Abstract
Background
Prolonged labour can lead to increased maternal and neonatal mortality and morbidity due to increased risks of maternal exhaustion, postpartum haemorrhage and sepsis, fetal distress and asphyxia and requires early detection and appropriate clinical response. The risks for complications of prolonged labour are much greater in poor resource settings. Active management of labour versus physiological, expectant management, has shown to decrease the occurrence of prolonged labour. Administering antispasmodics during labour could also lead to faster and more effective dilatation of the cervix. Interventions to shorten labour, such as antispasmodics, can be used as a preventative or a treatment strategy in order to decrease the incidence of prolonged labour. As the evidence to support this is still largely anecdotal around the world, there is a need to systematically review the available evidence to obtain a valid answer.
Objectives
To assess the effects of antispasmodics on labour in term pregnancies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (28 February 2013), the ProQuest dissertation and thesis database, the dissertation database of the University of Stellenbosch and Google Scholar (28 February 2013) and reference lists of articles. We also contacted pharmaceutical companies and experts in the field. We did not apply language restrictions.
Selection criteria
Randomised controlled trials comparing antispasmodics with placebo or no medication in women with term pregnancies.
Data collection and analysis
Two review authors independently screened abstracts and selected studies for inclusion, assessed risk of bias and extracted data. Data were checked for accuracy. We contacted trial authors when data were missing.
Main results
Twenty‐one trials (n = 3286) were included in the review. Seventeen trials (n = 2617) were included in the meta‐analysis. Antispasmodics used included valethamate bromide, hyoscine butyl‐bromide, drotaverine hydrochloride, rociverine and camylofin dihydrochloride. Most studies included antispasmodics as part of their package of active management of labour. Overall, the quality of studies was poor, as only four trials were assessed as low risk of bias. Thirteen trials (n = 1995) reported on the duration of first stage of labour, which was significantly reduced by an average of 74.34 minutes when antispasmodics were administered (mean difference (MD) ‐74.34 minutes; 95% confidence Interval (CI) ‐98.76 to ‐49.93). Seven studies (n = 797) reported on the total duration of labour, which was significantly reduced by an average of 85.51 minutes (MD ‐85.51 minutes; 95% CI ‐121.81 to ‐49.20). Six studies (n = 820) had data for the outcome: rate of cervical dilatation. Administration of antispasmodics significantly increased the rate of cervical dilatation by an average of 0.61 cm/hour (MD 0.61 cm/hour; 95% CI 0.34 to 0.88). Antispasmodics did not affect the duration of second and third stage of labour. The rate of normal vertex deliveries was not affected either. Only one study explored pain relief following administration of antispasmodics and no conclusions can be drawn on this outcome. There was significant heterogeneity for most outcomes and therefore, we undertook random‐effects meta‐analysis. Subgroup analysis was undertaken to explore heterogeneity, but remained largely unexplained. Maternal and neonatal adverse events were reported inconsistently. The main maternal adverse event reported was tachycardia. No serious neonatal adverse events were reported.
Authors' conclusions
There is low quality evidence that antispasmodics reduce the duration of first stage of labour and increase the cervical dilatation rate. There is very low quality evidence that antispasmodics reduce the total duration of labour. There is moderate quality evidence that antispasmodics do not affect the rate of normal vertex deliveries. There is insufficient evidence to make any conclusions regarding the safety of these drugs for both mother and baby. Large, rigorous randomised controlled trials are needed to evaluate the effect of antispasmodics on prolonged labour and to evaluate their effect on labour in a context of expectant management of labour.
Keywords: Female; Humans; Pregnancy; Labor Stage, First; Labor Stage, First/drug effects; Obstetric Labor Complications; Obstetric Labor Complications/drug therapy; Parasympatholytics; Parasympatholytics/adverse effects; Parasympatholytics/therapeutic use; Randomized Controlled Trials as Topic; Tachycardia; Tachycardia/chemically induced
Plain language summary
Antispasmodics for labour
A woman who is in active labour for too long (usually set at more than 12 hours) is at risk of becoming exhausted and developing complications such as infection and excessive bleeding. The unborn baby can also be harmed, showing distress and low oxygenation (asphyxia). It is common practice to intervene in the labouring process to avoid this by rupturing membranes (breaking the waters), giving medications to speed up contractions and providing ongoing support. Antispasmodics are drugs that are usually taken to relieve cramps. They work either by direct relaxation of muscle or by interfering with the message sent by the nerves to the muscle to contract. It is thought that these drugs may help with opening the womb (dilatation of the cervix), when given during labour as a preventative or a treatment strategy. This would shorten the time spent in labour. Evidence was sought to support this idea. Twenty‐one randomised controlled studies with a total of 3286 participants were included. The data were combined in an analysis to get an overall result. All types of antispasmodics were given at the beginning of established labour. They decreased the first stage of labour, the time from beginning of labour until the baby is about to be born, by 49 to 98 minutes, as well as the total duration of labour, from the beginning of labour until the delivery of the afterbirth, by 49 to 121 minutes. The drugs did not affect the number of women requiring emergency caesarean sections and did not have serious side effects for either mother or her baby. The most commonly reported adverse events for the mothers were fast heart rates and mouth dryness, but since both maternal and neonatal adverse events were poorly reported, more information is needed to make conclusions about the safety of these drugs during labour. The included studies were mostly of poor quality and good studies are needed to assess what happens when these drugs are given to women whose labour is already prolonged.
Summary of findings
Summary of findings for the main comparison. Antispasmodics versus control.
| Antispasmodics versus control | ||||||
| Patient or population: Women in labour Settings: Hospital setting Intervention: Antispasmodics Comparison: Control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antispasmodics | |||||
| Duration of first stage of labour minutes | 173 to 346.31 | The mean duration of first stage of labour in the intervention groups was 74.34 lower (98.76 to 49.93 lower) | 1995 (13 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Duration of second stage of labour minutes | 16.5 to 58 | The mean duration of second stage of labour in the intervention groups was 2.68 lower (5.98 lower to 0.63 higher) | 1297 (10 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Duration of third stage of labour minutes | 5.52 to8.3 | The mean duration of third stage of labour in the intervention groups was 0.06 lower (0.52 lower to 0.4 higher) | 765 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
|
Total duration of labour minutes |
192.2 to 413.9 | The mean total duration of labour (min) in the intervention groups was 85.51 lower (121.81 to 49.2 lower) | 797 (7 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Rate of cervical dilatation cm/h | 1.01 to 2.5 | The mean rate of cervical dilatation in the intervention groups was 0.61 higher (0.34 to 0.88 higher) | 820 (6 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Rate of normal vertex deliveries | Study population | RR 1.02 (1 to 1.05) | 2319 (16 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 905 per 1000 | 923 per 1000 (905 to 950) | |||||
| Moderate | ||||||
| 925 per 1000 | 943 per 1000 (925 to 971) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High risk for selection and incomplete reporting of outcomes in most studies 2 Significant, unexplained heterogeneity present 3 Wide 95% confidence interval
Background
Description of the condition
Improving maternal health (the fifth Millennium Development Goal) and decreasing maternal mortality remains one of the main health concerns worldwide. Some progress has been made towards achieving the target of reducing maternal mortality by three‐quarters between 1990 and 2015, as the global maternity mortality ratio (MMR) in 2008 was 260 maternal deaths per 100,000 live births, compared to 430/100,000 live births in 1990 (WHO 2010). However, in Sub‐Saharan Africa, the burden is significant, with an MMR of 640/100,000 live births in 2008 (WHO 2010).
According to an analysis conducted by the World Health Organization (WHO) (Khan 2006), 4.1% of maternal deaths in Africa were due to obstructed labour. The percentage for Asia, and Latin America and the Caribbean, was 9.4% and 13.4% respectively. The leading cause of maternal mortality worldwide is haemorrhage (developed countries 13.4%; Africa 33.9%; Asia 30.8%; Latin America and the Caribbean 20.8%). This includes postpartum haemorrhage, although the proportion was not specified (Khan 2006).
Prolonged labour can lead to increased maternal and neonatal mortality and morbidity due to increased risks of maternal exhaustion, postpartum haemorrhage and sepsis, fetal distress and asphyxia and requires early detection and appropriate clinical response. The causes of prolonged labour relate to maternal age, induction of labour, prelabour rupture of membranes, early admission to the labour ward, epidural analgesia and high levels of maternal stress hormones, but are unknown in most cases (Dencker 2009). The risks for complications of prolonged labour are much greater in poor resource settings (Neilson 2003). It is difficult to give a clear definition for prolonged labour. In practice, as recommended by the WHO maternal health and safe motherhood programme (WHO 1994), a woman should be transferred to a higher level of care if her rate of cervical dilatation (according to the partogram) becomes less than 1 cm/hour, and requires prompt, appropriate management if it is less than 1 cm in four hours (Lavender 2009). In addition, a recent review to determine the “slowest‐yet‐normal” dilatation rate amongst primigravid women (Neal 2010), determined that this dilatation rate approximates 0.5 cm/hour and that expectations of a faster dilatation rate (1 cm/hour) can lead to unnecessary interventions aiming to accelerate labour.
The concept of “active management of labour” (O'Driscoll 1973) was developed to assure a woman that her labour would not exceed 12 hours. Anything beyond that constituted prolonged labour. This package of care includes accurate and early diagnosis of the first stage of labour, early artificial rupture of membranes, ongoing support of the woman in labour by a professional caregiver and augmentation of labour with oxytocin (O'Driscoll 1994). Active management of labour versus physiological, expectant management, has shown to decrease the occurrence of prolonged labour (more than 12 hours). A Cochrane review found that active management significantly shortened the duration of labour by 1.27 hours, while the first stage of labour was significantly reduced by 1.56 hours. It also showed a small reduction in the rate of caesarean sections. There was no significant difference in maternal and neonatal morbidity (Brown 2008). Wei 2009 showed that early intervention with amniotomy and oxytocin augmentation, as a preventative strategy with mild delays in progress, leads to a reduction of 1.16 hours in the duration of labour.
Description of the intervention
Administering antispasmodics during labour could lead to faster and more effective dilatation of the cervix (Samuels 2009).
Antispasmodics are drugs that relieve spasms of smooth muscle tissue and have either musculotropic or neurotropic effects. The cervix is composed of connective tissue and smooth muscle, innervated by parasympathetic nerve fibres. Smooth muscle constitutes about 15% of the cervix (Leppert 1995), which is mainly found just below the internal os (Buhimschi 2003).
Musculotropic antispasmodics directly relax smooth muscles. They are phosphodiesterase type IV inhibitors, structurally related to papaverine, have mild Calcium (Ca)‐channel blocking effects, no anticholinergic effects and act directly on smooth muscle cells, inhibiting spasm (Sommers 2002).
Neurotropic antispasmodics break the connection between the parasympathetic nerve and the smooth muscle.They are parasympatholytics acting as antagonists of acetylcholine at muscarinic receptors, thus inhibiting muscle spasm (Samuels 2009; Sommers 2002).
Antispasmodics are commonly administered during labour in both developing and developed countries, although there is a paucity of scientific reports validating this. In India, drotaverine hydrochloride, an antispasmodic drug, forms part of their “Programmed Labour Protocol” to decrease the pain and the duration of labour (Yuel 2008). It is used in conjunction with amniotomy, oxytocin augmentation and administration of tramadol for pain relief.
There are a number of case‐reports, prospective studies and clinical trials describing the effects of antispasmodics during labour. A prospective study by Sirohiwal 2005 found that administration of hyoscine‐butyl bromide suppositories during labour significantly shortened the duration of the first stage of labour.
Adverse effects of these drugs are rare at therapeutic dosages and include dryness of the mouth, visual disturbances, tachycardia, drowsiness and fatigue. Some patients may experience paradoxical stimulation and excitation (Gibbon 2005).
How the intervention might work
As shown in studies conducted in India and elsewhere (Sirohiwal 2005; Yuel 2008), antispasmodics could be used as accelerators of labour. Although the exact mechanism of action of cervical dilatation and the influence of antispasmodics thereon is still somewhat unclear, more effective dilatation could lead to an accelerated labour.
Antispasmodics can be used in conjunction with the package of active management of labour, as has been done in India and across the world, or on its own. In the latter case, the general use of oxytocin would be reduced. Oxytocin, although widely used, has been described as the drug most commonly related to preventable adverse events during labour and has a very unpredictable therapeutic index (Clark 2009).
Furthermore, active management of labour as a whole package, or parts thereof, and other interventions to shorten labour, such as antispasmodics, can be used as a preventative strategy or a treatment strategy in order to decrease the incidence of prolonged labour. This depends on the setting, availability of resources and maternal preferences.
Why it is important to do this review
The use of antispasmodics as accelerators of labour is largely anecdotal around the world. The results of the first version of the review showed that there was low quality evidence that antispasmodics reduced the duration of the first stage of labour and very low quality evidence that they reduced the total duration of labour. The WHO is updating their augmentation of labour guidelines in 2013 and as this review was identified as one of the reviews to feed into the guideline, it is important to include all recent studies in this updated version of the review. Considering all studies addressing this question and interpreting the results in light of the quality of the studies will help policy makers and clinicians to make well‐informed decisions about the use of antispasmodics during labour.
Objectives
The aim of this review is to assess the effects of antispasmodics on labour in term pregnancies.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials for inclusion in this review and did not include quasi‐randomised controlled trials, cluster‐randomised trials or trials using a cross‐over design. We included studies available only as an abstract, if they reported on all the necessary information.
Types of participants
Primi‐ and multigravid women with term pregnancies (equal to or greater than 37 weeks' gestation) at the time of delivery; with spontaneous onset and induction of labour; low‐ and high‐risk pregnancies; active and expectant management of labour.
Types of interventions
We considered studies where any antispasmodic agent was administered during any stage of labour by means of oral, rectal, intramuscular or intravenous route compared with a control group (placebo or no medication) for inclusion in the review.
Types of outcome measures
Primary outcomes
Primary outcomes were the direct effect of antispasmodic drugs on labour.
Duration of labour.
Rate of cervical dilatation.
Pain relief.
Type of delivery (rate of normal vertex deliveries).
Secondary outcomes
Maternal adverse events: these included any maternal adverse event, defined in the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011) as any "unfavourable outcome that occurs during or after the use of a drug or other intervention but not necessarily caused by it".
Neonatal adverse events: this included any fetal or neonatal adverse event, defined in the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011), as any "unfavourable outcome that occurs during or after the use of a drug or other intervention but not necessarily caused by it".
Maternal satisfaction.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 Febuary 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the ProQuest dissertation and thesis database, the dissertation database of the University of Stellenbosch and Google Scholar (28 February 2013) (seeAppendix 1 for search terms used).
Searching other resources
We contacted experts in the field and relevant pharmaceutical companies that manufacture antispasmodics, but did not receive any additional study reports.
We also scanned reference lists of included papers for additional studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (either Anke Rohwer (AR) and Taryn Young (TY) or AR and Oswell Khondowe (OK)) independently reviewed the search output. We screened titles and abstracts of search results to exclude irrelevant studies. We then retrieved full text articles of seemingly relevant studies and examined them to see whether they met the inclusion criteria. We resolved any disagreement through discussion.
Data extraction and management
We designed a data extraction form. For eligible studies, two review authors (either AR and TY or AR and OK) extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2011) and checked it for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (either AR and TY or AR and OK) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011). We contacted authors for missing information and resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it as likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals, and for continuous data, we used the mean difference. All outcomes were measured in the same way between trials.
Unit of analysis issues
We dealt with studies with multiple treatment groups as recommended by the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011). If studies had two intervention groups with different antispasmodics and one control group, we included the two different intervention groups in the meta‐analysis as separate intervention groups, but divided the control group in two (Ramsay 2003).
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) using funnel plots when outcomes included more than 10 studies. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. For continuous outcomes we used the test proposed by Egger 1997, and for dichotomous outcomes we used the test proposed by Harbord 2006. If we detected asymmetry in any of these tests or by a visual assessment, we performed exploratory analyses to investigate it
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or when we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials.
When using random‐effects analyses, we presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We investigated substantial heterogeneity using subgroup analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following pre‐specified subgroup analyses.
Type of antispasmodic (musculotropic drugs versus neurotropic drugs).
Route of administration (intravenous administration versus intramuscular administration).
Gravidity of women (primigravidas versus multigravidas).
Type of labour (spontaneous labour versus induced labour.
We also added three more non pre‐specified subgroups:
Type of management of labour (active management versus expectant management).
Type of pregnancy (high risk versus low risk).
Studies excluding versus studies including caesarean sections in their analysis.
We used the following outcomes in subgroup analysis.
Duration of labour.
Rate of cervical dilatation.
Pain relief.
Type of delivery.
For fixed‐effect inverse variance meta‐analyses, we assessed differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicated a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We performed sensitivity analysis on primary outcomes, to see what effect the exclusion of studies with high risk of bias (for allocation concealment, and incomplete outcome data) had on the overall result of the meta‐analysis.
Results
Description of studies
Results of the search
September 2011 search
The search of the Cochrane Pregnancy and Childbirth Group's Register retrieved 42 trial reports. The search of the dissertation databases did not retrieve additional studies and a search on Google Scholar retrieved two extra studies. Screening reference lists yielded four extra studies, while contact with experts yielded another, unpublished study. An additional study was identified through the peer‐review process. After screening abstracts for eligibility, 23 studies were excluded (see Characteristics of excluded studies). Full text articles of seemingly relevant studies were obtained. Seven studies had insufficient information to be classified (Accinelli 1978; Ahmed 1982; Georges 2007; Rajani 2011; Ranka 2002; Recto 1997; Roy 2007), for more details, see Characteristics of studies awaiting classification. We contacted the study authors if a contact address was available to obtain additional information but none of the authors responded. One study was listed in the IRCT register and has not been published (Movahed 2010, see Characteristics of ongoing studies). We included one unpublished study (Mukaindo 2010) and 18 published studies (14 full texts, three conference abstracts and one letter to the editor), including 2798 participants, in the review (Table 1). Fifteen studies were included in the meta‐analysis. For a summary of the search results, see Figure 1.
1.
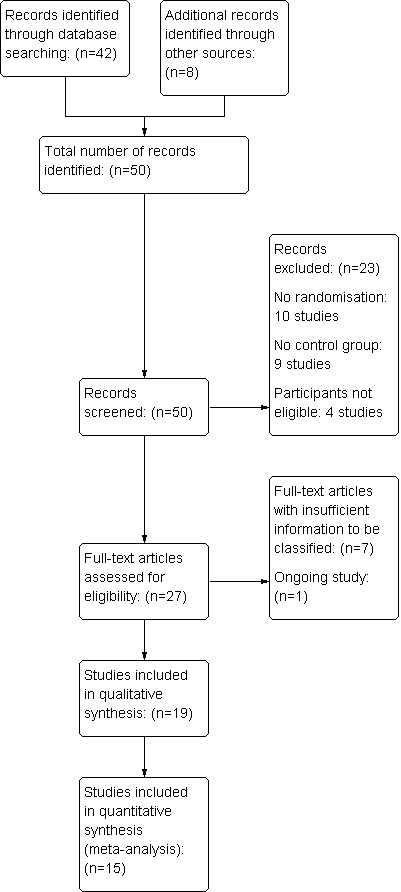
Study flow diagram ‐ September 2011 search
February 2013 search
This updated search retrieved one report from the Cochrane Pregnancy and Childbirth Group's Register and four from Google Scholar. After removing one duplicate study, we assessed full text of four. We excluded Fouedjio 2012 and included Dahal 2013 and Sekhavat 2012. Zagami 2012 is awaiting translation (see Figure 2).
2.
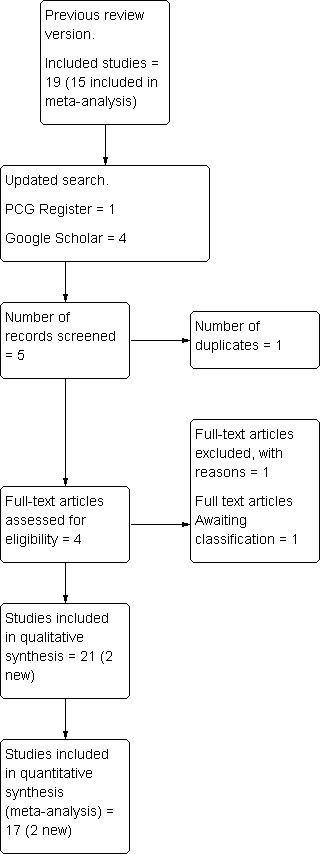
Study flow diagram ‐ February 2013 search
Included studies
Twenty‐one studies were included in this updated review of which 17 were included in the meta‐analysis. See Characteristics of included studies.
Study location
Nine of the studies were conducted in India (Ajmera 2006; Gupta 2008; Khosla 2003; Kuruvila 1992; Madhu 2010; Raghavan 2008; Sharma 2001; Singh 2004; Warke 2003); three in Iran (Azari 2010; Sekhavat 2012, Makvandi 2010); two in Turkey (Batukan 2006; Yilmaz 2009); two in Saudi Arabia (Al Matari 2007; Al Qahtani 2011); and one each in Jamaica (Samuels 2007); the USA (Taskin 1993); Kenya (Mukaindo 2010), Italy (Cromi 2011) and Nepal (Dahal 2013). All the studies took place in a hospital setting, mostly a tertiary or teaching hospital.
Types of antispasmodics/interventions
The antispasmodics used in the intervention groups were valethamate bromide (Ajmera 2006; Batukan 2006; Dahal 2013; Khosla 2003; Kuruvila 1992; Madhu 2010; Raghavan 2008; Sharma 2001; Taskin 1993; Yilmaz 2009), drotaverine hydrochloride (Ajmera 2006; Dahal 2013; Gupta 2008; Khosla 2003; Madhu 2010; Sharma 2001; Singh 2004), hyoscine butyl bromide ( Al Matari 2007; Al Qahtani 2011; Gupta 2008; Makvandi 2010; Mukaindo 2010; Raghavan 2008; Samuels 2007; Sekhavat 2012; Taskin 1993), rociverine (Cromi 2011) and camylofin dihydrochloride (Warke 2003). One study used a combination of hyoscine and atropine (Azari 2010).
Drotaverine hydrochloride, rociverine and camylofin dihydrochloride are musculotropic agents ‐ phosphodiesterase type IV inhibitors, structurally related to papaverine. They have mild Ca‐channel blocking effects, no anticholinergic effects and act directly on smooth muscle cells, inhibiting spasm (Romics 2003; Sommers 2002). Valethamate bromide and hyoscine butyl bromide (Buscopan) are anticholinergic agents, which act as antagonists of acetylcholine at muscarinic receptors, inhibiting muscle spasm of smooth muscles innervated by the parasympathetic nerves (Samuels 2009; Sommers 2002).
NIne of the studies were multi‐intervention studies (Ajmera 2006; Dahal 2013; Gupta 2008; Khosla 2003; Madhu 2010; Raghavan 2008; Sharma 2001; Taskin 1993; Yilmaz 2009), all with two or more intervention groups and one control group. Two multi‐intervention studies (Taskin 1993; Yilmaz 2009) had one intervention arm of meperidine, which is not classified as an antispasmodic and was therefore not included in the meta‐analysis. The other intervention arms of these two studies, valethamate bromide and hyoscine butyl bromide, were used for analysis. The other seven multi‐intervention studies (Ajmera 2006; Dahal 2013; Gupta 2008; Khosla 2003; Madhu 2010; Raghavan 2008; Sharma 2001) included two different antispasmodic agents in the intervention groups and a control group. All three groups were included in the meta‐analyses.
Antispasmodic drugs were administered intravenously (IV), intramuscularly (IM) or per rectum (PR). Hyoscine butyl bromide was administered IV or PR, while drotaverine hydrochloride was only administered IM. Valethamate bromide was administered either IV or IM. Rociverine as well as camylofin dihydrochloride were administered IM.
Fourteen studies (Al Qahtani 2011; Batukan 2006; Cromi 2011; Dahal 2013; Gupta 2008; Kuruvila 1992; Madhu 2010; Makvandi 2010; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Singh 2004; Taskin 1993; Yilmaz 2009) followed a protocol for active management of labour, which included artificial rupture of membranes (aROM), augmentation with oxytocin, or both. Two studies ( Sharma 2001; Warke 2003) avoided aROM and did not augment labour with oxytocin. Five studies (Ajmera 2006; Al Matari 2007; Azari 2010; Khosla 2003; Raghavan 2008) did not mention aROM or oxytocin.
