Abstract
Background/Aims
Methicillin-resistant Staphylococcus aureus (MRSA) is highly prevalent in hospitals, and has recently emerged in the community. The impact of methicillin-resistance on mortality and medical costs for patients with S. aureus bacteremia (SAB) requires reevaluation.
Methods
We searched studies with SAB or endocarditis using electronic databases including Ovid-Medline, Embase-Medline, and Cochrane Library, as well as five local databases for published studies during the period January 2000 to September 2011.
Results
A total of 2,841 studies were identified, 62 of which involved 17,563 adult subjects and were selected as eligible. A significant increase in overall mortality associated with MRSA, compared to that with methicillin-susceptible S. aureus (MSSA), was evidenced by an odds ratio (OR) of 1.95 (95% confidence interval [CI], 1.73 to 2.21; p < 0.01). In 13 endocarditis studies, MRSA increased the risk of mortality, with an OR of 2.65 (95% CI, 1.46 to 4.80). When three studies, which compared mortality rates between CA-MRSA and CA-MSSA, were combined, the risk of methicillin-resistance increased 3.23-fold compared to MSSA (95% CI, 1.25 to 8.34). The length of hospital stay in the MRSA group was 10 days longer than that in the MSSA group (95% CI, 3.36 to 16.70). Of six studies that reported medical costs, two were included in the analysis, which estimated medical costs to be $9,954.58 (95% CI, 8,951.99 to 10,957.17).
Conclusions
MRSA is still associated with increased mortality, longer hospital stays and medical costs, compared with MSSA in SAB in studies published since the year 2000.
Keywords: Methicillin resistance, Staphylococcus aureus, Bacteremia, Endocarditis, Mortality
INTRODUCTION
Hospital-acquired (HA) methicillin-resistant Staphylococcus aureus (MRSA) infections are a major cause of illness and death and impose serious economic costs on patients and hospitals. The estimated number of S. aureus-related hospitalizations increased by 62% from 294,570 to 477,927, and the estimated number of MRSA-related hospitalizations more than doubled, from 127,036 to 278,203, from 1999 through 2005 in the United States [1]. Published studies on mortality for patients with S. aureus bacteremia (SAB) indicated an increased risk of mortality for patients with MRSA compared to those with methicillin-susceptible S. aureus (MSSA) bacteremia [2]. Thus bacteremia due to HA-MRSA results in increased direct medical costs and hospital stays, compared with that due to MSSA [3].
Cases of MRSA have been documented among healthy community-dwelling persons without established risk factors for MRSA acquisition, lately defined as community-associated (CA)-MRSA [4]. Community-genotype strains carrying SCCmec type IV have now emerged as a significant cause of healthcare-associated (HCA) and hospital associated (HA) infections in the USA and European countries [5-9]. Despite the epidemiologic changes in hospital MRSA strains with the encroachment of CA-MRSA into healthcare settings [9,10], whether methicillin resistance adversely affects outcomes in patients with community-associated S. aureus bacteremia is unclear [11,12]. After the year 2000, newer antimicrobial agents active against MRSA have become available to treat MRSA and are in use as alternatives for treating serious MRSA infections. The efficacy of new antibiotics in terms of reducing mortality in patients infected with S. aureus, especially MRSA, has not been verified. Furthermore, progress in high-quality clinical management has been made in the last few years as evidenced by the fact that case fatality can be reduced by hospital infection control systems [13]. These factors, including the emergence of MRSA strains with reduced vancomycin susceptibility, enhanced the controversy regarding the clinical impact of methicillin resistance on outcomes in SAB [14].
Meta-analyses by Cosgrove et al. [2] and Whitby et al. [15] comparing the mortality rate of MRSA and MSSA bacteremia found that methicillin resistance was associated with an increased mortality. In a recent meta-analysis, a significant increase in mortality associated with MRSA bacteremia was evident in the odds ratio (OR) of 1.93 (95% confidence interval [CI], 1.54 to 2.42), when 31 articles were combined with data regarding mortality associated with both MSSA and MRSA bacteremia [2]. There were also worse outcomes in studies that involve nosocomial SAB, compared to those involving a significant proportion of CA-SAB [2]. However, in the era of the emergence of CA-MRSA and the advent of newer antimicrobial agents active against MRSA, the impact of methicillin-resistance on mortality and medical costs for patients with SAB needs to be reevaluated. Therefore, we performed a systematic review and meta-analysis to investigate the effect of methicillin-resistance on mortality, length of hospital stay and medical costs of patients with SAB based on reports published after the year 2000.
METHODS
Literature search and selection of eligible studies
We searched studies of SAB or endocarditis using electronic databases including Ovid-Medline, Embase-Medline, and the Cochrane Library, as well as five local databases providing information on Korean medical research, published from January 1, 2000 to September 15, 2011. We used the search filter recommended by the Scottish Intercollegiate Guidelines Network to efficiently identify cohort studies. We also reviewed the bibliographies of relevant articles to identify additional publications. A full-text search of eight databases in English or Korean were reviewed using the terms “Staphylococcus aureus” AND “bacteremia” OR “endocarditis.” Two reviewers (D.A.P. and S.M.L.) independently evaluated titles, abstracts and citations to assess relevance for full review. We applied no language restriction in the electronic database search, which was limited to studies involving humans.
The inclusion criteria were as follows: studies (1) targeting SAB or S. aureus endocarditis (SAE); (2) comparison of outcomes of MRSA and MSSA; (3) evaluating any type of mortality, the length of hospital stay (LOS) or medical costs; and (4) involving adults 18 years older. The exclusion criteria were as follows: (1) not original research; (2) animal or pre-clinical studies; (3) not cohort studies; (4) only an abstract; (5) studies not published in Korean or English; and (6) duplicate reports. Therefore, all cohort studies in adults with SAB or endocarditis were included if they compared outcomes of MRSA to those of MSSA. Outcomes of methicillin-resistance were analyzed in terms of all-cause mortality, in-hospital mortality, SAB-related mortality, and 30-day mortality. The LOS and medical costs were also compared between the MRSA and MSSA groups. Studies involving children or neonates and those of a case-control design were excluded. We also excluded studies involving the same population during an overlapping 1-year study period.
Since this study had evaluated the published data of applicable studies, it was not required to obtain approval by the Institutional Review Board. Obtaining written informed consent was not applicable in the performance of a meta-analysis where no foreseeable harm is expected to result from the study.
Data extraction
Using a standardized form developed in advance, two independent reviewers extracted the following pre-specified data: first author, publication year, country, study period, study setting, study design, total number of study participants, the number and proportion of individuals in the MRSA and MSSA groups, age, proportion of males, cases with nosocomial- and community-acquired bacteremia, SAE, and results of predetermined outcomes during the follow-up period. We also collected the adjusted estimates of mortality in SAB and endocarditis and confounding variables considered in the statistical models of each study. Agreement was obtained after discussion between the two reviewers. We did not assess the methodological quality of included studies because most did not differ in design or in the methods used for recruiting participants.
Data synthesis and analysis
We employed a random-effects model using the method described by DerSimonian and Laird [16] to synthesize data from included studies. For the outcome data on mortality, we calculated the OR and 95% CI as summary statistics. For continuous outcomes, such as the length of hospital stay and medical costs, weighted mean differences (WMDs) and 95% CIs were calculated.
