Abstract
In utero exposure to environmental factors can modify the development of allergies later in life whereby the mechanisms of the feto-maternal crosstalk still remain largely unknown. Murine studies revealed that inflammatory maternal signals elicited by chronic helminth infection within the placenta imprint a distinct gene expression profile related to the Vitamin-D-receptor (VDR)-inflammation-axis. We thus investigated whether pro- or anti- inflammatory immune responses as well as VDR and related gene expression within the placenta differ between women from helminth-endemic and non-endemic areas. A prospective pilot study was conducted in Munich, Germany (helminth non-endemic) and Lambaréné, Gabon (helminth-endemic). At delivery, clinical information alongside placenta tissue samples and maternal and cord blood were obtained for further laboratory analysis. Schistosoma haematobium infection was detected in 13/54 (23%) Gabonese women. RT PCR revealed significantly lower gene expression of VDR, Cyp27b1, Foxp3 and IL10 in Gabonese compared to German placentae as well as significantly lower levels of plasma IgG4 in newborns resulting in a significantly higher IgE/IgG4 ratio. These findings demonstrate that exposure in utero to different environments alters placental gene expression and thus possibly plays a role in the development and modulation of the immune system of the offspring.
Subject terms: Epidemiology, Genetics research
Introduction
Chronic inflammatory disorders, such as allergic airway inflammation, are negatively associated with high levels of helminth infection such as schistosomes, particularly in low-income countries1 where prevalence of allergic reactivity and autoimmune antibodies increases after anthelmintic treatment2–4. Approximately 220 million people are infected with schistosomes5 and thus, approximately 40 million women of childbearing age. Yet little is known about the specific morbidities inflicted on pregnant women and their offspring6. Complications such as anaemia and low-birth-weight7, as well as skewed immune responses, which can alter susceptibility for other diseases8, are just some examples of the potential impact of a pregnancy modulated by helminth infection. Such infections are known to modify host immune responses through a variety of mechanisms9, minimizing host-damage to enable survival of the parasite10. Stable, chronic helminth infections can endure for long periods (up to 20 years) in an individual host, facilitated through helminth-induced recalibration of host immunity towards a state of immune hyporesponsiveness that can be considered a form of immunologic tolerance11–13.
Recent research indicates that early exposure to such a modified environment during gestation already primes the fetal immune system in utero and leads to enhanced immunological maturity at birth14. Indeed, it was recently demonstrated, that experimental chronic infection with the helminth Schistosoma mansoni during pregnancy influences the outcome of allergic asthma in offspring15. This was further associated with downregulation of genes associated with either Vitamin-D-metabolism and –pathways such as the transcription factor Vitamin-D-receptor (VDR)16, the enzyme 1α-hydroxylase (Cyp27b1), responsible for vitamin D activation, as well as hydroxy-delta-5-steroid dehydrogenase (Hsd3b1)15, which is crucial for roles in the biosynthesis of all hormonal steroids17.
Therefore, defective VDR signalling in placental tissue might result in increased risk of placental inflammation and expression of inflammatory cytokines or dampening of anti-inflammatory and tolerogenic cytokines, respectively. Placental VDR expression, as well as Cyp27b1, has also been linked to regulation of key cytokines involved in inflammatory responses, namely interleukin 10 (IL-10) or interferon gamma (IFN-γ). Cyp27b1 hydroxylases’ 25(OH)D to the active form 1,25 (OH)2D, whereas Cyp24a1 is responsible for the inactivation. VDR and Cyp27b1 are expressed in almost all immune cells as well as in both decidua18 and trophoblast19 suggesting that the placenta itself converts 25(OH)D to the active form and may thereby function in an autocrine or paracrine fashion20. Indeed, the loss of 1,25 (OH)2D production in the fetal compartment of the placenta has been shown to cause generalized dysregulation of placental inflammation after immune challenge20, which are known to be induced during helminth infection21,22. IL-10 is produced by Forkhead-Box-Protein P3 (Foxp3) expressing regulatory T cells alongside other cell types such as the villous cytotrophoblasts within the placenta, where it appears to work as a key facilitator of successful pregnancy20.
The placenta plays a decisive role in pregnancy maintenance and the development and protection of the fetus. Besides the production of hormones, the placenta is a barrier between mother and fetus and maintains immunological tolerance. The mature, disc-shaped placenta can be divided into three zones. First the basal plate or decidua which is predominantly the maternal side of the placenta and consists of up to 30–40% leukocytes23 to avoid rejection of the fetus as well to protect it from maternal infections. The placental leukocyte population is compromised by around 70% uterine natural killer cells (uNKs), about 20% macrophages and 10% T-lymphocytes (with 10–15% regulatory T-cells), but also dendritic cells and mast cells can be found in the early placental bed24,25. The fetal side is composed of the chorionic plate. The feto-maternal zone in-between consists in the intervillous space with maternal blood and the villous trees providing the fetal blood.
Environmental triggers and maternal stress can lead to significant changes within to the placenta, with important outcomes for fetal health and development26. In multivariate models adjusted for geohelminths, maternal schistosomiasis was associated with increased levels of inflammatory cytokines in maternal peripheral blood, placental, and cord blood, as well as acute subchorionitis27. Granulomatous inflammation in the placenta28 and the cervix29 in the context of female genital schistosomiasis (FGS) can occur if immature worms or eggs directly become lodged in the placenta6. In general, placental inflammation is associated with substantially lower feto-maternal immunoglobin G (IgG) antibody transfer efficiency30 which is tightly regulated and mediated by neonatal Fc Receptor (FcRn)31. IgG is the only antibody subclass which is able to cross the placental barrier. IgG, especially its subclasse like IgG4, and IgE, which acts in a competitive way with IgG4, are strongly induced during helminth infections32. Strong anti-parasite IgE responses are associated with resistance to infection33, whereas high levels of IgG4 have been associated with susceptibility34. However, in contrast to IgG4, IgE is not able to cross the placental barrier. Thus, any detection of fetal IgE can be considered as evidence for in utero priming of the fetal immune system. Indeed, schistosome specific IgE have been detected in cord blood from S. haematobium infected mothers35.
