Abstract
To address the problem of resistance to paclitaxel treatment, we have investigated to which extent is possible to predict Breast Cancer (BC) patient response to this drug. We carried out a large-scale tumor-based prediction analysis using data from the US National Cancer Institute’s Genomic Data Commons. These data sets comprise the responses of BC patients to paclitaxel along with six molecular profiles of their tumors. We assessed 10 Machine Learning (ML) algorithms on each of these profiles and evaluated the resulting 60 classifiers on the same BC patients. DNA methylation and miRNA profiles were the most informative overall. In combination with these two profiles, ML algorithms selecting the smallest subset of molecular features generated the most predictive classifiers: a complexity-optimized XGBoost classifier based on CpG island methylation extracted a subset of molecular factors relevant to predict paclitaxel response (AUC = 0.74). A CpG site methylation-based Decision Tree (DT) combining only 2 of the 22,941 considered CpG sites (AUC = 0.89) and a miRNA expression-based DT employing just 4 of the 337 analyzed mature miRNAs (AUC = 0.72) reveal the molecular types associated to paclitaxel-sensitive and resistant BC tumors. A literature review shows that features selected by these three classifiers have been individually linked to the cytotoxic-drug sensitivities and prognosis of BC patients. Our work leads to several molecular signatures, unearthed from methylome and miRNome, able to anticipate to some extent which BC tumors respond or not to paclitaxel. These results may provide insights to optimize paclitaxel-therapies in clinical practice.
Keywords: biomarker discovery, machine learning, artificial intelligence, precision oncology, tumor profiling
Introduction
Breast cancer (BC) is the most common type of cancer in women worldwide resulting in half a million deaths annually (Golubnitschaja et al., 2016). BC is a disease presenting substantial inter-tumor heterogeneity (Russnes et al., 2011). Cytotoxic drugs are used to eradicate tumor cells, to complement surgery or radiotherapy as well as to alleviate cancer symptoms. Paclitaxel is a BC-approved cytotoxic drug from the taxane family, which acts by interfering with the normal function of microtubules during cell division (Perez, 1998). As with other cancer drugs (Brown and Böger-Brown, 1999; Cardoso et al., 2002; Ribeiro et al., 2012; Housman et al., 2014), resistance to paclitaxel have been regularly observed in BC patients (Flint et al., 2009; Ajabnoor et al., 2012).
Precision oncology requires predictors to guide the optimization of drug therapies for patients (Peck, 2016; Schwartzberg et al., 2017). Indeed, it is now well-established that gene polymorphisms and other genomic alterations play important roles in the observed heterogeneous response to drugs (Wang et al., 2011;Harper and Topol, 2012; Kadra et al., 2012). This has led to the identification of clinical biomarkers of drug response from molecular profiles of the patients’ tumors (Huang et al., 2014). These predictive biomarkers now guide patient-specific treatment selection during clinical trials and are also used in clinical practice (Mandrekar and Sargent, 2009; Biankin et al., 2015). Most commonly, single-gene markers are used to discriminate between therapy responders and non-responders (Prahallad et al., 2012; Rodríguez-Antona and Taron, 2015), typically consisting of an actionable mutation (e.g. single-nucleotide variant) of a specific gene in the tumor sample.
Single-gene markers that are able to predict the efficacy of cytotoxic drugs are rare (Felip and Martinez, 2012), especially for taxanes (Murray et al., 2012; Bartlett et al., 2015; Norimura et al., 2018). For instance, Marsh et al. (2007) have proposed that the point mutation CYP1B1*3 could be an important factor that helps to differentiate between sensitive and resistant BC patients to paclitaxel. However, Gehrmann et al. (2008) have raised doubts about the association between this alteration and paclitaxel-treated patient prognosis, concluding that CYP1B1 alone is not sufficient to predict tumor response to paclitaxel, and that it could interact with still unknown factors involved in paclitaxel sensitivity. This is an example of inter-patient variability in drug response not being fully captured by the mutational status of single gene, as it has also been seen in vitro in a range of drugs (Naulaerts et al., 2017).
Machine Learning (ML) can be used to build in silico models able to predict tumor response to a given drug by combining multiple tumor features in an optimal manner (Libbrecht and Noble, 2015; Ali and Aittokallio, 2018). The scarcity of suitable clinical data to build such predictors has been a major roadblock, which has made predictors based on cancer cell line data thrive (Costello et al., 2014; Ding et al., 2016). Fortunately, response data from paclitaxel-treated BC patients along with comprehensive molecular profiles of their tumors are increasingly available. Such datasets represent an opportunity to improve our ability to anticipate which BC patients will respond to paclitaxel. We obtained them from the recently created Genomic Data Commons (GDC) of the US National Cancer Institute (NCI) (Jensen et al., 2017). The GDC provides a unified data repository enabling data sharing across cancer genomic studies in support of precision medicine. The GDC feeds from several cancer genome programs at the NCI Center for Cancer Genomics, notably The Cancer Genome Atlas (TCGA) (Weinstein et al., 2013), and offers a range of information-rich genomic, transcriptomic and epigenomic profiles, as well as clinical drug response data.
These datasets, however, pose the challenge of being high-dimensional. Each profile typically contains between hundreds and many thousands of features, but only tens of profiled tumors of the same cancer type and treated with the same drug. For example, a community challenge intended to predict drug response employed 53 BC cell lines (Costello et al., 2014), while thousands of features from DNA copy-number variation, transcript expression, mutations, DNA methylation, and protein abundance profiles were considered. In another study (Tripathi et al., 2016), predictive models of response to cytotoxic drugs were achieved using 60 pancancer cell lines and gene variants as features. A further example of predictive drug-sensitivity models is a study employing 60 diverse cell lines and protein abundances as features (Ma et al., 2006). Small sample sizes are not only typical of preclinical studies, but also of clinical studies addressing the same problem. For instance, gene expression signatures were identified and evaluated using 81 melanoma patients to predict their response to PD-1 checkpoint inhibitors (Ayers et al., 2017).
In this study, we will investigate whether it is possible to anticipate the response of BC patients to paclitaxel using GDC data. We also aim at discovering the molecular factors that, collectively, best discriminate between paclitaxel-resistant and paclitaxel-sensitive BC patients. High-dimensional data promotes model overfitting, which in turn results in poorer predictions. As predictive performance differences between ML algorithms are strongly problem-dependent (Tan and Gilbert, 2003; Fernández-Delgado et al., 2014), considering a range of algorithms to identify those that are most suitable for paclitaxel-treated BC patients is appealing. To this end, we apply 10 ML methods to build predictive models in combination with each available molecular profile. Some of the resulting multi-variate predictors are highly interpretable in that they can answer questions such as why this particular patient is non-responsive. This information should permit formulating hypothesis about the molecular mechanisms of BC patient resistance to paclitaxel.
Material and Methods
GDC Data
GDC molecular profiling and clinical data from the TCGA Breast Invasive Carcinoma or BRCA (https://portal.gdc.cancer.gov/projects/TCGA-BRCA) provide the basis for this study. Molecular profiles and clinical data come from release version 4.0, except for miRNA and miRNA isoform (isomiR) expressions coming from release version 8.0 (Release Notes- GDC Docs).
