Abstract
Purpose
To investigate the effectiveness of a biphasic IVM culture strategy at improving IVM outcomes in oocytes from small follicles (< 6 mm) compared with routine Standard IVM in patients with polycystic ovaries.
Methods
This prospective pilot study was performed in 40 women with polycystic ovaries whose oocytes were randomized to two IVM culture methods. Patients received a total stimulation dose of 450 IU rFSH. Cumulus-oocyte complexes (COCs) from follicles < 6 mm and ≥ 6 mm were retrieved and cultured separately in either a prematuration medium with c-type natriuretic peptide followed by IVM (CAPA-IVM), or STD-IVM. Primary outcomes were maturation rate, embryo quality, and the number of vitrified day 3 embryos per patient.
Results
Use of the CAPA-IVM system led to a significant improvement in oocyte maturation (p < 0.05), to a doubling in percentage of good and top-quality day 3 embryos per COC, and to an increased number of vitrified day 3 embryos (p < 0.001), compared to STD IVM. Oocytes from follicles < 6 mm benefited most from CAPA-IVM, showing a significant increase in the amount of good and top-quality embryos compared to STD IVM. CAPA-IVM yielded significantly (p < 0.0001) less GV-arrested oocytes and larger oocyte diameters (p < 0.05) than STD IVM.
Conclusions
CAPA-IVM brings significant improvements in maturation and embryological outcomes, most notably to oocytes from small antral follicles (< 6 mm), which can be easily retrieved from patients with a minimal ovarian stimulation. The study demonstrates the robustness and transferability of the CAPA-IVM method across laboratories and populations.
Keywords: IVF, IVM, Embryo, Oocyte prematuration, C-type natriuretic peptide
Introduction
In vitro oocyte maturation (IVM) is a mild-approach–assisted reproductive technology (ART). The technology has mostly been applied in patients with polycystic ovaries [1]. Because of its simplicity, the more favorable financial implications (reduced cost) for patients, and the lower health risk profile of IVM compared to controlled ovarian stimulation (COS) in polycystic ovary syndrome (PCOS) patients, the use of IVM in selected patients may gain an increasing interest. Besides its application in patients with polycystic ovaries, IVM technology may have a role in fertility preservation, where young girls and adult women are facing infertility due to gonadotoxic chemotherapeutic treatment [2–4].
While IVM is being increasingly adopted in fertility clinics, its main challenge is still to overcome the reduced efficacy in terms of live birth rates in comparison to COS [5–7]. This is related to the fact that clinical and laboratory IVM protocols vary notably between fertility centers [8]. Up to date, following a short course of gonadotropin treatment to support follicular growth, many centers use hCG triggering prior to oocyte retrieval [8–10]. This strategy is a variant of the IVM protocol, referred as “hCG triggered IVM or truncated IVF” [11]. Exposure of follicles < 12 mm to an ovulatory hCG trigger results in a heterogeneous cohort of in vivo–matured metaphase (MII)-stage, MI-stage oocytes, and germinal-vesicle (GV)-stage oocytes [8] hampering standardization of IVM culture conditions.
A more homogenous cohort of oocytes can be retrieved after a short course of FSH [12] or highly purified (HP)-hMG stimulation without any hCG triggering [13]. With this strategy, compact cumulus-oocyte complexes (COCs) enclosing oocytes at the immature GV-stage are consistently retrieved from small and mid-antral follicles (2 to 10 mm) to be matured in vitro under standard conditions. In PCOS women, smaller follicles (< 6 mm) are abundant at the time of retrieval (~ 70–80%) and the oocytes from these have maturation rates lower than 50% [14, 15]. Previous characterization of the non-maturing population of oocytes arrested at the GV-stage revealed their nuclear and cytoplasmic immaturity [16]. This observation led to important adaptations of the IVM culture methodology with the aim to improve the developmental potential of oocytes from small follicles [15]. The overall intention of developing such advanced IVM culture methodologies is to eventually achieve the goal of developing a zero-stimulation IVM strategy, as has already been realized in animals [17].
Translational research in the field of oocyte maturation in livestock animals had indicated a prematuration culture strategy aiming to sustain synchronization of oocyte nuclear and cytoplasmic maturation [17]. The use of molecular compounds for in vitro modulation of cAMP levels within the cumulus-oocyte complex has been vital when using this new strategy [18–24]. High cAMP levels are required to maintain oocytes under meiotic arrest [25–28] for a sufficient length of time in appropriate media, to acquire developmental capacities. Under this premise, major improvements in the IVM approach have recently been reported and involve a longer culture period (pre-maturation or “capacitation” — CAPA — culture) in the presence of C-type natriuretic peptide (CNP), followed by IVM (CAPA-IVM), to induce the stage-dependent maturation features in the oocytes retrieved from small antral follicles [15, 29] and large antral follicles [30]. The principle components to develop a better pre-maturation culture system (i.e., CNP, E2, insulin, FSH, and AREG), were studied in an ovarian stimulation–free system that generated an increased yield of good quality embryos after IVM, by using COCs from juvenile mice [29]. Following translation of the mouse-model methodology into the human fertility clinic in a prospective (sibling) pilot study, significantly higher rates of oocyte maturation and yield of good quality embryos with a high euploidy rate have been accomplished [15].
