Abstract
Purpose
To investigate if the vaginal microbiome influences the IVF outcome.
Methods
Thirty-one patients undergoing assisted reproductive treatment (ART) with own or donated gametes and with cryotransfer of a single euploid blastocyst were recruited for this cohort study. Two vaginal samples were taken during the embryo transfer procedure, just before transferring the embryo. The V3 V4 region of 16S rRNA was used to analyze the vaginal microbiome, and the bioinformatic analysis was performed using QIIME2, Bioconductor Phyloseq, and MicrobiomeAnalyst packages. Alpha diversity was compared between groups according to the result of the pregnancy test.
Results
Fourteen (45.2%) patients did not and seventeen (54.8 %) did achieve pregnancy under ART. A greater index of alpha diversity was found in patients who did not achieve pregnancy comparing to those who did, although this difference was not significant (p = 0.088). In the analysis of beta diversity, no statistically significant differences were observed between groups established as per the pregnancy status. Samples from women who achieved pregnancy showed a greater presence of Lactobacillus spp. The cluster analysis identified two main clusters: the first encompassed the genera Lactobacillus, Gardnerella, Clostridium, Staphylococcus, and Dialister, and the second included all other genera. Women who achieved pregnancy were mainly detected microorganisms from the first cluster.
Conclusions
The vaginal microbiome can influence the results of ART. The profiles dominated by Lactobacillus were associated with the achievement of pregnancy, and there was a relationship between the stability of the vaginal microbiome and the achievement of pregnancy.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01564-0) contains supplementary material, which is available to authorized users.
Keywords: Infertility; Assisted reproductive techniques, Embryo transfer; pregnancy; Microbiota; Microbiome
Introduction
Any disruption in the balance of the human microbiome can severely alter its function, causing infections or other diseases [1]. The vaginal microbiome plays an important role in maintaining women’s overall health. The main bacteria are in the Lactobacillus genus, producers of lactic acid that helps preserving vaginal acidic pH, which acts as a defense against pathogens. In pregnant women, the vaginal microbiome differs from that of women in other life stages, both in the childbearing years and after menopause. However, while the relationship between the vaginal microbiome patterns and the evolution of pregnancy are well studied, research has only just begun on vaginal microbiota in relation to other aspects of women’s fertility [2].
About 10 to 15% of couples have trouble conceiving spontaneously [3], in part, due to delay in motherhood and current behavioral trends. Correct treatment for infertility depends on the cause. The association between vaginal flora and fertility has been well established throughout the past years; for example, bacterial vaginosis (Gardnerella vaginalis) is the alteration of vaginal flora associated with higher risk of miscarriages [4–6]. Other pathogenic microorganisms like Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma tuberculosis are related to a lower gestation rate, causing subclinical changes that are risk factors for subfertility [7].
The first longitudinal study that compared the microbiomes of healthy, full-term pregnant women versus healthy women who were not pregnant showed differences between the two groups, with the pregnant group showing a more stable microbiome [8]. Graspeuntner et al. [9] concluded that the microbiome demonstrates a specific pattern, associated not only with reproductive problems but also with a possible etiology. Thus, current evidence suggests that the microbiome could have an influence on infertility. A further inquiry along this research line would be to assess whether the microbiome—in addition to affecting fertility—has an impact on the outcome of in vitro fertilization treatment (IVF). Other studies aim to reveal differences in the vaginal microbiome as per pregnancy rates, including studies by Kyono et al. [10], Singer et al. [11], Bracewell-Milnes et al. [12], and Kroon et al. [13]. In Van Oostrum [7] et al. study, it was shown that the incidence of bacterial vaginosis is significantly higher in patients with tubal infertility compared with patients with non-tubal infertility. Bacterial vaginosis does not affect implantation rates, but it is significantly associated with pregnancy loss, although not with early miscarriages during the first trimester of pregnancy. Thus, the primary aim of this study is to assess the influence of the diversity, composition, and distribution of the vaginal microbiome on the IVF outcome.