We assessed statistical heterogeneity using the Cochrane Q-test (p < 0.10) and I2 statistic, with I2 > 50% indicating at least moderate heterogeneity [17]. To assess the potential explanations for heterogeneity, we performed subgroup analyses using pre-specified criteria including disease characteristics (bacteremia including mixed populations and endocarditis) and the type of infection (community-acquired infections and ≥ 70% vs. < 70% nosocomial infection). We also performed sensitivity analyses using summary estimates in studies adjusted for confounding variables. First, we used a funnel plot asymmetry approach to assess publication bias qualitatively, and then we confirmed the symmetry of the funnel plot using Begg and Mazumdar’s rank correlation test (Supplementary Fig. 1) [18,19]. If publication bias was suspected, we performed the Trim and Fill method to obtain symmetry in the funnel plot and to determine the effect of hypothetical studies on the pooled estimate [20]. Statistical analysis was performed using Review Manager version 5.0 (RevMan, The Cochrane Collaboration, Oxford, UK) and Stata software version 10.0 (SE, Stata Corp., College Station, TX, USA). A p value of 0.05 was regarded as statistically significant.
RESULTS
Study populations
A total of 2,841 studies were searched from January 2000 through September 2011. Of 2,075 studies from which duplicated reports were eliminated, 92 (eight studies in Korea and 84 in other countries) were selected after the first and second literature review. A flow diagram of identification of eligible studies is shown in Fig. 1. Of these, 62 cohort studies were selected as eligible that reported any outcome regarding mortality, LOS and medical costs after review of the full-text of articles (Table 1) [21-82]. Pooled data for 17,563 patients (6,390 MRSA and 11,173 MSSA) were included in the analysis. All were cohort studies, comprising 41 retrospective, 20 prospective and one both retro- and prospective study. The characteristics of the selected studies are shown in Table 1 according to year of publication.
Figure 1.

Flow diagram detailing reviewed articles and exclusion. DB, database.
Table 1.
Characteristics of the studies of Staphylococcus aureus bacteremia or endocarditis included in the systematic review
| No. | Author | Year | Study period | Country | Total no. of cases | Proportion of HAMRSA/CAMRSA/MSSAa,b,c | Population | IE (% of cases) | Types of outcomes | Mortality rate, % |
Crude OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | MSSA | |||||||||||
| 1 | Holmes [21] | 2011 | 2007.1–2008.11 | Australia | 532 | 22.2/15.8/62.0b | SAB | 30-Day mortality | 22.1 | 14.2 | 1.72 (1.09–2.71) | |
| New Zealand | ||||||||||||
| 2 | Kao [22] | 2011 | 2004.1–2004.12 | Taiwan | 137 | 42.3/5.8/51.8a | SAB | Hospital mortality | 43.9 | 21.1 | 2.93 (1.38–6.19) | |
| 3 | Lubart [23] | 2011 | 2004.1–2005.12 | Israel | 68 | 66.2/33.8c | SAB | 14-Day mortality | 40.0 | 13.0 | 4.44 (1.15–17.18) | |
| 4 | Park [24] | 2011 | 2003.1–2008.12 | Korea | 266 | 46.6/7.9/45.5a | SAB | 30-Day mortality | 21.4 | 28.9 | 0.67 (0.38–1.17) | |
| 5 | Big [25] | 2010 | 2004.1–2008.7 | USA | 76 | 60.5/39.5c | SAB | Hospital mortality | 34.8 | 20.0 | 2.13 (0.72–6.29) | |
| 6 | Kang [26] | 2010 | 2004.9–2006.8 | Korea | 709 | 46.4/53.6c | SAB/SAE | 31 (4.4) | 30-Day mortality | 33.1 | 17.1 | 2.40 (1.69–3.41) |
| 7 | Khan [27] | 2010 | 2007.7–2008.6 | Qatar | 53 | 13.2/86.8c | SAB | Hospital mortality | 28.6 | 28.3 | 1.02 (0.17–5.91) | |
| 8 | Kim [28] | 2010 | 2006.7–2008.12 | Canada | 684 | 27.9/72.1c | SAB | 30-Day mortality | 25.1 | 15.4 | 1.84 (1.22–2.77) | |
| 9 | Ponce-de-Le-on [29] | 2010 | 2003.1–2007.12 | Mexico | 172 | 45.9/0/54.1b | SAB | 30-Day mortality, hos- pital mortality, 7-day mortality, LOS | 21.5 | 21.5 | 1.0 (0.48–2.08) | |
| 10 | Takayama [30] | 2010 | 1990.1–2006.12 | Japan | 33 | 30.3/0/69.7b | SAE | 33 (100) | Hospital mortality | 70.0 | 34.8 | 4.38 (0.88–21.71) |
| 11 | Wehrhahn [31] | 2010 | 2-Year period | Australia | 81 | 0/22.2/77.8b | SAB/SAE | 15 (18.5) | 30-Day mortality | 22.2 | 11.1 | 2.29 (0.59–8.91) |
| 12 | Ammerlaan [32] | 2009 | 2007.1–2007.12 | Europe | 334 | 23.1/76.9c | SAB | 30-Day mortality | 26.0 | 23.3 | 1.15 (0.64–2.07) | |
| 13 | Ben-David [33] | 2009 | 2000.1–2003.8 | Israel | 182 | 52.2/0/47.8b | SAB | Hospital mortality, LOS, total medical cost | 25.3 | 18.4 | 1.5 (0.74–3.06) | |
| 14 | Khatib [34] | 2009 | 2002.1–2003.6 | USA | 78 | 78.2/21.8c | SAB | Hospital mortality | 34.4 | 5.9 | 8.4 (1.04–67.79) | |
