication; ABPI at rest measured by Doppler less than or equal to 0.70 on worst leg. Exclusion criteria: disorders preventing correct performance of treadmill test; abdominal aortic aneursym > 3 cm; occlusion or severe hamodynamic stenosis of pelvic arteries; history of gangliotomy or surgical revascularisation of the affected limb; serious endocrine disorders; type I diabetes; severe liver or kidney function impairment; severe heart disease; malignant arterial hypertension; any form of cancer; inflammatory vascular disease; history of hypersensitivity to extractive mucopolysaccharides; need for treatment with oral anticoagulants, ticlopidine or NSAIDs; pregnant and nursing mothers. | |
| Interventions | Treatment: sulodexide 60 mg i.m. daily (for 20 days), followed by 100 mg orally (for 6 months). Control: placebo. Duration: 27 weeks. | |
| Outcomes | Primary: doubling of the baseline pain‐free walking distance. Secondary: doubling the maximal walking distance; pain‐free and maximum walking distances. | |
| Notes | Safety data included. No data on biochemical outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Corsi 1985.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: randomisation list. Concealment of allocation: key to randomisation list provided by drug company in sealed envelopes. Exclusions post randomisation: none. Losses to follow up: none. Intention to treat: not specifically stated but all patients complied the assingned therapy and completed | |
| Participants | Country: Italy. Setting: hospital. No: 30, sulodexide 15; placebo 15. Age: (mean) sulodexide 65.8 ± 11.1; placebo 69.8 ± 12.6. Sex: sulodexide, males 12, females 3; placebo, males 10, females 5. Inclusion criteria: stage II peripheral disease (Fontaine's classification). Exclusion criteria: patients with diabetic disease, other non‐atheromatous vasculopathy, or chronic diseases. | |
| Interventions | Treatment: sulodexide (300 ULS) one vial twice daily i.m. for 20 days followed by 150 (ULS) 2 capsules twice daily for 70 days plus hypolipidaemic diet, if indicated. Control: identical placebo and diet, if indicated. Duration: 90 days. | |
| Outcomes | Primary: pain‐free walking distance; ABPI; blood flow; symptoms; side effects. Secondary: lipid profile and fibrinogen levels. | |
| Notes | Biochemical and haematologcial outcomes measured pre, during, and post study. Active treatment associated with reduced tChol and VLDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cospite 1985.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: supplied by manufacturer. Exclusions post randomisation: none. Losses to follow up: none. Intention to treat: not stated | |
| Participants | Country: Italy. Setting: hospital outpatient department. No: 30. Age: (mean) 58, range 40 to 72 years. Sex: males 20; females 10. Inclusion criteria: multi‐sited atherosclerosis confirmed by Doppler. Exclusion criteria: patients with severe liver disease, and severe endocrine dysfunction. | |
| Interventions | Treatment: one vial of sulodexide (300 LSU) twice daily i.m. for 10 days followed by 2 sulodexide capsules (150 LSU) twice daily for 80 days. Control: identical placebo Duration: 90 days. | |
| Outcomes | Total walking distance; subjective parameters (night pain intensity, cramps, tingling, burning, sense of coldness in lower limbs) confirmed using Doppler. Subjective parameters were confirmed using Doppler. | |
| Notes | Biochemical and haematologcial outcomes measured pre, during, and post study. Active treatment associated with reduced tChol and VLDL and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gans 1990.
| Methods | Study design: double blind, randomised controlled trial. Method of randomisation: not stated. Allocation concealment: numbered, sealed envelopes. Exclusions post randomisation: 3 (2 in treatment group and 1 in placebo group) Losses to follow up: 2 (1 in each group). Intention to treat: no. | |
| Participants | Country: Netherlands. Setting: hospital. No: 37 randomised, 32 completed the trial. Age: range 18 to 80 years. Sex: male 22, females 10. Inclusion criteria: intermittent claudication due to PAD, stable for 1 year according to treadmill and ABPI. Exclusion criteria: MI < 3 months; unstable angina; poorly controlled DM; CLI; hypertension; fish allergy; other lipid‐lowering treatment; antiplatelet treatment; and other medical disorders. | |
| Interventions | Treatments: six capsules fish oil daily (1.8 g eicosapentaenoic acod + 1.2 g docosahexaenoic acid). Control: six capsules corn oil (3 g linoleic acid) daily. Duration: 4 months. | |
| Outcomes | ABPI; walking distance (pain‐free and total). | |
| Notes | Biochemical and haematologcial outcomes measured pre, during, and post study. Active treatment associated with reduced TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
HPS.
| Methods | Study design: double blind, placebo‐controlled trial '2 x 2 factorial design' . Method of randomisation: central telephone system using minimisation. Concealment of allocation: centralised. Exclusions post randomisation: not known (sub group of a large study). Losses to follow up: not known (sub group of a large study) Intention to treat: yes. | |
| Participants | Country: UK. Setting: hospital outpatient department. No: 6748 Age: 40 to 80 years Sex: (with PAD) males 5014; females 1734. Inclusion criteria (for the whole HPS study): men and women aged 40‐80 years with non fasting blood total cholesterol concentrations of at least 3.5 mmol/l (135 mg/dl) were eligible provided they had a medical history coronary disease, PAD, cerebrovascular disease, diabetes, or treated hypertension (if also male and aged at least 65 years). PAD was defined as intermittent claudication, any peripheral revascularisation procedure, or aortic aneurysm. Exclusion criteria: anyone in whom statin therapy was considered to be clearly indicated; chronic liver disease; severe renal disease or evidence of impaired renal function; inflammatory muscle disease; concurrent treatment with ciclosporin, fibrates or high dose niacin; child bearing potential; severe heart failure. | |
| Interventions | Treatment: simvastatin 40 mg with or without antioxidant vitamins (650 mg vitamin E, 250 mg vitamin C, 20 mg beta carotene). Control: placebo with or without antioxidant vitamins (650 mg vitamin E, 250 mg vitamin C, 20 mg beta carotene). Duration: 5 year treatment period. | |
| Outcomes | All‐cause mortality; death due to vascular and non‐vascular causes; non‐fatal MI; non‐fatal stroke; coronary or non‐coronary revascularisation. | |
| Notes | Lipid profiles and blood vitamin concentrations. The trial included a wide range of patients at increased risk of coronary heart disease death (33% had PAD). Active treatment associated with reduced tChol, LDL and TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
LEADER.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Method of allocation: trial co‐ordinating centre. Exclusions post randomisation: none. Losses to follow up: bezafibrate 369 (47.1%); placebo 403 (51.3%). Intention to treat: yes. | |
| Participants | Country: UK. Setting: 85 general practices and 9 hospital vascular clinics. No: 1568, bezafibrate 783; placebo: 785. Age: bezafibrate (mean) 68.4 (SD 8.9); placebo (mean) 68.0 (SD 8.8). Sex : all male. Inclusion criteria: lower extremity arterial disease confirmed with the Edinburgh Claudication questionnaire. Exclusion criteria: Men with PAD were ineligible for the following reasons: unstable angina, unless or until it was controlled; total serum cholesterol less than 3.5 mmol/l or more than 8.0 mmol/l; significant renal or hepatic disease; a known hepatitis B or C or HIV positive status; malignant disease (other than non‐melanoma skin cancer) within the past 5 years; they were taking or likely to need lipid‐lowering agents or monamine oxidase inhibitors; they were unlikely to comply with trial treatment or procedures; they were in another trial; or at the discretion of the general practitioner for other reasons. | |
| Interventions | Treatment: bezafibrate 400 mg daily. Control: placebo tablets. Duration: minimum 3 years, maximum 4 years. | |
| Outcomes | Primary: combination of coronary heart disease and/or stroke. Secondary: all coronary events, fatal and non‐fatal coronary events separately, and strokes alone. | |
| Notes | Haematological and biochemical parameters measured. Active treatment associated with reduced tChol, LDL and TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Liguori 1993.
