Abstract
Background/Aims
Transarterial chemoembolization (TACE) is performed for single hepatocellular carcinoma (HCC) that are not eligible for surgery or ablation therapy. We investigated the clinical outcomes of patients with a single HCC ≤ 5 cm treated with TACE.
Methods
This study analyzed 175 consecutive patients who underwent TACE as an initial treatment for single HCC ≤ 5 cm. Predictive factors for complete response (CR), recurrence after CR, and overall survival (OS) were evaluated.
Results
Total 119 patients (68%) achieved CR after TACE. Tumor size < 3 cm and hepatitis B virus infection were significant predictors of CR (p < 0.05). Recurrent HCC was detected in 73 patients (61.3%) after CR. Age > 65 years and absence of liver cirrhosis were predictive factors for non-recurrence after CR (p < 0.05). The OS for all patients was 80.7 ± 5.6 months, and the 1-, 3-, and 5-year OS rates were 88.1%, 64.8%, and 49.9%, respectively. In multivariate analysis for OS, CR (hazard ratio [HR], 0.467; 95% confidence interval [CI], 0.292 to 0.747) and Child class A (HR, 0.390; 95% CI, 0.243 to 0.626) were significant factors. The OS for the CR and Child class A group were 92 and 93.6 months, respectively, and that of the non-CR and Child B, C group were 53.3 and 50.7 months, respectively (p < 0.001).
Conclusions
TACE can be a valid treatment in patients with a single HCC ≤ 5 cm not suitable for curative treatment, especially in patients with Child class A and CR after TACE.
Keywords: Carcinoma, hepatocellular; Chemoembolization, therapeutic; Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and the leading cause of death in patients with liver cirrhosis [1,2]. Liver transplantation (LT), surgical resection or radiofrequency ablation (RFA) are recommended as curative treatment options in patients with early-stage HCC [3,4]. LT improves survival in patients with HCC by removing the tumor and underlying cirrhosis, and is considered the best therapeutic option in selected patients [5,6]. However, some patients cannot be surgical candidates because of several associated clinical factors such as old age, severe comorbidities and lack of liver donors [5]. In patients with early stage HCC not eligible for LT, surgical resection is generally used as the main treatment for resectable HCC. However, because of the risk of postoperative hepatic dysfunction, RFA has been established as an alternative curative treatment with better safety and lesser invasiveness than hepatic resection. But, RFA is not generally recommended when lesions for RFA are located near large vessels, bile ducts, intestinal loops, or the liver capsule [7,8]. Therefore, in patients not eligible for LT, unresectable HCC, and unsuitable for local ablation, the transarterial chemoembolization (TACE) is performed, even though TACE is usually used as a palliative therapy for intermediate stage HCC [9-11]. Until now, results regarding the efficacy of TACE as an initial treatment for patients with single nodular HCC less than 5 cm are insufficient. The aim of this study was to investigate the clinical outcomes of patients with a single HCC less than 5 cm treated with TACE, including the factors affecting complete response (CR), recurrence, and overall survival (OS).
METHODS
Patients
A total of 1,726 consecutive patients were newly diagnosed with HCC at two tertiary hospitals (Soonchunhyang University Seoul Hospital, Seoul, Korea and Soonchunhyang University Bucheon Hospital, Bucheon, Korea) between January 2004 and December 2014. Among these, 701 HCC patients underwent TACE as an initial treatment. We retrospectively enrolled 175 patients who had a single HCC less than 5 cm at the time of their initial diagnosis (Fig. 1). Patients were excluded if they had (1) another treatment such as surgery, RFA, chemotherapy, or radiotherapy was performed before or after TACE, (2) insufficient data or loss to follow-up. Written consent was not obtained, because the participants remained anonymous and the data were analyzed anonymously. The study protocol was approved by the Institutional Review Boards of Soonchunhyang University Seoul Hospital, and Soonchunhyang University Bucheon Hospital. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of each hospital (SCHUH, 2016-05-011, SCHBC2018-01-007-001).
Figure 1.
