Abstract
Background/aims
To examine the association between rheumatoid arthritis (RA) and periodontitis or tooth loss.
Methods
The study used data from the fifth and sixth Korea National Health and Nutrition Examination Surveys conducted from 2010 to 2015. RA was defined as participant-reported physician-diagnosed RA that was being treated. Periodontitis and the number of natural teeth were determined by dental examination. Periodontitis was defined according to the community periodontal index (periodontal probing depth ≥ 4 mm). The association between RA and periodontitis or tooth loss was examined after controlling for confounding variables (e.g., age, smoking status, socioeconomic status, dental caries, frequency of toothbrushing, body mass index, alcohol consumption, and diabetes) in men and women. Subgroup analyses stratified by age were also performed.
Results
The study enrolled 20,297 participants aged ≥ 19 years (157 RA patients and 20,140 non-RA controls). There was no association between RA and periodontitis or tooth loss in men and women. Subgroup analyses in those aged < 60 years revealed a non-significant association between RA and periodontitis (adjusted odds ratio, 1.53; p = 0.162), but they revealed a significant association between RA and tooth loss (adjusted β, 0.20; p = 0.042).
Conclusions
RA was not associated with periodontitis, but was associated with tooth loss in younger adults. Younger RA patients are more likely to suffer tooth loss than general younger population; thus dental management is required.
Keywords: Arthritis, rheumatoid; Periodontitis; Tooth loss; Periodontal diseases; Epidemiologic studies
INTRODUCTION
Periodontitis is a chronic inflammatory disease initiated by bacterial infection between the teeth and their supporting tissues [1]. Periodontitis can lead to destruction of alveolar bone and is the most common cause of tooth loss in adults. Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the synovium lining the joints; severe disease results in destruction of cartilage and bone. In individuals with certain genetic backgrounds, RA is triggered by environmental factors such as smoking and infections. Periodontitis may be one such trigger; indeed, increasing evidence suggests an association between periodontitis and RA [2,3].
The pathogeneses of periodontitis and RA share environmental and genetic characteristics. For example, cigarette smoking increases the risk of both periodontitis and RA [4,5]. Several shared genetic factors are found in periodontitis and RA [6-8]. Smoking plus human leukocyte antigen (HLA)-DRB1 shared epitope alleles are associated with citrullination of peptides, which triggers anti-citrullinated peptide antibody (ACPA) responses in RA [9,10]. Periodontitis also shows an independent relationship with ACPA-positive RA [11]. Porphyromonas gingivalis, a unique periodontal pathogen that expresses the citrullinating enzyme peptidyl arginine deiminase (PAD), may explain this linkage with RA. A recent report shows that Aggregatibacter actinomycetemcomitans may trigger RA by inducing hypercitrullination [12]. Efforts to identify a causal relationship between periodontal pathogens and autoimmunity in RA are ongoing.
Epidemiologic studies support the relationship between periodontitis and RA. A study based on data from the third National Health and Nutrition Examination Survey (NHANES) in the USA reported a positive association of periodontitis and tooth loss with RA [13]. Another study based on national claims data from Taiwan also found an association between periodontitis and incidence of RA [14]. However, other population-based cohort studies do not show an association between periodontitis and RA [15-17]. These epidemiologic studies had some limitations in that they used inconsistent measures to define periodontitis or RA, or did not consider smoking status (the most important confounding factor).
The purpose of the present study was to examine the association between RA and periodontitis or tooth loss using data from Korean National Health and Nutrition Examination Survey (KNHANES). We assumed that patients with RA would be more likely to have periodontitis and tooth loss than non-RA controls. Possible confounding factors, including age, sex, smoking status, socioeconomic status, oral health status and behavior, body mass index (BMI), and diabetes, were considered.
METHODS
Data source
Data were obtained from the fifth and sixth KNHANES, which was conducted by the Korea Centers for Disease Control and Prevention during 2010 to 2015. KNHANES is a representative and non-institutionalized survey that monitors health behaviors, disease, and nutritional status of the South Korean population. Approximately 3,840 household units from 192 regions are sampled annually. Household sample units were based on the registered population in 2009 and the Population and Housing Census, 2010. Household members underwent interview and medical examinations by well-trained health professionals. Dental examinations were performed by trained dentists.
The number of candidates from the fifth (2010 to 2012) and sixth (2013 to 2015) KNHANES were 31,596 and 29,321, respectively. Response rates were 80.8% and 78.3%, respectively (https://knhanes.cdc.go.kr). In accordance with the Declaration of Helsinki, informed consent was obtained from each survey participant. The survey was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C).
