Abstract
Background
Anal canal intraepithelial neoplasia (AIN) is a pre‐malignant condition of the anal canal transitional epithelium that is associated with human papillomavirus (HPV) infection. The incidence and prevalence of AIN and anal cancer are increasing rapidly in HIV‐positive men who have sex with men (MSM). Other groups like HIV‐negative MSM, immunosuppressed patients and people affected by other HPV diseases like genital warts and cervical intraepithelial neoplasia (CIN) may also develop AIN. The condition is complicated by its multicentric and multifocal nature and high rates of relapse and morbidity. Targeted excisions using ablative treatments such as cautery, infrared coagulation (IRC) and cryotherapy have been used as first‐line therapeutic strategies, and there are many other options. There is no consensus about the optimal management of AIN.
Objectives
To evaluate the effects of therapeutic interventions for anal canal intraepithelial neoplasia (AIN).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 4), MEDLINE and EMBASE (to October 2011). We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of included studies, and contacted experts in the field and manufacturers of any AIN and HPV‐specific treatments.
Selection criteria
Randomized controlled trials (RCTs) that assessed any type of intervention for AIN.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. If it was possible, the data were synthesised in a meta‐analysis.
Main results
We found only one RCT, which included 53 patients, that met our inclusion criteria. This trial reported data on imiquimod versus placebo. There was no statistically significant difference in the risk of disease cure but there was a trend for imiquimod to downgrade the AIN to a low‐risk stage. The lack of statistical power of the trial may be due to the small number of patients in each group. The risk of bias was estimated as moderate.
Authors' conclusions
The included trial failed to demonstrate any statistically significant efficacy of imiquimod in the management of anal intraepithelial neoplasia (AIN). The absence of reliable evidence for any of the interventions used in AIN precludes any definitive guidance or recommendations for clinical practice. Prospective cohort studies and retrospective studies have not been included in this review as they are considered to provide lower quality evidence. Well designed RCTs are needed.
Keywords: Humans, Aminoquinolines, Aminoquinolines/therapeutic use, Anal Canal, Antineoplastic Agents, Antineoplastic Agents/therapeutic use, Anus Neoplasms, Anus Neoplasms/drug therapy, Carcinoma in Situ, Carcinoma in Situ/drug therapy, Imiquimod, Precancerous Conditions, Precancerous Conditions/drug therapy, Randomized Controlled Trials as Topic
Plain language summary
Comparison of interventions for patients diagnosed with pre‐cancerous changes of the anal canal (anal intraepithelial neoplasia)
Persistent infection with some types of human papillomavirus (HPV) can cause anal canal intraepithelial neoplasia (AIN), a condition which may become cancerous. HPV is transmitted via skin‐to‐skin contact. There are over 100 different types of HPV virus. At least 30 of these can be sexually transmitted. HPV transmission occurs easily among sexual partners. Despite this, spontaneous clearance of the infection is generally the rule.
In some cases, HPV may produce genital warts or even some anogenital pre‐malignant or malignant lesions. The latter is especially the case when some HPV types (which are named 'high‐risk' HPV, like HPV types 16, 18, 31 and 33) persist on anogenital mucosa or skin over long periods of time. Viral persistence is very common in an HIV‐positive population, particularly in the anal mucosa of men who have sex with men. The incidence of AIN is reaching epidemic proportions in this population group. AIN is usually asymptomatic. People at risk need to be under prevention screening programs in order to identify and treat those pre‐cancerous lesions.
To date there is no consensus on AIN management. The most used strategies are ablative treatments. Lesions in the anal canal are treated with cautery, infrared coagulation, laser or cryotherapy. Other available options include surgical excision, topical treatments (imiquimod, trichloroacetic acid, 5‐fluorouracil), interferon and HPV vaccines. We identified many studies that examined all the interventions mentioned above. Only one trial with 53 patients was randomized and placebo‐controlled and could be included in this review. The trial compared self‐application of imiquimod cream in the anal canal with placebo. The analysis of the findings could not demonstrate a definitive superior effect of imiquimod compared with placebo in terms or cure. Some effectiveness could be observed in terms of improvement, with high‐risk stages of AIN being reduced to low‐risk stages.
We conclude that the available information on AIN treatment does not meet evidence‐based medicine requirements. Future trials should be designed as randomized controlled trials, and should include data about HPV load and the types affecting the treated patients, recurrence rates, progression to anal cancer and quality of life of patients.
Background
Description of the condition
Dysplasia in the transitional epithelium of the area located between the anal verge and the linea dentata is known as anal canal intraepithelial neoplasia (AIN) or as anal squamous intraepithelial lesions (ASILs). Those lesions are the most common pre‐malignant lesions in the canal anal area. AIN and anal cancer are causally linked to persistent infection with human papillomaviruses (HPV). Persistent infection of high‐risk types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82) and expression of E6 and E7 proteins can induce several degrees of dysplasia in the squamous‐columnar transitional zone of the anus.
Most HPV infections (up to 90%) are cleared within two years (Veldhuijzen 2010). Only a few persist in people within the general population, but in HIV‐positive patients persistence is the rule. HIV seropositivity is the main risk factor for AIN and anal cancer. Other identified risk factors are the presence of multiple HPV genotypes, other HPV‐related diseases (genital intraepithelial neoplasia or cancer), low CD4 count, cigarette smoking, ano‐receptive intercourse, and immunosuppression following a solid organ transplant (Kreuter 2008b; Simpson 2011). Despite highly active antiretroviral therapy (HAART), the incidence of AIN and anal cancer is reaching epidemic proportions with 70 to 100 cases per 100,000 per year among HIV‐positive men who have sex with men (MSM) (Meys 2010). AIN diagnosed in HIV‐positive MSM can reach prevalences of 21% to 59% (Kreuter 2008a). Persistent infections with high‐risk HPV types, high recurrences rates after treatment and increased longevity of HIV patients may lead to further rises in anal cancer in the post‐HAART era (Crum 2010). The incidence and prevalence are higher than they were for cervical dysplasia and cervix cancer among women before systematic cytology screening was introduced in western countries. Marked similarities in the biological and pathological profiles of cervical cancer and anal cancer suggest that anal cancer should be preventable in a similar way. Regular anal screening is recommended for MSM to detect and treat anal dysplasia as early as possible.
Description of the intervention
The mainstay of AIN management is ablative treatment or targeted excision. Surgical excision of the whole transformation zone (as is done with the cervix conization procedure) is not usually considered as an option because it may lead to considerable morbidity (mainly anal stenosis and dyschezia). Ablative strategies are widely used, including laser, cryotherapy, infrared coagulation (IRC) and cautery. Several additional strategies have been developed such as topical application of 5‐fluorouracil (5‐FU), podophyllotoxin or trichloroacetic acid; photodynamic therapy; vaccination; and interferon.
All those interventions are associated with considerable recurrences rates (Chang 2002; Goldstone 2005, Kreuter 2010). Multifocal disease, immunosuppression but mainly HPV persistence have been suggested as possible causes of recurrences. Persistence of HPV has been documented in previously treated areas and in peri‐lesional, healthy tissue.
