Abstract
Objective:
The present study was conducted to investigate antibacterial properties of fruit and flower of Peganum harmala.
Material and Methods:
Column chromatography, followed by preparative thin layer chromatography (TLC) was used for final purification. The structure of pure alkaloids was determined using spectroscopic methods (1H-NMR, 13C-NMR, UV and MS). Smoke and extract of total alkaloids were investigated for antimicrobial activity against five different microorganisms (standards and hospital isolates). The antibacterial activity was evaluated using disc diffusion assay and minimum inhibitory concentration (MIC) was determined by serial dilution methods.
Results:
Chemical investigation of the chloroform extract of ripe fruit and flower of P. harmala led to identification of three alkaloids in ripe fruit and two alkaloids in the flower and leaves of this plant. Alkaloids identified in ripe fruit were harmine, peganine (vasicine) and harmaline. Two alkaloids, harmine and peganine, were detected in the flower of P. harmala, while harmaline was only found in the ripe fruit. The total alkaloids of flower were compared with total alkaloids of ripe fruit by TLC method. Fruits and flowers had 3.12 and 3.27% alkaloid contents, respectively.
Conclusion:
Our results showed that the alkaloids and smoke were specifically more effective on Candida albicans and Gram- positive bacteria (Micrococcus luteus and Staphylococcus aureus), while Gram- negative bacteria, especially Pseudomonas aeruginosa, were less sensitive.
Key Words: Alkaloid, Antimicrobial activity, Peganum harmala, Harmine, Harmaline, Smoke
Introduction
Peganum harmala L. (Zygophyllaceae) is a herb native to dry area from east Mediterranean to northern India and is widely spread in Iran (Branch, 2012). It is claimed that the plant has been used as an important medicinal plant in global folk medicine (Sharaf et al., 1997); also, burning its seeds was used in Iran as an antiseptic and disinfectant approach (Ghasemi Pirbalouti et al., 2013). While consuming the seeds can stimulate the central nervous system (CNS), can cause paralysis and is poisonous at high doses, the smoke of P. harmala seeds is used as an antimicrobial approach in folk medicine (Al-Shamma et al., 1981; Achour et al., 2012; Moloudizargari et al., 2013; Lamchouri, 2014; Mina et al., 2015).
P. harmala has various alkaloids of diverse structures that are difficult to be synthesized; however, the extract from its ripe fruit or flower contains structurally easier compounds. P. harmala contains β-carboline and quinazoline alkaloids which are responsible for the toxicological and pharmacological effects of the plant. β- carbolin alkaloids are abundant in many plant families and possess neurostimulant and monoamine oxidase (MAO) inhibitory activities (Herraiz et al., 2010).
Moreover, antihypertensive, hallucinogenic, and antidepressant effects have been reported for P. harmala (Moloudizargari et al., 2013). Traditionally, P. harmala has been used to treat various ailments including Parkinson's disease, gastrointestinal and cardiovascular disorders and certain types of malignancies (Moloudizargari et al., 2013). More recently, the antileishmanial activities of harmine were studied in detail (Mirzaie et al., 2007). Splettstoeser et al. reported that P. harmala alkaloids have protective effects on neurons against the excitotoxicity of dopamine and glutamate (Splettstoesser et al., 2005).
β-Carbolin alkaloids attracted the scientists' attention because of their potent inhibitory effects against certain antibiotic-resistant strains (Suzuki et al., 2018). In this article, we studied the antimicrobial activity of alkaloid extract and smoke of P. harmala against standard and hospital isolates.
Materials and Methods
P. harmala Collection and Extraction
P. harmala was collected from Neishabur (Khorasan Razavi Province) in July, 2017. The dried and powdered herb (3 kg) was macerated four times with 3 l of methanol for 24 hr. The extracts were combined and the solvent was evaporated to dryness, using a rotary evaporator. The residue was kept at -20°C until used.
