Abstract
Background
Advances in minimally invasive surgery for live kidney donors have led to the development of laparoendoscopic single site donor nephrectomy (LESS‐DN). At present, laparoscopic donor nephrectomy is the technique of choice for donor nephrectomy globally. Compared with open surgical approaches, laparoscopic donor nephrectomy is associated with decreased morbidity, faster recovery times and return to normal activity, and shorter hospital stays. LESS‐DN differs from standard laparoscopic donor nephrectomy; LESS‐DN requires a single incision through which the procedure is performed and donor kidney is removed. Previous studies have hypothesised that LESS‐DN may provide additional benefits for kidney donors and stimulate increased donor rates.
Objectives
This review looked at the benefits and harms of LESS‐DN compared with standard laparoscopic nephrectomy for live kidney donors.
Search methods
We searched the Cochrane Kidney and Transplant's Specialised Register to 28 January 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
We included randomised controlled trials (RCTs) that compared LESS‐DN with laparoscopic donor nephrectomy in adults.
Data collection and analysis
Three authors independently assessed studies for eligibility and conducted risk of bias evaluation. Summary estimates of effect were obtained using a random‐effects model and results were expressed as risk ratios (RR) or risk difference (RD) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes.
Main results
We included three studies (179 participants) comparing LESS‐DN with laparoscopic donor nephrectomy. There were no significant differences between LESS‐DN and laparoscopic donor nephrectomy for mean operative time (2 studies, 79 participants: MD 6.36 min, 95% CI ‐11.85 to 24.57), intra‐operative blood loss (2 studies, 79 participants: MD ‐8.31 mL, 95% CI ‐23.70 to 7.09), or complication rates (3 studies, 179 participants: RD 0.05, 95% CI ‐0.04 to 0.14). Pain scores at discharge were significantly less in the LESS‐DN group (2 studies, 79 participants: MD ‐1.19, 95% CI ‐2.17 to ‐0.21). For all other outcomes (length of hospital stay; length of time to return to normal activities; blood transfusions; conversion to another form of surgery; warm ischaemia time; total analgesic requirement; graft loss) there were no significant differences observed.
Although risk of bias was assessed as low overall, one study was assessed at high risk of attrition bias.
Authors' conclusions
Given the small number and size of included studies it is uncertain whether LESS‐DN is better than laparoscopic donor nephrectomy. Well designed and adequately powered RCTs are needed to better define the role of LESS‐DN as a minimally invasive option for kidney donor surgery.
Plain language summary
Laparoendoscopic single‐site donor nephrectomy (LESS‐DN) versus standard laparoscopic donor nephrectomy
Background
Kidney transplantation is well established and has a vital role in improving quality of life and longevity for people with end‐stage kidney disease (ESKD). Open surgical techniques lead to more postoperative pain, a longer hospital stay and a poorer cosmetic outcome for donors but advances in surgical techniques has meant that keyhole surgery is now the gold standard for removing donors' kidneys for transplantation. Keyhole surgery has shorter recovery times, minimal scarring and better outcomes for both donor and recipient. An alternative of this technique is for the surgeon to make a single incision (single site) in the patient's abdomen to enable removal of the kidney.
We aimed to assess if LESS‐DN provided benefits compared with standard laparoscopic donor nephrectomy.
Study characteristics
We searched to literature up to January 2016 and found three studies (reported in 5 publications that involved 179 participants) that compared these two types of kidney donor surgeries.
Key results
LESS‐DN was found to be as safe as standard keyhole surgical techniques; pain at discharge was significantly less with LESS‐DN, however there were no other discernible benefits over the standard technique.
Quality of the evidence
Overall, we found there was a low risk of bias for all studies; however, funding sources were not reported in two or three studies, and there was high risk of attrition bias in one study. The small number of studies with few participants eligible for inclusion indicates a need for future research in this area.
Background
Description of the condition
Kidney transplantation involves the removal of a kidney from one person and re‐implantation into another who has little or no native kidney function. Because nephrectomy for transplantation can involve live donors, reducing postoperative morbidity is paramount; hence, laparoscopic donor nephrectomy has become the technique of choice globally for obtaining kidney grafts from living donors for transplantation (Canes 2010; Kurien 2011; Ramasamy 2011; Walsh 2012). Compared with open nephrectomy, laparoscopic approaches are associated with decreased morbidity, shorter hospital stays, rapid recovery period, quicker return to normal daily activities, better cosmetic results, and reduced postoperative pain (Kok 2006; Kurien 2011; Tugcu 2010; Wilson 2011).
Description of the intervention
Laparoendoscopic surgery has evolved from laparoscopic surgery as technological advances have been made in the development of multichannel single ports and curved articulating instruments (Granberg 2010). Although various terms have been used to describe this method, the LaparoEndoscopic Single Site Surgery Consortium for Assessment and Research has agreed on laparoendoscopic single‐site surgery (LESS) (Gill 2010). Several centres have published results of LESS donor nephrectomy (LESS‐DN), partial nephrectomy, pyeloplasty, and other urological procedures (Canes 2010; Kurien 2011; Park 2010; Tugcu 2010; Walsh 2012).
