Abstract
Background
Atrophied T2 lesion volume at MRI is an imaging measure that reflects the replacement of T2 lesions by cerebrospinal fluid spaces in patients with multiple sclerosis (MS).
Purpose
To investigate the association of atrophied T2 lesion volume and development of disability progression (DP) and conversion to secondary progressive MS (SPMS).
Materials and Methods
This retrospective study included 1612 participants recruited from 2006 to 2016 and followed up for 5 years with clinical and MRI examinations. Accumulation of T2 lesion volume, atrophied T2 lesion volume, percentage brain volume change (PBVC), and percentage ventricular volume change (PVVC) were measured. Disability progression and secondary progressive conversion were defined by using standardized guidelines. Analysis of covariance (ANCOVA) adjusted for age and Cox regression adjusted for age and sex were used to compare study groups and explore associations between MRI and clinical outcomes.
Results
A total of 1314 patients with MS (1006 women; mean age, 46 years ± 11 [standard deviation]) and 124 patients with clinically isolated syndrome (100 women; mean age, 39 years ± 11) along with 147 healthy control subjects (97 women; mean age, 42 years ± 13) were evaluated. A total of 336 of 1314 (23%) patients developed DP, and in 67 of 1213 (5.5%) the disease converted from clinically isolated syndrome (CIS) or relapsing-remitting MS (RRMS) to SPMS. Patients with conversion to DP had higher atrophied T2 lesion volume (+34.4 mm3; 95% confidence interval [CI]: 17.2 mm3, 51.5 mm3; d = 0.27; P < .001) and PBVC (−0.21%; 95% CI: −0.36%, −0.05%; d = 0.19; P = .042) but not PVVC (0.36%; 95% CI: −0.93%, 1.65%; d = 0.04; P = .89) or T2 lesion volume change (−64.5 mm3; 95% CI: −315.2 mm3, 186.3 mm3; d = 0.03; P = .67) when compared with DP nonconverters. ANCOVA showed that atrophied T2 lesion volume was associated with conversion from CIS or RRMS to SPMS (+26.4 mm3; 95% CI: 4.2 mm3, 56.9 mm3; d = 0.23; P = .002) but not PBVC (−0.14%; 95% CI: −0.46%, 0.18%; d = 0.11; P = .66), PVVC (+0.18%; 95% CI: −2.49%, 2.72%; d = 0.01; P = .75), or T2 lesion volume change (−46.4 mm3; 95% CI: −460.8 mm3, 367.9 mm3; d = 0.03; P = .93). At Cox regression analysis, only atrophied T2 lesion volume was associated with the DP (hazard ratio, 1.23; P < .001) and conversion to SPMS (hazard ratio, 1.16; P = .008).
Conclusion
Atrophied brain T2 lesion volume is a robust MRI marker of MS disability progression and conversion into a secondary progressive disease course.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Chiang in this issue.
Summary
Together with whole-brain atrophy, atrophied brain T2 lesion volume seen with MRI is used to predict multiple sclerosis (MS) disability progression and is the only MRI feature related to conversion of clinically isolated syndrome and relapsing-remitting MS into secondary progressive MS.
Key Results
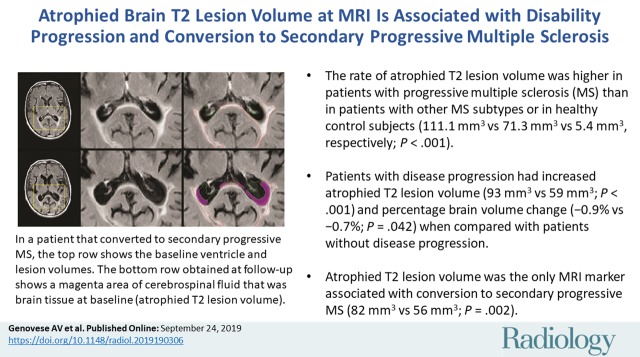
■ The annualized rate of atrophied T2 lesion volume was higher in patients with progressive multiple sclerosis (MS) than in patients with other MS subtypes or in healthy control subjects (111.1 mm3 vs 71.3 mm3 vs 5.4 mm3, respectively; P < .001).
■ Patients with disease progression had increased annualized atrophied T2 lesion volume (93 mm3 vs 59 mm3, P < .001) and percentage brain volume change (−0.9% vs −0.7%, P = .042) when compared with patients without disease progression.
■ Atrophied T2 lesion volume was the only MRI marker associated with conversion from clinically isolated syndrome or relapsing-remitting MS to secondary progressive MS (82 mm3 vs 56 mm3, P = .002).
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system. A secondary progressive clinical course develops in many individuals with MS, typically 20 years after onset, and sustained disability leads to impaired mobility and cognition (1).
Conventional MRI (ie, T2- or proton density–weighted, T2-weighted fluid-attenuated inversion recovery [FLAIR], or unenhanced and contrast material–enhanced T1-weighted imaging) is the main tool used in the diagnosis and routine surveillance of abnormal changes in patients with MS (2,3). Lesion measures (ie, number of new or enlarging or contrast-enhanced lesions and their respective volumes) enable quantitative assessment of active inflammation. They have been largely used as primary and secondary end points in many clinical trials (3,4). Monitoring the appearance of new lesions and their enlargement is routinely used as an indicator of disease activity and treatment efficacy in a clinical routine. However, the association between lesion MRI markers and MS clinical worsening or transition to secondary progressive MS (SPMS) disease course is weak to modest (5,6).
Assessment of brain atrophy is considered a more robust outcome reflecting neurodegeneration, the end stage of heterogeneous pathologic processes occurring in MS (7). Brain atrophy can be observed as early as in clinically isolated syndrome (CIS), and its development is a valid indicator of future clinical disease deterioration (3,7–12).
Despite growing interest in the use of neurodegenerative measures to monitor individuals with MS, there is still a lack of reliable MRI outcomes that can be used to detect early disease changes and predict future outcomes of disease progression and conversion to SPMS. A recently introduced MRI marker, brain atrophied T2 lesion volume, may aid in predicting the timing of MS disease progression (13–15). This quantitative measure reflects the amount of lesions being replaced by cerebrospinal fluid (CSF) spaces as a result of atrophy or direct lesion destruction (13–15). Parallel to the evolution of the disease, the inflammatory activity declines, and the neurodegenerative process accelerates (16). This could explain the plateauing of T2 lesion volume in more advanced disease stages (17) and could contribute to the poor correlation found between T2 lesion volume accumulation and clinical disability (5,7,14).