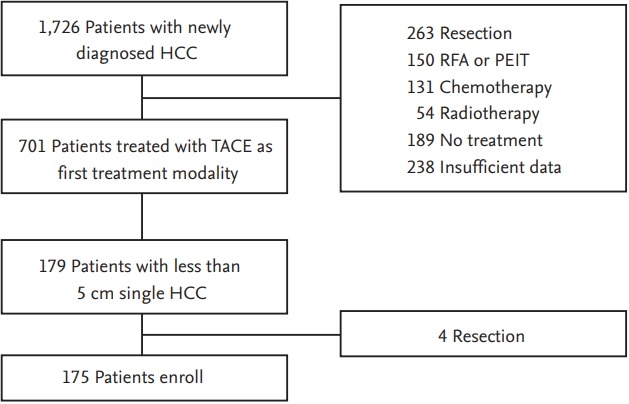
Flow diagram of the patients. HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; PEIT, percutaneous ethanol injection therapy; TACE, transarterial chemoembolization.
Diagnosis
Diagnosis and staging of HCC were based on the American Association for the Study of Liver Diseases criteria and Barcelona Clinic Liver Cancer (BCLC) and American Joint Committee on Cancer staging system 7th edition (AJCC-7) staging systems [12-14]. For the diagnosis of HCC, nodules found on ultrasound surveillance that are smaller than 1 cm should be followed up with ultrasound or helical multidetector computed tomography (CT) scan using contrast at 3 month intervals. Lesions larger than 1 cm in diameter should be evaluated by dynamic magnetic resonance imaging or helical multidetector CT scan using contrast. If the appearance is typical for HCC, no further investigation is required. Tumors were staged according to the modified Union Internationale Contre Le Cancer (modified UICC) classification system [14].
Procedure
All TACE procedures were performed by three experienced interventional radiologists. Hepatic angiography and indirect photography were performed using angiographic catheters, followed by selection of segmental arterial feeders. All procedures were performed under local analgesia. A mixture of iodized oil-doxorubicin hydrochloride emulsion was then administered into the feeding arteries. Once the arterial flow became sluggish, gelatine sponge particles mixed with mitomycin-C and contrast material was infected into the feeding arteries until blood flow stopped completely. TACE was performed at baseline and a multiphasic CT scan was performed at 4 weeks after every TACE. When CT scan after TACE detected viable tumors, repeat TACE was performed immediately. If viable tumors were not detected on CT scan, additional CT scans were conducted at 2-month intervals. The routine post-TACE follow-up protocol included biochemical liver function tests and serum alpha-fetoprotein (AFP) level measurements. Complications were documented according to the Society of Interventional Radiology guidelines [15].
Definition
CR was defined as the disappearance of any intratumoral arterial enhancement in all target lesions or compact lipiodol uptake after TACE according to modified Response Evaluation Criteria in Solid Tumors (RECIST) [16]. Tumor recurrence was determined when a new lesion appeared or when an enhancing portion was seen within or at the margin of the original mass on the next follow-up CT scan after initial CR. The recurrence pattern was classified as local recurrence (≤ 2 cm from the primary tumor or portal vein thrombosis) or distant recurrence (> 2 cm apart from the primary tumor or vascular invasion) according to the location of the recurred tumor. Time to local control was defined as the time from the date of initial treatment of HCC in group of CR and to the date of radiologic tumor recurrence. The OS was calculated from the diagnosis of HCC to the date of death and was censored at follow-up loss and at the last follow-up.
Data collection
Clinical, laboratory, and radiologic records of all patients were retrospectively reviewed. To determine affecting CR of TACE, we analyzed the clinical and biological factors that influenced initial CR using the following parameters: age, sex, size of tumors, viral markers (hepatitis B virus [HBV] surface antigen and hepatitis C virus antibody), AFP, model for end stage liver disease (MELD), UICC stage, prescence of liver cirrhosis, and Child-Pugh class. In addition, we analyzed the risk factors for recurrence after TACE-induced CR according to the recurrence pattern using the same parameters. Finally, we evaluated the predictive factors for OS.
Statistical analysis
Frequencies and percentages were used for descriptive statistics. Statistical differences between the two groups were investigated using the chi-square test and Student t test. Patient survival probability was estimated using the Kaplan-Meier method, and differences between the curves were compared using the log-rank test. The main analysis tool used for survival was the Cox proportional hazards model. Multivariate models were created using variables that were significant in a univariate analysis (p < 0.10) and clinically relevant. Backward selection was used for variable selection. All statistical analyses were performed using PASW version 18.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was defined as a p < 0.05.