| Methods | Study design: double blind, double‐dummy, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: not stated. Losses to follow up: withdrawals 22; 1 not recorded. Intention to treat: not stated. | |
| Participants | Country: Italy (mulitcentre). Setting:inpatients and outpatients. No: 248 randomised, 225 completed the treatment. Age: (range in years) treatment, 44 to 93; control, 43 to 93. Sex: treatment, males 124, females 62; control, males 46, females 16. Inclusion criteria: PAD (Fontaine stage II or III, confirmed by clinical examination and Doppler test). Exclusion criteria: Severe systemic infections or severe uncompensated hypertension; active gastroduodenal ulcer; recent haemorrhagic stroke; severe neurologic disorders; neoplastic diseases; renal, hepatic, or cardiovascular failure; known hypoersensitivity to extractive mucopolysaccharides; patients with any risk of haemorrhage; receiving fibrinolytic or anticoagulant treatment; and pregnant or breastfeeding women. | |
| Interventions | Treatment: sulodexide, 25 mg capsules twice daily, 50 mg enteric‐coated tablets twic daily, or 100 mg enteric‐coated tablets once daily. Patients in the treatment groups received dummy treatment of the two active treatments not provided for in that particular group. Control: placebo. Patients received dummy versions of active drug according to the approprioate dosage schedule. Duration: 90 days. | |
| Outcomes | Doppler analysis, Winsor Index, treadmill test (pain‐free walking distance), side effects. | |
| Notes | Haematological and biochemical analyses performed monthly. Effect on lipids not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mohler 2003.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: 10. Losses to follow up: none. Intention to treat: no. | |
| Participants | Country: USA. Setting: hospital outpatients. No: 354. Age: (mean) treatment (group 1) 69; (group 2) 68; control 67. Sex: males 273, treatment (group 1) 90, (group 2) 95, control 88; females 81 treatment (group 1) 30, (group 2) 25, control 26. Inclusion criteria: > 25 years; stable intermittent claudication for longer than 6 months; able to complete screening treadmill test; LDL less than or equal to 160 mg/dl (4.14 mmol/l. Exclusion criteria: MI; coronary revascularization; peripheral vascular surgery; percutaneous intervention procedure within 6 months; unstable angina within previous 3 months; stroke or TIA within 6 months; DVT within previous 3 months. | |
| Interventions | Treatment: atorvastatin (group1)10 mg daily; (group 2) 80 mg daily. Control: placebo. Duration: 12 months. | |
| Outcomes | Primary: change in maximal walking time at 12 months. Secondary: change from baseline in pain‐free walking time, or community‐based functional status by the SF‐36, WIQ, and LOPAR quality of life questionnaires, or ABPI; incidence of peripheral vascular e.g. worsening symtoms of claudication, development of rest ischaemia, peripheral revascularisation procedure, or limb amputation, or critical cardiovascular events e.g. MI, stroke and vascular death; lipid profiles. | |
| Notes | Biochemical parameters measured at baseline and after 12 months. Active treatment associated with reduced tChol, LDL and TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mondillo 2003.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: none. Losses to follow up: none. Intention to treat: not stated but all patients complied with assigned treatment, completed and analysed | |
| Participants | Country: Italy Settting: hospital outpatients. No: 86. Age: (mean) simvastatin 67 ± 6; placebo 67 ± 7. Sex: males 61; females 25. Inclusion criteria: symptomatic PAD; intermittent claudication; pain free at rest for at least 12 months; ultransonographic or angiographic evidence of obstructive PVD (i.e. arterial stenosis >50% in both lower limbs within previous 4 months); resting systolic ABPI less than or equal to 0.9; serum cholesterol level > 220 mg/dl; written informed consent. Exclusion criteria: previous surgery or angioplasty for PAD; >40% change in pain‐free walking distance in previous 2 months; renal or liver disease; any type of cancer or autoimmune disease; conditions that could limit ability to perform an exercise test; hyperthyroidism or hypothyroidism; uncontrolled hypertension; history of acute coronary syndromes or stroke within the previous 6 months; previous treatment with statins or other cholesterol‐lowering drugs. | |
| Interventions | Treatment: simvastatin 40 mg daily. Control: placebo. Duration: 6 months. | |
| Outcomes | Pain‐free walking distance; total walking distance; ABPI; blood pressure; lipid profile and biochemical tests; claudication self‐assessement questionnaire. | |
| Notes | Biochemical parameters measured pre, during, and at end of study. Active treatment associated with reduced tChol, LDL and TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nye 1973.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: none. Drop outs 22. Losses to follow up: none. Intention to treat: not evident from result and not stated | |
| Participants | Country: New Zealand. Setting: hospital. No: 68 randomised. Age: 40 to 74 years. Sex: male 64; female 4. Inclusion criteria: PAD of aorta or lower limb vessels (new referrals or those with previous surgery). Exclusions: DM and multiple drug therapy. | |
| Interventions | Treatment: betapyridyl carbinol up to 1.8 g plus advice (anti‐smoking, weight loss and exercise). Control: identical placebo plus advice. Duration: 2 yr. | |
| Outcomes | Mortality; walking distance; symptoms; side effects. | |
| Notes | Biochemical parameters measured pre, during, and at end of study. Active treatment associated with reduced tChol. Two male patients developed liver toxicity while on Betapyridyl carbinol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Palmieri 1984.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: none. Losses to follow up: none. Intention to treat: no (randomised 30 but analysed only 22 for interested outcome) | |
| Participants | Country: Italy. Setting: hospital outpatients. No: 30. Age: 20 to 60 years (mean) 42.5 ± 8.27. Sex: males 16; females 14. Inclusion criteria: PAD (Leriche's stages I‐II) confirmed by Doppler, plethysmography and ultrasonography. Exclusion criteria: not stated. | |
| Interventions | Treatment: sulodexide 600 units daily i.m. followed by oral treatment. Control: placebo i.m. followed by oral treatment. Duration: 20 days (i.m.) followed by 70 days oral treatment. | |
| Outcomes | Primary: pain‐free walking distance. At rest oscillometric index. | |
| Notes | Some patients had hyperliporoteinaemia. Biochemical and haematological outcomes measured pre and post study. Active treatment associated with reduced TG. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
PQRST.
| Methods | Study design: double blind, randomised, placebo‐controlled trial. Stratified randomisation by risk, sex and city. Method of randomisation: not stated. Concealment of allocation: not stated. Exclusions post randomisation: 29 patients were excluded because of inadequate primary endpoint measurements. Losses to follow up: none. Intention to treat: no. | |
| Participants | Country: Sweden. Setting: hospital. No: 303 randomised, 274 analysed. Age: < 71 years. Sex: males 158; females 116. Inclusion criteria: PAD on angiogram; hypercholesterolaemia (tChol > 265 mg/dl, LDL > 175 mg/dl, and TG < 350 mg/dl) and those who responded to lipid‐lowering drugs. Exclusion criteria: Type III hyperlipidaemia; DM, MI < 6 months; unstable angina; hypertension; and other medical disorders. | |
| Interventions | Treatment: probucol 1g, cholestyramine 8 to 16 g plus diet (P:S ratio 0.5). Control: identical placebo, cholestyramine 8 to 16 g plus diet as above. Duration: 3 years. | |
| Outcomes | Mortality; non‐fatal events; angiography (atheroma volume, edge roughness and minimal width); ABPI; walking distance; side effects. | |
| Notes | Total cholesterol significantly lower in treated than control group at end of trial. Significant reduction in HDL cholesterol in treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
St Thomas' Trial.