TCGA-BRCA project gathers data from 1,098 patients, resulting in almost 13,000 files (around 130 GB). These datasets were retrieved and downloaded using the GDC Application Programming Interface (API). Table S1 reports information about files collected from the GDC that have been used to generate datasets.
Processing Clinical Data for Modelling
Patient population included primary or secondary advanced breast cancer receiving single-agent paclitaxel. For some patients it was observed that different drugs have the same or very close treatment start and end time. These entries may form part of a drug combination. However, available drug response annotations do not allow to check this information. Therefore, possible effects due to drug combinations are ignored in this study when identifying paclitaxel-treated patients. Patients with missing paclitaxel response were not retained. To only consider baseline tumors’ molecular profiles, patient records were only retained if no treatment was administered before resection and the time of sample procurement is indicated. We assumed that a baseline molecular profile can explain drug responses observed in a given patient even if paclitaxel was administered at any time after sample resection (Geeleher et al., 2014). After these curation steps, 61 paclitaxel-treated BC patients with valid records remained (Table S2 reports information about treatments and biospecimens). Annotated patient responses are divided into four categories based on the RECIST standard (Therasse et al., 2000): “Complete Response” (CR), “Partial Response” (PR), “Stable Disease” (SD), and “Clinical Progressive Disease” (CPD). We further classified clinical responses into two categories, namely “responder” (CR or PR) and “non-responder” (SD or CPD).
Processing Molecular Profiles for Modelling
The GDC works on harmonization of raw genomic data developing specific workflows to provide consistent and up-to-date molecular profiles (GDC Reference Files| NCI Genomic Data Commons, Genomic Data Harmonization| NCI Genomic Data Commons). Available profiles comprise copy-number variation (CNV), DNA methylation, mRNA, miRNA and isomiR (Ameres and Zamore, 2013) expressions. In order to produce suitable inputs for ML algorithms and/or extract some specific information, we processed some of them. More details are available in the homonym section of Supplementary Methods. All the datasets produced from these molecular profiles are made of real-valued features.
Predicting Drug Response Using ML Algorithms With Embedded Feature Selection
Most classifiers were built with ML algorithms capable of embedded feature selection to mitigate the impact of high-dimensional data on their generalization on unseen data. Implementations of Classification And Regression Tree (CART) (Breiman et al., 1984) and Random Forest (RF) (Breiman, 2001) were taken from the python library Scikit-learn version 0.19.1, while the ones of XGBoost (XGB) (Chen and Guestrin, 2016) version 0.6 and LightGBM (LGBM) (Ke et al., 2017) version 2.0.10 were respectively downloaded from https://github.com/dmlc/xgboost and https://github.com/Microsoft/LightGBM. We also applied a Deep Neural Network (DNN) algorithm (Bengio, 2009) implemented with the python library Keras version 2.2.4 using the TensorFlow backend. In addition to these nonlinear models, linear models were generated with Logistic Regression (LR) (Ranstam et al., 2016), which is also implemented in Scikit-learn. The visualization of Decision Trees (DTs) was done with the python package dtreeviz version 0.2.2. The homonym section in Supplementary Methods provides more details.
Predicting Drug Response Using the Optimal Model Complexity (OMC)
OMC is a strategy to build ML models employing only the most relevant features (Nguyen et al., 2018). More details are available in the homonym section of Supplementary Methods.
Measuring the Predictive Performance of a Classifier
This is a binary classification problem, as each patient belongs to one of two classes, responder and non-responder, with the responder considered as the positive class. As it is customary with problems with a small number of data instances (Table S3), we are using LOO (Leave-One-Out) CV (Cross-Validation) to evaluate the developed classifiers. Several types of LOOCV will be used: standard for “all-features models”, nested for “OMC models”, and permutated for “permutation models”. As with any other CV (Kohavi, 1995), each data instance (patient here) is exactly once in the test set. Thus, CV performance of a model is exclusively based on the prediction of test instances that were not used in any way to train or select the model (any feature selection is hence carried out on training folds only). Employing nested CV on algorithms requiring model selection (those employing OMC) provides an unbiased estimate of model performance, as it has been shown elsewhere (Cawley and Talbot, 2010; Varma and Simon, 2006).
Once known and predicted classes are compared for all held-out samples, true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN) are counted among BC patients. These counts are used for calculating classification metrics that summarize the predictive performance reached by a classifier: precision, recall, F1-scores (Van Rijsbergen, 1979), and Matthews Correlation Coefficient (MCC) (Matthews, 1975; Boughorbel et al., 2017). More details about these metrics can be found in the homonym section of Supplementary Methods. Classification scores and contingency matrices obtained from all produced classifiers are stored in Tables S4 and S5, respectively.
Results
Benchmarking of All-Features Models (RF, XGB, LGBM, DNN, LR) Reveals Some Informative Molecular Profiles, But the Resulting Classifiers Perform Marginally Better Than Random
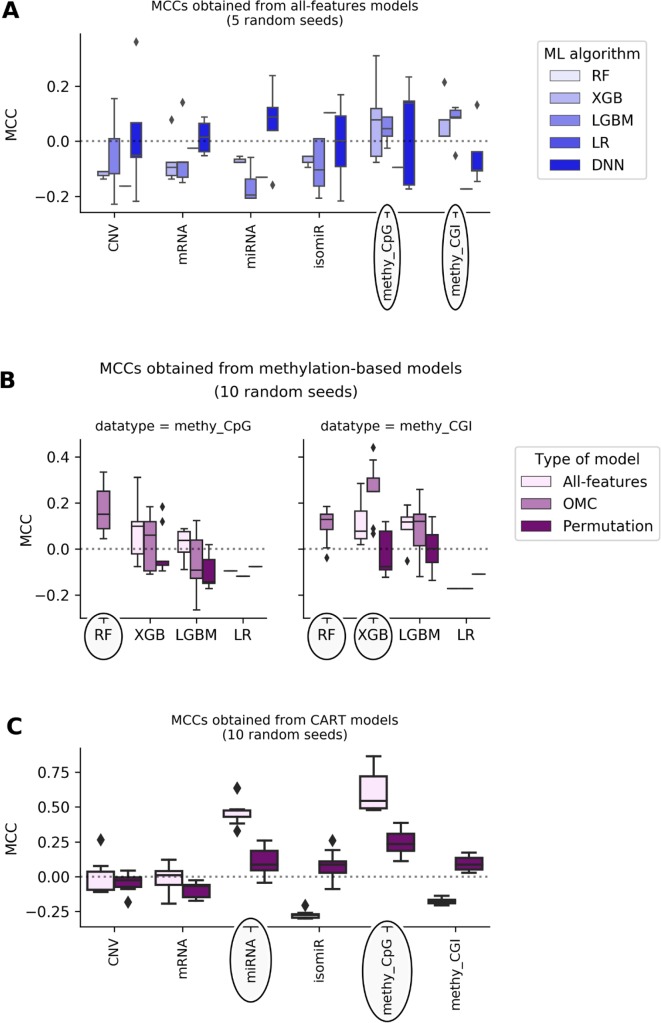
Figure 1 shows that most of the all-features ML classifiers perform worse than random classification reaching slightly negative median MCCs (from -0.19 to -0.05). Poor performance was also obtained when using linear models: LR models perform randomly at best (median MCCs range from -0.17 to 0.1 depending on the profile). Poor predictive performance is primarily caused by FPs (i.e. misclassification of non-responders). This problem was particularly noticeable in RF models, which misclassified every non-responsive patient regardless of the employed profile, leading to undefined MCCs. The latter shows that all-features RF handles class imbalance poorly on these particular problem instances.
