Abstract
Purpose
To explore clinical benefit of performing two intrauterine inseminations (IUI) 24 h apart—a double IUI vs. a single IUI among lesbian and single women.
Methods
Retrospective cohort study using electronic medical record review during a 17-year period (11/1999–3/2017). A total of 11,396 patients at a single academic-affiliated private practice were included in this study. All cycles with a single or double IUI were included. A sub-analysis of first cycles only (n = 10,413) was also performed. Canceled IVF cycles converted to IUI were excluded. T tests and Wilcoxon rank-sum tests were used for continuous data, and chi-square for categorical data. Multivariable logistic regression controlled for patient age, day 3 follicle-stimulating hormone (D3 FSH), body mass index (BMI), peak estradiol (E2), and post-wash total motile sperm counts to model the association between IUI number and ongoing pregnancy rate (OPR) according to sperm source (autologous vs. donor). Generalized estimating equations and mixed effect models accounted for multiple cycles from the same woman. Adjusted odds ratio (AOR) with 95% CI was determined. Sub-analyses of sexual orientation and partner status were performed to compare heterosexual couples with proven infertility to women with lesbian and single women.
Results
During the study period, 22,452 cycles met inclusion criteria (single IUI 1283 vs. double IUI 21,169). Mean patient age and BMI were similar between groups. For couples using autologous sperm, OPR was significantly higher with double IUI (12.0% vs. 14.1%; p = 0.0380). A similar increase was observed for donor sperm OPR among heterosexual couples (14.4% vs. 16.2%), though this did not reach statistical significance (p = 0.395). A sub-analysis restricted to donor sperm demonstrates a clinical benefit of second IUI in heterosexual couples, 8.5% vs. 17.6% OPR (AOR 2.94; CI 1.00–10.99; p = 0.0496). When lesbian and single patients were evaluated, there was no difference (17.2% vs. 15.2%; AOR 0.99; CI 0.59–1.70; p = 0.0958).
Conclusions
Double IUI is associated with a significantly higher OPR for heterosexual couples using an autologous or donor sperm source. The benefit of a second IUI is less clear in patients with undocumented fertility status using donor sperm, such as single and lesbian women.
Keywords: Pregnancy rate; insemination, Autologous, Donor, Fertility, Lesbian, LGBTQ, Intrauterine insemination
Introduction
In response to changes in demographic trends as well as family structure and dynamics, the ethics committee of the American Society for Reproductive Medicine (ASRM) has released opinions encouraging comparable access to fertility treatment for lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) persons as well as for single individuals and unmarried heterosexual couples [1, 2]. As similarly addressed in a report by the Institute of Medicine (IOM), these opinions highlight a paucity of data regarding the unique considerations in caring for the LGBTQ population [3]. Notably, a recent retrospective chart review of a university-based fertility center demonstrated that the majority of patients utilizing therapeutic donor insemination (TDI) were lesbians couples (41%) compared with only 0.7% in 1985 [4]. In this same study, 29% were single women and 28% were heterosexual couples [4]. Furthermore, as a growing number of women delay childbearing due to professional and educational goals, there has been an increase in the incidence of births to single and unmarried women [2].
Lesbian and single women presenting to fertility clinics to access assisted reproductive technologies (ART) may not meet standard criteria for infertility diagnoses. In fact, one could hypothesize that this population would have similar if not higher rates of reproductive success when compared with heterosexual couples seeing reproductive endocrinologists [5]. A prior study that controlled for age and hormonal stimulation showed that lesbian couples have higher pregnancy rates than single women [6].
