Abstract
Background
Generally, before being operated on, patients will be given informal information by the healthcare providers involved in the care of the patients (doctors, nurses, ward clerks, or healthcare assistants). This information can also be provided formally in different formats including written information, formal lectures, or audio‐visual recorded information.
Objectives
To compare the benefits and harms of formal preoperative patient education for patients undergoing laparoscopic cholecystectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2013), MEDLINE, EMBASE, and Science Citation Index Expanded to March 2013.
Selection criteria
We included only randomised clinical trials irrespective of language and publication status.
Data collection and analysis
Two review authors independently extracted the data. We planned to calculate the risk ratio with 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) with 95% CI for continuous outcomes based on intention‐to‐treat analyses when data were available.
Main results
A total of 431 participants undergoing elective laparoscopic cholecystectomy were randomised to formal patient education (215 participants) versus standard care (216 participants) in four trials. The patient education included verbal education, multimedia DVD programme, computer‐based multimedia programme, and PowerPoint presentation in the four trials. All the trials were of high risk of bias. One trial including 212 patients reported mortality. There was no mortality in either group in this trial. None of the trials reported surgery‐related morbidity, quality of life, proportion of patients discharged as day‐procedure laparoscopic cholecystectomy, the length of hospital stay, return to work, or the number of unplanned visits to the doctor. There were insufficient details to calculate the mean difference and 95% CI for the difference in pain scores at 9 to 24 hours (1 trial; 93 patients); and we did not identify clear evidence of an effect on patient knowledge (3 trials; 338 participants; SMD 0.19; 95% CI ‐0.02 to 0.41; very low quality evidence), patient satisfaction (2 trials; 305 patients; SMD 0.48; 95% CI ‐0.42 to 1.37; very low quality evidence), or patient anxiety (1 trial; 76 participants; SMD ‐0.37; 95% CI ‐0.82 to 0.09; very low quality evidence) between the two groups.
A total of 173 participants undergoing elective laparoscopic cholecystectomy were randomised to electronic consent with repeat‐back (patients repeating back the information provided) (92 participants) versus electronic consent without repeat‐back (81 participants) in one trial of high risk of bias. The only outcome reported in this trial was patient knowledge. The effect on patient knowledge between the patient education with repeat‐back versus patient education without repeat‐back groups was imprecise and based on 1 trial of 173 participants; SMD 0.07; 95% CI ‐0.22 to 0.37; very low quality evidence).
Authors' conclusions
Due to the very low quality of the current evidence, the effects of formal patient education provided in addition to the standard information provided by doctors to patients compared with standard care remain uncertain. Further well‐designed randomised clinical trials of low risk of bias are necessary.
Plain language summary
Formal education of patients about to undergo laparoscopic cholecystectomy
Background
The liver produces bile, which has many functions including elimination of waste processed by the liver and digestion of fat. The bile is temporarily stored in the gallbladder (an organ situated underneath the liver in the abdomen (belly) before it reaches the small bowel. Concretions in the gallbladder are called gallstones. Gallstones are present in about 5% to 25% of the adult western population. Between 2% and 4% become symptomatic in one year. The symptoms include pain related to the gallbladder (biliary colic), inflammation of the gallbladder (cholecystitis), obstruction to the flow of bile from the liver and gallbladder into the small bowel resulting in jaundice (yellowish discolouration of the body usually most prominently noticed in the white of the eye, which turns yellow), bile infection (cholangitis), and inflammation of the pancreas, an organ that secretes digestive juices and harbours the insulin‐secreting cells that maintain blood sugar level (pancreatitis). Removal of the gallbladder (cholecystectomy) is currently considered the best treatment option for patients with symptomatic gallstones. This is generally performed by key‐hole surgery (laparoscopic cholecystectomy). Generally, before being operated on, patients will be given informal information by the healthcare providers involved in the care of the patients (doctors, nurses, ward clerks, or healthcare assistants). This information is likely to include some information on the type of anaesthesia, expected duration of surgery, expected outcome of surgery including the complications, duration of hospital stay, wound dressing care (if applicable), return to normal activity, and return to work. This information can also be provided formally in different formats including written information, formal lectures, video, or computer presentations. The review authors set out to determine whether it is preferable to provide formal information to the patients before the operation.
Study characteristics
We searched the medical literature in order to identify studies that provided information on the above question. The authors obtained information from randomised trials only since such types of trials provide the best information if conducted well. Two review authors independently identified the trials and collected the information. The information is current to March 2013.
Key results
We found four trials including 431 patients undergoing elective laparoscopic cholecystectomy who received either formal patient education (215 participants) or standard care (216 participants). The choice of whether the patient received formal patient education or standard care was determined by a method similar to the toss of a coin in order to create comparable groups of patients. The patient education included providing information by just talking to the patient but in a more formal way or by using various method of presentation. All the trials were of high risk of bias (faults in study design that can result in erroneous conclusions). Only one trial including 212 participants reported deaths after surgery. There were no deaths in either group in this trial. There was no clear evidence of an effect on pain scores at 9 to 24 hours, patient knowledge, patient satisfaction, or patient anxiety associated with education. None of the trials reported surgical complications, quality of life, percentage of patients discharged as day‐procedure laparoscopic cholecystectomy, length of hospital stay, return to work, or the number of unplanned visits to the doctor.
A total of 173 participants undergoing elective laparoscopic cholecystectomy underwent patient education with repeat‐back (patients repeating back the information provided) (92 participants) or patient education without repeat‐back (81 participants) in one trial of high risk of bias. The only outcome reported in this trial was patient knowledge. The results we found for the effect onpatient knowledge between the patient education with repeat‐back and patient education without repeat‐back groups were uncertain and we could not exclude possible benefits of either education or control.
Due to the very low quality of the current evidence, we are uncertain as to whether formal patient education provided in addition to the standard information provided by doctors has any benefit to patients. Further well‐designed randomised clinical trials are necessary.
Quality of evidence
The overall quality of the evidence was very low.
