Abstract
The present study examined the acute effects of static stretching (SS) exercise order on cardiac responses. Seventeen individuals were submitted to two experimental SS session: Order “A” (larger to small muscles groups) and Order “B” (small to larger muscles groups). Heart rate (HR), systolic (SBP) and diastolic blood pressure (DBP), rate-pressure product (RPP) oxygen saturation (SpO2), and heart rate variability (HRV) were measured at rest, midpoint of the session, immediately after the session, and in 5, 10, and 20 minutes after. SS increased HR and RPP in both orders, while reducing the rMSSD index and SpO2. In the order “A”, the SBP and DBP increased at the midpoint of the session. In the order “B”, the SBP and DBP increased only immediately after the end of the session. DBP and RPP significantly higher in order “A” compared to order “B” in the midpoint of the session. It was also demonstrated higher values of DBP and minor mean R-R intervals in order “B” at 10 min-post session. SS increased cardiac overload in both performed orders. The overload generated by the SS of the larger muscles groups was greater when compared to the smaller muscles groups, suggesting that the exercise order interferes in cardiac overload.
Subject terms: Cardiology, Physiology
Introduction
According to American College of Sport Medicine1, flexibility is one of the most important components of physical fitness related to health. Accordingly, static muscle stretching (SS) is an important exercise for maintaining and developing joint range of motion adequate to a healthy life. Without involving osteoarticular impact, the use of this exercise serves as an important tool in clinical prescription. Nevertheless, although widely used in the therapeutic environment, there is conflicting evidence on the use of SS exercises. Recent studies have demonstrated decreases in overall muscle-tendon stiffness after stretching2–4 and a decreases in muscle oxygen saturation (SpO2)5, decreases in force development6–9 and in lower recruitment of motor units9,10.
Konrad et al.2 demonstrated trough ultrasonography and dynamometry that 5 sets of 1 minute of SS reduce muscle stiffness and maximal isometric torque until 10 minutes after exercise. Fowles et al.9 reported that from after 60 seconds of SS support, muscle strength reduction can be observed, and Costa e Silva et al.6 reported only 30 seconds of SS can already reduce the levels of strength. Neural and structural mechanisms capable of generating such an adverse effect on performance seem to generate metabolic compensation due to a possible cardiovascular overload, according to the studies with important clinical contribution of Farinatti et al.11 and Lima et al.12, where the authors found higher cardiac and pressure responses as a function of SS.
Farinatti et al.11 examined 22 asymptomatic young men and cautioned that SS should be avoided in subjects with high risk for adverse cardiovascular events, because they observed significant increases in systolic blood pressure (SBP) and rate-pressure product (RPP) during the stretching sessions with and without the use of Valsalva maneuver. In addition, Farinatti et al.11 reported that muscle size may influence the magnitude of these cardiovascular responses. According to the authors11, SS of the large muscle groups seems to generate greater cardiac overload compared to the SS of the smaller groups. Despite the absence of studies on the topic, the results would become of great value for the choice of exercises and manipulation of the respective orders.
Exercise order is among the main methodological variables of a training program1,12. In agreement with ACSM13, this variable has great appeal in exercise prescriptions and has been researched by several investigations14–26. However, no study has examined the effects of the order of the muscles stretching exercises on the cardiovascular system. Currently, the ACSM1 has recommendations about the number of sets and stretch durations, nevertheless no information has been provided about the order of exercises.
Thus, the present study focused to investigate the acute effects of SS exercise order (larger to small muscle groups vs. small to larger muscle groups) on cardiovascular responses of heart rate (HR), blood pressure (BP), RPP, SpO2, and heart rate variability (HRV) in healthy individuals.
Methods
Participants
Seventeen volunteers of both sexes (11 men and 6 women) were enrolled in the study (Table 1). The inclusion criteria were: age between 18 and 30 years, no history of injuries that limit joint range of motion, no hypo- or hypermobility, normotensive, familiarized with stretching exercises, no smokers and were advised for not drinking alcoholic beverages or caffeine in the 24 hours preceding the SS exercise bouts. The sample size was calculated using the G * Power 3.1 software. Based on an a priori analysis, we adopted a power of 0.80, α = 0.05, correlation coefficient of 0.5, correction nonsphericity of 1 and effect size of 0.26. This analysis of statistical power was performed to reduce the probability of type II error and to determine the minimum number of participants required for this investigation. We found that the sample size was sufficient to provide more than 81% statistical power. The research project was carried out in accordance to the recommendations of the National Health Council. We declare that all participants read and signed an informed consent form for study participation, and this study was approved by the Research Ethics Committee of the ABC Foundation School of Medicine (FMABC) (protocol number: 2.433.91) (Fig. 1).
Table 1.