Considering that the placental anti- as well as proinflammatory gene expression can be skewed by the environment including helminth infections, we compared placental gene expression as well as inflammation markers in maternal and cord blood of Germans and Gabonese cohorts in a cross-sectional study.
Results
Differences in maternal and neonatal characteristics between gabon and germany
For the study, 54 pregnant women in Gabon, and 47 in Germany fulfilled all inclusion criteria and were successfully included into the study (Table 1). At birth we registered data related to maternal health, pregnancy, birth outcome, and neonatal health as shown in Table 1. Significant differences between the two study populations emerged not only between the mothers but also between the newborns. Gabonese mothers were on average seven years younger than the mothers in the German control cohort. In both cohorts, the mothers delivered their newborns on average at 39 weeks of gestation and had the same number of deliveries, whereas the higher number of gravidities in Gabon indicates a higher rate of pregnancy loss (naturally and spontaneously) than in Germany. Furthermore, women in Gabon had a reduced haemoglobin (10.6 ± 2.21 vs. 12.15 ± 1.11 g/dl) rendering most women anaemic. CRP values at delivery were elevated in Gabonese women (1.09 ± [0.63;2.17] vs. 0.63 ± [0.24;1.13] mg/dl). Even though sampling was performed during the summertime in Germany, 25-OH-Vitamin D plasma concentration was significantly higher in Gabonese maternal peripheral blood samples (33.4 ± 8.7 ng/mL vs. 23.9 ± 13.8 ng/mL) as well as in Gabonese cord blood samples (36.0 ± 8.64 ng/mL vs 28.94 ± 15.66 ng/ml) (Table 1). The month of sample collection (Supplementary Fig. 2) in both countries nor infection status in the Gabonese cohort had an influence on 25-OH-Vitamin D concentrations in Gabon (Supplementary Fig. 2). No difference in gender distribution of neonates between both countries was seen. Gabonese newborns were, however, significantly shorter (3 cm) and on average 255 g lighter than their German counterparts.
Table 1.
Maternal and newborn characteristics at delivery in Gabon and Germany.
| Number n | Gabon | Germany | p value |
|---|---|---|---|
| 54 | 47 | ||
| Mother | |||
| Age [years] | 26 ± 7 | 33 ± 5 | <0.0001 |
| Parity | 2 ± 2 | 1.7 ± 0.7 | 0.51 |
| Gravidity | 4 ± 2 | 2.1 ± 1.5 | <0.0001 |
| Gestational age at delivery (weeks) | 39.3 ± 2 | 39.7 ± 1.2 | 0.20 |
| Haemoglobin [g/dL] | 10.6 ± 2.21 | 12.15 ± 1.11 | 0.0011 |
| White blood cells [10^3/mm^3] | 11.81 ± 5.15 | 13.11 ± 3.82 | 0.26 |
| Eosinophils [%] | 1.91 ± 1.91 | Not done | |
| 25-OHD-Vitamin D3 concentration [ng/mL] | 34.5 ± 8.8 | 23.9 ± 13.8 | <0.0001 |
| Calcium [mmol/L] | 2.29 ± 0.12 | 2.31 ± 0.11 | 0.42 |
| CRP [mg/dL] | 1.09 ± [0.63;2.17] | 0.63 ± [0.24;1.13] | 0.0022 |
| Newborn | |||
| Gender (male) | 30 (56) | 22 (47) | 0.38 |
| Length [cm] | 50 ± 2 | 53 ± 3 | <0.0001 |
| Birthweight [g] | 3076 ± 510 | 3331 ± 544 | 0.0012 |
| 25-OHD-Vitamin D3 concentration [ng/mL] | 36.0 ± 8.64 | 28.94 ± 15.66 | 0.0011 |
| Calcium [mmol/L] | 2.66 ± 0.22 | 2.79 ± 0.15 | 0.0004 |
| CRP [mg/dL] | 0.01 ± [0.01;0.02] | 0.01 ± [0.01;0.02] | 0.56 |
Data are presented as mean ± SD (n), median with IQR (n) for C-reactive Protein (CRP), values or as numbers (%) where indicated; T-test was performed where data are normal distributed; for data without normal distribution a two-tailed Mann Whitney U-test was performed; blood for plasma parameters was taken from maternal peripheral vein blood or from cord blood, respectively.
Comparing the Gabonese cohort, with participants with and without S. haematobium infection, none of the mentioned outcome parameters differed significantly (Supplementary Table 1).
Lower relative gene expression of steroid and vitamin D pathway associated genes in gabonese placenta tissue
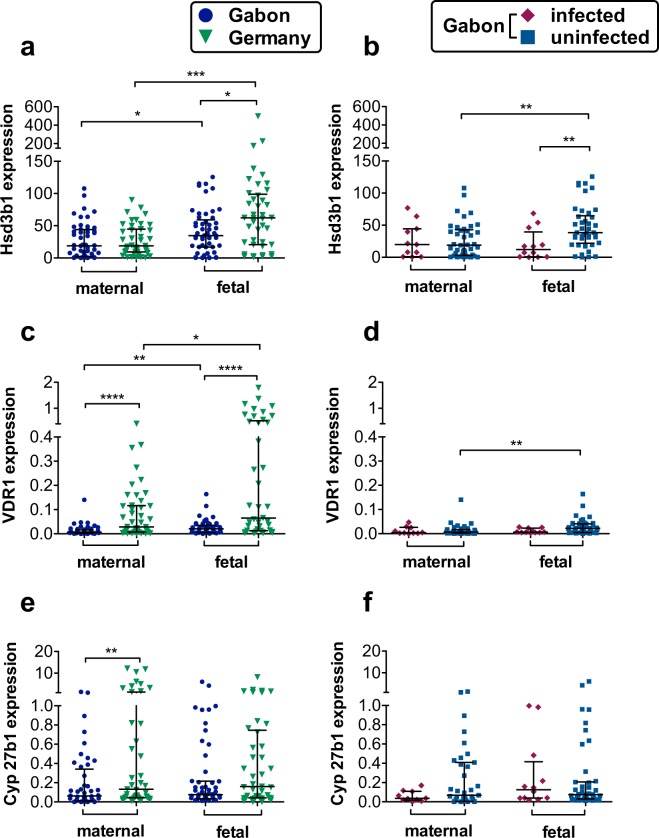
In order to study the potential effects of distinct geographical environments upon placental inflammation, in association with the effects of helminth infection, we compared the placental expression levels of genes known to be important for proper placental function through steroid and Vitamin D pathways between the Gabonese and German mothers. As shown in Fig. 1(a–f), comparative analyses of gene expression between Gabonese and German placenta samples revealed a significantly lower expression for VDR1 and Cyp27b1 on the Gabonese maternal placental side, whereas no differences were detected regarding Hsd3b1 expression. Regarding the fetal side of the placenta of the total Gabonese and German cohort, significantly lower expression for Hsd3b1 and VDR1 was found in the Gabonese when compared to the German samples and no differences in expression of Cyp27b1. However, these genes were expressed to a greater extent when compared to their corresponding maternal side (Fig. 1a,c). Within the Gabonese cohort, we compared placenta samples of mothers infected with S. haematobium to those uninfected Gabonese mothers (Fig. 1b,d,f), and showed a lower expression of Hsd3b1 on the fetal side of placenta from infected mothers. No differences for the VDR1 and Cyp27b1 expression between the infected and uninfected cohort was detected.
