Abstract
Purpose
Testicular tissue cryopreservation prior to gonadotoxic therapies is a method to preserve fertility in children. However, the technique still requires development, especially when the tissue is immature and rather susceptible to stress derived from in vitro manipulation. This study aimed to investigate the effects of vitrification with a new cryodevice (E.Vit) on cell membrane integrity and gene expression of prepubertal testicular tissue in the ovine model.
Methods
Pieces of immature testicular tissue (1 mm3) were inserted into “E.Vit” devices and vitrified with a two-step protocol. After warming, tissues were cultured in vitro and cell membrane integrity was assessed after 0, 2, and 24 h by trypan blue exclusion test. Controls consisted of non-vitrified tissue analyzed after 0, 2, and 24 h in vitro culture (IVC). Expression of genes involved in transcriptional stress response (BAX, SOD1, CIRBP, HSP90AB1), cell proliferation (KIF11), and germ- (ZBDB16, TERT, POU5F1, KIT) and somatic- (AR, FSHR, STAR) cell specific markers was evaluated 2 and 24 h after warming.
Results
Post-warming trypan blue staining showed the survival of most cells, although membrane integrity immediately after warming (66.00% ± 4.73) or after 2 h IVC (59.67% ± 4.18) was significantly lower than controls (C0h 89.67% ± 1.45). Extended post-warming IVC (24 h) caused an additional decrease to 31% ± 3.46 (P < 0.05). Germ- and somatic-cell specific markers showed the survival of both cell types after cryopreservation and IVC. All genes were affected by cryopreservation and/or IVC, and moderate stress conditions were indicated by transcriptional stress response.
Conclusions
Vitrification with the cryodevice E.Vit is a promising strategy to cryopreserve prepubertal testicular tissue.
Keywords: Cryopreservation, Testicular tissue, Gene expression, Prepubertal, In vitro culture
Introduction
Gonadotoxic therapies, including cancer treatments, may cause severe damage to gonads and potentially lead to infertility. While semen cryopreservation is recommended to adult patients to preserve ability to reproduce after the treatment, this option is not applicable to prepubertal patients, whose gonads do not yet produce spermatozoa. In this case, cryopreservation of spermatogonial stem cell (SSC) suspension or testicular tissue, which contains mainly SSCs, would offer a real option for preserving fertility prior to gonadotoxic treatments.
Different options to restore fertility can be considered when cured patients desire to conceive, including tissue auto-transplantation, xenografting, or in vitro spermatogenesis, followed by in vivo or in vitro fertilization [1]. These alternatives present advantages and disadvantages. Grafting or transplanting tissue to obtain sperm from samples of patients undergoing cancer treatments implies the risk of reintroduction of malignant cells to the patients [2]; on the other hand, the efficiency of in vitro testicular tissue culture and sperm differentiation is low and implies the use of in vitro fertilization to conceive (reviewed in [3]). The full clinical application of these options has not been achieved yet [4] and only prepubertal testicular tissue cryopreservation is currently clinically available [5] and recommended by the European Society of Human Reproduction and Embryology (ESHRE) and by the American Society for Reproductive Medicine (ASRM) [6]. Nevertheless, results are encouraging and the possibilities to reproduce for young patients who store testicular tissue for future use are promising.
Independently of the approach to restore spermatogenesis, tissue cryopreservation is the preliminary step that plays a crucial role to guarantee germ cell survival after long-term preservation for subsequent differentiation. While semen cryopreservation is widely practiced and routinely used in reproductive technologies, cryopreservation of testicular tissue still requires some development, especially when the tissue is immature and rather susceptible to stress derived from in vitro manipulation [7]. Numerous approaches have been tested in several species, with protocols that differ on the basis of cooling rate (ranging from controlled slow freezing [8] to procedures such as vitrification [9, 10]), type and concentration of cryoprotectants (CPA), device used to contain the sample, and amount of tissue [11]. The efficiency of this plethora of combinations has been evaluated by multiple approaches, including cell viability and apoptosis, morphological analysis, immunohistochemistry, and evaluation of oxidative stress [12, 13]. The birth of healthy live offspring, which represents the “gold standard” for reproductive procedures, was achieved in three species: in mice with sperm grown in vitro from cryopreserved immature testis [14, 15], in swine with cryopreserved prepubertal testicular tissue grafted into nude mice [16], and in the rhesus macaque with frozen and thawed prepubertal testicular tissue matured in vivo by autologous grafting [17]. Nevertheless, the abundant evidence in several species shows that the methods of cryopreservation affect the condition of the preserved tissues, leading to differences in spermatogenic efficiency during the following procedures [1]. As a result, there is still the need to optimize techniques and improve both cryopreservation and post-warming germ cell differentiation.
Despite the numerous studies focused on testicular tissue cryopreservation, the effects on gene expression were rarely evaluated [18–21]. The present study aims to fill this gap by combining the use of a novel vitrification protocol and the analysis of an extended panel of genes during a 24-h post-warming period. In order to dissociate the effect of in vitro culture from cryopreservation itself, control (non-cryopreserved) tissues cultured in vitro for 24 h were included in the analysis.
Materials and methods
Experimental design
Testicular tissue collected from regularly slaughtered lambs (n = 10) was subjected to cryopreservation with a novel vitrification system. After warming, tissue was cultured in vitro and evaluated in terms of cell vitality and expression of a panel of twelve genes.
To identify the potential specific effects of cryopreservation and warming, control tissues not subjected to cryopreservation were included in the analysis.
In summary, the following experimental groups were created:
C0h (n = 10): testicular tissue analyzed immediately after dissection.
C2h (n = 10): testicular tissue analyzed after 2 h of in vitro culture (post-dissection).
C24h (n = 10): testicular tissue analyzed after 24 h of in vitro culture (post-dissection).
V0h (n = 10): testicular tissue subjected to vitrification, warming, and immediately analyzed.
V2h (n = 10): testicular tissue subjected to vitrification, warming, and 2 h of post-warming in vitro culture.
V24h (n = 10): testicular tissue subjected to vitrification, warming, and 24 h post-warming in vitro culture.
Post-warming plasma membrane integrity was assessed in all experimental groups. Conversely, gene expression was analyzed in all groups except for V0h, because cells were allowed time to restore the transcriptional machinery before evaluating the effects of vitrification on the gene expression status.