While intrauterine insemination is often used as first-line treatment in single women and lesbian patients, there is still debate on how to best optimize protocols for this population. Specifically, patients may do natural IUI timed to their LH surge, or use oral medications (clomiphene citrate or letrozole) or injectable gonadotropins for ovulation induction or superovulation. Clinic protocols may vary in the timing and frequency of inseminations performed [6–9]. Specifically, performing two inseminations, double insemination, has been described as a method to increase the likelihood of success by increasing the opportunity for fertilization; however, a consensus has yet to be reached due to heterogeneity of studies and conflicting conclusions from systematic reviews [10, 11]. Notably, a Cochrane review initially dismissed the benefit of second insemination in an original report but when including an additional study [12] actually found an advantage to double IUI protocols [10, 11]. In the following years, additional studies reported benefits as high as a twofold increase in pregnancies with double IUI compared with single IUI whereas others only showed a non-significant trend towards a benefit [13, 14]. More recent data show increased pregnancy rate with double IUI in patient with male factor infertility and ovulatory dysfunction [15, 16]. Interestingly, almost all of these studies were focused on autologous sperm source; however, there are some smaller studies and one larger study using donor sperm with conflicting results [17–20]. In patients with unproven fertility status, it is not known whether double IUI improves pregnancy rates in patients using donor sperm. Additionally, while some previous analyses used aggregate data including single and same-sex female couples, this will be the first paper specifically investigating single vs. double intrauterine insemination in both single and lesbian women.
Materials and methods
Patients
Using electronically extracted retrospective data from an academic-affiliated private practice during a 17-year period (11/99–3/17), we investigated all cycles with a single or double IUI performed. A total of 22,452 cycles and 11,396 patients were included in this study. Inclusion criteria were all patients at our clinic undergoing a single or double IUI completed during 11/1999–3/2017 regardless of sperm source. We included all cycle types including natural IUI, ovulation induction (clomiphene citrate or letrozole) IUI, and gonadotropin IUI. Canceled in vitro fertilization (IVF) cycles converted to IUI were excluded as were any cycles with incomplete data. IUI cycle cancelation occurred after clinician counseling in the setting of an increased risk of higher order multiple gestation (e.g., age, estradiol level > 1000 pg/mL, > 3 lead follicles), inadequate response, or premature ovulation. Incomplete cycle data was most commonly due to D3 FSH values (8383 of 30,835 cycles). In order to identify lesbian patients, patient gender was compared with the gender of the linked partner’s electronic medical record. Single women were not linked to a partner’s record. All female patients in our practice, regardless of sexual orientation or partner status, undergo routine fertility evaluation, including day 3 hormones, sonogram for uterine pathology and antral follicle count, and tubal evaluation with hysterosalpingogram. If the patient has a male partner, a semen analysis is performed. In the case of donor sperm, a combination of anonymous sperm banks and known donors were used. The study procedures were exempt from review and no additional approval from Institutional Review Board (IRB) was needed.
Outcome
Our primary outcome was ongoing pregnancy rate (OPR) defined as fetal cardiac activity > 7 weeks.
Protocol
In our practice, the standard of care is to perform two inseminations in a given cycle. Those who elected to perform single IUI were left to the discretion of the patient and provider (e.g., financial barriers, scheduling conflicts, or surging without trigger). Trigger guidelines for ovulation induction or super ovulation with oral agents were based on a lead follicle greater than 20–22 mm in maximal dimension whereas 18–19 mm was used for injectable IUI cycles. Ovidrel® (premixed, prefilled syringe, 250 mcg of r-hCG in a 0.5 cc solution) was used regardless of insemination number. In double IUIs, the first insemination occurred 12 h after Ovidrel® trigger injection and the second was approximately 36 h. Single IUIs were performed 36 h after subcutaneous administration of Ovidrel®. For patients who surged on their own, one IUI was performed the following day. Autologous sperm refers to sperm donated from the patient’s sexually intimate partner as opposed to using sperm from a designated donor or sperm bank. For the purposes of this paper, the term “autologous sperm” will be used in place of “partner’s sperm.” Donor sperm came from both known donors and anonymous donor banks. During the study period, the same sperm wash and thaw protocols have been used at our practice, as overseen by the same andrology lab director.
