Abstract
Purpose
Almost every female classic galactosemia patient develops primary ovarian insufficiency (POI). The unique pathophysiology of classic galactosemia, with a severely reduced follicle pool at an early age, requires a new therapeutic approach. This study evaluated the effect of dehydroepiandrosterone (DHEA) on ovarian tissue in a galactose-induced POI rat model.
Methods
Pregnant rats were fed with either a normal or a 35% galactose-containing diet from day 3 of conception continuing through weaning of the litters. Galactose-exposed female offspring were further divided into 5 groups on PND21. The first group received no application. Treatment groups were fed orally by gavage once daily with sesame oil (group 2), or DHEA at doses of 0.1 mg/kg (group 3), 1 mg/kg (group 4) or 10 mg/kg (group 5) until PND70. Fertility rates of mothers with galactosemia, body weights (BWs), and ovarian weights of the litters from PND21 to PND70 were recorded. Ovarian follicle count, immunohistochemistry for proliferation and apoptosis marker expressions and TUNEL for cell death assessment were performed in offspring ovaries.
Results
Decreased fertility, ovarian/body weights were observed under galactosemic conditions, together with decreased follicle number and increased atresia. Improved postnatal development, primordial follicle recruitment and follicular growth were observed after DHEA treatment. After DHEA treatment, the expression of Ki67 protein was found to be increased; elevated expression of cleaved-caspase-3 under galactosemia was found to be reduced.
Conclusions
Our data suggests that DHEA treatment may be a potentially useful clinical therapy to improve ovarian ageing in women with POI-induced by galactosemia.
Keywords: Rat, Galactosemia, Primary ovarian insufficiency, DHEA
Introduction
Premature activation of ovarian follicles leads to early depletion of the ovarian reserve, known as primary ovarian insufficiency (POI), a non-reversible pathology leading to female infertility [1]. POI is most commonly associated with X chromosomal abnormalities [2]. However, various autoimmune and metabolic diseases such as adrenal insufficiency, autoimmune hypothyroidism, type 1 diabetes mellitus, pernicious anaemia and galactosemia have also been linked with POI [1, 3, 4].
Classical galactosemia is a hereditary disorder caused by a deficiency of one of the main enzymes of the major galactose assimilation pathway, galactose-1-phosphate uridyltransferase (GALT) [5]. The impairment of ovarian function in classical galactosemia has been known for over 25 years [6]. In galactosemic patients, direct toxic damage to the ovary can occur through the accumulation of either galactose and/or its metabolites, leading to ovarian cell apoptosis [7]. Additionally, there are some data regarding the toxic effect on the ovary using animal models. Chen at al. (1981) reported that when pregnant rats were fed with 50% galactose diet, there was a remarkable reduction in oocyte number in the offspring [8]. Swartz and Mattison (1988) showed signs of ovarian damage, such as a decrease in normal ovulatory response, increase in the interstitial tissue and the failure to respond to exogenous gonadotrophins after feeding adult female mice with a diet consisting of 50% galactose [9]. Another experimental galactosemia rat model confirming POI was reported by Bandyopadhyay et al. (2003) in which pregnant rats were fed with 35% galactose. Here, galactose toxicity led to delayed onset of puberty, and follicle depletion as a result of early follicular activation in female offspring [10].
Dehydroepiandrosterone (DHEA) is a ubiquitous steroid produced by the zona reticularis layers of the adrenal cortex and theca cells of the ovary. DHEA is produced by the conversion of cholesterol and is primarily important for the formation of androstenedione (A), testosterone (T) and estradiol (E2), the main androgens in women [11]. Because DHEA is known to be the first step for the production of A, T and E2 [12], the decrease in DHEA levels also leads to a decrease in the concentration of these hormones [11]. Therefore, DHEA is the essential prohormone for ovarian follicular steroidogenesis [11]. Although DHEA concentration is reported to be quite high during the reproductive years in women, it decreases with increased age leading to speculation that DHEA supplementation may improve follicular microenvironment and thus support fertility [13]. Indeed, a number of studies performed both in animal models and humans have shown that DHEA improves ovarian function in poor responders, reduces the atretic effect and increases the number of active oocytes [14, 15]. It was first described by Casson et al. that therapeutic benefits of DHEA in women with diminished ovarian reserve whose peak estradiol level tripled and their response to stimulation increased by two-folds after oral supplementation with DHEA [12, 15], and subsequently, many others reported the same outcome [13, 16–18]. Although the mechanism by which DHEA exerts its effects is still uncertain, it is suggesting that the DHEA may have a role in increasing serum concentrations of IGF-1, which in turn may improve the response to gonadotropins [12, 15, 19].