Figure 1.
DNA methylation and miRNA expression lead to the most predictive ML models. Each MCC of a given model is calculated by LOOCV. The experiment is repeated several times, each time with a different random seed, giving rise to a boxplot of MCCs for each case. Permutation models were generated after shuffling class labels on the considered training set. As RF models give undefined MCCs, blanks are found in bins where boxplots are expected. Substantial variability is observed, showing that this problem is both profile- and classification method-dependent. The dashed line shows the expected MCC from random classification. (A) Predictive performance of all-features models. All-features models are those in principle employing all the features in the profile to generate the prediction. Models are built with ML algorithms using the default operating threshold (0.5) to calculate the predicted class label from the predicted class probability. Five random seeds were set for each ML algorithm; thus, MCC values come from five runs of standard LOOCV. x-axis shows the employed molecular profile, while y-axis displays the MCCs obtained by classifiers. From the lightest to the darkest blue, boxplots summarize the distributions of MCCs obtained by XGB,LGBM, LR, and DNN models, respectively. Ellipses indicate which profiles employed by models obtain better-than-random predictive performance: DNA methylation profiles are the most predictive. This also suggests that the other profiles are less informative for the prediction of BC tumor response to paclitaxel. (B) Predictive performance applying OMC to methylation-based models. 10 random seeds were used to investigate further the most predictive profiles. OMC models had their hyperparameters complexity and operating threshold tuned and thus required nested LOOCV. Horizontal axes show the employed ML algorithms to process CpG site (left) and CGI (right) methylation datasets, while vertical axes display MCCs achieved by classifiers. Light-pink, medium-pink, and indigo boxplots summarize the distributions of MCCs obtained by all-features, OMC and permutations models, respectively. Circles indicate ML algorithms releasing models with predictive performance improved using OMC. This shows that predictive accuracy depends on both the molecular profile and the ML algorithm. Here, the best models found are CpG site methylation-based RF-OMC, CGI methylation-based RF-OMC, and CGI methylation-based XGB-OMC. (C) Predictive performance of CART models. These models (light-pink boxes) were built considering all features in the profile with no hyperparameter tuning. Permutation models (indigo boxes) were trained after that shuffling class labels in the training set. Each MCC is calculated by standard LOOCV, a process repeated with 10 different random seeds. x-axis shows the molecular profiles (‘CNV’ is short for copy-number variation, ‘methy_CpG’ for CpG site methylation, and ‘methy_CGI’ for CGI methylation), while y-axis displays the LOOCV MCCs achieved by each classifier. The dashed line shows the expected MCC from random classification. Ellipses indicate molecular profiles processed by CART models obtaining the highest predictive performance. These results reveal that CpG sites methylation-based and miRNA expression-based CART models are the most predictive. Predictive accuracy is substantially higher than that provided by all-features or OMC models (in A and B), which demonstrates that the CART learning algorithm is more suitable for these problem instances.
DNA methylation-based XGB, LGBM, and DNN models achieve median MCCs slightly higher than 0.0, and they perform hardly better than permutation models. On the one hand, CpG site methylation-based XGB, LGBM, and DNN models obtain a median MCC of 0.08 (p-value from two-sided paired Student’s t-test obtained by class-permutation test = 1.05·10-1), 0.04 (p-value = 1.41·10-1), and 0.14 (p-value = 4.08·10-1), respectively. On the other hand, CpG island (CGI) methylation-based XGB and LGBM models achieve a median MCC of 0.08 (p-value = 2.33·10-1) and 0.09 (p-value = 8.04·10-2), respectively. Moreover, miRNA and mRNA expression-based DNN models had a median MCC of 0.088 (p-value = 4.84·10-2) and 0.015 (p-value = 4.76·10-1), respectively.
Complexity-Optimized ML Models (RF-OMC, XGB-OMC, and LGBM-OMC) Provide Better Prediction and Extract Relevant Factors for Paclitaxel Response From CGI Methylation Data
Using OMC allows both to reduce considerably the number of features considered during model training and to adjust the operating threshold for assigning class labels to data instances. This leads to some OMC models that perform better than those considering all features from dataset (Table S12 in Supplementary Results). This is especially the case for some methylation-based models that have been improved using OMC (Figure 1), unlike for models based on other profiles (Figure S5). The improvement of OMC over the all-features approach is ML algorithm-dependent.
CGI methylation-based OMC models have obtained improved predictive performance, using either RF or XGB. For instance, XGB-OMC models obtain a median MCC of 0.25, which is significantly better than both permutation and all-features models (p-values equal 9.30 ×·10-4 and 2.16 ×·10-2, respectively). In order to extract a robust subset of molecular factors potentially involved in paclitaxel response, the most informative features selected by these models were investigated (Table S13 and S14). It results in 7 out of the 11,644 CGI coordinates encoded as CGI_ID.24217, CGI_ID.15915, CGI_ID.6919, CGI_ID.5276, CGI_ID.5459, CGI_ID.16043, and CGI_ID.11903. Moreover, we notice that 5 of them are common to the features used by the RF-OMC models. Consulting indices provided in Tables S7 and S8 (more details in Supplementary Methods), we found that these coordinates are related to the following 16 genes: CYP2D6, NDUFA6-AS1, RP4-669P10.19 (or C6orf108 pseudogene), MBTPS2, YY2, C2orf40 (or ECRG4), UXS1, IKZF1, APOBEC4, RGL1, ARPC5, NCF2, SMG7, C1orf177 (or LEXM), RP11-631M21.6 (or FAM166A pseudogene 7), and TUBB8 (Table S14).
Transparent ML Models (CART) Capture CpG Methylation Sites and Mature MIRNAS Relevant for the Sensitivity to Paclitaxel and Show How They Are Combined to Explain Drug Response
Most of the available profiles led to poor classification of test set patients when modelled with CART (Figure 1). By contrast, CART classifiers based on miRNA expression and CpG site methylation data provided high to very high predictive performance in the context of this problem (in 10 LOOCV runs, median MCCs of 0.43 and 0.54 were obtained, respectively) and performed significantly better than random models (p-values from two-sided paired Student’s t-test obtained by class-permutation test equal 4.57·× 10-6 and 2.86·× 10-4, respectively; see Figure 1 and Table 1). For each case, the best model is defined as that obtaining the highest MCC in 10 standard LOOCVs of the full dataset (i.e. all data instances and all available features). Figure S6 shows that the performance of these models is robust to different sizes of both training set and test set.
Table 1.