Study population
Among the 48,482 participants from the fifth and sixth KNHANES, 37,633 participants aged ≥ 19 years were included in the study (Fig. 1). Pregnant women and individuals with recent periodontal treatment (< 1 year) were excluded. Participants with missing data were also excluded. Thus, 20,297 participants were included in the final analysis.
Figure 1.
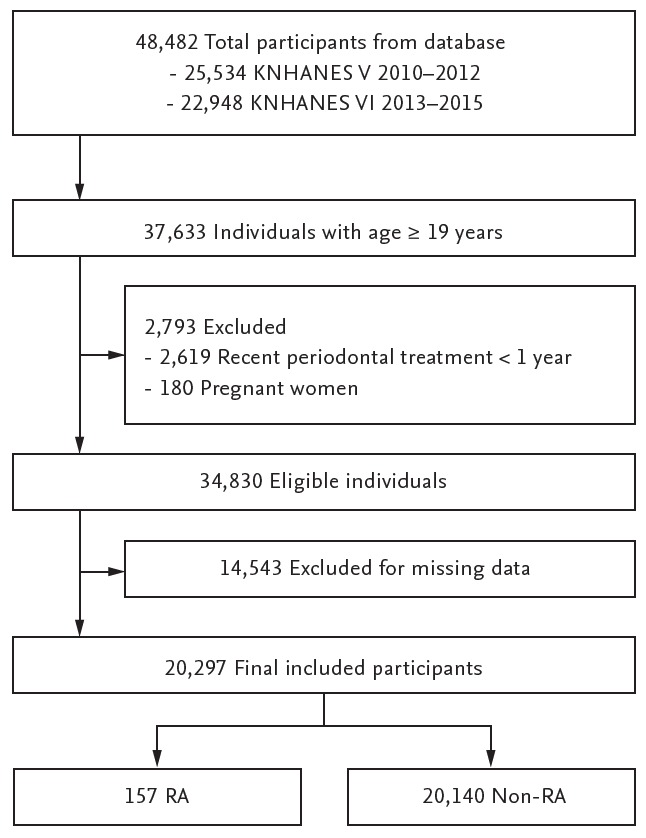
Flow chart showing the study design and populations. KNHANES, Korean National Health and Nutrition Examination Survey; RA, rheumatoid arthritis.
Classification of RA
Study participants were asked if (1) they had physician-diagnosed RA and (2) whether they were receiving treatment for RA. We defined RA as a physician-diagnosed disease that was being treated. Musculoskeletal examinations and laboratory tests (e.g., rheumatoid factor and ACPA levels) are not performed for KNHANES.
Dental examination and definition of periodontitis and tooth loss
Periodontal status was examined using a community periodontal index (CPI) probe, with a 20 g force applied to the periodontal pocket. Teeth were divided into six sections: right upper, upper front, left upper, left lower, lower front, and right lower. CPI scores were registered for each section. Periodontal probing was performed for ten index teeth (17, 16, 11, 26, 27, 37, 36, 31, 46, and 47, in that order). If there were no remaining index teeth in a particular section, other teeth were examined and the highest score was allocated as the CPI score for that section. Periodontitis was defined as a CPI score of 3 or 4 (range, 0 to 4) in at least one of the six sections. A CPI score of 3 meant a periodontal probing depth ≥ 4 mm and a CPI score of 4 meant a periodontal probing depth ≥ 6 mm.
The number of natural teeth (except wisdom teeth) was evaluated and categorized as 28, 20 to 27, or 0 to 19. Fewer than 28 (0 to 27) natural teeth was defined as tooth loss. Severe tooth loss was defined as fewer than 20 (0 to 19) natural teeth [18,19].
Assessment of other variables
Age, sex, BMI, socioeconomic status (education level and household income), smoking status, alcohol consumption, physical activity, presence of diabetes mellitus, and oral health status and behavior were evaluated. BMI was categorized as underweight (BMI < 18.5), normal (BMI ≥ 18.5 and < 25), and obese (BMI ≥ 25). Education level was determined according to whether a participant finished high school. Household income was divided into quartiles and classified as “lower income” (quartile 1 or 2) and “higher income” (quartile 3 or 4). Smoking status was classified as “never smokers” (< 100 cigarettes in their lifetime), “former smokers” (≥ 100 cigarettes in their lifetime but not currently smoking), and “current smokers” (≥ 100 cigarettes in their lifetime and currently smoking). Alcohol consumption was classified as “non-drinkers” (≤ once per month), “mild to moderate drinkers” (2 to 4 times per month or 2 to 3 times per week), and “heavy drinkers” (≥ 4 times per week). Physical activity was based on walking frequency (at least 10 minutes continuously) over a period of 1 week and was classified as “< 5 days per week” and “≥ 5 days per week.” Diabetes mellitus was diagnosed when fasting glucose levels were ≥ 126 mg/dL or the participant was receiving oral glucose-lowering agents and/or insulin. Presence of dental caries was evaluated and frequency of daily toothbrushing was classified as “< twice” and “≥ twice per day.”
