Abstract
There are many gaps between recommended urologic cancer care and real-world practice. While we increasingly define these quality gaps due to our growing health services research capacity in urologic oncology, we often fall short in translating these findings into effective interventions and strategies to reduce gaps in care. In this article, we highlight implementation research as a logical next step for translating our health services research findings into effective individual and organizational behavior change strategies to improve quality of care. We explain how implementation research focuses on different, upstream outcomes from our clinical outcomes to get the right care to the right patient at the right time. Last, we share information about resources and training for those interested in learning more about this emerging, trans-disciplinary field.
Keywords: implementation, dissemination, translation, oncology, voltage drop
There are many gaps between recommended urologic cancer care and real-world practice. Examples range from underuse of effective care (e.g., smoking cessation[1–3] and physical activity[4, 5] counseling, neoadjuvant chemotherapy for muscle-invasive bladder cancer,[6] adjuvant radiation therapy for prostate cancer[7]) and misuse of preference-sensitive care (e.g., failure to include patient values and preferences into treatment[8]) to overuse of supply-sensitive care (e.g., imaging for cancer surveillance[9], overtreatment[10]). While we increasingly define these quality gaps due to our growing health services research capacity in the specialty, we often fall short in translating these findings into effective interventions and strategies to reduce gaps in care. In this article, we highlight implementation research as a logical next step for translating our health services research findings into effective individual and organizational behavior change strategies to improve quality of care. We explain how implementation research focuses on different, upstream outcomes from our clinical outcomes to get the right care to the right patient at the right time. Last, we share information about resources and training for those interested in learning more about this emerging, trans-disciplinary field.
Trends in urologic health services research show a steady rise in peer-reviewed literature over the past decade. Thanks to many in the Urologic Oncology readership, we appear to have reached a critical mass in terms of capacity for examining cost, quality and access in urologic care. Nonetheless, our research does not directly improve patient care in a timely way. This is due, at least in part, to what has become a typical agenda in urologic health services research: first, we identify gaps in oncology care either through clinical experiences, our prior research, or findings from other fields. This motivates further research agendas. Next, we generate hypotheses, design studies to test these hypotheses, interpret the findings, and publish the results; increasingly in high impact journals. However, our research often stops here. Sometimes we complete the cycle by indicating further research is warranted given our new findings.
But where is the translation to improving urologic oncology practice to fill these gaps, and how do we translate the best of our research findings into improvements in urology practice? Many are familiar with the National Institutes of Health (NIH) roadmap outlining a ‘bench-to-bedside’ translation pipeline. As highlighted in a corresponding JAMA commentary, a third translational step involves dissemination and implementation research.[11] For most urologic oncology investigators, these aspects are an afterthought once the hard work of a clinical trial is over; for example, after a phase 3 clinical trial of a novel agent is completed, the findings are published, and the weekly challenges that occurred during enrollment, treatment, and follow up are long forgotten. This is also often accompanied by the unrealistic hope that we will get from published trial results to improved clinical practices and better outcomes for our patients without addressing the challenges of ensuring that new findings are adopted into practice. The lack of appreciation for the work required to change clinical practice is, at least in part, the reason it takes 17 years, by some estimates, for a minority of new scientific discoveries to enter day-to-day clinical practice.[12] We argue that more needs to be done to accomplish translation of important research findings, and that this requires new capacity building and training among urologic oncologists.