Studies into graft function after LESS‐DN have shown comparable results compared to laparoscopic donor nephrectomy (Afaneh 2011; Canes 2010; Inoue 2015; Kurien 2011)
How the intervention might work
LESS‐DN has been reported to be a safe and effective alternative to laparoscopic donor nephrectomy with better cosmetic results and less postoperative pain (Canes 2010; Kurien 2011; Park 2010; Ramasamy 2011; Tugcu 2010). Ramasamy 2011 found that LESS‐DN was as safe as laparoscopic donor nephrectomy performed using the modified Clavien classification system. However, reported advantages were derived from small cohort studies.
Laparoscopic donor nephrectomy is associated with lower rates of morbidity which is hoped will encourage the evaluation of more potential donors for surgery. It is anticipated that if LESS‐DN was found to demonstrate significant benefits and improved outcomes, this may further stimulate kidney donor rates and successes (Canes 2010).
Why it is important to do this review
Dialysis is a viable treatment alternative for patients with ESKD, particularly in settings with low donor rates. However, dialysis is associated with increased morbidity and mortality may significantly reduce long‐term survival (Gajdos 2013; Suzuki 2012; Unsal 2012). Kidney transplantation provides a viable option to improve long‐term survival, and approaches to reduce convalescence time, postoperative complications, and improve cosmetic results are highly desirable outcomes for kidney donors (Canes 2010; Soliman 2011). Faster recovery times and reduced pain attributed to LESS‐DN and laparoscopic nephrectomy can improve immediate postoperative quality of life for kidney donors and facilitate speedier return to normal activities such as work.
Objectives
This review looked at the benefits and harms of LESS‐DN compared with laparoscopic donor nephrectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) to look at comparisons of LESS‐DN and laparoscopic donor nephrectomy.
Types of participants
Inclusion criteria
Adults undergoing nephrectomy for live organ donation were considered for inclusion.
Exclusion criteria
We excluded children and patients undergoing nephrectomy for cancer or benign kidney disease.
Types of interventions
Studies comparing LESS‐DN versus laparoscopic donor nephrectomy were included. Comparisons of LESS‐DN with procedures other than laparoscopic donor nephrectomy were excluded.
Types of outcome measures
Primary outcomes
Operative times
Estimated intra‐operative blood loss
Postoperative pain scores
Complications.
Secondary outcomes
Length of hospitalisation
Length of time to return to normal activities
Blood transfusion rates
Conversion rates
Analgesic requirement postoperatively
Warm ischaemia time
Length of surgical wound, trocar size used
For donor nephrectomy: graft survival
Cost analysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant's Specialised Register up to 28 January 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by three authors who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on studies were retained initially. Three authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by three authors using standard data extraction forms. Studies reported in non‐English language journals were included. Where more than one publication of one study existed, reports were grouped together and only the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies between published versions were highlighted. Disagreements were resolved by consensus or arbitration by a third author where required.
Assessment of risk of bias in included studies
The following items were independently assessed by three authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes, such as patient demographics, complications, blood transfusion rates, conversion rates, and graft survival, were expressed as risk ratios (RR) with 95% confidence intervals (CI). Continuous outcomes, such as operative times, blood loss, postoperative pain scores, length of hospital stay, length of time to return to normal activities, and cost analysis were expressed as mean difference (MD) or SMD if different scales (e.g. pain scores) were used. Where possible, the risk difference with 95% CI was to be calculated for each outcome.
Unit of analysis issues
Only simple parallel group designs were available for comparison of these surgical techniques.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing the corresponding author) and any relevant information obtained in this manner was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
If possible, funnel plots were to be used to assess for the potential existence of small study bias (study effects versus study size) (Higgins 2011). This could not be performed due to the small number of included studies.
Data synthesis
Data were pooled using the random‐effects model and the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were to be used to explore possible sources of heterogeneity (e.g. participants, analyses of the impact of studies with poor methodology on the final result). Heterogeneity among participants could be related to demographics such as age and weight. Heterogeneity in treatments could be related to experience of the operating surgeon or assisting staff. Unfortunately there were insufficient studies to permit subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size, however there were in sufficient studies to permit these analyses.
Repeating the analysis taking account of risk of bias
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
-
Repeating the analysis excluding studies using the following filters:
diagnostic criteria
language of publication
source of funding (industry versus other)
country
conversion rate
donation versus kidney disease nephrectomy
extraction site
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The literature search identified six reports (four studies); one study was excluded after screening abstracts and titles. We identified three studies (five reports) that enrolled 179 participants which met our inclusion criteria (Figure 1).
1.