Statistics
T tests and Wilcoxon rank-sum and chi-square tests were utilized for continuous and categorical variables, respectively. Additionally, multivariable logistic regression controlled for patient age, D3 FSH, BMI, peak E2, and post-wash total motile counts. Lastly, generalized estimating equations (GEE) and mixed effect models (MEM) accounted for multiple cycles from the same woman. Adjusted odds ratio (AOR) with 95% CI was determined. A sub-analysis restricted to first cycle was performed only including complete cases (10,413 cycles from 10,413 patients). Incomplete cases (2721 cycles and patients) most commonly lacked data on D3 FSH. Odds ratio (OR) were computed by logistic regression and both Wald and likelihood-ratio test results were calculated. Sub-analyses of sexual orientation and partner status were also performed to compare heterosexual couples with proven infertility to women with lesbian and single women. Models were selected based on lowest Akaike information criterion (AIC). Post hoc power analysis was performed using with PS Power (http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize). Given our sample size, we had 80% power (α = 0.05) to detect a 2.3% difference in autologous OPR and a 5.5% difference in donor OPR.
Results
There were 22,452 cycles (11,396 patients) that met inclusion criteria (single IUI 1283 vs. double IUI 21,169) during the study period (Table 1). Demographic characteristics were similar between groups with mean patient age 33.7 ± 5.5 vs. 34.6 ± 4.5 years old and BMI 25.9 ± 6.5 vs. 26.1 ± 6.6 kg/m2. The majority of cycles were characterized by two inseminations per cycle using autologous sperm source.
Table 1.
Demographic characteristics and treatment parameters (all patients)
| Single IUI (n = 1283) | Double IUI (n = 21,169) | |
|---|---|---|
| Age (year) | 34.0 ± 5.38 | 34.7 ± 4.49 |
| BMI (kg/m2)1 | 26.0 ± 6.40 | 26.1 ± 6.56 |
| Cycle number, n (%) | ||
| • < 3 | • 954 (74%) | • 15,331 (72%) |
| • > 3 | • 329 (26%) | • 5838 (28%) |
| Donor sperm, n (%) | ||
| • Donor | • 187(15%) | • 1461(6.9%) |
| • Autologous | • 1096(85%) | • 19,708 (93%) |
| Stimulation type | ||
| • Clomiphene | • 357 (28%) | • 4140 (20%) |
| • Letrozole | • 51 (4.0%) | • 343 (1.6%) |
| • Natural | • 141 (11%) | • 822 (3.9%) |
| • Gonadotropin | • 711 (55%) | • 15,454 (73%) |
| • Unknown | • 23 (1.8%) | • 410 (1.9%) |
| Number of follicles (> 17 mm) | 0.943 ± 0.933 | 1.008 ± 0.941 |
| Peak estradiol (pg/mL) | 454.79 ± 415 | 508.81 ± 407 |
| Post-wash total motile sperm count #1 (M)2 | 36.42 ± 45.9 | 49.72 ± 53.2 |
| Post-wash total motile sperm count #2 (M)2 | – | 38.20 ± 38.2 |
