Abstract
A method for (hetero)arylboration of alkynes is presented. The reaction allows for the synthesis of densely functionalized tetrasubstituted alkenes with control of regioselectivity and diastereoselectivity. The conversion of the products to the corresponding α,α-bis(hetero)arylketones is also shown.
Alkyne carbofunctionalization reactions represent an important strategy for the synthesis of stereodefined alkenes.1 In recent years, alkyne carboboration has been demonstrated to work with a variety of substrates to prepare tri- and tetrasubstituted stereodefined alkenes.2,3 These reactions are valuable as the generated C–B bond can be elaborated to other groups by application of established strategies. Of the several catalysts systems applied toward alkyne carboboration, Cu-catalyzed variants have been investigated most expansively.3 These reactions have been shown to operate by addition of Cu-Bpin to an alkyne followed by capture of the generated alkenyl Cu-complex with an electrophile. In 2014, our group reported the first Cu-catalyzed arylboration of alkynes with aryliodides.4a The reactions worked well across a broad range of substrates; however, the regioselectivities suffered for some cases. Furthermore, reactions with substrates other than diarylalkynes resulted in lower yields (Scheme 1A).
Scheme 1.
Arylboration of alkynes.
In 2015, Cazin reported a Cu/Pd-catalyzed arylboration of alkynes with arylbromides. These reactions required the use of a Pd co-catalyst to activate the arylbromide component.4b More recently, Semba/Nakao have investigated a similar Cu/Pd-catalyzed arylboration of alkynes with primarily arylchlorides (Scheme 1B).4c These reactions likely operate by the catalytic cycles illustrated in Scheme 1C, in which an alkenyl-Cu-complex (III) is formed by borylcupration that then undergoes Pd-catalyzed cross coupling. This work is related to studies from the Semba/Nakao group as well as studies from our own lab towards Cu/Pd-catalyzed arylboration of activated alkenes.5 In this manuscript, we report our own efforts towards Cu/Pd-catalyzed arylboration of alkynes with arylbromides to specifically demonstrate reaction with heterocyclic substrates, as well as showcase a strategy for the synthesis of α,α-bis(hetero)arylketones (Scheme 1C).
Recently, our group reported the synthesis and use of Cu-pyridylidene complexes (e.g., 5) for heteroarylboration of heteroalkenylarenes.5h These conditions were also found to function well in the arylboration of an alkyne in one example. As shown in Scheme 2, arylboration of alkyne 2 with arylbromide 1 in the presence of Cu-catalyst 5 and APhosPdG3 worked well to provide 3 in excellent yield as a single observable regioisomer.6 It should be noted that in this example, SIMesCuCl (4) was also a competent catalyst; however, we elected to employ the Cu-pyridylidene complex 5 to further highlight the utility of these catalysts.
Scheme 2.
Comparison of Cu-Catalysts. Yield determined by analysis of the unpurified reaction mixture by 1H NMR with an internal standard.
With an optimized set of conditions in hand, we surveyed the scope of this transformation (Scheme 3). Internal alkynes that are substituted with aryl-, alkyl- and silyl-groups all function well. The regioselectivity of the reaction is established during the addition of Cu-Bpin, which occurs through a transition state to generate the more stable alkenyl-Cu-complex. The reaction was found to tolerate NHBoc and OTBDPS groups. In addition, heterocycles such as pyridines, furan, indoles and carbazoles can be utilized on both the arylbromide and/or alkyne components.
Scheme 3.
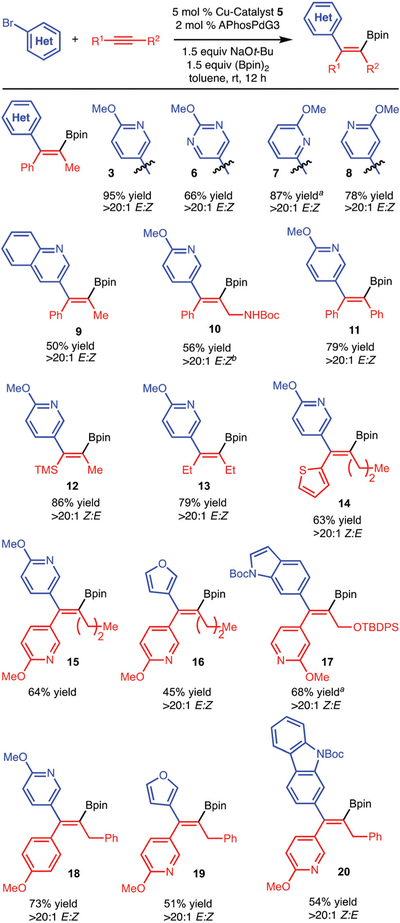
Reaction scope. Yields are the isolated, purified product and are reported as the average of at least two experiments. a Yield determined by analysis of the unpurified reaction mixture with an internal standard. b Reaction run at 45 °C.
Prior studies have demonstrated the utility of the C–B bond towards the synthesis of all carbon, stereodefined, tetrasubstituted alkenes by cross coupling.2d While oxidation of alkenylboronic esters has been demonstrated, use of this process to prepare α,α-bis(hetero)arylketones has not been shown. Thus simple treatment of the alkenylboronic esters with NaBO3 allows for smooth conversion to the ketones 21–24 (Scheme 4). The strategy outlined here for synthesis of α,α-bis(hetero)arylketones represents an orthogonal approach to this class of molecules that may offer advantages in some cases. For example, use of the related cross coupling approach, α-arylation,7 to prepare 22–24 would be complicated due to poor regioselectivity of reaction at one of two similarly substituted benzyl positions.
Scheme 4.
Synthesis of α,α-bis(hetero)arylketones. Yields are the isolated, purified product and are reported as the average of at least two experiments.