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) with survey procedures to produce nationally representative estimates and account for the complex sampling design. Data were expressed as the mean (standard error [SE]) for continuous variables and as the number (percentage) for categorical variables. Variables such as age, sex, BMI, socioeconomic status, smoking status, alcohol consumption, physical activity, presence of diabetes mellitus, and oral health status and behavior (including presence of periodontitis and the number of natural teeth) were compared between RA and non-RA controls. Group comparisons were assessed using the F-test in PROC SURVEYREG (continuous variables) or the Rao-Scott chi-square test in PROC SURVEYFREQ (categorical variables). The association between RA and periodontitis was examined by univariable and multivariable logistic regression analyses (PROC SURVEYLOGISTIC), setting periodontitis as a dependent variable and RA as an independent variable. Crude and adjusted odds ratios with 95% confidence intervals were determined. Simple and multiple linear regression analyses were conducted to investigate the association between RA and tooth loss, setting the log-transformed number of missing teeth as a dependent variable and RA as an independent variable. Standard regression coefficients (β) with SEs were reported. Multivariable regression analyses were performed adjusting for confounding factors such as age, sex, smoking status, education level, dental caries, frequency of toothbrushing, BMI, alcohol consumption, physical activity, and presence of diabetes mellitus. Subgroup analyses stratified according to sex (men and women) or age (< 60 and ≥ 60 years) were performed using less covariates in some analyses due to decreased population of subgroups. p values < 0.05 were considered statistically significant.
RESULTS
Characteristics of study participants
The 20,297 participants included 157 patients with RA and 20,140 non-RA controls. The weighted number of RA patients and non-RA controls was 146,913 and 22,992,779, respectively, based on nationally representative KNHANES data. All values were obtained by considering "weights" to make the results of this study can represent the whole national population.
The characteristics of study participants are described in Table 1. Patients with RA were older than non-RA controls. Education level and household incomes were significantly lower for patients with RA than for non-RA controls. RA patients were more likely to be obese than non-RA participants, but the difference was not significant in women. For male participants, never smokers were more common in the non-RA control group (13.7% RA vs. 28.2% non-RA) and former smokers were more common in the RA group (47.5% RA vs. 29.8% non-RA); however, the differences were not statistically significant (p = 0.087). Approximately 90% of female participants in the RA and non-RA groups were never smokers. Alcohol drinkers (mild to moderate and heavy) were more common in the non-RA group.
Table 1.
Characteristics of the study population
| Characteristic | Overall |
Male |
Female |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-RA (na = 20,140; nb = 22,992,799) | RA (na = 157; nb = 146,913) | p value | Non-RA (na = 8,564; nb = 11,499,711) | RA (na = 35; nb = 36,144) | p value | Non-RA (na = 11,576; nb = 11,493,068) | RA (na = 122; nb = 110,769) | p value | |
| Age, yr | |||||||||
| Mean ± SE | 43.9 ± 0.2 | 56.3 ± 1.4 | < 0.001 | 42.9 ± 0.2 | 56.1 ± 1.7 | < 0.001 | 44.8 ± 0.2 | 56.3 ± 1.7 | < 0.001 |
| < 60 | 14,421 (82.9) | 73 (60.8) | < 0.001 | 6,049 (84.7) | 21 (72.6) | < 0.001 | 8,372 (81.1) | 52 (56.9) | < 0.001 |