In order to endorse this novel IVM methodology as a safe fertility treatment, complementary studies showed no impact on imprinted gene DNA methylation or mRNA expression in blastocysts produced following the CAPA-IVM system in comparison to conventional COS followed by ICSI in age-matched PCOS patients [31].
The current prospective study focuses on the efficiency of CAPA-IVM at improving IVM outcomes in oocytes from small antral follicles (< 6 mm) compared with STD-IVM in PCOS patients.
Materials and methods
Prospective study design and patient characteristics
This study was carried out at My Duc hospital, Ho Chi Minh City, Vietnam, which has one of the largest IVM practices in the world with more than 500 patients treated yearly [10]. This also allowed us to validate the transferability of the CAPA-IVM system to another ART center. The current prospective study was performed on 40 infertile patients with polycystic ovaries who were referred to the clinic (My Duc Hospital, Ho Chi Minh City, Vietnam) to receive infertility treatment and were invited to participate if they fulfilled the inclusion criteria. All participants provided informed consent.
Inclusion and exclusion criteria and patient characteristics were as follows: patients < 38 years with polycystic ovarian morphology: at least 25 follicles (2–9 mm) per ovary and/or increased ovarian volume (> 10 ml) [32]. No major uterine or ovarian abnormalities. Exclusion criteria: medical contra-indication for pregnancy, high (> grade 2) grade endometriosis, and cases with extremely poor sperm quality.
Baseline patient characteristics are described in Table 1. Patients with polycystic ovaries involved in the study had no previous history of recurrent failure of ART (including recurrent miscarriages). Enrolments took place between March and June 2017. Patients were randomized to receive CAPA-IVM or standard IVM (STD IVM). The STD IVM protocol (non-hCG–primed) is one of the current clinical IVM protocols employed at My Duc Hospital and the objective of the study was to determine if CAPA-IVM is more or less efficient than the current STD IVM protocol. In total, 40 patients were involved (20 in each group, CAPA-IVM and STD IVM).
Table 1.
Basal patient characteristics and hormonal values on the day of oocyte retrieval
| CAPA-IVM | STD IVM | P value | |
|---|---|---|---|
| No of patients | 20 | 20 | – |
| BMI (kg/m2) | 21.1 ± 2.7 | 21.1 ± 1.9 | 0.857 |
| Age (y) | 29.1 ± 3.4 | 28.8 ± 2.9 | 0.952 |
| Gonadotropin priming: | Puregon | Puregon | – |
| Total injection dose (IU) | 450 | 450 | – |
| Days of stimulation | 3.1 ± 0.2 | 3.0 ± 0.0 | – |
| AMH (μg/L) | 10.4 ± 1.9 | 11.1 ± 2.2 | 0.527 |
| AFC (last ultrasound) | 41.9 ± 18.3 | 34.6 ± 11.8 | 0.317 |
| Total mean follicle size (mm), on last ultrasound | 5.9 | 6.2 | 0.243 |
| Proportion of COCs from follicles < 6 mm | 77% | 70% | – |
| Proportion of follicles ≥ 6 mm | 23% | 30% | – |
| Mean number of COCs per treatment | 15.2 ± 8.0 | 12.0 ± 6.9 | 0.193 |
| Hormones on day of OR | |||
| E2 (ng/L) | 1006 ± 987.5 | 1214 ± 1354 | 0.753 |
| Prog (μg/L) | 0.77 ± 1.3 | 0.54 ± 0.36 | 0.713 |
| FSH (IU/L) | 6.6 ± 1.2 | 6.6 ± 1.2 | 0.828 |
| LH (IU/L) | 6.2 ± 2.9 | 6.4 ± 2.9 | 0.995 |
Values are mean ± SD
AFC antral follicle count, AMH anti-Müllerian hormone, OR oocyte retrieval
Treatment regime
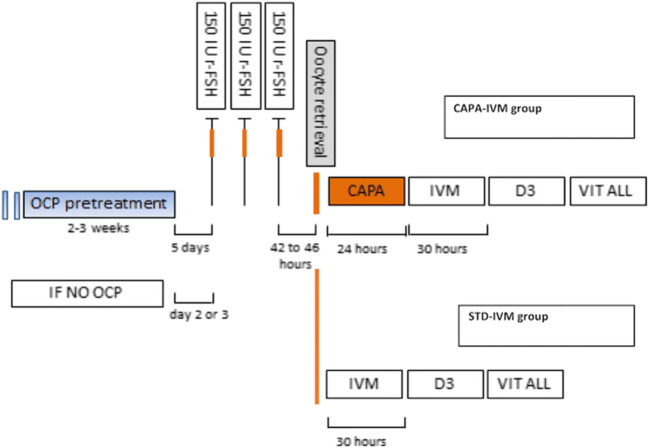
The stimulation protocol is presented in Fig. 1. Patients received three doses of r-FSH (150 IU daily) starting on day 1 to 3 after spontaneous menses or on day 5 after discontinuing oral contraceptives. A pelvic ultrasound scan was performed in the morning of the third stimulation day. The final injection of r-FSH was administered around 14:00 h and the oocyte retrieval (OR) was scheduled 2 days later, between 8:00 and 9:00 h (usually between 42 and 46 h after last r-FSH injection), without hCG trigger.
