Abstract
Background
Early thrombolysis for individuals experiencing a myocardial infarction is associated with better mortality and morbidity outcomes. While traditionally thrombolysis is given in hospital, pre‐hospital thrombolysis is proposed as an effective intervention to save time and reduce mortality and morbidity in individuals with ST‐elevation myocardial infarction (STEMI). Despite some evidence that pre‐hospital thrombolysis may be delivered safely, there is a paucity of controlled trial data to indicate whether the timing of delivery can be effective in reducing key clinical outcomes.
Objectives
To assess the morbidity and mortality of pre‐hospital versus in‐hospital thrombolysis for STEMI.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OVID), EMBASE (OVID), two citation indexes on Web of Science (Thomson Reuters) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for randomised controlled trials and grey literature published up to June 2014. We also searched the reference lists of articles identified, clinical trial registries and unpublished thesis sources. We did not contact pharmaceutical companies for any relevant published or unpublished articles. We applied no language, date or publication restrictions. The Cochrane Heart Group conducted the primary electronic search.
Selection criteria
We included randomised controlled trials of pre‐hospital versus in‐hospital thrombolysis in adults with ST‐elevation myocardial infarction diagnosed by a healthcare provider.
Data collection and analysis
Two authors independently screened eligible studies for inclusion and carried out data extraction and 'Risk of bias' assessments, resolving any disagreement by consulting a third author. We contacted authors of potentially suitable studies if we required missing or additional information. We collected efficacy and adverse effect data from the trials.
Main results
We included three trials involving 538 participants. We found low quality of evidence indicating uncertainty whether pre‐hopsital thrombolysis reduces all‐cause mortality in individuals with STEMI compared to in‐hospital thrombolysis (risk ratio 0.73, 95% confidence interval 0.37 to 1.41). We found high‐quality evidence (two trials, 438 participants) that pre‐hospital thrombolysis reduced the time to receipt of thrombolytic treatment compared with in‐hospital thrombolysis. For adverse events, we found moderate‐quality evidence that the occurrence of bleeding events was similar between participants receiving in‐hospital or pre‐hospital thrombolysis (two trials, 438 participants), and low‐quality evidence that the occurrence of ventricular fibrillation (two trials, 178 participants), stroke (one trial, 78 participants) and allergic reactions (one trial, 100 participants) was also similar between participants receiving in‐hospital or pre‐hospital thrombolysis. We considered the included studies to have an overall unclear/high risk of bias.
Authors' conclusions
Pre‐hospital thrombolysis reduces time to treatment, based on studies conducted in higher income countries. In settings where it can be safely and correctly administered by trained staff, pre‐hospital thrombolysis may be an appropriate intervention. Pre‐hospital thrombolysis has the potential to reduce the burden of STEMI in lower‐ and middle‐income countries, especially in individuals who have limited access to in‐hospital thrombolysis or percutaneous coronary interventions. We found no randomised controlled trials evaluating the efficacy of pre‐hospital thrombolysis for STEMI in lower‐ and middle‐income countries. Large high‐quality multicentre randomised controlled trials implemented in resource‐constrained countries will provide additional evidence for the efficacy and safety of this intervention. Local policy makers should consider their local health infrastructure and population distribution needs. These considerations should be taken into account when developing clinical guidelines for pre‐hospital thrombolysis.
Keywords: Humans, Emergency Medical Services, Thrombolytic Therapy, Thrombolytic Therapy/adverse effects, Thrombolytic Therapy/mortality, Fibrinolytic Agents, Fibrinolytic Agents/administration & dosage, Fibrinolytic Agents/adverse effects, Hemorrhage, Hemorrhage/chemically induced, Myocardial Infarction, Myocardial Infarction/drug therapy, Myocardial Infarction/mortality, Randomized Controlled Trials as Topic, Time‐to‐Treatment
Plain language summary
Delivering clot‐busting therapy before reaching hospital or in hospital to help people who are having heart attacks
Heart disease is the most common cause of death worldwide according to the World Health Organization. A heart attack can either be treated with a drug called a thrombolytic (clot buster) or with surgery. The earlier a thrombolytic is given, the less likely the individual is to die or have disabilities. Usually, thrombolysis is given in a hospital; however, the administration of this therapy before hospital, by paramedics, may be an effective intervention that may save time and reduce death and disability in people with heart attacks.
The aim of this review was to compare the effect of pre‐hospital and in‐hospital administration of thrombolytic therapy on all‐cause death and disability in individuals having a heart attack. We carried out a comprehensive search for all trials that have investigated this outcome. Two authors worked independently to ensure we found all of the trials and obtained the relevant information from them. Overall, we found three trials with 538 participants which could be included in this review. We found low‐quality evidence indicating uncertainty whether the numbers of people dying were different when therapy was given before hospital compared to in hospital (3 trials). We found high‐quality evidence that giving therapy before hospital reduced the time taken for an individual to receive thrombolytic therapy by more than 30 minutes (two studies) and generally low‐quality evidence that side effects, such as allergic reactions and bleeding, were similar whether therapy was given pre‐hospital or in hospital. The main limitations of the evidence were the unclear/high risk of bias in the studies and the low numbers of people recruited.
We conclude that clot‐busting therapy given before arriving at a hospital reduces the time taken for an individual to receive thrombolytic treatment. The limitations of the evidence we have found should be considered carefully, especially in settings where thrombolysis can be safely and correctly administered by trained staff. We found that there were no trials evaluating pre‐hospital thrombolytic therapy in poorer countries, and therefore further research in such settings will provide more information to advise on whether giving this therapy for heart attacks is safe and effective.
Summary of findings
Summary of findings for the main comparison. Pre‐hospital versus in‐hospital thrombolysis for ST‐elevation myocardial infarction.
| Pre‐hospital versus in‐hospital thrombolysis for ST‐elevation myocardial infarction | ||||||
| Participants or population: participants with ST‐elevation myocardial infarction Settings: USA, France and Germany Intervention: Pre‐hospital versus in‐hospital thrombolysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pre‐hospital versus in‐hospital thrombolysis | |||||
| All‐cause hospital mortality Follow up: 30 days1 | 73 per 1000 | 53 per 1000 (27 to 103) | RR 0.73 (0.37 to 1.41) | 538 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Follow up ranged from 15 days to 1 month 2 Downgraded by 1 level for risk of bias due to poor reporting of random sequence generation, allocation concealment (not described and concealment broken) and inadequate outcome reporting in Castaigne 1989 3 Downgraded by 1 level for imprecision as CI includes appreciable benefit and appreciable harm.
Summary of findings 2. Pre‐hospital versus in‐hospital thrombolysis for ST‐elevation myocardial infarction.
| Pre‐hospital versus in‐hospital thrombolysis for ST‐elevation myocardial infarction | ||||||
| Participant or population: participants with ST‐elevation myocardial infarction Settings: USA, France and Germany Intervention: Pre‐hospital versus in‐hospital thrombolysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pre‐hospital versus in‐hospital thrombolysis | |||||
| Time to thrombolytic treatment [minutes] | The mean time to thrombolytic treatment [minutes] in the intervention groups was 37.95 lower (61.12 to 14.77 lower) | 438 (2 studies) | ⊕⊕⊕⊕ high1 | |||
| Acute myocardial infarction functional outcomes ‐ ejection fraction [percentage] | The mean acute myocardial infarction functional outcomes ‐ ejection fraction [percentage] in the intervention groups was 1.18 lower (3.50 lower to 1.13 higher) | 416 (2 studies) | ⊕⊕⊝⊝ low2,3 | |||
| Adverse effects ‐ ventricular Fribrillation | 25 per 1000 | 67 per 1000 (17 to 268) | RR 2.73 (0.68 to 10.86) | 178 (2 studies) | ⊕⊕⊝⊝ low4 | |
| Adverse effects ‐ bleeding complications | 58 per 1000 | 51 per 1000 (24 to 112) | RR 0.88 (0.41 to 1.92) | 438 (2 studies) | ⊕⊕⊕⊝ moderate5 | |
| Adverse effects ‐ allergic reaction | 0 per 1000 | 0 per 1000 (0 to 0) | RR 0 (0.19 to 77.03) | 100 (1 study) | ⊕⊕⊝⊝ low4 | |
| Adverse effects ‐ Stroke | 11 per 1000 | 23 per 1000 (4 to 123) | RR 2.11 (0.39 to 11.4) | 360 (1 study) | ⊕⊕⊝⊝ low4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Methodological quality (e.g. no blinding in Castaigne 1989) not likely to effect this outcome, therefore not downgraded due to risk of bias 2 Downgraded by 1 level for risk of bias. Schofer 1990 had participants lost to follow up for this outcome (n = 17). Extent of non‐differential or differential loss to follow up unknown 3 Downgraded for imprecision, CI includes appreciable benefit and appreciable harm 4 Downgraded by 2 levels for imprecision. Low event rate and wide CI 5 Downgraded by 1 level for imprecision
Background
Description of the condition
The World Health Organization (WHO) reports that cardiovascular disease is the leading cause of death worldwide, with more than 80% of these deaths occurring in lower‐ and middle‐income countries (LMICs) (Mackay 2004; WHO 2011). Cardiovascular disease is responsible for more than 10% of disability‐adjusted life‐years lost in LMICs and for more than 18% of disability‐adjusted life‐years lost in high‐income countries (HICs) (Mackay 2004). ST‐segment elevation myocardial infarction (STEMI) falls under the umbrella classification of acute coronary syndromes (ACS), which also include non‐ST‐segment elevation myocardial infarction (NSTEMI) and unstable angina (Ruff 2011). A STEMI is the development of myocardial necrosis secondary to the interruption of the blood supply to an area of the myocardium identified by the presence of ST segment elevation on electrocardiography or the elevation of cardiac markers, or both. In the United States there has been a striking evolution in the epidemiology of ACS since the 1990s, with a steady decline in the incidence of STEMIs and a reciprocal incline in the incidence of NSTEMIs, as reported by Rogers 2008. In LMICs there is an increasing trend in ischaemic heart disease mortality (Mensah 2008) as these countries move through an epidemiological transition of increasing incidence and prevalence of cardiovascular disease (Gersh 2010). Acute myocardial infarction is defined as cardiac muscle death owing to prolonged lack of oxygenation (Thygesen 2007) caused by an abrupt reduction in coronary blood flow to part of the heart (Beers 2006). Symptoms of acute myocardial infarction may be more severe than those associated with angina and usually persist for longer (e.g. more than 15 to 20 minutes). Classic symptoms include chest discomfort or pain but can include other symptoms such as shortness of breath, nausea, sweating, dizziness and vomiting (Goodacre 2002; Goodacre 2003). Health costs relating to people suffering from acute myocardial infarction are diverse, with economic implications to the individual, family, healthcare system and country (IOM 2010).
Description of the intervention
STEMIs can be treated effectively using percutaneous coronary interventions (PCIs) or thrombolytic agents, or both (Bonnefoy 2009; Weaver 1993). Thrombolytic agents are enzymes that cause coronary thrombus dissolution through a cascade of effects to degrade fibrin thrombi and fibrinogen (SAMF 2010). These agents can be administered either in the pre‐hospital setting or, traditionally, in a hospital setting, and are most effective if given in the first few minutes to hours after onset of a STEMI (Beers 2006; Rawles 2003; Weaver 1993). Various thrombolytic agents are available, all with similar biological effects, efficacy and administration requirements. These include, but are not limited to, the following agents:
streptokinase, 1.5 million units intravenously (IV) over 30 to 60 minutes;
alteplase, 15 mg IV 0.75 mg/kg over 30 minutes followed by 0.5 mg/kg IV over 60 minutes;
reteplase, 10 U + 10 U IV given 30 minutes apart;
tenecteplase, single IV injection (weight dependent) (Van de Werf 2008).