Best CART models.
| Tumor profiling data | Number of considered features | Number of selected features | Median MCC(CART trained on original data) | Median MCC(CART trained on class-permutated data) | p-value(original vs permutated) |
|---|---|---|---|---|---|
| miRNA | 337 | 4 | 0.43 | 0.09 | 4.57·10-6 |
| methy_CpG | 22,941 | 2 | 0.54 | 0.23 | 2.86·10-4 |
The predictive performance of CART models was presented in Figure 1. Here we summarize the characteristics of the two best models (i.e. those exploiting miRNA expression and CpG methylation profiles). A median MCC was calculated with the 10 MCCs coming from LOOCV experiments (each with a different random seed). This five additional LOOCV runs with respect to those presented in Figure 3 were carried out to better characterize the performance of the best models found in our study. The small difference found in median MCC (0.52 in Figure 3 versus 0.54 here) suggests that this performance metric is quite robust to the number of LOOCV runs for CART. The training sets were also class-permutated during cross-validation as explained in the Methods section and CART trained on the resulting data to provide a second set of 10 MCCs per profile. The p-value (two-sided paired Student’s t-test) of this class-permutated test shows how likely are the MCCs of the CART models to arise by chance. The first model was trained on miRNA expression: 4 out of 337 mature miRNAs were retained to build this model reaching a median MCC of 0.43 and performing significantly better than models based on permutated data (p-value = 4.57·10-6). The second model is obtained processing CpG site methylation (shorten as ‘methy_CpG’): 2 out of 22,941 CpG sites were retained to build this model achieving a median MCC of 0.54 and performing significantly better than permutation models (p-value = 2.86·10-4).
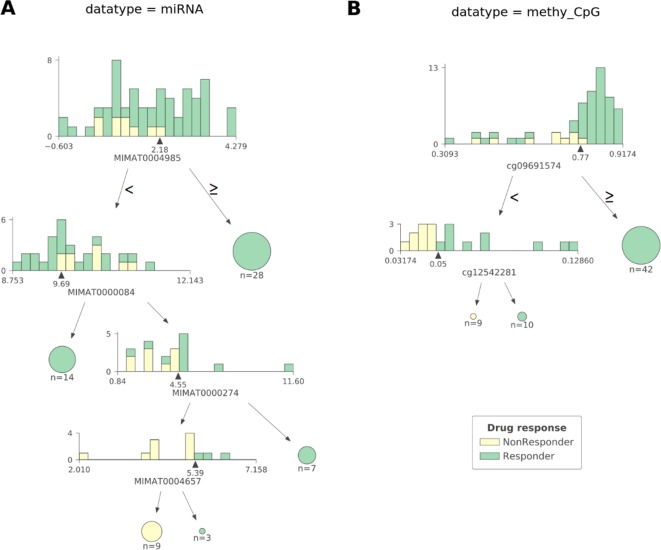
As observed in Figure 2 CART models strongly reduce the number of features involved in the predictions. The miRNA expression-based model found that 4 out of 337 mature miRNAs were the most informative features (MIMAT0004985 or miR-942-5p, MIMAT0000084 or miR-27a-3p, MIMAT0000274 or miR-217, and MIMAT0004657 or miR-200c-5p), while the CpG-site methylation model identified 2 out of 22,941 CpG sites as the most informative features (cg09691574, which is related to the protein coding genes MRGPRX4 and SAA2-SAA4, and to the lincRNA RP11-113D6.6 also called antisense to MRGPRX4; and cg12542281, which is related to the protein coding gene N4BP2L2). The DTs represented in Figure 2 show directly the interactions between independent features leading to the predictions. They also reveal the molecular types associated to paclitaxel-sensitive and paclitaxel-resistant BC tumors (the CpG site index is provided in Table S7).
Figure 2.
The most predictive CART models offer high interpretability of BC tumors response to paclitaxel. Visualization of the most predictive CART models seen in Figure 1 exploiting (A) miRNA expression and (B) CpG site methylation data. Each DT node has a histogram showing the distribution of patients at that node against the selected feature (the proportion of responders vs non-responders in each feature bin is also shown). The triangle under the histogram marks the value of the best split for the selected feature, whose name can be found under the histogram as well. Each node has two leaves: to the left (patients with a feature value lower than that of the best split) and to the right (the rest of the patients). Terminal nodes (or leaves) are displayed as pie charts. The proportions of non-responders and responders are respectively colored yellow and green. The log2-transformed miRNA expression-based DT shown in (A) reveals four different molecular types of sensitive BC tumors and one molecular type associated to resistant BC tumors involving four mature miRNAs: MIMAT0004985, MIMAT0000084, MIMAT0000274, and MIMAT0004657 (also known as miR-942-5p, miR-27a-3p, miR-217, and miR-200c-5p, respectively). Thus, a tumor is classified as responsive to paclitaxel if: 1) the expression value of MIMAT0004985 is higher than 2.18, or 2) the expression value of MIMAT0004985 is lower than 2.18 and that of MIMAT0000084 is lower than 9.69, or 3) the expression value of MIMAT0004985 is lower than 2.18, and that of MIMAT0000084 is higher than 9.69 and that of MIMAT0000274 is higher than 4.55, or 4) the expression value of MIMAT0004985 is lower than 2.18, and that of MIMAT0000084 is higher than 9.69, and that of MIMAT0000274 is lower than 4.55, and that of MIMAT0004657 is higher than 5.39. Otherwise, the tumor is classified as non-responsive. The DT based on CpG site methylation (shortened as ‘methy_CpG’) shown in (B) unveils two different molecular types of sensitive BC tumors and one type of resistant BC tumors involving two CpG sites represented by probes cg09691574, which is related to the protein coding genes, MRGPRX4 and SAA2-SAA4; and to the lincRNA RP11-113D6.6, also called antisense, to MRGPRX4; and to cg12542281, which is related to the protein coding gene N4BP2L2. Thus, a tumor is predicted to be sensitive to paclitaxel if: 1) the beta value associated to the methylation of cg09691574 is higher than 0.77, or 2) the beta value associated to the methylation of cg09691574 is lower than 0.77, and that of cg12542281 is higher than 0.05. Otherwise, the tumor is predicted to be resistant. Both DTs were found to give pure leaves (i.e. all data instances that are in terminal nodes belong to the same class).
Lastly, integrating different molecular profiles has sometimes been found to provide small predictive accuracy gains, e.g. see Figure 4 in this study (Costello et al., 2014). Thus, since both miRNA and methy_CpG profiles led to the most predictive models, it makes sense to integrate these data sets and train CART models on the features of the resulting hybrid profile. Using the same 10 random seeds as the methy_CpG-based CART models (median LOOCV MCC of 0.54), the hybrid CART models obtained slightly worse accuracy (median LOOCV MCC of 0.52). The resulting CART tree is identical to that in Figure 2, suggesting that miRNA features were overshadowed by methy_CpG features during CART induction.
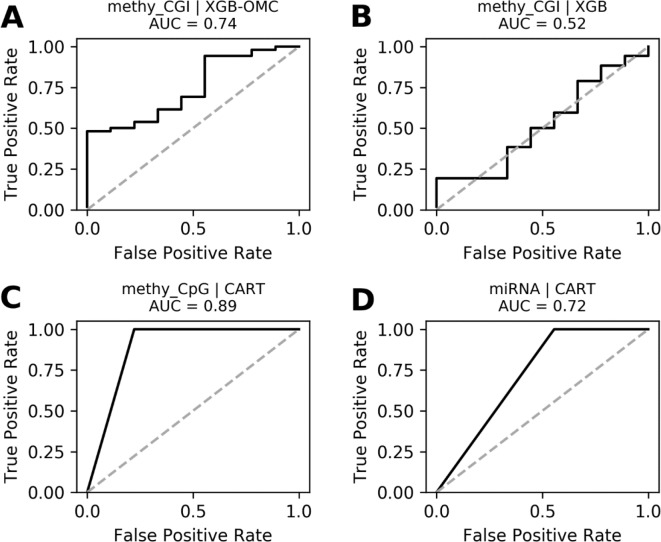
Figure 4.
