Abstract
Chronic obstructive pulmonary disease (COPD) is a multifactorial and heterogeneous disease that creates public health challenges worldwide. The underlying molecular mechanisms of COPD are not entirely clear. In this study, we aimed to identify the critical genes and potential molecular mechanisms of COPD by bioinformatic analysis. The gene expression profiles of lung tissues of COPD cases and healthy control subjects were obtained from the Gene Expression Omnibus. Differentially expressed genes were analyzed by integration with annotations from Gene Ontology and Kyoto Encyclopedia of Genes and Genomes, followed by construction of a protein‐protein interaction network and weighted gene coexpression analysis. We identified 139 differentially expressed genes associated with the progression of COPD, among which 14 Hub genes were identified and found to be enriched in certain categories, including immune and inflammatory response, response to lipopolysaccharide and receptor for advanced glycation end products binding; in addition, these Hub genes are involved in multiple signaling pathways, particularly hematopoietic cell lineage and cytokine‐cytokine receptor interaction. The 14 Hub genes were positively or negatively associated with COPD by wgcna analysis. The genes CX3CR1,PTGS2,FPR1,FPR2, S100A12,EGR1,CD163, S100A8 and S100A9 were identified to mediate inflammation and injury of the lung, and play critical roles in the pathogenesis of COPD. These findings improve our understanding of the underlying molecular mechanisms of COPD.
Keywords: bioinformatic analysis, chronic obstructive pulmonary disease, differentially expressed gene, epidemiology, GEO data
Abbreviations
- ARG1
arginase 1
- BP
biological process
- CC
cellular component
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CX3CR1
C‐X3‐C motif chemokine receptor 1
- DALY
disability‐adjusted life year
- DEG
differentially expressed gene
- EGR1
early growth response 1
- FC
fold change
- FGG
fibrinogen gamma chain
- FPR1
formyl peptide receptor 1
- FPR2
formyl peptide receptor 2
- GO
Gene Ontology
- GS
gene significance
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MF
molecular function
- MM
module membership
- ORM1
orosomucoid 1
- PPBP
proplatelet basic protein
- PPI
protein‐protein interaction
- PTGS2
prostaglandin‐endoperoxide synthase 2
- S100A12
S100 calcium binding protein A12
- S100A8
S100 calcium binding protein A8
- S100A9
S100 calcium binding protein A9
- VCAM1
vascular cell adhesion molecule 1
- wgcna
weighted gene coexpression network analysis
- YLLs
years of life lost
Chronic obstructive pulmonary disease (COPD), characterized by long‐term poorly reversible airway limitation and persistent respiratory symptoms, is a common and preventable disease 1. COPD is projected to become the third leading cause of all death by 2030 in the world 2. Globally, COPD affected 299.4 million people in 2017, with a 71.2% increase in the prevalence rate compared with 2015, ranking it as the fifth leading cause of disability‐adjusted life years (DALYs) and the seventh leading noncommunicable disease cause of years of life lost (YLLs) 3, 4, 5, 6. As shown in Fig. 1A, we observed a 12.3% increase in global all‐age deaths caused by COPD from 2.85 million in 1990 to 3.20 million in 2017 6, 7, 8, 9 and a predicted increase of 60% by 2020 compared with 1990. Figure 1B indicated that the all‐age standardized death rate of COPD in males, females, and both sexes separately decreased from 1990 to 2015 6, 8, which could be because of population growth and aging. Although the COPD death rate varies with different countries, more than 90% of COPD deaths occurred in low‐ and middle‐income countries 10. The global all‐age YLLs with COPD showed a small increase of 7.5% and 3.6% for both sexes and males, respectively, as well as a 21% decrease for females from 1990 to 2015 (Fig. 1C) 6, 8. In addition, as shown in Fig. 1D 3, 4, 5, 7, 11, 12, 13, 14, global all‐age DALYs caused by COPD had a small increase of 4.2% during 1990–2015 and was projected to decline to 57.6 million by 2020. The age‐standardized DALY rate caused by COPD in females was about twice as high as that of males, and that in low‐ and middle‐income countries was 6.7 times higher than in some high‐income countries 3. We observed that the global all‐age years lived with disability caused by COPD has grown 52.2% from 1990 to 2017. Taken together, COPD has presented a global public health challenge with high prevalence, mortality and disability rates, whereas the diagnosis of COPD is usually made based on spirometry values and clinical symptoms with a frequent underdiagnosis 15. Thus, it is important to explore the underlying molecular mechanisms and identify more effective early diagnostic methods and reliable biomarkers for this disease.
Figure 1.
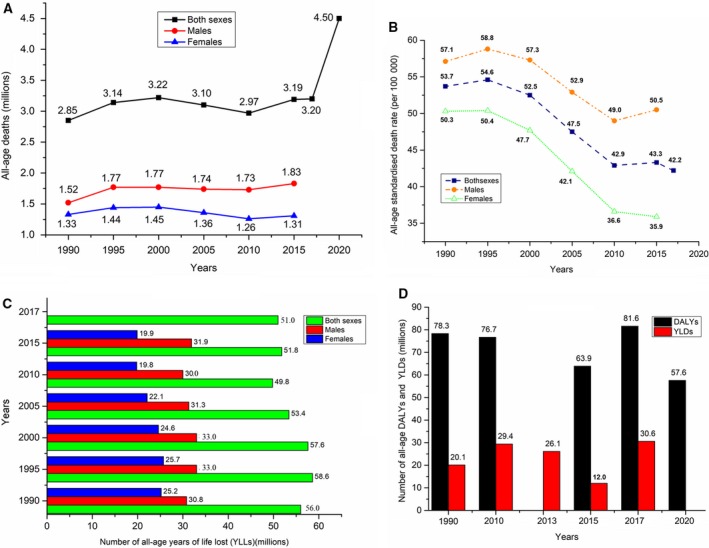
The global death and burden caused by COPD. (A) Global age‐related deaths (millions) caused by COPD in men and women, respectively, from 1990 to 2020 6, 7, 8, 9. (B) Global age‐related death rates (per 100 000) caused by COPD for both sexes, males and females, respectively, from 1990 to 2015 6, 8. (C) Global age‐related YLLs (millions) caused by COPD for both sexes, males and female, respectively, from 1990 to 2015 6, 8. (D) Global age‐related DALYs and years lived with disability (YLD) (millions) by COPD for both sexes from 1990 to 2020 3, 4, 5, 7, 11, 12, 13, 14.
As a large‐scale and efficient technique for acquiring genomic data, microarray‐based gene expression profiles have been widely used to seek new insights for biomarkers in many human diseases 16. Currently, many studies have been conducted on COPD gene expression profiles, and these studies have screened thousands of differentially expressed genes (DEGs) implicated in the development and progression of this disease 17, 18. However, the results for the identification of DEGs are discrepant among these studies due to sample heterogeneity and differences in technological detection platforms. In this study, we performed an integrated analysis on some of the gene expression profiling data based on lung tissues of COPD cases and control subjects using an unbiased approach aiming to identify the potential molecular mechanisms and biomarkers for COPD.
We selected two Gene Expression Omnibus microarray datasets on COPD ( GSE27597 and GSE106986). DEGs were identified by r software (Auckland, New Zealand) and subsequently analyzed using bioinformatic methods including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichments and construction of protein‐protein interaction (PPI) and weighted gene coexpression analysis (wgcna) networks. We screened the DEGs for potential association with the development and progression of COPD. Our work may further the understanding of the potential molecular mechanisms of COPD.
Materials and methods
Gene data
Two gene expression datasets, GSE27597 and GSE106986, were downloaded from the National Center for Biotechnology Information Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/). GSE27597 comprises the expression profile from 64 lung tissue samples from COPD cases and 16 samples from healthy donors 19. GSE106986 includes molecular profiling of 19 lung tissue samples, containing 14 samples from COPD cases and 5 from smokers 20. The experiments of GSE27597 and GSE106986 were conducted on the Affymetrix Human Exon 1.0 ST GeneChip (Affymetrix, Inc., Santa Clara, CA, USA; GPL5175 platform) and Agilent‐026652 Whole Human Genome Microarray 4x44K v2 (Agilent Technologies, Inc., Palo Alto, CA, USA; GPL13497 platform), respectively.#AuthorQuery Rep
Data preprocessing and screening for DEGs
The probe set IDs of two datasets were converted into gene symbols using the r software and annotation packages. The two datasets were merged into one array dataset and then batch normalized using r packages (sva and limma 3.40.6). The DEGs between COPD cases and control subjects were identified using the limma package in r 3.60. A P‐value <0.05 after being adjusted by false discovery rate and |log2FC > 1, where FC represents fold change, were applied together as the cutoff for DEGs screening.
GO and KEGG pathway enrichment analysis of DEGs
GO enrichment of the DEGs on the biological process (BP), molecular function (MF) and cellular component (CC) categories was performed using a david online tool (https://david.ncifcrf.gov/) 21, 22. KEGG pathway enrichment analysis was performed by using the KOBAS 3.0 online analysis database (http://kobas.cbi.pku.edu.cn/) 23.
Construction of the PPI network
STRING database (https://string-db.org/) is frequently used for identifying the protein interactions 16, 24. STRING database contains huge amounts of experimental and text mining data 25. cytoscape is an open source bioinformatic software platform used for integrating gene expression profiles and visualizing molecular interaction networks. cytoscape plugin cytoHubba provides multiple topological analysis methods on Hub genes, regulatory networks and drug targets for experimental biologists 26. In this study, we used STRING database to identify the interactions between the identified DEGs. A confidence score >0.4 was used as the cutoff criterion. In addition, Hub DEGs were identified with cytoscape version 3.6.1 (1999 Free Software Foundation, Inc., Boston, MA, USA) with cytoHubba plugin according to the rank of connection degree (number) for each gene, which is represented by the different degrees of color (from red to yellow): the role of the gene is greater in the PPI network with the darker color of the gene 26.
wgcna on COPD
wgcna may be used for screening modules (clusters) of highly correlated genes and for calculating module membership (MM) measures, in which the module eigengene or an intramodular Hub gene is used to summarize such modules, and eigengene network methodology is used for relating modules to one another and to external sample traits 27. In this study, the wgcna package was used to identify coexpression modules for the merged and standardized array datasets (GSE27597 and GSE106986). In brief, first, a weighted adjacency matrix containing pairwise connection strengths was constructed based on the selected soft threshold power (β = 11) on the matrix of pairwise correlation coefficients. Then, the connectivity measure per gene was calculated by summing the connection strengths with other genes; modules were defined as branches of a hierarchical clustering tree by using a dissimilarity measure, and each module was assigned a color. Subsequently, module preservation r function was used to assess the module structure preservation. Finally, the module eigengene was used for summarizing the gene expression profiles of each module, and each module eigengene was regressed on case trait (COPD) and smoking status by using the linear model in the limma r package.
Results
Identification of DEGs in COPD
After batch normalization on the integrated dataset from GSE27597 and GSE106986 by the sva and limma packages, 139 DEGs were identified using the limma package (corrected P < 0.05; |log2FC| > 1) (Tables 1 and 2). The cluster heatmap of the top 139 DEGs is shown in Fig. 2. Among them, 62 genes were up‐regulated and 77 genes were down‐regulated, which is shown in Fig. 3.
Table 1.