Participants
Ten studies included only primigravid women (Al Qahtani 2011; Azari 2010; Cromi 2011; Makvandi 2010; Mukaindo 2010; Raghavan 2008; Sharma 2001; Singh 2004; Warke 2003; Yilmaz 2009). Nine studies included both primi‐ and multigravid women (Ajmera 2006; Batukan 2006; Dahal 2013; Gupta 2008; Khosla 2003; Kuruvila 1992; Madhu 2010; Samuels 2007; Taskin 1993), the ratio of both not being statistically different in all the studies. Sekhavat 2012 only included multigravid women and Al Matari 2007 did not specify the gravidity of participants. Yilmaz 2009 included only women with induction of labour. Batukan 2006 and Dahal 2013 included women with induction of labour and women in spontaneous labour. Thirteen studies (Ajmera 2006; Al Qahtani 2011; Cromi 2011; Khosla 2003; Madhu 2010; Makvandi 2010; Mukaindo 2010; Raghavan 2008; Samuels 2007; Sekhavat 2012; Sharma 2001; Singh 2004; Warke 2003) only included participants in spontaneous labour. Five studies (Al Matari 2007; Azari 2010; Gupta 2008; Kuruvila 1992; Taskin 1993) did not specify whether participants were in spontaneous or induced labour.
Gupta 2008 included women with high‐risk pregnancies as well as women with low‐risk pregnancies. The rest of the studies only included women with low‐risk pregnancies.
Outcomes
For 17 studies (Ajmera 2006; Al Matari 2007; Al Qahtani 2011; Batukan 2006; Dahal 2013; Gupta 2008; Khosla 2003; Madhu 2010; Makvandi 2010; Mukaindo 2010; Raghavan 2008; Samuels 2007; Sekhavat 2012; Sharma 2001; Singh 2004; Warke 2003; Yilmaz 2009), the primary outcome was the duration of labour. This included duration of first stage of labour (active stage of labour, from 3 cm to 4 cm to full cervical dilatation), second stage of labour (from full cervical dilatation to expulsion of baby), third stage of labour (from delivery of baby to delivery of placenta) and total duration of labour.
Ten studies (Ajmera 2006; Batukan 2006; Cromi 2011; Gupta 2008; Khosla 2003; Kuruvila 1992; Madhu 2010; Sharma 2001; Singh 2004; Yilmaz 2009), excluded participants delivering via caesarean section from their calculation of the mean duration of labour, while seven studies (Al Qahtani 2011; Dahal 2013; Makvandi 2010; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Warke 2003) included participants undergoing caesarean section in the calculation of the mean duration of labour.
For three studies (Azari 2010; Cromi 2011; Kuruvila 1992), the primary outcome was rate of cervical dilatation. Singh 2004 reported on pain relief during labour. Taskin 1993 was only reported as an abstract and contains minimal information about methods, outcomes and results.
Excluded studies
Twenty‐four studies (Aggarwal 2008; Akleh 2010; Baracho 1982; Bhattacharaya 1984; Chan 1963; De Nobrega‐Correa 2010; Demory 1990; Fouedjio 2012; Guerresi 1981; Hamann 1972; Hao 2004; Kauppila 1970; Kaur 2003; Kaur 2006; Malensek 1985; Manpreet 2008; Maritati 1986; Mishra 2002; Mortazavi 2004; Rajkumar 2006; Sirohiwal 2005; Tabassum 2005; Tripti 2009; Von Hagen 1965) were excluded from the review. Reasons for exclusion were lack of randomisation, absence of a control group and inclusion of non‐eligible participants. These are summarised in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias for each study is presented in the 'Risk of bias' tables in the Characteristics of included studies table. Figure 3 and Figure 4 illustrate the summary of risk of bias in all the studies.
3.
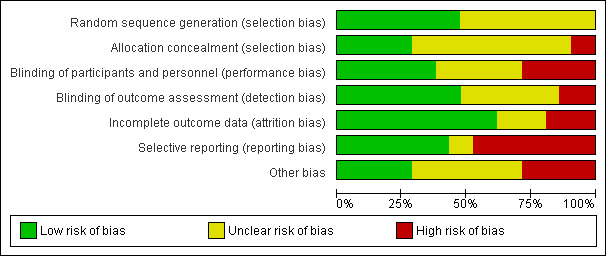
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
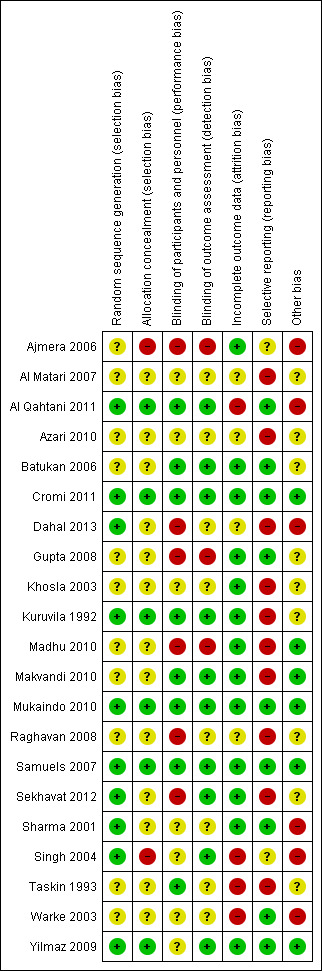
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation sequence generation was assessed as adequate in 10 studies (Al Qahtani 2011; Cromi 2011; Dahal 2013; Kuruvila 1992; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Sharma 2001; Singh 2004; Yilmaz 2009). Of these, seven (Cromi 2011; Dahal 2013; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Sharma 2001; Yilmaz 2009), used computer‐generated tables of random numbers; Kuruvila 1992 used a table of random numbers and two studies (Al Qahtani 2011; Singh 2004) drew paper slips from a box. Sequence generation was unclear in the remaining 11 studies (Ajmera 2006; Al Matari 2007; Azari 2010; Batukan 2006; Gupta 2008; Khosla 2003; Madhu 2010; Makvandi 2010; Raghavan 2008; Taskin 1993; Warke 2003).
Only six studies reported adequate allocation concealment (Al Qahtani 2011; Cromi 2011; Kuruvila 1992; Mukaindo 2010; Samuels 2007; Yilmaz 2009). Samuels 2007 and Mukaindo 2010 used sequentially numbered, pre‐filled syringes, Kuruvila 1992 used identical, sequentially numbered ampoules and Yilmaz 2009 used sequentially numbered, opaque, sealed envelopes to conceal allocation. Cromi 2011 used an independent resident physician who managed the randomisation process and drew up the injectable in a separate pharmacy room in the labour ward, while Al Qahtani 2011 used the nurse in charge to draw an envelope from a box and prepare the injectable accordingly. In Batukan 2006, it was unclear whether sealed envelopes were opaque and sequentially numbered, in Makvandi 2010 it was unclear whether suppositories were in identical packages and sequentially numbered, while in Ajmera 2006 and Singh 2004, allocation concealment was evidently absent. Allocation concealment was not reported in the rest of the studies (Al Matari 2007; Azari 2010; Dahal 2013; Gupta 2008; Khosla 2003; Madhu 2010; Raghavan 2008; Sekhavat 2012; Sharma 2001; Taskin 1993; Warke 2003).
Blinding
Eight studies had low risk of performance bias and adequately reported on blinding of participants and personnel (Al Qahtani 2011; Batukan 2006; Cromi 2011; Kuruvila 1992; Makvandi 2010; Mukaindo 2010; Samuels 2007; Taskin 1993). In Yilmaz 2009, participants were blinded, but nurses were not blinded. In six studies (Ajmera 2006; Dahal 2013; Gupta 2008; Madhu 2010; Raghavan 2008; Sekhavat 2012), blinding of participants and personnel was absent. In the remaining seven studies (Al Matari 2007; Azari 2010; Khosla 2003; Sekhavat 2012; Sharma 2001; Singh 2004; Warke 2003), it was unclear whether blinding of participants and personnel occurred due to poor reporting.
Ten studies (Al Qahtani 2011; Batukan 2006; Cromi 2011; Kuruvila 1992; Makvandi 2010; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Singh 2004; Yilmaz 2009) had low risk of detection bias and adequately reported on blinding of outcome assessors. Three studies (Ajmera 2006; Gupta 2008; Madhu 2010) did not blind outcome assessors and were considered to have a high risk of detection bias. The remaining studies ( Al Matari 2007; Azari 2010; Dahal 2013; Khosla 2003; Raghavan 2008; Sharma 2001; Taskin 1993; Warke 2003) were judged as unclear risk of detection bias, due to poor reporting of blinding of outcome assessors.
Incomplete outcome data
Four studies (Al Qahtani 2011; Singh 2004; Taskin 1993; Warke 2003) failed to adequately report on the outcomes of all the participants and were considered to have a high risk for attrition bias. Two studies (Singh 2004; Taskin 1993) did not report separate loss to follow‐up figures for control and intervention groups, while Warke 2003 did not report on the details of participant outcomes and the percentages did not add up. Al Qahtani 2011 did not report outcomes for 13 participants who were randomised to intervention and control groups, had received the corresponding treatment but were excluded from the analysis due to various reasons. Four studies (Al Matari 2007; Azari 2010; Dahal 2013; Raghavan 2008) could not be classified as high or low risk of attrition bias. Azari 2010 and Raghavan 2008 only reported the number of participants enrolled in the study; while in Al Matari 2007 it was unclear whether or not women who did not deliver after four hours were included in the analysis. Dahal 2013 did not report the number of participants with the results. The remaining studies (Ajmera 2006; Batukan 2006; Cromi 2011; Gupta 2008; Khosla 2003; Kuruvila 1992; Madhu 2010; Makvandi 2010; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Sharma 2001; Yilmaz 2009) reported adequately on all participants.
Selective reporting
Nine studies reported adequately on outcomes (Al Qahtani 2011; Batukan 2006; Cromi 2011; Gupta 2008; Mukaindo 2010; Samuels 2007; Sharma 2001; Warke 2003; Yilmaz 2009). Ten studies (Al Matari 2007; Azari 2010; Dahal 2013; Khosla 2003; Kuruvila 1992; Madhu 2010; Makvandi 2010; Raghavan 2008; Sekhavat 2012; Taskin 1993) were considered as having a high risk of reporting bias. Three of these studies (Azari 2010; Madhu 2010; Raghavan 2008), did not report on all the outcomes pre‐specified in the study report, Makvandi 2010 did not report on the primary outcomes as specified in the study protocol; while the rest (Al Matari 2007; Khosla 2003; Kuruvila 1992; Taskin 1993), did not pre‐specify which outcomes were being assessed in the methodology. In three studies (Ajmera 2006; Sekhavat 2012; Singh 2004), secondary outcomes were not clearly reported and Dahal 2013 omitted results of the control group for certain outcomes.
Other potential sources of bias
Six studies (Ajmera 2006; Al Qahtani 2011; Dahal 2013; Sharma 2001; Singh 2004; Warke 2003) had a high potential for other bias. Sharma 2001 reported a 100% rate of normal vertex deliveries (NVDs) although the hospital's normal rate of caesarean section is 12%, raising doubt about the validity of the study. Singh 2004 started the intervention at 3 cm to 6 cm cervical dilatation, but had more participants in the intervention group with 6 cm cervical dilatation than in the control group (18 versus three). In Al Qahtani 2011 , there was a significant baseline imbalance between groups regarding rupture of membranes at enrolment and Dahal 2013 failed to report on baseline characteristics of participants and had different results for the outcomes "duration of active phase of labour" and "duration of labour until the end of first stage of labour", although these are essentially the same. In nine studies (Al Matari 2007; Azari 2010; Batukan 2006; Gupta 2008; Khosla 2003; Kuruvila 1992; Raghavan 2008; Sekhavat 2012; Taskin 1993) it was unclear if any other bias was introduced. Three studies (Al Matari 2007; Azari 2010; Taskin 1993) were only reported as conference abstracts and lack detail and contact information, while Raghavan 2008 was reported as a letter to the editor and also lacks details and contact information. Data extraction of Batukan 2006 was carried out by a translator and judgement about other biases was therefore difficult. Gupta 2008 did not have the same starting point for all participants, but reported that this was not significantly different between treatment and intervention groups and Sekhavat 2012 did not specify which outcome was used to calculate the sample size and included only multigravid women in their study. Six studies (Cromi 2011; Madhu 2010; Makvandi 2010; Mukaindo 2010; Samuels 2007; Yilmaz 2009) were assessed as being free of other bias.
Bias introduced by drug company sponsorship was evidently absent in eight studies ( Al Qahtani 2011; Cromi 2011; Madhu 2010; Makvandi 2010; Mukaindo 2010; Samuels 2007; Warke 2003; Yilmaz 2009). In the remaining 13 studies (Ajmera 2006; Al Matari 2007; Azari 2010; Batukan 2006; Dahal 2013; Gupta 2008; Khosla 2003; Kuruvila 1992; Raghavan 2008; Sekhavat 2012; Sharma 2001; Singh 2004; Taskin 1993), it was unclear whether drug company sponsorship was present, since conflict of interest was not explicitly mentioned.
Effects of interventions
See: Table 1
A summary of the effects of the interventions is presented in Table 2.
1. Summary of included studies.
| Study | Country | Number of participants | Low‐/high‐risk pregnancy | Gravidity of participants | Time of intervention | Intervention (dose and route of administration) | Control | Non‐study interventions | Induced/spontaneous labour |
| Ajmera 2006 | India | 225 | Low risk | Primi‐and multigravidas | Active phase, 3 cm dilatation | Drotaverine hydrochloride (40 mg IMI¹) Valethamate bromide (8 mg IVI²) |
No medication | None specified | Spontaneous |
| Al Matari 2007 | Saudi Arabia | 199 | Not mentioned | Not mentioned | Not mentioned | Hyoscine‐N‐butyl bromide (not mentioned) | Placebo (not specified) | None specified | Not mentioned |
| Al Qahtani 2011 | Saudi Arabia | 110 | Low risk | Primigravidas | Established labour, 3‐4 cm dilatation | Hyoscine butyl bromide (40 mg IMI) | NaCl³ 0.9% (2 mL IMI) | Amniotomy, opioid analgesia | Spontaneous |
| Azari 2010 | Iran | 200 | Not mentioned | Primigravidas | 4 cm dilatation and ruptured membranes | Hyoscine and atropine (20 mg IVI) | Dextrose water (2 cc IVI) | Not specified | Not mentioned |
| Batukan 2006 | Turkey | 100 | Low risk | Primi‐and multigravida | 4‐5 cm dilatation | Valethamate bromide (8 mg IMI) | NaCl 0.9% (1 mL IVI) | Oxytocin if necessary, amniotomy if no spontaneous ROM⁴ at 8‐10 cm | Spontaneous and induced |
| Cromi 2011 | Italy | 120 | Low risk | Primigravida | 3‐5 cm dilatation | Rociverine (20 mg IMI) | NaCl 0.9% (2 mL IMI) | Amniotomy, augmentation with oxytocin, epidural analgesia | Spontaneous |
| Dahal 2013 | Nepal | 300 | Low risk | Primi‐ and multigravidas | Unclear | Valethamate bromide (8mg IMI) Drotaverine hydrochloride (40mg IMI) |
No medication | Amniotomy once cervical dilatation > 4cm, or if Bishop's score was favourable, oxytocin augmentation | Spontaneous and induced |
| Gupta 2008 | India | 150 | Low and high risk | Primi‐and multigravidas | 3 cm dilatation | Drotaverine hydrochloride (40 mg IMI) Hyoscine butyl bromide (20 mg IVI) |
No medication | Oxytocin if necessary, amniotomy | Not specified |
| Khosla 2003 | India | 300 | Low risk | Primi‐and multigravidas | 4 cm dilatation | Valethamate bromide (8 mg IMI) Drotaverine hydrochloride (40 mg IMI) |
No medication | Not specified | Spontaneous |
| Kuruvila 1992 | India | 120 | Low risk | Primi‐ and multigravidas | 2‐4 cm | Valethamate bromide (8 mg IMI) | NaCl 0.9% (1 mL IMI) | Oxytocin if necessary, amniotomy, pethidine 75 mg for primigravidas) | Not specified |
| Madhu 2010 | India | 150 | Low risk | Primi‐and multigravida | 4 cm dilatation | Drotaverine hydrochloride (40 mg IMI) Valethamate bromide (8 mg IMI) |
NaCl 0.9% (2 mL IMI) | Amniotomy, oxytocin if required, paracetamol and pethidine for labour analgesia | Spontaneous |
| Makvandi 2010 | Iran | 130 | Low risk | Primigravidas | 3‐4 cm dilatation | Hyoscine butyl bromide (20 mg PR⁵) | Placebo suppository | Amniotomy when presenting part was fixed | Spontaneous |
| Mukaindo 2010 | Kenia | 85 | Low risk | Primigravidas | 3‐6 cm dilatation | Hyoscine butyl bromide (40 mg IVI) | Sterile water (2 mL IVI) | Amniotomy and augmentation with oxytocin as per protocol | Spontaneous |
| Raghavan 2008 | India | 150 | Not mentioned | Primigravidas | Not mentioned | Hyoscine butyl bromide (PR) Valethamate bromide (IVI) |
No medication | Not specified | Spontaneous |
| Samuels 2007 | Jamaica | 129 | Low risk | Primi‐ and multigravidas | 4‐5 cm dilatation | Hyoscine butyl bromide (20 mg IVI) | NaCl 0.9% (1 mL IVI) | Routine amniotomy, opioid analgesia, oxytocin | Spontaneous |
| Sekhavat 2012 | Iran | 188 | Low risk | Multigravidas | 3‐4 cm dilatation | Hyoscine butyl bromide (20 mg IVI) | NaCl 0.9% (1 mL IVI) | Amniotomy, oxytocin | Spontaneous |
| Sharma 2001 | India | 150 | Low risk | Primigravidas | 4 cm dilatation | Drotaverine hydrochloride (40 mg IMI) Valethamate bromide (8 mg IMI) |
No medication | None | Spontaneous |
| Singh 2004 | India | 100 | Low risk | Primigravidas | 3‐6 cm dilatation | Drotaverine hydrochloride (40 mg IMI) | Distilled water 2 mL (IMI) | Amniotomy, oxytocin, pentazocine and promethazine, episiotomy | Spontaneous |
| Taskin 1993 | USA | 120 | Low risk | Primi‐and multigravidas | 4‐5 cm dilatation | Valethamate bromide (8 mg IVI) Hyoscine bromide (20 mg IVI) Meperidine (50 mg IVI) |
Placebo (not specified, IVI) | Not specified | Not mentioned |
| Warke 2003 | India | 100 | Low risk | Primigravidas | 3 cm dilatation | Camylofin dihydrochloride (50 mg IMI) | Placebo (not specified (IMI) | None | Spontaneous |
| Yilmaz 2009 | Turkey | 160 | Low risk | Primigravidas | 4‐6 cm dilatation | Valethamate bromide (16 mg IVI) Meperidine (50 mg IVI) |
NaCl 0.9% (10 mL) | Amniotomy, oxytocin, episiotomy | Induced only |
¹IVI: intravenous injection
²IMI: intramuscular injection
³NaCl: sodium chloride/saline
⁴ROM: rupture of membranes
⁵PR: per rectum
Although this review included 21 studies (3286 participants), only 17 studies (2617 participants) were included in the meta‐analysis. Four studies (Al Matari 2007; Azari 2010; Raghavan 2008; Taskin 1993) were not included in the meta‐analysis because they were only reported as abstracts and contained insufficient information on relevant data. In addition, they did not contain contact information of authors to obtain missing data.
Primary outcomes
1. Duration of labour
Duration of first stage of labour
Thirteen studies (Ajmera 2006; Al Qahtani 2011; Batukan 2006; Cromi 2011; Dahal 2013; Gupta 2008; Khosla 2003; Makvandi 2010; Samuels 2007; Sekhavat 2012; Singh 2004; Warke 2003; Yilmaz 2009) involving 1995 women were included in the random‐effects meta‐analysis (Analysis 1.1). Antispasmodics reduced the duration of first stage of labour by an average of 74.34 minutes (mean difference (MD) ‐74.34 minutes; 95% confidence interval (CI) ‐98.76 to ‐49.93; T² = 2083.23; I² = 83%).
1.1. Analysis.
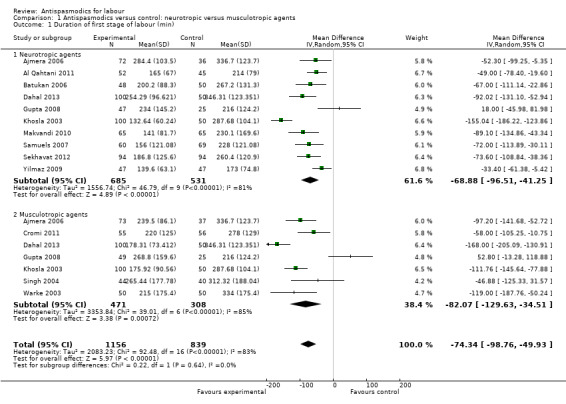
Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 1 Duration of first stage of labour (min).
Subgroup analysis showed significant differences between subgroups for management of labour (active versus expectant management of labour) (Chi² = 4.44, P = 0.04, I² = 77.5%) (Analysis 6.1) and type of pregnancy (low‐ versus high‐risk pregnancy) (Chi² = 21.48, P < 0.00001, I² = 95.3%) (Analysis 7.1). There were no significant differences between the other subgroups.
6.1. Analysis.
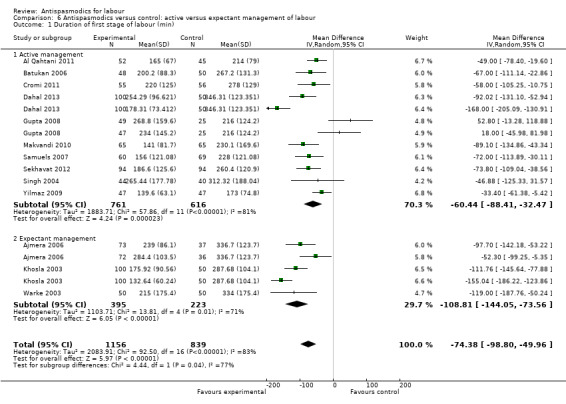
Comparison 6 Antispasmodics versus control: active versus expectant management of labour, Outcome 1 Duration of first stage of labour (min).
7.1. Analysis.
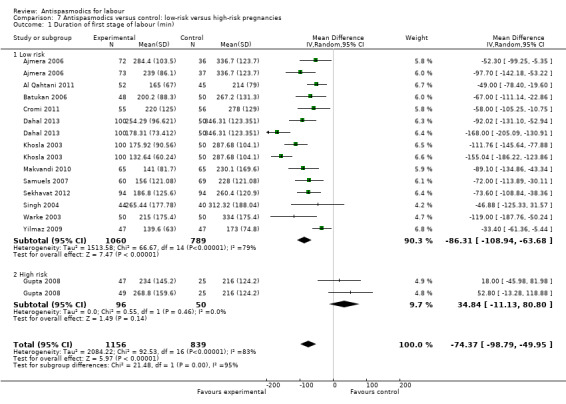
Comparison 7 Antispasmodics versus control: low‐risk versus high‐risk pregnancies, Outcome 1 Duration of first stage of labour (min).
Sensitivity analysis, excluding studies with high risk of bias (inadequate allocation concealment and incomplete outcome data), included only three studies (Cromi 2011; Samuels 2007; Yilmaz 2009; n = 334) and showed a smaller, yet still significant reduction in duration of first stage of labour (MD ‐47.78 minutes; 95% CI ‐68.66 to ‐26.91, fixed‐effect) with an absence of significant heterogeneity (Chi² = 2.48; P = 0.29; I² = 19%) (Analysis 8.1).
8.1. Analysis.
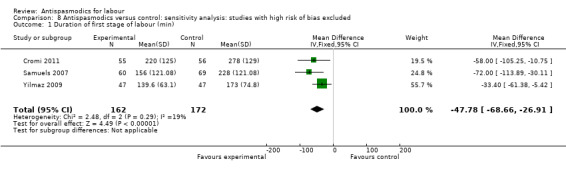
Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 1 Duration of first stage of labour (min).
