Abstract
Background
Charcot‐Marie‐Tooth disease (CMT) comprises a large group of different forms of hereditary motor and sensory neuropathy. The molecular basis of several CMT subtypes has been clarified during the last 20 years. Since slowly progressive muscle weakness and sensory disturbances are the main features of these syndromes, treatments aim to improve motor impairment and sensory disturbances to improve abilities. Pharmacological treatment trials in CMT are rare. This review was derived from a Cochrane review, Treatment for Charcot Marie Tooth disease, which will be updated via this review and a forthcoming title, Treatments other than ascorbic acid for Charcot‐Marie‐Tooth disease.
Objectives
To assess the effects of ascorbic acid (vitamin C) treatment for CMT.
Search methods
On 21 September 2015, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and LILACS for randomised controlled trials (RCTs) of treatment for CMT. We also checked clinical trials registries for ongoing studies.
Selection criteria
We included RCTs and quasi‐RCTs of any ascorbic acid treatment for people with CMT. Where a study aimed to evaluate the treatment of general neuromuscular symptoms of people with peripheral neuropathy including CMT, we included the study if we were able to identify the effect of treatment in the CMT group. We did not include observational studies or case reports of ascorbic acid treatment in people with CMT.
Data collection and analysis
Two review authors (BG and JB) independently extracted the data and assessed study quality.
Main results
Six RCTs compared the effect of oral ascorbic acid (1 to 4 grams) and placebo treatment in CMT1A. In five trials involving adults with CMT1A, a total of 622 participants received ascorbic acid or placebo. Trials were largely at low risk of bias. There is high‐quality evidence that ascorbic acid does not improve the course of CMT1A in adults as measured by the CMT neuropathy score (0 to 36 scale) at 12 months (mean difference (MD) ‐0.37; 95% confidence intervals (CI) ‐0.83 to 0.09; five studies; N = 533), or at 24 months (MD ‐0.21; 95% CI ‐0.81 to 0.39; three studies; N = 388). Ascorbic acid treatment showed a positive effect on the nine‐hole peg test versus placebo (MD ‐1.16 seconds; 95% CI ‐1.96 to ‐0.37), but the clinical significance of this result is probably small. Meta‐analyses of other secondary outcome parameters showed no relevant benefit of ascorbic acid. In one trial, 80 children with CMT1A received ascorbic acid or placebo. The trial showed no clinical benefit of ascorbic acid treatment. Adverse effects did not differ in their nature or abundance between ascorbic acid and placebo.
Authors' conclusions
High‐quality evidence indicates that ascorbic acid does not improve the course of CMT1A in adults in terms of the outcome parameters used. According to low‐quality evidence, ascorbic acid does not improve the course of CMT1A in children. However, CMT1A is slowly progressive and the outcome parameters show only small change over time. Longer study durations should be considered, and outcome parameters more sensitive to change over time should be designed and validated for future studies.
Plain language summary
Vitamin C for Charcot‐Marie‐Tooth (CMT) disease (hereditary motor and sensory neuropathy)
Review question
What are the benefits or harms of vitamin C (ascorbic acid) in the treatment of Charcot‐Marie‐Tooth (CMT) disease?
Background
CMT disease represents a broad spectrum of inherited peripheral neuropathies (conditions in which the nerves outside the brain and spinal cord are damaged), which in general progress slowly, and cause muscle wasting and loss of sensation. Muscle wasting and loss of sensation are caused by destruction of nerve fibres that go to the muscles or skin. Vitamin C has been proposed as a treatment for CMT, because vitamin C is necessary for myelination (development of the myelin, or insulation around the nerve fibres) in laboratory cultures of nerve cells and the peripheral nerves of mice.
Study characteristics
We searched the medical literature for trials of vitamin C in CMT disease and found six trials ‐ five in adults and one in children ‐ on the treatment of CMT type 1A (CMT1A) with vitamin C. All compared vitamin C doses of 1 to 4 grams per day with a placebo (a dummy or sugar pill disguised as vitamin C), and lasted for 12 or 24 months. The trials in adults included a total of 622 people. The other trial included 80 children. The main measure of the effects of vitamin C in this review was change in impairment. We also collected information on disability, nerve conduction studies, sensation, muscle strength, quality of life and harmful effects of vitamin C.
Key results and quality of the evidence
We found that ascorbic acid treatment did not improve impairment from CMT1A in adults as measured by the CMT neuropathy score (CMTNS). In children, the CMTNS was not reported, as it is a measure developed for adults with CMT. The measures used for children in this study did not show benefit from vitamin C. The studies were largely at low risk of bias, meaning they were well designed and the results were not easily influenced by chance. Adverse events were similar in nature and number in vitamin C and placebo groups.
There is high‐quality evidence for adults and low‐quality evidence for children that vitamin C does not improve the course of CMT1A. However, CMT progresses slowly, so the study durations of 12 or 24 months may not have been long enough to detect effects of treatment. Further research with longer study duration and more sensitive outcome parameters should be done, although any large effect in adults or children is unlikely.
Summary of findings
for the main comparison.
| Ascorbic acid treatment compared with placebo for CMT1A in adults | ||||||
|
Participants or population: adults with CMT1A Intervention: oral ascorbic acid (1 g to 4 g/day) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ascorbic acid | |||||
|
Impairment ‐ change in CMTNS at 24 months (0 to 36 points) |
The mean change in CMTNS ranged across control groups from ‐0.92 to 1 point | The mean change in CMTNS in the intervention groups was 0.21 lower (0.81 lower to 0.39 higher) | ‐0.21 (‐0.81 to 0.39) | 388 (3) | ⊕⊕⊕⊕ high | |
|
Impairment ‐ change in CMTES at 24 months (0 to 28 points) |
The mean change in CMTES ranged across control groups from ‐0.64 to 0.5 point | The mean change in CMTES in the intervention groups was 0.12 lower (0.67 lower to 0.42 higher) | ‐0.12 (‐0.67 to 0.42) | 337 (2) | ⊕⊕⊕⊝ moderate1 | |
|
Change in timed 10‐m walk at 12 months (seconds (s)) |
The mean change in timed 10‐m walk ranged across control groups from ‐0.15 to 0.56 s | The mean change in timed 10‐m walk in the intervention groups was 0.02 lower (0.32 lower to 0.29 lower) | ‐0.02 (‐0.32 to 0.29) | 401 (3) | ⊕⊕⊕⊕ high | |
|
Change in foot dorsiflexion at 12 months (N) |
The mean change in foot dorsiflexion force ranged across control groups from ‐5.1 to 3 N | The mean change in foot dorsiflexion force in the intervention groups was 1.1 higher (3.47 lower to 5.67 higher) | 1.1 (‐3.47 to 5.67) | 423 (4) | ⊕⊕⊕⊕ high | |
| Change in 9‐hole peg test (HPT) at 12 months (seconds) | The mean change in 9‐HPT ranged across control groups from ‐3.5 to 0.43 s | The mean change in 9‐HPT in the intervention groups was 0.39 lower (0.76 lower to 0.02 lower) | ‐0.39 (‐0.76 to ‐0.02) | 286 (3) | ⊕⊕⊕⊝ moderate1 | |
| Serious adverse events (SAE; %) | The relative abundance of SAE was 12% in the placebo group ((number of SAE/number of participants)*100) | The relative abundance of SAE was 11.7% in the intervention groups ((number of SAE/number of participants)*100) | Not estimable | 702 (6, including 1 study in children) | ⊕⊕⊕⊕ high | |
| *The assumed risk is based on the mean change or the range of mean change) across control groups in included studies.. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Key: 10‐m walk: 10‐metre walk; 9‐HPT: 9‐hole‐peg test; CMTES: CMT examination scale; CMTNS: CMT neuropathy scale; CI: confidence interval; RR: risk ratio; N: Newton; SAE: Serious adverse event | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence downgraded because of number of studies and participants, which was lower than for other outcomes. Further research may thus have an impact on our confidence in the estimate of effect and may change the estimate.
Background
Description of the condition
Charcot‐Marie‐Tooth disease (CMT) comprises a large group of inherited neuropathies, which primarily affect either the myelin sheath or the axon of the peripheral nerve (Shy 2005b). Primary loss of the myelin sheath, called demyelination, leads to secondary axonal degeneration, resulting in muscular atrophy and paresis (Krajewski 2000; Martini 2001). The main clinical motor signs are atrophy and weakness, starting in the feet and calves and later affecting the hands (Shy 2005b). The prevalence of CMT ranges from 10 to 80/100,000, depending on the population studied (Braathen 2012; Kurihara 2002). Sensory deficits and positive sensory symptoms are less prominent, but may cause discomfort and add to the disability. Skeletal deformities like scoliosis or foot deformities can be additional features (Shy 2005b). Depending on the underlying molecular genetic defect, the clinical severity varies from mild weakness to severe paralysis and disability (Baets 2014), but in general they progress very slowly over decades rather than years. Histopathological findings in peripheral nerve biopsies reveal demyelination or axonal degeneration, depending on the specific type of CMT (Gabreels‐Festen 1993). Demyelination results in slowed nerve conduction velocities (NCVs). Types of CMT with primary axonal loss and preserved myelin are characterised by normal or nearly normal NCVs, but variably reduced compound muscle action potentials (CMAPs).
Genetics
A broad spectrum of gene mutations have been identified as causes of CMT (Baets 2014). We will give a short introduction to the most frequent forms of CMT: autosomal‐dominant demyelinating CMT (CMT1), X‐chromosome linked CMT (CMTX), autosomal‐dominant axonal CMT (CMT2), and autosomal‐recessive CMT (CMT4).
CMT1 is primarily a demyelinating condition, and is the most common form of CMT (Nelis 1996). The most frequent genetic defect in CMT1 is an intrachromosomal duplication on chromosome 17p11.2 (Lupski 1991; Raeymakers 1991). The gene encoding peripheral myelin protein 22 (PMP22) is located within the duplicated region (Timmerman 1992). This gene has been shown to be the culprit for the genetic subgroup CMT1A (duplication) and CMT1E (point mutations). The presence of mutations in the gene encoding myelin protein zero (MPZ) defines CMT1B (Hayasaka 1993a; Hayasaka 1993b; Hayasaka 1993c).
The cause of CMTX1 is mutations in the gene encoding gap junction protein beta1 (GJB1), which is localised on the X‐chromosome. Histopathological signs of axonal degeneration and demyelination characterise CMTX1 (Hahn 2001; Nicholson 1993). These cause the typical electrophysiological features of mildly reduced NCV and reduction of CMAPs. In addition to CMTX1, other, rarer forms of X‐chromosomal CMT have been recently discovered (Bird 2014).
In addition to CMTX1, other intermediate CMT subtypes exist, which are inherited in an autosomal dominant or recessive fashion. These are termed dominant‐intermediate or recessive‐intermediate CMT (DI‐CMT, RI‐CMT). Several genes have been implicated in DI‐ and RI‐CMT (Liu 2014).
The purely axonal form of CMT is called CMT2. Mutations in the gene encoding mitochondrial fusion protein mitofusin 2 (MFN2) were shown to be a cause of CMT2A (Verhoeven 2006; Zuchner 2004). Many other genes for CMT2 have been described; these often contribute only a small proportion of the genetic spectrum.
The autosomal‐recessive form of CMT is CMT4. The autosomal‐recessive forms of CMT are generally much rarer than the autosomal‐dominant forms. Autosomal‐recessive forms account for only 10% of people with CMT in outbred populations, rising to 40% in populations with a higher degree of consanguinity. Among the many genes implicated in CMT4, some of the more common are SH3TC2, GDAP1 and HINT1 (Baxter 2002; Saporta 2011; Senderek 2003; Zimon 2012).
Hereditary neuropathy with liability to pressure palsies (HNPP) represents the most common form of inherited neuropathy with recurrent focal nerve palsies, although some people also display signs of a generalised demyelinating neuropathy with a more chronic disease course. The underlying genetic defect is the reciprocal state of CMT1A, namely the loss of one allele of PMP22 due to the loss of a chromosomal fragment on chromosome 17p11.2 (Chance 1993), or less commonly, PMP22 missense mutations.
Furthermore, hereditary neuropathies exist with either predominant motor involvement, termed distal hereditary motor neuropathies (dHMN), or predominant sensory involvement, termed hereditary sensory and autonomic neuropathies (HSAN). In recent years, there has been major progress in the elucidation of the genetics of these neuropathies (Auer‐Grumbach 2013; Rossor 2012).
Many rare dominant and recessive forms of CMT have now been classified (see the Inherited Peripheral Neuropathies Mutation Database www.molgen.ua.ac.be/CMTMutations or recent review articles (Baets 2014; Rossor 2013).
Pathophysiology
The pathophysiological mechanisms that lead to the clinical phenotype are still unclear for many of the genes implicated in CMT. In general, mutations in CMT‐associated genes lead to disturbed functions of either Schwann cells or axons. Disturbed functions of Schwann cells cause secondary axonal degeneration in the course of the disease. The molecular mechanisms leading to axonal degeneration in CMT are still elusive. Evidence is accumulating for not only a convergence of several forms of CMT, but also hereditary spastic paraplegia (HSP), and hereditary sensory and autonomic neuropathies (HSAN), owing to common mechanisms such as disorganisation of the axonal cytoskeleton, axonal transport, and mitochondrial dysfunctions (d'Ydewalle 2012; Pareyson 2014).