Descriptive data of the sample.
| Mass (Kg) | High (cm) | BMI (Kg/cm2) | Age (years) | |
|---|---|---|---|---|
| Men (n = 11) | ||||
| Mean ± SD | 85.93 ± 13.36 | 179.53 ± 5.12 | 26.59 ± 3.23 | 26.09 ± 4.18 |
| Women (n = 6) | ||||
| Mean ± SD | 60.16 ± 15.17 | 162.21 ± 7.41 | 22.69 ± 4.74 | 25.00 ± 4.33 |
| All (n = 17) | ||||
| Mean ± SD | 78.84 ± 18.56 | 173.42 ± 10.31 | 25.21 ± 4.15 | 25.70 ± 4.13 |
| CI 95% higher | 67.30 | 168.10 | 23.07 | 23.58 |
| CI 95% lower | 86.39 | 178.70 | 27.35 | 27.83 |
| SW p-value | 0.9374 | 0.1204 | 0.5002 | 0.8517 |
BMI = Body Mass Index; SD = Standard Deviation; CI = Confidence Interval; SW = Shapiro-Wilk.
Figure 1.
Volunteer Selection Flowchart.
Procedure
This study observed the cardiovascular and autonomic responses after two SS exercises orders based on the size of the stretched musculature. Participants were randomly submitted to two experimental stretching sessions with a minimum interval of 24 hours between sessions, named: Order “A” (from larger to small muscle groups): quadriceps, pectoralis, hamstrings, triceps, gastrocnemius, and biceps brachii. Order “B” (from small to larger muscle groups): biceps brachii, gastrocnemius, triceps, hamstring, pectoralis, and quadriceps. All volunteers participated in both experimental conditions. HR, SBP, diastolic blood pressure (DBP), SpO2, and HRV indexes were measured for 20 minutes at rest, at the midpoint of the session, immediately after the experimental session, and at 5, 10, and 20 minutes after the training session. The room temperature was approximately 22.06 ± 0.9 °C and relative air humidity was maintained at 60 ± 11.37%.
Flexibility test and static stretching protocol
Before the beginning of the experimental procedures, volunteers underwent a test and retest (minimum of 24 h interval) of flexibility measured by the maximum amplitude reached passively by a goniometer (CARCI, Brazil). The following movements were measured: knee flexion (stretching of quadriceps), horizontal flexion of the shoulder (stretching of the pectoralis and biceps brachii), hip flexion (stretching of the hamstrings), elbow flexion (stretching of the triceps), and dorsiflexion (stretching of gastrocnemius). Three 3 attempts were carried out unilaterally, and the largest value obtained in the goniometer was considered. The positions evaluated were corresponding to the sustained positions during the SS exercises performed in the experimental protocols.
The SS was performed passively and unilaterally to the maximum point of discomfort through a scale of 0–105. Three sets of 20 s were performed for each muscle group1, with a 20 s interval between sets and exercises. The SS for each muscle group was repeated using the maximum joint amplitude achieved and was measured with a goniometer. The breathing was guided with sound stimuli (2 seconds inspiration, 2 seconds expiration) during the entire session by the HRV Elite® software (Production Version 4.4.1, Asheville NC, USA).
Heart rate and heart rate variability measurements
HRV Elite® software (Production Version 4.4.1, Asheville NC, USA) validated by Perrota et al.27 was used to measure HR and HRV variables. The electrode was moistened and positioned directly on the sternum (at the level of the xiphoid process), transferring the beats via Bluetooth to the software. Regarding HR, the last value shown on the device screen was recorded at each moment after each measurement was completed. In accordance with the procedures of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology28, HRV analysis in the time domain was evaluated through the mean R-R intervals and square root of the mean squared differences of successive R-R intervals (rMSSD). The power signal intensity in the frequency range between 0.04–0.15 Hz (LF), and also in the frequency range between 0.15–0.40 Hz (HF), as well as the sympatho-vagal ratio (LF/HF) were used for HRV in the frequency domain.
Blood pressure and rate- pressure product (RPP)
The measurement of SBP and DBP values was performed using an ambulatory blood pressure monitor (Boso-medicus, D-72417, Germany) using the oscillometric technique29, thus enabling automatic recording of blood pressure values. To obtain the RPP values, the HR values were multiplied by the SBP values (RPP = HR × SBP).
Oxygen saturation
A finger pulse oximeter (digit Ox III N° AE-N2 Probasics, USA) was used to obtain oxygen saturation values. Finger pulse oximetry is considered an indirect measure of oxygen consumption30. To obtain the data, the probe was fixed on the index finger of the hand, which was supported on a fixed surface for stabilization.
Statistical analysis
A Shapiro-Wilk test was used to verify the normality of the sample’s descriptive data. Reliability between test and retest sessions was analyzed using a two-factor random effect model intraclass correlation coefficient (ICC) and paired t-tests. A two-way ANOVA (repeated measures) was performed to examine differences in HR, SBP, DBP, RPP, SpO2, and HRV indexes after the experimental treatments (Order “A” vs. Order “B” and rest vs. midpoint of the session vs. end of the session vs. 5 min-after vs. 10 min-after vs. 20 min-after). Bonferroni post hoc test was then performed when appropriate. Statistical analyses were conducted using the statistical software package GraphPad Prism 5.0 and the significance level was set at p < 0.05.
Results
The flexibility test and retest values presented excellent reproducibility (values between 0.90 and 0.99) and did not present a significant difference in the paired t test (p > 0.05).