RESULTS
Baseline characteristics
In total, 175 patients were included in this study. The baseline characteristics of these patients are summarized in Table 1. The mean age of the patients was 60.1 ± 11.1 years, and 63.4% were male. The most common etiology for liver cirrhosis was HBV (59.4%). One hundred and seven patients (66.9%) had Child-Pugh class A, while the others (n = 58, 33.1%) had advanced liver cirrhosis (Child-Pugh B/C, 54/4). Eighty-three patients (47.4%) had modified UICC stage I, 90 (51.4%) had stage II, and two (1.1%) had stage III. The mean tumor size was 22.7 ± 10.2 mm. The median follow-up duration was 87.3 months, and no major complications or deaths related to TACE occurred.
Table 1.
Baseline characteristics of patients (n = 175)
| Characteristic | Value |
|---|---|
| Age, yr | 60.1 ± 11.1 |
| Male sex | 111 (63.4) |
| Etiology | |
| HBV infection | 104 (59.4) |
| HCV infection | 30 (17.1) |
| Alcohol and others | 41 (23.4) |
| Liver cirrhosis at initial diagnosis | 155 (88.6) |
| Albumin, g/dL | 3.6 ± 0.6 (2.1–4.8) |
| Total bilirubin, mg/dL | 1.4 ± 1.8 (0.2–4.5) |
| Prothrombin time INR | 1.2 ± 0.3 (0.8–2.4) |
| Presence of ascites | 52 (30) |
| Child-Pugh class | |
| A | 117 (66.9) |
| B | 54 (34.8) |
| C | 4 (2.6) |
| MELD score | 10.7 ± 4.7 (6.4–21.3) |
| Tumor size, mm | 22.7 ± 10.2 |
| AFP, ng/mL | 216 (1.0–10,268) |
| Portal vein thrombosis | 10 (5.7) |
| Modified UICC stage at initial diagnosis | |
| I | 83 (47.4) |
| II | 90 (51.4) |
| III | 2 (1.1) |
| Complete response | 119 (68) |
| Recurrence after complete response | 73 (41.7) |
| Local recurrence | 51 (29.1) |
| Distant recurrence | 22 (12.6) |
Values are presented as mean ± SD, number (%), mean ± SD (range), or median (range).
HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end-stage liver disease; AFP, alpha-fetoprotein; UICC, Union Internationale Contre Le Cancer.
Complete response after TACE
After TACE, 119 patients (68%) showed CR and the repetition numbers of TACE for CR were as follows: one session, 97 patients; two sessions, 17 patients; three sessions, three patients; and four sessions, two patients. In a multivariate analysis, tumor size < 3 cm (odds ratio [OR], 2.024; 95% confidence interval [CI], 1.295 to 6.135; p = 0.049) and HBV infection (OR, 2.672; 95% CI, 1.207 to 5.125; p = 0.004) were significant predictors of CR (Table 2).
Table 2.
Predictive factors for complete response after transarterial chemoembolization
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age > 65 years | 0.602 | 0.312–1.60 | 0.129 | |||
| Male sex | 0.844 | 0.434–1.643 | 0.619 | |||
| Tumor size (< 3 cm) | 2.100 | 1.061–4.166 | 0.033 | 2.024 | 1.295–6.135 | 0.049 |
| Etiology | 2.735 | 1.423–5.256 | 0.003 | 2.672 | 1.207–5.125 | 0.004 |
| HBV infection | ||||||
| Non-HBV infection | ||||||
| AFP, ng/mL (≥ 20) | 1.206 | 0.609–2.389 | 0.591 | |||
| MELD score (> 10) | 0.630 | 0.332–1.196 | 0.158 | |||
| UICC stage I | 0.955 | 0.506–1.803 | 0.886 | |||
| Presence of liver cirrhosis | 1.073 | 0.532–2.163 | 0.845 | |||
| Child-Pugh class A | 1.492 | 0.768–2.898 | 0.238 | |||
OR, odds ratio; CI, confidence interval; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MELD, model for end-stage liver disease; UICC, Union Internationale Contre Le Cancer.