Much investment has been placed into treating fertility in POI. For example, hormonal therapies such as oestrogen pre-treatment and exogenous gonadotropin therapy have been tested as a potential strategy to improve ovulatory rates in women with POI, but no significant benefits have been obtained [20]. Recent experimental and clinical reports, however, have shown positive effects of DHEA supplementation on improving the ovarian response [15, 18, 21]. Having these previous findings in mind, we aimed to evaluate the potential role of DHEA on ovarian folliculogenesis and fertility outcomes in a galactose-induced POI rat model. For this purpose, galactosemic POI-induced females were treated with three different dosages of DHEA supplementation from postnatal day (PND) 21 to PND70. Our results delineate that while galactose toxicity leads loss of proliferation and premature depletion of the follicular reserve by triggering cell death in ovarian follicles, DHEA administration ameliorates these effects by stimulating primordial follicle recruitment and follicular growth. Here we provide new insights towards understanding the pathophysiological mechanisms of galactosemic gonadal dysfunction and its potential therapy.
Material and methods
Animals, food and DHEA administration
Sprague-Dawley rats were maintained under a 12-h light-dark cycle in the Experimental Animal Care and Production Unit of the Akdeniz University School of Medicine. Adult female rats were mated with proven fertile males of the same strain. The presence of sperm in the vaginal lavage was assigned as day 1 of gestation.
Female rats were fed with standard food pellets (carbohydrates, 65.5%; protein, 21%; fat, 5.5%; mineral mixture, 7%; and vitamin mixture, 1%) supplemented with 35% galactose (Sigma-Aldrich) (N = 60) or without galactose (control; N = 20), from day 3 of conception and continuing through weaning of the litters on PND21 (PND0; being the day of birth), as previously shown [10]. On PND21, the galactosemic female litters were separated into five further groups and maintained separately until PND70. No application was made to the first group to determine if there was a spontaneous recovery (group 1; gal-exposed, N = 10). For the treatment groups, female litters were dosed orally by gavage once daily with vehicle alone (sesame oil; group 2; N = 10) or with DHEA (Biosteron) suspended in the vehicle at doses of 0.1 mg/kg per day (low-dose group 3; N = 10), 1 mg/kg per day (mild-dose group 4; N = 10) or 10 mg/kg per day (high-dose group 5; N = 10). Individual dose volumes were calculated and adjusted weekly according to the most recent body weights. Doses were selected according to the results obtained in previous rat studies performed using DHEA [17, 22]. All experimental protocols were approved by the Akdeniz University Institutional Animal Care and Use Committee (Protocol number: 2012.07.02).
Assessment of fertility and litter growth
The number of pups per litter was recorded to determine the reproductive performance of mothers with galactosemia. After birth and lactation, body weights (BWs) of the litters were recorded every day from PND21 to PND70. Ovarian weights of litters were recorded on PND70 at the end of the experiment.
Ovarian follicle count
All ovarian tissue was fixed overnight in Bouin’s fixative and transferred into 70% ethanol until processing. Serial sections of 5 μm were cut for each paraffin-embedded ovarian block. Standard H&E staining was done on sections of all ovaries, then analysed under a bright-field microscope (Carl-Zeiss Inc.). Every fifth section was assessed, the total number of follicles on different stages was determined by counting the follicles containing oocytes with a visible nucleus. Primordial, primary, secondary, early antral and antral follicles were classified as described previously [23]. Briefly, primordial follicles were defined as an oocyte surrounded by a layer of squamous granulosa cells. Primary follicles possessed an oocyte surrounded by a single layer of cuboidal granulosa cells. Secondary follicles were surrounded by two or three layers of cuboidal granulosa cells with no visible antrum. Preantral follicles were surrounded by four or more layers of granulosa cells, forming the follicular antrum. Antral follicles contained a clearly defined single antral space. To determine the follicles undergone atresia, atretic follicles contained certain morphological phenomena such as pyknosis of cell nuclei and degenerated oocyte were counted.