| 15 | Kim [35] | 2009 | 1995.1.–2006.12 | Korea | 73 | 16.4/26.0/57.5b | SAE | 73 (100) | Hospital mortality, | 35.5 | 2.4 | 22.55 (2.72–187.07) |
| 16 | Rieg [36] | 2009 | 2002–2007 | Germany | 521 | 12.9/0/87.1a | SAB | Hospital mortality | 41.8 | 18.7 | 3.12 (1.82–5.35) | |
| 17 | Rubio-Terres [37] | 2009 | 2005.1–2005.12 | Spain | 366 | 26.8/6.3/66.9b | SAB | Hospital mortality, LOS (in wards), ICU stay, cost per episode bacteremia | 39.7 | 25.3 | 1.94 (1.22–3.09) | |
| 18 | Turnidge [38] | 2009 | 2007.6–2008.5 | Australia | 1865 | 24.1/75.9c | SAB | 30-Day mortality | 30.0 | 17.7 | 2.0 (1.57–2.55) | |
| New Zealand | ||||||||||||
| 19 | Allard [39] | 2008 | 1991–2005 | Canada | 815 | 8.3/0.1/91.5a | SAB | 30-Day mortality | 33.3 | 23.1 | 1.67 (0.98–2.83) | |
| 20 | Baroudi [40] | 2008 | 1990.1–2006.1 | USA | 27 | 55.6/44.4c | SAE | 27 (100) | Hospital mortality | 40.0 | 50.0 | 0.67 (0.14–3.09) |
| 21 | Libert [41] | 2008 | 2002.1–2004.12 | Belgium | 140 | 31.4/12.9/55.7b | SAB | Hospital mortality | 54.8 | 35.9 | 1.90 (0.97–3.76) | |
| 22 | Malani [42] | 2008 | 2004–2005 | USA | 68 | 52.9/47.1c | SAB | Hospital mortality | 25.0 | 12.5 | 3.88 (1.29–11.68) | |
| 23 | Bader [43] | 2007 | 2003.1–2004.12 | USA | 135 | 23.0/31.8/45.2b | SAB | Hospital mortality | 33.8 | 18.0 | 2.32 (1.03–5.22) | |
| 24 | Cagatay [44] | 2007 | 2001.10–2002.12 | Turkey | 57 | 80.7/19.3c | SAB | SAB-related mortality (30-day) | 54.3 | 63.6 | 0.68 (0.17–2.65) | |
| 25 | Das [45] | 2007 | 2001–11–2002–12 | UK | 140 | 49.3/10.7/40.0b | SAB | SAB-related mortality (within 10-day), LOS | 33.3 | 16.1 | 2.61 (1.12–6.08) | |
| 26 | Greiner [46] | 2007 | 1999.12–2005.5 | Germany | 109 | 18.3/7.4/74.3b | SAB | Total hospital cost | NR | NR | NR | |
| 27 | Hsu [47] | 2007 | 1995–2005 | Taiwan | 123 | 39.0/61.0c | SAE | 123 (100) | Hospital mortality | 41.7 | 16.0 | 3.75 (1.61–8.71) |
| 28 | Wang [48] | 2007 | 1990–2004 | Taiwan | 1148 | 74.1/25.9c | SAB | 30-Day mortality | 49.8 | 27.6 | 2.60 (1.95–3.47) | |
| 29 | Depuydt [49] | 2006 | 1992–2002 | Belgium | 32 | 59.4/40.6c | Bacteremic SAP | Hospital mortality | 72.2 | NR | NR | |
| 30 | Guilarde [50] | 2006 | 2000.1–2001.12 | Brazil | 111 | 55.0/45.0c | SAB | SAB-related mortality | 47.5 | 20.0 | 3.63 (1.54–8.53) | |
| 31 | Heo [51] | 2006 | 2000.1–2005.8 | Korea | 231 | 0/27.3/72.7b | SAB | Hospital mortality | 30.2 | 19.6 | 1.77 (0.91–3.41) | |
| 32 | Kim [52] | 2006 | 1999.1–2003.5 | Korea | 96 | 64.6/3.1/32.3a | SAB | Hospital mortality | 26.2 | 0.0 | 22.73 (1.32–391.68) | |
| 33 | Lesse [53] | 2006 | 1997.1–2003.12 | USA | 38 | 63.2/36.8c | SAB | Hospital mortality | 33.3 | 21.4 | 1.83 (0.4–8.49) | |
| 34 | Marra [54] | 2006 | 2003.12.15–2004.12.31 | USA | 91 | 46.2/53.8c | SAB | Hospital mortality | 26.2 | 4.1 | 8.69 (1.80–41.88) | |
| 35 | Nori [55] | 2006 | 1999.1–2004.2 | USA | 22 | 50.0/50.0c | SAE | 22 (100) | Hospital mortality | 54.5 | 45.5 | 1.44 (0.27–7.71) |
| 36 | Perovic [56] | 2006 | 1999.11–2002.10 | South Africa | 449 | 18.7/4.7/76.6b | SAB | SAB-related mortality (14-day) | 33.3 | 20.1 | 1.99 (1.23–3.23) | |
| 37 | Shorr [57] | 2006 | 2002–2003 | USA | 1540 | 21.2/6.2/72.6a | SAB | Hospital mortality | 23.5 | 16.4 | 1.57 (1.19–2.06) | |
| 38 | Wyllie [58] | 2006 | 1997.4–2004.3 | UK | 441 | 51.5/0/48.5b | SAB | 30-Day mortality | 33.5 | 27.1 | 1.35 (0.90–2.04) | |
| 39 | Cassettari [59] | 2005 | 1999.5–1999.8 | Brazil | 163 | 58.9/0/41.1b | SAB | Hospital mortality, SAB-related mortality (15-day) | 44.8 | 29.9 | 1.91 (0.99–3.69) | |
| 40 | DeRyke [60] | 2005 | 1999.1–2004.4 | USA | 60 | 70.0/0/30.0b | Bacteremic SAP | Hospital mortality, SAB-related mortality, infection-related LOS | 54.8 | 55.6 | 0.97 (0.32–2.94) | |
| 41 | Fowler [61] | 2005 | 2000.6–2003.12 | USA | 424 | 26.7/6.7/66.7a | SAE | 424 (100) | Hospital mortality | 29.8 | 23.3 | 1.39 (0.89–2.20) |
| Multicontinent | ||||||||||||
| 42 | Lodise [62] | 2005 | 1999.1–2001.1 | USA | 353 | 39.9/8.2/51.8b | SAB | SAB-related mortality, 30-day mortality, SAB-related LOS and hospital cost | 30.6 | 15.3 | 2.44 (1.45–4.10) | |
| 43 | Reed [63] | 2005 | 1996.7.–2001.8 | USA | 143 | 37.8/62.2 | SAB | Hospital-mortality, LOS, ICU stay, total hospital cost | 14.8 | 9.0 | 1.76 (0.62–5.01) | |
| 44 | Yoon [64] | 2005 | 1986.3–2004.3 | Korea | 32 | 18.8/12.5/68.8b | SAE | 32 (100) | Hospital mortality | 50.0 | 9.1 | 10.0 (1.48–67.55) |
| 45 | Chang [65] | 2004 | 1988.1–2002.12 | Taiwan | 12 | 66.7/33.3c | SAE | 12 (100) | Hospital mortality | 100 | 0 | 153.0 (2.58–9,077.05) |