Fig. 1.
Representative scheme showing the clinical protocol applied and the key steps following oocyte retrieval. The stimulation protocol used was similar for the two different groups of IVM systems
Oocyte retrieval and culture procedure
Cumulus-oocyte complexes (COCs) were retrieved 42–46 h after the last rFSH injection. Follicle size was measured before OPU by means of ultrasound scan. Larger follicles (≥ 6 mm) were punctured first, then the needle was flushed and the smaller follicles (< 6 mm) were punctured. COCs from two size ranges < 6 mm (smaller follicles) and ≥ 6 mm (larger follicles), were collected in separate tubes. In the laboratory, these two groups of COCs were processed and cultured separately.
For the STD IVM protocol, follicular aspirates were collected in Global collect medium (without heparin) and filtered through a cell strainer (Falcon®, 70 μm mesh size, BD Biosciences). After collection, COCs were washed and transferred to a four-well dish (Nunc, Denmark) containing IVM medium (IVM System, Medicult; Origio, Denmark) supplemented with 75 mIU/ml rFSH (Merc, Switzerland), 100 mIU/ml hCG (MSD, USA), 1 IU/mL Growth Hormone (Merck Switzerland), and 10 mg/ml human serum albumin (HSA) (SAGE, Denmark). Cumulus-oocytes-complexes were cultured for 30 h (and reassessed also at 32 h), 10 COCs/well in 500 μl IVM medium with oil overlay (Lite Oil, Life Global, Canada) at 37 °C under 6% CO2 in air.
The CAPA-IVM protocol was reported previously [15]. COCs were collected in Global collect medium (without heparin) containing 10 mg/ml HSA, 50 nM CNP (Tocris Bioscience; Abingdon, UK), and 20 nM Estradiol (Sigma; Schnelldorf, Germany). After collection, COCs were washed and transferred to a four-well dish, containing CAPA medium (IVM System, Medicult, Origio) supplemented with 1 mIU/ml rFSH (Puregon MSD, Australia), 5 ng/ml Insulin, 10 nM estradiol (E2) (both from Sigma; Schnelldorf, Germany), 10 mg/ml HSA (Vitrolife, Göteborg, Sweden), and 25 nM CNP (Tocris Bioscience; Abingdon, UK). COCs were cultured in 500 μl of CAPA medium, 10 COCs/well under oil for 24 h at 37 °C, 6% CO2 in air.
Following 24 h of incubation in CAPA media, COCs were thoroughly washed and transferred into Medicult IVM medium containing 5 ng/ml insulin, 10 nM E2, 100 ng/ml human recombinant amphiregulin (rhAREG; Tocris Bioscience), and 100 mIU/ml recombinant FSH. Cumulus-oocytes-complexes were cultured in IVM medium for 30 h (and reassessed also at 32 h).
In both STD IVM and CAPA-IVM protocols, oocytes without any surrounding corona-cumulus were not considered for further culture. Following follicle aspiration, COCs from follicles < 6 mm or ≥ 6 mm diameter were cultured separately after collection, each with the two IVM methods.
Fertilization, embryo culture, and embryo vitrification
Following IVM culture, oocytes were mechanically and enzymatically denuded from their cumulus layers under a stereomicroscope and oocyte maturation was assessed under the inverted microscope. Oocytes were classified as maturating to the metaphase II (MII) stage by the presence of the first polar body. Matured oocytes were fertilized using intracytoplasmic sperm injection (ICSI) and cultured at 37 °C under 5% CO2 and 5% oxygen. Fertilization was evaluated 16–18 h post-ICSI by the presence of two pronuclei. Embryos were cultured until day 3 in Global Total LP (Life Global, Canada) in groups of 2–3 embryos in one 30-μL microdroplet. Embryos that fulfilled the criteria for cryopreservation were vitrified (Cryotech, Japan) as cleaving day 3 embryos. Embryos of extremely poor quality (Istanbul consensus on embryo quality assessment) defined as fragmentation > 30%, < 6 cells, and multinucleation were not vitrified [33]. A total of 80 embryos were vitrified in the CAPA-IVM group and 44 embryos were vitrified in the STD IVM group.
Assessment of diameter in germinal vesicle (GV)–arrested oocytes
Oocytes at the GV-stage were measured (at 20× magnification) following the removal of cumulus cells post-IVM. Oocyte size was the average of the maximum and minimum oocyte diameter, without the zona pellucida.
Considering the previous report [16] on the heterogeneity of GV-stage oocyte size and the relationship between diameter and meiotic competence, our results were interpreted based on the three size categories considered earlier: small oocytes, measuring < 105 μm; medium oocytes, measuring ≥ 105 μm, and < 110 μm; and large oocytes, measuring ≥ 110 μm.
Study outcomes
The primary outcomes were oocyte maturation, embryo quality, and the number of vitrified Day 3 embryos of good morphology, per patient. Secondary outcomes were, fertilization, percentage, and diameter of GV-arrested oocytes post IVM.