Conclusions
In conclusion, our efforts towards the arylboration of alkynes is presented. We further demonstrate the utility of pyridylidene complexes in catalysis and showcase a strategy for the synthesis of α,α-bis(hetero)arylketones.
Supplementary Material
Acknowledgements
We thank Indiana University and the National Institutes of Health (R01GM114443 and R35GM128779) for generous financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund.
Footnotes
† Electronic supplementary information (ESI) available: Experimental procedures and copies of spectra. See DOI: 10.1039/c9ob00961b
Conflicts of interest
There are no conflicts to declare.
References
- 1.Flynn AB and Ogilvie WW, Chem. Rev, 2007, 107, 4698–4745. [DOI] [PubMed] [Google Scholar]
- 2:(a) Alfaro R, Parra A, Aleman J, Garcia Ruano JL and Tortosa M, J. Am. Chem. Soc, 2012, 134, 15165; [DOI] [PubMed] [Google Scholar]; (b) Yoshia H, Kageyuki I and Takaki K, Org. Lett, 2013, 15, 952; [DOI] [PubMed] [Google Scholar]; (c) Nagao K, Ohmiya H and Sawamura M, J. Am. Chem. Soc, 2014, 136, 10605; [DOI] [PubMed] [Google Scholar]; (d) Kubota K, Iwamoto H, Yamamoto E and Ito H, Org. Lett, 2015, 17, 620; [DOI] [PubMed] [Google Scholar]; (e) Su W, Gong T-J, Zhang Q, Zhang Q, Xiao B and Fu Y, ACS Catal, 2016, 6, 6417; [Google Scholar]; (f) Jing H, Feng X, Guo M, Zhou S, Li Y, Zhang J, Zhao W, Tang X and Wang G, Asian J. Org. Chem, 2017, 6, 1375; [Google Scholar]; (g) Itoh T, Shimizu Y and Kanai M, J. Am. Chem. Soc, 2016, 138, 7528; [DOI] [PubMed] [Google Scholar]; (h) Han JT and Yun J, Org. Lett, 2018, 20, 2104; [DOI] [PubMed] [Google Scholar]; (i) Kim-Lee S-H, Alonso I, Mauleon P, Gomez Arrayas R and Carretero JC, ACS Catal, 2018, 8, 8993; [Google Scholar]; (j) Zhang Q, Li M, Liu J-Q and Yu H-Z, J. Org. Chem, 2018, 871, 48; [Google Scholar]; (k) Cheng L-J and Mankad NP, Angew. Chem., Int. Ed, 2018, 57, 10328; [DOI] [PubMed] [Google Scholar]; (l) Rivera-Chao E and Fananas-Mastral M, Angew. Chem., Int. Ed, 2018, 57, 9945; [DOI] [PubMed] [Google Scholar]; (m) Rivera-Chao E and Fananas-Mastral M, Angew. Chem., Int. Ed, 2018, 57, 9945; [DOI] [PubMed] [Google Scholar]; (n) Liu S, Zeng X and Xu B, Adv. Synth. Catal, 2018, 360, 3249. [Google Scholar]
- 3. For reviews, see:; (a) Shimizu Y and Kanai M, Tetrahedron Lett, 2014, 55, 3727; [Google Scholar]; (b) Semba K, Fujihara T, Terao J and Tsuji Y, Tetrahedron, 2015, 71, 2183; [Google Scholar]; (c) Lazreg F, Nahra F and Cazin CSJ, Coord. Chem. Rev, 2015, 293–294, 48; [Google Scholar]; (d) Neeve EC, Geier SJ, Mkhalid IAI, Westcott SA and Marder TB, Chem. Rev, 2016, 116, 9091; [DOI] [PubMed] [Google Scholar]; (e) Semba K and Nakao Y, Tetrahedron, 2019, 75, 709. [Google Scholar]
- 4.(a) Zhou Y, You W, Smith KB and Brown MK, Angew. Chem., Int. Ed, 2014, 53, 3475; [DOI] [PubMed] [Google Scholar]; (b) Lesieur M, Bidal YD, Lazreg F, Nahra F and Cazin CSJ, ChemCatChem, 2015, 7, 2108; [Google Scholar]; (c) Semba K, Yoshizawa M, Ohtagaki Y and Nakao Y, Bull. Chem. Soc. Jpn, 2017, 90, 1340. [Google Scholar]
- 5:(a) Semba K and Nakao Y, J. Am. Chem. Soc, 2014, 136, 7567; [DOI] [PubMed] [Google Scholar]; (b) Smith KB, Logan KM, You W and Brown MK, Chem. – Eur. J, 2014, 20, 12032; [DOI] [PubMed] [Google Scholar]; (c) Logan KM, Smith KB and Brown MK, Angew. Chem., Int. Ed, 2015, 54, 5228; [DOI] [PubMed] [Google Scholar]; (d) Semba K, Ohtagaki Y and Nakao Y, Org. Lett, 2016, 18, 3956; [DOI] [PubMed] [Google Scholar]; (e) Logan KM and Brown MK, Angew. Chem., Int. Ed, 2017, 56, 851; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen B, Cao P, Yin X, Liao Y, Jiang L, Ye J, Wang M and Liao J, ACS Catal, 2017, 7, 2425; [Google Scholar]; (g) Bergmann AM, Dorn SK, Smith KB, Logan KM and Brown MK, Angew. Chem., Int. Ed, 2019, 58, 1719; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Huang Y and Brown MK, Angew. Chem., Int. Ed, 2019, 58, 6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Control experiments confirmed that the Pd-catalyst is necessary for this reaction, see the ESI† for details.
- 7.For a review regarding α-arylation, see: Johansson CCC and Colacot TJ, Angew. Chem., Int. Ed, 2010, 49, 676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