Sample collection
Testes were collected from regularly slaughtered lambs (40 days old). Immediately after collection, samples were placed in Dulbecco’s phosphate-buffered saline (PBS) with penicillin (50 IU/mL) and streptomycin (50 IU/mL) and maintained at 4 °C.
Testes were brought to the laboratory within 2 h of recovery and placed in a glass plate to be processed. The tunica albuginea was removed; the testes were washed twice in PBS and finally placed in dissection medium (DM) (TCM-199 with 25 mM N-2-hydroxyethylpiperazine-N-2-ethansulfonic acid (HEPES), 50 IU/mL streptomycin and 50 IU/mL penicillin, 0.005 M NaHCO3 and polyvinyl alcohol (PVA) 0.1% (w/v)) at pH 7.22 ± 0.1 at 4 °C. Testes were sectioned sagittally with a sterile microblade exposing the parenchyma, and tissue sections of 1 mm3 were collected.
Sections of each testis were processed according to the experimental design, as follows:
Immediately vitrified.
Immediately stored in RNAlater™ Stabilization Solution (Thermo Fisher Scientific) for subsequent gene expression analysis.
Immediately subjected to trypan blue exclusion test (post-dissection).
In vitro cultured for 2 or 24 h and then stored in RNAlater™ for gene expression analysis.
Trypan blue exclusion test
Cell plasma membrane integrity was estimated by trypan blue stain. Tissue sections were transferred to DM and mechanically fragmented with the help of a microblade. Further disruption was obtained by sequential pipetting with 1-mL and 200-μL pipettes, producing a suspension of isolated cells and fragments of tubules. Five milliliters of cell suspension was transferred into a 15-mL tube containing 5 mL DM supplemented with fetal calf serum (FCS; final concentration 10%) and incubated for 30 min to restore homeostasis.
Forty microliters of cell suspension was transferred to a 500-μL tube and stained with 8 μL trypan blue solution 0.4% (Sigma-Aldrich). After 2 min of incubation, staining was blocked by adding 200 μL DM. Ten microliters of cell suspension was put on a slide covered with a cover glass and observed under an inverted optical microscope (Olympus IX70) at × 40 magnification. Results are expressed as percentage of cells with intact plasma membrane.
Tissue vitrification and warming
Vitrification
The device used for vitrification, named E.Vit (FertileSafe LTD, Israel), consists of a 0.3-mL straw with a 50-μm pore polycarbonate grid applied at one of its open ends [22]. All cryopreservation procedures were performed in-straw: E.Vit allows the exit of excess cryoprotectant, but prevents the loss of the sample.
Samples were loaded into the straw (three 1-mm3 tissue sections per straw, all belonging to the same animal) with the aid of a syringe, and the grid was positioned at one free end. Samples were then exposed to two different solutions (equilibrating and vitrification solutions) following a two-step vitrification protocol at room temperature. First, samples were exposed for 5 min to 100 μL equilibrating solution (ES) constituting of TCM-199 with HEPES 25 mM, ethylene glycol (EG) 7.5%, dimethyl sulfoxide (DMSO) 7.5%, and + 20% FCS. Next, ES solution was removed from the straw by gentle blotting on sterile gauze and samples were exposed to 300 μL vitrification solution (VS: TCM-199 with HEPES 25 mM, EG 18%, DMSO 18%, bovine serum albumin (BSA) 0.6%, and trehalose 0.5 M) for 5 additional minutes.
The excess VS was eliminated from the straw by gentle blotting on sterile gauze to obtain a thin film of vitrification solution around the tissue, and straws were immediately plunged into liquid nitrogen.
Warming
Samples were warmed by sequentially immersing the straws in solutions of TCM-199 with 20% FCS and decreasing concentrations of sucrose (1 M, 0.5 M, and 0.25 M) at 38.6 °C. Samples were left inside each solution for 5 min and then removed from the straw. Tissue samples were transferred into culture medium (IVC: TCM-199 supplemented with 100 μM cysteamine, 10% FCS, 2.1 g/L sodium bicarbonate, 0.36 mM pyruvate) and stored in RNALater™ (Qiagen, Hilden, Germany) after 2 or 24 h in vitro culture. Samples were kept at − 80 °C until further processing.
Total RNA isolation and reverse transcription
Tissue samples for molecular analysis were put in 300 μL RNALater™ (Thermo Fisher Scientific) immediately after each treatment and stored at − 80 °C until RNA isolation. Total RNA was isolated using TRIzol reagent (Invitrogen Corporation, Carlsbad, CA) at 1 mL per 50 mg tissue and treated with DNase I (Invitrogen Corporation) according to manufacturer’s protocols. Resulting RNA quantity and purity were spectroscopically checked with NanoDropLite (Fisher Scientific S.A.S., Illkirch Cedex, France), while RNA integrity was evaluated by electrophoresis in a 1% agarose gel in Tris Borate EDTA buffer.
Five hundred nanograms of total RNA from each sample was reverse transcribed in 20 μL of reaction with 50 mM Tris HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 5 mM DTT, 1 mM dNTPs, 2.5 μM random hexamer primers, 20 U RNaseOUT™, and 100 U SuperScript™ III RT (Invitrogen Corporation). One tube without RNA and one with RNA, but without reverse transcriptase, were analyzed as negative controls. Reaction tubes were incubated at 25 °C for 10 min, at 42 °C for 1 h, and finally at 70 °C for 15 min to inactivate the reaction.