Screening up‐regulated and down‐regulated DEGs in COPD by integrated microarray
| DEGs | Gene symbol |
|---|---|
| Up‐regulated (62 genes) | PIEZO2, INHBA, VCAM1, RSPO2, CD1C, HSD17B6, AQP3, APLNR, GPD1, EGR2, KCNA, SLC18A2, FRZB, PTGS2, OLR1, EDNRA, SHISA2, ELF5, LUM, CYSLTR1, BMP5, HPGDS, MS4A2, DCC, FOSB, HHIP, MSMB, CD200, AMPD1, ICOS, RTKN2, CD83, FABP4, ISLR, GPR174, SLAMF7, WIF1, CTSW, CHIT1, CPA3, BMP1, EGR3, CX3CR1, EGR1, TREM2, ATF3, ICAM4, C8B, NR4A3, CCL8, HAS2, C4BPA, ITGB6, HBEGF, AGTR2, SERPIND1, CEACAM5, SFRP2, SELE, HLA‐DRB1, HLA‐DRB5, FGG |
| Down‐regulated (77 genes) | IL1R2, CEACAM4, ST6GALNAC3, ITGA10, MERTK, FKBP5, CLEC4E, LAMB1, MGAM, RPS6KA2, FLT1, IRAK3, SULT1B1, AOX1, SH3PXD2B, RNASE2, IL18R1, CD163, IL1RL1, GRASP, MT1A, S100A12, MMP8, GLT1D1, TMTC1, S100A8, IL4R, IL18RAP, FLT3, ANPEP, MT1M, SIGLEC10, SPARCL1, SMAP2, TIMP4, ANGPTL1, HIF3A, PKHD1L1, LILRB3, FPR1, SLED1, LDLRAD3, FAM150B, FPR2, ZBTB16, GCA, ARG1, CXCR2P1, S100A9, TMEM204, TINAGL1, ABCC8, VCAN, APOLD1, DDIT4, ARRDC2, SERPINA3, PIK3R3, ADM, PNMT, BTNL9, CRISPLD2, SLCO2A1, VNN2, TUBB1, PSAT1, PPBP, DEFA4, AQP9, TTN, PDK4, MT1L, MT1X, ORM1, CHRM2, PTX3, EIF1AY |
Table 2.
Screening 139 DEGs in COPD by integrated microarray. AveExpr, average expression; FDR, false discovery rate
| Gene symbol | logFC | AveExpr | t | P‐value | FDR |
|---|---|---|---|---|---|
| IL1R2 | −3.724170635 | 4.910345092 | −14.57070196 | 5.24E−25 | 8.38E−21 |
| CEACAM4 | −1.546190038 | 3.289938835 | −14.11578934 | 3.65E−24 | 2.92E−20 |
| ST6GALNAC3 | −1.509348297 | 4.115922297 | −13.72934365 | 1.93E−23 | 1.03E−19 |
| ITGA10 | −1.304452408 | 4.156071544 | −13.3424854 | 1.05E−22 | 4.18E−19 |
| MERTK | −1.371100619 | 5.743922084 | −13.22669655 | 1.74E−22 | 4.99E−19 |
| FKBP5 | −2.553152066 | 5.964790869 | −13.21010646 | 1.87E−22 | 4.99E−19 |
| CLEC4E | −2.102592383 | 3.712865442 | −12.2724444 | 1.22E−20 | 2.44E−17 |
| LAMB1 | −1.236900341 | 6.51036255 | −12.11282186 | 2.51E−20 | 4.31E−17 |
| MGAM | −1.918275604 | 3.743487329 | −12.0971852 | 2.69E−20 | 4.31E−17 |
| RPS6KA2 | −1.051171425 | 6.235125074 | −11.99287281 | 4.32E−20 | 6.28E−17 |
| FLT1 | −1.43735859 | 5.302047032 | −11.6588263 | 1.98E−19 | 2.43E−16 |
| IRAK3 | −1.440362949 | 5.407265256 | −11.53504535 | 3.48E−19 | 3.71E−16 |
| PIEZO2 | 1.165120322 | 5.154801689 | 11.4846069 | 4.38E−19 | 4.38E−16 |
| SULT1B1 | −1.966128566 | 3.712292447 | −11.44142282 | 5.34E−19 | 5.02E−16 |
| AOX1 | −1.498003567 | 4.380016656 | −10.80239392 | 1.02E−17 | 8.14E−15 |
| SH3PXD2B | −1.360072374 | 4.796109196 | −10.45348254 | 5.15E−17 | 3.92E−14 |
| RNASE2 | −2.214352488 | 3.502846585 | −10.41707921 | 6.11E−17 | 4.44E−14 |
| IL18R1 | −1.552709013 | 4.479557525 | −10.21566115 | 1.56E−16 | 1.04E−13 |
| CD163 | −2.353331128 | 6.483508901 | −10.18905044 | 1.77E−16 | 1.13E−13 |
| IL1RL1 | −2.008254697 | 5.747865421 | −10.06701534 | 3.13E−16 | 1.88E−13 |
| INHBA | 1.437565757 | 4.698423572 | 10.06451042 | 3.17E−16 | 1.88E−13 |
| VCAM1 | 1.58323448 | 4.54949487 | 9.975765691 | 4.80E−16 | 2.74E−13 |
| GRASP | −1.019125221 | 5.351837423 | −9.953904434 | 5.32E−16 | 2.93E−13 |
| RSPO2 | 1.22508185 | 4.281279622 | 9.943524241 | 5.59E−16 | 2.98E−13 |
| CD1C | 1.320908918 | 4.048138575 | 9.847836259 | 8.75E−16 | 4.51E−13 |
| HSD17B6 | 1.611303762 | 5.543589418 | 9.811225763 | 1.04E−15 | 5.03E−13 |
| MT1A | −2.003809136 | 5.341700669 | −9.802106469 | 1.08E−15 | 5.10E−13 |
| AQP3 | 1.465444106 | 6.255672988 | 9.709215286 | 1.68E−15 | 7.66E−13 |
| S100A12 | −2.381911789 | 3.793860618 | −9.658062064 | 2.13E−15 | 9.26E−13 |
| APLNR | 1.462761779 | 4.060794485 | 9.514044214 | 4.19E−15 | 1.76E−12 |
| GPD1 | 1.162408188 | 4.425391991 | 9.418745073 | 6.56E−15 | 2.65E−12 |
| EGR2 | 1.7756242 | 5.312493191 | 9.415498145 | 6.66E−15 | 2.65E−12 |
| MMP8 | −1.963376342 | 1.850422771 | −9.410996945 | 6.80E−15 | 2.65E−12 |
| KCNA3 | 1.127720579 | 5.181587794 | 9.376872801 | 7.99E−15 | 2.98E−12 |
| SLC18A2 | 1.155076678 | 4.861252305 | 9.375465524 | 8.04E−15 | 2.98E−12 |
| GLT1D1 | −1.354253187 | 4.134707596 | −9.333414043 | 9.80E−15 | 3.33E−12 |
| TMTC1 | −1.221922174 | 4.907718006 | −9.2268097 | 1.62E−14 | 5.28E−12 |
| S100A8 | −1.984436486 | 6.732435285 | −9.226677546 | 1.62E−14 | 5.28E−12 |
| IL4R | −1.016737627 | 6.13438495 | −9.220088063 | 1.67E−14 | 5.34E−12 |
| IL18RAP | −1.20706302 | 4.403623117 | −9.181902429 | 2.00E−14 | 6.26E−12 |
| FRZB | 1.108947075 | 4.570621342 | 9.054280215 | 3.64E−14 | 1.08E−11 |
| PTGS2 | 1.909599543 | 5.385732252 | 9.037623232 | 3.94E−14 | 1.14E−11 |
| FLT3 | −1.068728329 | 2.955444457 | −9.011875495 | 4.44E−14 | 1.24E−11 |
| OLR1 | 1.516024757 | 5.630156756 | 9.006280553 | 4.56E−14 | 1.24E−11 |
| ANPEP | −1.451754311 | 4.687966342 | −9.001861447 | 4.66E−14 | 1.24E−11 |
| MT1M | −2.585708871 | 5.454959847 | −8.990902454 | 4.90E−14 | 1.29E−11 |
| SIGLEC10 | −1.464287775 | 4.29164058 | −8.960779525 | 5.65E−14 | 1.46E−11 |
| EDNRA | 1.016233852 | 5.691095587 | 8.939250683 | 6.25E−14 | 1.56E−11 |
| SHISA2 | 1.00660372 | 5.288052754 | 8.886393339 | 8.02E−14 | 1.94E−11 |
| SPARCL1 | −1.096814178 | 7.567105051 | −8.760826399 | 1.45E−13 | 3.30E−11 |
| SMAP2 | −1.030733342 | 6.515681918 | −8.593715399 | 3.17E−13 | 6.73E−11 |
| TIMP4 | −1.656076369 | 4.229601784 | −8.456527093 | 6.04E−13 | 1.19E−10 |
| ELF5 | 1.521617319 | 4.673219557 | 8.448782695 | 6.26E−13 | 1.21E−10 |
| ANGPTL1 | −1.111036176 | 3.340161894 | −8.413321275 | 7.39E−13 | 1.37E−10 |
| HIF3A | −1.065614158 | 4.526630285 | −8.386705336 | 8.38E−13 | 1.52E−10 |
| PKHD1L1 | −1.402091953 | 3.533210448 | −8.281317436 | 1.37E−12 | 2.29E−10 |
| LUM | 1.008125744 | 6.388824425 | 8.261829056 | 1.50E−12 | 2.45E−10 |
| CYSLTR1 | 1.017621995 | 4.334713339 | 8.231060286 | 1.74E−12 | 2.78E−10 |
| LILRB3 | −1.182035723 | 3.930324211 | −8.201110624 | 2.00E−12 | 3.11E−10 |
| FPR1 | −1.497358339 | 5.123265733 | −8.165184027 | 2.36E−12 | 3.63E−10 |
| BMP5 | 1.082572 | 4.856866963 | 8.039056504 | 4.26E−12 | 6.14E−10 |
| HPGDS | 1.178976758 | 4.9661482 | 7.971057179 | 5.85E−12 | 8.07E−10 |
| SLED1 | −1.418643068 | 4.176703046 | −7.93529438 | 6.92E−12 | 9.42E−10 |