A thrombolytic agent is administered either by infusion or as a single bolus dose. This distinction is important to note as bolus doses are generally easier to administer, require less resources (e.g. an infusion pump) and expertise. Treatment of STEMIs is aimed at early diagnosis and risk stratification, with relief of pain, breathlessness and anxiety coupled with immediate coronary reperfusion either with a pharmacological or mechanical intervention depending on availability and on each individual's context (O'Connor 2010). The standard of care includes anti‐ischaemic therapy (oxygen, nitroglycerin, opioids and beta‐blockers), antiplatelet therapy (Aspirin, clopidogrel) (Fox 2004; ISIS‐2 1988), antithrombin therapies (heparin and low‐molecular‐weight heparins) (Armstrong 2006) and reperfusion strategies (O'Connor 2010; Van de Werf 2008).
How the intervention might work
Effective thrombolysis for individuals with STEMI is extremely time sensitive (Sayah 2008). The earlier a thrombolytic agent is initiated the better, with greatest benefit occurring within three hours from symptom onset (Bonnefoy 2009). The goal is to initiate thrombolysis within 30 to 60 minutes after symptom onset (Antman 2008). Despite this goal, achieving this in practice is challenging (Barbagelata 2007). Pre‐hospital initiation of thrombolysis has been reported to improve time to thrombolysis and reduce mortality compared with in‐hospital thrombolysis (Antman 2008 (narrative); Björklund 2006 (cohort study); Bonnefoy 2009; Brouwer 1996; Rawles 2003 (trials); Curtis 2006 (descriptive); Morrison 2000 (review)).
Why it is important to do this review
Early thrombolysis has been associated with better mortality and morbidity outcomes (Bonnefoy 2009). Pre‐hospital thrombolysis can provide improved time to thrombolysis (Björklund 2006) and a potential reduction in mortality and morbidity compared with in‐hospital treatment (Rawles 2003). A previous systematic review by Morrison 2000 found that pre‐hospital thrombolysis for acute myocardial infarction significantly decreased all‐cause hospital mortality based on a meta‐analysis of six randomised controlled trials (RCTs). This review incorporated any new evidence and utilised the GRADE assessment, together with Cochrane Heart Group methodology. It added to current knowledge of pre‐hospital thrombolysis by considering system and infrastructure needs for the successful implementation of the models of care and ascertained gaps in current research evidence. The results of this review may guide policy makers and other healthcare stakeholders to invest in the appropriate treatment strategy and health system/service requirements for individuals with STEMI needing thrombolysis, especially in LMICs where other treatment options for STEMI are scarce or not available. This review has important implications for areas where primary angioplasty is unavailable or where pre‐hospital transport times are long, such as rural areas ‐ specifically in LMICs.
Objectives
To assess the morbidity and mortality of pre‐hospital versus in‐hospital thrombolysis for STEMI.
Methods
Criteria for considering studies for this review
Types of studies
RCTs excluding cross‐over trials.
Types of participants
Adults (16 years and older) with STEMI diagnosed by a medical healthcare provider in either the pre‐hospital or in‐hospital setting. Diagnosis of STEMI will be defined according to the included studies' criteria for STEMI but should include at least two of the following three positive indicators: the individual's history and symptoms, electrocardiogram (ECG) findings and biochemical cardiac markers (cardiac makers are not mandatory for diagnosis, but may be used in certain pre‐hospital settings).
Types of interventions
Any thrombolytic agent used to treat STEMI in pre‐hospital and in‐hospital settings.
Types of outcome measures
Primary outcomes
All‐cause hospital mortality at one month (short term) and one year (mid term).
Secondary outcomes
Time to thrombolytic treatment, measured from symptom onset or first medical contact, or both (or as described by study authors) to the administration of a thrombolytic agent
Adverse effects. An adverse event is defined as an event for which a causal relationship between the intervention and the event is a reasonable possibility (e.g. ventricular fibrillation, pulseless ventricular tachycardia, cardiogenic shock, inappropriate use of thrombolytics, hypotension, bradycardia, re‐infarction, bleeding, or fatal and non‐fatal stroke)
-
Acute myocardial infarction functional outcomes including:
ejection fraction, measured using an echocardiogram;
classification of heart failure (New York Heart Association functional classification system);
time to discharge or days in hospital, measured from admission to discharge in days
Search methods for identification of studies
Electronic searches
In June 2014, we conducted comprehensive electronic searches for RCTs using the following key search terms ‐ thrombolysis, thrombolysis therapy, myocardial infarction, and pre‐hospital ‐ and using the Cochrane sensitivity‐precision maximising RCT filter (Lefebvre 2011), adapted for use with the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL, issue 5 of 12, September 2014, searched 5 June 2014, results: 1491);
MEDLINE (OVID, 1946 to May Week 4 2014, searched 10 June 2014, results: 1178);
EMBASE Classic + EMBASE (OVID, 1947 to 5 June 2014, searched 5 June 2014, results: 1196);
Science Citation Index Expanded (SCI‐EXPANDED, 1970 to 5 June 2014) and Conference Proceedings Citation Index‐ Science (CPCI‐S, 1990 to 5 June 2014) on Web of Science (Thomson Reuters) searched 5 June 2014; results: 2489;
CINAHL Plus with Full Text (EBSCO, 1936 to May 2014, searched 5 June 2014, results: 117).
We added no language or publication restrictions to the search strategies. The search strategies used can be found in Appendix 1.
In developing the search strategy we were assisted by the Cochrane Heart Review Group's Trials Search Co‐ordinator who conducted the main search.
Searching other resources
We searched grey literature, such as unpublished thesis sources, and the following additional databases: ProQuest Dissertations, Index to theses in Great Britain and Ireland, and DissOnline. We carried out no handsearching and contacted no pharmaceutical companies in order to identify additional studies due to operational time restraints.
We searched the reference lists of included studies and contacted the primary authors of included studies to identify additional relevant studies. We searched the following clinical trial registers: ClinicalTrials.gov (www.clinicaltrials.gov/), International Standard Randomised Controlled Trial Register (www.controlled‐trials.com/isrctn/) and the WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
We merged the results of the search using reference management software and removed duplicate records. Two review authors (MM and AL) independently examined titles and abstracts to remove obviously irrelevant reports and retrieved the full text of potentially relevant reports. They linked multiple reports of the same study and independently examined full‐text reports for compliance with eligibility criteria using a study eligibility form. MM and AL resolved any disagreements regarding study inclusion or exclusion with the assistance of the other author, TK. Neither author was blinded to the names of the study authors, institutions, journal of publication nor results, as this practice has uncertain benefit in protecting against bias (Higgins 2011). We created a PRISMA flow diagram (Moher 2009) to show the process of inclusion and exclusion of RCTs; potentially eligible studies that were excluded are noted in the 'Excluded studies' section.
Data extraction and management
Two review authors (MM and AL) independently extracted data from the studies using a data extraction form. We collected the following information:
study source (name of person extracting data, study ID, report ID, review author, citation and contact details);
eligibility (confirmation of eligibility for review as per protocol, reason for exclusion);
methods (study aims, study design, total study duration, unit of allocation, all information required for the 'Risk of bias' tool, ethics approval);
participants and setting (age, recruitment method, inclusion and exclusion criteria, baseline imbalances, informed consent obtained, number of participants randomised, time of first symptom onset, rural or urban setting, developing or developed country setting, subgroups measured, subgroups reported);
interventions (group name, number randomised to group, type of medication administered, method of administration, time of medication administration, place of administration, number and explanation for any dropouts, duration of follow up, economic variables);
outcome measures coupled with results (outcome definition/name, person measuring or reporting, all‐cause hospital mortality at 30 days and one year or longer where available, time to thrombolytic treatment, adverse effects, STEMI functional outcomes, comorbidities);
results (continuous variables of outcome data such as measures of variability, dichotomous data such as total number of events in each arm and numbers of participants), additional notes (key conclusions of study, correspondence with authors needed, clarification of queries, ethics or stated conflicts of interest, duplicate publication, translation required);
applicability (populations excluded, disadvantaged groups, applicability to developing countries).
We collated data from multiple reports of the same study into one data extraction form. MM collated and entered all data into Review Manager 5 (RevMan 2011). We resolved any disagreements by consensus.
Assessment of risk of bias in included studies
Two review authors (MM and AL) independently assessed the risk of bias of included studies using the Cochrane 'Risk of bias' assessment tool (six domains) (Higgins 2011), stating whether the risk of bias was low, high or unclear. The two authors independently pooled the results and discussed any differences with a third author (TK). We addressed the following bias domains: sequence generation, allocation concealment, blinding (blinding of participant and personnel, blinding of outcome assessors), incomplete outcome data, selective outcome reporting and other risks of bias. The review authors followed the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for assessing bias. We extracted information based on the published data and contacted the authors whenever descriptions were missing or unclear.
Measures of treatment effect
Dichotomous data
Dichotomous outcomes, such as all‐cause hospital mortality, were represented as risk ratios (RR) with 95% confidence intervals (CIs). Adverse effect data were measured as proportions or rates, respectively, depending on the study data.
Continuous data
Continuous effect measures included the time from symptom onset to thrombolysis, measured as the mean difference (MD) or standardised MD between individuals receiving thrombolytic therapy in a pre‐hospital or an in‐hospital setting. Time to discharge, number of days in hospital and ejection fraction were measured as MDs or standardised MDs between groups.
Unit of analysis issues
Only RCTs were included. The authors identified no cluster RCTs or multi‐arm RCTs. Hence, the unit of analysis was at an individual level.
Dealing with missing data
We asked the authors of one RCT (the European Myocardial Infarction Project (EMIP)) to provide missing data so that the study could potentially be included in the review. Unfortunately they were unable to provide any data and the trial was excluded from the study. We performed no imputing of missing data.
Assessment of heterogeneity
We performed a visual inspection of the forest plot for heterogeneity. Heterogeneity was assessed using the Chi2 test, with a P value < 0.1 considered indicative of significant heterogeneity, and the I2 statistic. As there was reasonable clinical and methodological similarity between trials, we were able to carry out a meta‐analysis. We sought possible reasons for any substantial heterogeneity.
Assessment of reporting biases
The use of a funnel plot to explore possible reporting biases was precluded due to the limited number of included studies (< 10).
Data synthesis
As the trials were clinically and methodologically similar, we undertook a meta‐analysis. We used a fixed‐effect meta‐analysis if studies were estimating the same treatment effect (no statistical heterogeneity) and a random‐effects meta‐analysis if studies showed substantial statistical heterogeneity. We used RevMan software to perform the meta‐analysis. If we performed a meta‐analysis in the presence of high levels of heterogeneity, we sought possible explanations for this heterogeneity.
Subgroup analysis and investigation of heterogeneity
We predefined several possible subgroups for meta‐analysis:
practitioner type: paramedic (basic versus advanced) versus physician (emergency versus cardiologist) thrombolytic administration on mortality outcome;
HIC versus LMIC settings;
rural versus urban settings;
remote telemetry with consultant communication versus independent paramedic thrombolytic administration;
automated versus manual ECG interpretation;
different types of thrombolytic medication administered compared for mortality and adverse effects;
anatomical location of STEMI;
mobile intensive care units compared with primary response;
adverse effects of pre‐hospital thrombolytic agents as administered by paramedics versus physicians.
However, we did not perform any subgroup analyses due to the limited number of included studies. The Cochrane Handbook for Systematic Reviews of Interventions recommends a minimum of 10 studies.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size:
fixed‐effect model versus a random‐effects model meta‐analyses;
exclusion of trials with a high risk of bias.
Results
Description of studies
Results of the search
The electronic database searches identified 6471 titles for potential inclusion. After the removal of duplications, 4111 titles remained of which 4027 titles were found not to be relevant. We retrieved full‐text articles for the remaining 84 titles which two authors independently screened for eligibility. We included three studies, reported in six papers, met the eligibility criteria. The trial registry searches revealed 146 potentially eligible studies of which all we excluded (Figure 1). Ten trials were translated with the help of the Cochrane Heart Group.
1.