Real-time polymerase chain reaction
Relative quantification of transcripts was performed by real-time polymerase chain reaction (RT-PCR) in a StepOne™ Real-Time PCR System (Applied Biosystems, Singapore). The PCR was performed in a 15 μL reaction volume containing 7.5 μL 2× SYBR Green PCR Master Mix (Applied Biosystems), 200 nM of each primer (Table 1), and cDNA equivalent to ∼ 25 ng RNA. The PCR protocol consisted of two incubation steps (50 °C for 5 min and 95 °C for 2 min), followed by 40 cycles of amplification program (95 °C for 15 s, gene-specific annealing temperature for 30 s; Table 1), a melting curve program (65–95 °C, starting fluorescence acquisition at 65 °C and taking measurements at 10-s intervals until temperature reached 95 °C), and finally a cooling step to 4 °C. Fluorescence data were acquired during the elongation step. To minimize handling variations, all samples to be compared were run on the same plate using a PCR Master Mix containing all reaction components apart from the sample. The PCR products were analyzed by generating a melting curve to check specificity and identity of the amplification product. For each primer pair, PCR efficiency was determined by building a standard curve with serial dilutions of a known amount of template, covering at least three orders of magnitude, so that the calibration curve’s linear interval included the interval above and below the abundance of the targets. Only primers achieving an efficiency of reaction between 90 and 110% (3.6 > slope > 3.1) and a coefficient of correlation r2 > 0.99 were used for the analysis. Target gene expression was normalized against the geometrical mean expression of four housekeeping genes: actin b (ACTB), ribosomal protein L19 (RPL19), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ), and succinate dehydrogenase complex flavoprotein, subunit A (SDHA).
Table 1.
Primers used for real-time PCR experiments. Ta, annealing temperature during PCR cycles; bps, size of the amplified gene fragment in base pairs
| Symbol | Gene name | Accession number | Primer sequence | Ta | bps |
|---|---|---|---|---|---|
| ACTB | Actin B | NM_001009784 |
5′ ttcctgggtatggatcctg 3′ 5′ ggtgatctccttctgcatcc 3′ |
60 °C | 162 |
| AR | Androgen receptor | KF227907 |
5′ atgtcctggaagccattgag 3′ 5′ caaacaccataagccccatc 3′ |
60 °C | 219 |
| BAX | BCL2-Associated X protein | XM_004015363 |
5′ aacatggagctgcagaggat 3′ 5′ ggacattggacttccttcga 3′ |
58 °C | 219 |
| CIRBP | Cold-inducible RNA-binding protein | XM_004008776 |
5′ gagggctgagttttgacacc 3′ 5′ atgggaagtctgtggatggg 3′ |
58 °C | 190 |
| FSHR | Follicle-stimulating hormone receptor | NM_001009289 |
5′ agtcttcctctgccaggaca 3′ 5′ cttctgggatgactcgaagc 3′ |
60 °C | 107 |
| HSP90AB1 | Heat shock protein 90 alpha family class B member 1 | XM_004018854 |
5′ tggagatcaaccctgacca 3′ 5′ gggatcctcaagcgagaag 3′ |
58 °C | 143 |
| KIF11 | Kinesin family member 11 | XM_004020034 |
5′ tgatcttgcaggcagtgaga 3′ 5′ ccctcttgactctgggaagg 3′ |
62 °C | 100 |
| KIT | KIT proto-oncogene receptor tyrosine kinase | NM_001308594 | 5′ cctgggatttcctcttcgtt 3′ | 60 °C | 86 |
| 5′ agacagttcccctggactca 3′ | |||||
|
POU5F1 (OCT4) |
POU domain, class 5, transcription factor 1 | XM_012101009 |
5′ gaggagtcccaggacatcaa 3′ 5′ ccgcagcttacacatgttct 3′ |
56 °C | 204 |
| RLP19 | Ribosomal protein L9 | XM_004012836 |
5′caactcccgccagcagat 3′ 5′ ccgggaatggacagtcaca 3′ |
56 °C | 127 |
| SDHA | Succinate dehydrogenase | XM_012125144 |
5′catccactacatgacggagca 3′ 5′ atcttgccatcttcagttctgcta 3′ |
60 °C | 90 |
| SOD1 | Superoxide dismutase 1 | NM_001145185 |
5′ ggcaatgtgaaggctgacaa 3′ 5′ aagaccagatgacttgggca 3′ |
58 °C | 130 |
| STAR | Steroidogenic acute regulatory protein | NM_001009243 |
5’ cccatggagaggctttatga 3′ 5′ cagccaactcgtgagtgatg 3′ |
60 °C | 130 |
| TERT | Telomerase reverse transcriptase catalytic subunit | EU139125 |
5′ ggagaccacgttccagaaga 3′ 5′gcctgacctctgcttctgac 3′ |
60 °C | 131 |
| YWHAZ | Tyrosine 3-monooxygenase | NM_001267887 |
5′ tgtaggagcccgtaggtcatct 3′ 5′ ttctctctgtattctcgagccatct 3′ |
60 °C | 168 |
| ZBDB16 (PLZF) | Zinc finger and BTB domain containing 16 | XM_012096530 | 5′ gtgtatgtggcgtggagctt 3′ | 60 °C | 84 |
| 5′ acacccgtacgtcttcatcc 3′ |
Statistical analysis
Data were analyzed with MINITAB Release 12.1 software package (Minitab, Inc., State College, PA, USA). Plasma membrane integrity data were examined by analysis of variance (ANOVA) followed by Tukey’s post hoc comparisons. After testing for normality using Kolmogorov–Smirnov test, gene expression data were analyzed by ANOVA repeated measures (including both treatment and individual lamb as factors) followed by Tukey’s post hoc comparison when significant differences between the groups, as a whole, were observed. Differences were considered significant when P < 0.05.
Results
Trypan blue exclusion test
Mean viability (n = 3 per experimental group) was estimated through a trypan blue dye exclusion assay. Results are summarized in Table 2.
Table 2.
Cell plasma membrane integrity after testicular tissue vitrification and warming, determined by trypan blue dye exclusion assay. Controls, testicular tissue analyzed immediately after dissection. Control 2 h and 24 h, testicular tissue analyzed after 2 and 24 h of in vitro culture. Vitrified 0 h, testicular tissue subjected to vitrification and analyzed immediately after warming. Vitrified 2 h and 24 h, testicular tissue subjected to vitrification and analyzed after 2 or 24 h post-warming in vitro culture. Different superscript letters indicate a significant difference (ANOVA; P < 0.05)
| Treatment | Plasma membrane integrity (mean ± SE) |
|---|---|
| Control 0 h | 89.67% ± 1.45 a |
| Control 2 h | 86.67% ± 1.33 a |
| Control 24 h | 81.00% ± 4.58 a |
| Vitrified 0 h | 66.00% ± 4.73 b |
| Vitrified 2 h | 59.67% ± 4.18 b |
| Vitrified 24 h | 31.00% ± 3.46 c |
Post-warming cell membrane integrity was significantly lower compared to controls. No differences were observed between vitrified samples immediately after warming (66.00% ± 4.73) or after 2 h of IVC (59.67% ± 4.18), but extended post-warming in vitro culture (24 h) caused an additional decrease to 31.00% ± 3.46 compared to controls and V0h and V2h (P < 0.05). Conversely, cell membrane integrity was not affected by IVC alone (P > 0.1).