ROC curves of the most predictive case of best models. ROC curves obtained plotting the true positive rates against the false positive rates calculated from the models presented in Figures S4. The AUCs were calculated from the predictions that came out from the nested and standard LOOCV runs and were respectively carried out for OMC and CART models. We notice that AUCs follow the same trend as MCCs and that models shown in (A), (C), and (D) are very robust. The dashed line delimitates the expected AUC from random classification.
Discussion
Owing to the wealth of curated data offered by the GDC, we could evaluate six profiles. The exhaustive evaluation of the 60 predictive models obtained, employing 10 ML algorithms with each profile, reveal strong variability in predictive performance (Figure 3). These results show the importance of considering multiple profiles and ML algorithms, the latter being always possible. For example, we could have carried out this study using the standard all-features versions of tree-ensemble, LR and DNN algorithms. However, this would have only resulted in models with near-random predictability despite using six profiles and thus, we could have concluded that precision oncology is not possible for paclitaxel-treated BC patients. Instead, we also tested algorithms generating models requiring only a handful of features (OMC-based and CART), which in addition, provided the best performance on these problem instances. Note that the most predictive of these models achieved an over 10,000-fold reduction in the number of features (Table 1).
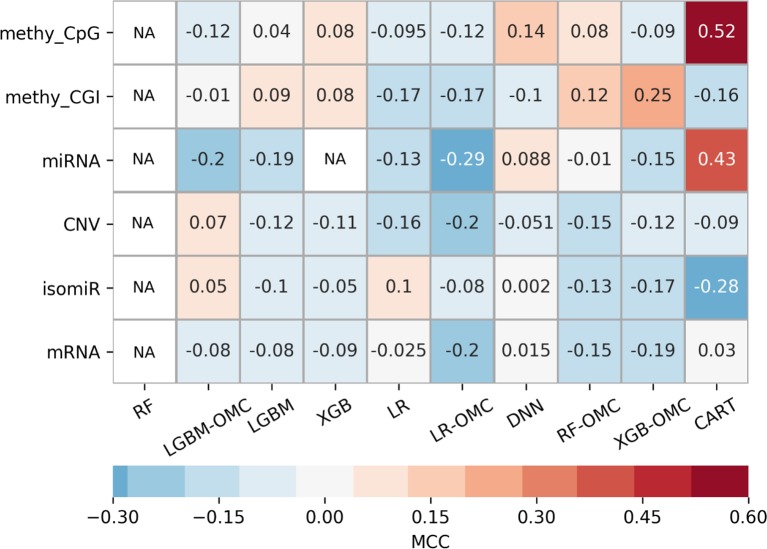
Figure 3.
Employing multiple ML algorithms and tumor profiles increase the likelihood of discovering models able to predict BC patient response to paclitaxel. ML algorithms include the unaltered version of tree-ensemble and linear algorithms using all available features (RF, XGB, LGBM, and LR) and their OMC versions (RF-OMC, XGB-OMC, LGBM-OMC, and LR-OMC). The 9th algorithm was CART, employed to generate simpler and more interpretable classification models. The 10th algorithm was DNN, employed to generate more sophisticated but less interpretable models. Each of these algorithms was evaluated on each of the six molecular profiles, which resulted in 60 classifiers on the same BC patients. LOOCV evaluation was performed 5 times setting a different random seed for the employed ML algorithm, leading to 5 MCC determinations quantifying predictive performance. The heatmap shows the median MCC per classifier. Rows show the processed molecular profiles (‘CNV’ is short for copy-number variation, ‘methy_CpG’ for CpG site methylation, and ‘methy_CGI’ for CGI methylation), while columns display the employed ML algorithms. Thus, each cell corresponds to the median MCC of a given predictive model. Cells are colored in light-blue and dark-blue when this model reaches a negative or very negative median MCC (i.e. classification worse than random); in grey when it reaches a median MCC very close to 0.0 (i.e. random classification); in light-brown and dark-brown when it reaches a positive or very positive median MCC (i.e. classification better than random or close to perfect); in white and labelled NA (i.e. not available) when it reaches an undefined median MCC (i.e. misclassification of non-responders within several or all iterations). These results show that DNA methylation is the most informative profile (it leads to 2 of the 3 classifiers with a median MCC of a least 0.25). The choice of ML algorithm also affects the predictive performance. For example, none of the RF or LGBM classifiers obtain an MCC of at least 0.10. Thus, predictive performance depends strongly on of algorithm- profile combination: only one XGB-OMC models is predictive (that based on CGI methylation) and it is among the best predictors (median MCC of 0.25). Two other examples are the CART classifiers based on CpG methylation and miRNA expression, with median MCC of 0.52 and 0.43, respectively. Figures S4 and S5 further characterizes the performance of the best classifiers.
Identifying a concise list of predictive molecular features is indeed beneficial for interpretability. The CGI methylation-based XGB-OMC model employs a dramatically reduced number of features (11 of the considered 11,644). The increased predictive performance comparing to all-features model (Figure 1) shows that the selected subset of features contains the information relevant for predictions (Figure S2). Therefore, applying OMC not only offers better predictivity, but also better interpretability of response to paclitaxel, as it revealed a molecular signature able to discriminate sensitive and resistant BC tumors from high-dimensional data. The best CART models reached the highest predictive performance among the generated predictors (Figure 1). Moreover, these models allow going further in the interpretation of response to paclitaxel (Figure 2). For example, the CpG-methylation DT unveils two rules employing only two features to predict which are the paclitaxel-sensitive BC tumors (Figure 2). The other example is the miRNA DT, which carries out these predictions using four induced rules based on only four features (Figure 2). Thus, the application of these rules to forthcoming tumors should improve paclitaxel treatment for BC patients. To facilitate such application, we are providing two python scripts in the supplementary materials, each implementing the rules for one of these predictive profiles.
Our best classifier obtained a median MCC of 0.54 in 10 LOOCV runs (an average MCC of 0.62, with MCC ranging from 0.48 to 0.87 in these runs as it can be seen in Figure 1). To put these predictive accuracies in the context of what is typically achieved when predicting tumor response to a drug from omics profiles, we have looked at other test set performances reported at the literature for this problem. One study (Kim et al., 2016) applied a range of ML algorithms to predict pancancer cell line response from transcriptomic profiles and obtained MCCs below 0.6 in all cases (see Figure 1 in that paper). Maximum MCCs slightly above 0.5 and 0.3 were also obtained using RF with transcriptomic profiles (Nguyen et al., 2017) and genomic profiles (Naulaerts et al., 2017), respectively. Another study (Xu et al., 2019) also predicted drug response using many hundreds of pancancer cell lines using several ML algorithms from various omics profiles (gene expression, copy-number alterations, single-nucleotide mutations). Depending on the considered data resource, average MCCs across drug and profiles range from 0.15 to 0.31 or from 0.22 to 0.45 (see Tables 2 and 3 in that paper). Yet another example is by (Tripathi et al., 2016) using gene variants as features, where MCCs range across drugs from 0.32 to 0.56 or from 0.30 to 0.44 depending on data resource (see Tables 1 and 2 in that paper). Lastly, single-gene drug response markers identified by MANOVA and Chi-Square tests on pancancer cell lines obtained maximum MCCs of 0.30 and 0.31, respectively (Dang et al., 2018).