| 46 | Cordova [66] | 2004 | 1997.7–1999.6 | Australia | 501 | 7.8/3.2/89.0a | SAB | Hospital mortality (within 16-day), 7-day mortality, LOS | 27.3 | 16.8 | 1.86 (0.98–3.53) | |
| 47 | Osmon [67] | 2004 | 2001.12–2002.9 | USA | 265 | 36.2/19.6/44.2a | SAB | Hospital mortality, LOS, ICU stay | 13.5 | 16.2 | 0.81 (0.41–1.59) | |
| 48 | Chang [68] | 2003 | 1994.8–1996.3 | USA | 64 | 15.6/15.6/68.8a | SAE | 64 (100) | 30-Day mortality, 14-Day mortality | 50.0 | 22.7 | 3.40 (1.10–10.47) |
| 49 | Kim [69] | 2003 | 1998.1–2002.3 | Korea | 29 | 48.3/51.7c | SAB | SAB-related mortality | 57.1 | 20.0 | 5.33 (1.02–27.76) | |
| 50 | Melzer [70] | 2003 | 1995.1–2000.12 | UK | 815 | 46.9/0/53.1b | SAB | SAB-related mortality, overall mortality | 29.6 | 13.6 | 2.66 (1.87–3.79) | |
| 51 | Na [71] | 2003 | 1990.1–2000.5 | Korea | 10 | 20.0/80.0c | SAE | 10 (100) | Hospital mortality | 100 | 25 | 13.00 (0.45–377.47) |
| 52 | Blot [72] | 2002 | 1992.1.–1998.12 | Belgium | 85 | 55.3/0/44.7b | SAB | Hospital mortality, 15-day mortality, 30-day mortality, ICU stay | 53.2 | 18.4 | 5.03 (1.85–13.69) | |
| 53 | Campillo [73] | 2002 | 1996.1–2001.3 | France | 83 | 90.4/0/9.6b | SAB/peritonitis | Hospital mortality | 60.0 | 75.0 | 0.5 (0.09–2.64) | |
| 54 | Talon [74] | 2002 | 1997.1–1998.12 | France | 99 | 11.1/19.2/69.7b | SAB | 51.7 | SAB-related mortality (14-day) | 43.3 | 20.3 | 3.00 (1.18–7.62) |
| 55 | Tumbarello [75] | 2002 | 1991.1–2000.12 | Italy | 129 | 24.8/7.0/68.2b | SAB | Hospital mortality, LOS | 34.1 | 11.4 | 4.04 (1.61–10.17) | |
| 56 | Cosgrove [76] | 2001 | 1997.7–2000.6 | USA | 348 | 27.6/72.4c | SAB | Hospital mortality, SAB-related mortality, SAB-related LOS and hospital charge | 22.9 | 19.8 | 1.20 (0.68–2.12) | |
| 57 | Morin [77] | 2001 | 1998.1–1998.12 | USA | 192 | 9.9/5.2/84.9a | SAB | Hospital mortality | 13.8 | 10.4 | 1.37 (0.43–4.42) | |
| 58 | Wisplinghoff [78] | 2001 | 1995.12–1997.5 | USA | 82 | 48.8/0/51.2b | SAB | Hospital mortality | 25.0 | 23.8 | 1.07 (0.39–2.92) | |
| 59 | Ibrahim [79] | 2000 | 1997.6–1999.7 | USA | 94 | 48.9/51.1c | SAB | Hospital mortality | 37.0 | 25.0 | 1.76 (0.73–4.27) | |
| 60 | Roghmann [80] | 2000 | 1995.10–1998.1 | USA | 125 | 22.7/7.0/70.3b | SAB | 30-Day mortality | 32.4 | 23.9 | 1.53 (0.66–3.57) | |
| 61 | Selvey [81] | 2000 | 1992–1997 | Australia | 504 | 37.3/0/62.7b | SAB | Hospital mortality, SAB-related mortality | 18.6 | 13.0 | 1.53 (0.94–2.51) | |
| 62 | Soriano [82] | 2000 | 1991.1–1998.12 | Spain | 908 | 19.9/4.8/75.2b | SAB/SAE | 31 (3.4) | SAB-related mortality (30-day), LOS | 21.8 | 8.9 | 2.84 (1.88–4.28) |
HA, hospital-acquired; MRSA, methicillin-resistant S. aurues; CA, community-acquired; MSSA, methicillin-susceptible S. aureus; IE, infective endocarditis; OR, odds ratio; CI, confidence interval; SAB, S. aureus bacteremia; SAE, S. aureus-associated infective endocarditis; LOS, length of hospital stay; ICU, intensive care unit; SAP, S. aureus-associated pneumonia.
Represents the proportion of cases which were epidemiologically defined according to CA- and HA-MRSA.
Represents the proportion of cases which were classified into community-onset and hospital-onset MRSA without definition of CA-MRSA.
Indicates the proportion of cases with MRSA, when onset of bacteremia was not defined according to the epidemiologic definition.
Mortality in patients with methicillin-resistant and methicillin-susceptible SAB and endocarditis
Of the 62 studies, 60 reported all-cause mortality including in-hospital mortality, 14- and 30-day mortality and SAB-related mortality. The clinical characteristics of all patients with MRSA and MSSA in the 62 studies are summarized in Table 1. A significant increase in all-cause mortality associated with MRSA was evident with a pooled OR of 1.95 (95% CI, 1.73 to 2.21; I2 = 44%) compared to that of MSSA (Fig. 2) [21-82]. The pooled OR for 40 studies that reported in-hospital mortality was 1.90 (95% CI, 1.57 to 2.28; I2 = 51%). In 13 studies that compared 30-day mortality rates in SAB, MRSA increased the odds of death 1.89-fold compared to MSSA (95% CI, 1.58 to 2.26; I2 = 40%). In the 16 studies that documented SAB or infection-related mortality, generic inverse variance methods were used. The pooled OR was 2.04 (95% CI, 1 63 to 2.55; I2 = 40%).
Figure 2.
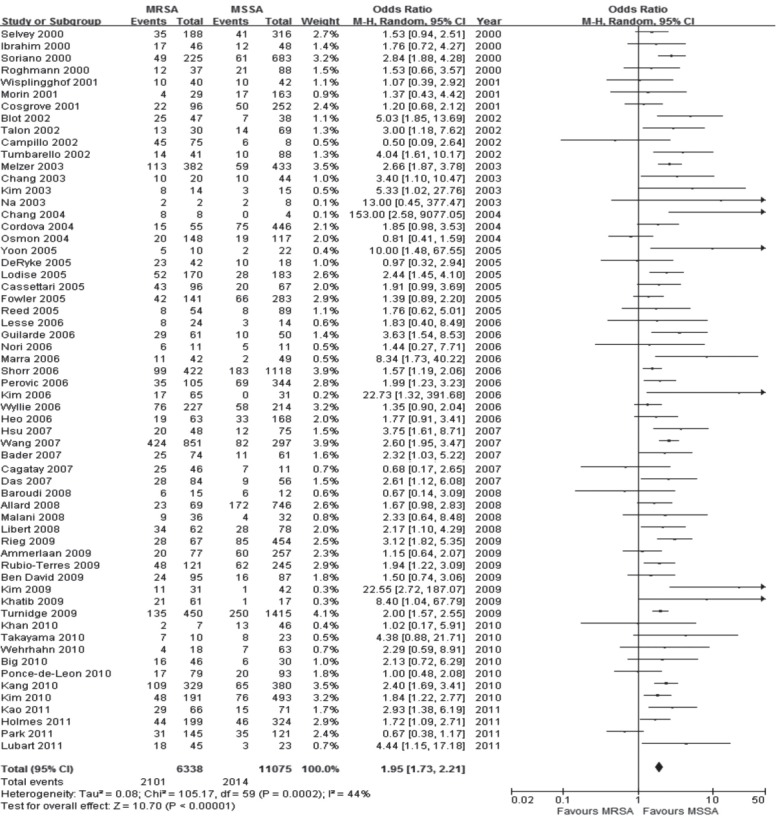
Forest plot summary of the results of 60 studies which reported all-cause mortality. MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; CI, confidence interval.