Statistical analysis
Differences in oocyte maturation rate, fertilization rate, and embryology between CAPA-IVM and STD-IVM, and in a subgroup of samples categorized by their follicular size < 6 versus ≥ 6 mm were recorded. For each outcome variable, a generalized linear mixed model for binary data using a logit link with patient as random factor was used to model the data. The fixed effects coefficients, variance-covariance matrix, and degrees of freedom of this model were used to compare treatment for each follicle size group separately and to compare follicle size groups for each treatment group. A correction for simultaneous hypothesis testing according to Sidak was applied.
Differences in diameter of GV-arrested oocytes (post IVM), hormone values, and the patient characteristics (BMI, AMH, AFC, mean follicle size, mean number of COCs) between CAPA-IVM and STD IVM were compared by applying a Mann-Whitney U test.
Values of p < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism version 8.00 software (GraphPad, San Diego, CA, USA).
Results
Demographics
There were no significant differences in basal patient characteristics between the CAPA-IVM or the STD IVM groups (Table 1). In general, the mean age was 28.9 ± 3.1 years, mean body mass index was ~ 21.2 ± 2.3 kg/m2, and patients had high AMH levels of ~ 10.7 ± 2.2 μg/L (on average). There was a weak but significant positive correlation between AMH levels and AFC on the day of the last ultrasound (r = 0.368).
The hormonal profiles on the day of oocyte retrieval showed high interpatient variability but without significant difference between the two groups (Table 1). No relationship between the fold differences of any of the hormones in the individual patients measured and the embryology outcomes could be evidenced.
Oocyte retrieval
Follicle size was measured before OPU by standard ultrasound scan. COCs from two size ranges < 6 mm (smaller follicles) and ≥ 6 mm (larger follicles), were retrieved in separate tubes for IVM culture. Overall, the mean follicle size was around 6 mm. A larger proportion (~ 73%) of COCs from follicles < 6 mm could be retrieved in comparison to follicles ≥ 6 mm. An average of 38 follicles per patient was counted on ultrasound on the day of oocyte retrieval. The average number of COC in each follicle class was similar in the two culture methods.
Oocyte maturation and embryology outcomes
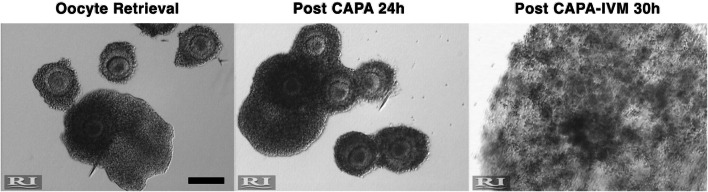
Figure 2 gives a view on how the COC developed in the two-step culture system as a representative example. A total of 238 COCs underwent STD IVM culture, whereas 305 COCs were incubated in CAPA-IVM culture.
Fig. 2.
Representative images of cumulus-oocyte complexes immediately after oocyte retrieval (left panel) and after undergoing the CAPA-IVM system. The figure shows a group of COCs < 6 mm from a single patient exposed to 24 h CAPA culture (post CAPA, middle panel) followed by 30 h IVM (post CAPA-IVM, panel on the right). Scale bar 200 μm
Maturation and embryology outcomes are shown in Table 2. The MII (maturation) rate was significantly higher in CAPA-IVM vs Standard IVM (62% vs 48%; p = 0.034). Rates of fertilization per mature oocyte and usable embryos per fertilized oocyte were similar among the groups. However, the rates of good quality embryos (EQ 1 + EQ 2) per mature oocyte or per cumulus-oocyte-complex (COC) were significantly higher in CAPA-IVM vs Standard IVM (38% vs 24%; p = 0.045, 24% vs 12%, p = 0.018, respectively). A similar trend was observed in the rate of good quality embryos per fertilized oocytes (p = 0.051). Likewise, the percentage of top-quality embryos on day 3 (EQ. 1) per COC was also significantly higher in the CAPA-IVM group (9.1% vs 3.9%, p = 0.028; Table 2). This resulted in a remarkable increase (1.8-fold higher) in the mean number of usable embryos (per patient), in CAPA-IVM vs Standard IVM (4.2% vs 2.2%; p = 0.0002, Table 2).
Table 2.
Main culture and embryology outcomes
| Oocyte developmental outcomes | CAPA-IVM N = 20 |
STD IVM N = 20 |
P value |
|---|---|---|---|
| Number of COCs | 305 | 238 | – |
| Maturation (MII) rate, % (n) | 62 (189) | 47.9 (114) | 0.034 |
| Fertilization rate per ICSI, % (n) | 85.7 (162) | 78.1 (89) | 0.244 |
| EQ1 + EQ2 rate per fertilized, % (n) | 43.8 (71) | 30.3 (27) | 0.051 |
| EQ1 + EQ2 rate per metaphase II, % (n) | 37.8 (71) | 24.1 (27) | 0.045 |
| EQ1 + EQ2 rate per COCs, % (n) | 24 (71) | 11.7 (27) | 0.018 |
| EQ1 rate per fertilized, % (n) | 16.7 (27) | 10.1 (9) | 0.169 |
| EQ1 rate per metaphase II, % (n) | 14.4 (27) | 8 (9) | 0.116 |
| EQ1 rate per COCs, % (n) | 9.1 (27) | 3.9 (9) | 0.028 |
| Vitrified d3 embryos of good morphological quality, mean (± SD) | 4.2 ± 0.5 | 2.2 ± 0.2 | 0.0002 |
Values are presented as percentages and total numbers, unless stated otherwise
COC cumulus-oocyte complex, MII metaphase II, EQ1 embryo quality 1 (top quality), EQ2 embryo quality 2
Effect of CAPA-IVM on competence of oocytes derived from different follicle sizes or variable COC morphology
As mentioned above, COCs were divided into two follicle size ranges < 6 mm and ≥ 6 mm.