Summary of findings
Summary of findings for the main comparison. Patient education compared with no patient education for patients undergoing laparoscopic cholecystectomy.
| Patient education compared with no patient education for patients undergoing laparoscopic cholecystectomy | |||
| Patient or population: patients undergoing laparoscopic cholecystectomy. Settings: secondary or tertiary hospital. Intervention: patient education. Comparison: no patient education. | |||
| Outcomes | Effect estimate | No of participants (studies) | Quality of the evidence (GRADE) |
| Patient knowledge | The mean patient knowledge in the intervention groups was 0.19 standard deviations higher (0.02 lower to 0.41 higher) | 338 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Patient satisfaction | The mean patient satisfaction in the intervention groups was 0.48 standard deviations higher (0.42 lower to 1.37 higher) | 305 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Patient anxiety | The mean patient anxiety in the intervention groups was 0.37 standard deviations lower (0.82 lower to 0.09 higher) | 76 (1 study) | ⊕⊝⊝⊝ very low1,3 |
| None of the trials reported surgery‐related morbidity, quality of life, proportion of people discharged as day‐procedure laparoscopic cholecystectomy, length of hospital stay, return to work, or the number of unplanned visits to the doctor. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 The trial(s) was (were) of high risk of bias. 2 There was severe heterogeneity as noted by the I2 statistic and the lack of overlap of confidence intervals. 3 The confidence intervals overlapped 0 and minimal clinically important difference. The total number of patients in the intervention and control group was fewer than 400.
Summary of findings 2. Patient education with repeat‐back compared with patient education without repeat‐back for patients undergoing laparoscopic cholecystectomy.
| Patient education with repeat‐back compared with patient education without repeat‐back for patients undergoing laparoscopic cholecystectomy | |||
| Patient or population: patients undergoing laparoscopic cholecystectomy. Settings: secondary or tertiary hospital. Intervention: patient education with repeat‐back. Comparison: patient education without repeat‐back. | |||
| Outcomes | Effect estimate | No of participants (studies) | Quality of the evidence (GRADE) |
| Patient knowledge | The mean patient knowledge in the intervention groups was 0.07 standard deviations higher (0.22 lower to 0.37 higher) | 173 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| This trial did not report surgery‐related morbidity, quality of life, proportion of people discharged as day‐procedure laparoscopic cholecystectomy, length of hospital stay, visual analogue pain scores, requirement for opiate analgesia, return to work, patient satisfaction, patient anxiety, or the number of unplanned visits to the doctor. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 The trial was of high risk of bias. 2 The confidence intervals overlapped 0 and minimal clinically important difference. The total number of patients in the intervention and control group was fewer than 400.
Background
Description of the condition
About 5% to 25% of the adult western population have gallstones (GREPCO 1984; GREPCO 1988; Bates 1992; Halldestam 2004). The annual incidence of gallstones is about 1 in 200 people (NIH 1992). Only 2% to 4% of people with gallstones become symptomatic with biliary colic (pain), acute cholecystitis (inflammation), obstructive jaundice, or gallstone pancreatitis in one year (Attili 1995; Halldestam 2004). Cholecystectomy (removal of gallbladder) is the preferred option in the treatment of symptomatic gallstones (Strasberg 1993). Every year, 1.5 million cholecystectomies are performed in the US and 60,000 in the UK (Dolan 2009; HES 2011). Approximately 80% of the cholecystectomies are performed laparoscopically (by key‐hole surgery) (Ballal 2009).
Description of the intervention
Generally, before being operated on, patients will be given informal information by the healthcare providers involved in the care of the patients (doctors, nurses, ward clerks, or healthcare assistants). This information is likely to include some information on the type of anaesthesia, expected duration of surgery, expected outcome of surgery including the complications, duration of hospital stay, wound dressing care (if applicable), return to normal activity, and return to work. This information can also be provided formally in different formats including written information, formal lectures, or audio‐visual recorded information (McDonald 2004).
How the intervention might work
By providing correct information to the patients in a formal way, the patients may know what to expect from the operation. This may decrease their anxiety (Stergiopoulou 2007), and improve their satisfaction. Preoperative education may also decrease postoperative pain (Cassady 1999; Stergiopoulou 2007). In addition, patients may be able to deal with minor problems on their own without requiring hospital visits.
Why it is important to do this review
Day‐patient laparoscopic cholecystectomy is safe and effective (Gurusamy 2008a; Gurusamy 2008b). Postoperative pain is one of the main reasons for failure of hospital discharge (Gurusamy 2008a; Gurusamy 2008b). There has been no systematic review on preoperative patient education for patients undergoing laparoscopic cholecystectomy in terms of reduction of pain and anxiety thereby improving the proportion of patients undergoing successful day‐patient laparoscopic cholecystectomy.
Objectives
To compare the benefits and harms of formal preoperative patient education for patients undergoing laparoscopic cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of blinding, language, publication status, or sample size. We planned to include quasi‐randomised studies (eg, allocation by date of birth, day of the week, etc) and observational studies for assessment of treatment‐related harms only.
Types of participants
Patients about to undergo laparoscopic cholecystectomy irrespective of whether the procedure was carried out in secondary care setting or tertiary care setting.
Types of interventions
Formal patient education (presented in any format including videos, interactive videos, audios, leaflets, formal face‐to‐face lectures; may or may not be web‐based) compared with no formal patient education.
Comparison of different methods of formal patient education.
We accepted any intervention continued postoperatively as long as the intervention was started preoperatively. Patient education to aid decision making was not included as this has been covered in another Cochrane review (O'Connor 2009).
Co‐interventions were allowed if used equally in both groups.
Types of outcome measures
Primary outcomes
Surgery‐related mortality and morbidity. We used the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ‐ Good Clinical Practice (ICH‐GCP) definition of serious adverse events (ICH‐GCP 1997). Serious adverse events are defined as any event that would increase mortality; is life threatening; requires hospitalisation; results in a persistent or significant disability; or any important medical event that might have jeopardised the patient or requires intervention to prevent it (ICH‐GCP 1997).
Quality of life (overall health‐related quality of life measured by a validated quality of life measurement tool such as EuroQol‐5 dimension (EQ‐5D) or Short Form‐36 (SF36)).
Secondary outcomes
-
Hospital stay.
Proportion discharged as day‐procedure laparoscopic cholecystectomy.
Length of hospital stay.
-
Pain.
Visual analogue scores (4 to 8 hours and 9 to 24 hours).
Requirement of opiate analgesia.
Return to work.
Patient knowledge.
Patient satisfaction/anxiety.
Number of unplanned visits to the doctor.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) to March 2013. We have given the search strategies with the time spans of the searches in Appendix 1.
Searching other resources
We searched the references of identified trials to identify further relevant trials. We also searched the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/mrct/). The meta‐register includes ISRCTN Register and NIH ClinicalTrials.gov Register among other registers.
Data collection and analysis
We performed the systematic review following the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2013).