The funnel plot for this outcome was asymmetrical (Figure 5).
5.
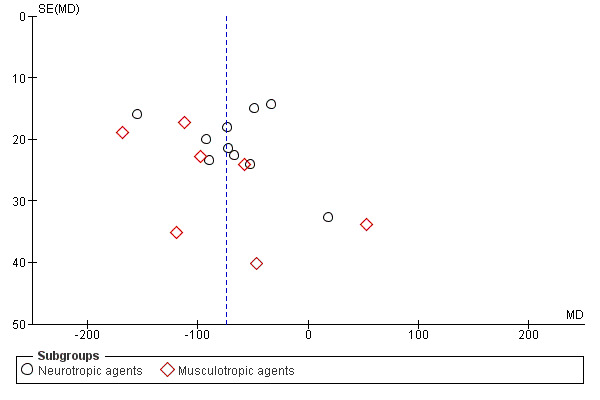
Funnel plot of comparison: 1 Antispasmodics versus control, outcome: 1.1 Duration of first stage of labour (min).
Duration of second stage of labour
Ten studies (involving 1297 women) (Ajmera 2006; Al Qahtani 2011; Batukan 2006; Cromi 2011; Gupta 2008; Makvandi 2010; Samuels 2007; Sekhavat 2012; Singh 2004; Yilmaz 2009) were included in the random‐effects meta‐analysis (Analysis 1.2). There was no significant reduction in the duration of the second stage of labour, with an average intervention effect of ‐2.68 minutes (95% CI ‐5.98 to 0.63, T² = 18.97; I² = 67%).
1.2. Analysis.

Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 2 Duration of second stage of labour (min).
Subgroup analysis showed significant differences between subgroups for studies excluding versus studies including caesarean sections in the analysis (Chi² = 9.65, P = 0.002, I² = 89.6%) (Analysis 2.2) and type of labour (spontaneous versus induced labour) (Chi² = 7.08, P = 0.008, I² = 85.9%) (Analysis 5.2).
2.2. Analysis.
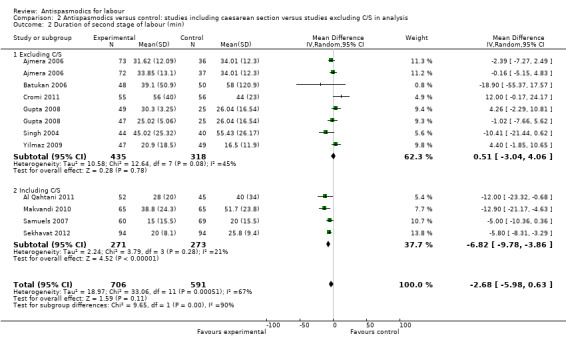
Comparison 2 Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis, Outcome 2 Duration of second stage of labour (min).
5.2. Analysis.
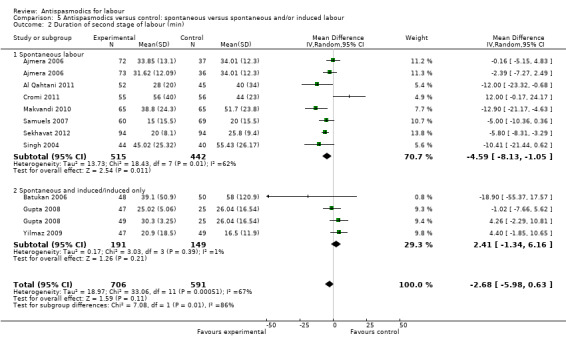
Comparison 5 Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour, Outcome 2 Duration of second stage of labour (min).
Sensitivity analysis included three studies (Cromi 2011; Samuels 2007; Yilmaz 2009) (involving 336 women) and did not show a significantly different effect (MD 2.64 minutes; 95% CI ‐6.34 to 11.61; I² = 78%) (Analysis 8.2).
8.2. Analysis.
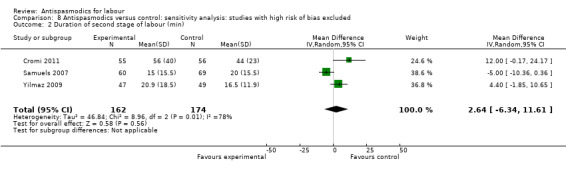
Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 2 Duration of second stage of labour (min).
Duration of third stage of labour
Five studies (involving 765 women) (Ajmera 2006; Gupta 2008; Samuels 2007; Sekhavat 2012; Singh 2004) were included in the random‐effects meta‐analysis (Analysis 1.3). Overall, there was no significant effect of antispasmodics on the third stage of labour (MD ‐0.06 minutes; 95% CI ‐0.52 to 0.40; T² = 0.17; I² = 52%). Subgroup analysis showed a significant difference between groups for gravidity of women (primigravidas versus primi‐and multigravidas versus multigravidas) (Chi² = 7.93, P = 0.02, I² = 74.8%) (Analysis 4.3).
1.3. Analysis.
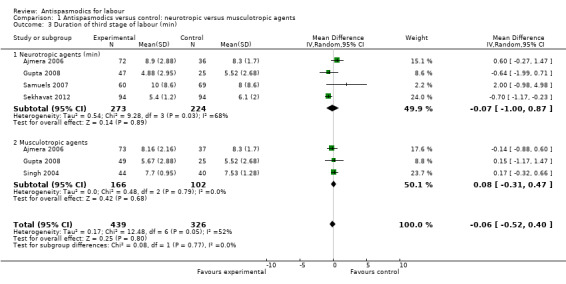
Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 3 Duration of third stage of labour (min).
4.3. Analysis.
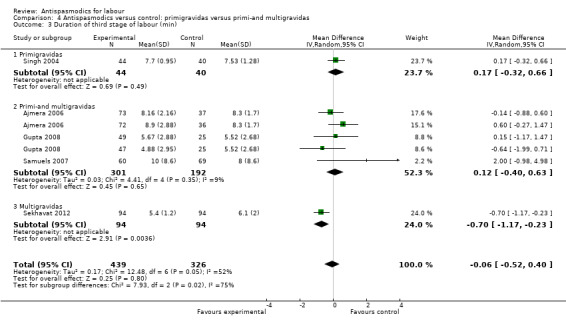
Comparison 4 Antispasmodics versus control: primigravidas versus primi‐and multigravidas, Outcome 3 Duration of third stage of labour (min).
Sensitivity analysis only included the Samuels 2007 study and did not show a significant effect on the duration of third stage of labour (MD 2.00 minutes; 95% CI ‐0.98 to 4.98; one study, 129 women) (Analysis 8.3).
8.3. Analysis.
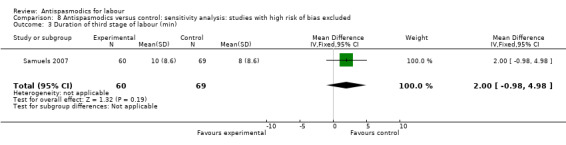
Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 3 Duration of third stage of labour (min).
Total duration of labour
Seven studies (involving 797 women) (Al Qahtani 2011; Madhu 2010; Mukaindo 2010; Samuels 2007; Sharma 2001; Warke 2003; Yilmaz 2009) assessed this outcome. Random‐effects meta‐analysis (Analysis 1.4) showed an average reduction of 85 minutes in the total duration of labour, but significant heterogeneity was also present (MD ‐85.51; 95% CI ‐121.81 to ‐49.20; T² = 2411.75; I² = 83%).
1.4. Analysis.
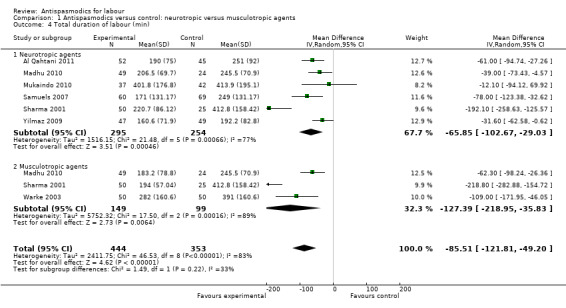
Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 4 Total duration of labour (min).
Subgroup analysis showed a significant difference between groups for route of administration (intravenous versus intramuscular administration) (Chi² = 4.01, P = 0.05, I² = 75.0%) (Analysis 3.4), type of labour (spontaneous versus induced labour) (Chi² = 5.70, P = 0.02, I² = 82.5%) (Analysis 5.4) and management of labour (active versus expectant management of labour) (Chi² = 12.76, P = 0.0004, I² = 92.2%) (Analysis 6.4).
3.4. Analysis.
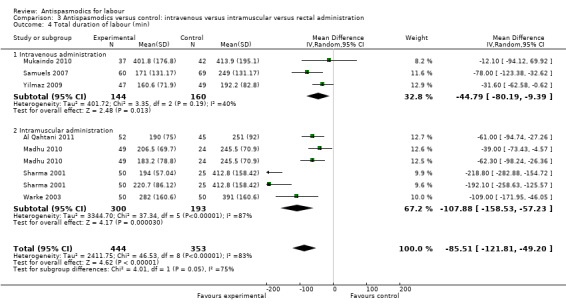
Comparison 3 Antispasmodics versus control: intravenous versus intramuscular versus rectal administration, Outcome 4 Total duration of labour (min).
5.4. Analysis.
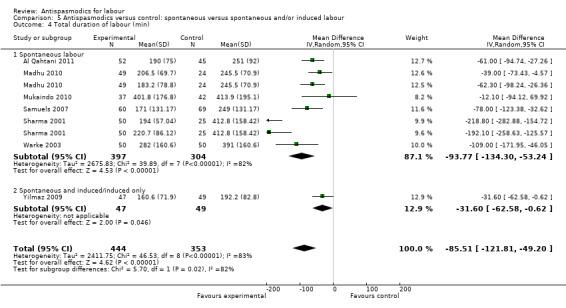
Comparison 5 Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour, Outcome 4 Total duration of labour (min).
6.4. Analysis.
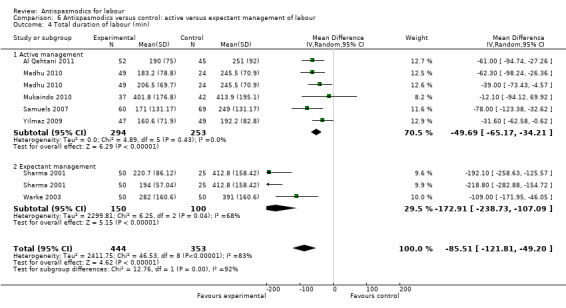
Comparison 6 Antispasmodics versus control: active versus expectant management of labour, Outcome 4 Total duration of labour (min).
Sensitivity analysis included three studies (involving 304 women) (Mukaindo 2010; Samuels 2007; Yilmaz 2009) with adequate allocation concealment and without incomplete reporting of data. The results showed a smaller, but significant reduction in the total duration of labour (MD ‐44.79 minutes; 95% CI ‐80.19 to ‐9.39; random‐effects, T² = 401.72, I² = 40%) (Analysis 8.4). Heterogeneity was reduced considerably.
8.4. Analysis.

Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 4 Total duration of labour (min).
2. Rate of cervical dilatation
Seven studies (Cromi 2011; Gupta 2008; Kuruvila 1992; Madhu 2010; Mukaindo 2010; Sekhavat 2012; Sharma 2001) reported on the cervical dilatation rate and six of these (involving 820 women) were included in the meta‐analysis (Analysis 1.5). Kuruvila 1992 reported data in four different subgroups and was not included. Random‐effects meta‐analysis showed a significant average increase in the rate of cervical dilatation of 0.61 cm/hour. Significant heterogeneity was observed (MD 0.61cm/hour; 95% CI 0.34 to 0.88; random‐effects, T² = 0.12; I² = 80%).
1.5. Analysis.
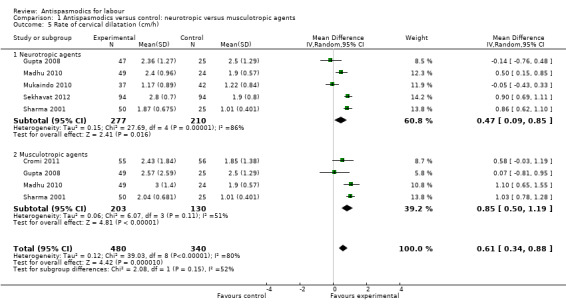
Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 5 Rate of cervical dilatation (cm/h).
Subgroup analysis showed a significant difference for type of labour (spontaneous versus induced labour) (Chi² = 7.34, P = 0.007, I² = 86.4%) (Analysis 5.5), management of labour (active versus expectant management of labour) (Chi² = 5.35, P = 0.02, I² = 81.3%) (Analysis 6.5) and type of pregnancy (low‐ versus high‐risk pregnancy) (Chi² = 7.34, P = 0.007, I² = 86.4%) (Analysis 7.4).
5.5. Analysis.
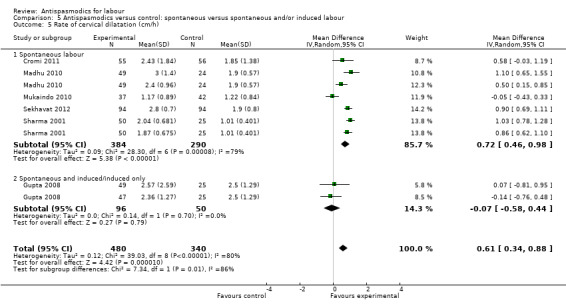
Comparison 5 Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour, Outcome 5 Rate of cervical dilatation (cm/h).
6.5. Analysis.
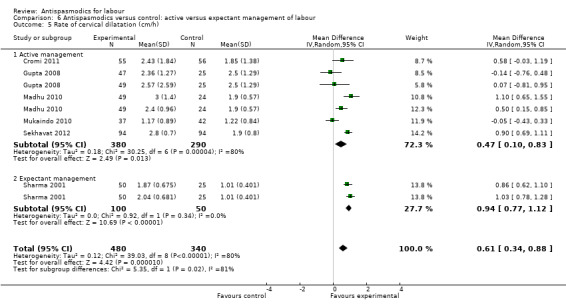
Comparison 6 Antispasmodics versus control: active versus expectant management of labour, Outcome 5 Rate of cervical dilatation (cm/h).
7.4. Analysis.
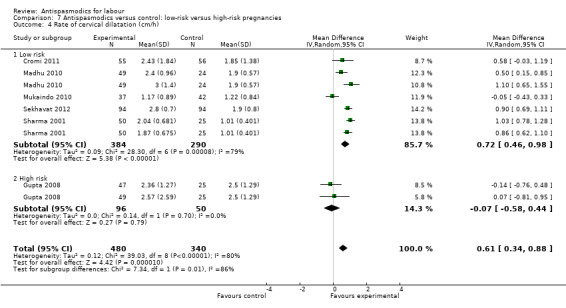
Comparison 7 Antispasmodics versus control: low‐risk versus high‐risk pregnancies, Outcome 4 Rate of cervical dilatation (cm/h).
Sensitivity analysis only included two studies (involving 190 women) (Cromi 2011; Mukaindo 2010) and showed a non‐significant effect of antispasmodics on the rate of cervical dilatation (MD 0.22 cm/hour; 95% CI ‐0.39 to 0.83; I² = 66%) (Analysis 8.5).
8.5. Analysis.
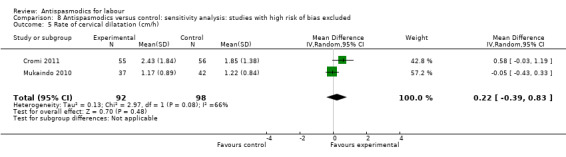
Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 5 Rate of cervical dilatation (cm/h).
3. Pain relief
Only one study (Singh 2004) assessed the effect of antispasmodics on pain relief (n = 100), by using both a visual analogue scale (VAS) with a zero to 100 score, and a verbal rating scale from zero to four. Pain was assessed hourly. There was a similar trend in the experience of pain in both groups. During the second stage, the intervention group had more pain than the placebo group, but in the fourth stage, the intervention group had less pain. Three participants asked for pain relief in the intervention group, compared with two in the control group. The effect of the intervention was thus not significant.
4. Rate of normal vertex deliveries (NVDs)
Sixteen studies (Ajmera 2006; Al Qahtani 2011; Cromi 2011; Dahal 2013; Gupta 2008; Khosla 2003; Kuruvila 1992; Madhu 2010; Makvandi 2010; Mukaindo 2010; Samuels 2007; Sekhavat 2012; Sharma 2001; Singh 2004; Warke 2003; Yilmaz 2009) (involving 2319 women) were included in the fixed‐effect meta‐analysis on rate of normal vertex deliveries (Analysis 1.6). Antispasmodics had no overall significant effect on the rate of normal vertex deliveries (risk ratio (RR) 1.02, 95% CI 1.00, 1.05). Subgroup analysis, however, showed a significant difference between groups for type of antispasmodic (neurotropic versus musculotropic) (Chi² = 4.97, P = 0.03, I² = 79.9%) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Antispasmodics versus control: neurotropic versus musculotropic agents, Outcome 6 Rate of normal vertex deliveries.
Sensitivity analysis included four studies (involving 425 women) (Cromi 2011; Mukaindo 2010; Samuels 2007; Yilmaz 2009) with low risk of bias and did not show a difference in effect (RR 1.03; 95% CI 0.96 to 1.10; I² = 34%) (Analysis 8.6).
8.6. Analysis.
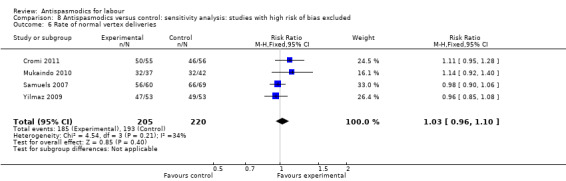
Comparison 8 Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded, Outcome 6 Rate of normal vertex deliveries.
The funnel plot for this outcome was symmetrical (Figure 6).
6.
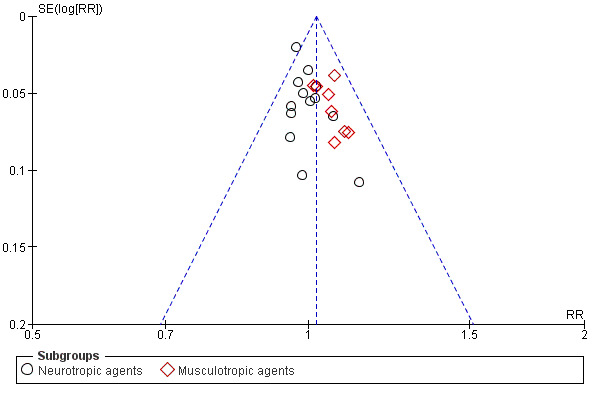
Funnel plot of comparison: 1 Antispasmodics versus control, outcome: 1.6 Rate of NVDs.
Secondary outcomes
1. Maternal adverse events
Maternal adverse events were reported using different approaches and are summarised in Table 3. Adverse events that were reported across studies were tachycardia, mouth dryness, headache, nausea, vomiting, dizziness, giddiness, cervical laceration, flushing of face and postpartum haemorrhage. Meta‐analysis was conducted for outcomes reported quantitatively.
2. Maternal adverse events.
| Study | Tachycardia | Mouth dryness | Nausea | Vomiting | Dizziness | Cervical laceration | Giddiness | Blood loss | Flushing of face | Headache |
|
Ajmera 2006 (Drotaverine hydrochloride) |
2.7% | 0 | 0 | 0 | 0 | 1.3% | 1.3% | Not reported | 0 | 4% |
|
Ajmera 2006 (Valethamtate bromide) |
14.6% | 2.7% | 0 | 0 | 0 | 0 | 0 | Not reported | 5.3% | 2.7% |
|
Ajmera 2006 (Placebo) |
5.3% | 1.3% | 0 | 0 | 0 | 4% | 4% | Not reported | 0 | 2.7% |
| Al Matari 2007 | Did not report on maternal adverse events | |||||||||
|
Al Qahtani 2011 (Hyoscine butyl bromide) |
PPH¹: 0/52; Tear: 2/50 | |||||||||
|
Al Qahtani 2011 (Placebo) |
PPH: 0/45; Tear: 0/45 | |||||||||
| Azari 2010 | Did not report on maternal adverse events | |||||||||
| Batukan 2006 | Women receiving Valethamate bromide experienced more dizziness and mouth dryness than women receiving placebo | |||||||||
|
Cromi 2011 (Rociverine) |
No maternal side effects reported | 300 mL (100‐1400) | ||||||||
|
Cromi 2011 (Placebo) |
No maternal side effects reported | 350 mL (50‐2000) | ||||||||
| Dahal 2013 | Dryness of mouth, vomiting, tachycardia and retention of urine were more pronounced in the valethamate bromide group than in the drotaverine hydrochloride and control groups | |||||||||
|
Gupta 2008 (Drotaverine hydrochloride) |
20% of participants experienced adverse events, the main ones being nausea and vomiting | |||||||||
|
Gupta 2008 (Hyoscine butyl bromide) |
10% | 0 | Main complaint | Main complaint | 24% of participants experienced adverse effects | |||||
|
Gupta 2008 (Placebo) |
No reported maternal adverse events | |||||||||
|
Khosla 2003 (Valethamate bromide) |
16% | 4% | Not reported | 4% | Not reported | |||||
|
Khosla 2003 (Drotaverine hydrochloride) |
No maternal adverse events reported | |||||||||
|
Khosla 2003 (Control) |
No maternal adverse events reported | |||||||||
| Kuruvila 1992 | Maternal pulse rate > 110/min observed in significantly more women receiving Valethamate bromide. Flushing of face and dryness of mouth not statistically significant between groups. | |||||||||
|
Madhu 2010 (Drotaverine hydrochloride) |
4% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10% |
|
Madhu 2010 (Valethamate bromide) |
81.6% | 20% | 0 | 0 | 0 | 0 | 0 | 0 | 10% | 2% |
|
Madhu 2010 (Control) |
6.30% | 4.2% | 0 | 0 | 0 | 0 | 2.1% | 0 | 0 | 0 |
|
Makvandi 2010 (Hyoscine butyl bromide) |
Mean heart rate:83.34 beats/min, SD:10.56 Mean systolic BP: 108.78 mmHg, SD: 12.34 |
|||||||||
|
Makvandi 2010 (Placebo) |
Mean heart rate:86.65 beats/min, SD:12.87 Mean systolic BP: 110.09 mmHg, SD:13.67 |
|||||||||
|
Mukaindo 2010 (Hyoscine butyl bromide) |
1 patient reported transient palpitations PPH in 5.7% participants |
|||||||||
|
Mukaindo 2010 (Placebo) |
No adverse events reported PPH in 7.3% of participants |
|||||||||
|
Raghavan 2008 (Hyoscine butyl bromide) |
Mild tachycardia, dryness of mouth and vomiting | |||||||||
|
Raghavan 2008 (Valethamate bromide) |
Mild tachycardia, dryness of mouth and vomiting | |||||||||
|
Raghavan 2008 (No medication) |
No adverse events reported | |||||||||
|
Samuels 2007 (Hyoscine butyl bromide) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 150 mL (50‐1800 mL) | 0 | 0 |
|
Samuels 2007 (Placebo) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 150 mL (50‐550 mL) | 0 | 0 |
| Sekhavat 2012 | No adverse events reported | |||||||||
|
Sharma 2001 (Drotaverine hydrochloride) |
4% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4% |
|
Sharma 2001 (Valethamate bromide) |
20% | 10% | 0 | 0 | 0 | 0 | 0 | 0 | 4% | 0 |
|
Sharma 2001 (Control) |
4% | 0 | 0 | 0 | 0 | 0 | 6% | 0 | 0 | 4% |
|
Singh 2004 (Drotaverine hydrochloride) |
18% had atonic PPH | |||||||||
|
Singh 2004 (Control) |
2.5% had atonic PPH | |||||||||
|
Warke 2003 (Camylofin dihydrochloride) |
1% | 5% | 5% | 6% | 0 | 2% | 0 | 0 | 0 | 0 |
|
Warke 2003 (Control) |
0 | 0 | 0 | 0 | 0 | 2% | 0 | 0 | 0 | 0 |
|
Yilmaz 2009 (Valethamte bromide) |
21.3% | 14.9% | 14.9% | 8.5% | 12.8% | 2.1% | 0 | 0 | 0 | 0 |
|
Yilmaz 2009 (Control) |
4.1% | 0 | 10.2% | 6.1% | 4.1% | 0 | 0 | 0 | 0 | 0 |
¹PPH: postpartum haemorrhage
Tachycardia (Analysis 9.1)
9.1. Analysis.
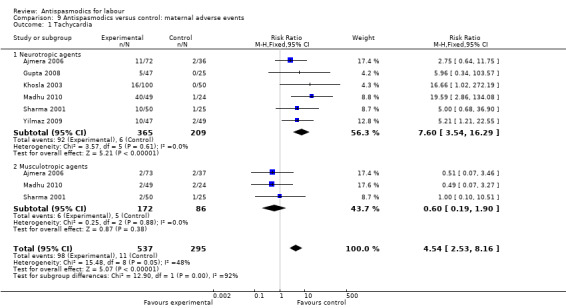
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 1 Tachycardia.