| Methods | Study design: randomised controlled trial. Outcome assessors blind. Method of randomisation: not stated Concealment of allocation: not stated. Exclusions post randomisation: 1 patient from the treatment group withdrawn due to . Losses to follow up: no losses stated. Intention to treat: no (did not count one excluded patient) | |
| Participants | Country: England. Setting: hospital. No: 25 randomised, 24 analysed. Age: < 65 years, (mean) treatment 54.8; control 56.1. Sex: males 21; females 3. Inclusion criteria: stable intermittent claudication > 6 months (due to femoro‐popliteal disease), and hyperlipidaemia (tChol > 6.5 mmol/l and TG >1.8 mmol/l, or both). Exclusions: imminent surgery; secondary hyperlipidaemia; DM; hypertension; and other medical disorders. | |
| Interventions | Treatment: depended on type of hyperlipidaemia (II ‐ cholestyramine 12 to 24 g plus nicotinic acid 3 to 6 g, if required; III ‐ clofibrate 1.5 to 2 g; IV ‐ nicotinic acid 3 to 6 g) plus "usual care" Control: "usual care" (anti‐smoking and weight‐loss advice). Duration: (mean) 19 months. | |
| Outcomes | Angiography (visual assessment and edge irregularity index, per segment). | |
| Notes | Total cholesterol significantly lower in treated than control group at end of trial. Active treatment also associated with reduced TG and increased HDL. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ABPI: ankle brachial pressure index ACD: absolute claudication distance CABG: coronary artery bypass graft CLI: critical limb ischaemia DM: diabetes mellitus DP: dorsalis pedis DVT: deep vein thrombosis HDL: high density lipoprotein ICD: inital claudication distance i.m.: intramuscular LDL: low density lipoprotein cholesterol LEAD: lower extremity arterial disease LRU: lipoproteinlipase releasing units MI: myocardial infarction NSAID: non steroidal anti‐inflammatory PAD: peripheral arterial disease PT: posterior tibial PVD: peripheral vascular disease tChol total cholesterol TG: triglycerides TIA: transient ischaemic attack VAS: visual analogue score VLDL: very low density lipoprotein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADMIT | Randomised controlled trial. No relevant clinical outcomes. |
| Aguilera 2004 | Randomised controlled trial. No relevant clinical outcomes. |
| Altmann 1986 | Double blind controlled clinical trial of pentoxifylline versus xantinol nicotinate. |
| Aronow 2003 | Randomised controlled trial. No relevant outcomes. |
| Attanasio 2001 | Randomised controlled trial. Patients randomised to Simvastatin or Atorvastatin. Cost effectiveness to determine annual maintenance costs of reducing LDL‐C in hypercholesterolemic patients. No relevant clinical outcomes. |
| Bartolo 1984 | Double blind, randomised controlled trial. Excluded due to study design. Treatment group received sulodexide parenterally for 20 days and the control group received placebo parenterally for 20 days; both groups then received oral sulodexide for 70 days. |
| Berrettini 1996 | Double blind randomised controlled study of eicosapentaenoic acid and docosahexaenoic acid versus corn oil. No relevant outcomes. |
| Betteridge 1999 | Double blind, randomised controlled trial. No relevant clinical outcomes. |
| Blann 2001 | Double blind, randomised controlled trial of cerivastatin versus simvastatin. No relevant clinical outcomes. |
| Bonanno 1985 | Double blind, randomised controlled trial. Excluded due to study design. Treatment group received sulodexide parenterally for 20 days and the control group received placebo parenterally for 20 days; both groups then received oral sulodexide for 70 days. |
| Broome 1967 | Double blind, randomised controlled trial. No relevant outcome measures. Measured blood flow. |
| Brown 1998 | Randomised controlled trial. No clinical outcomes measures. |
| Calabro 1985 | Controlled clinical trial. |
| Castano 2004 | Double blind, randomised controlled trial. Control group received antiplatelet therapy. |
| Castelluccio 1991 | Double blind, randomised controlled trial. No relevant outcomes. |
| Catania 1992 | Double blind, randomised controlled trial. No relevant outcomes. |
| Ciuffetti 1989 | Double blind, randomised controlled trial. No relevant outcomes. |
| CLAS | Randomised controlled trial. Participants included not only those suffering from lower limb atherosclerosis but also unaffected subjects. |
| Coffman 1983 | Randomised controlled trial. Patients with hyperlipoproteinaemia were given diet and/or Clofibrate. No relevant outcomes. Circulation tests and lipid levels measured. |
| Crepaldi 1986 | Double blind, randomised controlled trial. No relevant outcomes. |
| Crepaldi 1990 | Double blind, randomised controlled trial. No relevant outcomes. |
| Davis 1975 | Double blind, randomised controlled trial. No relevant outcomes. |
| Fischer 1980 | This double blind, randomised cross‐over trial of clofibrate vs placebo in patients with peripheral arterial disease was excluded for the following reasons: unrealistically short follow‐up period (6 weeks); no clinical outcome measures; and only 10 patients in each group with a mixed pattern of disease. |
| Gallino 2004 | Randomised controlled trial of patients post femoropopliteal angioplasty. |
| Giansante 1990 | Double blind, randomised study of xanthinol nicotinate versus antiplatelet agents did not have any relevant clinical outcomes. |
| Head 1986 | Double blind, randomised controlled trial. Uncertain lipid‐lowering property of inositol nicotinate. No relevant outcomes. |
| Heller 1989 | Randomised controlled trial. No relevant clinical outcome measures. |
| Hentzer 1967 | Double blind, randomised controlled trial. Uncertain lipid‐lowering property of inositol nicotinate |
| Hutchinson 1983 | Double blind, randomised controlled trial comparing two different lipid‐lowering diets, and therefore it does not have an adequate control group. |
| Ito 2001 | Randomised controlled trial. All participants received pravastatin. Only 5 patients with PAOD. |
| Kiff 1988 | Double blind, randomised controlled trial. Uncertain lipid‐owering property of inositol nicotinate |
| Kirk 1999 | Double blind, randomised controlled trial. No clinical outcome measures. |
| Kissin 1951 | Double blind, randomised controlled trial. Only one single dose given. |
| Larsen 1969 | Double blind, controlled clinical trial. |
| Leng 1998b | This was a 2 year randomised controlled trial of gamma‐linolenic acid and eicosapenataenoic acid in 120 patients with intermittent claudication. The sample size was calculated on the assumption that treatment with these polyunsaturated fatty acids would reduce cholesterol levels, but during the trial was there was no reduction in plasma cholesterol. Indeed, both treatment and control groups demonstrated an increase in lipid levels during the trial. This study could not therefore be included in a review of lipid‐lowering therapy. |
| Lunetta 1992 | Double blind randomised controlled cross over trial of Sulodexide versus placebo. No relevant outcomes. Blood viscosity and plasma fibrinogen concentrations measured. |
| Marzola 1985 | Double blind, randomised controlled trial. No relevant outcomes. |
| Maxwell 2000 | Double blind, randomised controlled trial. Dietary study not a lipid‐lowering treatment. |
| Mori 1992 | Double blind randomised controlled study of fish oil versus olive oil. No relevant outcomes. |
| O'Hara 1988 | Double blind, randomised controlled trial. Uncertain lipid‐lowering property of inositol nicotinate |
| Oxford Cholesterol | Randomised placebo controlled trial. Study of the effect of simvastatin on haemostatic variables. No relevant clinical outcomes. |
| Palmieri 1982 | Double blind, randomised controlled trial. No relevant outcomes. |
| Palmieri 1987 | Double blind, randomised controlled trial. No relevant outcomes. |
| Perego 1981 | Double blind, randomised controlled trial. No relevant outcomes. |
| Pisano 1986 | Double blind, randomised controlled trial. No relevant outcomes. |
| Ramirez‐Tortosa 1999 | Randomised controlled trial. No relevant outcomes. |
| Ramírez‐Tortosa 1999 | Randomised controlled trial. No relevant outcomes. |
| Saunders 2000 | Double blind, randomised controlled trial. No relevant outcomes. |
| Schumer 1963 | Double blind, randomised controlled trial. A single short report represents the only available results from this trial. Contact with the author confirmed it was randomised, but into two very unequal groups suggesting probable bias. In addition, the mode of action of gallogen is unclear, and although it is probably hypolipidaemic, lipid levels were not measured in this trial. |
| Shustov 1997 | Randomised controlled trial comparing the therapeutic efficacy of sulodexide versus pentoxifylline. |
| Smith 1981 | Double blind, randomised controlled trial. Muscle blood flow and skin temperature measured in six patients. |
| Smith 1999 | Double blind, randomised controlled trial. Cost analysis. Hypercholesterolaemic patients randomised to atorvastatin, fluvastatin, pravastatin or simvastatin. Lipid values measured every six weeks to ensure that LDL‐C target achieved. |
| Tyson 1979 | Double blind, controlled clinical trial. Outcomes included walking rate in paces/minute, pain and pulse scores. |
LDL‐C = low density lioprotein cholesterol
Characteristics of studies awaiting assessment [ordered by study ID]
Borreani 1993.