Selection of studies
Two review authors (KG and JV) independently identified the trials for inclusion. We have listed the excluded studies with the reasons for the exclusion in the Characteristics of excluded studies table. We resolved any differences through discussion.
Data extraction and management
Both review authors independently extracted the following data.
Year and language of publication.
Country.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Sample size.
Details of the preoperative education.
Outcomes (described in Primary outcomes and Secondary outcomes).
Risk of bias (described in Risk of bias in included studies).
We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials share the same patients ‐ completely or partially (by identifying common authors and centres) ‐ we planned to contact the authors of the trials to clarify whether the trial report has been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2013). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012; Savovic 2012a), the risk of bias of the trials were assessed based on the following bias risk domains:
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (eg, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
It is impossible to blind the participants.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were pre‐defined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: it is unclear whether all pre‐defined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For‐profit bias
Low risk of bias: the trial appears to be free of industry sponsorship or other type of for‐profit support (such as DVD or provision of recording facilities) that may manipulate the trial design, conductance, or results of the trial.
Uncertain risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by the industry or has received other type of for‐profit support.
We considered trials to have a low risk of bias if we assessed all the above domains as being at low risk of bias. In all other cases, the trials were considered to have a high risk of bias. Since it is impossible to blind the participants, we anticipated that all the trials be at high risk of bias.
Measures of treatment effect
For dichotomous variables, we planned to calculate the risk ratio (RR) with 95% confidence interval (CI). RR calculations do not include trials in which no events occurred in either group, whereas risk difference calculations do. We planned to report the risk difference if the conclusions using this association measure were different from RR. For continuous variables, we planned to calculate the mean difference (MD) with 95% CI for outcomes such as hospital stay and standardised mean difference (SMD) with 95% CI for quality of life (where different scales might be used).
Unit of analysis issues
The unit of analysis was the aggregate data on patients about to undergo laparoscopic cholecystectomy according to randomised group.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). We planned to impute data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, best‐case scenario, and worst‐case scenario (Gurusamy 2009; Gluud 2013).
For continuous outcomes, we used available patient analysis. We imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and we used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we imputed the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation would decrease the weight of the study for calculation of MDs and bias the effect estimate to no effect in case of SMDs (Higgins 2011).
For calculation of SMDs of outcomes such as patient satisfaction and patient knowledge, some trials reported the proportion of patients with adequate satisfaction (ie, as binary outcomes) and others reported the patient satisfaction scores (ie, as continuous outcomes). We calculated the natural logarithm of the odds ratio for the trials that reported the outcomes as binary outcomes and converted them into SMDs as described in Chapter 9.4.6 of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). We then combined the SMDs thus calculated with the continuous outcomes using the generic inverse variance method.
Assessment of heterogeneity
We explored heterogeneity using the Chi2 test with significance set at P value 0.10, and measured the quantity of heterogeneity using the I2 statistic (Higgins 2002). We planned to use overlapping of CIs on the forest plot to determine heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in case of at least 10 trials included (Egger 1997; Macaskill 2001). We planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry.
Data synthesis
We performed the meta‐analyses using the software package Review Manager 5 (RevMan 2012), and following the recommendations of The Cochrane Collaboration (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2013). We used both random‐effects model (DerSimonian 1986), and fixed‐effect model (DeMets 1987), meta‐analyses. In case of discrepancy between the two models, we have reported both results; otherwise, we have reported the results of the fixed‐effect model. We used the generic inverse variance method to combine the SMD when outcomes such as patient satisfaction were reported as mean differences rather than the scores in individual groups in the trial reports.
Trial sequential analysis
The underlying assumption of trial sequential analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We will add the trials according to the year of publication, and if more than one trial was published in one year, the trials will be added alphabetically according to the last name of the first author. On the basis of the required information size, trial sequential monitoring boundaries will be constructed. These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. In contrast, if the boundaries are not surpassed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010).
We planned to apply trial sequential analysis (CTU 2011; Thorlund 2011) using a required sample size calculated from an alpha error of 0.05, a beta error of 0.20, a control event proportion obtained from the results, and a relative risk reduction of 20% for binary outcomes if there were two or more trials reporting the outcome to determine whether more trials are necessary on this topic (if the trial sequential alpha‐spending monitoring boundary or the futility zone is crossed, then more trials may be unnecessary) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). Since trial sequential analysis cannot be performed for SMDs or rate ratios, we did not plan to perform the trial sequential analysis for quality of life, patient knowledge, patient satisfaction, or number of hospital visits. For pain, we planned to calculate the required sample size from an alpha error of 0.05, a beta error of 0.20, the variance estimated from the meta‐analysis results of low risk of bias trials, and an MD of 1 cm on the visual analogue scale (Todd 1996). For length of hospital stay and return to work, we planned to calculate the required sample size using an MD of one day with the remaining parameters kept the same as that for pain.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
Trials with low risk of bias compared to trials with high risk of bias.
Different types of preoperative education.
We planned to use the Chi2 test for subgroup differences to identify subgroup differences and planned to consider a P value < 0.05 as statistically significant.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, best‐case scenario, and worst‐case scenario (Gurusamy 2009; Gluud 2013). We performed a sensitivity analysis by excluding the trials in which either the mean or the standard deviation or both were imputed.
Summary of findings table
We have summarised the evidence in the Table 1 and Table 2 tables using GRADEpro (ims.cochrane.org/revman/other‐resources/gradepro).
Results
Description of studies
Results of the search
We identified 2425 references through electronic searches of CENTRAL (n = 424), MEDLINE (n = 977), EMBASE (n =365), and Science Citation Index Expanded (n = 659). We excluded 949 duplicates and 1468 clearly irrelevant references through reading abstracts. One reference was identified through scanning the reference list of the identified randomised trials. No reference was identified from mRCT. In total, we retrieved nine references for further assessment. We excluded two studies (three references) for the reasons stated in the Characteristics of excluded studies table. In total, six references of five trials met the inclusion criteria and provided data for this review (Blay 2005; Bollschweiler 2008; Wilhelm 2009; Fink 2010; Clark 2011). We have shown the reference flow in Figure 1. We have shown the details about the sample size, patient characteristics; the inclusion and exclusion criteria used in the trial; and the risk of bias of the included trials in the Characteristics of included studies table.
1.