The alteration of gene expression due to epigenetic modifications triggers the development of cancers, including BC. DNA methylation changes, occu rring both within and around CGIs, can impact transcriptional activity of genes or transcription factors involved in malignant phenotypes (Esteller, 2002; Irizarry et al., 2009; Levenson, 2010; Deaton and Bird, 2011; Manjegowda et al., 2017; Stirzaker et al., 2017). It has been shown that biomarkers for prognosis and treatment can be unearthed from DNA methylation profiles (Xiang et al., 2013; Mikeska and Craig, 2014; Stirzaker et al., 2014; Li et al., 2015; Pouliot et al., 2015). Furthermore, it has been found that DNA methylation can interfere in chemo-resistance to paclitaxel (Wang et al., 2012; Ignatov et al., 2014; Yun et al., 2015; He et al., 2016; Zhang et al., 2018). Our DNA-methylation-based predictors selected CpG sites and CGIs related to genes previously found individually involved in cancer development and with transcriptional activity regulated by methylation (e.g. MBTPS2, YY2, ECRG4, IKZF1). Selected features by these models are also related to genes associated to response to cytotoxic drugs such as N4BP2L2 (paclitaxel), CYP2D6 (tamoxifen), APOBEC4 (tamoxifen, doxorubicin, and etoposide), and TUBB8 (paclitaxel) (Table S15).
miRNAs also play a key role in cancer development by acting as tumor suppressors or oncogenes. These molecules can be used as biomarkers, and modulation of their specific activities provides insight for therapeutic investigations (Hayes et al., 2014; Peng and Croce, 2016). Furthermore, the expression of some miRNAs has been associated to the sensitivity to paclitaxel (Zhou et al., 2010; Chen et al., 2014; He et al., 2016; Lu et al., 2017). The miRNA expression-based CART model combines miR-27a-3p, miR-217, miR-200c-5p, and miR-942-5p to predict which BC tumors are paclitaxel-responsive with high accuracy (Figures 1 and 2A). Individually, each of these miRNAs have been linked to paclitaxel response and BC prognosis: the first three are related to paclitaxel resistance, whereas the last one is associated to shorter survival of BC patients (Table S15).
Our study has some limitations to be pointed out. First, for a given patient, molecular profiles were obtained from the primary tumor, while clinical response was registered later following tumor evolution. Both tumors may present some differences at the molecular level, due to temporal or spatial tumor heterogeneity, as well the possible impact of the treatment administered after tumor resection. Second, while we reported predictive accuracy on BC tumors not used in any way to build or select the model, an additional independent evaluation on a second cohort would shed further light into how general these models are. The latter is currently not possible due to the scarcity of paclitaxel-treated BC patients with DNA methylation or miRNA profiles of their tumors.
Yet, our work provides very predictive (in the context of the considered problem), robust (Figure S4 and Figure 4), and even interpretable models to identify primary BC tumors sensitive to paclitaxel. These results also suggest that tumor methylomes and miRNomes can be a source of multi-variate models to predict prognosis and treatment response. Indeed, our predictive models reveal molecular features that can collectively anticipate which BC tumors are sensitive or resistant to paclitaxel. Previous studies have experimentally validated the involvement in BC development, and even in the resistance to paclitaxel, of these molecular factors individually, which further supports the applicability of these classifiers. Furthermore, our results also suggest novel predictive factors such as the antisense to MRGPRX4; the pseudogenes (Poliseno et al., 2015; Xiao-Jie et al., 2015) C6orf108 and FAM166A; and the coding genes NDUFA6-AS1, UXS1, RGL1, and LEXM.
Data Availability Statement
The datasets generated for this study can be found in the https://portal.gdc.cancer.gov/.
Author Contributions
PB conceived the study and designed the experiments. AB and PB wrote the manuscript with the assistance of AG. AB carried out the numerical experiments. All authors analyzed the results and contributed to their discussion.
Funding
This work was supported by the Institut Paoli-Calmettes (grant number 305/2016 to PB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Computing resources for this study were partly provided by the computing facilities DISC (Datacenter IT and Scientific Computing) of the Centre de Recherche en Cancérologie de Marseille.
Supplementary Materials
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.01041/full#supplementary-material
References
- Ajabnoor G., Crook T., Coley H. (2012). Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 3, e260. 10.1038/cddis.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Aittokallio T. (2018). Machine learning and feature selection for drug response prediction in precision oncology applications. Biophys. Rev. 11, 1–9. 10.1007/s12551-018-0446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres S. L., Zamore P. D. (2013). Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 14, 475–488. 10.1038/nrm3611 [DOI] [PubMed] [Google Scholar]
- Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D. R., et al. (2017). IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. M. S., Nielsen T. O., Gao D., Gelmon K. A., Quintayo M. A., Starczynski J., et al. (2015). TLE3 is not a predictive biomarker for taxane sensitivity in the NCIC CTG MA.21 clinical trial. Br. J. Cancer. 113, 722–728. 10.1038/bjc.2015.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengio Y. (2009). “Learning Deep Architectures for AI,” in Found. Trends® Mach. Learn. Hanover, MA, USA. 10.1561/2200000006 [DOI] [Google Scholar]
- Biankin A. V., Piantadosi S., Hollingsworth S. J. (2015). Patient-centric trials for therapeutic development in precision oncology. Nature. 526, 361–370. 10.1038/nature15819 [DOI] [PubMed] [Google Scholar]
- Boughorbel S., Jarray F., El-Anbari M. (2017). Optimal classifier for imbalanced data using Matthews Correlation Coefficient metric. PLoS One. 12, e0177678. 10.1371/journal.pone.0177678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. (2001). Random Forests. Mach. Learn. Boca Raton, FL, USA. 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Breiman L., Friedman J., Stone C. J., Olshen R. A. (1984). Classification and Regression Trees. Chapman and Hall/CRC. [Google Scholar]
- Brown R., Böger-Brown U., (1999). Cytotoxic Drug Resistance Mechanisms. New Jersey: Humana Press. 10.1385/1592596878 [DOI] [Google Scholar]
- Cardoso F., Di Leo A., Lohrisch C., Bernard C., Ferreira F., Piccart M. J. (2002). Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann. Oncol. 13, 197–207. 10.1093/annonc/mdf101 [DOI] [PubMed] [Google Scholar]
- Cawley G. C., Talbot N. L. C. (2010). On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach. Learn. Res. 11, 2079–2107. [Google Scholar]
- Chen N., Chon H. S., Xiong Y., Marchion D. C., Judson P. L., Hakam A., et al. (2014). Human cancer cell line microRNAs associated with in vitro sensitivity to paclitaxel. Oncol. Rep. 31, 376–383. 10.3892/or.2013.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Guestrin C. (2016). “XGBoost,” in Reliable Large-scale Tree Boosting System. ACM; New York, NY, USA. 10.1145/2939672.2939785 [DOI] [Google Scholar]
- Costello J. C., Heiser L. M., Georgii E., Gönen M., Menden M. P., Wang N. J., et al. (2014). A community effort to assess and improve drug sensitivity prediction algorithms. Nat. Biotechnol. 32, 1202–1212. 10.1038/nbt.2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. C., Peón A., Ballester P. J. (2018). Unearthing new genomic markers of drug response by improved measurement of discriminative power. BMC Med. Genomics 11, 10. 10.1186/s12920-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton A. M., Bird A. (2011). CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022. 10.1101/gad.2037511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Zu S., Gu J. (2016). Evaluating the molecule-based prediction of clinical drug responses in cancer. Bioinformatics 32, 2891–2895. 10.1093/bioinformatics/btw344 [DOI] [PubMed] [Google Scholar]
- Esteller M. (2002). CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 21, 5427–5440. 10.1038/sj.onc.1205600 [DOI] [PubMed] [Google Scholar]
- Felip E., Martinez P. (2012). Can sensitivity to cytotoxic chemotherapy be predicted by biomarkers? Ann. Oncol. 23 Suppl 1, x189–x192. 10.1093/annonc/mds309 [DOI] [PubMed] [Google Scholar]
- Fernández-Delgado M., Cernadas E., Barro S., Amorim D. (2014). Do we need hundreds of classifiers to solve real world classification problems? J. Mach. Learn. Res. 15, 3133–3181. [Google Scholar]
- Flint M. S., Kim G., Hood B. L., Bateman N. W., Stewart N. A., Conrads T. P. (2009). Stress hormones mediate drug resistance to paclitaxel in human breast cancer cells through a CDK-1-dependent pathway. Psychoneuroendocrinology. 34, 1533–1541. 10.1016/j.psyneuen.2009.05.008 [DOI] [PubMed] [Google Scholar]
- GDC Reference Files | NCI Genomic Data Commons .