Six studies (Ajmera 2006; Gupta 2008; Khosla 2003; Madhu 2010; Sharma 2001; Yilmaz 2009) involving 832 women were included in the fixed‐effect meta‐analysis. There was an increased risk for tachycardia for participants receiving antispasmodics (RR 4.54; 95% CI 2.53 to 8.16). Subgroup analysis showed a significant difference between groups for type of antispasmodic (neurotropic versus musculotropic agents), with a greater risk with neurotropic agents (Chi² = 12.90,(P = 0.0003), I² = 92.3%).
Mouth dryness (Analysis 9.2)
9.2. Analysis.
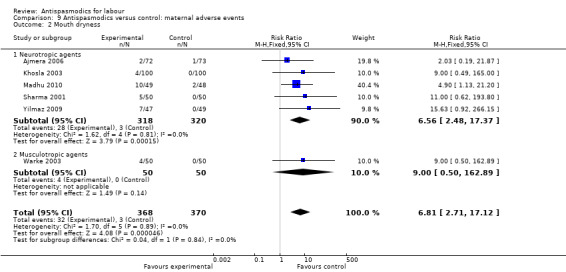
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 2 Mouth dryness.
Six studies (Ajmera 2006; Khosla 2003; Madhu 2010; Sharma 2001; Warke 2003; Yilmaz 2009) involving 738 women were included in the fixed‐effect meta‐analysis. There was an increased risk for mouth dryness for participants receiving antispasmodics (RR 6.81; 95% CI 2.71 to 17.12).
Headache (Analysis 9.3)
9.3. Analysis.
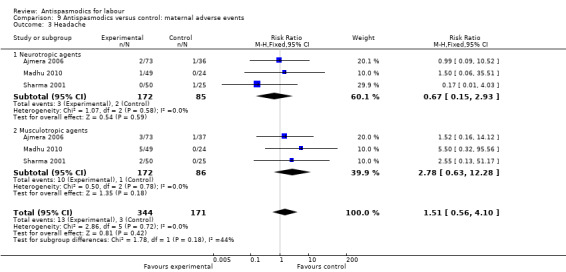
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 3 Headache.
Three studies (Ajmera 2006; Madhu 2010; Sharma 2001) involving 515 women were included in the fixed‐effect meta‐analysis.There was no significant difference in the risk for headaches between intervention and control groups (RR 1.51; 95% CI 0.56 to 4.10).
Nausea and vomiting (Analysis 9.4; Analysis 9.5)
9.4. Analysis.
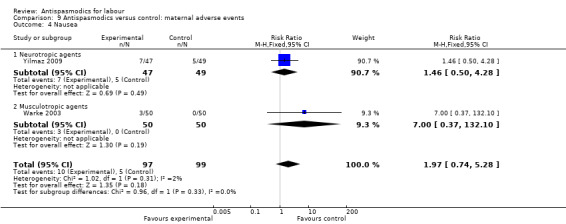
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 4 Nausea.
9.5. Analysis.
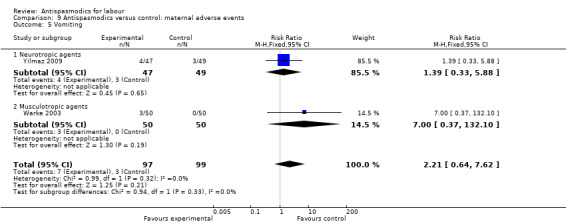
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 5 Vomiting.
Two studies (Warke 2003; Yilmaz 2009) involving 196 women had quantitative data on nausea and on vomiting and were included in the fixed‐effect meta‐analysis, showing no significant difference in risk between intervention and control groups for either nausea (RR 1.97; 95% CI 0.74, 5.28) or vomiting (RR 2.21; 95% CI 0.64 to 7.62).
Dizziness (Analysis 9.6)
9.6. Analysis.
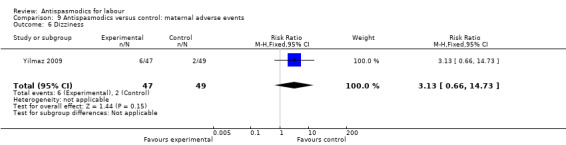
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 6 Dizziness.
Only one study (Yilmaz 2009) involving 96 women reported quantitative data on dizziness, showing no significant difference in risk for dizziness between intervention and control groups (RR 3.13; 95% CI 0.66 to 14.73).
Giddiness (Analysis 9.7)
9.7. Analysis.
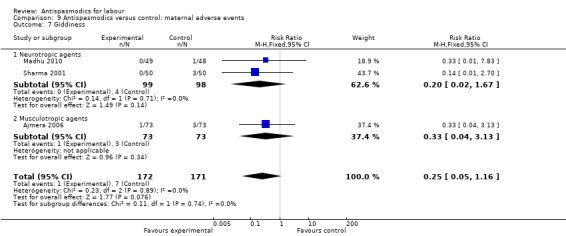
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 7 Giddiness.
Three studies (Ajmera 2006; Madhu 2010; Sharma 2001) involving 343 women were included in the fixed‐effect meta‐analysis, showing no significant difference in risk for giddiness between intervention and control groups (RR 0.25; 95% CI 0.05 to 1.16).
Cervical lacerations (Analysis 9.8)
9.8. Analysis.
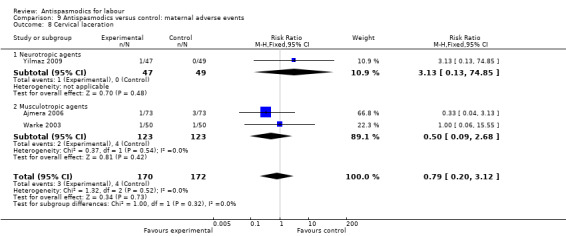
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 8 Cervical laceration.
Three studies (Ajmera 2006; Warke 2003; Yilmaz 2009) including 342 women were included in the fixed‐effect meta‐analysis, showing no significant difference in risk for cervical lacerations between intervention and control groups (RR 0.79; 95% CI 0.20 to 3.12).
Flushing of face (Analysis 9.9)
9.9. Analysis.
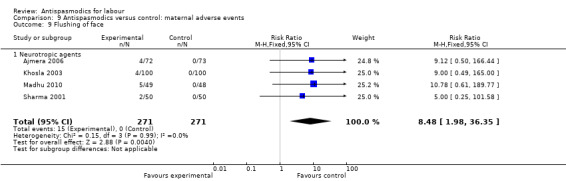
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 9 Flushing of face.
Four studies (Ajmera 2006; Khosla 2003; Madhu 2010; Sharma 2001) involving 542 women were included in the fixed‐effect meta‐analysis, showing a significant increased risk for flushing of the face in the intervention group (RR 8.48; 95% CI 1.98 to 36.35).
Postpartum haemorrhage (Analysis 9.10)
9.10. Analysis.
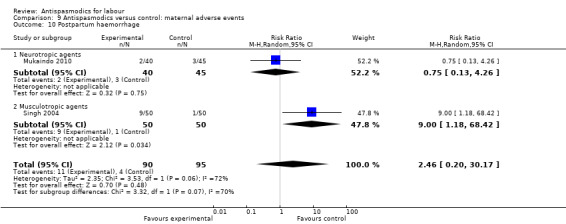
Comparison 9 Antispasmodics versus control: maternal adverse events, Outcome 10 Postpartum haemorrhage.
Two studies (Mukaindo 2010; Singh 2004) involving 185 women, were included in the random‐effects meta‐analysis, showing no significant difference in risk for postpartum haemorrhage between intervention and control groups (RR 2.46; 95% CI 0.20 to 30.17;T² = 2.35; I² = 72%)
2. Neonatal adverse events
As with maternal adverse events, neonatal adverse events were reported using different approaches and are summarised in Table 4. Adverse events reported admission to neonatal intensive care unit (NICU), fetal distress, fetal bradycardia, fetal tachycardia and presence of meconium‐stained liquor. Apgar scores at one and five minutes after delivery were also reported. A low Apgar score (less than seven at both one and five minutes) was also seen as an adverse event. Meta‐analysis was conducted for quantitatively reported outcomes.
3. Neonatal adverse events.
| Study | Apgar 1 min | Apgar 5 min | Meconium‐stained liquor | Fetal tachycardia | Fetal distress | Admission to NICU¹ | Need for resuscitation |
|
Ajmera 2006 (Drotaverine hydrochloride) |
No report on neonatal adverse events or Apgar scores | ||||||
|
Ajmera 2006 (Valethamate bromide) |
No report on neonatal adverse events or Apgar scores | ||||||
|
Ajmera 2006 (Control) |
No report on neonatal adverse events or Apgar scores | ||||||
|
Al Matari 2007 (Hyoscine butyl bromide) |
Mean score: 8.7 | Mean score: 9.4 | No other adverse events reported | ||||
|
Al Matari 2007 (Control) |
Mean score: 8.7 | Mean score: 9.3 | No other adverse events reported | ||||
|
Al Qahtani 2011 (Hyoscine butyl bromide) |
5/52 babies: score of 7‐8 |
Score: 8‐10 for all babies | No adverse events noted | ||||
|
Al Qahtani 2011 (Placebo) |
8/45 babies: score of 7‐8 |
Score: 8‐10 for all babies | No adverse events noted | ||||
| Azari 2010 | No reports of any neonatal adverse events | ||||||
| Batukan 2006 | No reported neonatal adverse events | ||||||
|
Cromi 2011 (Rociverine) |
Not reported | Score < 7: 0% | Arterial cord blood pH: 7.28 SD 0.11 | not reported | |||
|
Cromi 2011 (Placebo) |
Not reported | Score < 7: 1.8% | Arterial cord blood pH: 7.27 SD 0.08 | not reported | |||
|
Dahal 2013 (Valethamate bromide) |
Not reported | 4 | not reported | ||||
|
Dahal 2013 (Drotaverine hydrochloride) |
Not reported | 2 | not reported | ||||
|
Dahal 2013 (Control) |
Not reported | 2 | not reported | ||||
|
Gupta 2008 (Drotaverine hydrochloride) |
Mean score: 8 (7‐9) | Mean score: 9 (8‐9) | 0 | 0 | 0 | 2 | not reported |
|
Gupta 2008 (Hyoscine butyl bromide) |
Mean score: 8 (1‐9) | Mean score: 9 (3‐9) | 0 | 0 | 0 | 1 | not reported |
|
Gupta 2008 (Control) |
Mean score: 8 (4‐9) | Mean score: 9 (8‐9) | 0 | 0 | 0 | 3 | not reported |
| Khosla 2003 | "All babies had good Apgar scores and were discharged in good condition" | ||||||
| Kuruvila 1992 | No neonatal adverse events reported | ||||||
|
Madhu 2010 (Drotaverine hydrochloride) |
No report on neonatal adverse effects or Apgar scores | ||||||
|
Madhu 2010 (Valethamte bromide) |
Not reported | 10% | 0 | 0 | not reported | ||
|
Madhu 2010 (Control) |
No report on neonatal adverse effects or Apgar scores | ||||||
|
Makvandi 2010 (Hyoscine butyl bromide) |
Score < 7: 2 | Score < 7: 0 | Bradycardia: 3.10%; Tachycardia: 4.60% | not reported | |||
|
Makvandi 2010 (Control) |
Score < 7: 0 | Score < 7: 0 | Bradycardia: 4.60%; Tachycardia: 3.10% | not reported | |||
|
Mukaindo 2010 (Hyoscine butyl bromide) |
Not reported | 10 (8‐10) | No adverse events reported | ||||
|
Mukaindo 2010 (Placebo) |
Not reported | 10 (7‐10) | No adverse events reported | ||||
| Raghavan 2008 | No significant adverse events reported in any of the groups | ||||||
|
Samuels 2007 (Hyoscine butyl bromide) |
Mean score: 9 (2‐9) | Mean score: 9 (2‐10) | not reported | ||||
|
Samuels 2007 (Control) |
Mean score: 9 (3‐9) | Mean score: 9 (8‐10) | not reported | ||||
|
Sekhavat 2012 (Hyoscine butyl bromide) |
Mean score: 8.4 (SD 1.6) | Mean score: 7.8 (SD 1.2) | not reported | 1 | 2 | ||
|
Sekhavat 2012 (Placebo) |
Mean score: 8.1 (SD 1.8) | Mean score: 8.1 (SD 0.9) | not reported | 1 | 1 | ||
|
Sharma 2001 (Drotaverine hydrochloride) |
Not statistically different from other groups | not reported | |||||
|
Sharma 2001 (Valethamate bromide) |
Not statistically different from other groups | 0 | 0 | 4% | 0 | not reported | |
|
Sharma 2001 (Control) |
Not statistically different from other groups | 0 | 0 | 8% | 0 | not reported | |
|
Singh 2004 (Drotaverine hydrochloride) |
Mean score: 9.84 | Mean score: 9.96 | 0 | 0 | 0 | 0 | not reported |
|
Singh 2004 (Control) |
Mean score: 9.59 | Mean score: 9.93 | 0 | 0 | 0 | 0 | not reported |
| Taskin 1993 | not reported | ||||||
|
Warke 2003 (Camylofin dihydrochloride) |
Not reported | 0 | 0 | 0 | 2% | not reported | |
|
Warke 2003 (Control) |
Not reported | 0 | 0 | 0 | 2% | not reported | |
|
Yilmaz 2009 (Valethamate bromide) |
Score < 7: 8.5% | Score < 7: 2.1% | 10.6% | 0 | 0 | 0 | not reported |
|
Yilmaz 2009 (Control) |
Score < 7: 6.1% | Score < 7: 4.1% | 6.1% | 0 | 0 | 2% | not reported |
¹NICU: neonatal intensive care unit
Admission to NICU (Analysis 10.1)
10.1. Analysis.
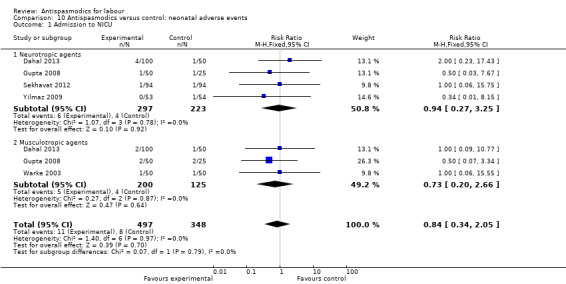
Comparison 10 Antispasmodics versus control: neonatal adverse events, Outcome 1 Admission to NICU.
Five studies (Dahal 2013; Gupta 2008; Sekhavat 2012; Warke 2003; Yilmaz 2009) involving 845 babies, were included in the fixed‐effect meta‐analysis, showing no significant difference in the risk for admission to NICU between intervention and control groups (RR 0.84; 95% CI 0.34 to 2.05).
Fetal distress (Analysis 10.2)
10.2. Analysis.
Comparison 10 Antispasmodics versus control: neonatal adverse events, Outcome 2 Fetal distress.
One study (Sharma 2001) involving 100 babies, reported quantitatively on this outcome and showed no difference in risk for fetal distress between intervention and control groups (RR 0.50; 95% CI 0.10 to 2.61).
Fetal bradycardia (Analysis 10.3)
10.3. Analysis.
Comparison 10 Antispasmodics versus control: neonatal adverse events, Outcome 3 Fetal bradycardia.
One study (Makvandi 2010) involving 130 babies, reported quantitatively on this outcome, showing no difference in risk for fetal bradycardia between intervention and control groups (RR 0.67; 95% CI 0.12 to 3.86).
Fetal tachycardia (Analysis 10.4)
10.4. Analysis.
Comparison 10 Antispasmodics versus control: neonatal adverse events, Outcome 4 Fetal tachycardia.
Two studies (Madhu 2010; Makvandi 2010) involving 230 babies were included in the fixed‐effect meta‐analysis, showing no significant difference in risk for fetal tachycardia between intervention and control groups (RR 3.40; 95% CI 0.85 to 13.67).
Meconium‐stained liquor (Analysis 10.5)
10.5. Analysis.
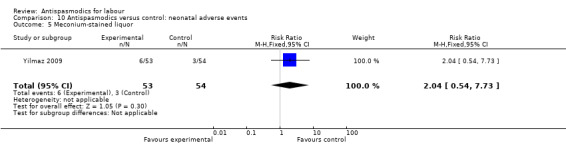
Comparison 10 Antispasmodics versus control: neonatal adverse events, Outcome 5 Meconium‐stained liquor.
One study (Yilmaz 2009) involving 107 babies, reported quantitatively on this outcome, showing no significant difference in risk for meconium‐stained liquor between intervention and control groups (RR 2.04; 95% CI 0.54, 7.73).
Low Apgar score
One‐ and five‐minute Apgar scores reported quantitatively, were either reported as means with the range of scores, or as proportion of babies with a score of less than seven. Results were thus not combined in meta‐analysis, but are presented in Table 4.
3. Maternal satisfaction
Only Mukaindo 2010 reported on maternal satisfaction. The investigators developed a questionnaire using Likert rating scale questions. Women in the study were asked to rate their satisfaction based on their expectations on four items (overall labour experience, pain control during labour, duration of labour, whether they would consider receiving the study drug in their next labour). A composite score out of 20 points was calculated. The mean score for the treatment group was 13.7, compared with 14.4 in the placebo group, showing no significant difference in maternal satisfaction (MD 0.7; 95% CI ‐2.5 to 1.1; one study, 58 women).
Discussion
Summary of main results
Twenty‐one studies were included in this updated review,17 of which were included in the meta‐analysis. Most studies were conducted in India. Valethamate bromide was the drug most commonly used in studies, followed by hyoscine‐butyl‐bromide. Both these drugs have anti‐cholinergic actions.
Including the two new studies did not change the overall results of this review. On average, antispasmodics decreased the duration of the first stage of labour, as well as the total duration of labour. For both outcomes, significant heterogeneity was observed that remained mostly unexplained. Sensitivity analysis, considering only studies with low risk of selection and attrition bias, showed a significant reduction in the duration of first stage of labour, with the absence of heterogeneity. The total duration of labour was also reduced with significantly less heterogeneity when only studies with low risk of bias were considered.
Two Cochrane reviews (Brown 2008; Wei 2009) reporting on the outcome 'duration of labour' also reported significant, unexplained heterogeneity. Calculation of the duration of labour can be very difficult and subjective. Firstly, differences in measurement of cervical dilatation exist, possibly introducing detection bias. Secondly, the course of labour differs immensely between labouring women. While some present with strong, painful contractions at 2 cm cervical dilatation, others will experience the same amount of pain only at 4 cm cervical dilatation. This influences the individual perception of the beginning of labour, which in effect cannot be determined. Most studies thus defined their measurement of the first stage of labour as the point of administration of the intervention until full cervical dilatation. This timing of the administration of the intervention varied from 3 cm to 6 cm cervical dilatation (Table 2), both within and between studies. Considering this, the rate of cervical dilatation would be a more accurate measurement. Random‐effects meta‐analysis of the results of the six studies that reported this outcome showed a significant increase in the rate of cervical dilatation. Significant, unexplained heterogeneity was also observed for this outcome and all results should thus be interpreted with caution.
Duration of second and third stage of labour was not affected by antispasmodics. Pain relief was only assessed by two studies and it remains unclear whether antispasmodics have an analgesic effect. The rate of normal vertex deliveries was not affected by antispasmodics. All but one study included only women with low‐risk pregnancies, mostly already in active labour which in itself presents favourable conditions for normal vertex deliveries.
Results for seven of the studies included in the meta‐analysis included patients who delivered via caesarean section in their calculation of the duration of labour. Subgroup analysis showed that there was no significant difference for the outcomes duration of first stage of labour and total duration of labour. There was a significant difference between subgroups for the outcome duration of second stage of labour. The rate of caesarean sections was similar in intervention and control groups in all these studies.
Maternal and neonatal adverse events were not reported consistently throughout studies. The most commonly reported maternal adverse events were tachycardia (six studies) and mouth dryness (six studies). For both, meta‐analyses showed an increased risk for the intervention group. Both of these are documented side effects of antispasmodic drugs.
Neonatal adverse events were also reported inconsistently. The most commonly reported adverse event (five studies) was admission to NICU. None of the adverse events included in the meta‐analyses showed a significant result. A low Apgar score was also seen as a neonatal adverse event. The one‐ and five‐minute Apgar scores were reported inconsistently but were almost identical in the intervention and control groups for all studies reporting these.
Overall completeness and applicability of evidence
Most studies included in the meta‐analysis were conducted in low‐ and middle‐income countries, although it is known that the use of antispasmodics during labour is widespread in high‐income countries as well. In most cases, labour is not a condition listed under the indications for the use of these drugs by their manufacturers, probably due to the lack of evidence regarding the safety for both mother and baby. Although there were no serious adverse events reported in any of the studies included in this review, less than half of the studies adequately reported on maternal and neonatal adverse events and there is thus insufficient information to make any conclusions about the safety of these drugs during labour.
Nine studies were conducted in India, where the administration of antispasmodics forms part of their protocol for programmed labour and is routinely administered (Daftary 2009; Yuel 2008). Yet, this still seems to be a widely debated topic. Valethamate bromide, a drug used quite commonly, is not even listed in the Indian Pharmacopoeia (Gitanjali 2010; Thirunavukkarasu 2010), again raising concerns about its safety when used during pregnancy.
Only one study (Mukaindo 2010) was carried out in an African country. Considering the selective availability of epidural analgesia in low‐ and middle‐income countries such as India and Kenya, antispasmodics could provide an alternative and cost‐effective method to shorten the duration and pain associated with labour. In high‐income countries, women opting to deliver without epidural analgesia, could benefit from the same alternative. Maternal views on artificial shortening of labour in the absence of prolonged labour were only addressed in one study measuring maternal satisfaction with treatment (Mukaindo 2010). Maternal views should be taken into consideration when deciding on a treatment strategy.
Most studies included antispasmodics as part of their package of active management of labour, and the studies where labour was managed expectantly were all at high risk of bias. The results should be interpreted keeping this in mind and mostly apply within a context of active management of labour. This included either artificial rupture of membranes (aROM), oxytocin augmentation or both. Subgroup analysis showed a significant difference between groups for outcomes: duration of first stage of labour, total duration of labour and rate of cervical dilatation. The effect was greater in the expectantly managed groups for all outcomes.
Quality of the evidence
Most studies included in this review lacked methodological rigour. Only four studies (Cromi 2011; Mukaindo 2010; Samuels 2007; Yilmaz 2009) were considered as having a low risk of bias. Sequence generation and allocation concealment were poorly reported in most studies thus introducing risk of selection bias. There was low risk of performance bias and detection bias in only eight and 10 studies respectively. It can be argued that progression of labour cannot be influenced by the participants' as well as the personnel's knowledge of the received intervention, but maternal relaxation and reassurance in any form aids the natural progression of labour while measurement of cervical dilatation is subjective to the person examining the woman. There was high risk of selective outcome reporting for 10 studies. Four of these were only reported as abstracts and thus only selectively reported on results. These were not included in the meta‐analysis. Judgement of risk of bias for this domain was mainly made by looking at the study report, since study protocols were available for three studies only.