How the intervention might work
Interventions for AIN are aimed at destroying the affected cells, directly or by enhancing the body’s immune response. The temporal relationship between the development of HPV infection, AIN and anal cancer has not been definitively proven. However, given the similarities between AIN and cervical intraepithelial neoplasia (CIN), the histologic similarities between the epithelium of the cervix and anus canal, and the role of HPV infection in these two diseases states, it is generally assumed that anal carcinoma evolves in a manner that is similar to cervical cancer, with worsening dysplasia progressing to invasive carcinoma. Eradication of dysplasia should lead to a decreased incidence of invasive cancer, a hypothesis confirmed in several observational studies on AIN patients. Scholefield et al, in a prospective study conducted between 1994 and 2003, observed 35 patients with high‐grade AIN (AIN‐III). Six patients were immunosuppressed and three of these patients (but none in the immunocompetent group) developed an invasive anal cancer (Scholefield 2005). In a larger series of 446 HIV‐positive MSM, five patients with high‐grade AIN who refused treatment developed anal cancer within a median of 8.6 months (Kreuter 2010).
Reduction of HPV DNA load, HPV types or eventual eradication of the infection may also achieve AIN remission, but currently this possibility is not supported by any evidence. Some interventions seem to induce a HPV‐specific immunity that may be associated with a decrease in DNA load and infecting HPV types, or even in eradication.
Why it is important to do this review
AIN and anal cancer are growing problems in the community, mainly among MSM and HIV‐infected individuals (Crum 2010). There is, however, a lack of data addressing the progression of AIN to anal cancer; likewise for the differences in treatment outcomes (for example on multifocal and monofocal AIN, high‐ and low‐grade AIN, HPV DNA load and HPV types, persistence of virus in peri‐lesional tissue, recurrences, etc).
There is no consensus nor clinical guidelines on the treatment of AIN that are supported by evidence‐based literature. An evaluation of the current evidence on the effects of interventions in AIN is therefore very important, and is also important for the design of future trials.
Objectives
To assess the effects of therapeutic interventions for anal canal intraepithelial neoplasia (AIN).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials of patients with AIN diagnosed by biopsy comparing topical, systemic, ablative, or surgical interventions to placebo or no treatment; and trials comparing various combinations of topical or systemic treatments to no treatment, placebo, or to other topical or systemic agents, ablative or surgical treatments. Inclusion was irrespective of publication year, publication status or language.
Types of participants
Patients with a confirmed histological diagnosis of AIN. Patients with a histological diagnosis of anal carcinoma, peri‐anal intraepithelial neoplasia, Paget's disease or Bowenoid papulosis were excluded.
Types of interventions
Any medical (topically or systemically administered), physical or surgical strategy:
i) electrocautery;
ii) cryotherapy, liquid nitrogen;
iii) infrared coagulation;
iv) radiotherapy;
v) surgical excision;
vi) imiquimod;
vi) photodynamic therapy;
vii) interferon;
viii) 5‐fluorouracil;
ix) trichloroacetic acid;
x) HPV vaccination;
xi) laser;
xii) any other intervention.
Types of outcome measures
Primary outcomes
AIN eradication defined by absence of histologic criteria after the intervention; the presence of a normal epithelium or scarring and the complete absence of dysplasia will be considered as the primary outcome
Human papillomavirus (HPV) eradication at weeks four, 12 and 48 after intervention
Development of invasive anal cancer
Secondary outcomes
Downgrading from AIN‐III or II to AIN‐I stage or from a high‐grade squamous intraepithelial lesion (HSIL) to a low‐grade squamous intraepithelial lesion (LSIL)
Adverse events requiring withdrawal of treatment
Quality of life
Recurrence, duration of remission or percentage of people with treated lesions that recur within 12 or 36 months
Any other adverse event according to the Common Terminology Criteria for Adverse Events (CTCAE 2009), which is a consensus language proposed by the National Cancer Institute (NCI)
Search methods for identification of studies
Our search strategy was developed to identify any relevant trials in the following databases. We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
Cochrane Colorectal Cancer Group (CCCG) Trials Register (up to October 2011). The register contains trials identified from MEDLINE and EMBASE and is included in the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
MEDLINE (up to October 2011)
EMBASE (up to October 2011)
The search strategies for MEDLINE and EMBASE are presented in Appendix 1 and Appendix 2.
Searching other resources
1. We searched for ongoing trials from the following sources:
WHO Trials Registry database: www.who.int/trialsearch;
Meta‐Register of Controlled Trials: www.controlled‐trials.com;
U.S. National Institutes of Health ongoing trials register: www.clinicaltrials.gov;
Australian and New Zealand Clinical Trials Registry: www.anzctr.org.au;
AIDS Clinical Trials Information Service (ACTIS): http://www.actis.org/;
National Research Register: http://www.nihr.ac.uk/Pages/NRRArchiveSearch.aspx;
Medical Research Council Clinical Trials Directory: http://www.ctu.mrc.ac.uk/.
Date of the most recent search of the register for this review: 27 October 2011.
2. References from published studies.
Handsearches were carried out of the reference lists in proctology and dermatology textbooks and from relevant articles. We also handsearched the following journals: British Journal of Surgery, Diseases of the Colon & Rectum.
3. Unpublished literature.
We handsearched conference proceedings for relevant abstracts, initiated correspondence with authors and made contact with pharmaceutical companies to identify unpublished data, confidential reports and raw data of published trials.
The possible pharmaceutical companies identified were MEDA (manufacturer of imiquimod), 3M (which investigated the molecule), GSK‐Stieffel (manufacturer of podophyllotoxin) and photodynamic therapy device manufacturers.
Data collection and analysis
Selection of studies
Two review authors independently checked all searches for eligibility. Titles and abstracts identified were checked. If it was clear that the study did not refer to an RCT on AIN, it was excluded. If it was unclear, then the full text of the study was obtained for independent assessment by two review authors (AM, MB). Authors decided which trials fitted the inclusion criteria. Disagreements were resolved by discussion between the review authors and, when necessary, by a third review author (AB). We listed the excluded studies and stated the reasons for exclusion in the Characteristics of excluded studies table. If additional information was necessary we tried to contact all the corresponding authors of the included studies and asked investigators for information about unpublished trials.
Data extraction and management
Data were extracted by two review authors (AM, MB) using a data extraction form, as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Extracted information included data on the following.
• Author, year of publication and journal citation (including language).
• Country.
• Setting.
• Inclusion and exclusion criteria.
• Study design, methodology.
• Study population:
total number enrolled;
patient characteristics;
age;
co‐morbidities;
previous treatment.
• AIN details:
unifocal or multifocal lesion;
grade;
size of lesion;
type of HPV.
• Details of dose and duration of any therapy used:
type of therapy;
dose (if appropriate);
duration (if appropriate).
• Risk of bias in study (see below). • Duration of follow‐up. • Outcomes: AIN eradication, HPV clearance, progression to anal cancer, downgrading from AIN‐III to AIN‐I, recurrence, quality of life (QoL), adverse events.
For each outcome:
outcome definition (with diagnostic criteria if relevant);
unit of measurement (if relevant);
for scales, upper and lower limits and whether high or low score is good;
for each outcome of interest, sample size; missing participants;
results, number of participants allocated to each intervention group.