Preliminary phytochemical screening
The qualitative analysis of the chemical constituents was carried out based on the methods previously described by Farnsworth (1996) with some modifications (Farnswort, 1996; Siddiqui et al., 1987; Kaskoos, 2014).
Alkaloids determination
Concentrated methanol extract (3g) was suspended in H2SO4 (3%) for 3 hr, stirred and filtrated. Then, it was basified using 10% NH4OH (pH 8-9) and extracted by CHCl3 (three times). Then, the extract was concentrated using rotary evaporator under vacuum, and spotted on a thin layer chromatography (TLC) plate. After development, the plate was dried, and sprayed with Dragendorff's reagent and orange spots were considered the alkaloids.
Saponins determination
To 1 g of methanol extract, 10 ml of water was added and the mixture was shaken vigorously for two minutes and after 30 min, the formation of a rich lather (which is stable for more than 10 min) was investigated.
Flavonoids determination
Metallic magnesium (100 g) was added to 1 g methanol extract in 10 ml water, and then, 5-6 drops of concentrated hydrochloride acid (HCl) were added slowly and dropwise. After adding 2 ml of amyl alcohol, the solution turns red if flavonoids are present in the extract.
Tannins determination
Two ml of 10% methanol extract was added to 0.1 g gelatin, 10 ml water and 1g sodium chloride. Precipitation is indicative of the presence of tannins.
Cyanogenetic glycosides determination
For qualitative detection of cyanogenetic glycosides in the plant, the Grignard test was employed. Briefly, 4 drops of toluene were added to about 2 g of moist plant material in a small test tube.
The Grignard reagent (a solution of 0.5 g picric acid, 5 g Na2CO3, and water q.s. 100 ml) was used as the indicator. The strips of filter paper were saturated with the solution, dried and introduced into the neck of the test tube containing the plant material. The test tube and contents were then warmed at 30-35οC for up to 3 hr. Any change in color of the yellow test paper to red, is indicative of cyanogenetic glycosides while absence of a red color after 3 hr is considered a negative result.
General experimental procedures
UV, NMR and mass spectra were recorded on Shimadzu UV1650 spectrometer, Bruker 100 MHz NMR spectrometer (Bruker, Germany) and Bruker Mass spectrometer (Bruker, Germany), respectively.
Extraction of alkaloids
The residue (obtained as was described in the extraction part) was acidified using 3% sulfuric acid (pH 1), filtered and washed with petroleum ether to remove high lipophilic compounds. The aqueous acid layer was basified to pH 8-9 using NH4OH (10%) and extracted several times using chloroform. The chloroform layer was mixed and the solvent was evaporated to dryness. Extraction of alkaloids was done by column chromatography with silica gel. For 1 g of the total alkaloid, 50 g silica gel (G60, mesh 230) was used. The polarity of the solvent system gradually increased from chloroform-methanol 95-5% to methanol 100%.
The collected fractions were tested for the presence of alkaloid by TLC using different solvent systems (chloroform/methanol/ammonia) and Dragendorff's reagent as indicator.
Final purification of alkaloids was done by preparative TLC and the structure of the purified compounds was elucidated using 1H-NMR, 13C-NMR, UV and MS (supporting information).
Antimicrobial effects of alkaloids and smoke
Preparation of alcoholic extract of alkaloids
The seeds of the plant were washed with petroleum ether, dried and ground into crude powder. In the first step, 100-150 g of dried powder was macerated in 1 L of 95% methanol for 12 hr at 50°C in water bath. After evaporating the solvent (i.e. methanol), the residue was dissolved in HCl (5%) until the pH of the final solution became 1, then the solution was filtered.
In the next step, the filtrate was extracted twice by 30 ml petroleum ether to remove highly lipophilic compounds. The residue was basified by 10% NH4OH (pH 9-10) and extracted by chloroform (30 ml); finally, the chloroform was evaporated to dryness.