Figure 1.
Steroid and Vitamin D pathway associated gene expression within the maternal and fetal side of the placenta. Relative Hsd3b1, VDR and Cyp27b1 expression, normalized to hypoxanthine-guanine phosphoribosyl transferase (HPRT) as house-keeping gene (HKG). All data are shown with median and interquartile range. P values are for Mann-Whitney U-tests. Comparison between the two maternal, or fetal groups, respectively. P value: *< 0,05; **< 0,01; ***< 0,001; ****< 0,0001; n (maternal, Gabon) = 50; n (maternal, Germany) = 44; n (fetal, Gabon) = 53; n (fetal, Germany) = 41; n (Gabon, maternal, uninfected) = 40; n (Gabon, maternal, infected) = 10 n (Gabon, fetal, uninfected) = 42; n (Gabon, fetal, infected) = 11. (a) Expression of Hsd3b1 in Gabonese and German placenta tissue. (b) Hsd3b1 gene expression in placentae from infected or uninfected Gabonese mothers. (c) Expression of VDR1 in Gabonese and German placenta tissue. (d) VDR1 gene expression in placentae from infected or uninfected Gabonese mothers. (e) Expression of Cyp27b1 in Gabonese and German placenta tissue. (f) Cyp27b1 gene expression in placentae from infected or uninfected Gabonese mothers.
Lower relative gene expression of Foxp3 and IL10 in Gabonese placental samples
Dynamic processes of tolerance and regulation of inflammatory signals take place within the placenta. As such, the expression of immune-related genes known to be expressed in the placenta, such as Foxp3, IFNG or IL10, was compared between the Gabonese and German samples (Fig. 2a,c,e). There was lower expression of Foxp3 and IL10 on the maternal side of the placenta in Gabonese samples compared to those from the German cohort (Fig. 2a,e), whereas IFNG was equally expressed in Gabonese and German placentae. Foxp3 and IL10 also exhibited significantly lower relative expression on fetal side of Gabonese placental samples compared to those from the German cohort (Fig. 2a,e). Comparing placental gene expression between infected and uninfected Gabonese mothers as shown in Fig. 2b,d,f showed no difference between the two cohorts. IFNG was lower expressed on the maternal side. Infection status within the Gabonese cohort had no influence on the gene expression (Fig. 2b,d,f).
Figure 2.
Expression of immunologically relevant genes within the maternal and fetal side of the placenta. Relative Foxp3, IFNG, and IL10 expression, normalized to HPRT. All data are shown with median and interquartile range. P values are for Mann-Whitney U-tests. Comparison between the two maternal, or fetal groups, respectively. P value: *< 0,05; **< 0,01; ***< 0,001; ****< 0,0001; n (maternal, Gabon) = 50; n (maternal, Germany) = 44; n (fetal, Gabon) = 53; n (fetal, Germany) = 41; n (Gabon, maternal, uninfected) = 40; n (Gabon, maternal, infected) = 10 n (Gabon, fetal, uninfected) = 42; n (Gabon, fetal, infected) = 11; (a) expression of Foxp3 in Gabonese and German placenta tissue. (b) Foxp3 gene expression in placentae from infected or uninfected Gabonese mothers. (c) expression of IFNG in Gabonese and German placenta tissue (d) IFNG gene expression in placentae from infected or uninfected Gabonese mothers. (e) expression of IL10 in Gabonese and German placenta tissue. (f) IL10 gene expression in placentae from infected or uninfected Gabonese mothers.
IgG4 correlation between Gabonese and German mother-child pairs and significantly higher schistosome-specific IgG4 in mother-child plasma samples from infected Gabonese mothers
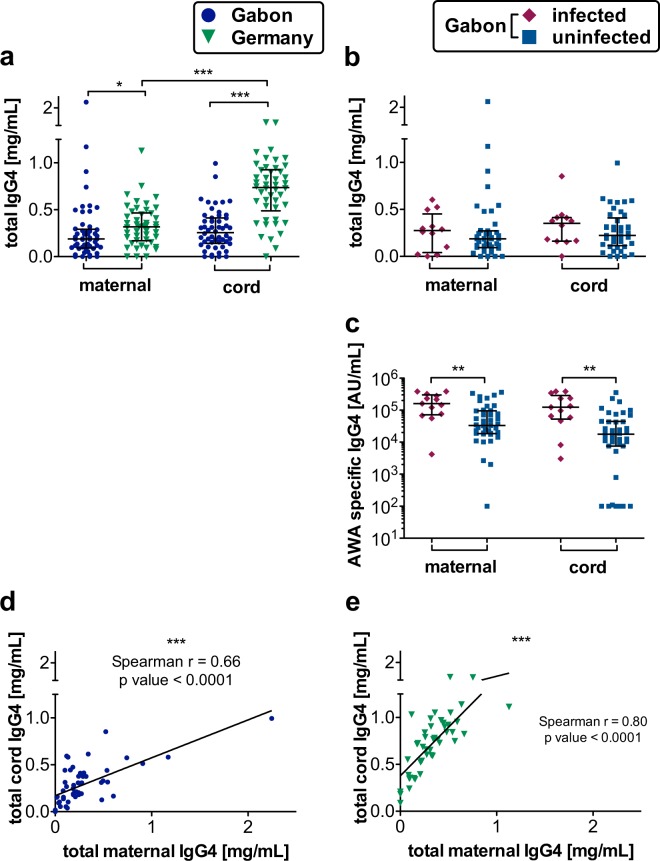
IgG4 plays an important role during helminth infections and its placental transfer can be diminished by placental inflammation. In German maternal and cord blood plasma samples, the total IgG4 levels were significantly higher compared to those from Gabon. However, the increase in fetal IgG4 which we observed in German cord blood, was not detectable in Gabonese samples, indicating that the active antibody-transport was diminished in the Gabonese placentas irrespective to their infection status (Fig. 3a,b). In the Gabonese cohort infection status had no influence on total IgG4 levels, but indeed on AWA specific IgG4 levels, which were significantly higher in maternal and cord blood in the infected group. Nevertheless, non-infected mothers still had substantial background antibodies possibly due to previous exposure to schistosomiasis or other helminths (Fig. 3c). Additionally, there is a strong correlation between maternal and cord blood total IgG4 concentrations in all groups (Fig. 3d,e) however stronger in the German cohort.