Data on outcomes were extracted as follows. For dichotomous outcomes (for example adverse events, response to treatment, HPV infection), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at the endpoint, in order to estimate a relative risk (RR). Where possible, all data extracted were relevant to an intention‐to‐treat (ITT) analysis in which participants were analysed in the groups to which they were assigned. The time points at which outcomes were collected and reported were noted.
Data were abstracted independently by two review authors (AB, CM) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AM), when necessary. One review author (AM) entered data into Review Manager (RevMan).
Assessment of risk of bias in included studies
The risk of bias in the included RCTs was assessed using the Cochrane Collaboration’s tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2008). This included assessment of the following.
• Sequence generation: was the allocation sequence adequately generated? 'Yes' (computer generated random numbers, table of random numbers, coin‐tossing or similar), 'No' (day of week, even or odd clinic record number, clinician judgment, participant preference, laboratory test result such as haemoglobin value, or similar), or 'Unclear' (insufficient information about the sequence generation process to permit judgment). • Allocation concealment: was allocation adequately concealed? 'Yes' (central independent unit, sequentially numbered drug containers or sealed envelopes of identical appearance, or similar), 'No' (alternation or rotation, date of birth, non‐opaque envelopes, open table of random numbers or similar), or 'Unclear' (randomisation stated but no information on method used available). • Blinding (of participants, healthcare providers and outcome assessors): was knowledge of the allocated intervention adequately prevented during the study? 'Yes' (identical placebo medication or similar), 'No' (tablets versus liquid or similar), or 'Unclear' (blinding stated but no information on method used available). • Incomplete outcome data: were incomplete data dealt with adequately by the researchers? 'Yes' (no missing outcome data, missing outcome data balanced in numbers across intervention groups and reasons for dropouts and withdrawals described or similar), 'No' (reason for missing outcome data likely to be related to true outcome or similar), or 'Unclear' (number or reasons for dropouts and withdrawals not described). ◦ We recorded the proportion of participants whose outcomes were not reported at the end of the trial, and we noted if loss to follow‐up was not reported. We coded a satisfactory level of losses to follow‐up for each outcome as: ⋄ 'Yes', if fewer than 10% of patients were lost to follow‐up and reasons for losses to follow‐up were similar in both treatment arms; ⋄ 'No', if more than 10% of patients were lost to follow‐up or reasons for losses to follow‐up differed between treatment arms; ⋄ 'Unclear', if loss to follow‐up was not reported. • Selective reporting of outcomes: are reports of the study free of suggestion of selective outcome reporting? 'Yes' (study protocol is available, published reports include all expected outcomes or similar), 'No' (not all of the study’s pre‐specified primary outcomes have been reported, one or more reported primary outcomes were not pre‐specified, or similar), or 'Unclear' (insufficient information to permit judgement of 'adequate’ or 'inadequate’). • Other possible sources of bias, such as stopping the study early or extreme baseline imbalance, were also explored.
The risk of bias tool was applied independently by two review authors (AB, CM) and differences were resolved by discussion or by appeal to a third review author (AM). Results are presented in both a risk of bias graph and a risk of bias summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We expressed the results as relative risk (RR) and 95% confidence interval (CI) for dichotomous outcomes.
Unit of analysis issues
The unit of analysis considered was individual patients.
Dealing with missing data
Where possible, we contacted the trial authors to request data on the study protocol and outcomes. For adverse effect outcomes, missing data were imputed using the 'rule of three' (Hanley 1983). The rule of three states that if 'none of n' patients showed the event, we can be 95% confident that the chance of this event is at most 3/n.
Assessment of heterogeneity
Heterogeneity between trials could not be assessed since only one study was suitable for inclusion in the review.
Assessment of reporting biases
We contacted the authors of Fox 2010 to request data on their original protocol in order to better describe the allocation concealment and sequence generation.
Data synthesis
We only found one RCT suitable for inclusion in our review, so it was not possible to perform meta‐analyses nor to assess heterogeneity between results of trials. We did not produce a funnel plot to assess the potential for small study effects.
Subgroup analysis and investigation of heterogeneity
The subgroup analysis could not be undertaken since only one RCT was included.
Sensitivity analysis
An ITT analysis was conducted for dichotomous outcomes.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
One RCT was included in this review (Fox 2010). It was carried out in the United Kingdom. Participants were HIV‐positive MSM.
Results of the search
After excluding duplicates, the search strategy identified 1109 unique references. The abstracts of these were read independently by two review authors. The articles which did not meet the inclusion criteria were excluded at this stage. Twenty‐three articles were retrieved in full. The full text screening of these 23 references excluded 22 of them for the reasons described in the table Characteristics of excluded studies. Only one completed RCT met our inclusion criteria, which is described in the table Characteristics of included studies. Searches of the grey literature did not identify any additional trials.
We have found two ongoing randomised trials (see Characteristics of ongoing studies):
NCT00622440 Clinical Trial (Clinicaltrials.gov) is a randomised, placebo‐controlled, double‐blinded study based in San Francisco, USA. It will assess the efficacy of a Chinese herbal topical cream (arnebia indigo jade pearl). At the time of writing this review, no interim results were available and we await completion of the trial in November 2012.
Netherlands Trial Register identifier NTR1236 is a three‐arm study (cautery versus imiquimod versus 5‐fluorouracil) in HIV‐positive patients.
Included studies
Design of studies
One RCT with a total of 53 participants was included in this review (Fox 2010). The objective of the study was to determine wether imiquimod was more effective than placebo for the treatment of high‐grade squamous intraepithelial lesion (HSIL). The trial used a parallel group design with two arms (imiquimod group and placebo group). It was conducted in two hospitals in the United Kingdom and was a prospective, randomised, double‐blinded, placebo‐controlled trial. A second phase followed with the use of open‐label imiquimod. The results of that phase have not been analysed in this review because blinding and control with a placebo were no longer applied.
Patient characteristics
HIV‐positive MSM with histologically proven HSIL were randomised. Recruitment commenced in October 2002 and was terminated in April 2005. Initially the authors intended to recruit 120 patients, but the manufacturer of imiquimod (3M Health Care Ltd, Loughborough, UK) stopped providing the randomized study drug when 64 patients had consented. All HIV‐positive MSM participants had been on HAART for at least three months prior to the study, had > 100 CD4 cells/µl and had not used imiquimod in the anal canal previously. Baseline demographics reported in the article included age, tobacco use, median duration of HSIL, median nadir CD4 cells count, mean time HIV‐positive, time on HAART, presence of ongoing warts, use of a protease inhibitor and HIV RNA copies.
Intervention
In the Fox 2010 trial 28 participants self‐applied, no further than 2 cm into the anal canal, half a sachet of 1 g of imiquimod three times a week during a period of four months, and 25 participants self‐applied half a sachet of a manufacturer‐prepared placebo. Patients experiencing soreness were instructed not to continue to apply cream until symptom free, and where symptoms were marked the dosage was reduced. A diary to document treatment compliance and any side effect was given to patients. High‐resolution anoscopy (HRA) and cytologic and histologic assessment were carried out eight weeks after the end of the treatment; and HRA and cytology again at 12 months.