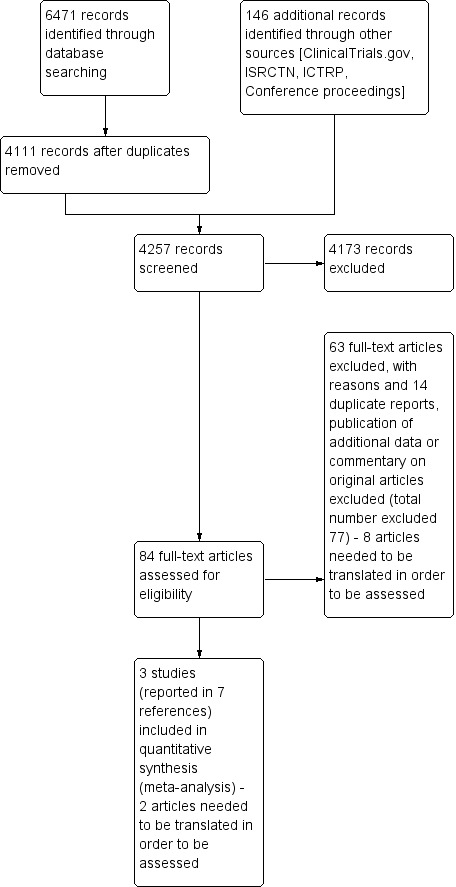
Study flow diagram.
Included studies
See: Characteristics of included studies
We identified three RCTs (538 participants), conducted in France, USA and Germany, which examined the efficacy of pre‐hospital versus in‐hospital thrombolysis for STEMI. Castaigne 1989 used 30 U anisoylated plasminogen streptokinase activator complex (APSAC) whereas Schofer 1990 used urokinase (2 million U IV) and Weaver 1993 used alteplase 100 mg as the thrombolytic agent in both the intervention and control arms. In Castaigne 1989 and Schofer 1990 physicians in mobile care units administered the pre‐hospital thrombolytic agents whereas in Weaver 1993 paramedics administered the thrombolytic. The primary outcomes were similar across all three trials and included mortality, time intervals, angiographic data, ejection fraction and complications.
Castaigne 1989 was a two phase study conducted in the Val de Marne district close to Paris, France. The first phase comprised a simulation pilot study and an education study; the latter evaluated anaesthesiologists' hypothetical decision to correctly thrombolyse individuals with chest pain possibly due to ACS in mobile care units. A total of 294 participants were reviewed over 1 year. The second phase of the study was a RCT comparing pre‐hospital versus in‐hospital thrombolysis conducted over 2 years using 30 U APSAC injected over more than four minutes. The researchers screened 320 individuals with STEMI, and 100 were included in the trial. The intervention in both treatment groups was administered by physicians (including that in mobile care units). The main outcome for phase one of the study was diagnostic accuracy; that for the second phase was the delay between at‐home and in‐hospital injection for participants having received placebo at home.
Schofer 1990 was an RCT conducted in Germany within the mobile care unit systems of AK Altona, Stadtische Kliniken Kiel and Darmstadt. The pre‐hospital group (40 participants) received urokinase (2 million U IV) at home and placebo at hospital whereas the in‐hospital group (38 participants) received placebo at home and urokinase (2 million U IV) at hospital, both followed by 1000 U/hour of heparin at hospital. Urokinase was diluted with 20 mL of injectable water. The mobile care units were staffed with a physician and two emergency medical technicians. The following study endpoints were reported: time intervals, angiographic data and creatine kinase levels, stress test before discharge and complications.
Weaver 1993 was an RCT of pre‐hospital versus in‐hospital initiated thrombolytic therapy conducted in Seattle metropolitan area and the surrounding King County, in the USA. The trial ran from November 1988 to December 1991, and involved 19 hospitals and all paramedical systems in the Metropolitan area. The pre‐hospital‐initiated group received Aspirin 325 mg and alteplase 100 mg at home and no placebo at hospital whereas the hospital‐initiated group received no placebo at home and Aspirin 325 mg and alteplase 100 mg at hospital. A total of 360 participants were included in the study, 175 and 185 in the pre‐hospital and in‐hospital treatment arms, respectively. Pre‐hospital thrombolysis was performed by paramedics (emergency care professionals) with physician guidance. Study endpoints included diagnostic accuracy of STEMI, time to treatment, pre‐hospital and in‐hospital complications, ejection fraction and infarct size.
Excluded studies
Risk of bias in included studies
See: 'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study (Figure 2).
2.
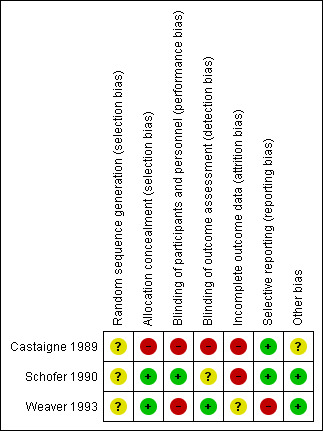
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
See: 'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies (Figure 3).
3.
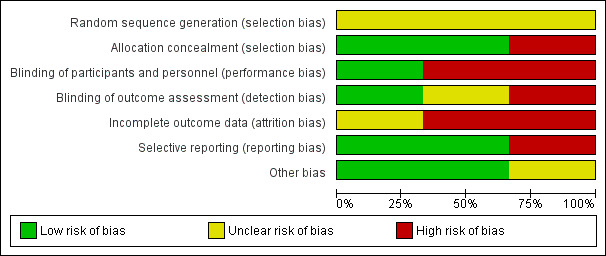
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Schofer 1990, Weaver 1993 and Castaigne 1989 provided no description of random sequence generation; as a result we considered the risk of bias to be unclear. We judged the risk of bias for allocation concealment in Castaigne 1989 to be high as the allocation code was broken. We considered Schofer 1990 and Weaver 1993 to have a low risk of bias for allocation concealment.
Blinding
We considered the risk of bias for the blinding of participants and personnel, as well as for outcome assessment (detection bias), in Castaigne 1989 to be high. The authors of this study state that the mobile care unit physicians were blinded. However, the blinding is not described and the code could be broken if the physician thought it necessary. Upon arrival at hospital the code was broken as all the other physicians and assessors would have knowledge of the treatment received. In Schofer 1990, we judged the risk of bias due to the blinding of participants and personnel to be low as numbered paired ampoules containing either placebo or thrombolysis were used. For outcome assessment we judged the risk to be unclear as no description was provided. We considered the risk of bias due to blinding of participants and personnel in Weaver 1993 to be high as alteplase was administered in an open manner; we judged the risk of bias for outcome assessment to be low as the groups were unknown to the assessor.
Incomplete outcome data
We judged the risk of bias for incomplete outcome data to be high in Castaigne 1989 and Schofer 1990, and unclear in Weaver 1993. Participants in Castaigne 1989 were not assessed according to intention‐to‐treat analysis and some outcome data were not reported. In Schofer 1990 some data were excluded from analysis and some were missing. Weaver 1993 did not report whether participants were lost to follow up or withdrawn from participation.
Selective reporting
We judged Castaigne 1989 and Schofer 1990 to have a low risk of bias for selective reporting. We considered Weaver 1993 to have a high risk as some prespecified complications were not reported in the intervention group.
Other potential sources of bias
Schofer 1990 and Weaver 1993 had no indications of other sources of bias and as a result we judged this risk to be low. We judged Castaigne 1989 to have an unclear risk as the report did not include a table of baseline characteristics.
Effects of interventions
Primary outcome
Mortality data were available for all three included RCTs. However, none of the studies presented the mortality data over the prespecified time periods (one month and one year); hence, no time period was used and we report the general all‐cause mortality rate.
There is low quality evidence indicating uncertainty about whether pre‐hospital compared to in‐hospital thrombolysis reduces mortality (Table 1) (RR 0.73, 95% CI 0.37 to 1.41, three RCTs; 538 participants) (Analysis 1.1). There was no heterogeneity between studies (Chi2 = 0.29; P value = 0.86; I2 = 0%) and we therefore used a fixed‐effect model for meta‐analysis (Analysis 1.1). Further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. It should be noted that the meta‐analysis included only 538 participants and thus one should interpret these results with caution. We rated the studies as having an overall unclear/high risk of bias (Figure 3).
1.1. Analysis.
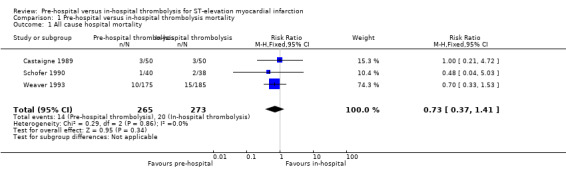
Comparison 1 Pre‐hospital versus in‐hospital thrombolysis mortality, Outcome 1 All cause hospital mortality.
A sensitivity analysis excluding the trial with a high risk of bias (Castaigne 1989) also found no significant difference between the pre‐hospital and in‐hospital thrombolysis groups (Analysis 3.1). Excluding Castaigne 1989, however, resulted in a shift of the pooled effect measure towards a stronger protective effect of pre‐hospital thrombolysis compared with the non‐sensitivity analysis (RR 0.68 compared with 0.73), although the difference between groups remained non‐significant.
3.1. Analysis.
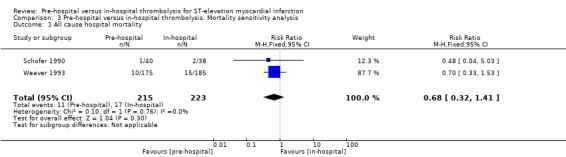
Comparison 3 Pre‐hospital versus in‐hospital thrombolysis: Mortality sensitivity analysis, Outcome 1 All cause hospital mortality.
Secondary outcomes
Time to thrombolysis
Schofer 1990 and Weaver 1993 presented data on the time from the onset of symptoms to thrombolysis. There was high‐quality evidence (Table 2) that pre‐hospital thrombolysis reduced the mean time to thrombolysis by 38 minutes (MD ‐37.95 minutes. 95% CI ‐61.12 to ‐14.77, two RCTs; 438 participants, Analysis 2.1). We found substantial heterogeneity (Chi2 = 3.53; P value = 0.06; I2 = 72%) and we therefore conducted a random‐effects meta‐analysis. Heterogeneity was not thought to be sufficiently significant to forgo meta‐analysis as a visual inspection revealed overlapping CIs and point estimates in a similar direction. We rated these two studies as having an overall low risk of bias (Figure 2).
2.1. Analysis.
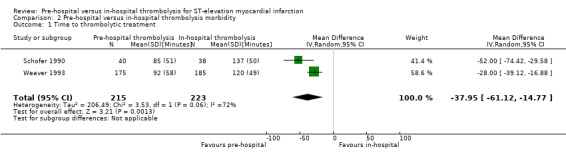
Comparison 2 Pre‐hospital versus in‐hospital thrombolysis morbidity, Outcome 1 Time to thrombolytic treatment.
Acute myocardial infarction functional outcomes
All three included RCTs reported mean percentage ejection fraction. However, Castaigne 1989 presented the mean percentage ejection fraction for pre‐hospital thrombolysis (56.7%) and in‐hospital thrombolysis (53.4%) without providing the standard deviations for the measurements. We therefore conducted a meta‐analysis including Schofer 1990 and Weaver 1993 only. We found low‐quality evidence (Table 2) that there may be no difference between the ejection fraction in pre‐hospital versus in‐hospital thrombolysis (MD ‐1.18, 95% CI ‐3.50 to 1.13, two RCTs; 416 participants, Analysis 2.2). As we found no heterogeneity (Chi2 = 0.16; P value = 0.69; I2 = 0%), we therefore used a fixed‐effect model for meta‐analysis. The low‐quality data indicate that further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We rated these two studies as having an overall low risk of bias (Figure 2).
2.2. Analysis.
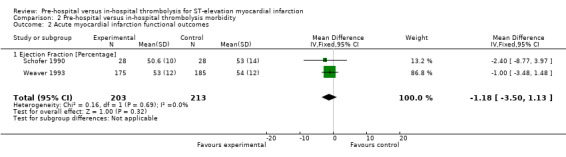
Comparison 2 Pre‐hospital versus in‐hospital thrombolysis morbidity, Outcome 2 Acute myocardial infarction functional outcomes.
None of the included RCTs reported data on the acute myocardial infarction functional outcomes, classification of heart failure (New York Heart Association functional classification system) and time to discharge or days in hospital, measured from admission to discharge (proposed secondary outcomes).