Of the 62 selected studies, 13 reported the outcomes of SAE, among which 10 involved a population with SAE [30,35,40,47,55,61,64,65,68,71], and the remaining three reported outcomes of SAE as part of SAB episodes [26,31,82]. Methicillin-resistance increased the risk of mortality by 2.65-fold in those patients (95% CI, 1.46 to 4.80; I2 = 50%). There was no significant heterogeneity among the results of these studies. Further analysis primarily involving the SAE population showed a pooled OR of 3.32 (95% CI, 1.68 to 6.59).
Mortality in patients with methicillin-resistant and methicillin-susceptible SAB and endocarditis in the Korean population
In a meta-analysis of eight studies which reported all-cause mortality in SAB and endocarditis in the Korean population, methicillin-resistance was associated with increases in mortality with a pooled OR of 3.14 (95% CI, 1.48 to 6.67) (Fig. 3) [24,26,35,51,52,64,69,71]. There was significant heterogeneity among the results of these studies (I2 = 76%). Of eight studies, three studies analyzed the outcomes of SAE in Korean populations [35,64,71]; in these, the mortality risk of MRSA increased 14.19-fold compared to that of MSSA (95% CI, 3.84 to 52.41).
Figure 3.
Forest plot summary of results of eight which reported all-cause mortality in Staphylococcus aureus bacteremia and endocarditis in the Korean population. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; CI, confidence interval.
Community- and hospital-acquired SAB
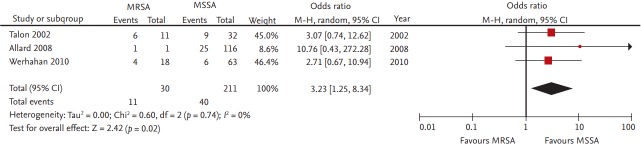
Twenty-two studies reported outcomes of CA-SAB; of these, only three studies compared mortality rates between CA-MRSA and CA-MSSA. MRSA increased the odds 3.23-fold, compared to MSSA (95% CI, 1.25 to 8.34) when the three studies were combined (Fig. 4) [31,39,74]. Forty-one studies reported the outcomes in patients of nosocomial SAB. In the 13 selected studies in which ≥ 70% of the cases of SAB were hospital-acquired, the pooled OR was 1.70 (95% CI, 1.29 to 2.25). In contrast, in 28 studies in which less than 70% were nosocomial, the OR was 1.95 (95% CI, 1.66 to 2.29).
Figure 4.
Forest plot summary of results of three studies which reported mortality rates between community-associated (CA)-methicillin-resistant Staphylococcus aureus (MRSA) and CA-methicillin-susceptible S. aureus (MSSA). CI, confidence interval.
LOS, ICU stay, and medical costs
LOS was divided into two categories for analysis—the total LOS and the length of stay after the onset of bacteremia. Eight studies reported total LOS (Table 2) [29,37,45,63,66,67,75,82]. Of them, four studies were combined for the meta-analysis of total LOS [37,63,67,75]. The average total LOS in the MRSA group was 10.03 days longer than that in the MSSA group; this difference was significant (WMD, 10.03; 95% CI, 3.36 to 16.70; I2 = 83%). The result of a sensitivity analysis, excluding the heterogeneous studies, indicated that patients with MRSA bacteremia stayed 6.72 days longer (WMD, 6.72; 95% CI, 3.38 to 10.0) than those with MSSA bacteremia without heterogeneity (I2 = 31%). Among six studies that reported length of stay after the onset of bacteremia, data from two studies [62,63] were included in the analysis and showed that the average stay was 5.02 days longer in the MRSA group than the MSSA group (WMD, 5.02; 95% CI, 2.66 to 7.38), with homogeneity (I2 = 0%). Four studies described the length of intensive care unit (ICU) stay. Patients with MRSA bacteremia stayed in the ICU 6.46 days longer (WMD, 6.46; 95% CI, 0.87 to 12.04), with heterogeneity among combined studies (I2 = 86%) than those with MSSA. Of six studies that reported medical costs (Table 3) [33,37,46,62,63,76], two were included in the analysis, and the estimated medical costs were $9,954.58 (WMD, 9,954.58; 95% CI, 8,951.99 to 10,957.17) with a statistically significant difference between groups and homogeneity between the two studies (I2 = 0%) [62,63].
Table 2.
Length of hospital stay
| No. | Author | Year | Populations | LOS, day |
p value | |
|---|---|---|---|---|---|---|
| MRSA | MSSA | |||||
| 1 | Ponce-de-Leon [29] | 2010 | SAB | 31 (1–585)a | 21 (0–140)a | 0.003 |
| 2 | Rubio-Terres [37] | 2009 | SAB | 24.8 (19.9–29.9)b | 22.66 (18.8–26.5)b | NR |
| 3 | Das [45] | 2007 | SAB | 14c | 8c | 0.004 |
| 4 | Reed [63] | 2005 | SAB | 16.6 ± 12.7d | 9.3 ± 8.5d | < 0.0001 |
| 5 | Cordova [66] | 2004 | SAB | 16 (6–25, 1–211)e | 14 (7–30, 1–273)e | NR |
| 6 | Osmon [67] | 2004 | SAB | 22.1 ± 24.9d | 13.2 ± 13.5d | 0.001 |
| 7 | Tumbarello [75] | 2002 | SAB | 49 ± 27d | 24 ± 16d | < 0.001 |
| 8 | Soriano [82] | 2000 | SAB | 18f | 8f | < 0.00001 |
LOS, length of hospital stay; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; SAB, S. aureus bacteremia; NR, not recorded.
Mean (range).
Mean (95% confidence interval).
Median.
Mean ± SD.
Median (interquartile range, range),
Mean.
Table 3.
Medical costs
| No | Author | Year | Medical costs |
p value | |
|---|---|---|---|---|---|
| MRSA | MSSA | ||||
| 1 | Rubio-Terres [37] | 2009 | €11,044.59/episodea | €9839.25/episodea | - |
| 2 | Ben-David [33] | 2009 | ICU origin: $113,852 (48,961–55,001)b | ICU origin: $42,137 (32,388–74,781)b | ICU origin: < 0.001 |
| General origin: $53,409 (32,945–84,053)b | General origin: $35,131 (18,340–50,896)b | General origin: 0.005 | |||
| 3 | Greiner [46] | 2006 | €24,931a | €10,573a | < 0.05 |
| 4 | Lodise [62] | 2005 | $21,577 (17,061–27,290)c | $11,668 (9,550–14,223)c | 0.001 |
| 5 | Reed [63] | 2005 | $28,297 ± 23,619d | $16,066 ± 16,337d | < 0.0001 |
| 6 | Cosgrove [76] | 2001 | $26,424 (14,006–50,484)b | $19,212 (9,999–36,548)b | 0.008 |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; ICU, intensive care unit.
Mean.
Median (interquartile range).
Mean (95% confidence interval).
Mean ± SD.
Publication bias
We generated contour-enhanced funnel plots to evaluate the presence of potential publication bias for the meta-analyses performed in our review (Supplementary Fig. 1). No evidence of publication bias was noted in the funnel plots and the adjusted rank correlation tests in the meta-analyses for all-cause mortality (p = 0.10), in-hospital mortality (p = 0.056), 30-day mortality (p = 0.714), or SAB-related mortality (p = 0.557).