Immunohistochemistry and quantitative analysis of immunohistological staining
Paraffin sections were deparaffinised in fresh xylene, then rehydrated in a series of decreasing ethanol concentrations. Epitope retrieval was done as follows: sections were first boiled at 750 W in 1 mM Tris-EDTA (TE) buffer (pH 9.0) and then warmed at 250 W for 25 min in the same buffer. Subsequently, endogenous peroxidase activity was quenched by incubating sections in 3% H2O2 prepared in methanol for 25 min at RT. Following several washes with phosphate-buffered saline (PBS), sections were blocked with Ultra V Block (LabVision) at RT for 5 min. Then, they were incubated overnight at 4 °C with primary antibodies specific for Ki-67 (1:500) (Thermo), cleaved caspase-3 (1:100) (Cell Signaling), or FOXO3a (1:50) (Millipore). Negative controls were performed by replacing the primary antibodies with normal rabbit serum at the same concentration. After several rinses in PBS, biotinylated goat anti-rabbit IgG (1/400) (Vector Lab) was applied for 1 h at RT. Following several PBS rinses, the slides were incubated with streptavidin–peroxidase complex for 20 min using a Dako LSAB kit (Dako). The antibody complex was visualised by incubation with diaminobenzidine (DAB) chromogen (BioGenex) prepared according to the manufacturer’s instructions. Slides were counterstained with Mayer’s haematoxylin (Dako) prior to permanent mounting and then evaluated under a bright-field microscope (Carl Zeiss Inc.). Quantitative analysis of immunohistological staining for each protein was performed by using Image J Fiji software with IHC image analysis toolbox and 3D object counter.
Terminal deoxynucleotidyl transferase deoxy-UTP-nick end labelling assay
Terminal deoxynucleotidyl transferase deoxy-UTP-nick end labelling (TUNEL) immunostaining of ovary sections from each group was performed using the In Situ Cell Death Detection kit from Roche (Roche, Basel, Switzerland), according to the manufacturer’s instructions. Briefly, tissue sections were washed twice in PBS for 5 min, incubated in permeabilisation solution (0.1% Triton X-100 in 0.1% sodium citrate) for 8 min at 4 °C and washed twice with PBS for 5 min. Samples were labelled using TUNEL-reagent and incubated for 1 h at 37 °C. Negative controls contained reagent without enzyme. Lastly, slides were mounted with mounting medium for fluorescence with DAPI (Vector Lab.) and examined using an Olympus BX61 fluorescence microscope. Quantitative analysis of TUNEL positive signals was performed by using Image J Fiji software with cell counter plugin.
Statistical analysis
All results analysed by one-way ANOVA (analysis of variance) test followed by a Tukey’s multiple comparisons test (Fig. 1d, Fig. 2A′–C′, Fig. 3, Fig. 4D, F). For comparison between two groups, Student’s t test was used (Fig. 1A, B, Fig. 4A, D). All error bars represent standard error mean (± SEM) values. P value < 0.05 was considered significant. Statistical calculations were performed using GraphPad Prism 8 software.
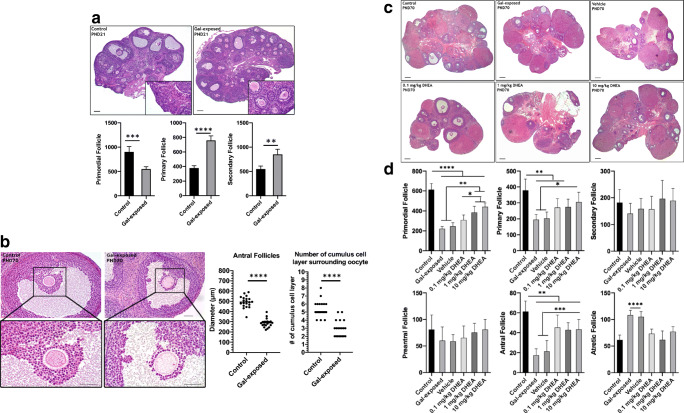
Fig. 1.