Clinical features
Although molecular genetic methods for the identification of new genes in CMT have rapidly expanded, CMT1A remains the most common subtype (Gess 2013; Murphy 2012; Saporta 2011). The most prominent symptom in all forms of CMT1 is progressive distal wasting of peroneal and calf muscles resulting in distal lower limb weakness, beginning in the first or second decade of life (Vallat 2013). Many people with CMT have foot deformities, such as high‐arched feet and hammer toes. Distal sensory loss is typically found but positive sensory symptoms, such as neuropathic pain, are more rare. Autonomic nervous system involvement is rare and not prominent. The need for a wheelchair is unusual.
The pathobiological hallmarks of CMT1 are demyelination and axonal degeneration with consequent length‐dependent muscle atrophy (Krajewski 1999; Krajewski 2000). In nerve conduction studies, the reduction of motor and sensory NCV can identify demyelination. However, the degree of reduction in NCV does not reflect the clinical severity of CMT1 (Krajewski 1999; Krajewski 2000). The diagnosis of CMT1 requires a reduced motor NCV of less than 38 m/s in the upper limbs (Shy 2005b). Axonal CMT2 cannot be differentiated by clinical means from CMT1. Electrophysiologically, the motor NCV is normal or only slightly reduced (greater than 38 m/s) in CMT2, but the CMAP amplitudes are significantly decreased (Harding 1980; Shy 2005b). Intermediate slowing of NCV (25 to 45 m/s), with mildly reduced CMAPs, characterise intermediate CMT (Harding 1980).
Description of the intervention
Established strategies for the treatment of CMT and its symptoms are lacking (Young 2008). Some trials that aimed to improve muscle strength or sensory deficits have been undertaken. Trials have been performed with corticosteroids and antioxidants, and with physiotherapy for improving muscle function (Grandis 2005; Young 2001).
Ascorbic acid (vitamin C) has been shown to be necessary for correct myelination of peripheral nerves in vitro and in vivo (Eldridge 1987; Gess 2010; Gess 2011). Ascorbic acid improved muscle function and reduced demyelination in a mouse model of CMT1A (Passage 2004). Based on these experimental data, several randomised controlled trials (RCTs) were undertaken on the treatment of CMT1A with ascorbic acid.
The recommended daily allowance of vitamin C is between 75 and 120 mg (depending upon sex and physiological status). Usual daily doses of ascorbic acid purchased over the counter are 500 mg/day.
Why it is important to do this review
Six RCTs on the treatment of CMT1A with ascorbic acid have been performed. It is important to analyse these trials, compare their data and summarise the results in order to reach a conclusion on the treatment of CMT1A with ascorbic acid.
This review and a forthcoming Cochrane review, Treatments other than ascorbic acid for Charcot‐Marie‐Tooth disease, which is currently in preparation, will update the previous Cochrane review, 'Treatment for Charcot‐Marie‐Tooth disease' (Young 2008).
Objectives
To assess the effects of ascorbic acid (vitamin C) treatment for CMT.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs of ascorbic acid for the treatment of people with CMT. Where a study aimed to evaluate the treatment of general neuromuscular symptoms of people with peripheral neuropathy including CMT, we included the study if we were able to identify the effect of treatment in the CMT group. We did not include observational studies and case reports on the treatment of people with CMT.
Types of participants
Because of the heterogeneity of CMT, we included people with all types of CMT regardless of which clinical, electrophysiological, histopathological or molecular genetic criteria were used. Due to the fast progress in the elucidation of the genetic basis of CMT, we considered rare forms of CMT. Since all trials on ascorbic acid treatment for CMT were conducted in people with CMT1A, the focus of this review is effectively on CMT1A only. We did not include experimental data from animal models.
Types of interventions
We included all ascorbic acid (vitamin C) treatments, regardless of daily dosage, form of application or dosage regimen. Treatment had to have extended for at least 12 successive months. We chose 12 months of treatment as a minimum because the very slow progressive course of CMT makes it unlikely that a shorter period of treatment would be adequate to test the efficacy of the intervention.
Types of outcome measures
We rescaled changes and numbers of events occurring over time to 'change or numbers of events in a fixed period', for example, three months, to permit the pooling of studies with differing follow‐up periods.
Primary outcomes
Change in impairment after at least 12 months.
We accepted any measure of impairment made with a validated scale, such as the MRC sum score, Neuropathy Impairment Score (NIS) (Dyck 2005b), Total Neuropathy Score (Cornblath 1999), CMT Neuropathy score (Shy 2005a), or CMT neuropathy score version 2 (Murphy 2011).
Secondary outcomes
Secondary outcome measures included:
Change in disability after at least 12 months. We accepted any measure of disability made with a validated scale such as the Rankin Scale (van Swieten 1988) or the Inflammatory Neuropathy Cause and Treatment Overall Disability Status Scale (Merkies 2000).
Change of maximum compound muscle action potential (CMAP) amplitudes of the ulnar, median or peroneal nerve, after at least 12 months.
Change in sensory impairment measured with a validated scale, after at least 12 months.
Change in sensory nerve action potential (SNAP) amplitude of the sural or other sensory nerves, after at least 12 months.
Change in muscle force as measured with the aid of a dynamometer or vigorimeter, after at least 12 months.
Change in quality of life assessment using validated scores, such as SF‐36 Health Survey questionnaire (Jenkinson 1993) or EuroQol (Anderson 1996), after at least 12 months.
Adverse events, whether minor or severe, caused by any form of treatment. We distinguished between minor adverse events and severe adverse events (those which were fatal, life‐threatening, required or prolonged hospitalisation or caused discontinuation of the treatment).
Search methods for identification of studies
Electronic searches
On 21 September 2015, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8 in theCochrane Library), MEDLINE (January 1966 to September 2015), EMBASE (January 1980 to September 2015) and LILACS (January 1982 to September 2015) for randomised controlled trials. The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register Appendix 1, CENTRAL Appendix 2, MEDLINE (Appendix 3), EMBASE (Appendix 4), LILACS (Appendix 5). In August 2015, we searched the database ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO international Clinical Trials Registry (www.who.int/ictrp/en/).
Searching other resources
We contacted authors from clinical trials and experts from the field to identify unpublished trials. We also checked the bibliographies of identified trials.
Data collection and analysis
Selection of studies
Three review authors (BG, PY and DP) checked the titles and abstracts of the articles retrieved by the search. The review authors worked independently. The review authors obtained full‐text reports of potentially eligible studies and independently selected studies for inclusion. In case of disagreement, the three authors were to make a consensual decision. We did not apply any language limitations.
Data extraction and management
Two review authors (BG and JB) independently extracted data onto a specially designed data extraction form and checked the extracted data for disagreements. In case of disagreement, the two review authors came to a consensus. BG performed the data entry into the Cochrane software Review Manager (RevMan) 5.3 (Review Manager 2014) and JB checked the data. We contacted trial authors to obtain missing data or raw data if necessary.
Assessment of risk of bias in included studies
Two review authors (BG and JB) graded the risk of bias using the Cochrane approach: high, low or unclear risk of bias (Higgins 2011). In case of disagreement, the two review authors discussed this further and came to a unanimous decision. The 'Risk of bias' assessment took into account security of randomisation, allocation concealment, participant blinding, observer blinding, selective reporting, incomplete outcome reporting (completeness of follow‐up), and the extent to which the study took into account any imbalance in important prognostic variables present at time of randomisation.
Measures of treatment effect
We summarised continuous data with mean differences (MDs) and 95% confidence intervals (CIs). If we had found outcomes measured with different scales, we would have used a standardised mean difference (SMD) with 95% CI.
None of our outcomes were dichotomised; however, we would have reported these using a risk ratio (RR) and 95% CI.
We reported all the data in terms of change over time.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial unexplained heterogeneity, we would have reported it and explored possible causes by pre‐specified subgroup analysis (Higgins 2011).
Data synthesis
When pooling data was possible and appropriate, we used RevMan to perform a fixed‐effect model meta‐analysis.
We reported the results for adults and children separately.
We assessed treatment effects by meta‐analysis of data using forest plots. We determined the overall treatment effect by the Z‐score. We considered a significance level of P < 0.05 as the threshold for statistically significant treatment effects.
In the Discussion, we considered adverse events and cost‐effectiveness, taking into account information from the non‐randomised literature.
'Summary of findings' tables
We generated 'Summary of findings' tables using the GRADE approach. Our selected outcomes were: Impairment ‐ change in the CMTNS at 24 months; Impairment ‐ change in CMTES at 24 months, change in timed 10‐metre walk at 12 months, change in foot dorsiflexion at 12 months, change in 9‐hole peg test at 12 months, and serious adverse events. The GRADE Working Group established the following grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
We used the five GRADE considerations (namely, study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence (all studies that contribute data for the pre‐specified outcomes). We followed methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and used GRADEpro software (GRADEpro 2015).
Subgroup analysis and investigation of heterogeneity
We had planned to compare CMT1, CMT2, and where possible, other CMT subgroups separately. However, all the trials of ascorbic acid treatment of CMT only included people with CMT1A (the most common form of CMT).
We assessed heterogeneity using forest plots. We determined the overall level of heterogeneity using the Chi² statistic. We set a significance level of P < 0.05 to be the threshold for significant heterogeneity. If we had found heterogeneity, we would have explored and described possible reasons for differences between studies such as types of participants, intervention or quality.
Sensitivity analysis
We performed sensitivity analyses by omitting trials which were at high risk of bias in one or more domains. If necessary, we would have used the random‐effects model to obtain the pooled estimate.
Results
Description of studies
Results of the search
The number of papers found by the searches outlined in the appendices were: Cochrane Neuromuscular Specialised Register 23, CENTRAL 21, MEDLINE 29, EMBASE 31, LILACS 0, DARE 0, HTA 0 and NHSEED 0. We identified no ongoing trials of ascorbic acid treatment for CMT in trial registers. One study was found by personal communication. Of the 105 studies found in total, 94 remained after removal of duplicates. After review of abstracts and titles, we selected seven of the 94 for full‐text assessment. One of these was excluded with reasons. Six studies fitted the inclusion criteria and were included in the quantitative analysis (see flow chart in Figure 1).
1.
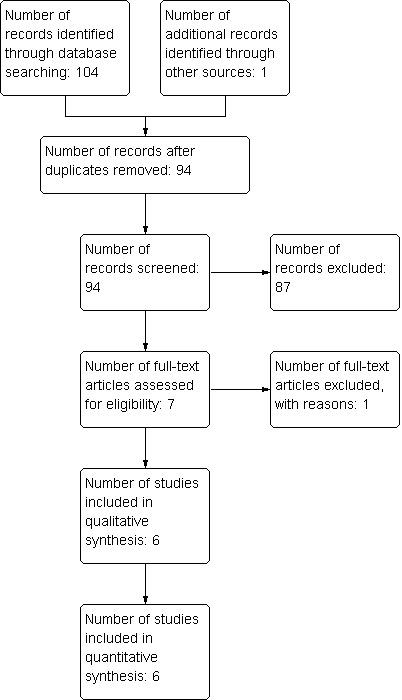
Study flow diagram.
Included studies
We identified seven controlled clinical trials on the treatment of CMT with ascorbic acid (see Characteristics of included studies). We excluded one study because it was not randomised (Toth 2009; Characteristics of excluded studies). The other studies fulfilled all inclusion parameters and we were thus able to include them in this review. One of these studies involved 80 children (Burns 2009), the other five included a total of 622 adults (Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a). Because the natural course of the disease differs between children and adults in terms of progression rates and outcome measures, we reported the study that was performed in children separately. None of the randomised studies defined a minimum level of impairment or disability at baseline. The study by Lewis et al used a futility design. Further, this study compared treated patients to historical controls, which were not randomised. Historical, natural history controls differ from placebo‐treated controls in their rate of progression. However, a placebo‐treated group was included too, so we included this study in this review (Lewis 2013).
All studies described interventions and the related procedures in detail, including the dosages, regimens and appearance of medication. The studies used different dosages (1 g to 4 g) and durations (12 to 24 months) of ascorbic acid treatment. All studies compared ascorbic acid to placebo treatment. Two studies continued treatment for 12 months (Micallef 2009; Verhamme 2009a), and three studies for 24 months (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011). To conduct a meta‐analysis of as many studies as possible, we took 12‐month results from the 24‐month trials, for a comparison of treatment at 12 months. In addition, we compared data at 24 months in a meta‐analysis of the three 24‐month trials. Five of the six included studies were published reports. One of the studies was not yet published, but we obtained data by personal communication with the principal investigator (Mazanec 2013 [pers comm]).
All the included studies provided definitions and descriptions of outcome criteria. All five trials in adults used the CMTNS as a composite disease‐specific score. Four studies used foot dorsiflexion force and SF‐36 physical functioning score. Three studies measured the CMTES (clinical component of the CMTNS (Shy 2005a)), nine‐hole peg test, 10‐metre walk test, hand grip force, three‐point pinch force, SF‐36 bodily pain and ascorbic acid concentration. Two studies used the ODSS, ONLS, SF‐36 energy, motor nerve conduction velocity (mean from ulnar and median nerves), and ulnar compound muscle action potential. Several other outcome parameters were used by only one study each.