11016 cycles were excluded due to either missing data or extreme BMI values (< 10 and > 70 kg/m2). 2314 cycles were excluded due to either missing data or extreme values for post tot mot (> 2000 M)
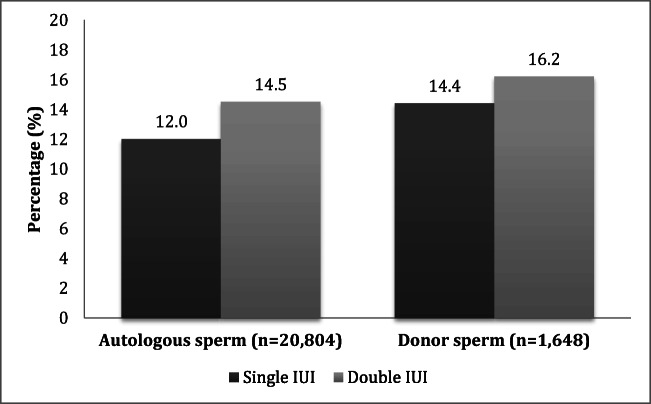
For couples using autologous sperm, Fig. 1 shows that OPRs were significantly higher with double IUI (12.0% vs. 14.5%; AOR 1.27; CI 1.01–1.61, p = 0.0380, GEE p = 0.0360). Sub-analysis of first cycles only demonstrated similar findings: the OPR among couples using autologous sperm (n = 9755) was 10.8% with one IUI and 14.9% with two IUI (AOR 1.46; CI1.09–1.95; p = 0.0100). A similar direction and magnitude of effect was observed for donor sperm OPR, though this did not reach statistical significance in the primary analysis of all cycles (n = 1648; AOR 1.23; CI 0.77–2.06; p = 0.400, GEE p = 0.435) or a sub-analysis of first cycles only (n = 658; AOR 1.25; CI 0.613–2.56; p = 0.536). Lesbian and single women were significantly more likely to undergo single IUI (RR 1.35, 95% CI 1.11–1.65; P = 0.00300).
Fig. 1.
Analysis of OPR from all cycles according to IUI number and sperm source
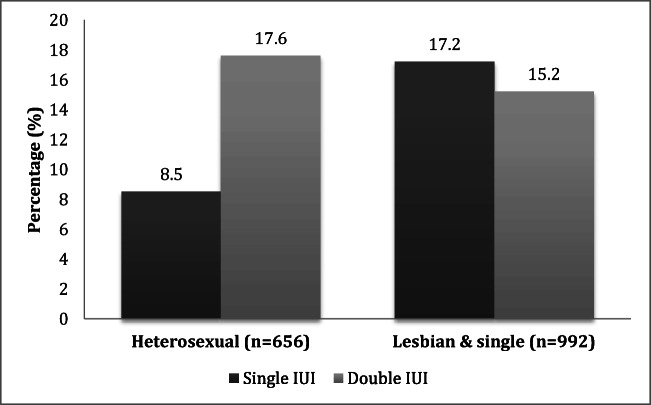
Figure 2 shows a sub-analysis restricted to donor sperm and demonstrates a clinical benefit of second IUI in heterosexual couples, 8.5% (n = 5/59) vs. 17.6% (n = 105/597) OPR (n = 656; AOR 2.94; CI 1.00–10.99; p = 0.0496; GEE p = 0.0660). The benefit of a second IUI persisted in the sub-analysis of heterosexual couples using donor sperm in their first cycle 9.5% (n = 2/21) vs. 16.7% (n = 38/228) OPR (n = 249; AOR 2.75; CI 0.424–17.8; p = 0.289). When lesbian and single patients were evaluated, there was no difference in OPR between one and two IUI in the primary analysis of all cycles (n = 992; 17.2% [n = 22/128] vs. 15.2% [n = 131/864]; AOR 0.99; CI 0.59–1.70; p = 0.0958; GEE p = 0.981) and the sub-analysis of first cycles only (n = 409; 16.9% vs. 15.1%; CI 0.46–2.22; p = 0.990).
Fig. 2.
Sub-analysis of OPR from donor IUI according to sexual orientation and partner status
Discussion
The primary finding of this study is that double IUI as compared with single IUI may not be necessary in those with unproven fertility status such as lesbian and single women. Notably, double IUI was shown to increase the OPR among infertile heterosexual couples regardless of sperm source. To the author’s knowledge, this will be one of the largest studies to date investigating single vs. double IUI using donor sperm.
Clinical protocols for IUI cycles vary from practice to practice. Specifically, some practitioners routinely perform one insemination per cycle (single IUI) whereas others perform two (double IUI) [8].