Figure 3.
Maternal and fetal plasma IgG4 levels and their correlation in Gabon and Germany. IgG4 levels were measured in plasma via ELISA. All data are shown with median and interquartile range. P values are for Mann-Whitney U-tests or Spearman’s rank correlation, respectively. Comparison between the two maternal, or cord blood groups, respectively. In the case additionally comparison between maternal and cord blood group each to show the placental transfer; P value: *< 0,05; **< 0,01; ***< 0,001; ****< 0,0001; n (maternal, Gabon) = 54; n (maternal, Germany) = 47; n (cord, Gabon) = 53; n (cord, Germany) = 47; n (Gabon, maternal, uninfected) = 42; n (Gabon, maternal, infected) = 12 n (Gabon, cord, uninfected) = 42; n (Gabon, cord, infected) = 12; (a) total IgG4 levels in all German maternal all Gabonese plasma samples (b) total IgG4 levels in Gabonese plasma samples divided into infected and uninfected group. (c) AWA specific IG4 levels in in Gabonese plasma samples divided into infected and uninfected group. (d) Correlation between general maternal and cord blood IgG4 levels in Gabonese plasma samples; number of pairs = 53; spearman r = 0,66, p value = < 0,0001. (e) Correlation between general maternal and cord blood IgG4 levels in German plasma samples; number of pairs = 45; spearman r = 0.80; p value = < 0,0001.
Significantly higher total and AWA specific IgE levels and IgE/IgG4 ratio in Gabonese plasma samples
Large amounts of specific and nonspecific IgE are a well-recognized feature of the immune response to parasitic helminth infections, including schistosomiasis. IgG4 and IgE influence susceptibility or protection against helminth infections. Comparing total IgE levels in maternal and cord blood plasma between Gabon and Germany yielded significant differences with strongly elevated levels in Gabonese samples which were further increased in the group of mothers infected with S. haematobium (Fig. 4a,b). Interestingly and again irrespective of the maternal infection status we detected IgE in 51 of 53 Gabonese cord blood samples but not in any German cord blood samples (Fig. 4a,b). AWA specific IgE levels were increased in maternal but not fetal plasma from infected Gabonese mothers (Fig. 4d). The ratio of total maternal IgE/IgG4 was significantly higher in Gabonese samples than in German samples (Fig. 4c).
Figure 4.
Maternal and fetal plasma IgE levels and the IgE/G ratio in Gabon and Germany. IgE levels were measured in plasma via ELISA. All data are shown with median and interquartile range. P values are for Mann-Whitney U-tests. Comparison between the two maternal, or cord blood groups, respectively. P value: *< 0,05; **< 0,01; ***< 0,001; ****< 0,0001; n (maternal, Gabon) = 54; n (maternal, Germany) = 47; n (cord, Gabon) = 53; n (cord, Germany) = 47; n (Gabon, maternal, uninfected) = 42; n (Gabon, maternal, infected) = 12 n (Gabon, cord, uninfected) = 42; n (Gabon, cord, infected) = 12; (a) Total IgE levels in all German maternal all Gabonese plasma samples (b) Total IgE levels in Gabonese plasma samples divided into infected and uninfected group. (c) IgE/IgG4 ratio in maternal plasma samples. (d) AWA specific IgE levels in Gabonese plasma samples divided into infected and uninfected group.
Discussion
In utero exposure to helminths and other environmental factors has recently been shown to alter placental gene expression36 and to influence the offspring’s propensity to develop allergies later in life14,15. Previous investigations of gene expression within the placental tissue have rarely considered potential expression differences between the fetal and maternal side of the placenta or possible differences in environmental exposure37,38. Indeed, Hsd3b1, the only isoform expressed in the placenta, and required for the biosynthesis of progesterone as well as for the maintenance of pregnancy and fetal growth17,39, was expressed to a significantly lower level within the fetal side of Gabonese placentas, and even lower within the sub-group from S. haematobium infected mothers. This might be connected to the higher number of previous pregnancy losses as well as lower birth weight and size of the children in this cohort. Furthermore, progesterone functions as an anti-inflammatory hormone and a decreased expression might thereby contribute to placental inflammation. In addition, the reduced expression of the Vitamin-D related genes VDR and Cyp27b1 on the maternal side of the Gabonese placenta samples alongside the overall differences in gene expression levels between the maternal and fetal side are indicative for independent regulation across these distinct tissue niches. Due to VDR’s important role as transcription factor and its presence in almost all kinds of immune cells, the reduction of VDR and Cyp27b1 as well as Foxp3 and IL10 expression in the Gabonese placentas could indicate that Gabonese mothers are more prone to host an inflammatory immune milieu in the placenta. The proinflammatory cytokine IFN-γ is present mainly in the early placenta and gestational endometrium and at later stages the trophoblasts dampen IFN-γ signalling strongly40 to avoid rejection of the fetus and possibly strong infection-mediated inflammation. No differences in placental IFNG gene expression, as one hallmark of inflammation, was found between both groups, although reports indicate that trophoblast cells only respond selectively to IFN-γ40 so rather pregnancy and related hormones than maternal infection influence its expression. In this study, elevated CRP levels in most women of the Gabonese cohort hinted towards low-grade inflammation. Except for Hsd3b1, infection with S. haematobium did not have an influence on Vitamin D pathway associated (VDR, Cyp27b1) or immunological (IL10, IFNG, Foxp3) genes as well as vitamin D plasma levels. Vitamin D has recently been shown to induce the antimicrobial peptide LL-37 which belongs to the cathelicidin family41–44 and has a potent antimicrobial activity against a wide assortment of bacterial, mycobacterial, protozoal, fungal and viral pathogens. Yet, until now, nothing is known about its role in helminthic diseases such as schistosomiasis. Within this study however, the schistosomiasis-infected group was too small to allow assessment of this parameter but will be included in future studies. In conclusion, environmental and perhaps genetic factors might suppress anti-inflammatory signals and alter the regulation and activation of vitamin D and the regulation of tolerogenic and anti-inflammatory markers.