| Methods | Randomised double‐blinded trial. |
| Participants | Chronic peripheral arterial occlusive disease; n = 100. |
| Interventions | Sulodexide versus placebo. |
| Outcomes | 1. Pain‐free walking distance 2. Maximum walking distance 3. Activated partial thromboplstin time 4. Fibrinogen levels 5. Platelet count |
| Notes | Requires further translation. |
Degni 1973.
| Methods | Article is coded as a randomised controlled trial in CENTRAL. Abstract states double‐blind, cross‐over study. |
| Participants | Patients with chronic obstructive atherosclerosis of the lower extremities; n = 20. |
| Interventions | ER‐113V (each capsule containing pyridinolcarvamate ‐ 160 mg and nicotinic acid ‐ 70 mg, 6 capsules daily. |
| Outcomes | 1. Paresthesias 2. Sensation of cold 3. Intermittent claudication 4. Cutaneous temperature 5. Oscillometry 6. Necrotic ulcer |
| Notes | Unable to obtain a copy |
Di Stefano 1984.
| Methods | Double‐blinded study. Title suggest that this is a controlled clinical trial. Unable to ascertain if this is a randomised trial. |
| Participants | 30 participants suffering from peripheral arterial occlusive disease of the lower limbs: treatment group 15; control group 15. |
| Interventions | Sulodexide i.m 20 days followed by oral dose for 70 days versus placebo. |
| Outcomes | Biochemical outcomes: triglycerides, HDL; oscillographic and photoplethysmographic tests of the lower limb. |
| Notes | Unable to get copy. Details taken from abstract in EMBASE |
Mayer 2001.
| Methods | Article is coded as a randomised controlled trial in MEDLINE. |
| Participants | Arterial occlusion of the lower extremities; n = 50. |
| Interventions | Statins (simvastatin) versus diet. |
| Outcomes | "Claudication interval". |
| Notes | Original article is in Czechoslovakian. Requires translation. |
Differences between protocol and review
The title of this review has been amended from 'Lipid‐lowering for lower limb atherosclerosis' to reflect more accurately the content of the review.
Contributions of authors
Updated version of the review (August 2007): Phyu Phyu Aung: selected trials for inclusion in the review, assessed trial quality, extracted data, and wrote the text.
Heather Maxwell: searched the PVD Group's Trials Register and The Cochrane Library for potential trials, selected trials for inclusion in the review, assessed trial quality, extracted data, and contributed to the text.
Ruth Jepson: selected trials for inclusion in the review, assessed trial quality, and extracted data.
Jackie Price: cross‐checked trials for inclusion and quality, confirmed data extraction, and contributed to the text.
Gillian Leng: commented on the text.
Original version of the review: Gillian Leng: identified possible trials, and assessed their eligiblity and quality; sought additional information from principal investigators of all trials that appeared to meet the inclusion criteria. Extracted data, wrote text.
Jackie Price: cross‐checked trials for inclusion in the review, and quality; extracted data.
Ruth Jepson: assisted in the identification of trials for the review, and provided general advice and guidance on methodological issues.
Sources of support
Internal sources
University of Edinburgh, UK.
External sources
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
British Heart Foundation, UK.
Declarations of interest
JP has received research funding from Pfizer plc and Bayer plc.
Edited (no change to conclusions)
References
References to studies included in this review
Bonalumi 1986 {published data only}
- Bonalumi F, Sarcina A, Bonadeo P, Maddinelli L. A randomized protocol for the management of chronic peripheral arterial diseases by means of sulodexide. European Review for Medical & Pharmacological Sciences 1986;8(2):123‐9. [Google Scholar]
Caramelli 1988 {published data only}
- Caramelli L, Mirchioni R, Carini A. Effectiveness of short‐term sulodexide treatment on peripheral vascular disease clinical manifestations. European Review for Medical Pharmacological Sciences 1988;10(1):55‐8. [PubMed] [Google Scholar]
Castano 1999 {published data only}
- Castano G, Mas R, Roca J, Fernandez L, Illnait J, Fernandez JC, et al. A double‐blind, placebo‐controlled study of the effects of policosanol in patients with intermittent claudication. Angiology 1999;50(2):123‐30. [DOI] [PubMed] [Google Scholar]
Castano 2001 {published data only}
- Castano G, Mas Ferreiro R, Fernandez L, Gamez R, Illnait J, Fernandez C. A long‐term study of policosanol in the treatment of intermittent claudication. Angiology 2001;52(2):115‐25. [DOI] [PubMed] [Google Scholar]
Castano 2003 {published data only}
- Castano G, Mas R, Fernandez L, Gamez R, Illnait J. Effects of policosanol and lovastatin in patients with intermittent claudication: a double‐blind comparative pilot study. Angiology 2003;54(1):25‐38. [DOI] [PubMed] [Google Scholar]
Coccheri 2002 {published data only}
- Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V. Arterial Arm of the Suavis (Sulodexide Arterial Venous Italian Study) group. Sulodexide in the treatment of intermittent claudication. Results of a randomized, double‐blind, multicentre, placebo‐controlled study. European Heart Journal 2002;23(13):1057‐65. [DOI] [PubMed] [Google Scholar]
Corsi 1985 {published data only}
- Corsi C, Bocci L, Cipriani C, Gazzini A, Marrapodi E. The effectiveness of glycosaminoglycans in peripheral vascular disease therapy: a clinical and experimental trial. Journal of International Medical Research 1985;13(1):40‐7. [DOI] [PubMed] [Google Scholar]
Cospite 1985 {published data only}
- Cospite M, Milio G, Ferrara F. Double‐blind study of the pharmacological effects of sulodexide in patients with multiple atherosclerotic vascular disease. European Review for Medical & Pharmacological Sciences 1985;7(1):97‐106. [Google Scholar]
Gans 1990 {published data only}
- Gans RO, Bilo HJ, Weersink EGL, Rauwerda JA, Fonk T, Popp‐Snijders C, et al. Fish oil supplementation in patients with stable claudication. American Journal of Surgery 1990;160(5):490‐5. [DOI] [PubMed] [Google Scholar]
HPS {published data only}
- Anonymous. MRC/BHF Heart Protection Study of cholesterol‐lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. European Heart Journal 1999;20(10):725‐41. [DOI] [PubMed] [Google Scholar]
- Collins R. The MRC/BHF heart protection study. HPS INFORMATION. www.ctsu ox.ac.uk 2001 (accessed 13 October 2004).