Material and methods
Prospective pilot case-control study in a private-assisted reproduction clinic in Spain in 2017 and 2018. This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and was approved by the institutional review board (Reference:16/318; 29 November 2016). The study population comprised patients attending the clinic from April 2017 to January 2018. Inclusion criteria were as follows: 18 to 50 years of age; diagnosis of infertility; indication for assisted reproductive treatment (ART) with the woman’s own or donated gametes and with cryotransfer of a single euploid blastocyst; at least 3 months since last antibiotic treatment; and signed informed consent form. Exclusion criteria were as follows: uterine malformations; untreated hydrosalpinx; known factors for implantation failure, defined as implantation failure following transfer of at least three good-quality embryos over at least two cycles.
Participants followed the usual ART protocol with controlled ovarian stimulation and intracytoplasmic sperm injection (ICSI). The embryos generated underwent to preimplantation genetic diagnosis techniques at the blastocyst stage to identify and select euploid embryos. Whole-genome amplification on each biopsy was performed using the Sureplex method and followed by NGS using Veriseq protocol (Illumina ®) with the MiSeq Sequencer (Illumina ®). Analyses for aneuploidy testing were performed using Bluefuse Multi Software (Illumina). Aneuploid embryos were discarded, while euploid embryos were frozen for a subsequent embryo transfer. In a cycle following the ovarian stimulation of the woman receiving the oocytes, endometrial preparation under the estrogen–progesterone replacement therapy was performed, and a single euploid embryo was transferred. A pregnancy test was performed 8 to 10 days after the embryo transfer by means of a human chorionic gonadotropin (hCG) blood test.
Vaginal samples
Two vaginal samples were taken during the embryo transfer procedure, just before transferring the embryo. The vaginal fluid was taken with a dry swab from the bottom of the rectouterine pouch, in the posterior fornix, visualized directly with the aid of a vaginal speculum and the patient being in the lithotomy position. After collecting the sample, we proceeded with the ultrasound-guided embryo transfer according to established protocols. All samples were preserved at − 80 °C for later analysis.
DNA extraction
DNA was extracted using the PureLink microbiome DNA purification kit (ThermoFisher) according to the manufacturer’s instructions. DNA was quantified with the Qubit 2.0 fluorometer (ThermoFisher). Extracted DNA was preserved at −20 °C for later use.
Amplification of the V3 V4 region of 16S rRNA
The 16S rRNA has nine less conserved hypervariable regions (V1 to V9), which provide the most useful information for phylogenetic and taxonomic studies. There is no consensus about which region is the most representative for each microorganism. Regarding the hypervariable regions of 16S rRNA used for analyzing the vaginal microbiome, the main ones are V1 V2, V3 V4, V4, and V4 V5. In the present study, we chose the hypervariable region V3 V4.
The oligonucleotides used for amplifying the V3 V4 region are detailed in Supplementary file 1. The amplification of the region by polymerase chain reaction (PCR) was performed with 1.5 units of Taq DNA polymerase (KAPA HiFi HotStart, Roche) in presence of deoxynucleotide triphosphates (dNTPs), the oligonucleotides 357F and 806R at a final concentration of 1 μM and a mean 100 ng of DNA, and at a final reaction volume of 25 μL. PCR was carried out in the thermocycler (Verity, Applied Biosystems) with the following time and temperature program: initial denaturation at 95 °C for 3 min, followed by 25 cycles at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, with a final step of elongation at 72 °C for 5 min. To validate the PCR technique, all the amplification reactions included positive and negative controls without a DNA mold. The PCR products were visualized via agarose electrophoresis, verifying that the amplified DNA band was of the correct size (449 pb). All the amplification products were stored at − 20 °C for later sequencing.
Sequencing of the V3 V4 region of rRNA 16S
Once we obtained the amplicon for sequencing, a library was generated with the identifying indexes of each sample as well as the sequences employed. We used the Nextera XT sequencing kit (Illumina), the MiSeq sequencer (Ilumina), and the metagenomics workflow.