Helminths provoke a subtle inflammation in their hosts and cause anaemia, also known as anaemia of infection or of chronic disease45–48. The main cause is proinflammatory cytokines such as TNF-α or IL-66. The women from Gabon, which is a highly endemic area for schistosomiasis49, indeed suffered from anaemia in contrast to the European women even though lower haemoglobin concentrations during pregnancy are physiologic (cut-off for pregnant women: haemoglobin <11 g/dL). This confirmed findings from another study with 998 pregnant women in Gabon, where 49.9% of the participants were anaemic7. Anaemia however is most likely to have multifactorial causes in this geographical region which besides schistosomiasis and pregnancy itself includes malaria, soil transmitted helminths and nutritional deficiencies such as iron deficiency.
Placental inflammation and pro-inflammatory cytokines might also contribute to fetal hypoxia6,45 and adverse birth outcomes, such as low birth weight7. Indeed, neonates in Gabon were significantly smaller (minus 3 cm) and lighter (minus 255 g) than those born in Germany. In addition, young maternal age has been shown to be associated to low birth weight, too50 and mothers in Gabon are younger than the Germans, but this might be an additional factor beside geographical difference.
Another consequence of placental inflammation is disorder of the placental barrier with a disruption of the active Fc receptor mediated antibody transfer. Cord blood IgG4 compared to maternal levels was significantly higher in Germany, whereas Gabonese cord and maternal IgG4 levels did not differ from each other. As this isotype represents a maternally-derived antibody, these results indicate disturbances to the processes involved in the transfer from maternal circulation in Gabon. Because of the small number of schistosome infected mothers (23%, n = 13) in our study, the noted differences are more likely to be influenced by other factors than acute schistosomiasis. Six Gabonese women (11%) stated to have had malaria during pregnancy whereas at time of delivery only one Gabonese mother had Plasmodium falciparum in her thick blood smear and was thus excluded from this study. Malaria could contribute to disturbed placental transfer taking into consideration that infections may be asymptomatic in high endemic areas for malaria and infections therefore obscure49.
Overall levels of IgG4 were significantly lower in Gabonese plasma, although the infected sub-group showed a trend towards higher IgG4. AWA-specific IgG4 levels were significantly higher in cord and maternal blood of the schistosome-infected group as expected in a population endemic for helminth infections since there exists substantial cross reactivity between helminth antigens32. IgG4 predominantly supports an immune-tolerant environment for helminths by counteracting helminth-specific IgE responses. High IgE levels are associated with resistance to infection33, whereas high levels of IgG4 have been associated with susceptibility34. In our study population total IgE levels were significantly higher in the Gabonese cohort and within this cohort also higher in the maternal plasma samples of the infected group. The high levels in some maternal German plasma samples were correlated to their history of allergy since severe autoimmune diseases were part of the exclusion criteria and therefore excluded. Regarding interactions between IgE and IgG4, a high IgE/IgG4 ratio correlates with protection against helminth infections. Indeed, in Gabon the maternal total IgE/IgG4 ratio is significantly higher than in Germany and lifelong exposures might induce protection against reinfection.
In contrast to IgG4, IgE cannot cross the placental barrier thus the IgE antibodies detected in cord blood are most likely already produced by the fetus in utero. Unlike the German cohort, about 98% Gabonese cord blood samples had detectable levels of IgE antibodies. In a study conducted in Coast Province, Kenya, cord blood lymphocytes were stimulated in vitro and about 36% of healthy newborns spontaneously produced polyclonal IgE and IgG51. Polyclonal and parasite-specific IgE were equally present in Kenyan cord and maternal sera whilst it remained undetectable in cord blood sera from North American infants52. Likewise, in Gabonese cord blood Schistosoma-specific IgE antibodies were detected previously35. However, in this study, even though levels of AWA-specific IgE were high in maternal blood, none could be detected in the cord blood. An explanation could be adult worm antigens may not cross the placenta in the same quantity as egg-related antigens35. The immune reaction to AWA is less strong than to the eggs and SEA53,54. Worms are able to survive for many years in the human host due to their ability to mask their surface tegument with host antigens55 and the excretion of an only small amount of antigenic molecules.
In summary, our results indicate that pregnant women in Gabon might have a higher risk for placental inflammation, with a lower anti-inflammatory milieu when compared to German women. This is associated with reduced transplacental IgG4 transfer whereby the detection of IgE in offspring could be a hint for early priming events such as the de novo antibody class-switching due to in utero exposure to (helminthic) antigens. Furthermore, as one of the first studies to compare inflammatory genes between geographically distinct populations, including subgroups based on helminth infection, as well as localized gene expression to placental and fetal placental subregions, we provide an initial setup for further investigation into how infection status can modify the complex crosstalk between placental inflammatory responses and healthy fetal development.
