Abstract
Purpose
In this study, we tested the hypothesis that, in PGT-A cycles, decreased semen quality is associated with increased rates of mosaic blastocysts.
Methods
In a retrospective analysis, three hundred and forty PGT-A cycles are divided into study groups according to semen quality. Cycles were initially divided into two groups, discerning couples with absence of male factor of infertility (non-male factor: NMF; N = 146 cycles) from couples with a male factor of infertility (MF; N = 173 cycles). Couples with severe male factor (SMF) infertility (n = 22) were assessed separately. Embryos were cultured to the blastocyst stage and chromosomally assessed by array comparative genomic hybridization (aCGH). The study did not involve specific interventions.
Results
The reproductive outcome of MF and NMF groups did not indicate statistically significant differences. However, while no differences were found between MF and NMF groups in terms of euploid or aneuploid blastocysts rates, a significantly higher rate of mosaic blastocysts was observed in the MF group (3.6% vs. 0.5%, respectively; P = 0.03). A similar pattern of results was observed in the SMF group when compared with those of the other PGT-A cycles taken together (no SMF). In particular, a significantly higher rate of mosaic blastocysts was observed in the SMF group (7.7% and 1.8%, respectively; P = 0.008).
Conclusions
The study outcome strongly suggests that compromised semen quality is associated with increased rates of mosaic blastocysts analysed in PGT-A cycles. Sperm assessment appears therefore as an important factor in the determination of embryo development and for a more precise prognostic assessment of PGT-A cases.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01584-w) contains supplementary material, which is available to authorized users.
Keywords: Aneuploidy, Mosaicism, Blastocyst, Semen, Infertility
Introduction
In assisted reproduction technology (ART) cycles, preimplantation genetic testing for aneuploidy (PGT-A) has long been proposed as an approach to identify and selectively use euploid embryos, in order to improve treatment efficiency and reduce time to pregnancy [1]. This strategy is justified by the fact that most of human conceptions are affected by different forms of aneuploidy, total or partial, that cause developmental failure at preimplantation or postimplantation stages or, in a few cases, very severe pathologies in the newborns [2]. The origins of such chromosomal disorders observed during development are well understood. While the paternal chromosome complement can account for a small fraction of chromosome segregation errors in the conceptus, over 90% of foetal aneuploidies are inherited from the maternal gamete as a consequence of chromosome mis-segregations occurring at meiosis I or meiosis II [3]. Therefore, maternal meiosis per se has been extensively investigated, not only for its prominent importance for embryo health, but also for the interest raised by the mechanistic factors that underpin chromosome segregation during oocyte meiosis [4].
Paternal- or maternal-derived aneuploidies are not the only factors that endanger the genetic integrity of the embryo. Chromosome segregation mechanisms may fail at post-fertilisation stages during the mitotic divisions of blastomeres. These errors generate a condition of chromosome mosaicism i.e. the simultaneous presence of two or more karyotipically distinct embryonic cell lines that may be incompatible with implantation and foetal development [5]. The study of mosaicism has a profound significance not only to understand human development, but also for the practice of ART. In fact, progress in genome analysis technologies has shown that mosaicism is not a negligible phenomenon in embryos able to develop to the blastocyst stage. Rather, it seems to affect a significant proportion of blastocysts, with important implications for embryo selection by PGT-A [6, 7]. Overall, the causes of this phenomenon remain rather obscure. Notably, however, the prevalence of embryo mosaicism does not seem to be determined by the same rules that govern embryo aneuploidy, as it is not associated with female meiosis and maternal age [8]. The data of the present study, in which we focused on a possible paternal impact on embryo genomic integrity, suggest that compromised semen quality is positively associated with increased rates of mosaic blastocysts analysed in PGT-A cycles.
Materials and methods
Patients, study design and study groups
This was a retrospective observational study carried out between May 2013 and December 2017 on 340 PGT-A cycles. Approval was obtained from the local Institution Review board. The couples included in the study were referred to PGT-A for severe male factor infertility, advanced maternal age, repeated implantation failure and recurrent unexplained pregnancy loss. Patients presenting with altered karyotype (e.g. balanced translocation) were excluded from the study.
The study groups were identified according to semen quality. In an initial analysis, the 340 PGT-A cycles were divided into two groups, discerning couples with absence of male factor of infertility (non-male factor: NMF; N = 146 cycles) from couples with a diagnosis of male factor of infertility (MF; N = 173 cycles). In our setup, male factor is assessed based on at least two consistent pre-treatment semen post-preparation (density gradient) results, by which clinicians can predispose a standard IVF or ICSI treatment. In particular, in the present study, male factor infertility (with an indication for ICSI) was excluded in cases where semen parameters were ALL above the following thresholds: total motile count < 2,000,000, total motility < 80%, progressive motility < 70%, normal morphology < 16%. Twenty-one cases were excluded from the study because semen values were below the thresholds, but not consistently in three different analyses (two pre-treatment assessments and one during treatment). Values reported in the relevant table are those detected on the day of treatment. Reproductive outcomes and blastocyst ploidy status of NMF and MF groups were compared. In a subsequent sub-analysis, couples with a PGT-A indication of severe male factor infertility (SMF: sperm concentration < 0.1 mil/ml; n = 22) were assessed separately. Reproductive outcomes and blastocysts ploidy status of SMF group were analysed and compared with those of all other PGT-A cycles taken together (N = 318 cycles).