We used GRADE Profiler software to assess the quality of the evidence by rating the quality of evidence for each outcome. Factors taken into consideration include study limitations, imprecision, inconsistency of results, indirectness of evidence and publication bias (Guyatt 2011). The quality of evidence ranged from "very low" for the outcome Total duration of labour; to "low" for the outcomes Duration of first stage of labour, Duration of second stage of labour, Duration of third stage of labour and Rate of cervical dilatation; and "moderate" for the outcome Rate of normal vertex deliveries (see Table 1) .
When considering only studies with low risk of bias in the sensitivity analysis, the quality of evidence for the outcome Duration of first stage of labour was rated as "high" since heterogeneity was absent in this meta‐analysis and no study limitations were present.
Potential biases in the review process
A comprehensive search strategy was established to include published and unpublished studies in all languages. Translators were involved to assist with studies in foreign languages.Two review authors independently selected studies for inclusion in the review, assessed the risk of bias and extracted the data in order to minimise bias. Funnel plots were used to assess reporting biases for the outcomes duration of first stage of labour (Figure 5) and rate of normal vertex deliveries (Figure 6). The funnel plot was symmetrical for the rate of normal vertex deliveries and asymmetrical for the duration of first stage of labour. The poor methodological design of the studies as well as the high level of unexplained heterogeneity between studies included in the meta‐analysis of duration of first stage of labour could be a source of funnel plot asymmetry.
Agreements and disagreements with other studies or reviews
No other systematic review on the effect of antispasmodics for labour was found. The results of all but two studies showed a reduction in the duration of labour with the administration of antispasmodics. Gupta 2008 was the only study that included women with low‐ and high‐risk pregnancies, while Mukaindo 2010 had a sample size of only 85 and did not show a significant effect.
Authors' conclusions
Implications for practice.
There is low quality evidence that administration of antispasmodics during the active phase of labour leads to a reduction in the duration of the first stage of labour and that there is an increase in the rate of cervical dilatation. There is very low quality evidence that the total duration of labour is reduced. When considering only studies with low risk of selection and attrition bias, there is high quality evidence that the duration of labour is reduced. There is evidence of moderate quality that antispasmodics are not effective in lowering caesarean section rates. There is insufficient evidence to make conclusions regarding maternal and neonatal adverse events of antispasmodics administration during labour.
Antispasmodics were administered at the beginning of established labour in all studies. The results of this review therefore, only apply when using antispasmodics in the light of prevention of prolonged labour, and not as treatment. Although there is a statistically significant reduction of approximately an hour in the duration of the first stage of labour, it is questionable whether this has any clinical relevance. However, considering limited human resources, crowded labour rooms and limited availability of epidural analgesia in low‐ and middle‐income countries, one hour per labouring woman could have a positive impact on both the birthing facilities and the labouring woman. In both low‐and middle‐income as well as high income countries, women should be allowed to make informed choices regarding management of labour.
Implications for research.
Since none of the included studies assessed the value of antispasmodics for the treatment of prolonged labour, a rigorously designed, well‐conducted randomised controlled trial (RCT) with a large sample size would be beneficial to answer this question. Only when the occurrence of prolonged labour is reduced, will there be a reduction in the associated complications such as postpartum haemorrhage.
Since most studies were conducted in a context of active management of labour and those that were conducted in the context of expectant management were all of poor quality, a methodologically sound RCT is needed to answer the question whether antispasmodics could be used as a replacement for active management of labour, including artificial rupture of membranes and oxytocin use.
The sample sizes of all studies were calculated relating to the effect of the duration of labour. It is unclear whether these samples are sufficient to conclude that there was no effect on the rate of caesarean sections as well as maternal and neonatal adverse events. Studies considering the mode of delivery and the safety of mother and baby as main outcomes with accordingly calculated sample sizes are required.
Conducting such studies in Sub‐Saharan Africa would be useful, because their outcomes could address the high burden of maternal morbidity and mortality experienced in these countries.
What's new
| Date | Event | Description |
|---|---|---|
| 8 April 2013 | New search has been performed | Search updated. Two additional studies have been included (Dahal 2013; Sekhavat 2012) and one excluded (Fouedjio 2012). |
| 8 April 2013 | New citation required but conclusions have not changed | Review updated. |
Acknowledgements
The 2013 updated systematic review was financially supported by the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) and the Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication. The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
For translation of non‐English language studies: Charles Okwundu (French studies), Baran Kalay (Turkish study), Ole Olsen (Danish study) and Marzia Lazzerini (Italian studies).
As part of the pre‐publication editorial process, the first version of this review was commented on by four peer referees (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms
We searched the Pro‐quest dissertation database, the dissertation database of Stellenbosch University and Google Scholar using following keywords: (antispasmodic OR parasympatholytic OR spasmolytic) AND (labour OR labor OR childbirth OR delivery)
Data and analyses
Comparison 1. Antispasmodics versus control: neurotropic versus musculotropic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.34 [‐98.76, ‐49.93] |
| 1.1 Neurotropic agents | 10 | 1216 | Mean Difference (IV, Random, 95% CI) | ‐68.88 [‐96.51, ‐41.25] |
| 1.2 Musculotropic agents | 7 | 779 | Mean Difference (IV, Random, 95% CI) | ‐82.07 [‐129.63, ‐34.51] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Neurotropic agents | 8 | 919 | Mean Difference (IV, Random, 95% CI) | ‐4.02 [‐7.71, ‐0.33] |
| 2.2 Musculotropic agents | 4 | 378 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐6.61, 7.72] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Neurotropic agents (min) | 4 | 497 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.00, 0.87] |
| 3.2 Musculotropic agents | 3 | 268 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.31, 0.47] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Neurotropic agents | 6 | 549 | Mean Difference (IV, Random, 95% CI) | ‐65.85 [‐102.67, ‐29.03] |
| 4.2 Musculotropic agents | 3 | 248 | Mean Difference (IV, Random, 95% CI) | ‐127.39 [‐218.95, ‐35.83] |
| 5 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 5.1 Neurotropic agents | 5 | 487 | Mean Difference (IV, Random, 95% CI) | 0.47 [0.09, 0.85] |
| 5.2 Musculotropic agents | 4 | 333 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.50, 1.19] |
| 6 Rate of normal vertex deliveries | 16 | 2319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [1.00, 1.05] |
| 6.1 Neurotropic agents | 13 | 1536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 6.2 Musculotropic agents | 8 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.02, 1.11] |
Comparison 2. Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.34 [‐98.76, ‐49.93] |
| 1.1 Excluding C/S | 7 | 1051 | Mean Difference (IV, Random, 95% CI) | ‐59.10 [‐95.81, ‐22.38] |
| 1.2 Including C/S | 6 | 944 | Mean Difference (IV, Random, 95% CI) | ‐93.09 [‐125.11, ‐61.08] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Excluding C/S | 6 | 753 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐3.04, 4.06] |
| 2.2 Including C/S | 4 | 544 | Mean Difference (IV, Random, 95% CI) | ‐6.82 [‐9.78, ‐3.86] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Excluding C/S | 3 | 448 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.23, 0.46] |
| 3.2 Including C/S | 2 | 317 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐2.28, 2.75] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Excluding C/S | 3 | 392 | Mean Difference (IV, Random, 95% CI) | ‐102.60 [‐164.12, ‐41.08] |
| 4.2 Including C/S | 4 | 405 | Mean Difference (IV, Random, 95% CI) | ‐68.85 [‐96.89, ‐40.81] |
| 5 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 5.1 Excluding C/S | 4 | 553 | Mean Difference (IV, Random, 95% CI) | 0.67 [0.39, 0.95] |
| 5.2 Including C/S | 2 | 267 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.49, 1.37] |
2.1. Analysis.
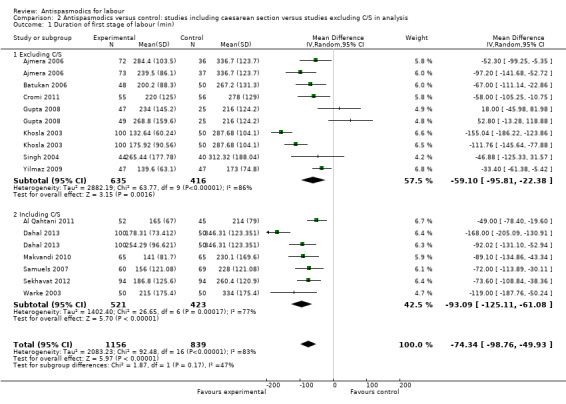
Comparison 2 Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis, Outcome 1 Duration of first stage of labour (min).
2.3. Analysis.
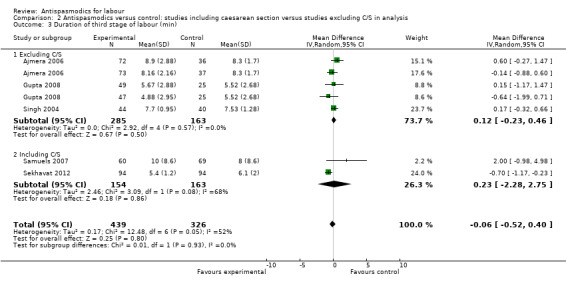
Comparison 2 Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis, Outcome 3 Duration of third stage of labour (min).
2.4. Analysis.
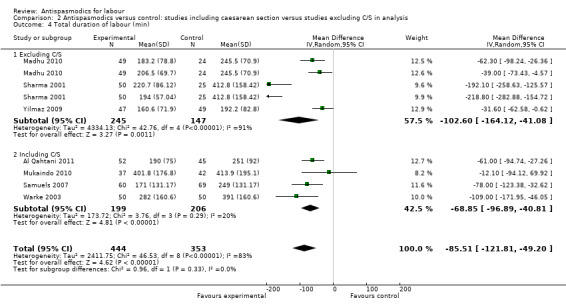
Comparison 2 Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis, Outcome 4 Total duration of labour (min).
2.5. Analysis.
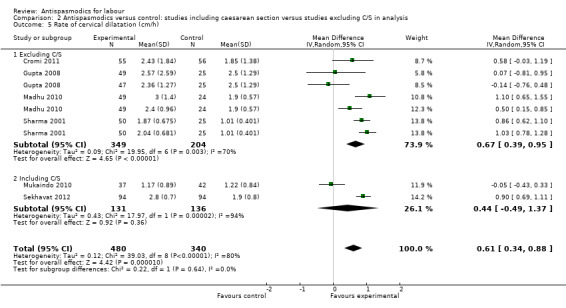
Comparison 2 Antispasmodics versus control: studies including caesarean section versus studies excluding C/S in analysis, Outcome 5 Rate of cervical dilatation (cm/h).
Comparison 3. Antispasmodics versus control: intravenous versus intramuscular versus rectal administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.37 [‐98.79, ‐49.95] |
| 1.1 Intravenous administration | 5 | 591 | Mean Difference (IV, Random, 95% CI) | ‐47.58 [‐74.09, ‐21.07] |
| 1.2 Intramuscular administration | 9 | 1274 | Mean Difference (IV, Random, 95% CI) | ‐86.34 [‐119.03, ‐53.65] |
| 1.3 Rectal administration | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐89.1 [‐134.86, ‐43.34] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Intravenous administration | 5 | 594 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐5.77, 1.72] |
| 2.2 Intramuscular administration | 6 | 573 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐8.86, 4.72] |
| 2.3 Rectal administration | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐21.17, ‐4.63] |
| 3 Duration of thrid stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Intravenous administration | 3 | 425 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐1.08, 1.44] |
| 3.2 Intramuscular administration | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.35, 0.40] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Intravenous administration | 3 | 304 | Mean Difference (IV, Random, 95% CI) | ‐44.79 [‐80.19, ‐9.39] |
| 4.2 Intramuscular administration | 4 | 493 | Mean Difference (IV, Random, 95% CI) | ‐107.88 [‐158.53, ‐57.23] |
| 5 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 5.1 Intravenous administration | 3 | 339 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.49, 1.03] |
| 5.2 Intramuscular administration | 4 | 481 | Mean Difference (IV, Random, 95% CI) | 0.79 [0.56, 1.03] |
3.1. Analysis.
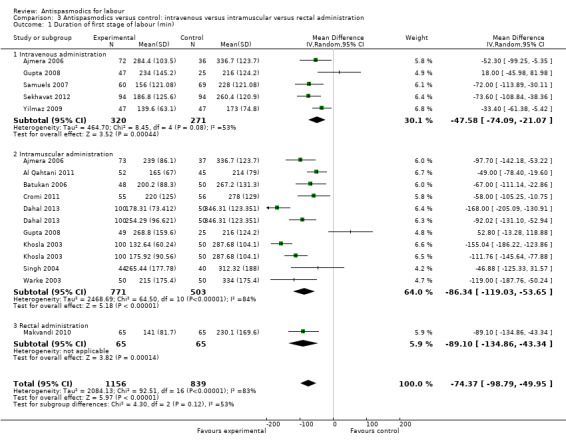
Comparison 3 Antispasmodics versus control: intravenous versus intramuscular versus rectal administration, Outcome 1 Duration of first stage of labour (min).
3.2. Analysis.
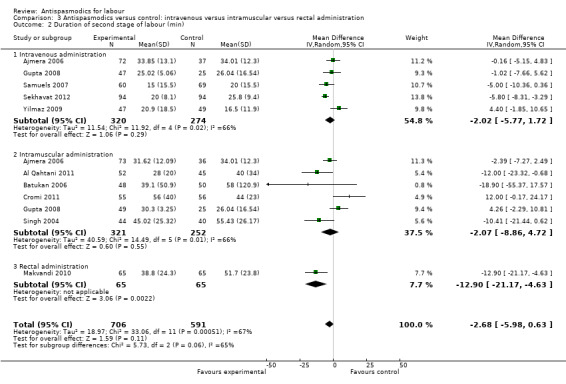
Comparison 3 Antispasmodics versus control: intravenous versus intramuscular versus rectal administration, Outcome 2 Duration of second stage of labour (min).
3.3. Analysis.
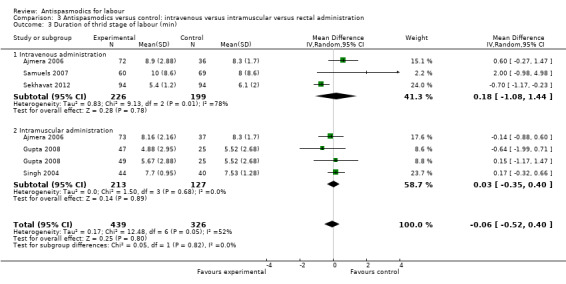
Comparison 3 Antispasmodics versus control: intravenous versus intramuscular versus rectal administration, Outcome 3 Duration of thrid stage of labour (min).
3.5. Analysis.
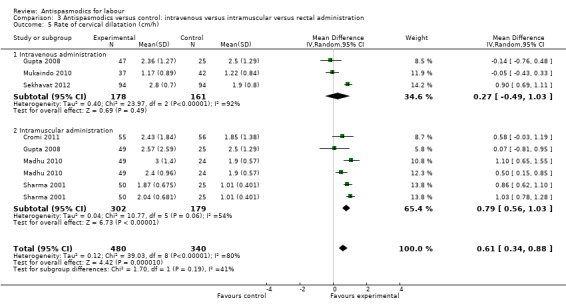
Comparison 3 Antispasmodics versus control: intravenous versus intramuscular versus rectal administration, Outcome 5 Rate of cervical dilatation (cm/h).
Comparison 4. Antispasmodics versus control: primigravidas versus primi‐and multigravidas.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.37 [‐98.79, ‐49.95] |
| 1.1 Primigravidas | 6 | 616 | Mean Difference (IV, Random, 95% CI) | ‐58.39 [‐80.74, ‐36.04] |
| 1.2 Primi‐ and multigravidas | 6 | 1191 | Mean Difference (IV, Random, 95% CI) | ‐79.18 [‐115.74, ‐42.62] |
| 1.3 Mulitgravidas | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐73.60 [‐108.84, ‐38.36] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.99, 0.59] |
| 2.1 Primigravidas | 5 | 518 | Mean Difference (IV, Random, 95% CI) | ‐3.81 [‐13.41, 5.79] |
| 2.2 Primi‐ and multigravidas | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐4.01, 1.32] |
| 2.3 Multigravidas | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐5.80 [‐8.31, ‐3.29] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Primigravidas | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.32, 0.66] |
| 3.2 Primi‐and multigravidas | 3 | 493 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.40, 0.63] |
| 3.3 Multigravidas | 1 | 188 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.17, ‐0.23] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Primigravidas | 5 | 522 | Mean Difference (IV, Random, 95% CI) | ‐102.50 [‐162.73, ‐42.26] |
| 4.2 Primi‐ and multigravidas | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐56.58 [‐78.38, ‐34.78] |
| 5 Rate of cervical dilatation (cm/h) | 5 | 632 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.22, 0.87] |
| 5.1 Primigravidas | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 0.63 [0.17, 1.08] |
| 5.2 Primi‐and multigravidas | 2 | 292 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.08, 0.97] |
4.1. Analysis.
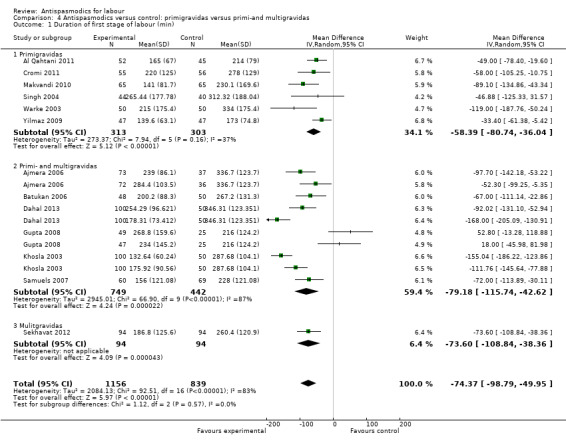
Comparison 4 Antispasmodics versus control: primigravidas versus primi‐and multigravidas, Outcome 1 Duration of first stage of labour (min).
4.2. Analysis.
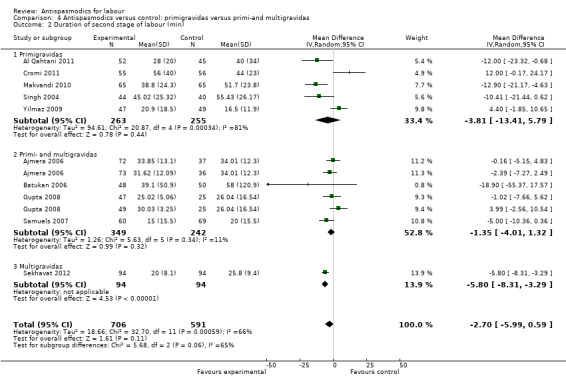
Comparison 4 Antispasmodics versus control: primigravidas versus primi‐and multigravidas, Outcome 2 Duration of second stage of labour (min).
4.4. Analysis.

Comparison 4 Antispasmodics versus control: primigravidas versus primi‐and multigravidas, Outcome 4 Total duration of labour (min).
4.5. Analysis.

Comparison 4 Antispasmodics versus control: primigravidas versus primi‐and multigravidas, Outcome 5 Rate of cervical dilatation (cm/h).
Comparison 5. Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1885 | Mean Difference (IV, Random, 95% CI) | ‐72.76 [‐98.57, ‐46.95] |
| 1.1 Spontaneous labour | 9 | 1247 | Mean Difference (IV, Random, 95% CI) | ‐84.09 [‐109.60, ‐58.58] |
| 1.2 Spontaneous and induced/induced only | 4 | 638 | Mean Difference (IV, Random, 95% CI) | ‐51.87 [‐109.47, 5.74] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Spontaneous labour | 7 | 957 | Mean Difference (IV, Random, 95% CI) | ‐4.59 [‐8.13, ‐1.05] |
| 2.2 Spontaneous and induced/induced only | 3 | 340 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐1.34, 6.16] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Spontaneous labour | 4 | 619 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.57, 0.57] |
| 3.2 Spontaneous and induced/induced only | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.18, 0.71] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Spontaneous labour | 6 | 701 | Mean Difference (IV, Random, 95% CI) | ‐93.77 [‐134.30, ‐53.24] |
| 4.2 Spontaneous and induced/induced only | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐31.60 [‐62.58, ‐0.62] |
| 5 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 5.1 Spontaneous labour | 5 | 674 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.46, 0.98] |
| 5.2 Spontaneous and induced/induced only | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.58, 0.44] |
5.1. Analysis.
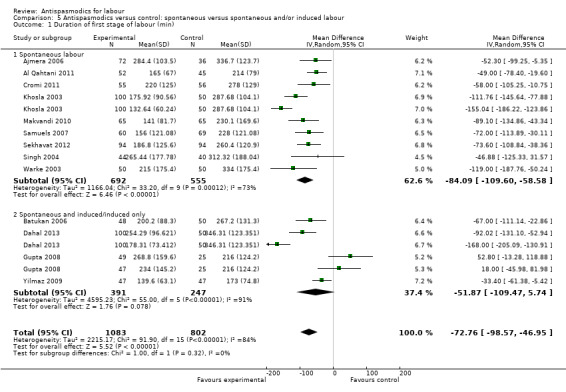
Comparison 5 Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour, Outcome 1 Duration of first stage of labour (min).
5.3. Analysis.
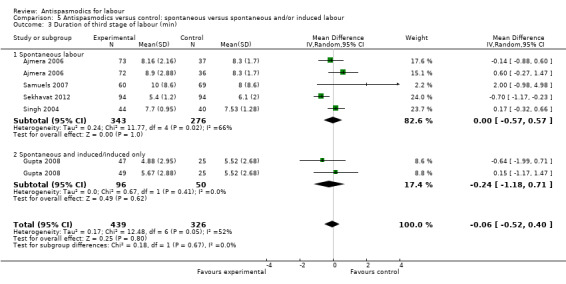
Comparison 5 Antispasmodics versus control: spontaneous versus spontaneous and/or induced labour, Outcome 3 Duration of third stage of labour (min).
Comparison 6. Antispasmodics versus control: active versus expectant management of labour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.38 [‐98.80, ‐49.96] |
| 1.1 Active management | 10 | 1377 | Mean Difference (IV, Random, 95% CI) | ‐60.44 [‐88.41, ‐32.47] |
| 1.2 Expectant management | 3 | 618 | Mean Difference (IV, Random, 95% CI) | ‐108.81 [‐144.05, ‐73.56] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Active management | 9 | 1079 | Mean Difference (IV, Random, 95% CI) | ‐3.12 [‐7.34, 1.10] |
| 2.2 Expectant management | 1 | 218 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐4.79, 2.19] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Active management | 4 | 547 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.78, 0.44] |
| 3.2 Expectant management | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.53, 0.92] |
| 4 Total duration of labour (min) | 7 | 797 | Mean Difference (IV, Random, 95% CI) | ‐85.51 [‐121.81, ‐49.20] |
| 4.1 Active management | 5 | 547 | Mean Difference (IV, Random, 95% CI) | ‐49.69 [‐65.17, ‐34.21] |
| 4.2 Expectant management | 2 | 250 | Mean Difference (IV, Random, 95% CI) | ‐172.91 [‐238.73, ‐107.09] |
| 5 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 5.1 Active management | 5 | 670 | Mean Difference (IV, Random, 95% CI) | 0.47 [0.10, 0.83] |
| 5.2 Expectant management | 1 | 150 | Mean Difference (IV, Random, 95% CI) | 0.94 [0.77, 1.12] |
6.2. Analysis.
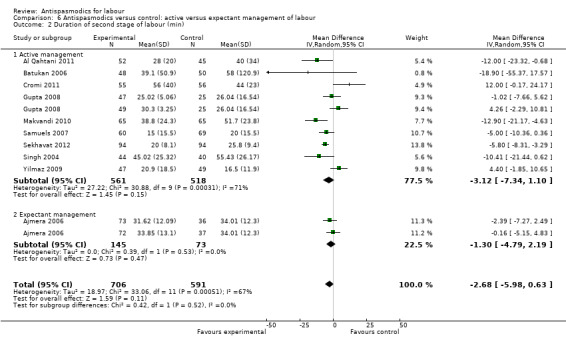
Comparison 6 Antispasmodics versus control: active versus expectant management of labour, Outcome 2 Duration of second stage of labour (min).