Adverse effects
Four adverse effects were prioritised as clinically important and incorporated in the GRADE assessment: ventricular fibrillation, stroke, allergic reaction and bleeding.
There was low‐quality evidence that there may be no difference in the occurrence of ventricular fibrillation (two RCTs), stroke (one RCT) or allergic reactions (one RCT) between groups. There was moderate‐quality evidence that was no difference in bleeding complications between groups (two RCTs, Table 2). We downgraded the evidence due to imprecision as the confidence interval included appreciable harm and appreciable benefit (Analysis 2.3).
2.3. Analysis.
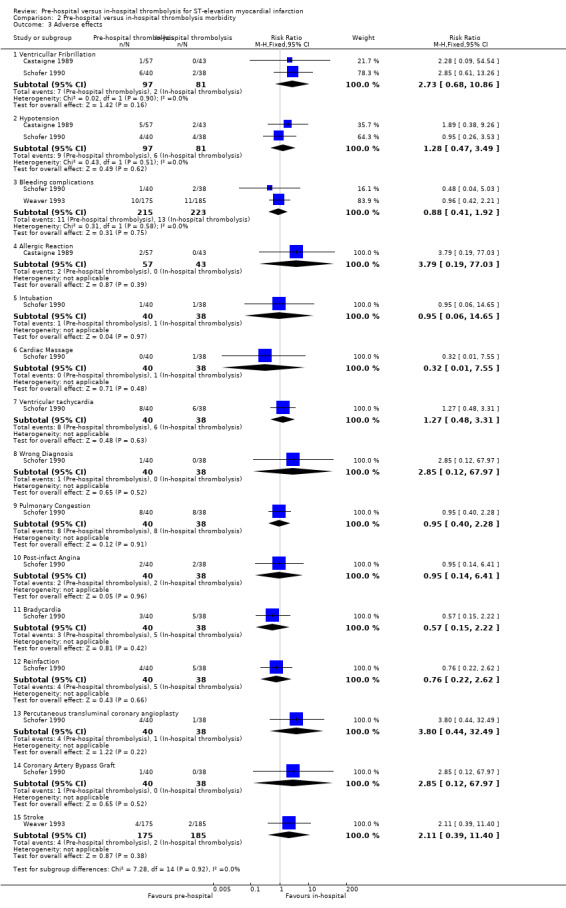
Comparison 2 Pre‐hospital versus in‐hospital thrombolysis morbidity, Outcome 3 Adverse effects.
Discussion
Summary of main results
There is low quality evidence indicating uncertainty about whether pre‐hospital compared to in‐hospital thrombolysis reduces mortality . Additional data may change this finding (Table 1). We rated the included studies as having an overall unclear/high risk of bias (Figure 3).
We found high‐quality evidence that the time to thrombolysis in those who were thrombolysed pre‐hospital compared with those thrombolysed in hospital was statistically significantly reduced by 38 minutes. We rated the studies included in this analysis as having an overall low risk of bias (Figure 3).
We found low‐quality evidence that there may be no difference in acute myocardial infarction functional outcomes (ejection fraction) between pre‐hospital and in‐hospital thrombolysis. We rated the relevant studies as having an overall low risk of bias (Figure 3).
There was low‐quality evidence that there may be no difference in adverse effects between pre‐hospital and in‐hospital thrombolysis (Table 2). We rated the relevant studies as having an overall low risk of bias (Figure 3).
Overall completeness and applicability of evidence
We were able to include only three relatively small trials in this review and this influences the external validity of our findings. We were unable to obtain requested data from a potentially eligible study and therefore have excluded it (EMIP). The excluded study could have contributed to the power of the meta‐analysis to detect a difference between groups for the primary outcome. None of the included studies presented data on heart failure classification and days in hospital or time to discharge. The results of this review are applicable to HICs but less so to LMICs, as all the included trials were conducted in developed country settings. We were unable to perform subgroup analyses due to the limited number of included studies.
The findings of this review have strong external validity when generalised to HICs; however, LMICs need to take into consideration their unique health and emergency medical care systems. Local policy makers and clinical directors should consider their local health infrastructure and population distribution needs (rural compared with urban), emergency care systems and availability of the intervention compared with surgical alternatives (e.g. availability of PCI). These considerations should be taken into account when developing clinical guidelines for pre‐hospital thrombolysis.
Quality of the evidence
We used GRADE methodology to explore the quality of the evidence. The primary outcome, mortality, was supported by low‐quality evidence only, which was attributable to a high risk of methodological bias and imprecision in the point estimate. Further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Secondary outcomes that were reported in the included studies were time to thrombolysis, ejection fraction and adverse effects. There was high‐quality evidence that time to thrombolysis is reduced when treatment is delivered pre‐hospital compared with in the hospital. Further research is unlikely to impact our confidence in the estimate. We rated the evidence for the outcome of ejection fraction as low quality, which we downgraded due to the risk of methodological bias and imprecision (the confidence interval includes appreciable benefit and appreciable harm). Only low‐quality evidence was available for all the adverse effect outcomes due to high levels of imprecision, with the exception of the evidence for bleeding complications, which we judged to be of moderate quality.
Potential biases in the review process
We used Cochrane methodology to conduct a comprehensive search to identify all the available trials in order to answer this review question. Data for one potentially eligible study could not be obtained as the authors did not provide the necessary data; hence, we may have omitted additional evidence that could have contributed to the results.
Agreements and disagreements with other studies or reviews
Morrison 2000 is a systematic review and meta‐analysis of pre‐hospital versus in‐hospital thrombolysis for acute myocardial infarction that assesses mortality. The study authors report a statistically significant difference in all‐cause hospital mortality in favour of pre‐hospital thrombolysis. Morrison 2000 included RCTs that assessed the efficacy of thrombolysis for both STEMI and NSTEMI. The current review specifically sought to investigate thrombolysis for STEMI as this type of therapy is not recommended for NSTEMI (O'Connor 2010). Morrison 2000 found a mean time difference of 60 minutes between pre‐hospital and in‐hospital thrombolysis for acute myocardial infarction. Our results are consistent with those of this previously published review.
Authors' conclusions
Implications for practice.
Pre‐hospital thrombolysis reduces time to thrombolytic treatment, based on the results of three studies conducted in HICs. In settings where it can be safely and correctly administered by trained staff, pre‐hospital thrombolysis may therefore be an appropriate intervention. We were unable to determine whether pre‐hospital thrombolysis is superior to in‐hospital thrombolysis with regard to mortality, ejection fraction or adverse effects. Pre‐hospital thrombolysis for STEMI has the potential to reduce the burden of disease in LMICs, especially in individuals who have limited access to in‐hospital thrombolysis or PCI (e.g. those living in rural areas). Local policy makers and clinical directors should consider their local health infrastructure and population distribution needs (rural compared with urban), emergency care system and the availability of thrombolytic therapy compared to surgical alternatives (e.g. the availability of PCI). These considerations should be taken into account when developing clinical guidelines for pre‐hospital thrombolysis. In Weaver 1993, pre‐hospital thrombolysis was performed by paramedics (emergency care professionals) with physician guidance, highlighting the advantage of a paramedic lead with physician teamwork as an alternative to a physician‐led thrombolysis team, especially when considering physician availability in LMICs.
Implications for research.
The implications of these findings for research into STEMI are less clear. Further research required may include studies that take STEMI into consideration as opposed to AMI in general. We found no RCTs that evaluated the efficacy of pre‐hospital thrombolysis for STEMI in LMICs. Large high‐quality multicentre RCTs implemented in LMICs have the potential to develop those countries' health infrastructure and service delivery capacity. A pragmatic approach to conducting these RCTs would be most advantageous in order to determine the efficacy and efficiency of pre‐hospital thrombolysis, especially taking into consideration the health challenges of LMICs. Pragmatic RCTs (including feasibility studies) would contribute to the required infrastructure, health system co‐ordination, training models and policy development necessary for the implementation and facilitation of pre‐hospital thrombolysis in LMICs.
Acknowledgements
The authors would like to acknowledge Dr Taryn Young for her review comments and input. We would like to thank The South African Cochrane Centre staff for their support and for providing their facilities during the production of this review. We are also very grateful for the support from the Cochrane Heart Group, including Nicole Martin who has aided us tremendously in obtaining foreign language translations. The authors would like to acknowledge and extend our gratitude to the following individuals for their assistance with foreign language translations: Aurelie Jeandron, Joerg Weber, Deirdre Beecher, Ela Gohil, Marina Karanikolos, Lunn Grignard and Nicole Martin.
Appendices
Appendix 1. Appendix
CENTRAL
#1 MeSH descriptor: [Myocardial Infarction] explode all trees
#2 "myocardial infarct*"
#3 "heart infarct*"
#4 ami
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Fibrinolytic Agents] this term only
#7 MeSH descriptor: [Thrombolytic Therapy] this term only
#8 thromboly*
#9 alteplase
#10 reteplase
#11 streptokinase
#12 tenecteplase
#13 urokinase
#14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 #5 and #14
#16 MeSH descriptor: [Hospitals] explode all trees
#17 hospital*
#18 prehospital*
#19 pre‐hospital*
#20 #16 or #17 or #18 or #19
#21 #15 and #20
1. exp Myocardial Infarction/
2. myocardial infarct$.tw.
3. heart infarct$.tw.
4. ami.tw.
5. 1 or 2 or 3 or 4
6. Fibrinolytic Agents/
7. Thrombolytic Therapy/
8. thromboly$.tw.
9. alteplase.tw.
10. reteplase.tw.
11. streptokinase.tw.
12. tenecteplase.tw.
13. urokinase.tw.
14. or/6‐13
15. 5 and 14
16. exp Hospitals/
17. hospital$.tw.
18. prehospital$.tw.
19. pre‐hospital$.tw.
20. 16 or 17 or 18 or 19
21. 15 and 20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. clinical trials as topic.sh.
27. randomly.ab.
28. trial.ti.
29. 22 or 23 or 24 or 25 or 26 or 27 or 28
30. exp animals/ not humans.sh.
31. 29 not 30
32. 21 and 31
MEDLINE OVID
1. exp Myocardial Infarction/
2. myocardial infarct$.tw.
3. heart infarct$.tw.
4. ami.tw.
5. 1 or 2 or 3 or 4
6. Fibrinolytic Agents/
7. Thrombolytic Therapy/
8. thromboly$.tw.
9. alteplase.tw.
10. reteplase.tw.
11. streptokinase.tw.
12. tenecteplase.tw.
13. urokinase.tw.
14. or/6‐13
15. 5 and 14
16. exp Hospitals/
17. hospital$.tw.
18. prehospital$.tw.
19. pre‐hospital$.tw.
20. 16 or 17 or 18 or 19
21. 15 and 20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. clinical trials as topic.sh.
27. randomly.ab.
28. trial.ti.
29. 22 or 23 or 24 or 25 or 26 or 27 or 28
30. exp animals/ not humans.sh.
31. 29 not 30
32. 21 and 31
EMBASE OVID
1. exp heart infarction/
2. myocardial infarct$.tw.
3. heart infarct$.tw.
4. ami.tw.
5. or/1‐4
6. fibrinolytic agent/
7. fibrinolytic therapy/
8. thromboly$.tw.
9. alteplase.tw.
10. reteplase.tw.
11. streptokinase.tw.
12. tenecteplase.tw.
13. urokinase.tw.
14. or/6‐13
15. 5 and 14
16. exp hospital/
17. hospital$.tw.
18. prehospital$.tw.
19. pre‐hospital$.tw.
20. or/16‐19
21. 15 and 20
22. random$.tw.
23. factorial$.tw.
24. crossover$.tw.
25. cross over$.tw.
26. cross‐over$.tw.
27. placebo$.tw.
28. (doubl$ adj blind$).tw.
29. (singl$ adj blind$).tw.
30. assign$.tw.
31. allocat$.tw.
32. volunteer$.tw.