Semen collection and preparation
Sperm samples were collected by masturbation at the day of oocyte pick-up. The samples were evaluated following the World Health Organization (WHO 2010, 5th edition) indications for sperm concentration, morphology and motility and were prepared using a discontinuous PureSperm gradient (Nidacon, Flöjelbergsgatan, Sweden) as previously described [9]. Briefly, sperm sample was layered upon a 40:80% PureSperm density gradient, processed by centrifugation at 600g for 15 min and resuspended in 1 ml of sperm culture medium (PureSperm wash; Nidacon). The 40:80% PureSperm gradient volumes were changed according to the total number of motile spermatozoa (motile spermatozoa total number < 15 mil: 0.5 ml PureSperm 40 and 0.5 ml Pure Sperm 80; motile spermatozoa total number ≥ 8 mil and ≤ 15 mil: 1 ml Pure Sperm 40 and 1 ml Pure Sperm 80; motile spermatozoa total number > 15 mil: 1.5 ml Pure Sperm 40 and 1.5 ml Pure Sperm 80). Immediately afterwards, a second evaluation of concentration, morphology and motility was carried out. The sperm suspension obtained with density gradient separation was used for oocyte insemination.
Ovarian stimulation, ICSI and embryo culture
Ovarian stimulation and oocyte retrieval were carried out as previously described [10]. In brief, ovarian stimulation was performed by recombinant FSH (Gonal F; Merck, Rome, Italy; Puregon; MSD, Rome, Italy) and monitored by plasma oestradiol and transvaginal ultrasonography. At 36 h before oocyte pick-up, 10,000 IU of human chorionic gonadotrophin (hCG), (Gonasi; Amsa, Rome, Italy) were administered. Oocyte retrieval was performed by vaginal ultrasound–guided aspiration, under general anaesthesia. On the day of oocytes retrieval, the sperm suspension obtained by density gradient centrifugation was used for oocyte intracytoplasmic sperm injection (ICSI) and ICSI was carried out as previously described [11]. At 16–18 h after insemination, oocytes were assessed for the appearance of two pronuclei and two polar bodies. Embryo culture was performed until day 5 or 6 utilising the EmbryoScope (Vitrolife, Sweden), an integrated embryo culture time-lapse microscopy system, at 37 °C in a N2/CO2/O2 (89:6:5, v/v) atmosphere, without control of humidity. Blastocyst grading procedures were carried out as previously described by [12].
Blastocyst biopsy, microarray comparative genomic hybridization and image interpretation
At 113–142 h after insemination, all fully expanded blastocysts with a clearly visible inner cell mass underwent trophectoderm (TE) biopsy. Blastocyst biopsy procedure was carried out as previously described [12]. Briefly, a diode laser (Saturn 3, Research Instruments, Cornwall TR11 4TA, UK) was used to assist the opening of a 10-20-μm hole in the zona pellucida on day 3. On days 5 and 6, the biopsy procedures were performed on a heated stage in a dish prepared with 20-μl droplets of Sydney IVF Blastocyst Medium (Cook IVF, Brisbane, Australia) overlaid with pre-equilibrated mineral oil. Five to nine trophectoderm cells were then aspirated into the biopsy aspiration pipette (Cook, Brisbane, Australia), followed by laser-assisted or mechanical removal of the trophectoderm cells from the epithelium.
Comprehensive chromosome analysis was carried out using microarray comparative genomic hybridization (aCGH) in combination with the 24SureTM Cytochip V3 microarray platform (Illumina Ltd., Cambridge, UK) for the detailed assessment of chromosomes in TE cells. The procedures were carried out as previously described by Fragouli et al. [13].
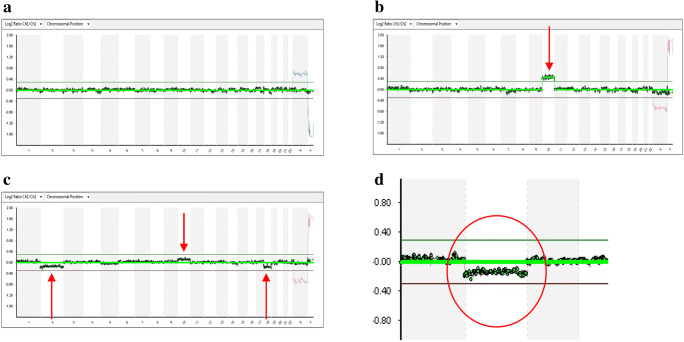
For image analysis and interpretation, ClearScan (Blugnome, Cambridge, UK) and Blue-Fuse multi software (Illumina, Cambridge, UK) were employed. Using algorithms and criteria recommended by the manufactures of the software, it was possible to classify blastocysts as euploid or aneuploid. Slight deviations of the aCGH profiles for one or more chromosomes, too subtle to be interpreted as a condition of aneuploidy, but at the same time not entirely consistent with an euploid profile, were considered indicative of mosaicism, as described by Fragouli et al. [14], (Fig. 1).