| MS4A2 | 1.127545704 | 4.501069641 | 7.934255426 | 6.95E−12 | 9.42E−10 |
| DCC | 1.107054924 | 4.066396917 | 7.874112823 | 9.20E−12 | 1.21E−9 |
| LDLRAD3 | −1.132183539 | 4.777010972 | −7.827670594 | 1.14E−11 | 1.42E−9 |
| FAM150B | −1.326763929 | 3.661484534 | −7.801467295 | 1.29E−11 | 1.57E−9 |
| FOSB | 2.16754811 | 6.605909434 | 7.722677313 | 1.86E−11 | 2.20E−9 |
| FPR2 | −1.585779616 | 4.015151074 | −7.702560188 | 2.04E−11 | 2.40E−9 |
| ZBTB16 | −1.704030574 | 5.808698383 | −7.660799671 | 2.48E−11 | 2.87E−9 |
| GCA | −1.036962474 | 4.831056628 | −7.646120016 | 2.65E−11 | 3.03E−9 |
| HHIP | 1.361681455 | 6.338309941 | 7.624501498 | 2.93E−11 | 3.28E−9 |
| CD200 | 1.045448111 | 4.105658515 | 7.579848564 | 3.61E−11 | 3.95E−9 |
| ARG1 | −1.363394352 | 2.024018729 | −7.54254425 | 4.29E−11 | 4.60E−9 |
| AMPD1 | 1.043721979 | 3.103940463 | 7.443735113 | 6.77E−11 | 6.85E−9 |
| ICOS | 1.222541378 | 4.277643563 | 7.41874473 | 7.59E−11 | 7.59E−9 |
| CXCR2P1 | −1.15616615 | 4.439580145 | −7.399724607 | 8.29E−11 | 8.23E−9 |
| S100A9 | −1.545635339 | 5.747693368 | −7.378920244 | 9.12E−11 | 9.01E−9 |
| RTKN2 | 1.696929199 | 6.166705232 | 7.344341435 | 1.07E−10 | 1.03E−8 |
| TMEM204 | −1.048529786 | 6.26406167 | −7.165974961 | 2.43E−10 | 2.11E−8 |
| CD83 | 1.134658456 | 5.71435664 | 7.163495775 | 2.45E−10 | 2.12E−8 |
| FABP4 | 1.58545563 | 4.778254935 | 7.12319994 | 2.95E−10 | 2.51E−8 |
| TINAGL1 | −1.026326535 | 5.532226715 | −7.093847539 | 3.38E−10 | 2.84E−8 |
| ABCC8 | −1.278473261 | 3.740007718 | −7.069781506 | 3.77E−10 | 3.09E−8 |
| VCAN | −1.162194417 | 5.676393323 | −7.050563618 | 4.11E−10 | 3.29E−8 |
| ISLR | 1.017944512 | 5.018753667 | 7.010420181 | 4.94E−10 | 3.80E−8 |
| APOLD1 | −1.385170588 | 5.098238511 | −6.976021904 | 5.78E−10 | 4.35E−8 |
| DDIT4 | −1.34772118 | 5.257280573 | −6.808572358 | 1.23E−9 | 8.33E−8 |
| ARRDC2 | −1.061269085 | 5.499220509 | −6.762488227 | 1.52E−9 | 1.01E−7 |
| GPR174 | 1.391314128 | 4.486280437 | 6.747714106 | 1.63E−9 | 1.07E−7 |
| SERPINA3 | −1.612709918 | 5.391772667 | −6.745585747 | 1.64E−9 | 1.07E−7 |
| PIK3R3 | −1.190376757 | 5.660292101 | −6.728449631 | 1.77E−9 | 1.14E−7 |
| SLAMF7 | 1.083604552 | 4.392800911 | 6.727219831 | 1.78E−9 | 1.14E−7 |
| WIF1 | 1.237156067 | 6.258872842 | 6.708747667 | 1.94E−9 | 1.24E−7 |
| ADM | −1.075662424 | 5.196278444 | −6.701236397 | 2.00E−9 | 1.28E−7 |
| PNMT | −1.051631588 | 4.068574241 | −6.553848929 | 3.88E−9 | 2.30E−7 |
| CTSW | 1.07957302 | 4.747480058 | 6.545368949 | 4.04E−9 | 2.38E−7 |
| BTNL9 | −1.132836349 | 5.578696855 | −6.494296937 | 5.07E−9 | 2.82E−7 |
| CHIT1 | 1.774209888 | 5.594573377 | 6.490067624 | 5.17E−9 | 2.87E−7 |
| CRISPLD2 | −1.078448778 | 5.7156417 | −6.459148182 | 5.93E−9 | 3.26E−7 |
| CPA3 | 1.002710147 | 5.278769406 | 6.398904433 | 7.75E−9 | 4.12E−7 |
| BMP1 | 1.049814522 | 5.450758141 | 6.3426456 | 9.95E−9 | 5.12E−7 |
| SLCO2A1 | −1.051736858 | 6.743524273 | −6.312993603 | 1.13E−8 | 5.69E−7 |
| VNN2 | −1.015536891 | 4.57616008 | −6.274690529 | 1.34E−8 | 6.55E−7 |
| TUBB1 | −1.110536854 | 3.804924924 | −6.264199999 | 1.41E−8 | 6.82E−7 |
| PSAT1 | −1.190066031 | 3.005323949 | −6.258737184 | 1.44E−8 | 6.94E−7 |
| EGR3 | 1.186935902 | 4.097335491 | 6.248573488 | 1.51E−8 | 7.22E−7 |
| CX3CR1 | 1.284828947 | 5.212105592 | 6.144966898 | 2.38E−8 | 1.08E−6 |
| EGR1 | 1.194905541 | 7.538328018 | 6.08564001 | 3.08E−8 | 1.35E−6 |
| TREM2 | 1.128703086 | 3.987081843 | 6.03163178 | 3.90E−8 | 1.67E−6 |
| ATF3 | 1.034162475 | 4.884273698 | 5.696410877 | 1.65E−7 | 5.92E−6 |
| PPBP | −1.424569764 | 3.860588827 | −5.646955582 | 2.04E−7 | 7.16E−6 |
| ICAM4 | 1.390911425 | 5.019641081 | 5.625030952 | 2.24E−7 | 7.74E−6 |
| DEFA4 | −1.206319405 | 2.718323142 | −5.614366702 | 2.34E−7 | 8.06E−6 |
| C8B | 1.199289417 | 3.724442441 | 5.595043977 | 2.54E−7 | 8.65E−6 |
| NR4A3 | 1.153028606 | 4.646253713 | 5.56155394 | 2.93E−7 | 9.79E−6 |
| CCL8 | 1.533954481 | 3.932933151 | 5.448623389 | 4.71E−7 | 1.48E−5 |
| AQP9 | −1.182290805 | 4.835881555 | −5.430616006 | 5.08E−7 | 1.56E−5 |
| HAS2 | 1.525864945 | 3.562127425 | 5.306311934 | 8.51E−7 | 2.44E−5 |
| TTN | −1.043207507 | 3.817413024 | −5.293238479 | 8.98E−7 | 2.56E−5 |
| C4BPA | 1.055176798 | 7.356691544 | 5.268476209 | 9.95E−7 | 2.79E−5 |
| ITGB6 | 1.140444449 | 6.076736655 | 5.260091655 | 1.03E−6 | 2.86E−5 |
| PDK4 | −1.021408815 | 6.733080202 | −5.134267178 | 1.72E−6 | 4.47E−5 |
| HBEGF | 1.032852155 | 5.680806271 | 5.061817586 | 2.31E−6 | 5.73E−5 |
| MT1L | −1.182918274 | 2.526143932 | −4.990697548 | 3.08E−6 | 7.38E−5 |
| MT1X | −1.137570423 | 3.368378253 | −4.974584006 | 3.29E−6 | 7.77E−5 |
| AGTR2 | 1.364930071 | 3.524442993 | 4.842112874 | 5.57E−6 | 0.000119643 |
| ORM1 | −1.331793001 | 2.918107873 | −4.800748967 | 6.56E−6 | 0.000138298 |
| CHRM2 | −1.011281909 | 2.379732249 | −4.689445874 | 1.01E−5 | 0.000200001 |
| SERPIND1 | 1.253485683 | 3.227485742 | 4.600275561 | 1.43E−5 | 0.000265554 |
| CEACAM5 | 1.121861431 | 3.16229753 | 4.495719542 | 2.14E−5 | 0.000368719 |
| SFRP2 | 1.129656517 | 4.14961175 | 4.486856217 | 2.21E−5 | 0.000377179 |
| SELE | 1.138034748 | 3.853029685 | 3.80214458 | 0.00026654 | 0.003035211 |
| PTX3 | −1.402248844 | 4.135304651 | −3.574413207 | 0.000577009 | 0.005747801 |
| HLA‐DRB1 | 2.096264176 | 4.01015487 | 3.496867141 | 0.000745469 | 0.007027456 |
| HLA‐DRB5 | 2.134896841 | 3.007061623 | 3.473802838 | 0.000803932 | 0.007481531 |
| EIF1AY | −1.506138016 | 3.020677733 | −3.35747922 | 0.00117078 | 0.010029593 |
| FGG | 1.286056757 | 4.918716551 | 2.965432506 | 0.003905804 | 0.026040868 |
| MSMB | 1.936339164 | 3.146192242 | 2.931036227 | 0.00432076 | 0.028265267 |
Figure 2.
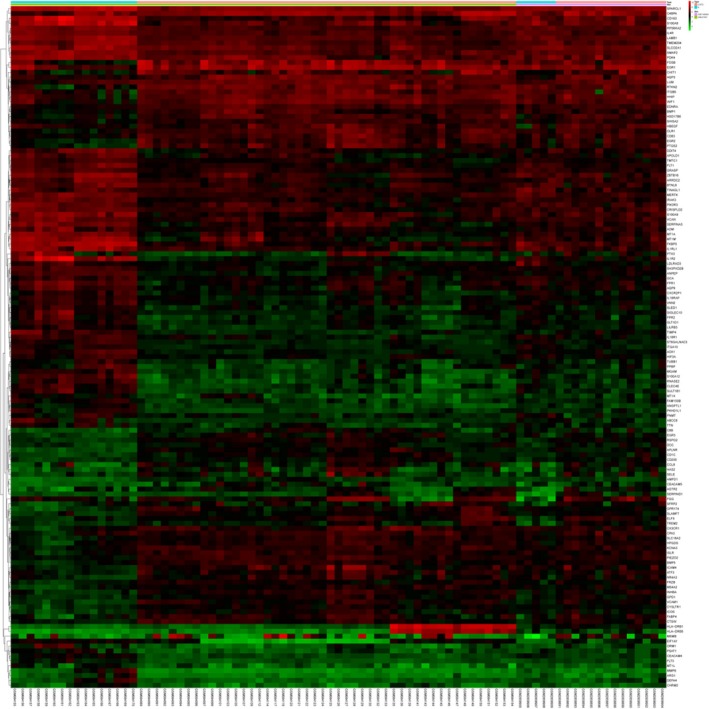
Hierarchical clustering heatmap of 139 DEGs screened on the basis of |FC| > 1 and a corrected P < 0.05. Red represents the up‐regulated DEGs, and green represents down‐regulated DEGs.
Figure 3.
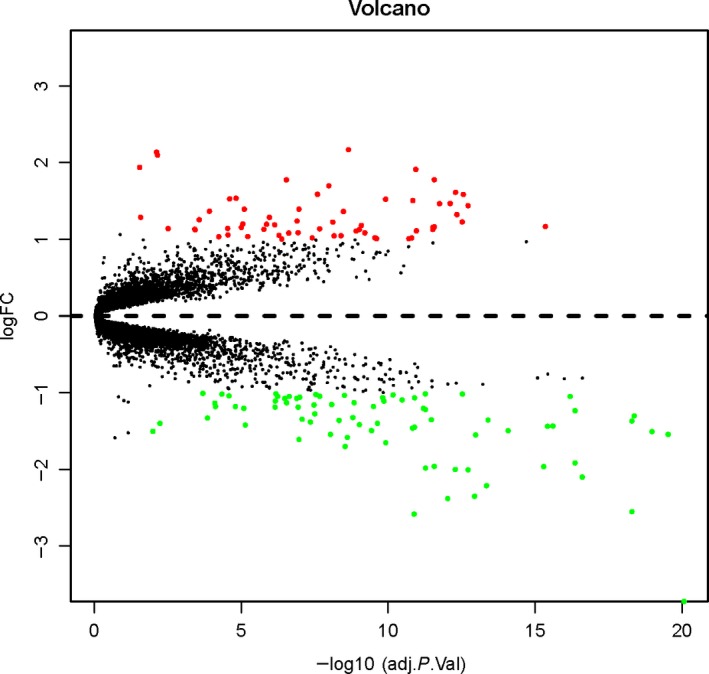
Volcano plots of differential gene expression data between two sets of samples. Red represents the up‐regulated DEGs, and green represents down‐regulated DEGs. adj.,P.Val, adjusted P‐value.