Gene expression
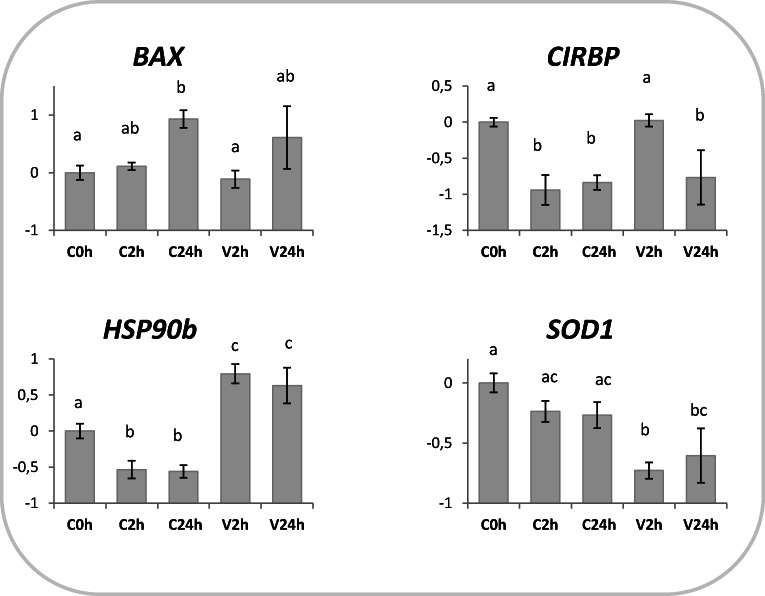
The relative quantification of the transcript is described in Figs. 1, 2, and 3. The expression of all genes was observed in the testicular tissue and was significantly affected by either cryopreservation and/or in vitro culture, but never by the lamb factor. SOD1 abundance was not affected by in vitro culture, but decreased after vitrification in V2h samples (Fig. 1). A decrease in HSP90b expression was observed in IVC groups, while vitrification caused an increase both at 2 and 24 h post-warming (Fig. 1). BAX transcript levels increased after 24 h of in vitro culture, both in vitrified (V24h) and in control cells (C24h; Fig. 1). CIRBP expression decreased in in vitro cultured cells (C2h and C24h), and showed a transient increase following vitrification (V2h) but significantly dropped after 24 h post-warming (V24h; Fig. 1).
Fig. 1.
Effects of vitrification and warming on genes involved in transcriptional stress response. Relative expression of BAX, CIRPB, HSP90b, and SOD1 in testicular tissue subjected to vitrification and in vitro cultured for 2 (V2h) or 24 h post-warming (V24h). Relative controls consisted in testicular tissue (not cryopreserved) analyzed immediately (C0h) or in vitro cultured for 2 (C2h) or 24 h (C24h). Relative abundance values are expressed as ΔCq and show the mean value ± SEM of 10 replicates per experimental group. Target gene abundance was normalized against the geometrical mean of four reference genes (ACTB, RPL19, SDHA, and YWHAZ). Different letters indicate a significant difference in relative mRNA abundance (ANOVA P < 0.05) among the groups
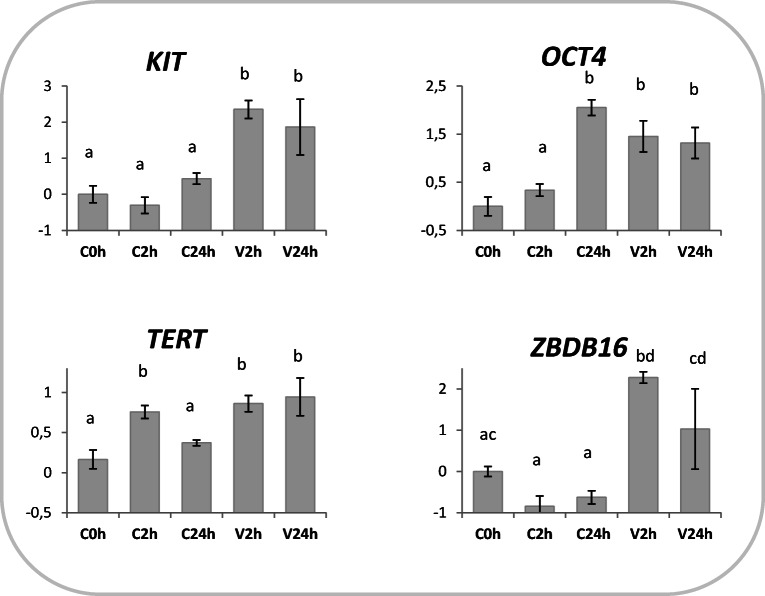
Fig. 2.