Heart rate
The intra-group analysis showed that the order “A” and the order “B” both generated significant increases in the HR responses (p < 0.05), already observed in the midpoint of the session (p < 0.001) and persists increased immediately after the end of the session (p < 0.001). The HR has returned to baseline levels 5 min after stretching and remained in this range at 20 min after stretching in both groups (p > 0.05). In addition, according to the inter-group analysis, no difference was observed in HR between the order “A” and order “B” (p > 0.05), according to Fig. 2.
Figure 2.
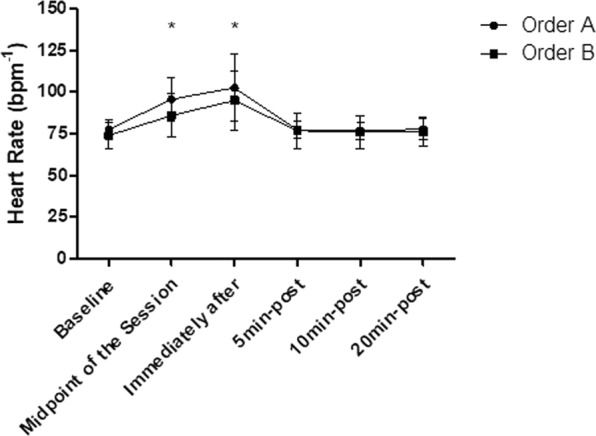
Mean and standard deviation of HR values in comparison between different exercise orders. *Represents significant difference from baseline.
Systolic blood pressure
The intra-group analysis showed that the order “A” and the order “B” both generated significant increases in the SBP responses (p < 0.05). In the order “A”, significant SBP increases were observed in the midpoint of the session (p < 0.01) and returned to baseline levels immediately after the end of the session, and remained in this range at 20 min after stretching (p > 0.05). In the order “B” significant SBP increases were observed immediately after the end of the session (p < 0.05) and returned to baseline levels 5 min after stretching and remained in this range at 20 min after stretching (p > 0.05). In addition, according to the inter-group analysis, no significant differences were observed between the order “A” and order “B” (p > 0.05), according to Fig. 3.
Figure 3.
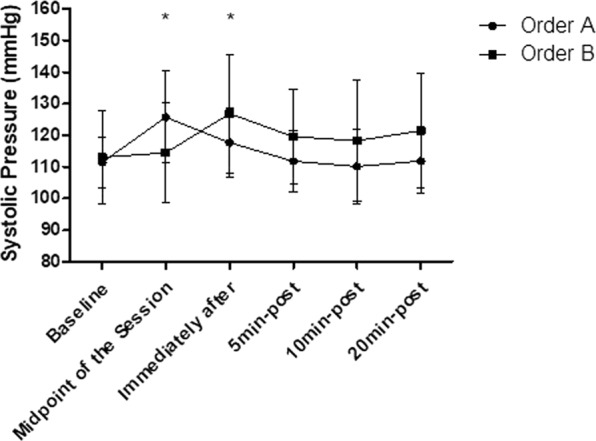
Mean and standard deviation of SBP values in comparison between different exercise orders. *Represents significant difference from the respective baseline.
Diastolic blood pressure
The intra-group analysis showed that the order “A” and the order “B” both generated significant increases in the DBP responses (p < 0.05). In the order “A”, significant increases in the DBP responses were observed in the midpoint of the session (p < 0.01), returned to baseline levels immediately after the end of the session and remained in this range at 20 min after stretching (p > 0.05). In relation to the analysis of the order “B”, we observed significant increases in the DBP responses immediately after the end of the session (p < 0.05), which remained increased (p < 0.05) until 20 min after stretching. In relation to the inter-group analysis, were found significant differences between order “A” and order “B” at the midpoint of the session (p < 0.05) and in 10 min-after SS (p < 0.01), according to Fig. 4.
Figure 4.
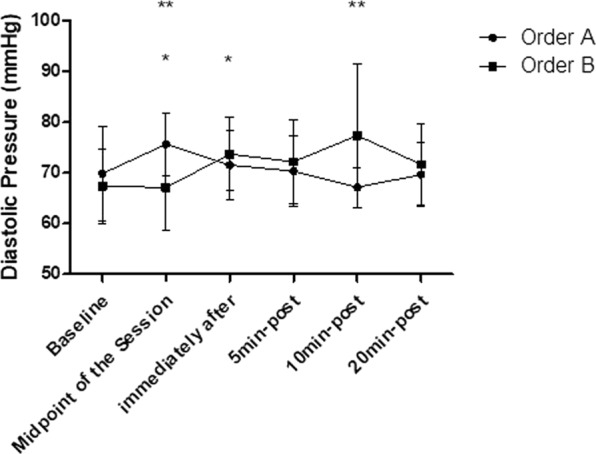
Mean and standard deviation of DBP values in comparison between different exercise orders. *Represents significant difference from the respective baseline. **Represents significant differences between different orders.
Rate-Pressure product
The intra-group analysis showed that the order “A” and the order “B” both generated significant increases in the RPP responses (p < 0.05), already observed in the midpoint of the session (p < 0.05) and persists increased immediately after the end of the session (p < 0.001). The RPP has returned to baseline levels 5 min after stretching and remained in this range at 20 min after stretching in both groups (p > 0.05). In addition, according to the inter-group analysis, were found significant differences between order “A” and order “B” at the midpoint of the session (p < 0.01), according to Fig. 5.