GO enrichment analysis of DEGs
GO analysis was done on the DEGs against BP, MF and CC terms. Biological annotation of the DEGs with COPD was identified using the david online analysis tool. As shown in Fig. 4, GO functional enrichments of the DEGs with a P‐value <0.05 were obtained. Significant results of the GO enrichment analysis of the DEGs associated with COPD were shown in Table 3. In the BP category, the DEGs were mainly involved in inflammatory response, immune response and response to lipopolysaccharide. In the MF category, the DEGs were mainly enriched in receptor activity and receptor for advanced glycation end products (RAGE) receptor binding. In the CC category, the DEGs were mainly involved in the extracellular region and space, the integral component of the plasma membrane, the plasma membrane and the external side of the plasma membrane (Fig. 5).
Figure 4.
GO enrichment analysis of DEGs in COPD. GO analysis divided DEGs into three functional groups: BPs, cell composition and MF. Green represents BP category, blue represents cell composition category and red represents MF category.
Table 3.
GO analysis of DEGs associated with COPD
| Term ID | Category | Description | Count | P‐value | Bonferroni |
|---|---|---|---|---|---|
| GO:0006954 | BP | Inflammatory response | 18 | 9.11E−9 | 0.0000088 |
| GO:0006955 | BP | Immune response | 18 | 4.26E−8 | 0.0000412 |
| GO:0032496 | BP | Response to lipopolysaccharide | 9 | 0.0000513 | 0.04832 |
| GO:0002523 | BP | Leukocyte migration involved in inflammatory response | 4 | 0.0000782 | 0.072795 |
| GO:0007155 | BP | Cell adhesion | 14 | 0.0000787 | 0.073195 |
| GO:0002576 | BP | Platelet degranulation | 7 | 0.000175 | 0.155654 |
| GO:0030198 | BP | Extracellular matrix organization | 9 | 0.000178 | 0.157584 |
| GO:0050729 | BP | Positive regulation of inflammatory response | 6 | 0.000292 | 0.245789 |
| GO:0006935 | BP | Chemotaxis | 7 | 0.000438 | 0.344834 |
| GO:0071294 | BP | Cellular response to zinc ion | 4 | 0.000438 | 0.345355 |
| GO:0045926 | BP | Negative regulation of growth | 4 | 0.000438 | 0.345355 |
| GO:0045600 | BP | Positive regulation of fat cell differentiation | 5 | 0.00053 | 0.400817 |
| GO:0050832 | BP | Defense response to fungus | 4 | 0.001263 | 0.705093 |
| GO:0060326 | BP | Cell chemotaxis | 5 | 0.001801 | 0.824787 |
| GO:0030593 | BP | Neutrophil chemotaxis | 5 | 0.001906 | 0.841641 |
| GO:0010043 | BP | Response to zinc ion | 4 | 0.002927 | 0.941058 |
| GO:0001501 | BP | Skeletal system development | 6 | 0.004843 | 0.990808 |
| GO:0007165 | BP | Signal transduction | 19 | 0.00503 | 0.992337 |
| GO:0002437 | BP | Inflammatory response to antigenic stimulus | 3 | 0.0062 | 0.997541 |
| GO:0030178 | BP | Negative regulation of Wnt signaling pathway | 4 | 0.008258 | 0.999668 |
| GO:0007263 | BP | Nitric oxide‐mediated signal transduction | 3 | 0.009889 | 0.999932 |
| GO:0071356 | BP | Cellular response to tumor necrosis factor | 5 | 0.011676 | 0.999988 |
| GO:0001666 | BP | Response to hypoxia | 6 | 0.012312 | 0.999994 |
| GO:0050776 | BP | Regulation of immune response | 6 | 0.014105 | 0.999999 |
| GO:0035924 | BP | Cellular response to vascular endothelial growth factor stimulus | 3 | 0.014331 | 0.999999 |
| GO:0002035 | BP | Brain renin‐angiotensin system | 2 | 0.015897 | 1 |
| GO:0070488 | BP | Neutrophil aggregation | 2 | 0.015897 | 1 |
| GO:0006952 | BP | Defense response | 4 | 0.016421 | 1 |
| GO:0032868 | BP | Response to insulin | 4 | 0.016421 | 1 |
| GO:0050900 | BP | Leukocyte migration | 5 | 0.016519 | 1 |
| GO:0001816 | BP | Cytokine production | 3 | 0.016818 | 1 |
| GO:0035987 | BP | Endodermal cell differentiation | 3 | 0.019474 | 1 |
| GO:0042476 | BP | Odontogenesis | 3 | 0.019474 | 1 |
| GO:0032689 | BP | Negative regulation of interferon‐gamma production | 3 | 0.020864 | 1 |
| GO:0010033 | BP | Response to organic substance | 3 | 0.020864 | 1 |
| GO:0071549 | BP | Cellular response to dexamethasone stimulus | 3 | 0.022294 | 1 |
| GO:0007204 | BP | Positive regulation of cytosolic calcium ion concentration | 5 | 0.022427 | 1 |
| GO:1900625 | BP | Positive regulation of monocyte aggregation | 2 | 0.023751 | 1 |
| GO:2001179 | BP | Regulation of interleukin‐10 secretion | 2 | 0.023751 | 1 |
| GO:0032602 | BP | Chemokine production | 2 | 0.023751 | 1 |
| GO:0070295 | BP | Renal water absorption | 2 | 0.023751 | 1 |
| GO:1902042 | BP | Negative regulation of extrinsic apoptotic signaling pathway via death domain receptors | 3 | 0.0284 | 1 |
| GO:0042742 | BP | Defense response to bacterium | 5 | 0.028971 | 1 |
| GO:0030307 | BP | Positive regulation of cell growth | 4 | 0.029638 | 1 |
| GO:0002793 | BP | Positive regulation of peptide secretion | 2 | 0.031543 | 1 |
| GO:0032673 | BP | Regulation of interleukin‐4 production | 2 | 0.031543 | 1 |
| GO:0032119 | BP | Sequestering of zinc ion | 2 | 0.031543 | 1 |
| GO:0072593 | BP | Reactive oxygen species metabolic process | 3 | 0.031676 | 1 |
| GO:0018108 | BP | Peptidyl‐tyrosine phosphorylation | 5 | 0.034254 | 1 |
| GO:0045786 | BP | Negative regulation of cell cycle | 3 | 0.035093 | 1 |
| GO:0042493 | BP | Response to drug | 7 | 0.03518 | 1 |
| GO:0007160 | BP | Cell‐matrix adhesion | 4 | 0.035316 | 1 |
| GO:0006953 | BP | Acute‐phase response | 3 | 0.038646 | 1 |
| GO:0002381 | BP | Immunoglobulin production involved in immunoglobulin‐mediated immune response | 2 | 0.039273 | 1 |
| GO:0008285 | BP | Negative regulation of cell proliferation | 8 | 0.039604 | 1 |
| GO:0043408 | BP | Regulation of mitogen‐activated protein kinase cascade | 3 | 0.042329 | 1 |
| GO:0002548 | BP | Monocyte chemotaxis | 3 | 0.044219 | 1 |
| GO:0045429 | BP | Positive regulation of nitric oxide biosynthetic process | 3 | 0.046139 | 1 |
| GO:0010042 | BP | Response to manganese ion | 2 | 0.046942 | 1 |
| GO:0038084 | BP | Vascular endothelial growth factor signaling pathway | 2 | 0.046942 | 1 |
| GO:0005576 | CC | Extracellular region | 44 | 2.35E−14 | 3.22E−12 |
| GO:0005615 | CC | Extracellular space | 40 | 4.29E−14 | 5.89E−12 |
| GO:0005887 | CC | Integral component of plasma membrane | 32 | 2.71E−8 | 0.00000371 |
| GO:0005886 | CC | Plasma membrane | 56 | 0.00000132 | 0.000181 |
| GO:0009897 | CC | External side of plasma membrane | 10 | 0.0000284 | 0.003882 |
| GO:0031093 | CC | Platelet alpha‐granule lumen | 5 | 0.000713 | 0.093083 |
| GO:0005578 | CC | Proteinaceous extracellular matrix | 8 | 0.003704 | 0.398509 |
| GO:0070062 | CC | Extracellular exosome | 32 | 0.011945 | 0.807247 |
| GO:0030666 | CC | Endocytic vesicle membrane | 4 | 0.012698 | 0.826358 |
| GO:0031012 | CC | Extracellular matrix | 7 | 0.02228 | 0.954358 |
| GO:0030669 | CC | Clathrin‐coated endocytic vesicle membrane | 3 | 0.03651 | 0.993875 |
| GO:0048471 | CC | Perinuclear region of cytoplasm | 10 | 0.039931 | 0.996238 |
| GO:0004872 | MF | Receptor activity | 10 | 0.0000321 | 0.009231 |
| GO:0050786 | MF | RAGE receptor binding | 4 | 0.0000611 | 0.017515 |
| GO:0004908 | MF | Interleukin‐1 receptor activity | 3 | 0.001097 | 0.271869 |
| GO:0005201 | MF | Extracellular matrix structural constituent | 4 | 0.013171 | 0.97833 |
| GO:0017147 | MF | Wnt protein binding | 3 | 0.02166 | 0.998215 |
| GO:0004896 | MF | Cytokine receptor activity | 3 | 0.028658 | 0.999776 |
| GO:0035662 | MF | Toll‐like receptor 4 binding | 2 | 0.029063 | 0.999801 |
| GO:0004982 | MF | N‐formyl peptide receptor activity | 2 | 0.029063 | 0.999801 |
| GO:0008201 | MF | Heparin binding | 5 | 0.030385 | 0.999866 |
| GO:0008083 | MF | Growth factor activity | 5 | 0.031597 | 0.999907 |
| GO:0004714 | MF | Transmembrane receptor protein tyrosine kinase activity | 3 | 0.031677 | 0.999909 |
| GO:0050544 | MF | Arachidonic acid binding | 2 | 0.036196 | 0.999976 |
| GO:0005160 | MF | Transforming growth factor‐beta receptor binding | 3 | 0.03807 | 0.999987 |
| GO:0005178 | MF | Integrin binding | 4 | 0.042233 | 0.999996 |
| GO:0042803 | MF | Protein homodimerization activity | 11 | 0.042565 | 0.999997 |
| GO:0004875 | MF | Complement receptor activity | 2 | 0.043278 | 0.999997 |
Figure 5.
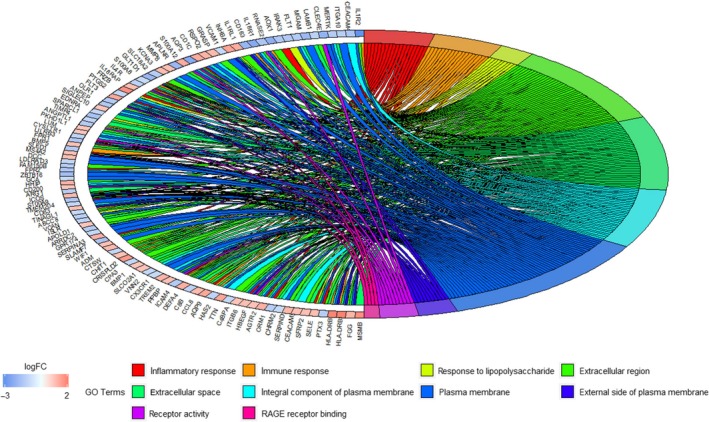
Distribution of DEGs in COPD for the most significant GO‐enriched functions.