Tested microorganisms
Micrococcus luteus (PTCC 9341), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (PTCC 1074) Staphylococcus aureus (ATCC 29737), and the yeast Candida albicans (ATCC 10231) were provided as lyophilized by Persian Type Culture Collection, Tehran, Iran. The pathogenic strains (hospital strains including one strain from E. coli, P. aeruginosa, S. aureus and C. albicans) were provided by University Hospital of Imam Reza, Mashhad, Iran.
Antimicrobial evaluations
Revival of microorganisms
Soybean-casein digest broth medium (0.5 ml) was added to lyophilized microorganisms and the suspension was used for preparation of master culture (soybean-casein digest agar) by streaking method. The culture medium was incubated (24 hr, at 37οC for bacteria and 48 hr, at 25οC for yeast) and then, the sub-master culture was prepared. From these sub-master cultures, using normal saline, the microbial count of 108 CFU/ml (comparing with 0.5 ml McFarland standard) was prepared.
Antimicrobial properties of total alkaloids
Alkaloids extract was added to the culture media (Mueller Hinton agar, 45οC) to prepare different concentrations of alkaloids (20-3000 µg/ml). The standard antibiotic powders, amikacin (from 0.25-100 µg/ml) and clotrimazole (from 0.25-20 µg/ml) were also prepared in the same manner. To inoculate each microorganism, 15 ml of culture medium was transferred to the plate and 0.5 ml from 105 CFU/ml suspension of microorganism was spread on the surface of the agar plate. Incubation of plates was performed at 37οC for 24 hr for bacteria and at 25οC for 48 hr for yeast; finally, the growth of microorganisms was investigated.
Smoke
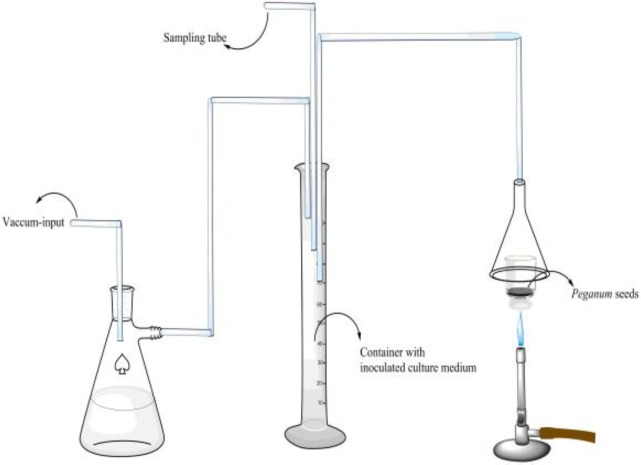
The smoke of burned seeds of P. harmala or formalin tablet in a watch glass was directed to a flask containing Mueller Hinton broth (150 ml) through a tube. One ml of each microbial suspension (equivalent to 0.5 ml McFarland standard, i.e. 108 CFU/ml) was added to the medium (Figure 1). Incubation of the flask was performed for 24 hr, at 37οC for bacteria and for 48 hr, 25οC for yeast and then, the growth of microorganisms was observed. All the steps were performed under aseptic conditions.
Figure 1.
A schematic view of the apparatus used in this study for investigation of the antibacterial activity of P. harmala seeds
Results
Phytochemical screening
The results of phytochemical screening of flowers and ripe fruit from P. harmala extracts are shown in Table 1. The phytochemical analysis was carried out to detect the presence of phytoconstituents (alkaloids, tannins, saponins and flavonoids) in methanol extracts of P. harmala which indicated the presence of saponins and alkaloids.
Table 1.