- Geeleher P., Cox N. J., Huang R. S. (2014). Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome Biol. 15, R47. 10.1186/gb-2014-15-3-r47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann M., Schmidt M., Brase J. C., Roos P., Hengstler J. G. (2008). Prediction of paclitaxel resistance in breast cancer: is CYP1B1*3 a new factor of influence? Pharmacogenomics. 9, 969–974. 10.2217/14622416.9.7.969 [DOI] [PubMed] [Google Scholar]
- Genomic Data Harmonization | NCI Genomic Data Commons .
- Golubnitschaja O., Debald M., Yeghiazaryan K., Kuhn W., Pešta M., Costigliola V., et al. (2016). Breast cancer epidemic in the early twenty-first century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumor Biol. 37, 12941–12957. 10.1007/s13277-016-5168-x [DOI] [PubMed] [Google Scholar]
- Harper A. R., Topol E. J. (2012). Pharmacogenomics in clinical practice and drug development. Nat. Biotechnol. 30, 1117–1124. 10.1038/nbt.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J., Peruzzi P. P., Lawler S. (2014). MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol. Med. 20, 460–469 10.1016/j.molmed.2014.06.005 [DOI] [PubMed] [Google Scholar]
- He D. X., Gu F., Gao F., Hao J. J., Gong D., Gu X. T., et al. (2016). Genome-wide profiles of methylation, microRNAs, and gene expression in chemoresistant breast cancer. Sci. Rep. 6, 24706. 10.1038/srep24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., et al. (2014). Drug resistance in cancer: an overview. Cancers (Basel). 6, 1769–1792. 10.3390/cancers6031769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Shen A., Ding J., Geng M. (2014). Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol. Sci. 35, 41–50. 10.1016/j.tips.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Ignatov T., Eggemann H., Costa S. D., Roessner A., Kalinski T., Ignatov A. (2014). BRCA1 promoter methylation is a marker of better response to platinum-taxane-based therapy in sporadic epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 140, 1457–1463. 10.1007/s00432-014-1704-5 [DOI] [PubMed] [Google Scholar]
- Irizarry R. A., Ladd-Acosta C., Wen B., Wu Z., Montano C., Onyango P., et al. (2009). The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 41, 178–186. 10.1038/ng.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. A., Ferretti V., Grossman R. L., Staudt L. M. (2017). The NCI genomic data commons as an engine for precision medicine. Blood. 130, 453–459. 10.1182/blood-2017-03-735654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadra G., Finetti P., Toiron Y., Viens P., Birnbaum D., Borg J.-P., et al. (2012). Gene expression profiling of breast tumor cell lines to predict for therapeutic response to microtubule-stabilizing agents. Breast Cancer Res. Treat. 132, 1035–1047. 10.1007/s10549-011-1687-8 [DOI] [PubMed] [Google Scholar]
- Ke G., Meng Q., Wang T., Chen W., Ma W., Liu T.-Y., et al. (2017). LightGBM: a highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 30, 3147–3155. [Google Scholar]
- Kim S., Sundaresan V., Zhou L., Kahveci T. (2016). Integrating domain specific knowledge and network analysis to predict drug sensitivity of cancer cell lines. PLoS One 11, e0162173. 10.1371/journal.pone.0162173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohavi R. (1995). A study of cross-validation and bootstrap for accuracy estimation and model selection. Proc. 14th Int. Jt. Conf. Artif. Intell. 2, 1137–1143. 10.1067/mod.2000.109031 [DOI] [Google Scholar]
- Levenson V. V. (2010). DNA methylation as a universal biomarker. Expert Rev. Mol. Diagn. 10, 481–488. 10.1586/erm.10.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Melnikov A. A., Levenson V., Guerra E., Simeone P., Alberti S., et al. (2015). A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer. 15, 417. 10.1186/s12885-015-1412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht M. W., Noble W. S. (2015). Machine learning applications in genetics and genomics. Nat. Rev. Genet. 16, 321–332. 10.1038/nrg3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Xie Z., Peng Q. (2017). MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am. J. Cancer Res. 7, 1863–1873. [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Ding Z., Qian Y., Shi X., Castranova V., Harner E. J., et al. (2006). Predicting cancer drug response by proteomic profiling. Clin. Cancer Res. 12, 4583–4589. 10.1158/1078-0432.CCR-06-0290 [DOI] [PubMed] [Google Scholar]
- Mandrekar S. J., Sargent D. J. (2009). Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J. Clin. Oncol. 27, 4027–4034. 10.1200/JCO.2009.22.3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjegowda M. C., Gupta P. S., Limaye A. M. (2017). Hyper-methylation of the upstream CpG island shore is a likely mechanism of GPER1 silencing in breast cancer cells. Gene. 614, 65–73. 10.1016/j.gene.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Marsh S., Somlo G., Li X., Frankel P., King C. R., Shannon W. D., et al. (2007). Pharmacogenetic analysis of paclitaxel transport and metabolism genes in breast cancer. Pharmacogenomics J. 7, 362–365. 10.1038/sj.tpj.6500434 [DOI] [PubMed] [Google Scholar]
- Matthews B. W. (1975). Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim. Biophys. Acta. Protein Struct. 405, 442–451. 10.1016/0005-2795(75)90109-9 [DOI] [PubMed] [Google Scholar]
- Mikeska T., Craig J. M. (2014). DNA methylation biomarkers: cancer and beyond. Genes (Basel). 5, 821–864. 10.3390/genes5030821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S., Briasoulis E., Linardou H., Bafaloukos D., Papadimitriou C. (2012). Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 38, 890–903. 10.1016/j.ctrv.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Naulaerts S., Dang C., Ballester P. J. (2017). Precision and recall oncology: combining multiple gene mutations for improved identification of drug-sensitive tumours. Oncotarget 8, 97025–97040. 10.18632/oncotarget.20923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Dang C. C., Ballester P. J. (2017). Systematic assessment of multi-gene predictors of pan-cancer cell line sensitivity to drugs exploiting gene expression data. Research 5, 2927. 10.12688/f1000research.10529.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Naulaerts S., Bomane A., Bruna A., Ghislat G., Ballester P. (2018). Machine learning models to predict in vivo drug response via optimal dimensionality reduction of tumour molecular profiles. bioRxiv 277772, 1–34. 10.1101/277772 [DOI] [Google Scholar]