In this study, we aimed to investigate the behavior of atrophied T2 lesion volume accumulation in a large cohort of patients with MS during long-term clinical routine 5-year follow-up. We explored the predictive value of atrophied T2 lesion volume in relation to the development of DP and conversion from CIS and relapsing-remitting multiple sclerosis (RRMS) course to SPMS. In addition, we included a group of control subjects to understand dynamics of atrophied T2-weighted lesion volume accumulation in healthy subjects.
Materials and Methods
This study was approved by the local institutional review board. Written informed consent was waived for the patients with MS because of the retrospective nature of this study. Written informed consent was obtained for the healthy control subjects prospectively, but these data were used retrospectively in this study. Retrospective chart review was conducted in accordance with Health Insurance Portability Accountability Act guidelines and was approved by the local institutional review board.
Study Participants
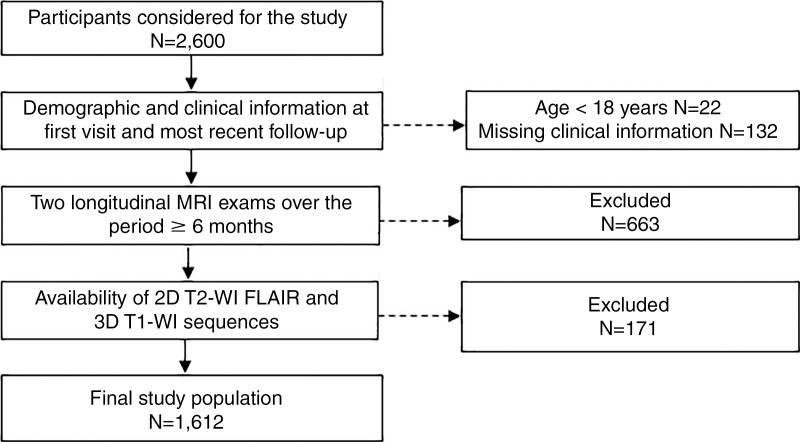
This retrospective study enrolled 1612 consecutive participants who were observed between 2006 and 2016 at Jacobs MS Center. The inclusion criteria (Fig 1) for the patients with MS and CIS included (a) being diagnosed with MS according to the 2010 McDonald criteria (just for MS) (18), (b) availability of images acquired with both two-dimensional T2-weighted FLAIR and three-dimensional T1-weighted MRI, (c) availability of results from at least two longitudinal MRI examinations within the same participant over a period of 6 or more months, and (d) availability of demographic and clinical information from the first and most recent follow-up examinations. The exclusion criteria included (a) a clinical relapse or corticosteroid treatment within 30 days after MRI and (b) being pregnant or a nursing mother. Healthy control subjects included nonfamilial relatives of patients with MS and participants recruited via local advertisements. Healthy control subjects were enrolled in the study if they had normal neurologic and MRI findings within clinical expectations for their age.
Figure 1:
Flowchart shows participant selection criteria. FLAIR = fluid-attenuated inversion recovery, T1-WI = T1-weighted imaging, T2-WI = T2-weighted imaging, 3D = three-dimensional, 2D = two-dimensional.
Age, sex, disease duration, and disease course, as defined by the revised Lublin criteria (19), were recorded at clinical assessment and cross-referenced with electronic medical records at the first and most recent follow-up examinations. Disease severity was evaluated with a standardized neurologic evaluation and scored with the Expanded Disability Status Scale (EDSS) (20).
MRI Acquisition and Analysis
Brain MRI was performed at baseline and follow-up by using either 1.5- or 3.0-T GE Signa Excite HD 12.0 Twin Speed eight-channel units (GE Healthcare, Milwaukee, Wis) and an eight-channel head and neck coil that did not undergo any hardware or software changes during the study (Table E1 [online]). Participants were consistently examined with the same 1.5- or 3.0-T unit and the same MRI protocol between 2006 and 2016. The 3.0-T MRI included (a) axial two-dimensional T2-weighted FLAIR (repetition time msec/echo time msec/inversion time msec, 8500/120/2100; flip angle, 75°; phase acquisition matrix, 1 × 1 × 3 mm) and (b) three-dimensional T1-weighted (5.9/2.8/900; flip angle, 10°; acquisition matrix, 1 × 1 × 1 mm) sequences. Similarly, the 1.5-T sequences used 8000/120/2000 and 7.7/3.7/900 for the two-dimensional T2-weighted FLAIR and three-dimensional T1-weighted sequences, respectively.
Image analyses were performed in a blinded manner with respect to disease and clinical status of study participants. The T2 lesion volume was quantified (D.P.R., 15 years of experience) by using a reliable semiautomated edge-detection contouring and thresholding technique with the T2-weighted FLAIR sequence with Jim software (version 6.0; http://www.xinapse.com) (21). The normalized brain volume at the first MRI examination was obtained with the SIENAX method (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl) (22), whereas the percentage brain volume change (PBVC) over the follow-up period was computed with SIENA (FMRIB Software Library, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA) (22) for three-dimensional T1-weighted images that were modified by using an inpainting technique to avoid tissue misclassification (23). The ventricular volume at the first MRI examination and the percentage ventricular volume change (PVVC) over the follow-up MRI examinations were measured on three-dimensional T1-weighted images by using SIENAX (22) and VIENA (FMRIB Software Library, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA) (24), respectively.
To calculate atrophied T2 lesion volume, images were preprocessed with N4 (25) to remove spatially varying intensity inhomogeneities and were standardized by using a piecewise histogram matching technique (26) linearly aligned to the baseline space by using FLIRT software (FMRIB Software Library, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT) (27). To calculate atrophied T2 lesion volume, rigidly aligned follow-up SIENA CSF maps were overlaid on baseline T2 lesion masks. Voxelwise follow-up CSF partial volume was then integrated over the baseline lesion regions (N.B., 15 years of experience) to determine total volume of periventricular and nonperiventricular lesion tissue subsequently replaced by CSF.