Phytochemical screening results
| Plant Part | Cyanogenic glycosides | Saponin | Tannin | Flavonoid | Alkaloid |
|---|---|---|---|---|---|
| Flower and leaves | _ | + | _ | _ | ++++ |
| Ripe fruit and leaves | _ | +++ | _ | _ | ++++ |
Antimicrobial activity
Minimum inhibitory concentration (MIC) of total alkaloids against standard microorganisms M. luteus, E. coli, P. aeruginosa and S. aureus was 31.25±1.65 µg/ml, 500±81.60 µg/ml, 1500±40.82 µg/ml and 125±2.04 µg/ml, respectively. MIC values for hospital isolates were 1500±16.32 µg/ml, 2000±40.82 µg/ml and 150±4.08 µg/ml for E. coli, P. aeruginosa, and S. aureus, respectively. In case of C. albicans, MIC was 62.5±2 µg/ml for both standard and hospital isolate (Table 2). The MIC test for each standard and hospital strain was performed in triplicate and the results are expressed as mean±standard deviation.
Table 2.
MICs (µg/ml) of total alkaloid extract, clotrimazole and amikacin against standard and pathogen microorganisms
| Microorganism |
Standard
(total alkaloid) |
Pathogen
(total alkaloid) |
Standard and pathogen
(clotrimazole) |
Standard
(Amikacin) |
Pathogen
(Amikacin) |
|---|---|---|---|---|---|
| Staphylococcus aureus | 125±2.04 | 150±4.08 | - | 8±0.163 | 15±0.408 |
| Escherichia coli | 500±8.160 | 1500±16.32 | - | 4±0.408 | 10±0.816 |
| Pseudomonas aeruginosa | 1500±40.820 | 2000±40.82 | - | 4±0.408 | 50±1.632 |
| Candida albicans | 62.5±2.04 | 62.5±2.04 | 8±0.204 | - | - |
| Micrococcus luteus | 31.25±1.650 | - | 4±0.408 | - |
The MIC of P. harmala smoke against standard microorganisms M. luteus, E. coli, P. aeruginosa, S. aureus and C. albicans was 2.25±0.04, 1±0.082, 6±0.122, 4±0.82 and 1±0.04 (smoke equivalent g of seed), respectively. The MIC for hospital isolates was 2±0.224, 8±0.408, 5±0.163, and 1±0.04 against E. coli, P. aeruginosa, S. aureus and C. albicans, respectively (smoke equivalent g of seed) (Table 3).
Table 3.
Antimicrobial activity (equivalent of g seeds) of smoke against standard and pathogenic microorganisms
| Microorganism (standard) |
Standard
(smoke) |
Pathogen
(smoke) |
Standard
(Formalin tablets) |
Pathogen
(Formalin tablets) |
|---|---|---|---|---|
| Staphylococcus aureus | 4±0.082 | 5±0.163 | 2±0.204 | 2±0.204 |
| Escherichia coli | 1±0.082 | 2±0.224 | 0.5±0.144 | 1.5±0.204 |
| Pseudomonas aeruginosa | 6±0.122 | 8±0.408 | 0.5±0.144 | 2±0.204 |
| Candida albicans | 1±0.04 | 1±0.04 | 1±0.163 | 1±0.163 |
| Micrococcus luteus | 2.25±0.04 | 0.5±0.144 | - |
Amikacin was the positive control for evaluation of antibacterial activity of total alkaloids and its MIC against standard microorganisms, M. luteus, E. coli, P. aeruginosa and S. aureus was 4±0.408 µg/ml, 4±0.408 µg/ml, 4±0.408 µg/ml and 8±0.163µg/ml, respectively. MIC of amikacin for hospital isolates was 15±0.408 µg/ml, 10±0.816 µg/ml and 50±1.632 µ/ml for S. aureus, E. coli and P. aeruginosa, respectively (Table 2).
Formalin was used as positive control for evaluation of the antimicrobial activity of smoke and its MIC against standard microorganisms, M. luteus, E. coli, P. aeruginosa, S. aureus and C. albicans was 0.5±0.144, 0.5±0.144, 0.5±0.144, 2±0.204 and 1±0.163 (Number of formalin pills), respectively. For hospital isolates, formalin MIC was 2±0.204, 1.5±0.204, 2±0.204 and 1±0.163 (Number of formalin 1000 mg pills, Table 3) for S. aureus, E. coli, P. aeruginosa and C. albicans, respectively.