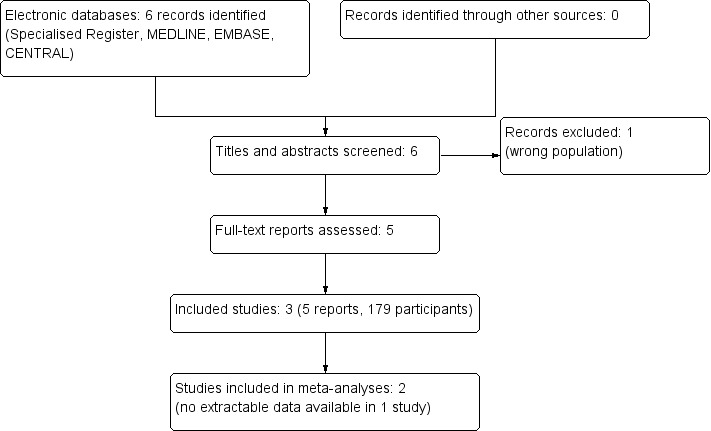
Study flow diagram
Included studies
We included three small RCTs (Aull 2014; Kurien 2011; Richstone 2013; 179 participants). Patient demographic data were comparable between arms in all studies. All studies measured postoperative pain using visual analogue pain scales/scores (VAPS/VA), length of hospital stay, analgesic requirement, estimated blood loss, operative duration, rates of conversion/complications and warm ischaemia time.
Aull 2014 (3 reports; 100 participants) conducted a parallel RCT that compared LESS‐DN and laparoscopic donor nephrectomy at a single centre in the USA. They assessed patient self‐reported "return to 100%" using de‐identified postal questionnaires. "Return to 100%" was measured by patients as the number of postoperative days they felt they were functioning at full capacity within 30 days. The study report did not include the questions asked, and the questionnaire was not standardised or validated.
Kurien 2011 (50 participants) performed a parallel RCT that compared LESS‐DN and laparoscopic donor nephrectomy in inpatient and outpatient settings of a single institution in India. The LESS‐DN procedure was carried out by a single surgeon. They measured difficulty of surgical steps (VAS, 1 to 10); reported patient cosmetic, quality of life and body image scores; measured estimated GFR (eGFR) and graft complication rates among kidney transplant recipients for one year after surgery.
Richstone 2013 (29 participants) reported outcomes of a parallel RCT that compared Pfannenstiel LESS‐DN and laparoscopic donor nephrectomy at a single centre in the USA. They blinded patients to the surgical intervention and attempted to blind postoperative caregivers by placing identical dressings and sutures on patients' abdomens.
Kurien 2011 and Richstone 2013 performed LESS‐DN for patients who required left sided donor nephrectomy; those deemed more suited to right donor nephrectomy were excluded. Aull 2014 had no exclusion criteria and patients requiring right donor nephrectomy were also included in this study.
Excluded studies
Tugcu 2010 compared single‐site nephrectomy with standard laparoscopic nephrectomy in the context of simple nephrectomy for benign kidney disease.
Risk of bias in included studies
Overall the included studies showed a low level of bias (Figure 2).
2.
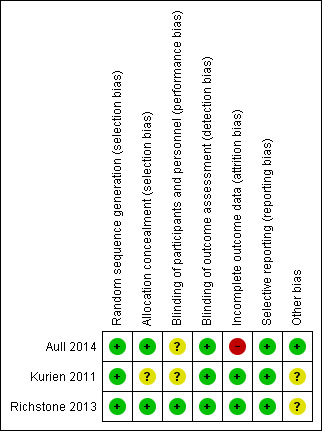
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
All studies used an established method of randomisation. Aull 2014 involved a research coordinator to allocate patients to study arms using a stratified and blocked randomisation developed by a statistician, which ensured that after four patients were enrolled in any given stratification two would be assigned to each study arm. Kurien 2011 used a chit method using block randomisation, which comprised of the assessor or participant taking a chit from a container indicating the study arm to which they were designated and then blocked to achieve equal numbers in each group. Richstone 2013 used opaque envelopes to randomise patients and allocation was concealed from participants, caregivers and data assessors.
There was no reported concealment of randomisation from either assessors or participants in Kurien 2011 and Aull 2014. Studies were assessed at low risk of allocation bias.
Blinding
Aull 2014 did not report blinding of participants or assessors. Kurien 2011 used de‐identified data collection forms to assess cosmetic and quality of life questionnaires. Richstone 2013 blinded participants and postoperative carers by placing sham sutures and dressings on the abdomens of both LESS‐DN and laparoscopic donor nephrectomy patients. We assessed that there was a low risk of detection bias among the included studies. Aull 2014 and Kurien 2011 were assessed as unclear level of performance bias because they did not state if this was considered; however, we do not believe this would have impacted the study's results or findings significantly.
Incomplete outcome data
Aull 2014 reported that three participants were lost to follow‐up from the laparoscopic donor nephrectomy group and that 25% of participants did not return questionnaires at the two month assessment point which introduced a high level of attrition bias. No participants withdrew or were removed from the study in Kurien 2011. Richstone 2013 reported that one patient required conversion to laparoscopic donor nephrectomy but results were analysed on an intention‐to‐treat basis.