In general, the analysis of the main outcomes demonstrated that in comparison to Standard IVM, CAPA-IVM system seemed to bring real benefit to COCs from follicles < 6 mm. CAPA-IVM led to a clear trend (p = 0.055) towards an increase (1.4-fold) in oocyte maturation rate and a doubling (p < 0.05) in the proportion of good and top-quality embryos per COC, with a 2.7-fold increase in top-quality embryos per COC (p < 0.05) (Table 3). Notably, none of these measures of improved outcomes from CAPA-IVM versus Standard IVM were evident in COCs from follicles ≥ 6 mm.
Table 3.
Main culture and embryology outcomes from follicles < 6 mm and ≥ 6 mm
| Oocyte developmental outcomes | CAPA-IVM < 6 mm N = 20 |
STD IVM < 6 mm N = 20 |
p value | CAPA-IVM ≥ 6 mm N = 14 |
STD-IVM ≥ 6 mm N = 15 |
p value |
|---|---|---|---|---|---|---|
| Number of COCs | 236 | 178 | – | 69 | 60 | – |
| Maturation (MII) rate, % (n) | 61 (144) | 44.4 (79) | 0.055 | 65.2 (45) | 58.3 (35) | 0.517 |
| Fertilization rate per ICSI, % (n) | 86.1 (124) | 84.8 (67) | 0.912 | 84.4 (38) | 62.9 (22) | 0.118 |
| EQ1 + EQ2 rate per fertilized, % (n) | 43.5 (54) | 29.9 (20) | 0.138 | 44.7 (17) | 31.8 (7) | 0.573 |
| EQ1 + EQ2 rate per MII, % (n) | 37.5 (54) | 25.3 (20) | 0.173 | 37.8 (17) | 21.2 (7) | 0.388 |
| EQ1 + EQ2 rate per COCs, % (n) | 23.7 (54) | 11.2 (20) | 0.033 | 25 (17) | 13.2 (7) | 0.456 |
| EQ1 rate per fertilized, % (n) | 16.9 (21) | 9 (6) | 0.258 | 15.8 (6) | 13.6 (3) | 0.258 |
| EQ1 rate per MII, % (n) | 14.7 (21) | 7.6 (6) | 0.241 | 13.3 (6) | 9.1 (3) | 0.810 |
| EQ1 rate per COCs, % (n) | 9.2 (21) | 3.4 (6) | 0.049 | 8.8 (6) | 5.7 (3) | 0.764 |
Values are presented as percentages and total numbers
COC cumulus-oocyte complex, MII metaphase II, EQ1 embryo quality 1 (top quality), EQ2 embryo quality 2
Regarding COC morphology at time zero, the majority of cumulus-oocyte complexes cultured in both CAPA-IVM and STD IVM derived from intact COCs, where oocytes were fully surrounded by cumulus cells. However, in both groups, a small fraction of oocytes was only partially surrounded by cumulus cells at retrieval (Fig. 3). Although the two COC types were not cultured separately in the current pilot study, it could be traced back that a proportion of the partially surrounded oocytes ended up totally isolated from their surrounding corona layer following the IVM culture step, which was more apparent in the CAPA-IVM group (Fig. 3).
Fig. 3.
Representative example of a pool of COCs after retrieval (left panel), with an oocyte that is only partially surrounded by cumulus cells (arrow). In the middle panel are COCs after CAPA culture. In the right panel a fully expanded COC is shown and an oocyte that has become totally disconnected from cumulus cells (arrow) after incubation in CAPA-IVM
Analysis of oocytes arrested at the germinal vesicle (GV)-stage
The use of CAPA-IVM culture yielded significantly (p < 0.0001) less GV-arrested oocytes compared to Standard IVM (~ 50% reduction, Table 4). The average diameters of oocytes that remained arrested at the GV-stage after IVM were measured. GV arrested oocytes in the CAPA-IVM group were significantly larger (by 3 μm, p = 0.019) than those in the STD IVM group (Table 4). A significantly (p = 0.002) larger proportion of oocytes in the CAPA-IVM group had oocytes ≥ 105 μm, whereas in the STD IVM group a significantly (p = 0.002) larger proportion of GV-arrested oocytes had remained small in diameter (< 105 μm).
Table 4.