Statistical Analyses
All statistical analyses were performed by authors (A.V.G., J.H., D.J.) and the biostatistician using the Statistical Package for Social Science (version 24.0; IBM, Armonk, NY). For the purposes of demographic, clinical, and MRI overall comparisons, the 217 patients with SPMS and the 35 patients with primary progressive MS were categorized into one group with progressive MS. Patients were divided into two groups based on the presence or absence of disability progression (DP) during follow-up. DP converters were classified as patients with an EDSS change of at least 1.5 if baseline EDSS was less than 1.0, those with an EDSS change of at least 1.0 if baseline EDSS was 1.0–5.5, and those with an EDSS change of at least 0.5 if baseline EDSS was 5.5 or higher, as previously reported (28). DP nonconverters were individuals who did not meet the criteria to be DP converters. Furthermore, on the basis of their conversion into SPMS disease phenotype (CIS and RRMS to SPMS), patients were classified as SPMS converters or SPMS nonconverters. We also investigated differences in MRI outcomes between clinically definite MS (CDMS) converters and nonconverters among patients with CIS.
To evaluate demographic and clinical differences, we used the Student t test, χ2 test, and Mann-Whitney U test, as appropriate. Baseline and longitudinal percentage changes in MRI measures were analyzed by using analysis of covariance (ANCOVA), adjusted for age. Because there were significant differences in time of follow-up between DP, SPMS, and CDMS converters and nonconverters, all longitudinal MRI analyses were corrected for time to follow-up, and results are presented as actual and annualized values between the first MRI and the most recent follow-up MRI. The Cohen d effect size and 95% confidence interval (CI) are provided for the primary longitudinal outcomes in the ANCOVA analyses. To determine the association between the MRI-derived markers (T2 lesion volume, atrophied T2 lesion volume, PBVC, and PVVC) and the conversion to DP or SPMS, we used binary logistic regression models. The significance and rank of the aforementioned variables was determined with forward stepwise criteria. The odds ratio, Wald coefficient, and P values are reported, as appropriate.
To further determine the temporal associations between atrophied T2 lesion volume and occurrence of DP (event) versus stable (censored) disease or transition to SPMS status (event) versus stable (censored) disease, age- and sex-adjusted time-to-event Cox regression analysis was performed. Moreover, similar survival analysis was used to determine the temporal associations with transition to the SPMS phenotype. Analogous survival analyses were performed for PBVC and PVVC. The hazard ratio and P values are reported.
Results were considered significant at P < .05 using two-tailed tests.
Results
Demographic and Clinical Characteristics at Baseline and Follow-up Visits
Demographic and clinical data for the whole study sample are reported in Table 1. A total of 1612 participants were included in this study; of these, 1341 were patients with MS (1006 women; mean age, 45.7 years ± 11.2 [standard deviation]), 124 were patients with CIS (100 women; mean age, 39 years ± 10.5), and 147 were healthy control subjects (97 women; mean age, 41.6 years ± 13). The cohort of 1341 patients with MS included 1089 patients with RRMS (81.2%), 217 with SPMS (16.2%), and 35 with primary progressive MS (2.6%). As expected, differences were found between patients with MS and those with CIS in EDSS scores at baseline (P < .001) and the most recent follow-up (P < .001). The patients with MS and healthy control subjects were not matched by age or sex (Table 1).
Table 1:
Demographic and Clinical Characteristics of Patients with MS, Patients with CIS, and Healthy Control Subjects
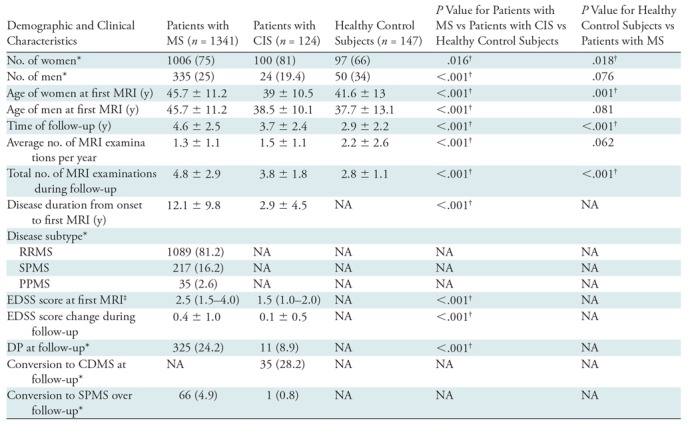
Note.—Unless otherwise indicated, data are mean ± standard deviation. P values were derived with the χ2 test, Student t test, or Mann-Whitney U test, as appropriate. CDMS = clinically definite MS, CIS = clinical isolated syndrome, DP = disease progression, EDSS = Expanded Disability Status Scale, MS = multiple sclerosis, NA = P value could not be obtained due to the healthy status of the control subject, PPMS = primary progressive MS, RRMS = relapsing-remitting MS, SPMS = secondary progressive MS.
* Data are numbers of participants, and data in parentheses are percentages.
† P value indicates a significant difference.
‡ Data are median and interquartile range.
Over the course of follow-up, of the 1465 patients with MS or CIS, 336 (22.9%) developed DP (246 had RRMS; 66, SPMS; 13, primary progressive MS; and 11, CIS), and 67 (4.6%) converted from RRMS (n = 66) or CIS (n = 1) to SPMS. Of the 124 patients with CIS, 35 (28.2%) converted to CDMS over the follow-up period. The median time to disability progression was 35.7 months (interquartile range, 18.2–59.9 months), and the median time for conversion from CIS or RRMS to SPMS was 31.1 months (interquartile range, 13.4–50.8 months).
MRI Characteristics at Baseline and Follow-up Visits
The baseline and follow-up MRI characteristics of the study participants are shown in Table 2.