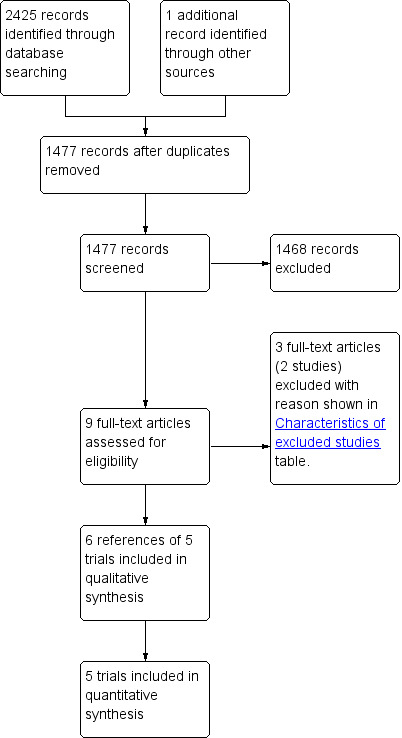
Study flow diagram.
Included studies
Formal patient education versus standard care
Participants
A total of 517 participants were randomised in four trials to formal patient education or standard care (Blay 2005; Bollschweiler 2008; Wilhelm 2009; Clark 2011). After excluding the post‐randomisation drop‐outs, 431 participants undergoing elective laparoscopic cholecystectomy were randomised to formal patient education (215 participants) or standard care (216 participants) in the four trials (Blay 2005; Bollschweiler 2008; Wilhelm 2009; Clark 2011). The mean age of the participants was 39 to 55 years (Blay 2005; Bollschweiler 2008; Wilhelm 2009; Clark 2011). The proportion of females was 62% (Bollschweiler 2008), 70% (Wilhelm 2009), and 84% (Blay 2005), in the three trials that provided this information.
Intervention
Patient education included verbal education during pre‐admission clinic (Blay 2005), multimedia DVD programme (Wilhelm 2009), computer‐based multimedia programme (Bollschweiler 2008), and PowerPoint presentation (Clark 2011).
Different methods of formal patient education
A total of 173 participants undergoing elective laparoscopic cholecystectomy were randomised to electronic consent with repeat‐back (patients repeating back the information provided) (92 participants) versus electronic consent without repeat‐back (81 participants) in one trial (Fink 2010). Although the intervention and control could be considered as decision‐making aids, both the groups received patient education and the patient knowledge after the intervention and control were reported in this trial and so this trial was included in this review (Fink 2010). This trial included other surgeries apart from elective laparoscopic cholecystectomy, which were excluded from this review. The mean age and the proportion of females were not available for patients who underwent elective laparoscopic cholecystectomy in this trial (Fink 2010).
Risk of bias in included studies
All the trials were of high risk of bias. The risks of bias in the individual domains are summarised in Figure 2 and Figure 3. All four trials were at low risk of bias in the allocation sequence generation domain (Blay 2005; Bollschweiler 2008; Wilhelm 2009; Fink 2010). Two trials were at low risk of bias in the allocation concealment domain (Bollschweiler 2008; Fink 2010). None of the trials were at low risk of bias in the blinding of participants and personnel, missing outcome data, and selective reporting domains. One trial was at low risk of bias in the blinding of outcome assessors domain (Wilhelm 2009). Two trials were at low risk of bias in the for‐profit bias domain (Wilhelm 2009; Fink 2010).
2.
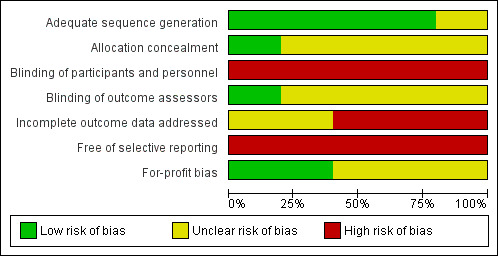
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
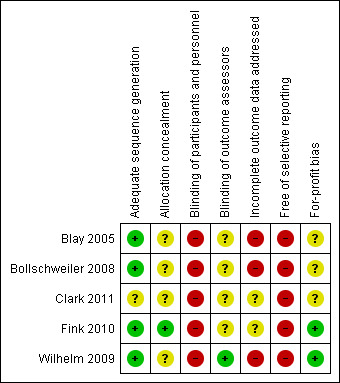
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
The results are summarised in Table 1 and Table 2.
Formal patient education versus standard care
The outcomes reported in the trials were mortality, pain, patient knowledge, and patient satisfaction/anxiety. None of the trials reported surgery‐related morbidity, quality of life, proportion of people discharged as day‐procedure laparoscopic cholecystectomy, length of hospital stay, return to work, or the number of unplanned visits to the doctor. We did not find non‐randomised studies that reported preoperative education‐related harms (we are not aware of any specific harms to the patient related to preoperative education).
Mortality
One trial reported mortality. There was no mortality in either group in 212 patients included in this trial (Wilhelm 2009).
Pain
None of the trials reported pain scores at four to eight hours. One trial reported the pain scores at 9 to 24 hours (Blay 2005). The pain scores were 5.05 cm in the intervention group versus 6.66 cm in the control group. There was insufficient information to calculate the standard deviation in each group. However, the authors mentioned that there was no significant difference in pain scores between the two groups. None of the trials reported requirement of opiate analgesia.
Patient knowledge
Three trials reported patient knowledge (Bollschweiler 2008; Wilhelm 2009; Clark 2011). There was no significant difference in patient knowledge between the two groups (SMD 0.19; 95% CI ‐0.02 to 0.41). Excluding the Wilhelm 2009 and Clark 2011 trials in which standard deviation was imputed did not change the results.
Patient satisfaction/anxiety
Two trials reported patient satisfaction (Blay 2005; Wilhelm 2009). Blay 2005 reported this as binary outcome while Wilhelm 2009 reported this as continuous outcome. These were combined as mentioned in the Dealing with missing data section. The raw data used in the calculation of the information required for generic inverse method of analysis is shown in Analysis 1.4 and Analysis 1.5. Patient satisfaction was significantly better in the patient education group than no patient education group using the fixed‐effect model (SMD 0.34; 95% CI 0.04 to 0.65) (Analysis 1.2). There was no significant difference between the groups using the random‐effects model (SMD 0.48; 95% CI ‐0.42 to 1.37). Excluding the Wilhelm 2009 trial in which standard deviation was imputed resulted in results similar to the fixed‐effect model. One trial reported patient anxiety (Bollschweiler 2008). There was no significant difference in patient anxiety between the two groups (SMD ‐0.37; 95% CI ‐0.82 to 0.09) (Analysis 1.3). The issue of fixed‐effect model versus random‐effects model did not arise because of the presence of only one trial for this comparison. This trial reported the mean and standard deviation (Bollschweiler 2008). Therefore, we did not perform a sensitivity analysis excluding trials in which either mean or standard deviation or both were imputed.