6.3. Analysis.
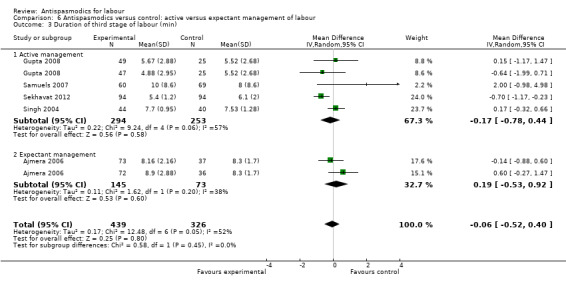
Comparison 6 Antispasmodics versus control: active versus expectant management of labour, Outcome 3 Duration of third stage of labour (min).
Comparison 7. Antispasmodics versus control: low‐risk versus high‐risk pregnancies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 13 | 1995 | Mean Difference (IV, Random, 95% CI) | ‐74.37 [‐98.79, ‐49.95] |
| 1.1 Low risk | 12 | 1849 | Mean Difference (IV, Random, 95% CI) | ‐86.31 [‐108.94, ‐63.68] |
| 1.2 High risk | 1 | 146 | Mean Difference (IV, Random, 95% CI) | 34.84 [‐11.13, 80.80] |
| 2 Duration of second stage of labour (min) | 10 | 1297 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐5.98, 0.63] |
| 2.1 Low risk | 9 | 1151 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐7.30, ‐0.03] |
| 2.2 High risk | 1 | 146 | Mean Difference (IV, Random, 95% CI) | 1.65 [‐3.52, 6.83] |
| 3 Duration of third stage of labour (min) | 5 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.40] |
| 3.1 Low risk | 4 | 619 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.57, 0.57] |
| 3.2 High risk | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.18, 0.71] |
| 4 Rate of cervical dilatation (cm/h) | 6 | 820 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.34, 0.88] |
| 4.1 Low risk | 5 | 674 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.46, 0.98] |
| 4.2 High risk | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.58, 0.44] |
7.2. Analysis.
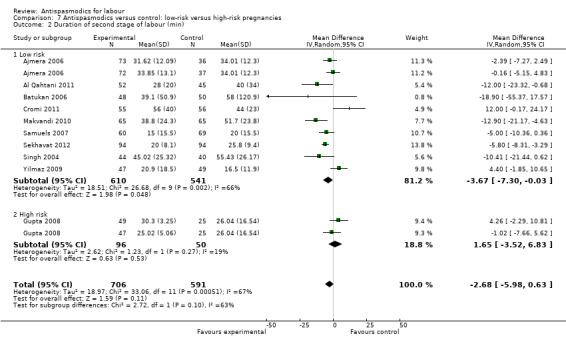
Comparison 7 Antispasmodics versus control: low‐risk versus high‐risk pregnancies, Outcome 2 Duration of second stage of labour (min).
7.3. Analysis.
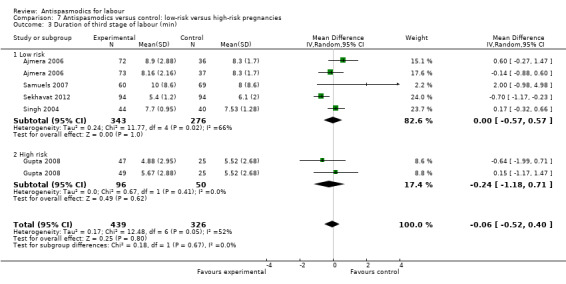
Comparison 7 Antispasmodics versus control: low‐risk versus high‐risk pregnancies, Outcome 3 Duration of third stage of labour (min).
Comparison 8. Antispasmodics versus control: sensitivity analysis: studies with high risk of bias excluded.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first stage of labour (min) | 3 | 334 | Mean Difference (IV, Fixed, 95% CI) | ‐47.78 [‐68.66, ‐26.91] |
| 2 Duration of second stage of labour (min) | 3 | 336 | Mean Difference (IV, Random, 95% CI) | 2.64 [‐6.34, 11.61] |
| 3 Duration of third stage of labour (min) | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.98, 4.98] |
| 4 Total duration of labour (min) | 3 | 304 | Mean Difference (IV, Random, 95% CI) | ‐44.79 [‐80.19, ‐9.39] |
| 5 Rate of cervical dilatation (cm/h) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.39, 0.83] |
| 6 Rate of normal vertex deliveries | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
Comparison 9. Antispasmodics versus control: maternal adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tachycardia | 6 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.54 [2.53, 8.16] |
| 1.1 Neurotropic agents | 6 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.60 [3.54, 16.29] |
| 1.2 Musculotropic agents | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.19, 1.90] |
| 2 Mouth dryness | 6 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.81 [2.71, 17.12] |
| 2.1 Neurotropic agents | 5 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.56 [2.48, 17.37] |
| 2.2 Musculotropic agents | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.50, 162.89] |
| 3 Headache | 3 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.56, 4.10] |
| 3.1 Neurotropic agents | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.15, 2.93] |
| 3.2 Musculotropic agents | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.63, 12.28] |
| 4 Nausea | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.74, 5.28] |
| 4.1 Neurotropic agents | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.50, 4.28] |
| 4.2 Musculotropic agents | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 5 Vomiting | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.64, 7.62] |
| 5.1 Neurotropic agents | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.33, 5.88] |
| 5.2 Musculotropic agents | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 6 Dizziness | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.66, 14.73] |
| 7 Giddiness | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.16] |
| 7.1 Neurotropic agents | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.67] |
| 7.2 Musculotropic agents | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.13] |
| 8 Cervical laceration | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.20, 3.12] |
| 8.1 Neurotropic agents | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 74.85] |
| 8.2 Musculotropic agents | 2 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.68] |
| 9 Flushing of face | 4 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.48 [1.98, 36.35] |
| 9.1 Neurotropic agents | 4 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.48 [1.98, 36.35] |
| 10 Postpartum haemorrhage | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.20, 30.17] |
| 10.1 Neurotropic agents | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.13, 4.26] |
| 10.2 Musculotropic agents | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.18, 68.42] |
Comparison 10. Antispasmodics versus control: neonatal adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission to NICU | 5 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.34, 2.05] |
| 1.1 Neurotropic agents | 4 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.27, 3.25] |
| 1.2 Musculotropic agents | 3 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.20, 2.66] |
| 2 Fetal distress | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] |
| 3 Fetal bradycardia | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.86] |
| 4 Fetal tachycardia | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.85, 13.67] |
| 4.1 Neurotropic agents | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.85, 13.67] |
| 5 Meconium‐stained liquor | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.54, 7.73] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ajmera 2006.
| Methods | Study design: randomised controlled trial. Allocation generation: random allocation. Allocation concealment: not done. Blinding: open trial. Loss to follow‐up: intervention: 3.3% control: 2.6%. |
|
| Participants | Total number of participants randomised: 225. Inclusion criteria: primi‐ and multigravidas. Gestational age 37‐41 weeks, singleton pregnancy, cephalic presentation, spontaneous labour, no obstetric or medical complications. Exclusion criteria: none specified. |
|
| Interventions | Intervention: 1. Drotaverine hydrochloride 40 mg IMI, administered in active phase at 3 cm dilatation and repeated every 2 h for max 3 doses. n = 75. 2. Valethamate bromide 8 mg IVI, administered in active phase, at 3 cm dilatation, repeated every half an hour for a max of 3 doses. n = 75. Control: No medication. n = 75. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (from 3 cm to full dilatation of cervix) 2. Duration of second and third stage of labour. 3. Mode of delivery. Secondary outcomes: Neonatal and maternal adverse events. |
|
| Notes | Ethics: ethical approval not mentioned, informed consent not mentioned. Location: India, Mumbai. No contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation ‐ not clearly described. |
| Allocation concealment (selection bias) | High risk | This is an open trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an open trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This is an open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, all participants accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes: 1. Duration of labour: adequately reported. 2. Mode of delivery: adequately reported. Secondary outcomes: 1. Maternal adverse events: adequately reported. 2. Neonatal adverse events: Apgar scores not reported. |
| Other bias | High risk | Authors did not mention spontaneous or artificial rupture of membranes, the use of other drugs to augment labour or analgesia. All these are known to influence the duration of labour and could have biased results. No contact details are present. Drug company sponsorship: not mentioned. |
Al Matari 2007.
| Methods | Study design: randomised controlled trial. Allocation generation: randomised (no details present). Allocation concealment:not described. Blinding: double‐blind (no details present). Loss to follow‐up: intervention: 3%. Control: 3%. |
|
| Participants | Total number of participants randomised:199. Inclusion criteria:patients at term with singleton pregnancy, cephalic presentation. Exclusion criteria: contraindication for vaginal delivery, previous caesarean section. |
|
| Interventions | Intervention: Buscopan Hyoscine‐N‐Butylbromide (no details about dosage). n = 99. Control: placebo (no details present). n = 100. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (from intervention to full dilatation). 2. Duration of first and second stage of labour (from intervention to delivery of baby). Secondary outcomes: 1. Apgar scores at 1 and 5 minutes. |
|
| Notes | Ethics: not mentioned. Location: Saudi Arabia. This study was only published as an abstract at the British International Congress of Obstetrics and Gynaecology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in detail, only mentioned the word "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in detail, only mentioned "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in detail, only mentioned "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether 3 participants who did not deliver after 4 hours were excluded from the analysis or not. not known how many participants were in each group. |
| Selective reporting (reporting bias) | High risk | No prespecified outcomes in the methods section. |
| Other bias | Unclear risk | This is only a conference abstract and lacks a lot of information. it is therefore difficult to judge whether any other biases are present. Drug company sponsorship: not mentioned. |
Al Qahtani 2011.
| Methods | Study design: randomised controlled trial. Allocation generation: cards with either "HBB" or "placebo" written on them placed in sealed envelopes. Envelopes were placed in a box, mixed and drawn by the nurse in charge. Allocation concealment: opaque sealed envelopes containing cards with either "HBB" or "placebo" written on them mixed in a box and drawn by the nurse in charge when the patient consented for participation in the study. Blinding: patients, nurses and physicians unaware of contents of syringe. Nurse in charge prepared syringe according to card in envelope. HBB and saline are both colourless and the contents of the syringes could thus not be established. Loss to follow‐up: intervention: 10%. Control: 13%. |
|
| Participants | Total number of participants randomised: 110. Inclusion criteria: singleton pregnancy, vertex presentation at term, no chronic or pregnancy‐induced illnesses, no contraindications to vaginal delivery, all in established, spontaneous labour with either intact or spontaneous rupture of membranes for less than 12 hours. Exclusion criteria: previous uterine scarring, malpresentation, antepartum haemorrhage, multiparity, twin pregnancy, induced delivery, any medical disease, oxytocin induction, prolonged premature rupture of membranes (more than 12 hours), epidural analgesia. |
|
| Interventions | Intervention: Buscopan Hyoscine Butylbromide 40 mg (2 mL) IMI; administered at 3‐4 cm cervical dilatation (once‐off dose) n = 58 (randomised) n = 52 (analysed). Control: Placebo (normal saline) 2 mL IMI; administered at 3‐4 cm cervical dilatation (once‐off dose) n = 52 (randomised) n = 45 (analysed). |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (from 4 cm cervical dilatation to full dilatation). 2. Duration of first and second stage of labour (from 4 cm cervical dilatation to delivery of baby). 3. Duration of second and third stage of labour. Secondary outcomes: 1. Postpartum haemorrhage. 2. Rate of caesarean sections. 3. Apgar score. |
|
| Notes | Ethics: informed consent signed by participants before randomisation, study approved by Ethical committee of the University of Dammam. Location: Saudi Arabia. Authors contacted to clarify number of participants randomised to each group and to clarify the measurement of duration of labour in case of a caesarean section. Authors responded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing envelopes containing cards either placebo or HBB written on them from a box |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes – mixed in box and drawn by nurse in charge once patient had signed consent. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurse in charge prepared syringes containing either placebo or HBB, which are both colourless fluids. She then attached the card from the envelope to the participants file after delivery. Participants, physicians and attending nurses were thus blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Principal investigator collected the raw data sheets from the labour rooms and was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear in study report whether 13 participants not included in the analysis were randomised to a group before being excluded. Author confirmed that seven of these received placebo and six received HBB and were excluded from analysis due to augmentation with oxytocin which indicates that there was attrition of 10% in the intervention group and 13% in the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol of the study found, but all outcomes prespecified in methods section addressed. |
| Other bias | High risk | Yes – 44% (23/52) participants in HBB group had spontaneous ROM at baseline, compared with 22% (10/42) in the placebo group – this is a statistically significant difference (P = 0.0039) which can influence the duration of labour |
Azari 2010.
| Methods | Study design: randomised controlled trial. Allocation generation: random division of patients. No details about method of sequence generation. Allocation concealment:not described. Blinding:double‐blind ‐ no details present. Loss to follow‐up: not mentioned. |
|
| Participants | Total number of participants randomised: 200. Inclusion criteria: primiparous women in labour. Exclusion criteria: not specified. |
|
| Interventions | Intervention: 20 mg Hyoscine and Atropine infusion. n = not mentioned. Control: 2 mL Dextrose water infusion. n = not mentioned. |
|
| Outcomes | Primary outcomes: 1. Cervical dilatation rate and effacement of cervix. 2. Duration of active phase of labour. 3. Duration of second stage of labour. 4. Duration of third stage of labour. Secondary outcomes: 1. Fetal heart rate. 2. Apgar scores at 1 and 5 minutes. |
|
| Notes | Ethics: not mentioned. Location: Iran. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in detail, only mentioned that the trial was "double‐blind". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in detail, only mentioned that the trial was "double‐blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, only total number of participants at beginning of study is present. |
| Selective reporting (reporting bias) | High risk | Outcomes prespecified in the methods section were not addressed in the results section (3rd stage of labour, Apgar scores). |
| Other bias | Unclear risk | This study is only published as a congress abstract and it is thus very difficult to judge whether it is free of other bias. Drug company sponsorship: not mentioned. |
Batukan 2006.
| Methods | Study design: randomised controlled trial. Allocation generation: not specified. Allocation concealment: done by sealed envelope technique. Blinding: doctors and observers were blinded. Loss to follow‐up: 2% had caesarean sections. |
|
| Participants | Total number of participants randomised: 100. Inclusion criteria: women with gestational age 38‐41 weeks in spontaneous labour or with induction of labour, vertex presentation, intact membranes. Exclusion criteria: cervical dilatation of more than 5 cm, placenta previa, spontaneous rupture of membranes at randomisation, intrauterine growth restriction, women with oligohydramnios, antepartum haemorrhage, pre‐eclampsia, hypertension, diabetes mellitus, fetal macrosomia (> 4000 g), cephalo‐pelvic disproportion, previous caesarean section, grande multipara. |
|
| Interventions | Intervention: Valethamate bromide 8 mg/mL IMI at the beginning of active phase of labour (4‐5cm cervical dilatation) at hourly intervals for a total of 3 doses. n = 49. Control: NaCl 0.9% 1 mL IMI at the beginning of active phase of labour (4‐5 cm cervical dilatation) at hourly intervals for a total of 3 doses. n = 51. |
|
| Outcomes | Primary outcomes: 1. Time elapsed to second stage of labour (from intervention to full cervical dilatation). 2. Duration of second stage of labour. 3. Need for augmentation with oxytocin. Secondary outcomes: Subjective impression of physician about efficacy of intervention and the associated adverse effects. |
|
| Notes | Ethics: ethical approval from institutional ethics committee, informed consent obtained from all patients. Location: Turkey. Study published in Turkish, with English abstract. Translator consulted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope technique. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Doctors blinded, not clear if participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, all participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes: 1. Time elapsed to second stage of labour: adequately reported. 2. Duration of second stage of labour: adequately reported. 3. Need for augmentation with oxytocin: adequately reported. Secondary outcomes: Subjective impression of physician about efficacy of intervention and the associated adverse effects: adequately reported. |
| Other bias | Unclear risk | Difficult to judge due to foreign language. Drug company sponsorship: unclear. |
Cromi 2011.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated randomisation sequence. Allocation concealment: independent resident was responsible for records of group allocation and drawing up of injectable, Rociverine and saline have similar appearances. Randomisation list was kept in a secure box in the labour ward. Injectable was drawn up by resident in a separate pharmacy room in the labour ward. Blinding: midwives, clinicians and patients were blinded. Loss to follow‐up: 8.3% in intervention group, 6.7% in placebo group. |
|
| Participants | Total number of participants randomised:120. Inclusion criteria: primiparous women with gestational age of more than 37 weeks, in spontaneous labour, with cervical dilatation between 3 and 5 cm, live fetus, singleton pregnancy with cephalic presentation. Exclusion criteria: contraindications to vaginal delivery, induced labour, twin pregnancies, gestational age < 37 weeks, non‐Italian speaking, labour diagnosed > 5 cm cervical dilatation. |
|
| Interventions | Intervention: Rociverine 20 mg (2 mL) IMI; administered between 3‐5 cm cervical dilatation. n = 60. Control: NaCl 0.9% 2 mL IMI; administered between 3‐5 cm cervical dilatation. n = 60. |
|
| Outcomes | Primary outcomes: 1. Cervical dilatation rate. Secondary outcomes: 1. Injection‐full dilatation time. 2. Oxytocin augmentation. 3. Length of second stage. 4. Operative delivery. 5. Epidural rate. 6. Blood loss. 7. Arterial cord blood pH. 8. 5‐minute Apgar scores. 9. Maternal side effects. |
|
| Notes | Ethics: written informed consent obtained from all participants, study approved by Institutional Review Board. Location: Italy. Authors contacted regarding outcomes and allocation procedure, responded to clarify. Authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Records of group allocation were maintained by an independent resident physician responsible for randomisation and drawing up of injectable and kept in a secure box in the labour war. Rociverine and saline have identical appearances. The resident drew up the injectable in a separate pharmacy room in the labour ward. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, midwives and clinicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Labour and delivery clinicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported on. |
| Other bias | Low risk | No other sources of bias identified. |
Dahal 2013.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated randomisation. Allocation concealment: not described. Blinding: not described whether participants, physicians or outcome assessors were blinded. The authors only state that this was a "single‐blind" RCT. Loss to follow‐up: not clearly described. |
|
| Participants | Total number of participants randomised: 300. Inclusion criteria: Women with term pregnancies in early active stage of labour (2‐3 contractions per 10 minutes lasting for 30 seconds), induced and spontaneous labour, vertex presentation. Exclusion criteria: Previous uterine scar, malpresentation, multiple pregnancies, antepartum haemorrhage, cephalopelvic disproportion, pre‐eclampsia and other hypertensive disorders, glaucoma, heart disease. |
|
| Interventions | Intervention: 1. Valethamate bromide 8mg, IMI every half hour for three doses. n = 100. 2. Drotaverine hydrochloride 40mg, IMI every two hours for a maximum of three injections. n =100. Control: No treatment. n = 100. |
|
| Outcomes | Primary outcomes: 1. Duration of active phase of labour. 2. Duration of second stage of labour. 3. Duration of third stage of labour. 4. Total duration of labour (injection to delivery time). Secondary outcomes: 1. Blood loss. 2. Adverse events. |
|
| Notes | Ethics: Informed consent obtained from all participants, study approved by BPKIHS ethical committee. Location: Nepal. Authors contacted to clarify who was blinded; and to request missing data of control group. Authors did not report whether any conflict of interest was present. Table 2: Missing in PDF (cervical dilatation at the start of the study). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No method of concealing allocation described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but both participants and personnel were aware which group they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. Authors describe study as being "single‐blind". Not clear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear because the number of participants analysed was not reported with the results. |
| Selective reporting (reporting bias) | High risk | Control group results not always reported. Primary and secondary outcomes not clearly pre‐specified. All results of pre‐specified outcomes not reported. |
| Other bias | High risk | Data for the "duration of active phase of labour" and "duration of labour until end of first stage" are not the same. It is not clear how authors distinguish between these two outcomes. No sample size calculation present. Characteristics of participants in all groups only partly described. |
Gupta 2008.
| Methods | Study design: randomised controlled trial. Allocation generation: participants randomised by simple randomisation. Allocation concealment: not described. Blinding: no blinding. Loss to follow‐up: Intervention: 4%. Control: 0%. |
|
| Participants | Total number of participants randomised: 150. Inclusion criteria:primi‐ and multigravidas at term, singleton pregnancy, cephalic presentation. High‐risk pregnancies were included. Hypertensive disorders, gestational diabetes, portal hypertension, tuberculosis, idiopathic thrombocytopenia, intra‐hepatic cholestasis, anaemia, IUGR and oligohydramnios. Exclusion criteria: preterm gestation, multiple pregnancy, CPD, non‐vertex presentation. |
|
| Interventions | Intervention: 1. Drotaverine hydrochloride 40 mg (2 mL) IMI in active labour at 3 cm dilatation, repeated every 2 h n = 50. 2. Hyoscine Butyl bromide 20 mg, (1 mL) IVI in active labour at 3 cm dilatation, repeated every 20 min n = 50. Control: No medication. n = 50. |
|
| Outcomes | Primary outcomes: 1. Duration of active phase of labour (3 cm to full cervical dilatation). 2. Rate of cervical dilatation (cm/h). 3. Duration of second stage of labour. Secondary outcomes: 1. Duration of third stage. 2. Mode of delivery. 3. Complications. |
|
| Notes | Location: India. Ethics: informed consent obtained, ethical approval not mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation, method not explicitly described. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, no missing data. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes: 1. Duration of active phase of labour ‐ adequately reported. 2. Rate of cervical dilatation ‐ adequately reported. 3. Duration of second stage ‐ adequately reported. Secondary outcomes: 1. Mode of delivery ‐ adequately reported. 2. Complications ‐ adequately reported. 3. Duration of 3rd stage ‐ adequately reported. |
| Other bias | Unclear risk | Medications were given via different routes and no placebo was used, the control group did not receive any medication Cervical dilatation was not the same at starting point in all the groups ‐ although it was shown not to be statistically significant (P value: 0.5). Drug company sponsorship: not mentioned. |
Khosla 2003.
| Methods | Study design: randomised controlled trial. Allocation generation: participants were randomly assigned (no details present). Allocation concealment: not mentioned. Blinding: not mentioned. Loss to follow‐up: not mentioned. |
|
| Participants | Total number of participants randomised: 300. Inclusion criteria: women with normal, singleton pregnancy at 38‐41 gestational age with vertex presentation, intact membranes and spontaneous onset of labour. Exclusion criteria: women with previous uterine scar, cephalopelvic disproportion, grand multiparity, antepartum haemorrhage, twin pregnancy. |
|
| Interventions | Intervention: 1. Valethamate bromide (Epidosin): 8 mg every half hour for 3 doses, intramuscular administration at 4 cm dilatation. n = 100. 2. Drotaverine hydrochloride (Drotin): 40 mg every 2 hours for a maximum of 3 doses, intramuscular administration at 4 cm dilatation. n = 100. Control: no medication. n = 100. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (not defined). 2. Rate of cervical dilatation. 3. Duration of second stage of labour. 4. Duration of third stage of labour. Secondary outcomes: 1. Maternal complications. 2. Neonatal condition at birth. |
|
| Notes | Ethics: not mentioned. Location: India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | High risk | Outcomes not prespecified in methods section. |
| Other bias | Unclear risk | Study was poorly reported. It is therefore difficult to judge whether it is free of other bias. Drug company sponsorship: not mentioned. |
Kuruvila 1992.