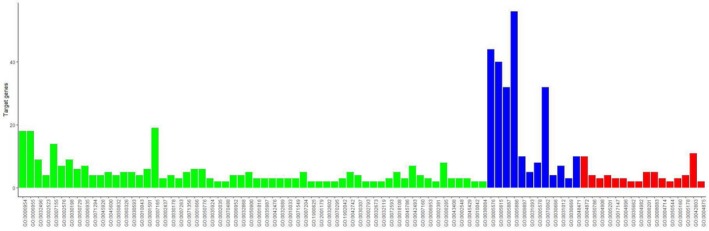
KEGG pathway analysis of DEGs
We analyzed the DEGs using the KOBAS 3.0 online analysis database. As shown in Table 4, the DEGs were enriched in 48 pathways, especially hematopoietic cell lineage and cytokine‐cytokine receptor interaction. The genes and pathway nodes are represented by cytoscape version 3.6.1 software that was used to calculate the topological characteristics of the network and determine each node (Fig. 6).
Table 4.
KEGG pathway analysis of DEGs associated with COPD
| ID | Pathway | Gene count | Corrected P‐value | DEGs |
|---|---|---|---|---|
| hsa04640 | Hematopoietic cell lineage | 7 | 4.07E−6 | FLT3, IL4R, HLA‐DRB1, HLA‐DRB5, IL1R2, CD1C, ANPEP |
| hsa04060 | Cytokine‐cytokine receptor interaction | 10 | 4.07E−6 | FLT3, FLT1, CCL8, IL4R, INHBA, CX3CR1, IL1R2, IL18RAP, PPBP, IL18R1 |
| hsa05150 | Staphylococcus aureus infection | 5 | 0.000147 | HLA‐DRB1, FPR2, FGG, FPR1, HLA‐DRB5 |
| hsa05166 | Human T‐lymphotropic virus type 1 infection | 8 | 0.000174 | PIK3R3, HLA‐DRB1, ATF3, HLA‐DRB5, VCAM1, IL1R2, EGR2, EGR1 |
| hsa05321 | Inflammatory bowel disease | 5 | 0.000174 | HLA‐DRB1, IL18RAP, IL4R, HLA‐DRB5, IL18R1 |
| hsa04514 | Cell adhesion molecules | 6 | 0.000456 | SELE, HLA‐DRB1, ICOS, HLA‐DRB5, VCAM1, VCAN |
| hsa04151 | Phosphoinositide 3‐kinase‐Akt signaling pathway | 8 | 0.000732 | CHRM2, PIK3R3, ITGA10, IL4R, LAMB1, DDIT4, ITGB6, FLT1 |
| hsa05146 | Amoebiasis | 5 | 0.000732 | C8B, PIK3R3, ARG1, LAMB1, IL1R2 |
| hsa04668 | Tumor necrosis factor signaling pathway | 5 | 0.001006 | VCAM1, PIK3R3, SELE, PTGS2, IL18R1 |
| hsa04080 | Neuroactive ligand‐receptor interaction | 7 | 0.00112 | CHRM2, FPR2, AGTR2, APLNR, EDNRA, CYSLTR1, FPR1 |
| hsa05200 | Pathways in cancer | 8 | 0.001401 | DCC, FLT3, ZBTB16, LAMB1, PIK3R3, HHIP, PTGS2, EDNRA |
| hsa04614 | Renin‐angiotensin system | 3 | 0.00144 | CPA3, AGTR2, ANPEP |
| hsa04610 | Complement and coagulation cascades | 4 | 0.002727 | FGG, C8B, C4BPA, SERPIND1 |
| hsa05310 | Asthma | 3 | 0.003037 | HLA‐DRB1, HLA‐DRB5, MS4A2 |
| hsa00750 | Vitamin B6 metabolism | 2 | 0.003805 | PSAT1, AOX1 |
| hsa05202 | Transcriptional misregulation in cancer | 5 | 0.005115 | FLT3, ZBTB16, FLT1, NR4A3, IL1R2 |
| hsa04933 | AGE‐RAGE signaling pathway in diabetic complications | 4 | 0.005115 | VCAM1, PIK3R3, SELE, EGR1 |
| hsa04510 | Focal adhesion | 5 | 0.007653 | PIK3R3, ITGA10, ITGB6, LAMB1, FLT1 |
| hsa04672 | Intestinal immune network for IgA production | 3 | 0.007653 | HLA‐DRB1, ICOS, HLA‐DRB5 |
| hsa05145 | Toxoplasmosis | 4 | 0.007715 | HLA‐DRB1, PIK3R3, LAMB1, HLA‐DRB5 |
| hsa04978 | Mineral absorption | 3 | 0.007715 | MT1X, MT1A, MT1M |
| hsa04923 | Regulation of lipolysis in adipocytes | 3 | 0.009035 | PIK3R3, PTGS2, FABP4 |
| hsa05221 | Acute myeloid leukemia | 3 | 0.009074 | FLT3, ZBTB16, PIK3R3 |
| hsa04380 | Osteoclast differentiation | 4 | 0.009515 | LILRB3, PIK3R3, FOSB, TREM2 |
| hsa01100 | Metabolic pathways | 12 | 0.00997 | PSAT1, HPGDS, ARG1, ST6GALNAC3, AOX1, PNMT, HSD17B6, AMPD1, PTGS2, CHIT1, MGAM, ANPEP |
| hsa04145 | Phagosome | 4 | 0.015537 | HLA‐DRB1, TUBB1, HLA‐DRB5, OLR1 |
| hsa05140 | Leishmaniasis | 3 | 0.015831 | HLA‐DRB1, PTGS2, HLA‐DRB5 |
| hsa04512 | Extracellular matrix‐receptor interaction | 3 | 0.02016 | ITGA10, ITGB6, LAMB1 |
| hsa05410 | Hypertrophic cardiomyopathy | 3 | 0.02016 | TTN, ITGA10, ITGB6 |
| hsa05222 | Small‐cell lung cancer | 3 | 0.021463 | PIK3R3, PTGS2, LAMB1 |
| hsa05414 | Dilated cardiomyopathy | 3 | 0.023455 | TTN, ITGA10, ITGB6 |
| hsa05323 | Rheumatoid arthritis | 3 | 0.023455 | HLA‐DRB1, FLT1, HLA‐DRB5 |
| hsa04062 | Chemokine signaling pathway | 4 | 0.023572 | CX3CR1, PIK3R3, CCL8, PPBP |
| hsa04915 | Estrogen signaling pathway | 3 | 0.027527 | FKBP5, PIK3R3, HBEGF |
| hsa04024 | cAMP signaling pathway | 4 | 0.027527 | CHRM2, PIK3R3, HHIP, EDNRA |
| hsa04810 | Regulation of actin cytoskeleton | 4 | 0.032602 | CHRM2, PIK3R3, ITGA10, ITGB6 |
| hsa00350 | Tyrosine metabolism | 2 | 0.032602 | AOX1, PNMT |
| hsa05020 | Prion diseases | 2 | 0.032602 | C8B, EGR1 |
| hsa05143 | African trypanosomiasis | 2 | 0.032602 | VCAM1, SELE |
| hsa05330 | Allograft rejection | 2 | 0.038438 | HLA‐DRB1, HLA‐DRB5 |
| hsa04722 | Neurotrophin signaling pathway | 3 | 0.038438 | IRAK3, PIK3R3, RPS6KA2 |
| hsa05332 | Graft‐versus‐host disease | 2 | 0.042328 | HLA‐DRB1, HLA‐DRB5 |
| hsa04940 | Type I diabetes mellitus | 2 | 0.04503 | HLA‐DRB1, HLA‐DRB5 |
| hsa04973 | Carbohydrate digestion and absorption | 2 | 0.047746 | MGAM, PIK3R3 |
| hsa05322 | Systemic lupus erythematosus | 3 | 0.048708 | HLA‐DRB1, C8B, HLA‐DRB5 |
| hsa04930 | Type II diabetes mellitus | 2 | 0.049139 | ABCC8, PIK3R3 |
| hsa05144 | Malaria | 2 | 0.049139 | VCAM1, SELE |
| hsa05030 | Cocaine addiction | 2 | 0.049139 | SLC18A2, FOSB |
Figure 6.
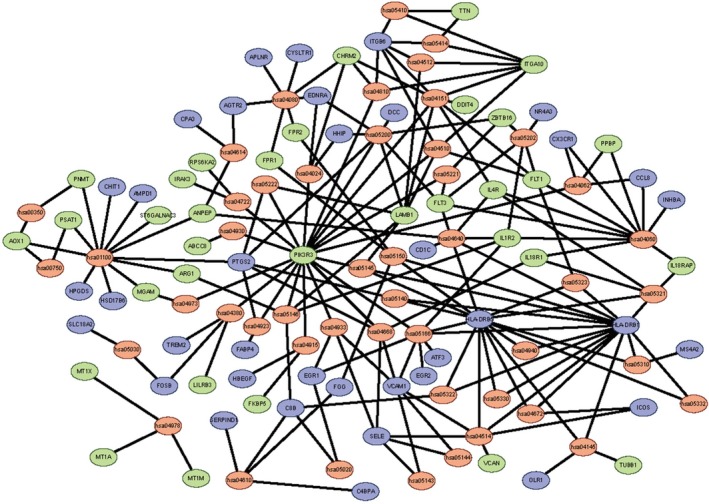
The significant KEGG pathways enrichment of DEGs. Green represents down‐regulated DEGs, blue represents up‐regulated DEGs and red represents the signaling pathway.
PPI network analysis of DEGs
The 139 DEGs were applied for construction of PPI networks using STRING. After removing the discrete and partially connected nodes, the PPI network data of DEGs were imported into the cytoHubba of cytoscape version 3.6.1, and a complex network of the DEGs was constructed. As shown in Figs 7 and 8, 14 Hub DEGs were obtained, including C‐X3‐C motif chemokine receptor 1 (CX3CR1), proplatelet basic protein (PPBP), prostaglandin‐endoperoxide synthase 2 (PTGS2), formyl peptide receptor 1 (FPR1), formyl peptide receptor 2 (FPR2), vascular cell adhesion molecule 1 (VCAM1), S100 calcium binding protein A12 (S100A12), arginase 1 (ARG1), early growth response 1 (EGR1), CD163, fibrinogen gamma chain (FGG), orosomucoid 1 (ORM1), S100 calcium binding protein A8 (S100A8) and S100 calcium binding protein A9 (S100A9).
Figure 7.
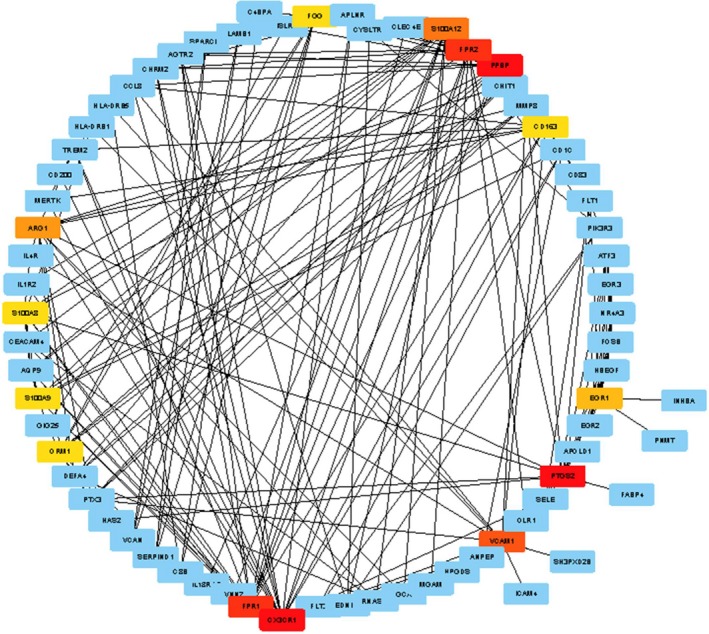
PPI network and Hub DEGs. Hub DEGs were identified with cytoscape version 3.6.1 with cytoHubba plugin, according to the rank of connection degree (number) for each gene, which is represented by the different degrees of color (from red to yellow): the role of the gene is greater in the PPI network with the darker color of the gene. Red, saffron yellow and yellow represent Hub DEGs.