Table 2:
MRI Outcomes of Patients with MS, Patients with CIS, and Healthy Control Subjects

Note.—Unless otherwise indicated, data are mean ± standard deviation. All P values are corrected for age and time to follow-up; between-group P values are derived from analysis of covariance. CIS = clinically isolated syndrome, MS = multiple sclerosis, NBV = normalized brain volume, PBVC = percentage brain volume change, PVVC = percentage ventricular volume change, RRMS = relapsing-remitting MS.
*P value indicates a significant difference.
T2 lesion volume (P < .001), as well as cumulative atrophied T2 lesion volume (P < .001), PVVC (P < .001), and PBVC (P < .001) were different between healthy control subjects and patients with MS or CIS. The annualized change of T2 lesion volume over follow-up was numerically but not statistically (P = .27) different among the groups. The lowest annualized atrophied T2 lesion volume was observed in healthy control subjects (mean, 5.4 mm3 ± 10.8), followed by patients with CIS (mean, 16.7 mm3 ± 34.7) and patients with MS (mean, 71.3 mm3 ± 140.4) (Table 2, P < .001) (Fig 2).
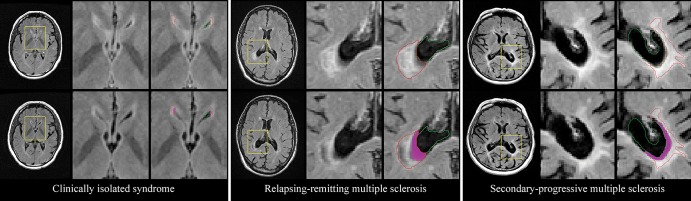
Figure 2:
Representative examples of atrophied T2 lesion volume in patients with clinically isolated syndrome, relapsing-remitting multiple sclerosis, and secondary progressive multiple sclerosis. Top row: Baseline images for each case. Left: Axial T2-weighted fluid-attenuated inversion recovery image. Yellow □ indicates the area of interest. Middle: Zoomed-in view of the corresponding area. Right: Outlines of the ventricle (green) and lesion volume (red). Bottom row: Corresponding follow-up images for each case, with the baseline ventricle and lesion volume outlines overlaid on top. The magenta area corresponds to cerebrospinal fluid that was lesioned tissue at baseline (atrophied T2 lesion volume).
Comparison between patients with RRMS and those with progressive MS showed substantial differences in baseline T2 lesion volume (P < .001), while annualized change in T2 lesion volume was not different over the course of follow-up (P = .35). Patients with progressive MS accumulated a greater amount of annualized atrophied T2 lesion volume over the course of follow-up (111.11 mm3) as compared with those with RRMS (62 mm3) (P = .001) (Fig 2). At post hoc analysis, there was no difference in the rate of annualized atrophied T2 lesion volume between patients with SPMS and those with primary progressive MS (115.1 mm3 vs 86.6 mm3, P = .32). At baseline, the normalized brain volume was lower (P < .001) and ventricular volume was higher (P < .001) in patients with progressive MS than in patients with RRMS. However, there were no differences between the two groups in annualized PVVC (P = .22) or PBVC (P = .93) over the course of follow-up.
Clinical and MRI Differences between DP Converters and Nonconverters
Table 3 provides demographic, clinical, and MRI characteristics of DP converters and nonconverters. The mean follow-up time was longer for DP converters than for nonconverters (P = .001). DP converters were older (P = .002) and had longer disease duration at first MRI (P < .001) when compared with nonconverters.
Table 3:
Demographic, Clinical, and MRI Characteristics for DP Converters versus DP Nonconverters
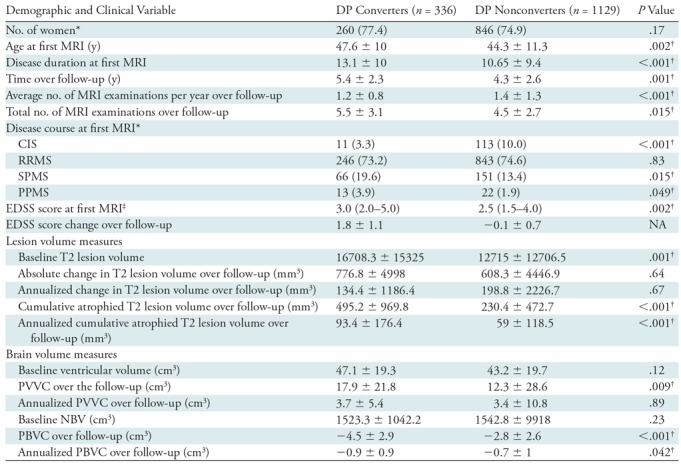
Note.—Unless otherwise indicated, data are mean ± standard deviation. P values represent DP converters versus DP nonconverters and were derived using the χ2 test, Student t test, and Mann-Whitney U test, as appropriate. For MRI measurements, all P values were corrected for age and time to follow-up; between-group P values were derived with analysis of covariance. NA indicates P values are not applicable, as classification of patients into DP converters and nonconverters was defined by EDSS score change. CIS = clinically isolated syndrome, DP = disability progression, EDSS = Expanded Disability Status Scale, NBV = normalized brain volume, PBVC = percentage brain volume change, PPMS = primary progressive multiple sclerosis, PVVC = percentage ventricular volume change, RRMS = relapsing-remitting multiple sclerosis, SPMS = secondary progressive multiple sclerosis.
* Data are numbers of participants, and data in parentheses are percentages.
† P value indicates a significant difference.
‡ Data are median and interquartile range.
At first MRI, DP converters had greater T2 lesion volume than did nonconverters (P < .001). Over the follow-up period, the DP converters had greater annualized atrophied T2 lesion volume (+34.4 mm3; 95% CI: 17.2 mm3, 51.5 mm3; d = 0.27; P < .001) but did not have annualized change in T2 lesion volume (−64.5 mm3; 95% CI: −315.2 mm3, 186.3 mm3; d = 0.03; P = .67). In terms of brain volume changes, the DP converters had greater annualized PBVC (−0.21%; 95% CI: −0.36%, −0.05%; d = 0.19; P = .042) but did not have greater annualized PVVC (0.36%; 95% CI: −0.93%, 1.65%; d = 0.04; P = .89).