Bioinformatic analysis
Once sequencing was finalized, the primary analysis of the obtained sequences was performed, consisting of their demultiplexation with the MiSeqReporter software (Illumina). The paired-end sequences of each sample were exported from the MiSeq system for analysis in the FASTA format. The bioinformatic analysis of the sequences was performed in the VirtualBox of the Ubuntu Linux operating system, with different commands in the QIIME2 package [14, 15]. For the subsequent data analysis, we used R software (version 3.4.2) along with the Bioconductor Phyloseq [16] and MicrobiomeAnalyst [17] packages.
The demultiplexed paired-end sequencing protocol was used for importing the sequences and the dada2 denoise-paired command to delete the low-quality ones. The sequences were grouped in operational taxonomic units (OTUs) with 97% similarity. To estimate microbial diversity, a rarefaction analysis was performed on 1000 sequences per sample for different alpha diversity indexes (phylogenetic distance, observed OTUs, and Shannon index).
Alpha diversity measures the richness of species in a given community. In our particular case, it is focused on the number of different species present and identified in a sample. Beta diversity, on the other hand, analyzes different composition, in terms of abundance of different taxa, between different samples. Beta diversity can be measured qualitatively or quantitatively, in the first case considering the abundance of microorganisms observed, while in the second one taking into account their presence or absence. The Chao1 analysis of alpha diversity was performed.
The beta diversity analysis was done by calculating the weighted UniFrac distance between each pair of samples. The taxonomic assignment used a classification based on a filtrate of the 99_otus sequence, from the Greengenes database to the V3 V4 region. The sequences obtained were filtered and assigned to at least one genus. Finally, a specific analysis for each taxon or group was performed as per to the results obtained. Clustering and ordination methods were employed to search for patterns associated with pregnancy.
Study variables
On the day of the enrolment in the study, the following variables were collected for each participant: age, weight, height, tobacco use (yes/no), number of previous pregnancies, previous miscarriage (yes/no), and their number as well as prior fertility treatments (yes/no). On the day of sample collection, we recorded the date and the endometrial thickness. Two weeks after the embryo transfer, we registered the result of the pregnancy test (positive/negative) according to the beta-hCG value.
Statistical analysis
Descriptive analysis of the data consisted of calculating the means for quantitative variables and the relative frequencies (%) for categorical variables. To determine the distribution of our variables, we performed a Shaphiro–Wilk test. The non-parametric test used was the Mann–Whitney U test and the parametric Student t test. We compared the mean values of quantitative variables by groups according to the result of the pregnancy. In regards to the variable distribution, parametric or non-parametric test was used. Alpha diversity between groups was compared by applying the Student t test using the Shannon and Chao1 indexes as both have normal distribution. We calculated the Pearson correlation coefficient between Lactobacillus and other types. A bivariable (chi-squared) analysis was performed to analyze the relative abundance of the different genera between samples from both groups. P values of less than 0.05 were considered significant.
Results
There were 31 included patients with a mean age of 40.0 years (standard deviation 4.3). Fourteen subjects (45.2%) did not and seventeen (54.8%) did achieve pregnancy under the ART. Table 1 shows participants’ characteristics. There were 7,089,699 total sequences for the 31 samples (mean 228,699; range 2285 to 1,142,892).
Table 1.