Dissemination and implementation research are rapidly evolving, trans-disciplinary fields of considerable relevance to the urologic oncology community. Growing our expertise in these fields will be critical to translating research findings into clinical practice improvements. As defined by the National Cancer Institute (NCI) and their team dedicated to implementation research: dissemination is “the targeted distribution of information and intervention materials to a specific public health or clinical practice audience” and implementation is “the use of strategies to adopt and integrate evidence-based health interventions and change practice patterns within specific settings.”[13]
Changing provider behavior and practice patterns across different settings and contexts is complex, and requires rigorous methods from a variety of disciplines including the social sciences, behavioral psychology, operations and human factors engineering, business, marketing, policy and organizational change.[14–19] Systematically approaching provider behavior change efforts (e.g., increasing the use of a one-time instillation after endoscopic surgery, not ordering a bone scan for low risk prostate cancer, AUA Choosing Wisely™ recommendations) using implementation research techniques can help prevent real-world delivery challenges, such as the ‘voltage drop’ experienced when products from efficacy trials are put into routine practice.[20]
Why we need implementation research to help us impact urologic health at a population level is shown in the Table. While this ‘voltage drop’ could happen in any recommended clinical intervention in urologic oncology, we will use the example of a breakthrough chemotherapy combination that improves bladder cancer survival (e.g., neoadjuvant methotrexate, vinblastine, doxorubicin, cisplatinum). After the publication of this breakthrough treatment in a high-impact journal, let’s suppose half of clinics have access to the drugs or are aware of the new findings, half of practitioners actually recommend the treatment, and half of patients accept the recommendation. Half of patients receive the correct regimen based on the clinical trial, perhaps less effective agents like carboplatin are substituted or there are missed doses. Because the patients treated with the regimen are not exactly like the patients from the clinical trial and due to heterogeneity of therapeutic effects, half of treated patients have substantial benefits, and so on. The ‘voltage drop’ demonstrated here is what will typically happen in real-world practice, despite breakthrough clinical findings, if we continue to be naïve to the importance of conducting implementation research alongside our clinical trials in preparation for broader population impact.
Table.
'Voltage drop' at the population level for interventions found to have efficacy in randomized clinical trials: An illustrative example for neoadjuvant chemotherapy for muscle-invasive bladder cancer with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC).[25]
| Dissemination | Concept | Impacted |
|---|---|---|
| 50% Clinics use MVAC | Adoption | 50% |
| 50% Practitioners recommend MVAC | Adoption | 25% |
| 50% Patients accept recommendation/attempt MVAC | Reach | 12.5% |
| 50% Follow MVAC regimen correctly | Implementation | 6.2% |
| 50% Implementing MVAC have substantial benefit | Effectiveness | 3.1% |
| 50% Continue to benefit/adhere to MVAC protocol (e.g., go on to radical cystectomy) after 6 months | Maintenance | 1.6% |
Based on the RE-AIM Framework by Glasgow et al.[26]
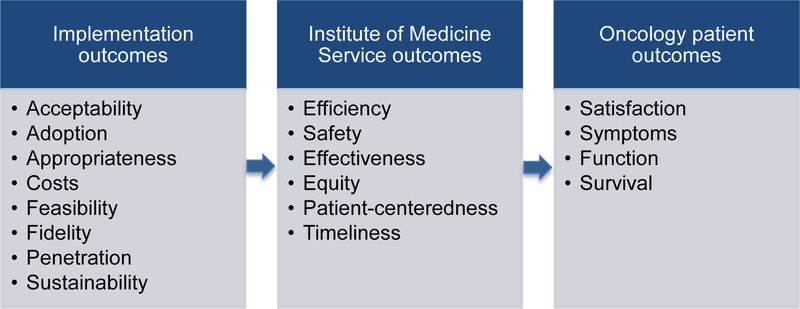
Unlike common clinical trial outcomes (e.g. patient function, symptoms, survival, and satisfaction), or Institute of Medicine service delivery outcomes (e.g., safety, timeliness, patient centeredness), implementation research focuses on outcomes that are further upstream to achieve these clinical and delivery system outcomes.[21] As illustrated in the Figure, implementation outcomes include the acceptability, adoption, appropriateness, costs, feasibility, fidelity, penetration and sustainability of an evidence-based clinical intervention in real-world practice. For example, implementation research might 1) investigate whether a recommended clinical intervention is acceptable to patients and providers and in which clinical contexts, 2) examine how much interventions cost to implement across different settings, 3) determine whether administering a new agent in a clinical setting is feasible, 4) study the extent to which fidelity is maintained with respect to the clinical trial protocols and the implications, and/or 5) compare different approaches to making a new evidence-based practice sustainable for clinicians, staff, patients and payers. These critical but neglected aspects of our current research agendas need to be addressed to deliver evidence-based care across settings and achieve high quality clinical and service outcomes for our urologic oncology patients.
Figure.