| Methods | Study design: randomised controlled trial. Allocation generation: random number tables used to number the batches of ampoules. Allocation concealment: identical ampoules containing either 1 mL of Valethamate bromide or 1 mL saline were prepared in batches of 3 and packed in 2 sets of 60X3. Each batch was sequentially numbered. Blinding: coding was only broken on completion of the study. Loss to follow‐up: Intervention: 8%. Control: 9%. |
|
| Participants | Total number of participants randomised: 120. Inclusion criteria: low‐risk pregnancy at term, vertex presentation, cervical dilatation 2‐4 cm on initial examination, no clinical suspicion of cephalopelvic disproportion. Exclusion criteria: not specified. |
|
| Interventions | Intervention: Valethamate bromide 8 mg (1 mL) IM at amniotomy, after 30 min and after 60 min. n = 62. Control: 1 mL normal saline IM at amniotomy, after 30 min and after 60 min. n = 58. |
|
| Outcomes | No primary outcomes specified, objective is to assess the usefulness of valethamate bromide in acceleration of labour. Mean rate of cervical dilatation was reported as an outcome regarding this objective. | |
| Notes | Ethics: Informed consent was signed by participants. Authors did not mention approval by ethics committee. Location: India. Contact authors regarding outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table of numbers. |
| Allocation concealment (selection bias) | Low risk | Identical ampoules, sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not explicitly stated, but the coding of the active ingredient was only broken after completion of the analyses. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not explicitly stated, but the coding of the active ingredient was only broken after completion of the analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | High risk | No outcomes prespecified in the methods section and no outcomes were explicitly reported on. |
| Other bias | Unclear risk | All primigravidae received pethidine, which could affect the outcomes. They did not mention that any multigravidas received analgesia, which is questionable. The outcomes are very poorly reported and it is difficult to judge whether there are any other biases. Drug company sponsorship: TKK Pharma limited sponsored drug and placebo, unclear whether there is conflict of interest. |
Madhu 2010.
| Methods | Study design: randomised controlled trial. Allocation generation: randomisation. Allocation concealment: sealed envelope technique Blinding: not described. Loss to follow‐up: Intervention: 2%. Control: 4%. |
|
| Participants | Total number of participants randomised: 150. Inclusion criteria: primi‐ and multigravidas, gestational age 37‐40 weeks, with low risk pregnancy, in spontaneous labour, cephalic presentation. Exclusion criteria: malpresentation, multiple pregnancy, intrauterine growth restriction, medical problems, previous caesarean section, antepartum haemorrhage, induction of labour, cervical dilatation of > 5 cm. |
|
| Interventions | Intervention: 1. Drotaverine hydrochloride 40 mg (2 mL) IMI at 4 cm dilation and every hour for a max of 3 doses n = 50. 2. Valethamate bromide 8 mg (1 mL) IMI at 4 cm dilation and every hour for a max of 3 doses. n = 50 Control: NaCl 0.9% 2 mL IMI at 4 cm dilation and every hour for a max of 3 doses. n = 50. |
|
| Outcomes | Primary outcomes: 1. Injection to delivery time. 2. Mean cervical dilatation rate (cm/h). 3. Duration of second stage. Secondary outcomes: Adverse maternal and neonatal events. |
|
| Notes | Ethics: ethics approval from medical research committee of the college. Location: India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by sealed envelope technique.Not described how random numbers were generated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope technique. Not clear how this was done and if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding due to lack of resources. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding due to lack of resources. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | High risk | Pre‐specified outcomes not reported: Duration of 2nd and 3rd stage of labour Apgar scores of newborns not reported No follow‐up report (mothers and babies were followed for 3 days and at day 10 postnatal). |
| Other bias | Low risk | The study appears to be free of other sources of bias. Confounding factors are not statistically significantly different between groups. Drug company sponsorship: no. |
Makvandi 2010.
| Methods | Study design: randomised controlled trial. Allocation generation: block randomisation. Allocation concealment: suppositories were prepared by a pharmaceutical technician who was not included in the trial. No details about packaging of suppositories. Blinding: patients and medical investigator were blinded. Loss to follow‐up: Intervention: 7.6% had caesarean sections. Control: 9.23% had caesarean sections. |
|
| Participants | Total number of participants randomised: 130. Inclusion criteria: primigravid women between 18 and 34 years of age with normal, singleton pregnancy, 37‐42 weeks gestational age, cephalic presentation and spontaneous onset of labour. Exclusion criteria: body mass index > 25, maternal tachycardia, antepartum haemorrhage, prolonged rupture of membranes, previous uterine scar, cephalopelvic disproportion, augmentation of labour with oxytocin, pre‐eclampsia, heart disease, any other serious medical conditions. |
|
| Interventions | Intervention: Hyoscine 20 mg suppository at beginning of active phase of labour (3‐4 cm cervical dilatation). n = 65. Control: Placebo suppository consisting of a suppocire AM‐15 (semi‐synthetic fatty acid glyceride) at beginning of active phase of labour. n = 65. |
|
| Outcomes | Primary outcomes: 1. Duration of active phase of labour (not defined). 2. Rate of cervical dilatation. 3. Duration of second stage of labour. Secondary outcomes: 1. Neonatal Apgar scores at 1 and 5 minutes after birth. 2. Fetal heart rate. 3. Maternal pulse rate. 4. Maternal blood pressure. |
|
| Notes | Ethics: study approved by Ethics Committee of Ahvaz Jundishapur University of Medical Sciences. Written consent obtained at antenatal visits. Location: Iran. Author contacted regarding randomisation and allocation concealment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation (blocks of 4) unclear what method of sequence generation was used. |
| Allocation concealment (selection bias) | Unclear risk | Random numbers were assigned to each package. They do not mention whether the packages were identical. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were unaware of the contents of the package, unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medical investigator was unaware of the contents of the packages. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | High risk | The primary outcome (pain relief), as specified in the protocol,was not at all addressed in the study report. |
| Other bias | Low risk | Unlikely that other biases are present. Drug company sponsorship: absent. No conflict of interest. |
Mukaindo 2010.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated random sequence of numbers. Allocation concealment: randomisation sequence was sequentially coded. The pharmacist, who was the only one with access to the code, prepared the syringes, which were only labelled with the randomisation number, accordingly and handed them over to the labour ward staff. Blinding: participants, labour ward staff and investigator were blinded. Loss to follow‐up: intervention: 8% were excluded from the analysis. Control: 7% were excluded from the analysis. |
|
| Participants | Total number of participants randomised: 85. Inclusion criteria: nulliparas above 18 years of age, at term, with singleton pregnancy, cephalic presentation in spontaneous labour and without contraindications to hyoscine butyl bromide. Exclusion criteria: multiparas, induced labour, preterm labour, contraindications to vaginal delivery or hyoscine butyl bromide, high‐risk pregnancies. |
|
| Interventions | Intervention: Hyoscine butyl bromide 40 mg (2 mL) IVI, administered between 3 and 6 cm cervical dilatation. n = 40. Placebo: Sterile water, 2 mL IVI, administered between 3 and 6 cm cervical dilatation. n = 45. |
|
| Outcomes | Primary outcomes: 1. Duration of labour (from diagnosis of active phase of labour to delivery). Secondary outcomes: 1. Rate of cervical dilatation (cm/h). 2. Maternal postpartum satisfaction scores. |
|
| Notes | Ethics: all participants required to sign informed consent. Study was approved by the ethics committee of the Aga Khan University Hospital. Location: Kenia. Author contacted regarding standard deviations of continuous measures, inclusion and exclusion criteria and flow diagram of study participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence of numbers. |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was sequentially coded. The pharmacist, who was the only one with access to the code, prepared the syringes, which were only labelled with the randomisation number, accordingly and handed them over to the labour ward staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and labour ward staff were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator was blinded until conclusion of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section reported on. |
| Other bias | Low risk | Unlikely that other bias is present. Funding: research grant, no funding received from any pharmaceutical company. |
Raghavan 2008.
| Methods | Study design: randomised controlled trial. Allocation generation: random allocation (not specified). Allocation concealment: not described. Blinding: "double blinded" but blinding not described. Loss to follow‐up: not mentioned. |
|
| Participants | Total number of participants randomised: 150. Inclusion criteria: primigravid women in spontaneous labour, at term, with singleton fetus. Exclusion criteria: cephalopelvic disproportion. |
|
| Interventions | Intervention: 1. Hyoscine butyl bromide suppository (dosage not specified). n = 50. 2. Valethamate bromide intravenous infusion (dosage not specified). n = 50. Control: No medication. n = 50. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour. 2. Duration of Total duration of labour. 3. Mode of delivery. Secondary outcomes: 1. Maternal adverse effects. 2. Complications of labour. 3. Neonatal adverse effects. |
|
| Notes | Ethics: study approved by Hospital ethics committee (Vani Villas Hospital, Bangalore). Location: India. This study was reported in a letter to the editor in response to the study by Samuels 2007. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specifically mentioned, but 1 group received intravenous medication, the other 1 suppositories, while the control group did not receive any medication. The patients and personnel could thus not have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | They only mention how many participants were enrolled at the beginning of the study. |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes were reported in the results. |
| Other bias | Unclear risk | This study was only reported in a letter to the editor and it is thus difficult to judge if other bias is present. Drug company sponsorship: unclear, not mentioned. |
Samuels 2007.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated random sequence of numbers. Allocation concealment: sequentially numbered syringes only PI knew correlation, which was only shown after analysis Blinding: participants, midwives and obstetricians were blinded. Loss to follow‐up: Intervention: 0% Control: 0%. |
|
| Participants | Total number of participants randomised: 129. Inclusion criteria: primi‐ and multigravidas, > 18 years old, at term, in established, spontaneous labour, with no pregnancy induced or chronic illness. Exclusion criteria: complicated pregnancies (not further specified). |
|
| Interventions | Intervention: Hyoscine butyl bromide 20 mg (1 mL) IVI, between 4‐5 cm dilatation. n = 60. Control: Placebo: NaCl 1 mL IVI, between 4‐5 cm dilatation. n = 69. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (time from intervention to full dilatation). Secondary outcomes: 1. Duration of second and third stages of labour. 2. Blood loss. 3. Rate of caesarean section. 4. Apgar scores. |
|
| Notes | Location: Jamaica. Other: standard deviations not reported. Author contacted. Could not provide standard deviations, hence they were imputed. Ethics: ethical approval obtained, informed consent obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence of numbers. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered syringes. Content of syringes was only known to PI during the study and was revealed after completion of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, no missing data. |
| Selective reporting (reporting bias) | Low risk | Primary outcome: duration of first stage of labour: adequately reported. Secondary outcomes: duration of 2nd and 3rd stages of labour, blood loss at delivery, rate of caesarean section, Apgar scores: all adequately reported 95% confidence intervals present. No standard deviations reported with the means. |
| Other bias | Low risk | No other sources of potential bias detected. Drug company sponsorship: no. |
Sekhavat 2012.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated random number list. Allocation concealment: not described. Blinding: Participants and caregivers/physicians not blind. Outcome assessors blind. Loss to follow‐up: Intervention: 0% Control: 0%. |
|
| Participants | Total number of participants randomised: 188. Inclusion criteria: Multigravidas only with normal, singleton pregnancy, gestational age 37‐42 weeks, vertex presentation, normal labour (spontaneous, presence of regular uterine contractions), active phase of labour (3‐4 cm cervical dilatation), intact membranes. Exclusion criteria: Chronic or pregnancy‐induced illnesses, contra‐indication to vaginal delivery, antepartum haemorrhage, multiple pregnancy, previous caesarean section, parity > 4. |
|
| Interventions | Intervention: Hyoscine butyl bromide 20 mg (1 mL) IVI, after admission to labour ward (at 3‐4 cm cervical dilatation). n = 94. Control: Placebo: NaCl 1 mL IVI, after admission to labour ward (at 3‐4 cm cervical dilatation). n = 94. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour. 2. Duration of second stage of labour. 3. Duration of third stage of labour. 4. Cervical dilatation rate. Secondary outcomes: 1. Delivery route. 2. Clinical side effects. 3. Neonatal Apgar score ar one and five minutes. |
|
| Notes | Location: Iran. Other: Authors did not address conflict of interest. Ethics: Ethical approval obtained by the ethics committee of Shadid Sadoughi University of Medical Sciences, Yazd, Iran, informed consent obtained from participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both participants and physicians were unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | High risk | Authors did not report on maternal adverse effects (prespecified in methods section). |
| Other bias | Unclear risk | Not clear what outcome authors used to calculate sample size. Study only included multiparous women. |
Sharma 2001.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated random numbers. Allocation concealment: not described. Blinding: not described. Loss to follow‐up: Intervention: 0%. Control: 0%. |
|
| Participants | Total number of participants randomised: 150. Inclusion criteria: primigravidae between 18‐30 years old, at term with healthy, singleton fetus, vertex presentation, intact membranes, in active phase of labour. Exclusion criteria: any medical, surgical or obstetric complications, like pre‐eclampsia, antepartum haemorrhage, induced labour, cervical dilatation of > 5 cm. |
|
| Interventions | Intervention: 1. Drotaverine hydrochloride 40 mg IMI at 4 cm dilatation and every 2 h for a max of 3 doses. n = 50. 2. Valethamate bromide 8 mg IMI at 4 cm dilatation and every hour for a max of 3 doses. n = 50 Control: No medication. n = 50. |
|
| Outcomes | Primary outcomes: 1. Duration of labour (injection to delivery time). Secondary outcomes: 1. Mode of delivery. 2. Side effects. 3. Neonatal outcome. |
|
| Notes | Ethics: study approved by institutional review board, written informed consent obtained. Location: India. Authors contacted: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. Placebo group did not receive any medication. It is thus assumed that no blinding was done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. Placebo group did not receive any medication. It is thus assumed that no blinding was done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, no missing data. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes: 1. Injection‐to‐delivery time: adequately reported. Secondary outcomes: 1. Mode of delivery: adequately reported. 2. Duration and complications of 3rd stage: adequately reported. 3. Apgar scoring: not adequately reported: "there was no statistically significant difference...although 2 newborns in group II and 4 in group III had fetal distress". |
| Other bias | High risk | All 150 patients in the study delivered vaginally. The study reports, that the hospital has a caesarean section rate of 12%. They claim that it was purely coincidental, but this raises questions about the selection of participants and introduction of selection bias. Drug company sponsorship: not mentioned. |
Singh 2004.
| Methods | Study design: randomised controlled trial. Allocation generation: identical paper slips with either drug or placebo written, on drawn from box, patient's serial number written on slip and kept in separate box. Allocation concealment: not clearly described. Blinding: patients and observers were blinded. Loss to follow‐up: 16% ‐ it is not specified to which group these participants were allocated to. |
|
| Participants | Total number of participants randomised:100. Inclusion criteria: primigravidae at term with singleton pregnancy, vertex presentation, established spontaneous labour, cervical dilatation of at least 3 cm. Exclusion criteria: any medical, surgical or obstetric complications. |
|
| Interventions | Intervention: Drotaverine hydrochloride 40 mg (2 mL) IMI in active labour, cervix at least 3 cm dilated. n = 50. Control: Distilled water 2 mL IMI in active labour, cervix at least 3 cm dilated.. n = 50. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage of labour (time of intervention to full cervical dilatation). 2. Pain. 3. Cervical dilatation rate. Secondary outcomes: 1. Maternal and neonatal outcome. 2. Side effects. |
|
| Notes | Ethics: approved by institutional ethical committee, informed consent obtained. Location: Delhi, India. Authors contacted: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Identical paper slips 50 with "drug" and 50 with "placebo" written on. A slip was drawn from the box and patient's serial no written on it, kept in separate box. |
| Allocation concealment (selection bias) | High risk | No allocation concealment took place. The nurse administering the intervention knew whether placebo or active ingredient was administered. The paper slips were not sequentially numbered, but explicitly stated "drug" or "placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant was blinded, nurse administering intervention was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 patients were enrolled, 16 were excluded before intervention was administered. 4 patients had caesarean section due to fetal distress, 5 patients opted out of the study and 7 had incomplete data. It is not mentioned to which group all these participants belonged to, which is inadequate. |
| Selective reporting (reporting bias) | Unclear risk | Duration of different stages of labour: adequately reported cervical dilatation rate: only reported in bar‐chart. The mean cervical dilatation rate of the intervention group is mentioned, but not of the control group. Pain assessment results are adequately reported It is not stated how many instrumental deliveries or caesarean section were done after the intervention. |
| Other bias | High risk | The starting point of the intervention was not the same in every participant. It varied from 3‐6 cm cervical dilatation. The number of participants in each group was not the same. In the group that received the intervention at 6 cm, there were 18 in the treatment group compared to 3 in the control group. The results presented are not statistically significant, but imbalances like that introduce bias. Drug company sponsorship: not mentioned. |
Taskin 1993.
| Methods | Study design: randomised controlled trial. Allocation generation: randomisation (not specified) Allocation concealment: not described. Blinding: patients and physicians were blinded. Not clear whether observers were blinded. Loss to follow‐up: 13% were excluded from the analysis. because they underwent caesarean section. Not specified to which group they belonged to. |
|
| Participants | Total number of participants randomised: 120. Inclusion criteria: term patients in labour with cervical dilatation of 5cm or less, with uncomplicated pregnancies. Exclusion criteria: patients with abnormal presentation, previous caesarean section, ruptured membranes, evidence of fetal or maternal compromise. |
|
| Interventions | Intervention: 1. Meperidine 50 mg intravenously at 4‐5 cm. n = 30. 2. Hyoscine bromide 20 mg intravenously at 4‐5 cm. n = 30. 3. Valethamate bromide 8 mg intravenously at 4‐5 cm. n = 30. Control: Placebo (not specified) intravenously at 4‐5 cm. n = 30. |
|
| Outcomes | Outcomes not specified. Outcome data on administration to delivery time is present. |
|
| Notes | Location: USA. Study was only published as an abstract and therefore minimal information is present. No contact details of authors are present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation ‐ not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians and participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No outcomes specified. |
| Selective reporting (reporting bias) | High risk | No details about participants. |
| Other bias | Unclear risk | Study only published as an abstract and therefore minimal information is present. Drug company sponsorship: not mentioned. |
Warke 2003.
| Methods | Study design: randomised controlled trial. Allocation generation: random sequence generation. Allocation concealment: not described. Blinding: not described. Loss to follow‐up: Intervention: 0%. Control: 0%. |
|
| Participants | Total number of participants randomised: 100. Inclusion criteria: primigravidas, gestational age 37‐40 weeks, between 18‐35 years old, in active phase of labour, singleton fetus, vertex presentation. Exclusion criteria: antenatal pregnancy complications like pre‐eclampsia, other medical disorders of pregnancy, CPD, premature rupture of membranes, induction or augmentation of labour. |
|
| Interventions | Intervention: Camylofin dihydrochloride 50 mg IMI in active phase of labour, at 3 cm dilatation. n = 50. Control: Placebo ‐ type of placebo and dose not specified. Given IMI in active phase of labour, at 3 cm dilatation. n = 50. |
|
| Outcomes | Primary outcomes: 1. Duration of active labour (time from onset of active labour (3 cm) to full cervical dilatation). 2. Rate of cervical dilatation (cm/h). 3. Effects on second and third stage of labour. Secondary outcomes: Side effects on mother and fetus. |
|
| Notes | Ethics: institutional ethics approval obtained, written, informed consent obtained. Location: India. Other: no contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation ‐ not adequately described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind trial." Not known who was blinded and if this was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind trial." Not known who was blinded and if this was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No numbers showing flow of participants. Percentages of modes of delivery do not add up, this could be a mistake or due to missing information. |
| Selective reporting (reporting bias) | Low risk | Time from onset of active labour to full cervical dilatation ‐ no SD reported with means. Rate of cervical dilatation ‐ no SD reported with means. Effects of treatment on second and third stage of labour ‐ no SD reported with means. Maternal side effects adequately reported. Neonatal side effects not specified. |
| Other bias | High risk | Tables are unclear and confusing. No contact details of the authors are present. Drug company sponsorship: no. |
Yilmaz 2009.
| Methods | Study design: randomised controlled trial. Allocation generation: computer‐generated random number. Allocation concealment: opaque, sealed envelopes that were consecutively numbered. Blinding: patients and physicians blinded. Loss to follow‐up: Intervention: 10%. Control: 9%. |
|
| Participants | Total number of participants randomised: 160. Inclusion criteria: primigravidae, 18‐30 years old, with gestational age 40+, healthy, with single fetus in vertex presentation, needing induction due to oligohydramnios, rupture of membranes or post‐term. Exclusion criteria: spontaneous labour, fetal weight > 4000 g, CPD, non‐reassuring CTG, allergy to meperidine or valethamate bromide, previous uterine surgery, active PV bleeding, placenta previa, use of analgesia prior to randomisation. |
|
| Interventions | Intervention: 1. Meperidine 50 mg (1 mL) in 9 mL NaCl IVI between 4 and 6 cm dilatation. n = 53. 2. Valethamate bromide 16 mg (2 mL) IVI in 8 mL of NaCl between 4 and 6 cm dilatation. n = 53. Control: NaCl 10 mL IVI between 4 and 6 cm dilatation. n = 54. |
|
| Outcomes | Primary outcomes: 1. Duration of first stage or labour (time from intervention to complete dilatation). 2. Duration of second stage. 3. Total duration of labour. Secondary outcomes: 1. Mode of delivery. 2. Cervical lacerations. 3. Adverse maternal and fetal events. |
|
| Notes | Ethics: approval by Human Research Review Committee Location: Turkey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed and consecutively numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded, nurses not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for, flow‐chart present. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported. Primary outcomes: time to complete dilation, duration of second stage of labour, labour duration ‐ no missing outcome data. Secondary outcomes: mode of delivery, presence of cervical lacerations and adverse maternal and neonatal events ‐ no missing outcome data. |
| Other bias | Low risk | No other potential bias detected. Drug company sponsorship: no. |
CPD: cephalopelvic disproportion CTG: cardiotocography h: hour(s) HBB: hyoscine butyl‐bromide IM: intramuscular IMI: intramuscular injection IVI: intravenous injection IUGR: intrauterine growth restriction NaCl: sodium chloride PI: principal investigator PV bleeding: vaginal bleeding RCT: randomised controlled trial ROM: rupture of membranes SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aggarwal 2008 | Study used consecutive randomisation and is thus not considered a truly randomised controlled trial. |
| Akleh 2010 | Study is not randomised. |
| Baracho 1982 | Study does not indicate that randomisation took place. |
| Bhattacharaya 1984 | Study is not randomised. |
| Chan 1963 | Study includes preterm labour. |
| De Nobrega‐Correa 2010 | Study compares hyoscine butyl‐bromide‐oxytocin and oxytocin. No control group. |
| Demory 1990 | All participants had cervical dystocia at randomisation. |
| Fouedjio 2012 | Authors used alternative randomisation and is thus not considered a truly randomised controlled trial. |
| Guerresi 1981 | No control group. |
| Hamann 1972 | No indication that randomisation took place. |
| Hao 2004 | Study compares Spasfon and Atropine ‐ no control group. |
| Kauppila 1970 | Study does not have control group. |
| Kaur 2003 | Study compares Drotaverine and Epidosin, no control group and no randomisation. |
| Kaur 2006 | Study comparing Anafortan and Epidosin, no control group and no randomisation. |
| Malensek 1985 | No randomisation. |
| Manpreet 2008 | No control group. |
| Maritati 1986 | Included women with post‐operative spasms. |
| Mishra 2002 | Study compares drotaverine and Epidosin, no control group and no randomisation present. |
| Mortazavi 2004 | Study is "semi‐experimental". |
| Rajkumar 2006 | Non‐randomised study. |
| Sirohiwal 2005 | Non‐randomised study. |
| Tabassum 2005 | Study included women with preterm deliveries (> 34 weeks' gestational age). |
| Tripti 2009 | Study compares drotaverine and valethamate bromide, no control group and no randomisation. |
| Von Hagen 1965 | Non‐randomised study. |
Characteristics of studies awaiting assessment [ordered by study ID]
Accinelli 1978.
| Methods | Participants were grouped according to a "randomized double‐blind scheme". The physician broke the code when he thought treatment was inadequate to decide on the next course of action. |
| Participants | Primiparae and multiparae, aged 17‐42, for whom normal labour was expected. |
| Interventions | Intervention: 1. DA 3177 (scopalamine‐N‐(cyclopropylmethyl) bromide). 2. Hyoscine‐N‐butyl bromide. 3. Placebo (not specified). |
| Outcomes | Duration of the period of dilatation. Duration of expulsive period. |
| Notes | Gestational age of participants not clear. |
Ahmed 1982.
| Methods | Double‐blind study. Sequence generation and allocation concealment not clear. |
| Participants | Primiparous women between 20 and 25 years old at term; no pregnancy related disease, 3 cm cervical dilatation, cephalic presentation. |
| Interventions | Intervention: 6 different types of antispasmodics. Control: placebo (NaCl). |
| Outcomes | The effect of antispasmodics on labour. |
| Notes | Not clear whether study is a RCT. Author contacted, awaiting reply. |
Georges 2007.