Characterization of alkaloids
Three known alkaloids, namely harmine, peganine and harmaline (Figure 2) were isolated from P. harmala and their structures were confirmed via comparing 1H-NMR, 13C-NMR, UV and MS spectra with those reported in the literature (Manske, 1954; Chatterjee and Ganguly, 1968; Brossi, 1985; Siddiqui et al., 1988; Ayoub et al., 1989). For detailed information on structure elucidation of the alkaloids, please see the data presented in supporting information.
Figure 2.
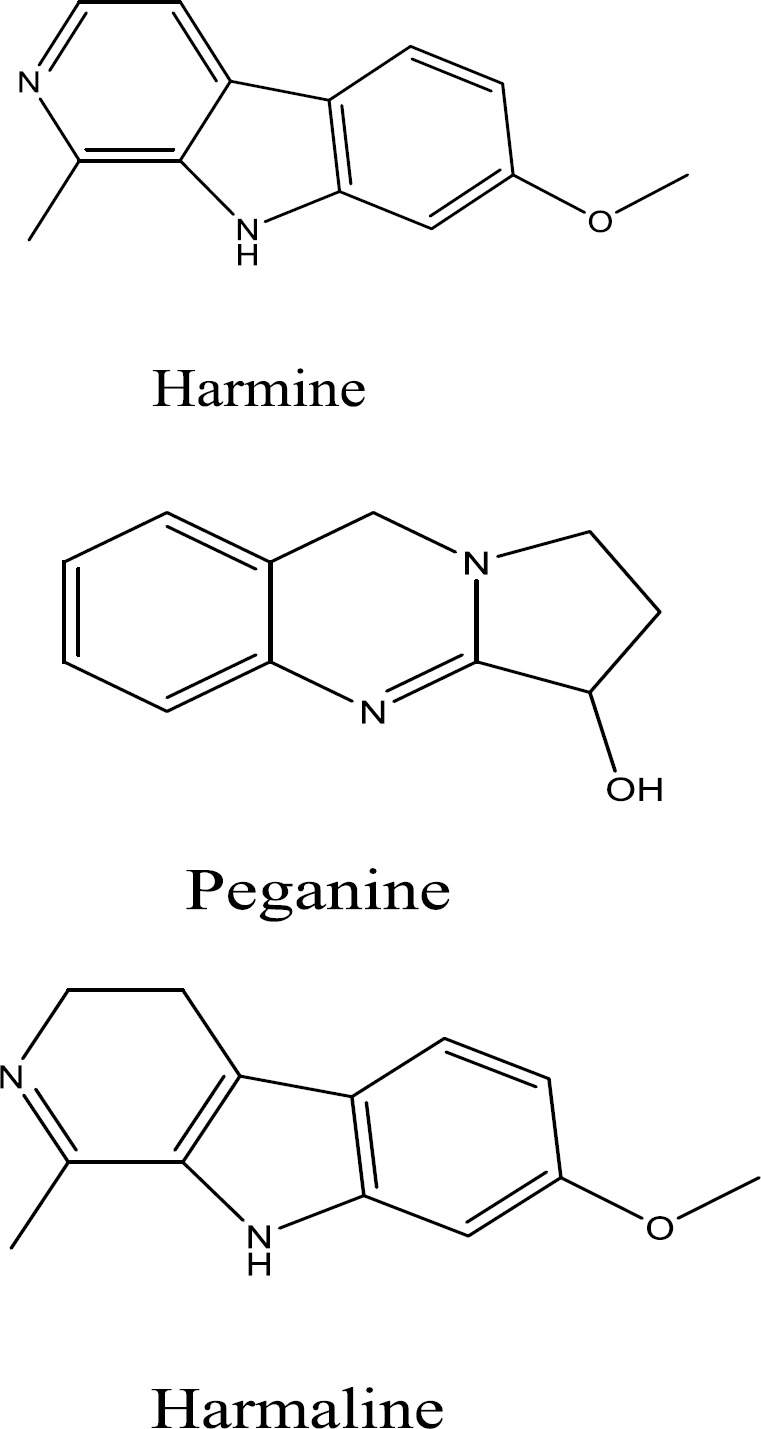
Chemical structures of alkaloids isolated from P. harmala
Discussion
For many years, medicinal plants have been considered a potential source of pharmaceutical natural products. Peganum harmala is one of the plants that has been extensively used in traditional medicine of Iran and various parts of this plant including its seeds, bark, and root have been used for treatment of different ailments (Mina et al., 2015).