Selective reporting
Our assessment found no evidence of selective reporting in any included study (Aull 2014; Kurien 2011; Richstone 2013).
Other potential sources of bias
Aull 2014 received partial financial support from the Clinical Translational Science Centre but this was considered to introduce low risk of bias. Neither Kurien 2011 nor Richstone 2013 reported funding sources, and were assessed as unclear.
Effects of interventions
Primary outcomes
Operative times
There was no significant difference in operative times between LESS‐DN and laparoscopic donor nephrectomy (Analysis 1.1 (2 studies, 79 participants): MD 6.36 min, 95% CI ‐11.85 to 24.57; I2 = 21%). Aull 2014 reported data as median and range figures and could not be incorporated into our analysis.
1.1. Analysis.
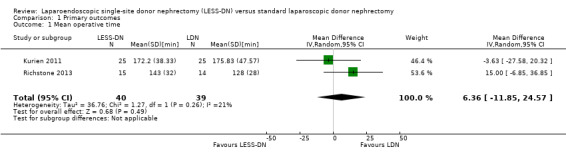
Comparison 1 Primary outcomes, Outcome 1 Mean operative time.
Estimated intra‐operative blood loss
There was no significant difference in estimated intra‐operative blood loss between LESS‐DN and laparoscopic donor nephrectomy (Analysis 1.2 (2 studies, 79 participants): MD ‐8.31 mL, 95% CI ‐23.70 to 7.09; I2 = 0%). Data from Aull 2014 could not be incorporated into our analysis.
1.2. Analysis.
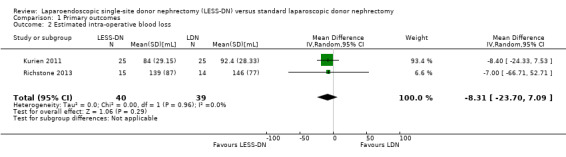
Comparison 1 Primary outcomes, Outcome 2 Estimated intra‐operative blood loss.
Postoperative pain scores at discharge
VAPS at the time of hospital discharge was significantly less in the LESS‐DN patients compared to laparoscopic donor nephrectomy patients (Analysis 1.3 (2 studies, 79 participants): MD ‐1.19, 95% CI ‐2.17 to ‐0.21). There was moderate heterogeneity (I2 = 57%) which could be accounted for by the use of non‐standardised scoring systems by each study. Data from Aull 2014 could not be incorporated into our analysis.
1.3. Analysis.
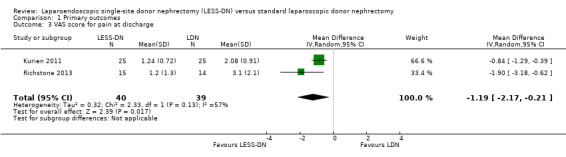
Comparison 1 Primary outcomes, Outcome 3 VAS score for pain at discharge.
Complications within 30 days of initial operation
Aull 2014 reported complications up to 30 days after the initial operation. There was no significant difference in complications between the two interventions and there was no significant heterogeneity (Analysis 1.4 (3 studies, 179 participants): RD 0.05, 95% CI ‐0.04 to 0.14; I2 = 0%). Kurien 2011 reported that seven patients sustained Clavien grade 1 complications (laparoscopic donor nephrectomy (4); LESS‐DN (3)). Two patients (one in each group) sustained Clavien grade 2 complications. Richstone 2013 reported that one participant sustained an intra‐operative serosal tear, which was identified immediately and over sewn using the LESS‐DN technique. This patient went on to develop postoperative ileus and increased length of stay (Richstone 2013).
1.4. Analysis.

Comparison 1 Primary outcomes, Outcome 4 Complications.
Secondary outcomes
Hospital stay duration
LESS‐DN did not result in significant reduction in length of hospital stay (Analysis 2.1 (2 studies, 79 participants): MD ‐0.20 days, 95% CI ‐1.12 to 0.72; I2 = 82%). Heterogeneity was high. There were no relevant data that could be included for analysis from Aull 2014.
2.1. Analysis.
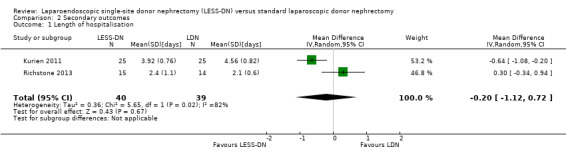
Comparison 2 Secondary outcomes, Outcome 1 Length of hospitalisation.
Length of time to return to normal activities
Aull 2014 found no reduction in time to return to normal activity between LESS‐DN and laparoscopic donor nephrectomy (Analysis 2.2 (100 participants): MD ‐3.00 days, 95% CI ‐11.35 to 5.35). Kurien 2011 and Richstone 2013 did not report on this outcome.