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet 2003;361(9374):2005‐16. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group. Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions. Lancet 2004;363(9411):757‐67. [DOI] [PubMed] [Google Scholar]
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. International Journal of Clinical Practice 2002;56(1):53‐6. [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. [see comments.]. Lancet 2002;360(9326):23‐33. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. [see comments.]. Lancet 2002;360(9326):7‐22. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol‐lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high‐risk conditions. Journal of Vascular Surgery 2007;45(4):645‐54. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause‐specific mortality and on cancer incidence in 20,536 high‐risk people: a randomised placebo‐controlled trial. BMC Medicine 2005;3:6. [Google Scholar]
- Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Heart Protection Collaborative Group. Cost‐effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals.[see comment]. Lancet 2005;365(9473):1779‐85. [DOI] [PubMed] [Google Scholar]
LEADER {published data only}
- Hogg CF, Zuhrie SR, Meade TW. Trial of bezafibrate in lower extremity arterial disease pilot study results. Thrombosis & Haemostasis 1995;73(6):1328‐Abstract No 1645. [Google Scholar]
- Jamshidi Y, Flavell DM, Hawe E, MacCallum PK, Meade TW, Humphries SE. Genetic determinants of the response to bezafibrate treatment in the lower extremity arterial disease event reduction (LEADER) trial. Atherosclerosis 2002;163(1):183‐92. [DOI] [PubMed] [Google Scholar]
- MRC Epidemiology and Medical Care Unit, MRC General Practice Research Framework. Lower extremity arterial disease event reduction. Trial of bezafibrate in lower extremity arterial disease. Unpublished work. 1996.
- Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ 2002;325(7373):1139‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade TW. Design and intermediate results of the Lower Extremity Arterial Disease Event Reduction (LEADER) trial of bezafibrate in men with lower extremity arterial disease. Current Controlled Trials in Cardiovascular Medicine 2001;2(4):195‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liguori 1993 {published data only}
- Liguori L, Saviano M, Lampugnani R, Spartera C, Bonavita E, Frasson P, et al. Efficacy, tolerability, and dose‐effect relationship of oral sulodexide in obstructive peripheral arterial disorders. Advances in Therapy 1993;10(2):52‐66. [Google Scholar]
Mohler 2003 {published data only}
- Mohler ER 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003;108(12):1481‐6. [DOI] [PubMed] [Google Scholar]
Mondillo 2003 {published data only}
- Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. American Journal of Medicine 2003;114(5):359‐64. [DOI] [PubMed] [Google Scholar]
Nye 1973 {published data only}
- Nye ER, Macbeth WA. The treatment of intermittent claudication with betapyridyl carbinol over two years. Atherosclerosis 1973;17(1):95‐106. [DOI] [PubMed] [Google Scholar]
Palmieri 1984 {published data only}
- Palmieri G, Nazzari M, Ambrosi G, et al. Sulodexide in the treatment of peripheral arterial disease. Clinical Trials Journal 1984;21(6):411‐27. [Google Scholar]
PQRST {published data only}
- Erikson U, Nilsson S, Stenport G. Probucol Quantitative Regression Swedish Trial: new angiographic technique to measure atheroma volume of the femoral artery. American Journal of Cardiology 1988;62(3):44B‐47B. [DOI] [PubMed] [Google Scholar]
- Holme I, Malmaeus I, Olsson AG, Nilsson S, Walldius G. Repeated measurements over time: statistical analysis of the angiographic outcomes in the Probucol Quantitative Regression Swedish Trial (PQRST). Clinical Trials & Meta‐Analysis 1993;28(2):95‐108. [PubMed] [Google Scholar]
- Johansson J, Olsson AG, Bergstrand L, Elinder LS, Nilsson S, Erikson U, et al. Lowering of HDL2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia. A Probucol Quantitative Regression Swedish trial (PQRST) Report. Arteriosclerosis, Thrombosis & Vascular Biology 1995;15(8):1049‐56. [DOI] [PubMed] [Google Scholar]
- Regnstrom J, Walldius G, Nilsson S, Elinder LS, Johansson J, Molgaard J, et al. The effect of probucol on low density lipoprotein oxidation and femoral atherosclerosis. Atherosclerosis 1996;125(2):217‐29. [PubMed] [Google Scholar]
- Walldius G, Carlson LA, Erikson U, Olsson AG, Johansson J, Molgaard J, et al. Development of femoral atherosclerosis in hypercholesterolemic patients during treatment with cholestyramine and probucol/placebo: Probucol Quantitative Regession Trial (PQRST): a status report. American Journal of Cardiology 1988;62(3):37B‐43B. [DOI] [PubMed] [Google Scholar]
- Walldius G, Erikson U, Olsson AG, Bergstrand L, Hadell K, Johansson J, et al. The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). American Journal of Cardiology 1994;74(9):875‐83. [DOI] [PubMed] [Google Scholar]
- Walldius G, Regnstrom J, Nilsson J, Johansson J, Schafer‐Elinder L, Moelgaard J, et al. The role of lipids and antioxidative factors for the developement of atherosclerosis. The Probucol Quantitative Regression Swedish Trial (PQRST). American Journal of Cardiology 1993;71(6):15B‐19B. [DOI] [PubMed] [Google Scholar]
St Thomas' Trial {published and unpublished data}
- Duffield RG, Lewis B, Miller NE, Jamieson CW, Brunt JN, Colchester AC. Treatment of hyperlipidaemia retards progression of symptomatic femoral atherosclerosis. A randomised controlled trial. Lancet 1983;2(8351):639‐42. [DOI] [PubMed] [Google Scholar]
- Duffield RG, Miller NE, Jamieson CW, Lewis B. A controlled trial of plasma lipid reduction in peripheral atherosclerosis ‐‐ an interim report. British Journal of Surgery 1982;69(Suppl):S3‐5. [DOI] [PubMed] [Google Scholar]
- Lewis B. Randomised controlled trial of the treatment of hyperlipidaemia on progression of atherosclerosis. Acta Medica Scandinavica Supplementum 1985;701:53‐57. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ADMIT {published data only}
- Chesney CM, Elam MB, Herd JA, Davis KB, Garg R, Hunninghake D, et al. Effect of niacin, warfarin, and antioxidant therapy on coagulation parameters in patients with peripheral arterial disease in the Arterial Disease Multiple Intervention Trial (ADMIT). American Heart Journal 2000;140(4):631‐6. [DOI] [PubMed] [Google Scholar]
- Egan DA, Garg R, Wilt TJ, Pettinger MB, Davis KB, Crouse J, et al. Rationale and design of the Arterial Disease Multiple Intervention Trial (ADMIT) pilot study. American Journal of Cardiology 1999;83(4):569‐75. [DOI] [PubMed] [Google Scholar]
- Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: A randomized trial. Arterial Disease Multiple Intervention Trial. JAMA 2000;284(10):1263‐70. [DOI] [PubMed] [Google Scholar]
- Garg R, Elam MB, Crouse JR 3rd, Davis KB, Kennedy JW, Egan D, et al. Effective and safe modification of multiple atherosclerotic risk factors in patients with peripheral arterial disease. American Heart Journal 2000;140(5):792‐803. [DOI] [PubMed] [Google Scholar]