Implementation outcomes upstream of delivery system and patient outcomes (Adapted from Proctor et al) [21]
In addition, implementation research can inform which implementation interventions and strategies work best and in what settings when we target a clinical practice to implement. It offers guidance regarding effective implementation strategies for creating behavior change, including how and when to choose from the smorgasbord of potential provider and organizational level interventions such as automated reminders, audit & feedback, education, coaching, modeling behavior, incentives, changing the environment, guidelines, marketing and communication strategies, legislation, stakeholder engagement, and quality collaboratives, among others. Using implementation frameworks can also help inform the process of implementation, what factors influence implementation outcomes, and evaluation of the implementation intervention or strategy in order to better understand why the intervention succeeds or fails, as well as to promote generalizable knowledge. Unfortunately, implementation strategies are often picked based on intuition rather than evidence, leading to wasted time and resources. Implementation research provides critical information about which implementation strategies will work best under what conditions. For example, audit and feedback interventions have been found to work best in changing behavior when they are timely, and have action-oriented, non-punitive, individualized feedback rather than the impersonal quarterly reports we commonly receive.[22] At the organizational level policies like formulary restriction can facilitate implementing evidence-based practice, but when, where, and how to use them is not easy to determine, nor are their intended and un-intended consequences. Moreover, failing to understand implementation planning and informed strategy selection not only leads to poor clinical intervention uptake, it can also thwart future efforts by creating resistance to change.
Common approaches in implementation research involve mixed methods, melding qualitative and quantitative approaches to identify gaps in care, and barriers and facilitators to implementation of an evidence-based clinical practice.[15] Rich data can help tailor implementation interventions to the appropriate context and promote increased uptake and effectiveness of the evidence-based practices being promoted. Qualitative data are often required to understand how interventions achieve their effect. In essence, to routinize evidence-based practices like neoadjuvant chemotherapy for bladder cancer, implementation strategies need to make it ‘easy’ for patients, providers, and organizations to deliver the practice, ultimately making it just part of how care is delivered.[23]
Implementation research and frameworks were recently highlighted in JAMA, indicating the relevance of this emerging field.[24] However, how do we grow capacity in the field of urologic oncology? Several resources can help.
The NCI has a team and website dedicated to implementation science (http://cancercontrol.cancer.gov/IS). Their mission is ‘To achieve the rapid integration of scientific evidence, practice, and policy, with the ultimate goal of improving the effect of research on cancer outcomes and promoting health across individual, organizational and community levels.’ As part of that mission they support a post-doctoral training program in collaboration with the Veterans Administration Health Services Research and Development and Washington University in St. Louis. This 2-year Mentored Training in Dissemination and Implementation Research in Cancer Fellowship (MT-DIRC) builds capacity in implementation research and consists of an excellent mix of didactic and practice-based learning alongside world-class mentors (www.mtdirc.org). Implementation Science (www.implementationscience.com), an open access, peer-reviewed journal dedicated to dissemination and implementation research, is one of a growing number of journals that specialize in publishing implementation and dissemination research. With respect to funding, the NIH has a standing implementation research review committee, and there are increasing numbers of funding opportunities available from VA, PCORI, AHRQ, foundations, and CMS.
It is clear that we’ve built significant urological health services research capacity thanks to many in the urologic oncology community. Going forward, the trans-disciplinary and mixed methods approaches used in implementation research will be critical to translating the best of our research findings into clinical practice. Taking advantage of increasing opportunities to grow capacity in this emerging field can to help close the gaps between recommended urologic cancer care for our patients and real-world practice.
HIGHLIGHTS.