Outcomes
The trial reported as its main outcome 'improvement'. Improvement was defined as a combination of the patients who cleared AIN and patients who downgraded to LSIL: "by cytology 6 months following commencement, and sustained at one year of follow‐up". While the diagnosis of AIN was made by biopsy, assessment of the results of the intervention was done by HRA and cytology. HRA with mapping of the lesions, cytology and biopsy were performed just prior to commencement and repeated eight weeks after the intervention. The authors referred the readers to a previous publication (Fox 2005) for the histological definition of AIN and the description of the methods used. They did not mention if biopsies at week eight were extracted from the area where the lesion had been at initial assessment. Two pathologists examined the slides. Where there was disagreement, the most abnormal finding was used. No definition of 'resolution' was found in the text.
Progression to invasive cancer was documented. The study did not report QoL, HPV viral load or genotype. Participants were asked to report any adverse effect in a diary.
Excluded studies
Twenty‐two references were excluded after obtaining the full text (see 'Characteristics of excluded studies') for the following reasons. Anderson 2009 was a blinded RCT investigating the safety and immunogenicity of a HPV15 E6/E7 protein in HIV‐positive participants with oncogenic HPV infection of the anus. Resolution of AIN was not an outcome of the study, but histologic data about AIN diagnosis and response to treatment were available in six patients in the full paper version. However, this information was not available in the placebo cohort so only a comparison between six patients in four active‐treatment groups could be made. Anderson 2009 stated that "therefore, the study could not assess the effect on disease". Further information was not obtained from the study authors even after repeated requests. For instance, the authors mentioned that “only 2 participants with HPV‐16 at baseline cleared the virus” and it would have been interesting to know their histologic data. Chang 2002 was a prospective, uncontrolled, unblinded study of surgical treatment of AIN. Four studies (Cranston 2008; Goldstone 2005; Goldstone 2011; Marks 2011) were uncontrolled, unblinded retrospective clinical studies treated with an infrared coagulator or electrocautery. One study (de Pokomandy 2011) was a prospective follow‐up study to identify risk factors for high‐grade AIN. Fiander 2006 was a non‐randomized phase II trial in women with vulval and vaginal intraepithelial neoplasia. Jay 2009 and Richel 2010 were open‐label, non‐randomized prospective studies on 5‐fluorouracil; Klencke 2002 was an open‐label, non‐randomised unblinded study of the efficacy of encapsulated plasmid DNA; Kreuter 2008a was a prospective follow‐up study with imiquimod in 19 patients, but only three of them had anal canal AIN; Palefsky 2006 was an unblinded, open‐label phase I/II study with a HspE7. Palefsky 2011 was an RCT studying prevention of AIN, and patients affected by AIN were excluded; Pelletier 2004 was a prospective, randomized, double‐blind and vehicle‐controlled study assessing the capacity of imiquimod to eradicate latent HPV infection in HIV‐infected patients, patients with condylomata or AIN were excluded; Sanclemente 2007 was an open‐label study on HPV viral load in HIV‐positive patients treated with imiquimod for anogenital warts or anal intraepithelial neoplasia, but patients with AIN were excluded. Singh 2009 was a retrospective study of trichloroacetic acid on AIN; Smyth 2004 an uncontrolled, non‐randomized study; and Stier 2008 was a prospective, multicentre, open‐label pilot study. Snyder 2011 was a retrospective study of only 11 patients treated with 5‐fluorouracil. Van der Snoek 2009 and Webber 2004 were before‐after, uncontrolled, non‐randomized trials assessing the effects of photodynamic therapy in AIN. Wieland 2006 was a prospective, non‐randomized, open‐label pilot study of 28 HIV‐positive MSM, only five of them had AIN.
Risk of bias in included studies
The included study was randomized, placebo‐controlled, prospective, and double‐blinded. We considered its risk of bias to be moderate.
Allocation
For sequence generation, the authors stated that “the randomizations sequence was only known to 3M Health Care”, without any other details. We considered it 'unclear'. Allocation concealment was also unclear as the authors only stated that “patients were allocated the next randomizations number in the study”.
Blinding
The study was double‐blinded.
Incomplete outcome data
Reasons for withdrawal from the study were documented, but not the allocation of each participant lost to follow‐up. An ITT analysis was done.
Selective reporting
We had no access to the protocol of the included trial. Published reports included all expected outcomes.
Other potential sources of bias
Baseline characteristics were different between the active and the placebo groups. The aim of the study was well defined.
We have also considered in our assessment that the study was terminated earlier than previously designed due to “inability to obtain further supplies of randomised study drug after 64 patients had consented”.
Effects of interventions
Topical imiquimod versus placebo
We found only one trial (Fox 2010), which included 53 patients, that met our inclusion criteria. This trial reported data on imiquimod versus placebo.
AIN eradication
(See Analysis 1.1)
1.1. Analysis.
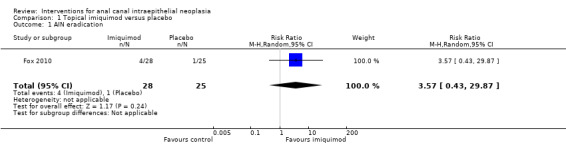
Comparison 1 Topical imiquimod versus placebo, Outcome 1 AIN eradication.
There was no statistically significant difference in terms of AIN eradication. Although AIN resolved in 4/28 patients in the imiquimod group and 1/25 in the placebo group, the RR was 3.57 (95% CI 0.43 to 29.87).
HPV eradication
This outcome was not studied in Fox 2010.
Progression to anal invasive cancer
(See Analysis 1.2)
1.2. Analysis.
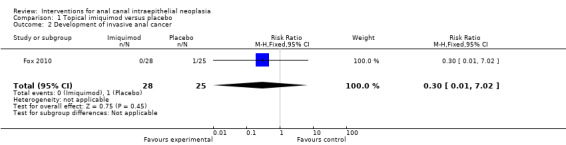
Comparison 1 Topical imiquimod versus placebo, Outcome 2 Development of invasive anal cancer.
There was no statistically significant difference in development of anal carcinoma (RR 0.30, 95% CI 0.01 to 7.02). Only one patient in the placebo group developed an anal squamous carcinoma.
Downgrading from AIN‐III or II to AIN‐I stage, or from HSIL to LSIL
(See Analysis 1.3)
1.3. Analysis.
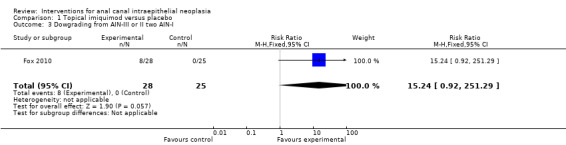
Comparison 1 Topical imiquimod versus placebo, Outcome 3 Dowgrading from AIN‐III or II two AIN‐I.
Eight participants in the active group downgraded to LSIL, while none did so in the placebo group. This was close to being a statistically significant difference (RR 15.24, 95% CI 0.92 to 251.29).
Adverse events
There was no description of adverse events. One patient abandoned the study because of side effects, but his allocated group was not given.
Recurrence
(See Analysis 1.4)
1.4. Analysis.
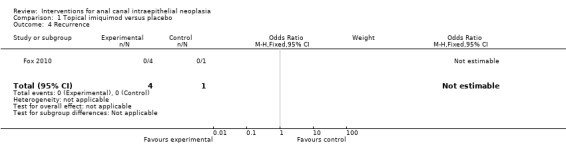
Comparison 1 Topical imiquimod versus placebo, Outcome 4 Recurrence.