- Garg R, Malinow M, Pettinger M, Upson B, Hunninghake D. Niacin treatment increases plasma homocyst(e)ine levels. American Heart Journal 1999;138(6 Pt 1):1082‐7. [DOI] [PubMed] [Google Scholar]
- Pettinger MB, Waclawiw MA, Davis KB, Thomason T, Garg R, Griffin B, et al. Compliance to multiple interventions in a high risk population. Annals of Epidemiology 1999;9(7):408‐18. [DOI] [PubMed] [Google Scholar]
- Philipp CS, Cisar LA, Saidi P, Kostis JB. Effect of niacin supplementation on fibrinogen levels in patients with peripheral vascular disease. American Journal of Cardiology 1998;82(5):697‐9. [DOI] [PubMed] [Google Scholar]
Aguilera 2004 {published data only}
- Aguilera CM, Mesa MD, Ramirez‐Tortosa MC, Nestares MT, Ros E, Gil A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clinical Nutrition 2004;23(4):673‐681. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altmann 1986 {published data only}
- Altmann E, Schmidt PKH, Nowak R, Hotzel M. Results of a clinical double blind study about the efficacy of pentoxifyllin (POF) for patients with peripheral arterial perfusional disturbances in the state II according to Fontaine. Zeitschrift fur Klinische Medizin 1986;41(9):667‐70. [MEDLINE: ] [Google Scholar]
Aronow 2003 {published data only}
- Aronow WS, Nayak D, Woodworth S, Ahn C. Effect of simvastatin versus placebo on treadmill exercise time until the onset of intermittent claudication in older patients with peripheral arterial disease at six months and at one year after treatment. American Journal of Cardiology 2003;92(6):711‐2. [DOI] [PubMed] [Google Scholar]
Attanasio 2001 {published data only}
- Attanasio E, Russo P, Allen SE. Cost‐minimization analysis of simvastatin versus atorvastatin for maintenance therapy in patients with coronary or peripheral vascular disease. Clinical Therapeutics 2001;23(2):276‐83. [DOI] [PubMed] [Google Scholar]
Bartolo 1984 {published data only}
- Bartolo M, Antignani PL, Eleuteri P. Experiences with sulodexide in the arterial peripheral diseases. Current Therapeutic Research, Clinical & Experimental 1984;36(51):979‐88. [Google Scholar]
Berrettini 1996 {published data only}
- Berrettini M, Parise P, Ricotta S, Iorio A, Peirone C, Nenci GG. Increased plasma levels of tissue factor pathway inhibitor (TFPI) after n‐3 polyunsaturated fatty acids supplementation in patients with chronic atherosclerotic disease. Thrombosis & Haemostasis 1996;75(3):395‐400. [MEDLINE: ] [PubMed] [Google Scholar]
Betteridge 1999 {published data only}
- Betteridge DJ. International multicentre comparison of cerivastatin with placebo and simvastatin for the treatment of patients with primary hypercholesterolaemia. International Cerivastatin Study Group. International Journal of Clinical Practice 1999;53(4):243‐50. [PubMed] [Google Scholar]
Blann 2001 {published data only}
- Blann AD, Gurney D, Hughes E, Buggins P, Silverman SH, Lip GY. Influence of pravastatin on lipoproteins, and on endothelial, platelet, and inflammatory markers in subjects with peripheral artery disease. American Journal of Cardiology 2001;88(1):A7‐8, 89‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonanno 1985 {published data only}
- Bonanno G, Bonaccorso R, Dell'Ali O, Salanitri G. Sulodexide in the treatment of atherosclerosis: A controlled clinical trial. Acta Therapeutica 1985;11(1):87‐98. [Google Scholar]
Broome 1967 {published data only}
- Broome A, Cederlund J, Eklof B. The effect of nicotinic acid on muscle blood flow in intermittent claudication measured with xenon‐133 clearance method. Scandinavian Journal of Clinical and Laboratory Investigation Supplement 1967;99:233‐40. [PubMed] [Google Scholar]
Brown 1998 {published data only}
- Brown AS, Bakker‐Arkema RG, Yellen L, Henley RW Jr, Guthrie R, Campbell CF, et al. Treating patients with documented atherosclerosis to National Cholesterol Education Program‐recommended low‐density‐lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. Journal of the American College of Cardiology 1998;32(3):665‐72. [DOI] [PubMed] [Google Scholar]
Calabro 1985 {published data only}
- Calabro A, Rossi A, Baiocchi MR, Coscetti G, Fellin R, Crepaldi G. Effect of sulodexide on hemorheological parameters in a group of patients with peripheral arteriosclerotic vascular disease [Effecto del sulodexide su alcuni parametri emoreologici in un gruppo di pazienti con vasculopatia arteriosclerotica periferica]. Ricerca in Clinica e in Laboratorio 1985;15(Suppl 1):455‐63. [PubMed] [Google Scholar]
Castano 2004 {published data only}
- Castano G, Mas R, Gamez R, Fernandez L, Illnait J. Effects of policosanol and ticlopidine in patients with intermittent claudication: a double‐blinded pilot comparative study. Angiology 2004;55(4):361‐71. [DOI] [PubMed] [Google Scholar]
Castelluccio 1991 {published data only}
- Castelluccio A, Bologna E. Effect of sulodexide on blood viscosity in patients with peripheral vascular disease. Current Medical Research & Opinion 1991;12(5):325‐31. [DOI] [PubMed] [Google Scholar]
Catania 1992 {published data only}
- Catania G, Salanitri T. Pharmacological treatment of intermittent claudication: double blind controlled study of Sulodexide vs placebo. European Review for Medical and Pharmacological Sciences 1992;14(3):149‐57. [PubMed] [Google Scholar]
Ciuffetti 1989 {published data only}
- Ciuffetti G, Mercuri M, Susta A, Lupattelli G, Pasqualini L, Lombardini R, et al. Effects of 3‐glucosaminoglycan sulfate on hemorheologic parameters in hyperlipidemic peripheral vascular disease (PVD) patients: A preliminary double‐blind crossover study. Angiology 1989;40(4):255‐9. [PubMed] [Google Scholar]
CLAS {published data only}
- Blankenhorn DH, Azen SP, Crawford DW, Nessim SA, Sanmarco ME, Selzer RH, et al. Effects of colestipol‐niacin therapy on human femoral atherosclerosis. Circulation 1991;83(2):438‐47. [DOI] [PubMed] [Google Scholar]
- Blankenhorn DH, Brooks SH. Angiographic trials of lipid‐lowering therapy. Arteriosclerosis 1981;1(4):242‐9. [Google Scholar]
- Blankenhorn DH, Johnson RL, Nessim SA, Azen SP, Sanmarco ME, Selzer RH. The Cholesterol Lowering Atherosclerosis Study (CLAS): design, methods and baseline results. Controlled Clinical Trials 1987;8(4):356‐87. [DOI] [PubMed] [Google Scholar]
- Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin‐Hemphill L. Beneficial effects of combined colestipol‐niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 1987;257(23):3233‐40. [PubMed] [Google Scholar]
- Mack WJ, Selzer RH, Pogoda JM, Lee PL, Shircore AM, Azen SP, et al. Comparison of computer‐ and human‐derived coronary angiographic end‐point measures for controlled therapy trials. Arteriosclerosis & Thrombosis 1992;12(3):348‐56. [DOI] [PubMed] [Google Scholar]
Coffman 1983 {published data only}
- Coffman JD, Rasmussen HM. The peripheral circulation and treatment of hyperlipoproteinemias. Atherosclerosis 1983;46(1):147‐59. [DOI] [PubMed] [Google Scholar]
Crepaldi 1986 {published data only}