Germ-cell marker gene expression in vitrified-warmed prepubertal testicular tissue. Relative expression of KIT, OCT4 (POUF5), TERT, and ZBDB16 (PLZF) in tissue subjected to vitrification and in vitro cultured for 2 (V2h) or 24 h post-warming (V24h). Relative controls consisted in testicular tissue (not cryopreserved) analyzed immediately (C0h) or in vitro cultured for 2 (C2h) or 24 h (C24h). Relative abundance values are expressed as ΔCq and show the mean value ± SEM of 10 replicates per experimental group. Target gene abundance was normalized against the geometrical mean of four reference genes (ACTB, RPL19, SDHA, and YWHAZ). Different letters indicate a significant difference in relative mRNA abundance (ANOVA P < 0.05) among the groups
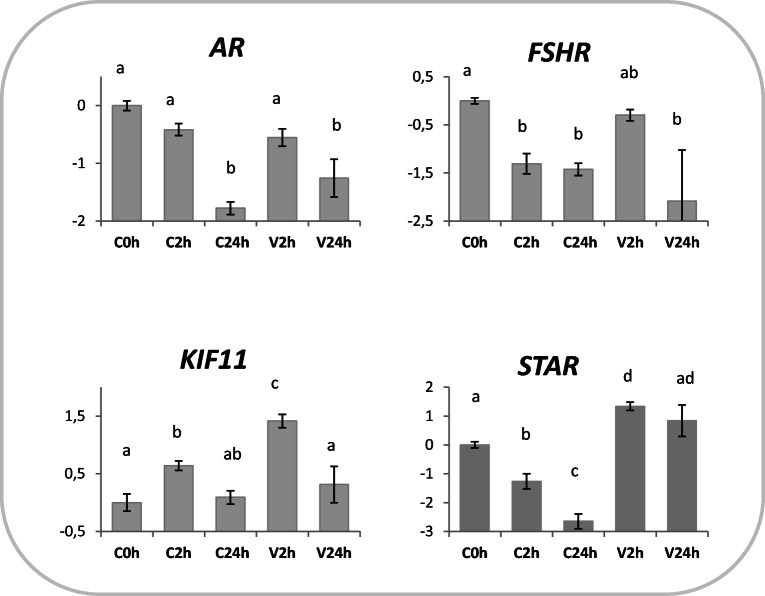
Fig. 3.
Relative expression of AR, FSHR, STAR (somatic cell markers), and KIF11 in prepubertal testicular tissue subjected to vitrification and in vitro cultured for 2 (V2h) or 24 h post-warming (V24h). Relative controls consisted in fresh testicular tissue analyzed immediately (C0h) or in vitro cultured for 2 (C2h) or 24 h (C24h). Relative abundance values are expressed as ΔCq and show the mean value ± SEM of 10 replicates per experimental group. Target gene abundance was normalized against the geometrical mean of four reference genes (ACTB, RPL19, SDHA, and YWHAZ). Different letters indicate a significant difference in relative mRNA abundance (ANOVA P < 0.05) among the groups
Higher OCT4 levels were observed in vitrified samples (V2h and V24h) and in control cells after 24 h in vitro culture (C24h; Fig. 2). TERT expression rose after 2 h and 24 h of IVC in the vitrified tissue, while transiently increased after 2 h in the samples subjected to IVC alone (Fig. 2). KIT mRNA abundance was not altered by IVC alone, but significantly increased following vitrification (Fig. 2). Similarly, PLZF/ZBDB16 transcription did not change during IVC, but was induced by vitrification (Fig. 2).
1pt?>KIF11 expression had a transient increase in vitrified (V2h) and control cells (C2h), which returned to basal levels after 24 h (C24h and V24h; Fig. 3). The expression of the androgen receptor (AR) decreased after 24 h independent of cryopreservation (Fig. 3). FSHR transcript abundance decreased in non-vitrified IVC groups (C2h and C24h) compared with control (C0h; P < 0.05); its level in V2h was similar to controls (C0h), but decreased in V24h compared with C0h (P < 0.05; Fig. 3). STAR mRNA abundance in vitrified-warmed tissues 2 h after warming (V2h) was significantly higher than C0h; conversely, it decreased during IVC of control tissues (C2h and C24h; Fig. 3).
Discussion
Successful cryopreservation of immature testicular tissue is still challenging, due to its particular susceptibility to in vitro manipulation [7].
In this study, we report a simple and rapid method to vitrify ovine prepubertal testicular tissues; we describe the effects of cryopreservation on gene expression addressing both somatic and spermatogenic cells, and we dissect the specific effect of post-warming in vitro culture from the vitrification procedure itself during a 24-h period. Such information is highly relevant to enhance cryopreservation protocols and improve post-warming testicular cell functionality, and has been lacking in previous studies.
The manual cryopreservation protocol we report combines a novel cryodevice “E.Vit” with a two-step vitrification protocol using EG and DMSO as cryoprotectants. E.Vit was recently designed to perform in situ straw dilution of all pre- and post-vitrification steps [22] that ensures a fast and simple method reducing the need for technical skills and minimizes operator manipulation errors. It has been successfully employed to vitrify murine and bovine oocytes and embryos, with high survival rates after warming [22]. In the present work, we confirmed the validity of the cryodevice to cryopreserve prepubertal testicular tissue, sustaining a plasma membrane integrity of 66.00% and 59.67% at 0 h and 2 h post-warming, respectively (Table 2), in line with observations in bovine immature testicular tissue cryopreserved by slow freezing (between 48.00 and 77.82%; [21]) and in vitrified prepubertal rat tissue (between 72.09 and 59.19%; [23]). Conversely, extending post-warming in vitro culture to 24 h negatively affected cell survival, suggesting a high susceptibility of the tissue to the suboptimal conditions of IVC (Table 2).
A critical point of cryopreservation protocols is the macroscopic physical dimension of the tissue [1], which affects preservation efficiency and potential cryo-injuries [24, 25]. In the present study, the tissue was cut in 1-mm3 cubes to allow rapid in and out diffusion of CPAs and uniform rates of temperature change and loading into the E.Vit straw, which has a diameter of 3 mm. Post-warming survival rates (Table 2) confirm that such dimensions are suitable to balance these critical parameters.
Degeneration of testicular tissue after cryopreservation and culture is non-negligible. A deeper understanding of the involved molecular mechanisms seems essential to improve cryopreservation and culture techniques. Here, we have attempted to understand the variation in gene expression after vitrification or IVC alone during a post-warming or post-dissection 24-h period. The selected gene panel comprises germ-cell specific markers (ZBDB16 (PLZF), TERT, POU5F1 (OCT4), KIT), genes specifically expressed in supporting somatic cells (in Sertoli or Leydig cells; AR, FSHR, STAR), and genes involved in cell stress response (BAX, SOD1, CIRBP, and HSP90AB1), which in turn are expressed in somatic or germ, or both types of cells (Table 3). KIF11, encoding a kinesin-like motor protein involved in chromosome positioning and spindle dynamics during mitosis [36], was included in the panel on the basis of its high expression in testicular tissue [37] and of its susceptibility to vitrification in bovine [38] and mouse oocytes [39]. Expression of all genes was observed in all experimental groups, clearly showing the survival of both cell types to cryopreservation and IVC (Figs. 1, 2, 3). One of the challenges of cryopreserving testicular tissue (and tissues in general) is the presence of various cell types, each differing in size, complexity, and membrane permeability. The cryopreservation optimum differs for each cell type; therefore, a specific cryopreservation protocol may fall short of preserving all cells [7]. Our results show that the proposed protocol supports survival of both germ and somatic cells, as all cell-specific genes are expressed in groups V2h and V24h.