Risk of bias in included studies
Figure 2 presents a summary of the 'Risk of bias' assessments of the included studies by the review authors. We considered only one of the selected studies to be at low risk of bias in all domains using the Cochrane approach (Pareyson 2011), but all studies were in general at low risk of bias.
2.
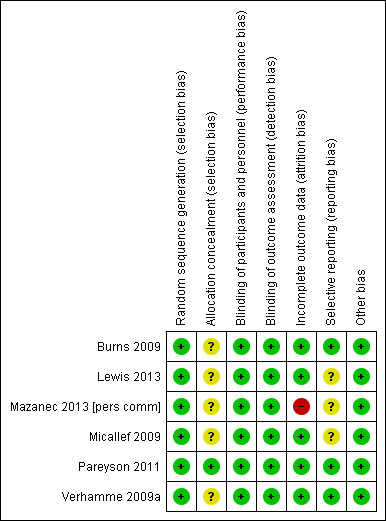
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.
Six of the studies described randomisation properly. Only one study supplied adequate information about allocation concealment.
Five of the studies explicitly considered differences in baseline characteristics. None of the study reports gave the age of onset of CMT. Two studies gave the duration of the disease. Five of the studies measured and stated disease severity. Four studies provided baseline CMTNS scores. In the trial involving children, for whom the CMTNS is not validated, trialists assessed other baseline parameters of severity, such as pain, pes cavus and regular trips and falls.
Five studies were at low risk of bias in terms of follow‐up. Five studies explicitly recorded the number and reasons for drop‐outs from the different experimental groups and used intention‐to‐treat analyses, while in one study, the number of drop‐outs was not given and data of dropped‐out patients were not included in the analysis (Mazanec 2013 [pers comm]). Three studies were at low risk for reporting bias (selective reporting of outcomes). Three studies were at unclear risk for reporting bias, as they showed minor differences between study protocol and reported outcomes.
All six included studies reported blinding of both the observer and the participant (Burns 2009; Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a).
Effects of interventions
See: Table 1
We performed meta‐analyses for outcome parameters used by two or more studies. We considered the trial of ascorbic acid treatment in children separately because of the different study population. We reported data from published results for four of the trials (Lewis 2013; Micallef 2009; Pareyson 2011; Verhamme 2009a) and from personal communication for one trial (Mazanec 2013 [pers comm]). For one trial, we obtained additional data by personal communication in order to make a comparison possible (Lewis 2013).
We have used 'no significant difference' when differences were neither clinically nor statistically significant.
Comparison: Oral ascorbic acid treatment versus placebo for adults with CMT1A (12 months)
Primary outcome ‐ impairment at 12 months on a validated scale
High‐quality evidence found no evidence of clinically significant differences between ascorbic acid and placebo at 12 months, on any of the validated measures for the primary outcome. Using the CMTNS (scale 0 to 36 points), the mean difference (MD) was ‐0.37; 95% CI ‐0.83 to 0.09; five studies; N = 533 (see Analysis 1.1; Figure 3). The CMTES (scale 0 to 28 points), which represents the CMTNS without the electrophysiological parameters, showed a significant benefit for ascorbic acid in one trial (Micallef 2009), but the meta‐analysis of all three trials that reported this outcome parameter found no significant difference (MD ‐0.15; 95% CI ‐0.57 to 0.27; three trials; N = 460; Analysis 1.2). For both outcome parameters, Pareyson 2011 dominated the meta‐analysis as it is the largest of the trials. The one study using the NIS reported no significant difference (MD 1.91; 95% CI ‐2.24 to 6.06; N = 110; Lewis 2013; Analysis 1.3). No study reported the MRC score, TNS, or CMTNS v2.
1.1. Analysis.
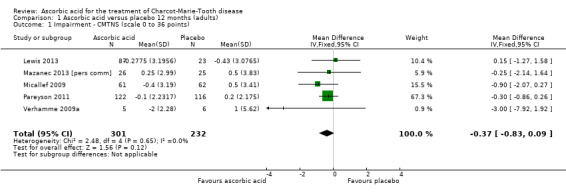
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 1 Impairment ‐ CMTNS (scale 0 to 36 points).
3.
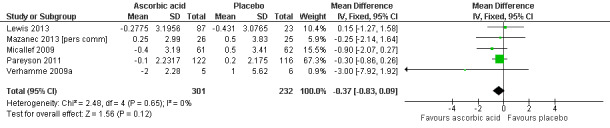
Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months, outcome: 1.1 CMTNS.
1.2. Analysis.
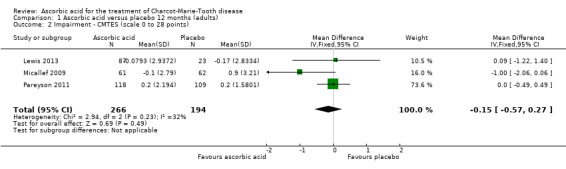
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 2 Impairment ‐ CMTES (scale 0 to 28 points).
1.3. Analysis.
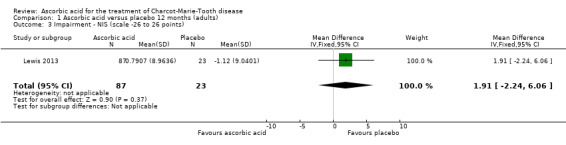
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 3 Impairment ‐ NIS (scale ‐26 to 26 points).
Secondary outcomes at 12 months
Change in disability on a validated scale
Treatment with ascorbic acid was not associated with any significant difference compared to placebo in the disability outcomes measured, including ODSS and ONLS, which were each measured in two trials (totals in analyses N = 134 and N = 289, respectively; Analysis 1.4; Analysis 1.5). These comparisons were dominated by single studies because of unequal study sizes. A single study (N = 11) reported the ALDS, with no significant change versus placebo after ascorbic acid (Verhamme 2009a; Analysis 1.6).
1.4. Analysis.
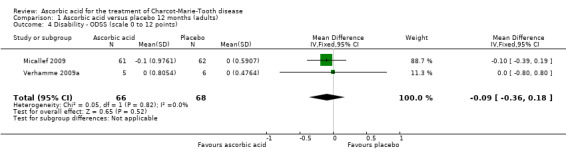
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 4 Disability ‐ ODSS (scale 0 to 12 points).
1.5. Analysis.
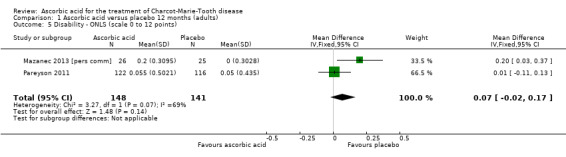
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 5 Disability ‐ ONLS (scale 0 to 12 points).
1.6. Analysis.
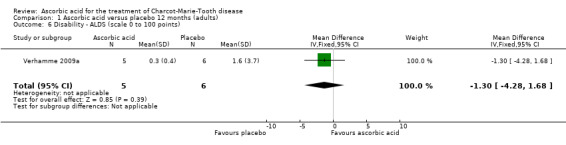
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 6 Disability ‐ ALDS (scale 0 to 100 points).
Change in CMAP amplitudes
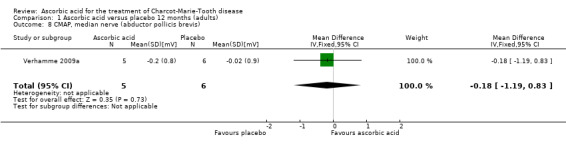
No significant differences were found between ascorbic acid and placebo in the electrophysiological parameters of CMAP amplitude in the ulnar nerve (two studies, N = 161) or median nerve (one study, N = 11), or in a summated amplitude measure (one study, N = 217; Analysis 1.7 to Analysis 1.9).
1.7. Analysis.
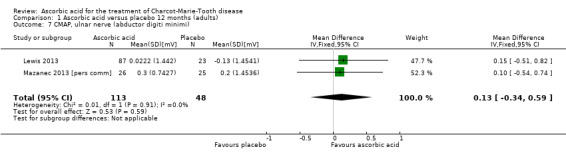
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 7 CMAP, ulnar nerve (abductor digiti minimi).
1.9. Analysis.
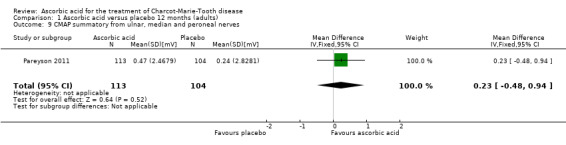
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 9 CMAP summatory from ulnar, median and peroneal nerves.
Change in sensory impairment measured with a validated scale
When measured semi‐quantitatively using the INCAT Sensory Sum (ISS) score in one study (N = 10), sensory function demonstrated no significant change with ascorbic acid versus placebo (Verhamme 2009a; Analysis 1.10).
1.10. Analysis.
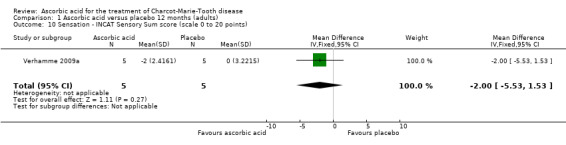
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 10 Sensation ‐ INCAT Sensory Sum score (scale 0 to 20 points).
Change in SNAP amplitude
One study (N = 123) reported data on SNAP amplitudes (Micallef 2009). SNAP amplitudes changed only mildly over the study period of 12 months. Micallef et al. reported a statistically significant effect favouring ascorbic acid when comparing all treatment groups (placebo, 1 g and 3 g ascorbic acid) by ANCOVA. However, when comparing placebo to the higher dosage of 3 g, there was no significant difference (Analysis 1.11).
1.11. Analysis.
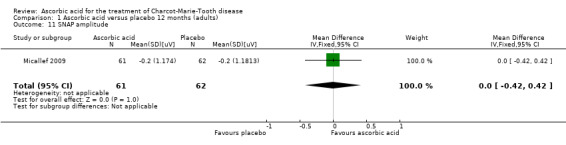
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 11 SNAP amplitude.
Change in grip force and other measures of force
Objective measures of force including hand grip force (N = 412; Mazanec 2013; Micallef 2009; Pareyson 2011), three‐point pinch (N = 300; Mazanec 2013; Pareyson 2011; Verhamme 2009a), and foot dorsiflexion force (N = 423; Mazanec 2013; Micallef 2009; Pareyson 2011; Verhamme 2009a) were not significantly changed after treatment with ascorbic acid versus placebo (Analysis 1.12; Analysis 1.13; Analysis 1.14).
1.12. Analysis.
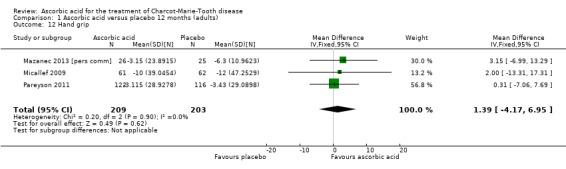
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 12 Hand grip.
1.13. Analysis.
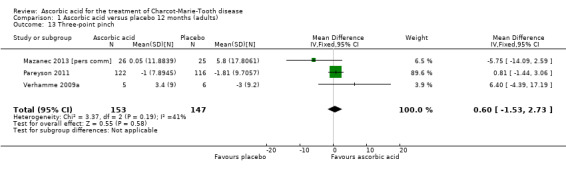
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 13 Three‐point pinch.
1.14. Analysis.
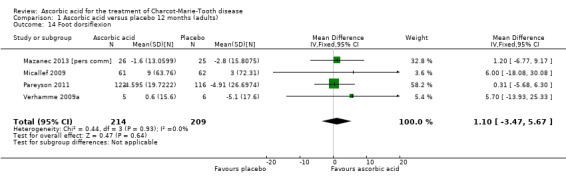
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 14 Foot dorsiflexion.
Change in quality of life
No significant changes were recorded in any of the SF‐36 measures (Analysis 1.15 to Analysis 1.20) when compared to placebo.
1.15. Analysis.
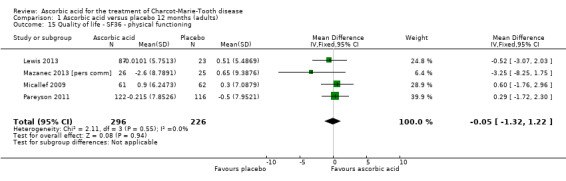
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 15 Quality of life ‐ SF36 ‐ physical functioning.
1.20. Analysis.
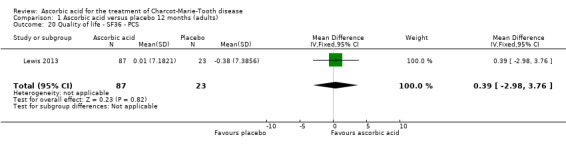
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 20 Quality of life ‐ SF36 ‐ PCS.
Other non pre‐specified secondary outcomes at 12 months
Change in the nine‐hole peg test
Three studies, with a total of 286 participants, reported data on the nine‐hole peg test as a secondary outcome parameter. Data were available for all 286 participants (Mazanec 2013 [pers comm]; Pareyson 2011; Verhamme 2009a). Ascorbic acid treatment showed a positive effect on the nine‐hole peg test over placebo; the effect was small and probably clinically insignificant (mean difference (MD) ‐0.39 seconds 95% CI ‐0.76 to ‐0.02). The studies were very unequal in size, so that the largest of the three trials (Pareyson 2011) achieved a weight of 82.2% (Analysis 1.21; Figure 4). Furthermore, the nine‐hole peg test is not a CMT‐specific outcome parameter and has not been a primary endpoint in any CMT treatment trial, although recently it was found to have good discrimination power between people with CMT who were mildly and severely affected (Mannil 2014).