- Crepaldi G, Fellin R, Calabro A, Baiocchi MR, Rossi A, Lenzi S, et al. Preliminary results of sulodexide treatment in patients with peripheral arteriosclerosis and hyperlipidemia. A multicentre trial. Monographs on Atherosclerosis 1986;14:215‐21. [PubMed] [Google Scholar]
Crepaldi 1990 {published data only}
- Crepaldi G, Fellin R, Calabro A, Rossi A, Ventura A, Mannarino E, et al. Double‐blind multicenter trial on a new medium molecular weight glycosaminoglycan. Current therapeutic effects and perspectives for clinical use. Atherosclerosis 1990;81(3):233‐43. [DOI] [PubMed] [Google Scholar]
Davis 1975 {published data only}
- Davis E, Rozov H. Xanthinol nicotinate in peripheral vascular disease. Practitioner 1975;215(1290):793‐8. [PubMed] [Google Scholar]
Fischer 1980 {published data only}
- Fischer M, Alexander K, Delbrueck A, Rahlfs V. Clofibrate‐induced lowering of plasma fibrinogen and calf blood flow in patients with arterial occlusive disease [Clofibrat‐induzierte plasmafibrinogensenkung und periphere durchblutung bei arterieller verschlusskrankheit]. Herz Kleislauf 1980;12(10):431‐4. [Google Scholar]
Gallino 2004 {published data only}
- Gallino A, Do DD, Alerci M, Baumgartner I, Cozzi L, Segatto JM, et al. Effects of probucol versus aspirin and versus brachytherapy on restenosis after femoropopliteal angioplasty: the PAB randomized multicenter trial. Journal of Endovascular Therapy: Official Journal of the International Society of Endovascular Specialists 2004;11(6):595‐604. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giansante 1990 {published data only}
- Giansante C, Calabrese S, Fisicaro M, Fiotti N, Mitri E. Treatment of intermittent claudication with antiplatelet agents. Journal of International Medical Research 1990;18(5):400‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Head 1986 {published data only}
- Head A. Treatment of intermittent claudication with inositol nicotinate. Practitioner 1986;230(1411):49‐54. [PubMed] [Google Scholar]
Heller 1989 {published data only}
- Heller RF, Elliott H, Bray AE, Alabaster M. Reducing blood cholesterol levels in patients with peripheral vascular disease: dietitian or diet fact sheet?. Medical Journal of Australia 1989;151(10):566‐8. [DOI] [PubMed] [Google Scholar]
Hentzer 1967 {published data only}
- Hentzer E. Treatment of peripheral arterial insufficiency with inositoli nicotinas (Hexanicit). Scandinavian Journal of Clinical and Laboratory Investigation Supplement 1967;99:226‐32. [PubMed] [Google Scholar]
Hutchinson 1983 {published data only}
- Hutchinson K, Oberle K, Crockford P, Grace M, Whyte L, Gee M, et al. Effects of dietary manipulation on vascular status of patients with peripheral vascular disease. JAMA 1983;249(24):3326‐30. [DOI] [PubMed] [Google Scholar]
Ito 2001 {published data only}
- Ito H, Ouchi Y, Ohashi Y, Saito Y, Ishikawa T, Nakamura H, Orimo H. A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: the pravastatin anti‐atherosclerosis trial in the elderly (PATE). Journal of Atherosclerosis & Thrombosis 2001;8(2):33‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kiff 1988 {published data only}
- Kiff RS, Quick CR. Does inositol nicotinate (Hexopal) influence intermittent claudication? A controlled trial. British Journal of Clinical Practice 1988;42(4):141‐5. [PubMed] [Google Scholar]
Kirk 1999 {published data only}
- Kirk G, McLaren M, Muir AH, Stonebridge PA, Belch JJ. Decrease in P‐selectin levels in patients with hypercholesterolaemia and peripheral arterial occlusive disease after lipid‐lowering treatment. Vascular Medicine 1999;4(1):23‐6. [DOI] [PubMed] [Google Scholar]
Kissin 1951 {published data only}
- Kissin M, Stein JJ, Adleman RJ. The effect of drugs used in the treatment of intermittent claudication on the exercise tolerance of individuals with obliterating arteriosclerosis. Angiology 1951;2(3):217‐24. [DOI] [PubMed] [Google Scholar]
Larsen 1969 {published data only}
- Larsen OA, Lassen NA. Medical treatment of occlusive arterial disease of the legs. Walking exercise and medically induced hypertension. Angiologica 1969;6(5):288‐301. [DOI] [PubMed] [Google Scholar]
Leng 1998b {published and unpublished data}
- Leng GC, Lee AJ, Fowkes FG, Jepson RG, Lowe GD, Skinner ER, et al. Randomized controlled trial of gamma‐linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clinical Nutrition 1998;17(6):265‐71. [DOI] [PubMed] [Google Scholar]
Lunetta 1992 {published data only}
- Lunetta M, Salanitri T. Lowering of plasma viscosity by the oral administration of the glycosaminoglycan sulodexide in patients with peripheral vascular disease. Journal of International Medical Research 1992;20(1):45‐53. [DOI] [PubMed] [Google Scholar]
Marzola 1985 {published data only}
- Marzola M, Donati D, Indelli M, Malacarne P. Sulodexide in the treatment of vasculopathic hyperlipidaemic patients [Il sulodexide nel trattamento del vasculopatico iperlipidemico: studio in doppio cieco]. European Review for Medical & Pharmacological Sciences 1985;7(2):273‐9. [Google Scholar]
Maxwell 2000 {published data only}
- Maxwell AJ, Anderson BE, Cooke JP. Nutritional therapy for peripheral arterial disease: a double‐blind, placebo‐controlled, randomized trial of HeartBar. Vascular Medicine 2000;5(1):11‐9. [DOI] [PubMed] [Google Scholar]
Mori 1992 {published data only}
- Mori TA, Vandongen R, Mahanian F, Douglas A. Plasma lipid levels and platelet and neutrophil function in patients with vascular disease following fish oil and olive oil supplementation. Metabolism: Clinical & Experimental 1992;41(10):1059‐1067. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Hara 1988 {published data only}
- O'Hara J. A double‐blind placebo‐controlled study of Hexopal in the treatment of intermittent claudication. Journal of International Medical Research 1985;13(6):322‐7. [DOI] [PubMed] [Google Scholar]
- O'Hara J, Jolly PN, Nicol CG. The therapeutic efficacy of inositol nicotinate (Hexopal) in intermittent claudication: a controlled trial. British Journal of Clinical Practice 1988;42(9):377‐83. [PubMed] [Google Scholar]
Oxford Cholesterol {published data only}
- Keech A, Collins R, MacMahon S, Armitage J, Lawson A, Wallendszus K, et al. Three‐year follow‐up of the Oxford Cholesterol Study: assessment of the efficacy and safety of simvastatin in preparation for a large mortality study. European Heart Journal 1994;15(2):255‐69. [DOI] [PubMed] [Google Scholar]
- Mitropoulos KA, Armitage JM, Collins R, Meade TW, Reeves BE, Wallendszus KR, et al. Randomized placebo‐controlled study of the effects of simvastatin on haemostatic variables, lipoproteins and free fatty acids. The Oxford Cholesterol Study Group. European Heart Journal 1997;18(2):235‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmieri 1982 {published data only}
- Palmieri G, Perego M, Nazzari M, Casalini F. Evaluation of a sulfomucopolysaccharide (3GS) in the treatment of hyperlipoproteinaemia. Clinica Terapeutica 1982;101(6):603‐14. [PubMed] [Google Scholar]
Palmieri 1987 {published data only}
- Palmieri G, Ambrosi G, Cantoni S, Agrati AM, Palazzini E. Clinical evaluation of a native low molecular weight heparin in the management of symptomatic peripheral vasculopathy in the elderly. Current Therapeutic Research, clinical & Experimental 1987;41(6):998‐1009. [Google Scholar]