| ≥ 60 | 5,719 (17.1) | 84 (39.2) | 2,515 (15.3) | 14 (27.4) | 3,204 (18.9) | 70 (43.1) | |||
| Sex | |||||||||
| Male | 8,564 (50.0) | 35 (24.6) | < 0.001 | - | - | - | - | - | - |
| Female | 11,576 (50.0) | 122 (75.4) | - | - | - | - | |||
| BMI, kg/m2 | |||||||||
| < 18.5 | 863 (4.8) | 9 (7.7) | 0.349 | 210 (2.7) | 0 | < 0.001 | 653 (6.9) | 9 (10.2) | 0.435 |
| ≥ 18.5 to < 25 | 12,805 (63.1) | 102 (58.6) | 5,185 (59.8) | 22 (52.1) | 7,620 (66.4) | 80 (60.7) | |||
| ≥ 25 | 6,472 (32.1) | 46 (33.7) | 3,169 (37.5) | 13 (47.9) | 3,303 (26.7) | 33 (29.1) | |||
| Educational level | |||||||||
| < High school | 6,370 (23.9) | 103 (55.1) | < 0.001 | 2,212 (18.6) | 18 (48.3) | < 0.001 | 4,158 (29.3) | 85 (57.4) | < 0.001 |
| ≥ High school | 13,770 (76.1) | 54 (44.9) | 6,352 (81.4) | 17 (51.7) | 7,418 (70.7) | 37 (42.6) | |||
| Household income | |||||||||
| Lower income | 8,529 (39.2) | 99 (62.0) | < 0.001 | 3,507 (37.2) | 20 (58.8) | 0.018 | 5,022 (41.3) | 79 (63.0) | < 0.001 |
| Higher income | 11,611 (60.8) | 58 (38.0) | 5,057 (62.8) | 15 (41.2) | 6,554 (58.7) | 43 (37.0) | |||
| Smoking status | |||||||||
| Never-smokers | 12,796 (59.3) | 115 (72.7) | 0.006 | 2,173 (28.2) | 4 (13.7) | 0.087 | 10,623 (90.3) | 111 (91.9) | 0.466 |
| Former smokers | 3,477 (16.9) | 23 (15.4) | 3,079 (29.8) | 18 (47.5) | 398 (3.9) | 5 (4.9) | |||
| Current smokers | 3,867 (23.9) | 19 (11.9) | 3,312 (42.0) | 13 (38.8) | 555 (5.8) | 6 (3.2) | |||
| Alcohol consumption | |||||||||
| Nondrinker | 11,230 (50.7) | 126 (74.8) | < 0.001 | 2,975 (33.3) | 19 (57.8) | 0.014 | 8,255 (68.2) | 107 (80.4) | 0.098 |
| Mild to moderate drinker | 7,567 (42.4) | 26 (21.5) | 4,488 (55.2) | 13 (34.3) | 3,079 (29.5) | 13 (17.4) | |||
| Heavy drinker | 1,343 (6.9) | 5 (3.6) | 1,101 (11.5) | 3 (7.9) | 242 (2.2) | 2 (2.2) | |||
| Physical activityc | |||||||||
| < 5 days of walking/week | 9,412 (45.3) | 90 (54.6) | 0.058 | 3,866 (43.9) | 18 (47.2) | 0.731 | 5,546 (46.7) | 72 (57.1) | 0.061 |
| ≥ 5 days of walking/week | 10,728 (54.7) | 67 (45.4) | 4,698 (56.1) | 17 (52.8) | 6,030 (53.3) | 50 (42.9) | |||
| Diabetes mellitus | |||||||||
| Normal or IFG | 18,175 (92.2) | 132 (86.0) | 0.015 | 7,934 (94.2) | 33 (94.1) | 0.313 | 10,616 (93.1) | 103 (86.0) | 0.012 |
| DM | 1,965 (7.8) | 25 (14.0) | 630 (5.8) | 2 (5.9) | 960 (6.9) | 19 (14.0) | |||
| Oral health status & behavior | |||||||||
| Periodontitis | |||||||||
| No | 14,647 (75.7) | 103 (67.8) | 0.046 | 5,681 (71.2) | 19 (56.9) | 0.103 | 8,966 (80.1) | 84 (71.3) | 0.042 |
| Yes | 5,493 (24.3) | 54 (32.2) | 2,883 (28.8) | 16 (43.1) | 2,610 (19.9) | 38 (28.7) | |||
| No. of natural teeth | |||||||||
| Mean ± SE | 25.7 ± 0.05 | 22.6 ± 0.6 | < 0.001 | 25.7 ± 0.1 | 23.2 ± 1.1 | 0.018 | 25.6 ± 0.1 | 22.5 ± 0.8 | < 0.001 |
| 28 | 9,280 (53.2) | 39 (31.2) | < 0.001 | 3,872 (54.4) | 9 (25.6) | 0.004 | 5,408 (52.1) | 30 (33.0) | < 0.001 |
| 20–27 | 8,459 (38.7) | 75 (48.4) | 3,615 (37.8) | 17 (58.7) | 4,844 (39.5) | 58 (45.0) | |||
| 0–19 | 2,401 (8.1) | 43 (20.4) | 1,077 (7.8) | 9 (15.8) | 1,324 (8.4) | 34 (22.0) | |||
| Dental caries of permanent teeth | |||||||||
| 0 | 14,042 (99.4) | 6,098 (99.3) | 0.226 | 5,613 (99.8) | 2,951 (99.5) | 0.021 | 8,429 (99.1) | 3,147 (98.9) | 0.544 |
| ≥ 1 | 101 (0.6) | 56 (0.7) | 17 (0.2) | 18 (0.5) | 84 (0.9) | 38 (1.1) | |||
| Frequency of daily toothbrushing | |||||||||
| < 2 | 2,283 (10.7) | 24 (13.8) | 0.264 | 1,367 (14.0) | 4 (8.0) | 0.285 | 916 (7.3) | 20 (15.7) | 0.003 |
| ≥ 2 | 17,857 (89.3) | 133 (86.2) | 7,197 (86.0) | 31 (92.0) | 10,660 (92.7) | 102 (84.3) | |||
Values are presented as number (%). Number is a crude number of study participants. Percentage is a weighted percentage from the national population.