Limitations
The neonates in Gabon and Germany were examined by different midwives. Except for Vitamin D, Ca++ and CRP levels, maternal hemogram was done in two different laboratories for each cohort and helminth diagnostic only for the Gabonese cohort, whereby we attempted to reduce the probability of infection to a maximum by using a questionnaire in the German group. Only 30 Gabonese women provided stool samples to investigate for geo-helminth infections.
The placenta samples were not washed, and gene expression analysis might be influenced by the presence of blood cells.
Materials and Methods
Study population
The study was approved by the Institutional ethic committee of CERMEL in BP 242 Lambaréné, Gabon, (CEI-007/2017) and the Ethikkommission der Fakultät für Medizin der Technischen Universität München, Ismaninger Straße 22, 81675 München, Munich, Germany, (22.11.2013, project number 385/13). It was financed by Deutsche Forschungsgemeinschaft DFG (CO 1469/14–1) and Deutsches Zentrum für Infektionsforschung (DZIF) e. V. (TI 07.003). The methods were carried out in accordance with the relevant guidelines and regulations. The study took place at two sites. First between June and August 2015 and Mai and August 2017 at the Klinikum rechts der Isar, Munich, Germany, where 57 pregnant women were screened and samples of 47 participants as European non-endemic control group could be collected and be included for analysis. All mothers were Caucasians. As the samples were all collected in Germany, we call them “German samples”. The second population consisted of 59 women living in the province of Moyen-Ogooue, Gabon, in central Africa and delivering their newborns between August and November 2017 either at the Albert Schweitzer Hospital or the Centre Hospitalier Rérgional Georges Rawiri (CHRGR) in Lambaréné. 54 women and their newborn fulfilled inclusion criteria. We call these samples “Gabonese samples”. The purpose of the study and the procedures involved were explained and only those mothers granting written informed consent were enrolled as participants. To participate, all adolescents were at least 18 years old, had ≥ 37 weeks of gestation, and were giving spontaneous vaginal birth. Caesarean section, as well as acute severe infections such as malaria or knowing of other chronic diseases such as hepatitis and HIV at time of delivery were exclusion criteria.
Sample collection
Paired umbilical cord and maternal peripheral venous blood samples were collected at delivery. To avoid admixture of maternal and cord blood, cord blood was obtained not by squeezing the umbilical cord but instead by direct needle aspiration, taking care to clean the cord of maternal blood beforehand. All blood samples were collected in 9 ml NH4-Heparin (BD Vacutainer S-Monovette®) tubes for further peripheral blood mononuclear cell- (PBMC) and cord blood mononuclear cell- (CBMC) processing and for plasma separation. Plasma (2,5 mL) was separated by centrifugation and frozen at – 20 °C. Moreover, for the Gabonese cohort, EDTA blood for parasitology diagnostic and for clinical analyses was taken at the same time point and immediately analysed for the presence of protozoan blood parasites.
Placenta tissue from both, maternal and fetal side, was collected. For the maternal side we took about 0,5 cm3 directly from a macroscopic not calcified region in the middle of the placenta. For the fetal samples, we prepared the chorionic plate away to take a 0,5 cm3 big sample from the intervillous space. The tissue was put into labelled cryotubes containing 700 μl RNAlater® solution to stabilize placental RNA. The samples were stored at 4 °C for 24 to 48 hours and then transferred to −20° for long time storage and shipment on dry ice to Munich, Germany.
Parasitological examination
These were performed in Gabonese specimens only. Thick blood smears of maternal peripheral blood were stained with Giemsa and examined microscopically for plasmodia and filaria. The mothers provided at least one urine sample for S. haematobium egg-count by microscopy; where possible the samples were frozen at −20 °C for shipment and further analysis. Furthermore, levels of schistosome circulating antigen (circulating anodic antigen [CAA]) were measured in cord and maternal peripheral plasma as well as in urine utilizing an immunochromatography based assay56.This lateral flow (LF) test applies luminescent upconverting particles (UCP) to quantitatively measure CAA levels with a 10 pg/mL lower limit threshold, analysing respectively 20 or 10 μL plasma or urine57. A total number of 13 Gabonese women were positive for S. haematobium by egg count and/or CAA in plasma (Supplemental Table 3). CAA was only detected in maternal plasma samples but not in cord blood.
30 women provided stool samples, which were prepared with standard methods for microscopic examination to determine the presence of intestinal helminth infections (Kato Katz, copro culture, McMaster, etc). Sample collection for parasitological examination was only performed at the time of delivery.
Biochemical analysis
The concentrations of 25(OH)D using the Diasorin assay (Diasorin, Stillwater, MN, USA), as well as CRP and Calcium levels in all plasma samples were measured at the Institute for Clinical Chemistry and Pathobiochemistry at the Klinikum rechts der Isar of the Technical University of Munich according to the manufacturer’s specifications.
Quantitative real-time Polymerase Chain Reaction (qRT-PCR)
Thawed fetal and maternal placenta samples were homogenized (using Precellys®24 tissue homogenizer) and RNA was isolated from using GenElute™ Mammalian Total RNA Miniprep Kit, according to manufacturer’s instructions. RNA yield and purity were measured using a NanoDrop® 1000 Spectrophotometer. The resulting RNA was stored at −80 °C until further processing. cDNA was prepared following the Promega usage information for first-strand cDNA synthesis with M-MLV RT (H-) Point Mutant and the resulting cDNA was stored at −20 °C until further processing. qRT-PCR was performed to measure the relative concentrations of certain genes, using Roche Universal Probe Library and HPRT as house-keeping gene. The LightCycler® Probes Master (Roche) is a ready-to-use reaction mix containing the Taq Polymerase and appropriate buffers. Gene sequences and probes are shown in Supplemental Table 2. The following qRT-PCR was performed in a Thermocycler C 1000 (Bio-Rad).