1.4. Analysis.
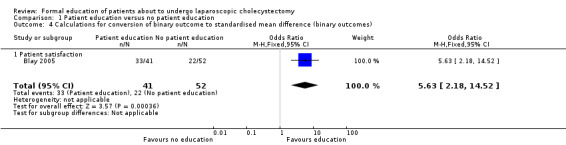
Comparison 1 Patient education versus no patient education, Outcome 4 Calculations for conversion of binary outcome to standardised mean difference (binary outcomes).
1.5. Analysis.
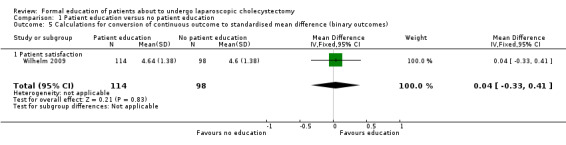
Comparison 1 Patient education versus no patient education, Outcome 5 Calculations for conversion of continuous outcome to standardised mean difference (binary outcomes).
1.2. Analysis.
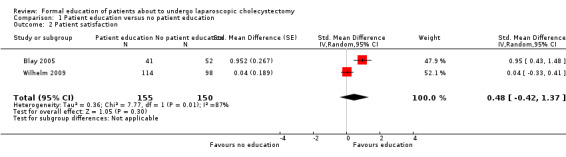
Comparison 1 Patient education versus no patient education, Outcome 2 Patient satisfaction.
1.3. Analysis.
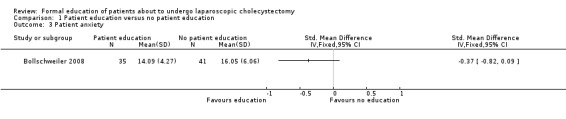
Comparison 1 Patient education versus no patient education, Outcome 3 Patient anxiety.
Subgroup analyses
We did not perform the planned subgroup analysis because of the few trials included in this review.
Trial sequential analysis
We did not perform trial sequential analysis because only one trial reported mortality (no mortality in either group) and pain. None of the trials reported morbidity, hospital stay, or return to work.
Reporting bias
We did not produce a funnel plot because of the inclusion of fewer than 10 trials in this review.
Different methods of patient education
The only outcome reported in the trial that compared different methods of patient education was patient knowledge (Fink 2010). There was no significant difference in patient knowledge between the patient education with repeat‐back and patient education without repeat‐back groups (SMD 0.07; 95% CI ‐0.22 to 0.37) (Analysis 2.1). The issue of fixed‐effect model versus random‐effects model did not arise because of the presence of only one trial for this comparison. This trial reported the mean and standard deviation (Fink 2010). Therefore, we did not perform the sensitivity analysis excluding trials in which either mean or standard deviation or both were imputed.
2.1. Analysis.
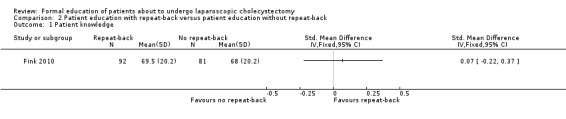
Comparison 2 Patient education with repeat‐back versus patient education without repeat‐back, Outcome 1 Patient knowledge.
Subgroup analyses
We did not perform the planned subgroup analysis because of the presence of only one trial for this comparison.
Trial sequential analysis
We did not perform trial sequential analysis because this trial did not report mortality, morbidity, hospital stay, pain, or return to work.
Reporting bias
We did not produce a funnel plot because of the inclusion of only one trial for this comparison.
Discussion
Summary of main results
This review has shown that there is currently no evidence to support or refute formal preoperative patient education for patients undergoing laparoscopic cholecystectomy. The four trials included in this review used different methods of patient education including verbal education during preadmission clinic (Blay 2005), multimedia DVD programme (Wilhelm 2009), computer‐based multimedia programme (Bollschweiler 2008), and PowerPoint presentation (Clark 2011), and compared these with no patient education. Only one trial reported mortality (Wilhelm 2009). None of the trials reported morbidity. By providing correct information to the patients, the patients may know what to expect from the operation. This may allow early reporting of potential complications and hence avoid the complications becoming severe or life threatening. However, the mortality and major morbidity of laparoscopic cholecystectomy is low (0.1% peri‐operative mortality and 0.3% bile duct injury) (Sinha 2013), and a sample size of approximately 725,000 patients is required to detect a 20% relative risk reduction in mortality and 240,000 patients to detect a 20% relative risk reduction in bile duct injury with an alpha error of 0.05 and beta error of 0.2. It is unlikely that trials can be conducted that measure differences in mortality or morbidity. Patient education involves significant use of resources to develop and maintain up‐to‐date information. While it is unlikely that any evidence of differences in mortality or morbidity are likely to be demonstrated in the foreseeable future, patient education may improve patient knowledge and satisfaction, and decrease anxiety, thereby improving the patient quality of life. However, patient quality of life was not reported in any of the trials. While patient satisfaction was better with patient education using the fixed‐effect model, there was no significant difference between the two groups using the random‐effects model. There were significant differences in the methods that patient education was delivered in the groups and this may have contributed to the differences in the effectiveness of patient education in the different trials. Patients' knowledge was assessed differently in different trials. The method of assessment of patient's knowledge could have been another source of heterogeneity. However, since different trials reported different outcomes, it was not possible to determine whether patient satisfaction was related to patient knowledge and whether a specific method of patient education was better than no education. Improved patient knowledge may avoid unnecessary hospital visits; however, none of the trials reported this outcome. Another potential benefit of patient education is to decrease pain and anxiety, and promote earlier discharge from hospital (including promotion of day‐surgery laparoscopic cholecystectomy), earlier return to normal activity, and earlier return to work. None of the trials reported hospital stay, return to normal activity, or return to work. Therefore, overall there is currently no evidence that patient education is of any benefit to patients.
One trial compared patient education with repeat‐back versus patient education without repeat‐back (Fink 2010). The only outcome reported in this trial was patient knowledge. There was no significant difference in patient knowledge between the two methods of patient education.