Figure 8.
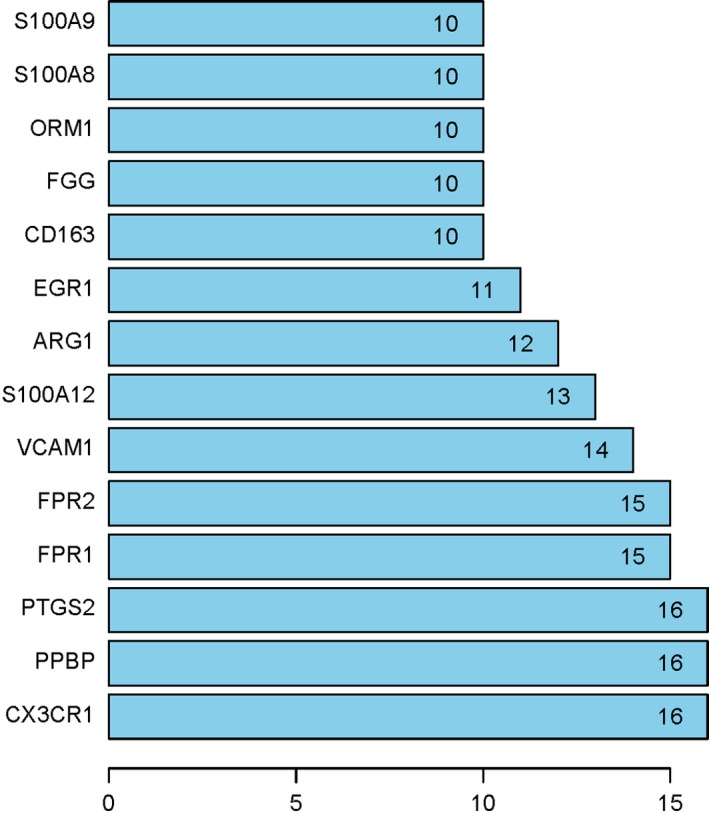
PPI network identified Hub DEGs. Numbers represent connection points of the 14 Hub genes identified by the cytoHubba plugin.
wgcna network construction in lung tissues
A wgcna network was first constructed using lung tissue expression data from cohorts GSE27597 and GSE106986, independent of COPD status and smoking status (ever/current smoking versus nonsmoking). A total of 2942 DEGs were selected (a corrected P < 0.05) and subsequently used to identify modules of coexpressed genes using a hierarchical clustering procedure. The corresponding branches of the resulting dynamic clustering tree and module are shown as colored bands underneath the cluster tree. We then merged the highly similar dynamic clustering modules into the merged dynamic modules (cut height = 0.25) (Fig. 9). We identified nine modules ranging in size from 113 genes in the Purple module to 1081 in the Grey module. A module eigengene, a weighted average of the module gene expression profiles, was used to summarize the expression profiles of transcripts in a given module through their first principal component.
Figure 9.
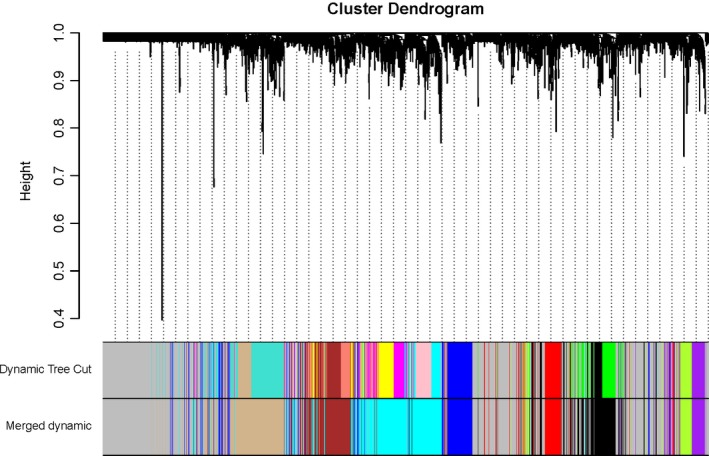
Network construction identifies distinct modules of coexpressed genes. The network was constructed using the lung tissue expression dataset of GSE27597 and GSE106986. The cluster dendrogram was produced by average linkage hierarchical clustering of genes using 1 − topological overlap as dissimilarity measure. Modules (Dynamic Tree Cut) and similarly merged modules (Merged dynamic) of coexpressed genes were assigned colors corresponding to the branches indicated by the horizontal bar beneath the dendrogram (merged cut height = 0.25).
Coexpression modules associated with COPD
To pinpoint modules associated with COPD and smoking status, we analyzed the association of each of the nine module eigengenes with the two traits. As shown in Fig. 10 and Table 5, all nine modules were significantly correlated with COPD and smoking status. Four modules were negatively associated with COPD and smoking status, marked Tan, Brown, Blue and Cyan, indicating that genes in these modules were predominantly down‐regulated in COPD cases and those who had a history of smoking. However, five modules, in Green yellow, Purple, Black, Red and Grey, were positively associated with COPD cases and smoking status, showing that genes in these modules are predominantly up‐regulated with the traits.
Figure 10.
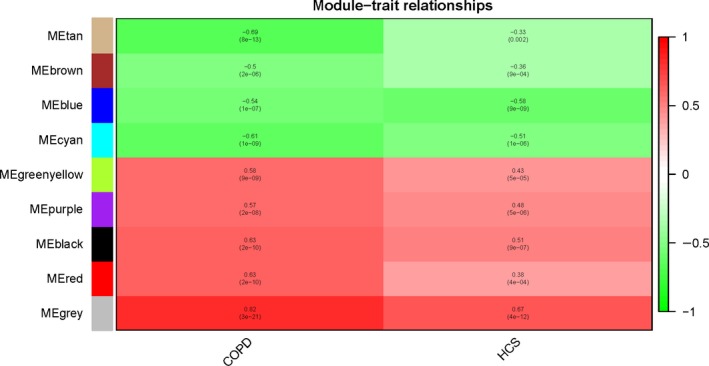
wgcna heatmap. Using the default parameter setting and all DEGs (n = 2942), we identified nine gene modules using wgcna that were positively or negatively associated with COPD and smoking trait. Each row corresponds to a module eigengene and each column to a clinical trait (COPD and smoking status). Positive associations are red, and negative associations are green. HCS, history of smoking.
Table 5.
Correlation of module eigengene with COPD and smoking status traits
| wgcna modules | Gene number | Merged COPD dataset | Smoking status | ||
|---|---|---|---|---|---|
| Correlation | P‐value | Correlation | P‐value | ||
| Tan | 353 | −0.69 | 8E−13 | −0.33 | 0.002 |
| Brown | 244 | −0.5 | 2E−6 | −0.36 | 9E−4 |
| Blue | 202 | −0.54 | 1E−7 | −0.58 | 9E−9 |
| Cyan | 467 | −0.61 | 1E−9 | −0.51 | 1E−6 |
| Green yellow | 105 | 0.58 | 9E−9 | 0.43 | 5E−5 |
| Purple | 113 | 0.57 | 2E−8 | 0.48 | 5E−6 |
| Black | 256 | 0.63 | 2E−10 | 0.51 | 9E−7 |
| Red | 121 | 0.63 | 2E−10 | 0.38 | 4E−4 |
| Grey | 1081 | 0.82 | 3E−21 | 0.67 | 4E−12 |
Four of these nine gene modules, in Cyan, Purple, Red and Grey, attracted our attention in that 14 Hub genes were identified as DEGs from the PPI analysis, including CX3CR1, PPBP, PTGS2, FPR1, FPR2, S100A12, ARG1, EGR1, CD163, VCAM1, FGG, ORM1, S100A8 and S100A9. We calculated gene significance (GS) versus each MM. We found that the 14 Hub genes were also either positively or negatively associated with COPD (Table 6). CX3CR1, PPBP, PTGS2, VCAM1, S100A12, ARG1, EGR1, CD163, S100A8 and S100A9 were significantly associated with each MM, whereas FPR1, FPR2 and ORM1 were correlated with each MM except Red MM, and FGG was correlated to each MM except the Purple MM. In addition, we found that the Purple (CX3CR1), Red (EGR1, VCAM1 and PTGS2) and Grey (ARG1, FGG, and PPBP) genes most significantly correlated with GS for COPD were also the important MM elements (Fig. 11).
Table 6.
Fourteen Hub genes positively or negatively associated with COPD and each MM. P, P‐value for COPD or each MM
| Gene | Located module | GS.COPD | P GS.COPD | P.MM Cyan | P.MM Grey | P.MM Purple | P.MM Red |
|---|---|---|---|---|---|---|---|
| CD163 | Cyan | −0.739974023 | 1.33E−15 | 4.23E−21 | 8.88E−10 | 0.00249 | 2.57E−5 |
| FPR1 | Cyan | −0.662223538 | 9.25E−12 | 7.92E−23 | 5.84E−7 | 6.61E−5 | 0.07339 |
| FPR2 | Cyan | −0.63983063 | 7.43E−11 | 1.37E−21 | 4.51E−7 | 6.73E−5 | 0.07508 |
| ORM1 | Cyan | −0.459721444 | 1.23E−5 | 3.81E−20 | 0.00014 | 0.00196 | 0.70155 |
| S100A12 | Cyan | −0.721577872 | 1.41E−14 | 1.50E−14 | 2.82E−7 | 4.13E−5 | 0.00278 |
| S100A8 | Cyan | −0.70598989 | 9.04E−14 | 6.83E−18 | 4.01E−7 | 0.00026 | 0.01347 |
| S100A9 | Cyan | −0.623468037 | 3.07E−10 | 7.84E−20 | 4.68E−6 | 0.00049 | 0.04290 |
| ARG1 | Grey | −0.632480057 | 1.42E−10 | 0.02374 | 2.47E−10 | 0.00054 | 2.19E−9 |
| FGG | Grey | 0.304097261 | 0.00519 | 0.00646 | 0.005969 | 0.2425 | 3.39E−8 |
| PPBP | Grey | −0.520314885 | 4.61E−7 | 5.44E−6 | 0.001842 | 0.0388 | 0.01072 |
| CX3CR1 | Purple | 0.553212675 | 5.84E−8 | 0.00133 | 9.38E−10 | 1.81E−13 | 4.46E−5 |
| EGR1 | Red | 0.549754835 | 7.33E−8 | 0.00127 | 2.09E−6 | 0.03903 | 1.80E−14 |
| PTGS2 | Red | 0.698684808 | 2.07E−13 | 0.00341 | 2.42E−11 | 4.99E−5 | 1.10E−28 |
| VCAM1 | Red | 0.7344482490 | 2.75E−15 | 0.00947 | 2.66E−12 | 5.70E−13 | 3.14E−16 |
Figure 11.