Participant characteristics and comparison between groups according to achievement of pregnancy
| Variable | Total (N = 31) | Pregnancy achieved (N = 17) | Pregnancy not achieved (N = 14) | P value |
|---|---|---|---|---|
| Age, median and range (years) | 40 (32-47) | 38.5 (32-47) | 42 (38-47) | 0.019* |
| BMI, mean (kg/m2) | 24.28 | 25.22 | 23.50 | 0.23 |
| % Smokers | 12.90 | 15.4 | 11.1 | 0.73 |
| Previous pregnancies, mean n | 0.77 | 0.82 | 0.69 | 0.66 |
| % with prior miscarriage | 70.96 | 70.6 | 76.9 | 0.69 |
| Prior miscarriages, mean n | 1.53 | 1.41 | 1.69 | 0.69 |
| Previous ART, % | 80.00 | 92.3 | 70.6 | 0.14 |
| Endometrial thickness, mean (mm) | 8.25 | 8.46 | 7.96 | 0.37 |
*Statistical significance
ART assisted reproductive treatment
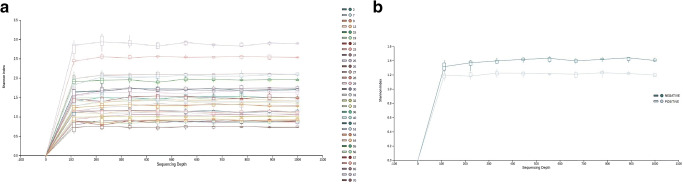
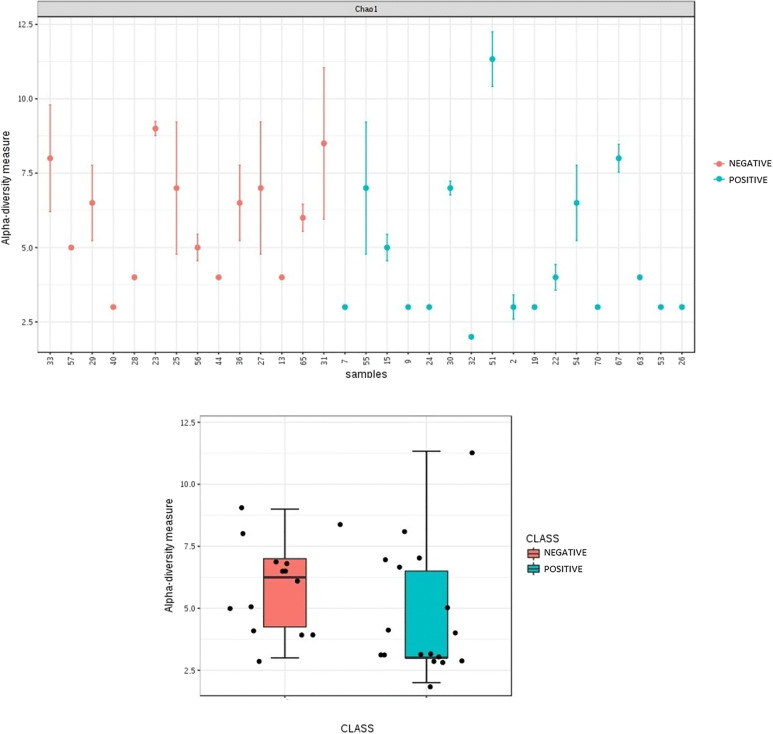
Figure 1 shows the rarefaction curves for the Shannon diversity index, both for all the samples and separating the groups by whether the patients achieved the pregnancy or not. The box-plot for the Shannon alpha diversity index showed a greater index of diversity in patients who did not achieve pregnancy, although the difference was not significant (p = 0.088). Finally, a comparative analysis was performed for the Chao1 diversity index (Fig. 2), yielding significant differences (p = 0.039).
Fig. 1.
Rarefaction curves for the Shannon index for a each sample and b according to result of pregnancy test (color)
Fig. 2.
Comparative analysis of Chao1 diversity index
In the analysis of beta diversity, using different indexes, no statistically significant differences were observed between groups according to pregnancy status. However, using the unweighted UniFrac index, a certain (non-significant) tendency was apparent (p = 0.088).