Figure 5.
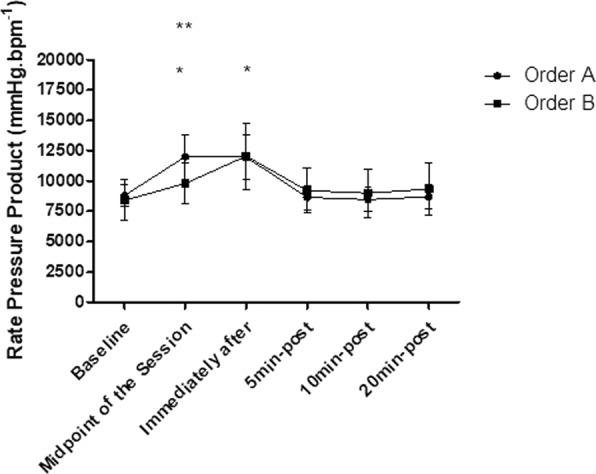
Mean and standard deviation of RPP values in comparison between different exercise orders. *Represents significant difference from the respective baseline. **Represents significant differences between different orders.
Oxygen saturation
The intra-group analysis showed that the order “A” and the order “B” both generated significant decreases in the SpO2 responses (p < 0.05), already observed in the midpoint of the session (p < 0.01) and persists decreased immediately after the end of the session (p < 0.01). The SpO2 has returned to baseline levels 5 min after stretching and remained in this range at 20 min after stretching in both groups (p > 0.05). In addition, according to the inter-group analysis, no significant differences were observed between the order “A” and order “B” (p > 0.05), according to Fig. 6.
Figure 6.
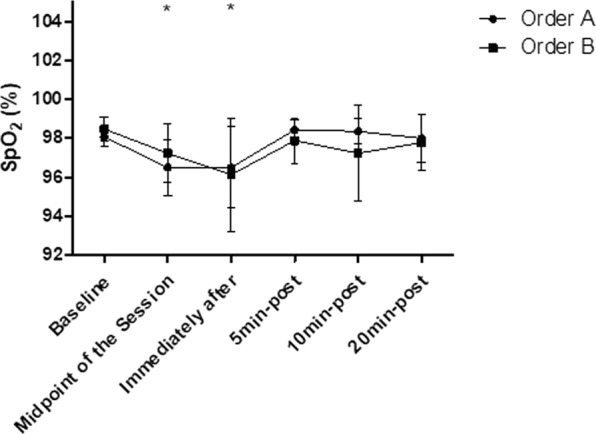
Mean and standard deviation of SpO2 values in comparison between different exercise orders. *Represents significant difference from the respective baseline.
rMSSD
The intra-group analysis showed that the order “A” and the order “B” both generated significant decreases (p < 0.05) in the rMSSD responses, already observed in the midpoint of the session (p < 0.01) and persists decreased immediately after the end of the session (p < 0.05). The rMSSD has returned to baseline levels 5 min after stretching and remained in this range at 20 min after stretching in both groups (p > 0.05). In addition, according to the inter-group analysis, no significant differences were observed between the order “A” and order “B” (p > 0.05), according to Fig. 7.
Figure 7.
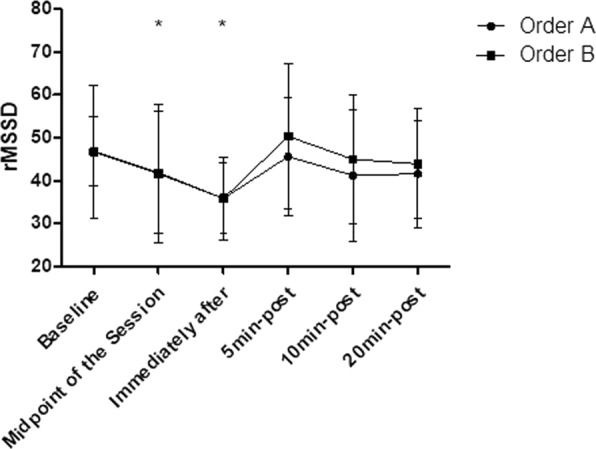
Mean and standard deviation of RMSSD values in comparison between different exercise orders. *Represents significant difference from the respective baseline.
LF
There are no significance in relation to the intra-group and inter-group LF analysis (p > 0.05), according to Fig. 8.
Figure 8.
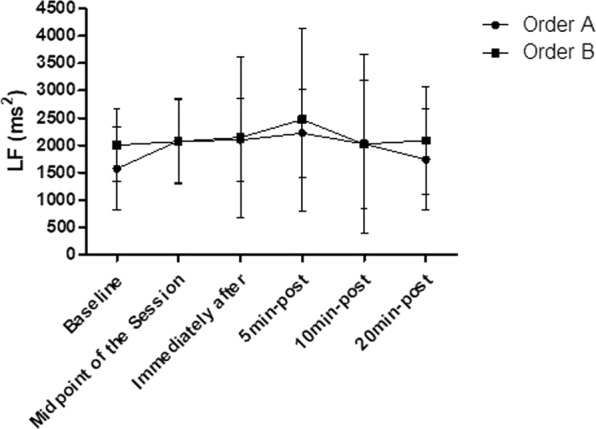
Mean and standard deviation of LF values in comparison between different exercise orders.