Prior systematic reviews have not agreed upon a definitive answer regarding the debate on single vs. double IUI; however, this study adds a large sample supporting the use of double IUI in heterosexual couples [10, 13, 21, 22]. Notably, this held true when using either an autologous or donor sperm source; however, in the latter case of donor sperm in a sub-analysis among hetersexual couples, the statistical significance was modest (p = 0.0496, CI 1.00–10.99). The benefit of a second insemination with donor sperm is similar to results from some [19, 20] but not all prior studies [17, 18]. One similar study with a large sample size (over 3000 cycles) highlighted small sample sizes and conflicting results in historic literature regarding IUI using donor sperm. Our study provides 1648 more donor IUI cycles to the existing literature and notably expands sub-analyses to include sexual orientation and partner status. Given a lack of infertility diagnosis for most single and lesbian women, one could hypothesize that fewer interventions would be necessary to achieve reproductive success in these populations. Interestingly, a recent study reported that lesbian couples may have even higher pregnancy rates than single women [6]. However, Nordqvist et al. only showed a significantly higher pregnancy rate in lesbian couples but not an increase in live birth rate [23]. Methodologic flaws have been highlighted including a small sample size and poorly controlling for confounders [5]. In the present study, we used a larger sample size and controlled for important confounders finding that an added benefit for second insemination was not seen in lesbian and single women.
This study is limited by its retrospective design with the intervention of choice (single vs. double IUI) being chosen by patient and/or physician possibly introducing selection bias. Our power analysis was post hoc of a convenience sample, and the study was only powered to detect a difference in donor OPR > 5.5%. Of note, 27% of IUI cycles (n = 8383/30,835) were excluded due to missing D3 FSH values. Some lesbian patients may be misclassified as single women if they withheld information regarding their partner status. Interestingly, lesbian patients in particular may be more likely to avoid medical intervention and only present for consultation after failed home vaginal insemination with donor sperm or samples from known acquaintances [6]. We did not collect data on whether or not patients had failed home insemination. Unfortunately, live birth outcomes were not available and thus not included in analysis.
To the authors’ knowledge, this is the largest study of IUI outcomes according to sexual orientation and partner status. Other strengths of the study include an improvement upon prior studies investigating single women and lesbian patients by using a large sample size and by using multivariable analysis controlled for multiple cycles contributed by the same woman, along with addressing important confounders such as age, D3 FSH, BMI, peak E2, and post-wash total motile counts [5]. Given that a majority (58%) of pregnancy losses after ART occur before 6 weeks’ gestation, we used OPR, defined as fetal cardiac activity > 7 weeks, as a surrogate marker in this study [24].
While previous research suggests that ovarian reserve, specifically antral follicle count, does not predict clinical pregnancy and miscarriage rate for unstimulated therapeutic donor insemination (TDI), a secondary analysis of two randomized-controlled trials demonstrated that patients with D3 FSH 10–15 mIU/mL were unlikely to achieve live birth after gonadotropin IUI [25, 26]. As a result, D3 FSH was included in our model to control for the heterogeneous group of patients included in the analysis. For heterosexual couples, a benefit of second IUI was observed that was not seen in lesbian and single patients. Our findings are consistent with an a priori assumption that OPR should be similar or higher in patients with unknown/presumed fertility given no medical indication for decreased fertility. Specifically, single individuals and lesbian patients often seek fertility treatment for family building not because they are infertile. Additionally, one would expect that among heterosexual couples using donor sperm, there may be other female factors contributing to their diagnosis of infertility. To account for multiple cycles per patient, we performed GEE and MEM taking into account important confounders and their interactions. Of note, a sub-analysis restricted to first cycle only to control for the effect of having the same patient in multiple cycles demonstrated similar findings.
Our results will also contribute to the ongoing conversation regarding how to best tailor IUI protocols not only for the infertile population-at-large but also for single women and the LGBTQ community. This study adds to the growing body of literature surrounding IUI optimization and provides good evidence for the use of double IUI in heterosexual couples using autologous sperm and possibly with the use of donor sperm as well.