1.21. Analysis.
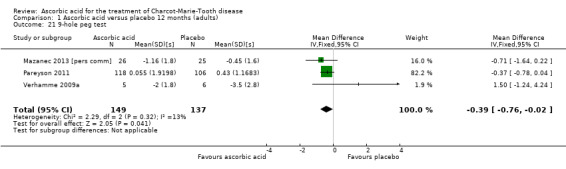
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 21 9‐hole peg test.
4.
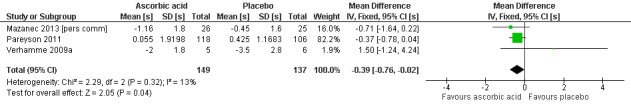
Forest plot of comparison: 1 Ascorbic acid versus placebo 12 months (adults), outcome: 1.21 9‐hole peg test [s].
Change in walking tests
There was no change with ascorbic acid compared to placebo in the 10‐metre walk in the three studies (N = 401) that measured this outcome (Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Analysis 1.22). A single study (N = 11) assessed 50‐metre walk and found no significant difference between ascorbic acid and placebo on this measure (Verhamme 2009a; Analysis 1.23).
1.22. Analysis.
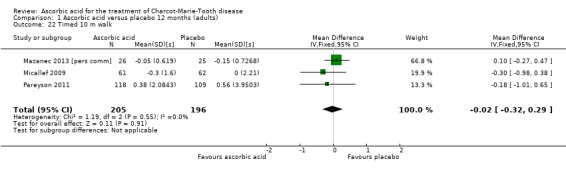
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 22 Timed 10 m walk.
1.23. Analysis.
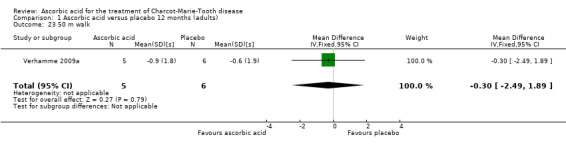
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 23 50 m walk.
Comparison: Oral ascorbic acid treatment versus placebo for adults with CMT1A (24 months)
Primary outcome ‐ impairment at 24 months on a validated scale
There was moderate‐quality evidence for the primary outcome that ascorbic acid treatment had no clinically meaningful effect over placebo using any of the validated outcome parameters (CMTNS in three studies, N = 388; CMTES in two studies, N = 337; Analysis 2.1; Analysis 2.2; Figure 5). It should be noted that these comparisons are strongly dominated by Pareyson 2011 because of its larger number of participants.
2.1. Analysis.
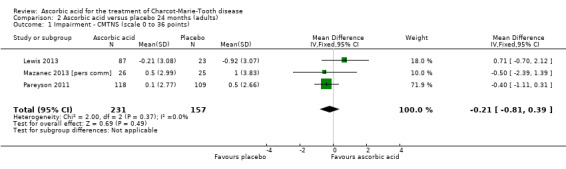
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 1 Impairment ‐ CMTNS (scale 0 to 36 points).
2.2. Analysis.
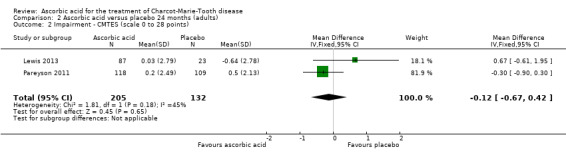
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 2 Impairment ‐ CMTES (scale 0 to 28 points).
5.
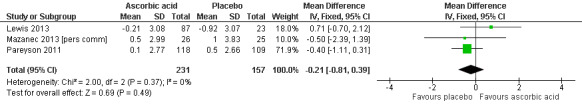
Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.1 Impairment ‐ CMTNS.
Secondary outcomes at 24 months
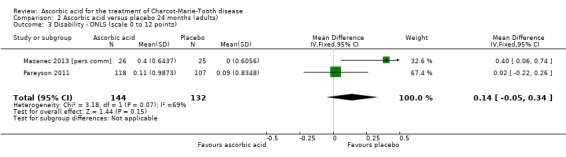
Change in disability on a validated scale
Treatment with ascorbic acid was not associated with any significant difference in the disability outcome ONLS (two studies, N = 276; Mazanec 2013 [pers comm]; Pareyson 2011;
Change in CMAP amplitudes
There was no evidence of significant differences in ulnar nerve (abductor digiti minimi) CMAP (two studies, N = 161; Lewis 2013; Mazanec 2013 [pers comm]; Analysis 2.4) when compared to placebo.
2.4. Analysis.
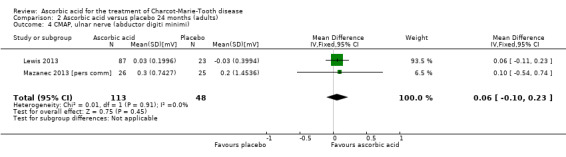
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 4 CMAP, ulnar nerve (abductor digiti minimi).
Change in sensory impairment measured with a validated scale
Sensory impairment was not separately measured by validated scales in the 24‐month trials.
Change in SNAP amplitude
SNAP amplitudes were not studied in the 24‐month trials.
Change in grip force and other measures of force
Objective measures of force including hand grip (N = 186), three‐point pinch (N = 276), and foot dorsiflexion (N = 278) showed no significant difference after treatment with ascorbic acid versus placebo (Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.5 to Analysis 2.7).
2.5. Analysis.
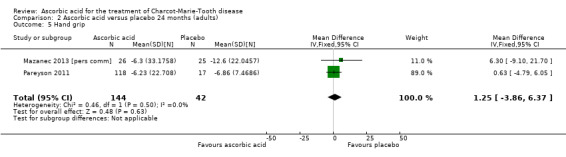
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 5 Hand grip.
2.7. Analysis.
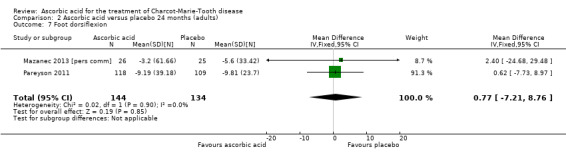
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 7 Foot dorsiflexion.
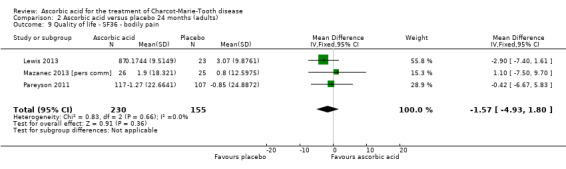
Change in quality of life
No significant changes were recorded in any of the SF‐36 measures when compared to placebo (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.8 to Analysis 2.10).
2.8. Analysis.
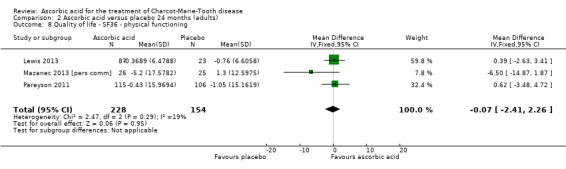
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 8 Quality of life ‐ SF36 ‐ physical functioning.
2.10. Analysis.
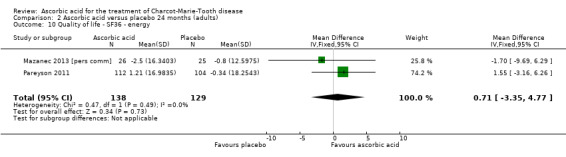
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 10 Quality of life ‐ SF36 ‐ energy.
Other non pre‐specified secondary outcome measures at 24 months
Change in the nine‐hole peg test
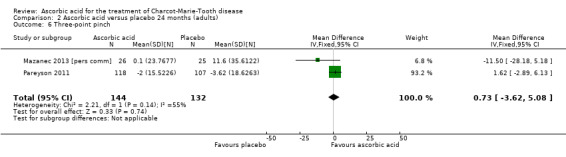
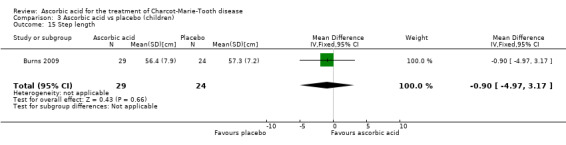
Two studies with a total of 275 participants used the nine‐hole peg test as a secondary outcome parameter (Mazanec 2013 [pers comm]; Pareyson 2011). As in the 12‐month data, ascorbic acid treatment did show a positive effect on the nine‐hole peg test versus placebo (P = 0.004). The MD was ‐1.16 seconds, 95% CI ‐1.96 to ‐0.37. The clinical significance of this result is probably small. The studies were very unequal in size, so that Pareyson 2011, the larger of the two trials, achieved a weight of 82.8% (Analysis 2.11, Figure 6).
2.11. Analysis.
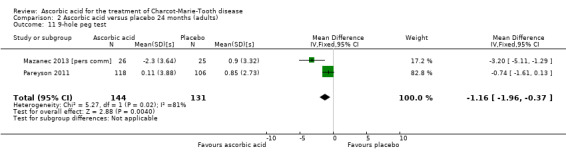
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 11 9‐hole peg test.
6.
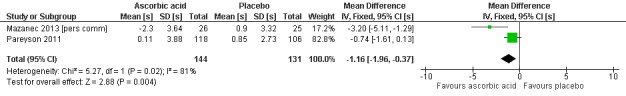
Forest plot of comparison: 2 Ascorbic acid versus placebo 24 months (adults), outcome: 2.11 Impairment ‐ 9‐hole peg test [s].
Change in walking test
The 10‐metre walk test was used by two studies with a total of 278 participants. The results showed no evidence of significant difference between ascorbic acid and placebo (Mazanec 2013 [pers comm]; Pareyson 2011; Analysis 2.12).
2.12. Analysis.
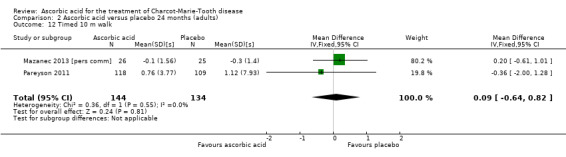
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 12 Timed 10 m walk.
Change in PMP22 mRNA
Skin biopsies of study participants did not show a clinically relevant difference in PMP22 mRNA (two studies, N = 114; Lewis 2013; Pareyson 2011; Nobbio 2014; Analysis 2.13).
2.13. Analysis.
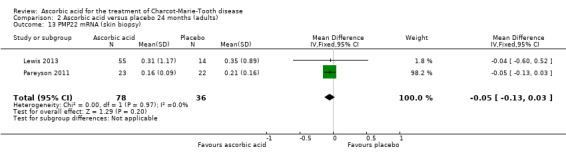
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 13 PMP22 mRNA (skin biopsy).
Comparison: Oral ascorbic acid treatment versus placebo for children with CMT1A
Children were studied in Burns 2009, N = 80.
Primary outcome ‐ impairment at 12 months on a validated scale
The trial did not report the CMTNS, CMTES or CMTNS v2 because they are not validated for children. A paediatric CMT scale has been recently developed (Burns 2012), but was not available at the time of the study.
Secondary outcomes at 12 months
Change in disability on a validated scale
No validated disability scales were used.
Change in CMAP amplitudes
The mean change in CMAP (abductor pollicis brevis) was not significantly different between ascorbic acid and placebo groups (Analysis 3.1).
3.1. Analysis.
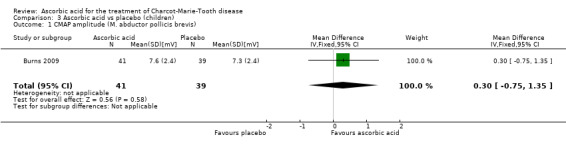
Comparison 3 Ascorbic acid vs placebo (children), Outcome 1 CMAP amplitude (M. abductor pollicis brevis).
Change in sensory impairment measured with a validated scale
Sensory impairment was not measured on a specific scale.
Change in SNAP amplitude
SNAP amplitudes were not reported.
Change of grip force and other measures of force
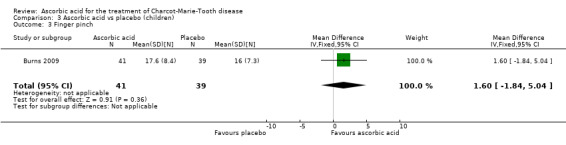
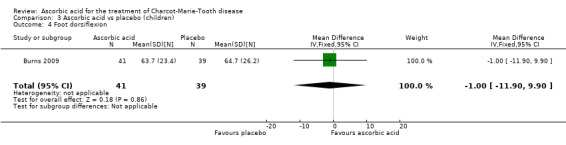
Muscle force measurements by dynamometry of hand grip, finger pinch, foot dorsiflexion and foot plantar flexion, did not show significant differences when compared to placebo (Analysis 3.2 to Analysis 3.5).
3.2. Analysis.
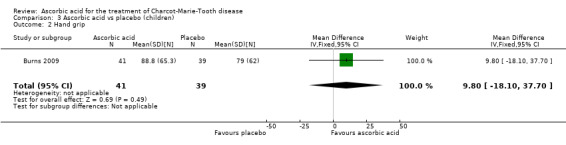
Comparison 3 Ascorbic acid vs placebo (children), Outcome 2 Hand grip.