DISCUSSION
This systematic review of 62 relevant reports published since 2000 that evaluated the outcomes of SAB and endocarditis in adults, suggested that methicillin-resistant isolates is associated with increased mortality, hospital stay and medical costs, compared with susceptible isolates. A significant increase in all-cause mortality associated with MRSA, compared to that with MSSA compromised of 17,565 patients from 62 combined studies was evident with a pooled OR of 1.95. This is consistent with the report in 2003 by Cosgrove et al. [2] which had combined 31 studies with a total of 3,962 patients of an OR of mortality associated with MRSA of 1.93, compared with MSSA. In 60 studies that reported mortality outcomes, the relative risk (RR) was estimated to be 1.59 based on the mean mortality rate of 33.1% (2,101/6,338) in the MRSA group and 18.2% (2,014/11,075) in the MSSA group. In studies of in-hospital mortality, the RR was estimated at 1.54. This is also similar to the RR of 1.42 reported by Cosgrove et al. [2]. In the analysis involving SAB-related mortality, the pooled OR was 2.04 (95% CI, 1.63 to 2.55; I2 = 40%). This was compatible with the OR of 2.2 (95% CI, 1.2 to 3.8) reported by Cosgrove et al. [2]. In this analysis reevaluating the impact of methicillin-resistance on mortality in the era of the changing epidemiology and treatment of MRSA infections, a similar trend for a strong association between methicillin-resistance and a significantly increased mortality risk was identified through a review of the literature. Through a systematic review using a database published since the year 2000, two studies reported length of stay after the onset of bacteremia; patients in the MRSA group stayed 5.02 days longer than those in the MSSA group (95% CI, 2.66 to 7.38).
We also intended to evaluate the risk of mortality in endocarditis by comparing the groups with MRSA and MSSA. Interestingly, methicillin resistance increased the risk of mortality in SAE by 2.65 (95% CI, 1.46 to 4.80). Further analysis, involving primarily the SAE population, showed a pooled OR of 3.32 (95% CI, 1.68 to 6.59), which is higher than that reported by Cosgrove et al. [2] 1.79 (95% CI, 0.84 to 3.81). This is different than our expectation that mortality would be lower among patients with MRSA endocarditis as a consequence of better management with new antibiotics. Although glycopeptides were the only treatment option for MRSA infections before 2000, new treatment agents, including daptomycin and linezolid, for MRSA have been introduced since that time. Hence, the outcomes in the MRSA group were expected to be better than those in the past, especially in SAE, which is a severe form of SAB. The increased risk of mortality in the SAE group is attributable in part to the delay between data collection and publication. More than half of the study population was collected before 2000. Besides, only fifteen studies specified the treatment regimens for SAB; the mainstay of therapy for these was limited to glycopeptides. There was no study evaluating the clinical outcomes of SAB according to the treatment regimens between glycopeptides and the new anti-MRSA agents among the 62 relevant studies. Thus, the estimated risk in our analysis does not fully reflect changes in treatment of MRSA infections using new antibiotics as alternatives to glycopeptides. Further studies are required to evaluate the risk of methicillin-resistance for mortality in the SAE population under treatments with antibiotics other than glycopeptides.
Traditionally, bacteremia and endocarditis are classified as either CA or HA (nosocomial). CA-MRSA infections have emerged in the past few years as an important medical problem, especially in children without traditional risk factors for healthcare-associated MRSA. To evaluate the risk for emergence of methicillin-resistance in the community, we examined the outcomes of 22 studies reporting outcomes for CA-SAB as part of SAB; of these, three studies reported outcomes by comparing CA-MRSA and CA-MSSA. Interestingly, methicillin-resistance increased the risk of death by 3.23 (95% CI, 1.25 to 8.34). Furthermore, in nosocomial SAB, methicillin-resistance had a relatively low risk of morality in adults with ≥ 70% HA-SAB, compared to those with < 70% HASAB. These findings are opposed to previous reports in adults, which have described non-severe outcomes in CA-SAB compared to those in HA-SAB with a few notable exceptions. Given the different distribution pattern of CA- and HA-SAB, empiric antimicrobial therapy for CA-SAB could be less appropriate than for patients with HA-SAB. Since clinical practice guidelines for the treatment of MRSA often do not recommend coverage for CA-MRSA, the association between the presence of CA-MRSA and mortality in SAB suggests that patients with CA-MRSA were more likely to have received antibiotics not effective against methicillin-resistant strains [83]. Heterogeneity among study results, however, was detected in subgroup analyses; thus, further studies are required to determine the impact of methicillin-resistance on outcomes in adults with CA-SAB.
This study had several limitations. First, we included all adult subjects irrespective of disease patterns and severity of illness in this meta-analysis; this wide distribution of subject characteristics may result in heterogeneity between the combined studies. In this study, however, the heterogeneity test results were considerably lower than those in the general meta-analysis by Cosgrove et al. [2]. When we assessed the statistical heterogeneity with I2 > 50% as the indication of at least moderate heterogeneity, between-study statistical heterogeneity was not found in this meta-analysis (I2 statistic, 44%). Twenty-two studies were selected as high-quality in the assessment of bias risk of 62 relevant papers; with these, the sensitivity analysis showed a pooled OR of 2.12 (95% CI, 1.76 to 2.55), a significantly increased risk of mortality of methicillin- resistance in SAB. Heterogeneity in the combined studies was not identified (I2 = 46%). Thus heterogeneity did not have a major impact on the results. Therefore, a wide distribution of subject characteristics between studies in this meta-analysis is not considered to have had a huge impact on the results. Second, this analysis included data in part collected before the year 2000. Given that our data were collected around 2000, the mainstay of therapy for MRSA in this analysis was confined to glycopeptides; this may not fully reflect current medical treatment, in which newer antimicrobial agents active against MRSA have become available. Further study of the effect of new antimicrobial agents on mortality of patients with SAB is required.
Despite these limitations, the present systematic review of studies published since 20 suggests that methicillin-resistance is associated with increased mortality and hospital stay compared with susceptible isolates in SAB and endocarditis. In the SAE and CA-SAB infection subgroups, methicillin-resistance was associated with increased mortality.
KEY MESSAGE
1. Methicillin-resistance is still associated with increased mortality and hospital stay, compared with susceptible isolates in Staphylococcus aureus bacteremia.
2. In comparison of outcome between community-acquired methicillin-resistant and methicillin-susceptible S. aureus bacteremia, methicillin-resistance increased the risk for mortality.
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Contour-enhanced funnel plot and Begg & Mazumdar’s rank correlation test for exploring publication bias for all-cause mortality (A), in-hospital mortality (B), 30-day mortality (C), and Staphylococcus aureus bacteremia-related mortality (D).