| Methods | Allocation generation: not known ("divided in two groups"). Allocation concealment: not described. Blinding: "double‐blind". Loss to follow‐up: not described. |
| Participants | Inclusion criteria: women in the labour ward. Exclusion criteria: not described. |
| Interventions | Intervention: Buscopan 20 mg IVI at 3 cm cervical dilatation. Controls: placebo (unknown) IVI. |
| Outcomes | Primary outcomes: 1. Duration of stages of labour. Secondary outcomes: 1. Maternal outcome. 2. Neonatal outcome. |
| Notes | Ethics: not mentioned. Location: Iraq. Notes: not clear whether this is a randomised study. Author contact details needed. |
Rajani 2011.
| Methods | Allocation generation: not known ("divided in.to 2 groups"). Allocation concealment: not described Blinding: not described. Loss to follow‐up: not described. |
| Participants | Inclusion criteria: women in labour with an unscarred uterus. Exclusion criteria: not described. |
| Interventions | Intervention: Valethamate bromide 1 mL IVI half hourly for a maximum of 3 doses starting at 3 cm cervical dilatation. Control: normal saline 1 mL, maximum of 3 doses half hourly. |
| Outcomes | Primary outcomes: 1. Duration of first stage of labour. 2. Duration of second stage of labour. 3. Duration of third stage of labour. Secondary outcomes: 1. Maternal side effects. 2. Fetal side effects. 3. Caesarean section rate. 4. Third stage bleeding. |
| Notes | Ethics: not mentioned. Location: India. Notes: not reported that participants were randomised. Trying to obtain author's contact details. |
Ranka 2002.
| Methods | Allocation generation: not described. Allocation concealment: not described. Blinding: not described. Loss to follow‐up: not described. |
| Participants | Inclusion criteria: primigravidae in labour. Exclusion criteria: not described. |
| Interventions | Intervention: Drotaverine hydrochloride. Control: no medication. |
| Outcomes | Progress and outcome of labour. |
| Notes | Ethics: not described. Location: India. Only reported as an abstract. Not clear whether this is a RCT. Trying to get author's contact details. |
Recto 1997.
| Methods | Allocation generation: "randomised" ‐ not clearly described. Allocation concealment: not described. Blinding: not described, but states "double‐blind". Loss to follow‐up: not described. |
| Participants | Primi‐ and multigravidas. No other information. |
| Interventions | Intervention: 10 mg of hyoscine‐N‐Butylbromide on admission and in 4‐hourly intervals until full cervical dilatation. Admission route not specified. Control: placebo ‐ not specified. |
| Outcomes | Primary outcomes: 1. Duration of first stage of labour. 2. Duration of second stage of labour. Secondary outcomes: 1. Maternal adverse effects. 2. Neonatal adverse effects. |
| Notes | Ethics: not mentioned. Location: Philipines. Not clear whether participants were really randomised: In both the treatment and control group there were 38 primi‐ and 38 multigravid women. The process of randomisation is not clearly described. Author contact details missing. |
Roy 2007.
| Methods | Prospective randomised study – not clear whether study was really randomised. |
| Participants | Women with spontaneous onset of labour. |
| Interventions | Intervention: Drotaverine hydrochloride 40 mg IVI. Control: No medication. |
| Outcomes | 1. Effects of drug on progress of labour. 2. Outcome of labour. |
| Notes | Methods not explicitly stated, not clear whether participants were randomised. Authors contacted, awaiting reply. |
Zagami 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Persian study ‐ no suitable translator found to date. |
IVI: intravenous injection RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Movahed 2010.
| Trial name or title | The effect of hyoscine on duration on the first stage of labour in term pregnancies. |
| Methods | Allocation generation: randomised. Allocation concealment: not described. Blinding: double‐blind. |
| Participants | Inclusion criteria: term pregnancy, cephalic presentation, singleton pregnancy. Exclusion criteria: contraindications for vaginal delivery. |
| Interventions | Intervention: hyoscine 20 mg (1 mL) intravenous injection, during the active phase of labour. Control: distilled water, 1 mL intravenous injection during active phase of labour. |
| Outcomes | Primary outcomes: 1. Duration of first stage of labour. Secondary outcomes: 1. Duration of second stage of labour. 2. Duration of third stage of labour. |
| Starting date | Date registered: 29 May 2010. |
| Contact information | Farideh Movahed: fmovahed@qums.ac.ir |
| Notes | Author contacted to request interim results. Not able to share these at this stage. |
Differences between protocol and review
Three additional subgroup analysis were added.
Type of management of labour (active management versus expectant management).
Type of pregnancy (high risk versus low risk).
Studies excluding versus studies including caesarean sections in their analysis.
Contributions of authors
Anke Rohwer (AR) developed the protocol and Taryn Young (TY) provided comments on the draft.
For the first version of the review, AR and TY; AR and Oswell Khondowe (OK) screened abstracts, selected studies for inclusion, assessed the risk of bias and extracted the data. OK adapted the methods section. AR performed the meta‐analysis, interpreted results, wrote the discussion and conclusion. TY provided input into all sections.
For the 2013 update, AR and OK screened abstracts, selected studies for inclusion, assessed risk of bias and extracted the data. AR updated the backgournd, results, discussion and conclusion. TY provided input into all sections.
Sources of support
Internal sources
University of Stellenbosch, South Africa.
External sources
Effective Health Care Research Consortium, UK.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) and the Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ajmera 2006 {published data only}
- Ajmera SK, Shah PK, Shinde GA. Comparative study of drotaverine vs. Valethamate in cervical phase of labour. Bombay Hospital Journal (http://www.bhj.org/journal/2006_4802_april/html/org_res_305‐309.html) (accessed 16 December 2011).
Al Matari 2007 {published data only}
- Al Matari F. Is buscopan (hyoscine‐N‐butylbromide) effective in shortening labor? [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:114.
Al Qahtani 2011 {published data only}
- Al Qahtani NH, Al Hajeri F. The effect of hyoscine butylbromide in shortening the first stage of labor: A double blind, randomized controlled trial. Therapeutics and Clinical Risk Management 2011;7:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azari 2010 {published data only}
- Azari M, Mardi A, Dargahi R. The effect of combination atropine and hyoscine progress of delivery in primiparous women in Ardabil ‐ Alavi Hospital 2008. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):226. [Google Scholar]
Batukan 2006 {published data only}
- Batukan AC, Ozgun MT, Turkyilmaz C, Dolanbay M, Muderris II. Effect of valethamate bromide in acceleration of labor: a double‐blind, placebo‐controlled trial [Valetamat bromurun dogum eylemini hizlandirmadaki etkisi: Plasebo kontrollu, cift kor bir calisma.]. Journal of the Turkish German Gynecology Association Artemis 2006;7(3):202‐5. [Google Scholar]
Cromi 2011 {unpublished data only}
- Cromi A, Ghezzi F, Agosti M, Uccella S, Piazza N, Serati M, et al. Use of an antispasmodic (Rociverine) to shorten the length of labor: a randomized, placebo‐controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2011;90(12):1371‐8. [DOI] [PubMed] [Google Scholar]
Dahal 2013 {published data only}
- Dahal P, Banerjee B, Uprety DK, Das BP, Thakur A, Agrawal A. Comparative study of efficacy of drotaverine hydrochloride and valethamate bromide with control in first stage of labour. Health Renaissance 2013;11(1):38‐42. [Google Scholar]
Gupta 2008 {published data only}
- Gupta B, Nellore V, Mittal S. Drotaverine hydrochloride versus hyoscine‐N‐butylbromide in augmentation of labor. International Journal of Gynecology & Obstetrics 2008;100(3):244‐7. [DOI] [PubMed] [Google Scholar]
Khosla 2003 {published data only}
- Khosla AH, Bala I, Dahiya K, Sangwan K. A comparative study of the efficacy of valethamate bromide with drotaverine in normal labor. Journal of Obstetrics and Gynecology of India 2003;53(6):568‐70. [Google Scholar]
Kuruvila 1992 {published data only}
- Kuruvila S, Jasper P, Peedicayil A, Mathai M. A randomized controlled trial of valethamate bromide in acceleration of labor. International Journal of Gynecology & Obstetrics 1992;38:93‐6. [DOI] [PubMed] [Google Scholar]
Madhu 2010 {published data only}
- Madhu C, Mahavarkar S, Bhave S. A randomised controlled study comparing drotaverine hydrochloride and valethamate bromide in the augmentation of labour. Archives of Gynecology and Obstetrics 2010;282:11‐5. [DOI] [PubMed] [Google Scholar]
Makvandi 2010 {published data only}
- Makvandi S. The effect of hyoscine ‐ N ‐ butyl bromide suppository on labor pain and process in nuliparous women. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Mukaindo 2010 {unpublished data only}
- Mukaindo AM, Stones WR (Department of Obstetrics and Gynaecology, Aga Khan University Hospital Nairobi). Hyoscine‐N‐butylbromide for the acceleration of labor in first time parturients: a randomized, double‐blind, placebo‐controlled trial. Personal communication via email 24 October 2011.
Raghavan 2008 {published data only}
- Raghavan R. The effect of hyoscine butyl bromide on the first stage of labour in term pregnancies. BJOG: an international journal of obstetrics and gynaecology 2008;115(8):1064‐5. [DOI] [PubMed] [Google Scholar]
Samuels 2007 {published data only}
- Samuels LA, Christie L, Roberts‐Gittens B, Fletcher H, Frederick J. The effect of hyoscine butylbromide on the first stage of labour in term pregnancies. BJOG: an international journal of obstetrics and gynaecology 2007;114(12):1542‐6. [DOI] [PubMed] [Google Scholar]
Sekhavat 2012 {published data only}
- Sekhavat L, Karbasi S, Fallah R, Mirowliai M. Effect of hyoscine butylbromide first stage of labour in multiparus women. African Health Sciences 2012;12(4):408‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2001 {published data only}
- Sharma JB, Pundir P, Kumar A, Murthy NS. Drotaverine hydrochloride vs. valethamate bromide in acceleration of labor. International Journal of Gynecology & Obstetrics 2001;74:255‐60. [DOI] [PubMed] [Google Scholar]
Singh 2004 {published data only}
- Singh KC, Jain P, Goel N, Saxena A. Drotaverine hydrochloride for augmentation of labor. International Journal of Gynecology & Obstetrics 2004;84:17‐22. [DOI] [PubMed] [Google Scholar]
Taskin 1993 {published data only}
- Taskin O, Saade G, Belfort M, Moise K. The effect of narcotics and spasmolytics on cervical dilatation in labor: a randomized placebo‐controlled study. American Journal of Obstetrics and Gynecology 1993;168:362. [Google Scholar]
Warke 2003 {published data only}
- Warke HS, Chauhan AR, Raut VS, Ingle KM. Efficacy of camylofin dihydrochloride in acceleration of labour: A randomised double blind trial. Bombay Hospital Journal 2003;45(3):420‐3. [Google Scholar]
Yilmaz 2009 {published data only}
- Yilmaz B, Kart C, Kelekci S, Gokturk U, Sut N, Tarlan N, et al. Meperidine versus valethamate bromide in shortening the duration of active labor. International Journal of Gynecology & Obstetrics 2009;107(2):126‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aggarwal 2008 {published data only}
- Aggarwal P, Zutshi V, Batra S. Role of hyoscine N‐butyl bromide (HBB, Buscopan) as labor analgesic. Indian Journal of Medical Science 2008;62(5):179‐84. [PubMed] [Google Scholar]
Akleh 2010 {published data only}
- Akleh HE, Al‐Jufairi ZA. Effect of hyoscine‐N‐bulyl bromide (Buscopan) in accelerating first stage of labor. Journal of the Bahrain Medical Society 2010;22(3):103‐7. [Google Scholar]
Baracho 1982 {published data only}
- Baracho HM, Kamat JR, Kunkalekar K, Jacob L. Hyoscine‐N‐Butylbromide (Buscopan) in acceleration of labour. Journal of Obstetrics and Gynaecology of India 1982;34:509‐12. [Google Scholar]
Bhattacharaya 1984 {published data only}
- Bhattacharaya P, Joshi SG. Acceleration of labour intramuscular "Buscopan" injection. Journal of Obstetrics and Gynaecology of India 1984;35:1014‐7. [Google Scholar]
Chan 1963 {published data only}
- Chan DPC. The use of Buscopan during the first stage of labour. The Bulletin of the Hong Kong Chinese Medical 1963;15:69‐71. [Google Scholar]
Demory 1990 {published data only}
- Demory JE, Firmin JM, Parot P. The value of magnesium pyrrolidone carboxylate in true cervix dystocia. A double blind vs placebo study. Revue Francaise de Gynecologie et d Obstetrique 1990;85:413‐6. [PubMed] [Google Scholar]
De Nobrega‐Correa 2010 {published data only}
- Nobrega‐Correa H, Guerra‐Velasquez M, Reyna‐Villasmil E, Mejia‐Montilla J, Reyna‐Villasmil N, Torres‐Cepeda D, et al. Effects of intravenous hyoscine butylbromide‐oxytocin or oxytocin on the duration of labor in term pregnancies [Spanish] [Efectos del butil bromuro de hioscina‐oxitocina u oxitocina intravenosos en la duracion del parto de embarazos a termino]. Progresos de Obstetricia y Ginecologia 2010;53(12):502‐6. [Google Scholar]
Fouedjio 2012 {published data only}
- Fouedjio JH, Ymele Fouelifack F, Kenfack B, Wache Sime LC, Nana NP, Temkou S, et al. Efficacité du phloroglucinol sur le raccourcissement de la phase active du travail à la maternité de l'Hôpital Central de Yaoundé, Cameroun. Clinics in Mother and Child Health 2012;9:C12502. [Google Scholar]
Guerresi 1981 {published data only}
- Guerresi E, Gori G, Beccari A, Farro M, Mazzanti C. Influence of spasmolytic treatment and amniotomy on delivery times: a factorial clinical trial. Clinical Therapeutics 1981;3(5):382‐8. [PubMed] [Google Scholar]
Hamann 1972 {published data only}
- Hamann GO. Avacan vs fortral. A controlled double blind investigation on parturient patients. Ugeskrift for Laeger 1972;134:2261‐5. [PubMed] [Google Scholar]
Hao 2004 {published data only}
- Hao Y, Zhai GR, Duan AH. Effects of spasfon on course of labor. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynecology] 2004;39(9):606‐8. [PubMed] [Google Scholar]
Kauppila 1970 {published data only}
- Kauppila O, Koistinen O, Vaalamo P. Drug‐induced acceleration of labour. Trials of an Indan derivative in childbirth. Annales Chirurgiae et Gynaecologiae Fenniae 1970;59:85‐9. [PubMed] [Google Scholar]
Kaur 2003 {published data only}
- Kaur D, Kaur R. Comparison of drotaverine and epidosin in the first stage of labor. Journal of Obstetrics and Gynecology of India 2003;53(5):449‐52. [Google Scholar]
Kaur 2006 {published data only}
- Kaur D, Kaur A. "Anafortan" an old drug with its newer use in acceleration of labour [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:59.
Malensek 1985 {published data only}
- Malensek B. The influence of spasmoanalgetics on uterine activity. Jugoslavenska Ginekologija i Perinatologija 1985;25:75‐9. [PubMed] [Google Scholar]
Manpreet 2008 {published data only}
- Manpreet K, Gouramba R, Jyothi K, Venkatesh S, Biradar R. A comparative study of hyoscine butylbromide versus drotaverine hydrochloride in first stage of labor. Journal of Obstetrics and Gynecology of India 2008;58(3):230‐4. [Google Scholar]
Maritati 1986 {published data only}
- Maritati V, Tanganelli E, Boccardo E, Camandona F, Pietrasanta R, Maganza C, et al. The use of sintropium bromide, a new spasmolytic, in obstetrics and gynecology. Minerva Ginecologica 1986;38(11):965‐9. [PubMed] [Google Scholar]
Mishra 2002 {published data only}
- Mishra SL, Toshniwal A, Banerjee R. Effect of drotaverine on cervical dilatation: a comparative study with epidosin (valethamate bromide). Journal of Obstetrics and Gynecology of India 2002;52(3):76‐9. [Google Scholar]
Mortazavi 2004 {published data only}
- Mortazavi F, Rakhshani MH. The effect of atropine, hyoscine and promethazine on the duration of labor stages and rate of labor progress in multiparous women. Journal of the Gorgan University of Medical Sciences 2004;6(2):92. [Google Scholar]
Rajkumar 2006 {published data only}
- Rajkumar K, Vedavalli R. Drotaverine hydrochloride vs valethamate bromide for cervical dilatation in active phase of labour [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:96.
Sirohiwal 2005 {published data only}
- Sirohiwal D, Dahiya K, Mandira DE. Efficacy of hyoscine‐N‐butyl bromide (Buscopan) suppositories as a cervical spasmolytic agent in labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45:128‐9. [DOI] [PubMed] [Google Scholar]
Tabassum 2005 {published data only}
- Tabassum S, Afridi B, Aman Z. Phloroglucinol for acceleration of labour: double blind, randomized controlled trial. JPMA ‐ Journal of the Pakistan Medical Association 2005;55(7):270‐3. [PubMed] [Google Scholar]
Tripti 2009 {published data only}
- Tripti N, Jyoti J. To compare and evaluate the efficacy and safety of drotaverine and valethamate bromide. Journal of Obstetrics and Gynaecology of India 2009;59(4):324‐31. [Google Scholar]
Von Hagen 1965 {published data only}
- Hagen H. Clinical trials with the spasmo‐analgesic Spasdolsin in obstetrics (with application of a new methodology). Zentralblatt fur Gynakologie 1965;36:1201‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Accinelli 1978 {published data only}
- Accinelli G, Olivieri T, Vibelli C. The effectiveness of a new spasmolytic drug (DA 3177) during the active phase of labour. Acta Therapeutica 1978;4:19‐27. [Google Scholar]
Ahmed 1982 {published data only}
- Ahmed LT, Merouana A, Nait B, Souiah N, Chenntouf M, Larbi LO. Antispasmodics in obstetrics: myth or reality [Les antispasmodiques en obstetrique: mythe ou realite]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1982;11(2):281‐4. [PubMed] [Google Scholar]
Georges 2007 {published data only}
- Georges R, Aldahan F, Alwaeely F. The evaluation of the effect of buscopan (hyoscine‐N‐butyl bromide) on the duration of labour [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:102.
Rajani 2011 {published data only}
- Rajani M, Umadevi N, Jayasree S. Efficacy of valethamate bromide on cervical dilatation during the labour in comparison with placebo. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:141.
Ranka 2002 {published data only}
- Ranka PR, Hishikar VA. Effect of drotaverine hydrochloride on normal labour ‐ a randomised study. Journal of Obstetrics and Gynecology of India 2002;52(6):28‐30. [Google Scholar]
Recto 1997 {published data only}
- Recto CP, Co TV, Clemente V. The effect of hyoscine‐n‐butylbromide on the first stage of labor: a clinical trial. Acta Obstetricia et Gynecologica Scandinavica 1997;76 Suppl(167:1):45. [Google Scholar]
Roy 2007 {published data only}
- Roy A, Patra KK, Mukhopadhyay S, Guha S. Study of drotaverine on first stage of labour and pregnancy outcome. Journal of the Indian Medical Association 2007;105(8):450. [PubMed] [Google Scholar]
Zagami 2012 {published data only}
- Zagami SE, Golmakani N, Saadatjoo SAR, Dadgar S, Baghbani B. Comparison of effects of hyoscine N‐butyl bromide and promethazine on length of active phase of first stage of labor. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(6):16‐21. [Google Scholar]
References to ongoing studies
Movahed 2010 {published data only}
- Movahed F. The effect of hyoscin on duration on the first stage of labor in term pregnancies. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Additional references
Brown 2008
- Brown HC, Paranjothy S, Dowswell T, Thomas J. Package of care for active management in labour for reducing caesarean section rates in low‐risk women. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhimschi 2003
- Buhimschi CS, Buhimschi IA, Malinow AM, Saade GR, Garfield RE, Weiner CP. The forces of labour. Fetal and Maternal Medicine Review 2003;14(4):273‐307. [Google Scholar]
Clark 2009
- Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. American Journal of Obstetrics and Gynecology 2009;200:35.e1‐35.e6. [DOI] [PubMed] [Google Scholar]
Daftary 2009
- Daftary SN, Desai SV, Thanawala U, Bhide A, Levi J, Patki A, et al. Programmed labor ‐ indegenous protocol to optimize labor outcome. South Asian Federation of Obstetrics and Gynecology 2009;1(1):61‐4. [Google Scholar]
Dencker 2009
- Dencker A, Berg M, Bergvist L, Ladfors L, Thorsen LS, Lilja H. Early versus delayed oxytocin augmentation in nulliparous women with prolonged labour ‐ a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116:530‐6. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbon 2005
- Gibbon CJ. South African Medicines Formulary. 6th Edition. Cape Town: Health and Medical Publishing, 2005. [Google Scholar]
Gitanjali 2010
- Gitanjali B. Valethamate bromide: Is there any proof of efficacy and safety for its use in labor?. Journal of Pharmacology and Pharmacotherapeutics 2010;1(1):2‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu M, Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066‐74. [DOI] [PubMed] [Google Scholar]
Lavender 2009
- Lavender T, Hart A, Smyth RMD. Effect of partogram use on outcomes for women in spontaneous labour at term. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005461.pub2] [DOI] [Google Scholar]
Leppert 1995
- Leppert PC. Anatomy and physiology of cervical ripening. Clinical Obstetrics and Gynecology 1995;38:267‐79. [DOI] [PubMed] [Google Scholar]
Neal 2010
- Neal JL, Lowe NK, Patrick TE, Cabbage LA, Corwin EJ. What is the slowest‐yet‐normal cervical dilatation rate among nulliparous women with spontaneous labor onset?. Journal of Obstetric, Gynecologic, and Neonatal Nursing : JOGNN 2010;39:361‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2003
- Neilson JP, Lavender T, Quenby S, Wray S. Obstructed labour. British Medical Bulletin 2003;67:191‐204. [DOI] [PubMed] [Google Scholar]
O'Driscoll 1973
- O'Driscoll K, Stronge JM, Minogue M. Active management of labour. British Medical Journal 1973;3:135‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Driscoll 1994
- O'Driscoll K. Active management of labour. True purpose has been misunderstood. BMJ 1994;309(6960):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay C. How do you include trials with more than two groups into a single meta‐analysis?. FAQs: methodology in the Cochrane Effective Practice and Organisation of Care Group 2003.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Romics 2003
- Romics I, Molnár DL, Timberg G, Mrklic B, Jelakovic B, Köszegi G, et al. The effect of drotaverine hydrochloride in acute colicky pain caused by renal and ureteric stones. BJU International 2003;92(1):92‐6. [DOI] [PubMed] [Google Scholar]
Samuels 2009
- Samuels L. Pharmacotherapy update: hyoscine butylbromide in the treatment of abdominal spasms. Clinical Medicine Insights: Therapeutics 2009;1:647‐55. [Google Scholar]
Sommers 2002
- Sommers De K. Sommers' Pharmacology. Pretoria: UP Drukkers, 2002. [Google Scholar]
Thirunavukkarasu 2010
- Thirunavukkarasu AB, Vijayan S. Valethamate bromide: Conflicting evidence and continuing use. Journal of Pharmacology and Pharmacotherapeutics 2010. [DOI] [PMC free article] [PubMed]
Wei 2009
- Wei S, Wo BL, Xu H, Luo ZC, Roy C, Fraser WD. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006794.pub2] [DOI] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization maternal health and safe motherhood programme. World Health Organization partograph in management of labour. Lancet 4 June 1994;343(8910):1399‐404. [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Trends in maternal mortality: 1990‐2008. Estimates developed by WHO, UNICEF, UNFPA and the World Bank. http://www.who.int/reproductivehealth/publications/monitoring/9789241500265/en/index.html (accessed 9 February 2010).
Yuel 2008
- Yuel VI, Kaur V, Kaur D. Programmed labor for optimizing labor and delivery. JK Science 2008;10(2):62‐4. [Google Scholar]
References to other published versions of this review
Rohwer 2012
- Rohwer AC, Khondowe O, Young T. Antispasmodics for labour. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009243.pub2] [DOI] [PubMed] [Google Scholar]