Fig. 1.
aCGH profiles of trophectoderm samples biopsied from three different blastocysts (a, b, c). a Profile of an euploid blastocyst. b Profile of an aneuploid blastocyst (chromosome 10: DNA copy number gain above the threshold value indicated by the green line). c Profile of a mosaic blastocyst (chromosomes 2, 10 and 18: DNA copy number gains and losses, but within the threshold values indicated by green and red lines). d Detail of the profile of the mosaic blastocyst shown in c (chromosome 2: DNA copy number loss, but within the threshold value indicated by red line) Figure 1 contains poor quality and small text inside the artwork. Please do not re-use the file that we have rejected or attempt to increase its resolution and re-save. It is originally poor, therefore, increasing the resolution will not solve the quality problem. We suggest that you provide us the original format. We prefer replacement figures containing vector/editable objects rather than embedded images. Preferred file formats are eps, ai, tiff and pdf.Done: I sent you the image 1 saved as docx and saved as a pdf. All the characters will be written so that they can be read more easil
Blastocyst vitrification, warming and embryo transfer
Blastocysts were cryopreserved using a vitrification protocol with a closed-system device (HSV straw, Cryo Bio System, France). Vitrification and warming procedures were carried out as previously described by Cobo et al. [15]. Solutions required for vitrification and warming were obtained from Kitazato (BioPharma Co., Japan). Embryo transfers were performed after freeze-all cycles or in fresh PGT-A cycles [16]. Assessment of clinical pregnancy was achieved by ultrasound detection of the gestational sac and visualisation of foetal heartbeat. Miscarriage was defined as pregnancy loss after ultrasound confirmation of embryo implantation and detection of foetal heartbeat.
Statistical analysis
Statistical analysis was performed with Statistics Package for Social Sciences (SPSS) for Windows software package version 10.1 (SPSS Inc., Chicago, IL, USA). The comparison between the different study groups, with regard to with regard to maternal and paternal age, body max index, FSH, trophectoderm cells collected and sperm parameters (sperm concentration, total sperm motility, progressive sperm motility and normal sperm morphology), was carried out using the t test. Levene’s test was applied before independent-samples t test to verify the equality of variances. The chi-square test was used to compare implantation, clinical pregnancy, miscarriage and live birth rates, and also the number of day 5 biopsy and the number of advanced maternal age (AMA), repeated implantation failures (RIF) and recurrent unexplained pregnancy loss (RM), in different groups of patients, where appropriate logistic regression analysis was performed using the XLMiner analysis ToolPak. Differences were considered significant at P < 0.05 and highly significant at P < 0.01.
Results
The present study focuses on the possible impact of semen quality on the generation of embryo mosaicism. For this purpose, a total of 340 PGT-A cycles were considered and divided into different study groups, identified according to semen quality. Overall, female age ranged between 26 and 48 years (mean 40.3 ± 4.4 years).
Impact of semen quality on embryo mosaicism: male factor infertility versus non-male factor infertility
In the initial study analysis, the 340 PGT-A cycles were divided into two groups, according to semen characteristics: (i) male factor (MF; N = 173); (ii) non-male factor (NMF; N = 146). Patient characteristics of such groups were comparatively assessed (Supplemental Table 1). The MF group was characterised by a younger female age and a smaller proportion of day 5 biopsies. Statistically significant differences between MF and NMF groups were observed with regard to all seminal features taken into consideration (P < 0.01): total sperm number (22.7 and 98.0 millions, respectively), sperm concentration (11.0 and 42.4 million/ml, respectively), total sperm motility (30.8% and 46.9%, respectively), progressive sperm motility (19.9% and 39.3%, respectively) and normal sperm morphology (11.3% and 20.0%, respectively), (Table 1). In the two groups, average maternal age between embryo transfer cycles was not statistically different (MF: 37.0 ± 3.9; NMF: 38.2 ± 2.3; P > 0.05). Moreover, comparison of the reproductive outcome of MF and NMF groups did not indicate statistically significant differences in terms of implantation, clinical pregnancy, miscarriage and live birth rates (P > 0.05) (Table 2). The two study categories were further compared with regard to euploid, aneuploid and mosaic blastocysts rate. Although no statistically significant difference was found between MF and NMF groups in terms of euploid blastocysts rates (45.9% and 42.9%, respectively; P > 0.05) and aneuploid blastocysts rates (50.5% and 56.5%, respectively; P > 0.05), a significantly higher rate of mosaic blastocysts was observed in the MF group (3.6% vs. 0.5%, respectively; P = 0.03), (Table 2). Notably, 91.7% (11/12) of the total number of mosaic blastocysts was found in the MF cycles.
Table 1.