RA, rheumatoid arthritis; SE, standard error; BMI, body mass index; IFG, impaired fasting glucose; DM, diabetes mellitus.
Crude number of study participants.
Weighted number of subjects, which represents the national population.
Physical activity was based on walking frequency (at least 10 minutes continuously) over a period of 1 week.
Periodontitis was significantly more common in RA patients than in non-RA controls in women (28.7% vs. 19.9%, respectively; p = 0.042). The number of natural teeth was significantly lower in RA patients than in non-RA controls. Tooth loss (number of natural teeth: 20 to 27 and 0 to 19) was significantly more common in RA patients than in non-RA controls. Dental caries were more common in RA patients than in non-RA controls, particularly in men.
Association between RA and periodontitis
Univariable analysis conducted for the female population revealed that periodontitis was associated with RA; however, this did not remain true in multivariable analyses adjusted for age, smoking status, education level, dental caries, frequency of toothbrushing, BMI, alcohol consumption, physical activity, and diabetes mellitus (Table 2). Multivariable analysis performed for male was adjusted for age, smoking status, education level, dental caries, and frequency of toothbrushing because of the relatively small sample size of men. Subgroup analysis stratified by age revealed no association between RA and periodontitis, although the adjusted OR of RA was approximately 1.2 for periodontitis in those aged < 60 years (Table 3).
Table 2.
Association between RA and periodontitis in the overall, male, and female populations
| Variable | Overall |
Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p value | Adjusted OR (95% CI)a | p value | Crude OR (95% CI) | p value | Adjusted OR (95% CI)b | p value | Crude OR (95% CI) | p value | Adjusted OR (95% CI)a | p value | |
| RA | ||||||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Yes | 1.48 (1.00–2.18) | 0.048 | 0.88 (0.57–1.36) | 0.556 | 1.87 (0.87–4.03) | 0.108 | 0.88 (0.41–1.86) | 0.73 | 1.62 (1.02–2.59) | 0.043 | 0.91 (0.54–1.53) | 0.724 |
Crude and adjusted OR (95% CI) of RA for periodontitis are presented.
RA, rheumatoid arthritis; OR, odds ratio; CI, confidence interval.
Adjusted for age, (sex), smoking, education level, dental caries, frequency of toothbrushing, body mass index, alcohol consumption, physical activity, and diabetes mellitus.
Adjusted for age, smoking, education level, dental caries, and frequency of toothbrushing.
Table 3.
Association between RA and periodontitis in populations stratified by age
| Variable | Age < 60 yr |
Age ≥ 60 yr |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p value | Adjusted OR (95% CI)a | p value | Crude OR (95% CI) | p value | Adjusted OR (95% CI)a | p value | |
| RA | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.34 (0.75–2.39) | 0.319 | 1.16 (0.58–2.33) | 0.684 | 0.96 (0.58–1.61) | 0.881 | 1.00 (0.59–1.71) | 0.995 |
Crude and adjusted OR (95% CI) of RA for periodontitis are presented.
RA, rheumatoid arthritis; OR, odds ratio; CI, confidence interval
Adjusted for sex, smoking, education level, dental caries, and frequency of toothbrushing.