Overall completeness and applicability of evidence
All the patients included in this review underwent elective laparoscopic cholecystectomy. Therefore, this review is applicable only for patients undergoing elective laparoscopic cholecystectomy. It should be noted that the patients were provided with information by doctors in the no patient education group as well and the findings of this review are applicable only when doctors provide information to the patients. Furthermore, the doctors or individuals providing the information in the control group are likely to be well trained in the trials. Some trials state this explicitly (Bollschweiler 2008; Clark 2011), while this is implied in some other trials (Blay 2005; Wilhelm 2009), since the individuals provided information as a part of 'standard pre‐admission procedure' and the individuals providing such information would be familiar with the information to provide the patients. Such standard pre‐admission procedures are likely to involve providing important information about the procedure to the patients.
Quality of the evidence
The overall quality of the evidence was very low as shown in Table 1 and Table 2. While it is impossible to blind patients regarding whether they received additional patient education, it is possible to blind the assessors. Patient knowledge can also be tested in an objective manner so that the bias due to lack of blinding of patients is minimised.
Potential biases in the review process
We performed a thorough search of the literature. However, trials conducted and not reported in the pre‐trial registration era may have been missed. It is likely that such unreported trials (if any) did not identify any significant advantage of patient education and so it is unlikely to change the conclusions of this review.
We performed a meta‐analysis despite the heterogeneity in the way that patients' knowledge was measured. The alternative was to summarise the information in a table, which would be even more difficult to interpret.
Agreements and disagreements with other studies or reviews
This is the only systematic review on this topic. Our conclusions are different from the conclusions of the authors of some of the trials included in this review (Blay 2005; Bollschweiler 2008; Wilhelm 2009), and other studies (Stergiopoulou 2007), who have suggested that patient education is beneficial in these patients. We agree with the conclusion of the authors of Clark 2011 who suggested that the method that they used for patient education was not effective.
Authors' conclusions
Implications for practice.
Due to the very low quality of the evidence currently available, the effects of formal patient education provided in addition to the standard information provided by doctors to patients compared with standard care remain uncertain.
Implications for research.
Further well‐designed randomised clinical trials of low risk of bias are necessary. Such trials should include quality of life, length of hospital stay, return to normal activity, and return to work as outcomes and ought to be designed according to the SPIRIT recommendations (www.spirit‐statement.org/) (SPIRIT 2013; SPIRIT 2013a) and reported according to the CONSORT recommendations (www.consort‐statement.org).
Notes
J Vaughan joined the authors team in the beginning of the review preparation stage.
Acknowledgements
To The Cochrane Hepato‐Biliary Group.
Peer reviewers: Giel Konig, The Netherlands; Richard ten Broek, The Netherlands. Contact editor: Frederik Keus, The Netherlands.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health'.
Appendices
Appendix 1. Search strategies for identification of studies
| Database | Time span of the search | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 2, 2013. | #1 laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*
#2 cholecystectom*
#3 MeSH descriptor Cholecystectomy, Laparoscopic explode all trees
#4 (( #1 AND #2 ) OR #3)
#5 MeSH descriptor Patient Education as Topic explode all trees #6 MeSH descriptor Preoperative Care explode all trees #7 information OR instruct* OR educat* OR advice* OR support* OR preoperativ* OR pre‐operativ* OR pre operativ* #8 #5 OR #6 OR #7 #9 #4 AND #8 |
| MEDLINE (PubMed) | 1987 to March 2013. | (((laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND (cholecystectom*)) OR “cholecystectomy, laparoscopic”[MeSH]) AND ("Patient Education as Topic"[Mesh] OR "Preoperative Care"[Mesh] OR information or instruct* OR educat* OR advice* OR support* OR preoperativ* OR pre‐operativ* OR pre operativ*) AND ((randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) |
| EMBASE (OvidSP) | 1987 to March 2013. | 1. (laparoscop* or coelioscop* or celioscop* or peritoneoscop*).af. 2. exp laparoscopic surgery/ 3. 1 or 2 4. cholecystectom*.af. 5. exp cholecystectomy/ 6. 4 or 5 7. (information or instruct* or educat* or advice* or support* OR preoperativ* or pre‐operativ* or pre operativ*).af. 8. patient education/ or preoperative education/ or preoperative care/ 9. 7 or 8 10. 3 and 6 and 9 11. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomised controlled trial/ or single‐blind procedure/ 12. (random* or factorial* or crossover* or placebo*).af. 13. 11 or 12 14. 10 and 13 |
| Science Citation Index Expanded (ISI Web of Knowledge) | 1987 to March 2013. | #1 TS=(laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) #2 TS=(cholecystectom*) #3 TS=(information or instruct* or educat* or advice* or support* OR preoperativ* or pre‐operativ* or pre operativ*) #4 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #5 #4 AND #3 AND #2 AND #1 |
| metaRegister of Controlled Trials (www.controlled‐trials.com/mrct/) | March 2013. | (laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND (cholecystectom*) AND (information or instruct* or educat* or advice* or support* OR preoperativ* or pre‐operativ* or pre operativ*) |
Data and analyses
Comparison 1. Patient education versus no patient education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient knowledge | 3 | 338 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.02, 0.41] |
| 2 Patient satisfaction | 2 | 305 | Std. Mean Difference (Random, 95% CI) | 0.48 [‐0.42, 1.37] |
| 3 Patient anxiety | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Calculations for conversion of binary outcome to standardised mean difference (binary outcomes) | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.63 [2.18, 14.52] |
| 4.1 Patient satisfaction | 1 | 93 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.63 [2.18, 14.52] |
| 5 Calculations for conversion of continuous outcome to standardised mean difference (binary outcomes) | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.33, 0.41] |
| 5.1 Patient satisfaction | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.33, 0.41] |
1.1. Analysis.
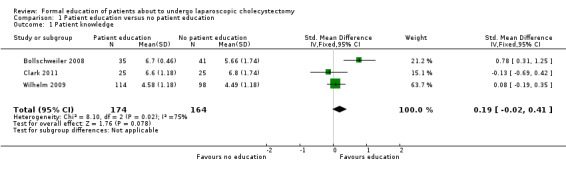
Comparison 1 Patient education versus no patient education, Outcome 1 Patient knowledge.