REFERENCES
- 1.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect Control Hosp Epidemiol. 1999;20:408–411. doi: 10.1086/501641. [DOI] [PubMed] [Google Scholar]
- 4.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 5.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 6.Davis SL, Rybak MJ, Amjad M, Kaatz GW, McKinnon PS. Characteristics of patients with healthcare-associated infection due to SCCmec type IV methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2006;27:1025–1031. doi: 10.1086/507918. [DOI] [PubMed] [Google Scholar]
- 7.Stranden AM, Frei R, Adler H, Fluckiger U, Widmer AF. Emergence of SCCmec type IV as the most common type of methicillin-resistant Staphylococcus aureus in a university hospital. Infection. 2009;37:44–48. doi: 10.1007/s15010-008-7430-7. [DOI] [PubMed] [Google Scholar]
- 8.Vidal PM, Trindade PA, Garcia TO, et al. Differences between “classical” risk factors for infections caused by methicillin-resistant Staphylococcus aureus (MRSA) and risk factors for nosocomial bloodstream infections caused by multiple clones of the staphylococcal cassette chromosome mec type IV MRSA strain. Infect Control Hosp Epidemiol. 2009;30:139–145. doi: 10.1086/593954. [DOI] [PubMed] [Google Scholar]
- 9.Valsesia G, Rossi M, Bertschy S, Pfyffer GE. Emergence of SCCmec type IV and SCCmec type V methicillin-resistant Staphylococcus aureus containing the Panton-Valentine leukocidin genes in a large academic teaching hospital in central Switzerland: external invaders or persisting circulators? J Clin Microbiol. 2010;48:720–727. doi: 10.1128/JCM.01890-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiman L, O’Keefe M, Graham PL, 3rd, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37:1313–1319. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 11.Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin Microbiol Infect. 2013;19:E291–E297. doi: 10.1111/1469-0691.12169. [DOI] [PubMed] [Google Scholar]
- 12.Wang JL, Chen SY, Wang JT, et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis. 2008;46:799–806. doi: 10.1086/527389. [DOI] [PubMed] [Google Scholar]
- 13.Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis. 2010;23:346–358. doi: 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 14.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 15.Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust. 2001;175:264–267. doi: 10.5694/j.1326-5377.2001.tb143562.x. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: a simple funnel-plotbased method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Holmes NE, Turnidge JD, Munckhof WJ, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis. 2011;204:340–347. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 22.Kao CH, Kuo YC, Chen CC, et al. Isolated pathogens and clinical outcomes of adult bacteremia in the emergency department: a retrospective study in a tertiary Referral Center. J Microbiol Immunol Infect. 2011;44:215–221. doi: 10.1016/j.jmii.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Lubart E, Segal R, Haimov E, Dan M, Baumoehl Y, Leibovitz A. Bacteremia in a multilevel geriatric hospital. J Am Med Dir Assoc. 2011;12:204–207. doi: 10.1016/j.jamda.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Son JS, Oh IH, Choi JM, Lee MS. Clinical impact of methicillin-resistant Staphylococcus aureus bacteremia based on propensity scores. Infection. 2011;39:141–147. doi: 10.1007/s15010-011-0100-1. [DOI] [PubMed] [Google Scholar]
- 25.Big C, Malani PN. Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J Am Geriatr Soc. 2010;58:300–305. doi: 10.1111/j.1532-5415.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang CI, Song JH, Chung DR, et al. Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect. 2010;61:299–306. doi: 10.1016/j.jinf.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Khan FY, Elshafie SS, Almaslamani M, et al. Epidemiology of bacteraemia in Hamad general hospital, Qatar: a one year hospital-based study. Travel Med Infect Dis. 2010;8:377–387. doi: 10.1016/j.tmaid.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Gregson DB, Ross T, Laupland KB. Time to blood culture positivity in Staphylococcus aureus bacteremia: association with 30-day mortality. J Infect. 2010;61:197–204. doi: 10.1016/j.jinf.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Ponce-de-Leon A, Camacho-Ortiz A, Macias AE, et al. Epidemiology and clinical characteristics of Staphylococcus aureus bloodstream infections in a tertiary-care center in Mexico City: 2003-2007. Rev Invest Clin. 2010;62:553–559. [PubMed] [Google Scholar]
- 30.Takayama Y, Okamoto R, Sunakawa K. Definite infective endocarditis: clinical and microbiological features of 155 episodes in one Japanese university hospital. J Formos Med Assoc. 2010;109:788–799. doi: 10.1016/S0929-6646(10)60124-6. [DOI] [PubMed] [Google Scholar]
- 31.Wehrhahn MC, Robinson JO, Pearson JC, et al. Clinical and laboratory features of invasive community-onset methicillin-resistant Staphylococcus aureus infection: a prospective case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:1025–1033. doi: 10.1007/s10096-010-0973-4. [DOI] [PubMed] [Google Scholar]
- 32.Ammerlaan H, Seifert H, Harbarth S, et al. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009;49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 33.Ben-David D, Novikov I, Mermel LA. Are there differences in hospital cost between patients with nosocomial methicillin-resistant Staphylococcus aureus bloodstream infection and those with methicillin-susceptible S. aureus bloodstream infection? Infect Control Hosp Epidemiol. 2009;30:453–460. doi: 10.1086/596731. [DOI] [PubMed] [Google Scholar]
- 34.Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis. 2009;41:4–9. doi: 10.1080/00365540802441711. [DOI] [PubMed] [Google Scholar]
- 35.Kim ES, Joo EJ, Ha YE, et al. Clinical characteristics of infective endocarditis caused by Staphylococcus aureus: a 12-year experience in a tertiary-care hospital. Korean J Med. 2009;76:329–337. [Google Scholar]
- 36.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation: a study of 521 patients in Germany. J Infect. 2009;59:232–239. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Rubio-Terres C, Garau J, Grau S, Martinez-Martinez L, Cast of Resistance Study group Cost of bacteraemia caused by methicillin-resistant vs. methicillin-susceptible Staphylococcus aureus in Spain: a retrospective cohort study. Clin Microbiol Infect. 2010;16:722–728. doi: 10.1111/j.1469-0691.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- 38.Turnidge JD, Kotsanas D, Munckhof W, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191:368–373. doi: 10.5694/j.1326-5377.2009.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 39.Allard C, Carignan A, Bergevin M, et al. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991-2005. Clin Microbiol Infect. 2008;14:421–428. doi: 10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 40.Baroudi S, Qazi RA, Lentine KL, Bastani B. Infective endocarditis in haemodialysis patients: 16-year experience at one institution. NDT Plus. 2008;1:253–256. doi: 10.1093/ndtplus/sfn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libert M, Elkholti M, Massaut J, Karmali R, Mascart G, Cherifi S. Risk factors for meticillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J Hosp Infect. 2008;68:17–24. doi: 10.1016/j.jhin.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc. 2008;56:1485–1489. doi: 10.1111/j.1532-5415.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 43.Bader MS. Hyperglycemia and mortality in elderly patients with Staphylococcus aureus bacteremia. South Med J. 2007;100:252–256. doi: 10.1097/01.smj.0000257383.66288.68. [DOI] [PubMed] [Google Scholar]