Comparison between semen parameters of male factor (MF) and non-male factor (NMF) groups. Values, expressed as mean ± SD, were compared by t-test
| MF (n = 173) |
NMF (n = 146) |
P value | |
|---|---|---|---|
| Sperm total number (mil) | 22.7 ± 37.1 | 98.0 ± 71.8 | < 0.01 |
| Sperm concentration (mil/ml) | 11.0 ± 18.2 | 42.4 ± 27.6 | < 0.01 |
| Total sperm motility (%) | 30.8 ± 14.0 | 46.9 ± 8.3 | < 0.01 |
| Progressive sperm motility (%) | 19.9 ± 12.7 | 39.3 ± 9.3 | < 0.01 |
| Normal sperm morphology (%) | 11.3 ± 6.3 | 20.0 ± 7.0 | < 0.01 |
Table 2.
Comparison by chi-square test of reproductive outcomes and rates (%) of euploid, aneuploid and mosaic blastocysts, between male factor (MF) and non-male factor (NMF) groups
| MF (n = 173) (95%CI) | NMF (n = 146) (95%CI) | P value | |
|---|---|---|---|
| Implantation | 37/123 (30.1%) (23.2–36.9) | 24/68 (35.3%) (27.5–43.1) | NS |
| Clinical pregnancy | 34/118 (28.8%) (22.1–35.5) | 24/68 (35.3%) (27.5–43.1) | NS |
| Miscarriage | 4/34 (11.8%) (7.0–16.0) | 8/24 (33.3%) (25.6–40.9) | NS |
| Live birth | 30/118 (25.4%) (18.9–31.9) | 16/68 (23.5%) (16.6–30.4) | NS |
| Clinical pregnancy/cycle | 34/74 (45.9%) (38.5–53.3) | 24/43 (55.8%) (47.7–63.9) | NS |
| Live birth/cycle | 30/74 (40.5%) (33.2–47.8) | 16/43 (37.2%) (29.4–45.0) | NS |
| Number of analysed blastocysts | 307 | 191 | |
| Euploid blastocysts | 141 (45.9%) (40.3–51.1) | 82 (42.9%) (35.9–49.9) | NS |
| Aneuploid blastocysts | 155 (50.5%) (44.8–56.0) | 108 (56.5%) (49.5–63.5) | NS |
| Mosaic blastocysts | 11 (3.6%) (1.5–5.7) | 1 (0.5%) (0.1–0.8) | 0.03 |
Impact of semen quality on embryo mosaicism: severe male factor infertility
To confirm and possibly more finely appraise the relationship between semen quality and embryo mosaicism, in a subsequent sub-analysis. attention was focused on patients characterised by severe male factor infertility (Supplemental Table 2). Characteristics of such patients, together with those of the remaining PGT-A cycles, are reported in Supplemental Table 2. The comparison indicated in the SMF groups a younger female age and a smaller proportion of day 5 biopsies.
Among the 340 PGT-A cycles, 22 cycles responded to the criterion of a severe male factor condition (SMF: sperm concentration < 0.1 mil/ml). The mean values of sperm parameters of the SMF group, representing a subgroup of MF cycles described in the initial analysis, are shown in Supplemental Table 3.
The reproductive outcomes of SMF group were evaluated and compared with those of the other PGT-A cycles taken together (no SMF). Maternal age of embryo transfer cycles was not statistically different (SMF: 35.9 ± 3.4; no SMF: 37.6 ± 3.7; P > 0.05). No statistically significant differences were found in terms of implantation, clinical pregnancy, miscarriage and live birth rates (P > 0.05) (Table 3). The comparison between SMF group and all other PGT-A cycles group was then extended to the rates of euploid, aneuploid and mosaic blastocysts. Although between the two groups no statistically significant difference was found in terms of euploid blastocysts rates (48.1% and 44.2%, respectively; P > 0.05) and aneuploid blastocysts rates (44.2% and 54.0%, respectively; P > 0.05), a significantly higher rate of mosaic blastocysts was observed in the SMF group in comparison to all other PGT-A cycles taken together (7.7% and 1.8%, respectively; P = 0.008), (Table 3). Despite the SMF cycles represented only 6.5% (22/340) of the PGT-A cycles, they included 33.3% (4/12) of total mosaic blastocysts. Importantly, further analysis by logistic regression confirmed that blastocyst mosaicism was associated to SMF (P = 0.01), but not female age (P > 0.1).
Table 3.
Comparison by chi-square test of reproductive outcomes and rates (%) of euploid, aneuploid and mosaic blastocysts, between severe male factor (SMF) cycles and all other PGT-A cycles taken together (no SMF).