2.2. Analysis.
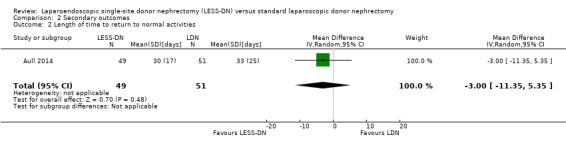
Comparison 2 Secondary outcomes, Outcome 2 Length of time to return to normal activities.
Blood transfusion rates
We found no significant reduction in blood transfusion rates between the interventions (Analysis 2.3 (3 studies, 179 participants): RD ‐0.01, 95% CI ‐0.05 to 0.03; I2 = 0%).
2.3. Analysis.
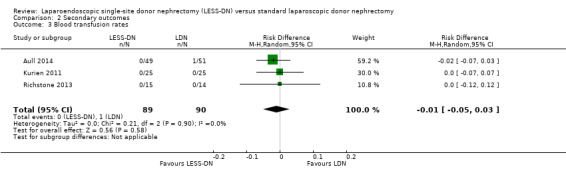
Comparison 2 Secondary outcomes, Outcome 3 Blood transfusion rates.
Conversion rates
No patients undergoing laparoscopic donor nephrectomy were reported to require conversion to open surgery; however Kurien 2011 and Richstone 2013 reported that two patients undergoing LESS‐DN required conversion to laparoscopic donor nephrectomy. Aull 2014 reported conversion to hand‐assisted laparoscopy (Analysis 2.4 (3 studies, 179 participants): RD 0.05, 95% CI ‐0.02 to 0.12; I2 = 0%).
2.4. Analysis.
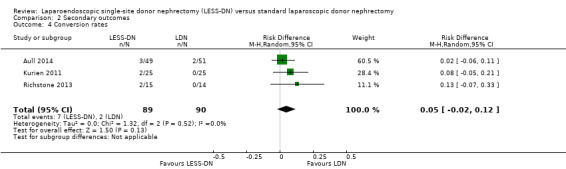
Comparison 2 Secondary outcomes, Outcome 4 Conversion rates.
Analgesia requirement postoperatively
All included studies used opiate (narcotic) based medication to manage postoperative pain in patients. Analgesia requirement and VAPS were recorded by all studies. We found no significant reduction in analgesia use in the LESS‐DN group (Analysis 2.6 (2 studies, 79 participants): MD 2.42 mg, 95% CI ‐38.10 to 42.94; I2 = 0%). Data from Aull 2014 could not be incorporated into our analysis.
2.6. Analysis.
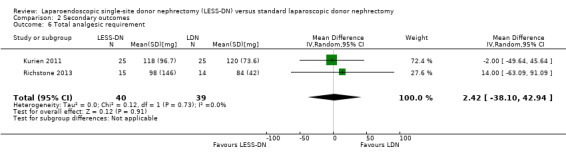
Comparison 2 Secondary outcomes, Outcome 6 Total analgesic requirement.
Warm ischaemia time
There was no significant difference in warm ischaemia time between the two groups (Analysis 2.5 (2 studies, 79 participants): MD 1.25 min, 95% CI ‐0.45 to 2.94). A high level of heterogeneity was demonstrated in this analysis (I2 = 80%). There is no evidence this is related to reporting bias and may be due to the experience of the operating surgeon or the different surgical techniques used. Data from Aull 2014 could not be incorporated into our analysis.
2.5. Analysis.
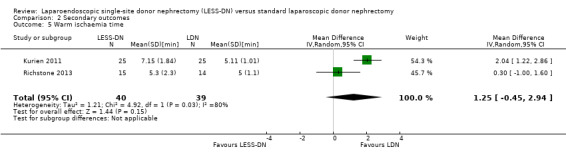
Comparison 2 Secondary outcomes, Outcome 5 Warm ischaemia time.
Length of surgical wound, trocar size used
Kurien 2011 investigated wound length and found this to be statistically shorter in the LESS‐DN group (P < 0.0001). This did not translate to a significant improvement in body image scores.
Graft survival
There was no significant difference in graft loss between LESS‐DN and laparoscopic donor nephrectomy (Analysis 2.7 (2 studies, 150 participants): RD 0.01, 95% CI ‐0.06 to 0.04; I2 = 0%). Three grafts were lost in total. One from a LESS‐DN patient and two from a laparoscopic donor nephrectomy. One death was due to Clostridium difficile colitis and the other two were attributed to cardiogenic causes. Richstone 2013 did not report graft loss.
2.7. Analysis.
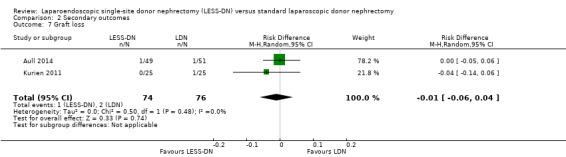
Comparison 2 Secondary outcomes, Outcome 7 Graft loss.
Cost analysis
Cost analyses were not performed by any of the included studies.