Enzyme-linked immunosorbent assay (ELISA) for detection of total and AWA specific IgE and IgG4 levels
Plasma samples were transported on dry ice to Munich, Germany, for quantification of total IgE and IgG4 and to Leiden, the Netherlands, for AWA specific IgE and IgG4. Levels of total IgE and IgG4 were measured in all European and African maternal and cord plasma samples using the Invitrogen IgE, or IgG4 Human ELISA Kit, respectively, according to manufacturer’s instructions (Thermo Fischer). The plasma was 1:10 diluted for IgE measurement and 1:1000 for IgG4. Acquisition of the multiplex immunoassay was performed by using the LSRII flow cytometer (Becton Dickinson). S. haematobium adult worm antigen (AWA) specific IgG4 and IgE were measured by ELISA modified from previous protocols58. Briefly, maxisorp plates (Nunc, Roskilde, Denmark) were coated overnight with 5 mg/ml AWA diluted in carbonate buffer pH 9.6. For IgG4, the plates were blocked with PBS-5%BSA while for IgE with PBS-2%BSA. The plasma was diluted in PBS-0.05%Tween-5% FCS (Fetal calf serum) or Tris-HCl–0.05% Tween for IgG4 and IgE ELISA, respectively. The presence of IgG4 was shown by using HRP-labelled anti human IgG4 (1:3000) (Sanquin, Amsterdam, the Netherlands). For IgE assay, the detection antibodies used were biotinylated goat anti human IgE (1:1000; Vector Laboratories, Burlingame, CA, USA) followed by streptavidin HRP conjugate (1:10000; Sanquin, Amsterdam, the Netherlands). The assays were developed with tetramethylbenzidine (TMB) and stopped with 10% H2SO4. Absorbances were measured at 450 nm. The levels of antibody present in a given sample are expressed in arbitrary units (AU/ml) according to the standard curve of pooled positive S. haematobium plasma.
Statistical analysis
Statistical analysis was performed by using SPSS for Windows version 23.0 (SPSS Inc., Chicago, IL) and PRISM® 5.01 (GraphPad Software Inc., San Diego, CA, USA). D’Agostino and Pearson omnibus normality tests were performed, and parametrically distributed data was analysed with unpaired T test (2 groups) and two-tailed Mann-Whitney-Test was used for non-parametric data. Statistical dependence was analysed by Spearman’s rank correlation coefficient. Categorical data were analysed by Pearson chi-squared test. Data are presented as median with interquartile range (IQR) or mean with standard deviation (SD).
In all cases, statistical significance was assumed with a p value < 0.05 and where significance level was reached, p values are indicated in individual graphs.
Supplementary information
Acknowledgements
We are grateful to all pregnant women who participated in the study. We thank all CERMEL personnel for their exceptional assistance, and we are indebted to the midwives at the General Hospital and those at HAS, as well as the co-workers at CERMEL. Claudia de Dood (LUMC, Cell and Chemical Biology department) is acknowledged for preparing the UCP-LF CAA test materials and performing the CAA assays on the plasma and urine samples. This work was supported and funded by DZIF TI 07.003 and DFG CO 1469/8-1.
Author contributions
C.P.d.C. initiated the study and together with A.A.A., M.E., S.M.L. and Y.J.H. generated the study protocol. Samples were collected by E.L. and J.H. and were analysed by E.L. G.J.D. and P.L.A.M.C. performed C.A.A. diagnostics and E.S. A.W.A.-specific Elisa. C.P.d.C. and M.L. helped with the development of the project and O.P.N. and M.L. with the discussion of the data. C.P.d.C. and E.L. interpreted the data, designed the figures and drafted the manuscript. Overall supervision was done by C.P.d.C. All authors revised the manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/28/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Supplementary information
is available for this paper at 10.1038/s41598-019-52074-z.
References
- 1.Feary J, Britton J, Leonardi-Bee J. Atopy and current intestinal parasite infection: a systematic review and meta-analysis. Allergy. 2011;66:569–578. doi: 10.1111/j.1398-9995.2010.02512.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood BM. Autoimmune disease and parasitic infections in Nigerians. Lancet. 1968;2:380–382. doi: 10.1016/S0140-6736(68)90595-3. [DOI] [PubMed] [Google Scholar]
- 3.Mutapi F, et al. Schistosome infection intensity is inversely related to auto-reactive antibody levels. PLoS One. 2011;6:e19149. doi: 10.1371/journal.pone.0019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Biggelaar AH, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 5.Combat, U. t. Bilharzia, https://unitingtocombatntds.org/ntds/schistosomiasis/ (2017).
- 6.Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23:159–164. doi: 10.1016/j.pt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Mombo-Ngoma G, et al. Urogenital schistosomiasis during pregnancy is associated with low birth weight delivery: analysis of a prospective cohort of pregnant women and their offspring in Gabon. Int J Parasitol. 2017;47:69–74. doi: 10.1016/j.ijpara.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell AD. Helminth infection during pregnancy: insights from evolutionary ecology. Int J Womens Health. 2016;8:651–661. doi: 10.2147/IJWH.S103529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Mpairwe H, Tweyongyere R, Elliott A. Pregnancy and helminth infections. Parasite Immunol. 2014;36:328–337. doi: 10.1111/pim.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King CL, et al. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maizels RM, Lawrence RA. Immunological tolerance: The key feature in human filariasis? Parasitol Today. 1991;7:271–276. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 13.Nutman TB, Kumaraswami V, Ottesen EA. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacorcia M, Prazeres da Costa CU. Maternal Schistosomiasis: Immunomodulatory Effects With Lasting Impact on Allergy and Vaccine Responses. Front Immunol. 2018;9:2960. doi: 10.3389/fimmu.2018.02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straubinger K, et al. Maternal immune response to helminth infection during pregnancy determines offspring susceptibility to allergic airway inflammation. J Allergy Clin Immunol. 2014;134:1271–1279 e1210. doi: 10.1016/j.jaci.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Suguitan AL, Jr., et al. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg. 2003;69:574–581. doi: 10.4269/ajtmh.2003.69.574. [DOI] [PubMed] [Google Scholar]
- 17.Ntostis P, et al. Genetic variation in the HSD3B1 gene and recurrent spontaneous abortions. J Matern Fetal Neonatal Med. 2012;25:408–410. doi: 10.3109/14767058.2011.582199. [DOI] [PubMed] [Google Scholar]