3.5. Analysis.
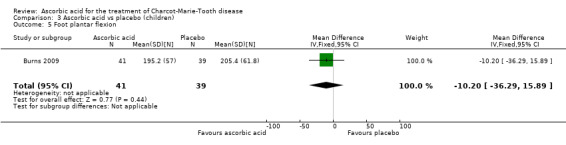
Comparison 3 Ascorbic acid vs placebo (children), Outcome 5 Foot plantar flexion.
Change in quality of life
Change in health‐related quality of life outcomes were reported in the web appendix of the study (Burns 2009). However, quality of life changes did not show any discernible differences between the treatment groups.
Other, non pre‐specified secondary outcome parameters
Change in median nerve conduction velocity
Change in median nerve conduction velocity was the primary outcome parameter of this study. However, there was no significant differences between the treatment groups (Analysis 3.6).
3.6. Analysis.
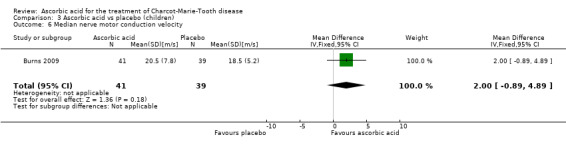
Comparison 3 Ascorbic acid vs placebo (children), Outcome 6 Median nerve motor conduction velocity.
Change in the nine‐hole peg test
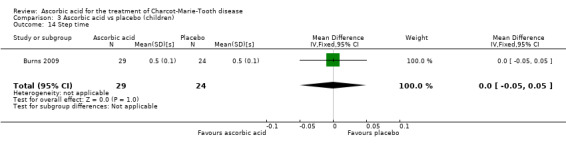
The nine‐hole peg test did not show a significant difference in ascorbic acid‐treated versus placebo‐treated children (Analysis 3.7, N = 78).
3.7. Analysis.
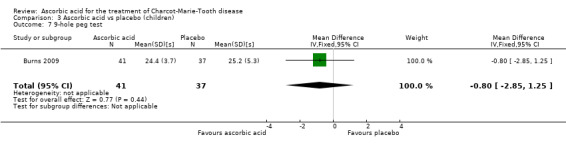
Comparison 3 Ascorbic acid vs placebo (children), Outcome 7 9‐hole peg test.
Change in walking tests
The six‐minute walk test did not show significant differences in ascorbic acid‐treated versus placebo‐treated children (Analysis 3.8, N = 65).
3.8. Analysis.
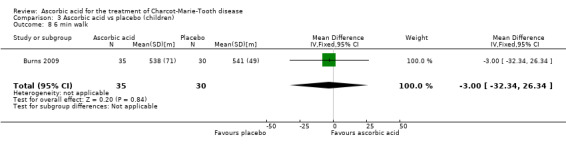
Comparison 3 Ascorbic acid vs placebo (children), Outcome 8 6 min walk.
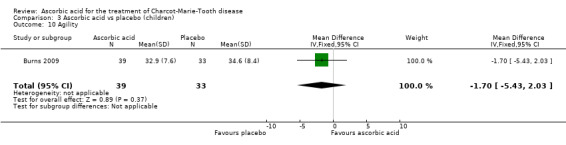
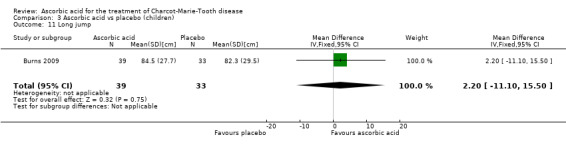
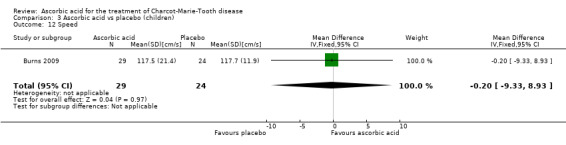
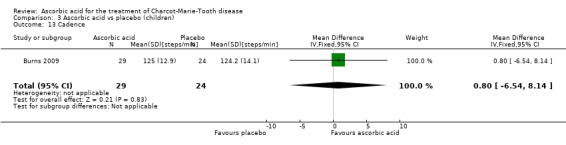
A number of further outcome parameters, particularly functional parameters assessing different aspects of motor function, such as balance, agility, long jump, speed, step time and length, were measured. None of these parameters showed evidence of differences between ascorbic acid and placebo (Analysis 3.9 to Analysis 3.16).
3.9. Analysis.
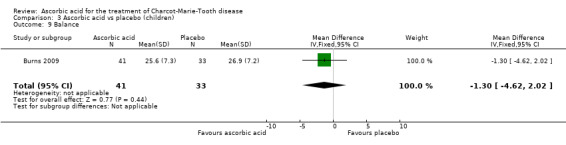
Comparison 3 Ascorbic acid vs placebo (children), Outcome 9 Balance.
3.16. Analysis.
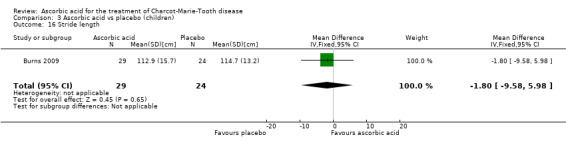
Comparison 3 Ascorbic acid vs placebo (children), Outcome 16 Stride length.
Adverse events, whether minor or severe, caused by ascorbic acid
We distinguished between minor and severe adverse events (those which were fatal, life‐threatening, required or prolonged hospitalisation or caused discontinuation of the treatment). Overall, ascorbic acid treatment was well tolerated. Most adverse events concerned nausea, gastro‐intestinal discomfort and constipation, but these complaints were not more common in the ascorbic acid groups compared to the placebo groups. Overall, 77 serious adverse events with ascorbic acid and placebo occurred in all studies combined (N = 702). The relative abundance of serious adverse events was 11.7% in the ascorbic acid and 12% in the placebo groups. (Participants can have more than one serious adverse event, so the percentages were calculated in the following way: (number of serious adverse events/number of participants) * 100.) (Burns 2009; Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a).
Discussion
Summary of main results
We identified a total of six different trials describing medical treatments of Charcot‐Marie‐Tooth disease (CMT) in a randomised controlled study design that fulfilled the inclusion criteria defined in the protocol. All of the six trials studied the effects of oral ascorbic acid versus placebo treatment in participants with CMT1A. The total number of participants in all trials amounted to 702 (Burns 2009; Micallef 2009; Verhamme 2009a; Pareyson 2011; Lewis 2013; Mazanec 2013 [pers comm]). One of these trial was conducted in children with CMT1A (N = 80, Burns 2009); the other five in adults (N = 622;Lewis 2013; Mazanec 2013 [pers comm]; Micallef 2009; Pareyson 2011; Verhamme 2009a). Overall, 414 participants were treated with ascorbic acid and 288 with placebo.
The six included RCTs all used a medical intervention in the form of an oral medication with ascorbic acid or placebo. Dosages of ascorbic acid were different, ranging from 1 g to 4 g per day. However, there was no significant dose‐response relationship in a regression analysis (data not shown).
These trials provide high‐quality evidence that ascorbic acid does not change the course of CMT1A over 12 or 24 months in adults (Table 1). The observed pooled effect size of ‐0.37 (95% CI ‐0.83 to 0.09) on the CMT neuropathy score (CMTNS) is probably not clinically relevant. Further research including a large number of participants and a trial period longer than 24 months may improve confidence in this estimate. There is low‐quality evidence that ascorbic acid does not improve the course of CMT1A in children over a treatment period of 12 months.
Overall completeness and applicability of evidence
The small number of trials that fulfilled the selection criteria for this review reflects the difficulties of undertaking clinical trials in CMT. Nonetheless, we did identify six trials conforming to the inclusion criteria, and it is very likely that we identified all available trials on ascorbic acid treatment of CMT. In terms of ascorbic acid treatment, this review comprises sufficient data to reach a meaningful conclusion. The consideration of possible effects of ascorbic acid on CMT1A is limited to a certain extent by the treatment period of the clinical trials (12 to 24 months). Due to the slow progression of the disease, the treatment period might have to be even longer than 24 months in order to detect changes in the disease course, but any large effect at longer time periods is unlikely.
Since CMT is biologically and genetically heterogenous and shows great variability in severity, analysis of different subgroups would have been appropriate, but the lack of data made this impossible. The ascorbic acid trials showed that the rate of change in CMTNS and other outcome parameters is very low, which makes a longer study duration, a higher number of participants or a more sensitive outcome measure necessary. The ascorbic acid trials also showed that a power analysis could account for slow rates of change of most outcome parameters. On the other hand, novel outcome parameters are being developed that are potentially more sensitive to change over time and therapeutic effects (Burns 2012; Komyathy 2013; Mannil 2014).
Although the trial in children appeared to provide good quality evidence in terms of trial design and reporting, we graded it as low quality according to GRADE criteria because it was a single trial with a relatively small number of participants, and the primary outcome parameter (motor nerve conduction velocity) was not ideal.
Quality of the evidence
The studies that were identified as RCTs used appropriate randomisation methods. Blinding and follow‐up were appropriate in all studies. All studies were placebo controlled. The treatment trial duration was two years in three studies (Lewis 2013; Mazanec 2013 [pers comm]; Pareyson 2011), and one year in three studies (Burns 2009; Micallef 2009; Verhamme 2009a). Follow‐up was generally good, with low numbers of drop‐outs. Most studies provided reasons for drop‐outs and all performed intention‐to‐treat analyses. One study did not include data of participants who dropped out and did not report the number of drop‐outs, therefore, it was not clear how many data were actually missing (Mazanec 2013 [pers comm]). The chosen outcome parameters were different between trials. Some trials used electrophysiologic outcome parameters (Burns 2009; Verhamme 2009a). The limitation of this approach is that electrophysiologic parameters change very little over time in CMT1A, especially in adults (Verhamme 2009b). However, most studies used the CMTNS as the primary outcome parameter. The CMTNS has been validated for people with CMT and was considered the most appropriate outcome parameter at the time the studies were done. Overall, the choice of outcome parameters was reasonable, being partly based on a workshop by the European Neuromuscular Centre (ENMC) on the conduct of trials in CMT (Reilly 2006). Importantly, there was good overlap of outcome measures between the different studies, so that we were able to perform meta‐analyses of results for several outcome measures. Taken together, we identified six RCTs with proper methodology and design, all studying the effects of ascorbic acid versus placebo on CMT1A.
Potential biases in the review process
BG and PY have been involved in basic research on ascorbic acid function in the peripheral nervous system (Gess 2010; Gess 2011). DP and MMR were investigators in one of the trials on ascorbic acid treatment in adults with CMT1A (Pareyson 2011). These involvements could present an intellectual bias, which we cannot altogether exclude. However, DP and MMR did not assess risk of bias in Pareyson 2011.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other studies or reviews, particularly no other meta‐analyses, on ascorbic acid treatment in Charcot‐Marie‐Tooth disease.
Outlook
Further studies of ascorbic acid in CMT disease with longer study duration and more sensitive outcome parameters may be sensible in the future. However, it is likely that ascorbic acid does not provide any significant benefit for CMT1A, and even with adaptation of study design or longer time periods, no significant effect is likely to be forthcoming. Further research into the mechanism of action of vitamin C in the peripheral nervous system may lead to novel therapeutic approaches. Active research on other medical and physical therapies of CMT is currently undergoing and will be reviewed in a separate Cochrane review on treatments of CMT other than ascorbic acid.
Authors' conclusions
Implications for practice.
There is high‐quality evidence that ascorbic acid does not improve the course of CMT1A in adults. There is low‐quality evidence that ascorbic acid does not improve the course of CMT1A in children.
Implications for research.
There is a need for further properly designed trials of interventions in CMT. Such trials should have adequate follow‐up of at least two years, use appropriate outcome measures and be powered to detect a clinically meaningful result. Furthermore, there is a need for new validated outcome parameters, sensitive to change over time.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2015 | Amended | Minor correction to punctuation |
Notes
The review 'Treatment for Charcot Marie Tooth disease' (Young 2008) has been split into two separate reviews: Ascorbic acid for Charcot‐Marie‐Tooth disease and Treatments other than ascorbic acid for Charcot‐Marie‐Tooth disease. Treatments other than ascorbic acid for Charcot‐Marie‐Tooth disease will be published shortly.