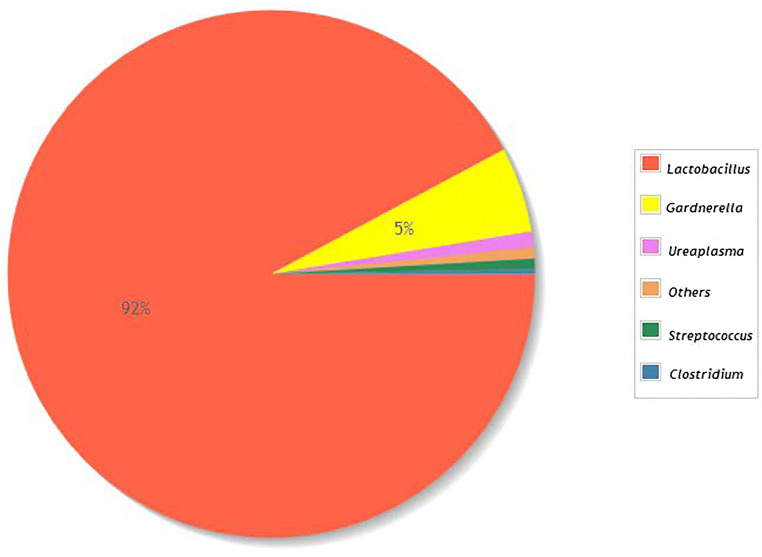
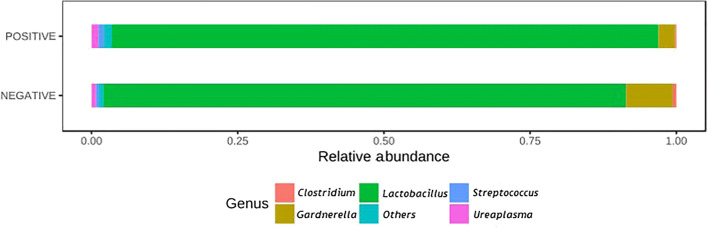
Regarding the taxonomic characterization, Fig. 3 shows the taxonomic diversity at the genus level in the analyzed samples, with Lactobacillus spp. standing out as the most prevalent genus. It was majorly represented by L. crispatus (47.05%), L. helveticus (22.85%), L. iners (21.95%), and L. jenseii (3.97%). Samples from women who achieved pregnancy showed a greater proportion of Lactobacillus spp. Although none of the results from bivariable analyses comparing the abundance of different genera between groups reached the level of significance, for Gardnerella, the p value was 0.11, and for Lactobacillus, it was 0.20 (Fig. 4).
Fig. 3.
Taxonomic diversity by genus in included study samples
Fig. 4.
Relative frequency of most abundant genera, according to the result of pregnancy test (positive/negative)
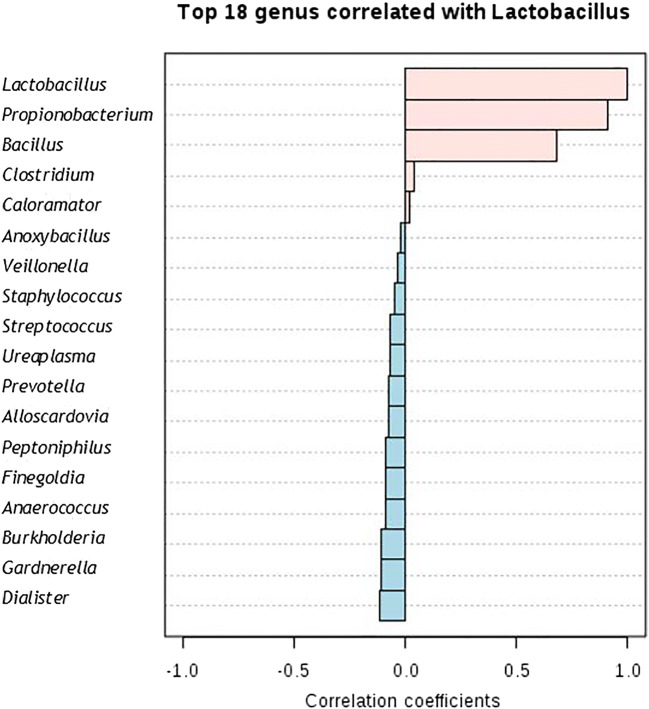
Finally, the cluster analysis identified two main clusters: the first encompassed the genera Lactobacillus, Gardnerella, Clostridium, Staphylococcus, and Dialister, and the second included all other genera. In women who achieved pregnancy, the microorganisms mainly from the first cluster were detected. The ordination analysis did not show any clear pattern except for that already observed for Lactobacillus and Gardnerella. The correlation graph (Fig. 5) indicates that Lactobacillus is positively correlated with the Propionibacterium, Bacillus, Clostridium, and Caloramator genera. On the other hand, it is mainly negatively correlated with Gardnerella, Dialister, Burkholderia, and Finegoldia.