Table 3.
Cell-specific expression of the analyzed genes
| Symbol | Gene name | Spermatogenic Cells | Supporting somatic cells | References |
|---|---|---|---|---|
| AR | Androgen receptor | No | Yes (Sertoli cells) | [26] |
| BAX | BCL2-Associated X Protein | Yes | Yes | [20] |
| CIRBP | Cold-inducible RNA-binding protein | Yes | No | [27] |
| FSHR | Follicle-stimulating hormone receptor | No | Yes (Sertoli cells) | [28] |
| HSP90AB1 | Heat shock protein 90 alpha family class B member 1 | Yes | No | [29] |
| KIF11 | Kinesin family member 11 | Unknown | Unknown | |
| KIT | KIT proto-oncogene receptor tyrosine kinase | Yes | Yes | [30] |
| POU5F1 (OCT4) | POU domain, class 5, transcription factor 1 | Yes | No | [31] |
| SOD1 | Superoxide dismutase 1 | Yes | Yes (Sertoli cells) | [32] |
| STAR | Steroidogenic acute regulatory protein | No | Yes (Leydig cell) | [33] |
| TERT | Telomerase reverse transcriptase catalytic subunit | Yes | No | [34] |
| ZBDB16 (PLZF) | Zinc finger and BTB domain containing 16 | Yes | No | [35] |
Evaluation of genes involved in cell stress response indicates moderate effects of IVC or cryopreservation on testicular cell gene expression. Signs of poor cell conditions after extended IVC are suggested by BAX abundance, which showed a significant increase after 24 h IVC, in both cryopreserved and control groups (Fig. 1). As widely known, BAX is a proapoptotic protein belonging to the Bcl2 superfamily involved in apoptosis incidence, whose accumulation in mitochondria outer membrane results in the release of cytochrome c and apoptosis [40]. In the present work, BAX expression pattern suggests an induction of apoptosis pathways more related to suboptimal IVC conditions than to cryopreservation itself. Furthermore, the vitrification protocol we propose does not seem to activate the mechanisms of antioxidant protection, according to SOD1 expression (Fig. 1). Superoxide dismutase 1 is the enzyme responsible for the elimination of free superoxide radicals by conversion to molecular oxygen and hydrogen peroxide; oxidative stress therefore stimulates SOD1 expression [41].
In testicular tissue, both CIRBP and HSP90AB1 are only expressed in germ cells. In mice, Cirbp is constitutively expressed and its level depends on the stage of differentiation [27]. Cirbp upregulation is induced by mild, but not severe, hypothermia [42], but also by cell stresses such as UV irradiation and hypoxia [43, 44]. In our experiments, CIRBP abundance decreased during IVC and remained similar to control after cryopreservation, indicating absence of severe cell stress. As expected, HSP90AB1 expression was induced in cryopreserved tissue (Fig. 1). HSP90ABs function as molecular chaperones that exert protective effects by binding to client proteins, supporting proper protein folding, and maintaining protein stability, especially after exposure to various kinds of cellular stress (i.e., heat or cold shock, hyperosmotic stress, or heavy metal toxicity [45–47]). HSP90AB1 upregulation following vitrification, and not after short or extended IVC, indicates a reaction specific to the cryopreservation procedure.
The preservation of spermatogenesis is the main objective of testicular tissue cryoconservation. Therefore, expression of germ-cell specific markers was analyzed to assess the effects of a novel vitrification protocol on spermatogenesis potential. The transcription repressor ZBTB16 (also known as PLZF) plays a crucial role in spermatogenesis and is expressed exclusively in gonocytes and undifferentiated spermatogonia [35]. Similarly, TERT mRNA is found only during germ cell differentiation [34]. TERT is the catalytic subunit of the enzyme telomerase that adds hexameric repeats to the telomeric DNA during replication [48], guaranteeing the transfer of full-length chromosomes in germline cells [49]. KIT gene is involved in the regulation of primordial germ cell proliferation and differentiation [50]. Interestingly, all markers consistently showed higher transcript abundance in vitrified tissues, both at 2 and 24 h post-warming (Fig. 2). Such increase may be the result of a preferential survival of germ cells (that would shift upwards germ cell marker expression compared with controls) or may be due to an effective gene upregulation, previously unreported and potentially due to a cold stress response. On the other hand, IVC exerted little effect on these markers: KIT and ZBDB16 were not affected at either time points, while TERT showed a transient increase 2 h post-dissection; only OCT4 mRNA synthesis showed an important increase after 24 h IVC (Fig. 2). OCT4 is a transcription factor highly expressed in pluripotent cells and specifically in SSC, where it is involved in SSC proliferation and differentiation [51, 52]. Besides being critical for pluripotency maintenance [53], OCT4 expression was induced by stress conditions in different cell types [54–56]. In accordance, we previously observed an increase in OCT4 transcription in sheep skin fibroblasts cryopreserved and cultured in vitro for 24 h [57]. In the present work, we hypothesize that cell stress induced by cryopreservation or extended IVC solicits OCT4 transcription, in line with previous evidence. The analysis of genes specifically expressed in supporting somatic cells (Table 3) confirmed the survival of this type of cells after vitrification. However, extended IVC seems to exert a negative effect on the genes involved in steroidogenesis in both cryopreserved (AR and FSHR) and control samples (AR, FSHR, and STAR; Fig. 3). A transient increase after 2 h IVC in vitrified (V2h) and control tissues (C2h; Fig. 3) indicates that KIF11 transcription is induced by both vitrification and in vitro culture, in partial accordance with previous studies in the vitrified oocytes [38, 39], where KIF11 upregulation was seen to be specific to vitrification procedures and not associated with in vitro environment alone.