HF
There are no significance in relation to the intra-group and inter-group HF analysis (p > 0.05), according to Fig. 9.
Figure 9.
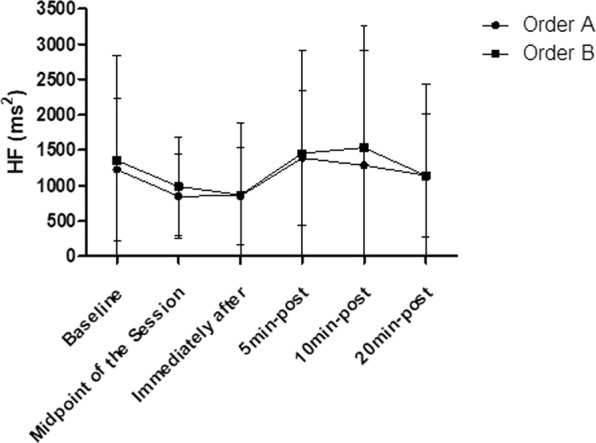
Mean and standard deviation of HF values in comparison between different exercise orders.
LF/HF ratio
There are no significance in relation to the intra-group and inter-group LF/HF ratio analysis (p > 0.05), according to Fig. 10.
Figure 10.
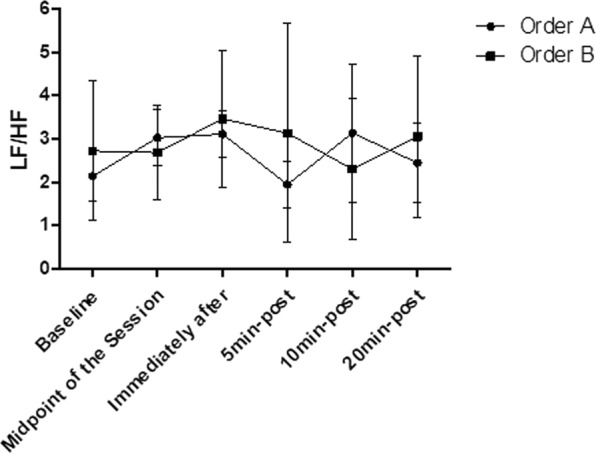
Mean and standard deviation of LF/HF values in comparison between different exercise orders.
Mean R-R intervals
There are no significant results in relation to the intra-group mean R-R intervals analysis in both orders (p > 0.05). However, in relation to the inter-group analysis were found significant differences between order “A” and order “B” at 10 min-after SS (p < 0.05), according to Fig. 11.
Figure 11.
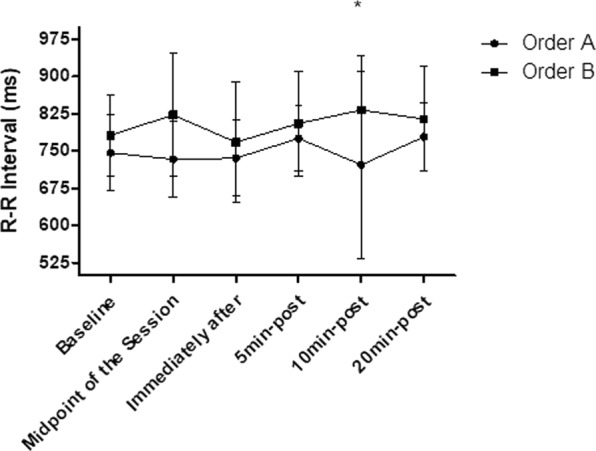
Mean and standard deviation of mean R-R intervals values comparing different orders of exercise. *Represents significant differences between different exercise orders.
Discussion
Through intra-group analysis, our best results demonstrated the SS session increase cardiac overload in both performed exercise order. Although inter-group results did not show significant differences immediately after the session in general, the data pointed to a higher cardiac overload in the SS of the larger muscle groups compared to the smaller muscle groups, suggesting that the exercise order interferes in cardiac overload. In the order “A”, SBP and DBP significantly increased at the midpoint of the session. Nevertheless, in the order “B”, the SBP and DBP significantly increased only immediately after the end of the session Accordingly, although the SS generated cardiac overload during and immediately after the experimental sessions, it was observed that even when performed up to the maximum joint amplitude, the values tend to return rapidly to the baseline levels within five minutes.
The present results demonstrated that SS does not allow the cardiovascular system to be overloaded for an extended period of time. Empirical knowledge and clinical prescriptions have usually recommended the accomplishment of stretching. Nevertheless, our findings showed that SS may increase heart overload and thereby, it should be prescribed with caution for individuals with a high cardiovascular risk. Additionally, in the present study the SS was performed to the maximum point of discomfort and probably the activation of peripheral pain pathways can enhance the HR and BP responses due to an increase in sympathetic modulation31.