Association between RA and tooth loss
Univariable analysis identified an association between tooth loss and RA, but the association was lost in multivariable analyses adjusted for confounding variables (Table 4). However, tooth loss was significantly associated with RA in those aged < 60 years (β = 0.20, p = 0.042 when adjusted for sex, smoking status, education level, dental caries, and frequency of toothbrushing) (Table 5).
Table 4.
Association between RA and tooth loss in the overall, male, and female populations
| Variable | Overall |
Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude β (SE) | p value | Adjusted β (SE)a | p value | Crude β (SE) | p value | Adjusted β (SE)b | p value | Crude β (SE) | p value | Adjusted β (SE)a | p value | |
| RA | ||||||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Yes | 0.54 (0.10) | < 0.001 | 0.08 (0.08) | 0.313 | 0.56 (0.17) | 0.001 | 0.07 (0.15) | 0.651 | 0.51 (0.12) | < 0.001 | 0.08 (0.09) | 0.375 |
Standard regression coefficients (β) with SE of RA for tooth loss are presented. Tooth loss variables were entered into linear regression models after log transformation [Ln (number of missing teeth + 1)].
RA, rheumatoid arthritis; SE, standard error.
Adjusted for age, (sex), smoking, education level, dental caries, and frequency of toothbrushing, body mass index, alcohol consumption, physical activity, and diabetes mellitus.
Adjusted for age, smoking, education level, dental caries, and frequency of toothbrushing.
Table 5.
Association between RA and tooth loss in populations stratified by age
| Variable | Age < 60 yr |
Age ≥ 60 yr |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude β (SE) | p value | Adjusted β (SE)a | p value | Crude β (SE) | p value | Adjusted β (SE)a | p value | |
| RA | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.30 (0.11) | 0.005 | 0.20 (0.10) | 0.042 | 0.18 (0.13) | 0.166 | 0.14 (0.13) | 0.314 |
Standard regression coefficients (β) with SE of RA for tooth loss are presented. Tooth loss variables were entered into linear regression models after log transformation [Ln (number of missing teeth + 1)].
RA, rheumatoid arthritis; SE, standard error.
Adjusted for sex, smoking, education level, dental caries, and frequency of toothbrushing.
DISCUSSION
Here, we examined the association between RA and periodontitis or tooth loss using nationally representative data from Korea. There was a strong association between RA and tooth loss in younger adults. Patients with RA aged < 60 years tended to have periodontitis, but the result was not statistically significant. There were no significant associations between RA and periodontitis or tooth loss in those aged ≥ 60 years.
Age seems to be an important confounding factor in the relationship between RA and periodontitis or tooth loss. The risk of periodontitis rises steadily with age and is highest in adults aged > 65 years [5]. Previous studies show that tooth loss is associated with several factors, including older age, smoking, low socioeconomic status, and comorbidities (RA and diabetes mellitus) [20-23]. Here, we found a significant association between tooth loss and RA after stratification by age. RA was an independent factor associated with tooth loss in younger adults, whereas factors other than RA were associated with tooth loss in older adults. The present study suggests that the association between RA and periodontitis or tooth loss may depend on age.
Periodontitis was not associated with RA, but tooth loss was significantly associated with RA in younger adults. Although dental caries and periodontitis are primary causes of tooth loss [24], the present study revealed the association between RA and tooth loss independent of dental caries. Our results support that periodontitis in younger RA patients may progress easily to tooth loss. Some factors might influence on the association between RA and tooth loss, such as reduced salivary flow rates in RA patients [25].
Although we found no significant association between RA and periodontitis, previous studies do demonstrate a relationship [2,3,26,27]. Some factors in the present study may make it difficult to identify an association between RA and periodontitis. First, periodontitis may be associated with specific types of RA, such as seropositive RA. Smoking increases the risk of developing seropositive, but not seronegative, RA [28]. Smoking is also a wellknown risk factor for periodontitis [5]. Based on these ideas, there may be an association between periodontitis and seropositive RA. A case-control study by Mikuls et al. [11] confirmed that periodontitis was not associated with RA overall but was associated with seropositive RA. The contribution of seropositivity to the relationship between RA and periodontitis has been reported by other studies [13,29]. Second, the association between RA and periodontitis may be restricted to the patients harboring specific genes. For example, smoking is a risk factor for ACPA only in patients with RA who carry shared epitope alleles [9,30]. In addition, different shared epitope subtypes contribute differently to development of ACPA and in their interaction with smoking [31]. From an ethnical perspective, patients with RA express different patterns of susceptibility alleles [32], and these genetic differences may explain the inconsistent findings regarding associations between RA and periodontitis.