Acknowledgements
We thank the Trials Search Co‐ordinator of the Cochrane Neuromuscular Disease Group, Angela Gunn, who provided the searches. We also thank Ruth Brassington of the Cochrane Foundation Neuromuscular Study Group for her excellent assistance with formal requirements and her much appreciated draft of the plain language summary.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Neuromuscular Disease Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health. The Cochrane Neuromuscular Disease Group is also supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. Neuromuscular Register (CRS) search strategy
#1 MeSH DESCRIPTOR Charcot‐Marie‐Tooth Disease [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Hereditary Sensory and Motor Neuropathy [REFERENCE] [STANDARD] #3 MeSH DESCRIPTOR Muscular Atrophy, Spinal [REFERENCE] [STANDARD] #4 (hereditary NEAR neuropathy):ti or (hereditary NEAR neuropathies):ti [REFERENCE] [STANDARD] #5 (hereditary NEAR neuropathy):ab or (hereditary NEAR neuropathies):ab [REFERENCE] [STANDARD] #6 peritoneal and atrophy [REFERENCE] [STANDARD] #7 distal and atrophy [REFERENCE] [STANDARD] #8 charcot [REFERENCE] [STANDARD] #9 inherited near neuropathy:ti or inherited near neuropathies:ti [REFERENCE] [STANDARD] #10 inherited near neuropathy:ab or inherited near neuropathies:ab [REFERENCE] [STANDARD] #11 #1 or #2 or #3 or #4 or #5 #6 or #7 or #8 or #9 or #10 [REFERENCE] [STANDARD] #12 MeSH DESCRIPTOR Ascorbic Acid Explode All [REFERENCE] [STANDARD] #13 "ascorbic acid" or "vitamin c" or ascorbate [REFERENCE] [STANDARD] #14 #11 and #13 [REFERENCE] [STANDARD] #15 (#11 and #13) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
#1MeSH descriptor: [Charcot‐Marie‐Tooth Disease] this term only #2charcot:ti or charcot:ab #3(hereditary near neuropathy):ti or (hereditary near neuropathy):ab #4(hereditary near neuropathies):ti or (hereditary near neuropathies):ab #5MeSH descriptor: [Hereditary Sensory and Motor Neuropathy] this term only #6peroneal and atrophy #7MeSH descriptor: [Muscular Atrophy, Spinal] explode all trees #8"spinal muscular atrophy" #9distal and atrophy #10#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 #11MeSH descriptor: [Ascorbic Acid] explode all trees #12"ascorbic acid" or "vitamin c" or ascorbate #13#11 or #12 #14#10 and #13
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to September Week 2 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (410388) 2 controlled clinical trial.pt. (91596) 3 randomized.ab. (302645) 4 placebo.ab. (157240) 5 drug therapy.fs. (1834137) 6 randomly.ab. (214353) 7 trial.ab. (314759) 8 groups.ab. (1352220) 9 or/1‐8 (3458228) 10 exp animals/ not humans.sh. (4113127) 11 9 not 10 (2948030) 12 Charcot‐Marie‐Tooth Disease/ (3093) 13 "Hereditary Sensory and Motor Neuropathy"/ (997) 14 (charcot or (heredit$ adj5 neuropath$)).tw. (7630) 15 (peroneal and atroph$).tw. (409) 16 muscular atrophy, spinal/ (2790) 17 (distal and atroph$).tw. (2475) 18 (inherit$ and neuropath$).tw. (2441) 19 or/12‐18 (14672) 20 exp Ascorbic Acid/ (37022) 21 ((vitamin adj1 c) or ascorbate).mp. (28872) 22 20 or 21 (48797) 23 11 and 19 and 22 (29) 24 remove duplicates from 23 (29)
Appendix 4. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2015 Week 38> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (44434) 2 double‐blind procedure.sh. (123428) 3 single‐blind procedure.sh. (20967) 4 randomized controlled trial.sh. (383450) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1183420) 6 trial.ti. (184803) 7 or/1‐6 (1326968) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1408726) 9 animal/ or nonanimal/ or animal experiment/ (3447477) 10 9 not 8 (2864089) 11 7 not 10 (1219469) 12 limit 11 to embase (1001797) 13 Hereditary Motor Sensory Neuropathy/ (7138) 14 charcot marie tooth.mp. (4386) 15 (charcot or (heredit$ adj5 neuropath$)).tw. (9998) 16 (peroneal and atroph$).tw. (484) 17 spinal muscular atrophy/ (4864) 18 (distal and atroph$).tw. (3542) 19 (inherit$ and neuropath$).tw. (3388) 20 or/13‐19 (22461) 21 exp ascorbic acid/ (74117) 22 ((vitamin adj c) or ascorbate).mp. (35012) 23 21 or 22 (82816) 24 12 and 20 and 23 (31) 25 remove duplicates from 24 (31)
Appendix 5. LILACS (IAHx) search strategy
(MH:"Charcot‐Marie‐Tooth Disease" or "Charcot‐Marie‐Tooth" or "Hereditary Sensory and Motor Neuropathy" or "Neuropatía Hereditaria Motora y Sensorial" or "Neuropatia Hereditária Motora e Sensorial" or "peroneal atrophy" or "Muscular Atrophy, Spinal" or "spinal muscular atrophy" or "Atrofia Muscular Espinal" or "distal atrophy" or "inherited neuropathy") and ("ascorbic acid" or "vitamin c" or ascorbate) and (((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos))))
Appendix 6. ClinicalTrials.gov
searched with the terms "Ascorbic acid Charcot", "Vitamin C Charcot", "Ascorbic acid neuropathy" and "Vitamin C neuropathy".
Appendix 7. WHO international Clinical Trials Registry
searched with the terms "Ascorbic AND acid AND Charcot", "Vitamin AND C AND Charcot", "Ascorbic AND acid AND neuropathy" and "Vitamin AND C AND neuropathy".
Data and analyses
Comparison 1. Ascorbic acid versus placebo 12 months (adults).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Impairment ‐ CMTNS (scale 0 to 36 points) | 5 | 533 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.83, 0.09] |
| 2 Impairment ‐ CMTES (scale 0 to 28 points) | 3 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.57, 0.27] |
| 3 Impairment ‐ NIS (scale ‐26 to 26 points) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [‐2.24, 6.06] |
| 4 Disability ‐ ODSS (scale 0 to 12 points) | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.36, 0.18] |
| 5 Disability ‐ ONLS (scale 0 to 12 points) | 2 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.02, 0.17] |
| 6 Disability ‐ ALDS (scale 0 to 100 points) | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐4.28, 1.68] |
| 7 CMAP, ulnar nerve (abductor digiti minimi) | 2 | 161 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.34, 0.59] |
| 8 CMAP, median nerve (abductor pollicis brevis) | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.19, 0.83] |
| 9 CMAP summatory from ulnar, median and peroneal nerves | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.48, 0.94] |
| 10 Sensation ‐ INCAT Sensory Sum score (scale 0 to 20 points) | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.53, 1.53] |
| 11 SNAP amplitude | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.42, 0.42] |
| 12 Hand grip | 3 | 412 | Mean Difference (IV, Fixed, 95% CI) | 1.39 [‐4.17, 6.95] |
| 13 Three‐point pinch | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.53, 2.73] |
| 14 Foot dorsiflexion | 4 | 423 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐3.47, 5.67] |
| 15 Quality of life ‐ SF36 ‐ physical functioning | 4 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐1.32, 1.22] |
| 16 Quality of life ‐ SF36 ‐ bodily pain | 3 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐2.69, 1.62] |
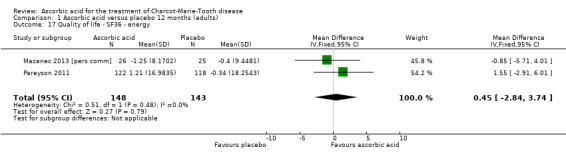
| 17 Quality of life ‐ SF36 ‐ energy | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐2.84, 3.74] |
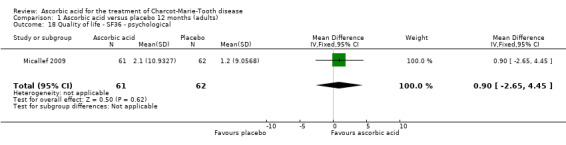
| 18 Quality of life ‐ SF36 ‐ psychological | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.65, 4.45] |
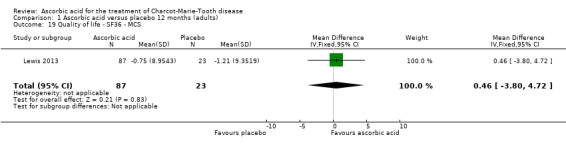
| 19 Quality of life ‐ SF36 ‐ MCS | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐3.80, 4.72] |
| 20 Quality of life ‐ SF36 ‐ PCS | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐2.98, 3.76] |
| 21 9‐hole peg test | 3 | 286 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.76, ‐0.02] |
| 22 Timed 10 m walk | 3 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.32, 0.29] |
| 23 50 m walk | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.49, 1.89] |
1.8. Analysis.
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 8 CMAP, median nerve (abductor pollicis brevis).
1.16. Analysis.
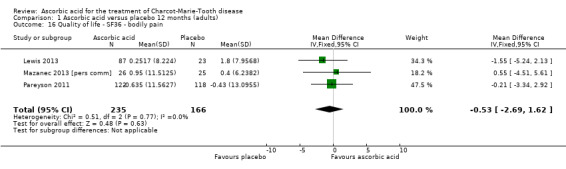
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 16 Quality of life ‐ SF36 ‐ bodily pain.
1.17. Analysis.
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 17 Quality of life ‐ SF36 ‐ energy.
1.18. Analysis.
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 18 Quality of life ‐ SF36 ‐ psychological.
1.19. Analysis.
Comparison 1 Ascorbic acid versus placebo 12 months (adults), Outcome 19 Quality of life ‐ SF36 ‐ MCS.
Comparison 2. Ascorbic acid versus placebo 24 months (adults).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Impairment ‐ CMTNS (scale 0 to 36 points) | 3 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.81, 0.39] |
| 2 Impairment ‐ CMTES (scale 0 to 28 points) | 2 | 337 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.67, 0.42] |
| 3 Disability ‐ ONLS (scale 0 to 12 points) | 2 | 276 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.05, 0.34] |
| 4 CMAP, ulnar nerve (abductor digiti minimi) | 2 | 161 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.10, 0.23] |
| 5 Hand grip | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐3.86, 6.37] |
| 6 Three‐point pinch | 2 | 276 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐3.62, 5.08] |
| 7 Foot dorsiflexion | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐7.21, 8.76] |
| 8 Quality of life ‐ SF36 ‐ physical functioning | 3 | 382 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐2.41, 2.26] |
| 9 Quality of life ‐ SF36 ‐ bodily pain | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐4.93, 1.80] |
| 10 Quality of life ‐ SF36 ‐ energy | 2 | 267 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐3.35, 4.77] |
| 11 9‐hole peg test | 2 | 275 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.96, ‐0.37] |
| 12 Timed 10 m walk | 2 | 278 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.64, 0.82] |
| 13 PMP22 mRNA (skin biopsy) | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.03] |
2.3. Analysis.
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 3 Disability ‐ ONLS (scale 0 to 12 points).
2.6. Analysis.
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 6 Three‐point pinch.
2.9. Analysis.
Comparison 2 Ascorbic acid versus placebo 24 months (adults), Outcome 9 Quality of life ‐ SF36 ‐ bodily pain.
Comparison 3. Ascorbic acid vs placebo (children).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CMAP amplitude (M. abductor pollicis brevis) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.75, 1.35] |
| 2 Hand grip | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 9.80 [‐18.10, 37.70] |
| 3 Finger pinch | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐1.84, 5.04] |
| 4 Foot dorsiflexion | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐11.90, 9.90] |
| 5 Foot plantar flexion | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐36.29, 15.89] |
| 6 Median nerve motor conduction velocity | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.89, 4.89] |
| 7 9‐hole peg test | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.85, 1.25] |
| 8 6 min walk | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐32.34, 26.34] |
| 9 Balance | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.62, 2.02] |
| 10 Agility | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐5.43, 2.03] |
| 11 Long jump | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐11.10, 15.50] |
| 12 Speed | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐9.33, 8.93] |
| 13 Cadence | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐6.54, 8.14] |
| 14 Step time | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 15 Step length | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐4.97, 3.17] |
| 16 Stride length | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐9.58, 5.98] |
3.3. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 3 Finger pinch.
3.4. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 4 Foot dorsiflexion.
3.10. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 10 Agility.
3.11. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 11 Long jump.
3.12. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 12 Speed.
3.13. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 13 Cadence.
3.14. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 14 Step time.