Corroborating the findings of Farinatti et al.11, both orders of SS were able to significantly elevate HR, SBP, DBP, and RPP. In addition, our experiment significantly reduced the SpO2 and rMSSD values, thus suggesting a possible blood occlusion5, accompanied by a suppression of vagal modulation. Possibly such effects were due to the activation of mechanoceptors of small muscle fibers by SS exercises32. According to Mizuno et al.33, the pressor reflex mechanism demonstrated that exercises such as SS can generate alterations in autonomic nervous system (ANS), especially via the increase of the sympathetic modulation accompanied by vagal suppression regulated by medullary neurons.
Because of the muscular afferents, especially type III fibers, SS cause an increase in cardiac responses. In the present study, although they were not significantly different due to the high variability of the results, we observed that the SS showed a tendency to increase the LF and the LF/HF ratio, whereas the HF had a tendency to decrease. The increased cardiovascular responses observed in our findings are likely mediated by the reflex from the skeletal muscle (i.e., pressor reflex). Accordingly, the increases in this function are evoked by small muscle fibers afferents associated with the metaboreceptors, which are activated slowly during ischemic muscle contraction and by mechanoreceptors, which respond rapidly to muscular deformation evoked from SS. The afferent sensory information is then processed in the central nervous system, specifically within the nucleus of the solitary tract (NTS) that integrates the the sympatho-vagal balance pathways34.
Venturelli et al.35, in very recent study, investigated the sympatho-vagal relationship and blood circulation in 8 healthy young men submitted to repeated unilateral SS sets (5 × 45 s of knee flexion and 15 seconds of knee extension). The authors verified HR, cardiac output (CO), mean arterial pressure (MAP), HRV, BP variability and blood flow in rest and during repeated SS series. According to a computerized analysis of HRV the results showed that SS increased sympathetic modulation (≈20%) and decreased parasympathetic activity (≈30%) with a parasympathetic withdrawal prevalence, corroborating our results. From the device of photoplethysmography and ultrasonography, the authors observed that the HR, CO and MAP increased significantly during the SS sets and noticed that the blood flow was occluded. The Venturelli et al.35 data indicate that the high hemodynamic responses of the heart to SS were mainly influenced by the parasympathetic suppression.
The vagal rMSSD suppression could also be observed in the present study. Thus, SS exercises do not appear to promote cardiovascular protection, elevating HR, BP and suppressing local blood flow and the vagal drive to the heart. In the meantime, the present study demonstrated a return of the variables to the baseline levels five minutes after the end of the session, thus having a vagal increase in the post-exercise recovery period agreeing with Farinatti et al.36. Farinatti et al.36 submitted 10 men with reduced levels of flexibility to a SS session (3 repetitions of 30 seconds) involving trunk and hamstring musculature up to the maximum point of joint amplitude. The authors measured HR and HRV before, during and 30 minutes after the end of the session. After analysis in the time and frequency domains, the results demonstrated sympathetic activity rises during static stretching exercise and decreases at the end of the exercise, despite a rapid vagal return. These observations were confirmed in the present study through the values of HR, SBP, DBP, RPP, and the rMSSD index.
Lima et al.12 reported that SS exercises increase heart overload, elevating HR and RPP, which is consistent with our findings. In addition, previous studies6,7,37 demonstrated that pre-exercise SS causes impairment in the performance of activities requiring strength, and may decrease the supply of oxygen to the stretched muscle5, suggesting that negative effects can generate metabolic and autonomic compensations38. In addition, Farinatti et al.11, after submitting apparently healthy non-athletes to two experimental conditions (4 sets of 30 seconds vs. 4 sets of 30 seconds with the Valsalva Maneuver) reported that SS significantly increased SBP and RPP, with or without performing a Valsalva Maneuver. Corroborating the present results, the authors demonstrated larger muscle groups are capable of generating more expressive responses when compared to the smaller groups.
The heart rate variability may be influenced by several factors, for example the differences between sexes. A study of 83 subjects of both sexes found higher heart rate variability in women. According to the authors, this difference is due to the protective effect of estrogen39. In this sense, Dutra et al.40 pointed that men have an increased sympathetic response compared to women, while women have a vagal predominance. Even with this possible vagal increase in women, our results demonstrated that static stretching was able to significantly suppress parasympathetic drive in both exercise orders.
Nevertheless, AIbinet et al.41, after 12 weeks of intervention with physical exercise, found similar improvement in vagal modulation through the standard deviation of R-R intervals (SDNN), rMSSD and HF indexes in men and women. Nagashima et al.42 showed significant improvement in heart rate recovery, which indirectly reflects post-exercise parasympathetic reactivation, also in men and women. The authors included men and women in the analysis, as well as Sandercock et al.43 who also evaluated young men and women in a study that demonstrated differences in autonomic modulation in individuals with higher levels of physical activity.
In our study, the Shapiro-Wilk test showed normality of data when analyzing men and women together. The procedures were randomized with counterbalanced input, each individual being compared to him- or herself. In addition to the fact that only six women do not justify a different statistical analysis, we chose to evaluate men and women together. The same choice was done by other authors2,3,9 who investigated the acute effects of static muscle stretching.
Our study is the first to show the effects of different orders of SS in small and large muscles groups on cardiovascular and autonomic responses. Thus, further studies involving different orders of stretching are still required to understand the mechanisms involved in the SS in order to be safety prescribed and widely diffused in the clinical setting.