| SMF (n = 22) (95%CI) | No SMF (n = 318) (95%CI) | P value | |
|---|---|---|---|
| Implantation | 8/26 (30.8%) (11.5–50.0) | 53/168 (31.5%) (26.4–36.6) | NS |
| Clinical pregnancy | 8/24 (33.3%) (13.6–53.0) | 50/164 (30.5%) (25.4–35.6) | NS |
| Miscarriage | 1/8 (12.5%) (1.3–26.3) | 11/50 (22.0%) (17.4–26.6) | NS |
| Live birth | 7/24 (29.2%) (10.2–48.2) | 39/164 (23.8%) (19.1–28.5) | NS |
| Clinical pregnancy/cycle | 8/12 (66.7%) (47.0–86.3) | 50/107 (46.7%) (41.2–52.2) | NS |
| Live birth/cycle | 7/12 (58.3%) (37.7–78.9) | 39/107 (36.4%) (31.1–41.7) | NS |
| Number of analysed blastocysts | 52 | 452 | |
| Euploid blastocysts | 25 (48.1%) (34.5–61.7) | 200 (44.2%) (39.6–48.8) | NS |
| Aneuploid blastocysts | 23 (44.2%) (30.7–57.7) | 244 (54.0%) (49.4–58.6) | NS |
| Mosaic blastocysts | 4 (7.7%) (0.4–14.9) | 8 (1.8%) (0.5–3.0) | 0.008 |
Likewise, the comparison between the SMF group and patients not affected by male factor of infertility (NMF group, assessed in the initial study analysis) showed no statistically significant difference in terms of euploid and aneuploidy blastocysts rates (P > 0.05) but a highly statistically significant difference with regard to mosaic blastocysts rate (7.7% and 0.5%, respectively; P = 0.0012). Finally, we performed a subgroup analysis: NMF, MF (excluding SMF) and SMF were compared in terms of aneuploid, euploid and mosaic blastocysts rates. This comparison did not indicate significant differences, but only a trend of an increase in the proportion of mosaic blastocysts with increasing severity of male factor infertility (Supplemental Table 4).
Discussion
In this study, we tested the hypothesis that, in cycles selected for PGT-A, decreased semen quality is associated with increased rates of mosaic embryos at the blastocyst stage. Indeed, the presented data are consistent with this postulate, emphasising the importance of paternal contribution to successful development.
Studies on the embryo chromosome constitution have overwhelmingly converged on full aneuploidy, in light of the concerns and prevalence associated with such phenomenon. As a result of intense investigation carried out in animal models and human material, embryo aneuploidy is now largely recognised as an age-dependent phenomenon originating from female meiosis and caused mainly by premature separation of kinetochores in bivalents and loss of cohesion between sister chromatids [17].
Conversely, much remains to be unraveled on embryo mosaicism, which has turned out to be biologically elusive and technically more difficult to appraise [6, 18, 19]. Non-disjunction and anaphase lagging are two major chromosome-dependent mechanisms by which mosaicism may possibly occur in human embryos [18, 19]. Non-disjunction, also occurring at early embryonic stages, is the failure of sister chromatids to separate [20]. Anaphase lagging is the failure of a single chromatid to attach to the spindle microtubules and leads to incorrect segregation in one of the two daughter cells. This phenomenon is thought to be at the basis of mosaicism during preimplantation development [21, 22].
Both intrinsic and extrinsic factors might trigger the molecular mechanisms that cause embryo mosaicism. Modifications in the normal course of oogenesis caused by ovarian stimulation have been postulated to produce chromosomal aberrations, including post-zygotic anomalies [23, 24]. However, data are contradictory. For example, Munné and colleagues [25] reported an association between ovarian stimulation protocols and mosaicism rates in cohorts of embryo generated in different IVF centres. On another hand, chromosomal abnormalities are also observed in embryos from unstimulated ovaries of young women [26]. Embryo culture condition (e.g. oxygen concentration, temperature fluctuations and culture medium composition) may also affect chromosome segregation. In particular, higher oxygen concentration was found to increase non-disjunction events in an embryo mouse model [27], suggesting that the IVF laboratory can be a source of factors influencing chromosome segregation.
Failure of correct chromosome segregation at post-fertilisation stages may be generated by elements that are intrinsic to the constitution of the female and male gametes. Mitochondria, as well as many of the proteins regulating chromosome segregation during the first cellular divisions, are provided by the oocyte. DNA repair, telomere shortening, chromosomes cohesion, cell cycle checkpoints and microtubule kinetics are examples of processes or events involving proteins of maternal origin [28–31]. In particular, cohesins, are proteins forming complexes responsible for binding sister chromatids together, thereby collaborating to correct chromosome segregation [32]. Animal and human studies demonstrated that advanced female age negatively correlates with cohesin abundance in oocytes, resulting in chromosome mis-segregation during meiosis [33, 34]. This suggests that, should cohesin complex function be affected in development during mitotic events, mosaicism could be generated at different embryonic stages. Surprisingly, however, while maternal age is the single most important factor associated with oocyte and embryo aneuploidy, the same does not hold true for mosaicism [35, 36].
Components of the fertilising sperm, such as the centrosome and associated proteins, also play an important role in chromosome segregation mechanisms at post-fertilisation stages [37]. The centrosome is the organising centre of the mitotic spindle. In somatic cells, it consists of two centrioles and, together with a plethora of ancillary proteins, is responsible for nucleation of spindle microtubules. At gamete fusion, the sperm centriolar module organises the sperm aster, which acts to juxtapose the female and male pronuclei [38]. Following duplication, centrosomes guide organisation of the first mitotic spindle and chromosome segregation in the two blastomeres. It is therefore plausible that sperm-borne functions affect chromosome constitution at fertilisation and post-fertilisation stages [39, 40]. This hypothesis was initially explored in treatment cycles involving sperm surgically retrieved from the testis and, therefore, possibly characterised by incomplete spermiogenesis. Indeed, in two separate studies, an increased incidence of aneuploidy and mosaicism was reported in embryos generated using sperm collected by TESE [41, 42]. However, such results cannot be considered conclusive or complete, involving only immature sperm and having been generated through an approach, based on embryo biopsy on day 3 and chromosome analysis by FISH that has not stood the test of time.