All four patients in the active group and one in the placebo group in which AIN eradication had been histologically proven were disease‐free after 12 months of follow‐up. In the placebo group, resolution of AIN persisted after 37 months of follow‐up. In the active group, the follow‐up period ranged from 20 to 42 months, with a median of 33 months. Analysis was not applicable with these data.
Quality of life, as measured by a validated scale
Not assessed in Fox 2010.
Discussion
Summary of main results
The near absolute absence of RCTs supports the view that there is no evidence on standard therapy for AIN. Only imiquimod has been subjected to a placebo‐controlled RCT with a small group of patients affected by high‐grade AIN (Fox 2010). When cure or downgrading were considered alone, this included trial could not demonstrate superiority of imiquimod to placebo. However, a combination of both outcomes (not pre‐defined in our review) achieved statistical significance. The authors of the trial state that "imiquimod was significantly associated with a positive outcome, combining those who cleared and those who downgraded, x 2 = 8.78 applying Yates’ correction with one degree of freedom, P = 0.003". We decided to use relative risk (RR) instead of odds ratio (OR) because RR is easier to interpret for clinicians. One of the outcomes would have been statistically significant using OR, but both OR and RR show very wide confidence intervals. This high uncertainty leads us to a more conservative interpretation of the statistical analysis (Katz 2006).
Overall completeness and applicability of evidence
Overall, the quality of the evidence was low (GRADE Working Group). The single identified RCT failed to demonstrate whether imiquimod is superior to placebo for cure of AIN, but showed a trend to statistical significance for downgrading of high‐grade AIN. An analysis combining both outcomes showed positive results, which makes it reasonable to undertake new, larger studies on imiquimod.
Quality of the evidence
We reviewed one RCT that evaluated imiquimod for the treatment of AIN (Fox 2010). The trial included 53 patients with the disease. Risk of bias may be considered moderate as the trial was randomized, double‐blinded, placebo‐controlled, and the period of follow‐up was long (median > 34 months). However, 17.18% (11/64) of the patients were not included in the analyses for different reasons. One patient developed an anal squamous carcinoma and three of five patients in the placebo arm were lost to follow‐up after commencing the therapy. Allocation of the remaining seven participants who were lost to follow‐up was not reported, so it is not possible to know if the reasons for withdrawal between the two arms of the study were similar. Neither is it possible to know the allocation of the patient who dropped out of the trial because of adverse events.
Sequence generation may have been well done but a description of the method used cannot be found in the text. Requests to the authors for further information have not been answered. Similarly with allocation concealment, "the next randomizations number sequence" was given to participants, it is not clear if the investigators could predict the allocation with the method used.
Finally, the premature termination of the study for extrinsic reasons is a well‐known source of bias.
Our judgement on the quality of the evidence of the included study is summarized in Figure 1 and Figure 2.
1.
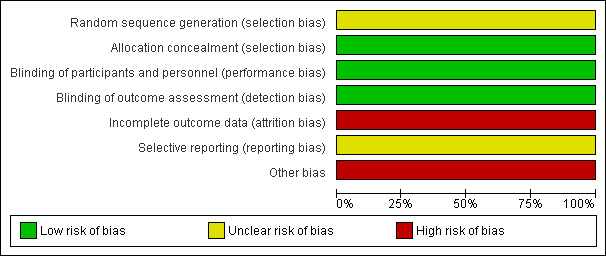
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Potential biases in the review process
Despite our efforts to locate relevant studies, it is possible that not all completed studies have been included in this review and that some unpublished trials were not found. In some instances, authors failed to respond to additional requests for information. Our pre‐specified criterion of excluding open‐label studies where placebo was used possibly meant that some studies were excluded.
Agreements and disagreements with other studies or reviews
A review of the use of imiquimod for anogenital intraepithelial neoplasia (Mahto 2010) and a review of diagnosis and management of AIN and anal cancer (Simpson 2011) are available. Mahto 2010 collected results from some of the studies we have excluded and added three case reports, but none of these studied AIN. Simpson 2011 did not collect any case reports or series on the treatment of AIN.
Authors' conclusions
Implications for practice.
There is a lack of evidence about the efficacy of available interventions for AIN. Efficacy, safety, optimal dosage and duration of treatment of imiquimod for the treatment of AIN are yet to be fully established. From the single trial analysis, imiquimod has not been proven to be superior to placebo in terms of AIN eradication. However, the available data seem to indicate that larger studies could find a statistically significant benefit.
The lack of prospective follow‐up studies assessing the spontaneous evolution of the distinct stages of HPV infection (from simple presence of the virus to anal cancer) makes it even more difficult to evaluate the sparse data that are available.
Implications for research.
When possible, well designed controlled, double‐blind randomised trials of sufficient power and duration are required to determine the efficacy of interventions in patients with AIN. Obviously some of the interventions may not be suitable for studies that are completely blinded (for example surgical interventions).
Research should also focus on different subgroups of patients since the prognosis and response to treatment is very different between HIV‐positive and HIV‐negative people.
The main outcome of future trials should be AIN resolution, defined by biopsy made after the intervention. As AIN is a histologic entity, it seems reasonable to determine its cure by biopsy and not by indirect data such as macroscopic or cytologic findings, which have lower sensitivity and specificity.
Other outcome measures should include improvement or downgrading of high‐risk to low‐risk stages, recurrence rates and disease‐free intervals, progression to high‐grade or invasive disease, HPV persistence, adverse events and effects on QoL. Sample size should be large enough to permit statistical analysis without a combination of different outcomes. Future trials should assess HPV types and the load before and after treatment. In terms of imiquimod trials, optimal dosage should be evaluated with dose ranging trials to assess its efficacy and safety. Multicentric trials comparing surgical management to medical interventions, therapeutic HPV vaccination or combinations of these treatments should be undertaken. It is very important to compare the cost of these interventions to the cost of general vaccination of males with HPV vaccines.
Acknowledgements
The authors wish to acknowledge: Marta Roqué from the Iberoamerican Cochrane Center (Barcelona) and Urbà González for their help in writing the protocol and the review; María Ángeles Mora and Rosa Amill for the bibliographical support.
Furthermore, Janet Wale from the Cochrane Consumer Network, for a thorough language revision and copy editing.
Appendices
Appendix 1. MEDLINE search strategy
Search strategy for MEDLINE (PubMed):
a) Search strategy to locate RCTs search terms 1‐36, as given in the Cochrane Handbook for Systematic Review of Interventions 5.0.2 (September 2009), appendix 5b.2 and 5c.2.
1. randomised controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trial.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. humans.sh. 10. 8 and 9
b) Search strategy to locate AIN search terms in MeSH.
11. MeSH descriptor Anal Neoplasms explode all trees.
12. (intraepithelial or dysplasia or in situ).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
13. 11 OR 12.
14. 10 AND 13.
c) Search strategy to locate AIN interventions:
15. Cryosurgery
16. Cryotherapy.
17. Curettage.
18. 5‐fluorouracil.
19. Aminolevulinic acid.
20. Photodynamic therapy.
21. Interferon.
22. Imiquimod
23. Aldara
24. Trichloroacetic acid
25. Liquid nitrogen
26. Metvix
27. Electrocautery
28. Infrared coagulation
29. Vaccination.
30. Gardasil.
31. Silgard.
32. Cervarix.
33. Laser.
34. OR/ 15‐33.
34. 14 AND 34.