33. crossover procedure/
34. double blind procedure/
35. randomized controlled trial/
36. single blind procedure/
37. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36
38. (animal/ or nonhuman/) not human/
39. 37 not 38
40. 21 and 39
41. limit 40 to embase
Web of Science
#19 #18 AND #17
#18 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
#17 #16 AND #12 AND #4
#16 #15 OR #14 OR #13
#15 TS=pre‐hospital*
#14 TS=prehospital*
#13 TS=hospital*
#12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5
#11 TS=urokinase
#10 TS=tenecteplase
#9 TS=streptokinase
#8 TS=reteplase
#7 TS=alteplase
#6 TS=thromboly*
#5 TS=fibrinolyt*
#4 #3 OR #2 OR #1
#3 TS=ami
#2 TS=(heart SAME infarct*)
#1 TS=(myocardial SAME infarct*)
CINAHL
S21 S20 Limiters ‐ Exclude MEDLINE records
S20 S17 and S18 and S19
S19 S13 or S14 or S15 or S16
S18 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12
S17 S1 or S2 or S3 or S4
S16 AB pre‐hospital* or TI pre‐hospital*
S15 AB prehospital* or TI prehospital*
S14 AB hospital* or TI hospital*
S13 (MH "Hospitals+")
S12 AB urokinase or TI urokinase
S11 AB tenecteplase or TI tenecteplase
S10 AB streptokinase or TI streptokinase
S9 AB reteplase or TI reteplase
S8 AB alteplase OR TI alteplase
S7 AB thromboly* or TI thromboly*
S6 (MH "Thrombolytic Therapy")
S5 (MH "Fibrinolytic Agents")
S4 AB ami or TI ami
S3 AB "heart infarct*" or TI "heart infarct*"
S2 AB "myocard* infarct*" or TI "myocard* infarct*"
S1 (MH "Myocardial Infarction+")
Data and analyses
Comparison 1. Pre‐hospital versus in‐hospital thrombolysis mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause hospital mortality | 3 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.41] |
Comparison 2. Pre‐hospital versus in‐hospital thrombolysis morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to thrombolytic treatment | 2 | 438 | Mean Difference (IV, Random, 95% CI) | ‐37.95 [‐61.12, ‐14.77] |
| 2 Acute myocardial infarction functional outcomes | 2 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐3.50, 1.13] |
| 2.1 Ejection Fraction [Percentage] | 2 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐3.50, 1.13] |
| 3 Adverse effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Ventricullar Fribrillation | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.68, 10.86] |
| 3.2 Hypotension | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.47, 3.49] |
| 3.3 Bleeding complications | 2 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.41, 1.92] |
| 3.4 Allergic Reaction | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [0.19, 77.03] |
| 3.5 Intubation | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 14.65] |
| 3.6 Cardiac Massage | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.55] |
| 3.7 Ventricular tachycardia | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.48, 3.31] |
| 3.8 Wrong Diagnosis | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.97] |
| 3.9 Pulmonary Congestion | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.40, 2.28] |
| 3.10 Post‐infact Angina | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.14, 6.41] |
| 3.11 Bradycardia | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.22] |
| 3.12 Reinfaction | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.22, 2.62] |
| 3.13 Percutaneous transluminal coronary angioplasty | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.8 [0.44, 32.49] |
| 3.14 Coronary Artery Bypass Graft | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.97] |
| 3.15 Stroke | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.39, 11.40] |
Comparison 3. Pre‐hospital versus in‐hospital thrombolysis: Mortality sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause hospital mortality | 2 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Castaigne 1989.
| Methods | Two‐phase study conducted in the Val de Marne district near Paris, France Phase 1: Simulation and education over 1 year Phase 2: Randomised trial conducted over 2 years |
|
| Participants | Criteria for inclusion in trial: 1) Age < 75 years 2) Typical ischaemic chest pain for > 30 minutes and < 3 hours that did not respond to nitrates 3) ST‐segment elevation of 0.2 mV or more in at least 2 standard leads (posterior infarction) or 3 precordial leads (anterior infarction) 4) No hypertension 5) No classic contraindication to thrombolytic therapy |
|
| Interventions | Pre‐hospital group: participants received nitrates and 30 U anisoylated plasminogen streptokinase activator complex (APSAC) injected over > 4 minutes or placebo In‐hospital group: on arrival in the coronary care unit, the code was broken and, if the individual had received placebo at home he/she received APSAC 30 U over > 4 minutes. The coronary care unit physician decided whether thrombolytic treatment was appropriate |
|
| Outcomes | Study endpoints: 1) Diagnostic accuracy, delay between phone call to emergency medical service and arrival in coronary care unit for individuals included in or excluded from the trial 2) Delay between at‐home and in‐hospital injection for individuals having received placebo at home 3) Events intervening during the pre‐hospital phase |
|
| Notes | Secondary reference (Dubois‐Rande 1989) translated by Aurelie Jeandron | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation. |
| Allocation concealment (selection bias) | High risk | No description of allocation concealment. Allocation codes were broken. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Mobile care unit physician was blinded to the treatment; however, no description of blinding provided. Furthermore, it is stated that the code could be broken if the physician thought it necessary or if a cardiologist was present when the ambulance reached the individual’s home. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Code broken upon arrival at hospital and therefore the assessor will have had knowledge of treatment received. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Individuals did not appear to be assessed according to intention to treat. Incomplete data. |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | Unclear risk | Did not report a baseline characteristics table – insufficient information to excluded other possible biases. |
Schofer 1990.
| Methods | Double‐blind randomised controlled trial in Germany | |
| Participants | Criteria for inclusion in trial: 1) Severe chest pain typical of myocardial ischaemia lasting > 30 minutes 2) Arrival of the ambulance doctor within 4 hours after the onset of symptoms 3) ≥2 mm of ST‐elevation in ≥2 ECG leads for inferior AMI and ≥ 3 mm of ST‐elevation in ≥2 precordial leads for anterior AMI 4) Age ≤ 70 years 5) No prior AMI 6) No contraindications against thrombolysis |
|
| Interventions | Pre‐hospital group: urokinase (2 million U intravenously) at home or placebo at hospital In‐hospital group: placebo at home or urokinase (2 million U intravenously) at hospital Followed by 1000 U/hour of heparin at hospital |
|
| Outcomes | Study end points: 1) Time intervals 2) Angiographic data and creatine kinase 3) Stress test before discharge 4) Complications |
|
| Notes | Secondary reference (Mathey 1990) translated by Joerg Weber | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation. Authors only state participants were randomly assigned to ampoule pairs. |
| Allocation concealment (selection bias) | Low risk | Randomly assigned to the next in the series of ampoule pairs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medac (Hamburg, Federal Republic of Germany) prepared numbered pairs of ampoules containing either urokinase in ampoule A and placebo in ampoule B, or vice versa. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 individual was diagnosed with pulmonary embolism and the data excluded. Some missing data for ejection fraction (see figure 3). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | Low risk | No indication of other bias. |
Weaver 1993.
| Methods | Randomised controlled clinical trial conducted in the city of Seattle and the surrounding King County from November 1988 to December 1991 | |
| Participants | Criteria for inclusion in trial: 1) Individuals with suspected acute myocardial infarction, pain for 6 hours or less 2) aged ≤ 75 years, with no risk of bleeding (no history of stroke, recent bleeding or uncontrolled systolic (less than 180 mmHg) or diastolic (less than 120 mmHg hypertension) . 3) 12‐lead ECG was obtained and a physician reviewed the findings over the telephone and made the final decision to randomise |
|
| Interventions | Pre‐hospital‐initiated group: Aspirin 325 mg and alteplase 100 mg at home and no placebo at hospital Hospital‐initiated group: no placebo at home and Aspirin 325 mg and alteplase 100 mg at hospital All participants received basic medical care including oxygen, rhythm monitoring devices and intravenous access. Additionally, morphine sulphate was used for pain, lidocaine and atropine for arrhythmias, and vasopressors and diuretics for treatment of hypotension and pulmonary oedema (prescribed by the remote physician) Sodium heparin administered to both groups on hospital arrival (5000 U bolus, followed by continuous intravenous infusion for at least 48 hours) |
|
| Outcomes | Study endpoints: 1) Accuracy of diagnosis 2) Time to treatment 3) Complications 4) Ejection fraction 5) Infarct size |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Sealed treatment kits were identical in terms of weight, balance and sound. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For participants allocated to hospital‐initiated treatment, no placebo was given in the field but an active treatment kit was available in the emergency department. Alteplase was infused in an open‐label manner. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For assessment of infarct size and ventricular function measures after hospital discharge the core laboratory personnel were blinded to the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It does not appear as if any participants withdrew or were lost to follow up; this is not reported in the text. |
| Selective reporting (reporting bias) | High risk | Some complications are not reported in intervention groups. |
| Other bias | Low risk | No indication of other bias. |
ECG, electrocardiogram
AMI, acute myocardial infarction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armstrong 2010 | Comparator percutaneous coronary intervention |
| Aufderheide 1992 | Not randomised controlled trial. No thrombolytic therapy was administered in the field during this study (feasibility study) |
| Bata 2009 | Participants randomly assigned to receive primary percutaneous coronary intervention or fibrinolysis |
| BEPS | Pilot study for GREAT trial ‐ no randomised trial, no hospital thrombolysis intervention |
| Brugemann 1992 | Data reported unclear ‐ no mortality data (missing outcome data) |
| Cannon 2000 | Not randomised controlled trial ‐ used historical controls |
| Castaigne 1987 | Outcome unclear ‐ does not report number of participants in intervention or control groups |
| Castaigne 1990 | Unclear mortality data, unclear time measurement and unclear ECG confirmation |
| Castle 2007 | Not randomised controlled trial ‐ retrospective descriptive analysis |
| Coccolini 1998 | Not pure randomised controlled trial ‐ consecutive participant allocation |
| Cuccia 1988 | Thombolysis (IV streptokinase) vs standard therapy. Translated by Deirdre Beecher |
| Danchin 2004 | French Nationwide Registry study |
| Doherty 2004 | Not randomised controlled trial |
| Dussoix 2003 | Compared pre‐hospital thrombolysis to usual hospital care (in‐hospital thrombolysis and mechanical intervention) |
| EMIP | Participants included those with non‐ST segment elevated myocardial infarction. Authors contacted for stratified (STEMI) data. Unable to provide missing data |
| Fokina 2008 | Not randomised controlled trial. Translated by Marina Karanikolos |