In this paper, phytochemical screening of the ripe fruit and flowers of P. harmala, was done. A summary of the results of the qualitative phytochemical analysis is shown in Table 1.
Our results showed that the content of total alkaloids in seeds and flowers was 3.12 and 3.27% respectively. So far, several studies have been done to extract and identify the alkaloids of this plant and most of these studies reported that the seed of the plant has the highest amount of alkaloids.
In 2012, Asgarpanah et al. reported that total alkaloid content of P. harmala varied between 2 and 5% which were consistent with our findings (Asgarpanah and Ramezanloo, 2012).
Herraiz et al. in 2010, reported that the highest levels of alkaloids are found in the seeds and roots followed by stems and leaves, but flowers lack such compounds (Herraiz et al., 2010).
Bukhari in 2008 reported that in the P. harmala, the harman alkaloids (harmine and harmaline) are specifically located in the seeds (Bukhari et al., 2008). Hemmateenejad et al. in 2006 reported an analysis of P. harmala seeds and the results indicated 1.84, 0.16, 0.25 and 3.90% content for harmine, harmane, harmaline and harmalol, respectively (Hemmateenejad et al., 2006).
The number of alkaloids in P. harmala ripe fruit and flower extract in our study, was limited. It is generally accepted that the geographical location and plant growth conditions as well as the conditions of plant gathering, affect the amount and type of plant compounds, so this finding may be due to such variations.
Thin layer chromatography only indicated three alkaloids with very too close Rf. The extracted alkaloids were polar, so the first solvent system used for column chromatography was chloroform- methanol (95:5). The polarity of the solvent gradually increased and fractions were purified using thin layer chromatography. The most suitable solvent system was chloroform- methanol-ammonia (60:30:0.5).
Thin layer chromatography showed that alkaloids in flower, leaves and ripe fruit are similar. These two alkaloids were harmine and peganine. Harmaline was the alkaloid that was only found in ripe fruit and did not exist in the flower of P. harmala. Spectroscopic properties of these alkaloids (assessed by UV, 1H-NMR, 13C-NMR and MS spectrum) are presented in supporting information.
The antibacterial activity of different parts of P. harmala has been investigated in many studies. Darabpour et al. in 2011 reported that the best antibacterial activity against Gram-positive bacterial species, including Bacillus anthracis, Bacillus cereus, Bacillus pumilus, Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes and Streptococcus pyogenes and Gram-negative bacterial species, including Pseudomonas aeruginosa, Brucella melitensis, Proteus mirabilis, Salmonella typhi, Escherichia coli and Klebsiella pneumoniae, were observed for the seed and root extracts among the studied parts of P. harmala. Even at the lowest concentration, the root and seed extracts exhibited antibacterial activity against all of tested bacteria. The antibacterial effect of leaves was moderate while stem and flower extracts showed relatively poor activity (Darabpour et al., 2011). Other studies also revealed the inhibitory effect of seed alkaloid extracts of P. harmala against some Gram-positive bacterial strains such as S. aureus and S. saprophyticus and Gram-negative including E. coli, K. pneumoniae, P. aeruginosa, Proteus mirabilis and Serratia spp. The results indicated that the diameters of inhibition zones ranged from 11 to 22 mm for all treatments (Benbott et al., 2012). Also, aqueous and ethanol extracts of P. harmala were effective against all Gram-positive bacteria tested in a study including Lactobacilli and Streptococcus mutans, respectively (Cowan, 1999). Other studies revealed the sensitivity of Aeromonas hydrophila strain to seed aqueous extract of P. harmala, since inhibition zone was 20.5 (Abutbul et al., 2005).