Overall, our study has shown differences in expression between germ-cell and somatic-cell markers, which may indicate a different response to vitrification or IVC. Somatic, but not germ, cell markers were largely downregulated by extended IVC (Figs. 2 and 3). On the other hand, vitrification caused a consistent upregulation of the genes specifically expressed in spermatogonia both at 2 (KIT, OCT4, TERT, and ZBDB16) and 24 (KIT, OCT, and TERT) hours post-warming, while AR and FSHR somatic markers were unaffected and only a transient increase occurred in STAR 2 h post-warming (V2h; Fig. 2).
The present work has some limitations. Firstly, no histological examination of testicular tissue was performed, so we could not describe the effects of vitrification on cell morphology and tissue structure. Secondly, the observed variations in gene expression are the results of two potential factors: a differential cell survival to cryopreservation or IVC, and an effective variation in mRNA synthesis. Nevertheless, the molecular analysis partly overcomes these issues, as the expression of both somatic and spermatogenic cell-specific markers confirms the survival of both types of cells in all treatment and time groups. Finally, the work would have benefited from a vitrification (e.g., a different vitrification device) or a cryopreservation control (e.g., slow freezing), and from a biological test to assess whether the vitrified tissues can actually support spermatogenesis and offspring production after warming.
Conclusion
We report a simple and rapid method for vitrifying ovine prepubertal testicular tissue and describe its effect on expression of spermatogenic and somatic testicular cell markers during a 24-h post-warming period. Such information is highly relevant to optimize cryopreservation protocols for fertility preservation or within genetic conservation programs of high-value animals or endangered species.
Funding
Research was supported by Regione Autonoma della Sardegna.- L.R. 7-MIGLIOVINGENSAR Project and by Bando Competitivo Fondazione di Sardegna – 2016, CUP J86C18000800005.
DB is the recipient of an RTDa contract at the University of Sassari, Italy, granted by “P.O.R. SARDEGNA F.S.E. 2014–2020” – CUP J86C18000270002.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Onofre J, Baert Y, Faes K, Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22:744–761. doi: 10.1093/humupd/dmw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- 3.Fattahi A, Latifi Z, Ghasemnejad T, Nejabati HR, Nouri M. Insights into in vitro spermatogenesis in mammals: past, present, future. Mol Reprod Dev. 2017;84:560–575. doi: 10.1002/mrd.22819. [DOI] [PubMed] [Google Scholar]
- 4.Gassei K, Orwig KE. Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril. 2016;105:256–266. doi: 10.1016/j.fertnstert.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, Mulhall J, Shnorhavorian M, Brinster RL, Kolon TF. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer. 2014;61:1673–1678. doi: 10.1002/pbc.25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez F, International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108:407–415.e11. doi: 10.1016/j.fertnstert.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Unni S, Kasiviswanathan S, D'Souza S, Khavale S, Mukherjee S, Patwardhan S, Bhartiya D. Efficient cryopreservation of testicular tissue: effect of age, sample state, and concentration of cryoprotectant. Fertil Steril. 2012;97:200–8.e1. doi: 10.1016/j.fertnstert.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, Donnez J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26:737–747. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- 9.Baert Y, Goossens E, van Saen D, Ning L, in’t Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97:1152–7.e1–2. doi: 10.1016/j.fertnstert.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28:578–589. doi: 10.1093/humrep/des455. [DOI] [PubMed] [Google Scholar]
- 11.Macente BI, Toniollo GH, Apparicio M, Mansano C, Thomé HE, Canella CL, Tozato M, Gutierrez RR. Evaluation of different fragment sizes and cryoprotectants for cryopreservation of feline testicular tissues. Reprod Domest Anim. 2017;52(Suppl 2):242–247. doi: 10.1111/rda.12828. [DOI] [PubMed] [Google Scholar]
- 12.Curaba M, Verleysen M, Amorim CA, Dolmans MM, Van Langendonckt A, Hovatta O, Wyns C, Donnez J. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril. 2011;95:229–34.e1. doi: 10.1016/j.fertnstert.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 13.Zarandi NP, Galdon G, Kogan S, Atala A, Sadri-Ardekani H. Cryostorage of immature and mature human testis tissue to preserve spermatogonial stem cells (SSCs): a systematic review of current experiences toward clinical applications. Stem Cells Cloning. 2018;11:23–38. doi: 10.2147/SCCAA.S137873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 15.Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, Hata K, Inoue K, Ogonuki N, Ogura A, Ogawa T. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5:4320. doi: 10.1038/ncomms5320. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, Ito J, Kashiwazaki N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8:e70989. doi: 10.1371/journal.pone.0070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C, Hanna C, Hennebold JD, Dobrinski I, Orwig KE. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–1319. doi: 10.1126/science.aav2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholami M, Hemadi M, Saki G, Zendedel A, Khodadadi A, Mohammadi-Asl J. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability. J Assist Reprod Genet. 2013;30:1271–1277. doi: 10.1007/s10815-013-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondanino C, Maouche A, Dumont L, Oblette A, Rives N. Establishment, maintenance and functional integrity of the blood-testis barrier in organotypic cultures of fresh and frozen/thawed prepubertal mouse testes. Mol Hum Reprod. 2017;23:304–320. doi: 10.1093/molehr/gax017. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XG, Li H, Hu JH. Effects of various cryoprotectants on the quality of frozen-thawed immature bovine (Qinchuan cattle) calf testicular tissue. Andrologia. 2017;49:9. doi: 10.1111/and.12743. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Bian YL, Schreurs N, Zhang XG, Raza SHA, Fang Q, Wang LQ, Hu JH. Effects of five cryoprotectants on proliferation and differentiation-related gene expression of frozen-thawed bovine calf testicular tissue. Reprod Domest Anim. 2018;53:1211–1218. doi: 10.1111/rda.13228. [DOI] [PubMed] [Google Scholar]