3.15. Analysis.
Comparison 3 Ascorbic acid vs placebo (children), Outcome 15 Step length.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burns 2009.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | 80 children with genetically proven CMT1A; aged 2 to 16 years; both sexes | |
| Interventions | Oral ascorbic acid dose (approximately 30 mg/kg body weight/day) (N = 41):
versus matching placebo (N = 39) for 12 months |
|
| Outcomes | Primary: change in median nerve conduction velocity (m/s) after 12 months Secondary: change in foot and hand strength, motor function, walking ability and quality of life after 12 months |
|
| Funding sources | "The University of Sydney Research and Development Scheme (#K2701 U3332), National Health and Medical Research Council of Australia (NHMRC #336705), James N Kirby Foundation, New South Wales Podiatrists Registration Board, Australian Podiatry Education and Research Foundation, and Charcot–Marie–Tooth Association of Australia" | |
| Conflicts of interest | No conflicts of interest reported | |
| Notes | Date study was performed: June 2007 to December 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned in a 1:1 ratio by a computer‐generated algorithm" |
| Allocation concealment (selection bias) | Unclear risk | "Randomisation data were retained by central pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The principal investigator (JB) and the other investigators, study coordinators, assessors, children, and their parents were unaware of the treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The principal investigator (JB) and the other investigators, study coordinators, assessors [...] were unaware of the treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analysis of treatment effect between groups was by intention to treat" |
| Selective reporting (reporting bias) | Low risk | Study protocol available on www.ANZCTR.org.au; outcome reporting according to protocol |
| Other bias | Low risk | None identified |
Lewis 2013.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | 110 participants between 14 and 69 years old with CMT1A proven genetically, both sexes | |
| Interventions | 4 g ascorbic acid per day (N = 87), versus matching placebo (N = 23) for 24 months | |
| Outcomes | Primary: CMTNS after 24 months Secondary: Neuropathy Impairment Score, nerve conduction studies, SF‐36, serum ascorbic acid levels, PMP22 mRNA in skin biopsy (RT‐PCR) after 24 months |
|
| Funding sources | "Muscular Dystrophy Association and the Charcot‐Marie‐Tooth Association. Wayne State University, the University of Rochester, and Johns Hopkins University were members of the Inherited Neuropathy Consortium of the Rare Disease Clinical Research Network supported by the National Institute of Neurological Disorders and Stroke and Office of Rare Diseases." | |
| Conflicts of interest | "Dr. Lewis has consulted for Baxter, CSL Behring, AxelaCare, Novartis, and Bristol‐Myers Squibb. Dr. Shy has received grant support from the National Institute of Neurological Disorders and Stroke, Muscular Dystrophy Association, and Charcot‐Marie‐Tooth Association." | |
| Notes | Study start date: April 2007; completion date: December 2012 (as stated on ClinicalTrials.gov) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization plan was computer generated" |
| Allocation concealment (selection bias) | Unclear risk | "Subjects were randomly assigned with 4:1 allocation to AA (80%) or placebo (20%)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Only a programmer in the Muscle Study Group Biostatistics Center and the investigational pharmacy had access to the treatment assignments during the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Only a programmer in the Muscle Study Group Biostatistics Center and the investigational pharmacy had access to the treatment assignments during the trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "This approach appropriately accounts for missing data when estimating the model parameters under the “missing at random” assumption." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available on www.ClinicalTrials.gov; all pre‐specified outcomes reported; some additional, not pre‐specified outcomes reported |
| Other bias | Low risk | None identified |
Mazanec 2013 [pers comm].
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | People with CMT1A genetically carrying the duplication on chromosome 17q11.2; between 18 and 70 years; both sexes | |
| Interventions | Ascorbic acid 1500 mg/day (N = 26) versus matching placebo (N = 25) for 24 months | |
| Outcomes | Primary: change in CMTNS after 24 months Secondary: change in MVIC, 10‐m walking, nine‐hole peg test, ONLS, VAS for pain and fatigue, SF36 after 24 months |
|
| Funding sources | "The clinical trial was funded by grant of The Czech Internal Grant Agency of Ministry of Health of Czech Republic (No NR/9517)" | |
| Conflicts of interest | No conflict of interest for any investigator | |
| Notes | Study not published yet, data obtained by communication with principal investigator Date study was performed: 1 January 2007 to 31 December 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization was performed by the pharmaceutical company FAVEA s.r.o, which prepared the study medication for this trial |
| Allocation concealment (selection bias) | Unclear risk | The investigator at each centre obtained the study medication and allocation concealment code from the pharmaceutical company |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The case report form included only initials and study number, not the names or further data about participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The two investigators in each centre (one examining and one treating neurologist) were not in contact about the participants; only the case report form documents were transmitted from examining to treating neurologist |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study evaluation did not include the data of participants who dropped out |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | Low risk | None identified |
Micallef 2009.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | 179 participants with CMT1A aged 34 to 58 years, genetically confirmed, both sexes | |
| Interventions | 1 g/day ascorbic acid (N = 56), 3 g/day ascorbic acid (N = 61), versus matching placebo (N = 62) for 12 months | |
| Outcomes | Primary: change in CMTNS after 12 months Secondary: change in CMTES, quantitative motor testing, SF‐36, ODSS, time to walk 10‐m, VAS, Clinical Global Impression Severity scale, ascorbic acid blood concentration, nerve conduction studies after 12 months |
|
| Funding sources | "French Ministry of Health (National PHRC 2004) and Association Française Contre les Myopathies." (p. 1) | |
| Conflicts of interest | "Ascorbic acid for the treatment of CMT1A is under patent and its application has been registered by INSERM, AFM, and Marseille II University. MF is one of the inventors of the patent, which has been licensed to Murigenetics. MF and OB participate in the activities of Murigenetics as scientific advisers and are each 15% shareholders in the company. The other authors have no conflict of interest." (p. 8) | |
| Notes | Date study was performed: recruitment from September 2005 to September 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was generated using randomization.com" |
| Allocation concealment (selection bias) | Unclear risk | "Active treatment and placebo were prepared by CLIPA Clinical Packaging." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both patients and investigators were unaware of the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both patients and investigators were unaware of the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analyses were done on the intention to treat population." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | Low risk | None identified |
Pareyson 2011.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | People with CMT1A genetically carrying the duplication on chromosome 17q11.2; between 18 and 70 years old; both sexes | |
| Interventions | 1500 mg/day ascorbic acid (N = 138) versus matching placebo (N = 133) for 24 months | |
| Outcomes | Primary: change in CMTNS after 24 months Secondary: change in MVIC, 10‐m walking, nine‐hole peg test, ONLS, VAS for pain and fatigue, SF‐36, skin biopsy (expression of PMP22) after 24 months |
|
| Funding sources | "Telethon‐UILDM (grant numbers GUP04002 and GUP05007) and AIFA (Italian Medicines Agency; grant number FARM53APAH) in Italy, and Muscular Dystrophy Campaign in the UK (grant number RA3/736/1). MMR also receives funding from CMTUK and the UK Medical Research Council. In the UK, this work was undertaken at University College London Hospitals and University College London, which received a proportion of funding from the UK Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme." | |
| Conflicts of interest | "RACH was a consultant for Octapharma, Baxter, Laboratoires Français de Fractionnement et des Biotechnologies (LFB), and Novartis. AS was a board member for Novartis, and has received speaker honoraria from Sanofi‐Aventis. All other authors declare that they have no conflicts of interest." | |
| Notes | Date study was performed: inclusion from March 2006 to September 2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was computer generated (by a pseudo‐random number generator)" |
| Allocation concealment (selection bias) | Low risk | "Treatment was allocated centrally by telephone, and was stratified by centre and disease severity (CMTNS ≤ 10 vs > 10, or CMTES ≤ 8 vs > 8 if electrophysiological assessment was not done in the previous 3 months), with a block size of four (unknown to investigators) within each centre. The sequence was available in opaque sealed envelopes at every centre for emergency unmasking." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, treating physicians, [...] were masked to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[...] physicians assessing outcomes with clinical scales at baseline and during follow‐up were masked to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The main analysis of the primary outcome included all randomised patients who took at least one dose of study treatment. [...] For patients who were unavailable, missing data were imputed according to Rubin's multiple imputation approach."; six participants dropped out after allocation due to withdrawal of consent. |
| Selective reporting (reporting bias) | Low risk | Study protocol available on www.ClinicalTrialsRegister.eu and separate publication; outcome reporting according to protocol |
| Other bias | Low risk | None identified |
Verhamme 2009a.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants | People with CMT1A, genetically carrying the duplication on chromosome 17q11.2, between 12 and 25 years, both sexes | |
| Interventions | Ascorbic acid 2 g/day (N = 5) versus matching placebo (N = 6) for 12 months | |
| Outcomes | Primary: change in motor nerve conduction velocity of the median nerve after 12 months Secondary: change in: minimal F response latency of the median nerve, compound muscle action potential amplitude and area, motor unit number estimation of the abductor pollicis brevis muscle, handgrip strength, strength of arm flexors, foot dorsiflexors, knee extensors and hip flexors, overall disability sum score, CMTNS, Academic Medical Center Linear Disability Scale score, serum ascorbic acid concentrations after 12 months | |
| Funding sources | "internally funded at the Department of Neurology and Clinical Neurophysiology, Academic Medical Centre, University of Amsterdam." | |
| Conflicts of interest | No competing interests declared | |
| Notes | Date study was performed: inclusion December 2005 and June 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The pharmacist, who did not have any further role in the study, generated a computerized randomization sequence." |
| Allocation concealment (selection bias) | Unclear risk | "Eligible patients were randomly allocated to oral ascorbic acid or placebo." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants and investigators were unaware of the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participants and investigators were unaware of the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Randomised patients with at least one follow‐up measure of the primary outcome were analyzed according to the intention‐to‐treat principle. In case of incomplete data, we used the last observation carried forward approach." |
| Selective reporting (reporting bias) | Low risk | Study protocol available on www.ClinicalTrials.gov; outcome reporting according to protocol |
| Other bias | Low risk | None identified |
N: number of participants
CMT1A: Charcot‐Marie‐Tooth disease type 1A
CMTES: Charcot‐Marie‐Tooth disease examination score
CMTNS: Charcot‐Marie‐Tooth disease neuropathy score
MVIC: maximal voluntary isometric contraction
ODSS: Overall Disability Sum Score
ONLS: Overall Neuropathy Limitations Scale
SF‐36: Short‐Form 36 Health Survey Questionnaire
PMP22: peripheral myelin protein 22 kDa
mRNA: messenger ribonucleic acid
RT‐PCR: reverse transcription polymerase chain reaction
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Toth 2009 | Controlled by untreated cohort group, not randomised |
Differences between protocol and review
B Gess, J Baets, MM Reilly and D Pareyson joined the author team for this review. F Stögbauer and T Butterfass‐Bahloul, who were authors of Young 2008, withdrew.
We added additional sections to the Methods according to current Cochrane requirements.
The time points of 12 and 24 months for our meta‐analyses were chosen post hoc, because we found that trials used either a 12‐month or a 24‐month study duration and these time points are more suitable than 12 weeks, given the slow progression of the disease.
'Explicit inclusion criteria' relate to the GRADE criterion of indirectness. We removed it from the 'Risk of bias' assessment.
Contributions of authors
B Gess wrote the first and all subsequent drafts. P Young and D Pareyson commented on the first and subsequent drafts. MM Reilly commented on the penultimate draft. P Young, M Reilly and D Pareyson assisted B Gess in the selection of studies. B Gess and J Baets independently extracted data from the studies. P Young assisted in evaluation of data and studies and overlooked the progress of the review. M Reilly supported the progress of the review. P de Jonghe assisted J Baets in extraction of data. B Gess analysed the data. All six authors agreed to the final text.
Sources of support
Internal sources
University of Münster, Germany.
University of Antwerp, Belgium.
External sources
European Neuromuscular Centre, Netherlands.
Declarations of interest
MM Reilly and D Pareyson were involved as investigators in one of the trials included in this review (Pareyson 2011). B Gess and P Young have been involved in basic research projects on the function of ascorbic acid in the peripheral nervous system (Gess 2010; Gess 2011).
Edited (no change to conclusions)
References
References to studies included in this review
Burns 2009 {published data only}
- Burns J, Ouvrier RA, Yiu EM, Joseph PD, Kornberg AJ, Fahey MC, et al. Ascorbic acid for Charcot–Marie–Tooth disease type 1A in children: a randomised, double‐blind, placebo‐controlled, safety and efficacy trial. Lancet Neurology 2009;8(6):537‐44. [PUBMED: 19427269] [DOI] [PubMed] [Google Scholar]
Lewis 2013 {published data only}
- Lewis RA, McDermott MP, Herrmann DN, Hoke A, Clawson LL, Siskind C, et al. for the Muscle Study Group. High‐dosage ascorbic acid treatment in Charcot‐Marie‐Tooth disease type 1A: results of a randomized, double‐masked, controlled trial. JAMA Neurology 2013;70(8):981‐7. [PUBMED: 23797954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazanec 2013 [pers comm] {unpublished data only}
- Mazanec R. CMT Cochrane Review [personal communication]. Email to: B Gess 12 August 2013.