Eighty of 104 patients with HBV infection showed CR. HBV viral load was assessed to identify whether it affected CR using a cut-off of HBV DNA < 2,000 IU/mL and a cut-off of HBV DNA < 20 IU/mL. Of the patients with HBV, 53 and 32 patients had HBV levels < 2,000 IU/mL and < 20 IU/mL, respectively, at TACE, and there was no association between viral load and CR for both cut-off values (p = 0.645 and p = 0.10, respectively).
Overall survival
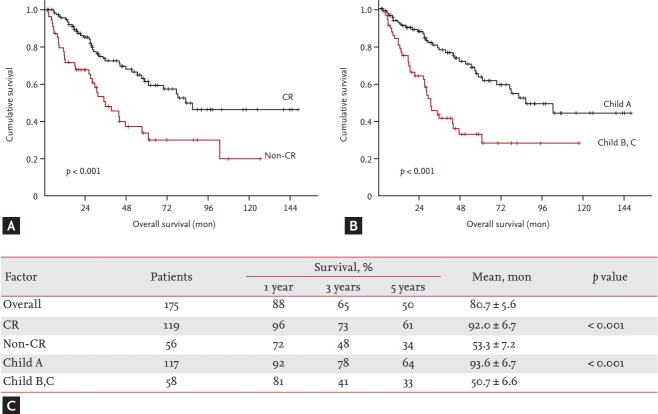
The mean OS for all patients was 80.7 ± 5.6 months. Total mortality included HCC-related mortality and cirrhosis complication-related mortality. The 1-, 3-, and 5-year OS rates were 88.1%, 64.8%, and 49.9%, respectively. In a multivariate analysis, CR (hazard ratio [HR], 0.467; 95% CI, 0.292 to 0.747; p = 0.001) and Child-Pugh class A (HR, 0.390; 95% CI, 0.243 to 0.626; p = 0.000) were independent risk factors for OS (Table 3). The mean OS was 92 months (95% CI, 78.876 to 105.107) for the CR group and 53.3 months (95% CI, 39.165 to 67.491) for the non-CR group (p < 0.001). The 1-, 3-, and 5-year OS rates were 96%, 73%, and 61% for the CR group, and 72%, 48%, and 34% for the in non-CR group, respectively. The mean OS was 93.6 months (95% CI, 80.443 to 106.766) for the Child-Pugh class A group and 50.7 months (95% CI, 37.795 to 63.775) for the Child-Pugh class B, C group (p < 0.001). The 1-, 3-, and 5-year OS rates were 92%, 78%, and 64% in Child-Pugh class A group, while 81%, 41%, and 33% in Child-Pugh class B,C group, respectively (Fig. 2).
Table 3.
Univariate and multivariate analyses of predictive factors for overall survival
| Variable | Univariate analysis |
Multivariate analys |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age > 65 years | 1.154 | 0.720–1.849 | 0.552 | |||
| Male sex | 1.041 | 0.640–1.694 | 0.870 | |||
| Complete response | 0.437 | 0.274–0.697 | 0.001 | 0.467 | 0.292–0.747 | 0.001 |
| AFP, ng/mL (> 20) | 1.140 | 0.704–1.845 | 0.594 | |||
| MELD score (> 10) | 1.507 | 0.947–2.396 | 0.083 | |||
| UICC stage I | 0.718 | 0.449–1.147 | 0.165 | |||
| Presence of liver cirrhosis | 1.073 | 0.532–2.163 | 0.845 | |||
| Child-Pugh class A | 0.369 | 0.231–0.592 | 0.000 | 0.390 | 0.243–0.626 | 0.000 |
HR, hazard ratio; CI, confidence interval; AFP, alpha-fetoprotein; MELD, model for end-stage liver disease; UICC, Union Internationale Contre Le Cancer.
Figure 2.
The cumulative overall survival rates according to the predictive factors. (A) The cumulative survival rates in patients with complete response (CR) was significantly higher than that of the patients with non-CR (p < 0.001). (B) The cumulative survival rates in patients with Child A was significantly higher than that of the patients with Child B and C (p < 0.001). (C) Table summarize the cumulative overall survival rates according to the tumor response and Child classification.