Histomorphometric evaluation of ovaries. A Representative low-magnification micrographs of control and gal-exposed ovaries on PND21. Below graphs show follicular count of ovaries from control and gal-exposed groups on PND21. The number of primordial follicles was significantly lower; primary, and secondary follicles was significantly higher in gal-exposed ovaries. Student t test, **P < 0.01; ***P < 0.001. (N = 4 per groups). B Representative high-magnification micrographs of antral follicles (top) and COCs (bottom) on PND70. Smaller antral follicles and fewer granulosa cell layers were observed in gal-exposed ovaries. Graphs show the measurement of antral follicles in size (largest cross-sectional diameter, left) and count data for number of cumulus cell layers surrounding oocytes within these antral follicles in control and gal-exposed groups on PND70 (right). Student t test, ***P < 0.001. (N = 4 ovaries, 5 antral follicles per groups). C Representative low-magnification micrographs of ovaries from all groups on PND70. D Follicular count of ovaries from control, gal-exposed, vehicle and DHEA-treated groups on PND70. ANOVA, *P < 0.05; **P < 0.01; **P < 0.001. (N = 8 per groups). All scale bars = 50 μm
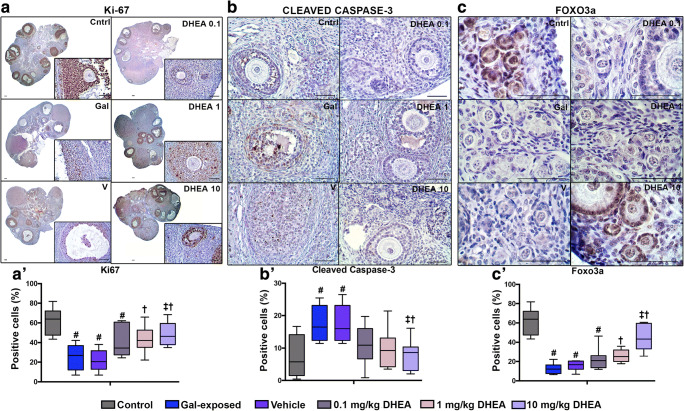
Fig. 2.
The expression of Ki-67, cleaved caspase-3 and FOXO3a proteins in PND70 ovaries. Whereas Ki-67 (A, A’) and FOXO3a (C, C′) expressions are lost and the expression of cleaved caspase-3 (B, B′) increased in gal-exposed ovaries; in the DHEA-supplemented rat ovaries, expression of cleaved caspase-3 decreases and expression of Ki-67 and FOXO3a increases (N = 6 per group). # indicates significant difference from control; † indicates significant difference from gal-exposed; ‡ indicates significant difference from the vehicle. ANOVA, P < 0.05. Scale bars = 50 μm
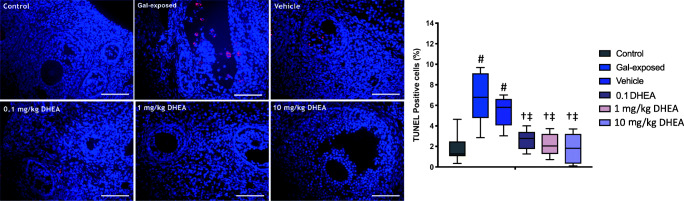
Fig. 3.
Representative images of TUNEL staining in ovarian tissues on PND70. Increased cell death was observed in ovarian follicles after galactose exposure. Strikingly, hardly any TUNEL-positive cells were detected after DHEA treatment. The red signal indicates TUNEL-positive staining, and the blue signal (DAPI) indicates nuclei. (N = 6 per groups). Graph shows quantification of average percentage of TUNEL-positive cells in ovaries per experimental group. # indicates significant difference from control; † indicates significant difference from gal-exposed; ‡ indicates significant difference from the vehicle. ANOVA, P < 0.05. Scale bars = 50 μm
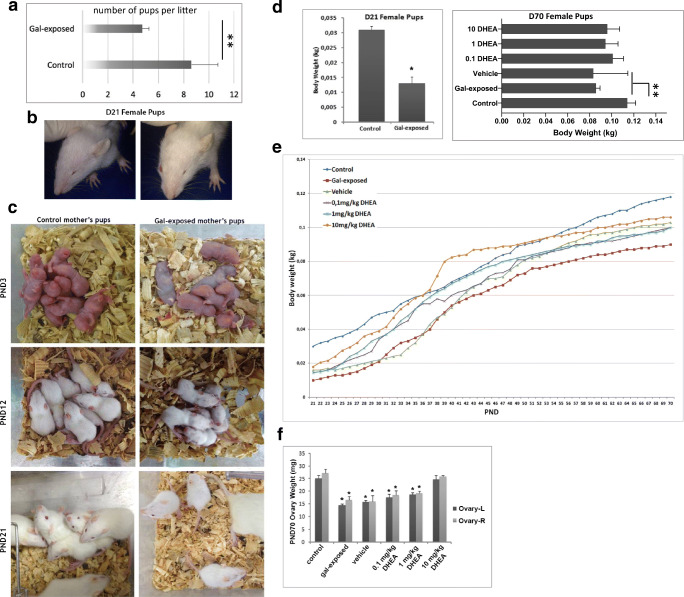
Fig. 4.