- Norimura S., Kontani K., Kubo T., Hashimoto S.-I., Murazawa C., Kenzaki K., et al. (2018). Candidate biomarkers predictive of anthracycline and taxane efficacy against breast cancer. J. Cancer Res. Ther. 14, 409–415. 10.4103/jcrt.JCRT_1053_16 [DOI] [PubMed] [Google Scholar]
- Peck R. W. (2016). The right dose for every patient: a key step for precision medicine. Nat. Rev. Drug Discovery. 15, 145–146. 10.1038/nrd.2015.22 [DOI] [PubMed] [Google Scholar]
- Peng Y., Croce C. M. (2016). The role of microRNAs in human cancer. Signal Transduct. Target. Ther. 1, 15004. 10.1038/sigtrans.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E. A. (1998). Paclitaxel in Breast Cancer. Oncologist 3, 373–389. [PubMed] [Google Scholar]
- Poliseno L., Marranci A., Pandolfi P. P. (2015). Pseudogenes in human cancer. Front. Med. 2, 68. 10.3389/fmed.2015.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M. C., Labrie Y., Diorio C., Durocher F. (2015). The role of methylation in breast cancer susceptibility and treatment. Anticancer Res. 35, 4569–4574. 10.1007/s13566-015-0216-5 [DOI] [PubMed] [Google Scholar]
- Prahallad A., Sun C., Huang S., Di Nicolantonio F., Salazar R., Zecchin D., et al. (2012). Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103. 10.1038/nature10868 [DOI] [PubMed] [Google Scholar]
- Ranstam J., Cook J. A., Collins G. S. (2016). Clinical prediction models. Br. J. Surg. 103, 1886. 10.1002/bjs.10242 [DOI] [PubMed] [Google Scholar]
- Release Notes – GDC Docs Available at: https://docs.gdc.cancer.gov/Data/Release_Notes/Data_Release_Notes/ [Accessed March 11, 2019].
- Ribeiro J. T., Macedo L. T., Curigliano G., Fumagalli L., Locatelli M., Dalton M., et al. (2012). Cytotoxic drugs for patients with breast cancer in the era of targeted treatment: back to the future? Ann. Oncol. 23, 547–555. 10.1093/annonc/mdr382 [DOI] [PubMed] [Google Scholar]
- Van Rijsbergen C. J. (1979) Information Retrieval. Butterworths, London, UK. 10.1016/j.pestbp.2006.07.008 [DOI] [Google Scholar]
- Rodríguez-Antona C., Taron M. (2015). Pharmacogenomic biomarkers for personalized cancer treatment. J. Int. Med. 277, 201–217. 10.1111/joim.12321 [DOI] [PubMed] [Google Scholar]
- Russnes H. G., Navin N., Hicks J., Borresen-Dale A. L. (2011). Insight into the heterogeneity of breast cancer through next-generation sequencing. J. Clin. Invest. 121, 3810–3818. 10.1172/JCI57088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg L., Kim E. S., Liu D., Schrag D. (2017). Precision oncology: who, how, what, when, and when not? Am. Soc. Clin. Oncol. Educ. B. 37, 160–169. 10.14694/EDBK_174176 [DOI] [PubMed] [Google Scholar]
- Stirzaker C., Song J. Z., Ng W., Du Q., Armstrong N. J., Locke W. J., et al. (2017). Methyl-CpG-binding protein MBD2 plays a key role in maintenance and spread of DNA methylation at CpG islands and shores in cancer. Oncogene. 36, 1328–1338. 10.1038/onc.2016.297 [DOI] [PubMed] [Google Scholar]
- Stirzaker C., Taberlay P. C., Statham A. L., Clark S. J. (2014). Mining cancer methylomes: prospects and challenges. Trends Genet. 30, 75–84. 10.1016/j.tig.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Tan A. C., Gilbert D. (2003). An empirical comparison of supervised machine learning techniques in bioinformatics. Proc. First Asia-Pacific Bioinforma. Conf. Bioinforma. 19, 219–222. [Google Scholar]
- Therasse P., Arbuck S. G., Eisenhauer E. A., Wanders J., Kaplan R. S., Rubinstein L., et al. (2000). New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 92, 205–216. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- Tripathi S., Belkacemi L., Cheung M. S., Bose R. N. (2016). Correlation between gene variants, signaling pathways, and efficacy of chemotherapy drugs against colon cancers. Cancer Inform. 15, 1–13. 10.4137/CIN.S34506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S., Simon R. (2006). Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics 7, 91. 10.1186/1471-2105-7-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., McLeod H. L., Weinshilboum R. M. (2011). Genomics and drug response. N. Engl. J. Med. 364, 1144–1153. 10.1056/NEJMra1010600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Zhang H., Yao Q., Wang Y., Dai S., Yang X. (2012). TGFBI promoter hypermethylation correlating with paclitaxel chemoresistance in ovarian cancer. J. Exp. Clin. Cancer Res. 31, 6. 10.1186/1756-9966-31-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Collisson E. A., Mills G. B., Shaw K. M., Ozenberger B. A., Ellrott K., et al. (2013). The cancer genome atlas pan-cancer analysis project. Nat. Genet. 45, 1113. 10.1038/ng.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T. X., Yuan Y., Li L. L., Wang Z. H., Dan L. Y., Chen Y., et al. (2013). Aberrant promoter CpG methylation and its translational applications in breast cancer. Chin. J. Cancer. 32, 12–20. 10.5732/cjc.011.10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao-Jie L., Ai-Mei G., Li-Juan J., Jiang X. (2015). Pseudogene in cancer: Real functions and promising signature. J. Med. Genet. 52, 17–24. 10.1136/jmedgenet-2014-102785 [DOI] [PubMed] [Google Scholar]
- Xu X., Gu H., Wang Y., Wang J., Qin P. (2019). Autoencoder based feature selection method for classification of anticancer drug response. Front. Genet. 10, 233. 10.3389/fgene.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun T., Liu Y., Gao D., Linghu E., Brock M. V., Yin D., et al. (2015). Methylation of CHFR sensitizes esophageal squamous cell cancer to docetaxel and paclitaxel. Genes Cancer. 6, 38–48. 10.18632/genesandcancer.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang J., Xu S., Zhang X., Wang P., Wu H., et al. (2018). Hypoxia-Induced TPM2 methylation is associated with chemoresistance and poor prognosis in breast cancer. Cell. Physiol. Biochem. 45, 692–705. 10.1159/000487162 [DOI] [PubMed] [Google Scholar]
- Zhou M., Liu Z., Zhao Y., Ding Y., Liu H., Xi Y., et al. (2010). MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J. Biol. Chem. 285, 21496–21507. 10.1074/jbc.M109.083337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the https://portal.gdc.cancer.gov/.