-
–
It can take 17 years for new scientific discoveries to enter clinical practice
-
–
‘Voltage drop’ occurs when efficacy trials are translated into routine practice
-
–
Dissemination and implementation (D&I) research informs changing practices
-
–
Conducting D&I research during clinical trials can hasten population impact
-
–
Growing D&I capacity in urologic oncology can help close gaps in cancer care
Acknowledgments
Funding: Dr. Skolarus is supported by a VA HSR&D Career Development Award-2 (CDA 12-171) and Mentored Training for Dissemination and Implementation Research in Cancer (MT-DIRC), National Cancer Institute, 1 R25 CA171994-01A1, Washington University in St. Louis, St. Louis, MO
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- CMS
Centers for Medicare and Medicaid Services
- D&I
Dissemination and implementation
- MT-DIRC
Mentored Training in Dissemination and Implementation Research in Cancer Fellowship
- MVAC
Methotrexate, vinblastine, doxorubicin, and cisplatin
- NIH
National Institutes of Health
- NCI
National Cancer Institute
- PCORI
Patient-Centered Outcomes Research Institute
Footnotes
Disclosures: Dr. Sales is Co-Editor-in-Chief, Implementation Science
References
- 1.Rink M, et al. , Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol, 2013. 63(4): p. 724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett JC, et al. , Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol, 2012. 30(15): p. 1871–8. [DOI] [PubMed] [Google Scholar]
- 3.Bjurlin MA, Goble SM, and Hollowell CM, Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol, 2010. 184(5): p. 1901–6. [DOI] [PubMed] [Google Scholar]
- 4.Friedenreich CM, et al. , Physical Activity and Survival After Prostate Cancer. Eur Urol, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Kenfield SA, et al. , Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol, 2011. 29(6): p. 726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandaglia G, et al. , The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy: a population-based study. Eur Urol, 2014. 66(3): p. 561–8. [DOI] [PubMed] [Google Scholar]
- 7.Kim SP, et al. , Variation in treatment recommendations of adjuvant radiation therapy for high-risk prostate cancer by physician specialty. Urology, 2013. 82(4): p. 807–12. [DOI] [PubMed] [Google Scholar]
- 8.Holmes-Rovner M, et al. , Informed Decision Making: Assessment of the Quality of Physician Communication about Prostate Cancer Diagnosis and Treatment. Med Decis Making, 2015. 35(8): p. 999–1009. [DOI] [PubMed] [Google Scholar]
- 9.Strope SA, et al. , Survival impact of followup care after radical cystectomy for bladder cancer. J Urol, 2013. 190(5): p. 1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs BL, et al. , Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA, 2013. 309(24): p. 2587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westfall JM, Mold J, and Fagnan L, Practice-based research--”Blue Highways” on the NIH roadmap. JAMA, 2007. 297(4): p. 403–6. [DOI] [PubMed] [Google Scholar]
- 12.Morris ZS, Wooding S, and Grant J, The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med, 2011. 104(12): p. 510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PAR-10–038: Dissemination and Implementation Research in Health (R01) (Domestic and International Funding Opportunity) http://grants.nih.gov/grants/guide/pa-files/PAR-13–055.html Accessed 2/26/2016.
- 14.Grol R, Wensing M, Eccles M. Improving Patient Care: The Implementation of Change in Clinical Practice. Published: NOV-2004, ISBN 10: 0–7506-8819-X [Google Scholar]
- 15.Complex Interventions in Health: An overview of research methods. Edited by Richards David A., Hallberg Ingalill Rahm. © 2015. – Routledge. [Google Scholar]
- 16.Brownson R, Colditz G, & Proctor E (Eds.) (2012). Dissemination and implementation research in health: Translating science to practice, New York, NY: Oxford University Press. [Google Scholar]
- 17.The Behaviour Change Wheel: A Guide To Designing Interventions. Copyright © Susan Michie, Lou Atkins and Robert West Silverback Publishing; 2014 [Google Scholar]
- 18.French SD, et al. , Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci, 2012. 7(1): p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michie S, et al. , Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care, 2005. 14(1): p. 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers DA, Glasgow RE, and Stange KC, The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci, 2013. 8: p. 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor E, et al. , Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health, 2011. 38(2): p. 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hysong SJ, Best RG, and Pugh JA, Audit and feedback and clinical practice guideline adherence: making feedback actionable. Implement Sci, 2006. 1: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May C, Towards a general theory of implementation. Implementation Science, 2013. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher ES, Shortell SM, and Savitz LA, Implementation Science: A Potential Catalyst for Delivery System Reform. JAMA, 2016. 315(4): p. 339–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman HB, et al. , Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med, 2003. 349(9): p. 859–66. [DOI] [PubMed] [Google Scholar]
- 26.Glasgow RE, Vogt TM, and Boles SM, Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health, 1999. 89(9): p. 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]