Postnatal development in litters. A Fertility outcomes of control (N = 20) and gal-exposed female rats (N = 30). Student t test, **P < 0.01. B A representative female litter with an eye abnormality after gal-exposure on PND21. C The appearance of the litters through weaning. D Body weight of the litters on PND21 (left) (N = 8 per group). Student t test *P < 0.05; PND70 (right) (N = 8 per group) ANOVA **P < 0.01. E Postnatal growth rates of the representative litters from each experimental group from PND21 to PND70. F Ovarian weight of the litters on PND70 (N = 8 per group) ANOVA *P < 0.05
Results
In this study, we first generated galactosemic pregnant rats by feeding with diets containing 35% galactose. Thus, a POI model was created in the offspring by induced galactose toxicity during the intrauterine and lactation periods. Then, to assess the effect of DHEA on the ovary, DHEA administration with three different doses was used in the litters.
Ovarian histomorphology
The evaluation of ovarian histomorphology after gal-exposure and subsequent DHEA treatment yielded several observations. First, on PND21, the control ovary exhibits follicles at different stages of maturity; however, the gal-exposed ovaries showed a significant reduction in primordial follicles and an increase in primary and secondary follicles (Fig. 1a). Second, the diameter of the antral follicles was observed as smaller in gal-exposed ovaries compared with those of controls both on PND21 and PND70 (Fig. 1a, b). This significantly smaller size and appearance suggest the impaired growth and differentiation during follicular development (Fig. 1b). Third, a lower number of the cumulus cells surrounding oocytes was consistently observed in gal-exposed ovaries (Fig. 1b).
It was observed that all type of follicles was present in the ovaries of all groups at PND70 (Fig. 1c), however, the number of primordial, primary, antral and atretic follicles were found significantly different among groups (Fig. 1d). The number of primordial follicles was found to be reduced significantly in gal-exposed ovaries displaying the ovarian reserve depletion (P < 0.05) (Fig. 1d). Additionally, the number of primary follicles and most remarkably antral follicles were reduced; atretic follicles were increased in gal-exposed ovaries (P < 0.05) (Fig. 1d). After DHEA administration, recovery was seen for primordial, primary and antral follicles, whereas secondary and preantral follicles showed no statistical change among groups (Fig. 1d). Importantly, the number of primordial follicles was found to be statistically higher in the ovaries after 1 mg/kg and 10 mg/kg DHEA treatment than in gal-exposed, vehicle and 0.1 mg/kg DHEA groups (P < 0.05) (Fig. 1d). Moreover, the number of antral follicles were counted to be significantly increased, and the number of atretic follicles was found to be significantly decreased in the ovaries after all DHEA treatments (Fig. 1d) (P < 0.05).
Ovarian reserve-related protein expression
Our findings of impaired development in gal-exposed ovaries with the decreased number of primordial and antral follicles directed us to further investigate the expression of molecular markers associated with different biomolecular pathways including proliferation, apoptosis and early follicle activation. To this end, we performed immunohistochemistry on ovaries at PND70.
We first assessed Ki-67 expression to evaluate the proliferation capacity of developing follicles in ovaries. Distinct nuclear expression of Ki-67 protein was found in granulosa cells from primordial to antral follicles of control ovaries (Fig. 2a, a′). A decreased expression for Ki-67 was observed in gal-exposed ovaries compared to those of control, whereas ovaries obtained after DHEA treatment at the doses of 1 mg/kg and 10 mg/kg showed remarkably higher expression of Ki-67 (P < 0.05) (Fig. 2a, a′).
The increased expression of the cleaved caspase-3 protein, a marker of apoptosis, was clearly observed as nuclear in the granulosa cells of primary, secondary, preantral and antral follicles in gal-exposed ovaries, but not in primordial follicles, thecal cells or oocytes (P < 0.05) (Fig. 2b, b′). Importantly, the expression level for cleaved caspase-3 in ovaries obtained after DHEA treatment was found as a similar level of control ovaries (Fig. 2b, b′) suggesting the protective effect of DHEA on follicular cells from apoptosis.