In this work, we examined the activity of total alkaloids extract of seeds of P. harmala against five different microorganisms (standards and hospital strains). Our results showed that P. harmala total alkaloids have remarkably higher activity against Gram-positive bacteria than Gram-negative ones and the MIC against Gram-positive bacteria was less than MIC against Gram-negative bacteria. This differences in MIC could be due to the external membrane of Gram-negative bacteria that prevents the molecules from penetration to the cell.
Total alkaloids at 62.5 µg/ml concentration showed activity equivalent to 8 µg/ml clotrimazole against standard and pathogenic C. albicans. This suggests a good activity of alkaloids against this yeast. Even the activity of total alkaloids against this yeast was more marked than its activity against Gram-positive and Gram-negative bacteria (except M. luteus). The smoke of P. harmala also showed higher activity against C. albicans compared to other microorganisms tested, with the exception of E. coli.
According to our results, the order of antimicrobial activity of total alkaloids and the smoke of P. harmala was yeasts, Gram-positive bacteria and Gram-negative bacteria, respectively.
Traditional medicine systems are still valuable sources of information to find biologically active plants and lead compounds for treatment of different diseases. In this study, the strong traditional background of P. harmala as a disinfectant, led us to unravel the antibacterial and especially antifungal activity of this plant. Future studies should mainly focus on isolation of compounds responsible for the observed activity and disclose the mechanism of action of such compounds. In addition, the results of this study propose the smoke of P. harmala seeds as a possible disinfectant that can be used safely for sanitary purposes. The chemical composition of the smoke of P. harmala seeds is another interesting topic remains to be elucidated in the future.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial supports. The results described in this paper, are part of two Pharm. D. theses.
We would also like to acknowledge Professor Mohammad Rahimizadeh, one of the leader of this project who passed away in 2017.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.The plant list. from http://www.theplantlist.org/tpl1.1/record/kew-2548698.
- 2.Abutbul S, Golan-Goldhirsh A, Barazani O, Ofir R, Zilberg D. Screening of desert plants for use against bacterial pathogens in fish. Israeli J of Aquacult. 2005;57: 71–80. [Google Scholar]
- 3.Achour S, Rhalem N, Khattabi A, Lofti H, Mokhtari A, Soulaymani A, Turcant A, Bencheikh RS. [Peganum harmala L poisoning in Morocco: about 200 cases] Therapie. 2012;67:53–58. doi: 10.2515/therapie/2012003. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shamma A, Drake S, Flynn DL, Mitscher L A, Park YH, Rao GS, Simpson A, Swayze JK, Veysoglu T, Wu ST. Antimicrobial agents from higher plants Antimicrobial agents from Peganum harmala seeds. J Nat Prod. 1981;44:745–747. doi: 10.1021/np50018a025. [DOI] [PubMed] [Google Scholar]
- 5.Asgarpanah J, Ramezanloo F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr J Pharm Pharmacol. 2012;6:1573–1580. [Google Scholar]
- 6.Ayoub MT, Rashan L, Khazraji A, Adaay M. An oxamide from Peganum harmala seeds. Phytochemistry. 1989;28:2000–2001. [Google Scholar]
- 7.Benbott A, Yahyia A, Belaidi A. Assessment of the antibacterial activity of crude alkaloids extracted from seeds and roots of the plant Peganum harmala L. J Nat Prod Plant Resour. 2012;2:568–573. [Google Scholar]
- 8.Branch S. Etymological review on chemical and pharmaceutical substances of the oriental origin. Int J Anim Veter Adv. 2012;4:40–44. [Google Scholar]