In individual logistic regression analysis, an increase of 1 mL in annualized atrophied T2 lesion volume was associated with five times higher odds of having DP (odds ratio = 5.04, Wald coefficient = 13.61, P < .001), whereas an additional 1% of annualized PBVC was associated with 21.3% higher odds of having DP (odds ratio = 0.78, Wald coefficient = 10.89, P = .001). Furthermore, in a stepwise forward analysis including all the significant MRI outcomes, both increase in annualized atrophied T2 lesion volume (odds ratio = 4.63, Wald coefficient = 10.16, P = .001) and increase in annualized PBVC (odds ratio = 0.89, Wald coefficient = 7.43, P = .006) remained associated with the presence of DP status.
These findings were further corroborated in the time-to-event Cox regression analyses. For every 1 mL of additional atrophied T2 lesion volume, there was a 22.8% higher chance of DP event occurrence (hazard ratio = 1.23, P < .001). Neither PBVC nor PVVC enabled prediction of a DP transition event in the respective survival analyses.
Clinical and MRI Differences between SPMS Converters and Nonconverters
Further analysis was performed among patients with MS who changed disease course from CIS or RRMS to SPMS. These clinical, demographic, and imaging data are shown in Table 4.
Table 4:
Demographic, Clinical, and MRI Characteristics of SPMS Converters versus SPMS Nonconverters in Patients with RRMS or CIS
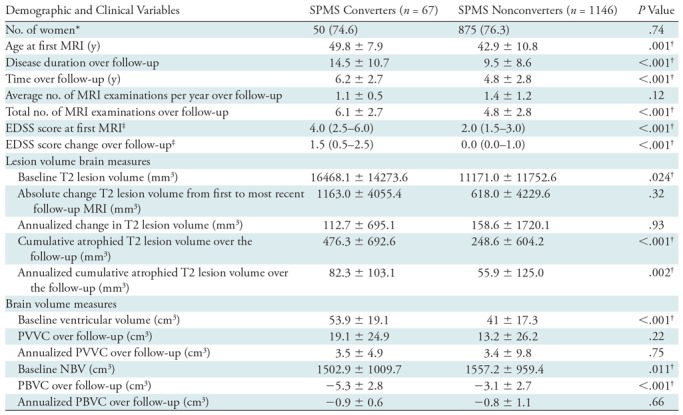
Note.—Unless otherwise indicated, data are mean ± standard deviation. P values represent secondary progressive multiple sclerosis (SPMS) converters versus SPMS nonconverters and were derived by using the χ2 test, Student t test, or Mann-Whitney U test, as appropriate. For MRI measurements, all P values were corrected for age and time to follow-up; between-group P values were derived with analysis of covariance. CIS= clinically isolated syndrome, EDSS = Expanded Disability Status Scale, NBV = normalized brain volume, PBVC = percentage brain volume change, PVVC = percentage ventricular volume change, RRMS = relapsing-remitting multiple sclerosis.
* Data are numbers of participants, and data in parentheses are percentages.
† P value indicates a significant difference.
‡ Data are median and interquartile range.
SPMS converters were followed up for a longer time than were nonconverters (P < .001), they were older (P < .001), and they had longer disease duration at first MRI (P < .001). Both at first MRI and at the most recent follow-up MRI, converters had higher EDSS scores (P < .001) than did nonconverters.
Over the follow-up period, SPMS converters had greater annualized atrophied T2 lesion volume than did nonconverters (+26.4 mm3; 95% CI: 4.2 mm3, 56.9 mm3; d = 0.23, P = .002) (Fig 3). There was no difference in annualized change of T2 lesion volume (−46.4 mm3; 95% CI: −460.8 mm3, 367.9 mm3; d = 0.03; P = .93). SPMS converters had higher ventricular volume (P = .001) and lower normalized brain volume (P = .012) at first MRI than did nonconverters. However, there were no differences in annualized PBVC (−0.14%; 95% CI: −0.46%, 0.18%; d = 0.11; P = .66) or PVVC (+0.18%; 95% CI: −2.49%, 2.72%; d = 0.01; P = .75) over the follow-up period between the two groups.
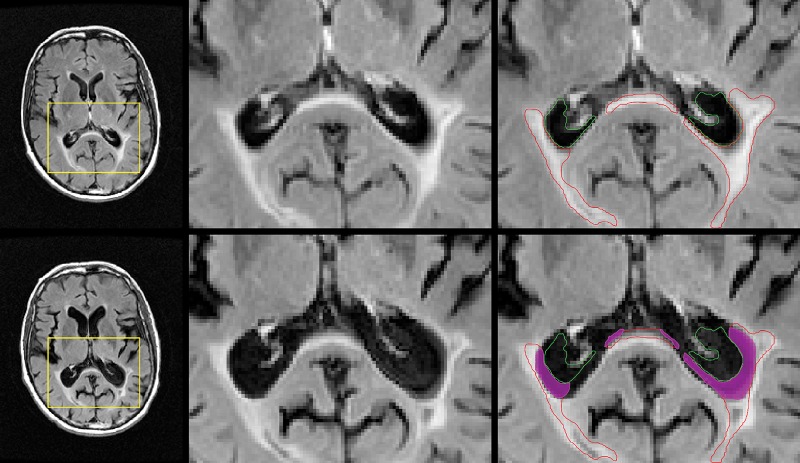
Figure 3:
Representative images of atrophied T2 lesion volume in a patient whose disease converted to secondary progressive multiple sclerosis over the course of follow-up. Top row: Baseline images. Left: Axial T2-weighted fluid-attenuated inversion recovery image. Yellow □ indicates the area of interest. Middle: Zoomed-in view of the corresponding area. Right: Outlines of the ventricle (green) and lesion volume (red). Bottom row: Corresponding follow-up images for each case, with the baseline ventricle and lesion volume outlines overlaid on top. The magenta area corresponds to cerebrospinal fluid that was lesioned tissue at baseline (atrophied T2 lesion volume).
Similar to the DP individual regression analyses, an increase of 1 mL in annualized atrophied T2 lesion volume was associated with a 4.7-fold higher chance to convert into the SPMS phenotype (odds ratio = 4.73, Wald coefficient = 4.17, P = .04). In a forward stepwise model of all MRI measures, only an increase in annualized atrophied T2 lesion volume was a significant predictor of SPMS conversion status (odds ratio = 4.87, Wald coefficient = 4.33, P = .04).