Recurrence after CR
Of the 119 patients with CR after TACE, recurrent HCC was detected in 73 patients (61.3%). The mean duration to recurrence in CR patients was 14.3 months (95% CI, 11.6 to 16.9). The overall 1-, 2-, and 3-year cumulative recurrence rates were 39.4%, 67.4%, and 75%, respectively. There were 51 cases (42.9%) of local recurrence and 22 cases (18.5%) of distant recurrence. The overall 1-, 2-, and 3-year cumulative local recurrence rates were 39.5%, 72.3%, and 85.6%, while those for distant recurrence were 18.5%, 45.7% and 52.4%, respectively. In a multivariate analysis, male sex (HR, 1.959; 95% CI, 1.164 to 3.297; p = 0.011) was the only predictive factor associated with HCC recurrence after TACE-induced CR (Table 4). We also investigated the predictive factors for patients without recurrence after CR during the entire follow-up period. Forty-six patients (38.7%) maintained CR without recurrence until the end of the follow-up period. In a multivariate analysis, age > 65 years (OR, 0.284; 95% CI, 0.100 to 0.808; p = 0.018,) and presence of liver cirrhosis (OR, 1.959; 95% CI 1.448 to 20.860; p = 0.012) were the predictive factors for patients without recurrence after CR (Table 5).
Table 4.
Predictive factors for recurrence after complete response
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age > 65 years | 0.528 | 0.305–0.912 | 0.022 | |||
| Male sex | 2.168 | 1.302–3.608 | 0.003 | 1.959 | 1.164–3.297 | 0.011 |
| Tumor size (< 3 cm) | 0.679 | 0.388–1.187 | 0.174 | |||
| Etiology | 1.127 | 0.679–1.872 | 0.644 | |||
| HBV infection | ||||||
| Non-HBV infection | ||||||
| AFP, ng/mL (≥ 20) | 0.862 | 0.529–1.406 | 0.551 | |||
| MELD score (> 10) | 0.888 | 0.551–1.431 | 0.624 | |||
| UICC stage I | 0.911 | 0.574–1.445 | 0.691 | |||
| Presence of liver cirrhosis | 1.585 | 0.638–3.938 | 0.322 | |||
| Child-Pugh class A | 0.739 | 0.448–1.219 | 0.237 | |||
HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MELD, model for end-stage liver disease; UICC, Union Internationale Contre Le Cancer.
Table 5.
Predictive factors for patients without recurrence after complete response
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age > 65 years | 0.253 | 0.093–0.687 | 0.007 | 0.284 | 0.100–0.808 | 0.018 |
| Male sex | 2.476 | 0.932–6.580 | 0.069 | |||
| Tumor size (< 3 cm) | 0.749 | 0.252–2.226 | 0.602 | |||
| Etiology | 2.476 | 0.932–6.580 | 0.069 | |||
| HBV infection | ||||||
| Non-HBV infection | ||||||
| AFP, ng/mL (≥ 20) | 1.050 | 0.378–2.915 | 0.926 | |||
| MELD score (> 10) | 1.258 | 0.456–3.471 | 0.658 | |||
| UICC stage I | 0.911 | 0.574–1.445 | 0.691 | |||
| Presence of liver cirrhosis | 6.347 | 1.771–22.749 | 0.005 | 1.959 | 1.448–20.860 | 0.012 |
| Child-Pugh class A | 2.913 | 0.783–10.840 | 0.111 | |||
OR, odds ratio; CI, confidence interval; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MELD, model for end-stage liver disease; UICC, Union Internationale Contre Le Cancer.
DISCUSSION
TACE is the alternative treatment of single nodular HCCs when other curative treatments are not possible. In this study we evaluated predictive factors for CR, recurrence after CR, and OS in patients treated with TACE as an initial treatment for single HCC ≤ 5 cm. Tumor size < 3 cm and HBV infection were significant predictive factors of CR, and tumor size was a significant predictor of CR in other studies [17-20], although these studies included patients with advanced stage of HCC. Terzi et al. [21] reported that only tumor size was found to be a statistically significant predictor of CR, in particular, a tumor diameter ≤ 3 cm and, more significantly, ≤ 5 cm in patients with a single nodule of HCC. Although that study included larger tumors (median, 3.0 cm [range, 0.8 to 15]) than our study, they similarly demonstrated that tumor size is significantly associated with CR.
In terms of HBV infection, HBV viral load did not affect the CR in our study. Although there is no reference for the effect of HBV infection to CR, we assume that the microcirculation of hepatic arterial blood flow might be different in HBV infection comparing with other causes, and it may afford effective superselective approach in TACE. However, it needs further study in the future.