Proportion and size of GV-arrested oocytes post IVM
| CAPA-IVM | STD IVM | P value | |
|---|---|---|---|
| Proportion of GV-arrested oocytes (n) | 17% (53) | 34% (80) | p < 0.0001 |
| Oocytes < 105 μm | 22% (8) | 54% (27) | p = 0.002 |
| Oocytes ≥ 105 < 110 μm | 54% (20) | 22% (11) | p = 0.002 |
| Oocytes ≥ 110 μm | 24% (9) | 24% (12) | p = 0.972 |
| Oocyte diameter (μm), mean ± SD | 107.5 ± 4.9 | 104.7 ± 6.2 | p = 0.019 |
Values are presented as percentages and total numbers unless stated otherwise. Oocyte diameter was measured in a total of 37 GV-arrested oocytes post CAPA-IVM and in 50 oocytes post STD IVM
Discussion
The successful application of IVM in a clinical setting depends on progress of translational research which explores culture conditions that simulate the physiological environment where the oocyte acquires meiotic and developmental potential. In recent years, notable advances contributing to the refinement of IVM settings have been published first in different animal models [22–24, 29, 34] and later in human, by introducing a prematuration step into the clinical IVM protocol [15]. The prematuration step, named “capacitation” (CAPA) followed by IVM culture (CAPA-IVM), is based on the use of physiologically relevant components: c-type natriuretic peptide (CNP) in the prematuration step and EGF-like peptides in the second IVM phase. CAPA-IVM has delivered remarkable positive results on oocyte maturation and embryo yield in mouse and human [15, 29, 30]. The current study focused on the efficacy of CAPA-IVM, at improving IVM outcomes in oocytes from small (< 6 mm) versus medium-size follicles (≥ 6 mm) and compared to routine STD-IVM in patients with polycystic ovaries.
Effect of CAPA-IVM on meiotic and developmental competence
The advantageous effect of the CAPA-IVM protocol on oocyte maturation rates in Vietnamese patients with polycystic ovaries was consistent with a previous report in which sibling oocytes from Caucasian patients with PCOS were exposed to CAPA or STD-IVM [15]. A gain in meiotic competence was revealed in oocytes cultured with CAPA-IVM protocol: oocytes showed a more synchronized meiosis resumption. All MII oocytes (except two oocytes) from the CAPA-IVM group, reached MII within 30 h post IVM (60% MII rate); whereas in the standard protocol, only 40% of oocytes were MII at 30 h, and at the second assessment time of 32 h, the denuded oocytes had reached a final maturation rate of 50%. A 50% increase in MII rate was achieved by using the CAPA-IVM protocol. While it is known that similar high rates have been reported in IVM systems using patient serum in the culture medium and/or when oocytes are derived from larger follicles, i.e., 10–12 mm [35, 36], the improvement reported in this study is substantial in the context of the long history of IVM using serum-free conditions, as the low MII rate of the human oocyte using standard IVM has stubbornly remained at ~ 50% for > 50 years [37] and is even lower for COCs from small antral follicles (~ 40%).
Fertilization rate was similar in the two IVM protocols, however, improvements in embryology outcomes measured by a significant increase in the number of usable embryos per patient and in embryo quality (especially top quality — EQ1 — embryos) were evident in the CAPA-IVM compared to the STD IVM protocol. In the current study, a particular emphasis was on oocytes from the smallest antral follicles (< 6 mm group). A clear benefit of CAPA-IVM in this subgroup was demonstrated, further translating into a significant increase of top-quality embryos. Similarity in the current approach and that reported by Dieci et al. [38] in the bovine shows that a tailored culture system for oocytes from small follicles increases developmental competence of oocytes at an earlier stage of differentiation. Meticulous characterization at the cellular and molecular level of both bovine [38] and human [16] oocytes was crucial before fine-tuning culture conditions that enhance developmental competence of immature oocytes from small follicles. Nevertheless, the fact that no benefit was apparent in oocytes from ≥ 6 mm follicles in the current study needs to be interpreted cautiously since this group was underrepresented, as a smaller fraction (max. 30%) of COCs were derived from follicles ≥6 mm. Furthermore, different to large animal research models where COCs are mostly retrieved from ovaries collected at an abattoir, several days of gonadotropin stimulus applied to the patient affects follicle recruitment and is likely to influence the initial developmental status of oocytes placed in culture.
Overall, the tangible gain of oocyte meiotic and developmental competence, leading to higher maturation rates, higher embryo yield, and the generation of embryos of superior quality, point to key improvements in IVM outcomes by using this biphasic IVM protocol. This led to adopting the term “capacitation” [39] in the current methodology, i.e., endowing oocytes with “capacity” during this extended prematuration culture period of supporting meiotic and developmental competence. An in-depth analysis of clinical outcomes after CAPA-IVM and STD IVM is part of a larger cohort study (Vuong et al. 2019, manuscript under review), however, preliminary results show that for the population presented in this study, clinical pregnancy rates after first embryo transfer are 58% (11/19) in CAPA-IVM and 35% (7/20) in STD IVM.
Analysis of GV-arrested oocytes demonstrates oocyte growth and acquisition of meiotic competence in vitro
In a previous study using STD-IVM [16], characterization of non-maturing oocytes following IVM culture revealed that the number of oocytes arrested at the GV stage and their oocyte diameter are important indicators of oocyte maturity and could be used as a post-hoc marker of the type of IVM method to apply for a certain class of follicles. In the present pilot study, a significant reduction by 50% in the proportion of oocytes remaining arrested at the GV-stage post IVM was observed in the CAPA-IVM group compared to STD-IVM. Furthermore, the CAPA-IVM culture conditions appear to support oocyte growth during culture; significantly larger diameters of GV arrested oocytes were observed in oocytes cultured under CAPA-IVM system compared to oocytes cultured in STD-IVM medium.