Fig. 5.
Analysis of correlation with Lactobacillus. Top 18 genera correlated with Lactobacillus
Discussion
The results of this study show that the vaginal microbiome could influence the results of ART, as the profiles dominated by Lactobacillus are associated with the achievement of pregnancy, and there is a relationship between the stability of the vaginal microbiome (Chao1 index) and the achievement of pregnancy.
The sequencing results from other studies on vaginal and/or endometrial microbiome populations vary in terms of the average number of OTUs filtered per sample, which range from 40 [18] to 250 [19]. Thus, factors such as the DNA extraction kit used, the hypervariable regions selected from the sequenced 16S rRNA gene, the pair of primers employed, and the kit or system for generating massive sequencing libraries could all strongly influence the sequencing of the 16S rRNA gene. In terms of the OTUs, we identified with the deblur protocol, the total of 116 within the range of values expected for this type of vaginal sample.
The vaginal microbiome has low levels of diversity according to studies performed in the framework of the human microbiome project [20, 21], with some variability existing in terms of the location in the vagina and the gestational week. Our analysis of alpha diversity showed generally low values; women who did not achieve pregnancy showed significantly more alpha diversity than those who did. Moreno et al. [22] performed this analysis in the endometrial microbiome and did not observe the same correlation. In previous studies that analyzed vaginal microbiome by week of gestation, the authors reported lower diversity indexes in patients with ongoing pregnancies [21]. This suggests that the vaginal microbiome changes during pregnancy; however, since we sampled the microbiome on the same day as the embryo transfer, the correct interpretation may be that a microbiome with little diversity favors the achievement of an ongoing pregnancy.
With regard to the beta diversity, previous studies describe differences in the vaginal microbiome according to gestational week and location in the vagina [20]. Moreover, some authors have observed differences in the beta diversity between pregnant and non-pregnant women [23]. We also observed differences, but these were not statistically significant, perhaps due to the small sample size.
The taxonomic characterization showed the unequivocal dominance of the Lactobacillus genus in the vaginal microbiome. The vaginal microbiome is mainly composed of microorganisms from the Lactobacillus genus [24], which creates an acidic environment that protects against sexually transmitted and other opportunistic infections. Moreno et al. [22] reported that an endometrial microbiota dominated by Lactobacillus spp. showed higher rates of embryo implantation, ongoing pregnancy, and lower rate miscarriage than this that was not. Nevertheless, the differences we found were not statistically significant.
Lactobacillus genus is the most abundant vaginal bacteria in women. They inhibit the binding of other bacteria to the epithelial cells and produce lactic acid that kills or inhibits the growth of many other bacteria. Lactic acid blocks histone deacetylases, improving gene transcription and DNA repair. Lactic acid induces autophagy in epithelial cells to degrade intracellular microorganisms and promote homeostasis. Lactobacilli are tolerated by vaginal epithelial cells and inhibit the induction of proinflammatory cytokines. Emotional stress can reduce the abundance of lactobacilli in the vaginal microbiota and increase inflammation. The ability of lactobacilli to inhibit infection without inducing inflammation can maximize fertility and the successful outcome of pregnancy in women [25].