Current trends demonstrate national increases in LGBTQ-identified patents pursuing assisted reproductive technologies for their family building needs [27]. Given that married same-sex couples are more likely to have children when compared with their unmarried counterparts, the landmark decision of Obergefell v. Hodges, guaranteeing the right to marry for same-sex couples, will likely even further increase the demand of LGBTQ fertility treatment across the country [27, 28]. Significantly, LGBTQ individuals are even more likely to be unaware of the risk factors of infertility as has been previously shown in reproductive-aged women [29]. This may further delay seeking out treatment and serves as a barrier to care that is only further exacerbated by a perceived lack of provider knowledge and support [30]. Clinics with LGBTQ website content are disproportionately located in the west or northeastern regions of the USA making access particularly difficult in certain geographical areas [27]. Notably, given similar clinical outcomes in single vs. double IUI, the routine use of single IUI in single individuals and lesbian couples could substantially reduce cost (~ $800 more per cycle) associated with donor sperm in these population [20]. The results from this study and additional studies could be used to generate evidence-based guidelines and provide reproductive endocrinologists with the opportunity to help improve health literacy and reproductive health outcomes as well as decreased cost in the LGBTQ patient population. Incorporating LGBTQ specific family building research on websites and clinical protocols may help reduce logistical and financial barriers.
Our study adds to a growing body of literature focused specifically on single and lesbian women; however, there are still many unanswered questions particularly with regard to LGBTQ fertility. Because lesbian couples have the unique ability to have two partners potentially capable of undergoing therapy, future studies could investigate success of the couple instead of the individual should multiple cycles with different partner be performed. Notably, a recent interview with a LGBTQ fertility expert and a subsequent separate study highlighted a deficit in research specifically dedicated to fertility preservation in the the transgender population despite the fact that as many as a quarter of transgender men express a desire to carry pregnancy [31, 32]. But without sufficient education and research, the LGBTQ population may only attempt treatment at home without presenting for a consultation with a reproductive endocrinologist. A qualitative study in Canada showed that lesbian and bisexual women choosing to have children were satisfied with care from midwives, doulas, and public health nurses but found fertility services unsupportive and were dissatisfied with physician care [30]. Healthcare providers and practices would benefit from additional LGBTQ focused training and research in order to develop evidence-based practices in caring for the special needs of this patient population.
In our present study, double IUI is associated with significantly higher OPR for heterosexual couples using an autologous or donor sperm source while the benefit of a second IUI was not found in patients with undocumented infertility using donor sperm, such as lesbian and single women. These data add to the growing literature about reproductive outcomes in the LGBTQ population. This work also highlights the importance of providing tailored medical care and the need for informed choices specifically in the setting of unproven fertility status. Our findings contribute to an ongoing discussion regarding the continued optimization of IUI protocols specifically within the context of single vs. double insemination when using either autologous or donor sperm.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