Perego 1981 {published data only}
- Perego M, Nazzari M, Palmieri G. Parenterally administered glycuronil‐glycosamine in atherosclerotic hyperlipidemic patients. Giornale della Arteriosclerosi 1981;6(3):289‐298. [Google Scholar]
Pisano 1986 {published data only}
- Pisano L, Moronesi F, Falco F, Stipa E, Fabbiani N, Dolfi R, et al. The use of sulodexide in the treatment of peripheral vasculopathy accompanying metabolic disease. Controlled study of hyperlipidemic and diabetic subjects. Thrombosis Research 1986;41(1):23‐31. [DOI] [PubMed] [Google Scholar]
Ramirez‐Tortosa 1999 {published data only}
- Ramirez‐Tortosa C, Lopez‐Pedrosa JM, Suarez A, Ros E, Mataix J, Gil A. Olive oil‐ and fish oil‐enriched diets modify plasma lipids and susceptibility of LDL to oxidative modification in free‐living male patients with peripheral vascular diseases: the Spanish Nutrition Study [see comments]. British Journal of Nutrition 1999;82(1):31‐9. [DOI] [PubMed] [Google Scholar]
Ramírez‐Tortosa 1999 {published data only}
- Ramírez‐Tortosa MC, Suárez A, Gómez MC, Mir A, Ros E, Mataix J, et al. Effect of extra‐virgin olive oil and fish‐oil supplementation on plasma lipids and susceptibility of low‐density lipoprotein to oxidative alteration in free‐living spanish male patients with peripheral vascular disease. Clinical Nutrition 1999;18(3):167‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saunders 2000 {published data only}
- Saunders E, Ferdinand K, Yellen LG, Tonkon MJ, Krug‐Gourley S, Poland, M. Efficacy and safety of cerivastatin and pravastatin in the treatment of primary hypercholesterolemia. Journal of the National Medical Association 2000;92(7):319‐326. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Schumer 1963 {published data only}
- Schumer W, Battung VR, Foa PP. A clinical evaluation of diethanolamine salt of p‐tolylmethylcarbinol camporic acid (Gallogen) in arteriosclerosis obliterans. Angiology 1963;14:381‐2. [DOI] [PubMed] [Google Scholar]
Shustov 1997 {published data only}
- Shustov SB. Controlled clinical trial on the efficacy and safety of oral sulodexide in patients with peripheral occlusive arterial disease. Current Medical Research & Opinion 1997;13(10):573‐82. [DOI] [PubMed] [Google Scholar]
Smith 1981 {published data only}
- Smith RS, Warren DJ. Effect of nicotinic acid and dipyridamole on tissue blood flow in peripheral vascular disease. Pharmatherapeutica 1981;2(9):597‐600. [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith DG, Leslie SJ, Szucs TD, McBride S, Campbell LM, Calvo C, et al. Cost of treating to a modified european atherosclerosis society LDL‐C target. Comparison of atorvastatin with fluvastatin, pravastatin and simvastatin. Clinical Drug Investigation 1999;17(3):185‐93. [Google Scholar]
Tyson 1979 {published data only}
- Tyson VC. Treatment of intermittent claudication. Practitioner 1979;223(1333):121‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Borreani 1993 {published data only}
- Borreani B, Brizio L, Cianfanelli G, Colotto P, Pastorelli M, Zepponi E. Activity evaluation of sulodexide in chronic peripheral scleroatheromatous arteriopathy. Gazzetta Medica Italiana 1993;152(1‐2):21‐24. [Google Scholar]
Degni 1973 {published data only}
- Degni M, Cilurzo O. Treatment of obstructive atherosclerosis of the lower extremities with the ER‐113V. Revista Brasileira Cardiovascular 1973;9(2):83‐98. [Google Scholar]
Di Stefano 1984 {published data only}
- Stefano F, Patane S, Vinci M. Medical treatment of atherosclerosis: Controlled clinical trial with new glycosaminoglycan: Sulodexide. European Review for Medical & Pharmacological Sciences 1984;6(3):525‐532. [Google Scholar]
Mayer 2001 {published data only}
- Mayer O, Hromadka M. Statins in the treatment of patients with arterial occlusive disease of the lower extremities. [Czech]. Vnitrni Lekarstvi 2001;47(10):664‐9. [PubMed] [Google Scholar]
Additional references
BNF
- BNF 53. Section 2.12 .lipid regulating drugs.. http://www.bnf.org/bnf/ (accessed 27 July 2007).
Cochrane Handbook
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 6. In: The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd..
Dormandy 1999
- Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Seminars in Vascular Surgery 1999;12(2):123‐37. [PubMed] [Google Scholar]
Duffield 1982
- Duffield RG, Miller NE, Jamieson CW, Lewis B. A controlled trial of plasma lipid reduction in peripheral atherosclerosis ‐‐ an interim report. British Journal of Surgery 1982;69(Suppl):S3‐S5. [DOI] [PubMed] [Google Scholar]
Duffield 1983
- Duffield RG, Lewis B, Miller NE, Jamieson CW, Brunt JN, Colchester AC. Treatment of hyperlipidaemia retards progression of symptomatic femoral atherosclerosis. A randomised controlled trial. Lancet 1983;2(8351):639‐42. [DOI] [PubMed] [Google Scholar]
Dumville 2004
- Dumville JC, Lee AJ, Smith FB, Fowkes FGR. The health related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. British Journal of General Practice 2004;54508:826‐31. [PMC free article] [PubMed] [Google Scholar]
Fowkes 1988
- Fowkes FGR. Epidemiology of atherosclerotic disease in the lower limbs. European Journal of Vascular and Endovascular Surgery 1988;2(3):283‐91. [DOI] [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FGR, Housley E, Cawood EHH, MacIntyre CCA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1919;20(2):384‐91. [DOI] [PubMed] [Google Scholar]
Heald 2006
- Heald CL, Fowkes FGR, Murray G, Price JF on behalf of the International ABI Collaboration. Risk of mortality and cardiovascular disease associated with the ankle‐brachial index: Systematic review. Atherosclerosis 2006;189(1):61‐9. [DOI] [PubMed] [Google Scholar]
Lewis 1985
- Lewis B. Randomised controlled trial of the treatment of hyperlipidaemia on progression of atherosclerosis. Acta Medica Scandinavica Supplementum 1985;701:53‐57. [DOI] [PubMed] [Google Scholar]
LIPID Study Group
- Anonymous. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The long term intervention with pravastatin in ischaemic disease (LIPID) study group. New England Journal of Medicine 1998;339(19):1349‐57. [DOI] [PubMed] [Google Scholar]
Sacks 1996
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New England Journal of Medicine 1996;335(14):1001‐9. [DOI] [PubMed] [Google Scholar]
SSS Study Group 1994
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383‐9. [PubMed] [Google Scholar]
References to other published versions of this review
Leng 1996
- Leng GC, Price JF, Jepson RG. Lipid‐lowering for lower limb atherosclerosis. Cochrane Database of Systematic Reviews 1996, Issue 3. [DOI] [PubMed] [Google Scholar]
Leng 1997
- Leng GC, Price JF, Jepson RG. Lipid‐lowering for lower limb atherosclerosis. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI] [PubMed] [Google Scholar]
Leng 1998
- Leng GC, Price JF, Jepson RG. Lipid‐lowering for lower limb atherosclerosis. Cochrane Database of Systematic Reviews 1998, Issue 4. [Art. No.: CD000123. DOI: 10.1002/14651858.CD000123] [DOI] [PubMed] [Google Scholar]