- 18.Delvin EE, Gagnon L, Arabian A, Gibb W. Influence of calcitriol on prolactin and prostaglandin production by human decidua. Mol Cell Endocrinol. 1990;71:177–183. doi: 10.1016/0303-7207(90)90023-2. [DOI] [PubMed] [Google Scholar]
- 19.Zehnder D, et al. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–114. doi: 10.1016/S0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu NQ, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186:5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe K, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77:676–682. doi: 10.4269/ajtmh.2007.77.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 24.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 25.Faas MM, De Vos P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta. 2018;69:125–133. doi: 10.1016/j.placenta.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54:507–523. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtis JD, et al. Maternal Schistosomiasis japonica is associated with maternal, placental, and fetal inflammation. Infect Immun. 2011;79:1254–1261. doi: 10.1128/IAI.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bittencourt AL, Cardoso de Almeida MA, Iunes MA, Casulari da Motta LD. Placental involvement in schistosomiasis mansoni. Report of four cases. Am J Trop Med Hyg. 1980;29:571–575. doi: 10.4269/ajtmh.1980.29.571. [DOI] [PubMed] [Google Scholar]
- 29.Youssef AF, Abdine FH. Bilharziasis of the pregnant uterus. J Obstet Gynaecol Br Emp. 1958;65:991–993. doi: 10.1111/j.1471-0528.1958.tb08594.x. [DOI] [PubMed] [Google Scholar]
- 30.McLean ARD, et al. P. falciparum infection and maternofetal antibody transfer in malaria-endemic settings of varying transmission. PLoS One. 2017;12:e0186577. doi: 10.1371/journal.pone.0186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 33.Turner JD, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–996. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Figueiredo JP, et al. Adult worm-specific IgE/IgG4 balance is associated with low infection levels of Schistosoma mansoni in an endemic area. Parasite Immunol. 2012;34:604–610. doi: 10.1111/pim.12001. [DOI] [PubMed] [Google Scholar]
- 35.Seydel LS, et al. Association of in utero sensitization to Schistosoma haematobium with enhanced cord blood IgE and increased frequencies of CD5- B cells in African newborns. Am J Trop Med Hyg. 2012;86:613–619. doi: 10.4269/ajtmh.2012.11-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangwiro YTM, et al. Exercise initiated during pregnancy in rats born growth restricted alters placental mTOR and nutrient transporter expression. J Physiol. 2019;597:1905–1918. doi: 10.1113/JP277227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 38.Sferruzzi-Perri AN, Camm EJ. The Programming Power of the Placenta. Front Physiol. 2016;7:33. doi: 10.3389/fphys.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simard J, et al. Molecular biology and genetics of the 3 beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. J Endocrinol. 1996;150(Suppl):S189–207. [PubMed] [Google Scholar]
- 40.Murphy SP, et al. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tangpricha V, et al. LL-37 concentrations and the relationship to vitamin D, immune status, and inflammation in HIV-infected children and young adults. AIDS Res Hum Retroviruses. 2014;30:670–676. doi: 10.1089/AID.2013.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rekha RS, et al. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–1699. doi: 10.1080/15548627.2015.1075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira Junior LR, et al. Association of vitamin D3, VDR gene polymorphisms, and LL-37 with a clinical form of Chagas Disease. Rev Soc Bras Med Trop. 2019;52:e20190133. doi: 10.1590/0037-8682-0133-2019. [DOI] [PubMed] [Google Scholar]
- 44.Honda JR, Connick E, MaWhinney S, Chan ED, Flores SC. Plasma LL-37 correlates with vitamin D and is reduced in human immunodeficiency virus-1 infected individuals not receiving antiretroviral therapy. J Med Microbiol. 2014;63:997–1003. doi: 10.1099/jmm.0.070888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Leenstra T, et al. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGarvey ST, et al. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. Am J Trop Med Hyg. 1996;54:498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 48.Friedman JF, Olveda RM, Mirochnick MH, Bustinduy AL, Elliott AM. Praziquantel for the treatment of schistosomiasis during human pregnancy. Bull World Health Organ. 2018;96:59–65. doi: 10.2471/BLT.17.198879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adegnika AA, et al. Epidemiology of parasitic co-infections during pregnancy in Lambarene, Gabon. Trop Med Int Health. 2010;15:1204–1209. doi: 10.1111/j.1365-3156.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 50.Kurth F, et al. Adolescence as risk factor for adverse pregnancy outcome in Central Africa–a cross-sectional study. PLoS One. 2010;5:e14367. doi: 10.1371/journal.pone.0014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King CL, et al. B cell sensitization to helminthic infection develops in utero in humans. J Immunol. 1998;160:3578–3584. [PubMed] [Google Scholar]
- 52.Malhotra I, et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest. 1997;99:1759–1766. doi: 10.1172/JCI119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Ridi R, Ismail S, Gaafar T, El Demellawy M. Differential responsiveness of humans with early-stage schistosomiasis haematobium to Schistosoma haematobium soluble adult-worm and egg antigens. Parasitol Res. 1997;83:471–477. doi: 10.1007/s004360050282. [DOI] [PubMed] [Google Scholar]
- 54.Hillyer GV, Nieves-Frau LL, Vazquez G. Identification of a genus-specific Schistosoma mansoni soluble egg antigen reactive with the serum of infected patients. Am J Trop Med Hyg. 1986;35:1198–1204. doi: 10.4269/ajtmh.1986.35.1198. [DOI] [PubMed] [Google Scholar]
- 55.Saunders N, Wilson RA, Coulson PS. The outer bilayer of the adult schistosome tegument surface has a low turnover rate in vitro and in vivo. Mol Biochem Parasitol. 1987;25:123–131. doi: 10.1016/0166-6851(87)90001-6. [DOI] [PubMed] [Google Scholar]
- 56.Corstjens PL, et al. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. Journal of clinical microbiology. 2008;46:171–176. doi: 10.1128/jcm.00877-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corstjens PL, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141:1841–1855. doi: 10.1017/s0031182014000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Biggelaar AH, Borrmann S, Kremsner P, Yazdanbakhsh M. Immune responses induced by repeated treatment do not result in protective immunity to Schistosoma haematobium: interleukin (IL)-5 and IL-10 responses. J Infect Dis. 2002;186:1474–1482. doi: 10.1086/344352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.