An abstract of this research was accepted and presented as a poster at the American Society of Reproductive Medicine (ASRM) annual meeting in San Antonio, TX in October 2017. A draft of this manuscript was given the Luigi Mastroianni Research Award from the Philadelphia Area Reproductive Endocrine Society.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104:1111–5. [DOI] [PubMed]
- 2.Access to fertility treatment by gays, lesbians, and unmarried persons: A committee opinion. Fertil Steril. 2013;100:1524–7. [DOI] [PubMed]
- 3.Temple-O’Connor MD, Wehr E, NIH LGBT. Wk. 6 consideration of the Institute of Medicine Report on the “health of lesbian, gay, biesexual, and transgender individuals”. 2011. Research coordinating committee. [Google Scholar]
- 4.Carpinello OJ, Jacob MC, Nulsen J, Benadiva C. Utilization of fertility treatment and reproductive choices by lesbian couples. Fertil Steril. 2016;106:1709–1713.e4. doi: 10.1016/j.fertnstert.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Tarín JJ, García-Pérez MA, Cano A. Deficiencies in reporting results of lesbians and gays after donor intrauterine insemination and assisted reproductive technology treatments: a review of the first emerging studies. Reprod Biol Endocrinol. 2015;13:52. doi: 10.1186/s12958-015-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara I, Balet R, Grudzinskas JG. Intrauterine donor insemination in single women and lesbian couples: a comparative study of pregnacny. Hum Reprod Englert. 2000;15:621–625. doi: 10.1093/humrep/15.3.621. [DOI] [PubMed] [Google Scholar]
- 7.Ombelet W. The revival of intrauterine insemination: evidence-based data have changed the picture. Facts, views Vis ObGyn. 2017;9:131–132. [PMC free article] [PubMed] [Google Scholar]
- 8.Bahadur G, Homburg R. Reappraisal of clinical data supports double IUI for improved pregnancy outcomes. Facts, views Vis ObGyn Vlaamse Vereniging voor Obstetrie en Gynaecologie. 2018;10:45–46. [PMC free article] [PubMed] [Google Scholar]
- 9.Bahadur G, Homburg R, Al-Habib A. A new dawn for intrauterine insemination: efficient and prudent practice will benefit patients, the fertility industry and the healthcare bodies. J Obstet Gynecol India. 2017;67:79–85. doi: 10.1007/s13224-016-0928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantineau AE, Heineman MJ, Cohlen BJ. Single versus double intrauterine insemination (IUI) in stimulated cycles for subfertile couples. Cochrane Database Syst Rev. 2003. [DOI] [PubMed]
- 11.Cantineau AEP, Heineman MJ, Cohlen BJ. Single versus double intrauterine insemination in stimulated cycles for subfertile couples: a systematic review based on a Cochrane review. Hum Reprod. Oxford University Press. 2003:941–6. [DOI] [PubMed]
- 12.Liu W, Gong F, Luo K, Lu G. Comparing the pregnancy rates of one versus two intrauterine inseminations (IUIs) in male factor and idiopathic infertility. J Assist Reprod Genet. 2006;23:75–79. doi: 10.1007/s10815-005-9017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garas A, Polyzos NP, Papanikolaou E, Anifandis G, Daponte A, Verykouki C, et al. Double versus single homologous intrauterine insemination for male factor infertility: a systematic review and meta-analysis. Asian J Androl. 2013;15:533–538. doi: 10.1038/aja.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagis T, Haydardedeoglu B, Kilicdag EB, Cok T, Simsek E, Parlakgumus AH. Single versus double intrauterine insemination in multi-follicular ovarian hyperstimulation cycles: a randomized trial. Hum Reprod. Oxford University Press. 2010;25:1684–1690. doi: 10.1093/humrep/deq112. [DOI] [PubMed] [Google Scholar]
- 15.Ghanem ME, Bakre NI, Emam MA, Al Boghdady LA, Helal AS, Elmetwally AG, et al. The effects of timing of intrauterine insemination in relation to ovulation and the number of inseminations on cycle pregnancy rate in common infertility etiologies. Hum Reprod. 2011;26:576–583. doi: 10.1093/humrep/deq362. [DOI] [PubMed] [Google Scholar]
- 16.Randall GW, Gantt PA. Double vs. single intrauterine insemination per cycle: use in gonadotropin cycles and in diagnostic categories of ovulatory dysfunction and male factor infertility. J Reprod Med. 2008;53:196–202. [PubMed] [Google Scholar]