| Goldstein 2005 | Not randomised controlled trial |
| Grajek 2007 | Not randomised controlled trial ‐ discusses mitral regurgitation. Translated by Ela Gohil |
| GREAT | Participation eligibility was not based on ECG findings (STEMI) but rather on strong clinical suspicion of acute myocardial infarction by physician |
| Grijseels 1995 | Not randomised controlled trial ‐ retrospective control arm |
| Herve 1988 | Randomised controlled trial with no description of participant selection, no inclusion criteria, no indication of age group or of how participants were diagnosed. Translated by Lynn Grignard |
| Hervé 1988a | Randomised controlled trial comparing home thrombolysis to placebo. Translated by Lynn Grignard. |
| Kasper 1999 | Not randomised controlled trial. Translated by Nicole Martin |
| Kelly 2003 | Not randomised controlled trial |
| Kelly 2010 | Compared pre‐hospital thrombolysis with half‐dose pre‐hospital thrombolysis and PCI |
| Khan 2009 | Not randomised controlled trial ‐ prospective cohort, no interventions |
| Koefoed‐Nielse 2002 | Quasi‐experimental study (before and after trial) |
| Kudenchuk 1998 | ECG study – outcomes focused on ECG abnormalities secondary to thrombolysis |
| Lamfers 1999 | Not randomised controlled trial ‐ retrospective review |
| Lamfers 2003 | Not randomised controlled trial. Study of two different thrombolysis medications administered in the pre‐hospital setting |
| Lamfers 2004 | Not randomised controlled trial |
| Linderer 1993 | Not randomised controlled trial |
| Liu 2003 | Randomised controlled trial comparing pre‐hospital thrombolysis versus immediate angiography |
| Mathew 2003 | Review of hospital data |
| McAleer 1992 | Open allocation ‐ not randomised controlled trial |
| McAleer 2006 | Open allocation ‐ not randomised controlled trial |
| McKendall 1991 | Not randomised controlled trial |
| McNiell 1989 | Some of the participants included in the trial were randomised for inclusion at the emergency department. Study does not present all‐cause mortality data, only cardiac‐cause mortality data |
| McNiell 1991 | Between 3 to 24 hours after administration of anistreplase, participants were randomised to receive either intervention or conventional therapy |
| Millin 2008 | Literature review |
| Morrison 2000 | Systematic review including randomised controlled trials of pre‐hospital vs in‐hospital thrombolysis in AMI with an outcome measure of all‐cause hospital mortality. Selection criteria for participants were not similar to those of the current systematic review |
| Morrow 2002 | Feasibility trial comparing pre‐hospital thrombolysis to sequential in‐hospital controls from 6 to 12 months before |
| Rawles 1999 | Randomised controlled trial reporting 5‐year mortality |
| Risenfors 1991 | Randomisation unclear ‐ ECG criteria not used to include participants but recorded only after administration and treatment |
| Roque 1995 | Not randomised controlled trial. Translated by Joerg Weber |
| Rosell‐Ortiz 2008 | Prospective database cohort |
| Rosenberg 2002 | Open‐label pilot study administering a first bolus of reteplase before emergency department arrival and the second bolus after emergency department arrival |
| Roth 1990 | Not randomised controlled trial ‐ used alternative monthly allocation |
| Rozenman 1994 | Not randomised controlled trial ‐ alternative allocation |
| Rozenman 1995 | Not randomised controlled trial |
| Ruda 2009 | Participants with spontaneous reperfusion were randomised to be treated with emergency coronary angiography and, in case of stenosis >50%, balloon angioplasty or conservative treatment. Translated by Marina Karanikolos |
| Smalling 2007 | Randomised controlled trial comparing pre‐hospital thrombolysis with PCI |
| Smith 2011 | Prospective data collection for all individuals admitted to Chesterfield Royal Hospital who received thrombolysis for a presumed myocardial infarction over a 12‐month period |
| Stewart 1993 | Not randomised controlled trial |
| Svensson 2003 | Feasibility study, no randomisation |
| Tatu‐Chitoiu 2002 | Pre‐hospital accelerated streptokinase combined with enoxaparin and in‐hospital accelerated streptokinase combined with enoxaparin compared to in‐hospital standard streptokinase with heparin |
| Topol 1986 | Not randomised controlled trial |
| Trent 1995 | Secondary analysis of GREAT data |
| Walletin 2003 | Pateints with STEMI in the pre‐hospital setting were randomised to receive tenecteplase and either (1) intravenous bolus of 30 mg enoxaparin followed by 1 mg/kg subcutaneously twice daily for a maximum of 7 days or (2) weight‐adjusted unfractionated heparin for 48 hours |
| Weaver 1994 | Not randomised controlled trial |
| White 1990 | Participants were randomised to receive intravenous streptokinase plus rt‐PA placebo over 3 hours or streptokinase placebo infused over 30 minutes plus rt‐PA infusion over 3 hours |
| Woollard 2005 | Randomised to telemetry and conventional treatment ‐ no active thrombolysis |
| Zeymer 2009 | Report on the data of the German Prehospital Myocardial Infarction Registry |
AMI, acute myocardial infarction
ECG, electrocardiogram
IV, intravenously
PCI, percutaneous coronary intervention
rt‐PA, recombinant tissue plasminogen activator
STEMI, ST‐elevation myocardial infarction
Differences between protocol and review
The title was changed from "Pre‐hospital versus in‐hospital thrombolysis for acute myocardial infarction" to "Pre‐hospital versus in‐hospital thrombolysis for ST‐elevation myocardial infarction". We were unable to find data on mortality at the times prespecified in the protocol and have therefore analysed mortality in general. No handsearching was done and pharmaceutical companies were not contacted due to operational time restraints.
Contributions of authors
Conceiving, designing and coordinating the review (MM). Data collection (MM and AL). Designing search strategies and undertaking searches (Cochrane Heart Group). Screening search results and organising, screening and appraisal of papers (MM and AL). Obtaining translations of papers and screening unpublished studies (MM, AL and Cochrane Heart Group). Data management and entering data into RevMan (MM). Analysis, interpretation of data and methodological, clinical and policy perspective (MM, AL and TK). TK provided overall general advice and guidance on the review.
Sources of support
Internal sources
-
South African Cochrane Centre, South Africa.
Exprertise, internal peer‐review and project working space
-
Stellenbosch University, South Africa.
Assess to electronic databases and library services
External sources
-
Cochrane Heart Group, UK.
Search strategy and primary electronic search, obtaining full text articles and translation of foreign language articles. Peer‐review and author support
Declarations of interest
None known. No direct funding was received for this project.
New
References
References to studies included in this review
Castaigne 1989 {published data only}
- Castaigne AD, Herve C, Duval‐Moulin AM, Gaillard M, Dubois‐Rande JL, Boesch C, et al. Prehospital use of APSAC: results of a placebo‐controlled study. The American Journal of Cardiology 1989;64(2):30A‐33A. [DOI] [PubMed] [Google Scholar]
- Dubois‐Rande JL, Herve C, Duval‐Moulin AM, Gaillard M, Boesch C, Louvard Y, et al. Prehospital thrombolysis. Evaluation of preliminary experiences at Val‐de‐Marne [Thrombolyse prehospitaliere, bilan d’une experience preliminaire menee dans le Val‐de‐Marne]. Arch Mal Coeur Vaiss 1989;82(12):1963‐6. [PubMed] [Google Scholar]
Schofer 1990 {published data only}
- Mathey DG, Buttner J, Geng G, Gutschmidt, Herden HN, Moecke H, et al. Pre‐hospital thrombolysis treatment of acute myocardial infarction: a randomized double‐blind study Deutsche [Thrombolyse‐Behandlung Des Akuten Myokardinfarktes Am Notfallort: Eine Randomisierte Doppelblindstudie]. Deutsche Medizinische Wochenschrift 1990;115(21):803‐8. [DOI] [PubMed] [Google Scholar]
- Schofer J, Buttner J, Geng G, Gutschmidt K, Herden HN, Mathey DG, et al. Prehospital thrombolysis in acute myocardial infarction. The American Journal of Cardiology 1990;66(20):1429‐33. [DOI] [PubMed] [Google Scholar]
Weaver 1993 {published data only}
- Brouwer MA, Martin JS, Maynard C, Wirkus M, Litwin PE, Verheugt FWA, et al. Influence of early prehospital thrombolysis on mortality and event‐free survival (the Myocardial Infarction Triage and Intervention [MITI] Randomized Trial). MITI Project Investigators. The American Journal of Cardiology 1996;78(5):497‐502. [DOI] [PubMed] [Google Scholar]
- Russell MT. [Commentary on] Prehospital‐initiated vs hospital‐initiated thrombolytic therapy: the myocardial infarction triage and intervention trial [original article by Weaver W et al appears in JAMA 1993;279(10):1211‐6]. ENA'S Nursing Scan in Emergency Care 1994; Vol. 4, issue 2:8. [PubMed]
- Weaver WD, Cerqueira M, Hallstrom AP, Litwin PE, Martin JS, Kudenchuk PJ, et al. Prehospital‐initiated vs hospital‐initiated thrombolytic therapy. The Myocardial Infarction Triage and Intervention Trial. JAMA 1993;270(10):1211‐16. [PubMed] [Google Scholar]
References to studies excluded from this review
Armstrong 2010 {published data only}
- Armstrong PW, Gershlick A, Goldstein P, Wilcox R, Danays T, Bluhmki E, et al. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. American Heart Journal 2010;160(1):30‐35.e1. [DOI] [PubMed] [Google Scholar]
Aufderheide 1992 {published data only}
- Aufderheide TP, Keelan MH, Hendley GE, Robinson NE, Hastings TE, Lewin RF, et al. Milwaukee Prehospital Chest Pain Project ‐ Phase I: feasibility and accuracy of prehospital thrombolytic candidate selection. The American Journal of Cardiology 1992;69(12):991‐6. [DOI] [PubMed] [Google Scholar]
Bata 2009 {published data only}
- Bata I, Armstrong PW, Westerhout CM, Travers A, Sookram S, Caine E, et al. Time from first medical contact to reperfusion in ST elevation myocardial infarction: a Which Early ST Elevation Myocardial Infarction Therapy (WEST) substudy. Canadian Journal of Cardiology 2009;25(8):463‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
BEPS {published data only}
- Prehospital thrombolysis in acute myocardial infarction: the Belgian eminase prehospital study (BEPS). BEPS Collaborative Group. European Heart Journal 1991;12(9):965‐7. [PubMed] [Google Scholar]
- Bossaert l, Demey H, Beaucourt L, Vrints C, Putzeys T, Lust P, et al. Prehospital Thrombolysis in Acute Myocardial‐Infarction ‐ The Belgian Eminase Prehospital Study (BEPS). European Heart Journal 1991;12(9):965‐7. [PubMed] [Google Scholar]
Brugemann 1992 {published data only}
- Brugemann J, Meer J, Graeff PA, Takens HL, Lie KL. Logistical problems in prehospital thrombolysis. European Heart Journal 192;13(6):787‐8. [DOI] [PubMed] [Google Scholar]
Cannon 2000 {published data only}
- Cannon CP, Sayah AJ, Walls RM. ER TIMI‐19: testing the reality of prehospital thrombolysis. Journal of Emergency Medicine 2000;19(3 Suppl 3):21S‐25S. [DOI] [PubMed] [Google Scholar]
Castaigne 1987 {published data only}
- Castaigne AD, Duval AM, Dubois‐Rande JL, Herve C, Jan F, Louvard Y. Prehospital administration of anisoylated plasminogen streptokinase activator complex in acute myocardial infarction. Drugs 1987;33(Suppl 3):231‐4. [DOI] [PubMed] [Google Scholar]
Castaigne 1990 {published data only}
- Castaigne AD, Herve C, Duval‐Moulin AM, Gaillard M, Dubois‐Rande JL, Lellouche D. Pre‐hospital thrombolysis, is it useful?. European Heart Journal 1990;11(Suppl F):43‐7. [DOI] [PubMed] [Google Scholar]
Castle 2007 {published data only}
- Castle NR, Owen RC, Hann M. Is there still a place for emergency department thrombolysis following the introduction of the amended Joint Royal Colleges Ambulance Liaison Committee criteria for thrombolysis?. Emergency Medicine Journal 2007;24(12):843‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coccolini 1998 {published data only}