These data on GV-arrested oocytes suggest that CAPA-IVM culture conditions support acquisition of oocyte meiotic and/or developmental competence, consistent with an increase in oocyte size in vitro. On the contrary, the higher proportion of GV-arrested oocytes following STD-IVM, which in addition, showed smaller diameters (majority < 105 μm) suggest that a single maturation incubation system is not capable of rescuing oocytes which are still at the very immature stage and still need to gain meiotic competence.
Status of cumulus-oocyte complexes at oocyte retrieval
A condition for CAPA-IVM to be supportive of oocyte growth and acquisition of oocyte maturation is to have an aspiration procedure which is non-disruptive for the cumulus-oocyte connections. CNP is a key component of the capacitation culture medium, and therefore, the generation of cGMP by cumulus cells via CNP/NPR2 system, and its diffusion into the oocyte via gap junctions is crucial to maintain oocytes under meiotic arrest [40, 41]. In the present report, most COCs cultured in CAPA-IVM and STD-IVM were intact GV oocytes fully surrounded by cumulus cells. Nevertheless, there was a minor fraction of COCs which were only partially surrounded by cumulus cells at retrieval. These partially surrounded COCs were cultured together with fully surrounded intact COCs with the intention that a larger mass of cumulus cells could aggregate with partially surrounded COCs during prematuration culture to further support oocyte-cumulus communication and meiotic arrest. In spite of this, such partially cumulus-connected oocytes became most often totally disconnected from their cumulus cells, but only after the IVM step. It is likely that oocytes poorly surrounded by cumulus cells may have insufficient cGMP to firmly maintain meiotic arrest throughout the pre-IVM period and may explain their release from the cumulus-oocyte complex during the second IVM culture step. In this study, individual culture of COCs was not done, and so, the impact of oocyte release on embryological outcomes could not be precisely recorded. Nonetheless, fast disconnection of the oocyte from the somatic compartment during maturation is non-physiological and is expected to be detrimental to future embryo development. Given the low incidence of these cases, we could not yet attribute oocyte release to a specific factor. A rescue strategy to compensate the low amount of somatic cells in the event of partially surrounded COCs at retrieval still requires further study.
Overall, the results of this study revealed an IVM method improving embryo quality and laid the basis for a large prospective randomized trial (My Duc Hospital at Ho Chi Minh City) to compare effectiveness of CAPA-IVM to current standard superovulation and IVF in patients with a high antral follicle count [42]. Follow-up of children born following the CAPA-IVM technique (Vuong et al. 2019, manuscript under review) is indispensable and is currently performed at My Duc Hospital (Ho Chi Minh city) to further evaluate the epigenetic safety of the technique by study of placenta, blood, and buccal smears. A study on the development of the children is also underway.
Conclusions
In conclusion, the use of CAPA-IVM resulted in a higher rate of oocyte maturation and translated into a higher number of good quality embryos per patient. CAPA-IVM was instrumental in maturing the oocytes from the smallest antral follicles (< 6 mm), which are the largest group in PCOS patients receiving minimal ovarian stimulation. This study proves also robustness and transferability of the CAPA-IVM method. Confirmation of the results on larger patient numbers could validate the CAPA-IVM strategy as a valuable first-line treatment in patients with a high follicle count.
Acknowledgments
The authors acknowledge Francesca Lolicato PhD for logistic support and advice during the study. The authors acknowledge Wim Coucke from Scientific Institute of Public Health (Brussels) for performing the statistical analysis.
Funding
The authors acknowledge the support of the Fund for Research Flanders (Fonds Wetenschappelijk Onderzoek-Vlaanderen-FWO, Project nr AL895) and of the Industrial Research Fund of the Vrije Universiteit Brussel (Industrieel onderzoeksfonds, IOF 2042) to the Project 4R-ART.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was performed in accordance with the ICH Harmonized Tripartite Guideline for GCP and the ethical principles of the Declaration of Helsinki. Ethics approval was obtained from the Review Board of the Research Center for Genetics and Reproductive Health and the Ethical Board of MyDuc Hospital (approval number 04/17/ĐĐ-BVMĐ, dated 28 March 2017).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Flor Sanchez and Anh H. Le contributed equally to this work.