Other outcomes
Kurien 2011 found no significant difference in patient‐perceived scores for cosmetic outcome, body image or quality of life using standardised patient self‐scoring systems. Aull 2014 tracked days to 100% recovery but found no significant differences between participants in either study arm.
Discussion
Summary of main results
LESS‐DN offers an interesting development in minimally invasive surgical techniques. However, we found a paucity of well‐designed RCTs comparing LESS‐DN with standard laparoscopic donor nephrectomy. Assessment demonstrated that LESS‐DN had no discernible technical, clinical or cosmetic superiority to laparoscopic donor nephrectomy. Kurien 2011 concluded that in the context of donor nephrectomy, cosmetic outcome was less important to patients than recipient outcomes.
Kurien 2011 and Aull 2014 monitored postoperative kidney function and graft data. Results from these studies showed no significant increase in graft loss. Although these findings bode well for LESS‐DN, without more robust evidence it was not possible to determine if LESS‐DN was as safe physiologically as standard practice. Our results show a high level of heterogeneity for the analysis of warm ischaemia times, which was likely due to surgical expertise and that studies used slightly different surgical techniques (Kurien 2011; Richstone 2013).
All included studies used VAPS to assess postoperative pain; we found significantly less pain at discharge with LESS‐DN (MD ‐1.19, 95% CI ‐2.17 to ‐0.21), with a moderate level of heterogeneity which is most likely due to use of different scoring systems. Kurien 2011 rated surgical difficulty for LESS‐DN and laparoscopic donor nephrectomy and reported that graft retrieval was less challenging using laparoscopic donor nephrectomy (1.32 ± 0.56 versus 3.48 ± 1.92; P < 0.0001). Limited data could be extracted from Aull 2014, and many were not compatible with our methods. Attempts to obtain raw data were unsuccessful.
Kurien 2011 was the only study to present complications using the Clavien classification system. Aull 2014 and Richstone 2013 listed the type of complication but did not provide specific details and so we could not grade these. As a result our analysis of complications within 30 days of operation was performed on all reported complication from each study rather than by grade of complication. As a result we can only comment that complication rates were similar between LESS‐DN and laparoscopic donor nephrectomy (RD 0.05, 95% CI ‐0.04 to 0.14), but cannot make a judgement on the grade of these events and if they affected patient outcome.
Further adequately powered RCTs are needed to assess potential benefits of LESS‐DN for live kidney donors. However, given the popularity of robotic techniques a recent meta‐analysis indicated that this approach may supersede LESS‐DN (Autorino 2014).
Overall completeness and applicability of evidence
The included studies involved small numbers of participants. Much of the operative data from Aull 2014 could not be extracted for meta‐analysis. Cost analyses were not reported in the included studies and should be considered in future studies because this is likely to be a key factor in its implementation. Future studies should also endeavour to apply the use of well‐recognised and established scoring systems to measure data variables. This would aid future meta‐analyses.
Quality of the evidence
Although the included studies were well designed and assessed at low risk of bias overall, their small size and limited number did not permit robust conclusions to be derived from the available data.
Potential biases in the review process
Although a thorough search of the literature was conducted for this review, a fundamental limitation was the small number of RCTs that were eligible for inclusion, which was further compounded further by the numbers of participants in each study. At a study level, Aull 2014 presented very few data that could be incorporated into our analysis. An unsuccessful attempt was made to obtain raw data from Aull 2014.
Agreements and disagreements with other studies or reviews
Autorino 2014 reported reduced analgesic requirements and lower postoperative pain levels, we found less pain at discharge with LESS‐DN but no difference in analgesic requirements.
Authors' conclusions
Implications for practice.
Standard laparoscopic donor nephrectomy remains the standard of care for living kidney donor surgeries. While LESS‐DN presents an alternative approach, we did not find to be superior to current practice. The decision to offer LESS‐DN to patients should continue to be driven by local experience and availability, patient choice and surgeon preference. Further research is required to establish the role of LESS‐DN in kidney donor surgery.
Implications for research.