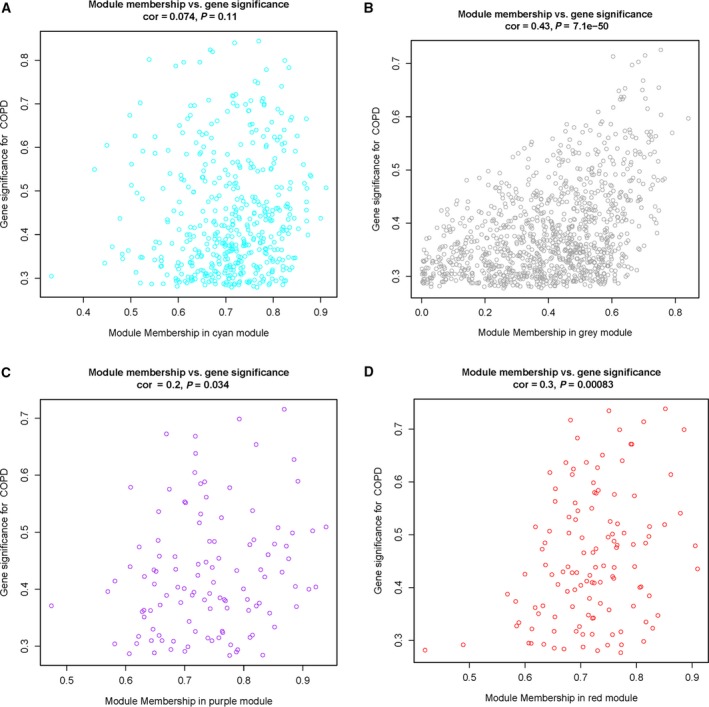
COPD absolute GS versus MM. wgcna calculation of GS to COPD versus MM. In oversimplified terms, MM is a measure of how ‘tight’ genes cluster within the module, or mathematically, how close gene expression is to the module eigenvalue. A gene with high MM and GS identifies Hub genes that are both key components to the underlying BP and highly associated with the trait of interest. The GS for COPD was plotted. (A) Cyan represents MM. (B) Grey represents MM. (C) Purple represents MM. (D) Red represents MM.
Discussion
In this study, we performed an integrated analysis on the gene expression profiles from lung tissues with or without COPD, aiming to identify the DEGs and related key signaling pathways for the disease. We identified 139 DEGs, including 62 up‐regulated genes and 77 down‐regulated genes. In addition, GO analysis showed that the 139 DEGs associated with COPD were involved in 60 BPs, 16 MFs and 12 CCs. Among these categories, the most important BPs included inflammatory response, immune response and response to lipopolysaccharide; the most important MFs included receptor activity and RAGE receptor binding; and the most important CCs included extracellular region and space, integral component of plasma membrane, plasma membrane and external side of plasma membrane. This finding accords with the knowledge that COPD is characterized by chronic inflammation in the lung and airways 28, 29; immune response mediates the development of COPD caused by the harmful stimuli 30, 31, 32; lipopolysaccharide may lead to increased airway and systemic inflammation, and contribute to the progressive deterioration of lung function 33, 34; and RAGE is a ‘driving force’ for cigarette smoke (CS)‐induced airway inflammation in COPD 35.
KEGG pathway analysis indicated that 48 pathways corresponded to these DEGs associated with COPD. Two pathways including hematopoietic cell lineage and cytokine‐cytokine receptor interaction were most important. This finding is in line with the results from previous studies 18, 32.
The PPI network of proteins encoded by DEGs identified 14 Hub DEGs associated with COPD, including CX3CR1, PPBP, PTGS2, FPR1, FPR2, VCAM1, S100A12, ARG1, EGR1, CD163, FGG, ORM1, S100A8 and S100A9. All of these Hub genes were involved in the most important two BPs, two MFs or five CCs revealed by GO analysis, and were mainly implicated in multiple pathways identified by KEGG analysis in this study. Those results indicate that these Hub DEGs are involved in the development and progression of COPD by playing important biological roles in multiple signaling pathways.
Using wgcna on the merged expression profile from two cohorts of lung tissues with COPD and healthy controls, we identified a set of gene signatures based on the 14 Hub genes. The increased expression of CX3CR1, FGG, EGR1, VCAM1 and PTGS2 is positively associated with COPD, and the underexpression of PPBP, FPR1, FPR2, S100A12, ARG1, CD163, ORM1, S100A8 and S100A9 is negatively associated with COPD.
CX3CR1 plays an important role in the development of chronic inflammatory lung diseases, such as COPD and emphysema, by contributing to structural destruction and remodeling. Chemoattractant inflammatory cells releasing CX3CR1, such as CD8−/CD4, dendritic cells, γδ T lymphocytes, natural killer cells and monocytes/macrophages, may lead to mononuclear cell accumulation in the parenchyma and lung vessel walls, release mediators to induce injury, stimulate proliferation and chemoattractant inflammatory cells 36. In addition, CX3CR1 + mononuclear phagocytes may induce an innate immune response to CS via producing interleukin‐6 and tumor necrosis factor‐α, and contribute to emphysema 37.
PTGS2 (COX‐2), an important mediator of inflammation, was shown to be involved in inflammation response and associated with COPD pathogenesis 38, 39, 40, 41. The decreased activity of PTGS2 may protect smokers against the development of COPD 40. Furthermore, FPR1 and FPR2 were reported to be involved in recruitment and activation of inflammatory cells induced by CS, and play important roles in lung inflammation, injury and the pathogenesis of COPD 42, 43, 44, 45.
S100A8, S100A9, and S100A12 might induce neutrophil and monocyte chemotaxis, adhesion to fibrinogen and diapedesis, and neutrophil migration to inflammatory sites 46, 47, and have been identified as key biomarkers in inflammatory diseases including COPD and neutrophil‐dominated infections 35, 48, 49. The mRNA levels of S100A8, S100A9 and S100A12 may be regulated by RAGE, which was shown to contribute to CS‐induced airway inflammation in COPD 35. This is consistent with our result in this study that the RAGE pathway including S100A8, S100A9 and S100A12 is important in the development of COPD.
EGR1 is an autophagy regulator gene that plays important roles in cellular homeostasis, airway remodeling and control of inflammatory immune response; it is also a significant risk factor for susceptibility to COPD 50, 51, 52. EGR1 may be induced by CS and involved in proinflammatory mechanisms that are likely associated with the development of COPD 51, whereas Egr‐1−/− mice were observed to resist CS‐induced autophagy, apoptosis and emphysema 53. These findings exhibit a critical role for EGR1 in CS‐induced inflammatory immune response and COPD, and effective inhibition of EGR1 may attenuate airway remodeling and inflammation associated with the pathology of COPD.
CD163, a carefully regulated component of the innate immune response to infection and a macrophage receptor for bacteria, was shown to play important roles in functional pulmonary defense elements and the inflammatory immune response of the respiratory system 54, 55. Overexpression of CD163 on lung alveolar macrophages may be implicated in the pathogenesis of COPD and poor lung function 56.
ARG1 was shown to contribute to asthma pathogenesis by inhibiting nitric oxide production, modulating fibrosis, regulating arginine metabolism and inhibiting T cell proliferation, and it involves the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation by regulating group 2 innate lymphoid cells 57, 58, 59, 60, whereas ARG1 ablation in the lung may enhance peripheral lung function but have little effect on airway inflammation 61. The role of ARG1 in COPD needs to be studied in the future.
ORM1 appears to function in regulating the activity of the immune system during the acute‐phase reaction and has been identified as an acute exacerbation of COPD‐specific immunomodulatory mediator 62. PPBP serves as a potent neutrophil chemoattractant and activator, and its elevated expression in the bronchial mucosa might be involved in the pathogenesis of COPD 63, 64. In addition, VCAM1 was shown to express in endothelial cells of atopic asthma cases, but not COPD cases 65, and present an association with lung function 66. FGG was found to be involved in blast lung injury resistance via promoting tissue‐protective adenosine signaling 67. In the lung tissues of COPD cases, its mRNA expression was reported to correlate with the burden of particulate matter in total lung and lung parenchyma 68.
In conclusion, we have identified 139 candidate DEGs associated with the progression of COPD. The results from bioinformatic analysis are in agreement with those from previous cell and animal models and human studies. Our results showed that nine Hub genes, CX3CR1, PTGS2, FPR1, FPR2, S100A12, EGR1, CD163, S100A8 and S100A9, potentially mediated inflammation and injury of the lung, and play critical roles in the pathogenesis of COPD. The roles of five Hub genes, including PPBP, ARG1, FGG, VCAM1 and ORM1, identified to be associated with COPD in this study need to be confirmed in the future. These findings could improve our understanding of the underlying molecular mechanisms of COPD and provide us with insights for drug target discovery for the disease.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
XK, FY and QW conceived and designed the project. XH and YL acquired the data. XG and ZZ analyzed and interpreted the data. XH and YL wrote the paper.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (81460007), Natural Science Foundation of Yunnan Province, China (2009CD130), and two funds of the Department of Education Scientific Research of Yunnan Province, China (09C0294 and 2015Z085).
Xinwei Huang and Yunwei Li contributed equally to this work
Contributor Information
Xiangyang Kong, Email: 3517826707@qq.com.
Fubing Yu, Email: yufb0402@163.com.
Qiang Wang, Email: tjzxwangqiang@163.com.