Appendix 2. EMBASE search strategy
Strategy for EMBASE. 1. randomised controlled trial/ 2. randomizations/ 3. controlled study/ 4. multicenter study/ 5. phase 3 clinical trial/ 6. phase 4 clinical trial/ 7. double blind procedure/ 8. single blind procedure/ 9. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab. 10. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab. 11. 6 or 3 or 7 or 9 or 2 or 8 or 4 or 1 or 10 or 5 12. "human*".ti,ab. 13. (animal* or nonhuman*).ti,ab. 14. 13 and 12 15. 13 not 14 16. 11 not 15
Data and analyses
Comparison 1. Topical imiquimod versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AIN eradication | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 3.57 [0.43, 29.87] |
| 2 Development of invasive anal cancer | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.02] |
| 3 Dowgrading from AIN‐III or II two AIN‐I | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.24 [0.92, 251.29] |
| 4 Recurrence | 1 | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fox 2010.
| Methods | RCT with 64 HIV‐positive patients (53 completed the study: 28 imiquimod arm and 25 patients in placebo arm) recruited in two anoscopy clinics. Initially planned recruitment of 120 patients, but after 64 patients had consented the manufacturer of imiquimod didn't supply more randomised study drug. | |
| Participants | Age: intervention group median 41.8, range 35.2‐48.4); placebo group 42.3 years, range 34.9‐49.7). Smoker: intervention group smokers n=13 and non‐smokers n=15. HAART for a minimum of 3 months prior to recruitment, >100 CD4 cells/ml or higher. No history of previous imiquimod use. Median CD4 cells count at enrolment (cells/µl): intervention group: 329 (258‐514); placebo group: 389 (262‐441). Median nadir CD4 cells counts (cells/µl): intervention group: 88 (60‐182); placebo group: 98 (44‐169). Median duration of HSIL (months): intervention group: 2.5 (2.0‐ 7.5); placebo group: 3 (2‐14). | |
| Interventions | ‐ imiquimod versus placebo. Self‐application three times a week by inserting a finger no further than 2 cm into the canal anal and not using more than half a sachet. ‐ treatment three times a week for 4 months. | |
| Outcomes | ‐ Response to treatment at 8 weeks after the end of treatment (HRA and cytology) and 12 months of follow‐up.
‐ Compliance to treatment.
‐ Local side effects. ‐ Recurrences at one year of follow‐up. |
|
| Notes | Partial and non‐responders were all offered 4 months of open‐label treatment with imiquimod and ongoing 6‐monthly surveillance. All participants were offered ongoing 6‐monthly HRA and cytology. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. “The randomization sequence was only known to 3M Health Care”. |
| Allocation concealment (selection bias) | Low risk | “Matching packs of imiquimod and placebo were supplied by 3M Health Care Ltd, Loughborough, UK. On recruitment, patients were allocated the next randomization number in the study”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The study was not unblinded until all patients had completed the randomized stage”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by people who were not aware of the patient’s treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/64 of participants is a 17.18%, a significant drop out rate. Description of reason for withdrawal of the patients who did not complete the study was partial (allocation of all drop outs is not reported). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | High risk | Study terminated earlier than previously designed due to “inability to obtain further supplies of randomized study drug after 64 patients had consented”. Knowgledge of a second phase with use of open‐label imiquimod may have influenced participants to withdrawal. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2009 | Randomized, blinded, controlled trial investigating the safety and immunogenicity of a HPV15 E6/E7 protein in HIV‐positive participants with oncogenic HPV infection of the anus. AIN was not an outcome of the study, but histologic data about AIN diagnosis and response to treatment were available in 6 patients in the full paper version. However, this information was not in the placebo cohort, so only comparisons between 6 patients in 4 active‐treatment groups could have been made. Anderson et al explicitly state that "therefore, the study could not assess the effect on disease". Repeated requests for more information from the study authors’ was not obtained. For instance, authors mention that “only 2 participants with HPV‐16 at baseline cleared the virus”. It would have been interesting to know their histologic data. |
| Chang 2002 | Prospective, uncontrolled, unblinded study of surgical treatment of AIN. |
| Cranston 2008 | Uncontrolled, unblinded retrospective clinical study. |
| de Pokomandy 2011 | Prospective, follow‐up study which goal was to identify risk factors for high‐grade AIN. Effects of HAART on AIN are assessed, but without control or randomization. |
| Fiander 2006 | Non‐randomized, uncontrolled trial. A report of the clinical findings from a previous open‐label study of the immunogenicity of an heterologous prime‐boost papillomavirus oncogene vaccination (three dose of HPV‐16 L2E6E7 fusion protein followed by a single dose of live recombinant vaccinia HPV‐16/18 E6/E7) in 29 women with high‐grade "anogenital intraepithelial neoplasia". 27 of them had vulval intraepithelial neoplasia and 2 of them vaginal intraepithelial neoplasia. |
| Goldstone 2005 | Uncontrolled, unblinded retrospective clinical study. |
| Goldstone 2011 | Uncontrolled, unblinded retrospective clinical study. |
| Jay 2009 | Open‐label, non‐randomized, prospective study with 5‐fluorouracil. |
| Klencke 2002 | Open‐label, not randomised, unblinded study of the efficacy of encapsulated plasmid DNA in AIN. |
| Kreuter 2008a | Prospective, uncontrolled, follow‐up study with imiquimod in 19 patients. Only three of them had anal canal AIN. |
| Marks 2011 | Uncontrolled, unblinded retrospective clinical study. |
| Palefsky 2006 | Unblinded, open‐label phase I/II study with an HspE7. |
| Palefsky 2011 | RCT studying prevention of AIN, and patients affected by AIN were excluded. |
| Pelletier 2004 | Prospective, randomized, double‐blind and vehicle controlled study assessing the capacity of imiquimod to eradicate latent HPV infection in HIV‐infected patients. Patients with condyloma or AIN were excluded. |
| Richel 2010 | Open‐label, non‐randomized, prospective study with 5‐fluorouracil. |
| Sanclemente 2007 | Open‐label study on HPV viral load in HIV‐positive patients treated with imiquimod for anogenital warts or anal intraepithelial neoplasia, but patients with AIN were excluded. |
| Singh 2009 | Retrospective study of thrichloroacetic acid on AIN. |
| Smyth 2004 | Uncontrolled, non‐randomized study. |
| Snyder 2011 | Retrospective study of 11 patients treated with 5‐fluorouracil. |
| Stier 2008 | Prospective, multicentric, open‐label study. |
| Van der Snoek 2009 | Before‐after, uncontrolled, non‐randomized trial assessing the effects of photodynamic therapy in AIN. |
| Webber 2004 | Before‐after, uncontrolled, non‐randomized trial assessing the effects of photodynamic therapy in AIN. |
| Wieland 2006 | Prospective, non‐randomized, open‐label pilot study of 28 HIV‐positive MSM. Only 5 of them had anal canal AIN. |
Characteristics of ongoing studies [ordered by study ID]
NCT00622440.