Large scale, multicentre RCTs providing operative and postoperative data are needed to evaluate LESS‐DN as an alternative to laparoscopic donor nephrectomy. We suggest that any future studies incorporate an objective and comprehensive analysis of cost, quality of life measures and length of time to return to work or full function. However, given the popularity of robotic techniques a recent meta‐analysis indicated that this approach may supersede LESS‐DN (Autorino 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2016 | Amended | Correction of typographical error in Plain Language Summary |
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review and the Cochrane Kidney and Transplant editorial team.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean operative time | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 6.36 [‐11.85, 24.57] |
| 2 Estimated intra‐operative blood loss | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐8.31 [‐23.70, 7.09] |
| 3 VAS score for pain at discharge | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.17, ‐0.21] |
| 4 Complications | 3 | 179 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.04, 0.14] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospitalisation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.12, 0.72] |
| 2 Length of time to return to normal activities | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐11.35, 5.35] |
| 3 Blood transfusion rates | 3 | 179 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 4 Conversion rates | 3 | 179 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 5 Warm ischaemia time | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 1.25 [‐0.45, 2.94] |
| 6 Total analgesic requirement | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 2.42 [‐38.10, 42.94] |
| 7 Graft loss | 2 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aull 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified and blocked randomisation was performed" Patients stratified according to three parameters: laterality, BMI > 30 and vascular complexity |
| Allocation concealment (selection bias) | Low risk | "Consenting subjects were randomised by the research coordinator using stratified and blocked randomisation generated by the study statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported but not relevant to outcome data reporting |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Missing data for three patients in the LDN group who reported not being fully recovered at 2 months, but who did not provide the actual time it took them to recover" Participants were lost from the laparoscopic donor nephrectomy arm which may have introduced attrition bias 12/51 and 13/49 participants did not return two month follow‐up questionnaires from LESS‐DN and laparoscopic donor nephrectomy groups respectively Questionnaires related to the study's primary outcome |
| Selective reporting (reporting bias) | Low risk | No bias identified |
| Other bias | Low risk | A study author received a grant from Clinical Translational Sciences Centre (UL1‐TR000457‐08) but it is unlikely that this influenced study outcomes |
Kurien 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised by chit method with block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding performed: de‐identified participant questionnaires assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Health survey and body image questionnaire were filled up by our trained transplant social worker who was blinded to the mode of nephrectomy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | Funding source not reported |
Richstone 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subject was randomised by the surgical team via opaque envelope method" |
| Allocation concealment (selection bias) | Low risk | "Patients were not informed of which approach would be used"; "LESS‐DN had 'sham' butterfly stitches" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and postoperative carers blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analysis performed on de‐identified data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None identified |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Unclear risk | Funding source not reported |
BMI ‐ body mass index; EBL ‐ estimated blood loss; eGFR ‐ estimated glomerular filtration rate; HCT ‐ haematocrit; IV ‐ intravenous; LESS‐DN ‐ laparoendoscopic single‐site donor nephrectomy; LOS ‐ length of stay; M/F ‐ male/female; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; VAPS ‐ visual analogue pain score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Tugcu 2010 | RCT looking at simple nephrectomy not donor nephrectomy |
RCT ‐ randomised controlled trial
Differences between protocol and review
The title of this review was changed from Laparoendoscopic single‐site donor nephrectomy (LESS‐N) versus standard laparoscopic donor nephrectomy to Laparoendoscopic single‐site donor nephrectomy (LESS‐DN) versus standard laparoscopic donor nephrectomy in keeping with widely used terminology.
Contributions of authors
Draft the protocol: OMA
Study selection: AG, OMA, KA
Extract data from studies: AG, OMA, KA
Enter data into RevMan: AG, OMA
Carry out the analysis: AG, OMA
Interpret the analysis: AG, OMA
Draft the final review: AG, OMA, KA, PD, PC
Disagreement resolution: PD, HK
Update the review: OMA
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Ameet Gupta: none known
Kamran Ahmed: none known
Howard G Kynaston: none known
Prokar Dasgupta: none known
Piotr L Chlosta: none known
Omar M Aboumarzouk: none known
Edited (no change to conclusions)
References
References to studies included in this review
Aull 2014 {published data only}
- Aull MJ, Afaneh C, Charlton M, Serur D, Douglas M, Christos PJ, et al. A randomized, prospective, parallel group study of laparoscopic versus laparoendoscopic single site donor nephrectomy for kidney donation. American Journal of Transplantation 2014;14(7):1630‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aull MJ, Afaneh C, Charlton MB, Rand E, Kapur S, Leeser DB, et al. Preliminary results of a randomized, controlled trial of laparoendoscopic single site donor nephrectomy vs. conventional laparoscopic donor nephrectomy for living kidney donors [abstract no: 423]. American Journal of Transplantation 2012;12(Suppl S3):153. [EMBASE: 70746370] [Google Scholar]