A pilot study (Kyono et al. 2019) aimed to analyze the results of pregnancy in patients under IVF treatment who presented Lactobacillus-dominated microbiota (LDM) and non-Lactobacillus-domesticated microbiota (NLDM) in the endometrium and reports cases that were treated for NLDM simultaneously with antibiotics and prebiotic/probiotic supplements in a Japanese infertile population [25]. The study of Singer et al. (2019) concludes that women with an abnormal vaginal microbiota have approximately 1.4 times less chance of pregnancy after an IVF treatment compared with women with normal microbiota [11].
The comparison of relative abundance (Fig. 4) provided more data on the relationship between the two main types of vaginal microbiomes. On one hand, those dominated by Lactobacillus and showing less diversity were characterized by greater reproductive success. The other profile is not dominated by Lactobacillus and contains the Gardnerella genus; according to previous reports, the proportion of Gardnerella genus bacteria exceeds 10% in patients with infection-related infertility. There is also a negative correlation between the abundance of this genus and of Lactobacillus [9].
Another factor evaluated in other studies, which differs from the methodology applied in the present one, opens the possibility of parallel analysis or complementary methods: it is bacterial contamination of the catheter tip [26]. The objective of that study was to evaluate the effect of bacterial colonization of the tip of the catheter used for embryo transfer on the clinical pregnancy rate in IVF treatments and concluded that, indeed, it is associated with the decrease of clinical pregnancy rate.
In another study of Selman et al. (2007), the presence of vaginal–cervical microbial contamination at the time of embryo transfer was analyzed. Samples were taken from the following different sources: the bottom of the vagina, the cervix, the culture medium of the embryo before and the after transfer procedure, the tip of the catheter, and the external sheet. This study also concluded that vaginal–cervical microbial contamination at the time of transfer can be associated with significantly lower pregnancy rates [27].
Limitations
The main limitation of this study is the small sample size. Future studies with more statistical power could confirm some of the tendencies that we observed. The cause of the infertility has not been analyzed either. Nevertheless, we have taken into account the patients diagnosed with recurrent implantation failure as they could alter the results in terms of embryo implantation and the maintenance of pregnancy. Another limitation of the study is the difficulty in the extrapolation of the results to other populations, since all patients analyzed are Caucasian. The fact that all subjects received the same endometrial preparation protocol helps us to normalize the population of the study and, therefore, also the validity of the results. At the same time, this uniformity makes it difficult to extrapolate the results to other endometrial preparation protocols.
Future studies with more statistical power are needed to confirm the tendencies that were observed in the present project.
Conclusions
The results of this study show that the vaginal microbiome has an influence on the results of assisted reproductive treatment. The microbiome profile that seemed to favor the achievement of pregnancy was dominated by Lactobacillus, while the notable presence of Gardnerella spp. was associated with the opposite outcome. The abundance of these genera shows a negative correlation. Moreover, there was a greater Chao1 alpha diversity in women who did not achieve pregnancy.
Electronic supplementary material
(DOCX 11 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrea Bernabeu, Email: abernabeu@institutobernabeu.com.
Belén Lledo, Email: blledo@institutobernabeu.com.
Ma. Carmen Díaz, Email: mdiaz@institutobernabeu.com.
Francisca M. Lozano, Email: fmlozano@institutobernabeu.com
Vicente Ruiz, Email: vruizcamara@institutobernabeu.com.
Ana Fuentes, Email: afuentes@institutobernabeu.com.
Adriana Lopez-Pineda, Email: adriannalp@hotmail.com.
Belen Moliner, Email: bmoliner@institutobernabeu.com.
Juan Carlos Castillo, Email: jccastillo@institutobernabeu.com.
Jose Antonio Ortiz, Email: jaortiz@institutobernabeu.com.
Jorge Ten, Email: jten@institutobernabeu.com.
Concepcion Carratala-Munuera, Email: atencion.primaria@umh.es.
Domingo Orozco-Beltran, Email: dorozcobeltran@gmail.com.
Jose A. Quesada, Email: jquesada@umh.es
Rafael Bernabeu, Email: rbernabeu@institutobernabeu.com.