- 44.Cagatay AA, Ozcan PE, Gulec L, et al. Risk factors for mortality of nosocomial bacteraemia in intensive care units. Med Princ Pract. 2007;16:187–192. doi: 10.1159/000100388. [DOI] [PubMed] [Google Scholar]
- 45.Das I, O’Connell N, Lambert P. Epidemiology, clinical and laboratory characteristics of Staphylococcus aureus bacteraemia in a university hospital in UK. J Hosp Infect. 2007;65:117–123. doi: 10.1016/j.jhin.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Greiner W, Rasch A, Kohler D, Salzberger B, Fatkenheuer G, Leidig M. Clinical outcome and costs of nosocomial and community-acquired Staphylococcus aureus bloodstream infection in haemodialysis patients. Clin Microbiol Infect. 2007;13:264–268. doi: 10.1111/j.1469-0691.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 47.Hsu RB, Lin FY. Methicillin resistance and risk factors for embolism in Staphylococcus aureus infective endocarditis. Infect Control Hosp Epidemiol. 2007;28:860–866. doi: 10.1086/518727. [DOI] [PubMed] [Google Scholar]
- 48.Wang FD, Chen YY, Chen TL, Liu CY. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control. 2008;36:118–122. doi: 10.1016/j.ajic.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Depuydt P, Benoit D, Vogelaers D, et al. Outcome in bacteremia associated with nosocomial pneumonia and the impact of pathogen prediction by tracheal surveillance cultures. Intensive Care Med. 2006;32:1773–1781. doi: 10.1007/s00134-006-0354-8. [DOI] [PubMed] [Google Scholar]
- 50.Guilarde AO, Turchi MD, Martelli CM, Primo MG. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J Hosp Infect. 2006;63:330–336. doi: 10.1016/j.jhin.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Heo ST, Peck KR, Ryu SY, et al. Analysis of methicillin resistance among Staphylococcus aureus blood isolates in an emergency department. J Korean Med Sci. 2007;22:682–686. doi: 10.3346/jkms.2007.22.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, Ryu JH, Kim MS, Choi HJ. The risk factors and prognosis of methicillin-resistant staphylococcus aureus bacteremia: focus on nosocomial acquisition. Korean J Med. 2006;71:405–414. [Google Scholar]
- 53.Lesse AJ, Mylotte JM. Clinical and molecular epidemiology of nursing home-associated Staphylococcus aureus bacteremia. Am J Infect Control. 2006;34:642–650. doi: 10.1016/j.ajic.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GM. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. 2006;44:1342–1346. doi: 10.1128/JCM.44.4.1342-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nori US, Manoharan A, Thornby JI, Yee J, Parasuraman R, Ramanathan V. Mortality risk factors in chronic haemodialysis patients with infective endocarditis. Nephrol Dial Transplant. 2006;21:2184–2190. doi: 10.1093/ndt/gfl200. [DOI] [PubMed] [Google Scholar]
- 56.Perovic O, Koornhof H, Black V, Moodley I, Duse A, Galpin J. Staphylococcus aureus bacteraemia at two academic hospitals in Johannesburg. S Afr Med J. 2006;96:714–717. [PubMed] [Google Scholar]
- 57.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34:2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 58.Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ. 2006;333:281. doi: 10.1136/bmj.38834.421713.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassettari VC, Strabelli T, Medeiros EA. Staphylococcus aureus bacteremia: what is the impact of oxacillin resistance on mortality? Braz J Infect Dis. 2005;9:70–76. doi: 10.1590/s1413-86702005000100012. [DOI] [PubMed] [Google Scholar]
- 60.DeRyke CA, Lodise TP, Jr, Rybak MJ, McKinnon PS. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005;128:1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 61.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 62.Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52:113–122. doi: 10.1016/j.diagmicrobio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2005;26:175–183. doi: 10.1086/502523. [DOI] [PubMed] [Google Scholar]
- 64.Yoon HJ, Choi JY, Kim CO, Kim JM, Song YG. A comparison of clinical features and mortality among methicillin- resistant and methicillin-sensitive strains of Staphylococcus aureus endocarditis. Yonsei Med J. 2005;46:496–502. doi: 10.3349/ymj.2005.46.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang CF, Kuo BI, Chen TL, Yang WC, Lee SD, Lin CC. Infective endocarditis in maintenance hemodialysis patients: fifteen years’ experience in one medical center. J Nephrol. 2004;17:228–235. [PubMed] [Google Scholar]
- 66.Cordova SP, Heath CH, McGechie DB, Keil AD, Beers MY, Riley TV. Methicillin-resistant Staphylococcus aureus bacteraemia in Western Australian teaching hospitals, 1997-1999: risk factors, outcomes and implications for management. J Hosp Infect. 2004;56:22–28. doi: 10.1016/j.jhin.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Osmon S, Ward S, Fraser VJ, Kollef MH. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125:607–616. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 68.Chang FY, MacDonald BB, Peacock JE, Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322–332. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 69.Kim SH, Park WB, Lee KD, et al. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis. 2003;37:794–799. doi: 10.1086/377540. [DOI] [PubMed] [Google Scholar]
- 70.Melzer M, Eykyn SJ, Gransden WR, Chinn S. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin Infect Dis. 2003;37:1453–1460. doi: 10.1086/379321. [DOI] [PubMed] [Google Scholar]
- 71.Na SH, Kim CH, Oh MD, Cho YS. Infective endocarditis in the elderly patients. J Korean Geriatr Soc. 2003;7:37–46. [Google Scholar]
- 72.Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002;162:2229–2235. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 73.Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1–10. doi: 10.1086/340617. [DOI] [PubMed] [Google Scholar]
- 74.Talon D, Woronoff-Lemsi MC, Limat S, et al. The impact of resistance to methicillin in Staphylococcus aureus bacteremia on mortality. Eur J Intern Med. 2002;13:31–36. doi: 10.1016/s0953-6205(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 75.Tumbarello M, de Gaetano Donati K, Tacconelli E, et al. Risk factors and predictors of mortality of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in HIV-infected patients. J Antimicrob Chemother. 2002;50:375–382. doi: 10.1093/jac/dkf126. [DOI] [PubMed] [Google Scholar]
- 76.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 77.Morin CA, Hadler JL. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J Infect Dis. 2001;184:1029–1034. doi: 10.1086/323459. [DOI] [PubMed] [Google Scholar]
- 78.Wisplinghoff H, Seifert H, Coimbra M, Wenzel RP, Edmond MB. Systemic inflammatory response syndrome in adult patients with nosocomial bloodstream infection due to Staphylococcus aureus. Clin Infect Dis. 2001;33:733–736. doi: 10.1086/322610. [DOI] [PubMed] [Google Scholar]
- 79.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 80.Roghmann MC. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med. 2000;160:1001–1004. doi: 10.1001/archinte.160.7.1001. [DOI] [PubMed] [Google Scholar]
- 81.Selvey LA, Whitby M, Johnson B. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infect Control Hosp Epidemiol. 2000;21:645–648. doi: 10.1086/501707. [DOI] [PubMed] [Google Scholar]
- 82.Soriano A, Martinez JA, Mensa J, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000;30:368–373. doi: 10.1086/313650. [DOI] [PubMed] [Google Scholar]
- 83.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contour-enhanced funnel plot and Begg & Mazumdar’s rank correlation test for exploring publication bias for all-cause mortality (A), in-hospital mortality (B), 30-day mortality (C), and Staphylococcus aureus bacteremia-related mortality (D).