Conclusions
The SS session increases cardiac overload in both performed orders, pointing to greater overload when we compare the largest muscle groups to the smaller muscle groups. Thus, the exercise order interferes in cardiac overload. It is possible to observe that even when the joint amplitude is maximal, values tend to return rapidly to baseline levels. However, such exercises should be prescribed with caution by individuals with a high cardiovascular risk when the goal is cardiac preservation, especially by increased HR, SBP, DBP, RPP, and vagal suppression accompanied by reduction of oxygen supply.
Acknowledgements
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for supporting this study.
Author contributions
G.C.S., R.L.A. and M.A.S. conceived and design research. G.C.S. performed the data collection, writing, text editing and data analysis. All authors proof read, contributed to editing, and approved the manuscript. Thus, we certify that all authors have participated sufficiently in the work to take public responsibility.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.(ACSM) American College of Sports Medicine Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Special Communications. Med Sci Sports Exerc. 43, 1334–59 (2011). [DOI] [PubMed]
- 2.Konrad, A., Reiner, M. M., Thaller, S. & Tilp, M. The time course of muscle-tendon properties and function responses of five-minute static stretching exercise. Eur J Sport Sci, [Epub ahead of print] (2019). [DOI] [PMC free article] [PubMed]
- 3.Konrad A, Budini F, Tilp M. Acute effect of constant torque and constant angle stretching on the muscle and tendon tissue properties. Eur J Appl Physiol. 2017;117:1649–1656. doi: 10.1007/s00421-017-3654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Key AD, Husbands-Beasley J, Blazevich AJ. Effect of contract-relax, static stretching, and isometric contractions on muscle-tendon mechanics. Med Sci Sports Exerc. 2015;47:2181–2190. doi: 10.1249/MSS.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 5.McCully KK. The Influence of Passive Stretch on Muscle Oxygen Saturation. Adv Exp Med Biol. 2010;662:317–322. doi: 10.1007/978-1-4419-1241-1_45. [DOI] [PubMed] [Google Scholar]
- 6.Costa e Silva G, et al. Acute effect of different stretching methods on isometric muscle strength. Acta Scientiarum Health Sci. 2014;36:51–57. doi: 10.4025/actascihealthsci.v36i1.15581. [DOI] [Google Scholar]
- 7.Costa e Silva G, et al. Acute effect of static stretching on isometric muscle strength performance. Conscientiae Saúde. 2012;11:274–280. doi: 10.5585/conssaude.v11n2.3124. [DOI] [Google Scholar]
- 8.Behm D, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol. 2011;111:2633–2651. doi: 10.1007/s00421-011-1879-2. [DOI] [PubMed] [Google Scholar]
- 9.Fowles J, Sale D, MacDougall J. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89:1179–1188. doi: 10.1152/jappl.2000.89.3.1179. [DOI] [PubMed] [Google Scholar]
- 10.Marek SM, et al. Acute effect of static and proprioceptive neuromuscular facilitation stretching on muscle strength and power output. J Athl Train. 2005;40:94–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Farinatti PTV, et al. Cardiovascular responses to passive static flexibility exercises are influenced by the stretched muscle mass and the Valsalva maneuver. Clinics. 2011;66:459–464. doi: 10.1590/S1807-59322011000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima TP, Farinatti PT, Rubini EC, Silva EB, Monteiro WD. Hemodynamic responses during and after multiple sets of stretching exercises performed with and without the Valsalva maneuver. Clinics. 2015;70:333–338. doi: 10.6061/clinics/2015(05)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(ASCM) American College of Sports Medicine. Position stand on progression models in resistance training for healthy adults. Med Sci Sports Exerc. 41, 687–708 (2009). [DOI] [PubMed]
- 14.Simão R, De Salles BF, Figueredo T, Dias I, Willardson M. Exercise order in resistance training. Sports Medicine. 2012;42:251–265. doi: 10.2165/11597240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Conceição RR, et al. Acute Endocrine Responses to Different Strength Exercise Order in Men. JHK. 2014;44:111–120. doi: 10.2478/hukin-2014-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaves CPG, et al. Influence of Exercise Order on Muscle Damage During Moderate-Intensity Resistance Exercise and Recovery. Res Sports Med: An Int J. 2013;21:176–186. doi: 10.1080/15438627.2012.738439. [DOI] [PubMed] [Google Scholar]
- 17.Dias I, De Salles BF, Novaes J, Costa PB, Simão R. Influence of exercise order on maximum strength in untrained young men. J Sci Med Sport. 2010;13:65–69. doi: 10.1016/j.jsams.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Miranda H, et al. Exercise order interacts with rest interval during upper body resistance exercise. J Strength Cond Res. 2010;24:1573–1577. doi: 10.1519/JSC.0b013e3181d8ea61. [DOI] [PubMed] [Google Scholar]