- Coccolini S, Berti G, Maresta A. The magnitude of the benefit from preCCU thrombolysis in acute myocardial infarction: a long term follow up. International Journal of Cardiology 1998;65(Suppl 1):S49‐56. [DOI] [PubMed] [Google Scholar]
Cuccia 1988 {published data only}
- Cuccia C, Gargano M, Berra P, Franzoni P, Gei P, Pagnoni N, et al. Is fibrinolytic therapy in acute myocardial infarction practicable outside the intensive care unit?. Rivista di Cardiologia Preventiva e Riabilitativa 1988;6(2):105‐8. [Google Scholar]
Danchin 2004 {published data only}
- Danchin N, Blanchard D, Steg PG, Sauval P, Hanania G, Goldstein P, et al. Impact of prehospital thrombolysis for acute myocardial infarction on 1‐year outcome: results from the French Nationwide USIC 2000 Registry. Circulation 2004;110(14):1909‐15. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. [Commentary on] Impact of prehospital thrombolysis for acute myocardial infarction on 1‐year outcome: results from the French Nationwide USIC 2000 Registry. ACC Current Journal Review. 2005; Vol. 14, issue 2:7. [DOI] [PubMed]
Doherty 2004 {published data only}
- Doherty DT, Dowling J, Wright P, Murphy AW, Bury G, Bannan L. The potential use of prehospital thrombolysis in a rural community. Resuscitation 2004;61(3):303‐7. [DOI] [PubMed] [Google Scholar]
Dussoix 2003 {published data only}
- Dussoix P, Reuille O, Verin V, Gaspoz JM, Unger PF. Time savings with prehospital thrombolysis in an urban area. European Journal of Emergency Medicine 2003;10(1):2‐5. [DOI] [PubMed] [Google Scholar]
EMIP {published data only}
- Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. The European Myocardial Infarction Project Group. The New England Journal of Medicine 1993;329(6):383‐9. [DOI] [PubMed] [Google Scholar]
- Boissel JP. The European Myocardial Infarction Project: an assessment of pre‐hospital thrombolysis. International Journal of Cardiology 1995;49(Suppl):S29‐37. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Haugh MC, Mercier C, Boissel JP. Pre‐hospital and hospital time delays in thrombolytic treatment in patients with suspected acute myocardial infarction. Analysis of data from the EMIP study. European Myocardial Infarction Project. European Heart Journal 1997;18(2):248‐53. [DOI] [PubMed] [Google Scholar]
- Nath SH, Daily EK. [Commentary on] Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction [original article by European Myocardial Infarction Project Group published in The New England Journal of Medicine 1993;329(6):383‐9. AACN Nursing Scan In Critical Care 1994;4(1):2‐3. [DOI] [PubMed] [Google Scholar]
Fokina 2008 {published data only}
- Fokina EG, Gratchev VG, Lipchenko AA, Kholkin IV, Bushuev AV, Kozlov SV. Prehospital thrombolytic therapy with tenecteplase in patients with ST‐elevation myocardial infarction. Kardiologiia 2008;48(4):14‐17. [PubMed] [Google Scholar]
Goldstein 2005 {published data only}
- Goldstein P, Wiel E. Management of prehospital thrombolytic therapy in ST‐segment elevation acute coronary syndrome (<12 hours). Minerva Anestesiologica 2005;71(6):297‐302. [PubMed] [Google Scholar]
Grajek 2007 {published data only}
- Grajek S, Araszkiewicz A, Gtowka A. Treatment delay in acute myocardial infarction ‐ the role of prehospital thrombolysis. Postepy W Kardiologii Interwencyjnej 2007;2(8):97‐104. [Google Scholar]
GREAT {published data only}
- Feasibility, safety, and efficacy of domiciliary thrombolysis by general practitioners: Grampian region early anistreplase trial. GREAT Group. BMJ 1992;305(6953):548‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawles J. Magnitude of benefit from earlier thrombolytic treatment in acute myocardial infarction: new evidence from Grampian region early anistreplase trial (GREAT). BMJ 1996;312(7025):212‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawles JM. GREAT: 10 year survival of patients with suspected acute myocardial infarction in a randomised comparison of prehospital and hospital thrombolysis. Heart 2003;89(5):563‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawles JM. Halving of mortality at 1 year by domiciliary thrombolysis in the Grampian Region Early Anistreplase Trial (GREAT). Journal of the American College of Cardiology 1994;23(1):1‐5. [DOI] [PubMed] [Google Scholar]
- Rawles JM. Myocardial salvage with early anistreplase treatment. Clinical Cardiology 1997;20(11 Suppl 3):III6‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawles JM. Quantification of the benefit of earlier thrombolytic therapy: five‐year results of the Grampian Region Early Anistreplase Trial (GREAT). Journal of the American College of Cardiology 1997;30(5):1181‐6. [DOI] [PubMed] [Google Scholar]
- Trent R, Adams J, Rawles J. Electrocardiographic evidence of reperfusion occurring before hospital admission. A Grampian Region Early Anistreplase Trial (GREAT) sub‐study. European Heart Journal 1994;15(7):895‐7. [DOI] [PubMed] [Google Scholar]
- Vale L, Silcock J, Rawles J. An economic evaluation of thrombolysis in a remote rural community. BMJ 1997;314(7080):570‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grijseels 1995 {published data only}
- Grijseels EW, Bouten MJ, Lenderink T, Deckers JW, Hoes AW, Hartman JA, et al. Pre‐hospital thrombolytic therapy with either alteplase or streptokinase. Practical applications, complications and long‐term results in 529 patients. European Heart Journal 1995;16(12):1833‐8. [DOI] [PubMed] [Google Scholar]
Herve 1988 {published data only}
- Herve C, Castaigne FJ. Pre‐hospital thrombolysis in myocardial infarction [Thrombolyse en pre‐hospitalier de l’infarctus du myocarde]. Therapie 1988;43:69‐80. [Google Scholar]
Hervé 1988a {published data only}
- Hervé C, Gaillard M, Dubois‐Rande JL, Boesch C, Duval AM, Lionnet F, et al. Home thrombolysis for myocardial infarction. A multicenter study of the feasibility and evaluation of short‐term prognosis. Presse médicale 1988;17(22):1143‐6. [PubMed] [Google Scholar]
Kasper 1999 {published data only}
- Kasper W, Furtwangler A, Martin U, Ott S, Drexler M. Prehospital thrombolysis with rt‐PS. A reperfusion strategy in the time management concept of acute myocardial infarct. Medizinische Klinik 1999;94(7):361‐6. [DOI] [PubMed] [Google Scholar]
Kelly 2003 {published data only}
- Kelly P. Thrombolysis in the pre‐hospital setting. British Journal of Cardiology 2003;10(5):395. [Google Scholar]
Kelly 2010 {published data only}
- Kelly DP, McCarthy JJ, Weirick T, Persse DE, Barker CM, Anderson HV, et al. 36: Out‐of‐Hospital Initiated Reperfusion for ST‐Elevation Myocardial Infarction Patients: Successful Implementation of an Out‐of‐Hospital Thrombolytic Strategy Coupled With Urgent PCI for Reducing Myocardial Ischemic Time In an Urban Environment. Annals of Emergency Medicine. 2010; Vol. 56, issue 3:S13.
Khan 2009 {published data only}
- Khan SN, Murray P, McCormick L, Sharples LS, Salahshouri P, Scott J, et al. Paramedic‐led prehospital thrombolysis is safe and effective: the East Anglian experience. Emergency Medicine Journal 2009;26(6):452‐5. [DOI] [PubMed] [Google Scholar]
Koefoed‐Nielse 2002 {published data only}
- Koefoed‐Nielsen J, Christensen EF, Melchiorsen H, Foldspang A. Acute myocardial infarction: does pre‐hospital treatment increase survival?. European Journal of Emergency Medicine 2002;9(3):210‐216. [DOI] [PubMed] [Google Scholar]
Kudenchuk 1998 {published data only}
- Kudenchuk PJ, Maynard C, Cobb LA, Wirkus M, Martin JS, Kennedy JW, et al. Utility of the prehospital electrocardiogram in diagnosing acute coronary syndromes: the Myocardial Infarction Triage and Intervention (MITI) Project.. Journal of the American College of Cardiology 1998;32(1):17‐27. [DOI] [PubMed] [Google Scholar]
Lamfers 1999 {published data only}
- Lamfers EJ, Hooghoudt TE, Uppelschoten A, Stolwijk PW, Verheugt FW. Effect of prehospital thrombolysis on aborting acute myocardial infarction. The American Journal of Cardiology 1999;84(8):928‐30. [DOI] [PubMed] [Google Scholar]
Lamfers 2003 {published data only}
- Lamfers EJ, Schut A, Hooghoudt TE, Hertzberger DP, Boersma E, Simoons ML, et al. Prehospital thrombolysis with reteplase: the Nijmegen/Rotterdam study. American Heart Journal 2003;146(3):479‐83. [DOI] [PubMed] [Google Scholar]
Lamfers 2004 {published data only}
- Lamfers EJ, Schut A, Hertzberger DP, Hooghoudt TE, Stolwijk PW, Boersma E, et al. Prehospital versus hospital fibrinolytic therapy using automated versus cardiologist electrocardiographic diagnosis of myocardial infarction: Abortion of myocardial infarction and unjustified fibrinolytic therapy. American Heart Journal 2004;147(3):509‐15. [DOI] [PubMed] [Google Scholar]
Linderer 1993 {published data only}
- Linderer T, Schroder R, Arntz R, Heineking ML, Wunderlich W, Kohl K, et al. Prehospital thrombolysis: beneficial effects of very early treatment on infarct size and left ventricular function. Journal of the American College of Cardiology 1993;22(5):1304‐10. [DOI] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu G, Zhao Y, Zhuang SJ. A clinical trial comparing primary angioplasty with a strategy of prehospital intravenous thrombolytic therapy with recombinant tissue plasminogen activator and immediate planned rescue angioplasty in patients with acute myocardial infarction. Circulation 2003;107(19):E142. [Google Scholar]
Mathew 2003 {published data only}
- Mathew TP, Menown IBA, McCarty D, Gracey H, Hill L, Adgey AA. Impact of pre‐hospital care in patients with acute myocardial infarction compared with those first managed in‐hospital. European Heart Journal 2003;24(2):161‐71. [DOI] [PubMed] [Google Scholar]
McAleer 1992 {published data only}
- McAleer B, Ruane B, Burke E, Cathcart M, Costello A, Dalton G, et al. Prehospital thrombolysis in a rural community: short‐ and long‐term survival. Cardiovascular Drugs and Therapy 1992;6(4):369‐72. [DOI] [PubMed] [Google Scholar]
- Varma MP, McAleer B. Pre‐hospital thrombolysis in acute myocardial infarction: improved survival in a rural area abstract. Irish Journal of Medical Sciences 1995;164(1):74. [Google Scholar]
McAleer 2006 {published data only}
- McAleer B, Varma MP. Feasibility and long term outcome of home vs hospital initiated thrombolysis. Irish Journal of Medical Science 2006;175(4):14‐19. [DOI] [PubMed] [Google Scholar]
McKendall 1991 {published data only}
- McKendall GR, Attibato MJ, Drew TM, Feit F, Sharaf BL, Thomas ES, et al. Safety and efficacy of a new regimen intravenous recombinant tissue‐type plasminogen‐activator potentially suitable for either prehospital or in‐hospital administration. Journal of the American College of Cardiology 1991;18(7):1774‐8. [DOI] [PubMed] [Google Scholar]
McNiell 1989 {published data only}
- McNiell AJ, Cunningham SR, Flannery DJ, Dalzell GW, Wilson CM, Campbell NP, et al. A double blind placebo controlled study of early and late administration of recombinant tissue plasminogen activator in acute myocardial infarction. British Heart Journal 1989;61(4):316‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
McNiell 1991 {published data only}
- McNeill AJ, Roberts MJ, Wilson CM, Dalzell GW, Dickey W, Flannery DJ, et al. Anistreplase in early acute myocardial infarction and the one‐year follow‐up. International Journal of Cardiology 1991;31(1):39‐49. [DOI] [PubMed] [Google Scholar]
Millin 2008 {published data only}
- Millin MG, Brooks SC, Travers A, Megargel RE, Colella R, Rosenbaum RA, et al. Emergency medical services management of ST‐elevation myocardial infarction. Prehospital Emergency Care 2008;12(3):395‐403. [DOI] [PubMed] [Google Scholar]
Morrison 2000 {published data only}
- Morrison LJ, Verbeek R, McDonald AC, Sawadsky BV, Cook DJ. Mortality and prehospital thrombolysis for acute myocardial infarction: a meta‐analysis. JAMA 2000;283(20):2686‐92. [DOI] [PubMed] [Google Scholar]
- Slawson D. Can prehospital thrombolysis decrease the risk of dying of an acute myocardial infarction (AMI)?. Evidence‐Based Practice 2000; Vol. 3, issue 8:6‐7.