- 22.Arav A, Natan Y, Kalo D, Komsky-Elbaz A, Roth Z, Levi-Setti PE, Leong M, Patrizio P. A new, simple, automatic vitrification device: preliminary results with murine and bovine oocytes and embryos. J Assist Reprod Genet. 2018;35:1161–1168. doi: 10.1007/s10815-018-1210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benvenutti L, Salvador RA, Til D, Senn AP, Tames DR, Amaral NLL, Amaral VLL. Wistar rats immature testicular tissue vitrification and heterotopic grafting. JBRA Assist Reprod. 2018;22:167–173. doi: 10.5935/1518-0557.20180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243–256. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- 25.Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67:81–89. doi: 10.1016/j.theriogenology.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 26.De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol. 2012;352:13–25. doi: 10.1016/j.mce.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, Okuno H, Millán JL, Matsuda T, Yoshida O, Fujita J. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152:289–296. [PMC free article] [PubMed] [Google Scholar]
- 28.Kliesch S, Penttilä TL, Gromoll J, Saunders PT, Nieschlag E, Parvinen M. FSH receptor mRNA is expressed stage-dependently during rat spermatogenesis. Mol Cell Endocrinol. 1992;84:R45–R49. doi: 10.1016/0303-7207(92)90039-9. [DOI] [PubMed] [Google Scholar]
- 29.Gruppi CM, Zakeri ZF, Wolgemuth DJ. Stage and lineage-regulated expression of two hsp90 transcripts during mouse germ cell differentiation and embryogenesis. Mol Reprod Dev. 1991;28:209–217. doi: 10.1002/mrd.1080280302. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Tang J, Haines CJ, Feng HL, Lai L, Teng X, Han Y. c-kit and its related genes in spermatogonial differentiation. Spermatogenesis. 2011;1:186–194. doi: 10.4161/spmg.1.3.17760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauls K, Schorle H, Jeske W, Brehm R, Steger K, Wernert N, Büttner R, Zhou H. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 2006;21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- 32.Selvaratnam J, Paul C, Robaire B. Male rat germ cells display age-dependent and cell-specific susceptibility in response to oxidative stress challenges. Biol Reprod. 2015;93:72. doi: 10.1095/biolreprod.115.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Saen D, Pino Sánchez J, Ferster A, van der Werff ten Bosch J, Tournaye H, Goossens E. Is the protein expression window during testicular development affected in patients at risk for stem cell loss? Hum Reprod. 2015;30:2859–2870. doi: 10.1093/humrep/dev238. [DOI] [PubMed] [Google Scholar]
- 34.Ravindranath N, Dalal R, Solomon B, Djakiew D, Dym M. Loss of telomerase activity during male germ cell differentiation. Endocrinology. 1997;138:4026–4029. doi: 10.1210/endo.138.9.5488. [DOI] [PubMed] [Google Scholar]
- 35.Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2001;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 36.Stolz A, Ertych N, Bastians H. A phenotypic screen identifies microtubule plus end assembly regulators that can function in mitotic spindle orientation. Cell Cycle. 2015;14:827–837. doi: 10.1080/15384101.2014.1000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y, Xie M, Chen W, Talbot R, Maddox JF, Faraut T, Wu C, Muzny DM, Li Y, Zhang W, et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science. 2014;344:1168–1173. doi: 10.1126/science.1252806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Yu XL, Guo XF, Zhang F, Pei XZ, Li XX, Han WX, Li YH. Effect of liquid helium vitrification on the ultrastructure and related gene expression of mature bovine oocytes after vitrifying at immature stage. Theriogenology. 2017;87:91–99. doi: 10.1016/j.theriogenology.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Wen Y, Zhao S, Chao L, Yu H, Song C, Shen Y, Chen H, Deng X. The protective role of antifreeze protein 3 on the structure and function of mature mouse oocytes in vitrification. Cryobiology. 2014;69:394–401. doi: 10.1016/j.cryobiol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Yan W, Suominen J, Samson M, Jegou B, Toppari J. Involvement of Bcl-2 family proteins in germ cell apoptosis during testicular development in the rat and pro-survival effect of stem cell factor on germ cells in vitro. Mol Cell Endocrinol. 2000;165:115–129. doi: 10.1016/s0303-7207(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 41.Song YS, Narasimhan P, Kim GS, Jung JE, Park EH, Chan PH. The role of Akt signaling in oxidative stress mediates NF-kappaB activation in mild transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1917–1926. doi: 10.1038/jcbfm.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1:243–255. [PubMed] [Google Scholar]
- 44.Wellmann S, Bührer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, Fujita J, Seeger K. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117:1785–1794. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- 45.Liu DH, Yuan HY, Cao CY, Gao ZP, Zhu BY, Huang HL, Liao DF. Heat shock protein 90 acts as a molecular chaperone in late-phase activation of extracellular signal-regulated kinase 1/2 stimulated by oxidative stress in vascular smooth muscle cells. Acta Pharmacol Sin. 2007;28:1907–1913. doi: 10.1111/j.1745-7254.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang XH, Kang L. Differences in egg thermotolerance between tropical and temperate populations of the migratory locust Locusta migratoria (Orthoptera: Acridiidae) J Insect Physiol. 2005;51:1277–1285. doi: 10.1016/j.jinsphys.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Haase M, Fitze G. HSP90AB1: helping the good and the bad. Gene. 2016;575(2 Pt 1):171–186. doi: 10.1016/j.gene.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 49.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 50.Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14:1926–1930. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 51.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Zhao Y, Xiao Z, Chen B, Wei Z, Wang B, Zhang J, Han J, Gao Y, Li L, Zhao H, Zhao W, Lin H, Dai J. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells. 2009;27:1265–1275. doi: 10.1002/stem.58. [DOI] [PubMed] [Google Scholar]
- 55.Byun K, Kim TK, Oh J, Bayarsaikhan E, Kim D, Lee MY, Pack CG, Hwang D, Lee B. Heat shock instructs hESCs to exit from the self-renewal program through negative regulation of OCT4 by SAPK/JNK and HSF1 pathway. Stem Cell Res. 2013;11(3):1323–1334. doi: 10.1016/j.scr.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Miki T, Wong W, Zhou E, Gonzalez A, Garcia I, Grubbs BH. Biological impact of xeno-free chemically defined cryopreservation medium on amniotic epithelial cells. Stem Cell Res Ther. 2016;7:8. doi: 10.1186/s13287-015-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Idda A, Bebbere D, Corona G, Masala L, Casula E, Cincotti A, Ledda S. Insights on cryopreserved sheep fibroblasts by cryomicroscopy and gene expression analysis. Biopreserv Biobank. 2017;15:310–320. doi: 10.1089/bio.2016.0100. [DOI] [PubMed] [Google Scholar]