The present study focuses precisely on the question of whether sperm quality, as assessed by standard semen analysis, has a reflection on embryo mosaicism, whose causes remain largely unknown or object of speculation. In doing so, compared with previous sporadic studies, overall, we adopted a significantly more advanced and reliable approach based on biopsy of trophectoderm cells at the blastocyst stage and comprehensive chromosome screening by aCGH. We acknowledge, however, that the approach to chromosome analysis has experienced significant progress in the few years. Therefore, we are planning to perform a similar investigation in the near future by using the next generation sequencing technology.
In an initial analysis assessing groups characterised by absence of male factor infertility or alternatively by compromised semen parameters, no relative differences were found in terms of reproductive outcome (implantation, clinical pregnancy, miscarriage and live birth rates) and proportion of euploid and aneuploid blastocysts. However, in the male factor group, although low in absolute terms, the rate of mosaic blastocysts was six times higher compared with the non-male factor group (3.6% vs. 0.5%). Strikingly, male factor cycles, which accounted for just over half of all PGT-A cycles, included 91.7% (11/12) of all mosaic blastocysts present in the data set. Analysis was then more specifically directed to compare cycles characterised by severe male factor with all other PGT-A cycles included in the study. Again, no statistically significant differences were found between these two groups in terms of reproductive outcome and rates of euploid and aneuploid blastocysts, while a much higher rate of mosaic blastocysts was observed in the severe male factor group (7.7% vs. 1.8%). The association between semen parameters and incidence of blastocyst mosaicism further emerges evident from our data if considered that although severe male factor cases cycles represented only 6.5% of all PGT-A cycles, they included 33.3% mosaic blastocysts. Finally, the comparison between severe male factor and non-male factor groups did not reveal statistically significant differences in the rates of euploid and aneuploidy blastocysts. However, again, it indicated a much higher rate of mosaic blastocysts in the severe male factor group, further confirming the positive relationship between compromised sperm parameters and embryo mosaicism. Furthermore, we performed a subgroup analysis: NMF, MF (excluding SMF) and SMF were compared in terms of aneuploid, euploid and mosaic blastocysts rates (data not shown). This comparison indicated only a trend of an increase in the proportion of mosaic blastocysts with increasing severity of male factor infertility. We reckon that this outcome is consistent with the study finding that the difference in the rate of mosaic blastocysts between the MF and NMF groups is mainly attributable to severe male factor cases.
Both in MF and SMF groups, female age was younger compared with the control group. This discrepancy, quite expected because male infertility is usually diagnosed earlier than other reproductive disorders, is not in conflict with the study main hypothesis. In fact, if anything, a younger age should involve a lower risk of chromosome segregation errors. Likewise, in the male factor groups, a smaller proportion of biopsied performed on day 5 blastocysts should not surprise, considering that sperm quality may well have an impact on the kinetics of development to the blastocyst stage [43].
Overall, the present analysis indicates a precise pattern involving decrease semen parameters, which represent a “proxy” for sperm quality and reduced genomic integrity of the preimplantation embryo. This relationship does not appear to impact the rate of aneuploid blastocysts, suggesting that chromosome segregation in the male germline during meiosis is unaffected by factors that vice versa can jeopardise other spermatogenetic processes. Consistent with such finding, spermatogenesis is known to have more robust meiotic and post-meiotic chromosome segregation check-points, compared with oogenesis [44]. On the contrary, an inverse relationship between semen quality and rate of mosaic embryos reported in this study points towards non-genomic sperm defects that may alter chromosome segregation at post-fertilisation stages. Correct distribution of sister chromatids during blastomere division is largely dependent on maternally inherited functions. However, crucially, sperm contribution to fertilisation and development includes also the centriole, from which a functional embryonic centrosome is formed after assembly with maternal proteins. As discussed above, the centrosome is essential for the formation of the mitotic spindle and ultimately chromosome segregation during cell division not only at fertilisation but also during preimplantation development. Astonishingly, decades-old, but crucial, evidence indicates that descendants of the sperm centriole can be detected during human preimplantation development and in fact traced from fertilisation through cleavage to the morula and hatching blastocyst stage [38]. These findings are compatible with the possibility that a defective sperm centriolar apparatus, possibly associated with decreased sperm quality, has the potential to impact spindle function and the fidelity of chromosome distribution not only during division of the zygotes into the first two blastomeres but also during successive cell divisions throughout development to the blastocyst stage. The introduction of next-generation sequencing (NGS) in the realm of PGT-A has raised even more awareness on the phenomenon of blastocyst mosaicism, irrespective of its origins, allowing a relatively more precise estimate of the mutual proportions of euploid/aneuploid cells in individual TE biopsies. While at this point in time the maximum rate of mosaicism in a blastocyst compatible with a healthy live birth is not known, there is a trend to rank mosaic blastocyst and prioritise for transfer those with smaller proportion of aneuploid cells.