Comparison 2. Patient education with repeat‐back versus patient education without repeat‐back.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient knowledge | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blay 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: Australia. Number randomised: 128. Post‐randomisation drop‐outs: 35 (27.3%). Revised sample size: 93. Mean age: 54 years. Females: 78 (83.9%). Inclusion criteria 1. Patients undergoing laparoscopic cholecystectomy who attended the pre‐admission clinic. Exclusion criteria 1. Patients undergoing day surgery. 2. Age < 14 years. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: patient education (n = 41). Further details: verbal education during pre‐admission clinic. Group 2: standard care (n = 52). | |
| Outcomes | Pain and patient satisfaction. | |
| Notes | Authors contacted in April 2013. No replies were received. Reasons for post‐randomisation drop‐outs (n): part of preoperative questionnaire pilot study (14); lost to follow‐up (12); surgery cancelled (6); self withdrawal (1); conversion to open cholecystectomy (1); awaiting surgery (1); groups to which the patients belonged were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Patients who attended the pre‐admission clinic (PAC) for LC [laparoscopic cholecystectomy] were invited to consent to participate in the study, allocated a study number and randomly assigned (using randomisation tables) to the standard pre‐admission program (provided by PAC staff) or standard program plus education intervention." |
| Allocation concealment | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel | High risk | Comment: It is impossible to blind the patients to the groups. |
| Blinding of outcome assessors | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data addressed All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Free of selective reporting | High risk | Comment: Important outcomes such as patient mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Bollschweiler 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 80. Post‐randomisation drop‐outs: 4 (5%). Revised sample size: 76. Mean age: 55 years. Females: 47 (61.8%). Inclusion criteria 1. Patients admitted for elective laparoscopic cholecystectomy. 2. Capable of understanding the printed literature in German. 3. Age > 18 years. Exclusion criteria 1. Patients undergoing emergency surgery or open cholecystectomy. 2. Cholecystectomy performed during another operation. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: patient education (n = 35). Further details: computer‐based multimedia programme. Group 2: standard care (n = 41). | |
| Outcomes | Patient anxiety and patient knowledge. | |
| Notes | Authors contacted in April 2013. They replied in April 2013. Reasons for post‐randomisation drop‐outs: refusal to participate (2 in intervention group and 1 in control group); unreadable questionnaire (1 in intervention group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Once the patient name was registered at Study headquarters, a random list was used to assign patients to the standard or Multimedia‐Based Information Program (MM‐IP) groups." |
| Allocation concealment | Unclear risk | Quote: "The patients were selected for participating in this study by the physician of the hospital. He informed the study‐nurse. The study nurse asked for the next envelope from central point" (author replies). |
| Blinding of participants and personnel | High risk | Comment: It is impossible to blind the patients to the groups. |
| Blinding of outcome assessors | Unclear risk | Quote: "Patients answered to the questions, but there was no blinding" (author replies). |
| Incomplete outcome data addressed All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Free of selective reporting | High risk | Comment: Important outcomes such as patient mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Clark 2011.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 50. Post‐randomisation drop‐outs: not stated. Revised sample size: 50. Mean age: 39 years. Females: not stated. Inclusion criteria 1. Patients undergoing elective laparoscopic cholecystectomy. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: patient education (n = 25). Further details: Power Point presentation. Group 2: standard care (n = 25). | |
| Outcomes | Patient knowledge. | |
| Notes | Authors contacted in April 2013. No replies were received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Comment: This information was not available. |
| Allocation concealment | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel | High risk | Comment: It is impossible to blind the patients to the groups. |
| Blinding of outcome assessors | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data addressed All outcomes | Unclear risk | Comment: This information was not available. |
| Free of selective reporting | High risk | Comment: Important outcomes such as patient mortality and morbidity were not reported. |
| For‐profit bias | Unclear risk | Comment: This information was not available. |
Fink 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 173. Post‐randomisation drop‐outs: not stated. Revised sample size: 173. Mean age: not stated Females: not stated Inclusion criteria 1. Patients undergoing various elective operations (only patients undergoing laparoscopic cholecystectomy were included for this review). Exclusion criteria 1. Inability to give informed consent as a result of incompetence. 2. Requirement for surrogate consent. 3. Non‐elective surgery. 4. Patients requiring > 1 surgical procedure. 5. Refusal to participate. 6. Inability to communicate in the English language. 7. Severe visual problems that limited ability to read written material. 8. Severe psychiatric illness limiting ability to consent or to meet study requirements including uncontrolled depression, psychosis, schizophrenia, bipolar disorder, mania, schizoaffective disorder, or other serious psychiatric illness. 9. Ongoing substance abuse. 10. Patients previously consented for their procedure using iMedConsent, who had to be re‐consented due to the expiration of the surgical consent after 30 days (per Veterans Health Administration policy). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: electronic consent procedure with repeat‐back (n = 92). Group 2: electronic consent without repeat‐back (n = 81). | |
| Outcomes | Patient knowledge. | |
| Notes | Authors contacted in April 2013. No replies were received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "Patients who gave consent and met eligibility criteria were randomized using an internet‐based program which used a concealed, computer‐generated simple randomization scheme without stratification." |
| Allocation concealment | Low risk | Quote: "The randomization sequence was concealed from each center's study personnel." |
| Blinding of participants and personnel | High risk | Comment: It is impossible to blind the patients to the groups. |
| Blinding of outcome assessors | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data addressed All outcomes | Unclear risk | Comment: This trial included different types of surgeries. While the post‐randomisation drop‐outs were presented in a flow‐chart, the types of procedures that these patients underwent were not stated and so it was impossible to determine whether there were any post‐randomisation drop‐outs in patients undergoing laparoscopic cholecystectomy. |
| Free of selective reporting | High risk | Comment: Important outcomes such as patient mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Quote: "Supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (Project no. IAF 05–308–01)." |
Wilhelm 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 259. Post‐randomisation drop‐outs: 47 (18.1%). Revised sample size: 212. Mean age: 53 years. Females: 148 (69.8%). Inclusion criteria 1. Patients undergoing laparoscopic cholecystectomy for cholecystolithiasis. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups. Group 1: patient education (n = 114). Further details: multimedia DVD programme. Group 2: standard care (n = 98). | |
| Outcomes | Patient satisfaction and patient knowledge. | |
| Notes | Authors contacted in April 2013. They replied in April 2013. Reasons for post‐randomisation drop‐outs: did not return questionnaire (groups not stated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Quote: "All patients were assigned randomly to either the DVD or the control group using a specifically built randomisation list and after having given informed consent concerning participating the study"; "We used a computer generated randomization list" (author replies). Comment: The information about the random list was generated was not stated. |
| Allocation concealment | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel | High risk | Comment: It is impossible to blind the patients to the groups. |
| Blinding of outcome assessors | Low risk | Quote: "Those who assessed the patients' education level did not have any information concerning the allocation to the respective study arms" (author replies). |
| Incomplete outcome data addressed All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Free of selective reporting | High risk | Comment: Important outcomes such as patient mortality and morbidity were not reported. |
| For‐profit bias | Low risk | Quote: "There was no funding of the study, the study was conducted as an internal project and in cooperation with the department of media sciences of the LMU Munich" (author replies). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Broadbent 2012 | Not a trial of patient education. |
| Stergiopoulou 2007 | Randomisation by a method similar to alternation. |
Differences between protocol and review
1. Patient knowledge was added as a secondary outcome since improvement in knowledge is an important aspect of patient education. 2. Minor changes to the 'Risk of bias' tool were made in line with the Cochrane Hepato‐Biliary Group module (Gluud 2013).