References
- 1.Peñalver Bernabé B, Cralle L, Gilbert JA. Systems biology of the human microbiome. Curr Opin Biotechnol. 2018;51:146–153. doi: 10.1016/j.copbio.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Witkin SS, Linhares IM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert JA, John S, Sobel JD, Akins RA. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: recognition of the conversion process. PLoS One. 2013;8(12):e82599. doi: 10.1371/journal.pone.0082599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 1999;319(7204):220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haahr T, Zacho J, Bräuner M, Shathmigha K, Skov Jensen J, Humaidan P. Reproductive outcome of patients undergoing in vitro fertilization treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG. 2018. [DOI] [PubMed]
- 7.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graspeuntner S, Bohlmann MK, Gillmann K, Speer R, Kuenzel S, Mark H, Hoellen F, Lettau R, Griesinger G, König IR, Baines JF, Rupp J. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS One. 2018;13(1):e0191047. doi: 10.1371/journal.pone.0191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyono K, Hashimoto T, Nagai Y, Sakuraba Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: a single-center pilot study. Reprod Med Biol. 2018;17:297–306. doi: 10.1002/rmb2.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Borg M, Ouburg S, Morré SA. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment—a meta-analysis. J Gynecol Obstet Hum Reprod. 2019;48(4):223–229. doi: 10.1016/j.jogoh.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Bracewell-Milnes T, Saso S, Nikolaou D, Norman-Taylor J, Johnson M, Thum MY. Investigating the effect of an abnormal cervico-vaginal and endometrial microbiome on assisted reproductive technologies: a systematic review. Am J Reprod Immunol. 2018;80(5):e13037. doi: 10.1111/aji.13037. [DOI] [PubMed] [Google Scholar]
- 13.Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril. 2018;110(3):327–336. doi: 10.1016/j.fertnstert.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Lawley B, Tannock GW. Analysis of 16S rRNA gene amplicon sequences using the QIIME software package. Methods Mol Biol. 2017;1537:153–163. doi: 10.1007/978-1-4939-6685-1_9. [DOI] [PubMed] [Google Scholar]
- 15.Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Microbiol. 2012; Chapter 1:Unit 1E.5. [DOI] [PMC free article] [PubMed]
- 16.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhariwal A, Chong J, Habib S, King I, Agellon LB, Xia J. MicrobiomeAnalyst - a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, Holmes E, Nicoholson JK, Marchesi JR, Bennett PR. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramaniam A, Kumar R, Cliver SP, Zhi D, Szychowski JM, Abramovici A, Biggio JR, Lefkowitz EJ, Morrow C, Edwards RK. Vaginal microbiota in pregnancy: evaluation based on vaginal flora, birth outcome, and race. Am J Perinatol. 2016;33(4):401–408. doi: 10.1055/s-0035-1565919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, Ramon D, Simon C. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 23.Walther-António MR, Jeraldo P, Berg Miller ME, Yeoman CJ, Nelson KE, Wilson BA, White BA, Chia N, Creedon DJ. Pregnancy's stronghold on the vaginal microbiome. PLoS One. 2014;9(6):e98514. doi: 10.1371/journal.pone.0098514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EA, Beasley DAE, Dunn RR, Archie EA. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol. 2016;7:1936. doi: 10.3389/fmicb.2016.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. 2019;18:72–82. doi: 10.1002/rmb2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maduka RN, Osaikhuwuomwan JA, Aziken ME. The effect of bacterial colonization of the embryo transfer catheter on outcome of in vitro fertilization–embryo transfer treatment. Afr J Med Health Sci. 2018;17(1):7–13. [Google Scholar]
- 27.Selman H, Mariani M, Barnocchi N, Mencacci A, Bistoni F, Arena S, Pizzasegale S, Brusco GF, Angelini A. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J Assist Reprod Genet. 2007;24(9):395–399. doi: 10.1007/s10815-007-9146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)