Some studies demonstrate linkage between RA and periodontal disease, and recent basic research has identified possible periodontal pathogens that can induce RA-associated autoimmunity. For example, P. gingivalis expresses its own PAD, which can generate citrullinated autoantigens in the host and auto-citrullinate bacteria. Studies show that this unique bacterium may initiate early citrullination, thereby triggering development of an ACPA response in patients with RA [33]. However, other bacteria can also trigger citrullination. Konig et al. [12] found that one type of oral bacterium, A. actinomycetemcomitans, might be an important trigger in RA patients. They studied the oral microbial composition of patients with periodontal disease. Among microbial pathogens associated with periodontal disease, only A. actinomycetemcomitans induced hypercitrullination which was similar to those found in RA joints. Pore-forming toxin was found to activate PAD enzymes in host neutrophils by affecting calcium influx. Thus, specific periodontal pathogens, rather than periodontal disease itself, might trigger citrullination and explain RA pathogenesis.
The present study has some limitations. First, classification of RA was based on patient self-reporting, which could be inaccurate. Self-reported arthritis yields variable results in terms of sensitivity, specificity, and positive and negative predictive values [34,35]. Although self-reported RA shows low accuracy, coupling this with self-reported medication data increases positive predictive value for RA [36,37]. Here, we based a diagnosis of RA on a combination of self-reported RA plus self-reported medication data. The prevalence of RA in our data was 0.8% which is similar to the known prevalence of RA [38]. Second, we did not determine the causal relationship between RA and periodontitis or tooth loss as the present study was cross-sectional in design.
Nevertheless, the data have several implications. We considered major confounding variables that influence the association between RA and periodontal disease. Older age and cigarette smoking increase the risk of RA and periodontitis. In addition, a larger proportion of Korean women than men are never smokers; thus, smoking status and sex had to be considered. Moreover, periodontal examination performed by trained dentists provides an accurate diagnosis of periodontitis. Previous epidemiologic studies are limited as they did not perform accurate diagnosis of periodontal disease, nor did they consider important confounding variables [14-16].
In conclusion, RA was not associated with periodontitis; however, it was associated with tooth loss in younger adults. Older age was an important factor in the association between RA and periodontitis or tooth loss. The results of this study suggest that RA patients with periodontitis may be more likely to experience tooth loss than non-RA controls. Further research should take into account seropositivity and/or genetic factors, which may help to explain the association between RA and periodontitis more precisely.
KEY MESSAGE
1. Rheumatoid arthritis is associated with tooth loss in younger adults.
2. Younger rheumatoid arthritis patients with periodontitis may progress easily to tooth loss.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0016). Statistical consultation was supported by the Department of Biostatistics of the Catholic Research Coordinating Center. We thank Mi Sun Park for her statistical support.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingham CO, 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013;25:345–353. doi: 10.1097/BOR.0b013e32835fb8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leech MT, Bartold PM. The association between rheumatoid arthritis and periodontitis. Best Pract Res Clin Rheumatol. 2015;29:189–201. doi: 10.1016/j.berh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16:R61. doi: 10.1186/ar4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eke PI, Wei L, Thornton-Evans GO, et al. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J Periodontol. 2016;87:1174–1185. doi: 10.1902/jop.2016.160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonfil JJ, Dillier FL, Mercier P, et al. A "case control" study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO) J Clin Periodontol. 1999;26:77–84. doi: 10.1034/j.1600-051x.1999.260203.x. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer AS, Jochens A, Dommisch H, et al. A large candidate-gene association study suggests genetic variants at IRF5 and PRDM1 to be associated with aggressive periodontitis. J Clin Periodontol. 2014;41:1122–1131. doi: 10.1111/jcpe.12314. [DOI] [PubMed] [Google Scholar]
- 8.Ishida K, Kobayashi T, Ito S, et al. Interleukin-6 gene promoter methylation in rheumatoid arthritis and chronic periodontitis. J Periodontol. 2012;83:917–925. doi: 10.1902/jop.2011.110356. [DOI] [PubMed] [Google Scholar]