Conclusions
We reckon that our data have clinical relevance. In the first place, they provide information by which the risk of formation of mosaic embryos can be more precisely assessed. This offers patients a better prognostic prediction of their PGT-A treatment, also on the basis of the conditions of the male partner. Secondly, the study confirms previous reports suggesting that male infertility does not affect the chances to obtain euploid blastocysts [45]. Moreover, our findings can at least partly explain inconsistencies in the rates of embryo mosaicism reported by different PGT-A programmes. Such differences have been the object of heated debate in recent years and generally explained as an effect of different sensitivity of alternative molecular approaches to chromosome analysis or biopsy modality. The present data suggest a diverse, although not mutually exclusive, origin of embryo mosaicism, highlighting the role of paternal contribution to successful development. Albeit plausible, the hypothesis of a direct involvement of the sperm centriole in the onset mosaicism remains unanswered in this study, suggesting new avenues for future research in this context. Also, to further test the study hypothesis, we are currently collecting further data in which mosaicism is being assessed by more advanced DNA analysis techniques. Future investigations will also overcome the limit of the present manuscript imposed by the relatively small size of study groups.
Electronic supplementary material
(DOCX 31 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Griffin DK, Ogur C. Chromosomal analysis in IVF: just how useful is it? Reproduction. 2018;156:F29–F50. doi: 10.1530/REP-17-0683. [DOI] [PubMed] [Google Scholar]
- 2.Fragouli E, Munne S, Wells D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update. 2019;25:15–33. doi: 10.1093/humupd/dmy036. [DOI] [PubMed] [Google Scholar]
- 3.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 4.Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi FM, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. 2017;23:706–722. doi: 10.1093/humupd/dmx026. [DOI] [PubMed] [Google Scholar]
- 5.McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–463. doi: 10.1016/j.tig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munné S, Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;107:1085–1091. doi: 10.1016/j.fertnstert.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Munné S. Origins of mosaicism and criteria for the transfer of mosaic embryos. Reprod BioMed Online. 2018;36:369–370. doi: 10.1016/j.rbmo.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Sekhon L, Feuerstein J, Nazem TG, Briton-Jones C, Lee JA, Grunfeld L, Copperman AB. The incidence of mosaicism is not associated with advanced maternal age or diminished ovarian reserve. Fertil Steril. 2017;108(3):e217. [Google Scholar]
- 9.Tarozzi N, Nadalini M, Bizzaro D, Serrao L, Fava L, Scaravelli G, Borini A. Sperm-hyaluronan-binding assay: clinical value in conventional IVF under Italian law. Reprod BioMed Online. 2009;19(Suppl 3):35–43. doi: 10.1016/s1472-6483(10)60282-9. [DOI] [PubMed] [Google Scholar]
- 10.Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82:601–605. doi: 10.1016/j.fertnstert.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Borini A, Bafaro MG, Bianchi L, Violini F, Bonu MA, Flamigni C. Oocyte donation programme: results obtained with intracytoplasmic sperm injection in cases of severe male factor infertility or previous failed fertilisation. Hum Reprod. 1996;11:548–550. doi: 10.1093/humrep/11.3.548. [DOI] [PubMed] [Google Scholar]
- 12.Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, Distratis V, Borini A. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod BioMed Online. 2017;34:137–146. doi: 10.1016/j.rbmo.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20:117–126. doi: 10.1093/molehr/gat073. [DOI] [PubMed] [Google Scholar]
- 14.Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, Wells D. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet. 2017;136:805–819. doi: 10.1007/s00439-017-1797-4. [DOI] [PubMed] [Google Scholar]
- 15.Cobo A, Bellver J, Domingo J, Pérez S, Crespo J, Pellicer A, Remohí J. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod BioMed Online. 2008;17:68–72. doi: 10.1016/s1472-6483(10)60295-7. [DOI] [PubMed] [Google Scholar]
- 16.Zacà C, Bazzocchi A, Pennetta F, Bonu MA, Coticchio G, Borini A. Cumulative live birth rate in freeze-all cycles is comparable to that of a conventional embryo transfer policy at the cleavage stage but superior at the blastocyst stage. Fertil Steril. 2018;110:703–709. doi: 10.1016/j.fertnstert.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68. doi: 10.1016/j.tcb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–581. doi: 10.1093/humupd/dmu016. [DOI] [PubMed] [Google Scholar]
- 19.Mantikou E, Wong KM, Repping S, Mastenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim Biophys Acta. 2012;1822:1921–1930. doi: 10.1016/j.bbadis.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Bean CJ, Hunt PA, Millie EA, Hassold TJ. Analysis of a malsegregating mouse Y chromosome: evidence that the earliest cleavage divisions of the mammalian embryo are non-disjunction-prone. Hum Mol Genet. 2001;10:963–972. doi: 10.1093/hmg/10.9.963. [DOI] [PubMed] [Google Scholar]