Morrow 2002 {published data only}
- Morrow DA, Antman EM, Sayah A, Schuhwerk KC, Giugliano RP, deLemos JA, et al. Evaluation of the time saved by prehospital initiation of reteplase for ST‐elevation myocardial infarction: results of The Early Retavase‐Thrombolysis in Myocardial Infarction (ER‐TIMI) 19 trial. Journal of the American College of Cardiology 2002;40(1):71‐7. [DOI] [PubMed] [Google Scholar]
Rawles 1999 {published data only}
- Rawles JM, Brinker JA. Benefits of earlier thrombolytic therapy. Cardiology Review 1999;16(6):22‐7. [Google Scholar]
Risenfors 1991 {published data only}
- Risenfors M, Gustavsson G, Ekstrom L, Hartford M, Herlitz J, Karlson BW, et al. Prehospital thrombolysis in suspected acute myocardial infarction: results from the TEAHAT Study. Journal of Internal Medicine. Supplement 1991;734:3‐10. [PubMed] [Google Scholar]
Roque 1995 {published data only}
- Roque M, Magrina J, Huguet M, Lopez A, Bosch X, Garcia A, et al. Comparative study by 99mTc‐MIBI cardiac perfusion scintigraphy of preadmission and intrahospital fibrinolysis in patients with acute myocardial infarction. Revista Espanola de Medicina Nuclear 1995;14(4):222‐6. [Google Scholar]
Rosell‐Ortiz 2008 {published data only}
- Rosell‐Ortiz F, Mellado‐Vergel FJ, Ruiz‐Bailén M, Perea‐Milla E. Out‐of‐hospital treatment and 1‐year survival in patients with ST‐elevation acute myocardial infarction. Results of the Spanish Out‐of‐Hospital Fibrinolysis Evaluation Project (PEFEX). Revista Espanola De Cardiologia 2008;61(1):14‐21. [PubMed] [Google Scholar]
Rosenberg 2002 {published data only}
- Rosenberg DG, Levin E, Lausell A, Brown A, Gardner J, Perez E, et al. Feasibility and timing of prehospital administration of reteplase in patients with acute myocardial infarction. Journal of Thrombosis and Thrombolysis 2002;13(3):147‐53. [DOI] [PubMed] [Google Scholar]
Roth 1990 {published data only}
- Roth A, Barbash GI, Hod H, Miller HI, Rath S, Modan M, et al. Should thrombolytic therapy be administered in the mobile intensive care unit in patients with evolving myocardial infarction? A pilot study. Journal of the American College of Cardiology 1990;15(5):932‐6. [DOI] [PubMed] [Google Scholar]
Rozenman 1994 {published data only}
- Rozenman Y, Gotsman M, Weiss T, Lotan C, Mosseri M, Sapoznikov D, et al. Very early thrombolysis in acute myocardial infarction ‐ a light at the end of the tunnel. Israel Journal of Medical Sciences 1994;30(1):99‐107. [PubMed] [Google Scholar]
Rozenman 1995 {published data only}
- Rozenman Y, Gotsman MS, Weiss AT, Lotan C, Mosseri M, Sapoznikov D, et al. Early intravenous thrombolysis in acute myocardial infarction: the Jerusalem experience. International Journal of Cardiology 1995;49(Suppl):S21‐S28. [DOI] [PubMed] [Google Scholar]
Ruda 2009 {published data only}
- Ruda Mla, Kuz'min AI, Merkulova IN, Samko AN, Merkulov EV, Sozykin AV, et al. Spontaneous reperfusion of the infarct‐related artery in patients with ST elevated myocardial infarction. Terapevticheskii Arkhiv 2009;81(5):20‐9. [PubMed] [Google Scholar]
Smalling 2007 {published data only}
- Smalling RW, Giesler GM, Julapalli VR, Denktas AE, Sdringola SM, Vooletich MT, et al. Pre‐hospital reduced‐dose fibrinolysis coupled with urgent percutaneous coronary intervention reduces time to reperfusion and improves angiographic perfusion score compared with prehospital fibrinolysis alone or primary percutaneous coronary intervention: results of the PATCAR Pilot Trial. Journal of the American College of Cardiology 2007;50(16):1612‐4. [DOI] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith AM, Hardy PJ, Sandler DA, Cooke J. Paramedic decision making: prehospital thrombolysis and beyond. Emergency Medicine Journal 2011;28(8):700‐2. [DOI] [PubMed] [Google Scholar]
Stewart 1993 {published data only}
- Stewart C. Prehospital thrombolysis. Emergency Medical Services 1993;22(10):46‐54. [Google Scholar]
Svensson 2003 {published data only}
- Svensson L, Karlsson T, Nordlander R, Wahlin M, Zedigh C, Herlitz J. Safety and delay time in prehospital thrombolysis of acute myocardial infarction in urban and rural areas in Sweden. American Journal of Emergency Medicine 2003;21(4):263‐70. [DOI] [PubMed] [Google Scholar]
Tatu‐Chitoiu 2002 {published data only}
- Tatu‐Chitoiu G, Oprisan M, Cismara O, Marinescu R, Marinescu A. Streptokinase and enoxaparin in the pre‐hospital management of the ST‐segment elevation acute myocardial infarction. Romanian Journal of Internal Medicine 2002;40(1‐4):11‐25. [PubMed] [Google Scholar]
Topol 1986 {published data only}
- Topol EJ, Fung AY, Kline E, Kaplan L, Landis D, Strozeski M, et al. Safety of helicopter transport and out‐of‐hospital intravenous fibrinolytic therapy in patients with evolving myocardial infarction. Catheterization and Cardiovascular Diagnosis 1986;12(3):151‐5. [PubMed] [Google Scholar]
Trent 1995 {published data only}
- Trent R, Adams J, Jennings K, Rawles J. Impact of resuscitation and thrombolysis on mortality rate from acute myocardial infarction. International Journal of Cardiology 1995;49(1):33‐7. [DOI] [PubMed] [Google Scholar]
Walletin 2003 {published data only}
- Wallentin L, Goldstein P, Armstrong PW, Granger CB, Adgey AA, Arntz HR, et al. Efficacy and safety of tenecteplase in combination with the low‐molecular‐weight heparin enoxaparin or unfractionated heparin in the prehospital setting: the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)‐3 PLUS randomized trial in acute myocardial infarction. Circulation 2003;108(2):135‐42. [DOI] [PubMed] [Google Scholar]
Weaver 1994 {published data only}
- Weaver WD. Prehospital thrombolysis in myocardial infarction. Hospital Practice 1994;29(4):77‐82, 85. [DOI] [PubMed] [Google Scholar]
White 1990 {published data only}
- White HD. The effects of streptokinase and tissue plasminogen activator on left ventricular function. Advances in Experimental Medicine and Biology 1990;281:383‐7. [DOI] [PubMed] [Google Scholar]
Woollard 2005 {published data only}
- Woollard M, Pitt K, Hayward AJ, Taylor NC. Limited benefits of ambulance telemetry in delivering early thrombolysis: a randomised controlled trial. Emergency Medicine Journal 2005;22(3):209‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeymer 2009 {published data only}
- Zeymer U, Arntz H, Dirks B, Ellinger K, Genzwurker H, Nibbe L, et al. Reperfusion rate and inhospital mortality of patients with ST segment elevation myocardial infarction diagnosed already in the prehospital phase: results of the German Prehospital Myocardial Infarction Registry (PREMIR). Resuscitation 2009;80(4):402‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Antman 2008
- Antman E. Time is muscle: translation into practice. Journal of the American College of Cardiology 2008;52(15):1216. [DOI] [PubMed] [Google Scholar]
Armstrong 2006
- Armstrong PW, Chang WC, Wallentin L, Goldstein P, Granger CB, Bogaerts K, et al. Efficacy and safety of unfractionated heparin versus enoxaparin: a pooled analysis of ASSENT‐3 and‐3 PLUS data. Canadian Medical Association Journal 2006;174(10):1421‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbagelata 2007
- Barbagelata A, Perna E, Clemmensen P, Uretsky F, Canella J, Califf M, et al. Time to reperfusion in acute myocardial infarction. It is time to reduce it!. Journal of Electrocardiology 2007;40(3):257‐64. [DOI] [PubMed] [Google Scholar]
Beers 2006
- Beers M, Porter R, Jones J, Kaplan J, Berkwits M, Albert R, et al. The Merck Manual of Diagnosis and Therapy. 18th Edition. Boston: Wiley‐Blackwell, 2006. [Google Scholar]
Björklund 2006
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre‐hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance‐transported real‐life patients with ST‐elevation myocardial infarction. European Heart Journal 2006;27(10):1146‐52. [DOI] [PubMed] [Google Scholar]
Bonnefoy 2009
- Bonnefoy E, Steg P, Boutitie F, Dubien P, Lapostolle F, Roncalli J, et al. Comparison of primary angioplasty and pre‐hospital fibrinolysis in acute myocardial infarction (CAPTIM) trial: a 5‐year follow‐up. European Heart Journal 2009;30(13):1598‐606. [DOI] [PubMed] [Google Scholar]
Brouwer 1996
- Brouwer MA, Martin JS, Maynard C, Wirkus M, Litwin PE, Verheugt FWA, et al. Influence of early prehospital thrombolysis on mortality and event‐free survival (the Myocardial Infarction Triage and Intervention [MITI] Randomized Trial). MITI Project Investigators. The American Journal of Cardiology 1996;78(5):497‐502. [DOI] [PubMed] [Google Scholar]
Curtis 2006
- Curtis, Portnay J, Wang E, Herrin Y, Bradley J, Magid E, et al. The pre‐hospital electrocardiogram and time to reperfusion in patients with acute myocardial infarction, 2000‐2002:findings from the National Registry of Myocardial Infarction‐4. Journal of the American College of Cardiology 2006;47(8):1544‐52. [DOI] [PubMed] [Google Scholar]
Fox 2004
- Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST‐elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110(10):1202‐8. [DOI] [PubMed] [Google Scholar]
Gersh 2010
- Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. European Heart Journal 2010;31(6):642‐8. [DOI] [PubMed] [Google Scholar]
Goodacre 2002
- Goodacre S, Locker T, Morris F, Campbell S. How useful are clinical features in the diagnosis of acute, undifferentiated chest pain?. Academic Emergency Medicine 2002;9(3):203‐8. [DOI] [PubMed] [Google Scholar]
Goodacre 2003
- Goodacre S, Angelini K, Arnold J, Revill S, Morris F. Clinical predictors of acute coronary syndromes in patients with undifferentiated chest pain. QJM 2003;96(12):893‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IOM 2010
- IOM (Institute of Medicine). Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
ISIS‐2 1988
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2. ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;332(8607):349‐60. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mackay 2004
- Mackay J, Mensah GA. Chapter 13: Global burden of coronary heart disease. In: The Atlas of Heart Disease and Stroke. www.who.int/cardiovascular_diseases/resources/atlas/en/. Gineva: WHO, 2004 (accessed 8 August 2014):46‐47. [Google Scholar]
Mensah 2008
- Mensah GA. Ischaemic heart disease in Africa. Heart 2008;94(7):836‐43. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2010
- O'Connor RE, Brady W, Brooks SC, Diercks D, Egan J, Ghaemmaghami C, et al. Part 10: acute coronary syndromes. Circulation 2010;122(18 Suppl 3):S787‐817. [DOI] [PubMed] [Google Scholar]
Rawles 2003
- Rawles JM. GREAT: 10 year survival of patients with suspected acute myocardial infarction in a randomised comparison of prehospital and hospital thrombolysis. Heart 2003;89(5):563‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rogers 2008
- Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non‐ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. American Heart Journal 2008;156(6):1026‐34. [DOI] [PubMed] [Google Scholar]
Ruff 2011
- Ruff C, Braunwald E. The evolving epidemiology of acute coronary syndromes. Nature Review Cardiology 2011;8(3):140‐70. [DOI] [PubMed] [Google Scholar]
SAMF 2010
- Rossiter D. South African Medicines Formulary. 9th Edition. Cape Town: South African Medical Association, 2010. [Google Scholar]
Sayah 2008
- Sayah A, Roe M. The role of fibrinolytics in the prehospital treatment of ST‐elevation myocardial infarction (STEMI). The Journal of Emergency Medicine 2008;34(4):405‐16. [DOI] [PubMed] [Google Scholar]
Thygesen 2007
- Thygesen K, Albert J, White H, Jaffe A, Apple F, Galvani M, et al. Universal definition of myocardial infarction. Circulation 2007;116(22):2636‐53. [DOI] [PubMed] [Google Scholar]
Van de Werf 2008
- Werf F, Bax J, Betriu A, Blomstrom‐Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation: the Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. European Heart Journal 2008;29(23):2909‐45. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Cardiovascular diseases (CVDs). Fact sheet No 317, 2011.. www.who.int/mediacentre/factsheets/fs317/en/index.html 2011 (accessed 18 September 2012).