| Trial name or title | Phase II Study for Treatment of Anal HSIL Through Use of a Chinese Herbal Topical Cream |
| Methods | Double‐blinded, randomized, controlled trial. |
| Participants | 70 patients. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Drug: AIJP (Arnebia Indigo Jade Pearl). Participants will administer their own treatment using 1/4 teaspoon of the cream twice daily for 48 weeks. Placebo: twice daily for 48 weeks. |
| Outcomes | Primary outcome measures: Pathologic response (progression, no change, or regression) of anal HSIL to treatment with the topical cream versus treatment with placebo. Clinical assessment at screen, week 24, week 48, and follow up at week 60. Secondary outcome measures: To evaluate treatment adherence and drop‐out rates, and obtain effect size for Phase 3 trial. Time frame: week 24, week 48, and week 60. |
| Starting date | March 2008 |
| Contact information | Fred Fishman, BS (415) 353‐7443; Fred.Fishman@ucsf.edu |
| Notes |
NTR1236.
| Trial name or title | Treatment of anal intraepithelial neoplasia in HIV‐positive patients, a triple‐arm randomized clinical trial |
| Methods | Randomized, not‐blinded. |
| Participants | 150 patients >18 years of age, HIV‐positive, MSM or woman. Exclusion criteria: history of anal carcinoma, chronic bowel disease, life expectancy <12 months, pregnancy or lactation, active i.v. drug user. |
| Interventions | ‐ Cauterisation, maximum 5 times in 16 weeks ‐ Imiquimod cream, 1 sachet 3 times a week ‐ 5‐Fluorouracil cream, 1 g, twice a week |
| Outcomes |
Primary: ‐ Histological resolution of AIN ‐ Relapse rate of AIN 24, 48 and 72 weeks after treatment Secondary: ‐ Side effects of treatment ‐ Qaly's, derived from the EQ‐5D questionnaire, questionnaire sexual functioning: FSFI and IIEf ‐ Costs of local treatment of precancerous lesions to prevent severe anal neoplasia ‐ HPV types and HPV load before and after treatment ‐ Single nucleotide polymorphisms (SNPs)in genes involved in the recognition of pathogens and the inflammatory response ‐ Presence of sexual transmitted co‐infections |
| Starting date | 1‐mrt‐2008 |
| Contact information | Prof Dr JM Prins. j.m.prins@amc.uva.nl. +31 (0)20 5664380 |
| Notes |
Differences between protocol and review
The most important difference was the addition of a secondary outcome. We considered that it would be very important to assess whether the different interventions achieved not only complete eradication of AIN but amelioration in terms of downgrading from a high‐risk AIN stage (III) to a low‐risk stage (AIN‐I). This outcome gives useful information about efficacy of the interventions. As it is not as useful as eradication of the disease, we considered it should be a secondary outcome.
Some studies use combinations of outcomes to achieve statistical significance of the efficacy of an intervention. This is the case with Fox 2010, which combined cure with downgrading, assessing an outcome named "improvement". We commented on this issue in the discussion section of our review and we decided to not include 'improvement' as a pre‐defined outcome since combinations of outcomes may be a source of bias.
We also changed the period of time for evaluating recurrence. We had previously pre‐defined a period of 48 weeks, but considerations made by the review authors and some experts on AIN about the high recurrence rate of the disease made us think it would be better to consider recurrence at 12 and 36 months.
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (AM).
Searching for trials (includes developing a search strategy, obtaining papers, contacting authors, investigators and drug companies) (AM, MB).
Selecting which trials to include and extracting data from trials (AM, MB, AB).
Enter data into RevMan (AM).
Carry out analysis (AM, AB).
Interpret data (AM, AB).
Draft final review (contribution from all).
Sources of support
Internal sources
-
Universitat Internacional de Catalunya, Spain.
Technical support
External sources
-
Centro Cochrane Iberolatinoamericano, Spain.
Technical support
Declarations of interest
None known
New
References
References to studies included in this review
Fox 2010 {published data only}
- Fox PA, Nathan M, Francis N, Singh N, Weir J, Dixon G, et al. A double‐blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high‐grade anal intraepithelial neoplasia in HIV‐positive MSM on HAART, with long‐term follow‐up data including th use of open‐label imiquimod. AIDS 2010;24:2331‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2009 {published data only (unpublished sought but not used)}
- Anderson JS, Hoy J, Hillman R, Barnden M, Eu B, McKenzie A, Gittleson C. A randomized, placebo‐controlled, dose‐escalation study to determine the safety, tolerability, and immunogenicity of an HPV‐16 therapeutic vaccine in HIV‐positive participants with oncogenic HPV infection of the anus. Journal of Acquired Immune Deficiency Syndromes 2009;52(3):371‐81. [DOI] [PubMed] [Google Scholar]
Chang 2002 {published data only}
- Chang GJ, Berry, MJ, Jay N, Palefsky, J, Welton ML. Surgical Treatment of High‐Grade Anal Squamous Intraepithelial Lesions: A Prospective Study. Diseases of the Colon and Rectum 2002;45(4):453‐8. [DOI] [PubMed] [Google Scholar]
Cranston 2008 {published data only}
- Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high‐grade anal dysplasia by infrared coagulation in a population of HIV‐positive men who have sex with men. International Journal of STD & AIDS 2008;19(2):118‐20. [DOI] [PubMed] [Google Scholar]
de Pokomandy 2011 {published data only}
- Pokomandy A, Rouleau D, Ghattas G, Trottier H, Vezina S, Cote P, et al. HAART and progression to high‐grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clinical Infectious Diseases 2011;52(9):1174‐81. [DOI] [PubMed] [Google Scholar]
Fiander 2006 {published data only (unpublished sought but not used)}
- Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime‐boost vaccination strategy in women with high‐grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. International Journal of Gynecological Cancer 2006;16(3):1075‐81. [DOI] [PubMed] [Google Scholar]
Goldstone 2005 {published data only}
- Goldstone SE, Kawalek AZ, Huyett JW. Infrared coagulator: a useful tool for treating anal squamous intraepithelial lesions. Diseases of the Colon and Rectum 2005;48:1042‐54. [DOI] [PubMed] [Google Scholar]
Goldstone 2011 {published data only}
- Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long‐term follow‐up of infrared coagulator ablation of anal high‐grade dysplasia in men who have sex with men. Diseases of the Colon and Rectum 2011;54(2):1284‐92. [DOI] [PubMed] [Google Scholar]
Jay 2009 {published data only}
- Jay N, Berry JM, Darrgh T, Palefsky J. Treatment of diffuse high grade anal intraepithelial neoplasia with 5%‐fluorouracil cream. 25th International Papillomavirus Conference Malmo Sweeden 2009; Vol. Poster 19.16.