- 21.Coonen E, Derhaag JG, Dumoulin JC, van WLC, Bras M, Janssen M, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19:316–324. doi: 10.1093/humrep/deh077. [DOI] [PubMed] [Google Scholar]
- 22.Capalbo A, Bono S, Spizzichino L, Biricik A, Baldi M, Colamaria S, Ubaldi FM, Rienzi L, Fiorentino F. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum Reprod. 2013;28:509–518. doi: 10.1093/humrep/des394. [DOI] [PubMed] [Google Scholar]
- 23.Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril. 2005;84:634–643. doi: 10.1016/j.fertnstert.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Baart EB, Martini E, Eijkemans MJ, Van OD, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilisation reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 25.Munne S, Magli C, Adler A, Wright G, de BK, Mortimer D, et al. Treatment-related chromosome abnormalities in human embryos. Hum Reprod. 1997;12:780–784. doi: 10.1093/humrep/12.4.780. [DOI] [PubMed] [Google Scholar]
- 26.Verpoest W, Fauser BC, Papanikolaou E, Staessen C, Van LL, Donoso P, et al. Chromosomal aneuploidy in embryos conceived with unstimulated cycle IVF. Hum Reprod. 2008;23:2369–2371. doi: 10.1093/humrep/den269. [DOI] [PubMed] [Google Scholar]
- 27.Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilisation in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod. 2002;17:2362–2367. doi: 10.1093/humrep/17.9.2362. [DOI] [PubMed] [Google Scholar]
- 28.Baumann C, Viveiros MM, De LFR. Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet. 2010;6:e1001137. doi: 10.1371/journal.pgen.1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3:230–234. doi: 10.1093/embo-reports/kvf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells D, Bermudez MG, Steuerwald N, Thornhill AR, Walker DL, Malter H, Delhanty JDA, Cohen J. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod. 2005;20:1339–1348. doi: 10.1093/humrep/deh778. [DOI] [PubMed] [Google Scholar]
- 31.Zheng P, Dean J. Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc Natl Acad Sci U S A. 2009;106:7473–7478. doi: 10.1073/pnas.0900519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooker AS, Berkowitz KM. The roles of cohesins in mitosis, meiosis, and human health and disease. Methods Mol Biol. 2014;1170:229–266. doi: 10.1007/978-1-4939-0888-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Höög C, Herbert M. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, Ohye T, Kato T, Fujii T, Kurahashi H. Age-related decrease of meiotic cohesins in human oocytes. PLoS One. 2014;9:e96710. doi: 10.1371/journal.pone.0096710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munne S. Chromosome abnormalities in human embryos. Hum Reprod Update. 1998;4(6):842–855. doi: 10.1093/humupd/4.6.842. [DOI] [PubMed] [Google Scholar]
- 36.Munné S, Cohen J, Sable D. Preimplantation genetic diagnosis for advanced maternal age and other indications. Fertil Steril. 2002;78:234–236. doi: 10.1016/s0015-0282(02)03239-9. [DOI] [PubMed] [Google Scholar]
- 37.Palermo G, Munné S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–1225. doi: 10.1093/oxfordjournals.humrep.a138682. [DOI] [PubMed] [Google Scholar]
- 38.Sathananthan AH, Kola I, Osborne J, Trounson A, Ng SC, Bongso A, Ratnam SS. Centrioles in the beginning of human development. Proc Natl Acad Sci U S A. 1991;88:4806–4810. doi: 10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terada Y, Nakamura S, Morita J, Tachibana M, Morito Y, Ito K, Murakami T, Yaegashi N, Okamura K. Use of mammalian eggs for assessment of human sperm function: molecular and cellular analyses of fertilisation by intracytoplasmic sperm injection. Am J Reprod Immunol. 2004;51:290–293. doi: 10.1111/j.1600-0897.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto-Kakoi T, Terada Y, Tachibana M, Murakami T, Yaegashi N, Okamura K. Assessing centrosomal function of infertile males using heterologous ICSI. Syst Biol Reprod Med. 2008;54:135–142. doi: 10.1080/19396360802043091. [DOI] [PubMed] [Google Scholar]
- 41.Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munné S. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79:30–38. doi: 10.1016/s0015-0282(02)04407-2. [DOI] [PubMed] [Google Scholar]
- 42.Magli MC, Gianaroli L, Ferraretti AP, Gordts S, Fredericks V, Crippa A. Paternal contribution to aneuploidy in preimplantation embryos. Reprod BioMed Online. 2009;18:536–542. doi: 10.1016/s1472-6483(10)60131-9. [DOI] [PubMed] [Google Scholar]
- 43.Borges E, Jr, Zanetti BF, Setti AS, Braga DPAF, Provenza RR, Iaconelli A., Jr Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil Steril. 2019;112:483–490. doi: 10.1016/j.fertnstert.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Templado C, Uroz L, Estop A. New insights on the origin and relevance of aneuploidy in human spermatozoa. Mol Hum Reprod. 2013;19:634–643. doi: 10.1093/molehr/gat039. [DOI] [PubMed] [Google Scholar]
- 45.Mazzilli R, Cimadomo D, Vaiarelli A, Capalbo A, Dovere L, Alviggi E, Dusi L, Foresta C, Lombardo F, Lenzi A, Tournaye H, Alviggi C, Rienzi L, Ubaldi FM. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108:961–72.e3. doi: 10.1016/j.fertnstert.2017.08.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 31 kb)