The most striking result was obtained from FOXO3a which is known to be a marker for follicle activation [24, 25]. In the control, FOXO3a was found to be expressed mainly in the nuclei of granulosa cells and oocytes of primordial follicles and early primary follicles (Fig. 2c). However, gal-exposed and vehicle groups, as well as the 0.1 mg/kg and 1 mg/kg DHEA treatment groups, have been found to be largely decreased of the FOXO3a expression which further supports POI (P < 0.05) (Fig. 2c, c′). Importantly, the expression of FOXO3a was clearly seen in ovaries obtained after 10 mg/kg DHEA treatment just as described for control ovaries (P < 0.05) (Fig. 2c, c′). Overall, these observations indicate that DHEA lessens the effect of galactose toxicity on ovaries by acting through the molecular pathways responsible for ovarian reserve preservation. Here we also provide an insight for understanding the molecular mechanisms underlying galactose-induced POI.
Cell death in ovarian follicles
To evaluate cell death in ovarian follicles among groups, TUNEL analysis indicating late-stage apoptosis was performed. The healthy (primordial to antral) follicles were observed as TUNEL-negative, whereas only the atretic follicles were observed as the TUNEL-positive in control ovaries (Fig. 3). However, increased TUNEL-positive expression was detected in granulosa cells in the gal-exposed ovaries (Fig. 3). Importantly, only a few TUNEL-positive follicular cells were found after DHEA treatments (Fig. 3).
Decreased fertility and growth rate in a consequence of galactose toxicity
The effect of galactose exposure on fertility outcomes of mothers was evaluated. We first observed that the average number of pups per litter was found to be significantly lower in galactosemic females (4.7 ± 2.11 pups/litter) than the control females (8.6 ± 0.54 pups/litter) (Fig. 4a) (P < 0.05). A large number of stillborn were obtained from the gal-exposed mothers. Also, a number of females did not give birth at all despite the presence of sperm in the vaginal lavage.
We then assessed in utero effects of galactose on newborns by examining their postnatal development. It is well known that galactosemia leads to eye abnormalities including cataracts [26]. Consistent with previous reports, we also observed that most of the litters from gal-exposed mothers had eye abnormalities (Fig. 4b). When we evaluated the developmental progress of the pups from PND0 to PND21, through weaning, it was observed that pups exposed to high galactose in utero were smaller in body size (Fig. 4c) and had significantly lower body weight (BW) on PND21 (Fig. 4d) than that of control counterparts (P < 0.05). At the end of weaning, the average BW of the gal-exposed pups (32.82 ± 2.11 g) was significantly lower than the control pups on PND21 (61.93 ± 1.03 g) (P < 0.05) (Fig. 4d). Although all the groups exhibited a steady rise in their BW during the treatment process from PND21 to PND70, the rate of growth was lower in gal-exposed pups (Fig. 4e). Additionally, pups treated with DHEA showed increased BW compared to non-treated pups suggesting DHEA treatment enhances growth rate (Fig. 4e). At PND70, the average BW of the gal-exposed and vehicle pups was significantly lower than the control pups, yet the increase on BW after DHEA treatments was not found significant (Fig. 4d). Finally, ovarian weight, both left and right, were found to be statistically lower in gal-exposed groups than in control on PND70 (Fig. 4f). It was observed that ovarian weight is similar to control and higher than gal-exposed only after 10 mg/kg DHEA treatment (Fig. 4f).
Discussion
A large majority of female patients with classic galactosemia have POI, necessitating a viable approach regarding fertility preservation. However, due to the unique and undetermined underlying pathophysiological mechanisms of POI induced by galactosemia, clinical treatments have remained elusive. In galactosemia, the defined mechanism of premature ovarian reserve depletion is not known and is thought to be caused by the toxic effects of galactose and galactose metabolites [27]. In order to obtain a better understanding of the pathophysiology of POI under galactosemic conditions, rodents exposed to high concentrations of galactose have been used as a model for several years [8, 26]. Indeed, it has been reported that prenatal exposure to galactose produces a sequela of abnormalities including POI in the offspring, characteristic of human galactosemia [8]. Consistent with the previous studies, we observed similar abnormalities including early follicular activation and accelerated follicle atresia. Increased proportion of growing follicles within the ovarian tissue at PND21 (short term), reduced number of primordial, growing and antral follicle at PND70 (long term) were observed in offspring after in utero galactose exposure. Therefore, our present study confirms that in utero galactose toxicity induces follicle reserve depletion and generates POI in offspring. Additionally, this study provides further insight towards an understanding of the molecular basis of galactosemic POI by evaluating the ovarian reserve-related protein expressions, including Ki-67, cleaved caspase-3 and Foxo3a, which was not shown in previous studies.