- 19.Spineti J, et al. Influence of exercise order on maximum strength and muscle volume in nonlinear periodized resistance training. J Strength Cond Res. 2010;24:2962–2969. doi: 10.1519/JSC.0b013e3181e2e19b. [DOI] [PubMed] [Google Scholar]
- 20.Bellezza PA, Hall EE, Miller PC, Bixby WR. The influence of exercise order on blood lactate, perceptual, and affective responses. J Strength Cond Res. 2009;23:203–208. doi: 10.1519/JSC.0b013e3181889156. [DOI] [PubMed] [Google Scholar]
- 21.Farinatti PTV, Simão R, Monteiro WD, Fleck SJ. Influence of exercise order on oxygen uptake during strength training in young women. J Strength Cond Res. 2009;23:1037–1044. doi: 10.1519/JSC.0b013e3181a2b3e4. [DOI] [PubMed] [Google Scholar]
- 22.Gentil P, et al. Effects of exercise order on upperbody muscle activation and exercise performance. J Strength Cond Res. 2007;21:1082–1086. doi: 10.1519/R-21216.1. [DOI] [PubMed] [Google Scholar]
- 23.Simão R, Farinatti PT, Polito MD, Viveiros L, Fleck SJ. Influence of exercise order on the number of repetitions performed and perceived exertion during resistance exercise in women. J Strength Cond Res. 2007;21:23–28. doi: 10.1519/00124278-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Spreuwenberg LPB, et al. Influence of exercise order in a resistance exercise session. J Strength Cond Res. 2006;20:141–144. doi: 10.1519/R-18185.1. [DOI] [PubMed] [Google Scholar]
- 25.Simão R, Farinatti PTV, Polito MD, Maior AS, Fleck SJ. Influence of exercise order on the number of repetitions performed and perceived exertion during resistance exercises. J Strength Cond Res. 2005;19:152–156. doi: 10.1519/1533-4287(2005)19<152:IOEOOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Sforzo GA, Touey PR. Manipulating exercise order affects muscular performance during a resistance exercise training session. J Strength Cond Res. 1996;10:20–24. [Google Scholar]
- 27.Perrota AS, Jeklin AT, Hives BA, Meanwell LE, Warburton DER. Validity of the Elite HRV Smartphone Application for examining Heart Rate Variability in a Field-Based Settings. J Strength Cond Res. 2017;31:2296–2302. doi: 10.1519/JSC.0000000000001841. [DOI] [PubMed] [Google Scholar]
- 28.European Society of Cardiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Circulation. 93, 1043–1065 (1996). [PubMed]
- 29.Sociedade Brasileira de Cardiologia (SBC). V Diretrizes Brasileiras de Hipertensão Arterial. Arq Bras Cardiol. 89, 24–79 (2007). [PubMed]
- 30.Martin D, et al. Validity of pulse oximetry during exercise in elite endurance athletes. J Appl Physiol. 1992;72:455–458. doi: 10.1152/jappl.1992.72.2.455. [DOI] [PubMed] [Google Scholar]
- 31.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci. 2015;188:19–23. doi: 10.1016/j.autneu.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno M, et al. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Reg Int Comp Physiol. 2016;311:39–48. doi: 10.1152/ajpregu.00124.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol. 2011;301:1191–1204. doi: 10.1152/ajpheart.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venturelli, M. et al. Heart and musculoeskeletal hemodynamic responses to repetitive bouts of quadriceps static stretching. J Appl Physiol, [Epub ahead of print] (2019). [DOI] [PubMed]
- 36.Farinatti PTV, Brandão C, Soares PPS, Duarte AF. Acute Effects of Stretching Exercise on the Heart Rate Variability in Subjects With Low Flexibility Levels. J Strength Cond Res. 2011;25:1579–1585. doi: 10.1519/JSC.0b013e3181e06ce1. [DOI] [PubMed] [Google Scholar]
- 37.Costa e Silva G, et al. Acute effects of static and proprioceptive neuromuscular facilitation stretching on sprint performance in male swimmers. Med Sport. 2014;67:119–128. [Google Scholar]
- 38.Costa e Silva G, et al. Does the combination of resistance training and stretching increase cardiac overload? Clinics. 2019;74:e1066. doi: 10.6061/clinics/2019/e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? Journal of the American College of Cardiology. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 40.Dutra Sabrina G. V., Pereira Ana Paula M., Tezini Geisa C. S. V., Mazon José H., Martins-Pinge Marli C., Souza Hugo C. D. Cardiac Autonomic Modulation Is Determined by Gender and Is Independent of Aerobic Physical Capacity in Healthy Subjects. PLoS ONE. 2013;8(10):e77092. doi: 10.1371/journal.pone.0077092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albinet CT, Boucard G, Bouquet CA, Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. European Journal of Applied Physiology. 2010;109:617–624. doi: 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- 42.Nagashima J, et al. Three-month exercise and weight loss program improves heart rate recovery in obese persons along with cardiopulmonary function. Journal of Cardiology. 2010;56:79–84. doi: 10.1016/j.jjcc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Sandercock GR, Hardy-Shepherd D, Nunan D, Brodie D. The relationships between self-assessed habitual physical activity and non-invasive measures of cardiac autonomic modulation in young healthy volunteers. Journal of Sports Science. 2008;26:1171–1177. doi: 10.1080/02640410802004930. [DOI] [PubMed] [Google Scholar]