- 17.Zarek SM, Hill MJ, Richter KS, Wu M, Decherney AH, Osheroff JE, et al. Single-donor and double-donor sperm intrauterine insemination cycles: does double intrauterine insemination increase clinical pregnancy rates? Fertil Steril. 2014;102:739–743. doi: 10.1016/j.fertnstert.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalifa Y, Redgment CJ, Tsirigotis M, Grudzinskas JG, Craft IL. The value of single versus repeated insemination in intra-uterine donor insemination cycles. Hum Reprod Oxford University Press. 1995;10:153–154. doi: 10.1093/humrep/10.1.153. [DOI] [PubMed] [Google Scholar]
- 19.Matilsky M, Geslevich Y, Ben-Ami M, Ben-Shlomo I, Weiner-Megnagi T, Shalev E. Two-day IUI treatment cycles are more successful than one-day IUI cycles when using frozen-thawed donor sperm. J Androl. 19:603–7. [PubMed]
- 20.Chavkin DE, Molinaro TA, Roe AH, Sammel MD, Dokras A. Donor sperm insemination cycles: are two inseminations better than one? J Androl. 2012;33:375–380. doi: 10.2164/jandrol.111.013276. [DOI] [PubMed] [Google Scholar]
- 21.Duran HE, Morshedi M, Kruger T, Oehninger S. Intrauterine insemination: a systematic review on determinants of success intrauterine insemination versus timed intercourse/intracervical insemination natural cycle versus ovarian stimulation in conjunction with intrauterine insemination timing/induction. Hum Reprod Update. 2002;8:373–384. doi: 10.1093/humupd/8.4.373. [DOI] [PubMed] [Google Scholar]
- 22.Osuna C, Matorras R, Pijoan JI, Rodríguez-Escudero FJ. One versus two inseminations per cycle in intrauterine insemination with sperm from patients’ husbands: a systematic review of the literature. Fertil Steril. 2004;82:17–24. doi: 10.1016/j.fertnstert.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Nordqvist S, Sydsjö G, Lampic C, Åkerud H, Elenis E, Skoog Svanberg A. Sexual orientation of women does not affect outcome of fertility treatment with donated sperm. Hum Reprod. 2014;29:704–711. doi: 10.1093/humrep/det445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol Narnia. 2007;165:1380–1388. doi: 10.1093/aje/kwm035. [DOI] [PubMed] [Google Scholar]
- 25.Ripley M, Lanes A, Léveillé M-C, Shmorgun D. Does ovarian reserve predict egg quality in unstimulated therapeutic donor insemination cycles? Fertil Steril. 2015;103:1170–1175. doi: 10.1016/j.fertnstert.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Kaser DJ, Goldman MB, Fung JL, Alper MM, Reindollar RH. When is clomiphene or gonadotropin intrauterine insemination futile? Results of the fast track and standard treatment trial and the forty and over treatment trial, two prospective randomized controlled trials. Fertil Steril. 2014;102:1331–1337.e1. doi: 10.1016/j.fertnstert.2014.07.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu HY, Yin O, Monseur B, Selter J, Collins LJ, Lau BD, Christianson MS. Lesbian, gay, bisexual, transgender content on reproductive endocrinology and infertility clinic websites. Fertil Steril. 2017;108:183–191. doi: 10.1016/j.fertnstert.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Gates GJ, Brown TNT. Marriage and same-sex couples after Obergefell. 2015. [Google Scholar]
- 29.Thomas S, Chung K, Paulson R, Bendikson K. Barriers to conception: LGBT individuals have worse fertility health literacy than their heterosexual female peers. Fertil Steril. 2018;109:e53–e54. [Google Scholar]
- 30.Ross LE, Steele LS, Epstein R. Service use and gaps in services for lesbian and bisexual women during donor insemination, pregnancy, and the postpartum period. J Obstet Gynaecol Canada. 2006;28:505–511. doi: 10.1016/S1701-2163(16)32181-8. [DOI] [PubMed] [Google Scholar]
- 31.Light A, Wang L-F, Zeymo A, Gomez-Lobo V. Family planning and contraception use in transgender men. Contraception Elsevier. 2018;98:266–269. doi: 10.1016/j.contraception.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Eyler AE, Pang SC, Clark A. LGBT assisted reproduction: current practice and future possibilities. [DOI] [PubMed]