- 9.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 10.Scally SW, Petersen J, Law SC, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. 2013;210:2569–2582. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikuls TR, Payne JB, Yu F, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–1100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konig MF, Abusleme L, Reinholdt J, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- 14.Chen HH, Huang N, Chen YM, et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: a nationwide, population-based, case-control study. Ann Rheum Dis. 2013;72:1206–1211. doi: 10.1136/annrheumdis-2012-201593. [DOI] [PubMed] [Google Scholar]
- 15.Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol. 2010;37:1800–1804. doi: 10.3899/jrheum.091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demmer RT, Molitor JA, Jacobs DR, Jr, Michalowicz BS. Periodontal disease, tooth loss and incident rheumatoid arthritis: results from the First National Health and Nutrition Examination Survey and its epidemiological follow-up study. J Clin Periodontol. 2011;38:998–1006. doi: 10.1111/j.1600-051X.2011.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson K, Nise L, Kats A, et al. Prevalence of periodontitis in patients with established rheumatoid arthritis: a Swedish population based case-control study. PLoS One. 2016;11:e0155956. doi: 10.1371/journal.pone.0155956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han DH, Khang YH, Lee HJ. Association between adult height and tooth loss in a representative sample of Koreans. Community Dent Oral Epidemiol. 2015;43:479–488. doi: 10.1111/cdoe.12175. [DOI] [PubMed] [Google Scholar]
- 19.Song IS, Han K, Ryu JJ, Park JB. Association between underweight and tooth loss among Korean adults. Sci Rep. 2017;7:41524. doi: 10.1038/srep41524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, Neiva R, Wang HL. Risk indicators for tooth loss due to periodontal disease. J Periodontol. 2005;76:1910–1918. doi: 10.1902/jop.2005.76.11.1910. [DOI] [PubMed] [Google Scholar]
- 21.Musacchio E, Perissinotto E, Binotto P, et al. Tooth loss in the elderly and its association with nutritional status, socio-economic and lifestyle factors. Acta Odontol Scand. 2007;65:78–86. doi: 10.1080/00016350601058069. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Okoro CA, Oh J, Fuller DL. Sociodemographic and health-related risk factors associated with tooth loss among adults in Rhode Island. Prev Chronic Dis. 2013;10:E45. doi: 10.5888/pcd10.110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando A, Ohsawa M, Yaegashi Y, et al. Factors related to tooth loss among community-dwelling middle-aged and elderly Japanese men. J Epidemiol. 2013;23:301–306. doi: 10.2188/jea.JE20120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapple IL, Bouchard P, Cagetti MG, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44 Suppl 18:S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 25.Silvestre-Rangil J, Bagan L, Silvestre FJ, Bagan JV. Oral manifestations of rheumatoid arthritis. A cross-sectional study of 73 patients. Clin Oral Investig. 2016;20:2575–2580. doi: 10.1007/s00784-016-1745-z. [DOI] [PubMed] [Google Scholar]
- 26.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 27.Detert J, Pischon N, Burmester GR, Buttgereit F. The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther. 2010;12:218. doi: 10.1186/ar3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson K, Nise L, Alfredsson L, et al. Seropositivity combined with smoking is associated with increased prevalence of periodontitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77:1236–1238. doi: 10.1136/annrheumdis-2017-212091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLADRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Helm-van Mil AH, Verpoort KN, le Cessie S, Huizinga TW, de Vries RR, Toes RE. The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum. 2007;56:425–432. doi: 10.1002/art.22373. [DOI] [PubMed] [Google Scholar]
- 32.Lee HS, Lee KW, Song GG, Kim HA, Kim SY, Bae SC. Increased susceptibility to rheumatoid arthritis in Koreans heterozygous for HLA-DRB1*0405 and *0901. Arthritis Rheum. 2004;50:3468–3475. doi: 10.1002/art.20608. [DOI] [PubMed] [Google Scholar]
- 33.Wegner N, Wait R, Sroka A, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bombard JM, Powell KE, Martin LM, Helmick CG, Wilson WH. Validity and reliability of self-reported arthritis: Georgia senior centers, 2000-2001. Am J Prev Med. 2005;28:251–258. doi: 10.1016/j.amepre.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Lo T, Parkinson L, Cunich M, Byles J. Discordance between self-reported arthritis and musculoskeletal signs and symptoms in older women. BMC Musculoskelet Disord. 2016;17:494. doi: 10.1186/s12891-016-1349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Formica MK, McAlindon TE, Lash TL, Demissie S, Rosenberg L. Validity of self-reported rheumatoid arthritis in a large cohort: results from the Black Women's Health Study. Arthritis Care Res (Hoboken) 2010;62:235–241. doi: 10.1002/acr.20073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walitt BT, Constantinescu F, Katz JD, et al. Validation of self-report of rheumatoid arthritis and systemic lupus erythematosus: The Women's Health Initiative. J Rheumatol. 2008;35:811–818. [PMC free article] [PubMed] [Google Scholar]
- 38.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4 Suppl 3:S265–S272. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]