Klencke 2002 {published data only (unpublished sought but not used)}
- Klencke B, Matijevic M, Urban RG, Lathey JL, Hedley ML, Berry M, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16‐associated anal dysplasia: A phase I study of ZYC101. Clinical Cancer Research 2002;8:1028‐37. [PubMed] [Google Scholar]
Kreuter 2008a {published data only}
- Kreuter A, Potthoff A, Brockmeyer NH, Gambichler R, Stücker M, Altmeyer P, et al. Imiquimod leads to a decrease of human papillomavirus and to a sustained clearance of anal intraepithelial neoplasia in HIV‐infected men. Journal of Investigative Dermatology 2008;128:2078‐83. [DOI] [PubMed] [Google Scholar]
Marks 2011 {published data only}
- Marks DS, Goldstone SE. Electrocautery ablation of high‐grade anal squamous intraepithelial lesions in HIV‐negative and HIV‐positive men who have sex with men. Journal of Acquired Immune Deficiency Syndromes 2011;59(3):259‐65. [DOI] [PubMed] [Google Scholar]
Palefsky 2006 {published data only}
- Palefsky JM, Berry JM, Jay N, Krogstad M, Costa M, Darragh TM, Lee JY. A trial of SGN‐00101 (HspE7) to treat high‐grade anal intraepithelial neoplasia in HIV‐positive individuals. AIDS 2006;20:1151‐5. [DOI] [PubMed] [Google Scholar]
Palefsky 2011 {published data only}
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. The New England Journal of Medicine 2011;365(17):1576‐85. [DOI] [PubMed] [Google Scholar]
Pelletier 2004 {published data only}
- Pelletier F, Drobacheff‐Thiebaut C, Aubin F, Venier AG, Mougin C, Laurent R. Effects of imiquimod on latent human papillomavirus anal infection in HIV‐infected patients [Effets de l’imiquimod sur l’infection périanalelatente à papillomavirus humainchez des malades infectés par le virusde l’immunodéficience humaine]. Annales de Dermatologie et de Vénéréologie 2004;131:947‐51. [DOI] [PubMed] [Google Scholar]
Richel 2010 {published data only}
- Richel O, Wieland U. Topical 5‐fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus‐positive men. British Journal of Dermatology 2010;163:1301–7. [DOI] [PubMed] [Google Scholar]
Sanclemente 2007 {published data only}
- Sanclemente G, Herrera S, Tyring SK, Rady PL, Zuleta J‐J, Correa L‐A, et al. Human papillomavirus viral load and HPV type in the clinical outcome of HIV‐positive patients treated with imiquimod for anogenital warts and anal intraepithelial neoplasia. Journal of the European Academy of Dermatology and Venereology 2007;21:1054‐60. [DOI] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh JC, Kuohung V, Palefsky JM. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV‐positive and HIV‐negative men who have sex with men. Journal of Acquired Immune Deficiency Syndromes 2009;52(4):474‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smyth 2004 {published data only}
- Smyth LJ, Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV‐16)‐associated anogenital intraepithelial neoplasia induced by heterologous prime‐boost HPV‐16 oncogene vaccination. Clinical Cancer Research 2004;10:2954‐61. [DOI] [PubMed] [Google Scholar]
Snyder 2011 {published data only}
- Snyder SM, Siekas L, Aboulafia DM. Initial experience with topical fluorouracil for treatment of HIV‐associated anal intraepithelial neoplasia. Journal of the International Association of Physicians in AIDS Care 2011;10(2):83‐8. [DOI] [PubMed] [Google Scholar]
Stier 2008 {published data only}
- Stier EA, Goldstone SE, Berry JM, Panther LA, Jay N, Krown SE, et al. Infrared coagulator treatment of high‐grade anal dysplasia in HIV‐infected individuals: an AIDS malignancy consortium pilot study. Journal of Acquired Immune Deficiency Syndromes 2008;47(1):56‐61. [DOI] [PubMed] [Google Scholar]
Van der Snoek 2009 {published data only}
- Snoek EM, Amelink A, Ende ME, Hollander JC, Hollander JG, Kroon FP, et al. Photodynamic therapy with topical metatetrahydroxychlorin (Fosgel) is ineffective for the treatment of anal intraepithelial neoplasia, grade III. Journal of Acquired Immune Deficiency Syndromes 2009;52(1):141‐3. [DOI] [PubMed] [Google Scholar]
Webber 2004 {published data only}
- Webber J, Fromm D. Photodynamic therapy for carcinoma in situ of the anus. Archives of Surgery 2004;139:259‐61. [DOI] [PubMed] [Google Scholar]
Wieland 2006 {published data only}
- Wieland U, Brockmeyer NH, Weissenborn S, Hochdorfer B, Stucker M, Swoboda J, et al. Imiquimod treatment of anal intraepithelial neoplasia in HIV‐positive men. Archives of Dermatology 2006;142:1438‐44. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00622440 {unpublished data only}
- Phase II Study for Treatment of Anal HSIL Through Use of a Chinese Herbal Topical Cream. Ongoing study March 2008.
NTR1236 {unpublished data only}
- Treatment of anal intraepithelial neoplasia in HIV‐positive patients, a triple‐arm randomized clinical trial. Ongoing study 1‐mrt‐2008.
Additional references
Crum 2010
- Crum‐Cianflone NF, Hullsiek KH, Marconi VC, et al. Anal cancers among HIV‐infected persons: HAART is not slowing rising incidence. AIDS 2010;24:535‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2009
- National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 (CTCAE). http://ctep.cancer.gov 2009.
Fox 2005
- Fox PA, Seet JE, Stebbing J, Francis N, Barton SE, Strauss S, et al. The value of anal cytology and human papillomavirus typing in the detection of anal intraepithelial neoplasia: a review of cases from an anoscopy clinic. Sexually Transmitted Infections 2005;81:142‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanley 1983
- Hanley JA, Lippman‐Hand A. If nothing is wrong, is everything allright?. JAMA 1983;259:1743‐5. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0. The Cochrane Collaboration February 2008.
Katz 2006
- Katz KA. The (relative) risks of using odds ratios. Archives of Dermatology 2006;142(6):761‐4. [DOI] [PubMed] [Google Scholar]
Kreuter 2008b
- Kreuter A, Brockmeyer NH, Weissenborn SJ, et al. Penile intraepithelial neoplasia is frequent in HIV‐positive men with anal dysplasia. The Journal of Investigative Dermatology 2008;128:2316‐24. [DOI] [PubMed] [Google Scholar]
Kreuter 2010
- Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Swoboda J, Stücker M, et al. German Competence Network HIV/AIDS. Anal carcinoma in human immunodeficiency virus‐positive men: results of a prospective study from Germany. British Journal of Dermatology 2010;162:1269‐77. [DOI] [PubMed] [Google Scholar]
Mahto 2010
- Mahto M, Nathan M, O'Mahony C. More than a decade on: review of the use of imiquimod in lower anogenital intraepithelial neoplasia. International Journal of STD & AIDS 2010;21:8‐16. [DOI] [PubMed] [Google Scholar]
Meys 2010
- Meys R, Gotch FM, Bunker CB. Human papillomavirus in the era of highly active antiretroviral therapy for human immunodeficiency virus: an immune‐reconstitution‐associated disease?. British Journal of Dermatology 2010;162:6‐11. [DOI] [PubMed] [Google Scholar]
Scholefield 2005
- Scholefield JH, Castle MT, Watson NF. Malignant transformation of high‐grade anal intraepithelial neoplasia. The British Journal of Surgery 2005;92:1133–6. [DOI] [PubMed] [Google Scholar]
Simpson 2011
- Simpson JAD, Scholefield JH. Diagnosis and management of anal intraepithelial neoplasia and anal cancer. BMJ 2011;343:d6818. [DOI] [PubMed] [Google Scholar]
Veldhuijzen 2010
- Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. Lancet Infectious Diseases 2010;10(12):862‐74. [DOI] [PubMed] [Google Scholar]