At Cox regression analysis, for every 1 mL of additional atrophied T2 lesion volume, there was a 16.3% greater chance of patients with MS transitioning from CIS or RRMS into SPMS (hazard ratio = 1.16, P = .008). Neither PBVC nor PVVC enabled us to predict the occurrence of an SPMS transition event in the respective survival analyses.
Clinical and MRI Differences between CDMS Converters and Nonconverters
The clinical, demographic, and imaging data of CDMS converters and nonconverters are shown in Table E2 (online). CDMS converters were observed for a longer time (P = .001) and had longer disease duration at first MRI (P = .045) when compared with nonconverters. They had greater EDSS score change over the follow-up period (P = .04). No MRI differences at first MRI or during follow-up were detected between CDMS converters and nonconverters.
Discussion
Atrophied T2 lesion volume may aid in predicting the timing of multiple sclerosis (MS) disease progression. Atrophied T2 lesion volume reflects the number of lesions being replaced by cerebrospinal fluid (CSF) spaces as a result of atrophy or direct lesion destruction. In this study, we aimed to investigate the behavior of atrophied T2 lesion volume accumulation in a large cohort of patients with MS during long-term routine clinical 5-year follow-up. This study corroborates the findings of initial reports regarding the utility of atrophied T2 lesion volume as a potential MRI-derived marker of disease progression in a large population-based cohort of patients with MS over the course of routine clinical follow-up. Together with whole-brain atrophy (d = 0.19, P = .042), atrophied T2 lesion volume (d = 0.27, P < .001) enabled us to predict development of disability progression (DP) over 5 years of follow-up. Furthermore, unlike percentage brain volume change (PBVC) (d = 0.11, P = .66) and percentage ventricular volume change (PVVC) (d = 0.01, P = .89), neither of which showed predictive power, atrophied T2 lesion volume (d = 0.23, P = .002) was the only MRI measure in the study that was related to conversion of clinically isolated syndrome (CIS) and relapsing-remitting MS (RRMS) into secondary progressive MS (SPMS). In contrast, atrophied T2 lesion volume did not enable prediction of conversion from CIS to clinically definite MS, enabling us to confirm that the processes leading to accumulation of atrophied T2 lesion volume occur later in the disease.
Atrophied T2 lesion volume represents the end stage of active inflammation (lesion accrual) and neurodegeneration (brain volume destruction). Because atrophied T2 lesion volume reflects more than one aspect in the heterogeneous pathologic process of MS, it may provide complementary information to explain changes leading to DP and conversion to SPMS beyond individual lesion and brain volume-related MRI outcomes. This hypothesis is supported by two recent studies that investigated whether atrophied T2 lesion volume explains additional independent variance in the prediction of DP (13,15). Thus, the current study extends previous findings from smaller samples of patients with MS.
Many different criteria have been proposed to distinguish between RRMS and SPMS disease courses, but a clear consensus remains elusive (19,29). Imaging and biologic markers may provide more objective criteria to separate these clinical phenotypes than clinical observation alone (19). In a recent study (15), atrophied T2 lesion volume was associated with the development of 10-year confirmed DP for almost all serial time points. This was not the case for whole-brain, cortical, or central atrophy, making atrophied T2 lesion volume a potentially more attractive MRI outcome for clinical monitoring on a year-to-year clinical routine basis in comparison with development of brain atrophy or lesion accrual. The same study showed that atrophied T2 lesion volume is an early predictor of DP, as it took only 6 months to show an association with the development of DP, whereas it took 2 years for whole-brain atrophy to show a comparable effect. Furthermore, atrophied T2 lesion volume accumulation accelerated from year 5 to year 10 of the follow-up period, whereas accumulation of total T2 lesion volume decelerated over the same period, suggesting that this MRI outcome could be of particular interest in monitoring the transition from RRMS to SPMS.
Lesion burden is the result of different processes, including demyelination with new lesion accrual, remyelination and repair, and neuroaxonal degeneration (16,30,31). Our results suggest that replacement of the lesions by CSF could be responsible for the decrease in lesion accumulation over time and could partially explain the plateau of T2 lesion volume (17) followed by an ultimate decrease in T2 lesion volume in the advanced stage of the disease (14).
Recent studies have shown a gradient of neuroinflammatory and neurodegenerative disease that is highest in areas close to ventricles and in proximity to CSF spaces (32,33). The presence of diffusible molecules in the CSF may explain greater neuroinflammation and neurodegeneration in periventricular areas. Locally secreted proinflammatory cytokines derived from meningeal and CSF compartments harbor B cells that reside within the CSF space, promoting greater regional disease (1,34). CSF-based ceramides have been shown to impair neuronal mitochondrial dysfunction and autophagy balance, leading to neuronal loss (35–37). Moreover, in patients with MS, atrophied T2 lesion volume may be partially a substrate of cardiovascular influence on the MS repair processes. Greater accumulation of destructive lesions has been shown in watershed regions, which are at greater risk of hypoperfusion. As the repair mechanisms involved in the lesions around the ventricles require a greater perfusion rate, the failure of such would lead to formation of T1 hypointensities and complete dissolvement into CSF. A recent study found greater central brain atrophy (increase in ventricular volume) in patients with cardiovascular comorbidities, which might be directly linked to greater atrophied T2 lesion volume (38). Related to this finding, our study was the first to include healthy control subjects. A minimal amount of atrophied T2 lesion volume was found in these study participants as well. This may be explained by the presence and evolution of white matter hyperintensities and periventricular bands in conjunction with age-related ventricular enlargement. The investigation of atrophied T2 lesion volume in relation to cerebrovascular disease may be the subject of future studies.