- Lee D, Otto B, Osterberg III EC, Aull M, Charlton M, Kapur S, et al. Randomized controlled trial of laparoendoscopic single site donor nephrectomy versus conventional laparoscopic donor nephrectomy for living kidney donors [abstract]. Journal of Urology 2013;189(4 Suppl 1):e869. [EMBASE: 71033060] [Google Scholar]
Kurien 2011 {published data only}
- Kurien A, Rajapurkar S, Sinha L, Mishra S, Ganpule A, Muthu V, et al. First prize: Standard laparoscopic donor nephrectomy versus laparoendoscopic single‐site donor nephrectomy: a randomized comparative study. Journal of Endourology 2011;25(3):365‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richstone 2013 {published data only}
- Richstone L, Rais‐Bahrami S, Waingankar N, Hillelsohn JH, Andonian S, Schwartz MJ, et al. Pfannenstiel laparoendoscopic single‐site (LESS) vs conventional multiport laparoscopic live donor nephrectomy: a prospective randomized controlled trial. BJU International 2013;112(5):616‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Tugcu 2010 {published data only}
- Tugcu V, Ilbey YO, Mutlu B, Tasci AI. Laparoendoscopic single‐site surgery versus standard laparoscopic simple nephrectomy: a prospective randomized study. Journal of Endourology 2010;24(8):1315‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Afaneh 2011
- Afaneh C, Aull MJ, Gimenez E, Wang G, Charlton M, Leeser DB, et al. Comparison of laparoendoscopic single‐site donor nephrectomy and conventional laparoscopic donor nephrectomy: donor and recipient outcomes. Urology 2011;78(6):1332‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Autorino 2014
- Autorino R, Brandao LF, Sankari B, Zargar H, Laydner H, Akca O, et al. Laparoendoscopic single‐site (LESS) vs laparoscopic living‐donor nephrectomy: a systematic review and meta‐analysis. BJU International 2014;115(2):206‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canes 2010
- Canes D, Berger A, Aron M, Brandina R, Goldfarb DA, Shoskes D, et al. Laparo‐endoscopic single site (LESS) versus standard laparoscopic left donor nephrectomy: matched‐pair comparison. European Urology 2010;57(1):95‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gajdos 2013
- Gajdos C, Hawn MT, Kile D, Robinson TN, Henderson WG. Risk of major nonemergent inpatient general surgical procedures in patients on long‐term dialysis. JAMA Surgery 2013;148(2):137‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gill 2010
- Gill IS, Advincula AP, Aron M, Caddedu J, Canes D, Curcillo PG 2nd, et al. Consensus statement of the consortium for laparoendoscopic single‐site surgery. Surgical Endoscopy 2010;24(4):762‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Granberg 2010
- Granberg CF, Gettman MT. Instrumentation for natural orifice translumenal endoscopic surgery and laparoendoscopic single‐site surgery. Indian Journal of Urology 2010;26(3):385‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Inoue 2015
- Inoue T, Tsuchiya N, Narita S, Tsuruta H, Akihama S, Saito M, et al. Successful introduction of laparoendoscopic single‐site donor nephrectomy after experience with laparoscopic single‐site plus‐one trocar donor nephrectomy. Journal of Endourology 2015;29(4):435‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kok 2006
- Kok NF, Lind MY, Hansson BM, Pilzecker D, Mertens zur Borg IR, Knipscheer BC, et al. Comparison of laparoscopic and mini incision open donor nephrectomy: single blind, randomised controlled clinical trial. [Reprint in Ned Tijdschr Geneeskd. 2007 Jun 16;151(24):1352‐60; PMID: 17665628]. BMJ 2006;333(7561):221. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2010
- Park YH, Park JH, Jeong CW, Kim HH. Comparison of laparoendoscopic single‐site radical nephrectomy with conventional laparoscopic radical nephrectomy for localized renal‐cell carcinoma. Journal of Endourology 2010;24(6):997‐1003. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramasamy 2011
- Ramasamy R, Afaneh C, Katz M, Chen X, Aull MJ, Leeser DB, et al. Comparison of complications of laparoscopic versus laparoendoscopic single site donor nephrectomy using the modified Clavien grading system. Journal of Urology 2011;186(4):1386‐90. [21855950] [DOI] [PubMed] [Google Scholar]
Soliman 2011
- Soliman SA, Shokeir AA, Kamal AI, El‐Hefnawy AS, Harraz AM, Kamal MM, et al. Long‐term outcome of grafts with multiple arteries in live‐donor renal allotransplantation: analysis of 2100 consecutive patients. Arab Journal of Urology 2011;9(3):171‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2012
- Suzuki H, Hoshi H, Inoue T, Kikuta T, Tsuda M, Takenaka T. Early start of combination therapy with hemodialysis and peritoneal dialysis prolongs survival and reduces cardiovascular events in male patients. Advances in Peritoneal Dialysis 2012;28:68‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Unsal 2012
- Unsal A, Basturk T, Koc Y, Sinangil A, Ahbap E, Sakaci T, et al. Factors associated with above and under 5‐year survival in peritoneal dialysis patients. Renal Failure 2012;34(9):1129‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 2012
- Wein A, Kavoussi L, Novick A, Partin A, Peters C (editors). Campbell‐Walsh Urology. 10th Edition. Vol. 2, Philadelphia: Elsevier Saunders, 2012. [Google Scholar]
Wilson 2011
- Wilson CH, Sanni A, Rix DA, Soomro NA. Laparoscopic versus open nephrectomy for live kidney donors. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD006124.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Aboumarzouk 2013
- Aboumarzouk OM, Ahmed K, Chlosta PL, Dasgupta P, Kynaston HG. Laparoendoscopic single‐site donor nephrectomy (LESS‐N) versus standard laparoscopic donor nephrectomy. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010850] [DOI] [PMC free article] [PubMed] [Google Scholar]