- 9.Brossi A. The alkaloids: Chemistry and pharmacology. Academic Press; 1985. pp. 140–143. [Google Scholar]
- 10.Bukhari N, Choi J, Jeon C, Park H, Kim W, Khan M, Leet S. Phytochemical studies of the alkaloids from Peganum harmala. Applied Chem. 2008;12:101–104. [Google Scholar]
- 11.Chatterjee A, Ganguly M. Alkaloidal constituents of Peganum harmala and synthesis of the minor alkaloid deoxyvascinone. Phytochemistry. 1968;7:307–311. [Google Scholar]
- 12.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darabpour E, Poshtkouhian Bavi A, Motamedi H, Seyyed Nejad SM. Antibacterial activity of different parts of Peganum harmala L growing in Iran against multi-drug resistant bacteria. EXCLIJ. 2011;10:252–263. [PMC free article] [PubMed] [Google Scholar]
- 14.Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1996;55:225–276. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi Pirbalouti A, Momeni M, Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan Districts, Ilam Province, Iran. Afr J Tradit Complement Altern Med. 2013;10:368–385. doi: 10.4314/ajtcam.v10i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmateenejad B, Abbaspour A, Maghami H, Miri R, Panjehshahin MR. Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts. Anal Chim Acta. 2006;575:290–299. doi: 10.1016/j.aca.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 17.Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ, Guillen H. beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem Toxicol. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Kaskoos R. Physico-chemical parameters, phytochemical screening and antioxidant activity of seeds of Peganum harmala collected from Iraq. Asian J Biomed Pharm Sci. 2014;4:20–24. [Google Scholar]
- 19.Lamchouri F. Antitumor properties and toxicity effects of Peganum harmala L. (Zygophyllaceae). Plant Sci Tod. 2014;1:192–195. [Google Scholar]
- 20.Manske RY, Holmes HL. The alkaloids. New York: Acad Press; 1954. p. 57. [Google Scholar]
- 21.Mina CN, Farzaei MH, Gholamreza A. Medicinal properties of Peganum harmala L in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med. 2015;35:104–109. doi: 10.1016/s0254-6272(15)30016-9. [DOI] [PubMed] [Google Scholar]
- 22.Mirzaie M, Nosratabadi SJ, Derakhshanfar A, Sharif I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Veterinarski Arhiv. 2007;77:365. [Google Scholar]
- 23.Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7:199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharaf M, el-Ansari MA, Matlin SA, Saleh NA. Four flavonoid glycosides from Peganum harmala. Phytochemistry. 1997;44:533–536. doi: 10.1016/s0031-9422(96)00531-6. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui S, Khan OY, Faizi S, Siddiqui BS. Studies in the chemical constituents of the seeds of Peganum harmala: isolation and structure elucidation of two β-carboline lactams―harmalanine and harmalacidine. Heterocycles. 1988;27:1401–1410. [Google Scholar]
- 26.Siddiqui S, Khan O Y, Siddiqui B S, Faizi S. Harmalidine, A β-carboline alkaloid from Peganum harmala. Phytochemistry. 1987;26:1548–1550. [Google Scholar]
- 27.Splettstoesser F, Bonnet U, Wiemann M, Bingmann D, Busselberg D. Modulation of voltage-gated channel currents by harmaline and harmane. Br J Pharmacol. 2005;144:52–58. doi: 10.1038/sj.bjp.0706024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K, Nomura I, Ninomiya M, Tanaka K, Koketsu M. Synthesis and antimicrobial activity of β-carboline derivatives with N2-alkyl modifications. Bioorg Med Chem Lett. 2018;28:2976–2978. doi: 10.1016/j.bmcl.2018.06.050. [DOI] [PubMed] [Google Scholar]