Among 119 patients with CR, recurrence of HCC occurred in 73 patients (61.3%). Recent studies have reported that non-compact lipiodol intake, tumor size, AFP > 20 ng/mL, and high serum des-gamma-carboxy prothrombin (DCP) levels were predictive factors for recurrence after CR [22,23].
In this study, age > 65 years and presence of liver cirrhosis were predictive factors for the maintenance of CR during the follow-up period. Prognosis according to age is controversial for HCC. Indeed, some authors have reported better survival rates in young patients than in older ones [24-27], while other studies have shown opposite or arbitrary results [28-31]. However, these studies are heterogeneous in relation to the treatment provided, the criteria defining old age, and the HCC stage. In our study, we assumed that patients with age > 65 years might have less aggressive tumor biology and less active tumor neo-angiogenesis than younger patients, as shown in a recent study [32].
In this study, the mean OS for all patients was 80.7 ± 5.6 months, and the 1-, 3-, and 5-year median OS rates were 88.1%, 64.8%, and 49.9%, respectively. Recent studies in which patients with early HCC or HCC < 4 cm received TACE as the initial treatment modality have shown that the 1-, 3-, and 5-year survival rates were 89% to 100%, 63% to 81%, and 43% to 52%, respectively [33-37]. The results of our study are in line with those of these studies. Another study investigated the long-term survival of patients with HCC who met the Milan criteria and underwent RFA (n = 315) or TACE (n = 215) as an initial treatment. The two groups did not show any significant differences in terms of long-term survival rates (the 1-, 3-, and 5-year survival rates were 85%, 60%, and 41% for RFA, and 86%, 55%, and 36% for TACE, respectively) [33]. Recently, Kim et al. [7] reported that TACE can be a viable alternative treatment modality for small HCCs (< 2 cm) when RFA is not indicated, although RFA showed better tumor response and tumor progression. These reports suggest that TACE plays an important role as a first-line therapy for early stage HCC. In our study, independent prognostic factors for OS were Child-Pugh class A and CR. Because tumor size < 3 cm and HBV infection were significant predictors of CR, they were excluded from the OS analysis to prevent overlapping with CR. Male sex, age > 65 years, and presence of liver cirrhosis were associated with recurrence after CR in this study; however, they did not show significance for OS. Previous studies have suggested that various factors, such as tumor stage, response to treatment, liver function, and performance status, are associated with prognosis after TACE [9,38]. In this study, there was a remarkable difference in OS according to CR and Child-Pugh class A. In patients with Child-Pugh class A and CR, it is expected that TACE will result in long-term OS and thus better prognosis. While several studies have investigated the effects of TACE for small single HCC or small multiple HCC, this is the first study to determine the effects of TACE as an initial treatment in patients with single HCC ≤ 5 cm. We identified CR and Child-Pugh class A as predictive factors of OS, and there were definite differences in OS between groups. The main limitations of this study are that data were obtained in a retrospective manner and only a small number of patients were included due to strict inclusion criteria.
In conclusion, our study showed that TACE may be an efficient alternative treatment option for single HCC ≤ 5 cm when patients are not eligible for curative treatment options. Specifically, TACE might offer better prognosis in patients with CR and a Child-Pugh class A.
KEY MESSAGE
1. In patients who underwent transarterial chemoembolization (TACE) as an initial treatment for single hepatocellular carcinoma (HCC) ≤ 5 cm, the mean overall survival (OS) was 80.7 ± 5.6 months, and the 1-, 3-, and 5-year OS rates were 88.1%, 64.8%, and 49.9%, respectively.
2. Tumor size < 3 cm and hepatitis B virus infection were significant predictors for complete response (CR), and age > 65 years and absence of liver cirrhosis were predictive factors for non-recurrence after CR.
3. CR and Child-Pugh class A were signif icant predictors of OS. TACE can be an alternative treatment option for a single nodular HCC not suitable for curative treatment, especially in patients with CR and a Child-Pugh class A.