Micallef 2009 {published data only}
- Micallef J, Attarian S, Dubourg O, Gonnaud PM, Hogrel JY, Stojkovic T, et al. Effect of ascorbic acid in patients with Charcot‐Marie‐Tooth disease type 1A: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet Neurology 2009;8(12):1103‐10. [PUBMED: 19818690] [DOI] [PubMed] [Google Scholar]
Pareyson 2011 {published data only}
- Pareyson D, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, et al. Ascorbic acid in Charcot‐Marie‐Tooth disease type 1A (CMT‐TRIAAL and CMT‐TRAUK): a double‐blind randomised trial. Lancet Neurology 2001;10(4):320‐8. [PUBMED: 23525455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhamme 2009a {published data only}
- Verhamme C, Haan RJ, Vermeulen M, Baas F, Visser M, Schaik IN. Oral high dose ascorbic acid treatment for one year in young CMT1A patients: a randomised, double‐blind, placebo‐controlled phase II trial. BMC Medicine 2009;7:70. [http://www.biomedcentral.com/1741‐7015/7/70; PUBMED: 19909499] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Toth 2009 {published data only}
- Toth C. Poor tolerability of high dose ascorbic acid in a population of genetically confirmed adult Charcot‐Marie‐Tooth 1A patients. Acta Neurologica Scandinavica 2009;120(2):134‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1996
- Anderson RT, Aaronson NK, Bullinger M, McBee WL. A review of the progress towards developing health‐related quality‐of‐life instruments for international clinical studies and outcomes research. Pharmacoeconomics 1996;10(4):336‐55. [DOI] [PubMed] [Google Scholar]
Auer‐Grumbach 2013
- Auer‐Grumbach M. Hereditary sensory and autonomic neuropathies. Handbook of Clinical Neurology 2013;115:893‐906. [DOI] [PubMed] [Google Scholar]
Baets 2014
- Baets J, Jonghe P, Timmerman V. Recent advances in Charcot‐Marie‐Tooth disease. Current Opinion in Neurology 2014;27(5):532‐40. [DOI] [PubMed] [Google Scholar]
Baxter 2002
- Baxter RV, Ben Othmane K, Rochelle JM, Stajich JE, Hulette C, Dew‐Knight S, et al. Ganglioside‐induced differentiation‐associated protein‐1 is mutant in Charcot‐Marie‐Tooth disease type 4A/8q21. Nature Genetics 2002;30(1):21‐2. [DOI] [PubMed] [Google Scholar]
Bird 2014
- Bird TD. Charcot‐Marie‐Tooth Hereditary Neuropathy Overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al. editor(s). GeneReviews [Internet]. Seattle: University of Washington, 1998 (updated 2014). [Google Scholar]
Braathen 2012
- Braathen GJ. Genetic epidemiology of Charcot‐Marie‐Tooth disease. Acta Neurologica Scandinavica Supplement 2012;193(iv):22. [DOI] [PubMed] [Google Scholar]
Burns 2012
- Burns J, Ouvrier R, Estilow T, Shy R, Laurá M, Pallant JF, et al. Validation of the Charcot‐Marie‐Tooth disease pediatric scale as an outcome measure of disability. Annals of Neurology 2012;71(5):642‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chance 1993
- Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 1993;72(1):143‐51. [DOI] [PubMed] [Google Scholar]
Cornblath 1999
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, et al. Total neuropathy score: validation and reliability study. Neurology 1999;53(8):1660‐4. [DOI] [PubMed] [Google Scholar]
d'Ydewalle 2012
- d'Ydewalle C, Benoy V, Bosch L. Charcot‐Marie‐Tooth disease: emerging mechanisms and therapies. International Journal of Biochemistry and Cell Biology 2012;44(8):1299‐304. [DOI] [PubMed] [Google Scholar]
Dyck 2005b
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments, and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: WB Saunders, 2005:1031‐52. [Google Scholar]
Eldridge 1987
- Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon‐related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. Journal of Cell Biology 1987;105(2):1023‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabreels‐Festen 1993
- Gabreels‐Festen A, Gabreels F. Hereditary demyelinating motor and sensory neuropathy. Brain Pathology 1993;3(2):135‐46. [DOI] [PubMed] [Google Scholar]
Gess 2010
- Gess B, Lohmann C, Halfter H, Young P. Sodium‐dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L‐ascorbic acid into Schwann cells. Glia 2010;58(3):287‐99. [DOI] [PubMed] [Google Scholar]
Gess 2011
- Gess B, Röhr D, Fledrich R, Sereda MW, Kleffner I, Humberg A, et al. Sodium‐dependent vitamin C transporter 2 deficiency causes hypomyelination and extracellular matrix defects in the peripheral nervous system. Journal of Neuroscience 2011;31(47):17180‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gess 2013
- Gess B, Schirmacher A, Boentert M, Young P. Charcot‐Marie‐Tooth disease: frequency of genetic subtypes in a German neuromuscular center population. Neuromuscular Disorders 2013;23(8):647‐51. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool [Software].(developed by Evidence Prime, Inc.). Available from gradepro.org. Computer program on www.gradepro.org]. McMaster University, 2015.
Grandis 2005
- Grandis M, Shy ME. Current therapy for Charcot‐Marie‐Tooth disease. Current Treatment Options in Neurology 2005;7(1):23‐31. [DOI] [PubMed] [Google Scholar]
Hahn 2001
- Hahn AF, Ainsworth PJ, Bolton CF, Bilbao JM, Vallat JM. Pathological findings in the x‐linked form of Charcot‐Marie‐Tooth disease: a morphometric and ultrastructural analysis. Acta Neuropathologica (Berlin) 2001;101(2):129‐39. [DOI] [PubMed] [Google Scholar]
Harding 1980
- Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 1980;103(2):259‐80. [DOI] [PubMed] [Google Scholar]
Hayasaka 1993a
- Hayasaka K, Himoro M, Takada G, Takahashi E, Minoshima S, Shimizu N. Structure and localization of the gene encoding human peripheral myelin protein 2 (PMP2). Genomics 1993;18(2):244‐8. [DOI] [PubMed] [Google Scholar]
Hayasaka 1993b
- Hayasaka K, Himoro M, Sawaishi Y, Nanao K, Takahashi T, Takada G, et al. De novo mutation of the myelin P0 gene in Dejerine‐Sottas disease (hereditary motor and sensory neuropathy type III). Nature Genetics 1993;5(3):266‐8. [DOI] [PubMed] [Google Scholar]
Hayasaka 1993c
- Hayasaka K, Ohnishi A, Takada G, Fukushima Y, Murai Y. Mutation of the myelin P0 gene in Charcot‐Marie‐tooth neuropathy type 1. Biochemical and Biophysical Research Communications 1993;194(3):1317‐22. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Jenkinson 1993
- Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993;306(6890):1437‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komyathy 2013
- Komyathy K, Neal S, Feely S, Miller LJ, Lewis RA, Trigge G, et al. Anterior tibialis CMAP amplitude correlations with impairment in CMT1A. Muscle & Nerve 2013;47(4):493‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krajewski 1999
- Krajewski K, Turansky C, Lewis R, Garbern J, Hinderer S, Kamholz J, et al. Correlation between weakness and axonal loss in patients with CMT1A. Annals of the New York Academy of Science 1999;883:490‐2. [PubMed] [Google Scholar]
Krajewski 2000
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, et al. Neurological dysfunction and axonal degeneration in Charcot‐Marie‐Tooth disease type 1A. Brain 2000;123(Pt 7):1516‐27. [DOI] [PubMed] [Google Scholar]
Kurihara 2002
- Kurihara S, Adachi Y, Wada K, Awaki E, Harada H, Nakashima K. An epidemiological genetic study of Charcot‐Marie‐Tooth disease in Western Japan. Neuroepidemiology 2002;21(5):246‐50. [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu L, Zhang R. Intermediate Charcot‐Marie‐Tooth disease. Neuroscience Bulletin 2014; Vol. 30, issue 6:999‐1009. [DOI] [PMC free article] [PubMed]
Lupski 1991
- Lupski JR, Oca‐Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, Trask BJ, et al. DNA duplication associated with Charcot‐Marie‐Tooth disease type 1A. Cell 1991;66(2):219‐32. [DOI] [PubMed] [Google Scholar]
Mannil 2014
- Mannil M, Solari A, Leha A, Pelayo‐Negro AL, Berciano J, Schlotter‐Weigel B, et al. Selected items from the Charcot‐Marie‐Tooth (CMT) Neuropathy Score and secondary clinical outcome measures serve as sensitive clinical markers of disease severity in CMT1A patients. Neuromuscular Disorders 2014;24(11):1003‐17. [DOI] [PubMed] [Google Scholar]
Martini 2001
- Martini R. The effect of myelinating Schwann cells on axons. Muscle & Nerve 2001;24(4):456‐66. [DOI] [PubMed] [Google Scholar]
Merkies 2000
- Merkies IS, Schmitz PI, Meche FG, Doorn PA. Psychometric evaluation of a new sensory scale in immune‐mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 2000;54(4):943‐9. [DOI] [PubMed] [Google Scholar]
Murphy 2011
- Murphy SM, Herrmann DN, McDermott MP, Scherer SS, Shy ME, Reilly MM, et al. Reliability of the CMT neuropathy score (second version) in Charcot‐Marie‐Tooth disease. Journal of the Peripheral Nervous System 2011;16(3):191‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2012
- Murphy SM, Laura M, Fawcett K, Pandraud A, Liu YT, Davidson GL, et al. Charcot‐Marie‐Tooth disease: frequency of genetic subtypes and guidelines for genetic testing. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(7):706‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelis 1996
- Nelis E, Broeckhoven C, Jonghe P, Lofgren A, Vandenberghe A, Latour P, et al. Estimation of the mutation frequencies in Charcot‐Marie‐Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. European Journal of Human Genetics 1996;4(1):25‐33. [DOI] [PubMed] [Google Scholar]
Nicholson 1993
- Nicholson G, Nash J. Intermediate nerve conduction velocities define X‐linked Charcot‐Marie‐Tooth neuropathy families. Neurology 1993;43(12):2558‐64. [DOI] [PubMed] [Google Scholar]
Nobbio 2014
- Nobbio L, Visigalli D, Radice D, Fiorina E, Solari A, Lauria G, et al. PMP22 messenger RNA levels in skin biopsies: testing the effectiveness of a Charcot‐Marie‐Tooth 1A biomarker. Brain 2014;127(6):1614‐20. [DOI] [PubMed] [Google Scholar]
Pareyson 2014
Passage 2004
- Passage E, Norreel JC, Noack‐Fraissignes P, Sanguedolce V, Pizant J, Thirion X, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot‐Marie‐Tooth disease. Nature Medicine 2004;10(4):396‐401. [DOI] [PubMed] [Google Scholar]
Raeymakers 1991
- Raeymaekers P, Timmerman V, Nelis E, Jonghe P, Hoogendijk JE, Baas F, et al. Duplication in chromosome 17p11.2 in Charcot‐Marie‐Tooth neuropathy type 1a (CMT 1a). The HMSN Collaborative Research Group. Neuromuscular Disorders 1991;1(2):93‐7. [DOI] [PubMed] [Google Scholar]
Reilly 2006
- Reilly MM, Jonghe P, Pareyson D. 136th ENMC International Workshop: Charcot‐Marie‐Tooth disease type 1A (CMT1A)8‐10 April 2005, Naarden, The Netherlands. Neuromuscular Disorders 2006;16(6):396‐402. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Center, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014.
Rossor 2012
- Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(1):6‐14. [DOI] [PubMed] [Google Scholar]
Rossor 2013
- Rossor AM, Polke JM, Houlden H, Reilly MM. Clinical implications of genetic advances in Charcot‐Marie‐Tooth disease. Nature Reviews Neurology 2013;9(10):562‐71. [DOI] [PubMed] [Google Scholar]
Saporta 2011
- Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot‐Marie‐Tooth disease subtypes and genetic testing strategies. Annals of Neurology 2011;69(1):22‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senderek 2003
- Senderek J, Bergmann C, Stendel C, Kirfel J, Verpoorten N, Jonghe P, et al. Mutations in a gene encoding a novel SH3/TPR domain protein cause autosomal recessive Charcot‐Marie‐Tooth type 4C neuropathy. American Journal of Human Genetics 2003;73(5):1106‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shy 2005a
- Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005;64(7):1209‐14. [DOI] [PubMed] [Google Scholar]
Shy 2005b
- Shy ME, Lupski JR, Chance PF, Klein CJ, Dyck PJ. Hereditary motor and sensory neuropathy. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. Vol. 2, Philadelphia: WB Saunders, 2005:1623‐58. [Google Scholar]
Timmerman 1992
- Timmerman V, Nelis E, Hul W, Nieuwenhuijsen B, Chen K, Wang S, et al. The peripheral myelin protein gene PMP‐22 is contained within the Charcot‐Marie‐Tooth disease type 1A duplication. Nature Genetics 1992;1(3):171‐5. [DOI] [PubMed] [Google Scholar]
Vallat 2013
- Vallat JM, Mathis S, Funalot B. The various Charcot‐Marie‐Tooth diseases. Current Opinion in Neurology 2013;26(5):473‐80. [DOI] [PubMed] [Google Scholar]
van Swieten 1988
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604‐7. [DOI] [PubMed] [Google Scholar]
Verhamme 2009b
- Verhamme C, Schaik IN, Koelman JH, Haan RJ, Visser M. The natural history of Charcot‐Marie‐Tooth type 1A in adults: a 5‐year follow‐up study. Brain 2009;132(Pt 12):3252‐62. [DOI] [PubMed] [Google Scholar]
Verhoeven 2006
- Verhoeven K, Claeys KG, Zuchner S, Schroder JM, Weis J, Ceuterick C, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot‐Marie‐Tooth type 2. Brain 2006;129(Pt 8):2093‐102. [DOI] [PubMed] [Google Scholar]
Young 2001
- Young P, Suter U. Disease mechanisms and potential therapeutic strategies in Charcot‐Marie‐Tooth disease. Brain Research. Brain Research Reviews 2001;36(2‐3):213‐21. [DOI] [PubMed] [Google Scholar]
Zimon 2012
- Zimoń M, Baets J, Almeida‐Souza L, Vriendt E, Nikodinovic J, Parman Y, et al. Loss‐of‐function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nature Genetics 2012;44(10):1080‐3. [DOI] [PubMed] [Google Scholar]
Zuchner 2004
- Zuchner S, Mersiyanova IV, Muglia M, Bissar‐Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot‐Marie‐Tooth neuropathy type 2A. Nature Genetics 2004;36(5):449‐51. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Young 2006
- Young P, Stögbauer F, Butterfass‐Bahloul T, Jonghe P. Treatment for Charcot‐Marie‐Tooth disease. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD006052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2008
- Young P, Jonghe P, Stögbauer F, Butterfass‐Bahloul T. Treatment for Charcot‐Marie‐Tooth disease. Cochrane Database of Systematic Reviews 2008, issue DOI: 10.1002/14651858.CD006052.pub2.. [DOI] [PMC free article] [PubMed]