Our study had limitations that must be considered. We did not investigate the spatial predilection of lesion accumulation and atrophy development. However, it has been previously observed that atrophied lesions are mostly located in periventricular areas and at cortical gyri borders (13,15). We also did not investigate the proportion of atrophied T2 lesion volume that was represented by T1 black hole lesions, which are usually characterized by a higher rate of tissue destruction (39,40). Further studies using advanced MRI techniques are needed to explore the underlying mechanism leading to the accumulation of atrophied T2 lesion volume. Another important limitation of the method described is that it cannot be used to distinguish between different causes for tissue motion, such as harmful mechanisms like axonal loss and potentially benign mechanisms like reduction in edema. Although it is unlikely that any current MRI technique could enable one to fully disentangle these contributors, more sophisticated methods, such as Jacobian determinant mapping, might at least enable one to better distinguish edge motion from true atrophy and could be an important avenue of future research. Related to this point, the approach does not currently distinguish between lesions that have been truly subsumed into the CSF and those that have been displaced by ventricular expansion but not fully destroyed. More advanced methods may aid in unraveling whether these two phenomena are equally indicative of clinical disability.
In conclusion, in this large population-based cohort study, we showed that atrophied brain T2 lesion volume represents a viable predictive MRI marker of the development of disability progression and conversion to secondary progressive multiple sclerosis disease course.
SUPPLEMENTAL TABLES
Supported by National Center for Advancing Translational Sciences (UL1TR001412).
Disclosures of Conflicts of Interest: A.V.G. disclosed no relevant relationships. J.H. disclosed no relevant relationships. N.B. disclosed no relevant relationships. D.J. disclosed no relevant relationships. M.G.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received consulting fees from Claret Medical and EMD Serono, institution received grant support from Novartis and Celgene. Other relationships: disclosed no relevant relationships. D.P.R. disclosed no relevant relationships. A.A.L. disclosed no relevant relationships. D.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Biogen, Genentech, Novartis, and EMD Serono; gave lectures for Biogen, Genentech, Novartis, and EMD Serono. Other relationships: disclosed no relevant relationships. C.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the advisory board of EMD Serono, Biogen, Genentech, Teva, Novartis, and Mallinckrodt. Other relationships: disclosed no relevant relationships. B.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the board of Biogen, Novartis, EMD Serono, Genentech, and Mallinckrodt; is a consultant for Biogen, Novartis, EMD Serono, Genentech, and Mallinckrodt; received grants from Biogen, Novartis, EMD Serono, Genentech, and Mallinckrodt; gave lectures for Biogen, Novartis, EMD Serono, and Genentech. Other relationships: disclosed no relevant relationships. R.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Novartis, Sanofi, Celgene, and EMD Serono; institution received grants from Novartis, Sanofi, Celgene, Mapi Pharma, V-WVE Medical, and Protembis; gave lectures for Novartis, Sanofi, and EMD Serono. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CDMS
- clinically definite MS
- CI
- confidence interval
- CIS
- clinically isolated syndrome
- CSF
- cerebrospinal fluid
- DP
- disability progression
- EDSS
- Expanded Disability Status Scale
- FLAIR
- fluid-attenuated inversion recovery
- MS
- multiple sclerosis
- PBVC
- percentage brain volume change
- PVVC
- percentage ventricular volume change
- RRMS
- relapsing-remitting MS
- SPMS
- secondary progressive MS
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. . Multiple Sclerosis . N Engl J Med 2018. ; 378 ( 2 ): 169 – 180 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson AJ, Banwell BL, Barkhof F, et al . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria . Lancet Neurol 2018. ; 17 ( 2 ): 162 – 173 . [DOI] [PubMed] [Google Scholar]
- 3. Wattjes MP, Rovira À, Miller D, et al . Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients . Nat Rev Neurol 2015. ; 11 ( 10 ): 597 – 606 . [DOI] [PubMed] [Google Scholar]
- 4. Sormani MP, Bonzano L, Roccatagliata L, De Stefano N. . Magnetic resonance imaging as surrogate for clinical endpoints in multiple sclerosis: data on novel oral drugs . Mult Scler 2011. ; 17 ( 5 ): 630 – 633 . [DOI] [PubMed] [Google Scholar]
- 5. Healy BC, Buckle GJ, Ali EN, et al . Characterizing Clinical and MRI Dissociation in Patients with Multiple Sclerosis . J Neuroimaging 2017. ; 27 ( 5 ): 481 – 485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kearney H, Rocca MA, Valsasina P, et al . Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration . Mult Scler 2014. ; 20 ( 1 ): 72 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zivadinov R, Jakimovski D, Gandhi S, et al . Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine . Expert Rev Neurother 2016. ; 16 ( 7 ): 777 – 793 . [DOI] [PubMed] [Google Scholar]
- 8. De Stefano N, Airas L, Grigoriadis N, et al . Clinical relevance of brain volume measures in multiple sclerosis . CNS Drugs 2014. ; 28 ( 2 ): 147 – 156 . [DOI] [PubMed] [Google Scholar]
- 9. Popescu V, Agosta F, Hulst HE, et al . Brain atrophy and lesion load predict long term disability in multiple sclerosis . J Neurol Neurosurg Psychiatry 2013. ; 84 ( 10 ): 1082 – 1091 . [DOI] [PubMed] [Google Scholar]
- 10. Bermel RA, Bakshi R. . The measurement and clinical relevance of brain atrophy in multiple sclerosis . Lancet Neurol 2006. ; 5 ( 2 ): 158 – 170 . [DOI] [PubMed] [Google Scholar]
- 11. Jacobsen C, Hagemeier J, Myhr KM, et al . Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study . J Neurol Neurosurg Psychiatry 2014. ; 85 ( 10 ): 1109 – 1115 . [DOI] [PubMed] [Google Scholar]