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion, and extracted data on included trials. J Vaughan independently assessed the trials for inclusion and extracted data on included trials. BR Davidson critically commented on the review and provided advice for improving the review.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR is the health research wing of the UK Government. It part funds Dr K Gurusamy's salary and funds all the materials needed for the preparation of this review.
Declarations of interest
None known.
New
References
References to studies included in this review
Blay 2005 {published data only}
- Blay N, Donoghue J. The effect of pre‐admission education on domiciliary recovery following laparoscopic cholecystectomy. Australian Journal of Advanced Nursing 2005;22(4):14‐9. [PubMed] [Google Scholar]
Bollschweiler 2008 {published data only}
- Bollschweiler E, Apitsch J, Obliers R, Koerfer A, Monig SP, Metzger R, et al. Improving informed consent of surgical patients using a multimedia‐based program? Results of a prospective randomized multicenter study of patients before cholecystectomy. Annals of Surgery 2008;248(2):205‐11. [DOI] [PubMed] [Google Scholar]
Clark 2011 {published data only}
- Clark S, Mangram A, Ernest D, Lebron R, Peralta L. The informed consent: a study of the efficacy of informed consents and the associated role of language barriers. Journal of Surgical Education 2011;68(2):143‐7. [DOI] [PubMed] [Google Scholar]
Fink 2010 {published data only}
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Annals of Surgery 2010;252(1):27‐36. [DOI] [PubMed] [Google Scholar]
- Fink AS, Prochazka AV, Henderson WG, Bartenfeld D, Nyirenda C, Webb A, et al. Predictors of comprehension during surgical informed consent. Journal of the American College of Surgeons 2010;210(6):919‐26. [DOI] [PubMed] [Google Scholar]
Wilhelm 2009 {published data only}
- Wilhelm D, Gillen S, Wirnhier H, Kranzfelder M, Schneider A, Schmidt A, et al. Extended preoperative patient education using a multimedia DVD‐impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial. Langenbeck's Archives of Surgery 2009;394(2):227‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Broadbent 2012 {published data only}
- Broadbent E, Kahokehr A, Booth RJ, Thomas J, Windsor JA, Buchanan CM, et al. A brief relaxation intervention reduces stress and improves surgical wound healing response: a randomised trial. Brain, Behavior and Immunity 2012;26(2):212‐7. [DOI] [PubMed] [Google Scholar]
Stergiopoulou 2007 {published data only}
- Stergiopoulou A, Birbas K, Katostara I, Mantas J. The effect of interactive multimedia on preoperative knowledge and postoperative recovery of patients undergoing laparoscopic cholecystectomy. Methods of Information in Medicine 2007;46(4):406‐9. [DOI] [PubMed] [Google Scholar]
- Stergiopoulou A, Birbas K, Katostaras T, Diomidous M, Mantas J. The effect of a multimedia health educational program on the postoperative recovery of patients undergoing laparoscopic cholecystectomy. Studies in Health Technology and Informatics 2006;124:920‐5. [PubMed] [Google Scholar]
Additional references
Attili 1995
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995;21(3):655‐60. [DOI] [PubMed] [Google Scholar]
Ballal 2009
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. [DOI] [PubMed] [Google Scholar]
Bates 1992
- Bates T, Harrison M, Lowe D, Lawson C, Padley N. Longitudinal study of gall stone prevalence at necropsy. Gut 1992;33(1):103‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Cassady 1999
- Cassady JF Jr, Wysocki TT, Miller KM, Cancel DD, Izenberg N. Use of a preanesthetic video for facilitation of parental education and anxiolysis before pediatric ambulatory surgery. Anesthesia and Analgesia 1999;88(2):246‐50. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 6 January 2014).
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dolan 2009
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2013
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 8. Art. No.: LIVER.
GREPCO 1984
- GREPCO. Prevalence of gallstone disease in an Italian adult female population. Rome group for the epidemiology and prevention of cholelithiasis (GREPCO). American Journal of Epidemiology 1984;119(5):796‐805. [PubMed] [Google Scholar]
GREPCO 1988
- GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part i. Prevalence data in men. The Rome group for epidemiology and prevention of cholelithiasis (GREPCO). Hepatology 1988;8(4):904‐6. [PubMed] [Google Scholar]
Gurusamy 2008a
- Gurusamy KS, Junnarkar S, Farouk M, Davidson BR. Day‐case versus overnight stay for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006798.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2008b
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Halldestam 2004
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. [DOI] [PubMed] [Google Scholar]
HES 2011
- HESonline. Hospital Episode Statistics. Main procedures and interventions: 3 character, 2011. www.hscic.gov.uk/hes (accessed 6 January 2014).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald S, Hetrick S, Green S. Pre‐operative education for hip or knee replacement. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003526.pub2] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NIH 1992
- National Institutes of Health. NIH consensus statement on gallstones and laparoscopic cholecystectomy, 1992. consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm (accessed 6 January 2014).
O'Connor 2009
- O'Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001431.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sinha 2013
- Sinha S, Hofman D, Stoker DL, Friend PJ, Poloniecki JD, Thompson MM, et al. Epidemiological study of provision of cholecystectomy in England from 2000 to 2009: retrospective analysis of Hospital Episode Statistics. Surgical Endoscopy 2013;27(1):162‐75. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPIRIT 2013a
- Chan A‐W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, et al. SPIRIT 2013 Explanation and Elaboration: Guidance for protocols of clinical trials. BMJ (Clinical Research Ed.) 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strasberg 1993
- Strasberg SM, Clavien PA. Overview of therapeutic modalities for the treatment of gallstone diseases. American Journal of Surgery 1993;165(4):420‐6. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 6 January 2014).
Todd 1996
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