References
- 1.Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:353–362. doi: 10.1016/s0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo”; method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano G, Dechène J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, Devreker F, Demeestere I. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod BioMed Online. 2017;34:575–558. doi: 10.1016/j.rbmo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, Mcveigh E, Child T. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case–control study of 194 treatment cycles. Fertil Steril. 2012;98:355–360. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Das M, Son WY, Buckett W, Tulandi T, Holzer H. In-vitro maturation versus IVF with GnRH antagonist for women with polycystic ovary syndrome: treatment outcome and rates of ovarian hyperstimulation syndrome. Reprod BioMed Online. 2014;29:545–551. doi: 10.1016/j.rbmo.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Julania S, Walls ML, Hart R. The place of in vitro maturation in PCO/PCOS. Int J Endocrinol. 2018;2028:5750298. doi: 10.1155/2018/5750298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16:675–689. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 9.Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15:165–170. doi: 10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- 10.Ho VNA, Pham TD, Le AH, Ho TM, Vuong LN. Live birth rate after human chorionic gonadotropin priming in vitro maturation in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:70. doi: 10.1186/s13048-018-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear – variations need defining. Hum Reprod. 2016;31:2411–2241. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 12.Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30:88–96. doi: 10.1093/humrep/deu248. [DOI] [PubMed] [Google Scholar]
- 13.De Vos M, Ortega-Hrepich C, Albuz FK, Guzman L, Polyzos NP, Smitz J, Devroey P. Clinical outcome of non-hCG-primed oocyte in vitro maturation treatment in patients with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2011;96:860–864. doi: 10.1016/j.fertnstert.2011.07.1108. [DOI] [PubMed] [Google Scholar]
- 14.Guzman L, Ortega-Hrepich C, Albuz FK, Verheyen G, Devroey P, Smitz J, De Vos M. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–507. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, Anckaert E, Smitz JEJ. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32:2056–2068. doi: 10.1093/humrep/dex262. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez F, Romero S, De Vos M, Verheyen G, Smitz J. Human cumulus- enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum Reprod. 2015;30:1396–1409. doi: 10.1093/humrep/dev083. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist RB, Luciano AM, Richani D, Zeng HT, Wang X, Vos MD, Sugimura S, Smitz J, Richard FJ, Thompson JG. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction. 2016;152(5):143–157. doi: 10.1530/REP-15-0606. [DOI] [PubMed] [Google Scholar]
- 18.Thomas RE, Armstrong DT, Gilchrist RB. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol. 2002;244:215–225. doi: 10.1006/dbio.2002.0609. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira D, Albano C, Adriaenssens T, Cortvrindt R, Bourgain C, Devroey P, Smitz J. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–1052. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 20.Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, Smitz J. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- 21.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011. doi: 10.1093/humrep/deq246. [DOI] [PubMed] [Google Scholar]
- 22.Luciano AM, Franciosi F, Modina SC, Lodde V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s) Biol Reprod. 2011;85:1252–1259. doi: 10.1095/biolreprod.111.092858. [DOI] [PubMed] [Google Scholar]
- 23.Franciosi F, Coticchio G, Lodde V, Tessaro I, Modina SC, Fadini R, Dal Canto M, Renzini MM, Albertini DF, Luciano AM. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol Reprod. 2014;91:61. doi: 10.1095/biolreprod.114.118869. [DOI] [PubMed] [Google Scholar]
- 24.Richani D, Wang X, Zeng HT, Smitz J, Thompson JG, Gilchrist RB. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol Reprod Dev. 2014;81:422–435. doi: 10.1002/mrd.22307. [DOI] [PubMed] [Google Scholar]
- 25.Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol. 1998;145:9–14. doi: 10.1016/s0303-7207(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 26.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 27.Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005;288:397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- 29.Romero S, Sanchez F, Lolicato F, Van Ranst H, Smitz J. Immature oocytes from unprimed juvenile mice become a valuable source for embryo production when using C-type natriuretic peptide as essential component of culture medium. Biol Reprod. 2016;95:64. doi: 10.1095/biolreprod.116.139808. [DOI] [PubMed] [Google Scholar]
- 30.Santiquet NW, Greene AF, Becker J, Barfield JP, Schoolcraft WB, Krisher RL. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol Hum Reprod. 2017;23(9):594–606. doi: 10.1093/molehr/gax032. [DOI] [PubMed] [Google Scholar]
- 31.Saenz-de-Juano MD, Ivanova E, Romero S, Lolicato F, Sanchez F, Van Ranst H, De Vos M, Smitz J, Kelsey G, Anckaert E. DNA methylation and mRNA expression of imprinted genes in blastocysts derived from an improved IVM method in PCOS patients. Hum Reprod. 2019 (in press). 10.1093/humrep/dez121 [DOI] [PubMed]
- 32.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update. 2014;20(3):334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 33.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 34.Sugimura S, Yamanouchi T, Palmerini MG, Hashiyada Y, Imai K, Gilchrist RB. Effect of pre-in vitro maturation with cAMP modulators on the acquisition of oocyte developmental competence in cattle. J Reprod Dev. 2018;64:233–241. doi: 10.1262/jrd.2018-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2012;98:888–892. doi: 10.1016/j.fertnstert.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 36.Walls M, Junk S, Ryan JP, Hart R. IVF versus ICSI for the fertilization of in-vitro matured human oocytes. Reprod BioMed Online. 2012;25(6):603–607. doi: 10.1016/j.rbmo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208(5008):349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 38.Dieci C, Lodde V, Labreque R, Dufort I, Tessaro I, Sirard MA, Luciano AM. Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Mol Hum Reprod. 2016;22(12):882–897. doi: 10.1093/molehr/gaw055. [DOI] [PubMed] [Google Scholar]
- 39.Hyttel P, Fair T, Callesen H, Greve T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology. 1997;47:23–32. [Google Scholar]
- 40.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology. 2011;152:4377–4385. doi: 10.1210/en.2011-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, Le AH, Pham TD, Wang R, Norman RJ, Smitz J, Gilchrist RB, Mol BW. Effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilisation in women with high antral follicle count: study protocol for a randomised controlled trial. BMJ Open. 2018;8(12):e023413. doi: 10.1136/bmjopen-2018-023413. [DOI] [PMC free article] [PubMed] [Google Scholar]