Acknowledgments
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(5 Suppl 1):S74–S83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M. Remodeling the surgical approach to hepatocellular carcinoma. Hepatogastroenterology. 2002;49:36–40. [PubMed] [Google Scholar]
- 4.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Kim JH, Sung KB, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109:1234–1240. doi: 10.1038/ajg.2014.152. [DOI] [PubMed] [Google Scholar]
- 8.Cho JY, Choi MS, Lee GS, et al. Clinical significance and predictive factors of early massive recurrence after radiofrequency ablation in patients with a single small hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:477–486. doi: 10.3350/cmh.2016.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, Practice Guidelines Committee. American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 10.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EW, Khan S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:265–272. doi: 10.3350/cmh.2017.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Gaba RC, Lewandowski RJ, Hickey R, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016;27:457–473. doi: 10.1016/j.jvir.2015.12.752. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304–1310. doi: 10.1016/j.jhep.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Vesselle G, Quirier-Leleu C, Velasco S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26:1640–1648. doi: 10.1007/s00330-015-3982-y. [DOI] [PubMed] [Google Scholar]
- 19.Ebied OM, Federle MP, Carr BI, et al. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042–1050. doi: 10.1002/cncr.11111. [DOI] [PubMed] [Google Scholar]
- 20.Golfieri R, Renzulli M, Mosconi C, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013;24:509–517. doi: 10.1016/j.jvir.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Terzi E, Piscaglia F, Forlani L, et al. TACE performed in patients with a single nodule of hepatocellular carcinoma. BMC Cancer. 2014;14:601. doi: 10.1186/1471-2407-14-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol. 2014;20:6995–7004. doi: 10.3748/wjg.v20.i22.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinugasa H, Nouso K, Takeuchi Y, et al. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47:421–426. doi: 10.1007/s00535-011-0492-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Choi MS, Lee H, et al. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2006;21:588–594. doi: 10.1111/j.1440-1746.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- 25.Chang PE, Ong WC, Lui HF, Tan CK. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol. 2008;43:881–888. doi: 10.1007/s00535-008-2238-x. [DOI] [PubMed] [Google Scholar]
- 26.Niederle IM, Worns MA, Koch S, et al. Clinicopathologic features and prognosis of young patients with hepatocellular carcinoma in a large German cohort. J Clin Gastroenterol. 2012;46:775–778. doi: 10.1097/MCG.0b013e31826102cc. [DOI] [PubMed] [Google Scholar]
- 27.Takeishi K, Shirabe K, Muto J, Toshima T, Taketomi A, Maehara Y. Clinicopathological features and outcomes of young patients with hepatocellular carcinoma after hepatectomy. World J Surg. 2011;35:1063–1071. doi: 10.1007/s00268-011-1017-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, Chang TT, Cheng KS, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26:766–773. doi: 10.1111/j.1478-3231.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 29.Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients? J Gastroenterol Hepatol. 2007;22:1226–1231. doi: 10.1111/j.1440-1746.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 30.Lam CM, Chan AO, Ho P, et al. Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients: implications for screening. Aliment Pharmacol Ther. 2004;19:771–777. doi: 10.1111/j.1365-2036.2004.01912.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki Y, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma in young adults: the clinical characteristics, prognosis, and findings of a patient survival analysis. Dig Dis Sci. 2007;52:1103–1107. doi: 10.1007/s10620-006-9578-2. [DOI] [PubMed] [Google Scholar]
- 32.Ha SY, Sohn I, Hwang SH, Yang JW, Park CK. The prognosis of hepatocellular carcinoma after curative hepatectomy in young patients. Oncotarget. 2015;6:18664–18673. doi: 10.18632/oncotarget.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu CY, Huang YH, Chiou YY, et al. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Liver Transpl. 2011;17:556–566. doi: 10.1002/lt.22273. [DOI] [PubMed] [Google Scholar]
- 34.Bargellini I, Sacco R, Bozzi E, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–1178. doi: 10.1016/j.ejrad.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Hsu KF, Chu CH, Chan DC, et al. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol. 2012;81:466–471. doi: 10.1016/j.ejrad.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 37.Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 38.Song YG, Shin SW, Cho SK, et al. Transarterial chemoembolization as first-line therapy for hepatocellular carcinomas infeasible for ultrasound-guided radiofrequency ablation: a retrospective cohort study of 116 patients. Acta Radiol. 2015;56:70–77. doi: 10.1177/0284185114520857. [DOI] [PubMed] [Google Scholar]