- 12. Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. . Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients . Mult Scler 2016. ; 22 ( 10 ): 1327 – 1336 . [DOI] [PubMed] [Google Scholar]
- 13. Dwyer MG, Bergsland N, Ramasamy DP, Jakimovski D, Weinstock-Guttman B, Zivadinov R. . Atrophied Brain Lesion Volume: A New Imaging Biomarker in Multiple Sclerosis . J Neuroimaging 2018. ; 28 ( 5 ): 490 – 495 . [DOI] [PubMed] [Google Scholar]
- 14. Zivadinov R, Bergsland N, Dwyer MG. . Atrophied brain lesion volume, a magnetic resonance imaging biomarker for monitoring neurodegenerative changes in multiple sclerosis . Quant Imaging Med Surg 2018. ; 8 ( 10 ): 979 – 983 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zivadinov R, Horakova D, Bergsland N, et al . A Serial 10-Year Follow-Up Study of Atrophied Brain Lesion Volume and Disability Progression in Patients with Relapsing-Remitting MS . AJNR Am J Neuroradiol 2019. ; 40 ( 3 ): 446 – 452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahad DH, Trapp BD, Lassmann H. . Pathological mechanisms in progressive multiple sclerosis . Lancet Neurol 2015. ; 14 ( 2 ): 183 – 193 . [DOI] [PubMed] [Google Scholar]
- 17. Li DK, Held U, Petkau J, et al . MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability . Neurology 2006. ; 66 ( 9 ): 1384 – 1389 . [DOI] [PubMed] [Google Scholar]
- 18. Polman CH, Reingold SC, Banwell B, et al . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria . Ann Neurol 2011. ; 69 ( 2 ): 292 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lublin FD, Reingold SC, Cohen JA, et al . Defining the clinical course of multiple sclerosis: the 2013 revisions . Neurology 2014. ; 83 ( 3 ): 278 – 286 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurtzke JF. . Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) . Neurology 1983. ; 33 ( 11 ): 1444 – 1452 . [DOI] [PubMed] [Google Scholar]
- 21. Zivadinov R, Heininen-Brown M, Schirda CV, et al . Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis: a case-control study . Neuroimage 2012. ; 59 ( 1 ): 331 – 339 . [DOI] [PubMed] [Google Scholar]
- 22. Smith SM, Zhang Y, Jenkinson M, et al . Accurate, robust, and automated longitudinal and cross-sectional brain change analysis . Neuroimage 2002. ; 17 ( 1 ): 479 – 489 . [DOI] [PubMed] [Google Scholar]
- 23. Gelineau-Morel R, Tomassini V, Jenkinson M, Johansen-Berg H, Matthews PM, Palace J. . The effect of hypointense white matter lesions on automated gray matter segmentation in multiple sclerosis . Hum Brain Mapp 2012. ; 33 ( 12 ): 2802 – 2814 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vrenken H, Vos EK, van der Flier WM, et al . Validation of the automated method VIENA: an accurate, precise, and robust measure of ventricular enlargement . Hum Brain Mapp 2014. ; 35 ( 4 ): 1101 – 1110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tustison NJ, Avants BB, Cook PA, et al . N4ITK: improved N3 bias correction . IEEE Trans Med Imaging 2010. ; 29 ( 6 ): 1310 – 1320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nyúl LG, Udupa JK, Zhang X. . New variants of a method of MRI scale standardization . IEEE Trans Med Imaging 2000. ; 19 ( 2 ): 143 – 150 . [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Jenkinson M, Woolrich MW, et al . Advances in functional and structural MR image analysis and implementation as FSL . Neuroimage 2004. ; 23 ( Suppl 1 ): S208 – S219 . [DOI] [PubMed] [Google Scholar]
- 28. Zivadinov R, Uher T, Hagemeier J, et al . A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients . Mult Scler 2016. ; 22 ( 13 ): 1709 – 1718 . [DOI] [PubMed] [Google Scholar]
- 29. Lorscheider J, Buzzard K, Jokubaitis V, et al . Defining secondary progressive multiple sclerosis . Brain 2016. ; 139 ( Pt 9 ): 2395 – 2405 . [DOI] [PubMed] [Google Scholar]
- 30. Frischer JM, Weigand SD, Guo Y, et al . Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque . Ann Neurol 2015. ; 78 ( 5 ): 710 – 721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zivadinov R. . Can imaging techniques measure neuroprotection and remyelination in multiple sclerosis? Neurology 2007. ; 68 ( 22 Suppl 3 ): S72 – S82 ; discussion S91–S96 . [DOI] [PubMed] [Google Scholar]
- 32. Brown JW, Pardini M, Brownlee WJ, et al . An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis . Brain 2017. ; 140 ( 2 ): 387 – 398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Z, Pardini M, Yaldizli Ö, et al . Magnetization transfer ratio measures in normal-appearing white matter show periventricular gradient abnormalities in multiple sclerosis . Brain 2015. ; 138 ( Pt 5 ): 1239 – 1246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magliozzi R, Howell OW, Nicholas R, et al . Inflammatory intrathecal profiles and cortical damage in multiple sclerosis . Ann Neurol 2018. ; 83 ( 4 ): 739 – 755 . [DOI] [PubMed] [Google Scholar]
- 35. Vidaurre OG, Haines JD, Katz Sand I, et al . Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics . Brain 2014. ; 137 ( Pt 8 ): 2271 – 2286 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurz J, Parnham MJ, Geisslinger G, Schiffmann S. . Ceramides as Novel Disease Biomarkers . Trends Mol Med 2019. ; 25 ( 1 ): 20 – 32 . [DOI] [PubMed] [Google Scholar]
- 37. Czubowicz K, Wójtowicz S, Wencel PL, Strosznajder RP. . The role of ceramide and SEW 2871 in the transcription of enzymes involved in amyloid b precursor protein metabolism in an experimental model of Alzheimer’s disease . Folia Neuropathol 2018. ; 56 ( 3 ): 196 – 205 . [DOI] [PubMed] [Google Scholar]
- 38. Jakimovski D, Gandhi S, Paunkoski I, et al . Hypertension and heart disease are associated with development of brain atrophy in multiple sclerosis: a 5-year longitudinal study . Eur J Neurol 2019. ; 26 ( 1 ): 87 – e88 . [DOI] [PubMed] [Google Scholar]
- 39. van Waesberghe JH, Kamphorst W, De Groot CJ, et al . Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability . Ann Neurol 1999. ; 46 ( 5 ): 747 – 754 . [DOI] [PubMed] [Google Scholar]
- 40. Tam RC, Traboulsee A, Riddehough A, Sheikhzadeh F, Li DK. . The impact of intensity variations in T1-hypointense lesions on clinical correlations in multiple sclerosis . Mult Scler 2011. ; 17 ( 8 ): 949 – 957 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.