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol 53, 128–149. [DOI] [PubMed] [Google Scholar]
- 2. Song Q, Christiani DC, Wang X and Ren J (2014) The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Int J Environ Res Public Health 11, 11822–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2015 Chronic Respiratory Disease Collaborators (2017) Global, regional, and national deaths, prevalence, disability‐adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 5, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBD 2017 DALYs and HALE Collaborators (2018) Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez AD and Murray CC (1998) The global burden of disease, 1990–2020. Nat Med 4, 1241–1243. [DOI] [PubMed] [Google Scholar]
- 8. GBD 2015 Mortality and Causes of Death Collaborators (2016) Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD 2016 Causes of Death Collaborators (2017) Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathers CD and Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3, e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2015 DALYs and HALE Collaborators (2016) Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez AD, Mathers CD, Ezzati M, Jamison DT and Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367, 1747–1757. [DOI] [PubMed] [Google Scholar]
- 13. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S et al (2012) Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223. [DOI] [PubMed] [Google Scholar]
- 14. Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortopassi F, Gurung P and Pinto‐Plata V (2017) Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med 33, 539–552. [DOI] [PubMed] [Google Scholar]
- 16. Yang X, Zhu S, Li L, Zhang L, Xian S, Wang Y and Cheng Y (2018) Identification of differentially expressed genes and signaling pathways in ovarian cancer by integrated bioinformatics analysis. Onco Targets Ther 11, 1457–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W, Hong YQ and Meng ZL (2014) Bioinformatics analysis of molecular mechanisms of chronic obstructive pulmonary disease. Eur Rev Med Pharmacol Sci 18, 3557–3563. [PubMed] [Google Scholar]
- 18. Zhao J, Cheng W, He X, Liu Y, Li J, Sun J, Li J, Wang F and Gao Y (2018) Chronic obstructive pulmonary disease molecular subtyping and pathway deviation‐based candidate gene identification. Cell J 20, 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO et al (2012) A gene expression signature of emphysema‐related lung destruction and its reversal by the tripeptide GHK. Genome Med 4, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinbockel L, Marwitz S, Schromm AB, Watz H, Kugler C, Ammerpohl O, Schnepf K, Rabe KF, Droemann D and Goldmann T (2018) Identification of novel target genes in human lung tissue involved in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 13, 2255–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. da Huang W, Sherman BT and Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57. [DOI] [PubMed] [Google Scholar]
- 22. Huang DW, Sherman BT and Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY and Wei L (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39, W316–W322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P et al (2017) The STRING database in 2017: quality‐controlled protein‐protein association networks, made broadly accessible. Nucleic Acids Res 45, D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P and Snel B (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and Lin CY (2014) cytoHubba: identifying hub objects and sub‐networks from complex interactome. BMC Syst Biol 8 (Suppl 4), S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langfelder P and Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barnes PJ (2019) Inflammatory endotypes in COPD. Allergy 74, 1249–1256. [DOI] [PubMed] [Google Scholar]
- 29. Barnes PJ (2017) Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond) 131, 1541–1558. [DOI] [PubMed] [Google Scholar]
- 30. Hopkins RJ and Young RP (2019) Mevalonate signaling, COPD and cancer: the statins and beyond. J Investig Med 67, 711–714. [DOI] [PubMed] [Google Scholar]
- 31. Bruno A, Cipollina C, Di Vincenzo S, Siena L, Dino P, Di Gaudio F, Gjomarkaj M and Pace E (2017) Ceftaroline modulates the innate immune and host defense responses of immunocompetent cells exposed to cigarette smoke. Toxicol Lett 279, 9–15. [DOI] [PubMed] [Google Scholar]
- 32. Bi H, Zhou J, Wu D, Gao W, Li L, Yu L, Liu F, Huang M, Adcock IM, Barnes PJ et al (2015) Microarray analysis of long non‐coding RNAs in COPD lung tissue. Inflamm Res 64, 119–126. [DOI] [PubMed] [Google Scholar]
- 33. Gupta V, Banyard A, Mullan A, Sriskantharajah S, Southworth T and Singh D (2015) Characterization of the inflammatory response to inhaled lipopolysaccharide in mild to moderate chronic obstructive pulmonary disease. Br J Clin Pharmacol 79, 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D'Anna C, Cigna D, Di Sano C, Di Vincenzo S, Dino P, Ferraro M, Bini L, Bianchi L, Di Gaudio F, Gjomarkaj M et al (2017) Exposure to cigarette smoke extract and lipopolysaccharide modifies cytoskeleton organization in bronchial epithelial cells. Exp Lung Res 43, 347–358. [DOI] [PubMed] [Google Scholar]
- 35. Li M, Guo L, Wang H, Wang T, Shen Y, Liao Z, Wen F and Chen L (2015) RAGE‐ligands axis: a new ‘driving force’ for cigarette smoke‐induced airway inflammation in COPD? Respirology 20, 998–999. [DOI] [PubMed] [Google Scholar]
- 36. Zhang J and Patel JM (2010) Role of the CX3CL1‐CX3CR36 axis in chronic inflammatory lung diseases. Int J Clin Exp Med 3, 233–244. [PMC free article] [PubMed] [Google Scholar]
- 37. Xiong Z, Leme AS, Ray P, Shapiro SD and Lee JS (2011) CX3CR37+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF‐alpha and IL‐6 and promote cigarette smoke‐induced emphysema. J Immunol 186, 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu L, Merrilees M, Young RP and Black PN (2011) The cyclooxygenase‐2‐765C promoter polymorphism protects against the development of chronic obstructive pulmonary disease. Respir Med 105, 506–510. [DOI] [PubMed] [Google Scholar]
- 39. Bonanno A, Albano GD, Siena L, Montalbano AM, Riccobono L, Anzalone G, Chiappara G, Gagliardo R, Profita M and Sala A (2016) Prostaglandin E(2) possesses different potencies in inducing vascular endothelial growth factor and interleukin‐8 production in COPD human lung fibroblasts. Prostaglandins Leukot Essent Fatty Acids 106, 11–18. [DOI] [PubMed] [Google Scholar]
- 40. Pietras T, Szemraj J, Panek M, Witusik A, Banasiak M, Antczak A and Gorski P (2012) Functional polymorphism of cyclooxygenase‐2 gene (G‐765C) in chronic obstructive pulmonary disease patients. Mol Biol Rep 39, 2163–2167. [DOI] [PubMed] [Google Scholar]
- 41. Zago M, Sheridan JA, Traboulsi H, Hecht E, Zhang Y, Guerrina N, Matthews J, Nair P, Eidelman DH, Hamid Q et al (2017) Low levels of the AhR in chronic obstructive pulmonary disease (COPD)‐derived lung cells increases COX‐2 protein by altering mRNA stability. PLoS One 12, e0180881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen YC, Lin MC, Lee CH, Liu SF, Wang CC, Fang WF, Chao TY, Wu CC, Wei YF, Chang HC et al (2018) Defective formyl peptide receptor 2/3 and annexin A1 expressions associated with M2a polarization of blood immune cells in patients with chronic obstructive pulmonary disease. J Transl Med 16, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardini S, Dalli J, Fineschi S, Perretti M, Lungarella G and Lucattelli M (2012) Genetic ablation of the fpr1 gene confers protection from smoking‐induced lung emphysema in mice. Am J Respir Cell Mol Biol 47, 332–339. [DOI] [PubMed] [Google Scholar]
- 44. Migeotte I, Communi D and Parmentier M (2006) Formyl peptide receptors: a promiscuous subfamily of G protein‐coupled receptors controlling immune responses. Cytokine Growth Factor Rev 17, 501–519. [DOI] [PubMed] [Google Scholar]
- 45. Hartt JK, Barish G, Murphy PM and Gao JL (1999) N‐formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N‐formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med 190, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryckman C, Vandal K, Rouleau P, Talbot M and Tessier PA (2003) Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 170, 3233–3242. [DOI] [PubMed] [Google Scholar]
- 47. Rouleau P, Vandal K, Ryckman C, Poubelle PE, Boivin A, Talbot M and Tessier PA (2003) The calcium‐binding protein S100A12 induces neutrophil adhesion, migration, and release from bone marrow in mouse at concentrations similar to those found in human inflammatory arthritis. Clin Immunol 107, 46–54. [DOI] [PubMed] [Google Scholar]
- 48. Kang JH, Hwang SM and Chung IY (2015) S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal‐regulated kinase and nuclear factor‐kappaB pathways. Immunology 144, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lorenz E, Muhlebach MS, Tessier PA, Alexis NE, Duncan Hite R, Seeds MC, Peden DB and Meredith W (2008) Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med 102, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kramer EL, Hardie WD, Mushaben EM, Acciani TH, Pastura PA, Korfhagen TR, Hershey GK, Whitsett JA and Le Cras TD (2011) Rapamycin decreases airway remodeling and hyperreactivity in a transgenic model of noninflammatory lung disease. J Appl Physiol (1985) 111, 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reynolds PR, Cosio MG and Hoidal JR (2006) Cigarette smoke‐induced Egr‐1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am J Respir Cell Mol Biol 35, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen CZ, Ou CY, Wang RH, Lee CH, Lin CC, Chang HY and Hsiue TR (2015) Association of Egr‐1 and autophagy‐related gene polymorphism in men with chronic obstructive pulmonary disease. J Formos Med Assoc 114, 750–755. [DOI] [PubMed] [Google Scholar]
- 53. Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali‐Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA et al (2008) Egr‐1 regulates autophagy in cigarette smoke‐induced chronic obstructive pulmonary disease. PLoS One 3, e3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weaver LK, Pioli PA, Wardwell K, Vogel SN and Guyre PM (2007) Up‐regulation of human monocyte CD163 upon activation of cell‐surface Toll‐like receptors. J Leukoc Biol 81, 663–671. [DOI] [PubMed] [Google Scholar]
- 55. Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD and van den Berg TK (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113, 887–892. [DOI] [PubMed] [Google Scholar]
- 56. Kaku Y, Imaoka H, Morimatsu Y, Komohara Y, Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M et al (2014) Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One 9, e87400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sy HY, Ko FW, Chu HY, Chan IH, Wong GW, Hui DS and Leung TF (2012) Asthma and bronchodilator responsiveness are associated with polymorphic markers of ARG1, CRHR2 and chromosome 17q21. Pharmacogenet Genomics 22, 517–524. [DOI] [PubMed] [Google Scholar]
- 58. Barron L, Smith AM, El Kasmi KC, Qualls JE, Huang X, Cheever A, Borthwick LA, Wilson MS, Murray PJ and Wynn TA (2013) Role of arginase 1 from myeloid cells in th2‐dominated lung inflammation. PLoS One 8, e61961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Halim TY, Steer CA, Matha L, Gold MJ, Martinez‐Gonzalez I, McNagny KM, McKenzie AN and Takei F (2014) Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation. Immunity 40, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait Wojno ED, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald HR et al (2016) Arginase 1 is an innate lymphoid‐cell‐intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cloots RH, Sankaranarayanan S, de Theije CC, Poynter ME, Terwindt E, van Dijk P, Hakvoort TB, Lamers WH and Kohler SE (2013) Ablation of Arg1 in hematopoietic cells improves respiratory function of lung parenchyma, but not that of larger airways or inflammation in asthmatic mice. Am J Physiol Lung Cell Mol Physiol 305, L364–L376. [DOI] [PubMed] [Google Scholar]
- 62. Shi L, Zhu B, Xu M and Wang X (2018) Selection of AECOPD‐specific immunomodulatory biomarkers by integrating genomics and proteomics with clinical informatics. Cell Biol Toxicol 34, 109–123. [DOI] [PubMed] [Google Scholar]
- 63. Smith NL, Bromley MJ, Denning DW, Simpson A and Bowyer P (2015) Elevated levels of the neutrophil chemoattractant pro‐platelet basic protein in macrophages from individuals with chronic and allergic aspergillosis. J Infect Dis 211, 651–660. [DOI] [PubMed] [Google Scholar]
- 64. Di Stefano A, Caramori G, Gnemmi I, Contoli M, Bristot L, Capelli A, Ricciardolo FL, Magno F, D'Anna SE, Zanini A et al (2009) Association of increased CCL5 and CXCL7 chemokine expression with neutrophil activation in severe stable COPD. Thorax 64, 968–975. [DOI] [PubMed] [Google Scholar]
- 65. Popper HH, Pailer S, Wurzinger G, Feldner H, Hesse C and Eber E (2002) Expression of adhesion molecules in allergic lung diseases. Virchows Arch 440, 172–180. [DOI] [PubMed] [Google Scholar]
- 66. Bhatt SP, Nath HP, Kim YI, Ramachandran R, Watts JR, Terry NLJ, Sonavane S, Deshmane SP, Woodruff PG, Oelsner EC et al (2018) Centrilobular emphysema and coronary artery calcification: mediation analysis in the SPIROMICS cohort. Respir Res 19, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagisawa K, Kinoshita M, Miyawaki H, Sato S, Miyazaki H, Takeoka S, Suzuki H, Iwaya K, Seki S, Shono S et al (2016) Fibrinogen gamma‐chain peptide‐coated adenosine 5′ diphosphate‐encapsulated liposomes rescue mice from lethal blast lung injury via adenosine signaling. Crit Care Med 44, e827–e837. [DOI] [PubMed] [Google Scholar]
- 68. Ling SH, McDonough JE, Gosselink JV, Elliott WM, Hayashi S, Hogg JC and van Eeden SF (2011) Patterns of retention of particulate matter in lung tissues of patients with COPD: potential role in disease progression. Chest 140, 1540–1549. [DOI] [PubMed] [Google Scholar]