Although there is still some uncertainty in the literature regarding when primordial follicles first appear, it is widely accepted that they are first formed at around 15–22 weeks of gestation in human foetuses, whereas the formation occurs within a few days of birth in rats and mice [28]. Normally, activation of some primordial follicles starts soon after their formation leading to the first wave of antral follicles around PND15 in rats and mice [28]. Previous studies performed in mice showed that FOXO3a deficiency drives primordial follicles to spontaneous global activation, with the primordial follicle reserve completely depleted 2 weeks after birth [29]. Therefore, FOXO3a is required to maintain the reproductive capacity. In humans, FOXO3a mutation is considered to have more severe phenotypic consequences than in mice [30, 31]. Our data showed that FOXO3a expression is dramatically downregulated in gal-exposed ovaries, whereas it was present DHEA-treated (10 mg/kg) ovaries. A clear dose-dependent effect of DHEA on FOXO3a expression indicates the correlation between the androgen amount and its selective control of follicle maturation, which was suggested before [32]. In addition to that, observing downregulation of Ki-67 in galactosemic offspring and augmentation following DHEA supplementation indicates increased granulosa cell proliferation and therefore healthy follicular development stimulated by DHEA under galactosemia. Together, we believe this data is important evidence showing that DHEA prevents early activation of primordial follicles.
Androgens, as oestrogen precursors, have been considered detrimental to women’s health for many decades. Androgen receptor (AR) is abundant in the preantral/antral stages in the murine ovary [33]. Likewise, it is expressed at all stages of follicular development beyond the primordial stage in human ovarian follicles [34]. Studies using androgen-receptor knockout (ARKO) mice, which eventually develop POI, have clearly demonstrated the importance of AR for normal ovarian function and female fertility [35]. Androgens induce ligand-activated AR and modulate FSH activities in developing granulosa cells. They act as crucial modulators of granulosa cell proliferation and follicle maturation, particularly at FSH-dependent stages. Therefore, androgenic effects appear pronounced at early stages of folliculogenesis both in mice and human [32]. Here, our finding of a possible stimulatory effect of DHEA on follicle development is supported by these previous observations. Data presented in this study signify that ovarian follicular maintenance requires AR-mediated androgen function in rats with galactosemia, and is likely to have a similarly significant role in humans.
Currently, there is no proven treatment available to fully restore normal function to the ovaries with diminished reserve. Few human clinical trial studies showed effective treatment of DHEA with the daily dose of 75–80 mg for at least 12 weeks to women with diminished ovarian reserve [15, 16, 18]. Here we present the significant improvement of ovarian reserve indicators in rats that received 1 mg/kg daily, which is compatible with human studies, and 10 mg/kg daily which is relatively high. Animal models have a great potential to improve our understanding of the cause, progression and potential therapeutic approaches of human diseases, yet physiological differences between rodents and humans must be considered before results are fully translated to humans. Data presented here supports the conclusions of previous case reports which show an improved ovarian function in poor responders after exogenous DHEA supplementation.
In conclusion, this study suggests a mechanism whereby DHEA treatment may be a clinically useful therapy to improve the ovarian ageing in women with POI induced by galactosemia. Future work will include both human randomised controlled trials and further experimentation with animal models to elucidate the effects of DHEA in search of galactosemic gonadal dysfunction.
Acknowledgements
The authors are grateful to Asli Okan (MSc) for the technical help and Andy Cox (PhD) for helpful comments and English proofreading on this article.
Authors’ roles
B.S., M.O., M.E., and T.G. designed the study. B.S. and M.O. created the gal-exposed animal model. B.S. performed data collection and statistical analysis. B.S. wrote the article with the help of M.E. and M.O. M.E., R.A. and N.D. provided a critical discussion of the data and manuscript.
Funding information
This study was supported by the Akdeniz University Scientific Research Fund (2013.01.0103.008)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Berna Sozen and Murat Ozekinci contributed equally to this work.
References
- 1.Shelling AN. Premature ovarian failure. Reproduction. 2010;140(5):633–641. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 2.Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34(2):357–366. doi: 10.1007/s10545-010-9221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva CA, Yamakami LYS, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E. Autoimmune primary ovarian insufficiency. Autoimmun Rev. 2014;13(4–5):427–430. doi: 10.1016/j.autrev.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PPCA, Wodzig WKWH, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2010;16(2):177–188. doi: 10.1093/humupd/dmp038. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman F, et al. Ovarian failure in galactosaemia. Lancet. 1979;2(8145):737–738. doi: 10.1016/s0140-6736(79)90658-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Hale GE, Hughes CL. Galactose metabolism and ovarian toxicity. Reprod Toxicol. 2000;14(5):377–384. doi: 10.1016/s0890-6238(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen YT, et al. Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science. 1981;214(4525):1145–1147. doi: 10.1126/science.7302587. [DOI] [PubMed] [Google Scholar]
- 9.Swartz WJ, Mattison DR. Galactose inhibition of ovulation in mice. Fertil Steril. 1988;49(3):522–526. [PubMed] [Google Scholar]
- 10.Bandyopadhyay S, et al. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod. 2003;18(10):2031–2038. doi: 10.1093/humrep/deg414. [DOI] [PubMed] [Google Scholar]
- 11.Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 12.Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–110. doi: 10.1016/s0015-0282(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 13.Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91(2):644–646. doi: 10.1016/j.fertnstert.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–2849. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- 15.Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–2132. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 16.Fusi FM, Ferrario M, Bosisio C, Arnoldi M, Zanga L. DHEA supplementation positively affects spontaneous pregnancies in women with diminished ovarian function. Gynecol Endocrinol. 2013;29(10):940–943. doi: 10.3109/09513590.2013.819087. [DOI] [PubMed] [Google Scholar]
- 17.Labrie C, et al. High bioavailability of dehydroepiandrosterone administered percutaneously in the rat. J Endocrinol. 1996;150 Suppl:S107–S118. [PubMed] [Google Scholar]
- 18.Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–245. doi: 10.1016/j.ijgo.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Yan Z, Lee GY, Anderson E. Influence of dehydroepiandrosterone on the expression of insulin-like growth factor-1 during cystogenesis in polycystic rat ovaries and in cultured rat granulosa cells. Biol Reprod. 1997;57(6):1509–1516. doi: 10.1095/biolreprod57.6.1509. [DOI] [PubMed] [Google Scholar]
- 20.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 21.Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2014;29(1):146–154. doi: 10.1093/humrep/det408. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Iemitsu M, Aizawa K, Mesaki N, Ajisaka R, Fujita S. DHEA administration and exercise training improves insulin resistance in obese rats. Nutr Metab (Lond) 2012;9:47. doi: 10.1186/1743-7075-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306(5703):1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Rajareddy S, Reddy P, du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134(1):199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63(1):94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Segal S. In utero galactose intoxication in animals. Eur J Pediatr. 1995;154(7):S82–S86. doi: 10.1007/BF02143810. [DOI] [PubMed] [Google Scholar]
- 27.Cramer DW, Harlow BL, Barbieri RL, Ng WG. Galactose-1-phosphate uridyl transferase activity associated with age at menopause and reproductive history. Fertil Steril. 1989;51(4):609–615. doi: 10.1016/s0015-0282(16)60608-8. [DOI] [PubMed] [Google Scholar]
- 28.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 29.Castrillon DH, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 30.Watkins WJ, et al. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril. 2006;86(5):1518–1521. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Gallardo TD, John GB, Bradshaw K, Welt C, Reijo-Pera R, Vogt PH, Touraine P, Bione S, Toniolo D, Nelson LM, Zinn AR, Castrillon DH. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod. 2008;23(1):216–221. doi: 10.1093/humrep/dem255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod Biol Endocrinol. 2011;9:116. doi: 10.1186/1477-7827-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137(10):4392–4397. doi: 10.1210/endo.137.10.8828500. [DOI] [PubMed] [Google Scholar]
- 34.Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92(3):1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- 35.Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111(8):3008–3013. doi: 10.1073/pnas.1318978111. [DOI] [PMC free article] [PubMed] [Google Scholar]